Summary
Atopic eczema and psoriasis are chronic, inflammatory dermatoses that can significantly affect the quality of life of those affected. Although both diseases are common, they rarely occur together. Severe psoriasis can be treated with biologic therapies targeting specific cytokine pathways involved in disease pathogenesis. There are reports of paradoxical eczema developing in biologic‐treated patients with psoriasis, sometimes necessitating treatment discontinuation and thus leading to poor disease control. This retrospective case series identified 36 such events occurring in 23 patients. All currently available biologic classes were implicated. Eosinophilia (n = 19) and elevated serum IgE (n = 3) were identified in some cases. Treatment strategies included no treatment, topical corticosteroids, broad‐acting systemic agents, and discontinuation or switch of biologic therapy. Two patients had persistent eczema and psoriasis despite discontinuation of all biologic therapies.
Atopic eczema and psoriasis are chronic, inflammatory dermatoses that can significantly affect the quality of life of those affected, although they rarely occur together. There are reports of paradoxical eczema developing in biologic‐treated patients with psoriasis, sometimes necessitating treatment discontinuation and thus leading to poor disease control. This retrospective case series describes 36 such events occurring in 23 patients, and summarizes the clinical features and treatments used.

Despite atopic eczema (AE) and psoriasis being genetically and immunologically discrete, 1 biologic therapy for either condition can lead to a paradoxical phenotypic switch to the other. 2 , 3 Paradoxical eczema (PE) occurring in patients with psoriasis treated with biologic therapy can significantly impact affected patients because treating one disease phenotype may flare or inadequately control, the other. A systematic review of PE occurring in patients with psoriasis receiving biologics found that 61% resulted in discontinuation of the culprit biologic. 4 It is important to report the clinical features and management of such adverse events and thereby add to the understanding of how to achieve optimal outcomes and to generate research hypotheses.
Report
In this retrospective case series, we identified 36 PE events occurring in 23 patients (13 men, 10 women) with plaque psoriasis at Salford Royal NHS Foundation Trust, Manchester, UK. The median age at first presentation was 49 years (range 26–67 years), and the median time of PE onset after biologic initiation was 109 days (range 5–1597 days). The implicated biologics were tumour necrosis factor (TNF) inhibitors [adalimumab (n = 12), etanercept (n = 1) and infliximab (n = 1)], anti‐interleukin (IL)‐17 drugs [(ixekizumab (n = 5), secukinumab (n = 4)], an anti‐IL‐23p19 drug (guselkumab; n = 3) and an anti‐IL‐12/23 drug (ustekinumab; n = 10). The distribution of eczema included: flexural (n = 8); generalized (n = 8); legs (n = 2); limbs not otherwise specified (n = 3); face (n = 4); hands (n = 2); trunk (n = 2); vulval (n = 1); neck/eyelids/limbs (n = 1); and no documentation of distribution in 5 cases. The morphologies observed included thin plaques, patches, xerosis, lichenification and nodular prurigo (Figs 1 and 2). Of the 23 patients, 9 had a history of at least 1 atopic disease [AE (n = 1), asthma (n = 3) and hay fever (n = 7)]. Laboratory findings included eosinophilia (n = 19) and elevated serum IgE (n = 3). Skin biopsies in three cases predominantly showed spongiosis (with coexisting features of psoriasis in one case; Fig. 1) and one showed evidence of excoriation.
Figure 1.
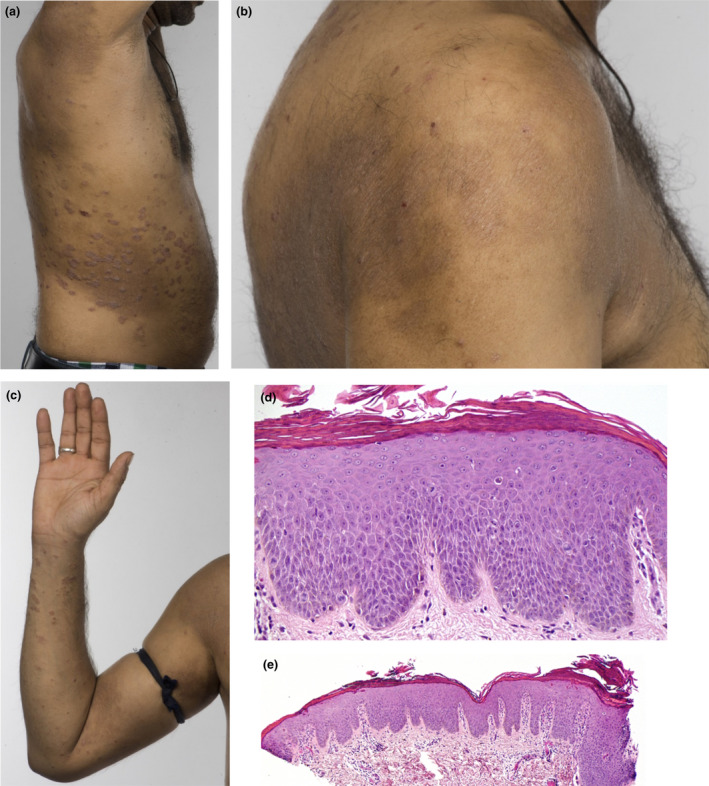
(a–e) Patient 4, a 34‐year‐old man with a 13‐year history of psoriasis, previously treated with ciclosporin, methotrexate and adalimumab, presented with flexural and periorbital eczema 81 days after starting ustekinumab. The eczema gradually resolved on stopping ustekinumab, although his psoriasis flared severely, reaching a Psoriasis Area Severity Index of 34.9. Infliximab resulted in a recurrence of paradoxical eczema (PE) 13 days after initiation, which persisted for > 2 years despite infliximab being stopped and treatment with ciclosporin and methotrexate initiated. Apremilast controlled the psoriasis, but not the PE. The patient was then started on secukinumab, resulting in recurrence of PE after 55 days, which persisted despite secukinumab being stopped and treatment with ciclosporin started. Tofacitinib, a Janus kinase inhibitor, also improved the PE but not the psoriasis. Finally, the patient was started on guselkumab while ciclosporin was continued, but the PE has persisted. (a‐c) Well‐defined, indurated plaques on the flanks and arms consistent with psoriasis, with coexisting eczema and lichenification affecting the shoulders and antecubital fossae, 18 months after stopping secukinumab. (d,e) Skin biopsy from the back showing (d) spongiosis and (e) psoriasiform hyperplasia. Haematoxylin and eosin, original magnification (d) × 200; (e) × 40. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
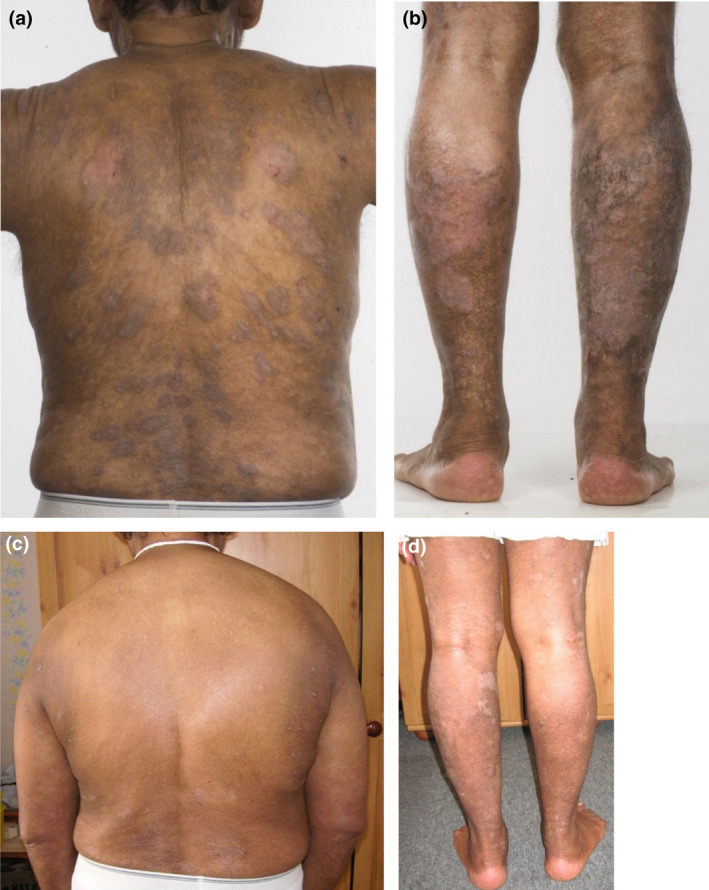
(a–d) Patient 5, a 61‐year‐old man with an 11‐year history of psoriasis previously treated with ciclosporin, methotrexate and etanercept, developed paradoxical eczema (PE) on three different biologic classes. The first episode presented with an eczematous reaction on the face 238 days after starting ustekinumab. The patient subsequently developed generalized eczema 25 days after starting secukinumab. Apremilast was ineffective in controlling the psoriasis. Both episodes of PE resolved with ciclosporin. PE recurred rapidly after starting guselkumab; the drug cleared the psoriasis completely and the PE is currently being managed with topical corticosteroids and antihistamines only. All episodes of PE were associated with eosinophilia, which was not present otherwise. (a,b) Severe plaque psoriasis affecting the back and legs 8 weeks after initiation of guselkumab; (c,d) significant improvement in psoriasis and the presence of hypopigmented atrophic scars from resolved pruriginous nodules 2 years after initiation of guselkumab. [Colour figure can be viewed at wileyonlinelibrary.com]
The treatments used in each case are summarized in Table 1. Oral immunomodulators, such as ciclosporin, methotrexate and apremilast were used with varying success. Tofacitinib, a Janus kinase inhibitor, resulted in control of PE but recurrence of psoriasis in Patient 4. Of the 36 PE events, 15 (42%) resulted in cessation of the causative biologic(s). Of these 15, 12 switched to a different biologic class and 1 switched within a class (from ixekizumab to brodalumab), resulting in persistence (n = 4), relapse (n = 3) or resolution or nonrecurrence of previously resolved PE (n = 6). Nine patients developed PE on > 1 class of biologic. Two patients had persistent eczema coexisting with psoriasis despite discontinuation of all biologic treatment.
Table 1.
Implicated biologics, timing of onset and management of patients with paradoxical eczema.
| Patient | Age, years | Sex | Biologic(s) (onset after biologic, days a ) | Management and treatment response |
|---|---|---|---|---|
| 1 | 27 F | F | ADA (60); UST (NR) | Topicals. Improved on stopping ADA. Recurred on UST and persists on GUS |
| 2 | 49 F | F | UST (271) | Topicals. UST continued. PE persists |
| 3 | 51 M | M | ADA (109); UST (1597); GUS (112) | Topicals and ADA/UST stopped; PE resolved. Recurred on GUS; controlled with topicals |
| 4 | 34 M | M | UST (81); INF (13); SEC (55); GUS (42) | UST, INF and SEC stopped; no resolution of PE. Partial improvement with ciclosporin and MTX. Apremilast ineffective for PE. Tofacitinib controlled PE but not psoriasis. PE persists on GUS |
| 5 | 61 M | M | UST (238); SEC (42); GUS (25) | Stopped UST and started ciclosporin; PE resolved. Stopped SEC; ciclosporin resolved PE. GUS continued; PE controlled with topicals |
| 6 | 66 M | M | IXE (85) | Topicals. IXE continued. Partial control of PE |
| 7 | 45 F | F | IXE (206) | Topicals; IXE switched to GUS. PE resolved |
| 8 | 43 M | M | ETA (5) | Topicals. Continued ETA. PE resolved |
| 9 | 54 M | M | UST (1056) | None. PE resolved |
| 10 | 67 M | M | UST (804) | Topicals. PE resolved |
| 11 | 63 F | F | ADA (331) | None. PE resolved |
| 12 | 39 F | F | ADA (959) | ADA stopped. PE resolved; psoriasis stable off biologic |
| 13 | 55 M | M | ADA (782); ADA (28) | ADA paused while investigated for breathlessness; PE resolved then recurred on restarting ADA. Controlled with topicals |
| 14 | 35 M | M | UST (91) | Topicals. UST continued. Ongoing PE flares |
| 15 | 52 F | F | ADA (977) | None. Outcome NR |
| 16 | 26 F | F | ADA (252); SEC (149) | MTX for 4 months. PE improved. ADA switched to SEC; PE controlled on SEC with topicals |
| 17 | 63 M | M | ADA (28) | Admission for topical treatments. ADA continued. PE resolved |
| 18 | 46 F | F | ADA (47); SEC (354) | Topicals and ciclosporin; PE improved. Recurred on SEC, PE resolved after admission and topical |
| 19 | 44 M | M | UST (40) | Topicals. UST continued. PE resolved |
| 20 | 61 M | M | ADA (642); IXE (161) | Topicals and MTX; PE persisted, switched to UST. Switched to IXE due to poor psoriasis control; PE controlled with topicals |
| 21 | 31 M | M | IXE (494) | Topicals and ciclosporin; PE improved then recurred. Switched to BRO; PE resolved |
| 22 | 36 F | F | ADA (62) | Topicals and ciclosporin; PE improved, but PE and psoriasis flare on tapering ciclosporin. Planned switch to IXE |
| 23 | 56 F | F | IXE (11); UST (7) | IXE stopped; ciclosporin; apremilast; prednisolone. PE improved on prednisolone. Switched to UST, which resulted in recurrence of PE; resolved with admission for topical treatments |
ADA, adalimumab; BRO, brodalumab; GUS, guselkumab; INF, infliximab; IXE, ixekizumab; MTX, methotrexate; NR, not reported; PE, paradoxical eczema; SEC, secukinumab; UST, ustekinumab.
For patients with > 1 episode, age at onset of first episode is recorded.
In our patients receiving biologic therapy for plaque psoriasis, both the clinical features of PE, such as the distribution and morphology, and the laboratory findings support the diagnosis of AE. This is of interest because AE and psoriasis rarely occur together. 5 A number of genetic risk loci have been identified that act in opposite directions in either condition; e.g. genetic variants associated with psoriasis are associated with the absence of AE and vice versa. 1 Eyerich et al. identified that even in patients who have concurrent psoriasis and AE, the individual lesions have distinct immunological profiles. 5 Thus, in such patients, psoriasis was characterized by T helper (Th)1/Th17 cells and higher filaggrin expression, while AE was characterized by Th2/Th22 cells, lower filaggrin expression and colonization with Staphylococcus aureus. 6 Furthermore, one of their patients was treated with infliximab, resulting in clearance of psoriasis, but exacerbation of AE, suggesting that targeting Th1/Th17 cytokines can flare Th2‐mediated diseases. 6
However, this is speculative and the pathogenesis of PE is unclear. In our cohort, an atopic diathesis was common but we did not compare its frequency with that of a control group. To date, there have been no epidemiological studies in patients with psoriasis to confirm an association of PE with atopy; however, a personal history of atopy has been shown to be associated with eczematous eruptions caused by biologics used in the management of inflammatory bowel disease (IBD). 7 Recurrence of PE on multiple biologic classes may indicate a genetic tendency to overexpress Th2 cytokines when the IL‐17/23 or Th1 axes are inhibited, although there have been no genetic studies and few molecular studies of PE. Ono et al. identified that eczematous lesions contained increased IL‐13 while psoriasis lesions contained increased IL‐23, IL‐17 and the psoriatic autoantigens LL‐37 and ADAMTSL5 (a disintegrin‐like and metalloprotease domain containing thrombospondin type 1 motif‐like 5) in a patient with PE secondary to ustekinumab. 8 Chicharro et al. reported a mixed cytokine profile in eczematous lesions of patients with psoriasis and PE, as did Stoffel et al. in TNF inhibitor‐treated patients with IBD. 9 , 10
PE can clearly have a major impact on the management of patients with psoriasis, with a significant proportion of affected patients having to switch biologic therapy, not always successfully. The persistence of PE for over 2 years after stopping infliximab in Patient 4 is far longer than can be accounted for by the drug's pharmacokinetic properties, suggesting that long‐lasting or even permanent immunological and phenotypic alterations can result from biologic therapy. Oral immunomodulators, such as methotrexate, which can be used to treat both AE and psoriasis, may be more suitable in these patients, although in our experience, success is inconsistent and impossible to predict.
To our knowledge, this is the largest case series of PE occurring in patients with psoriasis, and contains the first reports of persistent PE despite biologic discontinuation and the first report of using tofacitinib in this context. In‐depth characterization of the genetic and immunological basis of PE is required to understand its aetiology and to optimize treatment selection in such patients.
Learning points.
-
•
PE can be caused by any class of biologic currently available for psoriasis.
-
•
PE can recur on different biologic classes within the same patient.
-
•
PE can persist even after discontinuation of the culprit biologic.
-
•
Molecular and genetic studies are needed to understand the pathogenesis of PE.
Conflict of interest
ACF has received educational support to attend conferences from or acted as a consultant or speaker for AbbVie, Almirall, Celgene, Eli Lilly, Leo Pharma, Novartis, Pfizer, Janssen and UCB. CEMG has received honoraria or research grants from AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, Janssen, LEO Pharma, Novartis, Pfizer, Sandoz, Sanofi and UCB Pharma. RBW has received research grants from and leads clinical trials for AbbVie, Almirall, Amgen, Bristol‐Myers Squibb, Celgene, Eli Lilly, Janssen, LEO Pharma, Novartis, Pfizer and UCB Pharma. He has received consulting fees from AbbVie, Almirall, Amgen, Arena Pharmaceuticals, Avillion, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sanofi and UCB Pharma.
Acknowledgement
This work was supported by the National Institute for Health Research (NIHR) and NIHR Manchester Biomedical Research Centre. CEMG is an NIHR Emeritus Senior Investigator. We are grateful to Dr Ruth Green, Salford Royal Hospital NHS Foundation Trust for providing the histology images. Unfortunately, we are unable to share the data from this study.
A previous version of this work was presented at the British Association of Dermatologists 100th Annual Meeting, 2020.
References
- 1. Baurecht H, Hotze M, Brand S et al. Genome‐wide comparative analysis of atopic dermatitis and psoriasis gives insight into opposing genetic mechanisms. Am J Hum Genet 2015; 96: 104–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Napolitano M, Megna M, Fabbrocini G et al. Eczematous eruption during anti‐interleukin 17 treatment of psoriasis: an emerging condition. Br J Dermatol 2019; 181: 604–6. [DOI] [PubMed] [Google Scholar]
- 3. Varma A, Levitt J. Dupilumab‐induced phenotype switching from atopic dermatitis to psoriasis. JAAD Case Rep 2020; 6: 217–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al‐Janabi A, Foulkes AC, Mason K et al. Phenotypic switch to eczema in patients receiving biologics for plaque psoriasis: a systematic review. J Eur Acad Dermatol Venereol 2020; 34: 1440–8. [DOI] [PubMed] [Google Scholar]
- 5. Eyerich S, Onken AT, Weidinger S et al. Mutual antagonism of T cells causing psoriasis and atopic eczema. N Engl J Med 2011; 365: 231–8. [DOI] [PubMed] [Google Scholar]
- 6. Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol 1995; 32: 982–6. [DOI] [PubMed] [Google Scholar]
- 7. Esmailzadeh A, Pedram Y, Farhi D et al. Predictive factors of eczema‐like eruptions among patients without cutaneous psoriasis receiving infliximab: a cohort study of 92 patients. Dermatology 2009; 219: 263–7. [DOI] [PubMed] [Google Scholar]
- 8. Ono S, Honda T, Doi H et al. Concurrence of psoriasis vulgaris and atopic eczema in a single patient exhibiting different expression patterns of psoriatic autoantigens in the lesional skin. JAAD Case Rep 2018; 4: 429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chicharro P, Rodríguez‐Jiménez P, De la Fuente H et al. Mixed profile of cytokines in paradoxical eczematous eruptions associated with anti‐IL‐17 therapy. J Allergy Clin Immunol Pract 2020; 8: 3619–21.e1. [DOI] [PubMed] [Google Scholar]
- 10. Stoffel E, Maier H, Riedl E et al. Analysis of anti‐tumour necrosis factor‐induced skin lesions reveals strong T helper 1 activation with some distinct immunological characteristics. Br J Dermatol 2018; 178: 1151–62. [DOI] [PubMed] [Google Scholar]


