Abstract
Escherichia coli O157:H7 (strains ATCC 43895 and FO46) became nonculturable in sterile, distilled, deionized water or after exposure to chlorine. Recovery of nonculturable E. coli O157:H7 was examined by in vitro and in vivo methods. The decline in culturability of starved E. coli O157:H7 was measured by plate count on rich medium. Recovery in vitro of nonculturable cells was conducted with media amended with catalase or sodium pyruvate; however, there was no apparent increase over culturable cell counts on amended versus nonamended media. Although nonculturable E. coli O157:H7 did not recover under in vitro conditions, a mouse model was used to determine if in vivo conditions would provide sufficient conditions for recovery of nonculturable E. coli O157:H7. In separate studies, mice were orally challenged with starvation-induced nonculturable cells (FO46) or chlorine-induced nonculturable cells (43895 and FO46). Passage through the mouse gastrointestinal tract had no effect on recovery of nonculturable (starvation or chlorine induced) E. coli O157:H7 (43895 or FO46), based on analysis of fecal samples. Mouse kidneys were assayed for the presence of Shiga toxin using the Vero cell assay. Differences in cytotoxicity towards Vero cells from kidney samples of mice receiving nonculturable cells and control mice were not significant, suggesting a loss of virulence.
In recent years, a large number of outbreaks of Escherichia coli O157:H7 have been associated with consumption of contaminated municipal water, well water, and contact with recreational waters (4, 5, 6, 12, 13). In the fall of 1999, an outbreak of E. coli O157:H7 involving over 900 people at a county fair was linked to consumption of water or products made with water from a contaminated well (5). Swimming-related illnesses resulting from contaminated recreational lake water have occurred on more than one occasion (4, 6, 13). One of the largest waterborne outbreaks, with more than 2,000 cases, occurred in South Africa; E. coli O157 was detected in approximately 18% of surface water samples taken during the outbreak (12). The infectious dose of E. coli O157:H7 can be as low as 10 to 100 organisms; therefore, contamination of water sources can be significant even if the pathogen levels are low. However, detection and confirmation of E. coli O157:H7 are difficult if low-level contamination is present in water supplies (6, 16).
Several reports indicate that E. coli O157 becomes nonculturable during prolonged storage in water (18, 24, 27). Rigsbee et al. (24) reported that three different strains of E. coli O157:H7 became nonculturable after 70 days in sea salt medium (pH 7.4, 5°C). A five-strain cocktail of E. coli O157:H7 became nonculturable in reservoir (77 days) and lake water (50 and 70 days) held at 25°C (27). E. coli O157 suspended in water and held at 4°C became nonculturable in 21 days (18). Aside from E. coli O157, other bacteria have been reported to become nonculturable in water or buffered medium (3, 7, 20, 21, 22).
A number of in vitro methods have been evaluated for recovery of nonculturable bacteria. One recovery method relies on addition of peroxide-degrading agents (i.e., sodium pyruvate or catalase) to media; these agents serve to protect cells from oxidative stress. Recently, researchers reported that supplementation of agar medium with sodium pyruvate or catalase restored culturability to nonculturable E. coli O157 (18). Bogosian et al. (2) concluded that addition of peroxide-degrading agents permitted the recovery of a hydrogen peroxide-sensitive population of Vibrio vulnificus. While recovery of pathogens from environmental sources is important in determining the etiology of an outbreak, of greater concern from a human health perspective is in vivo recovery and retention of pathogenicity by nonculturable cells.
Pathogenic effects of nonculturable cells have not been clearly defined in that some authors have demonstrated pathogenicity, while others report loss of pathogenicity with viability. Nonculturable Vibrio cholerae and V. vulnificus were reported to retain pathogenicity upon recovering in the rabbit and mouse intestine, respectively (7, 21). Pathogenicity of nonculturable Salmonella enterica serovar Typhimurium was lost concomitantly with culturability, although cells were viable based on cytological testing (3). A recent report indicated that NaCl-stressed E. coli O157:H7 did not recover in the mouse intestine (15).
In the present study, we investigated the recovery and pathogenicity of E. coli O157:H7 experimentally stressed by long-term storage in water or exposure to chlorine. To determine recovery, nonculturable E. coli O157:H7 was administered orally to mice, and fecal samples were analyzed for culturable E. coli O157:H7. Retention of virulence was determined by Vero cell cytotoxicity of kidney homogenates.
MATERIALS AND METHODS
Bacterial strains.
E. coli O157:H7 strain FO46 was isolated from hamburger associated with an outbreak in Washington state and obtained from the Food and Drug Administration; in addition, this strain was used in the study by Rigsbee et al. (24). E. coli O157:H7 strain ATCC 43895 was also isolated from hamburger. Both 43895 and FO46 produce Shiga toxin 1 (Stx1) and Stx2. E. coli O157:H7 was grown in Trypticase soy broth (TSB) at 37°C until mid-log phase was reached. Cells were then harvested using centrifugation (10,000 × g 10 min, 4°C), washed, and resuspended in sterile, distilled, deionized water.
Water microcosms.
Distilled, deionized water was aliquoted (200 ml) into glass screw-top bottles and autoclaved. Microcosms were inoculated to achieve a final cell concentration of 106 CFU ml−1. After inoculation, the bottles were held static at 4°C.
Estimation of culturable cells.
Samples (1 ml) were removed directly from the microcosms, and culturable cell counts were determined using the drop plate method (four 20-μl drops onto Trypticase soy agar [TSA] plates). Plates were incubated at 37°C for 16 to 24 h, and colonies were counted. When colonies were no longer detected (<10 CFU ml−1), 10 ml of the microcosm was centrifuged, and the resulting pellet was plated onto TSA plates.
Recovery of nonculturable (starvation-induced) E. coli O157:H7 in vitro.
The method used for recovery of starvation-induced nonculturable E. coli O157:H7 was that of Bogosian et al. (2). Microcosms of strains 43895 and FO46 were set up and stored as indicated above. The hydrogen peroxide-degrading compounds catalase and sodium pyruvate were added to solid medium prior to plating. Sodium pyruvate and catalase solutions were filter sterilized (0.22-μm pore size) and aseptically spread onto the surface of TSA plates to achieve final concentrations of 80 mg and 200 U, respectively.
Recovery of nonculturable (starvation-induced) E. coli O157:H7 in vivo.
CF-1 male mice (22 to 25 g; SASCO, Wilmington, Mass.) were challenged by oral administration of 0.2 ml of a 20% (wt/vol) sucrose suspension using a stainless steel gavage needle. Five groups (four mice per group) received the following inocula: 20% sterile sucrose solution alone, culturable E. coli O157:H7 (FO46, 105 cells), ethanol-killed E. coli O157:H7, ampicillin-treated nonculturable E. coli O157:H7, and nonculturable E. coli O157:H7. Bacterial suspensions were prepared by centrifugation (10,000 × g, 20 min, 4°C), washed, and resuspended in a 20% sterile sucrose solution. Animals were housed individually in cages and permitted food and water ad libitum. Feces were collected daily from all animals, weighed, initially diluted (1:10) in phosphate-buffered saline (PBS), and serially diluted for plating on TSA and BCM O157:H7(+) (Biosynth, Naperville, Ill.). Plates were incubated at 37°C for 24 to 48 h. If no colonies formed, immunomagnetic separation of bacteria from fecal sample homogenates was performed to increase the detection of cells. Animal experiments were approved by the Rutgers University Animal Care and Facilities Committee and conducted in accordance with federal guidelines.
Chlorine-induced injury resulting in nonculturable E. coli O157:H7.
Strains of E. coli O157:H7 (43895 and FO46) were grown to stationary phase in TSB (37°C with shaking). Cells were washed twice in sterile distilled deionized water and resuspended in distilled deionized water at a final cell concentration of 106 CFU ml−1. Cell suspensions were held at 22°C for 7 days. The suspension was treated with 50 mg of sodium hypochlorite per liter for 30 s and neutralized with 0.1 M Na2S2O3. Aliquots of the treated suspension were plated onto TSA, TSA amended with sodium pyruvate, and mT7 agar to assess culturability. To quantify viability, the bacterial viability kit (BacLight) staining system (Molecular Probes, Inc., Eugene, Oreg.) was used according to the manufacturer's directions.
Recovery of nonculturable (chlorine-induced) E. coli O157:H7 in vivo.
CF-1 male mice (22 to 25 g) were challenged by oral administration of 0.2 ml of a 20% (wt/vol) sucrose suspension using a stainless steel gavage needle. Five groups (four mice per group) received the following inocula: 20% sterile sucrose solution, culturable E. coli O157:H7 (ATCC 43895, 106 cells); culturable E. coli O157:H7 (FO46, 106 cells); chlorine-treated E. coli O157:H7 (ATCC 43895, 106 cells); and chlorine-treated E. coli O157:H7 (FO46, 106 cells). Animals were housed individually and permitted food and water ad libitum. Feces were collected daily, weighed, initially diluted in PBS (1:10), and serially diluted for plating on TSA and Fluorocult E. coli O157:H7 agar (EM Science, Gibbstown, N.J.). If E. coli O157:H7 was not detected on differential agar, immunomagnetic separation and selective enrichment were performed on fecal samples. Animal experiments were approved by the Rutgers University Animal Care and Facilities Committee and conducted in accordance with federal guidelines.
Immunomagnetic separation of fecal homogenates.
Fecal homogenates (initial dilution, 1:10) were enriched according to previous methods (19). Briefly, samples were placed in a stomacher bag containing a filter and further diluted 1:2 with EC broth. Samples were homogenized and allowed to incubate at room temperature for 3 h. Immediately following incubation, novobiocin was then added to each sample (final concentration, 0.02 mg ml−1), and samples were allowed to incubate for 18 h at 42°C. Filtrates were removed and centrifuged (1,664 × g, 10 min, 4°C), the supernatant was decanted, and the pellet was resuspended in 1 ml of PBS-Tween (Sigma Chemicals, St. Louis, Mo.). Anti-E. coli O157:H7 Dynabeads (40 μl; Dynal Biotech Inc., USA, Lake Success, N.Y.) were added to each sample, and the sample was processed according to the manufacturer's directions. Immunomagnetic beads were plated onto Fluorocult agar, and presumptive positive colonies were confirmed by PCR.
Vero cytotoxicity of kidney filtrate.
In the first set of experiments assessing recovery of nonculturable O157, two mice from each group described above were euthanized on day 4 and day 8 (final day) of the experiment. In the second set of experiments examining in vivo recovery of chlorine-treated O157, all mice were euthanized on the final day of the experiment. Kidneys were excised following euthanization, flash frozen in liquid nitrogen, macerated with a mortar and pestle, and stored at −80°C until needed. Kidney samples were prepared for use in the Vero cell assay by resuspending samples in Vero cell growth medium, followed by vortexing, brief centrifugation, and passage of samples through a 0.22-μm-pore-size filter.
Cytotoxicity of kidney filtrates was determined by the Vero cell assay as described previously (29). Vero cells were seeded into wells (2 × 104 cells per well) of a 96-well microtiter plate and incubated for 24 h at 37°C and 5% CO2. Growth medium (Dulbecco's modified Eagle's medium supplemented with 10% [vol/vol] fetal bovine serum) was removed and replaced with fresh medium, and 100 μl of kidney suspension was added to the first row. Serial dilutions (1:2) were made, and plates were incubated at 37°C for 48 h. After 48 h, live cells were quantified using the tetrazolium salt 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (5 mg ml−1). Vero cells were incubated for 4 h at 37°C, the supernatant was discarded by inverting the plate, and acidified isopropanol was added to each well. Plates were incubated at room temperature with gentle shaking, and the optical density (OD) was determined at 570 nm. Controls included E. coli O157:H7 (FO46) supernatant, Stx1 (Toxin Technologies, Sarasota, Fla.), and PBS alone. Results are based on cell survival using the following formula: % live cells = (ODtreated cells/ODcontrol cells) × 100. Student's t test was used to determine differences between groups.
RESULTS
Loss of culturability of E. coli O157:H7.
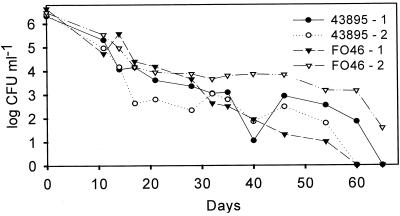
Survival of E. coli O157:H7 strains FO46 (n = 2) and ATCC 43895 (n = 2) in water during a 65-day period was determined by plating on TSA (Fig. 1). One microcosm of each strain (43895-2 and FO46-1) decreased to an undetectable level within 60 days, as determined by agar plate count. The cell population in microcosm 43895-1 decreased to an undetectable level by day 65. However, FO46-2 contained culturable cells, based on agar plate count, at the termination of the experiment.
FIG. 1.
Survival of E. coli O157:H7 strains in distilled, deionized water incubated at 4°C.
Recovery potential of E. coli O157:H7 in vitro.
Culturability and recovery of E. coli O157:H7 (43895 and FO46) were monitored using TSA, TSA amended with sodium pyruvate (80 mg per plate), and TSA amended with catalase (200 U per plate). No apparent increase was noted between culturable cell counts on TSA, TSA amended with sodium pyruvate, or TSA amended with catalase over a period of 55 days (data not shown).
Behavior of starvation-induced nonculturable E. coli O157:H7 in vivo.
Mice were orally challenged with nonculturable E. coli O157:H7 to determine if the pathogen would recover in the mouse gastrointestinal tract (GIT) or retain virulence (Table 1). The microcosm containing FO46 (Fig. 1) was used in this study. One treatment group received cells (FO46, nonculturable) treated with ampicillin. Ampicillin (20 mg ml−1) was added to the cell suspension to ensure that only nonculturable (nondividing) cells were present in the challenge suspension. Cells were washed to remove ampicillin prior to challenge of the mice. Other mouse groups received nonculturable cells without ampicillin treatment, culturable cells, and ethanol-killed cells. Fecal samples were collected from all mice in the study, and the presence of culturable E. coli O157:H7 was determined. Only feces from mice in the group receiving culturable E. coli O157:H7 were culture positive for the pathogen; moreover, shedding of E. coli O157:H7 was sporadic for all mice (Table 1). Aerobic plate counts did not change during the course of the study for any treatment group (data not shown).
TABLE 1.
Detection of E. coli O157:H7 in fecal samples from mice challenged with starvation-induced nonculturable cells
| Animal no. | Resulta on day postchallenge:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| 1 | + | − | − | + | NA | NA | NA | NA |
| 2 | − | − | + | − | ND | − | + | − |
| 3 | + | + | + | + | ND | − | + | − |
| 4 | − | + | + | + | NA | NA | NA | NA |
+, culture positive for E. coli O157:H7; −, culture negative for E. coli O157:H7; NA, animal euthanized on day 4; ND, not determined.
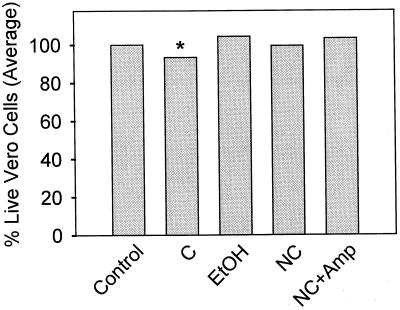
Kidney preparations from mice were tested for toxicity to Vero cells (Fig. 2). There was a significant increase (P < 0.05) in cytotoxicity of kidney from mice receiving culturable cells over that from the groups receiving nonculturable cells, nonculturable cells treated with ampicillin, and ethanol-killed cells. No significant differences in cytotoxicity were detected between kidney tissue from mice receiving culturable cells and control animals.
FIG. 2.
Vero cell cytotoxicity of kidney samples from mice receiving starvation-induced nonculturable E. coli O157:H7 (strain FO46; mice euthanized on day 4). Groups of four mice were orally fed (by gavage) ethanol-killed cells (EtOH), culturable cells (C), nonculturable cells (NC), nonculturable cells treated with ampicillin (NC+Amp), or a 20% sucrose solution (Control). All samples were run in triplicate, and group data were pooled and expressed as percent live cells. ∗, the group receiving culturable cells was significantly different from all other groups except control.
Chlorine treatment of E. coli O157:H7.
A recent study by Zhao and Matthews (30) showed that loss of culturability occurred following chlorine treatment, while a portion of cells remained viable, as determined by BacLight staining. Moreover, Lisle et al. (14) demonstrated that a population of E. coli O157:H7 cells exhibited a chlorine-resistant phenotype after 5 days of nutrient starvation. Based on those studies, the effect of starvation with subsequent exposure to chlorine on pathogenicity of E. coli O157:H7 was determined in an in vivo mouse model. Stationary-phase cells starved for 7 days in water (43895, 6.38 log CFU ml−1; FO46, 6.33 log CFU ml−1) were exposed to 50 mg of chlorine per liter for 30 s, and culturability and viability were determined. Chlorine treatment of microcosms containing 43895 or FO46 resulted in complete loss of culturability, as determined by plating on TSA, TSA amended with sodium pyruvate, and mT7 agar (data not shown). Viable cells (43895, 4.91; FO46, 4.78), based on Baclight staining, were detected in suspensions of both strains following exposure to chlorine.
Behavior of chlorine-treated E. coli O157:H7 in vivo.
In this study, mice were challenged with cells (either strain 43895 or FO46) exposed to chlorine. Fecal samples were collected from all animals during the 4-day experimental period and prepared for detection of culturable E. coli O157:H7. Fecal samples from animals receiving culturable cells without chlorine treatment were culture positive for the target pathogen (Table 2); however, fecal samples from animals in other groups (control and chlorine-treated cells) were culture negative. These results suggest that nonculturable E. coli O157:H7 exposed to chlorine does not recover upon passage through the mouse GIT. A greater number of mice receiving culturable E. coli O157:H7 strain 43895 shed the pathogen and for more days than mice receiving strain FO46.
TABLE 2.
Detection of E. coli O157:H7 in fecal samples from mice challenged with starved, chlorine-induced nonculturable cells
| Strain | Animal no. | Culture result on day postchallenge:
|
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| 43895 | 1 | + | − | − | − |
| 2 | − | + | + | + | |
| 3 | + | + | − | − | |
| FO46 | 2 | + | − | − | − |
| 4 | + | − | − | − | |
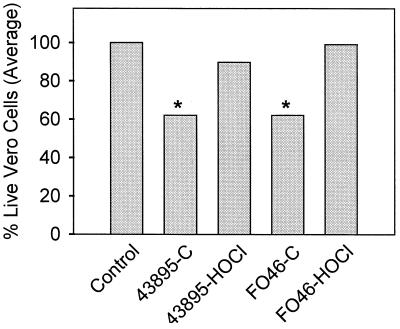
Kidney samples from mice receiving culturable cells of E. coli O157:H7 (strains 43895 and FO46) exhibited a significant increase in cytotoxicity compared to kidney samples from control mice and mice receiving chlorine-treated (43895 or FO46) cells (Fig. 3). There was no significant difference in cytotoxic activity between kidney samples from mice receiving culturable 43895 or FO46 or between control mice and mice receiving chlorine-induced nonculturable cells (43895 and FO46).
FIG. 3.
Vero cell cytotoxicity of kidney samples from mice receiving starved chlorine-treated E. coli O157:H7 (strain 43895 or FO46). Groups of four mice were orally fed (by gavage) culturable (C) or chlorine-induced nonculturable (HOCl) cells. Control animals received a 20% sucrose solution. All samples were run in triplicate and group data were pooled and expressed as percent live cells. ∗, the values for groups receiving culturable cells (43895 and FO46) were significantly different from those for the control group and the groups challenged with chlorine-treated cells (43895 and FO46).
DISCUSSION
The data presented here indicate that E. coli O157:H7 does indeed become nonculturable when exposed to starvation- or chlorine-induced stresses. Under starvation conditions, differences in time to nonculturability were observed under the set of conditions tested within and between 43895 and FO46. Starvation- and chlorine-induced stress is encountered by E. coli O157:H7 cycling from conditions in a farm environment (e.g., field run-off [1]) to a processing environment (e.g., chlorine treatment of water or minimally processed fruits and vegetables [14, 23]). In vitro culture of E. coli O157:H7 in amended media had no effect on recovery of nonculturable cells (43895 and FO46). In vivo experiments were conducted in an effort to understand whether nonculturable E. coli O157:H7 was recovered after passage through the mouse GIT; furthermore, mouse kidneys were assayed for Shiga toxin as an indicator of virulence. In vivo results indicate that E. coli O157:H7 does not recover after passage; furthermore, loss of culturability is related to a loss of virulence.
The culturability of E. coli O157:H7 following prolonged storage in water has been investigated (24, 27, 28, 30). Comparisons of studies are difficult because various temperatures, water sources, and strains of E. coli O157:H7 were used. Regardless, the pathogen does not behave consistently under conditions of nutrient starvation in water; similar results have been observed for E. coli by other investigators (20). In the present study, four water microcosms were evaluated using two strains, each run in duplicate (Fig. 1, strains 43895 and FO46). The decline in culturable cells for two microcosms, 43895-2 and FO46-1, occurred over a period of 60 days. The two other microcosms, 43895-1 and FO46-2, continued to decrease to a level of ≤0.01 CFU ml−1 in ≥65 days. Therefore, even microcosms started from the same culture and held under the same conditions varied with respect to cell culturability. In addition, E. coli O157:H7 strain FO46 was previously used in a study examining its survival in different water sources (24). Experimental results here and elsewhere (24) suggest that the water sources used (i.e., artifical seawater and river water versus distilled, deionized water) may account for the differences.
Prior to conducting in vivo studies designed to investigate the recovery of nonculturable E. coli O157:H7, in vitro studies were completed based on recovery of starvation-induced nonculturable cells on solid medium amended with catalase or sodium pyruvate. In previous studies, bacteria were placed under starvation conditions in water and held at low temperatures; culturable cells were measured by plate counts on amended or nonamended medium (2, 8, 18). Microcosms 43895-2 and FO46-1 were subjected to different amended media in an attempt to recover starvation-induced nonculturable cells. One milliliter from each microcosm was placed into minimal broth supplemented with glucose (0.001%), yeast extract (0.003%), and catalase (100 U ml−1) and incubated at room temperature for 24 h. After incubation, aliquots were plated onto TSA, plate count agar (PCA), or PCA amended with 0.1 or 1% sodium pyruvate, and incubated for an additional 24 h at room temperature. No turbidity in liquid medium or formation of colonies on solid medium was observed, indicating that recovery did not occur (data not shown). Recovery of starved cells (43895 or FO46) was again addressed using only solid media (i.e., TSA containing 80 mg of sodium pyruvate or 200 U of catalase). The number of culturable cells declined approximately 10,000-fold over the course of the study (55 days) with no apparent signs of recovery (i.e., increase in culturable counts on amended media). Bogosian et al. (2) suggested that higher cell counts on amended media were due to culturable hydrogen peroxide-sensitive cells, and, moreover, that these cells are in transit between an injured state and death, as indicated by the decline in culturable cells on amended media. Conversely, Mizunoe et al. (18) suggested that catalase and sodium pyruvate protect nonculturable cells from oxidative stress (i.e., H2O2) when placed on amended media, allowing cells to recover and form colonies.
Two distinct catalases, hydroperoxidase I (HPI) and HPII, function in E. coli to protect cells from oxidative stress (9, 10). HPI (encoded by katG) is part of the oxidative stress regulon oxyR, while HPII (encoded by katE) is under the control of the stationary-phase regulon rpoS (10). McCann et al. (17) demonstrated that HPII has a direct role in starvation-induced cross-protection to oxidative stress (i.e., H2O2). Moreover, HPI helps to maintain a basal level of oxidative resistance during growth and starvation. E. coli O157:H7 placed under starvation conditions has the potential to synthesize HPII, offering an explanation for the in vitro assays directed at recovery of the organism. If indeed HPII maintained proper function throughout the study, then addition of exogenous peroxide scavengers (i.e., catalase or sodium pyruvate) may have no additional effect on the recovery of cells. A functional catalase would protect cells on both amended and nonamended media, resulting in no apparent difference in culturable cells. Differences between our results and those of Mizunoe et al. (18) may be explained by the use of different strains of E. coli O157.
Recovery of nonculturable cells in vitro was not successful; however, others have reported the recovery of nonculturable cells when introduced into an in vivo system (7, 21). In addition, studies reporting maintained pathogenicity of nonculturable cells in an in vivo system have been conducted (7, 21, 22). The ability of nonculturable E. coli O157:H7 (starvation induced or chlorine induced) to recover during passage through the mouse GIT was evaluated in two separate studies; the study examining starvation-induced nonculturable cells is discussed here. Recovery was based on culture of E. coli O157:H7 from feces (Table 1). E. coli O157:H7 was detected only in fecal samples from mice receiving culturable FO46 cells; positive fecal samples were most prevalent on days 3 and 4, as detected by plating on selective agar. Samples from day 3 of the study were also processed using selective enrichment and immunomagnetic separation followed by plating on selective agar; there were no differences in detection of E. coli O157:H7 (data not shown).
The Vero cell assay was conducted with filtered kidney homogenates to determine the presence of Stx in association with kidney samples. A significant increase in cytotoxicity may be interpreted as an increase in Stx resulting from growth of recovered nonculturable E. coli O157:H7 during passage through the mouse GIT. Uchida et al. (25) definitively demonstrated that Stx is accumulated in the kidneys during hemolytic uremic syndrome attributable to an infection by E. coli O157:H7. The Vero cell assay was conducted to determine if kidney samples from mice receiving nonculturable E. coli O157:H7 differed significantly from those from mice receiving culturable cells. Results would indicate either maintenance of virulence by nonculturable cells or recovery of nonculturable cells (e.g., increased Stx production). The results shown in Fig. 2 are for animals (two per group) euthanized on day 4 of the study. Significant differences were noted between groups receiving culturable cells (FO46) and those receiving nonculturable, nonculturable treated with ampicillin, and ethanol-killed cells. In all instances, kidney samples from mice receiving culturable cells were more cytotoxic than kidney samples from other groups. Statistical analysis of the data also indicates a significant difference between the control group and the group receiving nonculturable cells treated with ampicillin, where kidney from the control group was more cytotoxic. Based on the comparison of data for the control group with that for the other groups, the significant difference detected between control and nonculturable cells treated with ampicillin was not expected. Examination of Vero cell data from individual kidney samples reveals that the raw data for one animal receiving nonculturable cells treated with ampicillin indicated less cytotoxicity than those for the control. When the data were pooled, the data for that mouse were sufficient to influence the group result.
Waterborne E. coli O157:H7 outbreaks occur on a yearly basis in different settings (4, 5, 12, 13). In some instances, water treated with chlorine (municipal water supplies and water parks) was linked to the outbreaks (5). Chlorine is commonly used to treat water to control the level of microorganisms in the water. The control of E. coli O157 in water is achievable by proper chlorination (23). In cases involving E. coli O157 outbreaks from treated water, improper chlorination was implicated (4). The food industry adds chlorine to water to achieve levels of 50 to 200 mg/liter for use in sanitizing raw fruits and vegetables, disinfecting beef carcasses, and sanitizing equipment (11). Researchers demonstrated that starved E. coli O157:H7 cells develop a chlorine-resistant phenotype; in addition, cells exposed to high levels of chlorine no longer form colonies on agar, but maintain viability (14, 30). To address this concern, starved E. coli O157:H7 cells were treated with 50 mg of chlorine per liter, resulting in a total loss of culturability as determined by plate counts on amended, nonamended, and mT7 agars. However, based on direct microscopic counts, only a 10-fold reduction occurred in the number of viable cells. Although cells were not recovered under in vitro conditions, the influence of in vivo conditions on the recovery of chlorine-induced nonculturable E. coli O157:H7 was tested.
In this study, groups of mice received culturable cells (43895 or FO46), chlorine-induced nonculturable cells (43895 or FO46), and no cells (control). E. coli O157:H7 was only isolated from fecal samples from mice receiving culturable cells (Table 2). Strain 43895 was recovered from a greater number of fecal samples than strain FO46; however, the reason for this difference was not investigated further. The Vero cell cytotoxicity of kidney samples from mice receiving nonculturable cells (43895 or FO46; Fig. 3) showed no significant difference from control samples. Conversely, cytotoxic activity was significantly greater in kidney samples from mice receiving culturable cells compared to other groups (control and nonculturable). These results are comparable to those from the Vero cell assay mentioned earlier.
In vivo experiments were designed to determine whether nonculturable cells (either starvation induced or chlorine induced) were capable of recovery upon passage through the mouse GIT. To preserve the normal intestinal flora, food was not withheld, and animals were not dosed with streptomycin prior to inoculation. Experiments were designed to simulate the potential scenario of a human consuming water or a minimally processed commodity (e.g., produce) contaminated with E. coli O157:H7 previously exposed to chlorine or starvation conditions. Nonculturable cells did not recover during passage through the GIT, as evidenced by lack of colony formation from fecal samples on agar plates. Kidney samples from mice receiving nonculturable cells did not cause significant cytotoxicity compared to kidney samples from mice receiving culturable cells (Fig. 2 and 3).
Routes of contamination of water and fresh produce with E. coli O157:H7 are numerous (1, 4, 5, 6, 12, 13). Although methods of testing for E. coli O157:H7 are routine, nonculturable cells retaining viability would be incorrectly classified as negative by conventional methods. These data suggest that consumption of food containing large numbers of nonculturable E. coli O157:H7 may not be sufficient to result in typical E. coli O157:H7-associated sequelae. Studies examining in vivo infectivity of Campylobacter spp. demonstrated that although metabolic activity of nonculturable cells was retained, cells did not colonize the research models examined and resulted in no signs of illness (26). Continued research focusing on survival at the molecular and physiological levels of E. coli O157:H7 is still of the utmost importance to public health.
ACKNOWLEDGMENTS
The work described in this paper was funded in part by the New Jersey Agricultural Experiment Station (project 10132).
We thank Charles Kaysner (FDA, Seattle, Wash.) for kindly providing strain FO46.
REFERENCES
- 1.Ackers M-L, Mahon B E, Leahy E, Goode B, Damrow T, Hayes P S, Bibb W F, Rice D H, Barrett T J, Hutwagner L, Griffin P M, Slutsker L. An outbreak of Escherichia coli O157:H7 infections associated with leaf lettuce consumption. J Infect Dis. 1998;177:1588–1593. doi: 10.1086/515323. [DOI] [PubMed] [Google Scholar]
- 2.Bogosian G, Aardema N D, Bourneuf E V, Morris P J L, O'Neil J P. Recovery of hydrogen peroxide-sensitive culturable cells of Vibrio vulnificus gives the appearance of resuscitation from a viable but nonculturable state. J Bacteriol. 2000;182:5070–5075. doi: 10.1128/jb.182.18.5070-5075.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caro A, Got P, Lesne J, Binard S, Baleux B. Viability and virulence of experimentally stressed nonculturable Salmonella typhimurium. Appl Environ Microbiol. 1999;65:3229–3232. doi: 10.1128/aem.65.7.3229-3232.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control. Surveillance for waterborne-disease outbreaks—United States, 1997–1998. Morb Mortal Wkly Rep. 2000;49:SS-4. [Google Scholar]
- 5.Centers for Disease Control. Outbreak of Escherichia coli O157:H7 and Campylobacter among attendees of the Washington county fair—New York, 1999. Morb Mortal Wkly Rep. 1999;48:803–804. [PubMed] [Google Scholar]
- 6.Chalmers R M, Aird H, Bolton F J. Waterborne Escherichia coli O157. J Appl Microbiol. 2000;88:124S–132S. doi: 10.1111/j.1365-2672.2000.tb05340.x. [DOI] [PubMed] [Google Scholar]
- 7.Colwell R R, Brayton P R, Grimes D J, Roszak D B, Huq S A, Palmer L M. Viable but non-culturable Vibrio cholerae and related pathogens in the environment; implications for release of genetically engineered microorganisms. Bio/Techniques. 1985;3:817–820. [Google Scholar]
- 8.Czechowicz S M, Santos O, Zottola E A. Recovery of thermally-stressed Escherichia coli O157:H7 by media supplemented with pyruvate. Int J Food Microbiol. 1996;33:175–184. doi: 10.1016/0168-1605(96)01116-6. [DOI] [PubMed] [Google Scholar]
- 9.Hassan H M, Fridovich I. Regulation of the synthesis of catalase and peroxidase in Escherichia coli. J Biol Chem. 1978;253:6445–6450. [PubMed] [Google Scholar]
- 10.Hennge-Aronis R. The role of rpoS in early stationary-phase gene regulation in Escherichia coli K12. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 171–200. [Google Scholar]
- 11.Hurst W C, Shuler G A. Fresh produce processing—an industry perspective. J Food Prot. 1992;55:824–827. doi: 10.4315/0362-028X-55.10.824. [DOI] [PubMed] [Google Scholar]
- 12.Isaacson M, Canter P H, Effler P, Aentzen L, Bomans P, Heenon R. Haemorrhagic colitis epidemic in Africa. Lancet. 1993;341:961. doi: 10.1016/0140-6736(93)91253-i. [DOI] [PubMed] [Google Scholar]
- 13.Keene W E, McAnulty J M, Hoesly F C, Williams P, Hedberg K, Oxman G L, Barret T J, Pfaller M A, Fleming D W. A swimming-associated outbreak of hemorrhagic colitis caused by Escherichia coli O157:H7 and Shigella sonnei. N Engl J Med. 1994;331:579–584. doi: 10.1056/NEJM199409013310904. [DOI] [PubMed] [Google Scholar]
- 14.Lisle J T, Broadway S C, Prescott A M, Pyle B H, Fricker C, McFeters G A. Effects of starvation on physiological activity and chlorine disinfection resistance in Escherichia coli O157:H7. Appl Environ Microbiol. 1998;64:4658–4662. doi: 10.1128/aem.64.12.4658-4662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makino S, Kii T, Asakura H, Shirahata T, Ikeda T, Takeshi K, Itoh K. Does enterohemorrhagic Escherichia coli O157:H7 enter the viable but nonculturable state in salted salmon roe? Appl Environ Microbiol. 2000;66:5536–5539. doi: 10.1128/aem.66.12.5536-5539.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maule A. Survival of verocytotoxigenic Escherichia coli O157 in soil, water and on surfaces. J Appl Microbiol. 2000;88:71S–78S. doi: 10.1111/j.1365-2672.2000.tb05334.x. [DOI] [PubMed] [Google Scholar]
- 17.McCann M P, Kidwell J P, Matin A. The putative ς factor KatF has a central role in development of starvation-mediated general resistance in Escherichia coli. J Bacteriol. 1991;173:4188–4194. doi: 10.1128/jb.173.13.4188-4194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizunoe Y, Wai S N, Takade A, Yoshida S. Restoration of culturability of starvation-stressed and low-temperature-stressed Escherichia coli O157:H7 cells by using H2O2-degrading compounds. Arch Microbiol. 1999;172:63–67. doi: 10.1007/s002030050741. [DOI] [PubMed] [Google Scholar]
- 19.Nakagawa H, Hara-Kudo Y, Kojima T, Ikedo M, Kodaka H, Konuma H, Kumagai S. Detection of freeze-injured Escherichia coli O157:H7 cells from foods by resuscitation prior to selective enrichment. Int J Food Microbiol. 2000;60:107–110. doi: 10.1016/s0168-1605(00)00325-1. [DOI] [PubMed] [Google Scholar]
- 20.Oliver J D. The public health significance of viable but nonculturable bacteria. In: Colwell R R, Grimes D J, editors. Nonculturable microorganisms in the environment. Washington, D.C.: American Society for Microbiology; 2000. pp. 277–300. [Google Scholar]
- 21.Oliver J D, Bockian R. In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl Environ Microbiol. 1995;61:2620–2623. doi: 10.1128/aem.61.7.2620-2623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pommepuy M, Butin M, Derrien A, Gourmelon M, Colwell R R, Cormier M. Retention of enteropathogenicity by viable but nonculturable Escherichia coli exposed to seawater and sunlight. Appl Environ Microbiol. 1996;62:4621–4626. doi: 10.1128/aem.62.12.4621-4626.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice E W, Clark R M, Johnson C H. Chlorine inactivation of Escherichia coli O157:H7. Emerg Infect Dis. 1999;5:461–463. doi: 10.3201/eid0503.990322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rigsbee W, Simpson L M, Oliver J D. Detection of the viable but nonculturable state in Escherichia coli O157:H7. J Food Safety. 1997;16:225–262. [Google Scholar]
- 25.Uchida H, Kiyokawa N, Horie H, Fujimoto J, Takeda T. The detection of shiga toxins in the kidney of a patient with hemolytic uremic syndrome. Pediatr Res. 1999;45:133–137. doi: 10.1203/00006450-199901000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Van De Giessen A W, Heuvelman C J, Abee T, Hazeleger W C. Experimental studies on the infectivity of non-culturable forms of Campylobacter spp. in chicks and mice. Epidemiol Infect. 1996;117:463–470. doi: 10.1017/s0950268800059124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang G, Doyle M P. Survival of enterohemorrhagic Escherichia coli O157:H7 in water. J Food Prot. 1998;61:662–667. doi: 10.4315/0362-028x-61.6.662. [DOI] [PubMed] [Google Scholar]
- 28.Warburton D W, Austin J W, Harrison B H, Sanders G. Survival and recovery of Escherichia coli O157:H7 in inoculated bottled water. J Food Prot. 1998;61:948–952. doi: 10.4315/0362-028x-61.8.948. [DOI] [PubMed] [Google Scholar]
- 29.Yaron S, Kolling G L, Simon L, Matthews K R. Vesicle-mediated transfer of virulence genes from Escherichia coli O157:H7 to other enteric bacteria. Appl Environ Microbiol. 2000;66:4414–4120. doi: 10.1128/aem.66.10.4414-4420.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao L, Matthews K R. Influence of starvation, temperature, and pH on culturability of Escherichia coli O157:H7. J Food Safety. 2000;20:193–208. [Google Scholar]