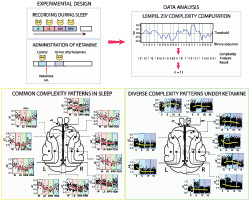
Abstract
There is increasing evidence that the level of consciousness can be captured by neural informational complexity: for instance, complexity, as measured by the Lempel Ziv (LZ) compression algorithm, decreases during anaesthesia and non‐rapid eye movement (NREM) sleep in humans and rats, when compared with LZ in awake and REM sleep. In contrast, LZ is higher in humans under the effect of psychedelics, including subanaesthetic doses of ketamine. However, it is both unclear how this result would be modulated by varying ketamine doses, and whether it would extend to other species. Here, we studied LZ with and without auditory stimulation during wakefulness and different sleep stages in five cats implanted with intracranial electrodes, as well as under subanaesthetic doses of ketamine (5, 10, and 15 mg/kg i.m.). In line with previous results, LZ was lowest in NREM sleep, but similar in REM and wakefulness. Furthermore, we found an inverted U‐shaped curve following different levels of ketamine doses in a subset of electrodes, primarily in prefrontal cortex. However, it is worth noting that the variability in the ketamine dose–response curve across cats and cortices was larger than that in the sleep‐stage data, highlighting the differential local dynamics created by two different ways of modulating conscious state. These results replicate previous findings, both in humans and other species, demonstrating that neural complexity is highly sensitive to capture state changes between wake and sleep stages while adding a local cortical description. Finally, this study describes the differential effects of ketamine doses, replicating a rise in complexity for low doses, and further fall as doses approach anaesthetic levels in a differential manner depending on the cortex.
Keywords: cats, complexity, cortex, ketamine, local field potential, psychedelics, sleep, thalamus
Previous studies have shown that Lempel Ziv complexity (LZ) decreases during anaesthesia and non‐rapid eye movement (NREM) sleep in humans and rats whereas it increases in REM sleep and under the effect of psychedelics. In this work, we show that in cats, LZ is lowest in NREM sleep, but similar in REM and wakefulness. We also found a ketamine inverted U‐shape dose–response curve only in the auditory and prefrontal cortex, with a much larger variability in the ketamine across cats and cortices when compared with the sleep cycle.
Abbreviations
- 5‐HT
5‐hydroxytryptamine
- BF
Bayes factors
- BIC
Bayesian information criterion
- DMT
N,N‐dimethyltryptamine
- ECG
electrocardiogram
- ECoG
electrocorticogram
- EEG
electroencephalography
- EMG
electromyogram
- ERPs
evoked related potentials
- GABA
gamma aminobutyric acid
- HCN channels
hyperpolarization‐activated cyclic nucleotide‐gated channels
- LS
light sleep
- LSD
lysergic acid diethylamide
- LZ
Lempel–Ziv
- NMDA
N‐methyl‐d‐aspartate
- NREM
non‐rapid eye movement
- PCI
perturbational complexity index
- PCIST
PCI state‐transition
- REM
rapid eye movements
- SWS
slow wave sleep
- TMS
transcranial magnetic stimulation
1. INTRODUCTION
There is increasing evidence for a strong association between neural information measures, such as electrophysiological signal complexity, and level of consciousness (Abásolo et al., 2015; Castro‐Zaballa et al., 2019; González et al., 2019; Mateos et al., 2018; Schartner et al., 2015; Schartner, Carhart‐Harris, et al., 2017; Zhang et al., 2001). One of the most studied neural complexity metrics is Lempel–Ziv (LZ) complexity, capturing the number of distinct substrings or patterns within a sequence (Lempel & Ziv, 1976; Ziv & Lempel, 1978). A decrease in complexity has been demonstrated for anaesthesia (Li & Mashour, 2019; Schartner et al., 2015; Zhang et al., 2001) and during non‐rapid eye movement sleep (NREM sleep) when compared with normal wakefulness. However, REM complexity has consistently been shown to be above NREM sleep and below normal wakefulness (Abásolo et al., 2015; Andrillon et al., 2016; Mateos et al., 2018; Schartner, Pigorini, et al., 2017). The increase in complexity during REM, where vivid dreaming often occurs, may lend credence to the hypothesis that complexity may not only be modulated by consciousness level but also signal the degree of contents of consciousness (Abásolo et al., 2015; Mateos et al., 2018).
Further evidence for LZ associated with an increase in the range of conscious contents comes from higher LZ during resting state in humans under the effect of psychedelics, specifically lysergic acid diethylamide (LSD), psilocybin, and subanaesthetic doses of the dissociative NMDA‐antagonist ketamine, compared with placebo (Li & Mashour, 2019; Mediano et al., 2020; Schartner, Carhart‐Harris, et al., 2017). These drugs have profound and widespread effects on conscious experiences, both internally and externally generated. More specifically, they appear to “broaden” the scope of conscious contents, vivifying imagination and positively modulating the flexibility of cognition (Carhart‐Harris et al., 2014, 2016). For all three drugs, reliably higher spontaneous signal diversity was reported. More recently, a higher level of complexity following a subanaesthetic dose of ketamine was also reported (Farnes et al., 2020; Li & Mashour, 2019) in spontaneous high‐density scalp electroencephalography (EEG) signals in healthy volunteers, but no increase was observed when auditorily stimulated.
Ketamine also appears to maintain spatiotemporal complexity, as measured through the perturbational complexity index (PCI) (Sarasso et al., 2015). PCI is the result of applying LZ to the spatiotemporal pattern of cortical activation evoked by transcranial magnetic stimulation (TMS) and has proven to be a reliable classifier of level of consciousness (Casali et al., 2013). PCI decreases during propofol, midazolam, and xenon anaesthesia (Casali et al., 2013) but maintains wakefulness baseline level during ketamine anaesthesia (Sarasso et al., 2015).
Despite this body of work, important questions remain unanswered. First, prior studies provide only a disjointed picture by investigating the effect of anaesthetic dose in TMS‐evoked cortical activation (Sarasso et al., 2015) or subanaesthetic dose in spontaneous magnetoencephalographic (MEG) signals (Schartner, Carhart‐Harris, et al., 2017). For a more complete understanding of ketamine's psychoactive effects, a systematic investigation of the dose‐dependent effects of ketamine on cortical complexity using the same modality is required. Therefore, in this work, we aimed to investigate the level of informational complexity during different stages of sleep in the cat as well as under subanaesthetic doses of ketamine in a dose‐dependent manner, compared with the control awake state. Additionally, we determined how the complexity measures under ketamine compared with baseline conditions, with or without the presence of sensory stimulation. Finally, we sought to understand the possible differences in informational complexity between resting‐state periods and sensory stimulation periods across conscious states. We aim to add to the characterization of the interaction between psychedelic states and perturbational states in intracranial recordings and via dose‐dependent manner because our own work suggests a modulation by task (Mediano et al., 2020, 2021) while others do not (Farnes et al., 2020). Accordingly (Pascovich et al., 2019), the following hypotheses were proposed: (1) LZ would reflect sleep level: LZ in wakefulness would be just above REM sleep. REM sleep would be above light sleep (LS), and NREM sleep would have the lowest complexity value; (2) LZ would be increased during the initial period of drug infusion compared with baseline wakefulness; (3) the level of complexity would be higher under sensory stimulation compared with baseline, for both conditions, with and without ketamine; and (4) stimulation‐induced complexity increase would be more evident under the effect of ketamine. This last hypothesis is in line with the entropic brain theory, assuming that under psychedelics the diversity of mental states is increased and the experience produced by a stimuli is amplified by the brain under this state (Carhart‐Harris et al., 2014, 2016).
2. RESULTS
2.1. Sleep shows a state‐dependent effect on LZ complexity
Cats underwent a polysomnographic recording in semirestricted conditions where they were adapted to sleep. Data were obtained during spontaneously occurring quiet wakefulness, LS, NREM sleep and REM sleep (Figure 1a). Examples of raw traces and power spectrum characterization in two cortices from one cat are presented in Figure 1d. For a more detailed description of power spectral density results, see Castro‐Zaballa et al. (2019).
FIGURE 1.
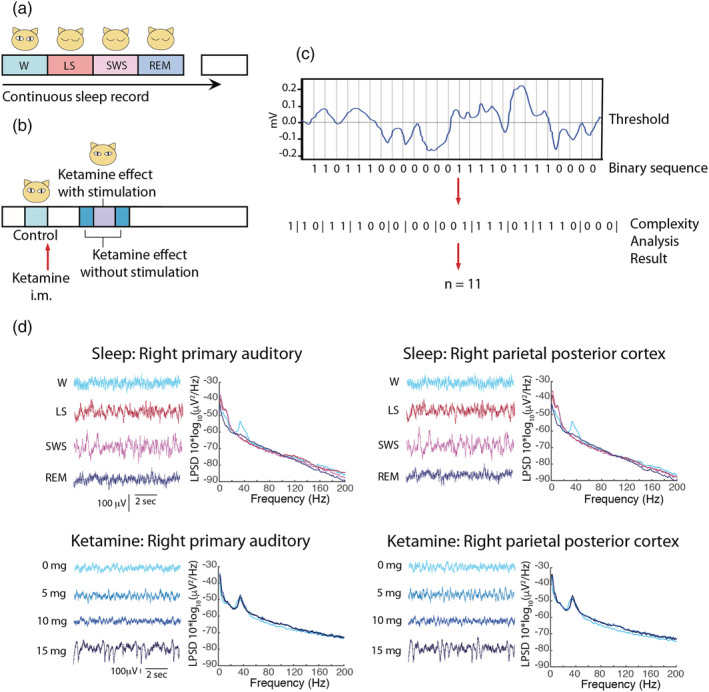
Schematic illustrating the experimental design for electrocorticographic recordings during the different states of sleep (a) and before, and after the different doses of ketamine (b). i.m., intramuscular. (c) Illustration showing how to transform a segment of ECoG signal series into a binary sequence and the result of the LZ complexity analysis on the binary sequence. (d) Raw traces and spectral characterization of sleep stages and different subanesthetic doses of ketamine in one animal
LZ was computed using the LZ78 algorithm (Ziv & Lempel, 1978; Figure 1c) from the different sleep stages for all the cortices available (Figure 2a). Effect sizes for differences between states at the single subject level are shown in Figure 2b. For all animals, LZ scored higher for wakefulness than NREM sleep (Cohen's d > 0.8) for most of the cortices. As predicted, LZ values were highest for REM and W, intermediate for LS, and lowest for NREM sleep.
FIGURE 2.
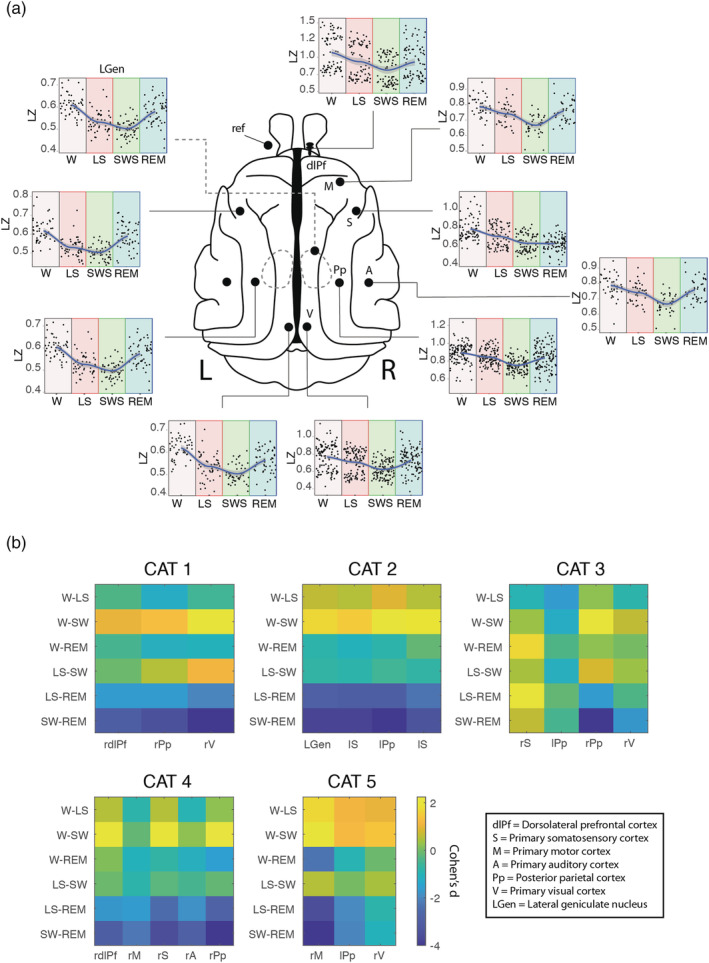
Cortical dynamic of LZ during sleep. Schematic representations of the cat brain are used to visualize the differential dynamics of LZ during wakefulness and different states of sleep (a), showing a U‐shaped complexity curve with state progression from W to LS, SWS and REM. “L” indicates Left side and “R” right side. (b) The differences in average LZ between sleep states, as measured by ANOVA and Tukey posthoc test. Effect sizes were calculated by Cohen's d and represented in a colour scale, where yellow means a positive difference and blue means a negative difference between the effect sizes of the pair of states compared. W = wakefulness; LS = light sleep; SWS = slow wave sleep; REM = rapid eye movements sleep; dlPf = dorsolateral prefrontal cortex; Pp = posterior parietal cortex; V = visual cortex; LGen = lateral geniculate nucleus; S = somatosensory cortex; M = motor cortex; A = auditory cortex; ref, reference electrode location. In b, “r” indicates right and “l” indicates left cortex
Additionally, mixed effects models were formulated for each cortex including the cat as a random effect when applicable. Thereafter, model selection was performed between linear and quadratic models using Bayes factors (BF) to decide between U‐shaped and linear fits. All model comparisons between linear and nonlinear quadratic fits showed the supremacy of the nonlinear fit (Table 1) in agreement with our previous hypotheses, where the REM sleep showed higher complexity values than the deep sleep—with the exception of the right somatosensory cortex, where the results showed a flattening of the curve compared with all other cortices (Figure 2).
TABLE 1.
Selection between linear and nonlinear models among different sleep stages for each cortex
| Cortex | No. of cats | Model | BF |
|---|---|---|---|
| Right dorsolateral prefrontal | 2 | Linear | |
| Quadratic * | 6.88 × 1018 | ||
| Right primary motor | 2 | Linear | |
| Quadratic * | 1.90 × 1017 | ||
| Right primary auditory | 1 | Linear | |
| Quadratic * | 4.42 × 106 | ||
| Right primary somatosensory | 2 | Linear | |
| Quadratic | 0.681 | ||
| Left primary somatosensory | 1 | Linear | |
| Quadratic * | 8.53 × 1020 | ||
| Right posterior parietal | 3 | Linear | |
| Quadratic * | 1.76 × 107 | ||
| Left posterior parietal | 1 | Linear | |
| Quadratic * | 7.19 × 109 | ||
| Right primary visual | 3 | Linear | |
| Quadratic * | 3.70 × 1011 | ||
| Left primary visual | 1 | Linear | |
| Quadratic * | 3.77 × 1017 | ||
| Right lateral geniculate | 1 | Linear | |
| Quadratic * | 7.00 × 1020 |
Note: Mixed effects models were formulated for each cortex including the cat as a random effect when applicable. Bayes factors (BF) were used to decide between U‐shaped and linear fits. With the exception of the right primary somatosensory cortex, all model comparisons showed the supremacy of the quadratic fit. The asterisks indicate substantial evidence for a quadratic fit (BF > 5).
2.2. Heterogeneous cortical dynamics across cortices under ketamine
For this experiment, the data were collected under the same experimental conditions as for sleep recordings in the same cats, and i.m. injections of ketamine of 5, 10, or 15 mg/kg were performed in separate nonconsecutive days as schematized in Figure 1b (see Section 4). The raw data shown in Figure 1d reveal the presence of slow waves with 15 mg/kg dose of ketamine, whereas the power spectral density plots show an increase in gamma power, as already had been reported by Castro‐Zaballa et al. (2019). Again, LZ was calculated in epochs before and after the administration of the drug. To address dose–response relationships, a multilevel model was used where LZ was predicted by dose (fixed effect), and cat and session were considered as random effects (with sessions nested within cats, and each dose of ketamine was repeated four times).
Considerably greater LZ variability was observed under ketamine than for the sleep results, especially during the lowest doses explored (Figure 3a). In some regions, the results are in agreement with our hypothesis, which predicted an increase in informational complexity after the lowest ketamine dose, followed by a decrease with the higher dose showing an inverted U‐shaped relationship. This can be observed clearly in the right rostral and dorsolateral prefrontal cortices as well as the right primary auditory cortex (BF = 1.70 × 1014, BF = 2.57 × 105, BF = 8.25 × 109, respectively). However, when we look at the individual effect per cortex in each animal, it can be seen that the inverted U‐shaped relationship is not systematic between cats and is present only in the cortices of two out of five cats (Figure 3b).
FIGURE 3.
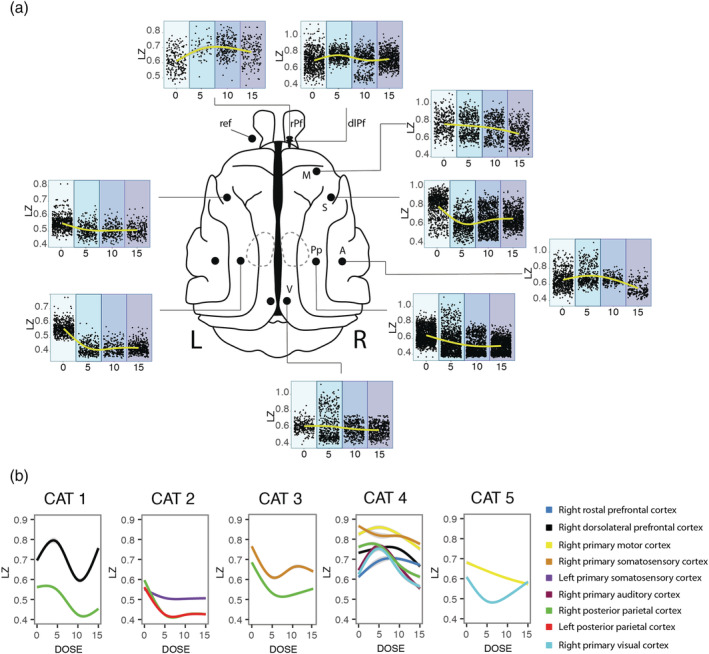
Curves dose‐response of the dose of ketamine on cortical dynamics of LZ. (a) Dose‐response curve of subanaesthetic doses of ketamine, showing an inverted U‐shaped curve only for prefrontal and auditory cortices, with monotonic decrease of complexity with concentration for the other cortices. Each plot represents the sum of the different sessions for each dose of the different cats which have that cortex, therefore the number of cats is different per cortex. (b) The curves are plotted per cat. It can be clearly seen that the variability in the informational complexity dynamic per cortex and per cat is evidenced more clearly when plotted individually, and shows that there is a dissociation of the dose‐response and the anatomical location. The doses are represented in mg/kg. rPf, rostral prefrontal cortex; dlPf, dorsolateral prefrontal cortex; M, primary motor cortex; S, primary somatosensory cortex; A, primary auditory cortex, Pp, posterior parietal cortex; V, visual cortex. “L” indicates the left side and “R” the right side
On the other hand, an opposite curve was obtained for somatosensory and posterior parietal cortices. Finally, for the visual cortex, the effects were less consistent among cats; in this last example, the two cats tested had different responses to ketamine with opposite effects (Figure 3b).
As for sleep, we studied ketamine effects on LZ using model fitting of the individual mixed effects models for each cortex. Model selection was performed in this case between linear, quadratic and cubic models using BF (Table 2).
TABLE 2.
Selection between linear and nonlinear models among different doses of ketamine for each cortex
| Cortex | No. of cats | Model | BF |
|---|---|---|---|
| Right dorsolateral prefrontal | 2 | Linear | |
| Quadratic * | 2.57 × 105 | ||
| Cubic | 288.73 | ||
| Right rostral prefrontal | 1 | Linear | |
| Quadratic * | 1.70 × 1014 | ||
| Right primary motor | 2 | Linear | |
| Quadratic | 0.04 | ||
| Right primary auditory | 2 | Linear | |
| Quadratic * | 8.25 × 109 | ||
| Right primary somatosensory | 3 | Linear | |
| Quadratic | 3.0 × 10−3 | ||
| Cubic | 0.66 | ||
| Left primary somatosensory | 1 | Linear | |
| Quadratic | 1.08 | ||
| Right posterior parietal | 4 | Linear | |
| Quadratic | 1.13 × 10−4 | ||
| Left posterior parietal | 2 | Linear | |
| Quadratic * | 46057.12 | ||
| Right primary visual | 2 | Linear | |
| Quadratic | 1.28 | ||
| Cubic | 3.0 × 10−3 |
Note: Mixed effects models were formulated for each cortex including the cat as a random effect when applicable. Bayes factors (BF) were used to decide between quadratic (U‐shaped), cubic and linear fits. Clear evidence towards a quadratic fit was found for right dorsolateral and rostral prefrontal cortices, right primary auditory cortex and left posterior parietal cortex. The asterisks indicate substantial evidence for a quadratic fit (BF > 5).
Finally, in order to show the possible inter‐areal differential effects of ketamine and dependencies to LZ in basal conditions among cortices, we studied both the baseline variance and its change per area. To study the basal conditions among cortices, we built a linear mixed effect model including cats and sessions as random effects, and cortex as fixed effect and that model is statistically reliable (BIC = −15,175) when contrasted against a null model (BIC = −12,941; p < 0.01) indicating that LZ vary among cortices. We further show that the effect of ketamine does not seem to be dependent on the LZ in basal conditions in wakefulness (see Figure S1). When looking into wakefulness and light sleep the effects in LZ were all in the same direction and comparable in intensity independently of the baselines variability. It seems that basal LZ does not predict whether the LZ increases or decreases with drug. Finally, an interesting exploratory finding showed higher baseline LZ for right parietal cortices when compared with left side ones (p < 0.01; BF = 3.3 × 1016) but not strong enough for primary somatosensory and primary visual.
2.3. Informational complexity is not modulated by auditory stimuli under ketamine
In three cats, modulation by auditory stimuli was studied. Under control conditions without ketamine, an increase in LZ was observed during stimulation in dorsolateral prefrontal (0.66 ± 0.04 to 0.70 ± 0.007, p < 0.01, η 2 = 0.044, BF = 10619.07) and auditory (0.63 ± 0.05 to 0.66 ± 0.007, p < 0.01, η 2 = 0.035, BF = 75.27) cortices, whereas the effect on other cortices studied were nonreliable, including right posterior parietal cortex (0.53 ± 0.02 to 0.53 ± 0.001, p < 0.01, η 2 = 0.008, BF = 7.0 × 10−5), right somatosensory cortex (0.62 ± 0.05 vs. 0.62 ± 0.002 with p = 0.61, η 2 = 0.005, BF = 1.0 × 10−4), left somatosensory cortex (0.52 ± 0.005 vs. 0.53 ± 0.002 with p < 0.01, η 2 = 0.002, BF = 0.09), and left posterior parietal cortex (0.47 ± 0.01 vs. 0.48 ± 0.001, p = 0.85, η 2 = 0.001, BF = 1.0 × 10−4, Figure 4a).
FIGURE 4.
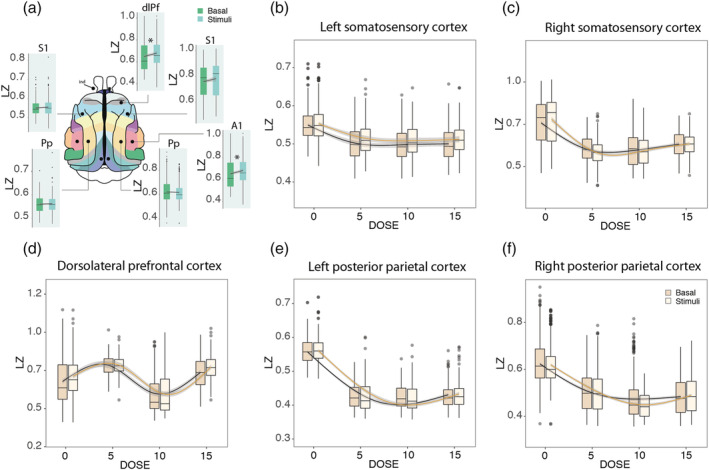
Modulation of LZ by auditory stimulation. (a) The effect of stimulation was shown without ketamine where an increase in LZ was observed during stimulation in dorsolateral prefrontal and auditory cortices. *Statistically reliable (p < 0.01; BF > 5). dlPf = dorsolateral prefrontal cortex; Pp = posterior parietal cortex; S1 = primary somatosensory cortex; A1 = primary auditory cortex. (b–f) Modulation by stimulation under the effect of ketamine in 5, 10, and 15 mg/kg doses. No stimulation by dose interaction was observed. The doses are represented in mg/kg
Initially, we hypothesized that the increment in complexity under the sensory stimulation versus nonstimulation conditions would be more evident under the effect of ketamine. However, there was no interaction between stimulation and ketamine. For left somatosensory cortex, nonreliable effect was observed during basal conditions in response to the stimuli (p = 3 × 10−4, BF = 0.09), as well as evidence for no interaction between dose and stimuli (p = 0.16; BF = 3.5 × 10−4, Figure 4b). For right somatosensory cortex, where nonreliable increase was evidenced in control conditions (Figure 4a), no reliable interaction was found during ketamine effect (p = 2.0 × 10−3; BF = 0.012) with no response to the stimuli (p = 0.64; BF = 1.5 × 10−4; Figure 4c). For the prefrontal cortex, where an increase was observed in control conditions, the same effect was found under ketamine (p = 9.4 × 10−8; BF = 335.92), with nonreliable interaction between stimuli and ketamine (p = 0.51; BF = 2.5 × 10−8; Figure 4d). For left posterior parietal cortex, nonreliable effect was found under baseline conditions, there was no effect of stimulation under ketamine (p = 0.85; BF = 1.2 × 10−4), and the interaction also remained unchanged under ketamine (p = 0.74; BF = 2.5 × 10−6; Figure 4e). Finally, for right posterior parietal cortex, no effect was found under basal conditions, there was no change with the stimuli under ketamine (p = 0.46; BF = 7.4 × 10−5), and the modulation by stimulation was nonreliable (p = 8 × 10−3; BF = 8 × 10−3; Figure 4f).
3. DISCUSSION
In this work, using LZ as a measure of dynamical complexity on direct intracranial recordings, we studied the effect of subanaesthetic doses of ketamine in a dose‐dependent manner. Ketamine elicited a diverse set of dynamics, with the lower doses showing the most variable effects. For prefrontal and auditory cortices an increase in LZ was observed from low to medium ketamine dose. However, a decrease was evidenced at the maximum dose, drawing an inverted U‐shape dose‐effect curve, whereas the opposite effect was observed for other cortices including somatosensory and posterior parietal cortices, where an initial decrease was followed by an increase in complexity at higher doses. Additionally, we also presented auditory stimulation to the cats, which elicited an increase in LZ in prefrontal and auditory cortices, but this effect was not modulated by ketamine. Finally, in the same animals, we studied LZ during sleep, which by contrast show a homogeneous pattern among cortices. We demonstrate that informational complexity in the cortex of the cat decreases in light and deep sleep compared with awake states and REM. The same effect was observed in the geniculate nucleus of the thalamus, but as it was tested in only one animal and hence more evidence is required to see convergence in subcortical structures. For most of the cortex, there is only marginal complexity difference between wakefulness and REM sleep. The results were consistent among cats and similar for all the cortices studied and, more importantly, confirm previous results in humans and rats.
As measures of neural signal diversity are known to be sensitive to conscious level in natural state changes (the sleep–wake cycle), they are also sensitive to the changes in brain dynamics associated with psychedelic and anaesthetic states. Specifically, Schartner et al. found increased global neural signal diversity for the psychedelic state induced by ketamine, psilocybin and LSD, as compared with placebo, across a range of measures (Schartner, Carhart‐Harris, et al., 2017). Other recent MEG and EEG studies have also demonstrated elevated signal diversity induced by canonical serotonergic psychedelics and ketamine (Tagliazucchi et al., 2014; Timmermann et al., 2019).
From the perspective of its effects on EEG signal diversity, the dissociative NMDA‐antagonist ketamine diverges from traditional anaesthetics at subanaesthetic concentrations, as it induces dissociative states characterized by a maintained or enhanced repertoire of brain states (Li & Mashour, 2019; Schartner, Carhart‐Harris, et al., 2017). This is in contrast to GABAergic anaesthetics such as propofol, which have been shown to degrade sensory integration and attenuate neural signal diversity in a dose‐dependent manner (Ferenets et al., 2006, 2007; Ishizawa et al., 2016). While those studies were based on EEG signals that had been low‐pass filtered at 55 Hz and lacked cortical dynamics in higher gamma frequencies, Pal et al. (2020) have recently demonstrated that this part of the signal is important. Using intracranial EEG data from frontal and parietal cortices of rats receiving ketamine or propofol anaesthesia, they demonstrated a reduction in broadband (0.5–175 Hz) EEG complexity during ketamine anaesthesia that is comparable to that induced by the GABAergic anaesthetic propofol. Bandwidth‐specific analyses restricted to higher gamma frequencies showed that ketamine anaesthesia is distinguished from propofol by suppression of EEG complexity in high gamma frequencies in the range of 65–175 Hz, which previous human studies using scalp EEG could not reveal (Pal et al., 2020). In the present study, by using intracranial electrodes in cats, we were able to study broadband (>0.5 Hz) signal complexity.
Contrary to the apparent convergence of psychedelics (LSD, N,N‐dimethyltryptamine or DMT, psilocybin) reported (Schartner, Carhart‐Harris, et al., 2017), some of us (González et al., 2021) have shown that the effects of ibogaine, a psychedelic alkaloid, induces high gamma power but are less coherent and less complex than control condition and similar to natural REM sleep. Although some differences in the complexity measure or animal model may explain the difference, it is key to highlight that the ibogaine local complexity patterns were more consistent than those found in the current study, pointing to a different mode of action between alkaloid, serotoninergic and N‐methyl‐d‐aspartate (NMDA) psychedelics.
Ketamine's primary mechanism of action is as an NMDA antagonist whose receptors are located quite ubiquitously across the cerebral cortex, as well as subcortically (Conti et al., 1994; Huntley et al., 1994). A differential interaction with various subtypes of NMDA receptors could explain the heterogeneity in cortical response under the effects of ketamine (Zanos et al., 2018). However, the non‐NMDA receptor effects of ketamine cannot be discounted, in particular its interactions with opioid receptors and hyperpolarization‐activated cyclic nucleotide‐gated (HCN) channels (Chen et al., 2009; Zanos et al., 2018; Zhou et al., 2013). Additionally, ketamine may indirectly exert effects through its interaction with other circuits. Previous work reported that subanaesthetic doses of ketamine increased the release of not only 5‐hydroxytryptamine (5‐HT) (Amargós‐Bosch et al., 2006; López‐Gil et al., 2012, 2019) but also noradrenaline (Lorrain et al., 2003) as well as glutamate (Moghaddam et al., 1997) in the medial prefrontal cortex, which may increase signal complexity. At the receptor level, ketamine blocks excitatory NMDA receptors on fast‐spiking cortical interneurons more effectively than those on pyramidal neurons. This results in down‐regulation of interneuron activity, and decreased gamma aminobutyric acid (GABA) release at the interneuron–pyramidal neuron synapse (Homayoun & Moghaddam, 2007; Seamans, 2008). This decrease in inhibitory tone (decreased GABA release) results in markedly excited pyramidal neurons. It has been proposed that this may explain why ketamine is associated with increased cerebral glucose utilization and blood flow (Langsjo et al., 2005; Långsjö et al., 2004) and increased EEG gamma oscillations (Blain‐Moraes et al., 2014; Castro‐Zaballa et al., 2019; Ferrer‐Allado et al., 1973; Lee et al., 2013; Schwartz et al., 1974) and may also help us understand the changes observed in the complexity of the signal. However, our results show a decrease of LZ in somatosensory and posterior parietal cortices after the lowest dose of ketamine (Figure 3b). As both of these cortices process somatosensory information, our results may be due to a reduction in the somatosensory information influx, as one of the main effects of subanaesthetic doses of ketamine is analgesia (Zanos et al., 2018).
A further possible outcome for subanaesthetic doses of ketamine effects that we did not find evidence for is the increased locomotion, as found in rats (Hetzler & Wautlet, 1985). An increase in complexity compared with baseline was found for prefrontal and motor cortices, thus, a connection between this regional increase of LZ complexity and putative increased motor activity could be proposed as a possible explanation, however, as reported in our previous publication using the same dataset (Castro‐Zaballa et al., 2019), the cats retained muscular tone but hyperlocomotion was not observed in our experiments, nor in previous studies in cats (Ambros & Duke, 2013; Issabeagloo et al., 2011).
The ongoing discussion about complexity as proxy to study integration in different consciousness states oscillates between perturbational and steady state studies. In a perturbational—complementary—study, Arena et al. (2021) quantified the complexity of electrocorticographic responses to intracranial electrical stimulation in rats, comparing wakefulness to propofol, sevoflurane, and ketamine anaesthesia using PCI and PCI state‐transition (PCIST) (Comolatti et al., 2019). They found ketamine‐induced evoked related potentials (ERPs) mixed features with a brief response followed by an OFF period (albeit long‐lasting deterministic activations in half of the animals), and the duration of the resulting phase‐locked response was close to that of wakefulness. The time course of PCIST revealed similarities to wakefulness but resulted in an overall reduction of complexity. These results from a perturbational study showed a similar feature to our “state” study in that the ketamine‐induced effects are cortically variable and not consistent between animals. It is, however, difficult to compare this study directly with our results because we used subanaesthetic doses of ketamine (5, 10, and 15 mg/kg), whereas Arena et al., 2021 used anaesthetic doses of ketamine (30 mg/kg), so our hypotheses of higher complexity with low doses cannot be addressed in their study. On the other hand, they explored the effect of perturbational complexity index (PCI) which is an electrophysiological metric for the capacity of cortical circuits to integrate information, whereas we studied the effect of auditory stimulation on the naturally occurring and ongoing cortical complexity and hence the complementarity of the findings should be encouraging for the field (Arena et al., 2021).
Neural diversity, assessed by LZ, is an attractive measure because of simplicity, practical applicability, and it is consistent with both complexity‐based (Tononi et al., 2016; Tononi & Edelman, 1998) and entropy‐based (Carhart‐Harris, 2018; Carhart‐Harris et al., 2014) theories of neural integration and consciousness. The measure is also useful in questions regarding local processing as it is computed at the electrode level, thus was able to demonstrate differential effects in distinct thalamic and cortical brain regions. Indeed, according to the dynamic core hypothesis (Tononi & Edelman, 1998) and subsequent theoretical developments such as Information Integration Theory (Tononi et al., 2016), only certain distributed subsets of the neuronal groups that are activated or deactivated in response to a given task are associated with conscious experience, therefore a large cluster of neuronal groups that together constitute, on a time scale of hundreds of milliseconds, a unified neural process of high complexity can be termed the “dynamic core.” In line with this idea, our results could be interpreted as the prefrontal and auditory cortices, where an increase in LZ was observed under the 5 mg dose of ketamine, constituting a part of the “dynamic core,” and somatosensory and posterior parietal cortices playing a different role in neural integration. However, our results do not necessarily provide strong evidence for the “dynamic core” over other theoretical interpretations such the entropic brain (Carhart‐Harris, 2018) or other complexity and consciousness frameworks (Sarasso et al., 2021), possibly because the predictions from most frameworks are less precise and hardly predict the specific pattern of results we present. Furthermore, there are complementary approaches to understand information and complexity dynamics, both using state and perturbational experimental and analyses models and frameworks that illustrate the underdeveloped integration of the theories and experiments in this subdiscipline. Another interpretation of the overall ketamine dose–response results, its variance and dynamics, is that it could reflect a level of connection to the external environment interacting with the pharmacological modulation. We however did not systematically assess this behavioural aspect, and hence, it is difficult to draw conclusions at that level.
Another useful framework for understanding these results is the neuroscience of arousal, including wakefulness, sleep, circadian rhythms, responsiveness, and alertness (Bekinschtein et al., 2009; Brown et al., 2011). Sleep shows a clear change in arousal throughout the day cycle; the intensity of the stimuli needed to wake up a person is maximal in deep sleep and lower in light sleep and REM. This pattern partially mimics the results obtained for informational complexity in this study using electrocorticogram recordings (ECoG), and several other nonlinear measures such as fractal dimension and other entropy methods (Ma et al., 2018), but not to other measures such as power in different bands and connectivity methods. This finding allows us to interpret that LZ may index behaviourally defined wakefulness, or arousability by stimuli (Bonnet et al., 1978). Although ketamine is used as an anaesthetic and creates unconsciousness in high doses and hence can be framed in terms of consciousness as wakefulness and arousal, the effects at lower doses require a multidimensional framework, able to accommodate neurological symptoms (dizziness, slurred speech), mood modulations, and psychedelic experiences. In principle, if ketamine had the classic profile of a sedative, responsiveness would monotonically decrease (Brown et al., 2011) and a similar profile would be expected for molecular and neural measures. However, ketamine has an interesting profile as it belongs to a group of hypnotics that show hallucinatory capacities and a hormetic or U‐shaped curve (Calabrese & Baldwin, 2001) in EEG and blood flow (Cavazzuti et al., 1987; Tsuda et al., 2007). The hormesis of the dose response allows for the comparison of not only conscious level in the sense of wakefulness but in terms of contents of consciousness in low ketamine and REM sleep. From humans, we know that the likelihood of increased richness in mental content during the sleep–wake cycle occurs during REM (Windt & Noreika, 2011) after a decrease in NREM (U‐shaped), and we know that the richness of mental content, including hallucinations, peaks early with ketamine before decreasing into sedation and anaesthesia (Powers et al., 2015) (an inverted U‐shaped curve). In both cases, the higher levels of content agree with the higher (or recovering) levels of informational complexity as measured by LZ (Abásolo et al., 2015; Mateos et al., 2018; Schartner et al., 2015; Schartner, Carhart‐Harris, et al., 2017; Schartner, Pigorini, et al., 2017). In this study, we compare the consistency of the complexity in the cortex in sleep and the diversity in the ketamine challenge as two putatively very different mechanisms of reaching a higher level of content in consciousness.
Recent findings by Mediano et al. (2020) provide strong quantitative evidence on how environmental conditions have a substantial influence on neural dynamics during a psychedelic experience in humans. This work showed how brain entropy is modulated by stimulus manipulation during a psychedelic experience by studying participants under the effects of LSD or placebo, either with gross state changes (eyes closed vs. open) or different stimuli (no stimulus vs. music vs. video). Results showed that while brain entropy increased with LSD in all the experimental conditions, it exhibited largest changes when subjects have their eyes closed, whereas the entropy enhancing effects of LSD were less marked when participants opened their eyes or perceived external stimuli—such as music or video (Mediano et al., 2020). In the present work, we studied the modulation of auditory stimulation on brain complexity in basal conditions and under increasing doses of ketamine in three cats using ECoG recordings with the hypothesis of observing a higher level of complexity under stimulation. However, only a slight increase in LZ was evidenced during stimulation in dorsolateral prefrontal and auditory cortices, whereas a complete lack of or very weak effect were found in the other cortices studied (Figure 4). This weak effect may be explained by the low relevance of the stimulus, as it failed to catch the attention of the animals, compared with extremely salient or meaningful stimuli such as music or video. Further evidence that stimulation studies should exploit more complex stimuli also comes from a recent study were TMS pulses also failed to increase complexity in low doses of ketamine in humans (Farnes et al., 2020). Furthermore, Nilsen et al. (2019) were unable to demonstrate an influence of attention in LZ complexity after stimulation, whereas we have reported (Mediano et al., 2020) that LZ is modulated when applying different types of stimulation (music and videos). Additionally, we have recently shown (Mediano et al., 2021) that LZ varies with the level of alertness and also depending on the task, not being restricted to measure the level of consciousness but cognitive and attentional demands.
New experiments using more appropriate stimuli in terms of relevance and salience are needed to better address this hypothesis and further the experimental understanding neural dynamics of information theory, complexity and entropy as the system is modulated pharmacologically.
Our sleep results are consistent with previous results in humans (Andrillon et al., 2016; Schartner, Pigorini, et al., 2017), as well as in rats (Abásolo et al., 2015). However, a closer read shows some differences: Andrillon et al. (2016) reported a small but reliable decrease in LZ during REM sleep compared with the waking state, possibly due to participants engaged in a task during the waking state, whereas the participants in the Schartner et al. study were simply at rest with eyes closed and not engaged or externally driven by task or stimuli. In our study the animals were also at rest but with eyes open and showed a decrease in LZ during LS and further decrease in SWS, which was similar for all cortices (Figure 2a) in line with previous findings (Andrillon et al., 2016; Schartner, Pigorini, et al., 2017). However, a greater variability was evident for REM sleep state where in some cortices LZ was equal in level of complexity to wakefulness whereas in others it was similar to LS or to SWS (Figure 2b). The complexity pattern among sleep stages observed in the cortex was also evidenced in the lateral geniculate nucleus (Figure 2a), lending clear convergent evidence to the common effects of informational complexity in the brain beyond the cortex for the sleep wake cycle.
In summary, our data demonstrate that there is a dose‐dependent ketamine effect on neural complexity. An increase in complexity compared with baseline was found for some cortices (prefrontal, motor, auditory, and visual) only in the lowest doses, whereas the higher dose frequently showed the lowest informational complexity. However, a decrease in complexity was also seen in somatosensory and posterior parietal cortex in the low doses. The heterogeneity of the ketamine effects between cats and cortices contrasts with the homogeneity of the changes in complexity seen for different stages of sleep, further highlighting the differences between natural and pharmacologically induced changes in consciousness. The individual and cortical variability in the neural complexity dynamics revealed by ketamine highlights the intricacy of the brain when altered by dissociatives and psychedelics, pushing for a multidimensional framework beyond simple arousal and alertness parameters to characterize the change in the states of consciousness from a neuropharmacological perspective.
4. METHODS
4.1. Animals
Five adult cats were used in this study, all of whom were also utilized in a previous report (Castro‐Zaballa et al., 2019). The animals were obtained from and determined to be in good health by the Institutional Animal Care Facility of the Faculty of Medicine (University of the Republic, Uruguay). All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (8th edition, National Academy Press, Washington DC, 2011) and were approved by Institutional and National Animal Care Commissions of the University of the Republic in Uruguay (Protocol No. 070153000089‐17). Adequate measures were taken to minimize pain, discomfort or stress to the animals. In addition, all efforts were made to use the minimum number of animals necessary to produce reliable scientific data.
4.2. Surgical procedure
Following general anaesthesia, the head was positioned in a stereotaxic frame and the skull was exposed. Stainless steel screw electrodes (1.4 mm diameter) were placed on the surface (above the dura matter) of different cortical areas including prefrontal, primary motor, primary somatosensory, and posterior parietal cortices. Note that because the animals were not prepared specifically for this work, we did not analyse the same cortices in all of them. The electrodes were connected to a Winchester plug, which together with two plastic tubes were bonded to the skull with acrylic cement in order to maintain the animals' head in fixed position without pain or pressure. After recovery from surgical procedures, they were adapted to the recording environment for a period of at least 2 weeks.
4.3. Data acquisition and preprocessing
Experimental sessions of 4 h were conducted between 11 AM and 3 PM in a temperature‐controlled environment (21–23°C). During these sessions (as well as during the adaptation sessions), the animals' head was held in a stereotaxic position by four steel bars that were placed into the chronically implanted plastic tubes, whereas the body rested in a sleeping bag (semirestricted condition).
The ECoG activity was recorded with a monopolar (referential) configuration, utilizing a common reference electrode located in the left frontal sinus. The experiments on sleep and ketamine were performed on the same cats but not the same cortices were recorded as they were originally designed for different studies. The electromyogram (EMG) of the nuchal muscles, which was recorded by means of an acutely placed bipolar electrode, was also monitored. The electrocardiogram (ECG), by electrodes acutely placed on the skin over the pre‐cordial region, and respiratory activity by means of a micro‐effort piezo crystal infant sensor were also recorded. Each cat was recorded daily for ∼30 days in order to obtain complete basal and treatment data sets. The animal retained muscular tone but hyperlocomotion was not observed in our experiments (Castro‐Zaballa et al., 2019), nor in previous studies in cats (Issabeagloo et al., 2011), an increase in motor activity was also absent in semirestricted condition, and ∼5 min following the injection of ketamine the animals lay down on the floor unable to stand up (i.e., an ataxia‐like effect), but responded to sound stimulus directing the gaze towards the sound source. In the absence of stimuli, the cats moved their head from one side to the other (i.e., a head‐weaving‐like behaviour, described in rodents, and defined as stereotypies characterized as lateral side‐to‐side movement of the head without locomotion).
Bioelectric signals were amplified (×1000), filtered (0.1–500 Hz), sampled (1024 Hz, 216 bits) and stored in a PC using the Spike 2 software (Cambridge Electronic Design).
Data were obtained after ketamine administration as well as during spontaneously occurring quiet W, LS, NREM sleep and REM sleep (Figure 1). Five, 10, and 15 mg/kg i.m. of ketamine (Ketonal®, Richmond Veterinaria S.A.) were administered to five animals in 4 different sessions. These three doses were administered in each animal in different experimental sessions performed in different days in a counterbalanced order. The scheme illustrated in Figure 1b corresponds to one session, in which only one bolus of ketamine was administered. In each session, the animal was recorded in resting conditions for around 30 min and then the bolus of ketamine was injected. After that, the recording continued for 4 h. Ten minutes after the injection the cat received auditory stimulation (in three of the five cats). The different doses of ketamine were administered in different days leaving 3 or 4 days in between. Additionally, each different dose was repeated four times. In each session, the whole experiment illustrated in Figure 1b was repeated; therefore, in total, the experiments were repeated 12 times (four per dose). Ketamine (50 mg/ml) was diluted in benzethonium chloride, hydrochloric acid, and water (solution for veterinary use). Basal recordings (without injections) were used as control. Sound stimuli were introduced ∼30 min after the beginning of the recording sessions in drug‐free condition and 10 min after ketamine injection. These sound stimuli had the same characteristics as those used to induce active W (Castro et al., 2013). Sound stimuli was presented for a period of 300 s, and consisted of 60–100 dB SPL clicks, with variable frequency of presentation (1–500 Hz), modified at random in order to avoid habituation (Castro et al., 2013; Torterolo et al., 2003). The mentioned frequency refers to the frequency of presentation of the clicks and not the sound frequency. There were no frequency steps, and the SPL had no steps. Sound stimuli during 300 s were also performed 10 min after ketamine injection in three cats. The stimuli were square pulses produced with an electric stimulator connected to a speaker which emit them as clicks.
For preprocessing, sleep stages were scored off‐line by visual inspection of 5‐s epochs in Spike2 software, where the ECoG and EMG were displayed simultaneously. In order to analyse LZ during sleep, a total of 300 artefact‐free seconds data were selected from each behavioural state. Additionally, to study LZ during the Ketamine effect 300 s duration segments, with and without stimulation, were selected before and after ketamine administration.
After scoring, for both experiments, the selected epochs were exported to MATLAB for further preprocessing. The MATLAB toolbox eeglab was used to filter the data (0.5–200 Hz band‐pass). Each epoch was visually inspected, and those with gross artefacts (e.g., movements) were removed from the analysis.
4.4. LZ complexity
In this study, we used LZ complexity to compute the complexity of measured neural signals (Lempel & Ziv, 1976). In particular, we used the LZ78 algorithm (Ziv & Lempel, 1978), which corresponds to the standard word‐dictionary implementation: given a binary string, the algorithm scans it sequentially looking for distinct structures or “patterns.” The more diverse the binary string, the more patterns are included in the dictionary (a sequence containing only zeros or only ones would lead to the minimal number of patterns being obtained). The total number of these patterns is a measure of signal diversity.
To compute LZ from our experimental data, the recording of each channel was split into segments of 5120 samples (5 s sampled at 1024 Hz). Then, to generate a discrete sequence from a real‐valued signal X of length T, X is detrended and binarized with a threshold of 0, and the resulting binary sequence is fed to the LZ78 algorithm. Finally, the resulting dictionary length L is normalized as
to yield a measure of complexity C.
Our choice on binarizing signals with a threshold of cero was driven by two factors: (1) LZ and related methods tend to be remarkably robust to the choice of discretisation procedure and number of bins (see, e.g., discussion in Mediano et al., 2020); and (2) the Hilbert transform of a very broadband signal is not easily interpretable, and to create a meaningful analytic signal, it would be necessary to bandpass filter the data in a particular frequency band of interest. Given these two arguments, we reasoned that the added analysis complexity introduced by the filter parameters, frequency bands, and so forth would not lead to substantially richer or more accurate results, and thus opted for the simple (yet probably effective) methodology.
4.5. Statistics
One way ANOVA, with Tukey post‐hoc test were used to compare LZ between sleep stages per cortex per animal (Figure 2b) where Cohen's d was used to address the size of the effect. Additionally, a multilevel approach as well as Bayes factors (BF) and Bayesian informational criterion (BIC) were used to find the most likely explanatory model within the hierarchical model in the group statistical analysis comparing linear, quadratic, and cubic models. For sleep study, the state of sleep was used as a fixed effect and the cat identity as a random effect. The same type of approach was used to study the ketamine effect among different cortices under control and stimulus conditions. In this case, the dose and stimulus (if present) were used as fixed effects; and cat identity and session as random effects. The interaction between stimuli and ketamine dose was also included in the model when studying the modulation by stimulus. All models were estimated via restricted maximum likelihood, using the open‐source packages lme4 v.1.1‐21 (Bates et al., 2015) and lmerTest v.3.1‐1 (Kuznetsova et al., 2017) on R v.3.6.1.
CONFLICT OF INTERESTS
The authors have declared that no conflict of interests exist.
AUTHOR CONTRIBUTION
P.T. and T.B. designed the study. C.Z.S. and P.T. performed the experiments and collected the data. C.P. and P.M. analysed data; M.P. and P.M. wrote LZ codes; C.P. wrote the manuscript; all authors participated in the interpretation of results and revision of the manuscript and approved the final version of the manuscript. P.T., T.B., and D.B. provided the financial support.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15646.
Supporting information
FIGURE S1. Comparison between LZ in wakefulness and LS (a), and wakefulness without ketamine vs. 5 mg of Ketamine (b) for the different cortices. RPf, rostral prefrontal cortex; DLPf, dorsolateral prefrontal cortex; M, primary motor cortex; S, primary somatosensory cortex; A, primary auditory cortex, Pp, posterior parietal cortex; V, visual cortex. “l” indicates left side, “r” right side and “1” indicates “primary cortex”.
ACKNOWLEDGEMENTS
This study was supported by the “Programa de Desarrollo de Ciencias Básicas, PEDECIBA” and the “Comisión Sectorial de Investigación Científica” (CSIC) I + D‐2020‐393 grant from Uruguay. PAM and DB are funded by the Wellcome Trust (grant no. 210920/Z/18/Z).
Pascovich, C. , Castro‐Zaballa, S. , Mediano, P. A. M. , Bor, D. , Canales‐Johnson, A. , Torterolo, P. , & Bekinschtein, T. A. (2022). Ketamine and sleep modulate neural complexity dynamics in cats. European Journal of Neuroscience, 55(6), 1584–1600. 10.1111/ejn.15646
Pablo Torterolo and Tristan A. Bekinschtein shared senior authors.
Edited by: Edmund Lalor
Funding information Comisión Sectorial de Investigación Científica, Grant/Award Number: I + D‐2020‐393; Wellcome Trust, Grant/Award Number: 210920/Z/18/Z; Programa de Desarrollo de Ciencias Básicas, PEDECIBA
DATA AVAILABILITY STATEMENT
The code for computation of LZ used for analysis is available in GitHub at the following link: https://gitlab.com/CPasco83/sleep-and-ketamine. Data are available upon reasonable request to the authors.
REFERENCES
- Abásolo, D. , Simons, S. , Morgado da Silva, R. , Tononi, G. , & Vyazovskiy, V. V. (2015). Lempel–Ziv complexity of cortical activity during sleep and waking in rats. Journal of Neurophysiology, 113(7), 2742–2752. 10.1152/jn.00575.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amargós‐Bosch, M. , López‐Gil, X. , Artigas, F. , & Adell, A. (2006). Clozapine and olanzapine, but not haloperidol, suppress serotonin efflux in the medial prefrontal cortex elicited by phencyclidine and ketamine. International Journal of Neuropsychopharmacology, 9(5), 565–573. 10.1017/S1461145705005900 [DOI] [PubMed] [Google Scholar]
- Ambros, B. , & Duke, T. (2013). Effect of low dose rate ketamine infusions on thermal and mechanical thresholds in conscious cats. Veterinary Anaesthesia and Analgesia, 40, 76–82. 10.1111/vaa.12057 [DOI] [PubMed] [Google Scholar]
- Andrillon, T. , Poulsen, A. T. , Hansen, L. K. , Léger, D. , & Kouider, S. (2016). Neural markers of responsiveness to the environment in human sleep. Journal of Neuroscience, 36(24), 6583–6596. 10.1523/JNEUROSCI.0902-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arena, A. , Comolatti, R. , Thon, S. , Casali, A. G. , & Storm, J. F. (2021). General anesthesia disrupts complex cortical dynamics in response to intracranial electrical stimulation in rats. ENeuro, 8(4), ENEURO.0343‐20.2021. 10.1523/ENEURO.0343-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. M. , & Walker, S. C. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bekinschtein, T. , Cologan, V. , Dahmen, B. , & Golombek, D. (2009). You are only coming through in waves: Wakefulness variability and assessment in patients with impaired consciousness. Progress in Brain Research, 177, 171–189. 10.1016/S0079-6123(09)17712-9 [DOI] [PubMed] [Google Scholar]
- Blain‐Moraes, S. , Lee, U. , Ku, S. , Noh, G. , & Mashour, G. A. (2014). Electroencephalographic effects of ketamine on power, cross‐frequency coupling, and connectivity in the alpha bandwidth. Frontiers in Systems Neuroscience, 8, 114. 10.3389/fnsys.2014.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet, M. , Hohnson, L. , & Webb, W. (1978). The reliability of arousal threshold during sleep. Psychophysiology, 15(5), 412–416. 10.1111/j.1469-8986.1978.tb01407.x [DOI] [PubMed] [Google Scholar]
- Brown, E. N. , Purdon, P. L. , & Van Dort, C. J. (2011). General anesthesia and altered states of arousal: A systems neuroscience analysis. Annual Review of Neuroscience (Palo Alto, CA), 34, 601–628. 10.1146/annurev-neuro-060909-153200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese, E. J. , & Baldwin, L. A. (2001). Hormesis: A generalizable and unifying hypothesis. Critical Reviews in Toxicology, 31(4–5), 353–424. 10.1080/20014091111730 [DOI] [PubMed] [Google Scholar]
- Carhart‐Harris, R. L. (2018). The entropic brain—Revisited. Neuropharmacology, 142, 167–178. 10.1016/j.neuropharm.2018.03.010 [DOI] [PubMed] [Google Scholar]
- Carhart‐Harris, R. L. , Kaelen, M. , Bolstridge, M. , Williams, T. M. , Williams, L. T. , Underwood, R. , Feilding, A. , & Nutt, D. J. (2016). The paradoxical psychological effects of lysergic acid diethylamide (LSD). Psychological Medicine, 46(7), 1379–1390. 10.1017/S0033291715002901 [DOI] [PubMed] [Google Scholar]
- Carhart‐Harris, R. L. , Leech, R. , Hellyer, P. J. , Shanahan, M. , Feilding, A. , Tagliazucchi, E. , Chialvo, D. R. , & Nutt, D. (2014). The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Frontiers in Human Neuroscience, 8(20), 1–22. 10.3389/fnhum.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali, A. G. , Gosseries, O. , Rosanova, M. , Boly, M. , Sarasso, S. , Casali, K. R. , Casarotto, S. , Bruno, M. A. , Laureys, S. , Tononi, G. , & Massimini, M. (2013). A theoretically based index of consciousness independent of sensory processing and behavior. Science Translational Medicine, 5(198), 198ra105. 10.1126/scitranslmed.3006294 [DOI] [PubMed] [Google Scholar]
- Castro, S. , Falconi, A. , Chase, M. H. , & Torterolo, P. (2013). Coherent neocortical 40‐Hz oscillations are not present during REM sleep. European Journal of Neuroscience, 37(8), 1330–1339. 10.1111/ejn.12143 [DOI] [PubMed] [Google Scholar]
- Castro‐Zaballa, S. , Cavelli, M. L. , Gonzalez, J. , Nardi, A. E. , Machado, S. , Scorza, C. , & Torterolo, P. (2019). EEG 40 Hz coherence decreases in REM sleep and ketamine model of psychosis. Frontiers in Psychiatry, 10, 1–14. 10.3389/fpsyt.2018.00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzuti, M. , Porro, C. A. , Biral, G. P. , Benassi, C. , & Barbieri, G. C. (1987). Ketamine effects on local cerebral blood flow and metabolism in the rat. Journal of Cerebral Blood Flow & Metabolism, 7, 806–811. 10.1038/jcbfm.1987.138 [DOI] [PubMed] [Google Scholar]
- Chen, X. , Shu, S. , & Bayliss, D. A. (2009). HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. Journal of Neuroscience, 29(3), 600–609. 10.1523/JNEUROSCI.3481-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comolatti, R. , Pigorini, A. , Casarotto, S. , Fecchio, M. , Faria, G. , Sarasso, S. , Rosanova, M. , Gosseries, O. , Boly, M. , Bodart, O. , Ledoux, D. , Brichant, J. F. , Nobili, L. , Laureys, S. , Tononi, G. , Massimini, M. , & Casali, A. G. (2019). A fast and general method to empirically estimate the complexity of brain responses to transcranial and intracranial stimulations. Brain Stimulation, 12(5), 1280–1289. 10.1016/j.brs.2019.05.013 [DOI] [PubMed] [Google Scholar]
- Conti, F. , Minelli, A. , Molnar, M. , & Brecha, N. C. (1994). Cellular localization and laminar distribution of NMDAR1 mRNA in the rat cerebral cortex. Journal of Comparative Neurology, 343(4), 554–565. 10.1002/cne.903430406 [DOI] [PubMed] [Google Scholar]
- Farnes, N. , Bjørn, E. J. , Nilsen, A. S. , Romundstad, L. G. , & Storm, J. F. (2020). Increased signal diversity/complexity of spontaneous EEG, but not evoked EEG responses, in ketamine‐induced psychedelic state in humans. PLoS ONE, 23, e0242056. 10.1371/journal.pone.0242056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenets, R. , Lipping, T. , Anier, A. , Jäntti, V. , Melto, S. , & Hovilehto, S. (2006). Comparison of entropy and complexity measures for the assessment of depth of sedation. IEEE Transactions on Biomedical Engineering, 53(6), 1067–1077. 10.1109/TBME.2006.873543 [DOI] [PubMed] [Google Scholar]
- Ferenets, R. , Vanluchene, A. , Lipping, T. , & Heyse, B. (2007). Behavior of entropy/complexity measures of the electroencephalogram during propofol‐induced sedation. Anesthesiology, 106(4), 696–706. [DOI] [PubMed] [Google Scholar]
- Ferrer‐Allado, T. , Brechner, V. L. , Dymond, A. , Cozen, H. , & Crandall, P. (1973). Ketamine‐induced electroconvulsive phenomena in the human limbic and thalamic regions. Anesthesiology, 38(4), 333–344. [DOI] [PubMed] [Google Scholar]
- González, J. , Cavelli, M. , Castro‐Zaballa, S. , Mondino, A. , Tort, A. B. L. , Rubido, N. , Carrera, I. , & Torterolo, P. (2021). EEG gamma band alterations and REM‐like traits underpin the acute effect of the atypical psychedelic ibogaine in the rat. ACS Pharmacology and Translational Science, 4(2), 517–525. 10.1021/acsptsci.0c00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González, J. , Cavelli, M. , Mondino, A. , Pascovich, C. , Castro‐Zaballa, S. , Torterolo, P. , & Rubido, N. (2019). Decreased electrocortical temporal complexity distinguishes sleep from wakefulness. Scientific Reports, 9(1), 1–9. 10.1038/s41598-019-54788-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzler, B. E. , & Wautlet, B. S. (1985). Ketamine‐induced locomotion in rats in an open‐field. Pharmacology Biochemistry and Behavior, 22, 653–655. [DOI] [PubMed] [Google Scholar]
- Homayoun, H. , & Moghaddam, B. (2007). NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. The Journal of Neuroscience, 27, 11496–11500. 10.1523/JNEUROSCI.2213-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley, G. W. , Vickers, J. C. , Janssen, W. , Brose, N. , Heinemann, S. F. , & Morrison, J. H. (1994). Distribution and synaptic localization of immunocytochemically identified NMDA receptor subunit proteins in sensory‐motor and visual cortices of monkey and human. Journal of Neuroscience, 14(6), 3603–3619. 10.1523/jneurosci.14-06-03603.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa, Y. , Ahmed, O. J. , Patel, S. R. , Gale, J. T. , Sierra‐Mercado, D. , Brown, E. N. , & Eskandar, E. N. (2016). Dynamics of propofol‐induced loss of consciousness across primate neocortex. Journal of Neuroscience, 36(29), 7718–7726. 10.1523/JNEUROSCI.4577-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issabeagloo, E. , Gharachorlou, A. A. , & Ghalahkandi, J. G. (2011). Comparison of sedative effects of oral ketamine & chlorpheniramine in the manner. Advances in Environmental Biology, 5(4), 784–789. [Google Scholar]
- Kuznetsova, A. , Brockhoff, P. B. , & Christensen, R. H. B. (2017). lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26. 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- Langsjo, J. W. , Maksimow, A. , Salmi, E. , Kaisti, K. , Aalto, S. , Oikonen, V. , Hinkka, S. , Aantaa, R. , Sipila, H. , Viljanen, T. , & Parkkola, R. (2005). S‐ketamine anesthesia increases cerebral blood flow in excess of the metabolic needs in humans. Anesthesiology, 103(2), 258–268. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed7&NEWS=N&AN=2005346193 [DOI] [PubMed] [Google Scholar]
- Långsjö, J. W. , Salmi, E. , Kaisti, K. K. , Aalto, S. , Hinkka, S. , Aantaa, R. , Oikonen, V. , Viljanen, T. , Kurki, T. , Silvanto, M. , & Scheinin, H. (2004). Effects of subanesthetic ketamine on regional cerebral glucose metabolism in humans. Anesthesiology, 100(5), 1065–1071. 10.1097/00000542-200405000-00006 [DOI] [PubMed] [Google Scholar]
- Lee, U. , Ku, S. , Noh, G. , Baek, S. , Choi, B. , & Mashour, G. A. (2013). Disruption of frontal‐parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology, 118, 1264–1275. 10.1097/ALN.0b013e31829103f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempel, A. , & Ziv, J. (1976). On the complexity of finite sequences. IEEE Transactions on Information Theory, 22(1), 75–81. 10.1109/TIT.1976.1055501 [DOI] [Google Scholar]
- Li, D. , & Mashour, G. A. (2019). Cortical dynamics during psychedelic and anesthetized states induced by ketamine. NeuroImage, 196, 32–40. 10.1016/j.neuroimage.2019.03.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Gil, X. , Jiménez‐Sánchez, L. , Campa, L. , Castro, E. , Frago, C. , & Adell, A. (2019). Role of serotonin and noradrenaline in the rapid antidepressant action of ketamine. ACS Chemical Neuroscience, 10(7), 3318–3326. 10.1021/acschemneuro.9b00288 [DOI] [PubMed] [Google Scholar]
- López‐Gil, X. , Jiménez‐Sánchez, L. , Romón, T. , Campa, L. , Artigas, F. , & Adell, A. (2012). Importance of inter‐hemispheric prefrontal connection in the effects of non‐competitive NMDA receptor antagonists. International Journal of Neuropsychopharmacology, 15(7), 945–956. 10.1017/S1461145711001064 [DOI] [PubMed] [Google Scholar]
- Lorrain, D. S. , Schaffhauser, H. , Campbell, U. C. , Baccei, C. S. , Correa, L. D. , Rowe, B. , Rodriguez, D. E. , Anderson, J. J. , Varney, M. A. , Pinkerton, A. B. , Vernier, J. M. , & Bristow, L. J. (2003). Group II mGlu receptor activation suppresses norepinephrine release in the ventral hippocampus and locomotor responses to acute ketamine challenge. Neuropsychopharmacology, 28(9), 1622–1632. 10.1038/sj.npp.1300238 [DOI] [PubMed] [Google Scholar]
- Ma, Y. , Shi, W. , Peng, C. K. , & Yang, A. C. (2018). Nonlinear dynamical analysis of sleep electroencephalography using fractal and entropy approaches. Sleep Medicine Reviews, 37, 85–93. 10.1016/j.smrv.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Mateos, D. M. , Guevara Erra, R. , Wennberg, R. , & Perez Velazquez, J. L. (2018). Measures of entropy and complexity in altered states of consciousness. Cognitive Neurodynamics, 12(1), 73–84. 10.1007/s11571-017-9459-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediano, P. A. M. , Ikkala, A. , Kievit, R. A. , Jagannathan, S. R. , Varley, T. F. , Stamatakis, E. A. , Bekinschtein, T. A. , & Bor, D. (2021). Fluctuations in neural complexity during wakefulness relate to conscious level and cognition. BioRxiv.
- Mediano, P. A. M. , Rosas, F. E. , Timmermann, C. , Roseman, L. , Nutt, D. J. , Feilding, A. , Kaelen, M. , Kringelbach, M. L. , Barrett, A. B. , Seth, A. K. , Muthukumaraswamy, S. , Bor, D. , & Carhart‐Harris, R. L. (2020). Effects of external stimulation on psychedelic state neurodynamics. BioRxiv. 10.1101/2020.11.01.356071 [DOI] [PMC free article] [PubMed]
- Moghaddam, B. , Adams, B. , Verma, A. , & Daly, D. (1997). Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. The Journal of Neuroscience, 17(8), 2921–2927. 10.1523/JNEUROSCI.17-08-02921.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen, A. S. , Juel, B. E. , & Storm, J. F. (2019). Measures of states of consciousness during attentional and cognitive load. BioRxiv. 10.1101/586149 [DOI]
- Pal, D. , Li, D. , Dean, J. G. , Brito, M. A. , Liu, T. , Fryzel, A. M. , Hudetz, A. G. , & Mashour, G. A. (2020). Level of consciousness is dissociable from electroencephalographic measures of cortical connectivity, slow oscillations, and complexity. Journal of Neuroscience, 40(3), 605–618. 10.1523/JNEUROSCI.1910-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascovich, C. , Castro, S. , Velasquez, N. , Bor, D. , Canales‐Johnson, A. , Torterolo, P. , & Bekinschtein, T. A. (2019). Complexity of cortical activity under subanesthetic doses of ketamine and during sleep. osf.io/dvpyr
- Powers, A. R. , Gancsos, M. G. , Finn, E. S. , Morgan, P. T. , & Corlett, P. R. (2015). Ketamine‐induced hallucinations. Psychopathology, 48(6), 376–385. 10.1159/000438675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasso, S. , Boly, M. , Napolitani, M. , Gosseries, O. , Charland‐Verville, V. , Casarotto, S. , Rosanova, M. , Casali, A. G. , Brichant, J. F. , Boveroux, P. , Rex, S. , Tononi, G. , Laureys, S. , & Massimini, M. (2015). Consciousness and complexity during unresponsiveness induced by propofol, xenon, and ketamine. Current Biology, 25(23), 3099–3105. 10.1016/j.cub.2015.10.014 [DOI] [PubMed] [Google Scholar]
- Sarasso, S. , Casali, A. G. , Casarotto, S. , Rosanova, M. , Sinigaglia, C. , & Massimini, M. (2021). Consciousness and complexity: A consilience of evidence. Neuroscience of Consciousness, 7, 1–24. 10.1093/nc/niab023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartner, M. , Seth, A. , Noirhomme, Q. , Boly, M. , Bruno, M. A. , Laureys, S. , & Barrett, A. (2015). Complexity of multi‐dimensional spontaneous EEG decreases during propofol induced general anaesthesia. PLoS ONE, 10(8), 1–21. 10.1371/journal.pone.0133532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartner, M. M. , Carhart‐Harris, R. L. , Barrett, A. B. , Seth, A. K. , & Muthukumaraswamy, S. D. (2017). Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Scientific Reports, 7, 1–12. 10.1038/srep46421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schartner, M. M. , Pigorini, A. , Gibbs, S. A. , Arnulfo, G. , Sarasso, S. , Barnett, L. , Nobili, L. , Massimini, M. , Seth, A. K. , & Barrett, A. B. (2017). Global and local complexity of intracranial EEG decreases during NREM sleep. Neuroscience of Consciousness, 2017, niw022. 10.1093/nc/niw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, M. S. , Viden, S. , & Scott, D. F. (1974). Effects of ketamine on the electroencephalograph. Anaesthesia, 29(2), 135–140. 10.1111/j.1365-2044.1974.tb00611.x [DOI] [PubMed] [Google Scholar]
- Seamans, J. (2008). Losing inhibition with ketamine. Nature Chemical Biology, 4(2), 91–93. 10.1038/nchembio0208-91 [DOI] [PubMed] [Google Scholar]
- Tagliazucchi, E. , Carhart‐Harris, R. , Leech, R. , Nutt, D. , & Chialvo, D. R. (2014). Enhanced repertoire of brain dynamical states during the psychedelic experience. Human Brain Mapping, 35(11), 5442–5456. 10.1002/hbm.22562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermann, C. , Roseman, L. , Schartner, M. , Milliere, R. , Williams, L. T. J. , Erritzoe, D. , Muthukumaraswamy, S. , Ashton, M. , Bendrioua, A. , Kaur, O. , Turton, S. , Nour, M. M. , Day, C. M. , Leech, R. , Nutt, D. J. , & Carhart‐Harris, R. L. (2019). Neural correlates of the DMT experience assessed with multivariate EEG. Scientific Reports, 9(1), 1–13. 10.1038/s41598-019-51974-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi, G. , Boly, M. , Massimini, M. , & Koch, C. (2016). Integrated information theory: From consciousness to its physical substrate. Nature Reviews Neuroscience, 17(7), 450–461. 10.1038/nrn.2016.44 [DOI] [PubMed] [Google Scholar]
- Tononi, G. , & Edelman, G. M. (1998). Consciousness and complexity. Science, 282(5395), 1846–1851. 10.1126/science.282.5395.1846 [DOI] [PubMed] [Google Scholar]
- Torterolo, P. , Yamuy, J. , Sampogna, S. , Morales, F. R. , & Chase, M. H. (2003). Hypocretinergic neurons are primarily involved in activation of the somatomotor system. Sleep, 26(1), 25–28. 10.1093/sleep/26.1.25 [DOI] [PubMed] [Google Scholar]
- Tsuda, N. , Hayashi, K. , Hagihira, S. , & Sawa, T. (2007). Ketamine, an NMDA‐antagonist, increases the oscillatory frequencies of α‐peaks on the electroencephalographic power spectrum. Acta Anaesthesiologica Scandinavica, 51(4), 472–481. 10.1111/j.1399-6576.2006.01246.x [DOI] [PubMed] [Google Scholar]
- Windt, J. M. , & Noreika, V. (2011). How to integrate dreaming into a general theory of consciousness—A critical review of existing positions and suggestions for future research. Consciousness and Cognition, 20(4), 1091–1107. 10.1016/j.concog.2010.09.010 [DOI] [PubMed] [Google Scholar]
- Zanos, P. , Moaddel, R. , Morris, P. J. , Riggs, L. M. , Highland, J. N. , Georgiou, P. , Pereira, E. F. R. , Albuquerque, E. X. , Thomas, C. J. , Zarate, C. A. , & Gould, T. D. (2018). Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharmacological Reviews, 70(3), 621–660. 10.1124/pr.117.015198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. S. , Roy, R. J. , & Jensen, E. W. (2001). EEG complexity as a measure of depth of anesthesia for patients. IEEE Transactions on Biomedical Engineering, 48(12), 1424–1433. 10.1109/10.966601 [DOI] [PubMed] [Google Scholar]
- Zhou, C. , Douglas, J. E. , Kumar, N. N. , Shu, S. , Bayliss, D. A. , & Chen, X. (2013). Forebrain HCN1 channels contribute to hypnotic actions of ketamine. Anesthesiology, 118(4), 785–795. 10.1097/ALN.0b013e318287b7c8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv, J. , & Lempel, A. (1978). Compression of individual sequences via variable‐rate coding. IEEE Transactions on Information Theory, 24(5), 530–536. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1. Comparison between LZ in wakefulness and LS (a), and wakefulness without ketamine vs. 5 mg of Ketamine (b) for the different cortices. RPf, rostral prefrontal cortex; DLPf, dorsolateral prefrontal cortex; M, primary motor cortex; S, primary somatosensory cortex; A, primary auditory cortex, Pp, posterior parietal cortex; V, visual cortex. “l” indicates left side, “r” right side and “1” indicates “primary cortex”.
Data Availability Statement
The code for computation of LZ used for analysis is available in GitHub at the following link: https://gitlab.com/CPasco83/sleep-and-ketamine. Data are available upon reasonable request to the authors.


