Summary
Background
A number of mutations related to malignant melanoma (MM) have been identified, and of the mutated genes, BRAF has been found to be altered in > 50% of cases. Most of these have been BRAF V600E mutations, whereas the incidence of BRAF V600K may vary from 10% to 30%. Little is known about the clinical prognostic correlations of BRAF V600K MMs. We evaluated the clinical and dermoscopic features, incidence, therapy response and outcomes in the medium to long term.
Aim
To compare the clinical and dermoscopic characteristics, the response to systemic therapies and the prognosis among MMs with BRAF V600E and BRAF V600K mutations.
Methods
We retrieved the data of patients tested in our centre for MM from 2012 to 2015, including clinical features, dermoscopic pictures, clinical history and tumour mutations. Only patients with BRAF V600E and BRAF V600K mutations were included. Any MMs positive for BRAF V600K mutation were collected, and the number of V600K cases and their features were used to extract the same number of patients with BRAF V600E from our database using a matching method. The clinical and dermoscopic presentation, therapy response and disease progression of the two groups were then evaluated.
Results
In total, 132 cases of BRAF V600E‐mutated MMs were identified, and then randomized with a propensity‐score method to match the 10 retrieved cases of BRAF V600K mutation. Both groups had a nodular appearance to the tumours and an advanced disease stage, and no significant differences in dermoscopic features were highlighted. During the follow‐up period, four patients with BRAF V600K died of disease‐specific causes. Moreover, we found a higher frequency of metastasis, a faster disease progression and more rapid mortality in patients with BRAF V600K.
Conclusion
Despite the small size of this study, the results show similar clinical and dermoscopic characteristics between V600E and V600K mutations, but compared with BRAF V600E MMs, BRAF V600K MMs seem to be less responsive to therapy and have a worse prognosis.
Our work shows that melanomas affected by the BRAF V600K variant have a similar clinical and dermoscopic presentation but a more aggressive behaviour than those with BRAF V600E.
Introduction
Malignant melanoma (MM) is a neoplasm that results from uncontrolled proliferation of melanocyte cells. 1 Although it is only the third most common cutaneous neoplasia, it has the highest mortality rate of all cutaneous malignancies.
Uncontrolled activation of the mitogen activated protein kinase (MAPK) signalling pathway is principally involved in MM pathogenesis. 2 In particular, this pathway can induce cell growth, proliferation and differentiation while inhibiting apoptosis by means of growth factors. Several key genes are involved in this pathway, including RAS, BRAF, MEK and ERK. 3 More than 90% of cutaneous MMs present constitutive MAPK pathway activity. In particular, the prevalence of mutations in codon 600 of BRAF ranges between 40% and 60% in patients with MM. The most prevalent mutations in MM are the BRAF V600E variant (about 80%) and BRAF V600K (5–30%), with other subtypes found at lower frequencies: V600M 4%, V600R 5% and V600D < 5%. 4
Heterogeneity in BRAF mutations within the same patient has been described, with differences between primary tumours and metastases, and between different metastases. 5 Testing for BRAF mutation status is usually performed on the most recently resected or biopsied tumours. It is important to note that although BRAF inhibitors show comparative efficacy in both the V600E and V600K mutations, the two tumour types have sometimes been considered as distinct entities. 6 Indeed, recent scientific studies reported that BRAF V600K MMs, in contrast to BRAF V600E MMs, seem to increase with age and have a higher risk for brain and lung metastases and a shorter time from diagnosis to metastasis onset and patient death. 7 Several reports of patients with BRAF V600K‐mutated MMs also described significant differences in sex, age, primary MM location, the interval from MM first diagnosis to Stage IV disease and the overall survival after the diagnosis of Stage IV disease. In particular, the V600K mutation was significantly associated with older age, male sex, head and neck as the primary MM site, short overall survival from the time of diagnosis of Stage IV disease and a higher degree of chronic sun damage, which might explain the variable geographical frequency of BRAF V600K. 8
Finally, although Pozzobon et al. 9 previously analysed the dermoscopic criteria associated with BRAF and NRAS mutation status in primary cutaneous MM, very little is known about the dermoscopic and prognostic differences between V600E and V600K variants and the correlation of dermoscopic features and mutational burden. 9 , 10 , 11 We therefore performed this study to compare the clinical and dermoscopic characteristics of MMs with these two mutations and the response to immunotherapies and targeted therapies.
Methods
The study was approved by the ethical committee at our institution (no. DERM‐MTC 2017). No informed consent was required.
Study design
We conducted a retrospective analysis of patients registered at our Melanoma Unit at the Policlinico Sant'Orsola‐Malpighi, Bologna, Italy. The data are available on request from the authors.
Patient collection
We built a database using data collected by primary search from our internal register of anatomopathological reports, collecting all the cases of MMs to analyse for BRAF mutations. Only patients with at least 5 years of follow‐up during the period 2012–2015 and treated with immune or targeted therapies were included.
We used next‐generation sequencing technique for mutation analysis, using a multigenic panel (Oncomine Focus Assay; ThermoFisher Scientific, Waltham, MA, USA). 12
Patients considered as having been treated with targeted and immunotherapies only if a cycle of treatment, consisting of the standard dosage and with a duration of at least 12 months, had been performed with at least three control computed tomography scans for evaluation.
Independent variables such as sex, age, tumour location on the skin, treatment type, TNM stage and Breslow thickness were registered in this purpose‐built database. Body sites for tumour location was included the limbs, trunk, face, scalp, back, hands, feet and anogenital area.
Melanoma collection
All MMs positive for BRAF V600K mutation were collected. The number of V600K cases and their features were used to extract the same number of patients with BRAF V600E from the database using a matched propensity score method. Matching criteria including patient sex and age, Breslow thickness, tumour site, TNM and therapies in use. Dermoscopic images of cases belonging to both groups were downloaded from our videodermoscopy database.
Comparison of the groups
We compared the two groups for the characteristics of interest. The clinical presentation of the tumour was assessed as one of three patterns: nodular, ulcerated nodular and superficial.
The evaluated dermoscopic characteristics included vascular pattern, blue–white veil, chrysalids, blue–grey blotches, reticular grey–blue areas, white regression structures, peppering, and presence of > 3 colours. Three trained dermoscopists have evaluated magnified images registered in our database. Each dermoscopic criteria have been singularly evaluated and each dermatologist was blinded to patient identity, clinical information and mutational status at the time of the assessment.
Response to therapy was evaluated by analysing the trend of metastasis numbers and volume on three consecutive CT scans after therapy administration. Outcomes considered were no metastasis; tumour in remission, stable, with partial improvement or no improvement; mortality rate; time to metastasis onset (months); and time to death (months).
Statistical analysis
The BRAF V600E group was extracted from a larger group using a matched propensity score method. Approximation values for independent variables with a tolerance of 20% were used to reach 1 : 1 ratios in both groups. The two groups were then rechecked for homogeneity using the Mann–Whitney U‐test for quantitative variables and Fisher exact test for qualitative variables. Quantitative data were then expressed as mean ± SD and an independent samples Mann–Whitney U‐test was used to compare the two groups. Qualitative data were described using the Fisher exact probability method. P < 0.05 was considered statistically significant. Kaplan–Meier curves were used to represent the mortality and metastasis onset between the two groups of patients. Data processing and statistical analysis were performed using an Excel (Microsoft Corp, Redmond, WA, USA) database and SPSS software (V26; IBM SPSS, Armonk, NY, USA).
Results
Patients
From the search in our hospital database, we found 223 patients positive for BRAF V600 mutations and with at least 5 years of follow‐up. Of these, 132 patients were positive for the BRAF V600E variant and 10 patients for the BRAF V600K mutation. Matching these groups, we identified 10 patients whose independent variables were not statistically different from that of patients with BRAF V600K.
Characteristics
The mean age was 57.7 years for the BRAF V600E group, and 65.90 years for the BRAF V600K groups (Table 1). There was a male predominance in both groups, with a male/female ratio of 7 : 3 for BRAF V600E and 8 : 2 for BRAF V600K.
Table 1.
Demographic characteristics, tumour thickness at diagnosis a and systemic therapies of the two groups.
| Parameter | BRAF mutation | P | |
|---|---|---|---|
| V600E | V600K | ||
| Age, years b | 57.70 ± 13.458 | 65.90 ± 3.139 | 0.14 c |
| Sex M : F | 7 : 3 | 8 : 2 | 1.00 d |
| Breslow thickness, mm b | 2.35 ± 10.52 | 2.73 ± 11.26 | 0.48 |
| Systemic therapies | 70% dabrafenib plus trametinib; 10% nivolumab; 10% ipilimumab; 10% NS | 70% dabrafenib plus trametinib; 10% nivolumab; 10% vemurafenib plus cobimetinib; 10% NS | 1.00 |
NS, not stated.
There was no difference between the groups in tumour site (arms, lower legs, thighs, feet, hands, face, scalp, trunk, genital area) or TNM stage (each TNM was assigned with a numeric number, e.g. IA = 1, IB = 2, etc.).
Mean ± SD.
Mann–Whitney U‐test.
Fisher exact test.
The macroscopic clinical analysis did not show any significant differences (Table 2). Both groups had the same frequency of affected areas (arms, legs, thighs, feet, hands, face, scalp, trunk and genitals).
Table 2.
Comparison of the clinical presentation, dermoscopic features response to therapies and outcomes of the two groups.
| Parameter | BRAF mutation | P | |
|---|---|---|---|
| V600E | V600K | ||
| Clinical presentation, n | 1.00 | ||
| Nodular | 3 | 3 | |
| Ulcerated nodular | 5 | 4 | |
| Superficial | 2 | 3 | |
| Dermoscopy findings, n a | |||
| Vessels | 4 | 3 | 0.31 |
| Blue–white veil | 3 | 4 | 0.61 |
| Chrysalids | 3 | 2 | 0.53 |
| Peppering | 1 | 2 | 0.44 |
| > 3 colours | 4 | 5 | 0.44 |
| Blue–grey blotches | 4 | 4 | 0.59 |
| Reticular grey–blue areas | 3 | 3 | 0.66 |
| White regression | 5 | 4 | 0.66 |
| Immune therapy response, n | 0.48 b | ||
| Not evaluable | 1 | 2 | |
| None | 0 | 5 | |
| Partial | 1 | 1 | |
| Stable | 8 | 2 | |
| Reduction | 0 | 0 | |
| Metastasis, n | |||
| Yes | 5 | 9 | 0.07 |
| No | 5 | 1 | |
| Died, n | |||
| Yes | 1 | 4 | 0.03 |
| No | 9 | 6 | |
Seven images of BRAF V600E (3) and BRAF V600K (4) were not found and thus could not be evaluated.
Mann–Whitney U‐test; all other P values were assessed using Fisher test.
The mean Breslow thickness was 2.35 mm for BRAF V600E and 2.73 mm for BRAF V600K. Based on the American Joint Committee on Cancer guidelines (eighth edition) there was no difference in TNM stage evaluated between the two groups for the same Breslow thickness.
Comparison of the two groups
The tumours were classified in accordance with their appearance, with ulcerated nodular being the most common, followed by nodular and then superficial.
There was lower statistical power for the dermoscopic feature due to the lack of three images in the BRAF V600K group and four images in the BRAF V600E group, making a total loss of seven cases (35%). However, analysis of the remaining samples did not demonstrate any noteworthy characteristics for either group.
In the BRAF V600E group, 70% of the cases were treated with a combination of dabrafenib and trametinib, while 10% were treated with nivolumab and 10% with ipilimumab; for the remaining 10% of patients, it was not possible to identify the therapy because of incomplete medical records. The BRAF V600K groups also had 70% treated with a combination of dabrafenib and trametinib, while 10% were treated with nivolumab and 10% with a combination of vemurafenib and cobimetinib; again, it was not possible to identify the therapy for the remaining 10% of patients because of incomplete medical records.
The study identified an interesting difference in the response to therapies, with a better outcome achieved in the BRAF V600E group; eight patients in this group had stable response, compared with partial or no response in six patients in the BRAF V600K group.
During the follow‐up period, four patients with BRAF V600E and nine patients with BRAF V600K died (Fig. 1a), which was statistically significant (P < 0.03). Finally, a similar but not statistically significant outcome was observed in metastasis onset, which developed more rapidly in the BRAF V600K group than in the BRAF V600E group (Fig. 1b).
Figure 1.
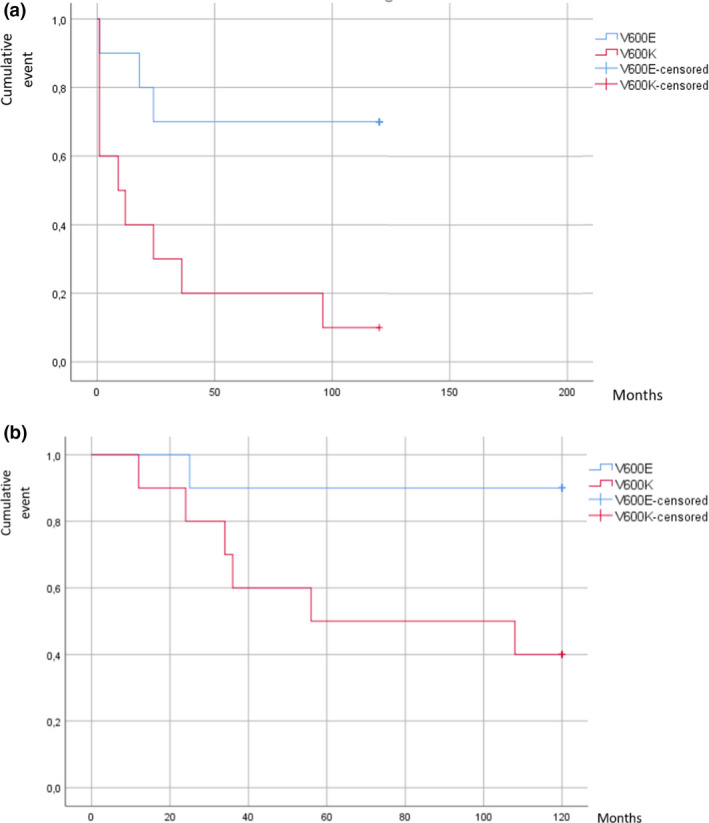
(a,b) Kaplan–Meier plots of (a) development of metastases diagnosed by computed tomography scan in the two groups, showing the faster progression in BRAF V600K cancers; and (b) disease‐specific mortality, showing that half of the patients with BRAF V600K died within 40 months. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
Specific mutations in MMs lead to differences in prognosis, therapy administration and response for wild‐type vs. BRAF‐mutated tumours. We know that BRAF‐mutated MMs are linked to a worse prognosis. 13 However, there is still controversy as to how different mutations of the same gene locus can change cancer prognosis, especially with regard to BRAF variants. Of the various BRAF mutations, the best‐known and most frequent variants are the V600E and V600K codon changes. Some studies have already explored the differences, clinical and histopathological characteristics, and predictive behaviour of these two mutation types, and have shown that BRAF V600K mutations appear to be more aggressive than the BRAF V600E mutations, 14 and our results are in agreement with these.
Despite the small size of our study, comparison of the two groups with the previous propensity matching technique 15 reduced any variability due to the independent parameters that could have influenced the results and could not have been excluded in later analysis. Once we established that the two groups did not show significant differences in characteristics and had undergone very similar therapeutic protocols, we were able to highlight the differences between them, excluding the most apparent confounding factors.
We analysed whether there was a difference in risk of metastases, micrometastases and sentinel lymph node involvement in patients with tumours of the same Breslow thickness, which would indicate more significant initial aggression. The analysis showed no distinct differences, suggesting that both mutations have a similar initial TNM stage.
Regarding the type of clinical presentation at macroscopic level, both groups had the same frequency of tumour types, namely superficial (flat) nodular or ulcerated appearance.
Because of the missing dermoscopic images, our sample appears too small for analysis to confirm a significant pattern between the two groups; however, the analysis of the remaining cases did not highlight any specific differences between the groups. Furthermore, also white regression and peppering has been reported by other groups, we did not find such a high prevalence of this feature in our study, casting doubt on its correlation with BRAF V600 mutations. 9
By contrast, the analysis of the response to systemic treatments was clearer; in the BRAF V600E group, although it was challenging to achieve remission of metastases, 8 of the 10 patients had at least a stable response, whereas in the BRAF V600K group, 6 of the 10 patients achieved little or no response. These results strongly suggest stronger resistance to targeted therapies by BRAF V600K‐mutated tumours compared with the more widespread BRAF V600E variant, which agrees with previous studies. 16 In addition, we observed more rapid spread of metastasis in the BRAF V600K, with 50% of the patients developing metastases in the first year after the first diagnosis, indicating a more aggressive course. The observed mortality rate was in accordance with the metastasis rate, with higher mortality from disease‐related causes occurring in the BRAF V600K compared with the BRAF V600E group, with a ratio of 4 : 1, respectively, and approximately half of the patients in the BRAF V600K group died within 3 years of diagnosis.
Conclusion
Despite the small size of our study being a limitation the results allowed us to identify some interesting elements and distinctive traits between the two groups. There were no differences in clinical features or dermoscopic patterns between the two variants. We could not identify any clinical feature that, without molecular analysis, could lead to one mutation being suspected over the other.
However, we did find differences in therapy response, metastasis development and mortality rate, with BRAF V600K‐mutated MMs showing lower response rate to therapies and a more rapid and frequent tendency to develop metastasis, implying greater resistance to the proposed treatments. Consequently, patients with BRAF V600K‐mutated MMs also had a more rapid and increased mortality rate during follow‐up.
In summary, this work shows that MMs with the BRAF V600K mutation have a similar clinical presentation but more aggressive behaviour than those with the BRAF V600E mutation.
What's already known about this topic?
-
•
BRAF V600K MMs have different clinical and dermoscopic features, a higher risk for developing metastases and a worse prognosis compared with BRAF V600E MMs.
-
•
BRAF V600K is more resistant to systemic therapies in comparison to BRAF V600E.
What does this study add?
-
•
We found nonsignificant differences in the clinical and dermoscopic presentations of the two principal mutations.
-
•
BRAF V600K tumours showed significantly greater resistance to systemic therapies and increased development and progression of metastases and mortality.
Acknowledgement
Open Access Funding provided by Universita degli Studi di Bologna within the CRUI‐CARE Agreement. [Correction added on 18 May, after first online publication: CRUI funding statement has been added.]
Conflict of interest: The authors declare that they have no conflicts of interest.
Contributor Information
Corrado Zengarini, Email: corrado.zengarini@yahoo.it.
Martina Mussi, Email: mussi.martina1809@gmail.com.
References
- 1. Lerner AB, McGuire JS. Melanocyte‐stimulating hormone and adrenocorticotrophic hormone – their relation to pigmentation. N Engl J Med 1964; 270: 539–46. [DOI] [PubMed] [Google Scholar]
- 2. Inamdar GS, Madhunapantula SV, Robertson GP. Targeting the MAPK pathway in melanoma: why some approaches succeed and other fail. Biochem Pharmacol 2010; 80: 624–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moreno S, Maiques O, Barcelo C et al. Differential immunoexpression of BRAF/V600E, senescence markers, PTEN, and T‐type calcium channels in acquired naevi according to their histopathological and dermoscopic classification. Acta Derm Venereol 2021; 101: adv00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng L, Lopez‐Beltran A, Massari F et al. Molecular testing for BRAF mutations to inform melanoma treatment decisions: a move toward precision medicine. Mod Pathol 2018; 31: 24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heinzerling L, Baiter M, Kühnapfel S et al. Mutation landscape in melanoma patients clinical implications of heterogeneity of BRAF mutations. Br J Cancer 2013; 109: 2833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reddy SM, Reuben A, Wargo JA. Influences of BRAF inhibitors on the immune microenvironment and the rationale for combined molecular and immune targeted therapy. Curr Oncol Rep 2016; 18: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lemech C, Infante J, Arkenau H‐T. The potential for BRAF V600 inhibitors in advanced cutaneous melanoma: rationale and latest evidence. Ther Adv Med Oncol 2012; 4: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bauer J, Büttner P, Murali R et al. BRAF mutations in cutaneous melanoma are independently associated with age, anatomic site of the primary tumor, and the degree of solar elastosis at the primary tumor site. Pigment Cell Melanoma Res 2011; 24: 345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pozzobon FC, Puig‐Butillé JA, González‐Alvarez T et al. Dermoscopic criteria associated with BRAF and NRAS mutation status in primary cutaneous melanoma. Br J Dermatol 2014; 171: 754–9. [DOI] [PubMed] [Google Scholar]
- 10. Gouillon L, Perier‐Muzet M, Amini‐Adle M et al. Dermoscopic features in BRAF and NRAS primary cutaneous melanoma: association with peppering and blue‐white veil. J Eur Acad Dermatol Venereol 2020; 34: e57–9. [DOI] [PubMed] [Google Scholar]
- 11. Bombonato C, Ribero S, Pozzobon FC et al. Association between dermoscopic and reflectance confocal microscopy features of cutaneous melanoma with BRAF mutational status. J Eur Acad Dermatol Venereol 2017; 31: 643–9. [DOI] [PubMed] [Google Scholar]
- 12. Siroy AE, Boland GM, Milton DR et al. Beyond BRAF(V600): clinical mutation panel testing by next‐generation sequencing in advanced melanoma. J Invest Dermatol 2015; 135: 508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moreau S, Saiag P, Aegerter P et al. Prognostic value of BRAF(V600) mutations in melanoma patients after resection of metastatic lymph nodes. Ann Surg Oncol 2012; 19: 4314–21. [DOI] [PubMed] [Google Scholar]
- 14. Li Y, Umbach DM, Li L. Putative genomic characteristics of BRAF V600K versus V600E cutaneous melanoma. Melanoma Res 2017; 27: 527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reeve BB, Smith AW, Arora NK, Hays RD. Reducing bias in cancer research: application of propensity score matching. Health Care Financ Rev 2008; 29: 69–80. [PMC free article] [PubMed] [Google Scholar]
- 16. Pires da Silva I, Wang KYX, Wilmott JS et al. Distinct molecular profiles and immunotherapy treatment outcomes of V600E and V600K BRAF‐mutant melanoma. Clin Cancer Res 2019; 25: 1272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


