Abstract
Background:
The ability of serial magnetic resonance imaging (MRI) to capture pathologic progression during active surveillance (AS) remains in question.
Objective:
To determine whether changes in MRI are associated with pathologic progression for patients on AS.
Design, setting, and participants:
From July 2007 through January 2020, we identified all patients evaluated for AS at our institution. Following confirmatory biopsy, a total of 391 patients who underwent surveillance MRI and biopsy at least once were identified (median follow-up of 35.6 mo, interquartile range 19.7–60.6).
Outcome measurements and statistical analysis:
All MRI intervals were scored using the “Prostate Cancer Radiologic Estimation of Change in Sequential Evaluation” (PRECISE) criteria, with PRECISE scores ≥4 considered a positive change in MRI. A generalized estimating equation–based logistic regression analysis was conducted for all intervals with a PRECISE score of <4 to determine the predictors of Gleason grade group (GG) progression despite stable MRI.
Results and limitations:
A total of 621 MRI intervals were scored by PRECISE and validated by biopsy. The negative predictive value of stable MRI (PRECISE score <4) was greatest for detecting GG1 to ≥ GG3 disease (0.94 [0.91–0.97]). If 2-yr surveillance biopsy were performed exclusively for a positive change in MRI, 3.7% (4/109) of avoided biopsies would have resulted in missed progression from GG1 to ≥ GG3 disease. Prostate-specific antigen (PSA) density (odds ratio 1.95 [1.17–3.25], p = 0.01) was a risk factor for progression from GG1 to ≥GG3 disease despite stable MRI.
Conclusions:
In patients with GG1 disease and stable MRI (PRECISE score <4) on surveillance, grade progression to ≥ GG3 disease is not common. In patients with grade progression detected on biopsy despite stable MRI, elevated PSA density appeared to be a risk factor for progression to ≥ GG3 disease.
Patient summary:
For patients with low-risk prostate cancer on active surveillance, the risk of progressing to grade group 3 disease is low with a stable magnetic resonance image (MRI) after 2 yr. Having higher prostate-specific antigen density increases the risk of progression, despite having a stable MRI.
Keywords: Active surveillance Fusion, biopsy Multiparametric, magnetic resonance, imaging Prostate Cancer, Radiologic Estimation of, Change in Sequential, Evaluation Prostate cancer
1. Introduction
As systematic biopsy alone has been shown to underdiagnose clinically significant disease and overdiagnose indolent disease [1], multiparametric magnetic resonance imaging (mpMRI) and MRI-targeted biopsies are used to improve the accuracy of the prostate cancer diagnostic pathway [1,2]. While MRI-targeted biopsies have been shown to improve risk stratification for patients electing active surveillance (AS) [3–5], the appropriate application of mpMRI in surveillance protocols remains unknown. A recent study by Chesnut et al [6] found that monitoring patients without MRI or clinical changes can avoid many surveillance biopsies, but does so at the expense of missing a significant amount of disease progression. This study, similar to others [7,8], sought to determine the ability of MRI to stratify patients prior to biopsy; however, each study used their own definitions of MRI changes, limiting the generalizability of their results. To address the inconsistencies of MRI reporting among different institutions, the European School of Oncology released the “Prostate Cancer Radiologic Estimation of Change in Sequential Evaluation” (PRECISE) criteria for reporting mpMRI findings of patients on AS [9]. Since this scoring system was made available in 2017, there have been very limited reports to date on the application of the PRECISE criteria to an AS cohort [10,11].
In this study, patients with multiple MRI examinations and combined MRI-targeted plus systematic biopsies were identified, and changes in each MRI interval were scored according to the PRECISE criteria. We aimed to determine whether changes in MRI (PRECISE score ≥4) were associated with pathologic cancer progression in our AS cohort.
2. Patients and methods
2.1. Patient population
Patients were enrolled in a prospective clinical study with institutional review board approval as part of an ongoing study on the use of electromagnetic tracking devices to locate disease during multimodality-navigated procedures (NCT00102544). Enrollment occurred between July 2007 and January 2020 with written informed consent. For our study, patients with a diagnosis of grade group (GG) 1 or GG2 prostate cancer on our AS protocol were identified. Patients were eligible for AS at our institution if diagnosed with GG1 disease without any threshold for prostate-specific antigen (PSA). In addition, patients with GG2 disease were eligible if they did not elect for definite therapy and MRI had no aggressive features on evaluation (ie, extraprostatic extension, seminal vesicle invasion, or invasion of adjacent anatomic structures). Only patients who received follow-up biopsy and were on surveillance for ≥1 yr were included in this study. Patients were excluded from AS analysis if confirmatory biopsy revealed ≥ GG3 disease, chose definitive treatment, or decided to follow up with an outside urologist. Surveillance MRI and biopsy was encouraged at 1–2-yr intervals based on clinical suspicion, PSA, changes in physical examination, or worsening changes in prostate MRI. In total, 391 patients were included in this study, all of whom were classified to have National Comprehensive Cancer Network very low risk, low risk, or favorable intermediate risk [12].
2.2. Imaging protocol
All patients initially underwent mpMRI of the prostate, including triplanar T2-weighted, dynamic contrast-enhanced, and diffusion-weighted imaging sequences performed on a 3 T MRI scanner (Achieva; Philips Healthcare, Andover, MA, USA) with a 16-channel cardiac surface coil (SENSE; Philips) positioned over the pelvis and an endorectal coil (BPX-30; Medrad Inc., Pittsburgh, PA, USA; Supplementary Table 1) [13]. These diagnostic mpMRI studies underwent blinded, centralized radiologic evaluation to identify lesions if present and to prospectively assign in-house suspicion scores according to previously described methods [14], as well as Prostate Imaging Reporting and Data System (PI-RADS) classifications starting in 2015. One of two genitourinary radiologists with a minimum of 13 yr of experience interpreting prostate MRI performed an independent review of all mpMRI findings in this series. All MRI intervals were retrospectively scored using the PRECISE criteria as listed in Table 1 [9]. A positive change in MRI was defined as a 1 mm increase in the greatest diameter of index lesion, an increase in overall number of lesions, an increase in suspicion score or PI-RADS score of index lesion, and a change of index lesion to new lesion with the same or a higher suspicion/PI-RADS score (Fig. 1).
Table 1 –
Definition of the PRECISE criteria [13] as well as a count of scores each MRI interval received in our cohort.
| PRECISE criteria | Definition | Intervals, N | Progression to ≥ GG2, N (%) | Progression to ≥ GG3, N (%) |
|---|---|---|---|---|
|
| ||||
| 1 | Resolution of features (no visible lesions) | 61 | 7 (11.5) | 5 (8.2) |
| 2 | Reduction in size/conspicuity of lesions | 173 | 30 (17.3) | 15 (8.7) |
| 3 | Stable MRI appearance; no new lesions | 119 | 21 (17.6) | 10 (8.4) |
| 4 | Increase in size/conspicuity of lesions | 251 | 59 (23.5) | 55 (21.9) |
| 5 | Definitive radiologic stage progression | 17 | 5 (29.4) | 3 (17.6) |
| Total | 621 | 122 (19.6) | 88 (14.2) | |
GG = Gleason grade group; MRI = magnetic resonance imaging; PRECISE = Prostate Cancer Radiologic Estimation of Change in Sequential Evaluation.
Fig. 1 -.
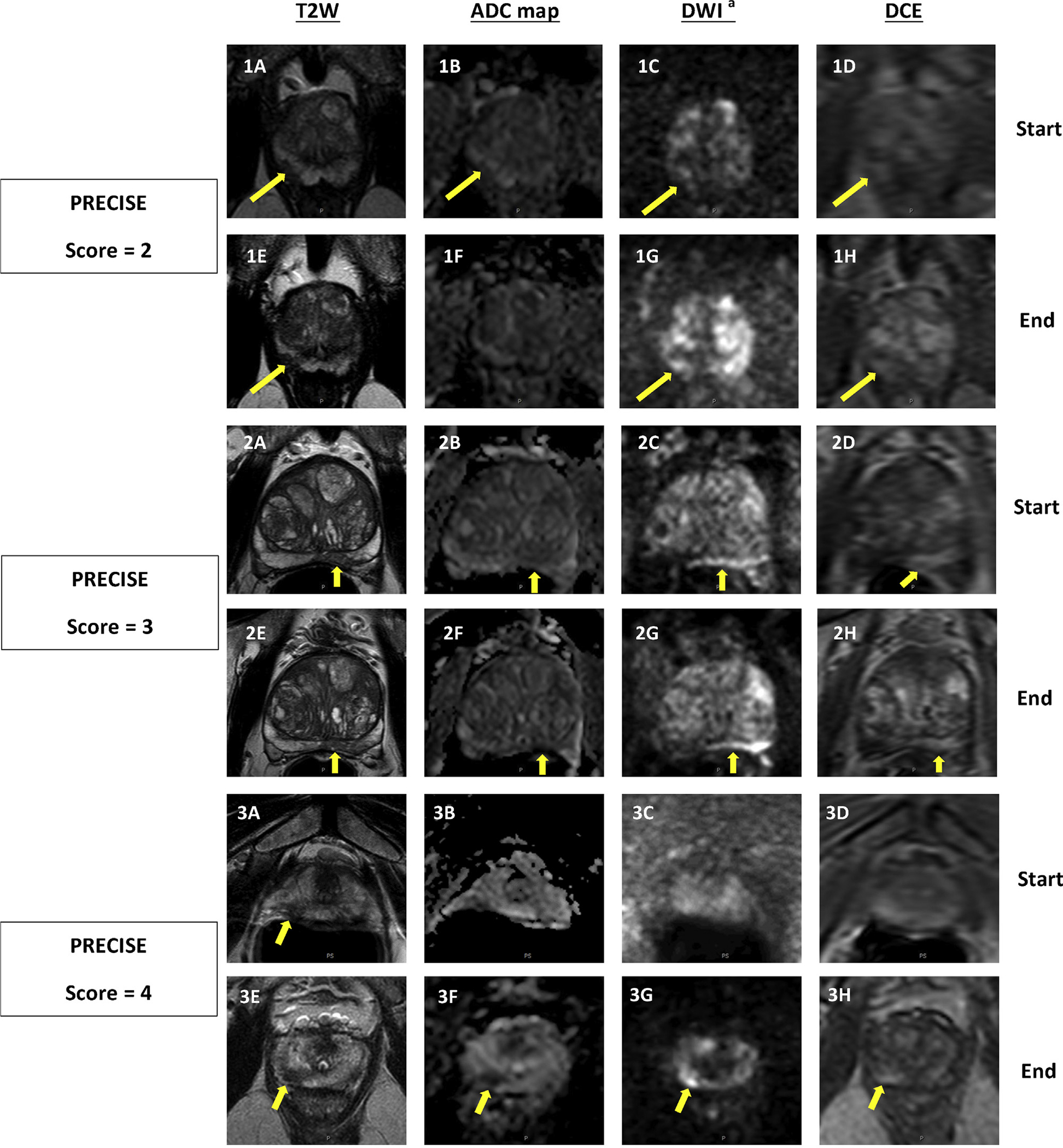
Three sets of intervals demonstrating how the PRECISE criteria are applied based on changing MRI features. Top row of each set (A–D) is at the beginning of the interval, bottom row (E–H) is corresponding to same pulse sequences at the end of the interval. (1) PI-RADS 3 lesion in right apical peripheral zone which was subsequently downgraded to PI-RADS 2 on follow-up MRI, as lesion is no longer visible on ADC map (PRECISE score = 2). PSA change (ng/mL): 5.23 → 9.00; PSAD change (ng/mL2): 0.11 → 0.15; MRI-targeted biopsy result: benign → benign. (2) PI-RADS 4 lesion in the left midbase peripheral zone remained stable on follow-up imaging (PRECISE score = 3). PSA change (ng/mL): 8.46 → 8.42; PSAD change (ng/mL2): 0.08 → 0.07; MRI-targeted biopsy result: benign → benign. (3) PI-RADS 4 lesion increased in size on T2W and became more prominent on DWI on follow-up MRI (PRECISE score = 4). PSA change (ng/mL): 5.16 → 5.81; PSAD change (ng/mL2): 0.15 → 0.14; MRI-targeted biopsy result: not targeted → GG1. ADC = apparent diffusion coefficient; DCE = dynamic contrast enhanced; DWI = diffusion-weighted imaging; GG = Gleason grade group; MRI = magnetic resonance imaging; PI-RADS = Prostate Imaging Reporting and Data System; PRECISE = Prostate Cancer Radiologic Estimation of Change in Sequential Evaluation; PSA = prostate-specific antigen; PSAD = prostate-specific antigen density; T2W = T2 weighted. a b = 1500s/mm2.
2.3. Biopsy protocol
Prior to biopsy, magnetic resonance images were reviewed, segmented, and lesion locations were recorded by a single experienced radiologist using a commercial biopsy software (DynaCAD; Philips). Patients with lesions identified on MRI underwent a targeted biopsy followed by a systematic biopsy. Using the UroNav MRI/ultrasound fusion device (Philips), or research iterations of the same device predating the commercially available device, the targeted biopsy was performed with the previously identified MRI lesions superimposed on the real-time transrectal ultrasound (TRUS) images. All MRI-visible lesions were sampled both in axial and in sagittal planes by an end-fire TRUS probe (Philips). The systematic biopsy was typically 12 cores collected in an extended sextant template from the lateral and medial aspects of the base, mid, and apical prostate on the left and right sides. More biopsy cores were obtained as part of the systematic biopsy if any abnormality was noted on ultrasound. All pathology specimens were reviewed by a single genitourinary pathologist with decades of experience.
2.4. Statistical analysis
For MRI interval-based analysis, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with respect to pathologic progression were calculated at a PRECISE score threshold of ≥4. Pathologic progression was defined as upgrading in GG on biopsy. Statistical inference for these estimates was obtained by the bootstrap resampling procedure with 2000 bootstrap samples drawn at the patient level. The 95% confidence limits were the 2.5% and 97.5% percentiles of the bootstrap samples. Difference in progression detection between PRECISE scores ≥4 and <4 was tested by a modified chi-square test [15]. A generalized estimating equation (GEE) analysis with logic link and exchangeable correlation was applied to MRI intervals with PRECISE score <4 to identify predictors for progression. Robust variance estimates were used to calculate the standard errors of the regression coefficients. Variables that were significant at the univariate analysis were considered for inclusion for the multivariable GEE analysis. Backward variable selection based on the quasi-likelihood under the independence model criterion (QICu) was used to select the best fitting multivariable model [16]. Effects of predictors on progression were measured by odds ratios and average marginal effects [17]. Per-patient detection of progression between PRECISE scores ≥4 and <4 was compared by Fisher’s exact test. Two-sided p < 0.05 was considered statistically significant for all analyses. Holm’s adjusted p values (padj) were used for tests on per-interval and per-patient progression detection with respect to the PRECISE score. All analyses were conducted using R 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
A total of 391 patients met the inclusion criteria of having at least one surveillance MRI scan with combined MRI-targeted plus systematic biopsy. At AS initiation, 73.4% of patients (287/391) had a diagnosis of GG1 and 26.6% (104/391) a diagnosis of GG2 disease. Complete baseline characteristics for included patients can be found in Table 2.
Table 2 –
Baseline clinical characteristics at the time of first mpMRI.
| Clinical characteristics, median (IQR) | |
|---|---|
|
| |
| Age (yr) | 63 (58–68) |
| PSA (ng/mL) | 5.38 (3.95–7.87) |
| Prostate volume (mL) | 51 (38–72) |
| PSAD (ng/mL2) | 0.10 (0.07–0.14) |
| No. of lesions | 2 (1–3) |
| Index lesion diameter (mm) | 10 (7–14) |
| NIH suspicion score, N (%) | |
| No lesion | 19 (4.9) |
| Low | 53 (13.5) |
| Low-moderate | 22 (5.6) |
| Moderate | 244 (62.4) |
| Moderate-high | 22 (5.6) |
| High | 19 (4.9) |
| NA | 12 (3.1) |
| PI-RADS classification, N (%) | |
| 1 | 9 (2.3) |
| 2 | 13 (3.3) |
| 3 | 25 (6.4) |
| 4 | 74 (18.9) |
| 5 | 21 (5.4) |
| NA | 249 (63.7) |
| Gleason grade group, N (%) | |
| 1 | 287 (73.4) |
| 2 | 104 (26.6) |
IQR = interquartile range; mpMRI = multiparametric magnetic resonance imaging; NA = not applicable; PI-RADS = Prostate Imaging Reporting and Data System; PSA = prostate-specific antigen; PSAD = prostate-specific antigen density.
The median follow-up in our cohort was 35.6 mo (interquartile range [IQR] 19.7–60.6). In total, 621 MRI intervals (time between consecutive surveillance MRI + biopsy) were evaluated for change using the PRECISE criteria. The median time between surveillance MRI intervals was 22 mo (IQR 13–31). The median time from MRI to surveillance biopsy was 1 mo (IQR 0–3). The majority of MRI intervals (353/621, 56.8%) did not demonstrate a change in MRI and were considered stable (PRECISE score <4; Table 1). Overall, 163/391 (41.7%) patients demonstrated pathologic progression during the time on surveillance. On a per–MRI interval analysis, progression was detected in 170/621 (27.3%) intervals. Among the 170 intervals with biopsy-confirmed pathologic progression, 122 demonstrated upgrading from GG1 to ≥ GG2, 48 from GG2 to ≥ GG3, and 40 from GG1 to ≥ GG3. When comparing progression rates based on GG, a positive change in MRI (PRECISE score ≥4) was associated with progression compared with stable MRI (PRECISE score <4) for GG1 to ≥ GG2 (37.9% vs 24.0%, padj = 0.004), GG2 to ≥ GG3 (32.3% vs 14.4%, padj = 0.027), and GG1 to ≥ GG3 (15.4% vs 5.8%, padj = 0.013). Overall, the NPV of MRI was highest for GG1 to ≥ GG3 progression (0.94 [CI 0.91–0.97]). The PPV, sensitivity, and specificity of MRI to predict any level of progression is presented in Table 3.
Table 3 –
Overall sensitivity, specificity, PPV, and NPV of a change in MRI capturing pathologic progression.
| Progression endpoints | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|
|
| |||||
| Change in MRI | GG1–≥GG2 | 0.53 (0.44–0.61) | 0.64 (0.58–0.70) | 0.38 (0.30–0.46) | 0.76 (0.71–0.81) |
| GG1–≥GG3 | 0.65 (0.50–0.80) | 0.62 (0.56–0.67) | 0.15 (0.10–0.21) | 0.94 (0.91–0.97) | |
| GG2–≥GG3 | 0.67 (0.53–0.80) | 0.59 (0.50–0.67) | 0.32 (0.24–0.42) | 0.86 (0.78–0.92) | |
GG = Gleason grade group; MRI = magnetic resonance imaging; NPV = negative predictive value; PPV = positive predictive value; PRECISE = Prostate Cancer Radiologic Estimation of Change in Sequential Evaluation.
A positive change in MRI is defined by a PRECISE score of ≥4, whereas negative/stable MRI is defined by a PRECISE score of <4.
On a per-patient analysis, 194/272 (71.3%) patients started with GG1 disease and 78/272 (28.7%) with GG2 who had a follow-up biopsy at 2 yr. In total, 28.4% (55/194) and 8.25% (16/194) of patients starting with GG1 progressed to GG2 and ≥ GG3 disease, respectively, while 33.3% (26/78) of patients starting from GG2 progressed to ≥ GG3. In total, 12/85 patients who had a detected change in MRI had GG1 to ≥ GG3 disease progression on biopsy, while 4/109 patients with stable MRI were found to have GG1 to ≥ GG3 progression (14.1% vs 3.67%, padj = 0.047). A change in MRI was not associated with progression from GG1 to ≥ GG2 (36.5% vs 22.0%, padj = 0.073) or from GG2 to ≥ GG3 (44.7% vs 22.5%, padj = 0.073). Table 4 summarizes the number of biopsies avoided if biopsy was performed only for a positive change in MRI (PRECISE score ≥4).
Table 4 –
Total number of biopsies performed and avoided if performing biopsy only for a positive change in MRI (PRECISE scores ≥4) at 2-yr follow-up.
| Biopsy threshold | Progression endpoints | Total biopsies, N | Total progressed, N (%) | Performed biopsies, N | Avoided biopsies, N | Missed progression in avoided biopsies, N (%) |
|---|---|---|---|---|---|---|
|
| ||||||
| Positive change in MRI | GG1–>GG2 | 194 | 55 (28.4) | 85 | 109 | 24 (22) |
| GG1–>GG3 | 194 | 16 (8.25) | 85 | 109 | 4 (3.7) | |
| GG2–>GG3 | 78 | 26 (33.3) | 38 | 40 | 9 (22.5) | |
GG = Gleason grade group; MRI = magnetic resonance imaging; PRECISE = Prostate Cancer Radiologic Estimation of Change in Sequential Evaluation.
For intervals with stable MRI, we found the combined effect of change in PSA with PSA density (PSAD) and the size of index lesion to be risk factors for progression from GG1 to ≥ GG2 on logistic regression. For progression from GG1 to ≥ GG3, PSAD was the only risk factor for progression. These results are summarized in Table 5. A similar table with marginal and incremental effects for these variables can be found in Supplementary Table 2. No clinically significant risk factors were identified for detecting progression from GG2 to ≥ GG3.
Table 5 –
Risk factors for progression among men with stable MRI (PRECISE score <4).
| Stable MRI (PRECISE score of <4) | Progression from GG1 to ≥ GG2 |
Progression from GG1 to ≥ GG3 |
||||
|---|---|---|---|---|---|---|
| Univariable |
Multivariable |
Univariable |
||||
| OR (95% CL) | p value | OR (95% CL) | p value | OR (95% CL) | p value | |
|
| ||||||
| Age (per decade) | 1.11 (0.705–1.748) | 0.651 | – | – | 1.664 (0.65–4.259) | 0.288 |
| PSA (log base 2) | 1.227 (0.915–1.645) | 0.172 | – | – | 1.811 (1.145–2.864) | 0.011 |
| PSAD (log base 2) | 1.676 (1.138–2.468) | 0.009 | 1.393 (0.894–2.172) | 0.143a | 1.951 (1.172–3.246) | 0.01 |
| ΔPSA (log base 2) | 2.174 (1.201–3.935) | 0.01 | 1.741 (0.904–3.354) | 0.097a | 1.961 (0.476–8.07) | 0.351 |
| ΔPSAD (log base 2) | 2.234 (1.117–4.467) | 0.023 | – | – | 1.409 (0.368–5.389) | 0.617 |
| Number of lesions | 1.32 (1.011–1.724) | 0.041 | – | – | 1.023 (0.583–1.795) | 0.936 |
| size of lesions (per mm) | 1.089 (1.024–1.159) | 0.007 | 1.07 (1.005–1.14) | 0.035 | 1.019 (0.904–1.148) | 0.76 |
| PI-RADS ≥4 vs <4 | 1.467 (0.677–3.178) | 0.331 | – | – | 0.94 (0.165–5.344) | 0.944 |
| NIH ≥ moderate vs < moderate | 1.555 (0.813–2.977) | 0.182 | – | – | 3.929 (0.812–19.004) | 0.089 |
CL = confidence limit; GG = Gleason grade group; MRI = magnetic resonance imaging; NIH = National Institutes of Health; OR=odds ratio; PI-RADS = Prostate Imaging Reporting and Data System; PRECISE = Prostate Cancer Radiologic Estimation of Change in Sequential Evaluation; PSA=prostate-specific antigen; PSAD = prostate-specific antigen density.
Combined effect of PSAD and ΔPSA was significant (p = 0.024).
4. Discussion
In this report, we investigated how changes in MRI, scored by PRECISE, were related to pathologic progression during AS. While there have been studies evaluating the interobserver reproducibility of PRECISE [18,19], there have been limited reports on the efficacy of this scoring system despite its release in 2017 [9–11,20]. Our study not only provides the largest AS cohort ever to be assessed with the PRECISE criteria, but also provides a comprehensive analysis on the efficacy of MRI to capture pathologic progression.
Our unique per-interval study design enabled us to analyze MRI data from each patient’s entire duration on AS and did not restrict us to a single time point. A PRECISE score of 4 represents a logical biopsy threshold as it not only has been used in previous studies [20], but, by definition, also describes MRI intervals with evidence of progression (Table 1) [9]. We found a stable MRI interval to have an overall NPV of 0.94 for progression from GG1 to ≥ GG3, suggesting that patients with stable surveillance MRI intervals (PRECISE scores <4) have low rates of progression when biopsied. When we analyzed our cohort on a per-patient basis at 2-yr follow-up, we were able to calculate the number of biopsies avoided if performing biopsy only for a positive change in MRI (PRECISE score ≥4; Table 4). Of the 109 avoided biopsies for patients with GG1 disease, only 3.7% (4/109) would have resulted in a missed progression to ≥ GG3 disease. This is a notable finding as urologists can have greater confidence that stable MRI at 2-yr follow-up for patients with GG1 disease is unlikely to result in upgrading to ≥ GG3.
As more than one in five stable MRI intervals (21.0%, 74/353) resulted in GG upgrading, we performed a logistic regression to identify clinical characteristics predictive of pathologic progression in the setting of stable MRI (PRECISE score <4). Most notably, PSAD was an important factor for progression from GG1 to both ≥ GG2 and ≥ GG3 disease (Table 5).
There is high-level evidence that supports the use of MRI for the selection of patients for AS [21]. In the 2-yr follow-up data from the ASIST trial, Klotz et al [21] reported that MRI and MRI-targeted biopsy at enrollment resulted in 50% reduction in AS failures compared with systematic biopsy. However, evidence is still developing on the ability of serial MRI examinations to capture pathologic progression and replace surveillance biopsy. The results from our study are in line with other studies that support the ability of negative MRI to rule out progression [7,10,22,23]. When specifically looking at 2-yr follow up, we found the NPV of negative MRI to be very high for detecting progression from GG1 to ≥ GG3 (0.96). This finding is very similar to the results from a recent study by Osses et al [20], which reported that only 4% (4/94) of patients with stable MRI (PRECISE score <4) progressed from GG1 to ≥ GG3 at 1-yr follow-up biopsy. While these data are encouraging, it is important to note that, in our study, avoiding biopsy for patients with stable MRI would have resulted in missing 25% (4/16) of progressions from GG1 to ≥ GG3 (Table 4). As this was a low-risk AS cohort, the overall prevalence of GG progression events in our study was low, which inherently drives the high NPV of stable MRI (PRECISE score <4). In addition, positive changes in MRI demonstrated moderate sensitivity for capturing pathologic progression, especially for GG1 to ≥ GG2 (0.53). A study by Chesnut et al [6] found that omitting biopsy for patients with no MRI change would have resulted in missing 53% of cancer progression from GG1 to ≥ GG2. However, as recent studies have shown that select patients with GG2 disease can safely be monitored on AS [24], we chose to also investigate progression to ≥ GG3 as an endpoint for our study. As the number of progressions from GG1 to ≥ GG3 missed by MRI cannot be ignored, other clinical factors can be used in conjunction with stable MRI to rule of pathologic progression. There have been several studies demonstrating that increased PSAD is more likely to result in the detection of clinically significant disease [25,26]. The results from our logistic regression support these findings and suggest that patients with elevated PSAD should have more regularly scheduled surveillance biopsies, regardless of MRI findings on AS.
Our study has some limitations. All MRI images were reviewed by one of two genitourinary radiologists in a dedicated MR facility with a minimum of 13 yr of experience in prostate mpMRI interpretation. Our definition of a change in MRI included a 1 mm change in tumor size, which may be an issue due to inter-reader variability in size measurements. All combined biopsies were performed by a single urologic oncologist with decades of experience in MRI-targeted biopsies. Thus, this single-institution study at an academic medical center may not be generalizable to the community setting, as there is a well-documented learning curve for both MRI-targeted biopsies and mpMRI interpretation [27]. While most patients received regular biopsies, some patients with negative MRI did not receive biopsy, which could be a potential confounder in our study results. Lastly, our definition of pathologic progression was strictly progression in GG without consideration of the percentage of Gleason pattern 4. We acknowledge that this may limit the generalizability of our results as many urologists use this to determine AS eligibility. While the results from our study suggest that stable MRI (PRECISE score <4) can be used to rule out pathologic progression, these findings need to be validated in a larger, multi-institutional cohort.
5. Conclusions
In this study, we report on the ability of changes in MRI, scored with PRECISE, to capture pathologic progression. Patients with stable MRI (PRECISE scores <4) have a low probability of detecting progression from GG1 to ≥ GG3 on biopsy. Elevated PSAD increases the risk of cancer progression despite stable MRI.
Supplementary Material
Funding/Support and role of the sponsor:
This research was made possible through the NIH Medical Research Scholars Program, a public private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, Genentech, the American Association for Dental Research, the Colgate-Palmolive Company, and other private donors. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E. NIH does not endorse or recommend any commercial products, processes, or services. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The views and personal opinions of authors expressed herein do not necessarily reflect those of the US Government, nor reflect any official recommendation or opinion of the NIH or NCI.
Footnotes
Financial disclosures: Peter A. Pinto certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: NIH and Philips have a cooperative research and development agreement. NIH has intellectual property in the field, including among other patents and patent applications, Patent: “System, methods, and instrumentation for image guided prostate treatment” US Patent number: 8948845, with inventors/authors including Peter L. Choyke, Bradford J. Wood, and Peter A. Pinto. NIH and Philips (InVivo Inc.) have a licensing agreement. NIH and authors Peter L. Choyke, Bradford J. Wood, and Peter A. Pinto receive royalties for a licensing agreement with Philips/InVivo Inc.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.euo.2020.09.004.
References
- [1].Ahdoot M, Wilbur AR, Reese SE, et al. MRI-targeted, systematic, and combined biopsy for prostate cancer diagnosis. N Engl J Med 2020;382:917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med 2018;378:1767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bjurlin MA, Carroll PR, Eggener S, et al. Update of the AUA policy statement on the use of multiparametric magnetic resonance imaging in the diagnosis, staging and management of prostate cancer. J Urol 2020;203:706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bloom JB, Hale GR, Gold SA, et al. Predicting Gleason group progression for men on prostate cancer active surveillance: role of a negative confirmatory magnetic resonance imaging-ultrasound fusion biopsy. J Urol 2019;201:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jayadevan R, Felker ER, Kwan L, et al. Magnetic resonance imaging guided confirmatory biopsy for initiating active surveillance of prostate cancer. JAMA Netw Open 2019;2:e1911019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chesnut GT, Vertosick EA, Benfante N, et al. Role of changes in magnetic resonance imaging or clinical stage in evaluation of disease progression for menwith prostate cancer on active surveillance. Eur Urol 2020;77:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Frye TP, George AK, Kilchevsky A, et al. Magnetic resonance imaging-transrectal ultrasound guided fusion biopsy to detect progression in patients with existing lesions on active surveillance for low and intermediate risk prostate cancer. J Urol 2017;197(3 Pt 1):640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mehralivand S, Shih JH, Rais-Bahrami S, et al. A magnetic resonance imaging-based prediction model for prostate biopsy risk stratification. JAMA Oncol 2018;4:678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Moore CM, Giganti F, Albertsen P, et al. Reporting magnetic resonance imaging in men on active surveillance for prostate cancer: the PRECISE recommendations—a report of a European School of Oncology Task Force. Eur Urol 2017;71:648–55. [DOI] [PubMed] [Google Scholar]
- [10].Dieffenbacher S, Nyarangi-Dix J, Giganti F, et al. Standardized magnetic resonance imaging reporting using the prostate cancer radiological estimation of change in sequential evaluation criteria and magnetic resonance imaging/transrectal ultrasound fusion with transperineal saturation biopsy to select men on active surveillance. Eur Urol Focus. In press. 10.1016/j.euf.2019.03.001. [DOI] [PubMed] [Google Scholar]
- [11].Ullrich T, Arsov C, Quentin M, et al. Multiparametric magnetic resonance imaging can exclude prostate cancer progression in patients on active surveillance: a retrospective cohort study. Eur Radiol 2020;30:6042–51. 10.1007/s00330-020-06997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2019;17:479–505. [DOI] [PubMed] [Google Scholar]
- [13].Mehralivand S, Shih JH, Harmon S, et al. A grading system for the assessment of risk of extraprostatic extension of prostate cancer at multiparametric MRI. Radiology 2019;290:709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gaur S, Harmon S, Mehralivand S, et al. Prospective comparison of PI-RADS version 2 and qualitative in-house categorization system in detection of prostate cancer. J Magn Reson Imaging 2018;48:1326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shih JH, Fay MP. Pearson’s chi-square test and rank correlation inferences for clustered data. Biometrics 2017;73:822–34. [DOI] [PubMed] [Google Scholar]
- [16].Pan W Akaike’s information criterion in generalized estimating equations. Biometrics 2001;57:120–5. [DOI] [PubMed] [Google Scholar]
- [17].Norton EC, Dowd BE. Log odds and the interpretation of logit models. Health Serv Res 2018;53:859–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Giganti F, Pecoraro M, Fierro D, et al. DWI and PRECISE criteria in men on active surveillance for prostate cancer: a multicentre preliminary experience of different ADC calculations. Magn Reson Imaging 2019;67:50–8. [DOI] [PubMed] [Google Scholar]
- [19].Giganti F, Pecoraro M, Stavrinides V, et al. Interobserver reproducibility of the PRECISE scoring system for prostate MRI on active surveillance: results from a two-centre pilot study. Eur Radiol 2020;30:2082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Osses DF, Drost FH, Verbeek JFM, et al. Prostate cancer upgrading with serial prostate magnetic resonance imaging and repeat biopsy in men on active surveillance: are confirmatory biopsies still necessary? BJU Int 2020;126:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Klotz L, Pond G, Loblaw A, et al. Randomized study of systematic biopsy versus magnetic resonance imaging and targeted and systematic biopsy in men on active surveillance (ASIST): 2-year postbiopsy follow-up. Eur Urol 2020;77:311–7. [DOI] [PubMed] [Google Scholar]
- [22].Panebianco V, Barchetti G, Simone G, et al. Negative multiparametric magnetic resonance imaging for prostate cancer: what’s next? Eur Urol 2018;74:48–54. [DOI] [PubMed] [Google Scholar]
- [23].Walton Diaz A, Shakir NA, George AK, et al. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol 2015;33:202, e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stavrinides V, Giganti F, Trock B, et al. Five-year outcomes of magnetic resonance imaging-based active surveillance for prostate cancer: a large cohort study. Eur Urol 2020;78:443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pagniez MA, Kasivisvanathan V, Puech P, Drumez E, Villers A, Olivier J. Predictive factors of missed clinically significant prostate cancers in men with negative MRI: a systematic review and meta-analysis. J Urol 2020;204:24–32. [DOI] [PubMed] [Google Scholar]
- [26].Washington SL 3rd, Baskin AS, Ameli N, et al. MRI-based prostate-specific antigen density predicts Gleason score upgrade in an active surveillance cohort. AJR Am J Roentgenol 2020;214:574–8. [DOI] [PubMed] [Google Scholar]
- [27].Kasabwala K, Patel N, Cricco-Lizza E, et al. The learning curve for magnetic resonance imaging/ultrasound fusion-guided prostate biopsy. Eur Urol Oncol 2019;2:135–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


