Abstract
The persistence of human astroviruses dried on representative porous (paper) and nonporous (china) surfaces was investigated. Long-term astrovirus survival on fomites was monitored by an integrated cell culture-reverse transcription-PCR procedure. Viruses were applied to inanimate surfaces in the presence and absence of fecal material, and their survival was assayed at 4 and 20°C with high relative humidity. Astroviruses exhibited a notable persistence when dried on porous and nonporous materials, particularly at low temperature. Short-term survival of astroviruses on fomites was compared to that of other enteric viruses significant for health, such as rotavirus, adenovirus, poliovirus, and hepatitis A virus. Overall, astroviruses persisted better than poliovirus and adenovirus, although they exhibited a shorter survival than rotavirus and hepatitis A virus. Astroviruses show a high level of persistence at the desiccation step, which is of major significance in determining the chance of subsequent virus survival dried on fomites. Astroviruses are able to survive on inert surfaces long enough to suggest that fomites may play a relevant role in the secondary transmission of astrovirus diarrhea.
Each year viral gastroenteritis causes 2 million to 4 million deaths worldwide (23). Diarrhea-causing viruses are excreted in high numbers in the feces of infected individuals, and one critical public health issue is whether once they are in the environment, gastroenteritis agents are able to persist long enough and in high enough numbers to pose an actual health hazard. Outbreaks of acute gastroenteritis are a matter of concern in institutions such as day care centers, hospitals, nurseries, schools, and military quarters. Many of these outbreaks may have been caused by vehicular transmission of gastroenteritis agents through fecally contaminated environmental surfaces (6).
Astroviruses (AsVs) were originally described in 1975 in association with outbreaks of gastroenteritis in newborns (3), and they have been established as a new family of nonenveloped single-stranded positive RNA viruses, the Astroviridae (22). AsV infections occur worldwide and are most frequent in young children, although illness rates rise again in the elderly (11, 12). AsVs are transmitted by the fecal-oral route, and outbreaks have been associated with consumption of sewage-polluted shellfish (11) and ingestion of water from contaminated sources (17). However, discrepancies still exist in information about their actual presence in the water environment (7). In the present study, we investigated the survival of AsVs when dried on representative porous (paper) and nonporous (china) materials at different temperatures. AsV behavior on fomites was compared to that of other enteric viruses significant for health, which were selected because of their acknowledged medical relevance. Group A rotavirus (RV) is the most common cause of infantile diarrhea and each year is responsible for 140 million cases (15). Moreover, institutional outbreaks of RV diarrhea have been shown to occur through fecally contaminated environmental surfaces (6). Epidemiological studies have demonstrated that enteric adenovirus (AdV) types 40 and 41 are also an important cause of acute diarrhea, contributing to 5 to 20% of hospitalizations for childhood diarrhea in developed countries (21). While most RV and AsV infections in temperate regions are detected in the winter months (14, 24), AdV infections are reported throughout the year (10). The medical significance of hepatitis A is sometimes neglected; nevertheless, it represents the most common type of hepatitis and continues to be a source of mortality in both developed and developing countries. Poliovirus (PV) was included in the present work since it may be regarded as the prototypical enteric virus for environmental studies (8). However, it has been previously shown that PV fails to provide an adequate indication of the behavior of other human enteric viruses significant for health in the environment under natural or disinfection conditions (1; A. Bosch et al., unpublished results).
MATERIALS AND METHODS
Viruses and virus assays.
Human AsV serotype 4 (kindly provided by W. D. Cubitt, Institute of Child Health, London, United Kingdom) and human enteric AdV type 40 (provided courtesy of W. O. K. Grabow, University of Pretoria, Pretoria, South Africa) were propagated and assayed in CaCo-2 cells as described elsewhere (18). MA-104 cell cultures were used to propagate and assay human RV type 3 Itor p13 (provided courtesy of T. H. Flewett, Regional Virus Laboratory, Birmingham, United Kingdom) as previously described (5). PV 1 strain LSc 2ab and the cytopathogenic HM-175 strain of hepatitis A virus (HAV) (provided courtesy of T. Cromeans, Centers for Disease Control and Prevention, Atlanta, Ga.) were propagated and assayed in BGM and FRhK-4 cells, respectively, as previously described (18).
The preparations of viruses used in these studies were deliberately not purified in order to be as close to natural as possible, as recommended by us (1) and other authors (13).
AsV RT-PCR.
Primers A1 (5′-CCTGCCCCGAGAACAACCAAGC-3′) and A2 (5′-GTAAGATTCCCAGATTGGTGC-3′) (25) were used for the development of a reverse transcription-PCR (RT-PCR) procedure for AsV detection (2, 17), which amplifies a fragment corresponding to nucleotides 2363 to 2599 of AsV serotype 2 (L13745). Briefly, primer A2 was used for RT, and both primers (A1 and A2) were used for PCR amplification. Ten microliters of sample was heated to 99°C for 5 min and immediately placed on ice. Salts, nucleotides, primer, and 6 U of reverse transcriptase (Expand Reverse Transcriptase; Roche) were added in 20 μl (final volume) to give working concentrations of 50 mM Tris-HCl (pH 8.3), 40 mM KCl, 5 mM MgCl2, 10 mM dithiothreitol, 0.5 mM Tween 20, 0.2 mM each deoxynucleoside triphosphate, and 1 μM primer. The samples were incubated for 60 min at 42°C for the RT reaction. Ten microliters of the RT product was added to a final volume of 50 μl of PCR mix containing 5 μl of Expand HF buffer (Roche), 2 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate, 0.5 μM each primer, and 0.525 U of the Expand High Fidelity PCR System enzyme mix (Roche). After a denaturation step of 3 min at 95°C, 40 cycles of amplification at 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s were performed, followed by a final extension for 7 min at 72°C. Ten-microliter portions of the PCR products were analyzed by electrophoresis on 1.5% agarose gels.
Experimental design for survival studies.
Viruses were applied onto fomites suspended in phosphate-buffered saline (PBS) or in a 20% fecal suspension. Feces, obtained from a healthy adult individual, were previously mixed with PBS, autoclaved, vortexed, and clarified by centrifugation at 1000 × g. Toilet china (Roca, Gavà, Spain) and cellulose filter paper (no. 1503; Albet, Barcelona, Spain) were selected as models of nonporous and porous materials, respectively. China was thoroughly washed with tap water and disinfected with 70% ethanol prior to use. Paper was employed without any pretreatment. The temperatures were 4 and 20°C, both with a high relative humidity of 90% ± 5%. The high level of relative humidity was achieved and maintained by placing ultrawet paper inside a closed chamber at 4 or 20°C. The air temperature and relative humidity level were monitored four times a week.
Roughly square pieces of paper (1 cm2) and china (3 cm2) were placed in tissue culture plates, inoculated with 20 and 50 μl, respectively, of each viral suspension, and allowed to dry (3 to 5 h in a flow cabinet at room temperature and at a flow pressure of 20 mm). Virus inocula ranged from 1 × 105 to 5 × 105 infectious units (see below). The plates were covered with aluminum foil and placed at the desired temperature and relative humidity level. At designated times, a piece of each surface inoculated with the different virus suspensions at the different ambient conditions was sampled. Viruses adsorbed to the fomites were eluted with 980 μl (paper) or 950 μl (china) of a solution of 3% beef extract in saline at pH 7.5, which after a 10-min contact time was vigorously pipetted 20 times to recover the sample. To evaluate the reduction in virus titer caused by the desiccation process, 20 μl (paper) or 50 μl (china) of each initial viral suspension was diluted with 980 or 950 μl, respectively, of 3% beef extract in saline and used as controls. All samples were stored at −75°C until assayed.
Survival of enteric viruses on fomites was determined by calculating the log10 (Nt/N0), where N0 is the initial virus titer eluted immediately after desiccation and Nt is the titer at the designated time interval. Enumeration of HAV, PV, RV, and AdV was performed by calculating the most probable number of cytopathogenic units per milliliter by infecting appropriate cell monolayers grown in 96-well microtiter plates (18). Eight wells were infected for each dilution, and 10 μl of inoculum was added to each well. Data were processed with a most-probable-number computer program (9). AsV numbers were figured in RT-PCR units (RT-PCRu), according to a previously described procedure based on combined infection of CaCo-2 cultured cell monolayers and RT-PCR (2). Briefly, AsV samples were pretreated with trypsin, and 10-fold dilutions (200 μl) were inoculated onto CaCo-2 cell monolayers grown in 60- by 15-mm dishes. Infected cells were collected at 5 days postinfection and resuspended in 300 μl of PBS. The total RNA from 50 μl was extracted by guanidine thiocyanate lysis, adsorption to silica (SiO2) particles, and elution with an aqueous low-salt buffer, essentially as described by Boom et al. (4). The nucleic acid-silica complexes were washed twice with a guanidine thiocyanate-containing buffer, twice with 70% ethanol, and once with acetone and finally dried. Nucleic acids were subsequently eluted with an aqueous low-salt buffer and employed for the above-described RT-PCR. In the case of AsV, the log10 (Nt/N0) is then figured as the log10 (RT-PCRut/RT-PCRu0), where RT-PCRu0 is the reciprocal endpoint dilution detectable by cell culture-RT-PCR at time zero and RT-PCRut is the reciprocal endpoint dilution detectable by cell culture-RT-PCR at the indicated time interval.
All experiments with HAV, PV, RV, and AdV were performed in duplicate, while AsV experiments were performed in triplicate to minimize the variability inherent in the RT-PCR titration. All samples from a given experiment were assayed at the same time and titrated at least in duplicate. The analysis of variance test (20) was performed with log-transformed data to determine significant differences generated by the type of material, environmental conditions, and virus strain.
RESULTS AND DISCUSSION
The potential of fomites in the vehicular transmission of human AsV was ascertained by employing paper and china, which in previous studies were shown to be good models of porous and nonporous surfaces, respectively, usually found in domestic and health care facilities (1). The study was carried out with viruses suspended in PBS and a 20% fecal suspension in order to mimic actual natural conditions, since the fecal-oral route is the common means of AsV transmission. AsV survival studies were conducted over 90 days and performed at temperatures of 4 and 20°C and a controlled relative humidity of 90% ± 5%. These conditions are commonly reported in January and September-October, respectively, in some areas of Catalonia, Spain. Astrovirus infection in Barcelona, Spain, shows a seasonal distribution, with the highest incidence in winter. Astrovirus cases start in October and reach a peak incidence in January (S. Guix et al., submitted for publication).
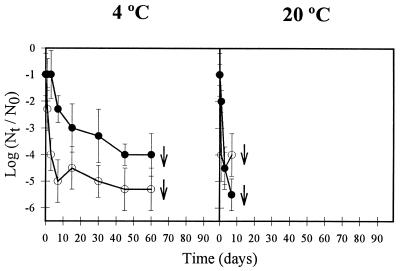
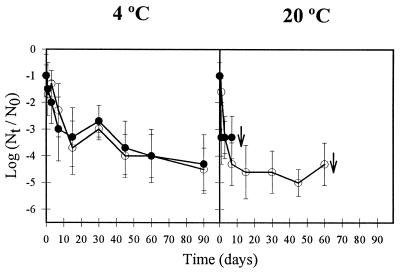
AsV exhibited considerable persistence when dried on porous and nonporous materials (Fig. 1 and 2). AsV showed a greatly enhanced survival on both types of fomites at low temperature (P < 0.05), suggesting that temperature, among other environmental factors, may be meaningful in the seasonal distribution of AsV outbreaks (24). At 4°C, AsV was able to persist for 60 days desiccated on china, showing 4- and 5.3-log-unit titer reductions in the presence and absence of fecal material, respectively (Fig. 1). When dried on paper at the same temperature, residual AsV infectivity was detected after 90 days, with reductions in titer of 4.3 to 4.5 log units (Fig. 2). Comparatively, a much faster decay was observed at 20°C, with AsV infectivity being detected only during the first 7 days after desiccation, except when AsV was applied to paper suspended in PBS, with residual infectivity titers detected for 60 days (Fig. 2).
FIG. 1.
Reduction of the infectivity of human AsV, expressed as log10 (RT-PCRut/RT-PCRu0), during 90 days dried on china at 4 and 20°C at a relative humidity of 90% ± 5% (open circles, PBS suspension; solid circles, 20% fecal suspension). Error bars indicate standard deviations. Arrows indicate the limit of detection.
FIG. 2.
Reduction of the infectivity of human AsV, expressed as log10 (RT-PCRut/RT-PCRu0), during 90 days dried on paper at 4 and 20°C at a relative humidity of 90% ± 5% (open circles, PBS suspension; solid circles, 20% fecal suspension). Error bars indicate standard deviations. Arrows indicate the limit of detection.
Fecal material induced different effects on AsV persistence on inanimate surfaces, depending on the type of fomites and temperature. AsV survival was not significantly affected (P < 0.05) by the presence of feces at 20°C when dried on china (Fig. 1) or at 4°C when dried on paper (Fig. 2). On the other hand, AsV survived significantly better (P < 0.05) at 4°C when applied on china as a 20% fecal suspension (Fig. 1), while fecal matter negatively influenced (P < 0.05) AsV survival at 20°C on paper (Fig. 2). In previous work (1), fecal material was shown to also induce a paradoxical effect on the persistence of other enteric viruses on fomites. However, in long-term survival studies of PV and AdV dried on fomites, these viruses exhibited similar behavior with regard to the presence of feces as observed in this work for AsV.
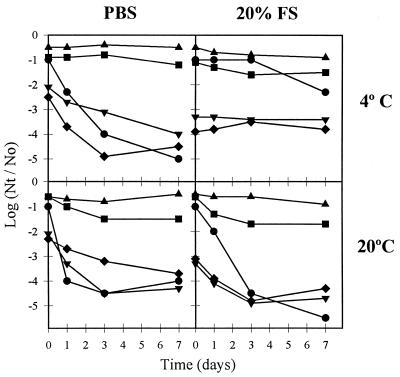
The comparative study of human enteric virus survival on surfaces was conducted for only 1 week, since in an actual case of fomites contamination by viruses, the likelihood of institutional outbreaks should depend on the capacity of the viruses to persist for the first few days after desiccation. Different patterns of behavior could be observed depending on the kind of virus and the type of material and temperature under study (Fig. 3 and 4). Overall, AsV persisted significantly longer than AdV and PV, although AsV is more readily inactivated (P < 0.05) than RV and HAV, which could be recovered from all types of surfaces, at both 4 and 20°C. It should be noted that AsV persisted extremely well dried on paper, in the presence of fecal material, at both 4 and 20°C (Fig. 4), which could correspond to actual spread of AsV infection through toilet tissue. AsV and AdV persisted longer at 4°C than at 20°C, while RV did not show increased survival at low temperature. These results suggest that temperature may be critical in the seasonal distribution of AsV outbreaks, while other factors must determine the seasonality of RV infections.
FIG. 3.
Comparative persistence of human enteric viruses suspended in PBS or in a 20% fecal suspension (FS) on china during the first 7 days after desiccation at 4 and 20°C (circles, AsV; triangles, HAV; inverted triangles, PV; squares, RV; diamonds, AdV). For the sake of clarity, error bars, which ranged from 0 to 1.2 log units, are not depicted.
FIG. 4.
Comparative persistence of human enteric viruses suspended in PBS or in a 20% fecal suspension (FS) on paper during the first 7 days after desiccation at 4 and 20°C (circles, AsV; triangles, HAV; inverted triangles, PV; squares, RV; diamonds, AdV). For the sake of clarity, error bars, which ranged from 0 to 1.2 log units, are not depicted.
We previously demonstrated (1) that the resistance to the desiccation step appears to be of major significance in determining the ability of a virus strain to persist dried on fomites. In the present work, AsV showed a high level of persistence on both china and paper, losing an average of only 1 log unit of its initial infectivity in either the presence or absence of fecal material. The actual AsV decay rates on china and paper were 1.0 ± 0.9 and 1.0 ± 0.3 log units in the absence and presence of fecal material, respectively. In the case of paper, decay rates were, respectively, 1.0 ± 0.6 and 1.0± 0.3 log units. AsV resistance to desiccation on porous and nonporous materials is on the same level as that exhibited by HAV and RV, which show maximum decay rates due to the desiccation process of 1.5 and 1.3 log units, respectively. In contrast, AdV and PV exhibited significantly higher rates of inactivation, ranging, depending of the assay conditions, from 2.4 to 3.7 and from 1.5 to 3.3 log units, respectively (1). This pronounced decrease in titer at this stage dramatically reduces the chance of subsequent survival of AdV and PV, both of which are frequently employed as models of enteric viruses in environmental studies (8, 16).
The procedure employed to elute viruses from fomites has been used in similar previous studies (1). It cannot be ruled out that the elution efficiency may differ from one virus to another. However, this may only influence the calculations of the inactivation due to the desiccation step. Moreover, in situations of actual virus contamination of inanimate surfaces, the ability of a virus to be eluted from this material will in some way determine the likelihood of its transmission through fomites. In any case, it is technically impossible to calculate the actual efficiency of recovery of desiccated viruses from the fomites.
Some viral infections transmitted through the fecal-oral route are spread by means of continual low-level transmission through the environment. The actual relative contribution of AsV to the total incidence of virus-associated diarrhea ranges between 5 and 10% (24; S. Guix et al., submitted for publication). Data on the occurrence and persistence of AsV in the environment are scarce. In a previous study (17), we detected AsV in water from an area where a concurrent gastroenteritis outbreak had been reported. Later (2), we verified that the survival of AsV in dechlorinated tap water was comparable to that of human RV and enteric AdV. In that work, a temperature effect was also observed, with the AsV decay being more pronounced at high temperature. In a recent study on the presence of AsV in Barcelona raw sewage, we observed that AsV prevalence in the environment is higher in winter months, although occasional summer peaks could also be observed (19). These data were in agreement with the clinical epidemiology of AsV in the same area (Guix et al., submitted for publication). AsV behavior in the presence of free chlorine is somewhat similar to that of AdV, being more readily inactivated than RV or HAV and far more resistant to chlorination than PV (2).
In the present work, we verified that AsV is able to survive on inert surfaces long enough to suggest that fomites may also act as vehicles for secondary transmission of AsV diarrhea. AsV is able to survive on inert surfaces long enough to represent a health threat. Control measures for outbreaks of viral gastroenteritis and hepatitis in high-risk settings such as hospital wards, day care centers, or restaurants should focus on the interruption of virus transmission through the adoption of adequate hygienic practices, such as proper sanitation and efficient disinfection.
ACKNOWLEDGMENTS
F. X. Abad is the recipient of a PQS contract from the Generalitat de Catalunya. This work was supported in part by grant 1997SGR 00224 from the Generalitat de Catalunya.
REFERENCES
- 1.Abad F X, Pintó R M, Bosch A. Survival of enteric viruses on environmental fomites. Appl Environ Microbiol. 1994;60:3704–3710. doi: 10.1128/aem.60.10.3704-3710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abad F X, Pintó R M, Villena C, Gajardo R, Bosch A. Astrovirus survival in drinking water. Appl Environ Microbiol. 1997;63:3119–3122. doi: 10.1128/aem.63.8.3119-3122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appleton H, Higgins P G. Viruses and gastroenteritis in infants. Lancet. 1975;i:1297. doi: 10.1016/s0140-6736(75)92581-7. [DOI] [PubMed] [Google Scholar]
- 4.Boom R, Sol C J, Salimans M M, Jansen C L, Wertheim-van Dillen P M, Van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosch A, Lucena F, Diez J M, Gajardo R, Blasi M, Jofre J. Waterborne viruses associated with an hepatitis A outbreak. J Am Water Works Assoc. 1991;83:80–83. [Google Scholar]
- 6.Butz A M, Fosarelli P, Dick J, Cusack T, Yolken R. Prevalence of rotavirus on high-risk fomites in day-care facilities. Pediatrics. 1993;92:202–205. [PubMed] [Google Scholar]
- 7.Eggleston S I, Caul E O, Vipond I B, Darville J M. Absence of human astrovirus RNA in sewage and environmental samples. J Appl Microbiol. 1999;86:709–714. doi: 10.1046/j.1365-2672.1999.00717.x. [DOI] [PubMed] [Google Scholar]
- 8.Gerba C P. Applied and theoretical aspects of virus adsorption to surfaces. Adv Appl Microbiol. 1984;30:133–168. doi: 10.1016/s0065-2164(08)70054-6. [DOI] [PubMed] [Google Scholar]
- 9.Hurley M A, Roscoe M E. Automated statistical analysis of microbial enumeration by dilution series. J Appl Bacteriol. 1983;55:159–164. [Google Scholar]
- 10.Kotloff K L, Losonsky G A, Morris J G, Wasserman S S, Singh-Naz N, Levine M M. Enteric adenovirus infection and childhood diarrhea: an epidemiologic study in three clinical settings. Pediatrics. 1998;84:219–225. [PubMed] [Google Scholar]
- 11.Kurtz J B, Lee T W. Astroviruses: human and animal. In: Bock G, Whelan J, editors. Novel diarrhoea viruses. New York, N.Y: John Wiley and Sons; 1987. pp. 92–107. [Google Scholar]
- 12.Lewis D C, Lightfoot N F, Cubitt W D, Wilson S A. Outbreaks of astrovirus type 1 and rotavirus gastroenteritis in a geriatric inpatient population. J Hosp Infect. 1989;14:9–14. doi: 10.1016/0195-6701(89)90128-x. [DOI] [PubMed] [Google Scholar]
- 13.Mbithi J N, Springthorpe V S, Sattar S A. Chemical disinfection of hepatitis A virus on environmental surfaces. Appl Environ Microbiol. 1990;56:3601–3604. doi: 10.1128/aem.56.11.3601-3604.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNulty M S. Rotaviruses. J Gen Virol. 1978;40:1–18. doi: 10.1099/0022-1317-40-1-1. [DOI] [PubMed] [Google Scholar]
- 15.Parashar U D, Bresee J S, Gentsch J R, Glass R I. Rotavirus. Emerg Infect Dis. 1998;4:561–570. doi: 10.3201/eid0404.980406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pina S, Puig M, Lucena F, Jofre J, Girones R. Viral pollution in the environment and in shellfish: human adenovirus detection by PCR as an index of human viruses. Appl Environ Microbiol. 1998;64:3376–3382. doi: 10.1128/aem.64.9.3376-3382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pintó R M, Abad F X, Gajardo R, Bosch A. Detection of infectious astroviruses in water. Appl Environ Microbiol. 1996;62:1811–1813. doi: 10.1128/aem.62.5.1811-1813.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pintó R M, Diez J M, Bosch A. Use of the colonic carcinoma cell line CaCo-2 for in vivo amplification and detection of enteric viruses. J Med Virol. 1994;44:310–315. doi: 10.1002/jmv.1890440317. [DOI] [PubMed] [Google Scholar]
- 19.Pintó R M, Villena C, Le Guyader F S, Guix S, Caballero S, Pommepuy M, Bosch A. Astrovirus detection in wastewater samples. Water Sci Technol. 2001;12:73–76. [PubMed] [Google Scholar]
- 20.Sokal R R, Rohlf E J. Introduction to biostatistics. W. H. San Francisco, Calif: Freeman; 1973. [Google Scholar]
- 21.Uhnoo I, Wadell G, Svensson L, Johansson M E. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol. 1984;20:365–372. doi: 10.1128/jcm.20.3.365-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Regenmortel M H V, Fauquet C M, Bishop D H L, Carstens E B, Estes M K, Lemon S M, Maniloff J, Mayo M A, McGeoch D J, Pringle C R, Wickner R B, editors. Virus taxonomy: the classification and nomenclature of viruses. The seventh report of the International Committee on Taxonomy of Viruses. San Diego, Calif: Academic Press; 2000. [Google Scholar]
- 23.Walsh J A, Warren K S. Selective primary health care: an interim strategy for disease control in developing countries. N Engl J Med. 1979;302:967–974. doi: 10.1056/NEJM197911013011804. [DOI] [PubMed] [Google Scholar]
- 24.Walter J E, Mitchell D K. Role of astroviruses in childhood diarrhea. Curr Opin Pediatr. 2000;12:275–279. doi: 10.1097/00008480-200006000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Willcocks M M, Ashton N, Kurtz J B, Cubitt W D, Carter M J. Cell culture adaptation of astrovirus involves a deletion. J Virol. 1994;68:6057–6058. doi: 10.1128/jvi.68.9.6057-6058.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]