Abstract
Background
Hematocrit (HCT) determination is an integral part of health and disease assessments in captive and wild white rhinoceroses. Several affordable automated hematology analyzers have been developed for in‐clinic and field use and have the advantage of being able to measure a large number of additional measurands. However, the accuracy of these analyzers for rhinoceros HCT measurements has not yet been investigated.
Objectives
We aimed to compare the HCT results generated by the EPOC portable analyzer system and the Abaxis VetScan HM5 with the gold standard of a manual packed cell volume (PCV) measured using the microhematocrit method.
Methods
Hematocrits were measured with the EPOC and the Abaxis VetScan HM5 (bovine setting) and compared with the PCVs of 69 white rhinoceros whole blood samples. Results were compared using Bland–Altman difference plots and Passing‐Bablok regression analysis. A total allowable analytical error of 10% was set as the performance goal.
Results
A significant positive bias, with a mean of 7.7% for the EPOC and 17.9% for the Abaxis, was found compared with the manual PCV method.
Conclusions
The allowable error goal of 10% was not exceeded with the EPOC analyzer. Although not analytically equivalent to the gold standard, the EPOC results could therefore be used as approximations in critical situations where manual measurements cannot be performed. The Abaxis exceeded this allowable error and overestimated HCTs in rhinoceroses. Therefore, method‐specific reference intervals should be used.
Keywords: Abaxis HM5, EPOC, method comparison, packed cell volume, point‐of‐care
1. INTRODUCTION
The southern white rhinoceros (Ceratotherium simum simum) is listed as “near threatened” by the International Union for Conservation of Nature (IUCN) Red List of Threatened Species. 1 Various conservation programs have been implemented to ensure the long‐term survival of viable and valued rhinoceroses in wild and zoo populations. 1 Determining the health status of rhinoceroses plays a central role in these conservation programs. 2 The packed cell volume (PCV) and hematocrit (HCT) are essential measurements in the health assessment of an individual. The packed cell volume is a directly measured value obtained from centrifuging blood in a microhematocrit tube and is considered the gold standard. 3 The HCT is a calculated value obtained from modern automated hematology analyzers that are widely distributed in veterinary practices and have the advantage of testing a larger number of measurands.
The EPOC portable analyzer system (Siemens Healthcare [PTY] Ltd, Midrand, South Africa) uses conductivity to measure HCTs, whereas the Abaxis VetScan HM5 (Abaxis Global Diagnostics, Griesheim, Germany) uses impedance technology to calculate the HCT from the RBC and the mean corpuscular volume (MCV). 4 , 5 Therefore, if there are any inaccuracies in the measurement of RBCs or MCVs, the HCT will reflect these.
Although automated HCT measurements are routinely performed during rhinoceros health checks in the field and at zoos, the accuracy of these HCT results has not yet been evaluated.
Accordingly, the objective of this study was to assess the accuracy of the two automated systems, the EPOC and Abaxis analyzers, for measuring HCTs in white rhinoceroses compared with the gold standard manual PCV method.
2. MATERIALS AND METHODS
Serial blood samples collected from 23 sub‐adult wild white rhinoceros bulls captured and transported within the Kruger National Park for reasons unrelated to this study were used (three samples per rhinoceros). Details concerning these rhinoceroses, as well as sample collection and handling, have been reported by Pohlin et al. 6 Ethical approval for this study was granted by the University of Pretoria Animal Ethics Committee (V067‐17) and the South African National Parks Animal Use and Care Committee (009/17).
Blood collected into lithium‐heparinized tubes (BD Vacutainer; Becton and Dickinson, Oxford, UK) was analyzed immediately using the EPOC Portable analyzer system (n = 68). Before measuring patient samples, an internal quality control check is included with each separate single‐use test cartridge, whereby calibration fluid included in the cartridge is used to test the sensors within the cartridge. Blood collected into EDTA tubes (BD Vacutainer) was stored in a cooler box that contained ice packs. After transport, samples were brought to room temperature and analyzed (within 6 h of collection) with the Abaxis VetScan HM5 hematology analyzer (n = 69). Tubes were inverted 10 times immediately prior to analysis. One level of commercial quality control material was run daily on the Abaxis before sample analysis, and results were within the manufacturer’s target range. Packed cell volumes were determined manually (n = 69), immediately after transport (within 6 h of collection), using a microhematocrit centrifuge (Hettich Mikrohematokrit 210, Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany). According to the American Society for Veterinary Clinical Pathology (ASVCP) guidelines, an EDTA blood sample was drawn into two microcapillary tubes filled to approximately three‐quarters of the tube length, and the tubes were centrifuged at 16 060g for 5 min. Packed cell volumes were measured using a microhematocrit reader (Hawksley, Lancing, Sussex, United Kingdom), and an average of the two measured PCVs was taken as the final PCV. The calculated HCT of the Abaxis was immediately compared with the manual PCV as a reference, using different species settings, and if there was a discrepancy larger than 0.05 L/L, the analysis was repeated on a different setting. Ultimately, the Abaxis “cattle” setting was used as it demonstrated the best and most consistent match between calculated HCTs and manual PCVs.
Statistical analysis was performed using MedCalc Statistical Software version 19.5.3. 7
Data obtained by the manual PCV and the EPOC and Abaxis analyzers were summarized and expressed as a multiple comparison plot (median plus data range) for descriptive purposes. Histograms and Q‐Q plots were used to assure normal distributions, followed by an ANOVA analysis to detect differences in hematocrit levels within method‐groups over time, as well as between the methods. Scheffé’s and Tukey’s post hoc tests were performed to determine significant differences.
Linearity was assessed visually and by performing a cumulative sum (CUSUM) test for each automated system (EPOC, Abaxis) compared with the manual PCV. Correlation between each automated method and the manual PCV was tested using Spearman’s coefficient of rank correlation (ρ). Subsequently, Passing‐Bablok regression analysis was performed. Statistically significant proportional error was present if the 95% confidence intervals of the slope from the regression equation did not include 1.0; significant constant bias was present if the 95% confidence intervals for the y‐intercept did not include 0.0. 8
Bland–Altman absolute mean‐difference plots for repeated measures with changing quantities were created. Plots included limits of total allowable error for HCT, which were calculated as 10% (as per the ASVCP guidelines) of the mean manual PCV, that is, 0.039 L/L. 9
The agreement between methods based on their combined inherent imprecision (CIP) was also assessed. 8 The coefficient of variation (CV, analytical imprecision) was calculated for the Abaxis (CVA) from results of internal quality control and was 5%. A CV of 4.3% was used for the EPOC (CVE) 10 and 4.0% for the manual PCV method (CVMM). 11 Limits of agreement derived from these CVs were calculated in MS Excel using the following formulae 8 , 12 :
The mean difference (absolute and percentage) for each paired result was calculated (manual PCV vs EPOC; manual PCV vs Abaxis), and upper and lower limits of acceptance were generated for each mean difference using the CIP percentage limits. The number of times a single absolute difference between the manual measurement and an automated method (EPOC, Abaxis) exceeded the calculated CIP limits was counted and expressed as a percentage of the sample size. Analytical equivalence was present if 95% or more of the mean difference values were within the calculated CIP limits of agreement.
The clinically allowable bias between the two methods (manual PCV vs EPOC; manual PCV vs Abaxis) was set at 10%, based on ASVCP total allowable error (TEa) goals for hematology in veterinary species. 9 The number of times a single percentage difference between two methods exceeded 10% was counted and expressed as a percentage of the sample size. Clinical equivalence was present if 95% or more of the percentage difference values were within 10%. 12
3. RESULTS
Sixty‐eight (EPOC) and 69 (PCV, Abaxis) blood samples originating from 23 white rhinoceros bulls collected during translocation were evaluated. A statistical summary and multiple comparison graph for HCT range, median, and mean values for each analytical method showed possible overestimations by the automated systems illustrated in Figure 1 and Table 1.
Figure 1.
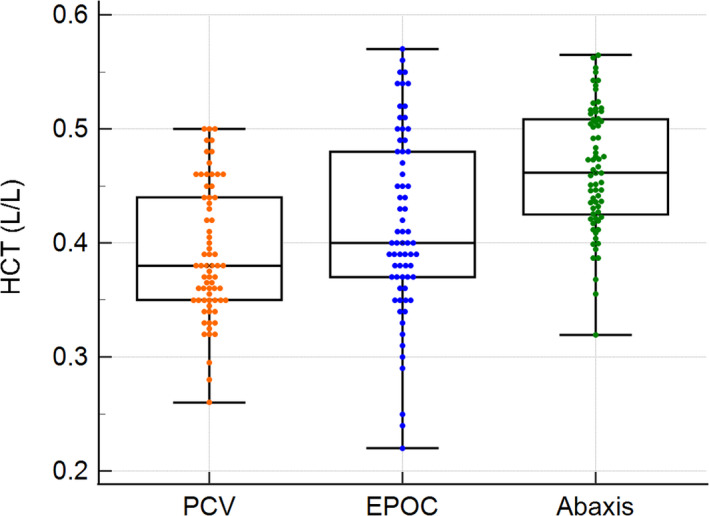
Box‐and‐whisker plot showing the comparison of the median and range for packed cell volumes (PCVs) and hematocrits (HCTs) measured with the EPOC and Abaxis Vetscan HM5 analyzers in white rhinoceros whole blood. The middle line in each box represents the median, the outlines of the box represent the 25th and 75th percentiles, and the whiskers represent the minimum and maximum values. The y‐axis shows the HCT in L/L.
Table 1.
Statistical summary of the hematocrit range, median, and mean in L/L, for white rhinoceros whole blood measured with the manual packed cell volume (PCV) method and two point of care analyzers, the EPOC and Abaxis Vetscan HM5
| Hematocrit in L/L | |||
|---|---|---|---|
| Manual PCV | EPOC | Abaxis | |
| Mean | 0.39 | 0.42 | 0.46 |
| Median | 0.38 | 0.40 | 0.46 |
| Minimum | 0.26 | 0.22 | 0.32 |
| Maximum | 0.50 | 0.57 | 0.57 |
The ANOVA showed significant differences between the three methods (F = 23.13, P < 0.001). Tukey tests for pairwise comparisons between the groups demonstrated that the Abaxis HM5 measurements were higher compared with the manual PCV (P < 0.01) and the EPOC (P < 0.01), thereby proving the suspected overestimation by the Abaxis. The EPOC system showed no significant difference from the manual measurements (P = 0.062). CUSUM tests for the EPOC and Abaxis analyzers showed no significant deviations from linearity with P‐values of 0.29 and 0.96, respectively. The automated measurements of both systems showed strong positive correlations with the manual PCV (ρ = 0.84 with 95% CI = 0.75 to 0.90, P < 0.001 for the EPOC; ρ = 0.90 with 95% CI = 0.845 to 0.94, P < 0.001 for the Abaxis).
The Passing–Bablok regression analysis performed for each automated system revealed the following regression equations:
Analysis of the regression lines indicated the presence of a significant constant bias of ‐0.15 L/L for the EPOC vs the PCV method and 0.08 L/L for the Abaxis vs the PCV method, as seen in Figure 2A and B, respectively. A proportional bias of 1.45 was present for the EPOC method.
Figure 2.
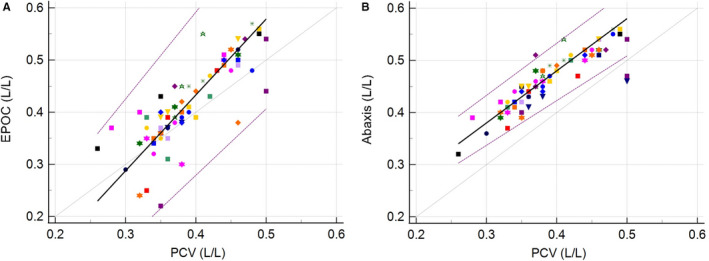
Passing‐Bablok regressions in L/L for the EPOC (A: left) and the Abaxis Vetscan HM5 (B: right) analyzers, including the 95% limits of agreement (purple). The gray lines depict the identity lines (x = y). The black lines represent the regression lines. The 23 individuals are represented using different symbols as data points
The Bland–Altman difference plot comparing the EPOC to the manual PCV method showed a mean difference of 0.03 L/L (7.7%) between the two methods, within the 0.039 L/L (10%) TEa limits depicted in Figure 3A. Acceptance limits based on the CIP were determined to be ±11.5%, and 60.3% of the differences between the two methods fell into these limits, while 54.4% were within TEa‐limits.
Figure 3.
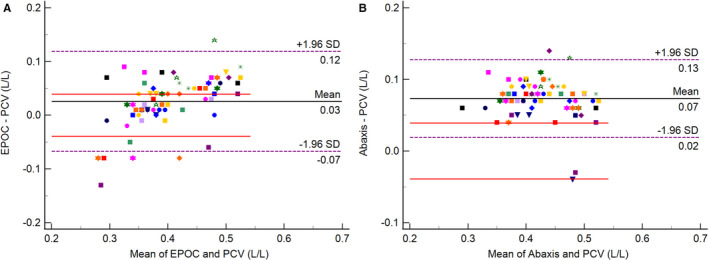
Bland–Altman plots depicting mean differences between the EPOC analyzer and manual packed cell volumes (PCVs; A: left) and between Abaxis Vetscan HM5 analyzer and manual PCVs (B: right). The black line represents the mean percentage difference. The dashed purple lines on either side indicate the limits of agreement (±1.96 standard deviation). The solid red lines represent the 10% total allowable error (TEa) limits. The 23 individuals are represented using different symbols as data points.
The Bland–Altman difference plot comparing the Abaxis to the manual PCV method showed the mean difference and a large amount of the scattergram outside the 0.039 L/L (10%) TEa limits, as illustrated in Figure 3B. There was also a mean positive bias of 0.07 L/L (17.9%). Acceptance limits based on the CIP were determined to be ±12.6%, and only 26.1% of the differences between the two methods fell within these limits, while only 11.6% were within the TEa limits.
4. DISCUSSION
Both the Abaxis and EPOC HCT methods showed significant analytical and clinical bias compared with the PCV method for white rhinoceros blood. The mean positive bias of 7.7% for the EPOC was at least within the TEa goal of 10%, while the mean bias of 17.9% for the Abaxis was not.
Results reported for HCT generated by the EPOC generally showed higher values and a wider range when compared with manual PCV measurements. These differences were probably attributable to atypical plasma osmolarity and protein concentrations, different inner body temperatures during the different phases of the translocation, 6 and rhinoceros erythrocyte characteristics (eg, erythrocyte diameter) that have altered conductivity through the sample. Notably, a positive proportional bias was present. The highest PCV results in our study were from samples collected at the time of capture, caused by stress hemoconcentration. Albumin was higher at capture than other time points. 6 As EPOC HCT is determined from the non‐conducting volume of the sample, which consists of all blood cells, lipids, and proteins, increased albumin concentrations could be the cause for the greater bias seen with higher PCV results using the EPOC method. Despite these significant differences between the two methods (EPOC and PCV), the overall agreement between results was evident in the Bland–Altman plot as the TEa of 10% was not exceeded by the mean difference.
Just over half of individual results fell within the 10% TEa range, which is not optimal, but we suggest that the HCT results of the EPOC could be used as approximations in critical situations in field laboratories where manual measurements cannot be performed.
The bias for the Abaxis method was unacceptable compared with the manual PCV with all the statistical analyses we performed, and the mean bias of 17.9% was clinically significant, as it was higher than the TEa of 10%. Similarly, Becker et al (2008) observed an extremely high bias for HCT on the Abaxis VetScan with blood samples from dogs and cats, which was associated with a strong positive bias for MCV due to constant systematic error. 13 The choice of the cattle setting probably introduced a similar error here. Based on these results, a prospective study aimed at validating the most appropriate species‐setting on this analyzer for white rhinoceros blood and the generation of analyzer‐specific reference intervals is needed. The results also show that the Abaxis HCT and PCV cannot be used interchangeably when monitoring trends within or between individual rhinoceroses.
The main limitation of this study was the lack of rhinoceros‐specific analytical CVs for each method. This data is not available. The value used for PCV was taken from a study using canine samples, 11 whereas the EPOC value was taken from a study on horses, 10 and the CV for the Abaxis was calculated from internal quality control measurements. Since horses are the closest domestic relatives to rhinoceroses, 14 values from equine hematology studies were preferred. Another limitation was that EPOC measurements were performed immediately after sample collection from heparinized blood, whereas the manual PCV and Abaxis measurements were conducted later the same day from EDTA‐anticoagulated specimens.
However, a delay of up to 24 hours is typical in clinical settings, 13 and previous studies have shown that valid reproducible results can be obtained within this time. 15 We, therefore, only expect minimal alterations due to the differences in storage time. It should also be noted that the PCV results presented here are using a centrifugation of 5 minutes at 16 060 g, and other settings may result in slightly different PCV values.
A limitation of our statistical analysis is that not all measurements are independent because three samples were taken for each of the 23 rhinoceros at different time points. There exists very little guidance in the veterinary or medical literature as to the statistical approach to method comparison for clustered observations. We addressed this by performing repeated measures Bland–Altman analysis, and graphically represented the data points from the 23 individual rhinoceros in the Bland–Altman and regression plots. 16
The sample size poses limitations as well, as the range of data is not wide for either measuring technique. The low range ratios (1.92 for PCV, 2.59 for the EPOC, and 1.78 for the Abaxis) implicate that larger numbers of tested individuals are needed. The samples used in this study do not cover the working range of the methods. 8
Currently, automated point‐of‐care hematology analyzers play an important role in the clinical work of wildlife veterinarians. Before adopting a method, species‐specific validation, or at least verification, should be performed. Method‐specific reference intervals should be established and used to avoid misinterpretation. Wildlife veterinarians should be aware of the limitations of hematologic methods developed for domestic species. By working together with industry and researchers in the field, it is hoped that methods can be adapted or improved to have similar standards of accuracy as are met for domestic animals. This may be of particular relevance for endangered species and could positively impact the field of conservation medicine.
ACKNOWLEDGMENTS
This work was supported by the International Rhino Foundation and the research fund of the Department of Paraclinical Sciences of the Faculty of Veterinary Science, University of Pretoria, as well as the Zebra Foundation, England (Registered Charity No: 1000452). We acknowledge the invaluable assistance of the Veterinary Wildlife Services: Kruger National Park, South African National Parks staff, and the team of the clinical pathology laboratory of the Onderstepoort Veterinary Academic Hospital.
Steyrer C, Pohlin F, Meyer LC, Buss P & Hooijberg EH. Comparison of three hematocrit measurement methods in the southern white rhinoceros (Ceratotherium simum simum). Vet Clin Pathol. 2022;51:225–230. doi: 10.1111/vcp.13076
REFERENCES
- 1. Emslie R. Ceratotherium simum. The IUCN Red List of Threatened Species 2020: e. T4185A45813880. 10.2305/IUCN.UK.2020-1.RLTS.T4185A45813880.en Accessed December 26, 2020. [DOI] [Google Scholar]
- 2. Metrione L, Eyres A. Rhino Husbandry Manual. International Rhino Foundation; 2014:49‐54. [Google Scholar]
- 3. Gebretsadkan TK, Ambachew G, Birhaneselassie H. The comparison between microhematocrit and automated methods for hematocrit determination. Int J Blood Res Disord. 2015;2:1‐3. [Google Scholar]
- 4. EPOC User Manual: https://cdn0.scrvt.com/39b415fb07de4d9656c7b516d8e2d907/1800000005894023/cd41cca52361/SH‐40‐18‐13374‐01‐76_epoc_WP_US_FINAL_SNG_1800000005894023.pdf Accessed December 26, 2020.
- 5. Abaxis Vetscan HM5 User Manual. Abaxis, Inc. https://www.abaxis.com/sites/default/files/resource‐packages/790‐7013%20Rev%20E%20HM5c%20User%20Manual.pdf Accessed December 26, 2020. [Google Scholar]
- 6. Pohlin F, Hooijberg E, Buss P, et al. A comparison of hematological, immunological and stress responses to capture and transport in wild white rhinoceros bulls (Ceratotherium simum simum) supplemented with azaperone or midazolam. Front Vet Sci. 2020;7. doi: 10.3389/fvets.2020.569576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MedCalc Statistical Software Version 19.2. 6 (MedCalc Software Bv, ; https://www.medcalc.org; 2020)
- 8. Jensen AL, Kjelgaard‐Hansen M. Method comparison in the clinical laboratory. Vet Clin Pathol. 2006;35:276‐286. [DOI] [PubMed] [Google Scholar]
- 9. Nabity MB, Harr KE, Camus MS, Flatland B, Vap LM. ASVCP guidelines: allowable total error hematology. Vet Clin Pathol. 2018;47:9‐21. [DOI] [PubMed] [Google Scholar]
- 10. Bardell D, West E, Senior JM. Evaluation of a new handheld point‐of‐care blood gas analyser using 100 equine blood samples. Vet Anaesth Analg. 2017;44:77‐85. [DOI] [PubMed] [Google Scholar]
- 11. Breheny CR, Salgado JPA, Bommer NX, Handel I, Gow AG. Standard operating procedure reduces interoperator variation and improves accuracy when measuring packed cell volume. Vet Rec. 2019;184:283‐283. [DOI] [PubMed] [Google Scholar]
- 12. Hooijberg EH, Cray C, Miller M, Buss P, Steenkamp G, Goddard A. Bias between two methods of albumin measurement in the white rhinoceros. Ceratotherium simum Vet Clin Pathol. 2020;49:91‐94. [DOI] [PubMed] [Google Scholar]
- 13. Becker M, Moritz A, Giger U. Comparative clinical study of canine and feline total blood cell count results with seven in‐clinic and two commercial laboratory hematology analyzers. Vet Clin Pathol. 2008;37:373‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Price SA, Bininda‐Emonds OR. A comprehensive phylogeny of extant horses, rhinos and tapirs (Perissodactyla) through data combination. Zoosyst Evol. 2009;85:277‐292. [Google Scholar]
- 15. Kirmizigul AH, Gokce E, Sozmen M. Effects of storage duration and temperature on the values of haematological parameters in bovine and ovine blood samples. J Hell Vet Med Soc. 2017;68:145‐154. [Google Scholar]
- 16. Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17(4):571‐582. [DOI] [PubMed] [Google Scholar]


