Abstract
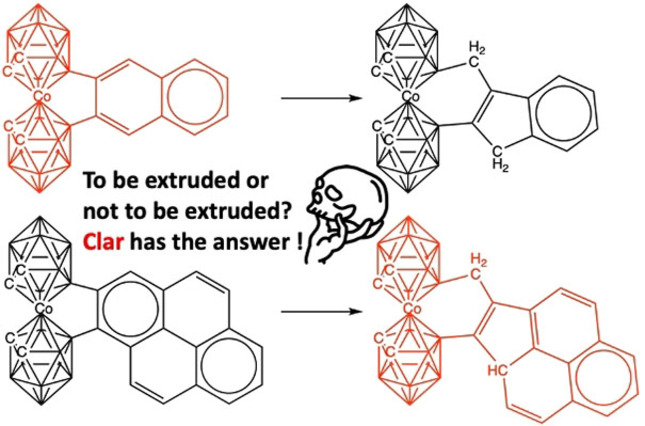
Benzene and pyrene can be synthetically linked to [o‐COSAN]− keeping their aromaticity. In contrast, naphthalene and anthracene are extruded in the same reaction. We have proven that extrusion is only favorable if the number of Clar's π‐sextets remains constant. Thus, Clar has the answer to whether an attached polycyclic aromatic hydrocarbon to [o‐COSAN]− is extruded or not.
Keywords: Aromaticity, Boron, COSAN, Clar π-Sextet, Extrusion
Clar has the answer! Clar's π‐sextets justify the reason why the linked system of [o‐COSAN]− and benzenoid drives to extrusion in the case of naphthalene or anthracene, but not for benzene, pyrene or perylene.
As Schleyer [1] and Hoffmann [2] pointed out, today there is a large proliferation of “types” of aromaticity, going far beyond the conventional confines of benzenoid hydrocarbons and their related heteroarenes. [3] However, in this work, we will restrict to the classical ones; to say these unsaturated chemical compounds made by planar rings, whose great stability is due to their aromaticity. This is associated with fully conjugated monocyclic compounds having a planar geometry, and with a number of π‐electrons that must be equal to 4n+2, in which n=1, 2, 3, etc. On the other hand, benzenoid compounds are assemblies of benzene rings that share at least a common side. One of the characteristics of these classical aromatic compounds is that the double bonds tend to not participate in addition reactions but instead undergo electrophilic substitution reactions, thus retaining the stability associated with the aromatic π‐electron system. Therefore, an aromatic fragment will tend not to lose the aromatic stabilization. However, there are examples where an arene has lost its aromaticity by C−C cleavage at high temperatures,[ 4 , 5 ] or by enzymes, [6] or by polymetallacomplexes.[ 7 , 8 ] Nonetheless, the extrusion of a CH unit from one aromatic benzene ring to yield a diradical 1‐methylcyclopenta‐1,3‐diene (Figure 1), a non‐aromatic fragment, is highly unlikely in the ground state. It is worth noting here that photoisomerization upon excitation of benzene to its antiaromatic S1 state leads to benzvalene and fulvene formation.[ 9 , 10 ]
Figure 1.
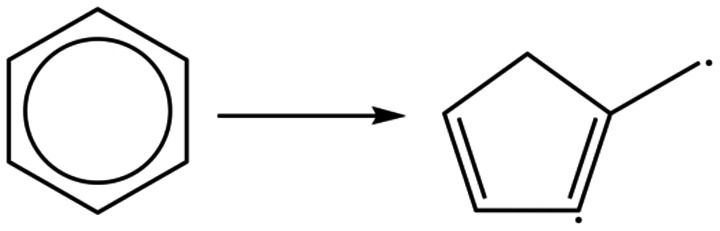
Hypothetic extrusion of benzene.
The word extrusion is not uncommon in organic synthesis, e.g. two‐fold extrusion reactions involve the loss of two small, typically inorganic fragments, bridging two atoms with the formation of a double bond between the atoms (most commonly affording an alkene or an imine). [11] This would be a conventional process in which no aromatic starting species would be involved. Neither would be the case where an aromatic species is being converted to another aromatic species, for example from a benzene to a cyclopentadienide. [12] The case we are discussing here is the conversion of an aromatic species into a non‐aromatic species, which is the shocking scenario at the outset.
The cobaltabis(dicarbollide), [3,3′‐Co(1,2‐C2B9H11)2]−, abbreviated as [o‐COSAN]−, [13] which displays global aromaticity, [14] is one of the most studied metallacarboranes. In 1978, Plešek and co‐workers succeeded in linking the two dicarbollide units in [3,3′‐Co(1,2‐C2B9H11)2]− with a benzene bridge (Figure 2A). [15] The structure was solved in 1988 and confirms the preservation of the aromatic cycle. [16] A second example was reported in 2011. [17] In 2020, while searching for a synthetic pathway for a similar [o‐COSAN]−‐naphthalene bridge (see Figure 2B), using the activation of the B−H group with the trityl cation on [3,3′‐Co(1,2‐C2B9H11)(8,8′‐C10H7‐1′,2′‐C2B9H10)]−, [18] we observed the formation of a B−C(sp2) bond, but it was not the one sought with the naphthyl, 8‐C10H7 − in the anion, but with the aryl group of the trityl. This led us to modify the reaction strategy and AlCl3 was used as a Lewis acid, mesitylene as a manageable high boiling solvent (165 °C) with sufficient symmetric steric hindrance to prevent electrophilic attack on its structure, and the benzenoid species and [3,3′‐Co(1,2‐C2B9H11)2]− as reagents (see Supporting Information).[ 17 , 18 ] First attempts were done with naphthalene, but did not lead to the naphthyl‐bridged [3,3′‐Co(1,2‐C2B9H11)2]− (Figure 2B), but to an unexpected 3‐methylindenyl bridge (Figure 2C), which had been earlier synthesized and structurally characterized.[ 19 , 20 , 21 ]
Figure 2.
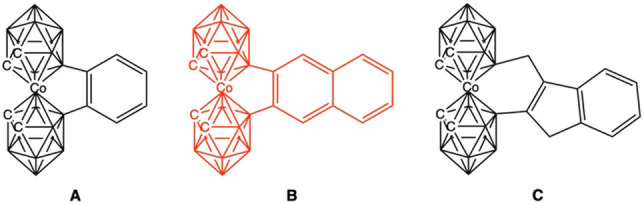
Structure of [Co(C2B9H11)2]− with a benzene (A), a naphthalene (B), and a 3‐methylindenyl (C) bridge. Structure B is shown in red because it was not obtained.
From these two reactions with arenes, the one leading to a benzene bridge and the one not leading to a naphthalene bridge but to an extrusion (Figure 2), we concluded the following: considering that the standing framework, the metallacarborane, and the dimensions of both connecting cycles are the same, the difference in behavior is not steric but electronic. Then, if both benzene and naphthalene are aromatic and both fulfil the 4n+2 conditions indicated above, what is the underlying mechanism that makes an aromatic ring lose this condition in favor of an extrusion and a non‐aromatic ring? What is the difference between the two aromatic compounds that can explain this anomalous behavior that goes against the reluctance of an aromatic fragment to lose this condition?
To get more details on this reaction, anthracene, pyrene, and perylene were also studied experimentally by us (see Supporting Information). Anthracene is an additional example to naphthalene of a linear benzenoid, whereas pyrene and perylene are examples of benzenoids where the cycles share more than one edge (Figure 3). Again, two different behaviors were found, anthracene goes into extrusion, whereas both pyrene and perylene do not.
Figure 3.
Localization of Clar's π‐sextets in the series of compounds under analysis.
All details of the synthesis and characterisation are given at the Supporting Information, but one detail is worth mentioning. Taking pyrene as the arene to bind to [o‐COSAN]−, several experiments were carried out by modifying temperature and reaction times. Thus, studies were carried out at 25, 80, and 160 °C and reaction times of 1 h, 2 h, and 5 h. At 2 h reaction time and 160 °C, 100 % of the material was converted into an arene bridge but, if the reaction time was extended to 5 h and 160 °C, the extrusion was dominant. This indicates that both the arene bridge and the extruded forms can be obtained depending on the used framework, which proves that for linear PAHs, extrusion is very easy to access and much more difficult for two‐dimensional PAHs. Thus, which is the difference between both sets of systems with respect to extrusion?
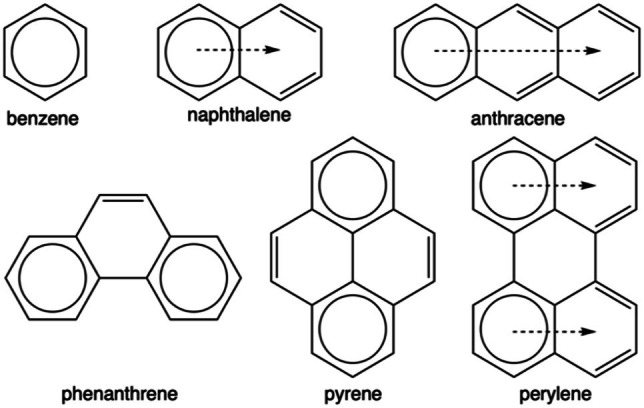
Erich Clar observed that resonance structures that maximize the number of rings with three double bonds are the most important. On this basis, he developed Clar structures, which restrict inscribed circles only to benzenoid rings that have six π‐electrons (π‐sextet). Once electrons have been assigned to a ring, they cannot be used in another; hence, rings with inscribed circles do not share an edge.[ 22 , 23 ] Thus now, if we go back to Figure 3, two types of benzenoids are found, those that may not have a Clar π‐sextet connected to the bridge of the [o‐COSAN]−‐benzenoid system and those that may have a Clar π‐sextet connected to the bridge. Benzene is a special case but ranks with those that have a Clar π‐sextet to the bridge. The above‐mentioned extrusion occurs with those that do not have a Clar π‐sextet linked to the bridge. In the following paragraphs, we will try to show how this unexpected extrusion and loss of aromaticity occurs.
Thus, can Clar's π‐sextets justify the reason why the linked system of [o‐COSAN]− and benzenoid drives to extrusion in case of naphthalene or anthracene, but not or with much more difficulty for benzene, pyrene or perylene as experimentally proven? For such, we will try to address the above hypothesis through a quantum chemical analysis of the aromaticity of the above synthesized series of compounds by means of the nucleus‐independent chemical shift (NICS)[ 24 , 25 , 26 , 27 ] and the multicenter index (MCI)[ 28 , 29 , 30 , 31 ] criteria, at the B3LYP(GD3BJ)/6–311++G** DFT level of theory (see Supporting Information for more details about computational methods used). Phenanthrene has also been added for completeness despite it has not been experimentally studied. First, the anionic cobaltabis(dicarbollide) cluster keeps its aromaticity when fused to any of the different benzenoids under analysis, with or without going into extrusion (Figures 4 and 5). This finding is in agreement with our recent work [14] in which we have proven the global aromatic character of this metallabis(dicarbollide). In particular, we have computationally found a strong diatropic ring current in the middle of the two five‐membered rings (5‐MRs) of the nido‐[C2B9H12]− carborane. This intense diatropic ring current, which is also found in the nido‐[C2B9H12]− carborane, explains the experimentally observed NMR chemical shifts displaced at high fields of the endocyclic proton and the boron in the vertex opposite to the open face of this nido carborane. And it is also supported by another recent work on the aromaticity of phenyl decorated closo‐monocarboranes. [32]
Figure 4.
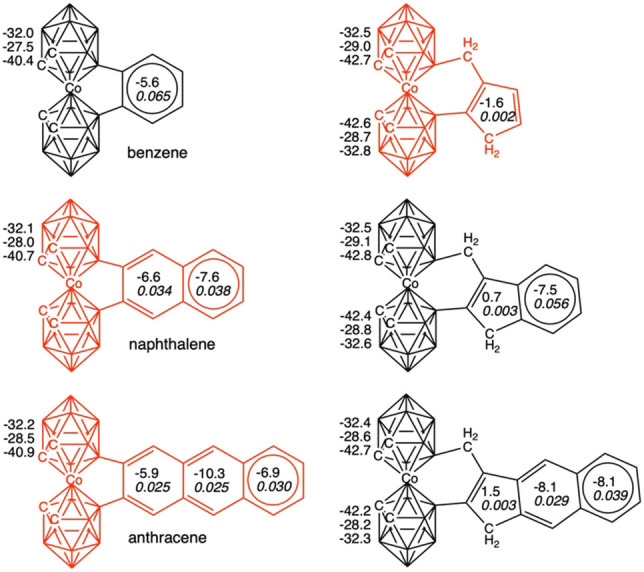
[o‐COSAN]− without (left) and with extrusion (right). NICS values for the upper 5‐membered ring (5‐MR), for the center of the cage, and for the lower 5‐MR (in ppm) and NICS and MCI values (in italics and in au) for the benzenoid moiety are also included. NICS for pristine [o‐COSAN]− are −32.9 (upper 5‐MR), −28.9 (center), and −42.7 (lower 5‐MR) ppm, respectively. Those not synthetically obtained systems are depicted in red.
Figure 5.
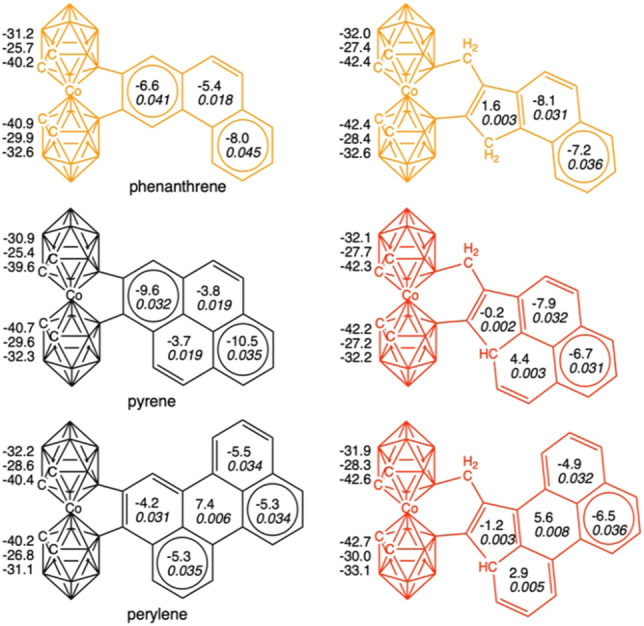
[o‐COSAN]− attached to phenanthrene, pyrene, and perylene driving to the possible products without (left) and with extrusion (right). NICS values for the upper 5‐MR, for the center of the cage, and for the lower 5‐MR (in ppm) and NICS and MCI values (in italics and in au) for the benzenoid moiety are also included. Those not synthetically obtained systems are depicted in red. In orange are shown these to which theory is not conclusive.
Then, the discussion on the aromaticity of benzenoids will be mainly based on MCI, as NICS suffers from the spurious contributions from the in‐plane tensor components and the large influence of the coupling of magnetic fields from different regions of the molecule.[ 33 , 34 , 35 , 36 ] Thus, if we first focus on naphthalene, it is observed how this benzenoid keeps the aromaticity of both rings if it is not extruded, but it loses the one of the five‐membered ring in case of extrusion (Figure 4). On the opposite site, pyrene does not go into extrusion (Figure 5), keeping both the internal (i.e., ring linked to [o‐COSAN]−) and the terminal rings aromatic, whereas in case of the extruded system, the middle and the terminal rings are aromatic, but not the 5‐MR and one of the 6‐MRs. Then, what is the reason naphthalene prefers to go through extrusion despite the consequent loss of aromaticity, whereas pyrene is not extruded?
As stated above, we must refer to Clar π‐sextets to get the most convincing answer.[ 22 , 23 ] Extrusion is only favorable if the number of π‐sextets remains constant, otherwise, if a π‐sextet is lost in the extrusion process, this does not take place. In other words, extrusion only takes place if aromaticity is not lost along such process. In this case, reduction of strain of the 5‐MR cobaltacycle (B−Co−B−C−C) that is transformed to a 6‐MR (B−Co−B−C−C−C) favors the extrusion. For instance, naphthalene has a migrating π‐sextet, and despite it goes through extrusion, it keeps a π‐sextet in the external ring, and the same applies to anthracene (Figure 4). In general, electronic structure of naphthalene can be described by a combination of two local aromatic π‐sextets (6‐MRs) and one global aromatic π‐dectet (10‐MR).[ 37 , 38 , 39 ] When it is extruded, naphthalene loses the π‐dectet, but it keeps the local aromaticity of the 6‐MR, and, therefore, the number of π‐sextets remains the same after extrusion. At difference, pyrene has two π‐sextets (the internal and the terminal rings), however its extruded counterpart would only present one migrating π‐sextet. Extrusion in benzene would make it reduce one π‐sextet to none, and in both phenanthrene and perylene, with two π‐sextets each, extrusion would also cause a loss of aromaticity, as it is observed from aromaticity criteria (Figure 5).
In addition, computed extrusion energies (Table 1) are consistent with the former aromaticity criteria. In this regard, the reaction [o‐COSAN]−‐benzenoid+H2→[o‐COSAN]−‐benzenoidextruded is particularly favorable for both naphthalene and anthracene (−11.0 and −15.7 kcal mol−1, respectively), that are keen to suffer extrusion. At difference, both pyrene and perylene show clearly unfavorable energies (+14.2 and +20.7 kcal mol−1, respectively). Same conclusion (even more clear) can be derived from the reaction [o‐COSAN]−+benzenoid→[o‐COSAN]−‐benzenoidextruded+H2.
Table 1.
Extrusion energies (in kcal mol−1) computed based on two different reactions: 1) [o‐COSAN]−‐benzenoid+H2→[o‐COSAN]−‐benzenoidextruded; and 2) [o‐COSAN]−+benzenoid→[o‐COSAN]−‐benzenoidextruded+H2. Distances [in Å] between the two B atoms fused to the benzenoid are also included.[a,b,c]
|
System |
ΔE ext 1 |
ΔE ext 2 |
d(B⋅⋅⋅B) |
d(B⋅⋅⋅B)extruded |
|---|---|---|---|---|
|
benzene |
−0.13 |
11.35 |
2.851 |
3.104 |
|
naphthalene |
−11.04 |
−2.07 |
2.858 |
3.094 |
|
anthracene |
−15.66 |
−8.23 |
2.861 |
3.092 |
|
phenanthrene |
−8.96 |
−1.44 |
2.856 |
3.097 |
|
pyrene |
14.23 |
23.79 |
2.853 |
3.095 |
|
perylene |
20.67 |
29.79 |
2.849 |
3.055 |
[a] The equivalent distance between the two B atoms in [o‐COSAN]− is 3.144 Å. [b] Extrusion energies calculated at the B3LYP(GD3BJ)/6–311++G** level of theory. [c] Gibbs energies are enclosed in Table S2.
Finally, we must refer to the balance between the loss of aromaticity upon extrusion in parallel with the loss of strain of the 5‐MR with cobalt (Table 1). Thus, naphthalene undergoes extrusion because it does not lose π‐sextets and at the same time the strain of the cobaltacycle is reduced. At difference, benzene, pyrene, and perylene give priority to not lose aromaticity. The case of phenanthrene is the most unique as it is arguably in a no man's zone. It is very clear that aromaticity indexes are very conclusive in the absence of extrusion, which is consistent with preserving the Clar π‐sextets. On the other hand, phenanthrene is the only one of the benzenoids presented in this work with two π‐Clar sextets whose rings, in this case the central ring, share at most two edges just as anthracene does. It is therefore not so surprising that the reaction enthalpies shown in Table 1 for phenanthrene are between those benzenoids which clearly maintain the aromaticity of the [o‐COSAN]−‐connected ring and those which clearly lead to extrusion. In the latter cases, both the aromaticity indices and the reaction enthalpies are convergent. In the case of phenanthrene, they are opposite. Therefore, taking into account the experimental data on pyrene indicated above, we believe that extrusion will require more time or more temperature than for anthracene but much less than for bidimendional PAHs. In other words, there will be mixtures of extruded and unextruded compounds at relatively low T, around 80 °C. For completeness, the aromaticity of the benzenoids has also been analyzed when [o‐COSAN]− is removed and substituted by hydrogen atoms. The trends are kept, which reinforces the determinant role of the aromaticity, i.e., keeping the number of Clar π‐sextets when extruded (Figures S6 and S7) is more determinant that the loss of strain. However, what is clear is that the [o‐COSAN]− has been the vehicle that has allowed this extrusion to be demonstrable for the first time, and it is quite possible that it can be visualised with other frameworks with similar characteristics or under appropriate conditions of temperature, pressure, and time. Finally, we have confirmed that our aromaticity data is not affected by the employed DFT functional (see Supporting Information).[ 39 , 40 , 41 ]
As a whole, experimentally, benzenoids linked to [o‐COSAN]− in some cases suffer extrusion (i.e., naphthalene and anthracene), whereas in other cases do not (i.e., benzene, pyrene, and perylene). We have shown that benzenoids linked to [o‐COSAN]− are prone to be extruded only in the case the number of π‐sextets is not reduced during the extrusion. This is consistent with two opposing forces that coexist at the junction between the metallacarborane and the aromatic ring. One tends to make the metallacarborane more comfortable, with less strain energy, and the other tends to make the aromatic ring more comfortable, with higher Aromatic Stabilization Energy, ASE. If the ASE is greater, the aromatic ring will be retained, but if part of the ASE can be secured by localizing it elsewhere in the molecule by sliding the aromaticity, then extrusion takes place. Therefore, the answer to the question to be or not to be extruded is found in a simple model such as the Clar's π‐sextet theory. Clar has the answer .
This research has shown that linear PAHs such as pentacene, of great interest as organic semiconductors, have a lower stability than more fused PAHs such as pyrene, with all the consequences that this may imply for molecular electronics. As for the [o‐COSAN]−, it can be effectively activated by light,[ 42 , 43 ] generating both a very strong reductant and oxidant that can reduce or oxidize a PAH intimately bound to it; modulating its electronic properties is of great importance in molecular electronics or catalysis. In this case, PAHs that remain intact would be more appropriate than those that give rise to extrusion. Therefore, apparently better perylene than pentacene, to give an example.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
This work has been supported by the Ministerio de Ciencia e Innovación of Spain (Projects PID2020‐113711GB‐I00, PID2019‐106832RB‐I00, PID2019‐106830GB‐I00, and MDM‐2017‐0767) and the Catalan Conselleria de Recerca i Universitats (projects 2017SGR39, 2017SGR1720, and 2017SGR348). Excellent service by the Supercomputer center of the Consorci de Serveis Universitaris de Catalunya (CSUC) is gratefully acknowledged. Open Access funding provided thanks to the CRUE‐CSIC agreement with Wiley.
J. Poater, C. Viñas, D. Olid, M. Solà, F. Teixidor, Angew. Chem. Int. Ed. 2022, 61, e202200672; Angew. Chem. 2022, 134, e202200672.
Contributor Information
Prof. Dr. Miquel Solà, Email: miquel.sola@udg.edu.
Prof. Dr. Francesc Teixidor, Email: teixidor@icmab.es.
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
References
- 1. Schleyer P. v. R., Chem. Rev. 2001, 101, 1115–1117. [DOI] [PubMed] [Google Scholar]
- 2. Hoffmann R., Am. Sci. 2015, 103, 18–22. [Google Scholar]
- 3. Solà M., Front. Chem. 2017, 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pope R. M., Vanorden S. L., Cooper B. T., Buckner S. W., Organometallics 1992, 11, 2001–2003. [Google Scholar]
- 5. Sattler A., Parkin G., Nature 2010, 463, 523–526. [DOI] [PubMed] [Google Scholar]
- 6. Bugg T. D. H., Winfield C. J., Nat. Prod. Rep. 1998, 15, 513–530. [Google Scholar]
- 7. Ellis D., McKay D., Macgregor S. A., Rosair G. M., Welch A. J., Angew. Chem. Int. Ed. 2010, 49, 4943–4945; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 5063–5065. [Google Scholar]
- 8. Hu S., Shima T., Hou Z., Nature 2014, 512, 413–415. [DOI] [PubMed] [Google Scholar]
- 9. Kaplan L., Wilzbach K. E., J. Am. Chem. Soc. 1968, 90, 3291–3292. [Google Scholar]
- 10. Ward H. R., Wishnok J. S., J. Am. Chem. Soc. 1968, 90, 5353–5357. [Google Scholar]
- 11. Guziec L. J., F. S. Guziec, Jr. , Org. React. 2012, 78, 411–549. [Google Scholar]
- 12. Zhou S., Schlangen M., Li J., Wu X.-N., Schwarz H., Chem. Eur. J. 2015, 21, 9629–9631. [DOI] [PubMed] [Google Scholar]
- 13. Wiesboeck R. A., Hawthorne M. F., J. Am. Chem. Soc. 1964, 86, 1642–1643. [Google Scholar]
- 14. Poater J., Viñas C., Bennour I., Escayola Gordils S., Solà M., Teixidor F., J. Am. Chem. Soc. 2020, 142, 9396–9407. [DOI] [PubMed] [Google Scholar]
- 15. Plešek J., Hermanek S., Collect. Czech. Chem. Commun. 1978, 43, 1325–1331. [Google Scholar]
- 16. Shelly K., Knobler C. B., Hawthorne M. F., New J. Chem. 1988, 12, 317–319. [Google Scholar]
- 17. Farràs P., Teixidor F., Rojo I., Kivekas R., Sillanpaa R., Gonzalez-Cardoso P., Viñas C., J. Am. Chem. Soc. 2011, 133, 16537–16552. [DOI] [PubMed] [Google Scholar]
- 18. Buades A. B., Kelemen Z., Arderiu V. S., Zaulet A., Viñas C., Teixidor F., Dalton Trans. 2020, 49, 3525–3531. [DOI] [PubMed] [Google Scholar]
- 19. Franken A., Plešek J., Nachtigal C., Collect. Czech. Chem. Commun. 1997, 62, 746–751. [Google Scholar]
- 20. Sivaev I. B., Bregadze V. I., Collect. Czech. Chem. Commun. 1999, 64, 783–805. [Google Scholar]
- 21. Plešek J., Chem. Rev. 1992, 92, 269–278. [Google Scholar]
- 22. Clar E., The Aromatic Sextet, Wiley, New York, 1972. [Google Scholar]
- 23. Solà M., Front. Chem. 2013, 1, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Z. F., Wannere C. S., Corminboeuf C., Puchta R., Schleyer P. V., Chem. Rev. 2005, 105, 3842–3888. [DOI] [PubMed] [Google Scholar]
- 25. Poater J., Solà M., Viñas C., Teixidor F., Chem. Eur. J. 2013, 19, 4169–4175. [DOI] [PubMed] [Google Scholar]
- 26. Poater J., Solà M., Viñas C., Teixidor F., Angew. Chem. Int. Ed. 2014, 53, 12191–12195; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 12387–12391. [Google Scholar]
- 27. Poater J., Solà M., Viñas C., Teixidor F., Chem. Eur. J. 2016, 22, 7437–7443. [DOI] [PubMed] [Google Scholar]
- 28. Bultinck P., Ponec R., Van Damme S., J. Phys. Org. Chem. 2005, 18, 706–718. [Google Scholar]
- 29. Feixas F., Matito E., Poater J., Solà M., Chem. Soc. Rev. 2015, 44, 6434–6451. [DOI] [PubMed] [Google Scholar]
- 30. Matito E., Duran M., Solà M., J. Chem. Phys. 2005, 122, 014109; Erratum: [DOI] [PubMed] [Google Scholar]; Matito E., Duran M., Solà M., J. Chem. Phys. 2006, 125, 059901. [DOI] [PubMed] [Google Scholar]
- 31. Matito E., Poater J., Solà M., Schleyer P. v. R. in Chemical Reactivity Theory (Ed.: Chattaraj P. K.), Taylor and Francis/CRC Press, Boca Raton, 2009. [Google Scholar]
- 32. Muñoz-Castro A., J. Phys. Chem. A 2021, 125, 4861–4866. [DOI] [PubMed] [Google Scholar]
- 33. Bultinck P., Faraday Discuss. 2007, 135, 347–365. [DOI] [PubMed] [Google Scholar]
- 34. Bultinck P., Fias S., Ponec R., Chem. Eur. J. 2006, 12, 8813–8818. [DOI] [PubMed] [Google Scholar]
- 35. Fallah-Bagher-Shaidaei H., Wannere C. S., Corminboeuf C., Puchta R., Schleyer P. V., Org. Lett. 2006, 8, 863–866. [DOI] [PubMed] [Google Scholar]
- 36. Feixas F., Matito E., Poater J., Solà M., J. Comput. Chem. 2008, 29, 1543–1554. [DOI] [PubMed] [Google Scholar]
- 37. Inostroza D., Garcia V., Yanez O., Torres-Vega J. J., Vasquez-Espinal A., Pino-Rios R., Baez-Grez R., Tiznado W., New J. Chem. 2021, 45, 8345–8351. [Google Scholar]
- 38. Sundholm D., Berger R. J. F., Fliegl H., Phys. Chem. Chem. Phys. 2016, 18, 15934–15942. [DOI] [PubMed] [Google Scholar]
- 39. Szczepanik D. W., Solà M., Krygowski T. M., Szatylowicz H., Andrzejak M., Pawelek B., Dominikowska J., Kukulka M., Dyduch K., Phys. Chem. Chem. Phys. 2018, 20, 13430–13436. [DOI] [PubMed] [Google Scholar]
- 40. Casademont-Reig I., Guerrero-Aviles R., Ramos-Cordoba E., Torrent-Sucarrat M., Matito E., Angew. Chem. Int. Ed. 2021, 60, 24080–24088; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 24282–24290. [Google Scholar]
- 41. Casademont-Reig I., Woller T., Contreras-Garcia J., Alonso M., Torrent-Sucarrat M., Matito E., Phys. Chem. Chem. Phys. 2018, 20, 2787–2796. [DOI] [PubMed] [Google Scholar]
- 42. Guerrero I., Kelemen Z., Viñas C., Romero I., Teixidor F., Chem. Eur. J. 2020, 26, 5027–5036. [DOI] [PubMed] [Google Scholar]
- 43. Guerrero I., Viñas C., Romero I., Teixidor F., Green Chem. 2021, 23, 10123–10131. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.


