Abstract
Diabetes mellitus (DM) is one of the most common complications in patients with ulcerative colitis (UC). Curcumin has a wide range of bioactive and pharmacological properties and is commonly used as an adjunct to the treatment of UC and DM. However, the role of curcumin in UC complicated by DM has not been elucidated. Therefore, this study was conducted to construct a model of UC complicating diabetes by inducing UC in DB mice (spontaneously diabetic) with dextran sodium sulfate. In this study, curcumin (100 mg/kg/day) significantly improved the symptoms of diabetes complicated by UC, with a lower insulin level, heavier weight, longer and lighter colons, fewer mucosal ulcers and less inflammatory cell infiltration. Moreover, compared to untreated DB mice with colitis, curcumin‐treated mice showed weaker Th17 responses and stronger Treg responses. In addition, curcumin regulated the diversity and relative abundance of intestinal microbiota in mice with UC complicated by DM at the phylum, class, order, family and genus levels. Collectively, curcumin effectively alleviated colitis in mice with type 2 diabetes mellitus by restoring the homeostasis of Th17/Treg and improving the composition of the intestinal microbiota
Keywords: colitis, curcumin, intestinal microbiota, Th17/Treg, type 2 diabetes mellitus
Abbreviations
- AUG
the area under the blood glucose curve
- CCR6
CC chemokine receptor 6
- CD
Crohn's disease
- CMC
carboxymethylcellulose
- Cur
curcumin
- DAI
disease activity index
- DB
C57BLKS/J(−/−) mice
- DM
diabetes mellitus
- DSS
dextran sodium sulfate
- ELISA
enzyme‐linked immunosorbent assay
- IBD
inflammatory bowel disease
- NMDS
non‐metric multidimensional scaling
- IPGTT
intraperitoneal glucose tolerance test
- OTU
operational taxonomic unit
- PCoA
principal co‐ordinates analysis
- PFA
paraformaldehyde
- PLS‐DA
partial least squares discriminant analysis
- RCTs
randomized controlled trials
- RIPA
radio immunoprecipitation assay
- T2DM
type 2 diabetes mellitus
- Th17
T helper cell 17
- Treg
regulatory T cells
- UC
ulcerative colitis
1. INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic inflammatory disease that includes Crohn's disease (CD) and ulcerative colitis (UC) (Caruso, Lo, & Nunez, 2020). CD affects the entire intestinal wall, while UC invades only the intestinal mucosa (Pittayanon et al., 2020). Diabetes mellitus (DM), usually classified as type 1 and type 2 diabetes mellitus (T2DM), is a chronic, relapsing metabolic disorder disease (Antonioli, Blandizzi, Csoka, Pacher, & Hasko, 2015; Yong, Johnson, Arvan, Han, & Kaufman, 2021). As healthcare grows and the number of patients with chronic diseases increases, there is the inevitable challenge of managing patients with multiple co‐morbidities (Tsai et al., 2015). DM is one of the most common co‐morbidities in people with UC (Maconi et al., 2014). It was reported that 5% of 2,810 patients with IBD had co‐morbid diabetes (Din et al., 2020) and that DM can also affect IBD outcomes and prognosis (Uwagbale et al., 2021). Meta‐analysis found that patients with CD or UC from specific regions may have a higher risk of T1DM than those without IBD, and a cohort study from Denmark showed an increased risk of T2DM in patients with IBD (incidence ratio 1.54; 95% confidence interval 1.49–1.60) (Jess, Jensen, Andersson, Villumsen, & Allin, 2020). These results are consistent with another cohort study from Korea, which found a similar association between IBD and DM (hazard ratio, 1.135; 95% confidence interval, 1.048–1.228) (Lai, Kuo, & Liao, 2020).
Moreover, DM and UC interfere with each other in the course of their treatment, which in turn affects the prognosis. Diabetes and hyperglycemia increase the risk of post‐operative complications and bowel pouch failure in UC. Dipeptidyl peptidase 4 inhibitors are the main drugs used in the clinical treatment of T2DM (Radel, Pender, & Shah, 2019), however, they can exacerbate the immune response thereby increasing the risk of IBD. Medical treatment of UC in diabetic patients is also particularly challenging (Din et al., 2020). Corticosteroids are the treatment of choice for active UC and the prolonged use of glucocorticoids in the treatment of UC can induce hyperglycemia and even diabetes (Curkovic, Egbring, & Kullak‐Ublick, 2013). Induction of hyperglycemia should be avoided during UC therapy, while the risk of UC should be considered during diabetes therapy. Therefore, the development of safe and effective treatments for diabetes co‐morbidity in UC is need.
Curcumin (Cur), diarylmethane, is the main polyphenol of the rhizome of C. longa and is a common herbal ingredient in many Asian dishes (Kotha & Luthria, 2019). In vitro and in vivo studies have reported a wide range of biopharmacological effects of Cur, including antioxidant, cardioprotective, anti‐inflammatory, anti‐microbial, renoprotective, anti‐tumor, hepatoprotective, immunomodulatory, hypoglycaemic and anti‐rheumatic effects (Burge, Gunasekaran, Eckert, & Chaaban, 2019; Patel et al., 2020). Previous studies revealed that Cur has the ability to inhibit inflammatory cell proliferation (Vecchi et al., 2014), invasion and angiogenesis and to mediate its anti‐inflammatory effects by down‐regulating inflammatory transcription factors, cytokines, redox status, protein kinases and enzymes (Shehzad, Rehman, & Lee, 2013; Sreedhar, Arumugam, Thandavarayan, Karuppagounder, & Watanabe, 2016). There is some evidence that supplements containing curcumin can inhibit the inflammatory response and analgesic effects in patients with IBD (Canistro et al., 2021; Razavi, Ghasemzadeh, & Hosseinzadeh, 2021). Mesalazine is a commonly used drug for the treatment of UC, and Cur supplementation is clinically safe and effective in relieving the development of colitis and preventing recurrence (Lang et al., 2015). In addition, a number of randomized controlled trials (RCTs) have shown that Cur improves blood glucose and reduces insulin resistance in subjects with pre‐diabetes or T2DM (Asadi et al., 2019). Notably, DM is one of the most common complications in patients with UC (Maconi et al., 2014). However, the role of Cur in UC complicated by DM has not been elucidated.
The balance between regulatory T cells (Treg) and helper T cell 17 (Th17) plays an important role in the adaptive immune response (Zhang et al., 2021c), antagonizing each other, and a breakdown in the balance between the two can lead to immune disease, including UC (Yan, Luo, Chen, & He, 2020) and T2DM (Zhang et al., 2014). In recent years, targeted modulation of Th17/Treg cell homeostasis has been an important strategy for the treatment or prevention of UC. Our previous study also demonstrated that Cur effectively alleviated DSS‐induced colitis by restoring Th17/Treg balance (Zhong et al., 2021). Recent evidence supports a clear role for Th17/Treg imbalance in the etiology of T2DM (Tao, Liu, & Gong, 2019), with patients exhibiting increased activation and proportion of Th17 and a significantly reduced proportion of Treg cells (Wang et al., 2018).
The gut microbiota consists of a large number of cells that are known as the body's second gene pool and form a complex symbiotic relationship with the host (Zhou et al., 2020). The intestinal microbiota plays a key role in maintaining healthy host homeostasis, and imbalances in its composition often involve in immune and metabolic disorders, such as colitis (Glassner, Abraham, & Quigley, 2020) and T2DM (Zhang et al., 2021a). Decreased diversity and imbalanced composition in the gut microbiota are typical of patients with CD and UC (Alam et al., 2020), the relative abundance of the beneficial bacteria Candidatus_Saccharimonas (Ge et al., 2021) and Eubacterium_xylanophilum_group were significantly reduced. Recent studies have shown that dysbiosis of the intestinal microbiota is also involved in the pathogenesis of T2DM. Disruptions in the relative proportions of gut microbial populations may contribute to insulin resistance, including alterations in Gammaproteobacteria and Verrucomicrobia, increased ratios of Firmicutes to Bacteroidetes, possible alterations in butyrate‐producing bacteria such as Faecalibacterium prausnitzii in DM (Barlow, Yu, & Mathur, 2015). Based on some clinical and preclinical evidence, improving the composition of the host intestinal microbiota is being considered as a potential new target for regulating the homeostasis of the internal environment. Our previous study demonstrated that Cur modulated the composition of the intestinal microbiota in DSS‐induced colitis mice (Zhong et al., 2021). Although Cur exhibits protective effects in patients with UC and DM, the treatment of UC complicated by DM has not been well studied. Therefore, in this study, we investigated the protective effects of Cur against UC with T2DM and its potential mechanisms of action
2. MATERIALS AND METHODS
2.1. Mice
Seven‐week‐old male C57BLKS/J(−/−) (DB) mice weighing 33–39 g were purchased from GemPharmatch Co. Ltd. (Nanjing, China) (Animal production license SCXK(Su) 2018‐0008) and housed in specific pathogen‐free conditions in Laboratory Animal Science and Technology Center at Jiangxi University of Traditional Chinese Medicine (Animal use license SYXK(Gan)2017‐0004). The protocol (Permit Number: JZLLSC2020‐334) was approved by the Jiangxi University of Traditional Chinese Medicine Animal Care and Use Committee and performed in accordance with the guidelines prescribed by the committee. All animals were acclimatized for 3 days before the experimental studies were performed.
2.2. Drugs
Cur (batch NO. GR‐133–140,421; purity ≥98% by high performance liquid chromatography; Formula, C21H20O6) (Figure S1) was obtained from GANGRUN Biotechnology (Nanjing, China), and dextran sodium sulfate (DSS) (batch NO. 160110; molecular weight: 36,000–50,000 Da) was obtained from MP Biomedicals (Santa Ana, CA). We asserted that the requirements considered relevant in recent best practice guidelines for pharmacological studies of natural products have been taken into account (Heinrich et al., 2020; Izzo et al., 2020).
2.3. Experimental colitis design
DB mice were acclimatized for 3 days and their blood glucose concentrations were measured twice. When the blood glucose concentration of the mice exceeded 16.7 mmol/L on both occasions (Liang et al., 2018), they were considered diabetic for inclusion in subsequent experiments. In conjunction with our previous studies (Kang et al., 2021) and with appropriate adjustments, DB mice were given 1.5% (w/v) DSS solution ad libitum to induce experimental colitis. Detailed experimental treatments are shown in Figure 1. Fresh 1.5% (w/v) DSS solutions were prepared every morning. The mice in the Control and Con+Cur groups received normal drinking water.
FIGURE 1.
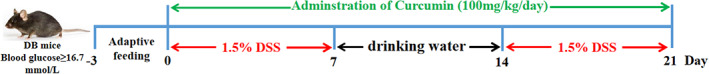
Colitis induction and curcumin (Cur) administration. The experiment lasted for 24 days, including 3 days of adaptive feeding, 2 × 7 days of 1.5% DSS treatment in drinking water and 7 days of free drinking water. Animals were divided into four groups: Control (n = 10), DSS (n = 14), DSS + Cur (100 mg/kg/day, n = 13) and Con+Cur (100 mg/kg/day, n = 10) groups. Cur was orally administered. Cur, Curcumin
2.4. Therapeutic protocols
Before administration, Cur was dissolved in 1.5% (w/v) sodium carboxymethylcellulose (CMC) solution at a dose of 100 mg/kg (purity >95% by HPLC) (Zhong et al., 2020; Zhong et al., 2021). Throughout the experiment, mice in the DSS + Cur and Con+Cur groups were orally administered with 100 mg/kg/day Cur and for 21 consecutive days, and mice in the DSS and Control groups were treated with equal volume of saline. On day 21, all mice were sacrificed under sodium pentobarbital (20 mg/kg i.p.) anesthesia.
2.5. Disease activity index (DAI)
The percentage of weight loss, changes in stool states including consistency, and blood presence in the stool of mice were evaluated daily and scored for each mouse over the experimental period. DAI scores = weight loss rate score + faecal consistency score + blood in stool score. DAI score was estimated using the following parameters: rate of weight loss (no significant loss, 0; loss of 1–5%, 1; loss of 6–10%, 2; loss of 11–20%, 3; decline of more than 20%, 4), degree of loose stools (normal stools, 0; loose stools (dry), 2; loose stools or diarrhea (watery stools), 4) and degree of bleeding (no bleeding, 0; positive fecal occult blood, 2; blood in stools, 3; anal bleeding, 4).
2.6. Histological evaluation
According to the literature (Chen et al., 2021), 2 cm of proximal colon was rapidly isolated in 4% paraformaldehyde (PFA) (Sorabio, Beijing, China) overnight at 4°C, dehydrated in an alcohol gradient (from 50% to 100%), xylene transparent, and paraffin embedded. The tissue was then cut into 4 μm thick slices. After dehydration in xylene and rehydration, sections were stained with hematoxylin (Sorabio, Beijing, China), dehydrated in an alcohol gradient (50–95%), stained with eosin (Sorabio, Beijing, China), dehydrated in 100% alcohol, cleared in xylene and sealed in neutral resin. Samples were imaged by biomicroscopy (Leica, Wetzlar, Germany).
2.7. Intraperitoneal glucose tolerance test (IPGTT)
In order to evaluate insulin resistance, fasting blood glucose concentration was measured in tail vein of mice after 12 hr fasting. Glucose was injected intraperitoneally (1.0 g/kg), and the level of blood glucose was measured at time points of 30, 60, 90 and 120 min, post injection. The area under the blood glucose curve (AUG) was calculated based on the IPGTT results.
2.8. Enzyme‐linked immunosorbent assay (ELISA)
The concentrations of serum insulin were analyzed using a commercial ELISA kit (Thermo Fisher Scientific, Waltham city, MA) according to the manufacturer's protocol. To evaluate the Th17/Treg immune response, Radio Immunoprecipitation Assay (RIPA) buffer (Cell Signaling Technology, Danvers, MA) was used to lyse colon tissue and the supernatant was extracted at 13000 rpm for 30 min at 4°C. The levels of IL‐17A and IL‐10 were measured in mouse colon tissue using a commercial ELISA kit (Thermo Fisher Scientific, Waltham city, MA).
2.9. RNA and real‐time PCR
The middle colon was taken in TRPIquick reagent and homogenized in a tissue homogenizer. Total RNA was extracted, nucleic acid electrophoresis was performed to detect RNA quality, and reverse transcription was performed to cDNA using TRUEscript first Strand cDNA Synthesis kit (Aidlab Biotechnlologies Co., Ltd., Beijing, China) according to the manufacturer's instructions. cDNA was normalized to the same concentration. Diluted cDNAs were used for real‐time PCR with SYBR Green reagents (Mei5 Biotechnology, Co., Ltd., Beijing, China) on a bioanalyzer (Roche LightCyler96, IN). The primer sequences are shown in Table S1. β‐actin was used as the housekeeping gene and the expression level of the target gene was calculated using the 2−ΔΔCt method.
2.10. Flow cytometry
Rapidly open the abdominal cavity and isolate the mesenteric lymph nodes on ice. Lymphocyte isolation solution (Cat No: MLSM1092, Multi Sciences Biotech, Co.,Ltd., Hangzhou, China) was used to isolate and prepare mononuclear cells from the mesenteric lymph nodes. Resuspend the precipitate in medium containing 10% fetal bovine serum to a concentration of 1 × 107/ml. 250 μl of the cell suspension is taken into a flow tube, followed by stimulation with leukocytes activation cocktail (InvivoGen, 00‐4975‐93) at 37°C in 5% CO2 for 4–6 hr. Then, the cells were fixed and permeabilized using a Cytofix/Cytoperm kit (BD Biosciences, Franklin Lakes, NJ, USA), followed by incubation with primary antibodies for 40 min at 4°C in the dark. Finally, these stained cells were subjected to FACSCanto II flow cytometry (BD Biosciences, Franklin Lakes, NJ). The following mAbs were used: BV510 rat anti‐mouse CD4 (1:200), PE‐Cy7 rat anti‐mouse Foxp3 (1:100), PE‐Cy7 rat anti‐mouse IL‐10 (1:100) and BV421 rat anti‐mouse CCR6 (1:100) (BD Biosciences, Franklin Lakes, NJ). Limits of quadratic labelling were set according to negative populations and isotype controls. Data were analyzed using FlowJo software V10 (TreeStar, Ashland, OR) to exclude inactive cells by gating.
2.11. Colonic microbiota analysis
Colonic cecal of all mice were collected in cryopreservation tubes and preserved at −80°C. The colonic microbiota analysis was conducted by the Majorbio Bio‐Pharm Technology (Shanghai, China). According to the manufacturer's instructions, cecal DNA was extracted using the QIAamp DNA Stool Mini Kit (Qiagen, Valencia, CA). The extracted DNA was used as template to amplify the V3‐V4 region of the bacterial 16S rRNA gene with primers: 338F (5′‐ACTCCTACGGGAGGCAGCAG‐3′) and 806R (5′‐GGACTACHVGGGTWTCTAAT‐3′). The PCR reaction consisted of 4 μl of 5 × FastPfu Buffer, 2 μl of 2.5 mM dNTPs, 0.8 μl of forward primer (5 μM), 0.8 μl of reverse primer (5 μM), 0.4 μl of FastPfu Polymerase (TransStart FastPfu DNA Polymerase, TransGen Biotech, Beijing, China), and 10 ng of template DNA. The amplification was performed on an Applied Biosystems PCR system (GeneAmp 9,700, ABI) as follows: 3 min of denaturation at 95°C, 27 cycles of 30 s at 95°C, 30 s at 55°C and 45 s at 72°C, with a final elongation at 72°C for 10 min. PCR products were purified using the AxyPrep DNA gel extraction kit (Axygen, Union City). High‐throughput sequencing was carried out on a MiSeq platform (Illumina, San Diego, CA, USA) according to the standard protocols by Majorbio Bio‐Pharm Technology. The quality control of raw sequence reads, deposited in the NCBI Sequence Read Archive (SRA) database (Accession Number: PRJNA655607), was conducted by FASTQC on FASTQ files. The operational taxonomic units (OTUs) were clustered with 97% similarity cutoff using UPARSE (version 7.1). The taxonomy of each 16S rRNA gene sequence was assigned by the Ribosomal Database Project (RDP) Classifier algorithm (http://rdp.cme.msu.edu/) against the SILVA (SSU123) 16S rRNA database using a confidence threshold of 70%. The sobs index and the Shannon index were calculated using Mothur (version v.1.30.1) to evaluate alpha diversity. Principal coordinates analysis (PCoA) was performed using Mothur, and statistical analysis was performed based on the values of PC1. Linear discriminant analysis (LDA) coupled with effect size (LEfSe) measurements (based on non‐parametric factorial Kruskal–Wallis sum‐rank test and the Wilcoxon rank‐sum test) was used to identify taxa that were significantly different (biomarkers) among groups, with p < 0.05 and an LDA score threshold of 4. Microbial difference analysis, correlation analysis, and co‐occurrence network analysis were performed using I‐sanger (Majorbio Bio‐Pharm Technology Co. Ltd.; www.i-sanger.com).
2.12. Statistical analysis
All data were expressed as mean ± standard error of the mean (SEM), and GraphPad Prism software version 8.0 (GraphPad Software Inc., La Jolla, CA, USA) was used for statistical analysis and plotting. One‐way analysis of variance (ANOVA), followed by Tukey–Kramer multiple comparisons test, was used to analysis significant difference among these four groups. All p < 0.05 was considered as statistically significant.
3. RESULTS
3.1. Curcumin inhibits DSS‐induced colitis in DB mice
To investigate the efficacy and action mechanism of Cur in colitis complicated by diabetes, spontaneously diabetic (DB) mice were selected and colitis was induced by the DSS free‐drinking method. First, we collected blood from the tail vein 2 times to detect the concentration of blood glucose before DSS induction. When the concentration of blood glucose was higher than 16.7 mmol/L on both occasions (Figure S2a), the DB mice were included in the subsequent experiments. Based on our previous study (Zhong et al., 2020; Zhong et al., 2021), DB mice were given 3% (w/v) DSS solution ad libitum and showed typical clinical symptoms of colitis starting from day 4 of the experiment, with significant weight loss (Figure S2b), anal blood (Figure S2c), generalized tremors and bradycardia, and all died on day 9 (Figure S2d), and post‐mortem examination revealed massive colonic bleeding (Figure S2e). This suggests that diabetes increases susceptibility to colitis in mice and is closely associated with deletion of the leptin receptor gene.
Subsequently, 1.5% (w/v) DSS in drinking water were used to induce colitis. Throughout the experiment, mice monitored daily for their body weight, stool consistency or diarrhea and presence of gross or occult blood in the rectum or stool. On days 3 to 21, the body weight of the DSS group was significantly lower than that of the Control group (Figure 2a). The survival rate of mice in the DSS group was lower than that of the Control group (Control group vs DSS group: 100% vs 64.3%) (Figure 2b). On the last day, mice were deeply euthanized and sacrificed, their colon was quickly separated, its length measured and weighed. Compared to the Control group, the DSS group showed a significant decrease in colonic length (Figure 2d, e) and a significant increase in colonic weight/colonic length (Figure 2g). Light microscopy (Figure 2h) revealed that the colonic mucosal epithelium of DB mice with colitis was detached, local ulcers were formed and a large number of inflammatory cells were infiltrated; in addition, the histopathological injury scores of the colon in the DSS group (Figure 2i) were significantly higher than that in the Control group.
FIGURE 2.
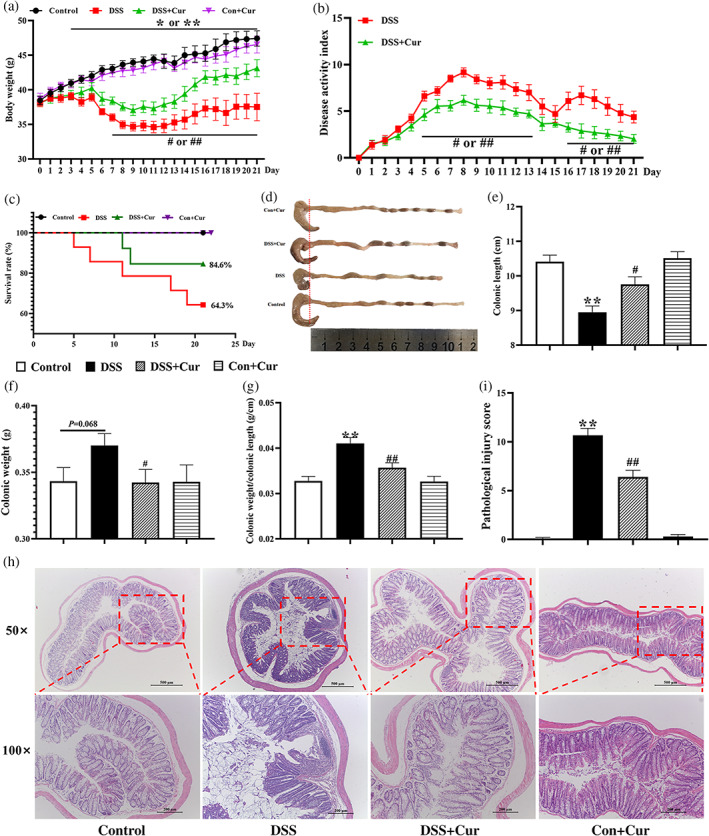
Curcumin (Cur) inhibited DSS‐induced colitis in DB mice. (a) Body weight. (b) Disease activity index (DAI). (c) Survival rate. (d) Changes in colonic length by naked eye. (e) Colonic length. (f) Colonic weight. (g) Colonic weight/Colonic length. (h) hematoxylin and eosin staining of colon (magnification: 50× or 100×). (i) Pathological injure score. Data were presented as mean ± SEM (n = 8–14). * p < 0.05 and ** p < 0.01 compared to the Control group; # p < 0.05 and ## p < 0.01 compared to the DSS group
After the simultaneous administration of Cur, the body weight of the DSS + Cur group (Figure 2a) was significantly higher than that of the DSS group from day 7 to day 21 of the experiment; the DAI of the DSS + Cur group (Figure 2b) was significantly lower than that of the DSS group from day 5 to day 13 and from day 16 to day 21; the survival rate of mice in the DSS + Cur group (Figure 2c) was higher than that of the DSS group (DSS + Cur group vs DSS group: 84.6% vs 64.3%). Compared to the DSS group, the DSS + Cur group showed a significant increase in colonic length (Figure 2d, e), and a significant decrease in colonic weight (Figure 2f) and colonic weight/colonic length (Figure 2g). The colonic mucosal epithelium of Cur‐treated DB mice with colitis was more intact, with occasional small infiltration of inflammatory cells (Figure 2h), and their pathological injure scores (Figure 2i) were also significantly lower than that of untreated DB mice with colitis. The above study showed that Cur was effective in alleviating DSS‐induced colitis in DB mice.
3.2. Curcumin regulates the levels of blood glucose and insulin in DB mice with colitis
To investigate the effects of Cur on the role of blood glucose and insulin in DB mice with colitis, the concentrations of blood glucose were measured by a Roche blood glucose meter (Figure 3a) and insulin levels were measured using an ELISA method (Figure 3b). On days 7 and 21, the concentrations of blood glucose in the DSS group (Figure 3a) were significantly lower than those in the Control group. Importantly, the concentrations of blood glucose (Figure 3a) were significantly lower in the DSS + Cur group than in the DSS group on day 14. The concentration of serum insulin in the DSS group (Figure 3b) was significantly higher than that in the Control and DSS + Cur groups. In addition, the intraperitoneal glucose tolerance test (IPGTT) was performed to observe the effect of Cur on disease‐related biomarkers in the serum of the DSS‐induced colitis in DB mice. The IPGTT curve is shown in Figure 3c and its area under the curve (AUC) is shown in Figure 3d. The AUC was obviously decreased in the DSS + Cur group, compared to the DSS group.
FIGURE 3.
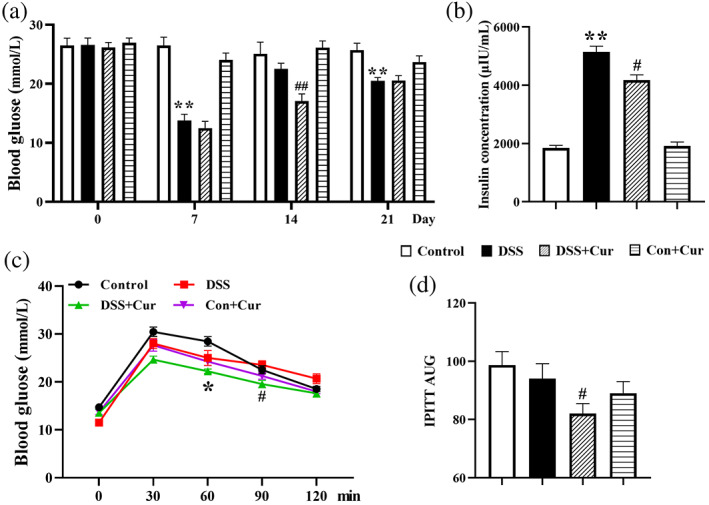
Curcumin (Cur) regulated the levels of blood glucose and insulin in DB mice with colitis. (a) Blood glucose concentrations of DB mice on days 0, 7, 14 and 21. (b) Insulin levels in serum of DB mice on day 21. (c) Intraperitoneal glucose tolerance test (IPGTT). (d) Area under the curve (AUC). Data were presented as mean ± SEM (n = 8–14). * p < 0.05 and ** p < 0.01 compared to the Control group; # p < 0.05 and ## p < 0.01 compared to the DSS group
3.3. Curcumin suppresses Th17 response in DB mice with colitis
Th17 cells are characterized by the production of pro‐inflammatory cytokines IL‐17A, and play a major role in autoimmune diseases, including UC (Bunte & Beikler, 2019) and DM (Abdel‐Moneim, Bakery, & Allam, 2018). CC chemokine receptor 6 (CCR6) is a specific marker for Th17 cells distinguishing them from other helper T cells (Sundrud & Trivigno, 2013). Compared to the Control group, the percentages of CD4+CCR6+ (Figure 4a, b) and CD4+IL‐17A+ (Figure 4c, d) Th17 cells in the mesenteric lymph nodes of the DSS group increased significantly, as did the concentration and the mRNA level of the cytokine IL‐17A (Figure 4e, f) secreted by Th17 cells. The key nuclear transcription factors BATF, C‐Maf, RORγt and RORɑ regulate Th17 cell differentiation and IL‐17 secretion (Lin et al., 2019). Compared with the Control group, the mRNA levels of BATF (Figure 4g), C‐Maf (Figure 4h), RORγt (Figure 4i) and RORɑ (Figure 4j) were significantly increased in the colonic tissues of the DSS group.
FIGURE 4.
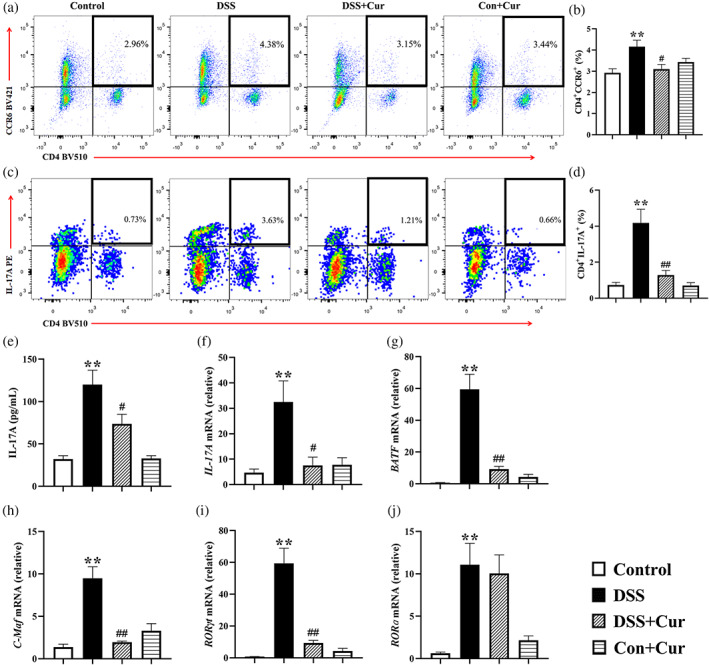
Curcumin (Cur) suppressed Th17 cell response in DB mice with colitis. (a) Percentage of CD4+CCR6+ Th17 cells. (b) Percentage of CD4+IL‐17A+ Th17 cells. Statistical analysis of CD4+CCR6+ Th17 cells (c) and CD4+IL‐17A+ Th17 cells (d) among the Control, DSS, DSS + Cur, Con+Cur groups. (e) The concentration of IL‐17A in colonic tissue. (f) The mRNA levels of IL‐17A in colonic tissue. The mRNA levels of Th17 cells‐related nuclear transcription factors BATF (g), C‐Maf (h), RORγt (i) and RORɑ (j) in colon tissue. Data were presented as mean ± SEM (n = 8–11). * p < 0.05 and ** p < 0.01 compared to the Control group; # p < 0.05 and ## p < 0.01 compared to the DSS group
After simultaneous administration of Cur, the percentages of CD4+CCR6+ (Figure 4a, b) and CD4+IL‐17A+ (Figure 4c, d) Th17 cells were significantly lower in the DSS + Cur group than in the DSS group. Compared with the DSS group, the concentration of IL‐17A (Figure 4e) was significantly lower in the colon tissue of the DSS + Cur group, as was its mRNA level (Figure 4f). Meanwhile, the mRNA levels of BATF (Figure 4g), C‐Maf (Figure 4h) and RORγt (Figure 4i) were significantly lower in the colon tissues of the DSS + Cur group than those of the DSS group. The above study showed that Cur inhibited the Th17 cell response in DB mice with colitis.
3.4. Curcumin promotes Treg response in DB mice with colitis
Treg cells play a critical role in maintaining self‐tolerance and promote the secretion of the key anti‐inflammatory cytokine IL‐10, which contributes to the relief of colitis (Izcue, Coombes, & Powrie, 2006) and DM (Qiao et al., 2016). Compared with the Control group, the percentages of CD4+Foxp3+ (Figure 5a, b) and CD4+IL‐10+ Treg cells (Figure 5c, d) in the mesenteric lymph nodes of the DSS group were significantly decreased. In addition, the concentrations and mRNA levels of IL‐10 (Figure 5e, f) were significantly lower in colonic tissues of the DSS group than those of the DSS + Cur group. The mRNA levels of Foxp3 (Figure 5g) and Eomes (Figure 5h), the key transcription factors regulating Treg cell differentiation and their cytokine secretion, were significantly decreased in colonic tissues of the DSS group than those of the DSS group.
FIGURE 5.
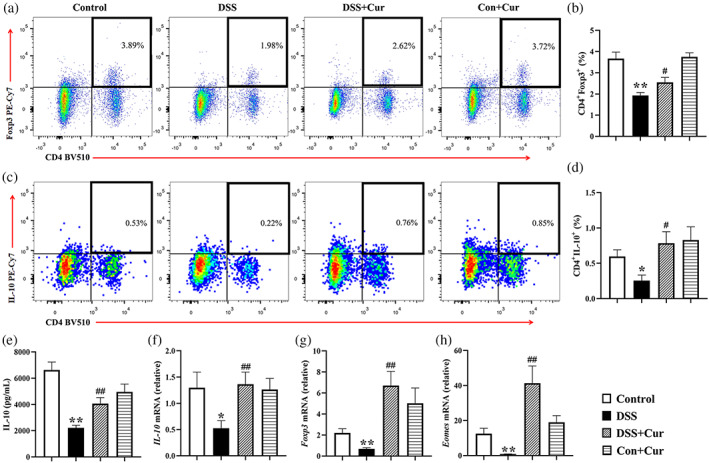
Curcumin (Cur) promoted Treg response in DB mice with colitis. (a) Percentage of CD4+Foxp3+ Treg cells. (b) Percentage of CD4+IL‐10+ Treg cells. Statistical analysis of CD4+Foxp3+ (c) and CD4+IL‐10+ (d) Treg cells among the Control, DSS, DSS + Cur, Con+Cur groups. (e) The concentration of IL‐10 in colonic tissue. (f) The mRNA levels of IL‐10 in colonic tissue. The mRNA levels of Treg cells‐related nuclear transcription factors Foxp3 (g) and Eomes (h) in colon tissue. Data were presented as mean ± SEM (n = 8–11). * p < 0.05 and ** p < 0.01 compared to the Control group; # p < 0.05 and ## p < 0.01 compared to the DSS group
After Cur was administered simultaneously for 21 days, the percentages of CD4+Foxp3+ (Figure 5a, b) and CD4+IL‐10+ Treg cells (Figure 5c, d) in the mesenteric lymph nodes of the DSS + Cur group were significantly higher than that of the DSS group, and their secreted cytokine IL‐10 (Figure 5e, f) was significantly lower in colon tissue in terms of concentration and mRNA levels. In addition, the mRNA levels of Foxp3 (Figure 5g) and Eomes (Figure 5h) in the DSS + Cur group were significantly higher than those in the DSS group. These studies suggested that Cur promoted Treg cell differentiation and effector functions in DB mice with colitis.
3.5. Curcumin improves the composition of intestinal microbiota in DB mice with colitis
A growing amount of evidence indicates that disturbed intestinal microbiota is implicated in different disease conditions, such as IBD (Martinez‐Guryn, Leone, & Chang, 2019) and T2DM (Lee, Chae, Jo, Jerng, & Bae, 2021). Recently, improving the composition of the intestinal microbiota is an effective measure in the treatment of IBD (Kedia, Rampal, Paul, & Ahuja, 2016) and DM (Wu et al., 2020). The rarefaction curve of Sobs index on the operational taxonomic unit (OTU) level (Figure 6a) flattens out and the sequencing data reach saturation, covering the vast majority of species in the gut microbiome community of DB mice. Single sample diversity (Alpha diversity) analysis reflects the abundance and diversity of the microbial community, including the microbial richness index Sobs (Figure 6b) and the microbial diversity index Shannon (Figure 6c). As shown in Figure 6b, there was a significant difference in sobs index on OTU level between the DSS group and the Control, DSS + Cur, and Con+Cur groups, respectively. Although there was no significant difference in Shannon index on OTU level (Figure 6c) among Control, DSS, DSS + Cur, and Con+Cur groups, Shannon index on OTU level was lower in the DSS group than in the Control, and DSS + Cur groups. Subsequently, Venn diagram analysis at the OTU level (Figure 6d) showed differences in the composition of the intestinal microbiota among the four groups, 633, 610, 647 and 640 OTUs in the Control, DSS, DSS + Cur and DSS + 5‐ASA groups, respectively. In addition, β‐diversity analyses were used to assess the differences in diversity of the intestinal microbiota among these four groups, including principal co‐ordinates analysis (PCoA) (Figure 6e) and non‐metric multidimensional scaling (NMDS) (Figure 6f). The PCoA (Figure 6e) and NMDS (Figure 6f) analyses showed that the distribution of species in the DSS and DSS + Cur groups was separate from the Control group, while the distance between the DSS + Cur group and the Control group were smaller than the distance between the DSS and Control groups.
FIGURE 6.
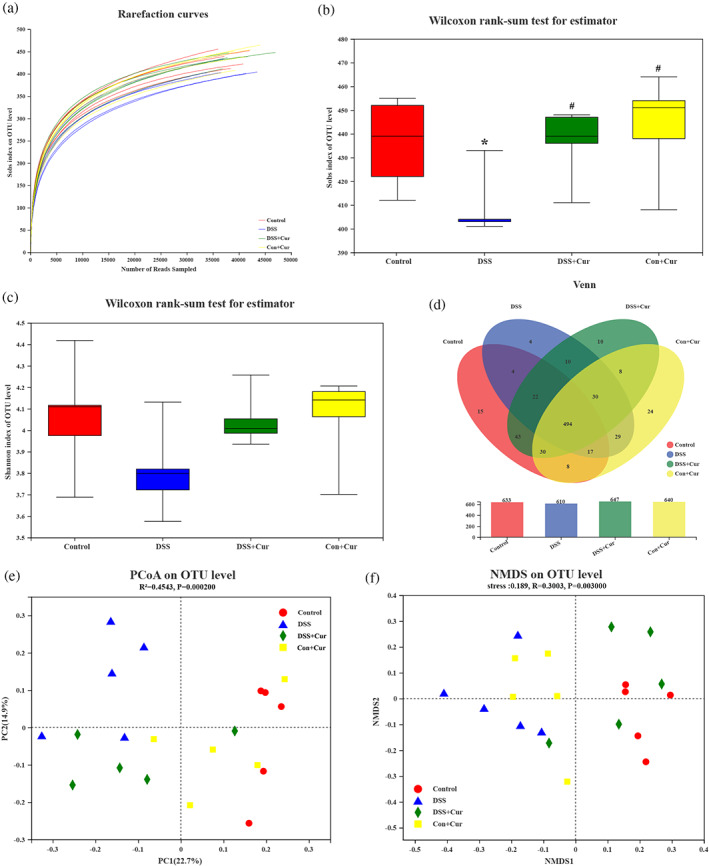
Curcumin (Cur) improved the composition of gut microbiota in DB mice with colitis. (a)The rarefaction curve of Sobs index on the operational taxonomic unit (OTU) level. Box plots indicate microbiome diversity differences of sobs index (b), and Shannon index (c) on OTU level followed with Wilcoxon rank‐sum test. (d) The Venn diagram depicted OTUs that differed in each group. (e) Partial least squares discriminant analysis (PLS‐DA) score on OTU level. (f) Non‐metric multidimensional scaling (NMDS) on OTU level. n = 5
3.6. Correlation analysis of intestinal microbiota
Based on the effective effects of Cur on Th17/Treg and intestinal microbiota in DB mice with colitis, Spearman correlation heat map and redundancy analysis (RDA) were used to further explore the correlation between Th17, Treg cells and intestinal microbiota. The Spearman correlation heat map at the genus level (Figure 7d) showed that Roseburia was negatively correlated with CD4+17A+Th17 cells and positively correlated with CD4+IL‐10+Treg cells; Erysipelatoclostridum was positively correlated with CD4+17A+Th17 cells and negatively correlated with CD4+IL‐10+Treg cells; Roseburia was negatively correlated with CD4+CCR6+Th17 cells and positively correlated with CD4+Foxp3+Treg cells; norank_f_Oscillospiraceae was positively correlated with CD4+CCR6+Th17 and negatively correlated with CD4+Foxp3+Treg. Meanwhile, CD4+IL‐17A+Th17, CD4+CCR6+Th17, CD4+IL‐10+Treg, CD4+Foxp3+Treg, colon index and pathological injury score were used as environmental factors to analyse their correlation with the intestinal microbiota. According to RDA analysis (Figure 7e), the abundance of CD4+IL‐17A+Th17 and CD4+CCR6+Th17 were consistent with the intestinal microbiota of the DSS group, while the abundance of CD4+IL‐10+Treg and CD4+Foxp3+Treg cells were consistent with the intestinal microbiota of the Control, DSS + Cur and Con+Cur groups. Regression analysis (Figure 7f, g) further revealed that CD4+IL‐17A+Th17 and CD4+CCR6+Th17 were inversely correlated with sobs, whereas CD4+IL‐10+Treg (Figure S4a) and CD4+Foxp3+Treg (Figure S4b) were not significantly correlated with sobs.
FIGURE 7.
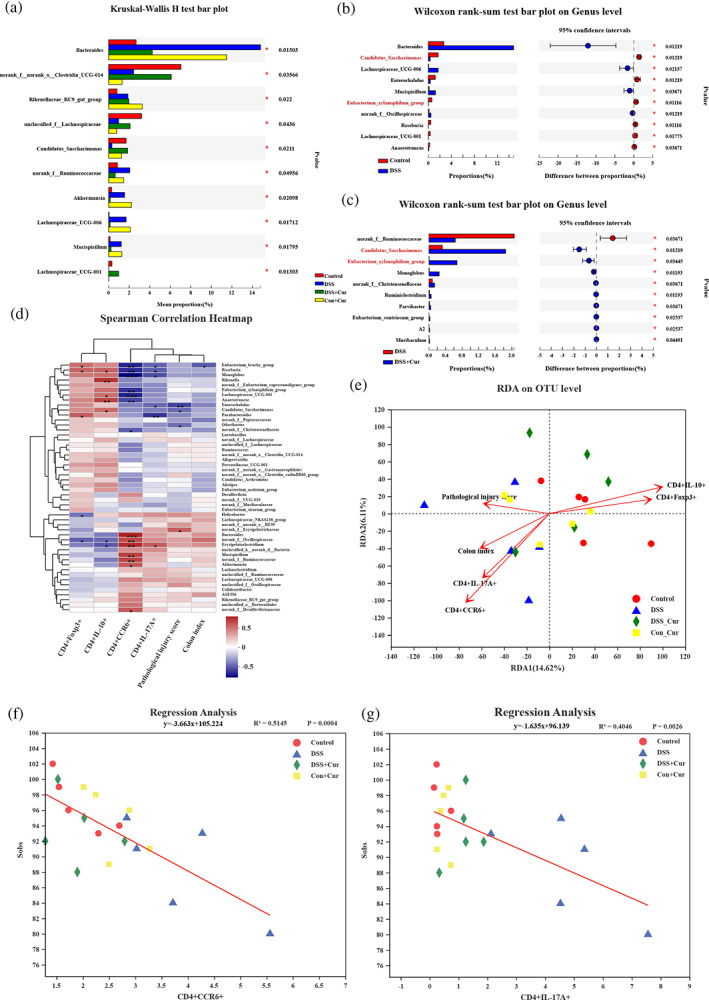
Species differences in intestinal microbiota and correlation analysis. (a) Differential analysis among these four groups at the genus level; (b) Phylotypes significantly different between Control and DSS groups at genus level; (c) Phylotypes significantly different between DSS and DSS + Cur groups at genus level; (d) Spearman's correlation heatmap of CD4+Foxp3+, CD4+IL‐10+, CD4+CCR6+, CD4+IL‐17A+, pathological injury score, colon index, and intestinal microbiota. (e) Linear regression analysis of relationship between CD4+IL‐17A+Th17 and gut microbiota. (f) Linear regression analysis of relationship between CD4+CCR6+Th17 and intestinal microbiota, n = 5
4. DISCUSSION
With advances in healthcare and an increase in the number of patients with chronic diseases, managing patients with multiple co‐morbidities is an inevitable challenge. DM is one of the most common complications in patients with UC (Bower, O'Flynn, Kakad, & Aldulaimi, 2021). Although the correlation between DM and IBD is under discussion, there is not enough research on the coexistence of DM and IBD. DB mice are spontaneous type 2 diabetic mice and are commonly used to investigate the pathogenesis of diabetes and to develop new drugs (Tesch & Lim, 2011; Wang, Burkhardt, Guan, & Yang, 2012). DSS‐induced experimental colitis is a classic model that mimics the pathogenesis of human ulcerative colitis (Bilsborough, Fiorino, & Henkle, 2021). C57BL/6 mice usually drink 3.0% DSS ad libitum in a replicated colitis model (Wirtz et al., 2017), but all DB mice in this study died from drinking 3.0% DSS ad libitum. This suggests that diabetes affects the survival of colitis mice. We induced colitis in DB mice by 1.5% DSS and observed typical signs of colitis in DB mice, prolapse, blood in stool, weight loss, shortened colon length, increased colon weight and microscopic ulcer formation and infiltration of inflammatory cells. This indicates that the 1.5% DSS replication of colitis in DB mice is successful. DB mice in this study were leptin receptor knockout mice (Guo et al., 2021) and were susceptible to DSS‐induced experimental colitis, implying that leptin receptor is resistant to colitis. This was also confirmed by clinical cases where children with at least one copy of the leptin receptor 223R mutation were susceptible to amoebic colitis (Mackey‐Lawrence & Petri, 2012). This provides a clue for our subsequent study of leptin receptor in the pathogenesis of colitis.
Metformin is a first‐line drug for the treatment of type 2 diabetes and is also used in the discovery of drugs for the treatment of diabetes (Foretz, Guigas, & Viollet, 2019; Forslund et al., 2017). Mesalazine is the drug of choice for the treatment of patients with mild‐to‐moderate colitis and is also commonly used to evaluate the development of drugs for UC (Singh, Feuerstein, Binion, & Tremaine, 2019; Urushidani, Kuriyama, & Matsumura, 2018). In this study, the disease—UC complicated with DM is complex and current drugs for this condition have not been reported. However, it is not appropriate to use metformin or mesalazine alone as a positive control. The combination of metformin and mesalazine was considered as a positive control without any negative interaction between metformin and mesalazine. But no studies have been reported in this research area, and this provides a new clue as to whether improving hyperglycemia in diabetes may enhance the anticolitis effect.
Elevated serum insulin concentrations imply increased insulin resistance and are one of the main markers to differentiate between metabolic disorders (e.g., diabetes and obesity) (Guest & Rahmoune, 2019). In T2DM mice, Cur improved insulin resistance and down‐regulated the levels of insulin and blood glucose (Pivari, Mingione, Brasacchio, & Soldati, 2019; Stefanska, 2012). Clinically Cur supplementation is effective in alleviating clinical symptoms in diabetic patients (Altobelli et al., 2021). In the present study, Cur reduced IPITT AUG levels and significantly down‐regulated insulin levels in colitis DB mice. In addition, our study found that Cur inhibited colitis in DB mice, reversed the increase in disease activity index, weight loss, shortened colon, increased colon weight and improved colonic mucosal damage. Cur, derived from turmeric (Curcuma longa L), has been shown to possess substantial health benefits. Nevertheless, due to its poor absorption in free form in the gastrointestinal tract, ingestion of 12 g of Cur was detected in human plasma at a concentration of only 0.051 mg/ml (Yang, Lin, Tseng, Wang, & Tsai, 2007). In the clinical management of UC and diabetes, the usual dose of Cur is 1,500 mg/day. High doses increase the absorption of Cur to some extent, and Cur supplements at doses below 12 g per day show no known toxic effects in humans (Lao et al., 2006). Consumption of the Cur (1,500 mg/day) supplement, along with drug therapy, is associated with significant improvement of the clinical outcomes, quality of life, hs‐CRP, and ESR in patients with mild‐to‐moderate UC (Sadeghi, Mansoori, Shayesteh, & Hashemi, 2020). A randomized, double‐blind, placebo‐controlled trial indicate that Cur (1,500 mg/day) consumption may reduce diabetes complications through decreasing TG level as well as indicators of inflammation (Adibian et al., 2019). However, clinical studies of Cur have not been reported in cases of UC complicated by DM. Based on body surface area (Nair & Jacob, 2016), the human dose of Cur was converted to a mouse dose of 307.5 mg/kg/day (1,500 mg/day ÷ 60 kg × 12.3), higher than the 100 mg/kg/day used in this study. This further suggests the potential of Cur to treat the complex disease of UC complicated by DM, which could be helpful in guiding the clinical dosing of Cur.
The immune homeostasis of Th17/Treg plays a key role in host health and its disruption often induces autoimmune diseases, including IBD (Huang et al., 2021) and DM (Zhang et al., 2021b). Th17 cells are pro‐inflammatory, secreting large amounts of IL‐17A upon activation, causing damage to the colonic mucosa (Owaga et al., 2015), killing pancreatic β‐cells and enhancing insulin tolerance (Kiernan & Maciver, 2020). In contrast, Treg cells exhibit anti‐inflammatory effects, secreting IL‐10 to promote colonic mucosal repair (Chang et al., 2021) and improving insulin tolerance (Bettini & Bettini, 2021). Targeted modulation of Th17/Treg balance is an effective strategy for the treatment of autochthonous diseases. Our previous study demonstrated the ability of Cur to reshape the Th17/Treg balance in DSS‐induced colitis mice (Zhong et al., 2021). In the present study, we found that Cur was able to down‐regulate CD4+CCR6+Th17, CD4+IL‐17A+Th17 and its cytokine IL‐17A, nuclear transcription factor BATF, C‐Maf, RORγt, and up‐regulate CD4+Foxp3+ Treg, CD4+IL‐10+ Treg and its cytokine IL‐10, nuclear transcription factor Foxp3, Eomes in mice with diabetic complications of colitis. This suggests that Cur has the ability to restore the balance of Th17/Treg in mice with colitis complicated by diabetes.
The human intestinal microbiota has emerged as a major player in human health and disease (Trebicka, Bork, Krag, & Arumugam, 2021). Targeted regulation of intestinal microbiota homeostasis is an emerging strategy for the treatment of autochthonous diseases. In the present study, a significant decrease in diversity and a significant change in the composition of the intestinal microbiota of mice with diabetes complicated by colitis, with lower relative abundance of Candidatus_Saccharimonas and Eubacterium_xylanophilum_groups. Not consistent with our previous study, DSS‐induced colitis mice did not show significant changes in Candidatus_Saccharimonas and Eubacterium_xylanophilum_group (Zhong et al., 2021). This may be strongly related to the different strains of mice, implying a strong relationship between leptin receptors on the composition of the intestinal microbiota of DSS‐induced colitis. Candidatus_Saccharimonas has an anti‐inflammatory profile and contributes to IL‐4 and IL‐10 secretion (Sang et al., 2020). Other studies reported that colitis mice with T2DM was alleviated via by intestinal microbiota, including Fermented Maillard Reaction Products by Lactobacillus gasseri 4M13 (Jeong, Park, Nam, & Lee, 2021) and Odd‐numbered agaro‐oligosaccharides (Wang et al., 2020). Notably, we found that Cur was effective in improving the diversity of intestinal microbiota and up‐regulating the relative abundance of Candidatus_Saccharimonas and Eubacterium_xylanophilum_group in DB mice with colitis. The role of Candidatus_Saccharimonas and Eubacterium_xylanophilum_group on diabetic complications of colitis can be further explored by fecal transplantation assays. In addition, Th17 cells were negatively correlated with Sobs, suggesting that Th17 cells disrupted the abundance of intestinal microbiota. Meanwhile, correlation analysis revealed that Roseburia was positively correlated with CD4+CCR6+Th17 and CD4+IL‐17A+Th17, and negatively correlated with CD4+Foxp3+ and CD4+IL‐10+Treg cells. This implies that Roseburia has potential value for the balance of Th17/Treg cells in DSS‐induced UC mice with T2DM and lays the foundation for subsequent studies.
In conclusion, Cur not only significantly improved insulin secretion and blood glucose levels in T2DM, but also alleviated DSS‐induced UC. Moreover, Cur regulated the balance of Th17/Treg cells and the homeostasis of intestinal microbiota in UC complicated with DM. These findings provide new insights and experimental evidence for the treatment and research of UC complicated with DM, which is a complex disease.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization, Duan‐Yong Liu; Data curation, You‐Bao Zhong and Hai‐Mei Zhao; Formal analysis, You‐Bao Zhong; Funding acquisition, You‐Bao Zhong, Hai‐Mei Zhao and Duan‐Yong Liu; Investigation, Qiu‐Ping Xiao, You‐Bao Zhong, Zeng‐Ping Kang, Jia‐Qi Huang, Wei‐Yan Fang, Si‐Yi Wei, Jian Long and Shan‐Shan Li; Methodology, Qiu‐Ping Xiao; Project administration, Hai‐Mei Zhao; Resources, Hai‐Mei Zhao and Duan‐Yong Liu; Supervision, Duan‐Yong Liu; Validation, Hai‐Mei Zhao; Writing – original draft, Duan‐Yong Liu; Writing – review & editing, You‐Bao Zhong and Duan‐Yong Liu.
ETHICS STATEMENT
The study (Permit Number: JZLLSC2020‐334) was approved by the Jiangxi University of Traditional Chinese Medicine Animal Care and Use Committee and performed in accordance with the guidelines prescribed by the committee.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (Grant Nos. 82060799 and 81760808), Natural Science Foundation of Jiangxi Province (Grant Nos. 20202ACBL206026 and 20192ACB20015), and Education Department of Jiangxi Province (Grant Nos. GJJ201261, GJJ196047 and GJJ196049), and Science and Technology Plan Project of Jiangxi Provincial Health and Health Commission (Grant No. 202110121), and 1,050 Young Talents Project (Grant No. 1141900603)
Xiao, Q.‐P. , Zhong, Y.‐B. , Kang, Z.‐P. , Huang, J.‐Q. , Fang, W.‐Y. , Wei, S.‐Y. , Long, J. , Li, S.‐S. , Zhao, H.‐M. , & Liu, D.‐Y. (2022). Curcumin regulates the homeostasis of Th17/Treg and improves the composition of gut microbiota in type 2 diabetic mice with colitis. Phytotherapy Research, 36(4), 1708–1723. 10.1002/ptr.7404
Qiu‐Ping Xiao and You‐Bao Zhong contributed equally to this work as co‐first authors.
Funding information 1050 Young Talents Project, Grant/Award Number: 1141900603; Education Department of Jiangxi Province, Grant/Award Numbers: GJJ196047, GJJ196049, GJJ201261; National Natural Science Foundation of China, Grant/Award Numbers: 81760808, 82060799; Natural Science Foundation of Jiangxi Province, Grant/Award Numbers: 20192ACB20015, 20202ACBL206026; Science and Technology Plan Project of Jiangxi Provincial Health and Health Commission, Grant/Award Number: 202110121
Contributor Information
Hai‐Mei Zhao, Email: haimei79@163.com.
Duan‐Yong Liu, Email: liuduanyong@163.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abdel‐Moneim, A. , Bakery, H. H. , & Allam, G. (2018). The potential pathogenic role of IL‐17/Th17 cells in both type 1 and type 2 diabetes mellitus. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 101, 287–292. 10.1016/j.biopha.2018.02.103 [DOI] [PubMed] [Google Scholar]
- Adibian, M. , Hodaei, H. , Nikpayam, O. , Sohrab, G. , Hekmatdoost, A. , & Hedayati, M. (2019). The effects of curcumin supplementation on high‐sensitivity C‐reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, double‐blind, placebo‐controlled trial. Phytotherapy Research, 33(5), 1374–1383. 10.1002/ptr.6328 [DOI] [PubMed] [Google Scholar]
- Alam, M. T. , Amos, G. , Murphy, A. , Murch, S. , Wellington, E. , & Arasaradnam, R. P. (2020). Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathogens, 12, 1. 10.1186/s13099-019-0341-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altobelli, E. , Angeletti, P. M. , Marziliano, C. , Mastrodomenico, M. , Giuliani, A. R. , & Petrocelli, R. (2021). Potential therapeutic effects of curcumin on glycemic and lipid profile in uncomplicated type 2 diabetes‐a meta‐analysis of randomized controlled trial. Nutrients, 13(2), 404. 10.3390/nu13020404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonioli, L. , Blandizzi, C. , Csoka, B. , Pacher, P. , & Hasko, G. (2015). Adenosine signalling in diabetes mellitus—Pathophysiology and therapeutic considerations. Nature Reviews. Endocrinology, 11(4), 228–241. 10.1038/nrendo.2015.10 [DOI] [PubMed] [Google Scholar]
- Asadi, S. , Gholami, M. S. , Siassi, F. , Qorbani, M. , Khamoshian, K. , & Sotoudeh, G. (2019). Nano curcumin supplementation reduced the severity of diabetic sensorimotor polyneuropathy in patients with type 2 diabetes mellitus: A randomized double‐blind placebo‐controlled clinical trial. Complementary Therapies in Medicine, 43, 253–260. 10.1016/j.ctim.2019.02.014 [DOI] [PubMed] [Google Scholar]
- Barlow, G. M. , Yu, A. , & Mathur, R. (2015). Role of the gut microbiome in obesity and diabetes mellitus. Nutrition in Clinical Practice, 30(6), 787–797. 10.1177/0884533615609896 [DOI] [PubMed] [Google Scholar]
- Bettini, M. , & Bettini, M. L. (2021). Function, failure, and the future potential of Tregs in type 1 diabetes. Diabetes, 70(6), 1211–1219. 10.2337/dbi18-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsborough, J. , Fiorino, M. F. , & Henkle, B. W. (2021). Select animal models of colitis and their value in predicting clinical efficacy of biological therapies in ulcerative colitis. Expert Opinion on Drug Discovery, 16(5), 567–577. 10.1080/17460441.2021.1851185 [DOI] [PubMed] [Google Scholar]
- Bower, J. , O'Flynn, L. , Kakad, R. , & Aldulaimi, D. (2021). Effect of inflammatory bowel disease treatments on patients with diabetes mellitus. World Journal of Diabetes, 12(8), 1248–1254. 10.4239/wjd.v12.i8.1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunte, K. , & Beikler, T. (2019). Th17 cells and the IL‐23/IL‐17 Axis in the pathogenesis of periodontitis and immune‐mediated inflammatory diseases. International Journal of Molecular Sciences, 20(14), 3394. 10.3390/ijms20143394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge, K. , Gunasekaran, A. , Eckert, J. , & Chaaban, H. (2019). Curcumin and intestinal inflammatory diseases: Molecular mechanisms of protection. International Journal of Molecular Sciences, 20(8), 1912. 10.3390/ijms20081912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canistro, D. , Chiavaroli, A. , Cicia, D. , Cimino, F. , Curro, D. , Dell'Agli, M. , … Martinelli, G. (2021). The pharmacological basis of the curcumin nutraceutical uses: An update. Pharmadvances, 3(2), 421–466. 10.36118/pharmadvances.2021.06 [DOI] [Google Scholar]
- Caruso, R. , Lo, B. C. , & Nunez, G. (2020). Host‐microbiota interactions in inflammatory bowel disease. Nature Reviews. Immunology, 20(7), 411–426. 10.1038/s41577-019-0268-7 [DOI] [PubMed] [Google Scholar]
- Chang, Y. , Zhai, L. , Peng, J. , Wu, H. , Bian, Z. , & Xiao, H. (2021). Phytochemicals as regulators of Th17/Treg balance in inflammatory bowel diseases. Biomedicine & Pharmacotherapy, 141, 111931. 10.1016/j.biopha.2021.111931 [DOI] [PubMed] [Google Scholar]
- Chen, L. , Xu, P. , Xiao, Q. , Chen, L. , Li, S. , Jian, J. M. , & Zhong, Y. B. (2021). Sunitinib malate inhibits intestinal tumor development in male Apc(Min/+) mice by down‐regulating inflammation‐related factors with suppressing beta‐cateinin/c‐Myc pathway and re‐balancing Bcl‐6 and Caspase‐3. International Immunopharmacology, 90, 107128. 10.1016/j.intimp.2020.107128 [DOI] [PubMed] [Google Scholar]
- Curkovic, I. , Egbring, M. , & Kullak‐Ublick, G. A. (2013). Risks of inflammatory bowel disease treatment with glucocorticosteroids and aminosalicylates. Digestive Diseases, 31(3–4), 368–373. 10.1159/000354699 [DOI] [PubMed] [Google Scholar]
- Din, H. , Anderson, A. J. , Ramos, R. C. , Proksell, S. , Koutroumpakis, F. , Salim, T. , … Binion, D. G. (2020). Disease characteristics and severity in patients with inflammatory bowel disease with coexistent diabetes mellitus. Inflammatory Bowel Diseases, 26(9), 1436–1442. 10.1093/ibd/izz305 [DOI] [PubMed] [Google Scholar]
- Foretz, M. , Guigas, B. , & Viollet, B. (2019). Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nature Reviews. Endocrinology, 15(10), 569–589. 10.1038/s41574-019-0242-2 [DOI] [PubMed] [Google Scholar]
- Forslund, K. , Hildebrand, F. , Nielsen, T. , Falony, G. , Le Chatelier, E. , Sunagawa, S. , … Pedersen, O. (2017). Corrigendum: Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature, 545(7652), 116. 10.1038/nature22318 [DOI] [PubMed] [Google Scholar]
- Ge, H. , Cai, Z. , Chai, J. , Liu, J. , Liu, B. , Yu, Y. , … Zhang, T. (2021). Egg white peptides ameliorate dextran sulfate sodium‐induced acute colitis symptoms by inhibiting the production of pro‐inflammatory cytokines and modulation of gut microbiota composition. Food Chemistry, 360, 129981. 10.1016/j.foodchem.2021.129981 [DOI] [PubMed] [Google Scholar]
- Glassner, K. L. , Abraham, B. P. , & Quigley, E. (2020). The microbiome and inflammatory bowel disease. The Journal of Allergy and Clinical Immunology, 145(1), 16–27. 10.1016/j.jaci.2019.11.003 [DOI] [PubMed] [Google Scholar]
- Guest, P. C. , & Rahmoune, H. (2019). Characterization of the db/db mouse model of type 2 diabetes. Methods in Molecular Biology, 1916, 195–201. 10.1007/978-1-4939-8994-2_18 [DOI] [PubMed] [Google Scholar]
- Guo, S. , Ouyang, H. , Du, W. , Li, J. , Liu, M. , Yang, S. , … Feng, Y. (2021). Exploring the protective effect of Gynura procumbens against type 2 diabetes mellitus by network pharmacology and validation in C57BL/KsJ db/db mice. Food & Function, 12(4), 1732–1744. 10.1039/d0fo01188f [DOI] [PubMed] [Google Scholar]
- Heinrich, M. , Appendino, G. , Efferth, T. , Furst, R. , Izzo, A. A. , Kayser, O. , … Viljoen, A. (2020). Best practice in research—Overcoming common challenges in phytopharmacological research. Journal of Ethnopharmacology, 246, 112230. 10.1016/j.jep.2019.112230 [DOI] [PubMed] [Google Scholar]
- Huang, J. , Yang, Z. , Li, Y. , Chai, X. , Liang, Y. , Lin, B. , … Zeng, J. (2021). Lactobacillus paracasei R3 protects against dextran sulfate sodium (DSS)‐induced colitis in mice via regulating Th17/Treg cell balance. Journal of Translational Medicine, 19(1), 356. 10.1186/s12967-021-02943-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue, A. , Coombes, J. L. , & Powrie, F. (2006). Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunological Reviews, 212, 256–271. 10.1111/j.0105-2896.2006.00423.x [DOI] [PubMed] [Google Scholar]
- Izzo, A. A. , Teixeira, M. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , … Ahluwalia, A. (2020). A practical guide for transparent reporting of research on natural products in the British Journal of Pharmacology: Reproducibility of natural product research. British Journal of Pharmacology, 177(10), 2169–2178. 10.1111/bph.15054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong, Y. J. , Park, H. Y. , Nam, H. K. , & Lee, K. W. (2021). Fermented Maillard reaction products by lactobacillus gasseri 4M13 alters the intestinal microbiota and improves dysfunction in type 2 diabetic mice with colitis. Pharmaceuticals (Basel), 14(4), 299. 10.3390/ph14040299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jess, T. , Jensen, B. W. , Andersson, M. , Villumsen, M. , & Allin, K. H. (2020). Inflammatory bowel diseases increase risk of type 2 diabetes in a nationwide cohort study. Clinical Gastroenterology and Hepatology, 18(4), 881–888.e1. 10.1016/j.cgh.2019.07.052 [DOI] [PubMed] [Google Scholar]
- Kang, Z. P. , Wang, M. X. , Wu, T. T. , Liu, D. Y. , Wang, H. Y. , Long, J. , … Zhong, Y. B. (2021). Curcumin alleviated dextran sulfate sodium‐induced colitis by regulating M1/M2 macrophage polarization and TLRs signaling pathway. Evidence‐Based Complementary and Alternative Medicine, 2021, 3334994. 10.1155/2021/3334994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedia, S. , Rampal, R. , Paul, J. , & Ahuja, V. (2016). Gut microbiome diversity in acute infective and chronic inflammatory gastrointestinal diseases in North India. Journal of Gastroenterology, 51(7), 660–671. 10.1007/s00535-016-1193-1 [DOI] [PubMed] [Google Scholar]
- Kiernan, K. , & Maciver, N. J. (2020). A novel mechanism for Th17 inflammation in human type 2 diabetes mellitus. Trends in Endocrinology and Metabolism, 31(1), 1–2. 10.1016/j.tem.2019.11.002 [DOI] [PubMed] [Google Scholar]
- Kotha, R. R. , & Luthria, D. L. (2019). Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules, 24(16), 2930. 10.3390/molecules24162930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, S. W. , Kuo, Y. H. , & Liao, K. F. (2020). Association between inflammatory bowel disease and diabetes mellitus. Clinical Gastroenterology and Hepatology, 18(4), 1002–1003. 10.1016/j.cgh.2019.09.016 [DOI] [PubMed] [Google Scholar]
- Lang, A. , Salomon, N. , Wu, J. C. Y. , Kopylov, U. , Lahat, A. , Har‐Noy, O. , … Ben‐Horin, S. (2015). Curcumin in combination with mesalamine induces remission in patients with mild‐to‐moderate ulcerative colitis in a randomized controlled trial. Clinical Journal of Gastroenterology, 13(8), 1444. 10.1016/j.cgh.2015.02.019 [DOI] [PubMed] [Google Scholar]
- Lao, C. D. , Ruffin, M. T. , Normolle, D. , Heath, D. D. , Murray, S. I. , Bailey, J. M. , … Brenner, D. E. (2006). Dose escalation of a curcuminoid formulation. BMC Complementary and Alternative Medicine, 6, 10. 10.1186/1472-6882-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. B. , Chae, S. U. , Jo, S. J. , Jerng, U. M. , & Bae, S. K. (2021). The relationship between the gut microbiome and metformin as a key for treating type 2 diabetes mellitus. International Journal of Molecular Sciences, 22(7), 3566. 10.3390/ijms22073566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, W. , Zhang, D. , Kang, J. , Meng, X. , Yang, J. , Yang, L. , … Gou, X. (2018). Protective effects of rutin on liver injury in type 2 diabetic db/db mice. Biomedicine & Pharmacotherapy, 107, 721–728. 10.1016/j.biopha.2018.08.046 [DOI] [PubMed] [Google Scholar]
- Lin, F. , Meng, X. , Guo, Y. , Cao, W. , Liu, W. , Xia, Q. , … Wang, L. (2019). Epigenetic initiation of the TH17 differentiation program is promoted by Cxxc finger protein 1. Science Advances, 5(10), eaax1608. 10.1126/sciadv.aax1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey‐Lawrence, N. M. , & Petri, W. J. (2012). Leptin and mucosal immunity. Mucosal Immunology, 5(5), 472–479. 10.1038/mi.2012.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maconi, G. , Furfaro, F. , Sciurti, R. , Bezzio, C. , Ardizzone, S. , & de Franchis, R. (2014). Glucose intolerance and diabetes mellitus in ulcerative colitis: Pathogenetic and therapeutic implications. World Journal of Gastroenterology, 20(13), 3507–3515. 10.3748/wjg.v20.i13.3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Guryn, K. , Leone, V. , & Chang, E. B. (2019). Regional diversity of the gastrointestinal microbiome. Cell Host & Microbe, 26(3), 314–324. 10.1016/j.chom.2019.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair, A. B. , & Jacob, S. (2016). A simple practice guide for dose conversion between animals and human. Journal of Basic and Clinical Pharmacy, 7(2), 27–31. 10.4103/0976-0105.177703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owaga, E. , Hsieh, R. H. , Mugendi, B. , Masuku, S. , Shih, C. K. , & Chang, J. S. (2015). Th17 cells as potential probiotic therapeutic targets in inflammatory bowel diseases. International Journal of Molecular Sciences, 16(9), 20841–20858. 10.3390/ijms160920841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, S. S. , Acharya, A. , Ray, R. S. , Agrawal, R. , Raghuwanshi, R. , & Jain, P. (2020). Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Critical Reviews in Food Science and Nutrition, 60(6), 887–939. 10.1080/10408398.2018.1552244 [DOI] [PubMed] [Google Scholar]
- Pittayanon, R. , Lau, J. T. , Leontiadis, G. I. , Tse, F. , Yuan, Y. , Surette, M. , & Moayyedi, P. (2020). Differences in gut microbiota in patients with vs without inflammatory bowel diseases: A systematic review. Gastroenterology, 158(4), 930–946.e1. 10.1053/j.gastro.2019.11.294 [DOI] [PubMed] [Google Scholar]
- Pivari, F. , Mingione, A. , Brasacchio, C. , & Soldati, L. (2019). Curcumin and type 2 diabetes mellitus: Prevention and treatment. Nutrients, 11(8), 1837. 10.3390/nu11081837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, Y. C. , Shen, J. , He, L. , Hong, X. Z. , Tian, F. , Pan, Y. H. , … Zhao, H. L. (2016). Changes of regulatory T cells and of Proinflammatory and immunosuppressive cytokines in patients with type 2 diabetes mellitus: A systematic review and meta‐analysis. Journal Diabetes Research, 2016, 3694957. 10.1155/2016/3694957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radel, J. A. , Pender, D. N. , & Shah, S. A. (2019). Dipeptidyl Peptidase‐4 inhibitors and inflammatory bowel disease risk: A meta‐analysis. The Annals of Pharmacotherapy, 53(7), 697–704. 10.1177/1060028019827852 [DOI] [PubMed] [Google Scholar]
- Razavi, B. M. , Ghasemzadeh, R. M. , & Hosseinzadeh, H. (2021). A review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders, pain, and their related patents. Phytotherapy Research, 35(12), 6489–6513. 10.1002/ptr.7224 [DOI] [PubMed] [Google Scholar]
- Sadeghi, N. , Mansoori, A. , Shayesteh, A. , & Hashemi, S. J. (2020). The effect of curcumin supplementation on clinical outcomes and inflammatory markers in patients with ulcerative colitis. Phytotherapy Research, 34(5), 1123–1133. 10.1002/ptr.6581 [DOI] [PubMed] [Google Scholar]
- Sang, H. , Xie, Y. , Su, X. , Zhang, M. , Zhang, Y. , Liu, K. , & Wang, J. (2020). Mushroom Bulgaria inquinans modulates host immunological response and gut microbiota in mice. Frontiers in Nutrition, 7, 144. 10.3389/fnut.2020.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad, A. , Rehman, G. , & Lee, Y. S. (2013). Curcumin in inflammatory diseases. BioFactors, 39(1), 69–77. 10.1002/biof.1066 [DOI] [PubMed] [Google Scholar]
- Singh, S. , Feuerstein, J. D. , Binion, D. G. , & Tremaine, W. J. (2019). AGA technical review on the management of mild‐to‐moderate ulcerative colitis. Gastroenterology, 156(3), 769–808.e29. 10.1053/j.gastro.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedhar, R. , Arumugam, S. , Thandavarayan, R. A. , Karuppagounder, V. , & Watanabe, K. (2016). Curcumin as a therapeutic agent in the chemoprevention of inflammatory bowel disease. Drug Discovery Today, 21(5), 843–849. 10.1016/j.drudis.2016.03.007 [DOI] [PubMed] [Google Scholar]
- Stefanska, B. (2012). Curcumin ameliorates hepatic fibrosis in type 2 diabetes mellitus—Insights into its mechanisms of action. British Journal of Pharmacology, 166(8), 2209–2211. 10.1111/j.1476-5381.2012.01959.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundrud, M. S. , & Trivigno, C. (2013). Identity crisis of Th17 cells: Many forms, many functions, many questions. Seminars in Immunology, 25(4), 263–272. 10.1016/j.smim.2013.10.021 [DOI] [PubMed] [Google Scholar]
- Tao, L. , Liu, H. , & Gong, Y. (2019). Role and mechanism of the Th17/Treg cell balance in the development and progression of insulin resistance. Molecular and Cellular Biochemistry, 459(1–2), 183–188. 10.1007/s11010-019-03561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesch, G. H. , & Lim, A. K. (2011). Recent insights into diabetic renal injury from the db/db mouse model of type 2 diabetic nephropathy. American Journal of Physiology. Renal Physiology, 300(2), F301–F310. 10.1152/ajprenal.00607.2010 [DOI] [PubMed] [Google Scholar]
- Trebicka, J. , Bork, P. , Krag, A. , & Arumugam, M. (2021). Utilizing the gut microbiome in decompensated cirrhosis and acute‐on‐chronic liver failure. Nature Reviews. Gastroenterology & Hepatology, 18(3), 167–180. 10.1038/s41575-020-00376-3 [DOI] [PubMed] [Google Scholar]
- Tsai, S. Y. , Yang, T. Y. , Lin, C. L. , Tsai, Y. H. , Kuo, C. F. , & Kao, C. H. (2015). Increased risk of varicella zoster virus infection in inflammatory bowel disease in an Asian population: A nationwide population‐based cohort study. International Journal of Clinical Practice, 69(2), 228–234. 10.1111/ijcp.12508 [DOI] [PubMed] [Google Scholar]
- Urushidani, S. , Kuriyama, A. , & Matsumura, M. (2018). 5‐aminosalicylic acid agents for prevention of recurrent diverticulitis: A systematic review and meta‐analysis. Journal of Gastroenterology and Hepatology, 33(1), 12–19. 10.1111/jgh.13846 [DOI] [PubMed] [Google Scholar]
- Uwagbale, E. , Adeniran, O. G. , Adeniran, O. A. , Onukogu, I. , Agbroko, S. , & Sonpal, N. (2021). In‐hospital outcomes of inflammatory bowel diseases in patients with diabetes mellitus: A propensity score matching analysis. Cureus, 13(7), e16566. 10.7759/cureus.16566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchi, B. L. , Marcuzzi, A. , Tricarico, P. M. , Zanin, V. , Girardelli, M. , & Bianco, A. M. (2014). Curcumin and inflammatory bowel disease: Potential and limits of innovative treatments. Molecules, 19(12), 21127–21153. 10.3390/molecules191221127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. , Burkhardt, B. R. , Guan, Y. , & Yang, J. (2012). Role of pancreatic‐derived factor in type 2 diabetes: Evidence from pancreatic beta cells and liver. Nutrition Reviews, 70(2), 100–106. 10.1111/j.1753-4887.2011.00457.x [DOI] [PubMed] [Google Scholar]
- Wang, M. , Chen, F. , Wang, J. , Zeng, Z. , Yang, Q. , & Shao, S. (2018). Th17 and Treg lymphocytes in obesity and type 2 diabetic patients. Clinical Immunology, 197, 77–85. 10.1016/j.clim.2018.09.005 [DOI] [PubMed] [Google Scholar]
- Wang, X. , Yang, Z. , Xu, X. , Jiang, H. , Cai, C. , & Yu, G. (2020). Odd‐numbered agaro‐oligosaccharides alleviate type 2 diabetes mellitus and related colonic microbiota dysbiosis in mice. Carbohydrate Polymers, 240, 116261. 10.1016/j.carbpol.2020.116261 [DOI] [PubMed] [Google Scholar]
- Wirtz, S. , Popp, V. , Kindermann, M. , Gerlach, K. , Weigmann, B. , Fichtner‐Feigl, S. , & Neurath, M. F. (2017). Chemically induced mouse models of acute and chronic intestinal inflammation. Nature Protocols, 12(7), 1295–1309. 10.1038/nprot.2017.044 [DOI] [PubMed] [Google Scholar]
- Wu, H. , Tremaroli, V. , Schmidt, C. , Lundqvist, A. , Olsson, L. M. , Kramer, M. , … Backhed, F. (2020). The gut microbiota in prediabetes and diabetes: A population‐based cross‐sectional study. Cell Metabolism, 32(3), 379–390.e3. 10.1016/j.cmet.2020.06.011 [DOI] [PubMed] [Google Scholar]
- Yan, J. B. , Luo, M. M. , Chen, Z. Y. , & He, B. H. (2020). The function and role of the Th17/Treg cell balance in inflammatory bowel disease. Journal of Immunology Research, 2020, 8813558. 10.1155/2020/8813558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, K. Y. , Lin, L. C. , Tseng, T. Y. , Wang, S. C. , & Tsai, T. H. (2007). Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC‐MS/MS. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 853(1–2), 183–189. 10.1016/j.jchromb.2007.03.010 [DOI] [PubMed] [Google Scholar]
- Yong, J. , Johnson, J. D. , Arvan, P. , Han, J. , & Kaufman, R. J. (2021). Therapeutic opportunities for pancreatic beta‐cell ER stress in diabetes mellitus. Nature Reviews. Endocrinology, 17(8), 455–467. 10.1038/s41574-021-00510-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Xiao, C. , Wang, P. , Xu, W. , Zhang, A. , Li, Q. , & Xu, X. (2014). The alteration of Th1/Th2/Th17/Treg paradigm in patients with type 2 diabetes mellitus: Relationship with diabetic nephropathy. Human Immunology, 75(4), 289–296. 10.1016/j.humimm.2014.02.007 [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Cai, Y. , Meng, C. , Ding, X. , Huang, J. , Luo, X. , … Zou, M. (2021a). The role of the microbiome in diabetes mellitus. Diabetes Research and Clinical Practice, 172, 108645. 10.1016/j.diabres.2020.108645 [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Gang, X. , Yang, S. , Cui, M. , Sun, L. , Li, Z. , & Wang, G. (2021b). The alterations in and the role of the Th17/Treg balance in metabolic diseases. Frontiers in Immunology, 12, 678355. 10.3389/fimmu.2021.678355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Liu, X. , Zhu, Y. , Liu, X. , Gu, Y. , Dai, X. , & Li, B. (2021c). Transcriptional and posttranslational regulation of Th17/Treg balance in health and disease. European Journal of Immunology, 51(9), 2137–2150. 10.1002/eji.202048794 [DOI] [PubMed] [Google Scholar]
- Zhong, Y. , Kang, Z. , Wang, M. , Long, J. , Wang, H. , Huang, J. , … Liu, D. (2021). Curcumin ameliorated dextran sulfate sodium‐induced colitis via regulating the homeostasis of DCs and Treg and improving the composition of the gut microbiota. Journal of Functional Foods, 86, 104716. 10.1016/j.jff.2021.104716 [DOI] [Google Scholar]
- Zhong, Y. B. , Kang, Z. P. , Zhou, B. G. , Wang, H. Y. , Long, J. , Zhou, W. , … Liu, D. Y. (2020). Curcumin regulated the homeostasis of memory T cell and ameliorated dextran sulfate sodium‐induced experimental colitis. Frontiers in Pharmacology, 11, 630244. 10.3389/fphar.2020.630244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, B. , Yuan, Y. , Zhang, S. , Guo, C. , Li, X. , Li, G. , … Zeng, Z. (2020). Intestinal flora and disease mutually shape the regional immune system in the intestinal tract. Frontiers in Immunology, 11, 575. 10.3389/fimmu.2020.00575 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


