Abstract
We have used molecular biological methods to study the distribution of microbial small-subunit rRNAs (SSU rRNAs), in relation to chemical profiles, in offshore Lake Michigan sediments. The sampling site is at a depth of 100 m, with temperatures of 2 to 4°C year-round. RNA extracted from sediment was probed with radiolabeled oligonucleotides targeting bacterial, archaeal, and eukaryotic SSU rRNAs, as well as with a universal probe. The coverage of these probes in relation to the present sequence database is discussed. Because ribosome production is growth rate regulated, rRNA concentrations are an indicator of the microbial populations active in situ. Over a 1-year period, changes in sedimentary SSU rRNA concentrations followed seasonal changes in surface water temperature and SSU rRNA concentration. Sedimentary depth profiles of oxygen, reduced manganese and iron, and sulfate changed relatively little from season to season, but the nitrate concentration was approximately fivefold higher in April and June 1997 than at the other times sampling was done. We propose that sediment microbial SSU rRNA concentrations at our sampling site are influenced by seasonal inputs from the water column, particularly the settling of the spring diatom bloom, and that the timing of this input may be modulated by grazers, such that ammonia becomes available to sediment microbes sooner than fresh organic carbon. Nitrate production from ammonia by autotrophic nitrifying bacteria, combined with low activity of heterotrophic denitrifying bacteria in the absence of readily degradable organic carbon, could account for the cooccurrence of high nitrate and low SSU rRNA concentrations.
Molecular microbiological methods based on nucleic acid (RNA and DNA) extraction can yield information about the in situ distribution and activities of multiple microbial groups simultaneously from a relatively small volume of sample. They are especially useful for sediments, where numerous different species are present and chemical composition and microbial populations can change on a scale of millimeters to centimeters. Molecular methods are also free of the well-known biases associated with traditional culture-based approaches, such as the preferential recovery of species that are well adapted to laboratory conditions, although issues such as extraction efficiency have yet to be fully resolved.
In this study, we have used oligonucleotide probes to characterize small-subunit rRNAs (SSU rRNAs) extracted from sediments at an offshore Lake Michigan site. SSU rRNA is an essential component of the ribosome, the RNA-protein complex responsible for protein synthesis in both prokaryotes and eukaryotes. rRNA-encoding genes (rDNAs) from thousands of species have now been sequenced and used to infer phylogenetic relationships based on nucleotide sequence divergence (67). Because the ribosome content generally increases with growth rate (reviewed in reference 33) and decreases with starvation (see, e.g., references 14, 23, and 46), rRNA quantitation by oligonucleotide probe hybridization can be used to estimate the composition of the actively growing population at different levels of phylogenetic resolution. Growth rate regulation of rRNA content differs among bacterial species (5, 7, 16, 17, 21) and even strains (30), however, so the precise relationship between SSU rRNA concentration and community growth rate will depend on the species present (32).
The activities of sediment microbes help determine how much of the carbon and nitrogen that reaches the bottoms of lakes and other bodies of water is buried and how much is returned to the water column or atmosphere. Deepwater sediments, with a relatively constant dark and cold physical environment, might be expected to have fairly constant and low levels of microbial activity. However, evidence has been accumulating that benthic sediments respond to seasonal changes nearer the water surface (see, e.g., references 11, 49, and 62). We show here that microbial SSU rRNA concentrations at our sampling site follow a seasonal pattern that appears to be closely linked to seasonal changes in the water column. In particular, we present evidence that nitrogen cycling in benthic Lake Michigan sediments may have a strong seasonal component.
MATERIALS AND METHODS
Site description and sample collection.
The Fox Point sampling site is approximately 27 km northeast of Milwaukee, Wis. (latitude 43°11′40"N, longitude 87°40′11"W) at a depth of ≈100 m. The bottom water temperature is between 2 and 4°C year-round. The sedimentation rate has been calculated as 0.24 cm year−1 (19). 137Cs profiles suggest that the Fox Point sediments are mixed to a depth of approximately 2 cm (50). Sediment was collected with a 30-cm2 box corer from the R/V Neeskay. Three-inch-diameter cylindrical subcores were taken for chemical analysis and nucleic acid extraction. The subcores were transported in an ice water bath or refrigerator protected from the light, stored in the dark at 4°C, and processed within 24 to 48 h. Prior to sectioning of a subcore for nucleic acid analysis, a disposable anaerobic glove bag (four-handed model; Sigma Chemical Co., St. Louis, Mo.) was taped over the end of the core liner. The glove bag atmosphere was exchanged three times and replaced with nitrogen, and slight positive pressure was maintained under continuous flow. The core was pushed up out of the liner with a water pressure-driven extruder and sectioned at 0.5-cm or greater intervals. Core slices were transferred to glass screw-cap vials, kept on ice under nitrogen until sectioning was completed, and then transferred to an anaerobic hood for the initial steps of RNA extraction. Water samples for RNA extraction were collected from depths of 0, 2, 5, 10, 15, 20, 25, 30, 60, 90, and ≈100 m with a Niskin bottle, transferred to Cubitainers (Hedwin Corp., Baltimore, Md.), and processed on deck within 1 to 12 h. Measured volumes of water (approximately 1 liter) were filtered through 0.2-μm-pore-size filters (Millipore Corp., Bedford, Mass.) which were placed in 50-ml Falcon tubes and frozen immediately on dry ice. Temperature, chlorophyll fluorescence, and oxygen saturation were measured with a SeaBird instrument package.
Oxygen measurement.
Oxygen was measured in the sediment cores with a Clark-style O2 microelectrode (model 737GC; Diamond General Corp., Ann Arbor, Mich.) positioned with a micromanipulator and coupled to an analog chemical microsensor (Diamond General).
Chemical methods.
Pore water was recovered by squeezing whole subcores into 5-ml plastic syringes (4). The water was subsequently filtered through 0.2-μm Acrodisc HT Tuffyrn syringe filters (Fisher Scientific). Nitrate and nitrite concentrations were determined by flow injection analysis (automated cadmium reduction method) (13). Sulfate was determined by ion chromatography (13). Ammonium was measured using the indolphenol blue method (34). All anion samples were run in duplicate unless the sample volume was too low. Dissolved manganese(II) and iron(II) were determined from pore water by atomic absorption spectroscopy. Squeezer syringes were acidified with approximately 50 μl of 1 N HCl for preservation until chemical analysis according to the manufacturer's instructions (IL Video 12 AAS; Allied Analytical Systems, Andover, Mass.). Methane concentrations were measured by gas chromatography using a flame ionization detector as described by Waples (65).
RNA extraction and membrane hybridization.
For sediment samples, 0.2-g aliquots of sediment were transferred with sterile 1-ml disposable syringes to screw-cap Eppendorf tubes containing low-pH buffer, buffer-equilibrated phenol (pH 5.1), sodium dodecyl sulfate, and 0.5 g of zirconium beads (53), shaken for 1 min with a Mini-Beadbeater (Biospec Products, Bartlesville, Okla.) in an anaerobic hood, and then transported on ice to our laboratory. RNA was isolated by bead beating, phenol-chloroform extraction, and ethanol precipitation as previously described (42, 53). RNA was resuspended in 200 μl of sterile deionized water. For water samples, frozen filters were later crushed with sterile, baked spatulas and transferred to screw-cap Eppendorf tubes and extracted as described for sediment samples. RNA was transferred to nylon membranes in triplicate and probed with radiolabeled oligonucleotides (Table 1) purchased from Operon Technologies Inc., Alameda, Calif. Membranes were prehybridized at 40°C and washed at the temperatures listed. Hybridization was measured with a PhosphorImager (model 400S or Storm; Molecular Dynamics Inc., Sunnyvale, Calif.).
TABLE 1.
Oligonucleotide probesa
| Probe name | Abbreviation | Sequence (5′ → 3′) | Wash temp (°C) | Reference species | Target group | Reference |
|---|---|---|---|---|---|---|
| S-∗-Univ-1390-a-A-18 | Uni 1390 | GAC GGG CGG TGT GTA CAA | 44 | Escherichia coli | All SSU rRNAs | 69 |
| S-D-Bact-0338-a-A-18 | Bact 338 | GCT GCC TCC CGT AGG AGT | 54 | Escherichia coli | Bacteria | 2 |
| S-D-Euca-1379-a-A-16 | Euk 1379 | TAC AAA GGG CAG GGA C | 42 | Saccharomyces cerevisiae | Eukarya | 27 |
| S-D-Arch-0915-a-A-20 | Arc 915 | GTG CTC CCC CGC CAA TTC CT | 56 | Methanosarcina frisius | Archaea | 3 |
Known amounts of RNA extracted from the reference species were used as standards in membrane blot hybridizations.
DNA extraction and amplification and reverse transcription-PCR (RT-PCR).
For the sequences designated LMBA, DNA was extracted from 5-g sediment samples, collected in July and August 1993, by the method of Fuhrman et al. (22) and purified over a Chroma Spin-100 column (Clontech Inc., Palo Alto, Calif.). High-molecular-weight DNA was excised from a 0.6% agarose gel and amplified using a 1605 Air Thermo-Cycler (Idaho Technology, Idaho Falls, Idaho) with the Bacteria-specific primers Bact11F and Bact1492AR (Table 2) according to the manufacturer's instructions. DNA was denatured (94°C for 4 min) and amplified for 30 cycles (92°C for 1 min, 50°C for 1 min, and 72°C for 1 min 30 s), and products were extended (72°C for 5 min) to facilitate cloning. Amplification products were cloned into the TA vector (Invitrogen Corp., San Diego, Calif.). Isolated colonies were streak purified and used to prepare plasmid DNA by an alkaline lysis miniprep procedure (V. Schulz and R. Karls, personal communication). Clones were designated LMBA (for Lake Michigan, bacterial amplification, Alm) followed by a number.
TABLE 2.
PCR primers
| Primer name | Abbreviation | Target group | Sequence (5′ → 3′)a | Reference |
|---|---|---|---|---|
| S-D-Bact-0011-a-S-17 | Bact11F | Bacteria | GTT TGA TCC TGG CTC AG | 31 |
| S-D-Bact-1492-a-A-21 | Bact1492AR | Bacteria | ACG GYT ACC TTG TTA CGA CTT | 31 |
| S-∗-Geo-0825-a-A-17 | GeoA | Geobacter group (delta proteobacteria) | TAC CCG CAA CAC CTA GT | 41 |
Y = C or T.
The sequences designated LMBGA (for Bact11F-Geo825RA) were obtained during an attempt to amplify Geobacter-like SSU rDNA sequences as part of another study. DNA was isolated from sediment collected on 3 August 1995 by lysozyme and freeze-thaw treatments, phenol-chloroform extraction, and ethanol precipitation, essentially by the method of Tsai and Olson (58). DNA was separated from brown humic substances on a 1% agarose gel and purified from agarose by centrifugation in 0.2-μm-pore-size SpinX centrifuge filter units (Costar Inc., Cambridge, Mass.). Oligonucleotide primers were purchased from Operon Technologies, Inc. DNA was amplified with a Hybaid PCR Express (Ashford, Middlesex, United Kingdom) according to the manufacturer's instructions, using the primer pair Bact11F-Geo825RA (Table 2). DNA was denatured (94°C for 30 s) and amplified for 30 cycles (92°C for 15 s, 53.3°C [calculated temperature] for 15 s, and 72°C for 45 s), and products were extended (72°C for 1 min) to facilitate cloning. Amplification products were cloned into the pCR4-TOPO vector (Invitrogen). Isolated colonies were streak purified and used to prepare plasmid DNA. Clones designated LMRTGA were obtained by RT-PCR of sediment-extracted RNA, collected on 3 August 1995, with the Access RT-PCR kit (Promega Inc., Madison, Wis.) using the primer pair Bact11F-Geo825RA. PCR was performed as described for the LMBA clones, and products were cloned in the pCR4-TOPO cloning vector for sequencing.
An automated DNA sequencer (model 4000L; LiCor Corp., Lincoln, Nebr.), a SequiTherm Long-Read Kit (LC) (Epicentre Technologies Inc., Madison, Wis.), and M13Fwd(-29) and M13Reverse IRD41-labeled primers (LiCor) were used for DNA sequencing. Sequence analysis was done using the Arb sequence database analysis package (55).
Nucleotide sequence accession numbers.
The SSU rRNA sequences presented here have been deposited in GenBank under accession numbers AF320917, AF320919 to -23, AF320930, AF320955 and -56, and AF320972 to -75.
RESULTS
Chemical profiles and universal probe hybridization.
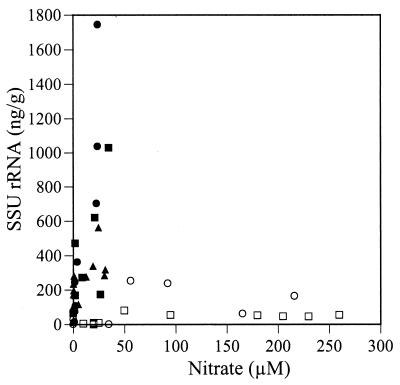
Sediment chemistry profiles of the Fox Point sediment cores generally showed the expected progression attributed to microbial utilization of terminal electron acceptors in order of their energy yield: oxygen depletion, nitrate depletion, Mn(II) accumulation, Fe(II) accumulation, and sulfate depletion (Fig. 1). The concentrations and depth profiles of oxygen, sulfate, Mn(II), Fe(II), and ammonia were relatively constant from sample to sample, where measured. Nitrate concentrations, however, were severalfold higher in April and June 1997. Sediment surface SSU rRNA concentrations also varied, ranging from 53 ng g of sediment−1 in April 1997 to 1,745 ng g of sediment−1 in September 1996. Thus, nitrate concentrations were highest when rRNA concentrations were lowest.
FIG. 1.
Sediment chemistry and universal probe (S-*-Univ-1390-a-A-18) hybridization. Chemical and SSU rRNA concentrations were determined as described in Results. ▪, 2 May 1996; ●, 4 September 1996; ▴, 13 November 1996; □, 2 April 1997; ○, 5 June 1997. Nitrite and oxygen data for April and ammonia data for June are not available.
Considering all samples for which both nitrate and rRNA concentrations were measured, nitrate concentrations of greater than or equal to 50 μM were found only with SSU rRNA concentrations of less than 100 ng g of sediment−1 (Fig. 2). There was a positive correlation between nitrate and SSU rRNA concentrations for the May, September, and November samples (correlation coefficient = 0.607; P < 0.01) (52), which is unsurprising since both of these parameters generally decrease with depth, but there was no significant correlation for the April and June samples or for all samples taken together.
FIG. 2.
Universal probe hybridization and nitrate concentration. ▪, 2 May 1996; ●, 4 September 1996; ▴, 13 November 1996; □, 2 April 1997; ○, 5 June 1997. There is a positive correlation between nitrate concentration and SSU rRNA concentration for the May, September, and November samples (correlation coefficient = 0.607; P < 0.01) (52) but no significant relationship for the April and June samples.
The nitrate distribution in June was unusual, being very low at the sediment surface and peaking at a depth of around 2 cm (Fig. 1). The nitrite concentration was also relatively high compared to that in September and November (nitrite was not measured in April). The SSU rRNA concentration in the 0- to 0.5-cm interval of this June sample was 414 ng g of sediment−1, compared to 53 ng g of sediment−1 in April, while the concentrations at 0.5 to 1 cm were 34 and 55 ng g of sediment−1, respectively. We have some evidence that the June profile may have been caused by some localized event (see Fig. 7; discussed below). One possibility is that a recent nutrient input resulted in increased near-surface microbial respiration and nitrogen demand, reflected in rRNA concentration, which consumed the near-surface nitrate.
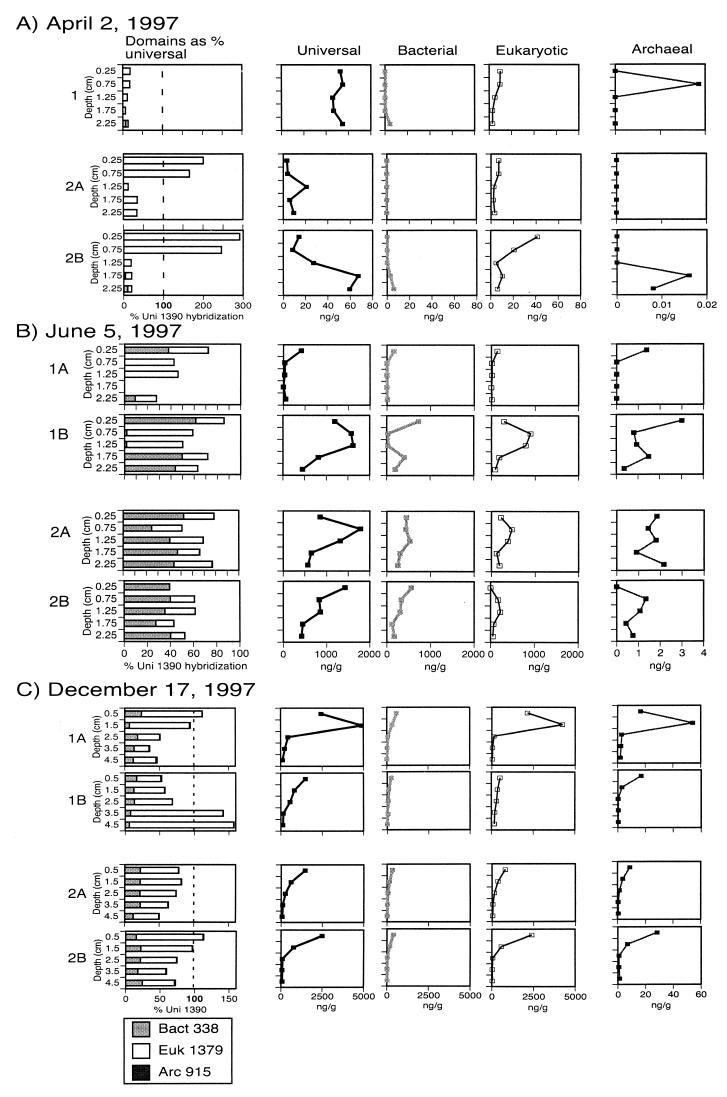
FIG. 7.
Replicate box cores. Two box cores were collected from sites approximately 1 km apart, and cylindrical subcores were taken for RNA extraction from the top 2.5 cm of sediment. Extracted RNA was hybridized with the universal and domain probes. Domain-level results are displayed both as concentrations and as percentages of universal probe hybridization. Because RNA concentrations ranged from picograms to micrograms per gram of sediment, different horizontal-axis scales were used for each sampling time and for the archaeal versus the universal, bacterial, and eukaryotic probes.
Comparison of sediment and water column rRNA concentrations.
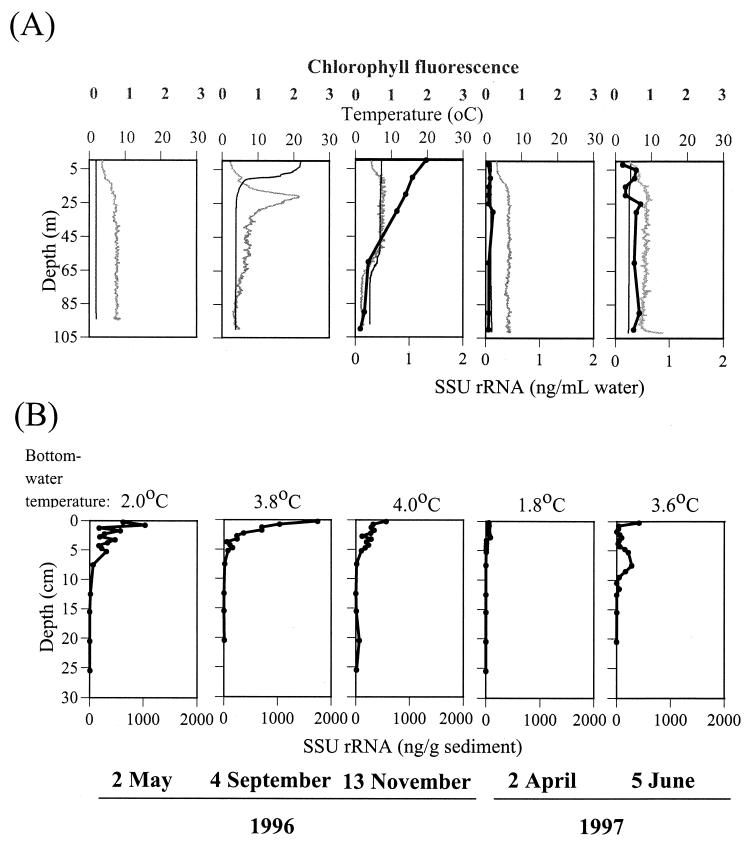
Sedimentary SSU rRNA concentrations appeared to be related to seasonal changes in the overlying water column (Fig. 3). They were highest in the stratified season (September) and lowest in early spring (April). Where water column RNA data are available, seasonal trends are also apparent, with highest concentrations in late fall (November) and lowest concentrations in early spring (April). Water column SSU rRNA concentrations for July and October 1997 were higher and more stratified by depth than those for the sampling times reported here (43, 63), in accordance with other seasonal cycles in the lake. Surface water temperatures ranged from 22.0°C in September to 1.8°C in April, while bottom water temperatures varied only between 1.8 and 4.0°C. Sediment SSU rRNA concentrations were highest in September, when water rather than sediment temperatures were highest.
FIG. 3.
Comparison of water column (A) and sediment (B) SSU rRNA concentrations. RNA extracted from water or sediment samples was quantitated by membrane hybridization with the universal probe (S-*-Univ-1390-a-A-18). Water temperature was measured with the SeaBird instrument package.
Domain-level probe hybridization.
Hybridization to all three domain-level probes (bacterial, eukaryotic, and archaeal), as well as to the universal probe, was lowest in April 1997 and highest at most depths in either September or November 1996 (Fig. 4). Note that different horizontal scales are used for each of the probes. Bacterial SSU rRNA predominated, except in April, followed by eukaryotic rRNA. Archaeal rRNA was a small fraction of the total at all times and depths sampled. For a given sample, the depth distributions for all four probes were similar.
FIG. 4.
Domain-level probe hybridization. RNA extracted from each 0.5- or 1-cm depth interval was transferred to nylon membranes and quantitated with a radiolabeled universal (S-*-Univ-1390-a-A-18), bacterial (S-D-Bact-0338-a-A-18), eukaryotic (S-D-Euca-1379-a-A-16), or archaeal (S-D-Arch-0915-a-A-20) oligonucleotide probe. Note that a different horizontal scale was used for each probe.
Hybridization to the archaeal probe was generally greatest in the oxic to suboxic zone. This is contrary to our initial expectation that archaeal rRNA would be contributed primarily by obligately anaerobic methanogens in the anoxic deeper sediments. Some of this archaeal signal can likely be attributed to the cold-water crenarchaeota (42; B. J. MacGregor, unpublished observations), which have been found in other oxic habitats (see, e.g., reference 12). Hybridization to probes targeting several different groups of methanogenic archaea has also been found in Fox Point samples from the oxic zone, suggesting the possibility of anaerobic niches there (1).
Ideally, hybridization to the universal probe should equal the sum of bacterial, eukaryotic, and archaeal probe hybridizations. Domain summations for May, September, and November were generally between 40 and 120% (Fig. 5). Both the lowest and the highest summations were obtained with low-rRNA samples collected from deep in the sediment or in April and June, and they may be due in part to uncertainties in measuring very low RNA concentrations. Chloroplast rRNA might also contribute to low domain summations (discussed below). Domain summations of greater than 100% may result from RNA degradation: the universal probe target site is located in a region of the SSU rRNA molecule with relatively little secondary structure (68) and may be especially subject to degradation (48). While RNA degradation is generally attributed to laboratory RNase contamination, nucleases are of course also found in natural environments. Partially degraded RNA might be especially abundant where cell lysis is frequent, due, for example, to grazing.
FIG. 5.
Domain summations. Hybridization to the bacterial, eukaryotic, and archaeal probes is expressed as a percentage of hybridization to the universal probe. Two summations over 200% are shown in the inset graphs. Gaps indicate depth intervals for which RNA extractions were not done.
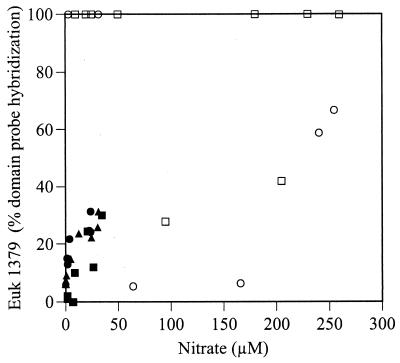
Bacterial SSU rRNA predominated, with several exceptions. The two deepest samples from May 1996 and the 15- to 16-cm sample from September hybridized only to the archaeal probe, suggesting that archaeal methanogens may be active at these greater depths. In April, eukaryotic rRNA was most abundant, although as a percentage of universal probe hybridization it was not more abundant than in other months. Eukaryotic rRNA was also proportionately abundant in December 1997 (see Fig. 7C) and between depths of 0.5 and 1.5 cm in box core 1 from June 1997 (Fig. 5; see Fig. 7B). The nitrate concentration and eukaryotic probe hybridization as proportions of total domain-level SSU rRNA were positively correlated overall (Fig. 6) (correlation coefficient, 0.402; P < 0.01) (52). Samples in which only eukaryotic RNA was detected (all of which were collected in April and June 1997) had a wide range of nitrate concentrations, however. An explanation for the cooccurrence of high nitrate levels and predominance of eukaryotic rRNA is suggested in Discussion.
FIG. 6.
Nitrate concentration and proportion of eukaryotic SSU rRNA. The eukaryotic rRNA concentration is expressed as a proportion of the combined eukaryotic, bacterial, and archaeal probe hybridization. ▪, 2 May 1996; ●, 4 September 1996; ▴, 13 November 1996; □, 2 April 1997; ○, 5 June 1997.
Spatial variability in probe hybridization.
Duplicate box cores and subcores were analyzed on three occasions to explore the degree of spatial as opposed to seasonal variation in the sediment community. RNA recovery from the 2 April 1997 box cores was low, and absolute site-to-site differences were small (Fig. 7A [note that horizontal scales differ from sampling time to sampling time and for the archaeal probe as opposed to the others]). Nearly all of the rRNA detected was eukaryotic. Replicate cores taken on 5 June 1997 differed (Fig. 7B). In box core 1, eukaryotic SSU rRNA accounted for nearly all probe hybridization in the 0.5- to 1.5-cm interval. Eukaryotic probe hybridization in box core 2 was more evenly distributed as a proportion of universal probe hybridization, but it was also greatest between 0.5 and 1.5 cm. This suggests a eukaryotic population (perhaps seasonal) concentrated at this depth. Subcore 1A was used for the full-length June hybridization profiles discussed above, and box core 1 was used for the chemical measurements, which showed high nitrate concentrations. The higher proportion of bacterial rRNA found in the summer and fall samples may develop unevenly across the lake floor, perhaps controlled by local rates of algal delivery and grazing.
The depth profiles of probe hybridization in the 17 December 1997 samples (Fig. 7C) were generally similar to each other, except that universal probe hybridization in the 1- to 2-cm interval of subcore 1A was severalfold higher than that in the other samples. Subcores 1A and 1B were taken from adjacent positions in box core 1, so this represents variability on a scale of <20 cm. Most of the difference is attributable to increased eukaryotic probe hybridization. Our sample size of one to several grams is not sufficient for representative sampling of larger eukaryotes, so peaks such as this are not unexpected. There was a peak of archaeal probe hybridization at the same depth, suggesting the possibility of a symbiotic association such as those found in anaerobic ciliates (18).
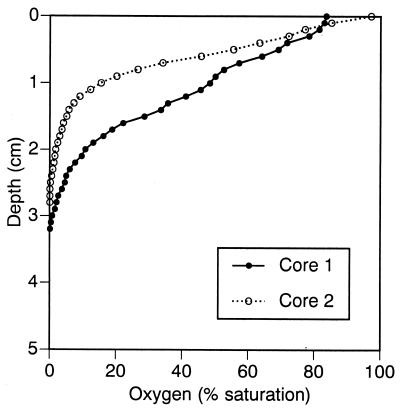
December oxygen profiles (Fig. 8) were measured on subcores separate from those used for RNA extraction. In box core 1, a subcore recovered from closer to subcore 1A than to subcore 1B was used. Oxygen penetration was approximately 1 cm deeper in core 1 than in core 2 or in any other of the Fox Point cores sampled to date (Fig. 1 and unpublished observations). The combination of a high proportion of eukaryotic probe hybridization and increased oxygen penetration suggests bioturbation and perhaps a response by microscopic eukaryotes to increased oxygen availability.
FIG. 8.
Replicate oxygen profiles from samples taken on 17 December 1997. Oxygen concentrations were measured by microelectrode on subcores taken from each of the two box cores.
Detection of potential nitrifying bacteria.
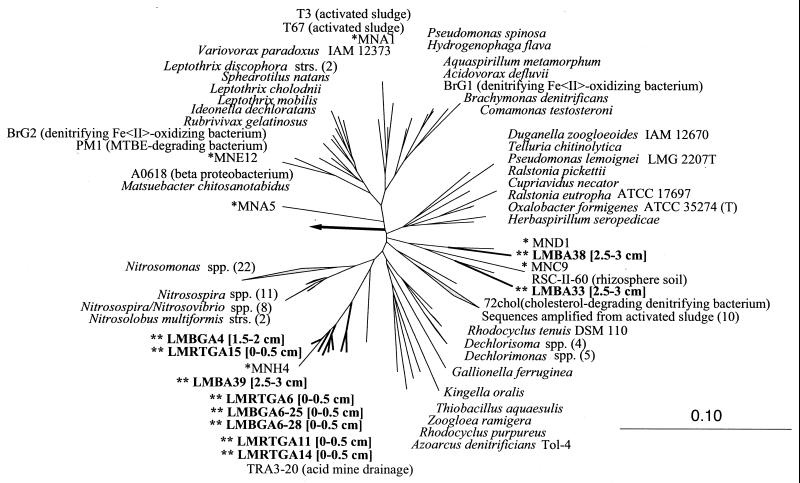
The most likely source of high sediment nitrate concentrations is biological oxidation of ammonia. Although we have not yet quantified known nitrifier groups, we identified a new clade of organisms closely affiliated with known beta-proteobacterial nitrifiers (Fig. 9). These sequences were recovered from several different sediment samples using two different primer sets not specifically targeting nitrifiers, suggesting a high abundance of this novel clade. A closely related sequence, MNH4, was isolated from Green Bay (Lake Michigan) sediments (Fig. 9) (54).
FIG. 9.
Beta-proteobacterial SSU rRNA sequences amplified from Lake Michigan sediment. Sequences in boldface with two asterisks were recovered from Fox Point sediments in this study. Sediment sampling intervals are shown in square brackets. Single asterisks indicate sequences amplified from Green Bay sediment by Stein et al. (61). Numbers in parentheses indicate the number of sequences included in a particular branch. The tree was calculated using the neighbor-joining method, as implemented in the sequence analysis program Arb (55). Gram-positive bacterial SSU rRNA sequences were used to root the tree. The scale bar represents one fixed mutation per 10 nucleotide positions.
DISCUSSION
Probe coverage.
Because the probes used were designed several years ago, and the number of SSU rRNA sequences available from GenBank is increasing rapidly, we first compare the probes to the present sequence database and show that their coverage remains substantially complete.
(i) Universal and bacterial probes.
Bacterial rRNA accounted for the majority of probe hybridization in the Fox Point samples, so differences in probe coverage between the universal (S-*-Univ-1390-a-A-18) and bacterial (S-D-Bact-0338-a-A-18) probes are a particular concern. Most bacterial groups presently recognized contain only a small proportion of nontarget species for either probe (Fig. 10 [due to computer limitations, only species missed by one or both probes are included]). Some of the mismatches, particularly the phylogenetically isolated ones, may be due to sequencing errors. The greatest concentration of universal probe mismatches is found in the epsilon proteobacteria, while bacterial probe mismatches are especially prevalent among the chlamydiales and planctomycetales (as a proportion of the presently described species). Outside of the deeply branching Thermotogales and Aquificales, few bacterial species are missed by both probes. The universal probe mismatches should be partially compensated for by the wash temperature (Table 1), which was experimentally chosen to allow detection of single-mismatch archaeal species without overrepresentation of the Bacteria and Eukarya (69). Daims et al. (10) have recently designed a set of probes that together target all presently known bacterial SSU rRNA sequences.
FIG. 10.
Bacterial species with mismatches to the universal (S-*-Univ-1390-a-A-18) and/or bacterial (S-D-Bact-0338-a-A-18) probe. The universal and bacterial probe sequences were checked against a collection of over 5,700 nearly full-length aligned prokaryotic SSU rRNA sequences, which include target regions with no ambiguities for both probes. Only species with mismatches to one or both probes are included in the tree shown here, which was calculated by neighbor joining with Olsen distance correction using the Arb sequence database program (55). Archaeal SSU rRNA sequences were used to root the tree. The scale bar represents one fixed mutation per 10 nucleotide positions.
(ii) Eukaryotic probe.
Eukaryotic rRNA was also a large proportion of the total in most of the Fox Point samples. Of 1,946 nearly full-length eukaryotic SSU rDNA sequences obtained from GenBank, 92 have mismatches to the eukaryotic probe (S-D-Euca-1379-a-A-16) only, 65 have mismatches to the universal probe only, and 19 have mismatches to both probes. Of aquatic microeukaryotes, rDNA sequences from the Euglenales in particular match the universal but not the eukaryotic probe. Without more information about the microeukaryotic populations at Fox Point, we cannot evaluate the possible contribution of these species to the domain summations.
(iii) Archaeal probe.
Nearly all archaeal SSU rDNA sequences have a perfect target site for the archaeal probe (S-D-Arch-0915-a-A-20) and a single mismatch with the universal probe, which is compensated for by a decreased wash temperature (69). One cluster of species with a single mismatch to the archaeal probe is found among the thermophiles (Fig. 11); the five other exceptional species are scattered among the archaeal clades. Archaeal probe hybridization generally represents only a small proportion of the total, so it should be a negligible source of error in any case.
FIG. 11.
Archaeal probe coverage. All of the sequences shown are targeted by both probes unless otherwise indicated. The tree was calculated by neighbor joining with Olsen distance correction using the Arb sequence database program (55). Bacterial SSU rDNA sequences were used to root the tree. The scale bar represents one fixed mutation per 10 nucleotide positions.
(iv) Organellar rRNA.
Mitochondria and chloroplasts, which are believed to have evolved from endosymbiotic bacteria, contain rRNA-encoding genes. Although the Fox Point sampling site is below the photic zone, the chloroplasts of photosynthetic organisms that settle to the bottom may contribute to the rRNA pool. Of 70 nearly full-length algal and higher-plant chloroplast rRNA sequences examined (Fig. 12), 39 are targeted by both the universal and bacterial probes, 24 are targeted by the universal probe only, 6 are targeted by the bacterial probe only, and 1 is targeted by neither probe. Chloroplast rRNAs that hybridized only with the universal probe might help account for some of the low domain summations, particularly in April 1997 (Fig. 5).
FIG. 12.
Bacterial and universal probe coverage of chloroplast SSU rDNA sequences. All sequences shown are targeted by both probes, unless otherwise indicated. The tree was calculated by neighbor joining using the Arb sequence database program (55). A delta-proteobacterial SSU rRNA sequence was used to root the tree. The scale bar represents one fixed mutation per 10 nucleotide positions.
Of 184 mitochondrial SSU rDNA sequences checked, nearly all had multiple mismatches to the universal probe and all of the domain probes (data not shown) and thus should have little impact on the domain summations. The only two sequences with perfect matches to any probe are both from aquatic species, however. The mitochondrial rDNAs of the red alga Porphyra purpurea and the freshwater amoeba Acanthamoeba castellani Neff include universal probe target sites.
Seasonal changes in SSU rRNA and nitrate concentration: a suggested role for grazers.
Because the temperature of the Fox Point sediments is nearly constant year-round, as were previously measured chemical profiles (K. H. Nealson, unpublished observations), we expected that microbial SSU rRNA concentrations would show little evidence of seasonality. However, we found at least a 10-fold variation in the upper sediment layers during the course of a year. We also found that while sulfate, iron, manganese, and oxygen profiles changed little, nitrate concentrations were severalfold higher in spring. Bottom water nitrate concentrations in southern Lake Michigan are between 5 and 30 μM year-round (8) and are lower in spring than in summer (Fig. 13), so this difference is unlikely to be caused by direct nitrate input from the water column. High nitrate levels were associated with low concentrations of SSU rRNA (Fig. 2).
FIG. 13.
Bottom water nitrite plus nitrate concentrations (data are from reference 59). Station 1115 GLSB/L. MICH 17 was chosen as the long-term Lake Michigan study site most similar to our Fox Point station. It is located offshore of Racine, Wis., at 42°44′0"N, 87°25′0"W; the water depth is 350 ft (107 m). Only measurements made at depths of greater than 80 m are shown. ▪, 1988; ●, 1989; ▴, 1990; ✖, 1991; ⋄, 1992; ○, 1993; ▵, 1996; ✚, 1997.
Nutrient availability is probably the primary seasonal factor at the Fox Point site. Sediment microbial communities can respond quickly to the settling of real or simulated algal blooms (reviewed in reference 26). Cold, unstratified water and seasonally low grazer populations allow a large proportion of the Lake Michigan spring diatom bloom to settle directly to the sediment (24, 51). Brooks and Torke (9) measured chlorophyll at Fox Point in 1973 and 1974. Integrated over the whole water column, the chlorophyll concentration began to increase in late winter, increased more quickly in March, and reached maximums in late May or early June each year. Those authors proposed that silica depletion and/or the onset of stratification may end the bloom in different years. Late-season primary production generally reaches the bottom of the lake only slowly. Nonsiliceous species sink more slowly than diatoms, and thermal stratification forms a density barrier. Thus, diatoms dominated the phytoplankton in deep sediment traps at a 100-m site in southeastern Lake Michigan even in summer, when they were only a small proportion of the algal population (51). Other seasonally varying sediment inputs may include thermal bar transport (15, 40, 44), calcium carbonate precipitation (whiting) events (28, 57, 61), vertical migrations by animals, lateral transport by deep currents, and anthropogenic inputs.
What might account for a combination of high nitrate concentration and low microbial SSU rRNA concentration in the spring? We suggest that benthic eukaryotes may modulate the timing of carbon and nitrogen delivery to the sediment microbial community. Eukaryotic grazers are proposed to quickly consume the bulk of the easily degraded new carbon and to excrete ammonia. The ammonia can be converted to nitrate by autotrophic nitrifying bacteria. Because of their generally low growth yield, a small number of nitrifiers can achieve a high conversion rate without causing a large increase in sediment SSU rRNA concentration. Nitrate consumption by heterotrophic denitrifiers may then await the slow hydrolysis of more recalcitrant carbon compounds. Preliminary modeling results (not shown) suggest that this mechanism is qualitatively possible. It may account for the seasonal association of high nitrate concentrations with a high proportion of eukaryotic SSU rRNA (Fig. 6), which could be contributed by diatoms (to the extent that they retain their rRNA) and the animals grazing on them.
One prediction from this scenario is that oxygen might be more quickly depleted when nitrifiers are active, because oxygen is required for ammonia oxidation. Oxygen profiles were unfortunately not obtained in April 1997, due to electrode malfunction. Oxygen concentrations measured on 22 April 1995 (not shown) decreased from 100% saturation at the sediment surface to 7% at the 1-cm depth, the steepest gradient yet found at Fox Point, but as there was no further decrease from 1 to 3 cm, this measurement may not be reliable.
Studies on soil, sediment, and aquatic systems have found both increased (29, 47, 56, 64) and decreased (37, 38) nitrification rates and/or nitrate concentrations in the presence of eukaryotes. Because the source environments, animals present, and laboratory conditions varied, it is difficult to draw general conclusions. Roles proposed for animals include mixing of ammonia into the oxic zone (64), ammonia production (47), and selection for bacterial aggregates with lower nitrification rates (37).
Benthic eukaryotes in Lake Michigan include Diporeia (Pontoporeia) hoyi (35, 45), Mysis relicta (opossum shrimp) (39), chironomid (midgefly) larvae (66), and Stylodrilus heringianus (36). The amphipod D. hoyi is the dominant benthic invertebrate (35, 45). We observed D. hoyi burrowing rapidly to and from a depth of several centimeters in most Fox Point sediment cores. D. hoyi is a detritivore, feeding in the top 2 cm of sediment, and may consume a major share of fresh detritus (20, 24). Gardner et al. (25) incubated gamma-irradiated or untreated Lake Michigan sediments with or without added D. hoyi. Ammonia accumulated significantly only in the treatment with animals and sterilized sediment, implying that ammonia was excreted by D. hoyi and then used by sediment organisms. Nitrate accumulated in the treatment with animals and unsterilized sediment, suggesting nitrifier activity, but the accumulation was small relative to the amount of ammonia production inferred from the other treatments. Flux measurements on separate cores showed that denitrification was significant and so probably accounted for the remainder of the nitrogen. Repeating these experiments with Fox Point sediments collected at different times of the year, or with and without added fresh detritus, might indicate whether grazers can in fact modulate the rate of nitrogen versus carbon input.
Conclusion.
Microbial rRNA concentrations in the sediment at the 100-m-deep Fox Point sampling site appear to depend on seasonal production nearer the surface. Although most physical and chemical profiles measured were relatively constant over the 1-year sampling period, SSU rRNA concentrations increased more than 10-fold between early spring and fall. Community composition changed as well, with eukaryotic rRNA predominating in winter (December) (Fig. 7C) and early spring (April) (Fig. 7A) and bacterial rRNA predominating the rest of the year.
The cooccurrence of high nitrate and low SSU rRNA concentrations in early spring was an unexpected finding. We propose that eukaryotes grazing on settled diatoms may release spring-bloom nitrogen, as ammonia, while consuming most of the readily degradable carbon compounds. Nitrate produced from this ammonia by slow-growing autotrophic nitrifying bacteria may then accumulate until enzymatic hydrolysis makes more recalcitrant carbon sources available to heterotrophic denitrifiers.
Further experiments are needed to test this model. An attempt should be made to isolate members of the beta-proteobacterial clade identified by 16S rDNA and rRNA amplification (Fig. 9) to determine whether they are nitrifiers, as the most closely related species are. Oligonucleotide probes and primers targeting the 16S rRNAs (reviewed in reference 60) and ammonia oxidation genes (reviewed in reference 6) of known nitrifying bacteria, as well as this new group, could then be used to look for seasonal trends in nitrifier activity. These could be related to surface production deposition by sediment trap studies or by measuring chlorophyll and pigment concentrations in sediment samples (62).
ACKNOWLEDGMENTS
We thank Captain Ron Smith, First Mate Greg Stamatelakys, and all of the crew of the R/V Neeskay for many pleasant (and some exciting) cruises. Michael Leonardo helped with sediment sampling. Benjamin Van Mooy, Changrui Gong, Joseph Werne, and David Hollander helped to collect water samples. Michael Dollhopf made most of the oxygen measurements. Thanks go to Art Brooks and the reviewers for helpful comments on the manuscript. The Storm PhosphorImager is located in the Keck Biophysics Facility, Northwestern University.
This work was supported by NSF grant DEB-961535 to D.A.S.
REFERENCES
- 1.Alm E W, Stahl D A. Critical factors influencing the recovery and integrity of rRNA extracted from environmental samples: use of an optimized protocol to measure depth-related biomass distribution in freshwater sediments. J Microbiol Methods. 2000;40:153–162. doi: 10.1016/s0167-7012(00)00120-2. [DOI] [PubMed] [Google Scholar]
- 2.Amann R I, Binder B J, Olson R J, Chisholm S W, Devereux R, Stahl D A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann R I, Krumholz L, Stahl D A. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol. 1990;172:762–770. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender M, Martin W, Hess J, Sayles F, Ball L, Lambert C. A whole-core squeezer for interfacial pore-water sampling. Limnol Oceanogr. 1987;32:1214–1225. [Google Scholar]
- 5.Binder B J, Liu Y C. Growth rate regulation of rRNA content of a marine Synechococcus (cyanobacterium) strain. Appl Environ Microbiol. 1998;64:3346–3351. doi: 10.1128/aem.64.9.3346-3351.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bothe H, Jost G, Schloter M, Ward B B, Witzel K P. Molecular analysis of ammonia oxidation and denitrification in natural environments. FEMS Microbiol Rev. 2000;24:673–690. doi: 10.1111/j.1574-6976.2000.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 7.Bremer H, Dennis P P. Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 1527–1542. [Google Scholar]
- 8.Brooks A S, Edgington D N. Biogeochemical control of phosphorus cycling and primary production in Lake Michigan. Limnol Oceanogr. 1994;39:961–968. [Google Scholar]
- 9.Brooks A S, Torke B G. Vertical and seasonal distribution of chlorophyll a in Lake Michigan. J Fish Res Board Can. 1977;34:2280–2287. [Google Scholar]
- 10.Daims H, Bruhl A, Amann R, Schleifer K H, Wagner M. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol. 1999;22:434–444. doi: 10.1016/S0723-2020(99)80053-8. [DOI] [PubMed] [Google Scholar]
- 11.Dell'Anno A, Fabiano M, Duineveld G C A, Kok A, Danovaro R. Nucleic acid (DNA, RNA) quantification and RNA/DNA ratio determination in marine sediments: comparison of spectrophotometric, fluorometric, and high-performance liquid chromatography methods and estimation of detrital DNA. Appl Environ Microbiol. 1998;64:3238–3245. doi: 10.1128/aem.64.9.3238-3245.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLong E F. Archaea in coastal marine environments. Proc Natl Acad Sci USA. 1992;89:5685–5689. doi: 10.1073/pnas.89.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaton A D, Clesceri L S, Greenberg A E, editors. Standard methods for the examination of water and wastewater. Washington, D.C.: American Public Health Association; 1995. [Google Scholar]
- 14.Eberl L, Givskov M, Sternberg C, Moller S, Christiansen G, Molin S. Physiological responses of Pseudomonas putida KT2442 to phosphate starvation. Microbiology. 1996;142:155–163. doi: 10.1099/13500872-142-1-155. [DOI] [PubMed] [Google Scholar]
- 15.Evans M S, Eadie B J, Glover R M. Sediment trap studies in southeastern Lake Michigan: fecal pellet express or the more traveled route? J Great Lakes Res. 1998;24:555–568. [Google Scholar]
- 16.Farewell A, Neidhardt F C. Effect of temperature on in vivo protein synthetic capacity in Escherichia coli. J Bacteriol. 1998;180:4704–4710. doi: 10.1128/jb.180.17.4704-4710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fegatella F, Lim J, Kjelleberg S, Cavicchioli R. Implications of rRNA operon copy number and ribosome content in the marine oligotrophic ultramicrobacterium Sphingomonas sp. strain RB2256. Appl Environ Microbiol. 1998;64:4433–4438. doi: 10.1128/aem.64.11.4433-4438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finlay B J, Fenchel T. Methanogens and other bacteria as symbionts of free-living anaerobic ciliates. Symbiosis. 1993;14:375–390. [Google Scholar]
- 19.Fitzgerald S A. The biochemistry of amino acids in sediments from the Great Lakes. Ph.D. thesis. Milwaukee: University of Wisconsin-Milwaukee; 1989. [Google Scholar]
- 20.Fitzgerald S A, Gardner W S. An algal carbon budget for pelagic-benthic coupling in Lake Michigan. Limnol Oceanogr. 1993;38:547–560. [Google Scholar]
- 21.Flärdh K, Cohen P S, Kjelleberg S. Ribosomes exist in large excess over the apparent demand for protein synthesis during carbon starvation in marine Vibrio sp. strain CCUG 15956. J Bacteriol. 1992;174:6780–6788. doi: 10.1128/jb.174.21.6780-6788.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuhrman J A, Comeau D E, Hagstrom A, Chan A M. Extraction from natural planktonic microorganisms of DNA suitable for molecular biological studies. Appl Environ Microbiol. 1988;54:1426–1429. doi: 10.1128/aem.54.6.1426-1429.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukui M, Suwa Y, Urushigawa Y. High survival efficiency and ribosomal RNA decaying pattern of Desulfobacter latus, a highly specific acetate-utilizing organism, during starvation. FEMS Microbiol Ecol. 1996;19:17–25. [Google Scholar]
- 24.Gardner W S, Nalepa T F, Frez W A, Cichocki E A, Landrum P F. Seasonal patterns in lipid content of Lake Michigan macroinvertebrates. Can J Fish Aquat Sci. 1985;42:1827–1832. [Google Scholar]
- 25.Gardner W S, Nalepa T F, Malczyk J M. Nitrogen mineralization and denitrification in Lake Michigan sediments. Limnol Oceanogr. 1987;32:1226–1238. [Google Scholar]
- 26.Goedkoop W, Gullberg K R, Johnson R K, Ahlgren I. Microbial response of a freshwater benthic community to a simulated diatom sedimentation event: interactive effects of benthic fauna. Microb Ecol. 1997;34:131–143. doi: 10.1007/s002489900043. [DOI] [PubMed] [Google Scholar]
- 27.Hicks R E, Amann R I, Stahl D A. Dual staining of natural bacterioplankton with 4′,6-diamidino-2-phenylindole and fluorescent oligonucleotide probes targeting kingdom-level 16S rRNA sequences. Appl Environ Microbiol. 1992;58:2158–2163. doi: 10.1128/aem.58.7.2158-2163.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodell D A, Schelske C L. Production, sedimentation, and isotopic composition of organic matter in Lake Ontario. Limnol Oceanogr. 1998;43:200–214. [Google Scholar]
- 29.Hondeveld B J M, Bak R P M, van Raaphorst W, Van Duyl F C. Impact of grazing by benthic eukaryotic organisms on the nitrogen sediment-water exchange in the North Sea. J Sea Res. 1999;41:255–268. [Google Scholar]
- 30.Kalpaxis D L, Karahalios P, Papapetropoulou M. Changes in ribosomal activity of Escherichia coli cells during prolonged culture in sea salts medium. J Bacteriol. 1998;180:3114–3119. doi: 10.1128/jb.180.12.3114-3119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kane M D, Poulsen L K, Stahl D A. Monitoring the enrichment and isolation of sulfate-reducing bacteria by using oligonucleotide hybridization probes designed from environmentally derived 16S rRNA sequences. Appl Environ Microbiol. 1993;59:682–686. doi: 10.1128/aem.59.3.682-686.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerkhof L, Kemp P. Small ribosomal RNA content in marine Proteobacteria during non-steady-state growth. FEMS Microbiol Ecol. 1999;30:253–260. doi: 10.1111/j.1574-6941.1999.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 33.Kerkhof L, Ward B B. Comparison of nucleic acid hybridization and fluorometry for measurement of the relationship between RNA/DNA ratio and growth rate in a marine bacterium. Appl Environ Microbiol. 1993;59:1303–1309. doi: 10.1128/aem.59.5.1303-1309.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koroleff F. Direct determination of ammonium as indolphenol blue. ICES Interlab Rep. 1970;3:19–22. [Google Scholar]
- 35.Landrum P F, Nalepa T F. A review of the factors affecting the ecotoxicology of Diporeia spp. J Great Lakes Res. 1998;24:889–904. [Google Scholar]
- 36.Lauritsen D D, Mozley S C, White D S. Distribution of oligochaetes in Lake Michigan and comments on their use as indexes of pollution. J Great Lakes Res. 1985;11:67–76. [Google Scholar]
- 37.Lavrentyev P J, Gardner W S, Johnson J R. Cascading trophic effects on aquatic nitrification: experimental evidence and potential implications. Aquat Microb Ecol. 1997;13:161–175. [Google Scholar]
- 38.Lee N M, Welander T. Influence of predators on nitrification in aerobic biofilm processes. Water Sci Technol. 1994;29:355–363. [Google Scholar]
- 39.Lehman J T, Bowers J A, Gensemer R W, Warren G J, Branstrator D K. Mysis relicta in Lake Michigan: abundances and relationships with their potential prey, Daphnia. Can J Fish Aquat Sci. 1990;47:977–983. [Google Scholar]
- 40.Likhoshway Y V, Kuzmina A Y, Potyemkina T G, Potyemkin V L, Shimaraev M N. The distribution of diatoms near a thermal bar in Lake Baikal. J Great Lakes Res. 1996;22:5–14. [Google Scholar]
- 41.Lonergan D J, Jenter H L, Coates J D, Phillips E J P, Schmidt T M, Lovley D R. Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J Bacteriol. 1996;178:2402–2408. doi: 10.1128/jb.178.8.2402-2408.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacGregor B J, Moser D P, Alm E W, Nealson K H, Stahl D A. Crenarchaeota in Lake Michigan sediment. Appl Environ Microbiol. 1997;63:1178–1181. doi: 10.1128/aem.63.3.1178-1181.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacGregor B J, Van Mooy B, Baker B J, Mellon M, Moisander P H, Paerl H W, Zehr J, Hollander D, Stahl D A. Microbiological, molecular biological and stable isotopic evidence for nitrogen fixation in the open waters of Lake Michigan. Environ Microbiol. 2001;3:205–219. doi: 10.1046/j.1462-2920.2001.00180.x. [DOI] [PubMed] [Google Scholar]
- 44.Mortimer C H. Large-scale oscillatory motions and seasonal temperature changes in Lake Michigan and Lake Ontario. University of WisconsinMilwaukee: Center for Great Lakes Studies; 1971. [Google Scholar]
- 45.Nalepa T F. Estimates of macroinvertebrate biomass in Lake Michigan. J Great Lakes Res. 1989;15:437–443. [Google Scholar]
- 46.Oda Y, Slagman S J, Meijer W G, Forney L J, Gottschal J C. Influence of growth rate and starvation on fluorescent in situ hybridization of Rhodopseudomonas palustris. FEMS Microbiol Ecol. 2000;32:205–213. doi: 10.1111/j.1574-6941.2000.tb00713.x. [DOI] [PubMed] [Google Scholar]
- 47.Pelegrí S P, Blackburn T H. Nitrogen cycling in lake sediments bioturbated by Chironomus plumosus larvae, under different degrees of oxygenation. Hydrobiologia. 1996;325:231–238. [Google Scholar]
- 48.Raskin L, Capman W C, Sharp R, Stahl D A. Molecular ecology of gastrointestinal ecosystems. In: Mackie R I, White B A, Isaacson R E, editors. Ecology and parasitology of gastrointestinal microbes. 2. Gastrointestinal microbiology and host interactions. London, United Kingdom: Chapman and Hall; 1997. [Google Scholar]
- 49.Rice A L, Thurston M H, Bett B J. The IOSDL DEEPSEAS programme: introduction and photographic evidence for the presence and absence of a seasonal input of phytodetritus at contrasting abyssal sites in the northeastern Atlantic. Deep-Sea Res I. 1994;41:1305–1320. [Google Scholar]
- 50.Robbins J A, Edgington D N. Determination of recent sedimentation rates in Lake Michigan using 210Pb and 137Cs. Geochim Cosmochim Acta. 1975;39:285–304. [Google Scholar]
- 51.Scavia D, Fahnenstiel G L. Dynamics of Lake Michigan phytoplankton: mechanisms controlling epilimnetic communities. J Great Lakes Res. 1987;13:103–120. [Google Scholar]
- 52.Sokal R R, Rohlf F J. Biometry: the principles and practice of statistics in biological research. 2nd ed. San Francisco, Calif: W. H. Freeman and Co.; 1981. [Google Scholar]
- 53.Stahl D A, Flesher B, Mansfield H R, Montgomery L. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl Environ Microbiol. 1988;54:1079–1084. doi: 10.1128/aem.54.5.1079-1084.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein L Y, La Duc M T, Grundl T J, Nealson K H. Bacterial and archaeal populations associated with freshwater ferromanganous micronodules and sediments. Environ Microbiol. 2001;3:10–18. doi: 10.1046/j.1462-2920.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- 55.Strunk O, Gross O, Reichel B, May M, Hermann S, Stuckman N, Nonhoff B, Lenke M, Ginhart A, Vilbig A, Ludwig T, Bode A, Schleifer K-H, Ludwig W. Arb: a software environment for sequence data. Munich, Germany: Technische Universität München; 2000. http://www.arb-home.de/ . ( http://www.arb-home.de/). ). [Google Scholar]
- 56.Svensson J M. Emission of N2O, nitrification and denitrification in a eutrophic lake sediment bioturbated by Chironomus plumosus. Aquat Microb Ecol. 1998;14:289–299. [Google Scholar]
- 57.Thompson J B, Schultze-Lam S, Beveridge T J, DesMarais D J. Whiting events: biogenic origin due to the photosynthetic activity of cyanobacterial picoplankton. Limnol Oceanogr. 1997;42:133–141. doi: 10.4319/lo.1997.42.1.0133. [DOI] [PubMed] [Google Scholar]
- 58.Tsai Y L, Olson B H. Rapid method for direct extraction of DNA from soil and sediments. Appl Environ Microbiol. 1991;57:1070–1074. doi: 10.1128/aem.57.4.1070-1074.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.U.S. Environmental Protection Agency. STORET Legacy Data Center. Washington, D.C.: U.S. Environmental Protection Agency; 2001. http://www.epa.gov/storet/ ( http://www.epa.gov/storet/). ). [Google Scholar]
- 60.Utaker J B, Nes I F. A qualitative evaluation of the published oligonucleotides specific for the 16S rRNA gene sequences of the ammonia-oxidizing bacteria. Syst Appl Microbiol. 1998;21:72–88. doi: 10.1016/S0723-2020(98)80010-6. [DOI] [PubMed] [Google Scholar]
- 61.Vanderploeg H A, Eadie B J, Liebig J R, Tarapchak S J. Contribution of calcite to the particle-size spectrum of Lake Michigan seston and its interactions with the plankton. Can J Fish Aquat Sci. 1987;44:1898–1914. [Google Scholar]
- 62.van Duyl F C, Kop A J. Bacterial production in North Sea sediments: clues to seasonal and spatial variations. Mar Biol. 1994;120:323–337. [Google Scholar]
- 63.Van Mooy B, MacGregor B J, Stahl D A, Nealson K H, Hollander D. Evidence for tight coupling between active bacteria and particulate organic carbon during seasonal stratification of Lake Michigan. Limnol Oceanogr. 2001;46:1202–1208. [Google Scholar]
- 64.Verhagen F J M, Laanbroek H J, Woldendorp J W. Competition for ammonium between plant roots and nitrifying and heterotrophic bacteria and the effects of protozoan grazing. Plant Soil. 1995;170:241–250. [Google Scholar]
- 65.Waples J T. Air-water gas exchange and the carbon cycle of Green Bay. Ph.D. thesis. University of Wisconsin-Milwaukee; 1998. , Milwaukee. [Google Scholar]
- 66.Winnell M H, White D S. The distribution of Heterotrissocladius oliveri Saether (Diptera, Chironomidae) in Lake Michigan. Hydrobiologia. 1986;131:205–214. [Google Scholar]
- 67.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woese C R, Gutell R, Gupta R, Noller H F. Detailed analysis of the higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol Rev. 1983;47:621–669. doi: 10.1128/mr.47.4.621-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng D D, Alm E W, Stahl D A, Raskin L. Characterization of universal small-subunit rRNA hybridization probes for quantitative molecular microbial ecology studies. Appl Environ Microbiol. 1996;62:4504–4513. doi: 10.1128/aem.62.12.4504-4513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]