Abstract
We evaluated sex differences in MRI-based volume loss and differences in predictors of this neurodegeneration in cognitively healthy older adults. Mixed-effects regression was used to compare regional brain volume trajectories of 295 male and 328 female cognitively healthy Baltimore Longitudinal Study of Aging participants, aged 55 to 92 years, with up to 20 years of follow-up and to assess sex differences in the associations of age, hypertension, obesity, APOE e4 carrier status, and HDL cholesterol with regional brain volume trajectories. For both sexes, older age was associated with steeper volumetric declines in many brain regions, with sex differences in volume loss observed in frontal, temporal and parietal regions. In males, hypertension and higher HDL cholesterol were protective against volume loss in hippocampus, entorhinal cortex, and parahippocampal gyrus. In females, hypertension was associated with steeper volumetric decline in gray matter, and obesity was protective against volume loss in temporal gray matter. Predictors of volume change may affect annual rates of volume change differently between men and women.
Keywords: neurodegeneration, cardiovascular risk factors, cognitively normal
1. Introduction
Evidence from post-mortem and in vivo imaging studies shows that advancing age is associated with brain atrophy and ventricular enlargement cross-sectionally and longitudinally (Coffey et al., 1998; Coffey et al., 1992; Courchesne et al., 2000; Gur et al., 1991; Murphy et al., 1996; Pfefferbaum et al., 1994; Resnick et al., 2003). It has also been suggested that in vivo imaging studies examining structural regional volumes conducted longitudinally may serve as proxies of brain atrophy (Jack Jr et al., 2017), thus representing a biomarker for neurodegeneration.
Among the factors that may affect age-related brain changes in cognitively normal older adults, sex could play a prominent role. Some cross-sectional studies have found that males have larger brain volumes than females after correcting for body size differences (Allen et al., 2003; Cosgrove et al., 2007; Lüders et al., 2002; Nopoulos et al., 2000; Ritchie et al., 2018), yet others found either null or opposite findings (Fjell et al., 2009; Greenberg et al., 2008; Lemaître et al., 2005; Raz et al., 1997; Salat et al., 2004; Sowell et al., 2007). On the other hand, a number of studies indicate that males have a lower ratio of gray to white matter volume than females (Allen et al., 2003; Goldstein et al., 2001; Gur et al., 1999). Additionally, males have lower cortical thickness and smaller frontal lobar volume than females, which may be suggestive of a sex-related vulnerability (Murphy et al., 1996; Xu et al., 2000).
In prior work, our group has reported that males show greater age-related cortical thinning and brain volume changes than females over a follow-up period of up to 10 years (Driscoll et al., 2009; Pacheco et al., 2015; Thambisetty et al., 2010). In contrast, other studies with fewer repeated observations or shorter follow-up intervals, and thus decreased power to detect differences, observed no sex differences in cortical thinning and volumetric change longitudinally (Persson et al., 2014; Persson et al., 2016; Raz et al., 2010; Raz et al., 2005; Yuan et al., 2018). Despite differences in the findings of these studies, it is possible that sex differences in cardiovascular disease (CVD) risk, especially among those who do not develop cognitive impairment, contribute to observations of sex differences in age-related brain volume changes. The effects of hypertension, obesity, and dyslipidemia on cardiovascular-related events are similar between men and women, yet prolonged smoking (Prescott et al., 1998) and diabetes (Huxley et al., 2006; Peters et al., 2014) are more detrimental to women than men. Information on possible effects of age-associated co-morbidities and cardiovascular risk factors on brain regional volumetric change by sex could elucidate potential preventative and treatment measures that differ between males and females. In our recent report, we identified age, hypertension, obesity, APOE e4 carrier status, and HDL cholesterol as predictors of volumetric change in a sample of cognitively normal older adults (Armstrong et al., 2019). Thus, in the current paper we focused on whether there were sex differences in the associations of these pre-determined predictors with volume change within a sample of older men and women who did not develop incident cognitive impairment during the follow-up period.
Since sex may play a prominent and independent role in both longitudinal brain volumetric changes and associations of predictors of volumetric change among older adults who remain cognitively normal, we investigated a sample of 617 community-dwelling older adults who remained cognitively healthy over follow-up of up to 20 years. We first investigated whether rates of longitudinal MRI-based tissue loss in the overall sample varied by sex. Based on the prior findings of greater cognitive decline in older men compared with women (McCarrey et al., 2016), we hypothesized that males would show greater age-related volume loss than females. We then stratified the sample by sex to determine whether differential patterns of predictors of volume change emerged in each group separately. We hypothesized that the patterns of these predictors of neurodegeneration would differ between males and females, with males showing greater vulnerability to potential effects of these predictors on volume change.
2. Materials and Methods
2.1. Characteristics of the Study Sample
There were 889 participants from the BLSA neuroimaging substudy who were followed from February 1994 to December 2015 for up to 23 years. The BLSA imaging and visit schedules have varied over time, and enrollment into BLSA has been continuous. Participants in the original imaging study had annual imaging assessments from 1994–2004, and they were enrolled based on enrollment procedures described elsewhere (Armstrong et al., 2019). Thereafter, participants aged 60–79 years had biennial BLSA and imaging visits, while participants aged ≥80 years had annual visits. Supplemental Figure 1 illustrates the inclusion and exclusion criteria of the study and defines cognitive status. The analytic sample consisted of 617 participants with 1,728 scans over a 20-year period. There were 57 deceased (9.2%) and 38 (6.2%) withdrawn participants. More males (n=42) than females (n=15) died during the period, but withdrawn rates were similar between males and females. The local Institutional Review Board approved the research protocol for this study, and written informed consent was obtained at each visit from all participants.
2.2. Predictors of Neurodegeneration
Based on a previous study using BLSA data that examined differences in associations of predictors of volume change by cognitive status (Armstrong et al., 2019), we examined predictors that were related to volume change. These predictors included mean-centered age, sex, race (white vs. non-white), APOE e4 carrier status (≥1 vs. 0 e4 alleles), obesity (body mass index ≥30 kg/m2 vs. <30 kg/m2 ), and hypertension (systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg or treatment with antihypertensive medications).
2.3. Image Acquisition
Scanning was performed on a General Electric (GE) Signa 1.5 T scanner (Milwaukee, WI) or a 3T Philips Achieva. GE 1.5-T scans used a high-resolution volumetric spoiled gradient recalled acquisition in a steady state (GRASS) series (axial acquisition, repetition time=35msec, echo time=5msec, flip angle=45°, field of view=24 cm, matrix=256×256, number of excitations=1, voxel dimensions=0.94×0.94×1.5 mm slice thickness). T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) scans were acquired on a 3T Philips Achieva (repetition time [TR]=6.8msec, echo time [TE]=3.2msec, flip angle=8°, image matrix=256×256, 170 slices, pixel size=1×1mm, slice thickness=1.2mm). There were 524 participants (1,055 scans) with 3-T MPRAGE images and 99 participants (692 scans) with 1.5-T SPGR images at baseline. Participants receiving 1.5-T scans comprised enrollees in the original BLSA neuroimaging substudy dating back to 1994 (Resnick et al. 2000).
2.4. Harmonization of MUSE Anatomical Labels across 1.5-T SPGR and 3-T MPRAGE
A new automated labeling method specifically designed to achieve a consistent parcellation of brain anatomy in longitudinal MRI studies with scanner and imaging protocol differences was used to harmonize BLSA MRI data. This method combines the MUSE anatomical labeling approach (Doshi et al., 2016) with harmonized acquisition-specific atlases (Erus et al., 2018). The approach is described in more detail in Erus et al. (2018). Briefly, using 35 labeled 3-T MPRAGE brain MRIs from the OASIS data set as atlases, we first performed the MUSE labeling method on 3-T MPRAGE images for 32 BLSA participants with 1.5-T SPGR at an earlier time point. Then, for each participant, we deformably registered their 1.5-T SPGR image to their 3-T MPRAGE image using a robust registration strategy that combines an ensemble of registrations obtained using two different algorithms and multiple smoothness parameters. From these steps, we obtained 32 pairs of 1.5-T SPGR and 3-T MPRAGE images in the same space with common anatomical labels, which served as atlases in the MUSE approach to obtain labels on the entire BLSA collection of 1.5-T SPGR and 3-T MPRAGE images. This workflow for anatomical labeling has been extensively validated on the BLSA MRI data set (Erus et al., 2018). Stability measures for longitudinal volumes were consistent over time, with intraclass correlations of volumes ranging from 0.89 to 0.99 within 1.5T and 3T scanners and 0.84 to 0.97 between 1.5-T and 3-T among the sample that remained cognitively normal.
2.5. Volumes of Regions of Interest
We examined volumes for whole brain, total gray matter (GM), total white matter (WM), ventricles, and lobar GM and WM (frontal, temporal, parietal, and occipital). Sex differences in volumetric change were reported as widespread in a previous analysis using BLSA data (Driscoll et al., 2009). We also examined the volume of the corpus callosum (Luders et al., 2014), as sex differences have been shown in this region. Lastly, we included volumes of the amygdala, hippocampus, entorhinal cortex, and parahippocampal gyrus, areas implicated in Alzheimer’s disease pathology.
2.6. Statistical Analysis
We characterized the sample using means and percentages, and we evaluated differences in baseline sample characteristics by sex, using two-sample t-tests for continuous variables and χ2 tests for categorical variables. Type I error level was set to 0.05 for ROI analyses, and we applied Bonferroni correction of p≤0.003 for multiple comparisons adjustment for 17 brain ROIs. Stata SE 15.0 (StataCorp, 2017) was used for all analyses.
2.6.1. Baseline and Longitudinal Brain Volumetric Change as a Function of Sex
Linear mixed-effects models were used to compare baseline and longitudinal changes in global and lobar regions in the overall sample. They account for the variability in visits, follow-up time, unequal number of measurements, and age, since BLSA participants enter in the study at different ages, have been in the study for various lengths of time, have unequally spaced follow-up visits, and have unequal numbers of measurements. The models use all available data (a snapshot in time) and do not rely on listwise deletion.
Our base model consisted of fixed effects, i.e., baseline intracranial volume (ICV), image type (1.5-T SPGR vs. 3-T MPRAGE), age, sex, race, time since first MRI, and two-way interactions of image type, age, sex, and race with time, and random effects (intercept and slope) with unstructured covariance. We used baseline rather than time-varying ICV to account for head size variation, as previously recommended for longitudinal studies (Barnes et al., 2010; Pengas et al., 2009). Random effects allowed individual-specific baseline brain volumes and rates of volumetric change to vary. Effect sizes (ES) for the difference in baseline ROI volumes were calculated by dividing the estimated difference in the baseline volumes between males and females by the estimated standard deviation (SD) at baseline. Additionally, ES for difference in rates of ROI volumetric changes by sex were calculated by dividing the estimated difference in annual rates of change by the estimated SD of the between-subject rates of change. Given that this analysis was exploratory, all results are reported in tables to help guide future research.
As a secondary analysis, we examined the association of sex with global and lobar volumetric change in a sample without baseline vascular burden. Vascular burden was a cumulative score of current smoking status, hypertension diagnosis, diabetes (fasting glucose >125 mg/dL, a pathologic oral glucose tolerance test, or a positive history of a diagnosis plus treatment with oral anti-diabetic drugs or insulin), obesity, and elevated total cholesterol (≥200 mg/dl vs. <200 mg/dl) (Gottesman et al., 2017).
2.6.2. Predictors of Volumetric Change as a Function of Sex
To evaluate the association of predictors of volumetric change among cognitively normal participants, we added the following fixed effects to our base model: hypertension, obesity, APOE e4 carrier status, HDL cholesterol, and two-way interactions of hypertension, obesity, APOE e4 carrier status, and HDL cholesterol with time. Individual three-way interactions (predictor*sex*time) were significant when evaluating the volume changes in certain, not all, regions of interest as a function of each predictor, sex, and time in the overall sample (Supplemental Table 2). However, when all three-way interactions were added to the base model, the three-way interactions were no longer significant, which could be due to the analysis being underpowered. For consistency and ease of interpretation across regions of interest, we then stratified the linear mixed effects models by sex to determine if there are differences in patterns of associations of predictors of volume change between males and females. We performed sensitivity analyses to examine the associations if we excluded 1.5-T scans from the sex-stratified models.
3. Results
3.1. Characteristics of Study Sample
Table 1 shows the baseline sample characteristics for the overall sample and by sex. On average, males (n=291) were older and had more years of education, lower HDL cholesterol, higher systolic and diastolic blood pressure, and greater ICV than females (n=326) (Table 1). Also, males were more likely to be white and current smokers as well as have diabetes and hypertension than females. Distributions of obesity, APOE e4 carrier status, vascular burden, use of any antihypertensive medication, and follow-up time were similar between males and females.
Table 1.
Sample Characteristics from the Baltimore Longitudinal Study of Aging (N=617)
| Baseline Characteristics | Overall N=617 | Females N=326 | Males N=291 | p-value for difference by sex |
|---|---|---|---|---|
| Age, in years, mean(SD) | 71.2 (8.7) | 70.3 (8.7) | 72.2 (8.5) | 0.008 |
| White, n(%) | 450 (72.9) | 214 (65.6) | 236 (81.1) | <0.001 |
| Education, in years, mean(SD) | 16.9 (2.5) | 16.6 (2.5) | 17.2 (2.5) | 0.005 |
| APOE e4 carrier status, n(%) | 127 (20.6) | 77 (23.6) | 50 (17.2) | 0.072 |
| Vascular Burden, n(%) | 0.055 | |||
| 0 conditions | 221 (35.5) | 105 (32.2) | 116 (39.3) | |
| 1 condition | 259 (42.0) | 151 (46.3) | 108 (37.1) | |
| 2+ conditions | 137 (22.2) | 70 (21.5) | 67 (23.0) | |
| Components of Vascular Burden, n(%) | ||||
| Diabetes | 26 (4.2) | 5 (1.5) | 21 (7.2) | <0.001 |
| Elevated Cholesterol | 231 (37.4) | 157 (48.1) | 74 (25.4) | <0.001 |
| Hypertension | 129 (20.9) | 51 (15.6) | 78 (26.8) | 0.001 |
| Obesity | 154 (25.0) | 85 (26.1) | 69 (23.7) | 0.643 |
| Current Smoker | 20 (3.2) | 4 (1.2) | 16 (5.5) | 0.003 |
| Systolic Blood Pressure, in mm Hg, mean(SD) | 119.0 (17.8) | 116.0 (16.4) | 122.3 (18.7) | <0.001 |
| Diastolic Blood Pressure, in mm Hg, mean(SD) | 67.6 (11.2) | 66.5 (10.7) | 68.8 (11.6) | 0.010 |
| Antihypertensive medications, n(%) | 265 (43.2) | 128 (39.3) | 137 (47.1) | 0.067 |
| HDL Cholesterol, in mg/dL, mean(SD) | 59.9 (17.5) | 67.1 (16.8) | 51.8 (14.5) | <0.001 |
| Baseline 3-T Scan, n(%) | 519 (84.1) | 285 (87.4) | 234 (80.4) | 0.017 |
| Number of 3-T Scans, n(%) | 1,045 (60.5) | 580 (65.8) | 465 (54.9) | <0.001 |
| Intracranial Volume, mean(SD) | 1397.8 (143.2) | 1305.7 (106.4) | 1488.3 (114.9) | <0.001 |
| Follow-up Time, mean (SD) | 3.5 (4.7) | 3.6 (5.0) | 3.4 (4.5) | 0.409 |
| Follow-up Time for those with ≥2 visits, mean (SD) | 4.1 (1.9) | 4.3 (5.2) | 3.9 (4.6) | 0.192 |
| Number of Follow-up Visits, n(%) | 0.786 | |||
| 1 | 617 (100.0) | 326 (100.0) | 291 (100.0) | |
| 2 | 368 (59.6) | 189 (58.0) | 179 (61.5) | |
| 3 | 172 (27.9) | 89 (27.3) | 83 (28.5) | |
| 4 | 101 (16.4) | 45 (13.8) | 56 (19.2) | |
| 5 | 83 (13.5) | 36 (11.0) | 47 (16.2) | |
| 6 | 73 (11.8) | 33 (10.1) | 40 (13.7) | |
| 7 | 67 (10.9) | 31 (9.5) | 36 (12.4) | |
| 8 | 59 (9.6) | 28 (8.6) | 31 (10.7) | |
| 9 | 49 (7.9) | 24 (7.4) | 25 (8.6) | |
| 10 | 42 (6.8) | 22 (6.7) | 20 (6.9) | |
| 11 | 34 (5.5) | 19 (5.8) | 15 (5.2) | |
| 12+ | 63 (10.2) | 39 (12.0) | 24 (8.2) |
SD – standard deviation, ICV – intracranial volume, CHF – congestive heart failure, HDL – high-density lipoprotein, LDL – low – density lipoprotein Note: We used t-tests for continuous variables and chi-squared tests for categorical variables. There were 142 (23.0%) missing for APOE e4 genotype, 3 (0.5%) missing for baseline elevated cholesterol, 4 (0.7%) missing for antihypertensive medications, and 3 (0.5%) missing for baseline obesity status.
3.2. Baseline and Longitudinal Brain Volumetric Change as Function of Sex
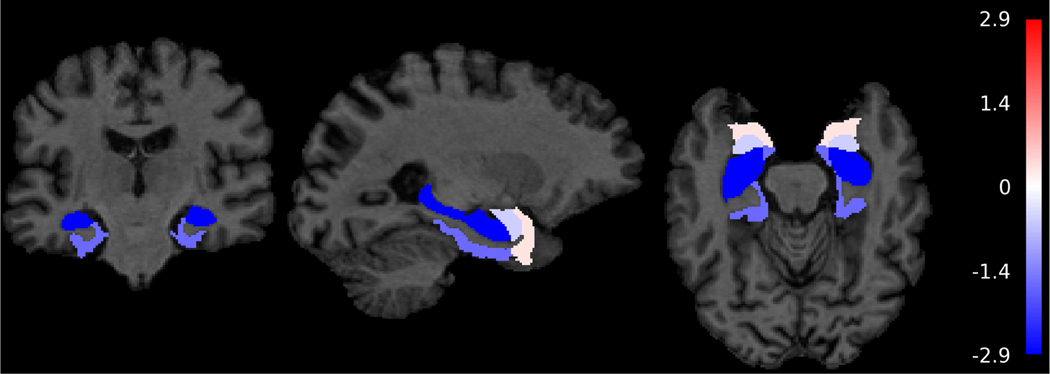
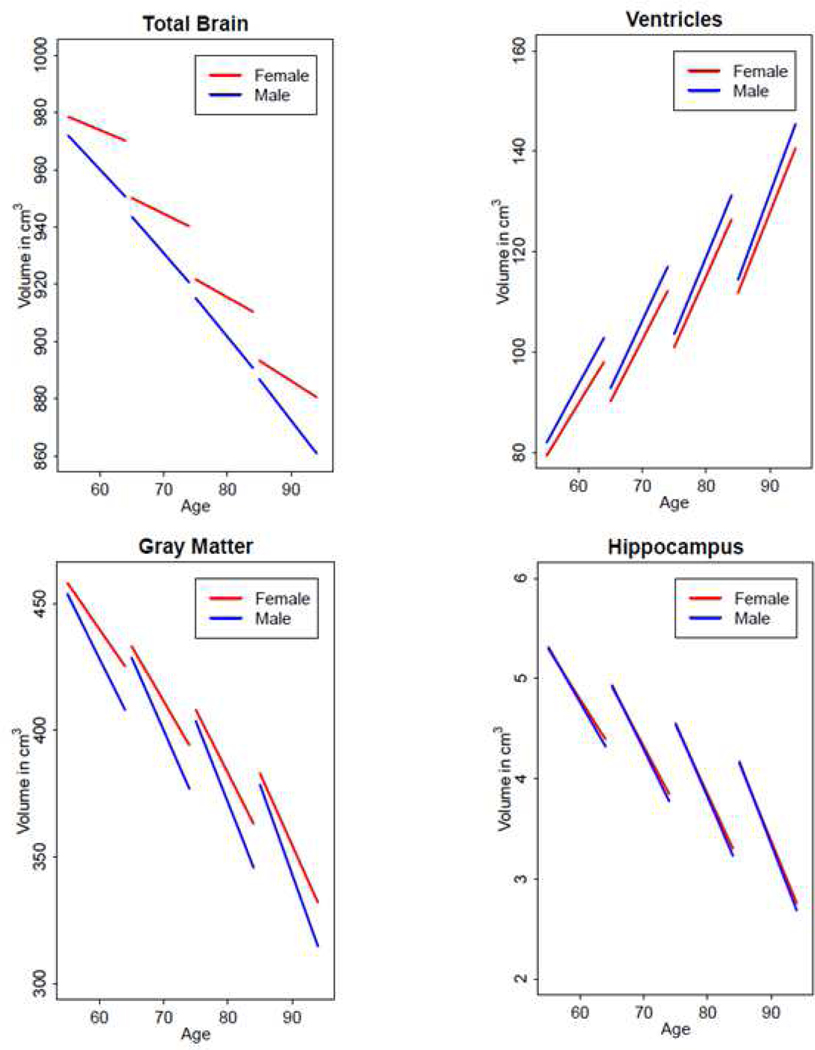
At baseline, males had larger ventricles, amygdala, entorhinal cortex, parahippocampal gyrus than females, while females had larger frontal GM and WM and parietal GM (Supplemental Table 1). Table 2 contains the annual rates of change in unstandardized and standardized regional brain volumes in the overall sample, males, and females and the difference in the annual rate of change in these volumes between males and females. Longitudinally, males had steeper volumetric declines in total brain (β=−1.677, SE=0.357, p<0.001), GM (β=−1.638, SE=0.281, p<0.001), and WM (β=−0.486, SE=0.151, p=0.001) and increased ventricular enlargement (β=0.452, SE=0.107, p<0.001) than females (Table 2). In terms of lobar GM and WM regions, males had steeper volumetric declines in frontal (β=−0.447, SE=0.102, p<0.001), temporal (β=−0.266, SE=0.050, p<0.001), parietal (β=−0.253, SE=0.055, p<0.001), and occipital (β=−0.180, SE=0.054, p=0.001) GM as well as frontal (β=−0.193, SE=0.060, p=0.001) and parietal (β=−0.102, SE=0.040, p=0.010) WM than females. Additionally, males had steeper volumetric declines in corpus callosum (β=−0.024, SE=0.005, p<0.001) and hippocampus (β=−0.012, SE=0.004, p=0.004) than females. However, there were no significant sex differences in rates of change in volumes of temporal WM, occipital WM, amygdala, entorhinal cortex, and parahippocampal gyrus (Table 2). Figure 1 depicts sex differences in volumetric change to highlight the finding that males had more volume loss across many ROIs than females. The unadjusted baseline volumes and annual rates of volume change are available in Supplemental Table 2. Supplemental Figure 2 shows the trajectories of brain volume change as a function of age between males and females in several key regions: total brain, GM, ventricles, and hippocampus.
Table 2.
Annual rates of change in regional brain volumes (in cm3) in the overall sample and between males and females in the Baltimore Longitudinal Study of Aging (N=617)
| Unstandardized Volumes | Standardized Volumes | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||||||
| Annual Rate of Change in Regional Brain Volumes in Overall Sample | Annual Rate of Change in Regional Brain Volumes in Males | Annual Rate of Change in Regional Brain Volumes in Females | Difference in Annual Rate of Change in Regional Brain Volumes between Men and Women | Annual Rate of Change in Regional Brain Volumes in Males | Annual Rate of Change in Regional Brain Volumes in Females | Difference in Annual Rate of Change in Regional Brain Volumes between Men and Women | |||||||||||||
| Brain Regions of Interest | β | SE | p-value | β | SE | β | SE | β | SE | p-value | Effect Size | β | SE | β | SE | β | SE | p-value | Effect Size |
| Total Brain | −4.375 | 0.423 | <0.001 | −5.213 | 0.480 | −3.536 | 0.438 | −1.677 | 0.357 | <0.001 | −0.891 | −0.042 | 0.004 | −0.029 | 0.004 | −0.014 | 0.003 | <0.001 | −0.891 |
| GM | −3.728 | 0.335 | <0.001 | −4.547 | 0.379 | −2.909 | 0.347 | −1.638 | 0.281 | <0.001 | −1.001 | −0.068 | 0.006 | −0.043 | 0.005 | −0.024 | 0.004 | <0.001 | −1.001 |
| Frontal GM | −1.337 | 0.119 | <0.001 | −1.561 | 0.136 | −1.114 | 0.124 | −0.447 | 0.102 | <0.001 | −0.772 | −0.075 | 0.006 | −0.053 | 0.006 | −0.021 | 0.005 | <0.001 | −0.772 |
| Temporal GM | −0.653 | 0.062 | <0.001 | −0.786 | 0.070 | −0.520 | 0.064 | −0.266 | 0.050 | <0.001 | −0.908 | −0.062 | 0.006 | −0.041 | 0.005 | −0.021 | 0.004 | <0.001 | −0.908 |
| Parietal GM | −0.597 | 0.065 | <0.001 | −0.723 | 0.074 | −0.470 | 0.067 | −0.253 | 0.055 | <0.001 | −0.895 | −0.067 | 0.007 | −0.044 | 0.006 | −0.024 | 0.005 | <0.001 | −0.895 |
| Occipital GM | −0.425 | 0.063 | <0.001 | −0.515 | 0.072 | −0.335 | 0.066 | −0.180 | 0.054 | 0.001 | −0.579 | −0.051 | 0.007 | −0.033 | 0.007 | −0.018 | 0.005 | 0.001 | −0.579 |
| WM | −1.693 | 0.183 | <0.001 | −1.936 | 0.206 | −1.450 | 0.189 | −0.486 | 0.151 | 0.001 | −0.583 | −0.036 | 0.004 | −0.027 | 0.004 | −0.009 | 0.003 | 0.001 | −0.583 |
| Frontal WM | −0.724 | 0.076 | <0.001 | −0.821 | 0.085 | −0.628 | 0.078 | −0.193 | 0.060 | 0.001 | −0.542 | −0.039 | 0.004 | −0.030 | 0.004 | −0.009 | 0.003 | 0.001 | −0.542 |
| Temporal WM | −0.298 | 0.050 | <0.001 | −0.334 | 0.056 | −0.261 | 0.051 | −0.073 | 0.042 | 0.079 | −0.371 | −0.027 | 0.005 | −0.021 | 0.004 | −0.006 | 0.003 | 0.079 | −0.371 |
| Parietal WM | −0.309 | 0.047 | <0.001 | −0.360 | 0.053 | −0.258 | 0.048 | −0.102 | 0.040 | 0.010 | −0.442 | −0.033 | 0.005 | −0.024 | 0.004 | −0.009 | 0.004 | 0.010 | −0.442 |
| Occipital WM | −0.109 | 0.030 | <0.001 | −0.111 | 0.035 | −0.106 | 0.032 | −0.005 | 0.026 | 0.855 | −0.030 | −0.019 | 0.006 | −0.018 | 0.005 | −0.001 | 0.004 | 0.855 | −0.030 |
| Ventricles | 1.199 | 0.110 | <0.001 | 1.425 | 0.130 | 0.973 | 0.114 | 0.452 | 0.107 | <0.001 | 0.465 | 0.068 | 0.006 | 0.047 | 0.005 | 0.022 | 0.005 | <0.001 | 0.465 |
| Corpus Callosum | −0.062 | 0.006 | <0.001 | −0.074 | 0.007 | −0.050 | 0.006 | −0.024 | 0.005 | <0.001 | −0.704 | −0.042 | 0.004 | −0.028 | 0.004 | −0.014 | 0.003 | <0.001 | −0.704 |
| Amygdala | −0.014 | 0.002 | <0.001 | −0.015 | 0.003 | −0.014 | 0.002 | −0.001 | 0.002 | 0.577 | −0.098 | −0.053 | 0.009 | −0.049 | 0.008 | −0.004 | 0.007 | 0.577 | −0.098 |
| Hippocampus | −0.046 | 0.005 | <0.001 | −0.053 | 0.006 | −0.040 | 0.005 | −0.012 | 0.004 | 0.004 | −0.435 | −0.062 | 0.007 | −0.047 | 0.006 | −0.015 | 0.005 | 0.004 | −0.435 |
| Entorhinal Cortex | −0.019 | 0.006 | 0.001 | −0.018 | 0.006 | −0.020 | 0.006 | 0.001 | 0.005 | 0.752 | 0.060 | −0.028 | 0.010 | −0.030 | 0.009 | 0.002 | 0.007 | 0.752 | 0.060 |
| Parahippocampal Gyrus | −0.026 | 0.006 | >0.001 | −0.030 | 0.007 | −0.021 | 0.007 | −0.009 | 0.005 | 0.093 | −0.283 | −0.034 | 0.008 | −0.024 | 0.008 | −0.010 | 0.006 | 0.093 | −0.283 |
GM – gray matter, WM – white matter, SE = standard error, Note: All bolded values mean p≤0.05. Linear mixed-effects models that included baseline ICV, scanner type, age, sex, race, time, and two-way interactions of scanner type, age, sex, and race with time were used to determine annual rates of change. Continuous variables were mean-centered, and sex was effect coded to obtain estimates for both males and females.
Figure 1.
Sex differences in volumetric change (in cm3) in cognitively normal participants in Baltimore Longitudinal Study of Aging (N=617)
Note: The color bar represents t-values from the results of the linear mixed effects models. These models consisted of fixed effects (baseline intracranial volume (ICV), image type [1.5-T SPGR vs. 3-T MPRAGE], age, sex, race, time since first MRI, and two-way interactions of image type, age, sex, and race with time) and random effects (intercept and time) with unstructured covariance. Note that the colors are uniform within regional labels since the figures depict ROI rather than voxel-based analyses.
As a secondary analysis, we examined longitudinal volume change in global and lobar regions as a function of sex in a sample without vascular burden (Supplemental Table 3). Although most associations were in the same direction and similar in magnitude to those from main analysis (Table 2), sex was not associated with greater ventricular enlargement or with volumetric change in the corpus callosum, frontal GM, and occipital GM in the sample without vascular burden.
3.3. Predictors of Volumetric Change as a Function of Sex
In these analyses, we first evaluated whether each predictor modified the association of sex with annual volume change. There were some three-way interactions at p<0.10 when we added each predictor, predictor*time, and predictor*time*sex to the base model (Supplemental Table 3). Males with increased baseline HDL cholesterol had less steep volume declines in hippocampus (β=0.001, SE=0.000, p=0.078), entorhinal cortex (β=0.001, SE=0.000, p=0.049), and parahippocampal gyrus (β=0.001, SE=0.000, p=0.082) than females with mean baseline HDL cholesterol. Male APOE e4 carriers had steeper volume declines in parietal GM (β=−0.599, SE=0.360, p=0.096) and frontal WM (β=−0.310, SE=0.121, p=0.011) as well as greater ventricular enlargement (β=0.255, SE=0.122, p=0.037) than female APOE e4 non-carriers. Hypertensive males had less steep volume declines in parahippocampal gyrus (β=0.028, SE=0.013, p=0.026) than females with low/normal BMI. Older males had less steep volume decline in corpus callosum (β=0.001, SE=0.001, p=0.042) than younger females. White males had steeper volumetric declines in temporal (β=−0.249, SE=0.130, p=0.094), parietal (β=−0.265, SE=0.098, p=0.007) and occipital (β=−0.131, SE=0.065, p=0.044) WM, and temporal GM (β=−0.249, SE=0.130, p=0.055) than non-white females. Based on observed trends toward three-way interactions, we performed analyses stratified by sex.
We evaluated the relationship of baseline age, hypertension, obesity, APOE e4 carrier status, and HDL cholesterol with volumetric change in global and lobar regions when stratifying by sex to determine the relationships in males and females separately. The results for these analyses are listed in Table 3. Some relationships between older baseline age and volume change were similar between males and females. In both males and females, older age was associated with volumetric declines in GM (males: β=−0.0786, SE=0.0342, p=0.021; females: β=−0.0488, SE=0.0210, p=0.020), temporal GM (males: β=−0.0189, SE=0.0060, p=0.002; females: β=−0.0104, SE=0.0039, p=0.008), amygdala (males: β=−0.0005, SE=0.0002, p=0.024; females: β=−0.0005, SE=0.0002, p=0.002), and hippocampus (males: β=−0.0021, SE=0.0005, p<0.001; females: β=−0.0017, SE=0.0004, p<0.001), as well as greater ventricular enlargement over time (males: β=0.0399, SE=0.0130, p=0.002; females: β=0.0378, SE=0.0070, p<0.001). After Bonferroni correction, age-related relationships with ventricles and hippocampus remained for both men and women. Also, APOE e4 carrier status was not associated with change in brain volumes by sex (Table 3).
Table 3.
Predictors of neurodegeneration between cognitively normal male and female older adults in the Baltimore Longitudinal Study of Aging.
| Brain Regions of Interest | Age * Time | Hypertension * Time | Obesity * Time | APOE e4 Status * Time | HDL * Time | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value | |
| Males Only (N=291, number of observations = 847) | |||||||||||||||
|
| |||||||||||||||
| Total Brain | 0.0017 | 0.0409 | 0.966 | −0.2943 | 0.5748 | 0.609 | −0.0981 | 0.4332 | 0.821 | −0.2514 | 0.6908 | 0.716 | −0.0436 | 0.0250 | 0.081 |
| GM | −0.0786 | 0.0342 | 0.021 | 0.0649 | 0.4815 | 0.893 | −0.1560 | 0.3559 | 0.661 | 0.1607 | 0.5788 | 0.781 | −0.0150 | 0.0209 | 0.471 |
| Frontal GM | −0.0065 | 0.0127 | 0.608 | 0.1057 | 0.1820 | 0.561 | −0.1507 | 0.1220 | 0.217 | 0.1807 | 0.2182 | 0.408 | −0.0065 | 0.0076 | 0.395 |
| Temporal GM | −0.0189 | 0.0060 | 0.002 * | 0.0456 | 0.0835 | 0.585 | 0.0546 | 0.0668 | 0.414 | 0.0530 | 0.1009 | 0.599 | −0.0039 | 0.0037 | 0.304 |
| Parietal GM | −0.0056 | 0.0064 | 0.386 | −0.0214 | 0.0909 | 0.814 | −0.0183 | 0.0698 | 0.793 | −0.0097 | 0.1091 | 0.929 | −0.0053 | 0.0039 | 0.180 |
| Occipital GM | −0.0103 | 0.0063 | 0.103 | −0.0982 | 0.0896 | 0.273 | 0.0250 | 0.0709 | 0.725 | −0.0174 | 0.1074 | 0.871 | −0.0041 | 0.0039 | 0.292 |
| WM | 0.0390 | 0.0180 | 0.030 | −0.2015 | 0.2537 | 0.427 | 0.0892 | 0.1974 | 0.651 | −0.0963 | 0.3042 | 0.751 | −0.0099 | 0.0110 | 0.372 |
| Frontal WM | 0.0136 | 0.0077 | 0.077 | −0.0295 | 0.1083 | 0.786 | −0.0177 | 0.0865 | 0.838 | 0.0801 | 0.1302 | 0.538 | −0.0022 | 0.0048 | 0.640 |
| Temporal WM | 0.0079 | 0.0047 | 0.094 | −0.1112 | 0.0661 | 0.093 | 0.0223 | 0.0526 | 0.672 | −0.1480 | 0.0795 | 0.063 | −0.0046 | 0.0029 | 0.116 |
| Parietal WM | 0.0116 | 0.0046 | 0.012 | 0.0135 | 0.0656 | 0.836 | 0.0459 | 0.0500 | 0.358 | −0.0179 | 0.0786 | 0.819 | −0.0032 | 0.0028 | 0.260 |
| Occipital WM | 0.0047 | 0.0030 | 0.121 | −0.0597 | 0.0432 | 0.166 | 0.0644 | 0.0328 | 0.049 | 0.0076 | 0.0517 | 0.884 | 0.0003 | 0.0019 | 0.877 |
| Ventricles | 0.0399 | 0.0130 | 0.002 * | −0.1322 | 0.2012 | 0.511 | 0.0053 | 0.0750 | 0.943 | −0.0886 | 0.2410 | 0.713 | −0.0105 | 0.0075 | 0.161 |
| Corpus Callosum | 0.0001 | 0.0006 | 0.831 | −0.0073 | 0.0080 | 0.362 | −0.0032 | 0.0062 | 0.604 | −0.0066 | 0.0096 | 0.492 | −0.0001 | 0.0004 | 0.734 |
| Amygdala | −0.0005 | 0.0002 | 0.024 | 0.0003 | 0.0032 | 0.930 | 0.0013 | 0.0022 | 0.557 | −0.0014 | 0.0038 | 0.721 | 0.0001 | 0.0001 | 0.488 |
| Hippocampus | −0.0021 | 0.0005 | <0.001 * | 0.0128 | 0.0064 | 0.046 | −0.0024 | 0.0050 | 0.627 | −0.0054 | 0.0077 | 0.482 | 0.0005 | 0.0003 | 0.072 |
| Entorhinal Cortex | −0.0013 | 0.0005 | 0.012 | −0.0057 | 0.0074 | 0.444 | 0.0068 | 0.0063 | 0.281 | 0.0031 | 0.0090 | 0.729 | 0.0009 | 0.0003 | 0.008 |
| Parahippocampal Gyrus | −0.0012 | 0.0006 | 0.057 | 0.0145 | 0.0086 | 0.091 | 0.0044 | 0.0070 | 0.527 | 0.0100 | 0.0103 | 0.334 | 0.0008 | 0.0004 | 0.030 |
|
| |||||||||||||||
| Females Only (N= 326, number of observations = 881) | |||||||||||||||
|
| |||||||||||||||
| Total Brain | −0.0286 | 0.0275 | 0.297 | −0.8579 | 0.5740 | 0.135 | 0.4631 | 0.3455 | 0.180 | −0.4046 | 0.4320 | 0.349 | 0.0074 | 0.0139 | 0.594 |
| GM | −0.0488 | 0.0210 | 0.020 | −0.9437 | 0.4418 | 0.033 | 0.4643 | 0.2735 | 0.090 | −0.2805 | 0.3289 | 0.394 | 0.0027 | 0.0106 | 0.802 |
| Frontal GM | −0.0180 | 0.0078 | 0.021 | −0.2318 | 0.1635 | 0.156 | 0.1835 | 0.0961 | 0.056 | 0.0236 | 0.1248 | 0.850 | −0.0019 | 0.0040 | 0.632 |
| Temporal GM | −0.0104 | 0.0039 | 0.008 | −0.2437 | 0.0817 | 0.003 * | 0.1000 | 0.0527 | 0.058 | 0.0024 | 0.0598 | 0.967 | 0.0025 | 0.0020 | 0.205 |
| Parietal GM | −0.0097 | 0.0043 | 0.023 | −0.1224 | 0.0893 | 0.171 | 0.0480 | 0.0546 | 0.379 | −0.0355 | 0.0668 | 0.596 | 0.0017 | 0.0022 | 0.423 |
| Occipital GM | −0.0070 | 0.0040 | 0.081 | −0.0756 | 0.0845 | 0.371 | 0.0732 | 0.0508 | 0.149 | −0.0450 | 0.0641 | 0.482 | 0.0007 | 0.0020 | 0.718 |
| WM | −0.0159 | 0.0099 | 0.109 | −0.1273 | 0.2036 | 0.532 | 0.0538 | 0.1393 | 0.699 | 0.0265 | 0.1405 | 0.850 | 0.0018 | 0.0049 | 0.719 |
| Frontal WM | −0.0036 | 0.0042 | 0.396 | −0.0346 | 0.0857 | 0.687 | −0.0439 | 0.0623 | 0.480 | 0.0196 | 0.0574 | 0.733 | 0.0005 | 0.0020 | 0.819 |
| Temporal WM | 0.0009 | 0.0031 | 0.777 | −0.0501 | 0.0655 | 0.444 | 0.0667 | 0.0400 | 0.096 | −0.0440 | 0.0486 | 0.365 | −0.0007 | 0.0016 | 0.651 |
| Parietal WM | −0.0011 | 0.0027 | 0.688 | 0.0076 | 0.0555 | 0.891 | 0.0485 | 0.0346 | 0.160 | 0.0267 | 0.0404 | 0.509 | 0.0021 | 0.0013 | 0.123 |
| Occipital WM | −0.0037 | 0.0018 | 0.045 | −0.0191 | 0.0382 | 0.617 | 0.0130 | 0.0240 | 0.587 | −0.0103 | 0.0281 | 0.714 | 0.0013 | 0.0009 | 0.175 |
| Ventricles | 0.0378 | 0.0070 | <0.001 * | −0.1728 | 0.1442 | 0.231 | −0.1504 | 0.0575 | 0.009 | −0.0074 | 0.1171 | 0.950 | −0.0063 | 0.0034 | 0.065 |
| Corpus Callosum | −0.0015 | 0.0004 | 0.001 * | −0.0076 | 0.0093 | 0.412 | −0.0050 | 0.0053 | 0.341 | −0.0102 | 0.0073 | 0.166 | 0.0001 | 0.0002 | 0.616 |
| Amygdala | −0.0005 | 0.0002 | 0.002 * | 0.0018 | 0.0035 | 0.620 | 0.0018 | 0.0018 | 0.318 | −0.0017 | 0.0029 | 0.554 | 0.0001 | 0.0001 | 0.149 |
| Hippocampus | −0.0017 | 0.0004 | <0.001 * | 0.0046 | 0.0078 | 0.553 | 0.0088 | 0.0044 | 0.047 | −0.0083 | 0.0061 | 0.174 | 0.0001 | 0.0002 | 0.565 |
| Entorhinal Cortex | −0.0007 | 0.0004 | 0.091 | 0.0072 | 0.0081 | 0.372 | −0.0018 | 0.0048 | 0.702 | −0.0020 | 0.0061 | 0.736 | 0.0002 | 0.0002 | 0.298 |
| Parahippocampal Gyrus | −0.0011 | 0.0005 | 0.022 | −0.0118 | 0.0102 | 0.246 | −0.0006 | 0.0057 | 0.917 | −0.0059 | 0.0080 | 0.463 | 0.0004 | 0.0002 | 0.082 |
GM – gray matter, WM – white matter, SE – standard error. Note: Linear-mixed effects models consisted of fixed effects, i.e., baseline intracranial volume (ICV), image type (1.5-T SPGR vs. 3-T MPRAGE), age, race, hypertension, obesity, APOE e4 carrier status, HDL cholesterol, time since first MRI, and two-way interactions of image type, age, race, hypertension, obesity, APOE e4 carrier status, and HDL cholesterol with time, and random effects (intercept and time) with unstructured covariance. The analyses were stratified by sex. All bolded values indicate p<0.05.
indicates significance at Bonferroni correction of p≤0.003. Predictor*Time indicates the association of predictor with annual rate of change in brain volume.
There were also differences in the patterns of associations between baseline age and volume change between males and females. Among males only, older age was associated with steeper declines in entorhinal cortex (β=−0.0013, SE=0.0005, p=0.012), as well as less steep volume declines in WM (β=0.0390, SE=0.0180, p=0.030), especially in parietal WM (β=0.0116, SE=0.0046, p=0.012) (Table 4). Among females only, older age was associated with volumetric declines in corpus callosum (β=−0.0015, SE=0.0004, p=0.001), frontal GM (β=−0.0180, SE=0.0078, p=0.021), and parietal GM (β=0.0097, SE=0.0043, p=0.023). After Bonferroni correction, age-related association with temporal GM remained for men, while age-related associations with corpus callosum and amygdala remained for women (Table 3).
Differences in patterns of associations for the other predictors, i.e. hypertension, obesity, and HDL cholesterol, also emerged between males and females (Table 3). Males with, compared to those without, hypertension had less steep volume decline in the hippocampus (β=0.0128, SE=0.0064, p=0.046), while hypertension was associated with steeper volumetric declines in GM (β=−0.9437, SE=0.4418, p=0.033), especially in temporal GM (β=−0.2437, SE=0.0817, p=0.003), where it survived Bonferroni correction, among females. While there were no significant associations of obesity with volume change among males, obesity was associated with less increase in ventricular volume (β=0.1504, SE=0.0575, p=0.009) and less steep declines in hippocampal volumes (β=−0.0088, SE=0.0044, p=0.047). Among males, higher HDL cholesterol was associated with less steep volume decline in entorhinal cortex (β=0.0009, SE=0.0003, p=0.008) and parahippocampal gyrus (β=0.0008, SE=0.0004, p=0.030), yet there were no associations of HDL cholesterol with volume change among females (Table 3).
3.4. Sensitivity Analyses
When we restricted the scans to 3-T images, the number of observations dropped by half for both men and women (Supplemental Table 4). Although the magnitudes of associations for some effects were diminished (perhaps due in part to shorter longitudinal follow-up), directions of effects remained the same.
4. Discussion
In this study, we found sex differences in regional volumetric change, with males having steeper volumetric declines than females, even in the absence of cardiovascular risk factors. When comparing patterns of predictors of volumetric change between males and females, distinct patterns emerged for males and females separately. While older age was associated with widespread volumetric declines in both males and females, the associations of hypertension, obesity, and HDL cholesterol with volume change differed between the groups. Hypertensive females had steeper volumetric declines in GM, especially in the temporal lobe, but this was not present in hypertensive males. While there were no associations between obesity and volume change among males, obesity was associated with decreased ventricular enlargement among females. Higher HDL cholesterol was associated with less steep volume declines in both the entorhinal cortex and parahippocampal gyrus among males, but not in females. APOE e4 carrier status was not associated with volume change in older men and women who maintained cognitive health over extended follow-up, suggesting that APOE e4 risk has a greater effect in those with greater vulnerability to cognitive decline (Armstrong et al., 2019). The lack of an APOE e4 effect in cognitively normal individuals is consistent with some prior studies (Persson et al., 2014; Raz et al., 2010), but differs from an earlier BLSA report (Moffat et al., 2000) over a shorter follow-up that would not have considered long-term cognitive status.
We found that sex differences in volume loss were widespread across the brain, with males having greater volume loss over time than females. Males had steeper rates of annual decline than females in most global and lobar regions, excluding temporal and occipital WM. Males also experienced greater ventricular enlargement than females. These results are consistent with our previous studies of the effects of sex on volume change (Driscoll et al., 2009; Thambisetty et al., 2010; Pacheco et al., 2015), and suggest that females may be less vulnerable to age-related atrophy. Similarly, Ritchie et al. (2015) reported that men had more volumetric decline in total brain and GM than females. Our findings are also consistent with cross-sectional observations of lower age-adjusted volumes of the medial temporal lobe in men compared with women in the Mayo Clinic Study of Aging (Jack et al., 1997).
The volumetric differences observed between males and females suggest that sex hormones may play a role in brain atrophy over time. It has been proposed that estrogen and progesterone may have a protective effect against brain volume loss in women (Green and Simpkins, 2000), although the WHIMS randomized trials with conjugated equine estrogens in older postmenopausal women do not support this hypothesis (Resnick et al., 2009). Conversely, greater WM volume decline in men may be related to the role that androgen, a sex hormone more predominant in males, plays in myelinogenesis. As age increases, levels of androgen decrease, thus reducing recruitment of astrocytes in the remyelination process (Bielecki et al., 2016).
Another possible explanation for the sex differences in rates of volume change relates to differential health risks and possible selection biases. There are well-known sex differences in CVD risk, with males having higher age-adjusted CVD mortality and morbidity rates than females (Mosca et al., 2011). Sex is associated with differential risk for age-related diseases, but males who remained cognitively normal were more likely to have fewer cardiovascular risk factors (elevated total cholesterol and obesity, in particular) overall as well as higher mean baseline HDL cholesterol than females. As noted by Raz et al. (1997), this type of selection bias may hide sex differences in secular trends. To address this possible bias, we evaluated rates of volumetric change in males and females without baseline vascular burden. Sex differences in rates and patterns of longitudinal volumetric change remained in the absence of CVD risk.
We performed sex-stratified analysis to determine whether associations of predictors of neurodegeneration showed similar associations with volumetric brain changes in males and females. Stratified analyses of pre-specified predictors of neurodegeneration revealed some sex differences in the patterns of these associations. Hypertensive males had less steep volume declines in hippocampus, while hypertensive females had greater declines in temporal GM. In prior work, we found that hypertension was associated with slower rates of hippocampal volume loss among those who remained cognitively normal, while hypertension was associated with steeper hippocampal volumetric declines in the subsequently impaired sample (Armstrong et al., 2019). Our current analyses suggest the association in cognitively normal individuals could be driven by the males. The findings in males differ from other studies reporting that hypertensive men have lower hippocampal volumes (Chen et al., 2006; Gianaros et al., 2006; Taki et al., 2004) or that there are no sex differences in the influence of hypertension on declines in hippocampal volume (Raz et al., 2005). In females, the association between hypertension and greater overall and temporal lobe GM may be associated with post-menopausal estrogen loss. In mid- to late midlife, pre-menopausal females generally have lower blood pressure than age-matched men likely due to the estrogen modulating effects on the renin-angiotensin-aldosterone system, which can result in beneficial effects on the cardiovascular and central nervous system (Fischer et al., 2002; Yang and Reckelhoff, 2011). The loss of estrogen post-menopause, however, can lead to higher blood pressure (Burt et al., 1995; Calhoun and Oparil, 1998), and cognitively normal women could be more sensitive to hypertension-associated GM volume loss.
Regarding obesity, we found differences in the association with volume change among females only. Obesity in females was associated with slower rate of ventricular enlargement and less steep volume declines in hippocampus. The apparent protective effect of obesity in older females in these brain regions is consistent with findings from the Women’s Health Initiative Memory Study in that lower, rather than higher, BMI was associated with reduced brain volumes in older females over time (Driscoll et al., 2016). Consistent with the interpretation in the WHIMS study, it is likely that weight loss, rather than weight gain, in older women is a marker of future disease. We did not find any associations of obesity with GM and ventricular volume change among older males, although the proportions of males and females with obesity were similar.
We found that HDL cholesterol was associated with less steep volume loss in temporal lobe cortical regions only among males. Previous findings suggest that high levels of HDL cholesterol may be protective against hippocampal atrophy (Wolf et al., 2004). Although we failed to see an association between HDL cholesterol and hippocampal volume change, we did see associations between higher HDL cholesterol levels and reduced volume loss in the parahippocampal gyrus and entorhinal cortex, areas that show early atrophic changes in the AD neurodegenerative process (de Leon et al., 2004). Men with lower HDL levels may be more at risk for volume change, because reduced HDL may contribute to the onset of the inflammatory response that occurs in the pathogenesis of atherosclerosis (Barter et al., 2007; Patel et al., 2009; Sampietro et al., 2006) or inflammaging, a state of increasing age-associated low-grade inflammatory state (Chung et al., 2009; Ferrucci et al., 2005). Greater HDL cholesterol has been inversely associated with lower levels of adiponectin and IL-6, markers of age-related inflammation, among healthy adult males (Miles et al., 2008).
There are many strengths of this study. First, this study consists of an extensively characterized large sample of older adults who remained cognitively healthy over lengthy follow-up. This limits generalizability to less selected cohorts but provides important information on sex differences in people who maintain cognitive health. Second, our image processing pipeline uses state-of-the-art and validated multi-atlas approaches for regional definition, yielding high measurement stability over time. There were also several limitations to our study. First, our sample is highly educated, mostly Caucasian, and has a higher socioeconomic status, thus limiting generalizability. Nevertheless, prior BLSA studies have shown similar rates of brain changes over time, relative to other studies (Resnick et al., 2003). Second, most participants were recruited in later life, so there is information missing on midlife risk factors. Third, as this is an ongoing study, 14.4% of the sample had only a single assessment at the time of this analysis, but are included in the analysis, as they contribute to stability of cross-sectional associations. Fourth, we did not detect any significant predictor*sex*time interactions after adding these to the base model. It is likely that larger sample sizes are necessary to determine higher order associations. Fifth, non-random missingness is always an issue in prospective studies. BLSA home visits minimize the impact of this concern with respect to long-term cognitive status, as participants continued to be followed with cognitive testing when they stop returning to the BLSA for clinic visits. Lastly, the study of specific indicators rather than a global construct of vascular burden is a limitation, yet we did not find any significant associations between a composite of cardiovascular risk factors, defined as vascular burden, and change in brain volumes among cognitively normal older adults (Armstrong et al., 2019).
In summary, we found widespread sex differences in the rates of regional volume loss, with men showing faster rates of neurodegeneration. We also found sex differences in the factors related to brain volume decline in men and women. Certain predictors were associated with less tissue loss in hippocampus, entorhinal cortex, and parahippocampal gyrus, as men with hypertension or high HDL cholesterol, and women with obesity were less susceptible to tissue volume decline in temporal lobe over time. Future investigations with longer follow-ups should include examination of sex differences in possible synergistic effects of risk factors on change in brain volumes. For instance, previous studies have found that APOE e4 carrier status and hypertension may have a synergistic effect on brain aging (Rast et al., 2017; Raz et al., 2009; Rodrigue et al., 2013), but it is unclear whether sex affects these associations. These findings highlight the importance of examining the differences in patterns of neurodegeneration among men and women in relation to risk factors, as these factors could differentially affect rates of tissue volume change over time.
Supplementary Material
Figure 2.
Volumetric changes (in cm3) in total brain, ventricles, gray matter, and hippocampus between males and females in the overall sample (N=617)
Note: Predicted values for volumes come from linear-mixed effects models consisting of fixed effects, i.e., baseline intracranial volume (ICV), image type (1.5-T SPGR vs. 3-T MPRAGE), age, sex, race, hypertension, obesity, APOE e4 carrier status, HDL cholesterol, time since first MRI, and two-way interactions of image type, age, sex, race, hypertension, obesity, APOE e4 carrier status, HDL cholesterol with time, and random effects (intercept and time) with unstructured covariance.
Highlights.
Older men show greater volume loss over time than older women.
Cardiovascular risk factors affect rates of volume change differently by sex.
Hypertension and HDL cholesterol are related to less steep volume declines in men.
Hypertension is related to steeper volume decline in gray matter in women.
Obesity is related to less steep volume decline in temporal gray matter in women.
Acknowledgments:
We would like to thank the participants and staff of the BLSA, the neuroimaging staff of the Laboratory of Behavioral Neuroscience, and the staff of the Johns Hopkins and NIA MRI facilities.
Funding:
This research was fully supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging. CD was supported, in part, by NIH grant RF1 AG054409. The authors of this manuscript include employees of the Intramural Research Program of the NIA, who participated in all aspects of the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
References
- Allen JS, Damasio H, Grabowski TJ, Bruss J, Zhang W, 2003. Sexual dimorphism and asymmetries in the gray–white composition of the human cerebrum. NeuroImage 18(4), 880–894. [DOI] [PubMed] [Google Scholar]
- Armstrong NM, An Y, Beason-Held L, Doshi J, Erus G, Ferrucci L, Davatzikos C, Resnick SM, 2019. Predictors of neurodegeneration differ between cognitively normal and subsequently impaired older adults. Neurobiology of Aging 75, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes J, Ridgway GR, Bartlett J, Henley SMD, Lehmann M, Hobbs N, Clarkson MJ, MacManus DG, Ourselin S, Fox NC, 2010. Head size, age and gender adjustment in MRI studies: a necessary nuisance? NeuroImage 53(4), 1244–1255. [DOI] [PubMed] [Google Scholar]
- Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart J-C, 2007. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. New England Journal of Medicine 357(13), 1301–1310. [DOI] [PubMed] [Google Scholar]
- Bielecki B, Mattern C, Ghoumari AM, Javaid S, Smietanka K, Abi Ghanem C, Mhaouty-Kodja S, Ghandour MS, Baulieu E-E, Franklin RJM, Schumacher M, Traiffort E, 2016. Unexpected central role of the androgen receptor in the spontaneous regeneration of myelin. Proceedings of the National Academy of Sciences of the United States of America 113(51), 14829–14834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D, 1995. Prevalence of hypertension in the US adult population: results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 25(3), 305–313. [DOI] [PubMed] [Google Scholar]
- Calhoun DA, Oparil S, 1998. The sexual dimorphism of high blood pressure. Cardiology in Review 6(6), 356–363. [DOI] [PubMed] [Google Scholar]
- Chen X, Wen W, Anstey KJ, Sachdev PS, 2006. Effects of cerebrovascular risk factors on gray matter volume in adults aged 60–64 years: a voxel-based morphometric study. Psychiatry Research: Neuroimaging 147(2–3), 105–114. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C, 2009. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Research Reviews 8(1), 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey C, Lucke JF, Saxton JA, et al. , 1998. Sex differences in brain aging: A quantitative magnetic resonance imaging study. Archives of Neurology 55(2), 169–179. [DOI] [PubMed] [Google Scholar]
- Coffey C, Wilkinson W, Parashos L, Soady S, Sullivan R, Patterson L, Figiel G, Webb M, Spritzer C, Djang W, 1992. Quantitative cerebral anatomy of the aging human brain: a cross-sectional study using magnetic resonance imaging. Neurology 42(3), 527–527. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK, 2007. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biological Psychiatry 62(8), 847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA, 2000. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 216(3), 672–682. [DOI] [PubMed] [Google Scholar]
- de Leon M, DeSanti S, Zinkowski R, Mehta P, Pratico D, Segal S, Clark C, Kerkman D, DeBernardis J, Li J, 2004. MRI and CSF studies in the early diagnosis of Alzheimer’s disease. Journal of Internal Medicine 256(3), 205–223. [DOI] [PubMed] [Google Scholar]
- Doshi J, Erus G, Ou Y, Resnick SM, Gur RC, Gur RE, Satterthwaite TD, Furth S, Davatzikos C, The Alzheimer’s Neuroimaging Initiative, 2016. MUSE: MUlti-atlas region Segmentation utilizing Ensembles of registration algorithms and parameters, and locally optimal atlas selection. NeuroImage 127, 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM, 2009. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology 72(22), 1906–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Gaussoin SA, Wassertheil-Smoller S, Limacher M, Casanova R, Yaffe K, Resnick SM, Espeland MA, 2016. Obesity and structural brain integrity in older women: The Women’s Health Initiative Magnetic Resonance Imaging Study. The Journals of Gerontology: Series A, Biological Sciences & Medical Sciences 71(9), 1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erus G, Doshi J, An Y, Verganelakis D, Resnick SM, Davatzikos C, 2018. Longitudinally and inter-site consistent multi-atlas based parcellation of brain anatomy using harmonized atlases. NeuroImage 166, 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL, 2005. The origins of age-related proinflammatory state. Blood 105(6), 2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Baessler A, Schunkert H, 2002. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovascular Research 53(3), 672–677. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB, 2009. Minute effects of sex on the aging brain: a multisample magnetic resonance imaging study of healthy aging and Alzheimer’s disease. The Journal of Neuroscience 29(27), 8774–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Greer PJ, Ryan CM, Jennings JR, 2006. Higher blood pressure predicts lower regional grey matter volume: consequences on short-term information processing. NeuroImage 31(2), 754–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS Jr, Faraone SV, Tsuang MT, 2001. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cerebral Cortex 11(6), 490–497. [DOI] [PubMed] [Google Scholar]
- Gottesman R, Schneider A, Zhou Y, Coresh J, Green E, Gupta N, Knopman D, Mintz A, Rahmim A, Sharrett A, Wagenknecht L, Wong D, Mosley T, 2017. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 317(14), 1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PS, Simpkins JW, 2000. Neuroprotective effects of estrogens: potential mechanisms of action. International Journal of Developmental Neuroscience 18(4), 347–358. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Messer DF, Payne ME, MacFall JR, Provenzale JM, Steffens DC, Krishnan RR, 2008. Aging, gender, and the elderly adult brain: an examination of analytical strategies. Neurobiology of Aging 29(2), 290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R, Turetsky B, Matsui M, Yan M, Bilker W, Hughett P, Gur R, 1999. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. Journal of Neuroscience 19(10), 4065–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Mozley PD, Resnick SM, Gottlieb GL, Kohn M, Zimmerman R, Herman G, Atlas S, Grossman R, Berretta D, 1991. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proceedings of the National Academy of Sciences 88(7), 2845–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley R, Barzi F, Woodward M, 2006. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 332(7533), 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E, 1997. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology 49(3), 786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Wiste HJ, Weigand SD, Therneau TM, Knopman DS, Lowe V, Vemuri P, Mielke MM, Roberts RO, Machulda MM, Senjem ML, Gunter JL, Rocca WA, Petersen RC, 2017. Age-specific and sex-specific prevalence of cerebral β-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross-sectional study. The Lancet Neurology 16(6), 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaître H, Crivello F, Grassiot B, Alpérovitch A, Tzourio C, Mazoyer B, 2005. Age- and sex-related effects on the neuroanatomy of healthy elderly. NeuroImage 26(3), 900–911. [DOI] [PubMed] [Google Scholar]
- Lüders E, Steinmetz H, Jäncke L, 2002. Brain size and grey matter volume in the healthy human brain. Neuroreport 13(17), 2371–2374. [DOI] [PubMed] [Google Scholar]
- Luders E, Toga AW, Thompson PM, 2014. Why size matters: differences in brain volume account for apparent sex differences in callosal anatomy: the sexual dimorphism of the corpus callosum. NeuroImage 84, 820–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, Resnick SM, 2016. Sex differences in cognitive trajectories in clinically normal older adults. Psychology and Aging 31(2), 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles EA, Rees D, Banerjee T, Cazzola R, Lewis S, Wood R, Oates R, Tallant A, Cestaro B, Yaqoob P, Wahle KWJ, Calder PC, 2008. Age-related increases in circulating inflammatory markers in men are independent of BMI, blood pressure and blood lipid concentrations. Atherosclerosis 196(1), 298–305. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Szekely CA, Zonderman AB, Kabani NJ, Resnick SM, 2000. Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology 55(1), 134. [DOI] [PubMed] [Google Scholar]
- Mosca L, Barrett-Connor E, Kass Wenger N, 2011. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation 124(19), 2145–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DG, DeCarli C, Mclntosh AR, Daly E, Mentis MJ, Pietrini P, Szczepanik J, Schapiro MB, Grady CL, Horwitz B, 1996. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Archives of General Psychiatry 53(7), 585–594. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Flaum M, O’Leary D, Andreasen NC, 2000. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Research: Neuroimaging 98(1), 1–13. [DOI] [PubMed] [Google Scholar]
- Pacheco J, Goh JO, Kraut MA, Ferrucci L, Resnick SM, 2015. Greater cortical thinning in normal older adults predicts later cognitive impairment. Neurobiology of Aging 36(2), 903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Puranik R, Nakhla S, Lundman P, Stocker R, Wang XS, Lambert G, Rye K-A, Barter PJ, Nicholls SJ, 2009. Acute hypertriglyceridaemia in humans increases the triglyceride content and decreases the anti-inflammatory capacity of high density lipoproteins. Atherosclerosis 204(2), 424–428. [DOI] [PubMed] [Google Scholar]
- Pengas G, Pereira J, Williams G, Nestor P, 2009. Comparative reliability of total intracranial volume estimation methods and the influence of atrophy in a longitudinal semantic dementia cohort. Journal of Neuroimaging 19(1), 37–46. [DOI] [PubMed] [Google Scholar]
- Persson J, Spreng RN, Turner G, Herlitz A, Morell A, Stening E, Wahlund L-O, Wikström J, Söderlund H, 2014. Sex differences in volume and structural covariance of the anterior and posterior hippocampus. NeuroImage 99, 215–225. [DOI] [PubMed] [Google Scholar]
- Persson N, Ghisletta P, Dahle CL, Bender AR, Yang Y, Yuan P, Daugherty AM, Raz N, 2016. Regional brain shrinkage and change in cognitive performance over two years: the bidirectional influences of the brain and cognitive reserve factors. NeuroImage 126, 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SAE, Huxley RR, Woodward M, 2014. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775 385 individuals and 12 539 strokes. The Lancet 383(9933), 1973–1980. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO, 1994. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology 51(9), 874–887. [DOI] [PubMed] [Google Scholar]
- Prescott E, Hippe M, Schnohr P, Hein HO, Vestbo J, 1998. Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ 316(7137), 1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rast P, Kennedy KM, Rodrigue KM, Robinson PR, Gross AL, McLaren DG, Grabowski T, Schaie KW, Willis SL, 2017. APOEε4 genotype and hypertension modify 8-year cortical thinning: five occasion evidence from the Seattle Longitudinal Study. Cerebral Cortex 28(6), 1934–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U, 2010. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. NeuroImage 51(2), 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD, 1997. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral cortex 7(3), 268–282. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD, 2005. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex 15(11), 1676–1689. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Land S, 2009. Genetic and vascular modifiers of age-sensitive cognitive skills: effects of COMT, BDNF, ApoE, and hypertension. Neuropsychology 23(1), 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick S, Pham D, Kraut M, Zonderman A, Davatzikos C, 2003. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. The Journal of Neuroscience 23(8), 3295–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Espeland MA, Jaramillo SA, Hirsch C, Stefanick ML, Murray AM, Ockene J, Davatzikos C, 2009. Postmenopausal hormone therapy and regional brain volumes: The WHIMSMRI Study. Neurology 72(2), 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM, Alloza C, Harris MA, Alderson HL, Hunter S, Neilson E, Liewald DCM, Auyeung B, Whalley HC, Lawrie SM, Gale CR, Bastin ME, McIntosh AM, Deary IJ, 2018. Sex differences in the adult human brain: evidence from 5216 UK Biobank participants. Cerebral Cortex 28(8), 2959–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SJ, Dickie DA, Cox SR, Valdes Hernandez MDC, Corley J, Royle NA, Pattie A, Aribisala BS, Redmond P, Muñoz Maniega S, Taylor AM, Sibbett R, Gow AJ, Starr JM, Bastin ME, Wardlaw JM, Deary IJ, 2015. Brain volumetric changes and cognitive ageing during the eighth decade of life. Human brain mapping 36(12), 4910–4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Sr, Diaz-Arrastia R, Park DC, 2013. Risk factors for β-amyloid deposition in healthy aging: vascular and genetic effects. JAMA Neurology 70(5), 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve D, Desikan RS, Busa E, Morris JC, Dale A, Fischl B, 2004. Thinning of the cerebral cortex in aging. Cerebral Cortex 14(7), 721–730. [DOI] [PubMed] [Google Scholar]
- Sampietro T, Bigazzi F, Dal Pino B, Puntoni M, Bionda A, 2006. HDL: The ‘new’ target of cardiovascular medicine. International Journal of Cardiology 108(2), 143–154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW, 2007. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cerebral Cortex 17(7), 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp, 2017. Stata Statistical Software: Release 15. StataCorp LLC, College Station, TX. [Google Scholar]
- Taki Y, Goto R, Evans A, Zijdenbos A, Neelin P, Lerch J, Sato K, Ono S, Kinomura S, Nakagawa M, 2004. Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiology of Aging 25(4), 455–463. [DOI] [PubMed] [Google Scholar]
- Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM, 2010. Longitudinal changes in cortical thickness associated with normal aging. NeuroImage 52(4), 1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H, Hensel A, Arendt T, Kivipelto M, Winblad B, Gertz HJ, 2004. Serum lipids and hippocampal volume: the link to Alzheimer’s disease? Annals of Neurology 56(5), 745–749. [DOI] [PubMed] [Google Scholar]
- Xu J, Kobayashi S, Yamaguchi S, Iijima K. i., Okada K, Yamashita K, 2000. Gender effects on age-related changes in brain structure. American Journal of Neuroradiology 21(1), 112–118. [PMC free article] [PubMed] [Google Scholar]
- Yang X-P, Reckelhoff JF, 2011. Estrogen, hormonal replacement therapy and cardiovascular disease. Current Opinion in Nephrology and Hypertension 20(2), 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Voelkle MC, Raz N, 2018. Fluid intelligence and gross structural properties of the cerebral cortex in middle-aged and older adults: a multi-occasion longitudinal study. NeuroImage 172, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.