Summary
Background
Despite high rates of depression and anxiety, little is known about the use of antidepressants amongst individuals diagnosed with inflammatory bowel disease (IBD).
Aims
To evaluate temporal trends in the use of antidepressants; rates of antidepressant initiation and adherence of antidepressant use to international guidelines amongst individuals with IBD.
Methods
This is a study of 14,525 incident IBD cases from 2004 to 2016 compared with 58,027 controls matched 1:4 for age and sex from the Clinical Practice Research Datalink. After excluding tricyclic antidepressants, we performed a Cox regression analysis to determine the risk associated with antidepressant use and logistic regression analysis to determine risk associated with antidepressant undertreatment.
Results
Antidepressant use amongst individuals with IBD increased by 51% during the 12‐year study period, who were 34% more likely to initiate antidepressants in the year after IBD diagnosis compared with controls (aHR:1.34, 95% CI 1.21‐1.49). In those with IBD starting antidepressants, 67% received treatment lasting less than the duration recommended in international guidelines, of which 34% were treated for 1 month or less.
18‐24 year olds were twice as likely to discontinue treatment within 1 month compared with those aged 40‐60 years (aHR:2.03, 95% CI 1.40‐2.95). Socioeconomic deprivation was also associated with early treatment discontinuation (aHR:1.40, 95% CI 1.07‐1.83).
Conclusions
In the year following IBD diagnosis individuals are significantly more likely to start antidepressants compared with controls, but treatment duration fell short of recommendations in the majority. Better integration of services may benefit individuals with IBD and psychiatric comorbidity.
Keywords: antidepressant medication, antidepressants, anxiety, clinical practice research datalink, Crohn’s disease, depression, incidence, inflammatory bowel disease, ulcerative colitis

1. INTRODUCTION
Depression and anxiety are approximately twice as common amongst individuals living with inflammatory bowel disease (IBD) relative to the general population, and these conditions may often go undetected or undertreated. 1 , 2 , 3 The importance of this mental health burden has been starkly highlighted by recent findings indicating an excess risk of suicide in the IBD population. 4 In its active state, IBD manifests with symptoms of abdominal pain, weight loss, diarrhoea and rectal bleeding, which may result in reduced quality of life, social functioning and mental well‐being. 5 Conversely, individuals with IBD who suffer from depression and anxiety are more likely to have adverse IBD outcomes and increased contact with healthcare providers. 6 , 7 , 8
Depression and anxiety are the most common comorbid psychiatric disorders diagnosed amongst individuals with IBD. 4 Antidepressant medications (ADM) are most frequently used to treat these conditions. 9 However, data regarding their use amongst individuals with coexistent IBD is lacking. In order for antidepressants to maintain remission and reduce the risk of relapse of depression and anxiety, international guidelines indicate a treatment course should continue for at least 6 months following symptom resolution. 10 , 11 , 12 , 13 , 14 Despite these recommendations, no previous studies have examined whether antidepressants are prescribed for an appropriate duration amongst IBD patients. Research is needed to identify risk factors predicting undertreatment with antidepressant medication in order to guide the development of appropriately targeted integrated care pathways.
We, therefore, aimed to: (1) compare rates of antidepressant initiation following IBD diagnosis with a matched control cohort without IBD; (2) determine the duration of antidepressant treatment and assess adherence to international guidelines; (3) examine risk factors associated with sub‐optimal antidepressant treatment duration and (4) examine temporal trends in antidepressant prescribing in line with guidelines.
2. METHODS
2.1. Design and data source
We obtained ethical and scientific approval for the use of the Clinical Practice Research Datalink (CPRD) for a comparison cohort study from the Independent Scientific Advisory Committee [ISAC Protocol number: 15_018R].
We analysed routinely collected primary care data from electronic health records from general practices that contributed to the CPRD, the largest validated primary care research database in the world. 15 It contains longitudinal, patient‐level, anonymised electronic health records of 18 million patients from more than 700 general practices and is broadly representative of the UK population. The median follow‐up for individuals registered on CPRD is 9.4 years, allowing the study of long‐term outcomes. Primary care physicians use clinical codes to record symptoms, diagnoses and prescriptions. Participating practices need to achieve and maintain “up to standard” status to continue contributing to the dataset. The coding system has been previously validated for use in IBD and mental health disorders. 16 , 17
2.2. Selection of IBD (Crohn’s disease and ulcerative colitis) and control cohorts
We defined incident cases of IBD, using a previously validated and published methodology, as individuals who had a first diagnostic Read code for either Crohn’s disease (CD) or Ulcerative colitis (UC) at least 1 year after registering with an “Up To Standard” practice between 1 January 2004 and 31 December 2015.
We excluded individuals if they had codes for both CD and UC, or indeterminate codes such as “non‐specific colitis.” We matched the IBD cohort by age and sex with four randomly selected controls without a record of IBD at any stage of their follow‐up to form a control cohort. Members of the control cohort were assigned the IBD diagnosis date of their matched IBD case termed as “pseudo‐diagnosis date.”
Individuals were followed forward from an index date 2 years prior to their recorded date of IBD diagnosis in CPRD. Our recent study demonstrated a significant excess of depression in the 2 years prior to diagnosis of IBD. 17 Therefore we considered the index date as 2 years prior to the IBD diagnosis/pseudo‐diagnosis date, in order to also capture incident psychiatric morbidity that develops in the peri‐diagnostic period requiring treatment with antidepressant medication. Follow‐up time started and continued from the index date up to the first recorded date of antidepressant use, when an individual left the practice or died, if these occurred before that time, or at the study end date. All individuals regardless of whether they had a record for a psychiatric comorbidity prior to the study index date were included in the cohort, in order to ensure that the entire at‐risk population was considered.
2.3. Outcome definition
Our main outcomes were the incidence of antidepressant use following the index date in relation to diagnosis or pseudo‐diagnosis with IBD and the proportion of antidepressant episodes which had a duration of 7 months or more as recommended in guidelines. Excluding tricyclic antidepressants, we examined temporal trends in the rates of initiation of seven most commonly prescribed antidepressants accounting for over 99% of the total antidepressant prescriptions in 2012. 18 , 19 We excluded tricyclic antidepressants from our main analysis since we previously found they are primarily prescribed at low dose for indications other than anxiety and depression. 19 The incidence of tricyclic antidepressant use was considered in separate sub‐group analyses.
We defined incident antidepressant use as the first recorded prescription for an antidepressant medication following the index date in relation to diagnosis or pseudo‐diagnosis with IBD (Supplemental appendix F ‐ ADM Code List). Individuals were excluded from the incident antidepressant cohort if they had a prior record of antidepressant use at any time before the study index date.
We used the term “antidepressant episode” to describe the period from initiating to discontinuing an antidepressant. We determined the proportion of antidepressant episodes lasting at least 7 months, as this is the minimum recommended duration of antidepressant treatment. 10 , 11 , 12 , 13 , 14 This duration is based on international guidance that a course of antidepressant medication should continue for at least 6 months after full symptom resolution, which normally takes at least 1 month, irrespective of the presence of comorbid chronic medical conditions, including IBD. 10 , 11 , 12 , 13 , 14
We calculated the duration of an “antidepressant episode” as any period of continuous antidepressant use with less than 90 days between antidepressant prescriptions. We chose the cut‐off of 90 days since we found the majority of individuals received prescriptions every 2 months. The cut‐off allowed for late collection of prescriptions without being considered to have discontinued treatment.
We determined the rate of new antidepressant prescriptions by individual drug, to explore antidepressant prescribing trends between 2004 and 2015. In this analysis, we considered prescribing episodes involving two or more prescriptions.
We determined the incidence of depression and anxiety following the index date in relation to diagnosis or pseudo‐diagnosis with IBD. We defined incident depression and anxiety as individuals with a first‐ever record of depression, anxiety or those with symptoms of depression or anxiety (Supplemental appendix G ‐ Depression and Anxiety Code List). Individuals were excluded from the incident depression and anxiety cohort if they had a record for these conditions at any time before their index date.
2.4. Covariates
To identify risk factors associated with antidepressant medication undertreatment a priori, we identified relevant variables based on clinical knowledge and published literature as previously described. 19 , 20 We explored potential risk factors for undertreatment with antidepressant medication and adjusted for the following covariates: sex, age at IBD diagnosis (<18, 18‐24, 25‐39, 40‐59, and >60 years), socio‐economic deprivation (SED), smoking status, corticosteroid use and era of IBD diagnosis.
We used the Index of Multiple Deprivation (IMD), a postcode‐linked measure of socio‐economic deprivation, to assign individuals to one of five groups defined using IMD quintile cut‐off points, from IMD group 1 (least deprived) to 5 (most deprived).
We defined individuals as “smokers,” “ex‐smokers” or “non‐smokers” based on codes for smoking status preceding diagnosis. Individuals whose most recent code indicated active smoking were classed as “smokers” and those with codes indicating previous but not current smoking were classed as “ex‐smokers”; individuals with only “non‐smoker” codes were classed as “non‐smokers.”
We defined corticosteroid use by identifying individuals who were prescribed at least one episode of corticosteroids at any point after the study index date. To examine changes in antidepressant prescribing practice over the study period, we adjusted for era of IBD diagnosis (era 1: 2004‐2007; era 2: 2008‐2011; era 3: 2012‐2015).
3. STATISTICAL ANALYSIS
Baseline characteristics of the cohort were summarised using frequencies and percentages. We used t‐tests and the one‐way analysis of variance (ANOVA) to determine differences between groups of continuous data, and chi‐squared test for comparisons of categorical data. We calculated crude incidence rates of antidepressant use amongst the IBD and control cohorts. We first used a univariable Cox proportional hazards model to calculate hazard ratios (HR) for the risk of incident antidepressant use followed by a multivariable regression analysis adjusting for sex, age at IBD diagnosis, socioeconomic deprivation, smoking status, corticosteroid use and era of IBD diagnosis. We also adjusted for clustering by general practice to account for variation in diagnosis, prescribing and coding by practice. We then calculated the proportion of antidepressant medication episodes which lasted the recommended minimum duration of 7 months. We used simple and multiple ordered logistic regression analysis to identify risk factors associated with antidepressant medication undertreatment. We conducted further analysis to examine the risk of discontinuing an antidepressant medication following a single prescription, to gain an understanding of the proportion of the IBD cohort who were considered to have psychiatric comorbidity severe enough to warrant treatment but did not continue treatment beyond 28 days. We calculated the rate of new antidepressant prescriptions per 100‐person years at risk by each drug during each year of the study period.
We calculated the crude and adjusted incidence rates of depression and anxiety from the study index date, amongst the IBD and control cohort. We developed a Cox regression model for a multivariable regression analysis to determine risk factors for the incidence of depression and anxiety amongst individuals diagnosed with IBD adjusting for sex, age at IBD diagnosis, socioeconomic deprivation, smoking status, corticosteroid use and era of IBD diagnosis. We conducted a sensitivity analysis where individuals with a record for depression, anxiety and antidepressant use prior to the index date were included in order to obtain estimates of the proportion of the IBD population who experienced depression, anxiety or antidepressant use in the period either side of the study index date (Supplemental appendix A). All analyses were performed using STATA 16 (Statacorp LP, College Station, TX, USA).
4. RESULTS
We identified 14 525 incident cases of IBD diagnosed between 1 January 2004, and 31 December 2015. Of these, 4436 had CD and 10 089 had UC. We identified 58,100 age and sex‐matched controls. Following the initial matching process, 73 individuals of the control cohort (<0.001%) were later diagnosed with IBD during the study follow up period and were therefore excluded from the study, leaving a control cohort of 58 027 individuals (Table 1).
TABLE 1.
Baseline characteristics of study population
| IBD status | Crohn’s disease | Ulcerative colitis | Controls | P value |
|---|---|---|---|---|
| 4,436 | 10,089 | 58,027 | ||
| Demographics | ||||
| Men: n (%) | 2096 (47) | 5396 (53) | 29 930 (52) | 0.99 |
| Age at diagnosis: n (%) | ||||
| <18 years | 492 (12) | 372 (4) | 3452 (6) | 0.99 |
| 18‐25 years | 504 (12) | 553 (6) | 4226 (8) | 0.98 |
| 25‐40 years | 1092 (26) | 2377 (24) | 13 866 (25) | 0.97 |
| 40‐60 years | 1216 (29) | 3267 (34) | 17 910 (32) | 0.99 |
| ≥60 years | 958 (22) | 3204 (33) | 16 617 (30) | 0.96 |
| Social deprivation: n (%) | ||||
| IMD* 1‐3 | 1614 (37) | 4131 (41) | 21 337 (37) | <0.0001 |
| IMD 4‐5 | 918 (21) | 1780 (18) | 12 151 (21) | <0.0001 |
| Unknown | 1904 (42) | 4178 (41) | 24 539 (42) | <0.0001 |
| Smoking status n (%) | ||||
| Smoker | 1110 (25) | 1044 (10) | 5506 (9) | <0.0001 |
| Ex‐smoker | 1037 (23) | 3752 (37) | 6591 (11) | <0.0001 |
| Never | 1198 (27) | 2895 (29) | 22 458 (38) | <0.0001 |
| Missing | 1091 (25 | 2398 (24) | 23 472 (40) | <0.0001 |
IMD, Index of Multiple Deprivation; IMD 1 represents the least deprived and IMD 5 the most deprived. Data are available only for individual’s resident in England.
4.1. Antidepressant use in IBD vs control cohort
During a median follow up of 7.7 years we found the incidence rate of antidepressant use was 19.54 vs 16.94/1000 person‐years amongst individuals with IBD and the control cohort without IBD, respectively. The highest risk of incident antidepressant use was observed during the first year after IBD diagnosis (aHR = 1.34, 95% CI, 1.21‐1.49). The excess risk persisted for 10 years after diagnosis (aHR = 1.11, 95% CI, 1.04‐1.17) (Table 2). The incidence rate of tricyclic antidepressant use, considered separately, was 17.76 vs 8.41/1000 person‐years amongst individuals with IBD and the control cohort without IBD, respectively. Similarly, the highest risk of incident tricyclic antidepressant use was observed during the first year after IBD diagnosis (aHR = 1.59, 95% CI, 1.42‐1.77) (Supplemental appendix B).
TABLE 2.
Risk of first ADM use in the first year and 10 years following diagnosis amongst individuals with IBD compared with the control cohort
| Outcome | First year | Ten years | ||
|---|---|---|---|---|
| Unadjusted HR (95% CI; P value) | Adjusted* HR (95% CI; P value) | Unadjusted HR (95% CI; P value) | Adjusted* HR (95% CI; P value) | |
| Cohort | ||||
| Matched controls | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| IBD | 1.49 (1.34‐1.64; 0.000) | 1.34 (1.21‐1.49; 0.000) | 1.20 (1.13‐1.27; 0.000) | 1.11 (1.04‐1.18; 0.001) |
| Sex | ||||
| Male | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| Female | 1.46 (1.32‐1.61; 0.000) | 1.43 (1.29‐1.58; 0.000) | 1.44 (1.37‐1.52; 0.000) | 1.45 (1.38‐1.53; 0.000) |
| Age at diagnosis (years) | ||||
| <18 | 0.14 (0.09‐0.24; 0.000) | 0.24 (0.15‐0.41; 0.000) | 0.56 (0.49‐0.65; 0.000) | 0.67 (0.57‐0.78; 0.000) |
| 18‐25 | 1.29 (1.08‐1.53; 0.007) | 1.42 (1.19‐1.70; 0.000) | 1.57 (1.44‐1.72; 0.000) | 1.67 (1.52‐1.84; 0.000) |
| 25‐40 | 1.22 (1.08‐1.39; 0.002) | 1.25 (1.10‐1.42; 0.000) | 1.29 (1.20‐1.38; 0.000) | 1.29 (1.21‐1.39; 0.000) |
| 40‐60 | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| ≥60 | 0.90 (0.79‐1.02; 0.103) | 0.91 (0.80‐1.04; 0.162) | 0.95 (0.89‐1.02; 0.192) | 0.97 (0.91‐1.04; 0.437) |
| Social deprivation | ||||
| IMD 1‐3 | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| IMD 4‐5 | 1.18 (1.02‐1.35; 0.018) | 1.09 (0.95‐1.25; 0.214) | 1.19 (1.10‐1.28; 0.000) | 1.12 (1.04‐1.21; 0002) |
| Unknown | 1.04 (0.93‐1.68; 0.442) | 1.02 (0.91‐1.14; 0.735) | 1.06 (0.99‐1.12; 0.067) | 1.00 (0.95‐1.07; 0.815) |
| Era of IBD diagnosis | ||||
| Era 1: 2004‐2007 | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| Era 2: 2008‐2011 | 1.13 (1.00‐1.28; 0.045) | 1.02 (0.90‐1.15; 0.772) | 1.30 (1.22‐1.38; 0.000) | 1.27 (1.19‐1.35; 0.000) |
| Era 3: 2012‐2015 | 1.56 (1.39‐1.77; 0.000) | 1.35 (1.19‐1.53; 0.000) | 1.82 (1.69‐1.97; 0.000) | 1.76 (1.63‐1.91; 0.000) |
| Smoking status, n (%) | ||||
| Never | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| Smoker | 2.02 (1.77‐2.30; 0.000) | 1.94 (1.70‐2.21; 0.000) | 1.74 (1.62‐1.89; 0.000) | 1.71 (1.58‐1.84; 0.000) |
| Ex‐smoker | 1.25 (1.10‐1.42; 0.001) | 1.26 (1.10‐1.45; 0.001) | 1.17 (1.08‐1.26; 0.000) | 1.24 (1.15‐1.34; 0.000) |
| Missing | 0.46 (0.39‐0.54; 0.000) | 0.53 (0.45‐0.63; 0.000) | 0.89 (0.84‐0.96; 0.003) | 0.99 (0.92‐1.07; 0.797) |
| Corticosteroid use | ||||
| No | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| Yes | 0.83 (0.57‐1.21; 0.328) | 1.11 (0.76‐1.64; 0.585) | 0.78 (0.63‐0.94; 0.010) | 0.89 (0.73‐1.10; 0.260) |
All covariates in the table were included within the adjusted analysis. Abbreviations: ADM (Antidepressant medication); HR (Hazard Ratio); CI (Confidence Interval) * IBD (Inflammatory Bowel Disease) *IMD ‐ Index of Multiple Deprivation; IMD 1 represents the least deprived and IMD 5 the most deprived. IMD ‐ Data are available only for individual’s resident in England.
Bold values indicate statistical significance.
4.2. Antidepressant episode duration and predictors of undertreatment
The median duration of an antidepressant prescribing episode was 98 days (Interquartile range: 28‐317 days; total range 28‐4977 days). Amongst individuals with IBD who started on an antidepressant medication, two‐thirds (67%) received treatment for less than the recommended minimum duration of 7 months. Individuals aged 18‐24 years at IBD diagnosis were twice as likely to discontinue antidepressant treatment early compared with individuals aged between 40 and 60 years at diagnosis (aHR = 2.03; 95% CI, 1.40‐2.95). Amongst individuals initiating an antidepressant, 78% of 18‐24‐year‐olds received an antidepressant treatment course lasting less than recommended guidance compared with 61% of 40‐60‐year‐olds (Table 3).
TABLE 3.
Predictors of first ADM episode duration amongst the IBD population
| ADM episode lasting <6 months | ADM episode lasting ≤28 days | |||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI; P value) | Unadjusted OR (95% CI) | Adjusted OR (95% CI; P value) | |
| IBD Cohort | ||||
| UC | 1 (‐) | 1 (‐) | 1 (‐) | 1 (‐) |
| CD | 1.06 (0.87‐1.29) | 0.95 (0.77‐1.17; 0.621) | 1.05 (0.87‐1.29) | 1.42 (0.81‐1.25; 0.950) |
| Sex | ||||
| Male | 1 (‐) | 1 (‐) | 1 (‐) | 1 (‐) |
| Female | 0.96 (0.79‐1.16) | 0.97 (0.80‐1.17; 0.747) | 0.91 (0.75‐1.11) | 0.92 (0.76‐1.11; 0.393) |
| Age at diagnosis (years) | ||||
| <18 | 1.32 (0.77‐2.24) | 1.42 (0.81‐2.47; 0.220) | 1.20 (0.70‐2.06) | 1.27 (0.73‐2.21; 0.413) |
| 18 < 25 | 2.15 (1.52‐3.04) | 2.03 (1.40‐2.95; 0.000) | 1.91 (1.39‐2.60) | 2.03 (1.44‐2.84; 0.000) |
| 25 < 40 | 1.10 (0.86‐1.39) | 1.12 (0.88‐1.41; 0.349) | 1.11 (0.88‐1.43) | 1.12 (0.87‐1.43; 0.375) |
| 40 < 60 | 1 (‐) | 1 (‐) | 1 (‐) | 1 (‐) |
| ≥60 | 1.38 (1.07‐1.79) | 1.40 (1.07‐1.82; 0.012) | 1.50 (1.16‐1.94) | 1.47 (1.13‐1.92; 0.004) |
| Social deprivation | ||||
| IMD 1‐3 | 1 (‐) | 1 (‐) | 1 (‐) | 1 (‐) |
| IMD 4‐5 | 1.07 (1.01‐1.40) | 1.05 (0.80‐1.38; 0.739) | 1.40 (1.07‐1.82) | 1.40 (1.07‐1.83; 0.013) |
| Unknown | 0.81 (0.65‐0.99) | 0.79 (0.64‐0.98; 0.033) | 0.98 (0.75‐1.15) | 0.94 (0.76‐1.17; 0.576) |
| Era of IBD diagnosis | ||||
| Era 1 2004‐2007 | 1 (‐) | 1 (‐) | 1 (‐) | 1 (‐) |
| Era 2 2008‐2011 | 1.06 (0.86‐1.32) | 1.07 (0.86‐1.33; 0.533) | 1.06 (0.85‐1.31) | 1.04 (0.83‐1.30; 0.744) |
| Era 3 2012‐2015 | 1.10 (0.84‐1.37) | 1.07 (0.84‐1.37; 0.584) | 0.97 (0.76‐1.24) | 0.96 (0.75‐1.24; 0.762) |
| Smoking status | ||||
| Never | 1 (‐) | 1 (‐) | 1 (‐) | 1 (‐) |
| Smoker | 1.81 (0.62‐1.06) | 1.06 (0.80‐1.41; 0.654) | 1.14 (0.92‐1.42) | 1.17 (0.88‐1.55; 0.229) |
| Ex‐smoker | 0.98 (0.74‐1.29) | 0.85 (0.66‐1.09; 0.214) | 1.21 (0.92‐1.59) | 1.19 (0.90‐1.57; 0.891) |
| Missing | 0.80 (0.59‐1.07) | 0.86 (0.65‐1.14; 0.311) | 0.91 (0.67‐1.24) | 0.96 (0.70‐1.32; 0.151) |
| Corticosteroid use | ||||
| No | 1 (‐) | 1 (‐) | 1 (‐) | 1 (‐) |
| Yes | 1.24 (0.83‐1.85) | 1.19 (0.79‐1.78; 0.409) | 1.00 (0.66‐1.51) | 0.96 (0.63‐1.46; 0.874) |
All covariates described were included within the adjusted analysis. Abbreviations: HR, Hazard ratio; CI, confidence interval; *IBD, inflammatory bowel disease; *IMD ‐ Index of Multiple Deprivation; IMD 1 represents the least deprived and IMD 5 the most deprived. IMD ‐ Data are available only for individual’s resident in England.
Bold values indicate statistical significance.
One in three (34%) individuals started on an antidepressant medication received only a single prescription in their first treatment episode, meaning they received treatment for 28 days or less (Supplemental appendix E). Of these, only 7% went on to receive a further antidepressant course lasting 7 months duration or longer. Amongst individuals starting antidepressant treatment, we found 11% switched to an alternative antidepressant class within their first treatment episode.
Individuals aged 18‐24 years at IBD diagnosis were significantly more likely to discontinue treatment after just one prescription than older individuals aged between 40 and 60 years (aHR = 2.03, 95% CI, 1.44‐2.84). Those living in areas of greater socioeconomic deprivation were also more likely to discontinue treatment after a single prescription (IMD 4‐5 vs IMD 1‐3: aHR = 1.40; 95% CI, 1.07‐1.83) (Table 3).
4.3. Trends in antidepressant prescribing in the IBD population
Antidepressant use increased for individuals diagnosed with IBD between the two eras 2004‐2007 and 2012‐2015 (aHR = 1.51, 95% CI, 1.33‐1.71) (Table 4). There were temporal changes in the prescription rates of each antidepressant medication (Figure 1). Overall, the most frequent antidepressant used was citalopram. The incidence of citalopram prescribing decreased from 2.1 per 100‐person years to 1.6 (95% CI 1.4‐2.8); whereas the rate of sertraline initiation increased steadily from 0.5 to 1.4 (95% CI, 1.2‐2.2) between 2004 and 2015.
TABLE 4.
Predictors of incident depression, anxiety and ADM use amongst the IBD population
| Depression | Anxiety | ADM | ||||
|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR (95% CI; P value) | Unadjusted HR (95% CI) | Adjusted HR (95% CI; P value) | Unadjusted HR (95% CI) | Adjusted HR (95% CI; P value) | |
| IBD cohort | ||||||
| UC | 1 (−) | 1 (−) | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| CD | 1.29 (1.16‐1.44) | 1.10 (0.98‐1.24; 0.116) | 1.15 (1.02‐1.29) | 1.04 (0.91‐1.17; 0.570) | 1.19 (1.08‐1.32) | 1.05 (0.95‐1.17; 0.299) |
| Sex | ||||||
| Male | 1 (−) | 1 (−) | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| Female | 1.54 (1.39 ‐1.71) | 1.51 (1.36‐1.68; 0.000) | 1.53 (1.37‐1.71) | 1.52 (1.36‐1.70; 0.000) | 1.34 (1.22‐1.47) | 1.31 (1.19‐1.43; 0.000) |
| Age at diagnosis (year) | ||||||
| <18 | 0.93 (0.73‐1.19) | 1.21 (0.94‐1.56; 0.140) | 0.88 (0.67‐1.15) | 0.98 (0.74‐1.31; 0.916) | 0.55 (0.42‐0.73) | 0.68 (0.51‐0.90; 0.007) |
| 18‐25 | 1.76 (1.49‐2.08) | 1.80 (1.49‐2.16; 0.000) | 1.61 (1.34‐1.94) | 1.69 (1.37‐2.07; 0.000) | 1.79 (1.54‐2.08) | 1.78 (1.50‐2.09; 0.000) |
| 25‐40 | 1.28 (1.1‐1.46) | 1.27 (1.11‐1.45; 0.000) | 1.40 (1.21‐1.61) | 1.39 (1.21‐1.60; 0.000) | 1.40 (1.24‐1.57) | 1.38 (1.23‐1.55; 0.000) |
| 40‐60 | 1 (−) | 1 (−) | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| ≥60 | 0.78 (0.68‐0.91) | 0.81 (0.70‐0.93; 0.004) | 0.78 (0.68‐0.89) | 0.79 (0.67‐0.92; 0.003) | 0.89 (0.79‐1.01) | 0.92 (0.81‐1.04; 0.192) |
| Social deprivation | ||||||
| IMD 1‐3** | 1 (−) | 1 (−) | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| IMD 4‐5 | 1.18 (1.02‐1.36) | 1.10 (0.95‐1.27; 0.200) | 1.04 (0.88‐1.22) | 0.98 (0.85‐1.17; 0.845) | 1.18 (1.03‐1.34) | 1.09 (0.97‐1.21; 0.179) |
| Unknown | 1.02 (0.91‐1.15) | 0.99 (0.88‐1.11; 0.850) | 1.21 (1.06‐1.36) | 1.18 (1.04‐1.34; 0.008) | 1.07 (0.97‐1.19) | 1.01 (0.91‐1.12; 0.926) |
| Era of IBD diagnosis | ||||||
| Era 1: 2004‐2007 | 1 (−) | 1 (−) | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| Era 2: 2008‐2011 | 0.91 (0.81‐1.02) | 0.88 (0.84‐1.31; 0.143) | 0.98 (0.86‐1.12) | 0.97 (0.85‐1.10; 0.652) | 1.11 (0.99‐1.24) | 1.11 (0.99‐1.24; 0.140) |
| Era 3: 2012‐2015 | 0.99 (0.86‐1.15) | 0.97 (0.76‐1.25; 0.644) | 1.22 (1.05‐1.42) | 1.19 (1.01‐1.38; 0.026) | 1.56 (1.36‐1.75) | 1.51 (1.33‐1.71; 0.000) |
| Smoking status | ||||||
| Never | 1 (−) | 1 (−) | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| Smoker | 1.54 (1.34‐1.78) | 1.47 (1.27‐1.71; 0.000) | 1.25 (1.05‐1.48) | 1.21 (1.02‐1.44; 0.029) | 1.45 (1.28‐1.66) | 1.44 (1.26‐1.65; 0.000) |
| Ex‐smoker | 0.94 (0.82‐1.08) | 1.12 (0.96‐1.27; 0.116) | 1.01 (0.88‐1.17) | 1.19 (1.02‐1.37; 0.024) | 0.96 (0.85‐1.08) | 1.06 (1.02‐1.27; 0.523) |
| Missing | 0.74 (0.64‐0.87) | 0.77 (0.66‐0.90; 0.002) | 0.92 (0.79‐1.08) | 0.99 (0.84‐1.17; 0.913) | 0.69 (0.60‐0.79) | 0.78 (0.68‐0.90; 0.001) |
| Corticosteroid use | 0.98 (0.77‐1.24) | 1.03 (0.81‐1.31; 0.747) | 0.84 (0.66‐1.07) | 0.88 (0.69‐1.12; 0.289) | 0.86 (0.70‐1.05) | 0.90 (0.74‐1.10; 0.349) |
*All covariates in the table were included within the adjusted analysis. Abbreviations: *HR, Hazard ratio; *CI, confidence interval; *IBD, inflammatory bowel disease; *CD, Crohn’s disease *UC, ulcerative colitis; *Depression, depression diagnostic and/or depressive symptom code; *Anxiety, anxiety diagnostic and/or anxiety symptom code; *ADM, antidepressant medication; *IMD, Index of Multiple Deprivation; IMD 1 represents the least deprived and IMD 5 the most deprived. IMD data are available only for individual’s resident in England.
Bold values indicate statistical significance.
FIGURE 1.
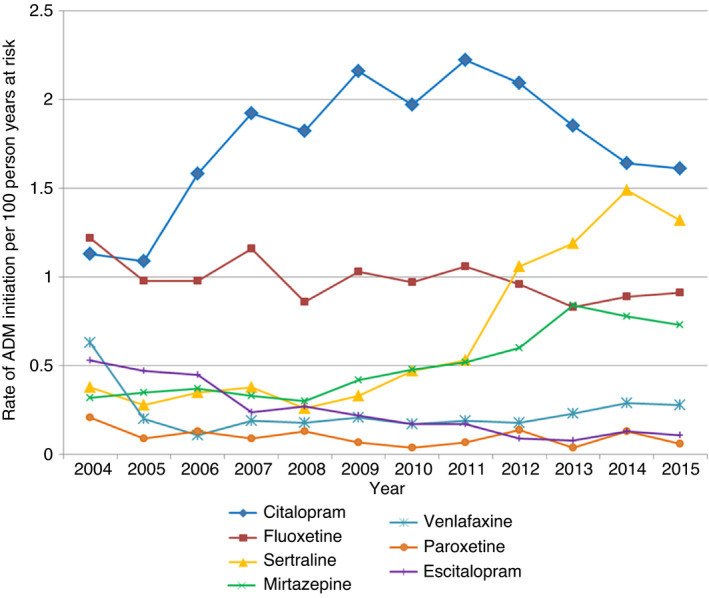
Rate of ADM initiation by drug type following IBD diagnosis
4.4. New‐onset depression and anxiety in IBD
In tandem with the differences observed for antidepressant use, we found the incidence rate of depression was 14.81 and 11.99/1000 person‐years in the IBD cohort and matched control cohort respectively. Likewise, the incidence rate of anxiety was 12.99 and 10.30/1000 person‐years in the IBD cohort and matched control cohort respectively. In keeping with our findings with respect to incident antidepressant use, we found the highest risk of incident depression and anxiety was observed during the first year after IBD diagnosis (Depression aHR = 1.26, 95% CI, 1.13‐1.40: Anxiety aHR = 1.36, 95% CI, 1.21‐1.52) (Table 5). The close relationship between antidepressant use and depression or anxiety is underscored when inter‐relations were reported as conditional frequencies (Supplemental appendix D).
TABLE 5.
Risk of incident depression and anxiety in the first year following diagnosis amongst individuals with IBD compared with the control cohort
| Outcome | Depression | Anxiety | ||
|---|---|---|---|---|
| Unadjusted HR (95% CI; P value) | Adjusted* HR (95% CI; P value) | Unadjusted HR (95% CI; P value) | Adjusted* HR (95% CI; P value) | |
| Cohort | ||||
| Matched Controls | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| IBD | 1.37 (1.23‐1.52; 0.000) | 1.26 (1.13‐1.40; 0.000) | 1.50 (1.35‐1.68; 0.000) | 1.36 (1.21‐1.52; 0.000) |
| Sex | ||||
| Male | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| Female | 1.80 (1.61‐2.00; 0.000) | 1.77 (1.59‐1.97; 0.000) | 1.68 (1.50‐1.87; 0.000) | 1.67 (1.49‐1.86; 0.000) |
| Age at diagnosis (years) | ||||
| <18 | 0.36 (0.25‐0.50; 0.000) | 0.59 (0.42‐0.83; 0.003) | 0.39 (0.28‐0.55; 0.000) | 0.55 (0.38‐0.77; 0.001) |
| 18 < 25 | 1.23 (1.02‐1.47; 0.028) | 1.37 (1.14‐1.64; 0.001) | 1.20 (0.99‐1.46; 0.094) | 1.30 (1.06‐1.58; 0.011) |
| 25 < 40 | 1.17 (1.02‐1.32; 0.019) | 1.19 (1.04‐1.35; 0.010) | 1.16 (1.01‐1.33; 0.028) | 1.18 (1.03‐1.35; 0.019) |
| 40 < 60 | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| ≥60 | 0.77 (0.67‐0.90; 0.000) | 0.79 (0.69‐0.91; 0.001) | 0.83 (0.72‐0.98; 0.008 | 0.82 (0.71‐0.95; 0.007) |
| Social deprivation | ||||
| IMD 1–3 | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| IMD 4–5 | 1.24 (1.07‐1.42; 0.002) | 1.14 (0.99‐1.31; 0.056) | 1.07 (0.92‐1.24; 0.380) | 1.02 (0.88‐1.18; 0.787) |
| Unknown | 1.06 (0.94‐1.19; 0.335) | 1.06 (0.93‐1.19; 0.333) | 1.12 (0.99‐1.25; 0.068) | 1.11 (0.98‐1.25; 0.098) |
| Era of IBD diagnosis | ||||
| Era 1 2004–2007 | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| Era 2 2008–2011 | 1.21 (1.07‐1.36; 0.002) | 1.11 (1.02‐1.29; 0.102) | 1.02 (0.90‐1.16; 0.809) | 0.95 (0.86‐1.12; 0.470) |
| Era 3 2012–2015 | 1.23 (1.08‐1.40; 0.002) | 1.08 (1.00‐1.31; 0.230) | 1.41 (1.24‐1.61; 0.000) | 1.27 (1.12‐1.46; 0.000) |
| Smoking status n (%) | ||||
| Never | 1 (−) | 1 (−) | 1 (−) | 1 (−) |
| Smoker | 2.03 (1.77‐2.32; 0.000) | 1.96 (1.71‐2.24; 0.000) | 1.65 (1.41‐1.91; 0.000) | 1.60 (1.37‐1.86; 0.000) |
| Ex‐smoker | 1.23 (1.08‐1.41; 0.002) | 1.32 (1.15‐1.51; 0.000) | 1.32 (1.15‐1.53; 0.000) | 1.37 (1.18‐1.58; 0.000) |
| Missing | 0.57 (0.48‐0.66; 0.000) | 0.61 (0.52‐0.72; 0.000) | 0.70 (0.60‐0.82; 0.000) | 0.78 (0.67‐0.92; 0.003) |
| Corticosteroid use | ||||
| No | 1 (−) | 1(−) | 1(−) | 1(−) |
| Yes | 0.89 (0.59‐1.32; 0.564) | 1.14 (0.76‐1.72; 0.525) | 0.63 (0.44‐0.91; 0.013) | 0.84 (0.58‐1.22; 0.367) |
*All covariates in the table were included within the adjusted analysis. Abbreviations: *ADM, antidepressant medication; *OR, odds ratio; *CI, confidence interval; *IBD, inflammatory bowel disease;*CD, Crohn’s disease *UC, ulcerative colitis; *IMD ‐ Index of Multiple Deprivation; IMD 1 represents the least deprived and IMD 5 the most deprived. IMD ‐ Data are available only for individual’s resident in England * Depression (depression diagnostic and/or depressive symptom code) * Anxiety (anxiety diagnostic and/or anxiety symptom code).
Bold values indicate statistical significance.
5. DISCUSSION
5.1. Main findings
The risk of antidepressant use amongst individuals with IBD increased by more than half during the 12‐year study period. In the year following IBD diagnosis individuals are 34% more likely to initiate an antidepressant compared with the general population. Two‐thirds of individuals who started an antidepressant did not receive the adequate duration of treatment recommended by international guidelines. Moreover, a third of individuals with IBD who initiated an antidepressant received just a single prescription. Individuals diagnosed with IBD between the ages of 18 and 24 years and those living in areas of higher socioeconomic deprivation were at greatest risk of treatment duration falling short of recommendations.
5.2. Findings in relation to previous studies
Our study demonstrates individuals diagnosed with IBD are significantly more likely to initiate an antidepressant medication compared with matched controls. This is the first nationally representative study to report the incidence of antidepressant use in IBD. A Finish and a Canadian study have reported the prevalence of antidepressant use amongst individuals with IBD but these studies preceded the introduction of international guidelines and the more recent wider use of selective serotonin reuptake inhibitors (SSRIs). 21 , 22
We found the greatest frequency of antidepressant use occurred in the first year following IBD diagnosis, and the likelihood of initiating an antidepressant in the years following IBD diagnosis increased by 51% between 2004 and 2015. This is consistent with findings that report the highest risk of common psychiatric morbidity occurs in the first year after IBD diagnosis. 4
Our study is the first to examine the duration of antidepressant treatment and adherence to published recommendations in IBD. International guidelines indicate antidepressant should be continued for a minimum of 6 months after symptom resolution of depression or anxiety, which takes a minimum of 1 month to achieve. 10 , 11 , 12 , 13 , 14 We found two thirds of antidepressant treatment courses prescribed fell short of this duration, leaving individuals inadequately treated. This is important since treatment of depression and anxiety lasting less than 6 months following symptom resolution carries a high risk of relapse. 22 , 23 Meta‐analysis indicates continuing antidepressants for at least 6 months after successful treatment of depression is associated with a significantly lower rate of relapse. 23 In turn, untreated psychiatric co‐morbidity may adversely impact the disease course of IBD. 6 , 7 , 24 , 25 However, it is important to stress our analysis could not adjust for either non‐response to treatment or potential drug‐related adverse events, which may necessitate appropriate treatment discontinuation.
Our study found those living in areas of greater socioeconomic deprivation and younger individuals, at IBD diagnosis, were at particular risk of antidepressant undertreatment. Young adults frequently relocate in pursuit of education and employment, and the absence of a consistent point of health care contact may be a contributing factor. Furthermore, the affordability of prescriptions has been reported as a barrier to obtaining medications for those on lower incomes. 26
We found a third of individuals with IBD that started an antidepressant received only a single prescription, consistent with findings in the general population. 20 Reasons for early discontinuation are likely to be varied and include resolution of precipitating stressors; lack of timely response; side effects and concerns about dependency. Whilst there is good evidence for the efficacy of antidepressant in treating comorbid mood disorders in people with other physical illnesses, in IBD, despite their common use, evidence is limited. 27 We found amongst individuals with IBD who were prescribed a single course of antidepressants, 83% had a record for either diagnosis or symptoms of depression or anxiety following treatment. Three small trials have been conducted exploring the efficacy of antidepressants in the treatment of comorbid depression and anxiety in IBD. 28 , 29 , 30 Whilst Tianeptine and Duloxetine reduced symptoms of depression and anxiety in comparison with placebo, fluoxetine had no effect. Our study demonstrated temporal shifts in the choice of antidepressant used during the 12 year study period, changes consistent with that in the general population. 20 We found SSRIs made up the majority of antidepressant prescriptions with a switch from citalopram to sertraline amongst IBD patients in recent years.
5.3. Strengths and limitations
Our study used data from a large, nationally representative primary care research database, using previously validated methodology to establish the duration of an antidepressant episode. 20 CPRD data is collected at the time of consultation or prescription and is independent of referral centre, recall or participant selection bias. We used validated diagnostic codes for depression and anxiety. In common with other observational studies using routinely collected data, inaccuracies in coding and completeness may occur. Previous studies suggest depression and anxiety may not always be detected in primary care and thus our findings may underestimate their occurence. 31
The CPRD dataset does not record the indication for antidepressant prescriptions, therefore we cannot be certain that antidepressants were prescribed for a diagnoses or symptoms of depression or anxiety. However, we observed a strong inter‐relationship between antidepressant use and mood disorders. Previous studies report three quarters of antidepressant prescriptions are for either depression or anxiety, and still greater for SSRIs, which comprised the majority of antidepressant prescriptions in our study. 9 We acknowledge individuals may start antidepressant treatment in primary care when they present with distress, which may then resolve quickly, accounting for some early discontinuation.
Unlike previous studies, we excluded the use of tricyclic antidepressant prescriptions from our main analysis since they are primarily prescribed at low dose for disorders of brain‐gut axis, functional syndromes, chronic pain and a number of other conditions.
Antidepressant prescriptions may be initiated in secondary care but, in the UK, prescriptions then continue to be issued in the primary care setting. 32 We were unable to determine the severity of depression or anxiety using a standardised psychiatric tool since these are not routinely used in primary care. Neither were we able to ascertain associations with IBD phenotype or severity, which may have influenced the risk of psychiatric co‐morbidity. Nor were we able to evaluate the rate of initiation of psychological therapy since these episodes are not coded in the dataset. 33 Corticosteroid use has been associated with an increased risk of psychiatric morbidity. 34 For this reason we identified and adjusted for the occurrence of corticosteroids prescribing. We were unable to adjust for anti‐TNF use or other biologics since these are not coded in the dataset.
5.4. Implications
Despite the heavy burden of depression and anxiety amongst individuals diagnosed with IBD the duration of antidepressant treatment falls short of recommended international guidance in more than two thirds. This raises concern since individuals discontinuing antidepressant treatment continue to have a risk of relapse even when continued long term. 35 Some evidence suggests comorbid depression in the context of other chronic conditions may be less likely to respond to antidepressants. 36 , 37 Early referral for psychological therapy may offer a better alternative but access to such services is often limited. 38 , 39 “The IBD Benchmarking Exercise” reported that only 2% of adult IBD units in the UK meet the benchmark for adequate access to psychological and psychiatric support. 39 Moreover, only a quarter of 10,000 IBD patients surveyed reported being asked about their mental health or emotional wellbeing in the clinic. In order to improve antidepressant adherence, psychological well‐being and IBD outcomes there is a need for better integration of IBD and mental health services at the point of diagnosis and beyond. 40 , 41
6. CONCLUSION
In the year following IBD diagnosis individuals are significantly more likely to initiate an antidepressant medication compared with controls. Two‐thirds of individuals with IBD who initiate antidepressant treatment do not complete an adequate course. Better integration of services may benefit individuals with IBD and psychiatric comorbidity.
CONFLICT OF INTEREST
None declared.
ETHICS STATEMENT
Independent Scientific Advisory Committee (ISAC) Protocol number: 15_018R.
AUTHORSHIP
Guarantor for the article: Richard C. G. Pollok
Author contributions: The POP‐IBD study group is a collaboration between St George’s University of London, Imperial College London, University College London and King’s College London, conducting population‐based studies in the field of Inflammatory Bowel Disease. NJ and JB contributed equally to this project and are joint first authors. NJ, JB, SS, AB, IP, HC, MH & RP conceived and designed this study. NJ and JB prepared the data and carried out statistical analysis overseen by IP and AB. All authors contributed to the development of the analysis, interpreting data and preparing the manuscript.
All authors approved the final version of the manuscript
Personal and funding interest: JB was funded by Crohn’s and Colitis UK Grant [grant number: SP2018/3] RP received support by a Wellcome Trust Institute Strategic Support Fund (ISSF) grant. SS is funded by the National Institute for Health Research (NIHR) School for Public Health Research (SPHR) and NIHR Northwest London Applied Research Collaboration (ARC). [Grant number: PD‐SPH‐2015]. The School for Public Health Imperial College London is also grateful for support from the Imperial NIHR Biomedical Research Centre. The NIHR School for Public Health Research is a partnership between the Universities of Sheffield; Bristol; Cambridge; Imperial; and University College London; The London School for Hygiene and Tropical Medicine (LSHTM); LiLaC – a collaboration between the Universities of Liverpool and Lancaster; and Fuse ‐ The Centre for Translational Research in Public Health a collaboration between Newcastle, Durham, Northumbria, Sunderland and Teesside Universities MH acknowledges support from the National Institute of Health Research Biomedical Research Centre at the Maudsley and is an NIHR Senior Investigator. The Dr Foster Unit at Imperial is affiliated with the National Institute of Health Research (NIHR) Imperial Patient Safety Translational Research Centre. The NIHR Imperial Patient Safety Translational Centre is a partnership between the Imperial College Healthcare NHS Trust and Imperial College London. The Dr Foster Unit at Imperial College are grateful for support from the NIHR Biomedical Research Centre funding scheme. The views expressed in this publication are those of the authors and not necessarily those of Crohn’s & Colitis UK, the NHS, the NIHR or the Department of Health
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
JB was funded by Crohn’s and Colitis UK Grant [grant number: SP2018/3]. RP received support by a Wellcome Trust Institute Strategic Support Fund (ISSF) grant. MH acknowledges support from the National Institute of Health Research Biomedical Research Centre at the Maudsley and is an NIHR Senior Investigator. SS is funded by the National Institute for Health Research (NIHR) School for Public Health Research (SPHR) and NIHR Northwest London Applied Research Collaboration (ARC). [Grant number: PD‐SPH‐2015]. The School for Public Health Imperial College London is also grateful for support from the Imperial NIHR Biomedical Research Centre. The NIHR School for Public Health Research is a partnership between the Universities of Sheffield; Bristol; Cambridge; Imperial; and University College London; The London School for Hygiene and Tropical Medicine (LSHTM); LiLaC—a collaboration between the Universities of Liverpool and Lancaster; and Fuse ‐ The Centre for Translational Research in Public Health a collaboration between Newcastle, Durham, Northumbria, Sunderland and Teesside Universities. The Dr Foster Unit at Imperial is affiliated with the National Institute of Health Research (NIHR) Imperial Patient Safety Translational Research Centre. The NIHR Imperial Patient Safety Translational Centre is a partnership between the Imperial College Healthcare NHS Trust and Imperial College London. The Dr Foster Unit at Imperial College are grateful for support from the NIHR Biomedical Research Centre funding scheme. The views expressed in this publication are those of the authors and not necessarily those of Crohn’s & Colitis UK, the NHS, the NIHR or the Department of Health.
Jayasooriya N, Blackwell J, Saxena S, et al. Antidepressant medication use in Inflammatory Bowel Disease: a nationally representative population‐based study. Aliment Pharmacol Ther. 2022;55:1330–1341. doi: 10.1111/apt.16820
Funding informationThis work was supported by the Living with IBD Research Programme at Crohn’s & Colitis UK [grant number: SP2018/3]. This funding source had no role in the design or execution of this study or in the analysis and interpretation of the data. The views expressed are those of the authors and not necessarily those of Crohn’s & Colitis UK.
The Handling Editor for this article was Dr Cynthia Seow, and it was accepted for publication after full peer review.
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. Data were obtained from CPRD GOLD.
REFERENCES
- 1. Bernstein CN, Hitchon CA, Walld R, et al. Increased burden of psychiatric disorders in inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:360‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bennebroek Evertsz’ F, Thijssens NAM, Stokkers PCF, et al. Do inflammatory bowel disease patients with anxiety and depressive symptoms receive the care they need? J Crohn’s Colitis. 2012;6:68‐76. [DOI] [PubMed] [Google Scholar]
- 3. Barberio B, Zamani M, Black CJ, Savarino EV, Ford A. Prevalence of anxiety and depression in inflammatory bowel disease: systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2021;6:359‐370. [DOI] [PubMed] [Google Scholar]
- 4. Ludvigsson JF, Olén O, Larsson H, et al. Association between inflammatory bowel disease and psychiatric morbidity and suicide: a Swedish Nationwide population‐based cohort study with sibling comparisons. J Crohn’s Colitis. 2021;2021:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knowles SR, Graff LA, Wilding H, Hewitt C, Keefer K, Mikocka‐Wallus A. Quality of life in inflammatory bowel disease: a systematic review and meta‐analyses‐part I. Inflamm Bowel Dis. 2018;24:742‐751. [DOI] [PubMed] [Google Scholar]
- 6. Gracie DJ, Guthrie EA, Hamlin PJ, Ford AC. Bi‐directionality of brain–gut interactions in patients with inflammatory bowel disease. Gastroenterology. 2018;154:1635‐1646.e3. [DOI] [PubMed] [Google Scholar]
- 7. Ananthakrishnan AN, Gainer VS, Perez RG, et al. Psychiatric co‐morbidity is associated with increased risk of surgery in Crohn’s disease. Aliment Pharmacol Ther. 2013;37:445‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dubinsky MC, Dotan I, Rubin DT, et al. Burden of comorbid anxiety and depression in patients with inflammatory bowel disease: a systematic literature review. Expert Rev Gastroenterol Hepatol. 2021;15:985‐997. [DOI] [PubMed] [Google Scholar]
- 9. Petty DR, House A, Knapp P, Raynor T, Zermansky A. Prevalence, duration and indications for prescribing of antidepressants in primary care. Age Ageing. 2006;35:523‐526. [DOI] [PubMed] [Google Scholar]
- 10. Royal College of Psychiatrists PS04/19 . Position statement on antidepressants and depression; 2019. https://www.rcpsych.ac.uk/docs/default‐source/improving‐care/better‐mh‐policy/position‐statements/ps04_19‐‐‐antidepressants‐and‐depression.pdf?sfvrsn=ddea9473_5]. Accessed March 5, 2020.
- 11. NICE National Institute for Health and Clinical Excellence Generalised anxiety disorder and panic disorder in adults: management NICE Clin Guidel 113 Guid; 2011. https://www.nice.org.uk/guidance/cg113 .Accessed March 5, 2020.
- 12. National Institute for Health and Care Excellence NICE Guideline on the Treatment and Depression the Treatment and Management of Depression; 2009. https://www.nice.org.uk/guidance/cg90. Accessed March 5, 2020.
- 13. Kennedy SH, Lam RW, McIntyre RS, et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61:540‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gelenberg AJ. A review of the current guidelines for depression treatment. J Clin Psychiatry. 2010;71:e15. [DOI] [PubMed] [Google Scholar]
- 15. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44:827‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewis JD, Brensinger C, Bilker WB, Strom BL. Validity and completeness of the general practice research database for studies of inflammatory bowel disease. Pharmacoepidemiol Drug Saf. 2002;11:211‐218. [DOI] [PubMed] [Google Scholar]
- 17. Blackwell J, Saxena S, Petersen I, et al. Depression in individuals who subsequently develop inflammatory bowel disease: a population‐based nested case–control study. Gut. 2021;70:1642‐1648. [DOI] [PubMed] [Google Scholar]
- 18. Ilyas S, Moncrieff J. Trends in prescriptions and costs of drugs for mental disorders in England, 1998‐2010. Br J Psychiatry. 2012;200:393‐398. [DOI] [PubMed] [Google Scholar]
- 19. Blackwell J, Alexakis C, Saxena S, et al. Association between antidepressant medication use and steroid dependency in patients with ulcerative colitis: a population‐based study. BMJ Open Gastroenterol. 2021;8(1):e000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCrea RL, Sammon CJ, Nazareth I, Petersen I. Initiation and duration of selective serotonin reuptake inhibitor prescribing over time: UKcohort study. Br J Psychiatry. 2016;209:421‐426. [DOI] [PubMed] [Google Scholar]
- 21. Haapamäki J, Tanskanen A, Roine RP, et al. Medication use among inflammatory bowel disease patients: excessive consumption of antidepressants and analgesics. Scand J Gastroenterol. 2013;48:42‐50. [DOI] [PubMed] [Google Scholar]
- 22. Fuller‐Thomson E, Sulman J. Depression and inflammatory bowel disease: findings from two nationally representative Canadian surveys. Inflamm Bowel Dis. 2006;12(8):697‐707. [DOI] [PubMed] [Google Scholar]
- 23. Kato M, Hori H, Inoue T, et al. Discontinuation of antidepressants after remission with antidepressant medication in major depressive disorder: a systematic review and meta‐analysis. Mol Psychiatry. 2021;26:118‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burcusa SL, Iacono WG. Risk for recurrence in depression. Clin Psychol Rev. 2007;27:959‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scholten WD, Batelaan NM, Van Balkom AJ, Wjh. Penninx B, Smit JH, Van Oppen P. Recurrence of anxiety disorders and its predictors. J Affect Disord. 2013;147:180‐185. [DOI] [PubMed] [Google Scholar]
- 26. Schafheutle EI, Hassell K, Noyce PR, Weiss MC. Access to medicines: cost as an influence on the views and behaviour of patients. Heal Soc Care Community. 2002;10:187‐195. [DOI] [PubMed] [Google Scholar]
- 27. Rayner L, Price A, Evans A, Valsra K, Higginson IJ, Hotopf M. Antidepressants for depression in physically ill people. Cochrane Database Syst Rev. 2010;(3):CD007503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Daghaghzadeh H, Naji F, Afshar H, et al. Efficacy of duloxetine add on in treatment of inflammatory bowel disease patients: a double‐blind controlled study. J Res Med Sci. 2015;20:595‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mikocka‐Walus A, Hughes PA, Bampton P, et al. Fluoxetine for maintenance of remission and to improve quality of life in patients with Crohn’s disease: a pilot randomized placebo‐controlled trial. J Crohn’s Colitis. 2017;11:509‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chojnacki C, Walecka‐Kapica E, Klupińska G, et al. Evaluation of the influence of tianeptine on the psychosomatic status of patients with ulcerative colitis in remission. Pol Merkur Lek. 2011. Aug;31:92‐96. [PubMed] [Google Scholar]
- 31. Wittchen HU, Mühlig S, Beesdo K. Mental disorders in primary care. Dialogues Clin Neurosci. 2003;5:115‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnson CF, Dougall NJ, Williams B, MacGillivray SA, Buchanan AI, Hassett RD. Patient factors associated with SSRI dose for depression treatment in general practice: a primary care cross sectional study. BMC Fam Pract. 2014;15:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mikocka‐Walus A, Ford AC, Drossman DA. Antidepressants in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:184‐192. [DOI] [PubMed] [Google Scholar]
- 34. Bhangle SD, Kramer N, Rosenstein ED. Corticosteroid‐induced neuropsychiatric disorders: review and contrast with neuropsychiatric lupus. Rheumatol Int. 2013;33:1923‐1932. [DOI] [PubMed] [Google Scholar]
- 35. Lewis G, Lewis G. Maintenance or discontinuation of antidepressants in primary care. N Engl J Med. 2021;14:1257‐1267. [DOI] [PubMed] [Google Scholar]
- 36. O’Connor CM, Jiang W, Kuchibhatla M, et al. Safety and efficacy of sertraline for depression in patients with heart failure: results of the SADHART‐CHF (sertraline against depression and heart disease in chronic heart failure) trial. J Am Coll Cardiol. 2010;56:692‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hedayati SS, Gregg LP, Carmody T, et al. Effect of sertraline on depressive symptoms in patients with chronic kidney disease without dialysis dependence: the CAST randomized clinical trial. JAMA. 2017;318:1876‐1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Royal College of Physicians . National audit of inflammatory bowel disease (IBD) service provision UK IBD audit. Heal care Improv Partnersh; 2014. https://www.rcplondon.ac.uk. Accessed June 6, 2020
- 39. IBD UK . Crohn’s and Colitis Care in the UK: the hidden cost and a vision for change; 2021. https://ibduk.org/reports/crohns‐and‐colitis‐care‐in‐the‐uk‐the‐hidden‐cost‐and‐a‐vision‐for‐change. Accessed May 21, 2021
- 40. Sirey JA, Banerjee S, Marino P, et al. Adherence to depression treatment in primary care: a randomized clinical trial. JAMA Psychiatry. 2017;74:1129‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lores T, Goess C, Mikocka‐Walus A, et al. Integrated psychological care reduces health care costs at a hospital‐based inflammatory bowel disease service. Clin Gastroenterol Hepatol. 2021;19:96‐103.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. Data were obtained from CPRD GOLD.


