Abstract
Background
Diabetic ketoacidosis (DKA) patients present with low serum bicarbonate (), and an increase in its level to ≥15 mEq/L is considered one of the criteria for DKA resolution. Both proton pump inhibitors and histamine‐2 receptor antagonists inhibit downstream functioning of H+/K+ ATPase in the gastric parietal cells, which results in the decreased secretion of into the bloodstream.
Objectives
We aimed to introduce the hypothesis that DKA patients on acid‐suppressive medications may have a delayed rise in serum to >15 mEq/L that may cause increased hospital length of stay (LOS) and sought to compare the outcomes of such patients. For the sake of simplicity, conditions requiring acid suppression are grouped under the term peptic ulcer disease (PUD) in this study.
Methods
This is a retrospective study using Nationwide Inpatient Sample employing International Classification of Diseases (ICD‐10) codes for adult patients with a primary diagnosis of DKA. Analyses were performed using STATA, proportions were compared using Fisher exact test, and continuous variables using Student's t‐test. Confounding variables were adjusted using propensity matching, multivariate logistic, and linear regression analyses.
Results
DKA patients with PUD had higher adjusted LOS, intensive care unit admission, and total hospital costs. Mortality and morbidity indicators were similar in both groups. The variables found to be independent predictors of increased LOS were malnutrition, Clostridium difficile infection, pneumonia, Glasgow Coma Scale score of 3–8, and higher Charlson comorbidity score.
Conclusion
We found that Clostridium difficile and pneumonia predicted longer LOS in DKA patients with concomitant PUD, hinting at the possible role of acid suppression in prolonging the LOS in such patients. However, further studies are needed to examine the effect of lesser generation on LOS.
Keywords: histamine H2 blockers, hospital costs, length of stay, morbidity, mortality, proton pump Inhibitors

Introduction
The leading causes of upper gastrointestinal bleeding (UGIB) in approximate order of descending frequency include peptic ulcer disease (PUD), erosive disease of the upper gastrointestinal (GI) mucosa, and variceal bleeding [1, 2]. Mortality from UGIB has decreased significantly over the years, attributed to advances in endoscopic treatment and the widespread use of proton pump inhibitors (PPIs). Mishuk et al., using Medical Expenditure Panel Survey data, reported that the proportion of PPI users was 6.3% in 2016–2017, increasing from prior numbers [3].
Diabetic ketoacidosis (DKA) presents with GI symptoms, including nausea, vomiting, and abdominal pain. In the majority of the hospitals across the states, it is considered a criterion for intensive care unit (ICU) admission, a practice also endorsed by the American Diabetes Association [4, 5]. Diagnostic criteria of DKA include serum glucose >250 mg/dL, serum bicarbonate () <10–15 mEq/L, and pH <7.30–7.00 depending upon the severity of DKA [4].
Bicarbonate use is controversial in DKA but is still practiced in most institutes across the states in severely acidotic patients [6]. In addition to the increase in PPI use, histamine‐2 receptor antagonists (H2RAs) use is also rising overall in recent years for acid suppression [7, 8]. Both PPI and H2RAs inhibit downstream functioning of H+/K+ adenosine triphosphatase (ATPase) in the gastric parietal cells, which physiologically, if not blocked, results in the secretion of hydrogen ion (H+) into the gastric lumen and concomitant secretion of into the bloodstream towards the basal side of parietal cells due to the action of parietal cell carbonic anhydrase [9]. Therefore, we aimed to introduce the hypothesis that DKA patients on acid‐suppressive medications may have a delayed rise in serum to >15 mEq/L (one of the criteria for DKA resolution) that may cause increased hospital length of stay (LOS) and sought to compare the outcomes of such patients.
For the sake of simplicity, conditions requiring acid suppression are grouped under the term PUD in this paper.
Materials and methods
Study design and database description
This is a retrospective cohort study of adult patients hospitalized with DKA in acute‐care hospitals across the USA. The combined releases of the year 2016 through 2018 of the Nationwide Inpatient Sample (NIS) database were used to select patients. In NIS, a 20% probability sample of patients from all hospitals is collected. Each discharge is then weighted (weight = total number of discharges from all acute care hospitals in the United States divided by the number of discharges included in the 20% sample), making it nationally representative. The dataset consists of more than 7 million discharges each year, which is a 20% stratified sample of patients from over 4500 nonfederal acute care hospitals of more than 45 states of the United States. This estimates to about 35 million yearly discharges nationwide when weighted and represents 95% of hospital discharges nationwide.
Study patients
Patients with principal International Classification of Diseases, Clinical Modification (ICD‐10‐CM) diagnosis‐specific codes for DKA were included in the study. Using ICD‐10‐CM codes, patients with upper GI inflammatory conditions were selected. The specific ICD‐10‐CM codes that were included are listed in the Supporting Information Appendix. Patients were excluded if they were younger than 18 years of age. Other upper GI pathologies that require comparatively short‐term PPI use were not included. The Institutional Review Board of Loyola University Medical Center authorized this study and deemed the research project exempt from approval because it was a retrospective review of already collected de‐identified data.
Study variables and outcomes
The primary outcome was the hospital LOS. The secondary outcomes were: (1) inpatient mortality, (2) total hospitalization charges, (3) acute kidney injury (AKI), (4) ICU admission, and (5) independent predictors of LOS. The potential confounders that were collected and adjusted for were: age in years; sex as male or female; race as White, Black, Hispanic, Asian or Pacific Islander, Native American, or other; admission day as the weekend (12:00 AM Saturday to 11:59 PM Sunday) or weekday; median income in the patient's ZIP code as four hierarchical categories; patient comorbidities as measured by the Deyo adaptation of the Charlson Comorbidity Index for administrative data (modified to exclude PUD); hospital location as rural or urban; hospital region as Northeast, Midwest, West, or South; hospital teaching status as teaching or nonteaching based on the American Hospital Association annual survey of hospitals; and hospital bed size as small, medium, and large [10]. Total hospital charges depict the amount that the hospitals billed for the overall hospital stay but do not reflect the actual cost of care. Time to reach serum >15 mEq/L was not included as an outcome due to unavailable data on the laboratory values in the database. Similarly, baseline pH, , and pCO2 were inaccessible in the dataset.
Statistical analysis
Analyses were performed using STATA, version MP 14.2 (StataCorp, College Station, Texas, USA). The weighting of patient‐level observations was applied to procure estimates for the entire population in the United States of hospitalized patients with DKA. We used univariable logistic regression analysis to compute unadjusted odds ratios (ORs) for the primary and secondary outcomes. We used two distinct approaches to adjust for confounders in our analysis: propensity score matching and multivariate regression analysis. Propensity scores were employed to match patients with DKA who had coexistent PUD to those who did not. A nonparsimonious multivariate logistic regression model was developed to estimate the propensity score using the following variables: age, race, sex, Charlson Comorbidity Index, income in patient's ZIP code, insurance status, hospital bed size, hospital urban location, hospital teaching status, and hospital region. During model building for propensity score, the family specified was binomial and link was logit [11]. The double robust method was then used to generate treatment weights, and the inverse probability of treatment weighting was used to match cases with controls using generalized linear models [11]. The match variables were age, gender, race, and relevant comorbidities identified from the literature search (heart failure, hypertension, hyperlipidemia, diabetes and its complications, malnutrition, pneumonia, Clostridium difficile infection, and vitamin B 12 deficiency). In the second analysis, multivariable regression models were built by including all confounders significantly associated with the outcome on univariable analysis with a cutoff p‐value of 0.2 [12]. Variables deemed clinically important to the outcome based on literature were included in the model irrespective of whether they were significantly associated with the outcome on univariable analysis. A logistic regression model was used for binary outcomes, and a linear regression model was used for continuous outcomes. For the other calculations, proportions were compared using the Fisher exact test, and continuous variables were compared using Student's t‐test. All p‐values were two sided, with 0.05 as the threshold for statistical significance.
Missing data
Hospital characteristic variables did not have any missing data (Table 1). Four variables pertaining to the patient characteristics had missing data—most of the variables had a very low percentage of missing data (<0.05%) except for race (2.77%), elective admission (0.13%), median income in the patient's ZIP code (1.9%), and insurance (0.18%). To test whether missing data could introduce bias into the study, we assumed that data were not missing at random and applied a multivariate imputation by chained equations (i.e., MICE) method estimated from sequential multivariable models with fully conditional specifications [13]. Overall, 10 imputed datasets were constructed using information from all covariates used in the regression models, as well as other covariates in the database without missing information. Results with and without imputation were not meaningfully different. Thus, results without imputation were reported.
Table 1.
Missing data
| Variables | Data missing (%) |
|---|---|
| Age (years) | 0.007 |
| Gender | 0.02 |
| Race | 2.77 |
| Charlson Comorbidity Index | 0.00 |
| Elective admission | 0.13 |
| Weekend admission | 0.00 |
| Median income in patient's ZIP code | 1.9 |
| Hospital region | 0.00 |
| Hospital bed size | 0.00 |
| Hospital location/teaching status | 0.00 |
| Insurance | 0.18 |
Results
Patient characteristics
Figure 1 shows the flow diagram for study inclusion. The total number of patients in the studied NIS cohort was 107,000,000, among whom 589,734 (0.55%) were diagnosed with DKA (Table 2). Among patients with DKA, 121,529 (20.61% of DKA patients) had coexisting PUD. Patients with PUD were more likely to be older and female, more likely to be White, had higher Charlson Comorbidity Index score, were more likely to be insured by Medicare, less likely to have Medicaid or private insurance, and had very little difference in median annual income compared with patients without PUD. There were also numerically small but statistically significant differences in hospital characteristics between the two groups; patients with PUD were more likely to be admitted to a large hospital and less likely to be admitted to small and medium‐sized hospitals than patients without PUD. Patients without PUD were more likely to be admitted to nonteaching hospitals in urban areas compared to PUD patients. The other characteristics were similar between the two patient groups or had differences numerically too small to be clinically significant.
Fig. 1.
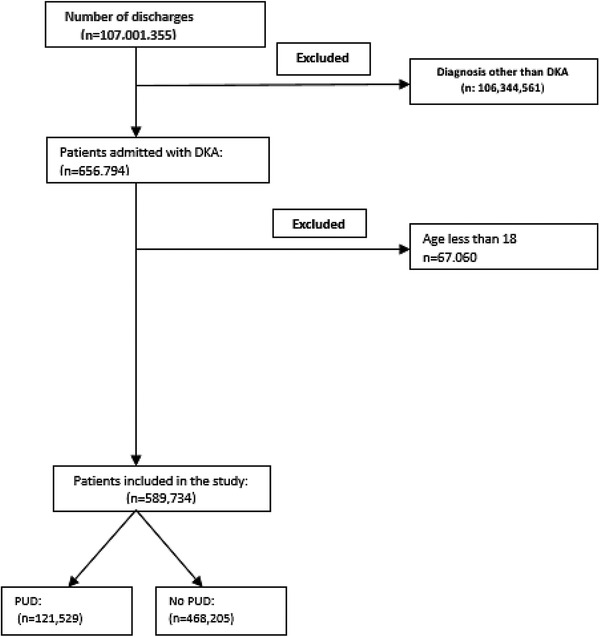
Patient selection flow diagram (DKA, diabetic ketoacidosis; PUD, peptic ulcer disease).
Table 2.
Patient and hospital characteristics
| DKA (n = 589,734) | |||
|---|---|---|---|
| Baseline characteristics | PUD a (n = 121,529) | No PUD (n = 468,205) | p‐value |
| Age (mean [95% confidence interval]) (years) | 45.18 (44.96–45.39) | 40.38 (40.26–40.51) | <0.001 |
| Female gender (n [%]) | 63,704 (52.43) | 225,774 (48.23) | <0.001 |
| Race (n [%]) | <0.001 | ||
| White | 74,674 (63.04) | 250,864 (55.14) | <0.001 |
| Black | 28,995 (24.48) | 124,280 (27.32) | <0.001 |
| Hispanic | 10,734 (9.06) | 58,044 (12.76) | <0.001 |
| Asians | 939 (0.79) | 5499 (1.21) | 0.05 |
| Native Americans | 1074 (0.91) | 4754 (1.05) | 0.69 |
| Others | 2034 (1.72) | 11,499 (2.53) | <0.001 |
| Charlson Comorbidity Index (n [%]) | <0.001 | ||
| 0 | 80 (0.07) | 445 (0.09) | 0.17 |
| 1 | 39,294 (32.33) | 263,949 (56.37) | <0.001 |
| 2 | 37,334 (30.72) | 108,639 (23.2) | <0.001 |
| ≥3 | 44,819 (36.88) | 95,169 (20.33) | <0.001 |
| Admission day is weekend (n [%]) | 32,999 (27.15) | 123,999 (26.48) | 0.04 |
| Median household income b (quartile) (n [%]) | 0.001 | ||
| First (0–25th) | 46,924 (39.27) | 178,599 (38.93) | 0.24 |
| Second (26th to 50th) | 33,864 (28.34) | 125,219 (27.29) | 0.001 |
| Third (51st to 75th) | 25,074 (20.98) | 95,624 (20.84) | 0.51 |
| Fourth (76th to 100th) | 13,635 (11.41) | 59,369 (12.94) | <0.001 |
| Insurance status (n [%]) | <0.001 | ||
| Medicare | 38,285 (31.55) | 97,514 (20.87) | <0.001 |
| Medicaid | 40,080 (33.03) | 158,469 (33.91) | 0.02 |
| Private | 27,805 (22.92) | 130,594 (27.95) | <0.001 |
| Uninsured | 10,775 (8.88) | 61,889 (13.24) | <0.001 |
| Hospital region (n [%]) | <0.001 | ||
| Northeast | 17,930 (14.75) | 67,310 (14.38) | 0.25 |
| Midwest | 30,375 (24.99) | 98,325 (21.00) | <0.001 |
| South | 52,665 (43.34) | 208,040 (44.43) | 0.02 |
| West | 20,559 (16.92) | 94,530 (20.19) | <0.001 |
| Hospital bed size (n [%]) | 0.06 | ||
| Small | 26,569 (21.86) | 104,650 (22.35) | 0.18 |
| Medium | 36,475 (30.01) | 143,230 (30.59) | 0.17 |
| Large | 58,485 (48.12) | 220,325 (47.06) | 0.02 |
| Hospital teaching status (n [%]) | 0.07 | ||
| Rural | 15,169 (12.48) | 57,195 (12.22) | 0.34 |
| Urban nonteaching | 29,535 (24.3) | 117,740 (25.15) | 0.03 |
| Urban teaching | 76,825 (63.21) | 293,270 (62.64) | 0.18 |
Abbreviations: DKA, diabetic ketoacidosis; PUD, peptic ulcer disease.
Includes other upper gastrointestinal (GI) inflammatory conditions requiring anti‐acid therapy with either proton pump inhibitors or H2 blockers, for example, gastroesophageal reflux disease (GERD), esophagitis, upper GI angiodysplastic condition, increased gastrin secretion, and so on.
Median household income for the patient's ZIP code.
Length of stay
The overall mean LOS was 3.31 days for patients hospitalized with DKA. It was 3.84 days for those who had coexisting PUD and 3.17 days for those who did not (Table 3). After adjusting for confounders, patients with concurrent PUD had a significantly longer mean LOS (mean adjusted additional LOS: 0.28, 95% confidence interval [CI]: 0.21–0.34, p < 0.001).
Table 3.
Primary and secondary outcomes
| With PUD | Without PUD | Adjusted OR | |||
|---|---|---|---|---|---|
| Outcomes | Overall | (95% CI) | (95% CI) | (95% CI) | p‐value |
| Mean LOS, days (95% CI) | 3.31 (3.28–3.33) | 3.84 (3.79–3.90) | 3.17 (3.14–3.19) | 0.28 (0.21–0.34) | <0.001 |
| Mortality, % | 0.42 (0.39–0.46) | 0.88 (0.51–1.50) | 0.42 (0.38–0.45) | 1.58 (0.88–2.84) | 0.12 |
| Total hospitalization charges, mean, USD | 31,718 (31,279–32,157) | 34,740 (34,048–35,431) | 30,933 (30,477–31,390) | 944 (251–1637) a | 0.008 |
| Total hospitalization costs, mean, USD | 7738 (7666–7810) | 8649 (8523–8774) | 7502 (7425–7579) | 413 (281–546) a | <0.001 |
| Bicarbonate infusion, % | 0.46 (0.36–0.59) | 0.48 (0.36–0.60) | 0.39 (0.26–0.53) | 0.87 (0.67–1.15) | 0.33 |
| AKI, % | 37.51 (37.04–37.97) | 40.6 (39.87–41.33) | 36.71 (36.21–37.19) | 1.006 (0.97–1.04) | 0.73 |
| AKI requiring dialysis, % | 0.23 (0.20–0.26) | 0.27 (0.21–0.35) | 0.22 (0.19–0.25) | 1.02 (0.76–1.37) | 0.89 |
| ICU admission | 1.69 (1.61–1.77) | 4.26 (3.35–5.40) | 1.66 (1.58–1.73) | 1.94 (1.48–2.55) | <0.001 |
Abbreviations: AKI, acute kidney injury; CI, confidence interval; ICU, intensive care unit; LOS, length of stay; OR, odds ratio; PUD, peptic ulcer disease; USD, United States Dollar.
Adjusted mean difference in USD.
Independent predictors of LOS
The strength of association of LOS with multiple variables was tested individually using univariate regression analysis. Multiple patient and hospital‐level variables were tested. The final model is presented in Table 4. The variables screened to assess whether they were independent predictors of LOS were as follows: (1) patient level: age, sex, race, insurance provider, median income in the ZIP code, Glasgow Coma Scale (GCS) score on presentation, and Charlson comorbidity score; (2) hospital level: hospital number of beds, teaching status/location, and admission over the weekend; and (3) comorbidities: including heart failure, hypertension, hyperlipidemia, malnutrition status, vitamin B 12 deficiency, Clostridium difficile infection, toxic megacolon, pneumonia, and complications of diabetes mellitus. The variables found to be independent predictors of increased LOS were malnutrition, Clostridium difficile infection, pneumonia, and a higher Charlson comorbidity score. The variables associated with lower odds of LOS were hypertension, hyperlipidemia, heart failure, older age, female gender, and admission to a larger teaching hospital. GCS score of 3–8 on presentation predicted increased LOS, while scores higher than 8 did not affect LOS. The rest of the variables had no influence on LOS.
Table 4.
Independent predictors of length of stay
| Variable | Adjusted odds ratios (95% confidence interval) | p‐value |
|---|---|---|
| Patient‐level variables | ||
| Age | 0.03 (0.02–0.30) | <0.001 |
| Female gender | 0.15 (0.11–0.20) | <0.001 |
| Race | ||
| White | Reference | Reference |
| Black | 0.22 (0.17–0.28) | <0.001 |
| Hispanic | 0.21 (0.14–0.29) | <0.001 |
| Asian or Pacific Islander | 0.37 (0.13–0.61) | 0.003 |
| Native American | 0.07 (−0.08–0.22) | 0.39 |
| Other race | 0.30 (0.16–0.45) | <0.001 |
| Insurance provider | ||
| Medicare | Reference | Reference |
| Medicaid | 0.32 (−1.11–0.26) | 0.26 |
| Private | 0.21 (−1.47–1.49) | 0.34 |
| Uninsured | 0.93 (0.47–2.9) | 0.80 |
| Median income in patient's ZIP code (quartile) a | ||
| First (0–25th) | Reference | Reference |
| Second (26th to 50th) | 0.98 (0.92–1.03) | 0.40 |
| Third (51st to 75th) | 0.95 (0.89–1.003) | 0.07 |
| Fourth (76th to 100th) | 0.93 (0.86–1.01) | 0.09 |
| Glasgow Coma Scale (GCS) | ||
| GCS 13–15 | Reference | Reference |
| GCS 9–12 | 1.23 (−0.46–2.93) | 0.15 |
| GCS 3—8 | 6.79 (1.89–11.71) | 0.007 |
| Hospital‐level variables | ||
| Hospital bed size | ||
| Small | Reference | Reference |
| Medium | 0.20 (0.14–0.25) | <0.001 |
| Large | 0.45 (0.39–0.50) | <0.001 |
| Hospital location/teaching status | ||
| Rural | Reference | Reference |
| Urban nonteaching | 0.37 (−1.15–1.89) | 0.63 |
| Urban teaching | 0.85 (−0.95–2.65) | 0.35 |
| Weekend admission | 0.98 (0.93–1.02) | 0.30 |
| Comorbidities | ||
| Malnutrition | 2.05 (1.76–2.33) | <0.001 |
| Hypertension | 0.07 (0.03–0.12) | 0.002 |
| Hyperlipidemia | 0.81 (0.75–0.87) | <0.001 |
| Heart failure | 0.64 (0.45–0.84) | <0.001 |
| Vitamin B 12 deficiency | 2.51 (−0.51–5.53) | 0.10 |
| Clostridium difficile infection | 3.49 (2.75–4.25) | <0.001 |
| Toxic megacolon | 0.66 (−1.98–3.30) | 0.62 |
| Pneumonia | 1.46 (1.19 [0.56] to 2.36) | 0.001 |
| DM complicated with nephropathy | 0.02 (−2.34–2.29) | 0.39 |
| DM complicated with retinopathy | 0.48 (−1.13–2.08) | 0.52 |
| DM complicated with neuropathy | 0.53 (−1.07–2.12) | 0.56 |
| DM complicated with vascular disease | 0.40 (−2.6–1.86) | 0.22 |
Abbreviation: DM, diabetes mellitus.
First quartile: $1–$42,999, $1–$43,999, and $1–$45,999 for Nationwide Inpatient Sample (NIS) 2016, 2017, and 2018, respectively. Second quartile: $43,000–$53,999, $44,000–$55,999, and $46,000–$58,999 for NIS 2016, 2017, and 2018, respectively. Third quartile: $54,000–$70,999, $56,000–$73,999, and $59,000–$78,999 for NIS 2016, 2017, and 2018, respectively. Fourth quartile: >$71,000, >$74,000, and >$79,000 for NIS 2016, 2017, and 2018, respectively.
Inpatient mortality
A total of 2510 (0.42%) patients died during hospitalization admitted with a principal diagnosis of DKA. There were 0.88% of deaths for those who had coexisting PUD and 0.42% for those who did not (unadjusted). After adjusting for confounders, DKA patients with concurrent PUD did not differ with regard to inpatient mortality compared with those who did not (adjusted odds ratio [aOR]: 1.58, 95% CI: 0.88–2.84, p < 0.12).
Morbidity
The overall rate of AKI was 36.71% and 40.6% among non‐PUD patients compared to PUD, respectively, but this difference was not significant after adjusting for confounders (aOR: 1.006, 95% CI: 0.97–1.04, p = 0.73). Similarly, AKI requiring dialysis was not different between the two groups (aOR: 1.02, 95% CI: 0.76–1.37, p = 0.89).
Resource utilization
Markers of resource utilization used were: total hospitalization costs, total hospitalization charges, and ICU admission. Evaluation of the mean total hospitalization charges showed that these were $29,727 (95% CI: $ 28,634–$30,820) for the overall study population, $30,933 (95% CI: $30,477–$31,390) for non‐PUD patients, and $34,740 ($34,048–$35,431) for PUD patients. After adjusting for the confounders, total hospitalization charges were significantly higher for PUD patients. Similar results were found when examining total hospitalization costs, with the overall study population, non‐PUD, and PUD patients having mean total hospitalization costs of $8,049 (95% CI: $7,811–$8,288), $7,502 (95% CI: $7,425–$7,579), and $8,649 (95% CI: $8,523–$8,774), respectively. PUD patients had significantly higher adjusted mean total hospitalization costs than non‐PUD patients. In our study, 4.26% of DKA patients having PUD required ICU compared to 1.66% in the non‐PUD group. After adjusting for confounding factors, including hospital‐level factors, the odds of ICU admission were higher in the PUD group (aOR: 1.94, 95% CI: 1.48–2.55, p < 0.001).
Discussion
By analyzing a large nationally representative database, we concluded that the presence of PUD in DKA patients is associated with increased LOS and identified predictors responsible for this. We also found significantly increased odds of total hospital charges and cost. The presence of PUD in patients with DKA did not increase the inpatient mortality or incidence of AKI, but it was associated with higher odds of ICU admission.
PPI and H2RAs inhibit H+/K+ ATPase in the gastric parietal, which physiologically, if not blocked, results in the secretion of hydrogen ion (H+) into the gastric lumen and concomitant secretion of bicarbonate () into the bloodstream towards the basal side of the parietal cells due to the action of parietal cell carbonic anhydrase [9]. Therefore, we based our study on the hypothesis that DKA patients taking PPIs or H2 blockers for acid suppression will have comparatively lesser generation and take more time to meet DKA resolution criteria, resulting in a prolonged hospital stay.
We found that DKA patients with concomitant PUD had higher LOS than patients without PUD. Mean LOS was 3.84 days (95% CI: 3.79–3.90) in the PUD group while 3.17 days (95% CI: 3.14–3.19) in patients without PUD. After controlling for confounding factors, the mean adjusted LOS was 0.28 days more in the PUD group. Historically, predictors known to prolong LOS in DKA independently include age, concurrent infection, hypokalemia, lower pH on presentation, precipitating cause as infection including urinary tract infection and pneumonia, and severity of DKA based on biochemical markers including serum osmolality, anion gap apart from many others [14, 15, 16, 17, 18]. Studies reporting the association of quantitative on presentation found contradictory results with both positive and no association reported [14, 17]. We found that malnutrition and Clostridium difficile infection predicted longer LOS in DKA patients with PUD. At the same time, complications from diabetes—including nephropathy, retinopathy, neuropathy, and vascular disease—did not affect LOS in this patient population. However, PPI use is associated with the risk of Clostridium difficile infection and pneumonia, which can potentially be among the reasons for longer LOS in the PUD group in addition to the hypothesized role of less generation [19, 20].
To our knowledge, no prior studies have reported PUD association with mortality in DKA patients. PUD in DKA patients was not a predictor of mortality in our study. PUD resulted in higher resource utilization measured by total hospitalization charges and costs resulting from DKA. Desai et al. reported an increasing trend of the mean total charges for DKA admissions from $18,987 in 2003 to $26,566 in 2014 (p < 0.001) [21]. We found it to be $30,933 in combined DKA hospitalization from 2016 to 2018, whereas DKA patients with PUD had $34,740. Similarly, total hospitalization costs are more in the PUD group. The American Diabetes Association recommends that patients with DKA should be treated in the ICU until DKA resolution criteria are met, and then care can be transitioned down to other units in the hospital [4, 6]. We found that 4.26% of DKA patients having PUD required the ICU compared to 1.66% in the non‐PUD group. After adjusting for confounding factors, including hospital‐level variables, the odds of ICU admission were higher in the PUD group. Greater LOS and ICU stay contributed to more hospital costs and charges in the PUD group and potentially explain the difference between the two groups. AKI commonly complicates DKA due to hyperglycemia‐induced diuresis commonly treated with intravenous fluid administration [22, 23]. As expected, we found no difference in the proportion of AKI between the two groups in DKA patients (aOR: 1.006, p = 0.73) as PUD does not influence the presence of AKI. Intravenous fluid administration to correct the deficit is a cornerstone in DKA management, and medical providers are good at managing AKI in DKA irrespective of the presence or absence of PUD [24].
There are various shortcomings to our study. First, the retrospective nature of our study limits the complete randomization of the exposure. We relied on propensity matching and multivariate regression models to control for confounders. The results obtained from both methods were similar, reducing the likelihood of confounding, but residual confounding can still exist. Second, an administrative database was used to acquire the data. Claims‐based databases such as NIS are intrinsically susceptible to missing codes or erroneously entered data [25]. Nonetheless, the frequency of missing data among the variables used in the study was less than 2.5%, with one exception, and the multiple imputations method was used to account for the missing data. The use of ICD‐10 codes as opposed to clinical parameters can result in misclassification of the diagnosis. ICD‐10‐CM codes were used to derive the data, which are more specific than ICD‐9‐CM and have demonstrated high sensitivity and specificity to study GI diseases [26, 27]. However, DKA itself is prone to coding inaccuracies, as shown by VanderWeele et al. [28]. Third, the data on medical treatments is not captured in the NIS; therefore, we assumed that all patients included in the study were taking acid‐suppressive medications. We included only those disease processes which require a relatively long course of acid suppression. Noncompliance is also an issue, but by excluding individuals for which transient acid suppression is adequate, we believe that the population included in the study will adequately represent people who are on acid suppression (ICD‐10 codes available in the Supporting Information). Finally, due to unavailable data on the laboratory values in the database, the severity of DKA on presentation could not be assessed; instead, we used Charlson Comorbidity Index, a generalized validated prognostic scale, as was employed in previous studies [29]. We believe further randomized trials are required to overcome the limitations encountered in this study.
In spite of these impediments, our study has many strengths. This study is the first to our knowledge that reports the effect of PUD on LOS in DKA at a national level. Additionally, we introduced the hypothesis of possible effects of concomitant acid suppression on LOS in DKA that may drive prospective studies on specific factors, mechanisms, and predictors of LOS in DKA. We used NIS, which contains data on patients at diverse hospital‐level characteristics from over 45 states, as described in the methods section. This results in improved external validity and generalizability; therefore, we believe that the results obtained should reflect the patient population admitted to the hospitals across the United States. Moreover, NIS eradicates the frequently experienced constraint of single‐center studies by allowing the use of a large sample size as it is the largest publicly available all‐payer database comprising the inpatient population. This increases the power of the study, lowering the likelihood of type II error in the analysis drawn. Moreover, the characteristic variables in the database granted the opportunity to explore variables such as household income estimates, hospitalization cost, and hospital factors, which are not commonly possible in single‐center studies. Propensity matching is a powerful tool while analyzing administrative databases and attempts to control confounding by indication [30]. It relies on a vast array of empirically derived covariates that serve as surrogates for unmeasured confounding variables while matching cases with controls [31, 32].
Conclusion
The renal contribution to acid–base balance during DKA resolution is small in addition to other factors that delay the normalization of , including vomiting of gastric acid contents and urinary excretion of ketone anions leading to urinary loss [33]. We found that concurrent PUD increases the LOS in DKA patients by 0.28 days. The conjecture that formed the basis of our study was that patients taking PPIs or H2RAs will have relatively less generation and will take more time to meet the criteria for DKA resolution. We found that Clostridium difficile and pneumonia predicted longer LOS in DKA patients with concomitant PUD, hinting at the possible role played by PPIs and acid suppression in prolonging the LOS in such patients. Increased LOS also drives the more hospital cost burden in this patient population. Even though the results are interesting for LOS, owing to the limitations inherent to the database, the hypothesis of increased LOS with acid‐suppressive medications remains a hypothesis and demands further studies. Whether sucralfate would help reduce the cost of the stay in severe DKA patients who were on acid‐suppressive medications or those who need stress ulcer prophylaxis is yet to be elucidated in future studies. Further research is warranted to test interventions to reduce the hospital resource utilization gap between these two groups.
Conflict of interest
None of the authors has any financial, industrial, or commercial conflict of interest to disclose.
Author contributions
Umer Farooq: Conceptualization; data curation; formal analysis; writing – original draft; writing – review and editing. Zahid Ijaz Tarar: Data curation; formal analysis. Faisal Kamal: Writing – original draft. Adnan Malik: Formal analysis. Joseph Bresnahan: Conceptualization. Ayokunle T. Abegunde: Critical revision of the manuscript for important intellectual content; study supervision.
Supporting information
Appendix
Farooq U, Tarar ZI, Kamal F, Malik A, Bresnahan J, Abegunde AT. Is acid suppression associated with the increased length of stay in diabetic ketoacidosis patients? A nationwide analysis. J Intern Med. 2022;292:136–145.
References
- 1. Wuerth BA, Rockey DC. Changing epidemiology of upper gastrointestinal hemorrhage in the last decade: a nationwide analysis. Dig Dis Sci. 2018;63(5):1286–93. [DOI] [PubMed] [Google Scholar]
- 2. Boonpongmanee S, Fleischer DE, Pezzullo JC, Collier K, Mayoral W, Al‐Kawas F, et al. The frequency of peptic ulcer as a cause of upper‐GI bleeding is exaggerated. Gastrointest Endosc. 2004;59(7):788–94. [DOI] [PubMed] [Google Scholar]
- 3. Ahmed Ullah Mishuk LC, Gaillard P, Westrick S, Hansen RA, Qian J. National trends in prescription proton pump inhibitor use and expenditure in the United States in 2002–2017. J Am Pharm Assoc. 2021;61(1):87–94.E7. [DOI] [PubMed] [Google Scholar]
- 4. Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, et al. Management of hyperglycemic crises in patients with diabetes. Diabet Care. 2001;24(1):131–53. [DOI] [PubMed] [Google Scholar]
- 5. Mendez Y, Surani S, Varon J. Diabetic ketoacidosis: treatment in the intensive care unit or general medical/surgical ward? World J Diabet. 2017;8(2):40–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabet Care. 2009;32(7):1335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abrahami D, McDonald EG, Schnitzer M, Azoulay L. Trends in acid suppressant drug prescriptions in primary care in the UK: a population‐based cross‐sectional study. BMJ Open. 2020;10(12):e041529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Faden HS, Ma CX. Trends in oral antibiotic, proton pump inhibitor, and histamine 2 receptor blocker prescription patterns for children compared with adults: implications for Clostridium difficile infection in the community. Clin Pediatr. 2016;55(8):712–6. [DOI] [PubMed] [Google Scholar]
- 9. Puscas I, Buzás G, Puscas JC, Persa F. Carbonic anhydrase inhibitors: antisecretory and cytoprotective properties. Acta Physiol Hung. 1989;73(2–3):167–77. [PubMed] [Google Scholar]
- 10. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. [DOI] [PubMed] [Google Scholar]
- 11. Dugoff EH, Schuler M, Stuart EA. Generalizing observational study results: applying propensity score methods to complex surveys. Health Serv Res. 2014;49(1):284–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eric Vittinghoff DVG, Shiboski SC, McCulloch CE. Regression methods in biostatistics. New York, NY; Heidelberg: Springer Nature; 2012. [Google Scholar]
- 13. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–99. [DOI] [PubMed] [Google Scholar]
- 14. Freire AX, Umpierrez GE, Afessa B, Latif KA, Bridges L, Kitabchi AE. Predictors of intensive care unit and hospital length of stay in diabetic ketoacidosis. J Crit Care. 2002;17(4):207–11. [DOI] [PubMed] [Google Scholar]
- 15. Lee MH, Calder GL, Santamaria JD, MacIsaac RJ. Diabetic ketoacidosis in adult patients: an audit of factors influencing time to normalisation of metabolic parameters. Intern Med J. 2018;48(5):529–34. [DOI] [PubMed] [Google Scholar]
- 16. Idampitiya C, Sithole J, Idris I. Age is the only independent predictor for the length of hospital stay in patients admitted to a UK district general hospital with diabetic ketoacidosis. Eur J Intern Med. 2006;17(8):585. [DOI] [PubMed] [Google Scholar]
- 17. Azevedo LC, Choi H, Simmonds K, Davidow J, Bagshaw SM. Incidence and long‐term outcomes of critically ill adult patients with moderate‐to‐severe diabetic ketoacidosis: retrospective matched cohort study. J Crit Care. 2014;29(6):971–7. [DOI] [PubMed] [Google Scholar]
- 18. Wu XY, She DM, Wang F, Guo G, Li R, Fang P, et al. Clinical profiles, outcomes and risk factors among type 2 diabetic inpatients with diabetic ketoacidosis and hyperglycemic hyperosmolar state: a hospital‐based analysis over a 6‐year period. BMC Endocr Disord. 2020;20(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McDonald EG, Milligan J, Frenette C, Lee TC. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med. 2015;175(5):784–91. [DOI] [PubMed] [Google Scholar]
- 20. Trifan A, Stanciu C, Girleanu I, Stoica OC, Singeap AM, Maxim R, et al. Proton pump inhibitors therapy and risk of Clostridium difficile infection: systematic review and meta‐analysis. World J Gastroenterol. 2017;23(35):6500–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Desai D, Mehta D, Mathias P, Menon G, Schubart UK. Health care utilization and burden of diabetic ketoacidosis in the U.S. over the past decade: a nationwide analysis. Diabet Care. 2018;41(8):1631–8. [DOI] [PubMed] [Google Scholar]
- 22. Chen J, Zeng H, Ouyang X, Zhu M, Huang Q, Yu W, et al. The incidence, risk factors, and long‐term outcomes of acute kidney injury in hospitalized diabetic ketoacidosis patients. BMC Nephrol. 2020;21(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spira A, Gowrishankar M, Halperin ML. Factors contributing to the degree of polyuria in a patient with poorly controlled diabetes mellitus. Am J Kidney Dis. 1997;30(6):829–35. [DOI] [PubMed] [Google Scholar]
- 24. Gosmanov AR, Gosmanova EO, Dillard‐Cannon E. Management of adult diabetic ketoacidosis. Diabet Metab Syndr Obes. 2014;7:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klabunde CN, Warren JL, Legler JM. Assessing comorbidity using claims data: an overview. Med Care. 2002;40(8 Suppl):IV‐26–35. [DOI] [PubMed] [Google Scholar]
- 26. Cartwright DJ. ICD‐9‐CM to ICD‐10‐CM codes: what? Why? How? Adv Wound Care. 2013;2(10):588–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. CDC . International Classification of Diseases, (ICD‐10‐CM/PCS) transition—background. https://www.cdc.gov/nchs/icd/icd10cm_pcs_background.htm (2015). Accessed 11/3/2021
- 28. VanderWeele J, Pollack T, Oakes DJ, Smyrniotis C, Illuri V, Vellanki P, et al. Validation of data from electronic data warehouse in diabetic ketoacidosis: caution is needed. J Diabet Complications. 2018;32(7):650–4. 10.1016/j.jdiacomp.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 29. Abougergi MS, Travis AC, Saltzman JR. Impact of day of admission on mortality and other outcomes in upper GI hemorrhage: a nationwide analysis. Gastrointest Endosc. 2014;80(2):228–35. [DOI] [PubMed] [Google Scholar]
- 30. Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf. 2005;14(7):465–76. [DOI] [PubMed] [Google Scholar]
- 31. Austin PC, Wu CF, Lee DS, Tu JV. Comparing the high‐dimensional propensity score for use with administrative data with propensity scores derived from high‐quality clinical data. Stat Methods Med Res. 2020;29(2):568–88. [DOI] [PubMed] [Google Scholar]
- 32. Yu R, Silber JH, Rosenbaum PR. Matching methods for observational studies derived from large administrative databases. Stat Sci. 2020;35(3):338–55. [Google Scholar]
- 33. Oh MS, Carroll HJ, Uribarri J. Mechanism of normochloremic and hyperchloremic acidosis in diabetic ketoacidosis. Nephron. 1990;54(1):1–6. 10.1159/000185800 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix


