Abstract
Objectives
To establish the prevalence of pain and functional disability in Irish adults with moderate and severe haemophilia, and to examine demographic and lifestyle influences.
Methods
Males ≥18 years with moderate or severe haemophilia participated. Pain and function were examined using the PROBE questionnaire.
Results
Of 49 participants [median age 44 (IQR 32, 52) years], most had severe haemophilia (Factor VIII = 30; Factor IX = 13) and were on regular prophylaxis (88%). Those with moderate haemophilia (Factor VIII = 5; Factor IX = 1) treated on demand (12%). Acute (72%) and chronic pain (71%), functional difficulties (58%), and analgesic requirements (92%) were prevalent. Age was significantly associated with more advanced haemophilic arthropathy (p = .002), chronic pain (p = .029) and functional difficulties (p = .036). Adults who reported chronic pain commenced prophylaxis significantly later in life [32 (20, 51) vs. 8 (1, 23) years; p = .004]. Physical activity was significantly lower in those with functional difficulties (p < .05). A disparity between self‐perceived ‘target joints’ and clinically defined target joints was also identified (76% vs. 23%).
Conclusion
Haemophilic arthropathy, pain and functional disability were prevalent amongst Irish adults with moderate and severe haemophilia. Age‐dependent lifestyle, analgesic and treatment influences on pain and function warrant further investigation.
Keywords: function, haemophilia, pain, physical activity, prophylaxis
1. INTRODUCTION
Haemophilia is a bleeding disorder resulting from a deficiency in procoagulant Factor VIII (FVIII) or Factor IX (FIX), also known as haemophilia A and B, respectively. 1 Disease severity is stratified according to basal clotting factor levels, resulting in severe (<1%), moderate (1%–5%) or mild (>5%–40%) haemophilia. 1 People with Haemophilia (PWH) may experience traumatic or spontaneous bleeding into joints, resulting in significant pain, swelling and reduced function. 1 In the longer term, repeated haemarthroses may result in synovitis and osteochondral destruction causing chronic haemophilic arthropathy (HA), which characteristically affects the elbows, knees and ankles. 2
For people with moderate or severe haemophilia, significant phenotypic variability exists, resulting in differing rates and severity of bleed frequency, HA and functional disability. 3 PWH with a severe bleeding phenotype are typically treated via regular intravenous administration of clotting factor concentrates, which aims to prevent bleeding and subsequent HA. Treatment regimens are classified as primary, secondary or tertiary prophylaxis. Primary prophylaxis is commenced before the second clinically evident joint bleed, and before three years of age. 4 Secondary prophylaxis is commenced after two or more joint bleeds, before the onset of evident HA. 4 Tertiary prophylaxis is typically commenced in adulthood, after the onset of clinically evident HA. 4 With wider use of prophylaxis, novel replacement therapies and gene therapy, bleed rates have markedly decreased. 5 However, for adults on secondary and tertiary prophylaxis, the burden of pre‐existing HA and pain may continue to impact physical and social functioning. Difficulties with activities of daily living (ADLs) due to pain have been described in PWH, and increased age and intensity of pain have been identified as predictors of functional disability. 6 , 7 , 8 Functional disability contributes to lower levels of physical activity (PA), and pain presents a barrier to exercising for some, 9 further perpetuating disability. Chronic pain in the general population has been associated with elevated cardiometabolic risk factors, including obesity, 10 which should also be considered in PWH who may be less physically active due to HA.
This study aimed to provide an in‐depth analysis of the experience and prevalence of pain amongst Irish people with moderate and severe haemophilia. Functional disability, analgesic requirements, demographic and lifestyle factors (age, treatment, body composition, joint health and PA) were also examined, providing a comprehensive analysis of the complications resulting from HA.
2. METHODS AND MATERIALS
2.1. Study design and participants
Recruitment and data collection for this cross‐sectional study took place between April 2018 and March 2020 at the National Coagulation Centre, St. James's Hospital Dublin. In 2017, there were 330 people with moderate and severe haemophilia in Ireland. 11 Patients were approached during routine clinical visits and were provided with an information leaflet inviting them to voluntarily participate. Eligibility criteria included males ≥18 years with diagnosed moderate (1%–5%) or severe (<1%) FVIII or FIX deficiency, without active inhibitors. Individuals who lacked capacity to provide informed consent were not eligible for inclusion in the iPATH study. Additionally, those with acute medical concerns, recent bleeds or who were non‐ambulatory were not eligible for the iPATH Physical Activity and Health assessment. Ultimately, the iPATH study enrolled 105 patients, and 91 were invited to participate in the Physical Activity and Health assessment (Figure 1). This study received ethical approval from St. James's Hospital/Tallaght University Hospital Joint Research Ethics Committee.
FIGURE 1.
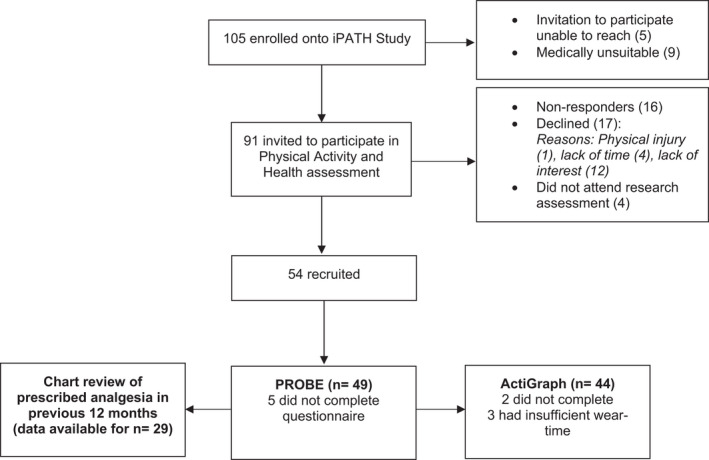
Recruitment flow chart
2.2. Participant demographics
Age, type/severity of haemophilia, treatment regimen, age at which prophylaxis was commenced, prescribed analgesia within the previous year (where available), and inhibitor history were recorded. Height was measured using a stadiometer, and Body Mass Index (BMI) was calculated using the SECA mBCA Multi‐Frequency Body Composition Analyser [Seca, Hamburg].
Joint health was assessed using the Haemophilia Joint Health Score (HJHS), which was determined by clinical specialist physiotherapists in haemophilia. The currently used version (HJHS 2.1) scores individual elbow, knee and ankle joints in the following domains: Swelling (0–3); Duration of swelling (0–1); Muscle atrophy (0–2); Crepitus on motion (0–2); Flexion loss (0–3); Extension loss (0–3); Joint pain (0–2); Strength (0–4). 12 A Global Gait Score (0–4) also assesses functional mobility. The maximum achievable score is 124, with higher scores indicating more severe HA. 12
2.3. Pain and functional disability
A validated tool for assessing patient reported outcomes in PWH, the Patient Reported Outcomes Burdens and Experiences (PROBE) questionnaire, was used to examine pain, difficulties with ADLs and their impact on activities and quality of life. 13 , 14 , 15 It assesses the prevalence, causes and impact of acute and chronic pain, analgesia, difficulties with ADLs and the presence of target joints. The PROBE defines acute pain as “…pain that arises in response to an event (like an injury or bleeding episode)”; and chronic pain as “…pain from a persistent cause…” which “…can vary in frequency and intensity (like back pain, pain from sore joints, or arthropathy)”, over the previous 12 months. Target joints were defined as three or more consecutive, spontaneous bleeds into a single joint within the previous six months. 16 The PROBE includes questions on self‐perceived target joints (without formal definition), as well as a separate question on the presence of three or more consecutive, spontaneous bleeds into any one joint in the previous six months.
2.4. Physical activity
International guidelines recommend that 150–300 minutes per week (mins/wk) of moderate intensity aerobic PA, or 75–150 mins/wk of vigorous intensity aerobic PA (combined or alone), are related to substantial health benefits for adults. 17 Moderate PA is defined as the equivalent of activity performed ≥3 to <6 times the intensity of rest on an absolute scale, and vigorous PA is performed ≥6.0 times the intensity of rest. 17 PA was measured objectively using the ActiGraph GT3X‐BT triaxial accelerometer (Actigraph Corp, Pensacola, Florida, USA). The ActiGraph is a valid and reliable device for measuring PA in both laboratory and field settings. 18 , 19 Participants wore the accelerometer on an elastic belt above their right hip for seven days during waking hours, except during bathing or swimming. They were provided with an activity diary to document ‘on’ and ‘off’ wear‐time for validation purposes. It was emphasised to participants that they should carry out a typical week of PA. As recommended, only data that met wear‐time inclusion criteria of ≥10 hours per day on ≥4 days (including one weekend day) were analysed. 20 Cut‐points established by Troiano et al. were used to classify PA as light, moderate and vigorous PA, 21 and results were compared with PA guidelines. 17 Bouts of moderate‐vigorous PA (MVPA) lasting ≥10 min were also analysed.
2.5. Statistical analysis
Data were analysed using the Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, Version 25.0. IBM Corp.). Continuous data are presented as median and interquartile range (IQR; Q1, Q3). Categorical data are presented as frequency counts and percentages, and were also separated by age groups. Chi‐square tests were used to examine relationships between categorical data. Fisher's exact test was run when the expected cell count of one or more cells was less than five. Due to the small sample size and non‐parametric distribution of continuous data, non‐parametric statistical tests were chosen. Between‐group differences were assessed using the Mann‐Whitney U test for dichotomous variables, and the Kruskall‐Wallis H test for variables with more than two groups. Dunn's post hoc pairwise comparisons were generated for statistically significant results of the Kruskall‐Wallis H test. The shape of data distribution between groups was dissimilar for the majority of comparisons, thus mean rank values are additionally reported. The following parameters were compared by categories of acute and chronic pain in the previous 12 months, current difficulties with ADLS and target joint prevalence: Age; Age at which prophylaxis was commenced; BMI; HJHS; PA. Spearman's rank‐order correlations using coded dichotomous variables were used to explore the relationship between pain and functional disability with demographic and lifestyle variables. A p‐value <.05 (two‐tailed) was deemed statistically significant.
3. RESULTS
A total of 54 participants were recruited, representing 59% of those invited to partake. The PROBE was completed by 49 participants, and data on prescribed analgesia were available for 29 participants. A high level of compliance with the ActiGraph accelerometer was achieved by 44 participants. Recruitment flow and reasons for non‐inclusion are presented in Figure 1.
3.1. Participant demographics
Descriptive statistics for demographic variables are presented in Table 1. The median age of the sample was 44 (range = 18–71) years, and overall 61% had severe FVIII deficiency. Those with severe haemophilia were treated with regular prophylaxis, whilst those with moderate were treated on demand. The median age at which prophylaxis was commenced was 26 (13, 49) years. A small proportion (12%) had a past history of inhibitors, but these were inactive during the study period. History of significant HA was evident with a median HJHS of 28 (20, 36). HA was significantly influenced by age [H(2) = 12.731; p = .002]. Post hoc pairwise comparisons revealed adults in the youngest age group had a significantly lower HJHS than adults in the middle‐aged (p = .044) and oldest age group (p < .0005). Adults in the oldest group also had a significantly higher HJHS than the middle‐aged group (p = .030). The ankles were the most severely affected joints, followed by the elbows and knees. Over half of participants met guideline recommended levels of PA (70%); however, only 20% achieved this via sustained MVPA in bouts ≥10 min. The majority of the group (67%) were overweight or obese as per the World Health Organization cut‐off values for BMI. 22 There were no significant differences according to type of haemophilia for age (mean rank = FVIII 23.24, FIX 29.39; U = 306.5, z = 1.362, p = .173), age at which prophylaxis was commenced (mean rank = FVIII 17.69, FIX 23.42; U = 203.0, z = 1.477, p = .140), BMI (mean rank = FVIII 24.76, FIX 25.61; U = 253.5, z = .188, p = .851) or total HJHS (mean rank = FVIII 22.45, FIX 22.62; U = 203.0, z = .039, p = .969).
TABLE 1.
Demographic information
| Total | FVIII deficiency | FIX deficiency | |
|---|---|---|---|
| n | 49 (100) | 35 (71) | 14 (29) |
| Age (years) | 44 (32, 52) | 39 (29, 50) | 48 (33, 56) |
| Age groups | |||
| 18–32 years | 13 (26) | 11 (85) | 2 (15) |
| 33–52 years | 24 (49) | 18 (75) | 6 (25) |
| 53–71 years | 12 (25) | 6 (50) | 6 (50) |
| Severity | |||
| Severe | 43 (88) | 30 (70) | 13 (30) |
| Moderate | 6 (12) | 5 (83) | 1 (17) |
| Treatment history | |||
| Prophylaxis | 43 (88) | 30 (70) | 13 (30) |
| On demand | 6 (12) | 5 (83) | 1 (17) |
| Age prophylaxis commenced (years) a | 26 (13, 49) | 23 (12, 42) | 41 (17, 52) |
| Age prophylaxis commenced by age group | |||
| <3 years | 5 (13) | 4 (80) | 1 (20) |
| 3–17 years | 9 (24) | 7 (78) | 2 (22) |
| ≥18 years | 24 (63) | 15 (63) | 9 (37) |
| BMI (kg/m2) | 27.0 (24.8, 29.6) | 26.8 (24.1, 30.6) | 28.0 (24.8, 29.2) |
| HJHS b | |||
| Total | 28 (20, 36) | 29 (20, 38) | 24 (20, 35) |
| Left ankle | 5 (2, 8) | 5 (2, 7) | 6 (4, 8) |
| Right ankle | 7 (4, 8) | 6 (4, 7) | 7 (6, 9) |
| Left knee | 1 (0, 5) | 1 (0, 5) | 1 (1, 7) |
| Right knee | 1 (0, 3) | 1 (0, 3) | 1 (1, 4) |
| Left elbow | 3 (0, 7) | 4 (0, 7) | 1 (0, 4) |
| Right elbow | 4 (0, 8) | 4 (0, 8) | 4 (0, 9) |
| Global gait score | 4 (4, 4) | 4 (4, 4) | 4 (4, 4) |
| HJHS by age category b | |||
| 18–32 years | 20 (4, 23) | 21 (7, 26) | 1 c |
| 33–52 years | 27 (21, 38) | 31 (21, 41) | 22 (18, 23) |
| 53–71 years | 34 (32, 46) | 32 (32, 40) | 35 (32, 53) |
| History of inhibitors | |||
| Inactive | 6 (12) | 5 (83) | 1 (17) |
| No history | 43 (88) | 30 (70) | 13 (30) |
Data are presented as median (IQR‐ Q1, Q3); Categorical variables are presented as n (%).
Abbreviations: BMI, body mass index; HJHS, haemophilia joint health score.
n = 38 (FVIII = 26; FIX = 12, remaining did not answer).
n = 44 (no HJHS available for 5 participants with moderate haemophilia).
n = 1, thus raw value is reported.
3.2. Pain and analgesic requirements
Both acute (72%) and chronic (71%) pain were prevalent. There was no significant association between age and acute pain, but participants in the youngest age group reported significantly less chronic pain compared to the middle‐aged group (p = .011) and the oldest group (p = .041) (Table 2). Reports were not significantly different between the middle and oldest age groups (p = 1.000). The use of pharmacological analgesia was high (92%) and similar across age groups (Table 2). Pharmacological analgesia was reportedly taken less frequently, according to percentage of time, by younger adults compared to older adults (p = .114; Figure 2).
TABLE 2.
Pain prevalence, analgesic requirements and functional difficulty prevalence by age group
| 18–32 years | 33–52 years | 53–71 years | ||||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Yes | No | Yes | No | Yes | No | p‐value | |
| Prevalence (n) | ||||||||
| Acute pain (47) | 34 (72) | 10 (77) | 3 (23) | 17 (77) | 5 (23) | 7 (58) | 5 (42) | .454 |
| Chronic pain (48) | 34 (71) | 5 (39) | 8 (61) | 19 (83) | 4 (17) | 10 (83) | 2 (17) | .016* |
| Use of pharmacological analgesia (48) | 44 (92) | 11 (85) | 2 (15) | 23 (96) | 1 (4) | 10 (91) | 1 (9) | .437 |
| Difficulties with ADLs (48) | 28 (58) | 4 (31) | 9 (69) | 14 (61) | 9 (39) | 10 (83) | 2 (17) | .035* |
| Pharmacological analgesia (n = 29) | ||||||||
| COX−2 inhibitors | 23 (79) | 2 (67) | 1 (33) | 15 (83) | 3 (17) | 6 (75) | 2 (25) | .665 |
| Weak opioid analgesics a | 11 (38) | 1 (33) | 2 (67) | 7 (39) | 11 (61) | 3 (38) | 5 (62) | 1.000 |
| Paracetamol | 11 (38) | 2 (67) | 1 (33) | 6 (33) | 12 (67) | 3 (38) | 5 (62) | .627 |
| Steroid injections | 6 (21) | 2 (67) | 1 (33) | 4 (22) | 14 (78) | 0 | 8 (100) | − |
| Strong opioid analgesics a | 2 (7) | 0 | 3 (100) | 2 (11) | 16 (89) | 0 | 8 (100) | ‐ |
| NSAIDs | 1 (3) | 0 | 3 (100) | 1 (6) | 17 (94) | 0 | 8 (100) | ‐ |
| Other a | 3 (10) | 1 (33) | 2 (67) | 1 (6) | 17 (94) | 1 (13) | 7 (87) | ‐ |
| Taking >1 analgesic medication | 13 (45) | 2 (67) | 1 (33) | 9 (50) | 9 (50) | 2 (25) | 6 (75) | .459 |
Data are presented as n (%). Fischer's Exact analyses of pain and pharmacological analgesia by age group is presented due to expected cell counts less than five. (‐) indicates test no statistical comparison interpreted due to very limited sample size. Missing data were excluded from the analyses.
Abbreviations: ADLs, activities of daily living; Cox‐2, cyclooxygenase‐2; NSAIDs, non‐steroidal anti‐inflammatory drugs.
Weak opioid analgesics = E.g. Tramadol, Co‐codamol; Strong opioid analgesics = E.g. Oxycodone, Morphine; Other = Gabapentin, lidocaine, herbal remedies, topical gels.
Significant at p < .05 (two‐tailed).
FIGURE 2.
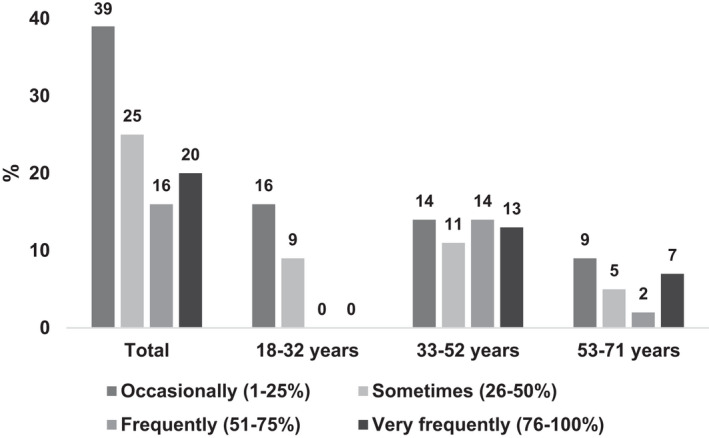
Use of pharmacological analgesia by age group
Details of prescribed pharmacological analgesia were available for 29 participants [Age: 45 (35, 55) years; BMI: 28.3 (25.4, 31.1) kg/m2; HJHS: 31 (21, 36)]. More than one pain medication was prescribed in 45%. COX‐2 inhibitors (e.g. Etoricoxib) were the most commonly prescribed medications (79%), followed by paracetamol (38%) and weak opioid analgesics (38%). Age‐related associations were not statistically significant (Table 2).
Participants reported various causes of acute and chronic pain, with common causes of both involving walking, stair climbing, exercising/playing sport, amongst others (Table 3). Pain also interfered with quality of life and a number of activities including general activity levels, exercise/playing sport, mobility, mood, sleep and overall enjoyment of life (Table 3).
TABLE 3.
Causes and impact of pain
| Causes of pain onset | Acute pain | Chronic pain |
|---|---|---|
| Walking | 13 (38) | 29 (85) |
| Stair climbing | 9 (26) | 18 (53) |
| At night (such as waking you up/keeping you awake) | 2 (6) | 16 (47) |
| Resting | 3 (9) | 13 (38) |
| Weight bearing | 5 (15) | 22 (65) |
| Playing or participating in sports/exercise a | 11 (32) | 21 (62) |
| After falling or a trauma | 16 (47) | 3 (9) |
| Impact on activities and quality of life: | Acute pain b | Chronic pain |
|---|---|---|
| General activity | 22 (67) | 26 (76) |
| Mood | 16 (48) | 23 (68) |
| Walking ability | 24 (73) | 29 (85) |
| Normal work (both outside the home and housework) | 13 (39) | 19 (56) |
| Attending school | 1 (3) | 0 |
| Relations with others | 5 (15) | 9 (26) |
| Sleep | 10 (30) | 16 (47) |
| Enjoyment of life | 16 (48) | 23 (68) |
| Playing or participating in sports/exercise a | 17 (52) | 21 (62) |
| Lifting | 14 (42) | 13 (38) |
Acute pain (n = 34); Chronic pain (n = 34). Data are presented as n (%).
Includes playing with children.
Acute pain = 33 (n‐1, did not answer).
3.3. Demographic and lifestyle influences on pain
Descriptives of demographic and lifestyle influences by categories of acute and chronic pain prevalence are presented in Table 4. Differences between individuals who reported acute pain compared to those who did not were not significant for age (mean rank = Yes 23.00, No 26.62; U = 187.0, z = ‐.809, p = .418), age at which prophylaxis was commenced (mean rank = Yes 17.24, No 21.36; U = 106.0, z = −1.083, p = .279), BMI (mean rank = Yes 24.06, No 23.85; U = 223.0, z = .048, p = .962) or HJHS (mean rank = Yes 20.30, No 24.50; U = 144.0, z = −1.004, p = .316). Correlations between acute pain with demographic and PA variables were weak (Table S1).
TABLE 4.
Influences of demographic and lifestyle factors on pain and functional disability
| Acute pain | No acute pain | Chronic pain | No chronic pain | Difficulties with ADLs | No difficulties with ADLs | |
|---|---|---|---|---|---|---|
| Age (years) | 44 (32, 51) | 49 (31, 57) | 45 (33, 55)* | 29 (23, 50) | 45 (35, 58)* | 36 (25, 50) |
| Age prophylaxis commenced (years) a | 23 (11, 43) | 44 (23, 50) | 32 (20, 51)* | 8 (1, 23) | 34 (19, 52) | 15 (7, 40) |
| BMI (kg/m2) | 27.4 (24.6, 29.5) | 26.8 (24.8, 31.9) | 27.0 (24.8, 29.5) | 27.4 (24.0, 33.0) | 27.5 (24.8, 31.3) | 27.1 (25.0, 28.7) |
| HJHS b | 23 (19, 35) | 32 (22, 35) | 29 (21, 34) | 22 (5, 35) | 32 (21, 39) | 22 (16, 35) |
| Light PA (mins/wk) c | 2012 (1511, 2582) | 1727 (1375, 2136) | 1883 (1338, 2429) | 1969 (1626, 2335) | 1830 (1335, 2264)* | 2268 (1837, 2919) |
| Moderate PA (mins/wk) c | 183 (104, 261) | 284 (196, 353) | 197 (104, 265) | 208 (173, 359) | 164 (99, 232) | 265 (187, 363) |
| Vigorous PA (mins/wk) c | 0 (0, 1) | 0 (0, 2) | 0 (0, 1) | 1 (0, 13) | 0 (0, 0) | 1 (0, 8) |
| MVPA (mins/wk) c | 204 (104, 265) | 285 (196, 353) | 202 (104, 271) | 248 (198, 360) | 164 (99, 242)* | 271 (223, 364) |
| MVPA of at least 10‐min bouts (mins/wk) c | 31 (11, 78) | 81 (27, 170) | 28 (11, 96) | 70 (40, 118) | 28 (11, 77)* | 81 (33, 173) |
Data are presented as median (IQR‐ Q1, Q3).
Abbreviations: ADLs, activities of daily living; BMI, body mass index; HJHS, haemophilia joint health score; mins/wk, minutes per week; MVPA, moderate‐vigorous physical activity; PA, physical activity.
n = 36 (n‐13 did not answer).
n‐5 with moderate haemophilia (no HJHS available).
PA analysis n = 42 for acute pain and n = 43 for chronic pain and ADLs.
Significant at p < .05 (two‐tailed).
Those who experienced chronic pain demonstrated no significant differences to those who did not in BMI (mean rank = Yes 24.04, No 25.61; U = 222.5, z = −.352, p = .725) or the HJHS (mean rank = Yes 23.63, No 17.79; U = 236.5, z = 1.369, p = .171); however, they were significantly older (mean rank = Yes 27.32, No 17.64; U = 334.0, z = 2.179, p = .029) and commenced prophylaxis at a significantly older age (mean rank = Yes 22.27, No 11.27; U = 228.0, z = 2.827, p = .005). Chronic pain was moderately correlated with age at which prophylaxis was commenced, but weakly correlated with remaining demographic and PA variables (Table S1).
ActiGraph data were available for 44 participants [Age: 45 (33, 55) years; BMI: 27.4 (24.8, 30.4) kg/m2; HJHS: 27 (21, 34)]. Those who experienced acute pain demonstrated no significant differences to those who did not for time spent in light PA (mean rank = Yes 22.71, No 18.09; U = 208.0, z = 1.073, p = .283). Participants who reported acute pain spent less time in total MVPA, and MVPA sustained in bouts ≥10 min, than participants without acute pain, although differences were not significant (mean rank = Yes 19.61, No 26.82; U = 112.0, z = −1.674, p = .094; mean rank = Yes 19.68, No 26.64; U = 114.0, z = −1.621, p = .105, respectively).
Those who experienced chronic pain also demonstrated no significant differences to those who did not for time spent in light PA (mean rank = Yes 21.42, No 23.50; U = 168.0, z = −.487, p = .626), total MVPA (mean rank = Yes 20.35, No 26.25; U = 135.0, z = −1.381, p = .167) and sustained bouts of MVPA ≥10 min (mean rank = Yes 20.29, No 26.42; U = 133.0, z = −1.439, p = .150).
3.4. Functional disability
Descriptive statistics of demographic and lifestyle influences by category of functional disability are presented in Table 4. Over half of adults reported difficulties with ADLs (58%), which was significantly associated with age (Table 2). Adults with functional difficulties were significantly older than those who denied difficulties (mean rank = Yes 28.07, No 19.50; U = 380.0, z = 2.093, p = .036). Specifically, adults in the youngest age group reported significantly less functional difficulties compared to adults in the oldest group (p = .015), but not the middle‐aged group (p = .083). Reports were not significantly different between the middle and oldest age groups (p = .259). There were no significant differences between those who reported difficulties with ADLs and those who did not for age at which prophylaxis was commenced (mean rank = Yes 21.90, No 15.19; U = 229.0, z = 1.872, p = .061), BMI (mean rank = Yes 25.46, No 23.15; U = 307.0, z = .565, p = .572) and the HJHS (mean rank = Yes 24.35, No 18.41; U = 282.0, z = 1.517, p = .129). A significantly lower duration of time spent in PA was found in adults who reported functional difficulties for light PA (mean rank = Yes 18.46, No 27.41; U = 129.0, z = −2.285, p = .022), total MVPA (mean rank = Yes 17.38, No 29.06; U = 101.0, z = −2.981, p = .003) and sustained bouts of MVPA ≥10 min (mean rank = Yes 18.81, No 26.88; U = 138.0, z = −2.067, p = .039). Weak to moderate correlations were demonstrated between functional difficulties with demographic and PA variables (Table S1).
3.5. Patient reported ‘target joints’ vs. clinically defined target joints
Target joint prevalence was examined according to age, and results are presented in Table S2. Current target joints were reported by 76% and were more frequently reported by adults in the youngest and middle age groups compared to the oldest age group, although differences were not significant. Chronic pain resulting from target joints was reported by 58%; however, there were no significant associations with age. Contrastingly, when asked specifically about three or more spontaneous bleeds into any one joint within the previous six months (i.e. the clinical definition of a target joint), only 23% answered yes. Age was not significantly associated. There was no significant association between self‐perceived “target joint” reports and clinically defined target joint reports (Table S3). Age, BMI, HJHS and PA were not significantly different in adults who reported self‐perceived target joints compared to those who denied them (Table S4); however, they did commence prophylaxis at a significantly younger age (mean rank = Yes 17.63, No 27.79; U = 50.5, z = −2.186, p = .029).
4. DISCUSSION
This study highlights patient lived experiences of pain associated with HA, and the resultant impact on ADLs and PA. High rates of acute and chronic pain, and functional disability were reported. Age was significantly associated with more advanced HA, chronic pain and functional difficulties. Lower levels of objectively measured PA were significantly associated with functional difficulties. Adults who reported chronic pain commenced prophylaxis at a significantly later age, and more frequent analgesic requirements were also evident in older adults. Chronic pain attributable to self‐perceived target joints was prevalent; however, a disparity between self‐perceived ‘target joints’ and clinically defined target joints was also identified.
The considerable levels of pain reported in this study are similar to previous studies who have reported a high prevalence of both acute and chronic pain in other populations with haemophilia. 23 , 24 Specifically, the prevalence of chronic pain in adults with moderate and severe haemophilia from the present cohort was far higher than the prevalence of chronic pain in the general Irish population, which is estimated to be between 13 and 36%. 25 , 26 Chronic pain in particular was significantly related to older age and later commencement of regular prophylaxis, which reflects the impact of improved treatments in more recent years on bleeds, severity of HA and associated pain in younger PWH. 27 , 28 Although limited data was available regarding the age that prophylaxis was initiated in this cohort, the majority of participants were treated with secondary or tertiary prophylaxis. Future comparisons with younger cohorts on primary prophylaxis and novel treatments would be of interest to ascertain the impact of these therapies on HA and resultant pain. Additionally, further comparison is warranted in populations without optimal access to prophylaxis.
Interestingly, chronic pain was perceived in part to be attributable to target joints; however, a disparity between self‐perceived and clinically defined target joints was highlighted. Younger adults reported a significantly higher prevalence of current target joints, despite a lower recall of clinically defined target joints. A disconnect between the clinical definition of a target joint and what patients identify as their ‘target joint’ is not unexpected. Perceptions of problematic joints resulting from HA are more common than frequent spontaneous haemarthroses, especially in patients treated with secondary or tertiary prophylaxis.
As expected, older adults demonstrated significantly more advanced HA compared to younger adults. HA is of course a major cause of pain for many PWH, although the HJHS did not differ significantly by category of pain or functional difficulties. As the HJHS was developed for children and younger PWH, 29 the interpretation of the analysis of HA in older adults from the present study is limited. However, in addition to the complex, multi‐faceted nature of haemophilia‐related pain, and the potential variation in phenotypic presentation, this confirms that the HJHS is not fully reflective of the severity of pain and disability experienced by individuals. Conflicting evidence has been described between the correlation of pain symptoms and the severity of radiographically measured HA in PWH, 7 , 30 which has also been demonstrated extensively in other arthritic populations, including osteoarthritis. 31 , 32 Findings from the present study therefore support the concept that traditional measures of joint health do not fully reflect the severity of pain and disability experienced by an individual, and the use of functional and patient‐reported outcomes are additionally important in the treatment and management of chronic HA in PWH.
Holistic pain coping strategies and the optimisation of pain management have been advised to improve health‐related quality of life for PWH who experience chronic pain 33 ; however, the frequent use of pharmacological analgesia was an important finding in this study. Despite discouragement of the long‐term use of COX‐2 inhibitors and other pharmacological analgesia, it has been suggested that the benefits outweigh the potential for unfavourable side effects in the short term when appropriately managed and monitored. 34 However, the long‐term pharmacological management of pain is limited amongst older adults who may develop other cardiovascular risk factors with age. Therefore, individually tailored PA and exercise programmes are ever more pertinent for PWH across the lifespan, as these therapies offer multi‐faceted benefits, including the potential to treat and manage chronic pain, 35 reduce cardiometabolic risk factors 17 and even optimise the potential for successful post‐operative outcomes for those who require orthopaedic joint replacement surgery. 36 Analgesia was prescribed in the tertiary healthcare setting in this cohort; however, information regarding the use of ‘over the counter’ analgesia and that prescribed in the primary healthcare setting should also be examined in future studies.
Our findings that pain was not significantly related to objectively measured PA are in keeping with similar research by Timmer et al., who found no significant relationship between pain and PA measured by accelerometry in adult PWH 37 ; however, the possibility of a type II error cannot be ignored in light of the small sample size in the present study. Despite this, time spent in any intensity of PA was significantly lower in individuals who reported having difficulties with ADLs. Participants further reported that pain was commonly caused by certain functional and physical activities, and pain simultaneously impacted various aspects of quality of life. Any causal inference between pain, functional disability and PA in an individual is difficult to establish due to the complex interrelationship between these parameters; however, pain and functional disability commonly present barriers to exercise and PA. Some evidence has been found to support water‐ and land‐based exercise for the treatment and management of pain in PWH, although more studies of improved methodological quality are recommended by McLaughlin et al. 38
Increased mechanical stress may exacerbate pain, and increased adiposity is also speculated to contribute to systemic inflammation via increased levels of adipokines, which may also compound pain. 39 , 40 Elevated BMI has been identified as a significant determinant of chronic pain in individuals with osteoarthritis and rheumatoid arthritis, 39 , 40 , 41 although surprisingly, BMI was not significantly related to pain or functional difficulties in adults with HA in this study, despite 68% being classified as overweight or obese. This is in keeping with the prevalence of overweight and obesity in the general male Irish population (~68%). 42 Weight loss via diet, exercise and behaviour modification has been shown to reduce pain, functional disability and inflammation in other disease populations and, despite the study findings, may offer a potential alternative treatment for pain in PWH. 43 , 44
Given the cross‐sectional nature of this study, there is no means to establish temporality or causality between variables. In addition to the small sample size, another limitation of this study was that demographic information about non‐responders was not available; therefore, a potential non‐response bias could not be avoided. Findings based on self‐reported methods may also be affected by potential recall bias. Lastly, although the PROBE questionnaire was the most appropriate tool to measure pain for this study, it did not grade the intensity of pain or assess the additional multi‐faceted aspects of haemophilia‐related pain.
5. CONCLUSION
In conclusion, this study found a high prevalence of acute and chronic pain, and functional disability amongst Irish adults with moderate and severe haemophilia impacted by chronic HA. Older adults had more years without adequate treatment especially as children; therefore, the extent of pain and functional impairment appears to be age‐dependent and may affect PA participation amongst other aspects of quality of life. This has potential implications for the physical health and wellbeing of the ageing population with haemophilia and requires further investigation. Additionally, the frequent use and efficacy of pharmacological analgesia amongst different age groups, and the influence of treatment regimens and novel therapies on the development of chronic pain across the lifespan, warrant further consideration in future longitudinal studies.
CONFLICT OF INTEREST
M.L. has served on an advisory board to Tremeau Pharmaceuticals and as a consultant to Sobi. She received indirect funding from Takeda for the development of educational material. N.M. O’C. has received research support from SOBI; participated in advisory boards for F. Hoffmann‐La Roche Ltd, UniQure, SOBI, Freeline; and participated in speakers’ bureaus for F. Hoffmann‐La Roche Ltd, SOBI and Novo Nordisk. J.S. O’D. has served on the speaker's bureau for Baxter, Bayer, Novo Nordisk, Boehringer Ingelheim, Leo Pharma, Takeda and Octapharma. He has also served on the advisory boards of Baxter, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, Takeda and Pfizer. J.S. O’D. has received research grant funding awards from Baxter, Bayer, Pfizer, Shire (now part of Takeda), Takeda and Novo Nordisk. P.L.T. is full‐time employee of Baxalta Innovations GmbH, a member of the Takeda group of companies, and shareholder of Takeda Pharmaceutical Company Limited. M.K. has served on an advisory council to Takeda. The remaining authors have no competing interests to declare.
Supporting information
Tab S1‐S4
ACKNOWLEDGEMENTS
P.L.T., J.S. O’D., M.L., N.M. O’C., S.W.P., B. O’M. and J.G. all contributed to the research conception and design, data analysis and interpretation. M.K. was involved in data acquisition, analysis and interpretation. S.R., M. McG., E.S. and K.R. were involved in data acquisition and interpretation. M.K. and J.G. drafted the paper. All authors contributed to drafting and revising of the paper and have approved the final version. The authors would like to thank the participants who volunteered their time and effort to this research. Further thanks are extended to the clinical and research teams at the National Coagulation Centre, St. James’s Hospital and to the Irish Haemophilia Society for their support with this study. The authors would also like to acknowledge the assistance and support of the Wellcome – HRB Clinical Research Facility at St. James’s Hospital in providing a dedicated environment for the conduct of high‐quality clinical research activities. The iPATH study is supported in part by a research grant from Science Foundation Ireland (SFI) under the SFI Strategic Partnership Programme Grant (16/SPP/3303) and research support from Shire US Inc., a Takeda company, Lexington, MA, USA. Open access funding provided by IReL. [Correction added on 25 May 2022, after first online publication: IReL funding statement has been added.]
Kennedy M, O’ Mahony B, Roche S, et al; the iPATH study group . Pain and functional disability amongst adults with moderate and severe haemophilia from the Irish personalised approach to the treatment of haemophilia (iPATH) study. Eur J Haematol. 2022;108:518–527. 10.1111/ejh.13763
Novelty Statement
1. The new aspect of this work:
Chronic pain experienced by adult males with moderate and severe haemophilia was significantly associated with age at which prophylaxis was commenced, and additionally, objectively measured physical activity was significantly associated with functional disability.
2. The central finding of this work:
Age, age at which prophylaxis was commenced and physical activity were all related to patient lived experiences of pain and functional disability arising from haemophilic arthropathy, which were highly prevalent in males with moderate and severe haemophilia.
3. The clinical relevance of this work:
Age‐dependent lifestyle, analgesic and treatment influences on pain and function across the lifespan of people with haemophilia warrant further investigation, especially in the era of newly available treatments and novel therapies.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
REFERENCES
- 1. Mannucci PM, Tuddenham EG. The hemophilias–from royal genes to gene therapy. N Engl J Med. 2001;344(23):1773‐1779. [DOI] [PubMed] [Google Scholar]
- 2. Raffini L, Manno C. Modern management of haemophilic arthropathy. Br J Haematol. 2007;136(6):777‐787. [DOI] [PubMed] [Google Scholar]
- 3. Franchini M, Mannucci PM. Modifiers of clinical phenotype in severe congenital hemophilia. Thromb Res. 2017;156:60‐64. [DOI] [PubMed] [Google Scholar]
- 4. Srivastava A, Santagostino E, Dougall A, et al. Guidelines for the management of hemophilia. Haemophilia. 2020;26(S6):1‐158. [DOI] [PubMed] [Google Scholar]
- 5. Mancuso ME, Mahlangu JN, Pipe SW. The changing treatment landscape in haemophilia: from standard half‐life clotting factor concentrates to gene editing. Lancet. 2021;397(10274):630‐640. [DOI] [PubMed] [Google Scholar]
- 6. Wallny T, Hess L, Seuser A, Zander D, Brackmann HH, Kraft CN. Pain status of patients with severe haemophilic arthropathy. Haemophilia. 2001;7(5):453‐458. [DOI] [PubMed] [Google Scholar]
- 7. van Genderen FR, Fischer K, Heijnen L, et al. Pain and functional limitations in patients with severe haemophilia. Haemophilia. 2006;12(2):147‐153. [DOI] [PubMed] [Google Scholar]
- 8. Santavirta N, Solovieva S, Helkama O, Lehto S, Konttinen YT, Santavirta S. Musculoskeletal pain and functional ability in haemophilia A and B. Physiotherapy and rehabilitation in haemophilia patients. Rheumatol Int. 2001;21(1):15‐19. [DOI] [PubMed] [Google Scholar]
- 9. Flaherty LM, Schoeppe J, Kruse‐Jarres R, Konkle BA. Balance, falls, and exercise: beliefs and experiences in people with hemophilia: a qualitative study. Res Pract Thromb Haemost. 2018;2(1):147‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goodson NJ, Smith BH, Hocking LJ, et al. Cardiovascular risk factors associated with the metabolic syndrome are more prevalent in people reporting chronic pain: results from a cross‐sectional general population study. Pain. 2013;154(9):1595‐1602. [DOI] [PubMed] [Google Scholar]
- 11. WFH . World Federation of Hemophilia Report on the Annual Global Survey 2009. World Federation of Hemophilia; 2017. [Google Scholar]
- 12. Gouw SC, Timmer MA, Srivastava A, et al. Measurement of joint health in persons with haemophilia: a systematic review of the measurement properties of haemophilia‐specific instruments. Haemophilia. 2019;25(1):e1‐e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skinner MW, Chai‐Adisaksopha C, Curtis R, et al. The patient reported outcomes, burdens and experiences (PROBE) project: development and evaluation of a questionnaire assessing patient reported outcomes in people with haemophilia. Pilot Feasibility Study. 2018;4(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chai‐Adisaksopha C, Skinner MW, Curtis R, et al. Psychometric properties of the patient reported outcomes, burdens and experiences (PROBE) questionnaire. BMJ Open. 2018;8(8):e021900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chai‐Adisaksopha C, Skinner MW, Curtis R, et al. Test‐retest properties of the patient reported outcomes, burdens and experiences (PROBE) questionnaire and its constituent domains. Haemophilia. 2019;25(1):75‐83. [DOI] [PubMed] [Google Scholar]
- 16. Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935‐1939. [DOI] [PubMed] [Google Scholar]
- 17. Bull FC, Al‐Ansari SS, Biddle S, et al. World health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kelly LA, McMillan DG, Anderson A, Fippinger M, Fillerup G, Rider J. Validity of actigraphs uniaxial and triaxial accelerometers for assessment of physical activity in adults in laboratory conditions. BMC Med Phys. 2013;13(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aadland E, Ylvisåker E. Reliability of the actigraph GT3X+ accelerometer in adults under free‐living conditions. PLoS One. 2015;10(8):e0134606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Migueles JH, Cadenas‐Sanchez C, Ekelund U, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. 2017;47(9):1821‐1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181‐188. [DOI] [PubMed] [Google Scholar]
- 22. WHO . Body mass index ‐ BMI. World Health Organisation Regional Office for Europe. https://www.euro.who.int/en/health‐topics/disease‐prevention/nutrition/a‐healthy‐lifestyle/body‐mass‐index‐bmi. Accessed February 23, 2022.
- 23. Kempton CL, Recht M, Neff A, et al. Impact of pain and functional impairment in US adults with haemophilia: patient‐reported outcomes and musculoskeletal evaluation in the pain, functional impairment and quality of life (P‐FiQ) study. Haemophilia. 2018;24(2):261‐270. [DOI] [PubMed] [Google Scholar]
- 24. Lorenzato CS, Santos RB, Fagundes GZZ, Ozelo MC. Haemophilia experiences, results and opportunities (HERO study) in Brazil: assessment of the psychosocial effects of haemophilia in patients and caregivers. Haemophilia. 2019;25(4):640‐650. [DOI] [PubMed] [Google Scholar]
- 25. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287‐333. [DOI] [PubMed] [Google Scholar]
- 26. Raftery MN, Sarma K, Murphy AW, De la Harpe D, Normand C, McGuire BE. Chronic pain in the Republic of Ireland—Community prevalence, psychosocial profile and predictors of pain‐related disability: results from the prevalence, impact and cost of chronic pain (PRIME) study, part 1. Pain. 2011;152(5):1096‐1103. [DOI] [PubMed] [Google Scholar]
- 27. Manco‐Johnson MJ, Lundin B, Funk S, et al. Effect of late prophylaxis in hemophilia on joint status: a randomized trial. J Thromb Haemost. 2017;15(11):2115‐2124. [DOI] [PubMed] [Google Scholar]
- 28. Manco‐Johnson MJ, Soucie JM, Gill JC. Prophylaxis usage, bleeding rates, and joint outcomes of hemophilia, 1999 to 2010: a surveillance project. Blood. 2017;129(17):2368‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hilliard P, Funk S, Zourikian N, et al. Hemophilia joint health score reliability study. Haemophilia. 2006;12(5):518‐525. [DOI] [PubMed] [Google Scholar]
- 30. Wallny T, Lahaye L, Brackmann HH, Heß L, Seuser A, Kraft CN. Clinical and radiographic scores in haemophilic arthropathies: how well do these correlate to subjective pain status and daily activities? Haemophilia. 2002;8(6):802‐808. [DOI] [PubMed] [Google Scholar]
- 31. Wang K, Kim HA, Felson DT, et al. Radiographic knee osteoarthritis and knee pain: cross‐sectional study from five different racial/ethnic populations. Sci Rep. 2018;8(1):1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635‐646. [DOI] [PubMed] [Google Scholar]
- 33. Auerswald G, Dolan G, Duffy A, et al. Pain and pain management in haemophilia. Blood Coagul Fibrinolysis. 2016;27(8):845‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arachchillage DRJ, Makris M. Choosing and using non‐steroidal anti‐inflammatory drugs in haemophilia. Haemophilia. 2016;22(2):179‐187. [DOI] [PubMed] [Google Scholar]
- 35. Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of cochrane reviews. Cochrane Database Syst Rev. 2017;4:CD011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santa Mina D, Clarke H, Ritvo P, et al. Effect of total‐body prehabilitation on postoperative outcomes: a systematic review and meta‐analysis. Physiotherapy. 2014;100(3):196‐207. [DOI] [PubMed] [Google Scholar]
- 37. Timmer MA, Veenhof C, de Kleijn P, de Bie RA, Schutgens REG, Pisters MF. Movement behaviour patterns in adults with haemophilia. Ther Adv Hematol. 2020;11:2040620719896959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McLaughlin P, Hurley M, Chowdary P, Khair K, Stephensen D. Physiotherapy interventions for pain management in haemophilia: a systematic review. Haemophilia. 2020;26(4):667‐684. [DOI] [PubMed] [Google Scholar]
- 39. Arranz L‐I, Rafecas M, Alegre C. Effects of obesity on function and quality of life in chronic pain conditions. Curr Rheumatol Rep. 2013;16(1):390. [DOI] [PubMed] [Google Scholar]
- 40. Daïen CI, Sellam J. Obesity and inflammatory arthritis: impact on occurrence, disease characteristics and therapeutic response. RMD Open. 2015;1(1):e000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ajeganova S, Andersson ML, Hafström I, Group, f.t.B.S . Association of obesity with worse disease severity in rheumatoid arthritis as well as with comorbidities: a long‐term followup from disease onset. Arthritis Care Res (Hoboken). 2013;65(1):78‐87. [DOI] [PubMed] [Google Scholar]
- 42. Department of Health/Ipsos MRBI, Healthy Ireland 2015 Survey Summary of Findings . Department of Health, 2015. https://assets.gov.ie/16210/525a06d3aaef4f23889c8fbdcc40d40a.pdf. Accessed February 22, 2022.
- 43. Janke EA, Collins A, Kozak AT. Overview of the relationship between pain and obesity: what do we know? Where do we go next? J Rehabil Res Dev. 2007;44(2):245‐262. [DOI] [PubMed] [Google Scholar]
- 44. Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti‐inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607‐615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab S1‐S4
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.


