Abstract
Background
Acute infection/inflammation increases the risk of acute vascular events (AVEs). Invasive dental treatments (IDTs) trigger short‐term acute inflammation.
Purpose
The aim of this work is to critically appraise the evidence linking IDTs and AVEs.
Data Sources
Six bibliographical databases were searched up to 31 August 2021. A systematic review following PRISMA guidelines was performed.
Study Selection
Intervention and observational studies reporting any AVEs following IDT were included.
Data Extraction
Two reviewers independently extracted data and rated the quality of studies. Data were pooled using fixed‐effect, inverse variance weights analysis.
Risk of Bias
Risk of bias was assessed by the Newcastle–Ottawa Quality Assessment Scale for observational studies and the Cochrane Handbook–Rob 2.0 for randomized controlled trials.
Data Synthesis
In 3 out of 16 clinical studies, a total of 533,175 participants, 124,344 myocardial infarctions, and 327,804 ischaemic strokes were reported. Meta‐analysis confirmed that IDT did not increase incidence ratios (IR) for combined vascular events either at 1‐4 weeks (IR of 1.02, 95% CIs: 0.92 to 1.13) and at 5‐8 weeks (IR of 1.04, 95% CIs: 0.97 to1.10) after treatment.
Limitations
A high level of heterogeneity (study designs and time point assessments) was found.
Conclusion
Patients who received IDT exhibited no substantial increase in vascular risk over 8 weeks post treatment.
Keywords: cardiovascular diseases, dental treatment, extraction, inflammation, periodontitis
Clinical Relevance.
Scientific rationale for study: We wanted to investigate whether there is a risk of suffering an acute vascular event within 8 weeks after an invasive dental procedure.
Principal findings: This comprehensive review with meta‐analysis of self‐control case studies found no substantial increases in the incidence of myocardial infarction or ischaemic stroke (or both combined) in the first 8 weeks after an invasive dental treatment.
Practical implications: Routine invasive dental treatments are among the most common procedures worldwide. Patients undergoing invasive dental procedures such as an extraction are not at an increased risk of acute vascular events during the first 8 weeks post treatment.
1. INTRODUCTION
Cardiovascular diseases (CVDs) are the leading cause of death worldwide (World Health Organization, 2014). Endothelial dysfunction is the earliest step in the development of atherosclerosis (Ross, 1993), and it precedes the intra‐vascular inflammatory and prothrombotic changes linked to atheroma formation and evolution (Davignon & Ganz, 2004). Acute and chronic inflammation (Smeeth et al., 2004) are the major drivers of endothelial dysfunction and plaque evolution (Libby, 2013) leading to acute cardiovascular events such as myocardial infarction (MI), ischaemic stroke (IS), and peripheral arterial disease (PAD) (Hansson, 2005; Herrington et al., 2016; Yang et al., 2018). The risk of an acute cardiovascular event increases considerably during periods of acute infection (Smeeth et al., 2004) or influenza seasons (Nguyen et al., 2016).
Chronic dental infections have been linked to systemic inflammation (Schillinger et al., 2006), endothelial dysfunction (Higashi et al., 2009; Mendes & Fernandes, 2016), and raised incidence of future cardiovascular events (Dietrich et al., 2017; Yang et al., 2018; Sanz et al., 2020). Little evidence, however, exists on the effects of invasive dental treatment (IDT) on acute cardiovascular risk. The aim of most dental treatments is to resolve acute/chronic infections. Even simple dental procedures, however, could trigger an acute host response (i.e., sub‐gingival tooth cleaning [Nordendahl et al., 2018; Chen et al., 2019], tooth extraction [Haheim et al., 2011], oral surgical procedures [Minassian et al., 2010], dental implant placement [Nordendahl et al., 2018], and endodontic surgeries [Nordendahl et al., 2018]). Local inflammation, microbial dissemination (Kinane et al., 2005), and systemic inflammation have been reported after these simple invasive dental procedures (D'Aiuto, Nibali, et al., 2004).
Invasive dental procedures have also been linked to infective endocarditis. This association, however, remains controversial (Chen et al., 2018). Current evidence confirms that IDTs result in a week‐long acute inflammatory response (D'Aiuto, Nibali, et al., 2004; Luthra et al., 2019) with transient impairment of vascular function (Tonetti et al., 2007) and the creation of a pro‐thrombotic state (D'Aiuto et al., 2007). While it is now accepted that the risk of distant vascular infections (i.e., endocarditis) is negligible except in some limited circumstances (Cahill et al., 2017), some evidence indicates that IDTs could result in an increased risk of acute vascular events (AVEs) in the first 4 weeks post treatment (Minassian et al., 2010). The strength of the evidence, however, is questionable (Nordendahl et al., 2018; Chen et al., 2019). Our aim was therefore to perform a critical appraisal of all the available evidence on the link between IDT and incidence of AVEs over the first 2 months after treatment.
2. MATERIALS AND METHODS
2.1. Protocol and registration
A systematic review protocol was registered with PROSPERO (CD42019131124, Appendix 1) and followed the PRISMA 2020 guidelines (Appendix 2).
The focused research question posed was as follows: “What is the effect of IDTs on the risk of AVEs compared to non‐treatment or controlled intervention in adults?”
2.2. Eligibility: Inclusion and exclusion criteria
The following PICO outline was used:
2.2.1. Population
Individuals older than18 years.
2.2.2. Intervention/exposure
IDTs (non‐surgical periodontal therapy, surgical periodontal therapy, single, multiple, or wisdom tooth extractions, endodontic surgery, and dental implant therapy).
2.2.3. Comparison
No treatment or intervention was provided; only oral hygiene instruction was provided, only supragingival cleaning was performed, or only community (non‐specialist) dental care was provided.
2.2.4. Outcome
Any measure of the prevalence of AVEs including MI, stroke, or death within 8 weeks from the intervention.
2.2.5. Study design and duration
Both experimental and observational studies were included if they reported any AVEs following IDTs and reported in English language. Non‐human studies, reviews, letters to the editors, and studies with non‐invasive dental procedures were excluded.
2.3. Information sources and searches
Six bibliographical databases were searched up to 31 August 2021: MEDLINE OVID SP, EMBASE OVID SP, Cochrane library, CINAHL Ebsco Host, Web of Sciences Core Collection, and System for Information on Grey Literature in Europe using dedicated strategies (full details in Appendix 3: T1).
2.4. Study selection and data extraction
Titles and abstracts of all search results were independently screened by two reviewers (Shailly Luthra and Roberto Rotundo) to select relevant studies. Disagreements were resolved by discussion, and if necessary, involving a third reviewer (Yago Leira). Full‐text articles of the selected studies were appraised. When manuscripts lacked information/data, the authors were contacted to provide missing information. Detailed reasons for exclusion/inclusion of manuscript were recorded (Appendix 3: T2 and T3). Kappa score was used to assess the agreement between the reviewers based on full‐text screening. The selected studies were combined for qualitative and quantitative analysis.
2.5. Risk of bias in individual studies
Quality assessment of included studies was undertaken independently and in duplicate by two reviewers (Shailly Luthra and Yago Leira) as a part of the data extraction process. The Newcastle–Ottawa Quality Assessment Scale (NOS) was used to assess observational studies (Stang, 2010). Scores ranging from 4/9 to 9/9 were used, and studies were categorized as high, medium, or low risk of bias within the respective study design. The NOS scores were converted into Agency for Health Research and Quality standards as good, fair, and poor quality of evidence based on the number of starts achieved in three different domains (selection, comparability, and outcome/exposure). The Cochrane Handbook–Rob 2.0 (Cumpston et al., 2019) tool was used to assess randomized controlled trials (RCTs).
2.6. Summary measures
The main outcome variables were reports of the odds ratio (OR), incidence ratio (IR), hazard ratio (HR), or risk ratio.
2.7. Data analysis and synthesis of results
Descriptive statistics were performed to comment on the evidence retrieved to define the quantity of data, examining further for study variations in terms of study characteristics and results. This assisted in corroborating the appropriateness of further synthesis methods. Statistical analyses to generate forest plots were performed using the statistical software R.
AVEs reported over common time periods (1–4 and 5–8 weeks) were combined when possible. Analyses with ORs and IRs were computed separately using a fixed‐effect analysis. When a limited number of studies were identified, random effect models were also used. If the study outcome was reported over days, inverse weighted variance assessment was done to combine the data from the time points and convert days to weeks.
2.8. Risk of bias across studies: Publication bias
Owing to the limited number of studies, a formal publication bias assessment could not be performed.
3. RESULTS
3.1. Study selection
The combined electronic search identified 12,426 potentially relevant studies. After removal of duplicates (n = 80), 12,346 manuscripts were screened based on their title and abstract, resulting in 110 studies eligible for full‐text screening. Ninety‐four studies were excluded (Appendix 3: T2). Sixteen studies (Yoshimura, 1983; Niwa et al., 2000; Beck et al., 2008; Minassian et al., 2010; Haheim et al., 2011; Skaar et al., 2012; Lee et al., 2013, 2015; Smith et al., 2014; Nordendahl et al., 2018; Chen et al., 2019; Lin et al., 2019; Aarabi et al., 2020; Chang et al., 2021; Cho et al., 2021; Nordendahl et al., 2021) met the inclusion criteria and were included in the qualitative analysis (Appendix 4: F1). Evidence tables were generated according to the study design and in chronological order (Appendix 3: T3).
The selected studies were conducted across three continents and included several ethnicities. Data drawn from healthcare and insurance databases (4 out of 5 case–control studies and 10 out of 11 observational studies) or hospital outpatients (1 case–control and 1 observational study) were retrieved. Reviewers achieved good agreement when selecting/extracting data (Cohen's κ = 0.86).
Bias assessment confirmed that four case–control studies were scored as of high quality, while one was scored as of fair quality. For the observational studies, nine studies were of high quality, whereas two studies were considered of fair quality (Appendix 3: T4). The only RCT identified was based on a pilot trial, reporting three serious cardiovascular adverse events, but there was no information about the group in which these events occurred. The timeline of these events could not be established even after contacting the authors. There was low risk of bias for randomization and selection reporting; however, assignment, adherence, and missing data and outcome contributed to an overall high risk of bias (Appendix 3: T5).
3.2. Qualitative analysis
Sixteen studies, including 15 observational studies and 1 randomized control trial, were assessed.
Cho et al. (2021) performed a retrospective cohort study and reported an association between periodontitis and raised risk of acute MI (HR = 1.11; 95% confidence interval [CI]: 1.02–1.20) and IS (1.04; 95% CI: 1.01–1.07) over a 10‐year follow‐up.
Chang et al. (2021) conducted a 5‐year population‐based study and confirmed a protective effect of oral hygiene maintenance by toothbrushing (HR = 0.81; 95% CI: 0.76–0.87) and of oral prophylaxis (HR = 0.94; 95% CI: 0.88–1.10) in decreasing the risk of IS.
Lin et al. (2019) conducted a 13‐year population‐based cohort study, while Lee et al. (2013) performed a 10‐year case–control study. Both studies confirmed that maintaining good periodontal health (HR = 0.95; 95% CI: 0.91–0.99) and providing periodontal treatment (HR = 0.79; 95% CI: 0.69–0.92) were associated with a long‐term decrease in the risk of IS.
Nordendahl et al. (2021) completed a 3‐year Swedish registry‐based case–control study and reported no association between MI and periodontitis as assessed by one or more sessions of dental cleaning or gum surgery (OR = 1.02; 95% CI: 1.00–1.05). In a separate report by the same group, it was concluded that IDTs were not associated with an increased risk of MI (OR = 0.98; 95% CI: 0.91–1.06) (Nordendahl et al., 2018). Haheim et al. (2011) used a nested case–control study with 1173 participants but reported a positive association between IDTs and AVEs (OR = 1.64; 95% CI: 1.24–2.16). Data from a large insurance dataset including 720,343 participants monitored over 10 years suggested that diagnosis of periodontitis was associated with future raised long‐term risk of MI (Lee et al., 2015). Interestingly, participants who had no treatment (HR = 1.23; 95% CI: 1.13–1.35) or intensive treatment of periodontitis (HR = 1.09; 95% CI: 1.03–1.15) were at greater risk of MI when compared to participants who underwent routine dental prophylaxis (HR = 0.90; 95% CI: 0.86–0.95).
Aarabi et al. (2020) conducted a retrospective analysis using German health insurance data claims including patients who were hospitalized for symptomatic PAD. They concluded that patients who did not receive treatment for the gum problems had 1.97‐times greater odds of presenting with PAD (p < .0001; 95% CI: 1.83–2.13) when compared to those who had received treatment of periodontitis and after adjustments for age, diabetes, and gender differences.
Smith et al. (2014) performed a retrospective evaluation of the incidence of any adverse events within 30 days of dental clearance/extraction procedures. Their conclusions were suggestive of an increased risk of major adverse outcomes (such as death, bleeding, acute coronary syndrome, cerebrovascular accident, transient‐ischaemic attack, and renal failure requiring dialysis) following IDTs.
Skaar et al. (2012) investigated the risk of a second vascular event in 50,329 patients with established CVDs who underwent invasive dental procedures between 30 and 180 days after a first event. They concluded that patients were not at an increased risk of experiencing a second event during the given period (HR < 1).
Niwa et al. (2000) assessed the cardiovascular complications during and after an invasive dental procedure in 63 patients with unstable angina pectoris or acute MI. Most patients tolerated dental treatment well, but a small proportion (10%) experienced post‐operative adverse events such as chest pain.
Yoshimura (1983) conducted a clinical trial reporting any anginal attacks during dental extractions. Upon evaluation of 17 patients with angina pectoris or any other cardiomyopathies—aortitis syndrome, hypertension, or previous MI—dental treatment was well tolerated, with only one patient experiencing an anginal attack.
Minassian et al. (2010) conducted a data‐based self‐control case study on 20,369 individuals who were hospitalized for MI or IS. They assessed the possible risk of increased vascular events after IDTs. Their results showed that the rate of vascular events was raised during the first 4 weeks after the IDT but gradually returned to the baseline rate within 6 months (n = 1152, overall IR: 1.50; 95% CI: 1.09–2.06; IR for MI, n = 525, 1.56; 95% CI: 0.98–2.47; and IR for IS, n = 650, 1.39; 95% CI: 0.89–2.15). They concluded that IDTs may be associated with a transient increase in the risk for vascular events.
Chen et al. (2019) conducted studies using the Taiwanese National Health Care Claim database. They used two separate study designs—one being a case–control study, and the other a self‐control case series—using patients who experienced burns as negative controls. They screened 123,819 MI patients, 327,179 IS patients, and 73,247 patients with burns in the case‐crossover design, while including 117,655 MI, 298,757 IS, and 84,239 patients with burns in the self‐controlled case series design. Their results suggested that IDTs were not associated with a transient risk of MI and IS in their population group.
Lastly, the Periodontitis and Vascular Events pilot study (PAVE‐2008) was designed as a feasibility trial of secondary prevention of major cardiovascular events in the US population (Beck et al., 2008). The authors reported a total of 15 serious adverse events occurring in the community care group versus the treatment group (6.6% vs. 3.3%; p = .19) but provided limited information on the type of procedures performed and the exact timings of the occurrence of these severe events.
3.3. Quantitative analyses
Of the 16 studies identified, only 3 reported data (as case‐control or self‐controlled case series) according to the pre‐specified timeline after IDTs which could then be combined for meta‐analyses (Table 1). Demographic and study designs differences, however, were observed (Table 2).
TABLE 1.
Summary of quantitative estimates of meta‐analysis when comparing outcome of acute vascular events after treatment
| Outcome | Studies | WMD (95% CI) | p‐Value | I 2 (%) | p‐Value |
|---|---|---|---|---|---|
| OR 2–8 weeks | |||||
| 2 Weeks, MI | 2 | 0.99 (0.88–1.09) | .845 | 67 | .081 |
| 4 Weeks, MI | 2 | 0.99 (0.90–1.07) | .806 | 0 | .828 |
| 8 Weeks, MI and IS | 2 | 0.97 (0.94–1.01) | .142 | 0 | .627 |
| IR 1–4 weeks | |||||
| IS | 2 | 1.04 (0.98–1.10) | .191 | 15 | .277 |
| MI | 2 | 0.97 (0.87–1.07) | .557 | 58 | .121 |
| MI and IS (all) | 2 | 1.02 (0.92–1.13) | .722 | 61 | .031 |
| IR 5–8 weeks | |||||
| IS | 2 | 1.05 (1.01–1.09) | .014 | 0 | .682 |
| MI | 2 | 1.05 (0.95–1.07) | .744 | 0 | .345 |
| MI and IS (all) | 2 | 1.04 (0.97–1.10) | .191 | 55 | .132 |
Note: The fixed‐effect, inverse variance weighed pooled analysis was used, data given as WMD, p‐value, I 2 (heterogeneity), and CI. OR 1–7 days MI and IS and OR 2–8 weeks. IS could not be calculated due to different time periods. τ 2 could not be calculated because there were only two studies and fixed‐effect analysis was used.
Abbreviations: CI, confidence interval; IS, ischaemic stroke; MI, myocardial infarction; OR, odds ratio; WMD, weighed mean difference.
Bold value is statistically significant, pointing to increase in risk.
TABLE 2.
Summary of demographics and study design difference between studies selected for meta‐analyses
| Studies with OR outcome | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year/country | Study design | Study population | Type of invasive dental procedure | Study duration | Adjustment of confounders | Outcome reported OR | ||||
| Age (years) | Gender | Control group | No. of subjects | ||||||||
| Male | Female | ||||||||||
| Type of event, MI | |||||||||||
| Chen et al |
2019 Taiwan |
Case crossover | 20 to ≥90 | 83,752 (67.6%) | 40,067 (32.4%) | Burn patients |
MI = 123,819 Burns = 73,247 |
SRP Dentoalveolar surgery/tooth extractions Odentectomy Periodontal surgery |
24 weeks |
Diabetes Hypertension Hyperlipidaemia Congestive heart failure Age Sex |
3 days = 1.05; 95% CI: 1.08–1.58 7 days = 1.00; 95% CI: 1.01–1.31 2 weeks = 1.02; 95% CI: 0.96–1.08 4 weeks = 0.99; 95% CI: 0.94–1.04 8 weeks = 0.98; 95% CI: 0.95–1.02 |
| Nordendahl et al |
2018 Sweden |
Case–control study |
Case = 72.6 ± 13 Controls = 72.3 ± 13 |
Cases = 32,107 (62%) Controls = 151,636 (61%) |
Cases = 19,773 (38%) Controls = 95,342 (39%) |
5 control subjects—randomly selected, free from prior MI matched for age, sex, and geographic area of residence. |
MI = 51,880 Controls = 246,978 |
SRP Dentoalveolar surgery/tooth extractions Implant surgery Periodontal surgery Apical surgery |
4 weeks |
Diabetes Previous CVD CVD Drugs Education Income Age Sex Geographic area |
2 days =0.71; 95% CI: 0.55–0.93 2 weeks = 0.92; 95% CI: 0.83–1.02 4 weeks = 0.98; 95% CI: 0.91–1.06 |
| Type of event, IS | |||||||||||
| Chen et al |
2019 Taiwan |
Case crossover | 20 to ≥90 | 189,659 (58%) | 137,520 (42%) | Burn patients |
IS = 327,179 Burns = 73,247 |
SRP Dentoalveolar surgery/tooth extractions Odentectomy Periodontal surgery |
24 weeks |
Diabetes Hypertension Hyperlipidaemia Congestive heart failure Age Sex |
3 days = 0.93; 95% CI: 0.86–1.01 7 days = 0.97; 95% CI: 0.92–1.02 2 weeks = 0.97; 95% CI: 0.93–1.01 4 weeks = 0.97; 95% CI: 0.94–1.00 8 weeks = 0.97; 95% CI: 0.95–0.99 |
| Studies with IR outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year/country | Study design | Study population | Type of invasive dental procedure | Study duration | Adjustment of confounders | Outcome reported IR | |||
| Age (years) | Gender (WP/IP) | No. of subjects | ||||||||
| Male | Female | |||||||||
| Type of event, MI | ||||||||||
| Chen et al | 2019 | Self‐controlled case series | 20 to ≥90 | 84,448 (71.8%) | 33,207 (28.2%) | MI = 117,655 |
SRP Dentoalveolar surgery/tooth extractions Odentectomy Periodontal surgery |
24 weeks |
Diabetes Hypertension Hyperlipidaemia Congestive heart failure Age Sex |
1–3 days = 1.05; 95% CI: 0.98–1.13 4–7 days = 1.02; 95% CI: 0.95–1.08 8–14 days = 1.00; 95% CI: 0.95–1.06 3–4 weeks = 0.94; 95% CI: 0.91–0.98 5–8 weeks = 1.01; 95% CI: 0.98–1.04 |
| Minassian et al | 2010 | Self‐controlled case series | 20 to ≥90 | 236 (45%) | 289 (55.1%) | MI = 525 |
SRP Dentoalveolar surgery/tooth extractions Implant surgery Periodontal surgery Apical surgery |
24 weeks | Age |
1–4 weeks = 1.56; 95% CI: 0.98–2.47 5–8 weeks = 1.35; 95% CI: 0.82–2.23 |
| Type of event, IS | ||||||||||
| Chen et al | 2019 | Self‐controlled case series | 177,175 (59.3%) | 121,582 (40.7%) | IS = 298,757 |
SRP Dentoalveolar surgery/tooth extractions Odentectomy Periodontal surgery |
24 weeks |
Diabetes Hypertension Hyperlipidaemia Congestive heart failure Age Sex |
1–3 days = 0.94; 95% CI: 0.90–0.99 4–7 days = 1.06; 95% CI: 1.01–1.10 8–14 days = 1.05; 95% CI: 1.01–1.08 3–4 weeks = 1.05; 95% CI: 1.02–1.07 5–8 weeks = 1.05; 95% CI: 1.03–1.07 |
|
| Minassian et al | 2010 | Self‐controlled case series | 20 to ≥90 | 233 (35.9%) | 417 (64.2%) | IS = 650 |
SRP Dentoalveolar surgery/tooth extractions Implant surgery Periodontal surgery Apical surgery |
24 weeks | Age |
1–4 weeks = 1.39; 95% CI: 0.89–2.15 5–8 weeks = 0.94; 95% CI: 0.55–1.60 |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; IP, incident population; IS, ischaemic stroke; MI, myocardial infarction; OR, odds ratio; SRP, scaling and root planing; WP, whole population.
3.3.1. AVEs over 1–14 days post treatment (ORs)
Participants who underwent IDTs presented with decreased odds of MI after 2 days (0.71, 95% CI: 0.55–0.93) (Nordendahl et al., 2018), but no other changes were noted up to 2 weeks (Chen et al., 2019). The overall odds for MI after IDTs at 2 weeks was 0.99 (95% CI: 0.89–1.09) (Appendix 4: F2). Pooled analysis across studies could not be carried out because of inconsistent reporting of time points.
3.3.2. AVEs over 4–8 weeks post treatment (ORs)
The overall odds for MI after IDTs at 4 weeks was 0.99 (95% CI: 0.90–1.07). Similar estimates were reported for IS (OR = 0.97; 95% CI: 0.94–1.00) but pooled analysis could not be carried out, as data at this time point were reported in only one study (Chen et al., 2019).
No differences were observed even when analysing odds for combined events (MI and IS) (Appendix 4: F3).
3.3.3. AVEs over 1–4 weeks post treatment (IRs)
No substantial changes in the incidence of MI or IS (as well as combined) in the first 4 weeks following IDTs were observed (Figure 1).
FIGURE 1.
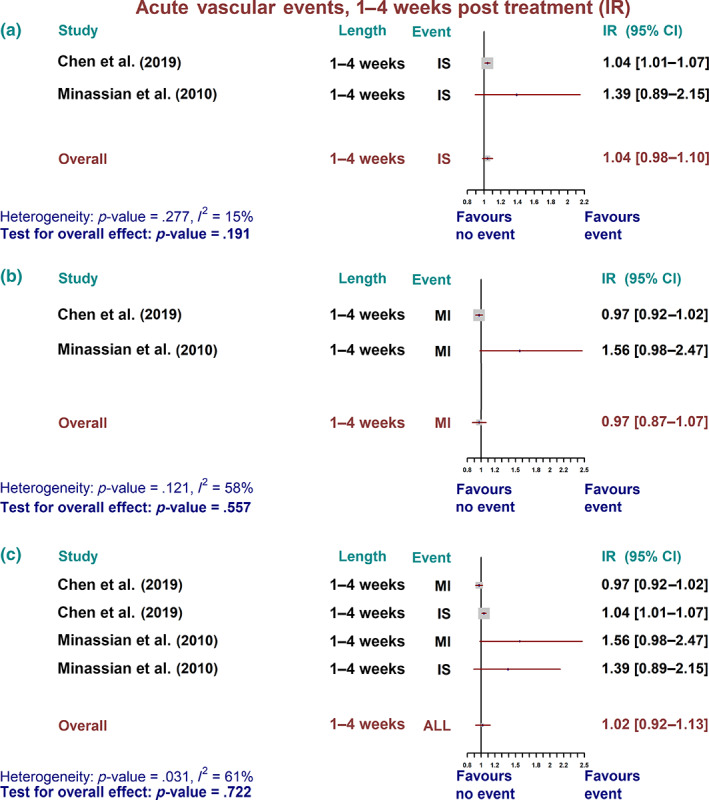
Acute vascular event over 1–4 weeks post treatment—incidence ratio (IR). Summary forest plot for incidence ratio of acute vascular events. (a) Ischaemic stroke (IS), (b) myocardial infarction (MI), and (c) all combined vascular events over 1–4 weeks post treatment in self‐controlled case series studies. The fixed‐effect, inverse variance weighed pooled analysis was used, and the data markers indicate the time length and events in each study. CI, confidence interval
3.3.4. AVEs over 5–8 weeks post treatment (IRs)
A minor but statistically not significant increase in overall IR for all vascular events was reported at 5‐8 weeks after invasive dental treatments. This was attributable to a statistically increased IR for IS over the same period (1.05; 95% CI: 1.01–1.09; p = .014) (Figure 2).
FIGURE 2.
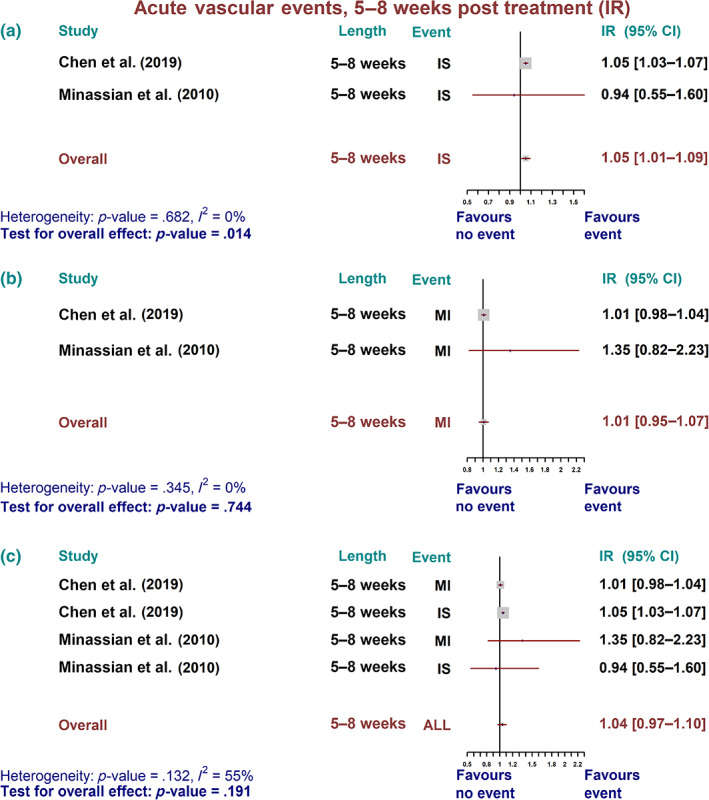
Acute vascular event over 5–8 weeks post treatment—incidence ratio (IR). Summary forest plot for incidence ratio of acute vascular events. (a) Ischaemic stroke (IS), (b) myocardial infarction (MI), and (c) all combined vascular events over 5–8 weeks post treatment in self‐controlled case series studies. The fixed‐effect, inverse variance weighed pooled analysis was used and the data markers indicates the time length and events in each study. CI, confidence interval
3.4. Risk of bias within studies
The bias assessment across case–control and cohort studies was low. Publication bias could not be carried out.
4. DISCUSSION
This systematic review demonstrates that there is lack of evidence to support an association between IDTs and the risk of AVEs over 8 weeks after treatment.
This is the first comprehensive review combining evidence related to 1,356,278 participants and 921,617 combined vascular events. No previous reports were available to contrast and compare our findings. On the contrary, there is sufficient evidence linking acute infections, bacterial dissemination, or surgical procedures and increased incidence of AVEs or mortality. Bergh et al. (2017) reported a higher risk of CVD following hospital admission for sepsis or pneumonia over a prolonged period (up to 5 years post infection). Indeed, 4389 inpatients with confirmed bacteraemia presented with a doubled risk of MI or IS when compared to those without bacteraemia (3.6% vs. 1.7%) and with a tripled risk when compared to healthy controls (0.2%) (Dalager‐Pedersen et al., 2014). Daily activities such as toothbrushing or chewing (Kinane et al., 2005; Forner et al., 2006; Crasta et al., 2009) can also be associated with bacteraemia similar to that seen after an IDT (Yoshimura, 1983; Heimdahl et al., 1990; Lockhart et al., 2008). This would suggest that IDT could trigger a short‐term acute increased vascular risk.
Periodontitis is a very common inflammatory disease initiated by a dysbiotic dental biofilm, but it is also a common trigger of systemic inflammation (D'Aiuto, Nibali, et al., 2004). Effective treatment of periodontitis does improve oral health and resolve systemic inflammation (D'Aiuto et al., 2006). This treatment could involve tooth extractions and/or intensive sessions of dental cleaning. A transient impairment of endothelial function (Tonetti et al., 2007), acute inflammation (D'Aiuto et al., 2007; Habbab et al., 2019), increased leukocyte counts (Luthra et al., 2019), and short‐lived bacteraemia (Kinane et al., 2005) are commonly observed within 24 h of intensive treatment of periodontitis (D'Aiuto, Nibali, et al., 2004; Luthra et al., 2019). Similar acute changes plus a rise in serum levels of high‐sensitivity cardiac troponin T, fibrinogen, and endotoxin activity have been reported in patients with established cardiac problems undergoing dental extractions (Habbab et al., 2019). Other common dental procedures such as root canal treatment (Debelian et al., 1995) or dental implant placement (Piñeiro et al., 2010) have been associated with short‐term bacterial dissemination (Schenkein et al., 2020). An increased inflammatory burden or bacterial dissemination could represent plausible mechanisms linking common dental treatments, endothelial dysfunction, and altered vascular risk (Figure 3). The idea that acute infection/inflammation is linked to vascular events was first reported by Valtonen et al. (1993) (4% increased risk of MI and 10% of IS). Even a brief exposure to bacterial endotoxins or transient acute inflammation impairs endothelium‐dependent relaxation for several days (Bhagat et al., 1996). There is evidence to suggest that increased thrombotic activity and endothelial dysfunction can result from an acute inflammatory response following IDTs (D'Aiuto et al., 2005; Joshipura et al., 2004). Further evidence that infectious agents trigger systemic inflammation and increase the risk of vascular events comes from recent reports confirming raised levels of myocardial injury biomarkers Tn1 (Shi et al., 2020) and TnT (Guo et al., 2020) in patients affected by the coronavirus disease (COVID‐19).
FIGURE 3.
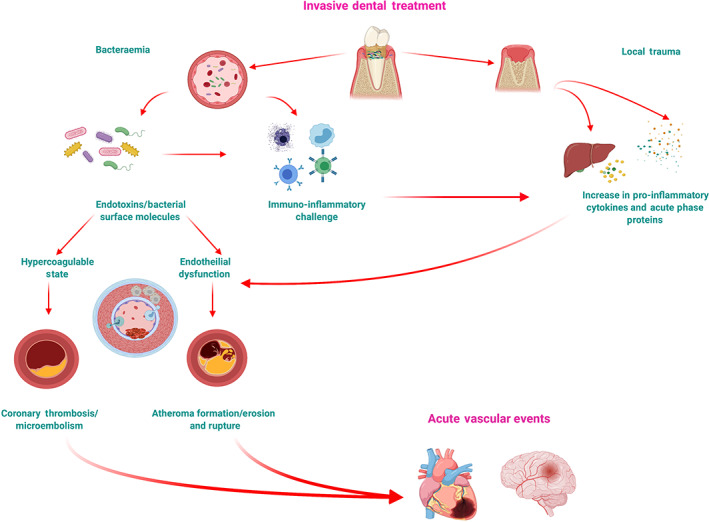
Plausible mechanistic links between invasive dental treatment and acute vascular events. Putative pathways through which oral infection and inflammation can increase the risk of an acute vascular event
A separate unanswered question is whether individuals who already suffer from a medical condition might be at a greater risk of AVEs when facing an acute inflammatory status or infection. A large retrospective cohort study (Wilcox et al., 2021) (n = 3,848,501) confirmed that the 30‐day event rate of perioperative MI increased in a step‐wise manner with the presence of additional CVD risk factors (0.41% for one, 0.81% for two, and 1.07% for three; p < .001) (Wilcox et al., 2021). Similar evidence was reported on the effect of periodontitis and its treatment on the risk of PAD (Yang et al., 2018; Aarabi et al., 2020). We hypothesized that IDTs could influence the acute vascular risk of patients with other co‐morbidities (e.g., diabetes or established CVDs). The only study that investigated this question confirmed no greater acute vascular risk (Chen et al., 2019). Even though more evidence should be produced on this important topic, we speculate that performing invasive dental procedures does not change the acute risk of cardiovascular events, but caution should be applied when planning and delivering such procedures in patients with unstable health and co‐morbidities.
The latest consensus report from the World Heart and European Periodontology Federations (Sanz et al., 2020) on the safety of dental extractions and dental cleaning concludes that dental care professionals should liaise with direct medical teams when planning invasive dental procedures. This review further confirms that such IDTs are not associated with AVEs. A minor increase in the incidence of stroke between 5 and 8 weeks following treatment was observed. However, no other notable increases in AVEs have been reported. Caution, however, should be taken when interpreting our findings within the wider context of different public health systems and countries. Owing to the high volumes of dental procedures performed worldwide, even a 5% increase in the risk of AVEs could result in a relatively large number of patients being affected. Future surveillance studies should be considered and performed to confirm our findings. Future efforts should also focus on investigating the risk of AVEs following any type of IDTs by clearly defining them using the Centers for Disease Control or MeSH database controlled vocabulary. Researchers must also be vigilant of the time points for analysis, including changes in well‐established biomarkers or blood cell levels and rheology, which have been linked to AVEs.
4.1. Limitations and strengths
There are some limitations in this review that should be highlighted. A significant variation in the study designs including several dental treatments and inconsistent time points used to record events inevitably increased the heterogeneity of the evidence retrieved. Only a limited number of studies could be included in the quantitative analysis. One study in particular (Chen et al., 2019) had the largest weight in the analysis and hence it influenced the overall results and interpretation. We urge, therefore caution in interpreting the data and hope that more studies will be performed and published in the future. This review, however, included data on more than 5 million participants across three continents using a robust methodology to screen and extract the available evidence, with particular emphasis in performing quantitative analyses.
5. CONCLUSIONS
This review does not support an association between IDTs and AVEs. As a large number of dental procedures are performed worldwide, healthcare systems should enable better integration and facilitate communication between dental care professionals and medical teams when essential or advanced dental treatments are planned, especially in patients with multiple co‐morbidities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Shailly Luthra, Yago Leira, and Francesco D'Aiuto had the original idea for the project. Shailly Luthra and Yago Leira wrote the first draft of the research protocol. All authors discussed and formulated the final research design. Desta Bokre and Debora Marletta helped with the search strategy. Shailly Luthra and Roberto Rotundo identified research reports and extracted data in agreement with Yago Leira. Simon Harden did statistical analyses, which were discussed and interpreted by all authors. Shailly Luthra, Simon Harden, Marco Orlandi, and Francesco D'Aiuto contributed to data analysis and manuscript preparation. Shailly Luthra, Marco Orlandi, and Francesco D'Aiuto drafted the final report, which was edited and revised by all authors.
Supporting information
Data S1. Supporting information.
Data S2. Supporting information.
Data S3. Supporting information.
Data S4. Supporting information.
ACKNOWLEDGEMENTS
This study was completed at the UCL Biomedical Research Centre, which receives funding from the National Institute for Health Research (NIHR). Dr. Marco Orlandi holds an NIHR Clinical Lectureship. Dr. Yago Leira holds a Senior Clinical Research Fellowship supported by the UCL Biomedical Research Centre, which receives funding from the NIHR (NIHR‐INF‐0387), and a research contract with the Fundación Instituto de Investigación Sanitaria de Santiago de Compostela. Figure 3 (Plausible mechanistic links between invasive dental treatments and acute vascular events) image was created using the BioRender Software.
Luthra, S. , Orlandi, M. , Leira, Y. , Bokre, D. , Marletta, D. , Rotundo, R. , Harden, S. , & D'Aiuto, F. (2022). Invasive dental treatment and acute vascular events: A systematic review and meta‐analysis. Journal of Clinical Periodontology, 49(5), 467–479. 10.1111/jcpe.13600
Registration: The systematic review protocol was registered with PROSPERO with ID: CD42019131124; URL: https://www.crd.york.ac.uk/prospero/.
Funding information NIHR‐BRC at UCL/UCLH, Grant/Award Number: NIHR‐INF‐0387
DATA AVAILABILITY STATEMENT
All data relevant to the study are included in the article or uploaded as supplementary information.
REFERENCES
- Aarabi, G. , Raedel, M. , Kreutzburg, T. , Hischke, S. , Debus, E. S. , Marschall, U. , Seedorf, U. , & Behrendt, C.‐A. (2020). Periodontal treatment and peripheral arterial disease severity – A retrospective analysis of health insurance claims data. VASA. Zeitschrift für Gefässkrankheiten, 49, 128–132. 10.1024/0301-1526/a000846 [DOI] [PubMed] [Google Scholar]
- Beck, J. D. , Couper, D. J. , Falkner, K. L. , Graham, S. P. , Grossi, S. G. , Gunsolley, J. C. , Madden, T. , Maupome, G. , Offenbacher, S. , Stewart, D. D. , Trevisan, M. , Van Dyke, T. E. , & Genco, R. J. (2008). The Periodontitis and Vascular Events (PAVE) pilot study: Adverse events. Journal of Periodontology, 79, 90–96. 10.1902/jop.2008.070223 [DOI] [PubMed] [Google Scholar]
- Bergh, C. , Fall, K. , Udumyan, R. , Sjöqvist, H. , Fröbert, O. , & Montgomery, S. (2017). Severe infections and subsequent delayed cardiovascular disease. European Journal of Preventive Cardiology, 24, 1958–1966. 10.1177/2047487317724009 [DOI] [PubMed] [Google Scholar]
- Bhagat, K. , Moss, R. , Collier, J. , & Vallance, P. (1996). Endothelial “stunning” following a brief exposure to endotoxin: A mechanism to link infection and infarction? Cardiovascular Research, 32, 822–829. [PubMed] [Google Scholar]
- Cahill, T. J. , Dayer, M. , Prendergast, B. , & Thornhill, M. (2017). Do patients at risk of infective endocarditis need antibiotics before dental procedures? BMJ (Clinical research ed.), 358, j3942. 10.1136/bmj.j3942 [DOI] [PubMed] [Google Scholar]
- Chang, Y. , Woo, H. G. , Lee, J. S. , & Song, T.‐J. (2021). Better oral hygiene is associated with lower risk of stroke. Journal of Periodontology, 92, 87–94. 10.1002/JPER.20-0053 [DOI] [PubMed] [Google Scholar]
- Chen, T. T. , D'Aiuto, F. , Yeh, Y. C. , Lai, M. S. , Chien, K. L. , & Tu, Y. K. (2019). Risk of myocardial infarction and ischemic stroke after dental treatments. Journal of Dental Research, 98, 157–163. 10.1177/0022034518805745 [DOI] [PubMed] [Google Scholar]
- Chen, T. T. , Yeh, Y. C. , Chien, K. L. , Lai, M. S. , & Tu, Y. K. (2018). Risk of infective endocarditis after invasive dental treatments: Case‐only study. Circulation, 138(4), 356–363. [DOI] [PubMed] [Google Scholar]
- Cho, H. J. , Shin, M. S. , Song, Y. , Park, S. K. , Park, S. M. , & Kim, H. D. (2021). Severe periodontal disease increases acute myocardial infarction and stroke: A 10‐year retrospective follow‐up study. Journal of Dental Research, 100, 706–713. 10.1177/0022034520986097 [DOI] [PubMed] [Google Scholar]
- Crasta, K. , Daly, C. G. , Mitchell, D. , Curtis, B. , Stewart, D. , & Heitz‐Mayfield, L. J. (2009). Bacteraemia due to dental flossing. Journal of Clinical Periodontology, 36, 323–332. 10.1111/j.1600-051X.2008.01372.x [DOI] [PubMed] [Google Scholar]
- Cumpston, M. , Li, T. , Page, M. J. , Chandler, J. , Welch, V. A. , Higgins, J. P. , & Thomas, J. (2019). Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Database of Systematic Reviews, 10, ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aiuto, F. , Nibali, L. , Mohamed‐Ali, V. , Vallance, P. , & Tonetti, M. S. (2004). Periodontal therapy: A novel non‐drug‐induced experimental model to study human inflammation. Journal of Periodontal Research, 39, 294–299. 10.1111/j.1600-0765.2004.00741.x [DOI] [PubMed] [Google Scholar]
- D'Aiuto, F. , Parkar, M. , Nibali, L. , Suvan, J. , Lessem, J. , & Tonetti, M. S. (2006). Periodontal infections cause changes in traditional and novel cardiovascular risk factors: Results from a randomized controlled clinical trial. American Heart Journal, 151, 977–984. 10.1016/j.ahj.2005.06.018 [DOI] [PubMed] [Google Scholar]
- D'Aiuto, F. , Parkar, M. , & Tonetti, M. S. (2005). Periodontal therapy: A novel acute inflammatory model. Inflammation Research, 54, 412–414. 10.1007/s00011-005-1375-4 [DOI] [PubMed] [Google Scholar]
- D'Aiuto, F. , Parkar, M. , & Tonetti, M. S. (2007). Acute effects of periodontal therapy on bio‐markers of vascular health. Journal of Clinical Periodontology, 34, 124–129. 10.1111/j.1600-051X.2006.01037.x [DOI] [PubMed] [Google Scholar]
- Dalager‐Pedersen, M. , Søgaard, M. , Schønheyder, H. C. , Nielsen, H. , & Thomsen, R. W. (2014). Risk for myocardial infarction and stroke after community‐acquired bacteremia: A 20‐year population‐based cohort study. Circulation, 129, 1387–1396. 10.1161/circulationaha.113.006699 [DOI] [PubMed] [Google Scholar]
- Davignon, J. , & Ganz, P. (2004). Role of endothelial dysfunction in atherosclerosis. Circulation, 109(23_suppl_1), III‐27‐III‐32. 10.1161/01.CIR.0000131515.03336.f8 [DOI] [PubMed] [Google Scholar]
- Debelian, G. J. , Olsen, I. , & Tronstad, L. (1995). Bacteremia in conjunction with endodontic therapy. Endodontics & Dental Traumatology, 11, 142–149. 10.1111/j.1600-9657.1995.tb00476.x [DOI] [PubMed] [Google Scholar]
- Dietrich, T. , Webb, I. , Stenhouse, L. , Pattni, A. , Ready, D. , Wanyonyi, K. L. , White, S. , & Gallagher, J. E. (2017). Evidence summary: The relationship between oral and cardiovascular disease. British Dental Journal, 222, 381–385. 10.1038/sj.bdj.2017.224 [DOI] [PubMed] [Google Scholar]
- Forner, L. , Larsen, T. , Kilian, M. , & Holmstrup, P. (2006). Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. Journal of Clinical Periodontology, 33, 401–407. 10.1111/j.1600-051X.2006.00924.x [DOI] [PubMed] [Google Scholar]
- Guo, T. , Fan, Y. , Chen, M. , Wu, X. , Zhang, L. , He, T. , Wang, H. , Wan, J. , Wang, X. , & Lu, Z. (2020). Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiology, 5, 811–818. 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habbab, K. M. , D'Aiuto, F. , Habbab, M. A. , & Porter, S. R. (2019). Molecular markers relevant to myocardial injury following dental extraction in patients with and without coronary artery disease. BDJ Open, 5, 9. 10.1038/s41405-019-0018-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haheim, L. L. , Olsen, I. , & Ronningen, K. S. (2011). Association between tooth extraction due to infection and myocardial infarction. Community Dentistry and Oral Epidemiology, 39, 393–397. 10.1111/j.1600-0528.2011.00616.x [DOI] [PubMed] [Google Scholar]
- Hansson, G. K. (2005). Inflammation, atherosclerosis, and coronary artery disease. New England Journal of Medicine, 352, 1685–1695. 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- Heimdahl, A. , Hall, G. , Hedberg, M. , Sandberg, H. , Soder, P. O. , Tuner, K. , & Nord, C. E. (1990). Detection and quantitation by lysis‐filtration of bacteremia after different oral surgical procedures. Journal of Clinical Microbiology, 28, 2205–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington, W. , Lacey, B. , Sherliker, P. , Armitage, J. , & Lewington, S. (2016). Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circulation Research, 118, 535–546. [DOI] [PubMed] [Google Scholar]
- Higashi, Y. , Goto, C. , Hidaka, T. , Soga, J. , Nakamura, S. , Fujii, Y. , Hata, T. , Idei, N. , Fujimura, N. , Chayama, K. , Kihara, Y. , & Taguchi, A. (2009). Oral infection‐inflammatory pathway, periodontitis, is a risk factor for endothelial dysfunction in patients with coronary artery disease. Atherosclerosis, 206, 604–610. 10.1016/j.atherosclerosis.2009.03.037 [DOI] [PubMed] [Google Scholar]
- Joshipura, K. J. , Wand, H. C. , Merchant, A. T. , & Rimm, E. B. (2004). Periodontal disease and biomarkers related to cardiovascular disease. Journal of Dental Research, 83, 151–155. 10.1177/154405910408300213 [DOI] [PubMed] [Google Scholar]
- Kinane, D. F. , Riggio, M. P. , Walker, K. F. , MacKenzie, D. , & Shearer, B. (2005). Bacteraemia following periodontal procedures. Journal of Clinical Periodontology, 32, 708–713. 10.1111/j.1600-051X.2005.00741.x [DOI] [PubMed] [Google Scholar]
- Lee, Y. L. , Hu, H. Y. , Chou, P. , & Chu, D. (2015). Dental prophylaxis decreases the risk of acute myocardial infarction: A nationwide population‐based study in Taiwan. Clinical Interventions in Aging, 10, 175–182. 10.2147/cia.S67854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. L. , Hu, H. Y. , Huang, N. , Hwang, D. K. , Chou, P. , & Chu, D. (2013). Dental prophylaxis and periodontal treatment are protective factors to ischemic stroke. Stroke, 44, 1026–1030. 10.1161/strokeaha.111.000076 [DOI] [PubMed] [Google Scholar]
- Libby, P. (2013). Mechanisms of acute coronary syndromes and their implications for therapy. The New England Journal of Medicine, 368(21), 2004–2013. 10.1056/NEJMra1216063 [DOI] [PubMed] [Google Scholar]
- Lin, H. W. , Chen, C. M. , Yeh, Y. C. , Chen, Y. Y. , Guo, R. Y. , Lin, Y. P. , & Li, Y. C. (2019). Dental treatment procedures for periodontal disease and the subsequent risk of ischaemic stroke: A retrospective population‐based cohort study. Journal of Clinical Periodontology, 46, 642–649. 10.1111/jcpe.13113 [DOI] [PubMed] [Google Scholar]
- Lockhart, P. B. , Brennan, M. T. , Sasser, H. C. , Fox, P. C. , Paster, B. J. , & Bahrani‐Mougeot, F. K. (2008). Bacteremia associated with toothbrushing and dental extraction. Circulation, 117, 3118–3125. 10.1161/circulationaha.107.758524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthra, S. , Grover, H. , Singh, A. , Lall, A. , & Masamatti, S. (2019). Comparative evaluation of C‐reactive protein and complete blood count in chronic periodontitis patients following Phase I therapy: A serological and hematological study. Journal of Indian Society of Periodontology, 23, 525–533. 10.4103/jisp.jisp_639_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes, R. , & Fernandes, D. (2016). Endothelial dysfunction and periodontitis: The role of inflammatory serum biomarkers. Dental Hypotheses, 7, 4–11. 10.4103/2155-8213.177401 [DOI] [Google Scholar]
- Minassian, C. , D'Aiuto, F. , Hingorani, A. D. , & Smeeth, L. (2010). Invasive dental treatment and risk for vascular events: A self‐controlled case series. Annals of Internal Medicine, 153, 499–506. 10.7326/0003-4819-153-8-201010190-00006 [DOI] [PubMed] [Google Scholar]
- Nguyen, J. L. , Yang, W. , Ito, K. , Matte, T. D. , Shaman, J. , & Kinney, P. L. (2016). Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiology, 1, 274–281. 10.1001/jamacardio.2016.0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa, H. , Sato, Y. , & Matsuura, H. (2000). Safety of dental treatment in patients with previously diagnosed acute myocardial infarction or unstable angina pectoris. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 89, 35–41. 10.1016/s1079-2104(00)80011-6 [DOI] [PubMed] [Google Scholar]
- Nordendahl, E. , Fored, M. , Kjellström, B. , Ekbom, A. , Norhammar, A. , & Gustafsson, A. (2021). Periodontitis, assessed using periodontal treatment as a surrogate marker, has no association with a first myocardial infarction in a Swedish population. Journal of Periodontology, 92, 1730–1737. 10.1002/JPER.20-0758 [DOI] [PubMed] [Google Scholar]
- Nordendahl, E. , Kjellström, B. , Fored, C. M. , Ekbom, A. , Svensson, T. , Norhammar, A. , & Gustafsson, A. (2018). Invasive dental treatment and risk for a first myocardial infarction. Journal of Dental Research, 97, 1100–1105. 10.1177/0022034518767834 [DOI] [PubMed] [Google Scholar]
- Piñeiro, A. , Tomás, I. , Blanco, J. , Alvarez, M. , Seoane, J. , & Diz, P. (2010). Bacteraemia following dental implants' placement. Clinical Oral Implants Research, 21, 913–918. 10.1111/j.1600-0501.2010.01928.x [DOI] [PubMed] [Google Scholar]
- Ross, R. (1993). The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature, 362, 801–809. 10.1038/362801a0 [DOI] [PubMed] [Google Scholar]
- Sanz, M. , Marco del Castillo, A. , Jepsen, S. , Gonzalez‐Juanatey, J. R. , D'Aiuto, F. , Bouchard, P. , Chapple, I. , Dietrich, T. , Gotsman, I. , Graziani, F. , Herrera, D. , Loos, B. , Madianos, P. , Michel, J.‐B. , Perel, P. , Pieske, B. , Shapira, L. , Shechter, M. , Tonetti, M. , … Wimmer, G. (2020). Periodontitis and cardiovascular diseases: Consensus report. Journal of Clinical Periodontology, 47, 268–288. 10.1111/jcpe.13189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein, H. A. , Papapanou, P. N. , Genco, R. , & Sanz, M. (2020). Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontology, 2000(83), 90–106. 10.1111/prd.12304 [DOI] [PubMed] [Google Scholar]
- Schillinger, T. , Kluger, W. , Exner, M. , Mlekusch, W. , Sabeti, S. , Amighi, J. , Wagner, O. , Minar, E. , & Schillinger, M. (2006). Dental and periodontal status and risk for progression of carotid atherosclerosis: The inflammation and carotid artery risk for atherosclerosis study dental substudy. Stroke, 37, 2271–2276. 10.1161/01.STR.0000236495.82545.2e [DOI] [PubMed] [Google Scholar]
- Shi, S. , Qin, M. , Shen, B. , Cai, Y. , Liu, T. , Yang, F. , Gong, W. , Liu, X. , Liang, J. , Zhao, Q. , Huang, H. , Yang, B. , & Huang, C. (2020). Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiology, 5, 802–810. 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaar, D. , O'Connor, H. , Lunos, S. , Luepker, R. , & Michalowicz, B. S. (2012). Dental procedures and risk of experiencing a second vascular event in a Medicare population. Journal of the American Dental Association, 143, 1190–1198. 10.14219/jada.archive.2012.0063 [DOI] [PubMed] [Google Scholar]
- Smeeth, L. , Thomas, S. L. , Hall, A. J. , Hubbard, R. , Farrington, P. , & Vallance, P. (2004). Risk of myocardial infarction and stroke after acute infection or vaccination. The New England Journal of Medicine, 351, 2611–2618. 10.1056/NEJMoa041747 [DOI] [PubMed] [Google Scholar]
- Smith, M. M. , Barbara, D. W. , Mauermann, W. J. , Viozzi, C. F. , Dearani, J. A. , & Grim, K. J. (2014). Morbidity and mortality associated with dental extraction before cardiac operation. The Annals of Thoracic Surgery, 97, 838–844. 10.1016/j.athoracsur.2013.10.034 [DOI] [PubMed] [Google Scholar]
- Stang, A. (2010). Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. European Journal of Epidemiology, 25, 603–605. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- Tonetti, M. S. , D'Aiuto, F. , Nibali, L. , Donald, A. , Storry, C. , Parkar, M. , Suvan, J. , Hingorani, A. D. , Vallance, P. , & Deanfield, J. (2007). Treatment of periodontitis and endothelial function. The New England Journal of Medicine, 356, 911–920. 10.1056/NEJMoa063186 [DOI] [PubMed] [Google Scholar]
- Valtonen, V. , Kuikka, A. , & Syrjänen, J. (1993). Thrombo‐embolic complications in bacteraemic infections. European Heart Journal, 14 Suppl K, 20–23. [PubMed] [Google Scholar]
- Wilcox, T. , Smilowitz, N. R. , Xia, Y. , Beckman, J. A. , & Berger, J. S. (2021). Cardiovascular risk factors and perioperative myocardial infarction after noncardiac surgery. The Canadian Journal of Cardiology, 37, 224–231. 10.1016/j.cjca.2020.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Global status report on noncommunicable diseases 2014. https://www.who.int/nmh/publications/ncd-status-report-2014/en/
- Yang, S. , Zhao, L. S. , Cai, C. , Shi, Q. , Wen, N. , & Xu, J. (2018). Association between periodontitis and peripheral artery disease: A systematic review and meta‐analysis. BMC Cardiovascular Disorders, 18, 141. 10.1186/s12872-018-0879-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura, Y. (1983). The relation of tooth extraction to anginal attack. Journal of Oral and Maxillofacial Surgery, 41, 365–376. 10.1016/s0278-2391(83)80007-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.
Data S2. Supporting information.
Data S3. Supporting information.
Data S4. Supporting information.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.


