Abstract
Angelman syndrome (AS) is a genetic neurodevelopmental disorder characterized by developmental delay, lack of speech, seizures, intellectual disability, hypotonia, and motor coordination deficits. Motor abilities are an important outcome measure in AS as they comprise a broad repertoire of metrics including ataxia, hypotonia, delayed ambulation, crouched gait, and poor posture, and motor dysfunction affects nearly every individual with AS. Guided by collaborative work with AS clinicians studying gait, the goal of this study was to perform an in‐depth gait analysis using the automated treadmill assay, DigiGait. Our hypothesis is that gait presents a strong opportunity for a reliable, quantitative, and translational metric that can serve to evaluate novel pharmacological, dietary, and genetic therapies. In this study, we used an automated gait analysis system, in addition to standard motor behavioral assays, to evaluate components of motor, exploration, coordination, balance, and gait impairments across the lifespan in an AS mouse model. Our study demonstrated marked global motoric deficits in AS mice, corroborating previous reports. Uniquely, this is the first report of nuanced aberrations in quantitative spatial and temporal components of gait in AS mice compared to sex‐ and age‐matched wildtype littermates followed longitudinally using metrics that are analogous in AS individuals. Our findings contribute evidence toward the use of nuanced motor outcomes (i.e., gait) as valuable and translationally powerful metrics for therapeutic development for AS, as well as other genetic neurodevelopmental syndromes.
Lay Summary
Movement disorders affect nearly every individual with Angelman Syndrome (AS). The most common motor problems include spasticity, ataxia of gait (observed in the majority of ambulatory individuals), tremor, and muscle weakness. This report focused on quantifying various spatial and temporal aspects of gait as a reliable, translatable outcome measure in a preclinical AS model longitudinally across development. By increasing the number of translational, reliable, functional outcome measures in our wheelhouse, we will create more opportunities for identifying and advancing successful medical interventions.
Keywords: Angelman syndrome, animal models, autism, behavior, gait, genetics, longitudinal, motor, mouse models, neurodevelopment
INTRODUCTION
Angelman syndrome (AS) is a rare genetic neurodevelopmental disorder (NDD) characterized by developmental delay, impaired receptive and expressive communication skills, motor disability, severe intellectual disabilities, and seizures (Williams, 2010; Williams et al., 2010; Williams & Franco, 2010). Movement disorders affect nearly every individual with AS and are more prevalent than other commonly associated symptoms. These movement abnormalities are not the sole result of weakness, albeit, accompanying contributions of weak muscle tone are a key component (Bird, 2014; Gentile et al., 2010; Schlaggar & Mink, 2003; Tan et al., 2011; Wheeler et al., 2017). AS is caused by the loss of maternal expression of the gene UBE3A (ubiquitin‐protein ligase E3A/E6AP) (Jiang et al., 1998; Matsuura et al., 1997; Sutcliffe et al., 1997). Due to brain‐specific imprinting, the paternal allele is silenced; thus, loss of the maternal expression causes UBE3A deficiency isolated to the central nervous system (Sutcliffe et al., 1997; Yamasaki et al., 2003).
The AS motor profile includes gross and fine motor skill impairments and motor problems including spasticity, ataxia of gait, tremor, and muscle weakness. Delays in meeting motor milestones such as neck control, limb coordination, and crawling are often noted in the first year of life, and additional motor deficits such as hypotonia, delays, and impairments in independent walking abilities, and uncoordinated and ‘jerky’ movements are observed as children develop (Gentile et al., 2010; Micheletti et al., 2016; Wheeler et al., 2017). The delay in or lack of typical motor skill acquisition likely contributes to poor daily living skills, delay or failure to ambulate unassisted, and adds to maladaptive cognitive development observed later in life (Peters et al., 2004; Wheeler et al., 2017). Data from studies of other neurodevelopmental disorders also show a strong relationship between the degree of motor deficit and other behavioral domains such as social communication and cognition (Champion et al., 2014; Gernsbacher et al., 2008; Leonard, 2016). Prior research in AS has reported motor aberrations (DeLorey et al., 1998; Heck et al., 2008; Jiang et al., 1998; Landers et al., 2005; Sinkkonen et al., 2003; Sonzogni et al., 2020). Gait abnormalities such as ataxic gait with broad, unstable stance have been well reported in AS children who walk independently, affecting upward of 80% of patients in one report (Tan et al., 2011; Wheeler et al., 2017). Yet, to date, there is only one study that clinically quantified gait in AS (Grieco et al., 2018). Preclinically, the use of the hind limb clasp (Homanics et al., 1997) and walking with painted paws have been reported but provide limited subjective data (Carter et al., 2001). Grieco et al. used the Zeno Walkway, an electronic pressure mat covered with sensors, which analyzes gait parameters, and found that AS children walked with small, fast steps. Moreover, at 6–9 years old, AS children had poor gait skills, more similar to typically developing children aged 1–3 years (Grieco et al., 2018). To improve preclinical objectivity and accuracy, we moved away from paw painting to the DigiGait treadmill for enhanced reliability and translation. The translational power of the DigiGait system lies in its ability to precisely and automatically collect the same gait outcome metrics in rodent models of AS that are collected clinically in human AS patients (e.g., stance width, stride frequency, double support, etc.).
In vivo models are essential for testing therapeutic delivery methods and clinically relevant outcome measures in those models are required to demonstrate the efficacy of new treatments, whether they be innovative designs or traditional medicinal therapies being repurposed (Thurm et al., 2020). Motor development is highly conserved across species, which is exemplified by gene expression profiles in the cortex and cerebellum (Strand et al., 2007). For humans and rodent models alike, motor deficits are the most consistently reported phenotype in AS. In mice, this behavioral phenotype has been well reported as reduced locomotor activity, poor balance and coordination, and impairment of performance on the accelerated rotarod (Born et al., 2017; Huang et al., 2013; Jiang et al., 1998; Miura et al., 2002). Recently, a novel rat model of a full deletion of Ube3a recapitulated these motor deficits including the aforementioned accelerated rotarod motor learning deficit as well as additional nuanced impairments in rearing and fine forelimb motor skills (Berg et al., 2020, 2021; Dodge et al., 2020).
There is a plethora of data on motor ability impairment in earlier reports of AS preclinical models. Homanics et al. (1997) performed the hindlimb clasp (i.e., retraction of all four paws), which is observed in numerous models of NDD (e.g., Rett) and some unaltered control wildtype littermates. In the first behavioral phenotyping of their AS mouse, Jiang et al. (1998) used hind‐paw footprint analysis to assess ataxia and noted a slightly reduced stride length in maternally deficient Ube3a animals (Homanics, et al., 1997; Jiang et al., 1998). Footprint analysis via paw painting sustains its role in numerous behavioral neuroscience laboratories, to date (Brooks et al., 2004; Carter et al., 2001). Heck et al. and Mulherkar and Jana convincingly used various metrics of rope climbing, beam walking, and rotarod for coordination, balance, fine motor skills, and strength. Additionally both conducted paw painting down a runway of paper with manual scoring of metrics on both limbs and found elongated stride length and a wider stance only in the hindlimbs, identifying similar gait phenotypes observed in AS clinically (Brooks et al., 2004; Carter et al., 2001; Grieco et al., 2014; Mulherkar & Jana, 2010). Herein, we applied treadmill walking to corroborate and extend earlier work and to illuminate a variety of gait metrics while utilizing a longitudinal design to detect phenotypes across the lifespan. Our longitudinal approach allowed us to evaluate the phenotype's trajectory and determine whether a consistent worsening over time (decline), a loss of skills (regression), or no change (stagnation) occurred. We confirmed that DigiGait is test–retest reliable and introduced gait's potential for informing clinical trials by its translatability via indices of spatial and temporal gait. Initial characterizations and validations needed to be performed using the DigiGait hardware and software to quantify disrupted walking and gait across development in a mouse model of AS. This work extends previous findings of impaired motor abilities in AS mouse models by (a) using DigiGait to quantify gait parameters in a more nuanced way, using spatial and temporal indices, and (b) assessing gait across development, from weaning to adulthood.
In this study, we focused on numerous indices of gait, from physical metrics to spatial and temporal properties, analogous to clinical gait studies in AS, and discovered many maladaptive gait patterns in AS model mice. Temporal gait metrics in AS mice have not been reported, to date. Furthermore, we compared the gait of AS mice to sex‐ and aged‐matched wildtype littermate controls across the lifespan to identify onset, progression, and severity of abnormal gait patterns. Our data revealed numerous gait alterations in AS mice that were penetrant early in life, highlighting a potential quantitative biomarker that could be correlated with gait analysis in human subjects.
METHODS
Animal subjects
All animals were housed at the University of California Davis School of Medicine in Sacramento in a temperature‐controlled vivarium on a 12:12 light–dark cycle. All procedures were conducted in compliance with the NIH Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of UC Davis. A colony was maintained by breeding male B6.129S7‐Ube3atm1Alb/J (Jackson Laboratory, Stock No. 016590) with female C57BL6/J mice, maintaining paternal transmission of the mutant allele. Angelman syndrome (AS) model mice used in behavioral experiments had maternal transmission of the mutant allele and were generated by crossing heterozygous dams with C57BL6/J males to produce maternally inherited mutant Ube3a m−/p+ (AS, N = 20) mice and wildtype littermate controls (WT, N = 30). Mice were tested in the gait task from postnatal day (PND) 22 (weaning) to PND180 and motor assays were conducted at 4 months of age. Both sexes were assayed.
Motor behavior assays
Motor behavior was tested at 4 months of age, during adulthood when both gait and global motor deficits have been previously described (Adhikari et al., 2021). Animals were habituated in a 30 lux room, separate and away from the testing room, for 1 h prior to every assay. Seventy percent ethanol was used to clean equipment between each subject and trials in all described assays.
Open field
Open field was used to detect locomotive activity and general explorative behavior, as previously described (Adhikari et al., 2018; Bales et al., 2014; Copping et al., 2016, 2019). Individual mice were placed in a novel open arena for 30 min at 30 lux. EthoVision XT (Noldus) software was used to record sessions and analyze the total distance traveled during the 30 minutes and the average velocity of the subject during the session.
Beam‐walking
To assess motor coordination and balance abilities, the beam‐walking task was conducted. Subjects were placed, one by one, at one end of a 59‐cm long beam, elevated 68 cm above a cushion, at the end of which a darkened goal box (12‐cm diameter cylinder) was placed on the far end of the beam to motivate subjects to walk across. On the first training day, three trials on a large diameter (35‐mm) beam were conducted for mice to become accustomed to the task. Animals that had scores of 60 s for all three trials on the training day were excluded from analysis; no animals were excluded during this experiment. On the next day, animals were placed on three beams of different diameters (35‐, 18‐, and 13‐mm) in order of least to most difficult (widest to narrowest). Two trials per beam were conducted with an inter‐trial rest interval of at least 30 min. Each trial was a maximum of 60 s. Any falls off the beam were recorded as 60 s. The average latency to traverse the beam for the two trials was recorded.
Rotarod
An accelerating rotarod task was employed to further assess motor coordination and motor learning (Ugo Basile, Schwenksville, PA). Mice were placed on an accelerating cylinder that slowly accelerated from 4 to 40 revolutions per minute over a 5‐min period. Latency to fall off the cylinder or no longer comply with the task (i.e., cling to rod for more than two full rotations instead of walking forward) was recorded per trial with a maximum of 300 s. Mice were tested for three consecutive days with three trials per day separated with an hour inter‐trial interval to prevent fatigue. Results from each day's trials were averaged, as is the field's standard approach (Yang et al., 2012).
Gait analysis
The DigiGait imaging system was used to analyze gait (Mouse Specifics Inc, Boston, MA). DigiGait utilizes a ventral plane camera underneath a transparent treadmill belt that generates digital paw prints as a subject walks or runs (Hampton et al., 2004). Initial characterizations and validations were performed using previously published protocols (Amende et al., 2005; Hampton & Amende, 2010; Hampton et al., 2004). Protocols were adapted for AS using published methods (Grieco et al., 2018) as well as personal communications with community members of the Foundation for Angelman Syndrome Therapeutics (FAST) and Dr. Jessica Duis.
The day after weaning, mice were habituated in the walking chamber for 1 min, and then the belt was turned on and slowly increased from 5 cm/s to the target belt speed of 20 cm/s. On all subsequent testing days, mice were placed in the walking chamber for 1 min and the belt speed was set to 20 cm/s. Subjects unable to walk at the target speed for at least 5 s were allowed to rest and were retested. Subjects were recorded walking for 4–5 s, allowing for at least 10 full strides. Videos were taken when animals were running forward and were retaken if there were instances of subjects jumping, using the front bumper as an aid, walking diagonally across the camera view, or sliding backwards into the back bumper.
Frames were digitized, and relevant gait parameters were analyzed using DigiGait analysis software as previously described (Hampton, 2004; Hampton et al., 2004). Quality control steps in the analysis were conducted blind to genotype. Left and right fore‐ and hindlimbs were averaged together per subject for each metric.
Statistical analysis
All statistical analysis was conducted using GraphPad Prism software. Open field, beam walking, rotarod, and adult DigiGait data were analyzed using two‐way repeated‐measures ANOVA with planned posthoc multiple comparisons assessing performance between genotypes at each time bin, rod, day, and limb, respectively. Longitudinal DigiGait data were analyzed using repeated measures mixed‐effects models with planned Sidak posthoc multiple comparisons of genotype performance at each time point as well as multiple comparisons between all timepoints within genotype for each parameter studied. Data were analyzed for sex differences before pooling; there were no sex differences found in motor assays or gait metrics (Figure S5). Additionally, a full report of statistics is available in Table S1.
RESULTS
Global motor deficits
Adult AS mice exhibited global motor deficits as assessed by three gold‐standard rodent behavioral assays at 4 months of age. As previously reported, AS mice showed reduced activity in a novel open arena exploration task (Figure 1a,b). AS mice traveled less distance during the exploration period, traversing, on average, 25% less than WT (Figure 1a, p =0.003). Moreover, during the same period, AS mice showed reduced velocity, on average producing only 80% of the velocity of WT mice (Figure 1b, p=0.026). In addition to gross locomotive ability, we assayed other motor abilities such as balance, coordination, and motor learning using the beam walking and accelerating rotarod tasks. AS mice had impairments in both tasks (Figure 1c,d). AS mice demonstrated characteristic poor performance on accelerated rotarod on all three days (Figure 1cgenotype, p <0.05). AS mice showed significantly increased latencies to cross more difficult beams, demonstrating an impairment in balance and coordination (Figure 1d, Holm‐Sidak posthoc analysis, AS versus WT, rod 1: ns, rod 2: p =0.0162, rod 3: p <0.0001).
FIGURE 1.
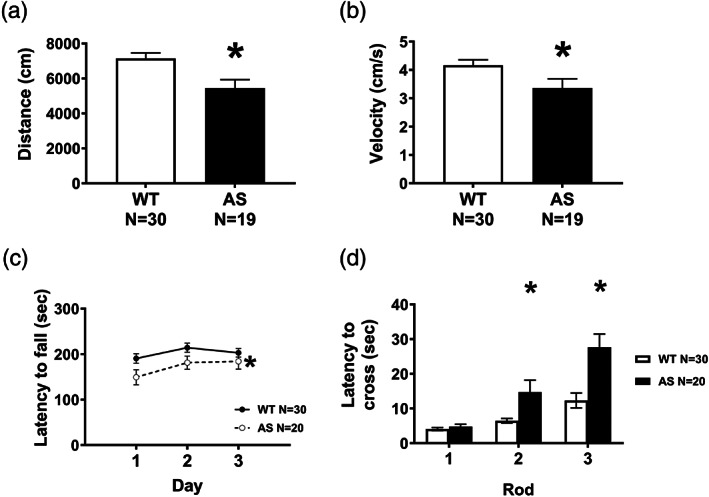
Adult AS mice exhibit severe motor deficits in locomotor exploration, speed, motor coordination, balance, and motor learning. (a,b) Adult AS mice were given a 30‐minute exploration period in a novel chamber. (a) AS mice demonstrated low locomotive activity, as they traversed less distance, compared to wildtype mice. (b). Moreover, during the exploration period, AS mice moved significantly more slowly than WT. (c) In the accelerating rotarod, AS mice showed a significant decrease in latency to fall across all 3 days, suggesting an impairment in motor learning and coordination. (d) Motor balance and coordination were assessed in a beam walking assay. Mice were trained to traverse a beam and their latency to cross the beam was recorded on progressively thinner and more difficult beams. AS mice took significantly longer than WT mice to cross the more difficult beams. *p <0.05 in posthoc comparison between WT and AS. (a–d) Data represented as mean ± SEM. (a,b) *p <0.05 Student's t‐test between genotypes. (c,d) *p <0.05 main effect of genotype in repeated measures two‐way ANOVA
Adult gait
AS mice showed abnormal spatial and temporal subcomponents of gait compared to age‐ and sex‐matched WT littermate controls. Spatial parameters included stance width, step length, and stepping frequency, also known as cadence. Stance width, defined as the distance between right and left limbs when limbs are grounded, was wider in the forelimbs and trended wider in the hindlimbs of AS mice, compared to WT (Figure 2a, AS versus WT Holm‐Sidak post‐hoc analysis, fore: p <0.0001, hind: p =0.07). Additionally, AS mice took longer steps, with an average 20% longer step or stride length (Figure 2b, AS versus WT Holm‐Sidak post‐hoc analysis, fore: p <0.0001, hind: p <0.0001). Accordingly, stride frequency was decreased in AS fore‐ and hindlimbs (Figure 2b, AS versus WT Holm‐Sidak posthoc analysis, fore: p <0.0001, hind: p <0.0001). The abnormal wider and elongated gait of adult AS mice may indicate lower ability during ambulation. Total stride duration and its four phases of temporal gait pattern were analyzed: swing, during which a paw has no contact with the ground, braking, during which a paw transitions from the swing phase and steps onto the ground into the stance phase, propulsion, during which a paw lifts off the ground and into the swing phase, and stance when the paw makes full contact with the ground. AS mice required more time to make one stride as the stride duration was significantly increased (Figure 3a, AS versus WT Holm‐Sidak posthoc analysis, fore: p <0.0001, hind: p <0.0001). Within the stride, both stance and swing times were increased as AS mice spent more time with their paws on the ground and in the air compared to WT mice (Figure 2b,c, both swing and stance AS versus WT Holm‐Sidak posthoc analysis, fore: p <0.0001, hind: p <0.0001), which suggests the increase in stride time was not determined by a single component of the gait cycle. In the transitional phases of gait, we observed elevated propulsion time in AS gait (Figure 3d, AS versus WT Holm‐Sidak posthoc analysis, fore: p <0.0001, hind: p <0.0001) whereas time required to decelerate and brake was only detected in the forelimbs (Figure 3e, AS vs WT Holm‐Sidak posthoc analysis, fore: p <0.0001, hind: ns). Time spent in each phase of the cycle, as a function of the whole stride, was abnormal in the hindlimbs of AS mice (Figure S3). We also looked at the percent of the stance phase where both paws are on the treadmill, referred to as % shared time and % double support. Mammals with poor balance tend to have increased double support as it increases stability; however, we observed that the AS mice actually had a decreased % shared time (Figure 2f, t (47) =3.485, p =0.0011). These phenotypes were replicated in a second, independent cohort (Figure S4). No sex differences were observed in WT or AS (Figure S5).
FIGURE 2.

AS mice exhibit deficits in spatial metrics of gait compared to WT controls. (a). During ambulation, stance width between left and right limbs during maximum stance was wider in AS mice in both fore and hindlimbs, potentially indicating an instable posture. (b) AS mice took longer strides in both fore and hindlimbs and therefore, conversely, (c) the step frequency, or cadence, was significantly reduced. Bars represent mean ± SEM *indicates p <0.05, student t‐test between genotypes
FIGURE 3.
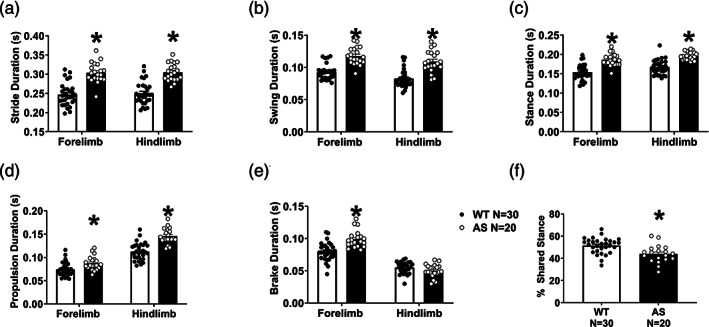
AS mice exhibit deficits in temporal metrics of gait compared to WT controls. (a) Stride duration was elevated in both fore and hindlimbs of adult AS mice, compared to WT controls, requiring more time to make a single step. (b) AS mice showed an increased duration spent in the swing phase, when the paw has no contact with the ground, compared to WT. (c) Converse to swing, it was expected that the AS mice spent less time in the stance phase of the stride. (d) Within the stance phase, propulsion is the lift‐off of the paw from the ground into swing, propelling the animal forward. The AS mice show increased time spent in this phase. (e) At the opposite end of the stance is braking, when the paw exits the swing and meets the ground, which was also shorter in AS, but only in the forelimbs. This is likely related to the specialized role of steering forelimbs are primarily used for in quadrupeds. (f) Curiously, AS mice showed reduced time spent in % shared stance, also known as double support. Bars represent mean ± SEM * indicates p <0.05 (a–e) between genotype post hoc comparison, (f) Student's t‐test
Gait across the lifespan
Next, we wanted to elucidate the progression of observable gait abnormalities in AS mice and determine whether we could observe an onset and specific developmental pattern of nuanced motor disabilities. We assessed gait from weaning until 6 months of age in AS mice to investigate the trajectory of the abnormal gait we observed in adulthood. Over this time course, AS mice showed reliably wider stance widths (Figure 4a, fore: F genotype (1,48) = 22.02, p <0.0001, hind: F genotype (1,48) = 11.65, p <0.0001). As previously, we utilized Holm‐Sidak multiple comparison tests to evaluate changes in gait parameters within genotypes, between time points. To that end, interestingly, while AS mice had wider stances, both AS and WT mice showed the same developmental pattern of stance width with steady increases over development and a sharp increase between PNDs 120 and 180. A longer step length was observed as early as the time of weaning in AS hindlimbs, and PND30 in forelimbs, and it continued to increase throughout development, whereas WT mice showed a slight but significant increase in step length between PNDs 22 and 30 and then showed a stable, mature step length until after PND120 (Figure 4b, fore: F genotype (1,48) = 75.78, p <0.0001, hind: F genotype (1,48) = 76.48, p <0.0001). As expected, we saw the converse in step frequency; AS had a decreased cadence that worsened throughout their lives, while WT mice showed only moderate changes between PNDs 22 and 30 and between 120 and 180 (Figure 4c, fore: F genotype (1,48) = 63.75, p <0.0001, hind: F genotype (1,48) = 70.80, p <0.0001). In humans, as gait matures from first steps into independent walking, the stance width between the feet decreases; step length increases as limb lengths also increase; and cadence, or step frequency, increases as a child's speed increases. Here, speed is held constant, therefore, while stride lengths increase as mice grow, the step frequency inversely decreases.
FIGURE 4.
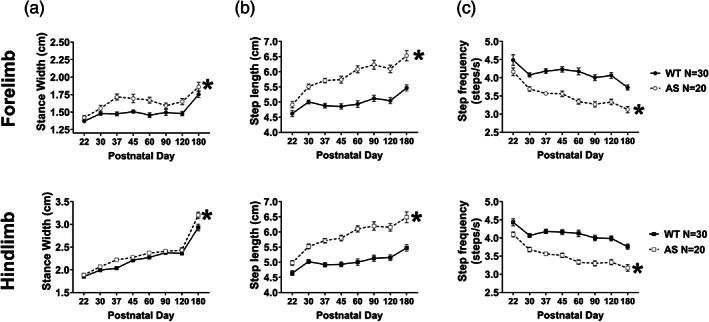
Across development, AS mice exhibited slower progression across all spatial gait indices. (a) Stance width between right and left limbs in both fore (top) and hindlimbs (bottom) increased slightly throughout development but was wider in the AS mice, compared to WT mice, throughout. At the oldest time point, both WT and AS mice showed a sharp increase in stance width with a similar slope. (b) Similarly, AS mice exhibited increased step lengths throughout the experiment and the discrepancy in step length was present at weaning at PND22. Additionally, while WT mice had a stabilized step length after PND30, step length continued to increase in AS mice and began stabilizing around after PND60. The same pattern was observed in both fore and hindlimbs. (c) Expectedly, the stepping frequency showed the converse: In both limbs, AS mice showed reduced step frequency that was present at weaning and progressed throughout development, unlike the WT mice which showed a relatively stable, mature cadence after PND30. *p <0.05, main effect of group of two‐way repeated measured mixed effects model
When we looked at temporal subcomponents of gait, we saw that AS mice had disrupted developmental gait trajectories, with complex temporal patterns. Across all the temporal parameters reported, we observed that AS mice displayed a significantly elongated stride time in both the fore‐ and hindlimbs that was continuously prolonged while WT stride time stabilized after PND30 and until PND120, at which time both groups showed an age‐related increase in stride time (Figure 5a, fore: F genotype (1,48) = 75.92, p <0.0001, hind: F genotype (1,48) = 76.54, p <0.0001). AS mice spent more time in the stance phase than WT mice with both groups showing similar developmental patterns (Figure 5b, fore: F genotype (1,48) = 55.22, p <0.0001, hind: F genotype (1,48) = 55.03, p <0.0001). AS swing time was also detectably longer in the forelimbs at PND30, but this aberration was present at weaning in the hindlimbs (Figure 5c, fore: F genotype(1,48) = 84.17, p <0.0001, hind: F genotype (1,48) = 64.94, p <0.0001). Corroborating our earlier observations, we detected a developmental pattern: a stabilization of gait metrics in WT mice after PND30 and an age‐related increase between PND120 and 180 that was also observed in AS mice. AS mice spent more time in the propulsion phase of gait compared to WT, with both groups showing the same pattern across time (Figure 5d, fore: F genotype(1,48) =34.07, p <0.0001, hind: F genotype (1,48) = 58.53, p <0.0001). AS mice deviated from WT in braking of the hindlimbs, and had increased braking time in the forelimbs (Figure 5e, fore: F genotype (1,48) = 29.31, p <0.0001, hind: F genotype (1,48) =4.599, p =0.371). The pattern of braking duration across time was unique compared to the other temporal metrics. A steep increase in braking time between PND22 and 30 was revealed in both groups; WT had a stabilization until PND120, while the AS mice continued to spend more time in the braking phase until PND60. At PND60, AS mice showed reduced time in double support when both paws are simultaneously on the ground (Figure 2f), however, we saw that this was not the case across the developmental time window we studied (Figure 5f). There was a significant difference between the genotypes (F genotype (1,48) =3.485, p =0.0011), although Holm‐Sidak multiple comparisons could not detect that double support was different from WT, perhaps resulting from more variability and a less robust signal: noise ratio at the earliest and latest time points collected.
FIGURE 5.
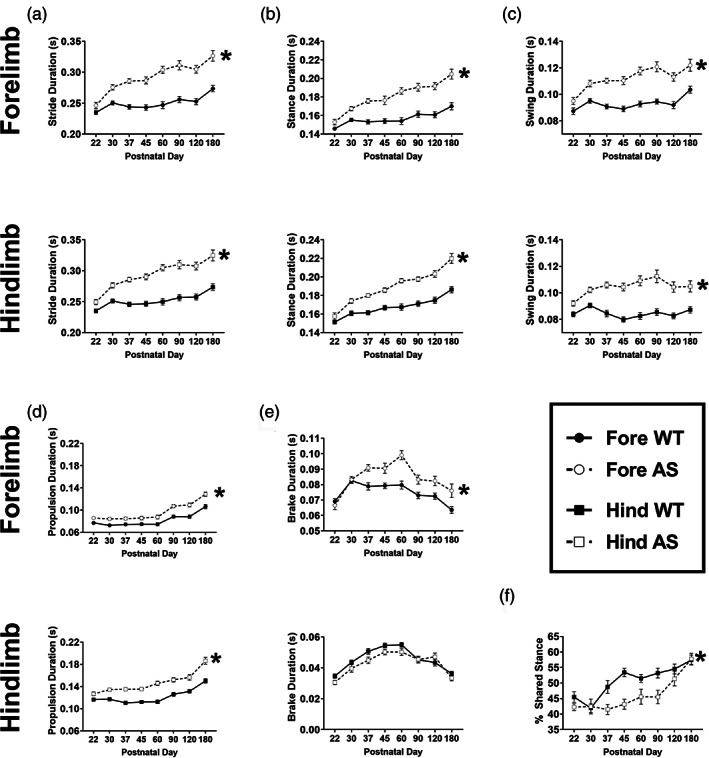
AS mice exhibited aberrant temporal metrics of gait beginning at PND30 with progressive worsening over time. Temporal subcomponents of gait were detectably different in AS mice as early as weaning, but typically at PND30, and continued to progress while WT mice showed maturation and stabilization of gait parameters. (a) Stride duration was significantly higher in the fore and hindlimbs of AS mice, compared to WT. (b) Aberrant stance time was observed in AS mice, in both limbs. (c) Similar to stance time, swing time was also elevated in AS mice. Interestingly, elevated hindlimb swing time was detected earlier at PND22. (d) Propulsion time was increased in the AS group PND30 onwards, which suggests more time is required to produce force necessary to initiate the next step. (e) Time spent in the braking phase was only significantly higher in the forelimbs of AS mice, likely due to the specific use of forelimbs in steering. This difference emerged at PND37. (f) Double support, or % shared stance time, in the hindlimbs, was significantly different between AS and WT mice over the lifespan. *p <0.05, main effect of group of two‐way repeated measured mixed‐effects model
We observed that many abnormalities in AS gait present as early as the first time point, weaning. Before PND22, mice of either genotype were unable to walk consistently and continuously long enough at the requisite speed to capture high‐quality videos for analysis. We attempted to observe whether ambulation abnormalities arose earlier by performing a neonatal circle transverse task and saw that AS pups gained proficient crawling and walking skills later than WT, suggesting motor deficits are present early in life and progress (Figure S2).
DISCUSSION
This is the first report of precisely quantified spatiotemporal indices of gait and motor patterns across development in AS mice, potentially providing reliable and translational functional outcome metrics. Motor deficits have been key in the study of rodent models of AS, as dysfunction on the rotarod and reduced activity have been the most consistently reported behavioral phenotypes, likely related to cerebellar dysfunction (Adhikari et al., 2021; Berg et al., 2020; Born et al., 2017; Cheron et al., 2005; Dodge et al., 2020; Heck et al., 2008; Huang et al., 2013; Sonzogni et al., 2018). These phenotypes are intra‐ and inter‐laboratory reproducible observations in the AS field. Our report extends earlier work on global motor deficits in AS mice. For the first time, we confirm reliable, substantial clinically‐relevant gait aberrations in AS mice, using both spatial and temporal indices, while also providing evidence that gait abnormalities are detectable early in life and progressively decline with age. This study leveraged DigiGait technology which allowed for 1) automation, 2) a longitudinal design to detect phenotypes across the lifespan, when they onset and if they regress and/or decline 3) inherent enhanced reliability, 4) higher throughput, 5) confirmation of test–retest reliability, 6) the ability to collect spatial and temporal metrics, and 7) an emphasis on a uniquely translational aspect of a behavioral phenotype.
We corroborated earlier work that demonstrated moderate to severe global motor deficits in AS mice. Importantly, we discovered numerous quantitative gait parameters that indicated instability and poor gait, similar to clinic reports by Dr. Jessica Duis (Duis, 2021; Duis et al., in review) and others (Grieco et al., 2018; Sadhwani et al., 2021). We observed wider stances in both sets of limbs in juveniles and adults, an indicator of instability since wider stances serve as a compensatory measure for imbalance during ambulation. Abnormalities in gait, via spatial indices, were further detectable by the number of steps taken by the AS subjects. Specifically, the use of longer, but fewer steps, compared to WT. One conjecture is that the speed chosen (20 cm/s) represents a walk for normal WT mice but may be more difficult for the AS mice. In humans, this would be reflected by a gait pattern signature for running, characterized by longer strides and reduced stride frequency (Cappellini et al., 2006). At a higher treadmill speed of 36 cm/s, both genotypes were running, demonstrated by longer stride lengths (Figure S6 ). Double support, when both feet or paws are on the ground at the same time, is informative about balance and stability; in humans, a less stable, balanced gait typically has more time with both limbs on the ground. Double support also typically decreases as a function of speed, in both bipedal humans and quadrupeds: as speed increases, there are fewer instances when both feet, or paws, are on the ground (Cappellini et al., 2006). Children with AS, known to have poor balance, exhibit more double support and less single support while walking and have demonstrably slower walking speeds than age‐matched neurotypical children (Grieco et al., 2018). However, in our study, speed is fixed as the animals are moving on a treadmill, therefore the reduced double support we observed in AS mice may represent the adoption of a running gait pattern to maintain treadmill speed. A limitation of this report is that we did not explore other treadmill speeds with full power for statistical comparisons, comprehensively analyze endurance, or compare to a control group with known normal muscular strength but impoverished motor coordination. More work will need to be performed clinically and preclinically to collect data to answer this query with support. We are currently optimizing developmental protocols to determine if the DigiGait can be utilized to capture the onset and/or development of walking milestones.
Temporal indices of gait, a novel outcome measure unable to be easily captured by previous methodologies, were also altered in AS mice, exhibited by lengthened components of the gait cycle. Stride time, which includes the components of swinging, braking, propulsion, drag, and stance, was increased overall, but not as a function of any singular index. Swing is captured when the paw is in the air, and stance when it is on the ground. Swing and stance times were increased in both sets of limbs in AS mice. Propulsion is the time a paw requires to transition from stance to swing, effectively, by pushing off the ground and propelling forward. We discovered longer propulsion times and elevated paw drag in AS mice (Figure 1). Paw drag is calculated as the area under the curve during the propulsion phase and these two indices are intercorrelated. In quadrupeds, hindlimbs are primarily used to push off and propel the animal forward while the forelimbs are preferentially used for steering and controlling deceleration. Combined, the augmented braking and propulsion/drag suggested that AS mice may lack either fine motor control or may have reduced muscular strength, as more time is required to propel into the next step. Future studies will inform us. One limitation of the current work is that such detailed and nuanced motor deficits have yet to be commonly identified or reported in preclinical models of NDDs, which limited our ability to compare the AS mouse to other NDD models. Our goal is to continue this nuanced examination of spatial and temporal gait indices to confirm and corroborate if these metrics are useful as a rigorous, reliable translational outcome measure, beyond our observations in AS.
For the first time, this study assessed a motoric metric, gait, from weaning to adulthood. We performed this assessment at the urging of AS clinicians, as translational motor measures, to date, remain underdeveloped (Duis, 2021; personal communications with Drs. Duis, Anderson, Jeste, Thurm, and Berry‐Kravis). An earlier report on reliable phenotypes using new and meta‐data reinforced the high signal:noise ratio of behavioral outcome measures of rotarod, nest building, marble burying, and forced swim (Sonzogni et al., 2018). However, these assays each have a pronounced motor component, while only one is typically classified as a motor task (i.e., rotarod); thus, when motor is severely deficient, as we and others have illustrated repeatedly, the mouse cannot explore and dig to bury marbles, build complex nests, nor swim effectively in the forced swim cylinder. Therefore, hesitation was raised regarding the “reliability” and underlying functional components being evaluated in this earlier report of an AS‐tailored behavioral battery.
We found that many abnormal metrics were identifiable at the earliest age tested, PND22 or weaning age. This age was chosen as a first time point because mice younger than PND21 were not able to successfully walk at the requisite speed of 20 cm/s in preliminary studies. Most preclinical studies of NDDs are performed in adults, which, unfortunately, foregoes detection of early phenotypic onset; regression, if present; and progression, or decline, over time. Also, testing different ages at which times the degree of impairment is not ascertained and well‐known can lead to both false‐positive (type I) and false‐negative (type II) errors. Thus, the development and reliability of longitudinal outcomes is key to this research field, as some treatments may only be successful when delivered during “critical windows” (Sonzogni et al., 2019; Sonzogni et al., 2020); however, our laboratory and others have shown functional efficacy and behavioral rescue at multiple time points including adulthood (Adhikari et al., 2021; Bailus et al., 2016; Ciarlone et al., 2016; Daily et al., 2011).
Genotype differences were usually apparent by PND30 or earlier. This corroborates preclinical and clinical findings of global developmental delay (Berg et al., 2020). To support this in the current work, we measured the development of ambulatory skills using the circle traverse assay during the neonatal period (Berg et al., 2018; Ellegood et al., 2021). We observed delayed exiting from the circle in AS pups (i.e., successful ambulation); however, we were unable to delineate the circuits or physical cause underpinning these data by this single rudimentary neonatal index (Figure S2).
In this study, gait analysis proved a reliable, translational assay that can accomplish within‐subject lifespan development, regression, progression and/or decline without confounding test–retest effects. Rotarod, on the other hand, does not account for the inherent confound of increased body weight and lethargy over time nor test–retest effects (i.e., signal is changed with repeated exposure). DigiGait avoids many of these pitfalls and appears to be a versatile tool, sensitive to various treatments, and has an established record of use as a strong longitudinal/repeat testing outcome measure (Hansen & Pulst, 2013). Compared to rotarod, DigiGait is more sensitive to severe deficits and can be used to collect motor data in animals unable to stand on a rod (Lee et al., 2021). Treadmill gait analysis, using ventral imaging, such as DigiGait and the Noldus CatWalk, allows for 1) unhindered access to an ambulating mouse, 2) walking that is unaffected by the experimenter's grasping or “scruffing,” and 3) detection of nuanced and fine‐grained phenotypes (Lei et al., 2014). Quantifiable automated assays with increased sensitivity and high translational value used in corroboration with gold standard assays, such as exploration in a novel open field, may also provide a greater understanding of the pathological profile and more precise signal detection and accurate interpretations.
Many pharmaceutical clinical trials have failed to move forward successfully despite preclinical “rescues”; our work suggests it is possible that tasks lacking translational specificity and analogous neural circuitry contribute to this phenomenon (Gordon, 2019). In other words, the weaknesses of the outcome measures preclinically are equally as vital as the lack of quantifiable outcome measures clinically. We predict that outcome measures with enhanced translational specificity and analogous circuitry will reduce the “valley of death” or “lost in translation” gap in bench‐to‐bedside research. An example is the latency to fall off the rotarod, which is confounded by weight, age, background genetic strain, repeated testing, and lack of complexity in its behavior (Deacon, 2013). This has also been true for other models of NDDs where underdeveloped and insensitive outcome measures have failed to show meaningful change in potentially promising drug studies (Erickson et al., 2017). Automated gait analysis may overcome limitations by presenting a reliable quantitative method of assessing components to clinical gait analysis. Indices and metrics congruent to human gait studies should be more readily included in future behavioral phenotyping deep dives (Hampton et al., 2004; Hansen & Pulst, 2013; Lei et al., 2016, 2019).
Our study showcases the longitudinal use of quantitative motor metrics for a genetic NDD. Clinically, quantitative gait research has gained traction and the idea to tailor attention on gait in preclinical studies is now gaining momentum (FAST and ABOM consortia, IDDRC working group, AGENDA working group, personal communications) for Rett syndrome, neurofibromatosis, Down syndrome, and other NDDs (Hampton et al., 2004). Data from our work showed analogous measures of gait phenotypes in rodents that are being quantified currently in AS clinics (Dr. Jessica Duis, personal communication). We identified a set of parameters that demonstrate maladaptive gait progression, potentially linked to reduced limb strength, instability, and/or lack of capability. We plan to expand this work to other genetic NDDs for which there is a growing body of evidence that there exists a relationship between degree of motor deficit and other behavioral domains, such as social communication and cognition (Copping et al., 2016; DiStefano et al., 2016; Finucane et al., 2016; Shumway et al., 2011; Soorya et al., 2018). In summary, gait is a versatile quantitative outcome measure with great potential for use in therapeutic evaluation.
Supporting information
Supplemental Figure 1 AS mice exhibited elevated paw drag, inferring poor hindlimb propulsion, compared to WT controls.
Supplemental Figure 2 Gait development is delayed in AS mice
Supplemental Figure 3 PND60 AS mice show abnormal portion of stride in temporal subcomponent gait metrics in hindlimbs
Supplemental Figure 4 Gait aberrations in AS mice replicated in independent cohort
Supplemental Figure 5 No sex differences observed in gait metrics in either WT or AS mice
Supplemental Figure 6 Gait aberrations in AS mice are consistent at slower speed, but not higher, running, speed
Supplemental Table 1
ACKNOWLEDGMENTS
This work was supported by generous funding from the Foundation for Angelman Syndrome Therapeutics (FAST; SP, AA, EB, TF, and JLS), NIH R01NS097808 (SP, JLS), and the MIND Institute's Intellectual and Developmental Disabilities Research Center (IDDRC; P50HD103526; PI LA).
Petkova, S. P. , Adhikari, A. , Berg, E. L. , Fenton, T. A. , Duis, J. , & Silverman, J. L. (2022). Gait as a quantitative translational outcome measure in Angelman syndrome. Autism Research, 15(5), 821–833. 10.1002/aur.2697
Funding information Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: P50HD103526; National Institute of Neurological Disorders and Stroke, Grant/Award Number: R01NS097808; Intellectual and Developmental Disabilities Research Center; Foundation for Angelman Syndrome Therapeutics
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Adhikari, A. , Copping, N. A. , Beegle, J. , Cameron, D. L. , Deng, P. , O'Geen, H. , Segal, D. J., Fink, K. D., Silverman, J. L., & Anderson, J. S. (2021). Functional rescue in an Angelman syndrome model following treatment with lentivector transduced hematopoietic stem cells. Human Molecular Genetics, 30(12), 1067–1083. 10.1093/hmg/ddab104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari, A. , Copping, N. A. , Onaga, B. , Pride, M. C. , Coulson, R. L. , Yang, M. , Yasui, D. H., LaSalle, J. M., & Silverman, J. L. (2018). Cognitive deficits in the Snord116 deletion mouse model for Prader‐Willi syndrome. Neurobiology of Learning and Memory, 165, 106874. 10.1016/j.nlm.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amende, I. , Kale, A. , McCue, S. , Glazier, S. , Morgan, J. P. , & Hampton, T. G. (2005). Gait dynamics in mouse models of Parkinson's disease and Huntington's disease. Journal of Neuroengineering and Rehabilitation, 2, 20 doi:10.1186/1743‐0003‐2‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailus, B. J. , Pyles, B. , McAlister, M. M. , O'Geen, H. , Lockwood, S. H. , Adams, A. N. , Nguyen, J. T., Yu, A., Berman, R. F., & Segal, D. J. (2016). Protein delivery of an artificial transcription factor restores widespread Ube3a expression in an Angelman syndrome mouse brain. Molecular Therapy, 24(3), 548–555. 10.1038/mt.2015.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales, K. L. , Solomon, M. , Jacob, S. , Crawley, J. N. , Silverman, J. L. , Larke, R. H. , Sahagun, E., Puhger, K. R., Pride, M. C., & Mendoza, S. P. (2014). Long‐term exposure to intranasal oxytocin in a mouse autism model. Translational Psychiatry, 4, e480. 10.1038/tp.2014.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, E. L. , Copping, N. A. , Rivera, J. K. , Pride, M. C. , Careaga, M. , Bauman, M. D. , Berman, R. F., Lein, P. J., Harony‐Nicolas, H., Buxbaum, J. D., Ellegood, J., Lerch, J. P., Wöhr, M., & Silverman, J. L. (2018). Developmental social communication deficits in the Shank3 rat model of Phelan‐McDermid syndrome and autism spectrum disorder. Autism Research, 11(4), 587–601. 10.1002/aur.1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, E. L. , Jami, S. A. , Petkova, S. P. , Berz, A. , Fenton, T. A. , Lerch, J. P. , Segal, D. J., Gray, J. A., Ellegood, J., Wöhr, M., & Silverman, J. L. Silverman, J. L. (2021). Excessive laughter‐like vocalizations, microcephaly, and translational outcomes in the Ube3a deletion rat model of Angelman syndrome. The Journal of Neuroscience, 41(42), 8801–8814. 10.1523/JNEUROSCI.0925-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, E. L. , Pride, M. C. , Petkova, S. P. , Lee, R. D. , Copping, N. A. , Shen, Y. , Adhikari, A., Fenton, T. A., Pedersen, L. R., Noakes, L. S., Nieman, B. J., Lerch, J. P., Harris, S., Born, H. A., Peters, M. M., Deng, P., Cameron, D. L., Fink, K. D., Beitnere, U., O'Geen, H., … Silverman, J. L. (2020). Translational outcomes in a full gene deletion of ubiquitin protein ligase E3A rat model of Angelman syndrome. Translational Psychiatry, 10(1), 39. 10.1038/s41398-020-0720-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird, L. M. (2014). Angelman syndrome: Review of clinical and molecular aspects. The Application of Clinical Genetics, 7, 93–104. 10.2147/TACG.S57386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born, H. A. , Dao, A. T. , Levine, A. T. , Lee, W. L. , Mehta, N. M. , Mehra, S. , Weeber, E. J., & Anderson, A. E. (2017). Strain‐dependence of the Angelman syndrome phenotypes in Ube3a maternal deficiency mice. Scientific Reports, 7(1), 8451. 10.1038/s41598-017-08825-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, S. P. , Pask, T. , Jones, L. , & Dunnett, S. B. (2004). Behavioural profiles of inbred mouse strains used as transgenic backgrounds. I: Motor tests. Genes, Brain, and Behavior, 3(4), 206–215. 10.1111/j.1601-183X.2004.00072.x [DOI] [PubMed] [Google Scholar]
- Cappellini, G. , Ivanenko, Y. P. , Poppele, R. E. , & Lacquanti, F. (2006). Motor patterns in human walking and running. J. Physio, 95(3), 3426–3437. 10.1152/jn.00081.2006 [DOI] [PubMed] [Google Scholar]
- Carter, R. J. , Morton, J. , & Dunnett, S. B. (2001). Motor coordination and balance in rodents. Current Protocols in Neuroscience, 8, 12. 10.1002/0471142301.ns0812s15 [DOI] [PubMed] [Google Scholar]
- Champion, J. A. , Rose, K. J. , Payne, J. M. , Burns, J. , & North, K. N. (2014). Relationship between cognitive dysfunction, gait, and motor impairment in children and adolescents with neurofibromatosis type 1. Developmental Medicine & Child Neurology, 56(5), 468–474. 10.1111/dmcn.12361 [DOI] [PubMed] [Google Scholar]
- Cheron, G. , Servais, L. , Wagstaff, J. , & Dan, B. (2005). Fast cerebellar oscillation associated with ataxia in a mouse model of Angelman syndrome. Neuroscience, 130(3), 631–637. 10.1016/j.neuroscience.2004.09.013 [DOI] [PubMed] [Google Scholar]
- Ciarlone, S. L. , Grieco, J. C. , D'Agostino, D. P. , & Weeber, E. J. (2016). Ketone ester supplementation attenuates seizure activity, and improves behavior and hippocampal synaptic plasticity in an Angelman syndrome mouse model. Neurobiology of Disease, 96, 38–46. 10.1016/j.nbd.2016.08.002 [DOI] [PubMed] [Google Scholar]
- Copping, N. A. , Adhikari, A. , Petkova, S. P. , & Silverman, J. L. (2019). Genetic backgrounds have unique seizure response profiles and behavioral outcomes following convulsant administration. Epilepsy & Behavior, 101(Pt A, 106547. 10.1016/j.yebeh.2019.106547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copping, N. A. , Berg, E. L. , Foley, G. M. , Schaffler, M. D. , Onaga, B. L. , Buscher, N. , Silverman, J. L., & Yang, M. (2016). Touchscreen learning deficits and normal social approach behavior in the Shank3B model of Phelan‐McDermid syndrome and autism. Neuroscience, 345, 155–165. 10.1016/j.neuroscience.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily, J. L. , Nash, K. , Jinwal, U. , Golde, T. , Rogers, J. , Peters, M. M. , Burdine, R. D., Dickey, C., Banko, J. L., & Weeber, E. J. (2011). Adeno‐associated virus‐mediated rescue of the cognitive defects in a mouse model for Angelman syndrome. PLoS One, 6(12), e27221. 10.1371/journal.pone.0027221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon, R. M. (2013). Measuring motor coordination in mice. Journal of Vision, Exp(75), e2609. 10.3791/2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey, T. M. , Handforth, A. , Anagnostaras, S. G. , Homanics, G. E. , Minassian, B. A. , Asatourian, A. , Fanselow, M. S., Delgado‐Escueta, A., Ellison, G. D., & Olsen, R. W. (1998). Mice lacking the beta3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. The Journal of Neuroscience, 18(20), 8505–8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano, C. , Gulsrud, A. , Huberty, S. , Kasari, C. , Cook, E. , Reiter, L. T. , Thibert, R., & Jeste, S. S. (2016). Identification of a distinct developmental and behavioral profile in children with Dup15q syndrome. Journal of Neurodevelopmental Disorders, 8, 19. 10.1186/s11689-016-9152-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge, A. , Peters, M. M. , Greene, H. E. , Dietrick, C. , Botelho, R. , Chung, D. , Willman, J., Nenninger, A. W., Ciarlone, S., Kamath, S. G., Houdek, P., Sumová, A., Anderson, A. E., Dindot, S. V., Berg, E. L., O'Geen, H., Segal, D. J., Silverman, J. L., Weeber, E. J., & Nash, K. R. (2020). Generation of a novel rat model of Angelman syndrome with a complete Ube3a gene deletion. Autism Research, 13(3), 397–409. 10.1002/aur.2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duis, J. (2021). Multidisciplinary clinic findings on gait in individuals with Angelman syndrome and application of an observational gait scale. Presented at the FAST translational research symposium and Angelman syndrome biomarker and outcome consortium scientific meeting. TX. [Google Scholar]
- Ellegood, J. , Petkova, S. P. , Kinman, A. , Qiu, L. R. , Adhikari, A. , Wade, A. A. , Fernandes, D., Lindenmaier, Z., Creighton, A., Nutter, L., Nord, A. S., Silverman, J. L., & Lerch, J. P. (2021). Neuroanatomy and behavior in mice with a haploinsufficiency of AT‐rich interactive domain 1B (ARID1B) throughout development. Molecular Autism, 12(1), 25. 10.1186/s13229-021-00432-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, C. A. , Davenport, M. H. , Schaefer, T. L. , Wink, L. K. , Pedapati, E. V. , Sweeney, J. A. , Fitzpatrick, S. E., Brown, W. T., Budimirovic, D., Hagerman, R. J., Hessl, D., Kaufmann, W. E., & Berry‐Kravis, E. (2017). Fragile X targeted pharmacotherapy: Lessons learned and future directions. Journal of Neurodevelopmental Disorders, 9, 7. 10.1186/s11689-017-9186-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane, B. M. , Lusk, L. , Arkilo, D. , Chamberlain, S. , Devinsky, O. , Dindot, S. , Spurling Jeste, S. , LaSalle, J. , Reiter, L. , Schenan, N. C. , Spence, S. , Thibert, R. , Calvert, G. , Luchsinger, K. , & Cook, E. (2016). 15q duplication syndrome and related disorders (pp. 1993–2016). University of Washington. [Google Scholar]
- Gentile, J. K. , Tan, W. H. , Horowitz, L. T. , Bacino, C. A. , Skinner, S. A. , Barbieri‐Welge, R. , Bauer‐Carlin, A., Beaudet, A. L., Bichell, T. J., Lee, H. S., Sahoo, T., Waisbren, S. E., Bird, L. M., & Peters, S. U. (2010). A neurodevelopmental survey of Angelman syndrome with genotype–phenotype correlations. Journal of Developmental and Behavioral Pediatrics, 31(7), 592–601. 10.1097/DBP.0b013e3181ee408e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gernsbacher, M. , Sauer, E. , Geye, H. , Schweigert, E. , & Hill Goldsmith, H. (2008). Infant and toddler oral‐ and manual‐motor skills predict later speech fluency in autism. Journal of Child Psychology and Psychiatry, 49(1), 43–50. 10.1111/j.1469-7610.2007.01820.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, J. https://www.nimh.nih.gov/about/director/messages/2019/what-can-animals-tell-us-about-mental-illnesses
- Grieco, J. C. , Ciarlone, S. L. , Gieron‐Korthals, M. , Schoenberg, M. R. , Smith, A. G. , Philpot, R. M. , Philpot, R. M., Heussler, H. S., Banko, J. L., & Weeber, E. J. (2014). An open‐label pilot trial of minocycline in children as a treatment for Angelman syndrome. BMC Neurology, 14(1), 232. 10.1186/s12883-014-0232-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco, J. C. , Gouelle, A. , & Weeber, E. J. (2018). Identification of spatiotemporal gait parameters and pressure‐related characteristics in children with Angelman syndrome: A pilot study. Journal of Applied Research in Intellectual Disabilities, 31(6), 1219–1224. 10.1111/jar.12462 [DOI] [PubMed] [Google Scholar]
- Hampton, T. (2004). Shuffling mouse's genetic deck may illuminate causes of complex diseases. JAMA, 292(21), 2568, 10.1001/jama.292.21.2568. [DOI] [PubMed] [Google Scholar]
- Hampton, T. G. , & Amende, I. (2010). Treadmill gait analysis characterizes gait alterations in Parkinson's disease and amyotrophic lateral sclerosis mouse models. Journal of Motor Behavior, 42(1), 1, 10.1080/00222890903272025–4. [DOI] [PubMed] [Google Scholar]
- Hampton, T. G. , Stasko, M. R. , Kale, A. , Amende, I. , & Costa, A. C. (2004). Gait dynamics in trisomic mice: Quantitative neurological traits of down syndrome. Physiology & Behavior, 82(2–3), 381–389. 10.1016/j.physbeh.2004.04.006 [DOI] [PubMed] [Google Scholar]
- Hansen, S. T. , & Pulst, S. M. (2013). Response to ethanol induced ataxia between C57BL/6J and 129X1/SvJ mouse strains using a treadmill based assay. Pharmacology, Biochemistry, and Behavior, 103(3), 582–588. 10.1016/j.pbb.2012.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck, D. H. , Zhao, Y. , Roy, S. , LeDoux, M. S. , & Reiter, L. T. (2008). Analysis of cerebellar function in Ube3a‐deficient mice reveals novel genotype‐specific behaviors. Human Molecular Genetics, 17(14), 2181–2189. 10.1093/hmg/ddn117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homanics, G. E. , DeLorey, T. M. , Firestone, L. L. , Quinlan, J. J. , Handforth, A. , Harrison, N. L. , Krasowski, M. D., Rick, C. E., Korpi, E. R., Mäkelä, R., Brilliant, M. H., Hagiwara, N., Ferguson, C., Snyder, K., & Olsen, R. W. (1997). Mice devoid of gamma‐aminobutyrate type a receptor beta3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proceedings of the National Academy of Sciences of the United States of America, 94(8), 4143–4148. 10.1073/pnas.94.8.4143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. S. , Burns, A. J. , Nonneman, R. J. , Baker, L. K. , Riddick, N. V. , Nikolova, V. D. , Riday, T. T., Yashiro, K., Philpot, B. D., & Moy, S. S. (2013). Behavioral deficits in an Angelman syndrome model: Effects of genetic background and age. Behavioural Brain Research, 243, 79–90. 10.1016/j.bbr.2012.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y. H. , Armstrong, D. , Albrecht, U. , Atkins, C. M. , Noebels, J. L. , Eichele, G. , Sweatt, J. D., & Beaudet, A. L. (1998). Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long‐term potentiation. Neuron, 21(4), 799–811. [DOI] [PubMed] [Google Scholar]
- Landers, M. , Calciano, M. A. , Colosi, D. , Glatt‐Deeley, H. , Wagstaff, J. , & Lalande, M. (2005). Maternal disruption of Ube3a leads to increased expression of Ube3a‐ATS in trans. Nucleic Acids Research, 33(13), 3976–3984. 10.1093/nar/gki705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. , Miller, M. R. , Fernandez, M. A. , Berg, E. L. , Prada, A. M. , Ouyang, Q. , Schmidt, M. , Silverman, J. L. , Young‐Pearse, T. L. , & Morrow, E. M. (2021). Early lysosome defects precede neurodegeneration with amyloid‐β and tau aggregation in NHE6‐null rat brain. Brain, 10.1093/brain/awab467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, C. , Sunzi, K. , Dai, F. , Liu, X. , Wang, Y. , Zhang, B. , He, L., & Ju, M. (2019). Effects of virtual reality rehabilitation training on gait and balance in patients with Parkinson's disease: A systematic review. PLoS One, 14(11), e0224819. 10.1371/journal.pone.0224819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, H. , Toosizadeh, N. , Schwenk, M. , Sherman, S. , Karp, S. , Sternberg, E. , & Najafi, B. (2016). A pilot clinical trial to objectively assess the efficacy of Electroacupuncture on gait in patients with Parkinson's disease using body worn sensors. PLoS One, 11(5), e0155613. 10.1371/journal.pone.0155613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, P. , Ayton, S. , Moon, S. , Zhang, Q. , Volitakis, I. , Finkelstein, D. I. , & Bush, A. I. (2014). Motor and cognitive deficits in aged tau knockout mice in two background strains. Molecular Neurodegeneration, 9, 29 doi: 10.1186/1750-1326-9-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard,, H. C. (2016). The Impact of Poor Motor Skills on Perceptual, Social and Cognitive Development: The Case of Developmental Coordination Disorder. Frontiers in Psychology, 7, 10.3389/fpsyg.2016.00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura, T. , Sutcliffe, J. S. , Fang, P. , Galjaard, R. J. , Jiang, Y. H. , Benton, C. S. , Rommens, J. M., & Beaudet, A. L. (1997). De novo truncating mutations in E6‐AP ubiquitin‐protein ligase gene (UBE3A) in Angelman syndrome. Nature Genetics, 15(1), 74–77. 10.1038/ng0197-74 [DOI] [PubMed] [Google Scholar]
- Micheletti, S. , Palestra, F. , Martelli, P. , Accorsi, P. , Galli, J. , Giordano, L. , Trebeschi, V. , & Fazzi, E. (2016). Neurodevelopmental profile in Angelman syndrome: more than low intelligence quotient. Italian Journal of Pediatrics, 42(1), 91. 10.1186/s13052-016-0301-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura, K. , Kishino, T. , Li, E. , Webber, H. , Dikkes, P. , Holmes, G. L. , & Wagstaff, J. (2002). Neurobehavioral and Electroencephalographic Abnormalities in Ube3aMaternal‐Deficient Mice. Neurobiology of Disease, 9(2), 149–159. 10.1006/nbdi.2001.0463 [DOI] [PubMed] [Google Scholar]
- Mulherkar, S. A. , & Jana, N. R. (2010). Loss of dopaminergic neurons and resulting behavioural deficits in mouse model of Angelman syndrome. Neurobiology of Disease, 40(3), 586–592. 10.1016/j.nbd.2010.08.002 [DOI] [PubMed] [Google Scholar]
- Peters, S. U. , Beaudet, A. L. , Madduri, N. , & Bacino, C. A. (2004). Autism in Angelman syndrome: implications for autism research. Clinical Genetics, 66(6), 530–536. 10.1111/j.1399-0004.2004.00362.x [DOI] [PubMed] [Google Scholar]
- Sadhwani, A. , Wheeler, A. , Gwaltney, A. , Peters, S. U. , Barbieri‐Welge, R. L. , Horowitz, L. T. , Noll, L. M., Hundley, R. J., Bird, L. M., & Tan, W. H. (2021). Developmental skills of individuals with Angelman syndrome assessed using the Bayley‐III. Journal of Autism and Developmental Disorders. 26(1), 2–9. 10.1007/s10803-020-04861-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar, B. L. , & Mink, J. W. (2003). Movement disorders in children. Pediatrics in Review, 24(2), 39–51. 10.1542/pir.24-2-39 [DOI] [PubMed] [Google Scholar]
- Shumway, S. , Thurm, A. , Swedo, S. E. , Deprey, L. , Barnett, L. A. , Amaral, D. G. , Rogers, S. J., & Ozonoff, S. (2011). Brief report: Symptom onset patterns and functional outcomes in young children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 41(12), 1727–1732. 10.1007/s10803-011-1203-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkkonen, S. T. , Homanics, G. E. , & Korpi, E. R. (2003). Mouse models of Angelman syndrome, a neurodevelopmental disorder, display different brain regional GABA(a) receptor alterations. Neuroscience Letters, 340(3), 205–208. 10.1016/s0304-3940(03)00123-x [DOI] [PubMed] [Google Scholar]
- Sonzogni, M. , Hakonen, J. , Bernabe Kleijn, M. , Silva‐Santos, S. , Judson, M. C. , Philpot, B. D. , van Woerden, G. M., & Elgersma, Y. (2019). Delayed loss of UBE3A reduces the expression of Angelman syndrome‐associated phenotypes. Molecular Autism, 10, 23. 10.1186/s13229-019-0277-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonzogni, M. , Wallaard, I. , Santos, S. S. , Kingma, J. , du Mee, D. , van Woerden, G. M. , & Elgersma, Y. (2018). A behavioral test battery for mouse models of Angelman syndrome: A powerful tool for testing drugs and novel Ube3a mutants. Molecular Autism, 9, 47. 10.1186/s13229-018-0231-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonzogni, M. , Zhai, P. , Mientjes, E. J. , van Woerden, G. M. , & Elgersma, Y. (2020). Assessing the requirements of prenatal UBE3A expression for rescue of behavioral phenotypes in a mouse model for Angelman syndrome. Molecular Autism, 11(1), 70. 10.1186/s13229-020-00376-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soorya, L. , Leon, J. , Trelles, M. P. , & Thurm, A. (2018). Framework for assessing individuals with rare genetic disorders associated with profound intellectual and multiple disabilities (PIMD): The example of Phelan McDermid syndrome. The Clinical Neuropsychologist, 32(7), 1226–1255. 10.1080/13854046.2017.1413211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand, A. , Aragaki, A. , Baquet, Z. , Hodges, A. , Cunningham, P. , Holmans, P. , Jones, K. , Jones, L. , Kooperberg, C. , & Olson, J. (2007). Conservation of Regional Gene Expression in Mouse and Human Brain. PLoS Genetics, 3(4), e59. 10.1371/journal.pgen.0030059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe, J. S. , Jiang, Y. H. , Galijaard, R. J. , Matsuura, T. , Fang, P. , Kubota, T. , Christian, S. L., Bressler, J., Cattanach, B., Ledbetter, D. H., & Beaudet, A. L. (1997). The E6‐Ap ubiquitin‐protein ligase (UBE3A) gene is localized within a narrowed Angelman syndrome critical region. Genome Research, 7(4), 368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, W. H. , Bacino, C. A. , Skinner, S. A. , Anselm, I. , Barbieri‐Welge, R. , Bauer‐Carlin, A. , Beaudet, A. L., Bichell, T. J., Gentile, J. K., Glaze, D. G., Horowitz, L. T., Kothare, S. V., Lee, H. S., Nespeca, M. P., Peters, S. U., Sahoo, T., Sarco, D., Waisbren, S. E., & Bird, L. M. (2011). Angelman syndrome: Mutations influence features in early childhood. American Journal of Medical Genetics. Part A, 155A(1), 81–90. 10.1002/ajmg.a.33775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurm, A. , Kelleher, B. , & Wheeler, A. (2020). Outcome measures for Core symptoms of intellectual disability: State of the field. American Journal on Intellectual and Developmental Disabilities, 125(6), 418–433. 10.1352/1944-7558-125.6.418 [DOI] [PubMed] [Google Scholar]
- Wheeler, A. C. , Sacco, P. , & Cabo, R. (2017). Unmet clinical needs and burden in Angelman syndrome: A review of the literature. Orphanet Journal of Rare Diseases, 12(1), 164. 10.1186/s13023-017-0716-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, C. , & Franco, L. (2010). Angelman syndrome at the synapse: Meeting report of the Angelman Syndrome Foundation's 2009 scientific symposium. Journal of Child Neurology, 25(2), 254–261. 10.1177/0883073809353450 [DOI] [PubMed] [Google Scholar]
- Williams, C. A. (2010). The behavioral phenotype of the Angelman syndrome. American Journal of Medical Genetics. Part C, Seminars in Medical Genetics, 154C(4), 432–437. 10.1002/ajmg.c.30278 [DOI] [PubMed] [Google Scholar]
- Williams, C. A. , Driscoll, D. J. , & Dagli, A. I. (2010). Clinical and genetic aspects of Angelman syndrome. Genetics in Medicine, 12(7), 385–395. 10.1097/GIM.0b013e3181def138 [DOI] [PubMed] [Google Scholar]
- Yamasaki, K. , Joh, K. , Ohta, T. , Masuzaki, H. , Ishimaru, T. , Mukai, T. , Niikawa, N., Ogawa, M., Wagstaff, J., & Kishino, T. (2003). Neurons but not glial cells show reciprocal imprinting of sense and antisense transcripts of Ube3a. Human Molecular Genetics, 12(8), 837–847. [DOI] [PubMed] [Google Scholar]
- Yang, M. , Bozdagi, O. , Scattoni, M. L. , Wohr, M. , Roullet, F. I. , Katz, A. M. , Abrams, D. N., Kalikhman, D., Simon, H., Woldeyohannes, L., Zhang, J. Y., Harris, M. J., Saxena, R., Silverman, J. L., Buxbaum, J. D., & Crawley, J. N. (2012). Reduced excitatory neurotransmission and mild autism‐relevant phenotypes in adolescent Shank3 null mutant mice. The Journal of Neuroscience, 32(19), 6525–6541. 10.1523/JNEUROSCI.6107-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 AS mice exhibited elevated paw drag, inferring poor hindlimb propulsion, compared to WT controls.
Supplemental Figure 2 Gait development is delayed in AS mice
Supplemental Figure 3 PND60 AS mice show abnormal portion of stride in temporal subcomponent gait metrics in hindlimbs
Supplemental Figure 4 Gait aberrations in AS mice replicated in independent cohort
Supplemental Figure 5 No sex differences observed in gait metrics in either WT or AS mice
Supplemental Figure 6 Gait aberrations in AS mice are consistent at slower speed, but not higher, running, speed
Supplemental Table 1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


