Abstract
Pyridine-2,6-dithiocarboxylic acid (pdtc) is a metal chelator produced by Pseudomonas spp. It has been shown to be involved in the biodegradation of carbon tetrachloride; however, little is known about its biological function. In this study, we examined the antimicrobial properties of pdtc and the mechanism of its antibiotic activity. The growth of Pseudomonas stutzeri strain KC, a pdtc-producing strain, was significantly enhanced by 32 μM pdtc. All nonpseudomonads and two strains of P. stutzeri were sensitive to 16 to 32 μM pdtc. In general, fluorescent pseudomonads were resistant to all concentrations tested. In competition experiments, strain KC demonstrated antagonism toward Escherichia coli. This effect was partially alleviated by 100 μM FeCl3. Less antagonism was observed in mutant derivatives of strain KC (CTN1 and KC657) which lack the ability to produce pdtc. A competitive advantage was restored to strain CTN1 by cosmid pT31, which restores pdtc production. pT31 also enhanced the pdtc resistance of all pdtc-sensitive strains, indicating that this plasmid contains elements responsible for resistance to pdtc. The antimicrobial effect of pdtc was reduced by the addition of Fe(III), Co(III), and Cu(II) and enhanced by Zn(II). Analyses by mass spectrometry determined that Cu(I):pdtc and Co(III):pdtc2 form immediately under our experimental conditions. Our results suggest that pdtc is an antagonist and that metal sequestration is the primary mechanism of its antimicrobial activity. It is also possible that Zn(II), if present, may play a role in pdtc toxicity.
Under conditions of iron stress, most aerobic microorganisms produce at least one siderophore (26), and in some cases, a single bacterial strain can produce two or more. Each siderophore probably has a specific role in metal acquisition. One molecule may be important for the acquisition of iron, while another may be responsible for transport of some other metal. Pyochelin, one of two siderophores produced by Pseudomonas aeruginosa PAO1, has a relatively low affinity for iron. However, pyochelin binds a variety of metals (7), and regulation of pyochelin synthesis correlates with its relative affinity for Mo(VI), Co(II), and Fe(III) (37). Pyoverdine, the other siderophore produced by PAO1, demonstrates a binding affinity and a regulatory response typical of a transport molecule specific for iron (24, 38).
There are a variety of biochelators produced by microorganisms which do not function as siderophores (23), and some rather well-known siderophores appear to have additional activities apart from transport of metals, such as antioxidant and antibiotic action (10, 25, 28, 30). The antimicrobial activity of siderophores can have significant ecological effects. For example, the siderophores of fluorescent pseudomonads are responsible for antagonism toward various strains of fungi and some Pseudomonas spp. that are pathogenic to plants (5, 11). In addition, microbial siderophores can serve as iron sources for plants (3, 6, 15), and the production of a siderophore by Pseudomonas putida has been shown to enhance the yield of potato tubers (2).
Pyridine-2,6-dithiocarboxylic acid (pdtc) is a compound known to be secreted by at least three strains of Pseudomonas spp. (13, 20, 27). It has a remarkably strong affinity for various metals. Measurements by potentiometric and spectrophotometric titration were used to determine stability constants for iron, cobalt, and nickel of 1033, 1034, and 1033, respectively (J. C. Stolworthy, A. J. Paszczynski, R. A. Korus, and R. L. Crawford, submitted for publication). Based on metal-metal competition studies, the affinity of pdtc for copper was found to be comparable. The highly reactive Cu:pdtc complex has been shown to degrade carbon tetrachloride, primarily forming CO2 (21). The genetic locus (pdt) that codes for the production of pdtc has been characterized by Lewis et al. (22), who isolated pdt mutants and determined that pdtc is not essential for normal growth, even under metal-limiting conditions.
It is unlikely that pdtc is the cell's primary siderophore. In experiments using a radioactive 59Fe tracer (Cortese et al., unpublished data), we demonstrated that pdtc alone does not deliver iron to the cell, and in the presence of another extracellular factor, pdtc only modestly improves iron uptake. Dybas et al. (8) detected both hydroxamate and catechol-type siderophore activities in a 500- to 10,000-Da supernatant fraction of strain KC cultures, and evidence was presented for the presence of a high-molecular-weight (>10 kDa) molecule which may interact with pdtc. Clearly, strain KC has other iron transport systems which are adequate for survival under iron stress conditions, although pdtc may play a supplementary role in this process. To date we are aware of no reports that explain the physiological function of pdtc in pseudomonads. Here we examine the antimicrobial effects of pdtc and attempt to explain the mechanism of this activity.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The strains and plasmids used in this study are shown in Table 1. All strains were maintained on plates of tryptic soy agar (TSA) (Difco). All media were prepared with deionized water purified to <18 megaohm-centimeters resistivity using a Water Pro PS filtration unit (Labconco). Growth and competition experiments were performed in a minimal succinate medium (SM), which contained (per liter of deionized water) 6.0 g of K2HPO4, 3.0 g of KH2PO4, 1.0 g of (NH4)2SO4, and 4.0 g of succinic acid. The pH of the medium was adjusted to 7.5 with 10 M NaOH. Sterile 1 M MgSO4 (1 ml) and 1.0 ml of sterile 45 mM CaCl2 were added after autoclaving. Tetracycline, kanamycin (Sigma), Fe(III), Co(III), Cu(II), Zn(II), and synthetic pdtc were added as necessary. Metals used were of the highest available purity (inductively coupled plasma [ICP]) standards in 2.0% HNO3; Fisher Scientific) unless otherwise noted. Pdtc was synthesized according to Hildebrand et al. (12), and a 20 mM stock solution was prepared in N,N-dimethylformamide (DMF). For analysis by mass spectrometry, SM was modified (DM) by removing succinate and adding 20% DMF. For plate counts, serial dilutions of bacterial cultures were prepared in phosphate-buffered saline (PBS), and plate counts were performed on TSA (for Pseudomonas stutzeri) and Luria-Bertani (LB) medium (for Escherichia coli). M9 citrate medium was prepared by adding 10.0 g of sodium citrate per liter to 5 × M9 minimal medium (pH 8.0) (19) and autoclaving. M9 citrate plates were prepared by autoclaving 15 g of Noble agar (Difco) in 800 ml of deionized water and adding 200 ml of 5 × M9 citrate, 1.0 ml of 1 M MgSO4, and 1.0 ml of 45 mM CaCl2 after tempering all solutions in a 50°C waterbath.
TABLE 1.
Strains and plasmids used
| Strain or plasmid | Description | Reference or sourcea |
|---|---|---|
| P. stutzeri | ||
| KC | Wild type, aquifer isolate, pdt+ | C. Criddle, Stanford University (5a) |
| KC657 | Tn5 derivative of strain KC, Kmr, pdt | L. Sepulveda-Torres, Michigan State University (34a) |
| CTN1 | Spontaneous mutant of strain KC (Δ178 kb), pdt | Lewis et al. (22) |
| 14405 | Wild type, marine isolate | ATCC |
| 17588 | Wild type, clinical isolate (type strain) | ATCC |
| P. putida | ||
| DSM3601 | Wild type, pdt+ | DSMZ |
| MT-2 | Wild type, toluene degrader | University of Idaho culture collection (39) |
| P. fluorescens F 113 | Wild-type soil isolate | F. O'Gara, University of Cork, Ireland |
| P. aeruginosa PAO1 | Wild type | N. Schiller, University of California, Riverside |
| Escherichia coli | ||
| 25922 | Wild type, clinical isolate (type strain) | ATCC |
| S17-1 | Chromosomal RP4 integrant, mob+, Smr | K. Timmis, GBF, Braunschweig, Germany |
| Arthrobacter sp. 33790 | Wild type, clinical isolate | ATCC |
| Staphylococcus epidermidis 35547 | Wild type, soil isolate | ATCC |
| Plasmids | ||
| pRK311 | Broad-host-range cosmid, Mob−, Tra+, Tcr | M. Kahn, Washington State University (7a) |
| pT31 | pRK311 containing a 25.7-kb insert of strain KC genomic DNA, pdt+ | Accession no. AF196567 |
ATCC, American Type Culture Collection, Manassas, Va.; DSMZ, Deutsche Sammlung für Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany.
Pdtc sensitivity assays.
From overnight cultures grown in SM, several bacterial strains were transferred (0.5%, vol/vol) to 96-well microtiter dishes containing SM amended with various concentrations of pdtc. Where appropriate, metals were added to an apparent final concentration of 10 μM in the case of Fe(III), Co(III), and Zn(II) and 1 μM in the case of Cu(II). We sought to avoid interference from stabilizing chelators such as citrate. Therefore, the only measure taken to enhance the solubility of iron was to add pdtc to the media prior to adding metals. Control plates were prepared to which only DMF was added. Microtiter dishes were incubated unshaken at 30°C for 48 h. The optical density at 595 nm (OD595) was measured using a microplate reader (ELx800; Bio-Tek).
Competition assays.
Strains of P. stutzeri (KC, KC657, and CTN1) and E. coli were grown overnight in SM and adjusted to a density of ∼108 CFU/ml. Then, 25 ml of SM was inoculated (5.0%, vol/vol) with P. stutzeri, with or without a 5% coinoculum of E. coli, and each culture was shaken (250 rpm) in a 250-ml Erlenmeyer flask at 30°C. After incubation for 16 h, serial dilutions were made in PBS, and plate counts were performed. M9 citrate agar (pH 8.0) was used as a selection for P. stutzeri (grown at 30°C). LB at pH 7.0 was used as a selection for E. coli (grown at 37°C).
ES−/MS.
Solutions of 0.2 mM Fe(III), Co(III), and Zn(II) and 0.4 mM Cu(II) were prepared in SM containing 0.4 mM pdtc. These concentrations correspond to the exact stoichiometry (metal-pdtc ratio) of the respective pdtc-metal complexes. Samples were analyzed by negative electrospray-ionization mass spectrometry (ES−/MS) (Quattro II; Micromass Ltd.). Samples were delivered into the MS source at a flow rate of 5 μl/min using a syringe pump (Harvard Apparatus, South Natick, Mass.). A potential of 2.5 to 3 kV was applied to the electrospray needle. The sample cone voltage was maintained at 12 V. The counterelectrode, skimmer, and radio frequency lens potentials were tuned to maximize the ion beam for the given solvent. Detector resolution was set at 15,000, and source temperature was kept constant at 80°C. The instrument was calibrated using a polyethylene glycol solution. All spectra were an average of 10 to 15 scans. In order to increase the signal-noise ratio, analyses were repeated using solutions prepared in DM.
Measurement of pdtc.
Pdtc concentrations were determined using specific absorbance of the Fe(II)-pdtc2 complex at 687 nm (4). Reagents were prepared immediately before analysis. Sample (0.9 ml) and 0.05 ml of 5 mM FeCl3 were added to a 1.0-cm disposable cuvette and mixed thoroughly. Then, 0.05 ml of 1 M NaS2O2 was added, and absorbance was measured at 687 nm. The concentration of Fe(II):pdtc2 was calculated using the molar extinction coefficient ɛ687 = 8,435 (22).
RESULTS
Comparison of sensitivity of various bacteria to pdtc.
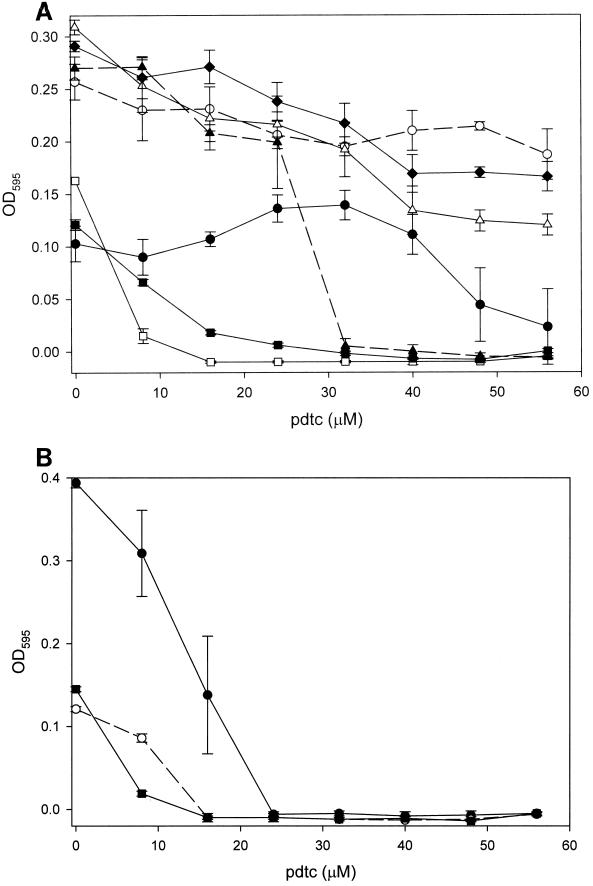
We investigated the possibility that pdtc may function as an antibacterial agent. Several species of bacteria were examined for their responses to the presence of pdtc. Pdtc significantly enhanced the growth of P. stutzeri strain KC at a concentration of 32 μM, above which growth was supressed (Fig. 1A). P. fluorescens F113, P. aeruginosa PAO1, and P. putida DSM 3601 tolerated higher concentrations of pdtc than did strain KC; however, no growth promotion was evident. The MIC of pdtc was 32 μM for P. putida MT-2 and ≤24 μM for P. stutzeri strains 14405 and 17588 and all nonpseudomonads (Fig. 1B). No growth inhibition occurred in controls to which only DMF was added (data not shown).
FIG. 1.
Comparison of the sensitivity of various Pseudomonas spp. to pdtc. Cultures were inoculated (0.5%) into 96-well microtiter plates containing SM and various concentrations of pdtc and grown for 48 h at 30°C. (A) Pseudomonas strains: ●, P. stutzeri strain KC; □, P. stutzeri 14405; ■, P. stutzeri 17588; ○, P. putida DSM3601; ▴, P. putida MT-2; ▵, P. fluorescens F113; ⧫, P. aeruginosa PAO1. (B) Nonpseudomonads: ●, E. coli 25922; ○, Arthrobacter sp.; ■, Staphylococcus epidermidis. Values represent the mean of three replicates, and error bars represent the standard deviation (error bars may be obscured by symbols).
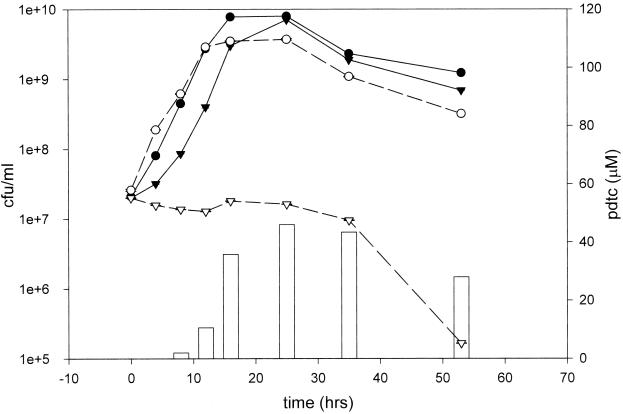
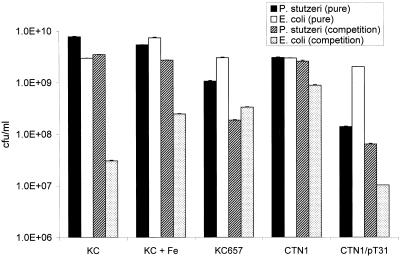
We next investigated whether pdtc production by strain KC would lead to competitive inhibition of the model organism E. coli. When grown in iron-limited SM, E. coli and strain KC exhibited normal sigmoidal growth curves in pure culture (Fig. 2). When strain KC and E. coli were grown in mixed culture, strain KC had a profound antagonistic effect on E. coli. In addition, the death phase of E. coli followed the appearance of pdtc in the medium. Competitive inhibition of E. coli by strain KC was partially relieved when 100 μM FeCl3 was added to the medium (Fig. 3). Antagonistic activity was reduced in strains KC657 and CTN1, which are derivatives of strain KC that lack the ability to produce pdtc. When CTN1 was transformed with cosmid pT31, which restores pdtc production, antagonistic activity toward E. coli was reestablished. With the exception of CTN1/pT31, which tended to grow in clumps, no significant flocculation occurred during the 16-h incubation with any strain. Most likely, flocculation of strain CTN1/pT31 is the reason for its apparently low plate counts.
FIG. 2.
Growth kinetics for strain KC and E. coli in competition. A 5% inoculum of strain KC (●) and E. coli (▾) was made to 25 ml of SM in pure culture (——) and in mixed culture (–––). At ∼4-h intervals, plate counts were made using media selective for each strain, and the pdtc concentration of the mixed culture (vertical bars) was measured. Values represent the mean of three plate counts, and error bars represent the standard deviation.
FIG. 3.
Competition between E. coli and strain KC or pdt mutant derivatives of strain KC. Values represent the mean plate count of three cultures, and error bars represent the standard deviation.
Enhancement of pdtc resistance by cosmid pT31.
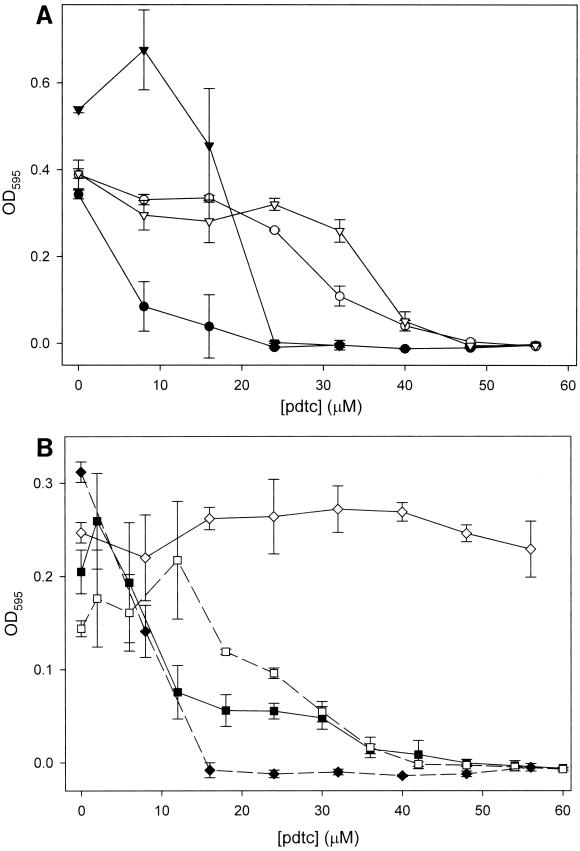
Cosmid pT31 contains a 25.8-kb insert that includes a gene cluster (pdt) which confers pdtc production on various Pseudomonas spp. (22). We examined whether genes within the insert of pT31 were responsible for resistance to pdtc. We transformed pdtc-sensitive strains CTN1, E. coli 25922, P. stutzeri 17588, and P. putida MT-2 with pT31. Using the pdtc sensitivity assay described above, we examined the sensitivity of these strains to pdtc. Strains containing only the vector pRK311 exhibited the same sensitivity as the wild-type strains (Fig. 4), while pT31 significantly enhanced resistance to pdtc. At lower concentrations of pdtc (< 24 μM), pT31 repressed the growth of strain CTN1 and P. putida MT-2.
FIG. 4.
Plasmid pT31 encodes elements responsible for resistance to pdtc. Various pdtS strains of Pseudomonas were transformed with plasmid pT31, and the resistance of these strains to pdtc was examined using the pdtc sensitivity assay described in the legend to Fig. 1. (A) ●, P. stutzeri 17588/pRK311; ○, P. stutzeri 17588/pT31; ▾, P. putida MT-2/pRK311; ▿, P. putida MT-2/pT31. (B) ■, CTN1/pRK311; □, CTN1/pT31; ⧫, E. coli/pRK311; ◊, E. coli/pT31.
Effect of metals on antagonism or toxicity of pdtc.
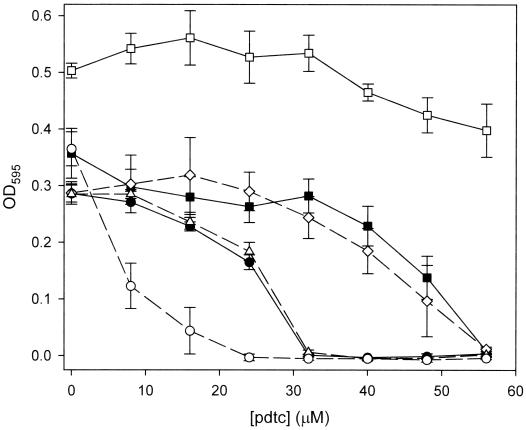
Since it is likely that the mechanism of pdtc toxicity is related to its chelation of trace metals and the resulting inability of cells to access sequestered metals from within a pdtc complex, we examined the effect of trace metals on the MIC of pdtc in cultures of E. coli. With no metal present, the MIC of pdtc was determined to be ∼32 μM (Fig. 5). Iron at 10 μM relieved inhibition by pdtc, while 10 μM cobalt and 1 μM copper increased the MIC to 56 μM. NaCl at 10 μM had no effect, and 10 μM zinc lowered the MIC to 24 μM. Copper at 10 μM was toxic at all levels of pdtc (data not shown).
FIG. 5.
Effect of metals on the antimicrobial activity of pdtc. The sensitivity of E. coli to pdtc was examined in the presence of various metals at 10 μM. Symbols: ●, no metal; □, Fe(III); ■, Co(III); ○, Zn(II); ▵, NaCl. Cu(II) (◊) was added to a final concentration of 1 μM.
MS of pdtc-metal complexes.
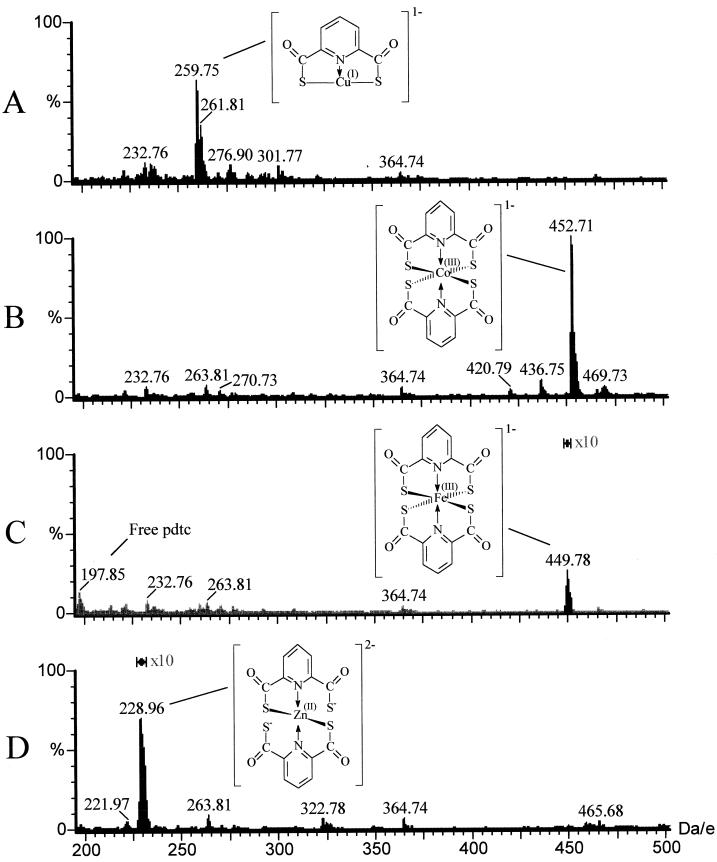
To help explain the different biological effects observed with each metal described above, we used ES−/MS to examine the formation of metal complexes in the media. In order to increase the signal-noise ratio for ES−/MS analyses, a modified medium (DM) was used. Stoichiometric amounts of copper, cobalt, iron, and zinc were added separately to DM containing 0.4 mM pdtc. The pH of these mixtures was determined to be ∼7.4. After preparation, samples were immediately analyzed by ES−/MS. Both Co(III):pdtc2 and Cu(I):pdtc formed immediately (Fig. 6A and B). A precipitate formed in the copper reaction mixture after ∼15 min. Complexes of pdtc with iron and zinc were detected only after solutions remained at room temperature for 48 h (Fig. 6C and D). Identical results were obtained using SM; however, the level of background noise was increased (data not shown). When the same experiments were performed in deionized water, all complexes formed immediately (data not shown). The structures shown in Fig. 6 confirm our previous observations of pdtc-metal complexes, though this is the first observation of a [Cu(I):pdtc]−1 complex (m/z = 260). The previously described [Cu(II)Cl:pdtc]−1 complex (m/z = 295) was not detected
FIG. 6.
MS analysis of pdtc-metal complexes. Stoichiometric amounts of metal were added to DM containing 400 μM pdtc, and samples were immediately analyzed by ES−/MS. Results shown for Fe(III) and Zn(II) are after a 48-h incubation. (A) Time zero, 400 μM Cu(II); (B) time zero, 200 μM Co(III); (C) at 48 h, 200 μM Fe(III); (D) at 48 h, 200 μM Zn(II).
Stability of pdtc in the presence of Co(III) or Cu(II).
Growth studies with E. coli indicated that copper “inactivates” pdtc in greater than stoichiometric amounts, suggesting that copper might catalyze some degradation or precipitation of pdtc. Therefore, we compared the stability of pdtc in the presence of Co(III) and Cu(II). In separate reactions, 10 μM Co(III) and 10 μM Cu(II) were added to 1.0 ml of deionized water containing 100 μM pdtc. The resulting pH of both mixtures was 6.0. Reactions were held at room temperature, and free pdtc was measured after 1 h. In the cobalt reaction, 74.6 ± 2.0 μM pdtc remained. In the copper reaction, 1.8 ± 0.6 μM pdtc remained, indicating that ≥88% of the pdtc had precipitated or degraded. A precipitate was collected from this reaction by centrifugation, rinsed once with deionized water, and dissolved in DMF. ES−/MS analysis of the supernatant fraction showed that Cu(I):pdtc had disappeared from the reaction and no degradation products were evident (data not shown). The precipitated fraction was analyzed by ES−/MS, and it was found to contain pdtc and a lesser amount of Cu(I):pdtc (data not shown).
DISCUSSION
If pdtc is involved in iron uptake, it is probably not the primary iron transport mechanism of strain KC. pdt mutant strains grow as well as KC even under iron limitation, suggesting that other iron uptake systems are adequate for growth. Assays for various siderophores indicate that strain KC also produces hydroxamate and catechol siderophores (8). Interestingly, strain KC can produce pdtc at concentrations (>40 μM) that are self-inhibitory.
Pdtc sensitivity assays provided clear evidence that pdtc exhibits antagonistic activity toward some species of bacteria. Of the strains examined, nonpseudomonads were generally the most sensitive. However, the two ATCC strains of P. stutzeri examined were sensitive to low concentrations of pdtc as well. These observations suggest that P. stutzeri may not be an archetypal host of the pdt locus; perhaps this DNA was obtained by strain KC through horizontal transfer from other organisms. In support of this theory is our prior observation that the mutant strain CTN1 was the result of a spontaneous ∼170-kb deletion of DNA (22). This indicates that the pdt locus, along with other nearby elements, is unstable under certain conditions, such as during growth in a nutrient-rich environment. Under other conditions, such as iron stress and the presence of pdtc, a positive selection may be created for pdtc-resistant bacteria. Analysis of the complete sequence of pT31 revealed that the pdt locus contains an open reading frame, ORF K, that is homologous to the siderophore receptor fyuA, part of a mobile genetic element found in Yersinia entercolitica and Yersinia pesits (9, 29, 34). If the genes encoding pdtc production were part of a similar genetic element, it is possible that they may be present in a variety of bacteria. We are attempting to isolate additional mutants of strain KC in order to better characterize this deletion event. In addition, we are attempting to isolate new strains of bacteria which possess the ability to produce pdtc. It is possible that the pdt locus is part of a pathogenicity island, a region of mobile DNA that may be transferred between different species of bacteria and enhance competitiveness.
P. stutzeri strain CTN1, a deletion mutant of strain KC, lacks resistance to pdtc. Pdtc resistance was restored to this and other pdtcS strains by cosmid pT31, which also restored pdtc production to other Pseudomonas spp. but not to Rhizobium meliloti or E. coli (22). These results suggest that the insert of pT31 encodes some means of obtaining essential metals in the presence of pdtc, most likely a pdtc-specific transporter. Efforts are under way to identify the specific genetic element(s) involved in pdtc resistance. Plasmid pT31 inhibited the growth of strain CTN1 and P. putida MT-2 at lower concentrations of pdtc. The production of pdtc, which this plasmid allows, may place a burden on the normal metabolism of these cells. It is also possible that the pdt locus encodes a receptor which initiates a quorum-sensing response to pdtc in a manner similar to the bacteriocin receptors of E. coli and Yersinia species (9, 33). In support of this possibility is the observation that fyuA, the homologue of ORF K of the pdt locus, encodes a dual-function receptor involved in both transport of iron and sensitivity to a bacteriocin produced by Y. pestis (32).
Inhibition of the growth of E. coli by pdtc can be relieved by the addition of iron, copper, and cobalt, suggesting that pdtc's mode of inhibition is through chelation of metals. Cobalt, copper, and zinc did not affect growth in the absence of pdtc (Fig. 5). In the absence of pdtc, the iron-enriched culture grew to a density almost twice that of the nonsupplemented control, confirming that iron is a major limiting factor for growth under these conditions and that a significant amount of the Fe3+ that we added was available for growth.
The biological effects of various pdtc-metal complexes can perhaps be explained by their chemical structures. The moderating effects of cobalt on pdtc sensitivity of E. coli suggest that Co(III) competitively inhibits the binding of essential metals by pdtc. In the presence of 10 μM cobalt, significant inhibition of E. coli occurs at a pdtc concentration of ≥40 μM. E. coli can normally tolerate only 16 μM pdtc, and thus −24 μM pdtc must be “inactivated” by the addition of 10 μM cobalt. Direct measurement of pdtc concentrations in water after chelation with cobalt verifies this conclusion, and the 2:1 stoichiometry of the cobalt-pdtc complex (previously described by Hildebrand et al. [14]) was confirmed by MS (Fig. 6). The inactivation of pdtc may therefore result simply from the formation of a biologically inactive complex, Co(III):pdtc2.
Since copper forms a complex with pdtc in 1:1 stoichiometry, one would expect 1 μM copper to inactivate an equal molar amount of pdtc. This would have a negligible effect on the growth E. coli at the pdtc concentrations used. However, 1 μM copper relieved inhibition to the same extent as 10 μM cobalt. MS and stability measurements suggest that, in these particular conditions, copper mediated the precipitation of pdtc. We observed that upon addition of copper, there was rapid formation of a Cu(I):pdtc complex, followed by the formation of a precipitate and disappearance of Cu(I):pdtc from the solution. No degradation products of pdtc could be detected, and the precipitate was found to contain primarily uncomplexed pdtc. The stoichiometry of the disappearance of pdtc suggests that this is a catalytic process rather than simply the precipitation of the Cu(I):pdtc complex. Also, precipitation of pdtc cannot be attributed to acidification of the solution by trace nitric acid [coming from our stock solution of Cu(II)], since the pH of SM after reagents were added was 7.4. The copper-catalyzed oxidation of thiols has been described previously (17, 18). Our observations are consistent with these studies, which describe the formation of a cuprous complex, followed by the oxidation of the thiol by atmospheric O2 and the release of copper.
In SM, iron and zinc complexes with pdtc were scarcely detectable by MS. When these mixtures were prepared in water, a strong signal was instantly detected from both complexes by MS. Either these complexes were slow to form in SM, or some component of the medium interferes with their detection. Our growth studies suggest that both metals have a significant effect on pdtc toxicity. However, it is not clear whether this effect involves the formation of a pdtc complex with iron or zinc. If Fe(III):pdtc2 and Zn(II):pdtc2 are not stable under our experimental conditions, the toxicity of pdtc may be attributed to sequestration of some other metal.
Certain siderophore complexes with copper and scandium are extremely toxic to bacteria (1, 31), and Zn(II) in complex with the antibiotic cephalexin increases the antimicrobial activity of this antibiotic (16). Zinc and pdtc may behave in a similar manner. A concentration of 10 μM Zn(II) profoundly enhanced the toxicity of pdtc, though it had no inhibitory effect by itself. Zinc alone is toxic at higher concentrations; therefore, pdtc may somehow enhance the delivery of Zn to the cell. Another possibility is that the complex itself may be toxic. The structure of Zn:pdtc2 is unique compared to other metal-pdtc complexes, because Zn(II) is coordinated by only two sulfur atoms, leaving two thiocarboxylic groups free. It is possible that this structure increases the reactivity of the metal. Also, the reactive free thiol groups may add to the toxicity of this complex.
In addition to its antimicrobial properties, the redox activity of pdtc in complex with copper or another transition metal may be important to its biological function. The reactive nature of pdtc has been described previously. Copper is essential to the biodegradation of carbon tetrachloride by strain KC (36). Lewis et al. (21) described a mechanism for this transformation in which the transforming agent is a Cu(II)Cl:pdtc complex. The reaction of pdtc with CCl4 is probably fortuitous; however, Cu:pdtc may interact with other molecules. The reaction of Cu:pdtc with CCl4 is inhibited by a combination of iron and a high-molecular-weight secreted molecule (8), suggesting that another extracellular factor may act as a “preferred substrate” of Cu:pdtc. More work is needed to determine if electrons can be transferred between a metal-pdtc complex and another biological molecule. It is also important to determine if Cu(I):pdtc is active toward CCl4.
Analysis of the complete genome of P. aeruginosa has revealed unusual genetic and functional complexity which allows this species to thrive in diverse ecological niches (35), and the same is likely for other Pseudomonas spp. The genes that encode synthesis of pdtc may constitute an example of one such specialized system involved in ecological competition. Based on the knowledge that pdtc is induced under iron-limited conditions and that it promotes the growth of P. stutzeri strain KC, it might reasonably be designated a siderophore. Yet its toxicity to many bacterial species and the reactivity of its copper complex place it in a unique category of extracellular reactive molecules, characterized by an affinity for a broad range of metals, by significant antimicrobial activity, and by the ability to promote complex chemical transformations in the environment. Such a diversity of activities may provide a host with the ability to influence the chemical composition of the environment and the species composition of the microbial community.
It is not clear whether antibiosis is the primary function of pdtc. Perhaps its physiological function may be as a redox agent or as a metal shuttle to a another transport molecule. Antagonism toward various microorganisms may simply be a failure of some species to compete with pdtc producers for the limited supply of metals. Nevertheless, pdtc clearly provides a host cell with an ecological advantage, and in terms of evolution, nothing is more important.
ACKNOWLEDGMENT
This work was funded by the U.S. Department of Energy NABIR Program under grant DE-FG03-96ER62273.
REFERENCES
- 1.Arceneaux J E, Boutwell M E, Byers B R. Enhancement of copper toxicity by siderophores in Bacillus megaterium. Antimicrob Agents Chemother. 1984;25:650–652. doi: 10.1128/aac.25.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakker P A H M, Lamers J G, Bakker A W, Marugg J D, Wesbeek P J, Schippers B. The role of siderophores in potato tuber yield increase by Pseudomonas putida in a short rotation of potato. Neth J Plant Pathol. 1986;92:249–256. [Google Scholar]
- 3.Bar-ness E, Chen Y, Hadar Y, Marschener H, Romheld V. Siderophores of Pseudomonas putida as an iron source for dicot and monocot plants. Plant Soil. 1991;130:231–241. [Google Scholar]
- 4.Budzikiewicz H, Hildebrand U, Ockels W, Reiche M, Taraz K. Weitere aus dem Kulturmedium von Pseudomonas putida isolierte Pyridinderivate—Genuine Metaboliten oder Artefackte? Z Naturforsch. 1983;386:516–520. [Google Scholar]
- 5.Buyer J S, Leong J. Iron transport-mediated antagonism between plant growth-promoting and plant-deleterious Pseudomonas strains. J Biol Chem. 1986;261:791–794. [PubMed] [Google Scholar]
- 5a.Criddle C S, DeWitt J T, Grbic-Galic D, McCarty P L. Transformation of carbon tetrachloride by Pseudomonas sp. strain KC under denitrifiction conditions. Appl Environ Microbiol. 1990;56:3240–3246. doi: 10.1128/aem.56.11.3240-3246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowley D E, Reid C P, Szanszlo P J. Microbial siderophores as iron sources for plants. In: Winkelmann G, van der Helm D, Neilands J B, editors. Iron transport in microbes, plants and animals. Weinheim, Germany: VCH Verlagsgesellschaft; 1987. pp. 375–386. [Google Scholar]
- 7.Cuppels D A, Stipanovic R D, Stoessl A, Sothers J B. The constitution and properties of a pyochelin-zinc complex. Can J Chem. 1987;65:2126–2130. [Google Scholar]
- 7a.Ditta G, Schmidhauser T, Yakobson E, Lu P, Liang X W, Finlay D R, Guiney D, Helinski D R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985;13:149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- 8.Dybas M J, Tatara G M, Criddle C S. Localization and characterization of the carbon tetrachloride transformation activity of Pseudomonas sp. strain KC. Appl Environ Microbiol. 1995;61:758–762. doi: 10.1128/aem.61.2.758-762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fetherston J D, Lillard J W, Perry R D. Analysis of the pesticin receptor from Yersinia pestis: role in iron-deficient growth and possible regulation by its siderophore. J Bacteriol. 1995;177:1824–1833. doi: 10.1128/jb.177.7.1824-1833.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartzen S H, Frimodt-Moller N, Frolund T. The antibacterial activity of a siderophore. 1. In vitro activity of deferoxamine alone and in combination with ascorbic acid on Staphylococcus aureus. APMIS. 1989;97:419–424. [PubMed] [Google Scholar]
- 11.Hemming B C, Orser C, Jacobs D L, Sands D C, Strobel G A. The effects of iron on microbial antagonism by fluorescent Pseudomonads. J Plant Nutr. 1982;5:683–702. [Google Scholar]
- 12.Hildebrand U, Ockels W, Lex J, Budzikiewicz H. Zur Struktur eines 1:1-Adduktes von Pyridin-2,6-Dicarbothiosäure und Pyridin. Phosphorus Sulfur. 1983;16:361–364. [Google Scholar]
- 13.Hildebrand U, Taraz K, Budzikiewicz H. Dicyano-bis(pyridin-2,6-dicarbothioato)-ferrat(II)/ferrat(III), ein weiteres eisenhaltiges Redoxsystem aus der Kulturlösung eines Pseudomonas. Z Naturforsch. 1985;40C:201–207. [Google Scholar]
- 14.Hildebrand U W, Lex J. Untersuchungen zur Struktur von Co(III)-und Ni(II)-Komplexen der Pyridin-2,6-di(monothiocarbonsäure) Z Naturforsch. 1989;44b:480. [Google Scholar]
- 15.Hordt W, Romheld V, Winkelmann G. Fusarinines and dimerum acid, mono- and dihydroxamate siderophores from Penicillium chrysogenum, improve iron utilization by strategy I and strategy II plants. Biometals. 2000;13:37–46. doi: 10.1023/a:1009234612486. [DOI] [PubMed] [Google Scholar]
- 16.Iqbal M S, Ahmad A R, Sabir M, Asad S M. Preparation, characterization and biological evaluation of copper(II) and zinc(II) complexes with cephalexin. J Pharm Pharmacol. 1999;51:371–375. doi: 10.1211/0022357991772556. [DOI] [PubMed] [Google Scholar]
- 17.Kachur A V, Held K D, Koch C J, Biaglow J E. Mechanism of production of hydroxyl radicals in the copper-catalyzed oxidation of dithiothreitol. Radiat Res. 1997;147:409–415. [PubMed] [Google Scholar]
- 18.Kachur A V, Koch C J, Biaglow J E. Mechanism of copper-catalyzed autoxidation of cysteine. Free Radic Res. 1999;31:23–34. doi: 10.1080/10715769900300571. [DOI] [PubMed] [Google Scholar]
- 19.Lech K, Brent R. Escherichia coli, plasmids, and bacteriophages. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Short protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1992. pp. 1-3–1-52. [Google Scholar]
- 20.Lee C H, Lewis T A, Paszczynski A, Crawford R L. Identification of an extracellular agent [correction of catalyst] of carbon tetrachloride dehalogenation from Pseudomonas stutzeri strain KC as pyridine-2, 6-bis(thiocarboxylate) Biochem Biophys Res Commun. 1999;261:562–566. doi: 10.1006/bbrc.1999.1077. . (Erratum, 265:770). [DOI] [PubMed] [Google Scholar]
- 21.Lewis T A, Paszczynski A J, Gordon-Wylie S W, Jeedigunta S, Lee C-H, Crawford R L. Carbon tetrachloride dechlorination by the bacterial transition metal chelator pyridine-2,6-bis(thiocarboxylic acid) Environ Sci Technol. 2001;35:552–559. doi: 10.1021/es001419s. [DOI] [PubMed] [Google Scholar]
- 22.Lewis T A, Cortese M S, Sebat J L, Green T L, Lee C-H, Crawford R L. A Pseudomonas stutzeri gene cluster encoding biosynthesis of the CCl4-dechlorination agent pyridine-2,6-bis(thiocarboxylic acid) Environ Microbiol. 2000;2:407–416. doi: 10.1046/j.1462-2920.2000.00122.x. [DOI] [PubMed] [Google Scholar]
- 23.Matzanke B F. Structures, coordination chemistry and functions of microbial iron chelates. In: Winkelman G, editor. Handbook of microbial iron chelates. Boca Raton, Fla: CRC Press; 1991. pp. 15–64. [Google Scholar]
- 24.Meyer J M, Abdallah M A. The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physicochemical properties. J Gen Microbiol. 1978;107:319–328. [Google Scholar]
- 25.Morel I, Cillard J, Lescoat G, Sergent O, Pasdeloup N, Ocaktan A Z, Abdallah M A, Brissot P, Cillard P. Antioxidant and free radical scavenging activities of the iron chelators pyoverdin and hydroxypyrid-4-ones in iron-loaded hepatocyte cultures: comparison of their mechanism of protection with that of desferrioxamine. Free Radic Biol Med. 1992;13:499–508. doi: 10.1016/0891-5849(92)90144-6. [DOI] [PubMed] [Google Scholar]
- 26.Neilands J B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- 27.Ockels W, Römer A, Budzikiewicz H. An Fe(III) complex of pyridine-2,6-di-(monothiocarboxylic acid)—a novel bacterial metabolic product. Tetrahedron Lett. 1978;1978:3341–3342. [Google Scholar]
- 28.Pal K K, Tilak K V, Saxena A K, Dey R, Singh C S. Antifungal characteristics of a fluorescent Pseudomonas strain involved in the biological control of Rhizoctonia solani. Microbiol Res. 2000;155:233–242. doi: 10.1016/S0944-5013(00)80038-5. [DOI] [PubMed] [Google Scholar]
- 29.Pelludat C, Rakin A, Jacobi C A, Schubert S, Heesemann J. The yersiniabactin biosynthetic gene cluster of Yersinia enterocolitica: organization and siderophore-dependent regulation. J Bacteriol. 1998;180:538–546. doi: 10.1128/jb.180.3.538-546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penyalver R, Oger P, Lopez M M, Farrand S K. Iron-binding compounds from Agrobacterium spp.: biological control strain Agrobacterium rhizogenes K84 produces a hydroxamate siderophore. Appl Environ Microbiol. 2001;67:654–664. doi: 10.1128/AEM.67.2.654-664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plaha D S, Rogers H J, Williams G W. Studies of the antibacterial effect of the scandium complex of enterochelin. J Antibiot (Tokyo) 1984;37:588–595. doi: 10.7164/antibiotics.37.588. [DOI] [PubMed] [Google Scholar]
- 32.Rakin A, Saken E, Harmsen D, Heesemann J. The pesticin receptor of Yersinia enterocolitica: a novel virulence factor with dual function. Mol Microbiol. 1994;13:253–263. doi: 10.1111/j.1365-2958.1994.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 33.Riley M A, Gordon D M. The ecological role of bacteriocins in bacterial competition. Trends Microbiol. 1999;7:129–133. doi: 10.1016/s0966-842x(99)01459-6. [DOI] [PubMed] [Google Scholar]
- 34.Schubert S, Rakin A, Karch H, Carniel E, Heesemann J. Prevalence of the “high-pathogenicity island” of Yersinia species among Escherichia coli strains that are pathogenic to humans. Infect Immun. 1998;66:480–485. doi: 10.1128/iai.66.2.480-485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.Sepulveda-Torres L C, Rajendran N, Dybas M J, Criddle C S. Generation and initial characterization of Pseudomonas stutzeri KC mutants with impaired ability to degrade carbon tetrachloride. Arch Microbiol. 1999;171:424–429. doi: 10.1007/s002030050729. [DOI] [PubMed] [Google Scholar]
- 35.Stover C K, Pham X Q, Erwin A L, Mizoguchi S D, Warrener P, Hickey M J, Brinkman F S, Hufnagle W O, Kowalik D J, Lagrou M, Garber R L, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody L L, Coulter S N, Folger K R, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong G K, Wu Z, Paulsen I T. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 36.Tatara G M, Dybas M J, Criddle C S. Effects of medium and trace metals on kinetics of carbon tetrachloride transformation by Pseudomonas sp. strain KC. Appl Environ Microbiol. 1993;59:2126–2131. doi: 10.1128/aem.59.7.2126-2131.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visca P, Serino L, Orsi N. Isolation and characterization of Pseudomonas aeruginosa mutants blocked in the synthesis of pyoverdin. J Bacteriol. 1992;174:5727–5731. doi: 10.1128/jb.174.17.5727-5731.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wendenbaum S, Demange P, Dell A, Meyer J M, Abdallah M A. The structure of pyoverdin Pa, the siderophore of Pseuodomonas aeruginosa. Tetrahedron Lett. 1983;24:4877–4880. [Google Scholar]
- 39.Williams P A, Murray K. Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J Bacteriol. 1974;120:416–423. doi: 10.1128/jb.120.1.416-423.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]