Abstract
Background:
Human papillomavirus (HPV)-associated cervical cancer is a leading cause of death among Indian women. Indian women living with HIV (WLWH) may be at especially high risk. The quadrivalent HPV vaccine (qHPV) is effective in prevention of initial infection with HPV-6/11/16/18 in HIV-negative women. Little is known about previous exposure to HPV-6/11/16/18, safety and immunogenicity of qHPV in Indian WLWH.
Methodology:
150 WLWH with different CD4 levels and HIV viral load (VL) were vaccinated at 0/2/6 months at CART-CRS-IDMC, Chennai, India. Serology was performed at weeks 0, 28 and 52 for HPV-6/11/16/18 using a competitive Luminex immunoassay (cLIA) and for HPV-16/18 using a pseudovirion-based neutralization assay (PBNA).
Results:
Mean age was 30.8 years (range, 19–44 years). 71/87/73/81% of women were naïve (sero-negative and DNA-negative) to HPV-6/11/16/18 at baseline, respectively. Among per-protocol women naïve to HPV-6/11/16/18 at baseline, 100/99/99/90%, respectively, seroconverted at week 28. 95/96/98/71% were sero-positive at week 52, respectively. PBNA identified more sero-conversion to HPV-18 than cLIA. There were no significant differences in the proportion seroconverting by baseline or nadir CD4 or HIV VL; however, there was a trend for increased proportion sero-converting to HPV-18 among women with higher baseline CD4 level (p=0.052). There were no qHPV-related serious adverse events and no change in CD4 level or HIV VL among women on ART.
Conclusions:
qHPV vaccine was safe and immunogenic in Indian WLWH. A high proportion were naïve to HPV-6/11/16/18 and may benefit from vaccination even though many were married and several years post-initiation of sexual activity.
Keywords: Indian, women living with HIV, human papillomavirus, vaccination, quadrivalent HPV vaccine, cervical cancer
Introduction
More than one-fourth of all cases of cervical cancer worldwide occur in India and over 60,000 women die each year1. As in many other countries, HPV16 and 18 are found in more than 80% of cervical cancers in India2. The incidence of cervical cancer has been substantially reduced in the developed world through routine cervical cytology screening and treatment of cervical squamous intraepithelial lesions (CSIL) prior to progression to invasive cancer. India and many other developing countries have not instituted a routine screening systems because of the high cost, and better measures to prevent cervical cancer are urgently needed in these locations.
Cervical cancer occurs at higher rates in WLWH than in the general population and is an AIDS-defining illness3–5. There is evidence for interactions between the plasma HIV VL, CD4+ level, HPV infection and CSIL6. Antiretroviral therapy (ART) may have a beneficial but incomplete effect on the prevalence and incidence of CSIL, and on the incidence of cervical cancer7,8, and WLWH remain at high risk of cervical cancer even if on effective ART. In the absence of routine cervical cancer screening, HPV vaccination assumes a particularly important role in cervical cancer prevention strategies. Most current HPV vaccination strategies focus on girls under the age of 20 years and studies are needed to determine if older Indian WLWH may benefit from HPV vaccination. It is also important to know if WLWH may benefit from HPV vaccination after acquiring HIV. The efficacy of HPV vaccination among Indian WLWH will depend on several factors: 1) The degree of their prior exposure to the HPV types in the vaccine, since qHPV is a prophylactic vaccine effective only to prevent initial HPV infection; 2) Effect of CD4 level or HIV VL on response to vaccination; 3) Adherence to the complete 3-injection vaccination regimen, and 4) Risk of new exposures to vaccine HPV types in the future.
Compared with women in the United States and sub-Saharan Africa, a higher proportion of Indian women acquire HIV infection from their husbands or steady male partner. They initiate sexual activity at an older age, have a lower lifetime number of sexual partners and have fewer sexual partners other than their husband9,10. Indian WLWH may therefore have higher rates of being naïve to vaccine HPV types defined as being sero-negative and DNA-negative to those types, but with ongoing exposure to new HPV types associated with the sexual activities of their main partner, may potentially benefit more from HPV vaccination than similarly aged WLWH in other parts of the world. The objectives of this study were to study the safety and immunogenicity of the qHPV vaccine and to determine baseline rates of prior exposure to qHPV vaccine types among WLWH in Chennai, India. Since many Indian WLWH did not initiate ART until their CD4 levels fell below 350 cells/mm3, we also examined the relationship between vaccine response, HIV VL, CD4 nadir and current CD4 levels. Finally we compared the results of competitive Luminex immunoassay (cLIA) serology testing for HPV-16 and 18 with pseudovirion-based neutralization assay (PBNA) in our study population since sensitivity for HPV-18titers using cLIA may be lower than for the other qHPV types and neutralizing HPV-18 antibodies may still be present in the absence of a positive cLIA titer11.
Methods
The study was approved by the institutional review boards of YRG Care Medical Centre and the University of California, San Francisco. 150 WLWH aged 18 years or older under care at the YRGCare Medical Centre in Chennai, India were enrolled on this study between 2009 and 2011. At the time the study was enrolled, the Indian standard of care was that ART was initiated if the CD4 level was below 350 cells/mm3 regardless of HIV VL. The inclusion/exclusion criteria were designed to be concordant with this practice. Based on the nadir CD4 level, current CD4+ level and history of ART use, women were assigned to one of the following 3 groups: Group 1) CD4 nadir ≤ 350 cells/mm3, on ART; Group 2) CD4 nadir >350 cells/mm3, current CD4 between 350 cells/mm3 and 500 cells/mm3, not on ART; and Group 3) CD4 nadir > 350 cells/mm3, current CD4 >500 cells/mm3, not on ART. The first 50 women assigned to these groups were consecutively enrolled into the study.
After informed consent, women underwent a complete physical exam. Exclusion criteria included being pregnant at the time of study entry; current or history of high-grade squamous intraepithelial lesions (HSIL) or cervical cancer; inability to provide informed consent; or allergy to yeast or any of the components of qHPV. Women had a cervical swab for cervical cytology and HPV testing. Women with abnormal cytology underwent colposcopy with biopsy of visible lesions. Medication history was obtained, including HIV treatment history in the 6 months prior to study entry. HIV-1 infection was documented using a licensed ELISA test kit and confirmed by Western blot prior to study entry. Nadir CD4 was obtained from medical records. Blood was obtained for CD4+ level and HIV VL, complete blood count and liver function tests.
The qHPV vaccine was administered at 0, 8 and 24 weeks. Women were seen at weeks 28, 36 and 52 following initiation of the vaccine series. Each participant was contacted by the study nurse by telephone 24 to 48 hours following each vaccination to determine if any side effects occurred. Data on adverse events were collected at each visit. This study utilized the Common Terminology Criteria for Adverse Event (CTCAE) for adverse event reporting.
Serology was obtained prior to vaccination at week 0, and at weeks 28 and 52. Geometric mean titers were measured using cLIA for HPV-6, 11, 16 and 1812 and PBNA for HPV-16 and 1813. Cervical HPV DNA was detected using MY09/MY11 PCR followed by typing for 39 individual HPV types, as described previously12. Women who were sero-negative and HPV DNA-negative for a vaccine HPV type and who adhered to all protocol visits and procedures were included in the per-protocol (PP) analysis for that HPV type; all women who had at least one vaccine dose were included in an intent-to treat (ITT analysis).
Statistical methods
Sample size calculation.
As a pilot study, type-specific antibody response rates greater than 50% in this population would be considered successful and supportive of a future efficacy trial. It was estimated that one-third to one-half of women would be seropositive or DNA-positive for a given HPV type at baseline. 150 participants would yield approximately 80 sero-negative participants for each HPV type. With 80, the null hypothesis that the antibody response rate for each HPV type was 50% can be tested against the alternative that it was 65% at the one-sided significance level of 10% with power of 82%. No adjustment for multiple testing was planned.
Statistical analysis.
Analyses were conducted for both ITT and PP populations. Descriptive statistics were calculated for participant characteristics, HPV DNA positivity, and sero-positivity. The percentage of participants who fell into one of four categories defined by baseline sero-positivity and cervical DNA-positivity were calculated overall and according to three groups defined by CD4 and ART status. Groups were compared using exact chi-square tests. To determine antibody responses to the vaccine, the proportion of women who were sero-negative at baseline who had detectable titers for each qHPV type were calculated. Changes in titers from baseline to each visit for each HPV type, CD4+ levels and HIV VL were tested to determine if they were different from zero using the Wilcoxon signed rank test. A logistic regression model assessed the effect of group and age on antibody response at week 28.
Poisson rates (per 100 participants) and their 95% confidence intervals (CIs) were used to estimate the rate of adverse events of grade 2 or higher and grade 3 or higher. Binomial proportions and their two-sided 95% CIs were estimated for the proportion of women experiencing a > 1 log increase in HIV VL from baseline on 2 consecutive occasions and the proportion of women experiencing a decrease in CD4 absolute count to less than 75% of baseline on 2 consecutive occasions; the probability that the proportion was >0.30 was computed using binomial calculations. Per the protocol, this level of HIV VL increase or CD4+ decrease occurring in 30% or more of participants was a priori deemed clinically significant.
Results
213 women were screened to enroll 150 women. 126 women (84%) met the criteria for the PP analysis. The main reasons for screen failures were unwillingness to participate in the study after providing consent or not meeting the protocol-required criteria. The demographics of the ITT and PP populations are shown in Table 1. The mean age of the women was 30.8 years, and most acquired HIV through heterosexual sex. The median CD4 count among women in the PP group was 505 cells/mm3. The median CD4 levels in Groups 1, 2 and 3 were 367, 432 and 712 cells/mm3 respectively. The median HIV copy number was 5600 copies/mL. The median HIV copy numbers in Groups 1, 2 and 3 were ≤400 (undetectable), 57,300 and 3520 copies/mL, respectively. None of the women had cervical LSIL, HSIL or cancer on cytology at baseline.
Table 1.
Demographics and baseline characteristics of Indian women living with HIV
| Intent-to-treat# N (%) | Per-protocol& N (%) | |
|---|---|---|
| Number of participants | 150 (100) | 126 (100) |
| Group | ||
| 1 – CD4 nadir≤ 350, on HAART | 49 (32.7) | 47 (37.3) |
| 2 – CD4 nadir>350, current CD4 350–500, not on HAART | 50 (33.3) | 33 (26.2) |
| 3 – CD4 nadir>350, current CD4 >500, not on HAART | 51 (34.0) | 46 (36.5) |
| Age in years | ||
| Mean (±SD) | 30.8±5.2 | 31.2±5.1 |
| Median (IQR) | 30.0 (27–34) | 30.5 (28–34) |
| CDC HIV Risk Group | ||
| Homosexual contact | 0 | 0 |
| Heterosexual contact | 147 (98.0) | 124 (98.4) |
| IV drug user | 0 | 0 |
| Transfusion recipient | 1 (0.7) | 1 (0.7) |
| Other – health care worker | 1 (0.7) | 1 (0.7) |
| Unknown | 1 (0.7) | 0 |
| CD4 level (cells/mm 3 ) | ||
| Mean (±SD) | 538.7±257.7 | 552.2±272.4 |
| Median (IQR) | 484.5 (390–686) | 504.5 (390–704) |
| HIV VL (copies/ mm 3 ) | ||
| Geometric Mean (95% CI) | 6967 (4722–10279) | 5393 (3533–8235) |
| Median (IQR) | 7440 (400–52800) | 5600 (400–36000) |
1 participant missing HIV VL at baseline and 1 participant had an undetectable HIV VL (<400 copies/mL) at baseline.
Intent to treat population: all women who received at least one vaccination injection
Per-protocol population: women who were eligible, received all vaccinations, and attended all protocol visits
Table 2 shows the distribution of cervical DNA HPV types at baseline. In the ITT population, HPV-16 was the most commonly detected oncogenic HPV type at baseline, found in 5.9%. HPV-18 DNA was found in 1.5% of women. An additional 8.1% were positive for one or more of HPV-31/33/45/52/58, the additional HPV types included in the nonavalent vaccine
Table 2.
Distribution of HPV types at baseline in the intent-to treat and per-protocol populations
| HPV Type | ITT# | Naïve PP# | ||
|---|---|---|---|---|
| Week 0 N=135 | Week 0 N=104 | |||
| n | % | n | % | |
| 6*,** | 0 | 0.0 | 0 | 0.0 |
| 11*,** | 1 | 0.7 | 0 | 0.0 |
| 16*,** | 8 | 5.9 | 0 | 0.0 |
| 18*,** | 2 | 1.5 | 0 | 0.0 |
| 31 ** | 0 | 0.0 | 0 | 0.0 |
| 33 ** | 4 | 3.0 | 2 | 1.9 |
| 45 ** | 1 | 0.7 | 0 | 0.0 |
| 52 ** | 1 | 0.7 | 0 | 0.0 |
| 58 ** | 5 | 3.7 | 2 | 1.9 |
| 26/69 | 0 | 0.0 | 0 | 0.0 |
| 30 | 2 | 1.5 | 2 | 1.9 |
| 32/42 | 0 | 0.0 | 0 | 0.0 |
| 35 | 2 | 1.5 | 2 | 1.9 |
| 39 | 0 | 0.0 | 0 | 0.0 |
| 51 | 4 | 3.0 | 1 | 1.0 |
| 53 | 4 | 3.0 | 2 | 1.9 |
| 56 | 3 | 2.2 | 2 | 1.9 |
| 57/2/27 | 0 | 0.0 | 0 | 0.0 |
| 59 | 1 | 0.7 | 1 | 1.0 |
| 61 | 3 | 2.2 | 1 | 1.0 |
| 62 | 3 | 2.2 | 2 | 1.9 |
| 66 | 1 | 0.7 | 0 | 0.0 |
| 67 | 0 | 0.0 | 0 | 0.0 |
| 68 | 3 | 2.2 | 3 | 2.9 |
| 70 | 1 | 0.7 | 1 | 1.0 |
| 71 | 2 | 1.5 | 1 | 1.0 |
| 72 | 2 | 1.5 | 2 | 2.9 |
| 73 | 1 | 0.7 | 1 | 1.0 |
| 81 | 2 | 1.5 | 1 | 1.0 |
| 82/subtype | 1 | 0.7 | 1 | 1.0 |
| 83 | 0 | 0.0 | 0 | 0.0 |
| 84 | 1 | 0.7 | 0 | 0.0 |
| 85 | 4 | 3.0 | 3 | 2.9 |
| 86/87 | 0 | 0.0 | 0 | 0.0 |
| 90/106 | 4 | 3.0 | 2 | 1.9 |
| 97 | 0 | 0.0 | 0 | 0.0 |
| 102/89 | 1 | 0.7 | 0 | 0.0 |
ITT, intent to treat; Naïve PP, per protocol population and naïve to quadrivalent vaccine HPV types
Included in the quadrivalent HPV vaccine
Included in the nonavalent HPV vaccine
Table 3 shows HPV DNA-positivity and sero-positivity to the qHPV types at baseline by group. Although not statistically significant, the proportion of women who were sero-negative and HPV DNA-negative at baseline was generally lower among the women in Group 1 than the other 2 groups, and there were few differences between Groups 2 and 3.
Table 3.
Sero-positivity using competitive Luminex Immunoassay and cervical DNA-positivity to quadrivalent HPV vaccine types at baseline by group
| n (%) | ||||
|---|---|---|---|---|
| Overall | HPV-6 N=135 | HPV-11 N=135 | HPV-16 N=135 | HPV-18 N=135 |
| Sero-positive and DNA-positive | 0 (0) | 1 (1) | 6 (4) | 1 (1) |
| Sero-positive and DNA-negative | 39 (29) | 16 (12) | 29 (22) | 24 (18) |
| Sero-negative and DNA-positive | 0 (0) | 0 (0) | 2 (2) | 1 (1) |
| Sero-negative and DNA-negative | 96 (71) | 118 (87) | 98 (73) | 109 (81) |
| Group 1 CD4 nadir<=350, on HAART | HPV-6 N=43 | HPV-11 N=43 | HPV-16 N=43 | HPV-18 N=43 |
| Sero-positive and DNA-positive | 0 (0) | 1 (2) | 3 (7) | 1 (2) |
| Sero-positive and DNA-negative | 15 (35) | 7 (16) | 8 (19) | 10 (23) |
| Sero-negative and DNA-positive | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Sero-negative and DNA-negative | 28 (65) | 35 (81) | 32 (74) | 31 (72) |
| Group 2 CD4 nadir > 350, current CD4 350–500, not on HAART | HPV-6 N=46 | HPV-11 N=46 | HPV-16 N=46 | HPV-18 N=46 |
| Sero-positive and DNA-positive | 0 (0) | 0 (0) | 2 (4) | 0 (0) |
| Sero-positive and DNA-negative | 9 (20) | 4 (9) | 10 (22) | 7 (15) |
| Sero-negative and DNA-positive | 0 (0) | 0 (0) | 1 (2) | 0 (0) |
| Sero-negative and DNA-negative | 37 (80) | 42 (91) | 33 (72) | 39 (85) |
| Group 3 CD4 nadir>350, current CD4 > 500, not on HAART | HPV-6 N=46 | HPV-11 N=46 | HPV-16 N=46 | HPV-18 N=46 |
| Sero-positive and DNA-positive | 0 (0) | 0 (0) | 1 (2) | 0 (0) |
| Sero-positive and DNA-negative | 15 (33) | 5 (11) | 11 (24) | 7 (15) |
| Sero-negative and DNA-positive | 0 (0) | 0 (0) | 1 (2) | 0 (0) |
| Sero-negative and DNA-negative | 31 (67) | 41 (89) | 33 (72) | 39 (85) |
Among per-protocol women naïve to HPV-6/11/16/18 at baseline, 100/99/99/90%, respectively, seroconverted at week 28. 95/96/98/71% were sero-positive at week 52, respectively. Table 4 shows the results of cLIA and PBNA serology testing to HPV-16 and 18 at baseline and relationship to HPV DNA status. When including all samples across the study there was high agreement between cLIA and PBNA for HPV-16 and 18 (kappa=.85 (95% CI, 0.79–0.92) and .75 (95% CI, 0.68–0.82), respectively). Among 114 women HPV-18 DNA-negative at baseline, 19 (17%) were HPV-18-positive by cLIA, and 22 (20%) were HPV-18-positive by PBNA.
Table 4.
Serostatus to HPV-16 and 18 by competitive Luminex immunoassay (cLIA) and pseudovirion-based immunoassay (PBNA) positivity at baseline among all women and after vaccination
| N | cLIA*−/PBNA#− | cLIA+/PBNA+ | cLIA+/PBNA− | cLIA-/PBNA+ | |
|---|---|---|---|---|---|
| Women in intent-to-treat group at baseline (% seropositive) | |||||
|
| |||||
| HPV-16 DNA-positive | 5 | 1 (20%) | 3 (60%) | 0 | 1 (20%) |
|
| |||||
| HPV-16 DNA-negative | 111 | 74 (67%) | 21 (19%) | 5 (5%) | 11 (10%) |
|
| |||||
| HPV-18 DNA-positive | 2 | 1 (50%) | 1 (50%) | 0 | 0 |
|
| |||||
| HPV-18 DNA-negative | 114 | 85 (75%) | 12 (11%) | 7 (6%) | 10 (9%) |
|
| |||||
|
| |||||
| Women in per-protocol group who were DNA negative at baseline (% seropositive) | |||||
|
| |||||
| HPV-16 | |||||
|
| |||||
| Baseline | |||||
| DNA-negative | 102 | 68 (67%) | 19 (19%) | 5 (5%) | 10 (10%) |
|
| |||||
| Week 28 | |||||
| DNA-negative | 93 | 0 | 92 (99%) | 0 | 1 (1%) |
| DNA-positive | 2 | 0 | 2 (100%) | 0 | 0 |
|
| |||||
| Week 52 | |||||
| DNA-negative | 97 | 1 (1%) | 95 (98%) | 0 | 1 (1%) |
| DNA-positive | 0 | 0 | 0 | 0 | 0 |
|
| |||||
| HPV-18 | |||||
|
| |||||
| Baseline | |||||
| DNA-negative | 104 | 77 (74%) | 11 (11%) | 7 (7%) | 9 (9%) |
|
| |||||
| Week 28 | |||||
| DNA-negative | 96 | 5 (5%) | 88 (92%) | 1 (1%) | 2 (2%) |
| DNA-positive | 1 | 0 | 1 (100%) | 0 | 0 |
|
| |||||
| Week 52 | |||||
| DNA-negative | 97 | 15 (16%) | 69 (71%) | 4 (4%) | 9 (9%) |
| DNA-positive | 2 | 0 | 0 | 0 | 2 (100%) |
cLIA, competitive Luminex immunoassay (cLIA)
PBNA, pseudovirion-based immunoassay (PBNA)
Among 95 PP women naïve to HPV-16 at baseline with analyzable samples at week 28, all seroconverted at week 28 by PBNA or cLIA, with one being positive only on PBNA. Among 97 PP women with analyzable samples at week 52, all but one remained sero-positive, and with one sero-positive on PBNA only. Among the 97 PP women naïve to HPV-18 DNA at baseline with analyzable samples at week 28, 90 (93%) and 91 (94%) seroconverted to HPV-18 using cLIA and PBNA, respectively. Of these women with detectable HPV-18 at week 28, 67 of 87 (77%) evaluable women and 73 of 87 (84%) remained cLIA- and PBNA-sero-positive at week 52, respectively. One woman who was seronegative at week 28 seroconverted by week 52 based on PBNA, but none had seroconverted based on cLIA. All women naïve to HPV-6 or 11 at baseline were sero-positive to HPV-6 and 99% to HPV-11 at week 28, respectively. At week 52, 95% and 96% remained sero-positive, respectively.
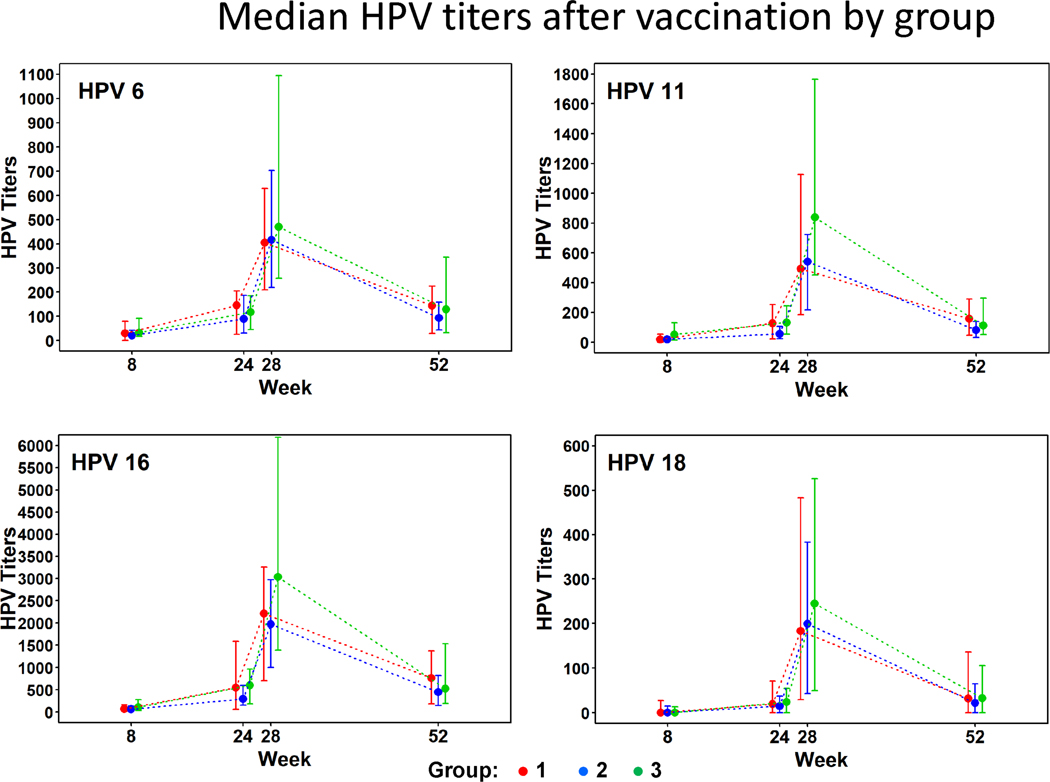
Significant increases in antibody titer from baseline were detected for each HPV type at each post-baseline study visit and for each group (Fig 1). Peak titers were observed at week 28. Median titers declined for all four types by week 52. Peak titers at week 28 were highest for Group 3 but there was little difference among groups in median titer by Week 52.
Figure 1.
Competitive Luminex immunoassay titers to HPV-6/11/16 and 18 at baseline, week 28 and week 52 in the per-protocol population. Points represent median; error bar IQR
Group 1: CD4 nadir ≤ 350 cells/mm3, on ART
Group 2: CD4 nadir >350 cells/mm3, current CD4 between 350 cells/mm3 and 500 cells/mm3, not on ART
Group 3: CD4 nadir > 350 cells/mm3, current CD4 > 500 cells/mm3, not on ART
Logistic regression analyses among those who were sero-negative at baseline were performed to evaluate the association between group and age with seropositivity at week 28. Group and age were not significant, but in a model with nadir CD4 count, baseline CD4 count, baseline ART and age, there was a trend for a positive association between higher baseline CD4 count and proportion of per-protocol women sero-positive to HPV-18 at week 28 (P=0.052). All or almost all participants had detectable HPV-6, −11, or-16 titers at week 28, precluding evaluation of associations for these types.
Safety.
There were no grade 3, 4, or 5 adverse events (AES) that were related to vaccination among the 150 participants in the ITT population. (0% [1-sided 95% CI, 2.0%]). No participants died while on study. Five serious adverse events (SAEs) were reported. Four were grade 3 (anemia, cryptococcal meningitis, leptospirosis, pulmonary tuberculosis) and one (seizures) was grade 2. All required hospitalization but none were attributed to qHPV. Seven (5%) participants had a grade 3 AE or higher. The incidence of grade 3 or higher AEs was 6.0 per 100 participants and the incidence of grade 2 or higher AEs was 102.7 per 100 participants (Table, supplemental digital content). The most frequent AEs were cough (39% of women), pain in extremity (30%), upper respiratory infection (29%), and pruritus (27%). No participants discontinued treatment due to an AE. There were no treatment delays or reductions in dose due to AEs.
The effect of qHPV on CD4 and CD8 levels and HIV VL was examined. There was no significant decrease in CD4 or CD8 level or increase in HIV VL among women in Group 1 who were all on ART. Among women in Groups 2 and 3, none of who were on ART, statistical comparisons were not made given the difficulty of interpretation in these groups.
Conclusions
This is the first study of the safety and immunogenicity of the qHPV vaccine in Indian WLWH and baseline exposure to qHPV types as measured by DNA- and sero-positivity. An understanding of the performance of qHPV in the Indian context is important for several reasons. While the growth of the HIV epidemic has reportedly slowed in India and the overall proportion of the population that is living with HIV is small, the absolute number of cases of HIV infection is substantial given the size of the Indian population.
Results of HPV DNA testing in the women in our study suggest that they were similar to those of other Indian WLWH. In one study of WLWH from eastern India with a mean age of 35 years, the most prevalent HPV type was HPV-16 in 7.9% and 1.4% had HPV-185. The women in our study had a mean age of 31 years, 5.9% had HPV-16 and 1.5% had HPV-18. They are also similar to WLWH in other parts of the world. In a meta-analysis of WLWH across 5 continents without cytological abnormalities, 4.5% had HPV-16 and 3.1% had HPV-1814.
Compared with WLWH in other countries, Indian women are predominantly infected by heterosexual transmission from their husbands. A high proportion is married and they have relatively fewer sexual partners than women in other countries. Consistent with this, our study population had a relatively low baseline level of exposure to the qHPV types, with more than 70% being defined as “naïve” to each of the HPV types, and all participants had normal cervical cytology at baseline. WLWH in India thus represent a group that may benefit from qHPV if shown to be safe and immunogenic even if given at an age older than recommended in Western guidelines (routine up to age 26 years, case-by-case basis 27–45 years).
This study was performed using the qHPV vaccine, which remains one of two HPV vaccines available in India along with the bivalent vaccine. qHPV was previously shown to be safe in children living with HIV15, men living with HIV (MLWH) over the age of 26 years16 and WLWH in the U.S.17. The bivalent HPV vaccine was also shown to be safe and immunogenic in South African women18. Although the nonavalent vaccine is not currently available in India, baseline DNA positivity rates for the 5 additional oncogenic HPV types compared with qHPV were low, but we did not measure seropositivity to these types.
Nearly all women who were sero-negative at baseline seroconverted post-vaccination to all four HPV types and titers were well above levels expected to be protective against initial HPV infection. The titers observed in Indian WLWH were generally lower than those seen in younger, HIV-negative populations15–17. Titers among Indian WLWH naïve to HPV-6/11/18 at baseline overall were similar to those reported in U.S. WLWH, although those to HPV-16 appeared to be higher in our study19. Week 28 titers in our study were generally higher than those reported in among MLWH naïve to qHPV types at baseline12. Similar to our study, declines in titers after week 28 were reported in U.S. WLWH at week 7219 and studies of HIV-negative populations20,21.
As seen in other studies Indian WLWH had lower titers to HPV-18 compared with other HPV types and a higher proportion did not seroconvert to HPV-1817,19. However, in part this reflects lower sensitivity of this assay for HPV-18, and PBNA was positive in 10% of women who were negative using cLIA at week 52. Some of this has been attributed to technical issues with the cLIA assay for HPV-18, consistent with the higher positivity rate in our PBNA assay. The cLIA assay has also been shown to be less sensitive for HPV-18 than the IgG Luminex assay22. Despite lower titers and higher rates of sero-reversion, clinical protection against HPV-18 in vaccinated populations has been durable for up to 14 years and as high as for the other HPV types22.
Our study shows that qHPV vaccine is generally well tolerated in Indian WLWH spanning a range of CD4 level and HIV VLs. Although the overall rate of AEs reported by our participants was high, almost all were grade 2 or less and there were no SAEs attributed to the vaccine. In a study of qHPV in U.S. WLWH, 17% had a grade 3 or higher AEs, a rate higher than that observed in our study17. Likewise qHPV was not associated with any grade 3 or higher AEs in two studies of MLWH12,16. No participants discontinued treatment in our study due to an AE. There were no treatment delays or reductions in dose due to AEs.
There was no effect of qHPV on CD4 level. Significant increases in HIV VL and log VL were observed for all ITT and PP women in Groups 1, 2 and 3 combined, but this effect was not observed among women in Group 1 who were on ART. Although some of this increase among women in Groups 2 and 3 may have been related to vaccination, it is more likely that the increase reflects changes in CD4 level and VL associated with the natural history of untreated HIV infection, in a population not on ART.
There are several limitations to this study. Since the study was performed, World Health Organization recommendations on initiation of ART were adopted in India, and ART is now recommended for all WLWH. We followed women only for one year after study entry, and the long-term levels of antibody titers are not known. The study was not designed to not assess vaccine efficacy and the degree to which they remain at risk of future HPV exposure and development of cervical cancer is unknown. Since we did not have an unvaccinated control, the direct effect of vaccination on CD4 levels or HIV VL cannot be determined with certainty. A recent study showed that while rates of vaccine failure were low in Canadian WLWH, vaccinated WLWH may be at higher risk for vaccine failure than vaccinated women without HIV23. Studies are needed to establish the efficacy of vaccinating Indian WLWH over the age of 26 years. Women who are < 26 years should be vaccinated if possible regardless of HIV status. Future studies should include the use of the nonavalent HPV vaccine which will likely become available in India in the future. Finally the significance of being “naïve” to a given HPV type in this study context is not known. Sero-negative women may have been sero-positive in the past and reverted to sero-negative. It is not clear if qHPV affords the same protection in these women compared with those who were never exposed to that HPV type.
Overall, with their high rate of being naïve to qHPV vaccine types, and with high levels of safety and immunogenicity, our data suggest that a high proportion of Indian WLWH over the age of 26 years could potentially benefit from HPV vaccination. Efficacy studies of HPV vaccination in Indian WLWH are needed, and if future studies show clinical efficacy, policy makers should strongly consider addition of HPV vaccination as a standard of care for Indian WLWH.
Supplementary Material
Acknowledgments
Special thanks to the participants and the staff of YRGCARE and special thanks to Emmes Corporation for excellent study support.
Supported by: NIH National Cancer Institute grant number U01 CA121947.
References
- 1.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray. https://www.hpvcentre.net/parser.php?xml=M2_Cervical%20Cancer_Number%20of%20Deaths&iso=IND&title=M2.%20Disease%20burden%20estimates%20-%20Cervical%20cancer%20-%20Number%20of%20deaths. Updated 2018. Accessed 08/06, 2019.
- 2.Saranath D, Khan Z, Tandle AT, et al. HPV16/18 prevalence in cervical lesions/cancers and p53 genotypes in cervical cancer patients from India. Gynecol Oncol. 2002;86:157–62. [DOI] [PubMed] [Google Scholar]
- 3.Denny LA, Franceschi S, de Sanjose S, Heard I, Moscicki AB, Palefsky J. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine. 2012;30 Suppl 5:F168–74. [DOI] [PubMed] [Google Scholar]
- 4.Joshi S, Chandorkar A, Krishnan G, et al. Cervical intraepithelial changes & HIV infection in women attending sexually transmitted disease clinics in Pune, India. Indian J Med Res. 2001;113:161–169. [PubMed] [Google Scholar]
- 5.Chakravarty J, Chourasia A, Thakur M, Singh AK, Sundar S, Agrawal NR. Prevalence of human papillomavirus infection & cervical abnormalities in WLWHin eastern India. Indian J Med Res. 2016;143(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97:577–86. [DOI] [PubMed] [Google Scholar]
- 7.Menon S, Rossi R, Zdraveska N, et al. Associations between highly active antiretroviral therapy and the presence of HPV, premalignant and malignant cervical lesions in sub-saharanafrica, a systematic review: Current evidence and directions for future research. BMJ Open. 2017;7(8):e015123–2016-015123. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly H, Weiss HA, Benavente Y, de Sanjose S, Mayaud P, ART and HPV Review Group. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: A systematic review and meta-analysis. Lancet HIV. 2018;5(1):e45–e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newmann S, Sarin P, Kumarasamy N et al. Marriage, monogamy and HIV: a profile of HIV-infected women in south India. Int J STD AIDS. 2000; 11 (4): 250–253. [DOI] [PubMed] [Google Scholar]
- 10.Vickerman P, Foss AM, Pickles M. et al. To what extent is the HIV epidemic in southern India driven by commercial sex? A modelling analysis. AIDS 2010, 24:2563–2572. [DOI] [PubMed] [Google Scholar]
- 11.Roberts C, Swoyer R, Bryan J. Evaluation of the HPV-18 antibody response in gardasil(R) vaccinees after 48 mo using a pseudovirion neutralization assay. Hum Vaccin Immunother. 2012;8(4):431–434. [DOI] [PubMed] [Google Scholar]
- 12.Wilkin T, Lee JY, Lensing SY, et al. Safety and immunogenicity of the quadrivalent human papillomavirus vaccine in HIV-1-infected men. J Infect Dis. 2010;202:1246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma R, Efird JT, Chein A, et al. Prevalence and risk factors for neutralizing antibodies to human papillomavirus types 16 and 18 in HIV-positive men who have sex with men. J Acquir Immune DeficSyndr. 2013;64(5):479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clifford GM, Goncalves MAG, Franceschi S et al. Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS 2006; 20: 2337–2344. [DOI] [PubMed] [Google Scholar]
- 15.Levin MJ, Moscicki AB, Song LY, et al. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. J Acquir Immune DeficSyndr. 2010;55:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkin TJ, Chen H, Cespedes MS, et al. A randomized, placebo-controlled trial of the quadrivalent HPV vaccine in HIV-infected adults age 27 years or older: AIDS clinical trials group protocol A5298. Clin Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojic EM, Kang M, Cespedes MS, et al. Immunogenicity and safety of the quadrivalent human papillomavirus vaccine in HIV-1-infected women. Clin Infect Dis. 2014;59(1):127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denny L, Hendricks B, Gordon C, et al. Safety and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine in WLWH in South Africa: A partially-blind randomised placebo-controlled study. Vaccine. 2013;31(48):5745–5753. [DOI] [PubMed] [Google Scholar]
- 19.Cespedes S, Kang M, Kojic E et al. Anogenital human papillomavirus virus DNA and sustained response to the quadrivalent HPV vaccine in women living with HIV-1. Papillomavirus Research 2018; 6: 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown DR, Garland S, Ferris DG et al. The humoral response to Gardasil over four years as defined by total IgG and competitive Luminex immunoassay. Hum Vaccin 2011; 7:230–8. [DOI] [PubMed] [Google Scholar]
- 21.Godi A, Panwar K, Haque, et al. Durability of the neutralizing antibody response to vaccine and non-vaccine HPV types 7 years following immunization with either Cervarix® or Gardasil® vaccine. Vaccine 2019; 37: 2455–2462 [DOI] [PubMed] [Google Scholar]
- 22.Kjaer SK, Nygard M, Sundstrom K et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine 23 (2020) 100401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClymont E, Lee, Raboud J et al. The Efficacy of the Quadrivalent Human Papillomavirus Vaccine in Girls and Women Living With Human Immunodeficiency Virus. Clin Infect Dis 2019; 68: 788–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.