Abstract
Background
Antiplatelet agents may be useful for the treatment of deep venous thrombosis (DVT) when used in addition to best medical practice (BMP), which includes anticoagulation, compression stockings, and clinical care such as physical exercise, skin hydration, etc. Antiplatelet agents could minimise complications such as post‐thrombotic syndrome (PTS) and pulmonary embolism (PE). They may also reduce the recurrence of the disease (recurrent venous thromboembolism (recurrent VTE)). However, antiplatelet agents may increase the likelihood of bleeding events.
Objectives
To assess the effects of antiplatelet agents in addition to current BMP compared to current BMP (with or without placebo) for the treatment of DVT.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register, CENTRAL, MEDLINE, Embase and CINAHL databases and World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov trials registers to 7 December 2021. The review authors searched LILACS and IBECS databases (15 December 2021) and also checked the bibliographies of included trials for further references to relevant trials, and contacted specialists in the field, manufacturers and authors of the included trials.
Selection criteria
We considered randomised controlled trials (RCTs) examining antiplatelet agents compared to BMP following initial standard anticoagulation treatment for DVT. We included studies where antiplatelet agents were given in addition to current BMP compared to current BMP (with or without placebo) for the treatment of DVT (acute: treatment started within 21 days of symptom onset; chronic: treatment started after 21 days of symptom onset). We evaluated only RCTs where the antiplatelet agents were the unique difference between the groups (intervention and control).
Data collection and analysis
We used standard Cochrane methodological procedures. Two review authors independently extracted data and assessed risk of bias of the trials. Any disagreements were resolved by discussion with a third review author. We calculated outcome effects using risk ratio (RR) or mean difference (MD) with a 95% confidence interval (CI) and the number needed to treat to benefit (NNTB).
Main results
We included six studies with 1625 eligible participants, with data up to 37.2 months of follow‐up. For one preplanned comparison (i.e. antiplatelet agents plus BMP versus BMP plus placebo) for acute DVT we identified no eligible studies for inclusion.
In acute DVT, antiplatelet agents plus BMP versus BMP alone was assessed by one study (500 participants), which reported on four outcomes until 6 months of follow‐up. There were no deaths and no cases of major bleeding reported. The participants who received antiplatelet agents showed a lower risk of PTS (RR 0.74, 95% CI 0.61 to 0.91; 1 study, 500 participants; very low‐certainty evidence). The control group presented a lower risk of adverse events compared to the intervention group (RR 2.88, 95% CI 1.06 to 7.80; 1 study, 500 participants; very low‐certainty evidence). This study did not provide information for recurrent VTE or PE.
In chronic DVT, antiplatelet agents plus BMP versus BMP alone was assessed by one study (224 participants). The study authors reported four relevant outcomes, three of which (major bleeding, mortality and adverse events) showed no events during the 3 years of follow‐up. Therefore, an effect estimate could only be reported for recurrent VTE, favouring antiplatelet agents plus BMP versus BMP alone (RR 0.12, 95% CI 0.05 to 0.34; 1 study, 224 participants; very low‐certainty evidence). For the outcomes PE and PTS, this study did not present information which could be used for analysis.
In chronic DVT, antiplatelet agents plus BMP versus BMP plus placebo was assessed by four studies (901 participants). The meta‐analysis of this pooled data showed a lower risk of recurrent VTE for the antiplatelet agents group (RR 0.65, 95%, CI 0.43 to 0.96; NNTB = 14; low‐certainty evidence). For major bleeding, we found no clear difference between placebo and intervention groups until 37.2 months of follow‐up (RR 0.98, 95% CI 0.29 to 3.34; 1 study, 583 participants; moderate‐certainty evidence). In PE fatal/non‐fatal outcome, we found no clear difference with the use of antiplatelet agents (RR 0.52, 95% CI 0.23 to 1.14; 1 study, 583 participants; moderate‐certainty evidence). For all‐cause mortality, the overall effect of antiplatelet agents did not differ from the placebo group (RR 0.48, 95% CI 0.21 to 1.06; 3 studies, 649 participants; moderate‐certainty evidence). The adverse events outcome did not show a clear difference (RR 1.57, 95% CI 0.34 to 7.19; 2 studies, 621 participants; moderate‐certainty evidence). There is no assessment of PTS in these studies.
We downgraded the certainty of evidence for risk of bias, indirectness, imprecision and publication bias.
Authors' conclusions
In chronic DVT settings, following the initial standard treatment with anticoagulants, there is low‐certainty evidence that antiplatelet agents in addition to BMP may reduce recurrent VTE, (NNTB = 14) when compared to BMP plus placebo. Moderate‐certainty evidence shows no clear difference in adverse events, major bleeding and PE when antiplatelet agents are used in addition to BMP compared to BMP plus placebo.
In acute and chronic DVT settings, following the initial standard treatment with anticoagulants, we can draw no conclusions for antiplatelet agents in addition to BMP compared to BMP alone due to very low‐certainty evidence.
Trials of high methodological quality, that are large and of sufficient duration to detect significant clinical outcomes are needed. Trials should ideally last more than 4 years in order to estimate the long‐term effect of antiplatelet agents. Trials should include people with acute and chronic DVT and provide relevant individual data, such as the outcome for each index event (DVT or PE), the use of an inferior vena cava (IVC) filter, whether the DVT is provoked or unprovoked, and the age of participants.
Plain language summary
Aspirin and similar drugs for the treatment of deep vein thrombosis (DVT)
Key messages
‐ When used after standard initial treatment with anticoagulants, aspirin and similar drugs (antiplatelet agents) in addition to best medical practice (BMP), may reduce recurrent venous thromboembolism (VTE), i.e. deep vein thrombosis (DVT) or pulmonary embolism (PE), when compared to BMP plus placebo in a chronic DVT setting. There is no clear difference in side effects, major bleeding or PE with the use of antiplatelet agents.
‐ Studies of high methodological quality that are large and long enough to detect significant clinical outcomes are needed to assess the long‐term effect of antiplatelet agents. Studies should include people with acute and chronic DVT and include information about important outcomes such as DVT, PE and major bleeding, the use of an inferior vena cava (IVC) filter, and the age of the participants.
What is DVT?
DVT is a blood clot formed inside the venous system of the body, blocking the flow of blood.
How is DVT treated?
After initial treatment with anticoagulants, people receive follow‐up treatment (known as BMP) which include drugs for preventing new clots, compression stockings and clinical care (such as physical exercise and skin hydration). Antiplatelets such as aspirin, are medicines that stop cells in the blood (platelets) from sticking together and forming a clot. They could therefore be seen as a potential additional intervention to the current BMP for treating DVT. Antiplatelets could be used to minimise complications such as post‐thrombotic syndrome (PTS, a situation in which a blood clot in the vein causes impaired function in the affected vessels), and PE (when the blood clot travels through the bloodstream to the lung and blocks its flow). Antiplatelets may also reduce the recurrence of DVT or PE. One drawback of the use of antiplatelets is the potential for an increase in bleeding.
What did we want to find out?
We wanted to know whether giving antiplatelets to people to treat DVT, following initial treatment, reduced the number of recurrent VTEs, bleeding or PE compared to people who received only BMP or BMP plus placebo (an identical‐seeming treatment but with no medical effect). We also wanted to determine whether antiplatelets reduced death, whether people developed PTS, whether they experienced side effects, whether their quality of life improved and whether there was a change in the length of time spent in hospital.
What did we do?
We searched for studies that assessed antiplatelets given to people to treat DVT. Studies should have a randomised design (when participants are randomly allocated to treatment groups) as long as they compared an antiplatelet plus BMP with BMP alone or BMP with placebo. These treatments were started after initial standard anticoagulation for DVT. We pooled the results when appropriate.
What did we find?
The results are based on six studies with 1625 participants from the USA, Canada, Europe, India, Argentina, Australia and New Zealand. Two large groups of participants were studied: participants with acute DVT (treatment started up to 21 days after symptoms), and participants with chronic DVT (treatment started after 21 days of symptoms). All studies used BMP as a comparison, or BMP plus placebo. Each comparison investigated the effects of antiplatelets on the recurrence of DVT, PE, death and side effects.
We have limited confidence that antiplatelets in addition to BMP may have an effect on reducing the risk of a new episode of VTE when compared to BMP with placebo in a chronic DVT setting. It is necessary to treat 14 patients to avoid one VTE event. When only PE was studied, however, antiplatelets did not achieve a difference between the groups. The use of antiplatelets as an additional treatment does not appear to add any harm or risks, such as death, bleeding, or other side effects.
Although our confidence in the evidence is very limited, people who receive antiplatelets may have lower rates of recurrent VTE compared to those who did not receive antiplatelets in a chronic DVT setting. Similarly, although our confidence in the evidence is very limited, people who receive antiplatelets may have a lower rate of PTS and increased side effects in an acute DVT setting.
We can draw no conclusions from the very limited available evidence for using antiplatelets as an additional treatment to BMP compared with BMP alone in acute and chronic DVT settings.
What are the limitations of the evidence?
Our confidence in the evidence was limited or very limited because few people experienced the outcomes, and some studies' limitations could introduce errors, for example randomisation and selective reporting concerns, poorly‐defined outcomes and duplicate publication.
Future high‐quality studies may produce important data, especially for results such as death and side effects, as well as the treatment of acute DVT.
How up to date is this evidence?
The evidence is up to date to 7 December 2021.
Summary of findings
Background
See Appendix 1 for a glossary of terms.
Description of the condition
Venous thromboembolism (VTE) describes the formation of a thrombus (blood clot) in the deep veins, most commonly in the legs (deep venous thrombosis or DVT) or the subsequent embolisation of all or part of the thrombus in the pulmonary circulation (pulmonary embolism or PE), or both. DVT is caused by the acute formation of a thrombus in veins situated under the muscular fascia of the limbs or in the deep central veins. In general, DVT situated between the popliteal vein and the iliac and inferior cava veins is designated a proximal DVT (Vedantham 2009). The clinical signs may vary depending on the extent of the clot and which veins are affected, but include localised pain, swelling and erythaema. Although DVT may cause discomfort, of utmost importance are the complications of this disorder: PE in the short term and post‐thrombotic syndrome (PTS) in the long‐term (Avila 2022; Broderick 2021; Flumignan 2015). PE occurs when a thrombus breaks away in the limb and follows the bloodstream to the lungs, blocking the circulation in part of the lung tissue. At least one‐third and sometimes one‐half of people with VTE develop PTS due to the damage to the vessel wall. PTS comprises a wide variety of symptoms ranging from relatively light symptoms (slight oedema, venous eczema, pigmentation and minor complaints) to more severe symptoms (severe swelling, venous claudication, pain and chronic recurrent ulcers). PTS can develop after DVT at any time, but mainly during the two years following diagnosis of DVT (Avila 2022; Guanella 2013). The incidence of recurrent DVT in the PTS population is between 20% and 50% (Avila 2022; de Wolf 2012; Guanella 2013).
After stroke and myocardial infarction, VTE is the third most common cardiovascular disease in the world (Goldhaber 2012; ISTH 2014). In the USA and Europe, an estimated 50,000 deaths and 300,000 hospitalisations per year are due to DVT, and 600,000 hospitalisations to PE (Nicolaides 2001). The incidence of DVT is growing with the ageing population, and the estimated rates vary from 1 to 2 per 1000 people per year, with approximately 100,000 to 180,000 deaths per year due to PE (Galanaud 2012; Goldhaber 2012; Matielo 2008; Naess 2007; Tagalakis 2013). Chronic cardiovascular diseases continue to be a major cause of health loss for all regions of the world, and VTE is one of the 10 leading causes of death (Roth 2017).
DVT is a multifactorial disease in which genetic and environmental factors interact with each other, leading to the onset of the disease (Rosendaal 2005). Risk factors for DVT include older age (Anderson 1991), immobilisation (Sevitt 1961), previous VTE (Samama 2000), obesity (Tsai 2002), type of anaesthesia (Urwin 2000), cancer (Heit 2000), chemotherapy (Falanga 1999), pregnancy and delivery (McColl 1997), hormonal contraceptives (Gomes 2004), hormone replacement (Rossouw 2002), and infection by the SARS‐CoV‐2 virus (COVIDSurg 2022; Flumignan 2022; Santos 2022).
Anticoagulants are the standard mode of treatment for DVT (COVIDSurg 2022; Flumignan 2022; Kakkos 2021; Santos 2022; Stevens 2021). These drugs reduce the formation of clots and prevent further VTE. Other options for additional treatment of DVT include thrombolysis, mechanical thrombectomy, angioplasty and stenting (Broderick 2021; Flumignan 2015; Robertson 2016). After initial treatments, follow‐on treatments including further anticoagulation, compression stockings and clinical care, such as physical exercises, skin hydration, etc. are implemented as per BMP for individual clinical settings (Avila 2022; COVIDSurg 2022; Flumignan 2022; Kakkos 2021; Santos 2022; Stevens 2021).
Description of the intervention
Antiplatelet agents are drugs commonly used to decrease platelet aggregation and inhibit thrombus formation. Various antiplatelet agents are available, such as acetylsalicylic acid (ASA; aspirin), clopidogrel, phosphodiesterase inhibitors (dipyridamole and cilostazol) and glycoprotein IIb/IIIa inhibitors (tirofiban and abciximab).
Antiplatelet agents, mostly ASA, are already routinely used in clinical practice to treat various cardiovascular problems. The routine use of ASA has already been established in cases of arterial thrombosis with a cardiac, cerebrovascular or peripheral origin. In coronary artery disease, ASA is part of the routine management of a wide spectrum of patients with coronary artery disease (Braunwald 2012). Clinical and experimental evidence suggests that antiplatelet agents can prevent the onset and spread of venous thrombus, minimising the adverse consequences of PE and DVT (Castro 2006). In one multicentre, double‐blind study, over 400 people with a first unprovoked VTE who had completed six to 18 months of anticoagulation, were randomised to ASA or placebo. The study found a statistically significant difference in the reduction of recurrent VTE with ASA after discontinuing anticoagulation (Becattini 2012). Brighton 2012 used a similar protocol (ASA after the end of anticoagulation therapy) and found no significant reduction in the rate of VTE recurrence, but did find a reduction in the rate of major vascular events. Similar to treatment with anticoagulants, antiplatelet therapy may require monitoring of blood tests, mainly due to the high incidence of resistance to ASA and clopidogrel, as well as resistance to glycoprotein IIb/IIIa (Brar 2011; El‐Atat 2011; Lugo 2008; Matzdorff 2005). However, monitoring blood tests for antiplatelet agents are not routinely available in most practices.
How the intervention might work
Platelets are unique to mammals and are the principal effector cells on haemostasis, coagulation and thrombosis. They are a large part of the ‘haemostatic plug’, a combination of platelets and proteins that adheres to the site of vascular lesions. The secretion of von Willebrand factor by the damaged cells of the endothelium and other mediators of thrombosis from intracellular granules lead to the amplification of platelet adhesion and its aggregation. This receptor‐mediated activation event has been intensely studied because it is a fundamental part of the physiopathology of several diseases, including DVT (Vieira‐de‐Abreu 2012).
ASA inhibits platelet aggregation by arachidonic acid metabolism through inactivation of the enzyme cyclooxygenase, which is an irreversible event during the life of the platelet. Other antiplatelet agents, such as clopidogrel have different mechanisms of action. Clopidogrel is a thienopyridine and is chemically and pharmacologically similar to ticlopidine, which acts by selectively and irreversibly modifying the P2Y12 adenosine diphosphate (ADP) receptor on the platelet surface, thus inhibiting platelet aggregation. ADP is an important activator of platelet aggregation and is found in high concentrations in the platelet granules. ADP induces platelet aggregation by activating a specific receptor located on the outer surface of its membrane, which results in changes in intracellular calcium concentration and the expression of receptors for fibrinogen on the platelet surface (Lugo 2008).
Because antiplatelet agents act in Virchow’s triad as inhibitors of clot formation, they may be a complement to current treatment by potentially changing morbidity and mortality related to DVT, PE and PTS.
Why it is important to do this review
This review is critical at this time. VTE is a growing public health concern mainly due to an ageing population (Tagalakis 2013). The most serious complication of DVT is PE, where almost one‐quarter of the initial clinical presentation is sudden death (Heit 2008). Therefore, any attempt to improve the standard treatment for DVT should be studied. Potential improvements include the reduction of the incidence of acute complications, i.e. PE and chronic complications, i.e. PTS. The treatment of a first unprovoked DVT episode in people with low or moderate risk of bleeding relies on therapeutic anticoagulation with direct oral anticoagulants preferred over heparin or vitamin K antagonists (VKAs) for at least 3 months (Kakkos 2021). The use of inferior vena cava filters is generally reserved for high‐risk people or those with active bleeding. Additional interventions such as compression stockings are recommended for those who do not have any contraindication and aim to avoid PTS. After the initial 3 months of anticoagulation, physicians revise the risk of bleeding and examine the status of the vein with duplex ultrasound to consider either extending the anticoagulation period or stopping anticoagulation (Kakkos 2021).
Although some guidelines and expert consensus recognise the importance of antiplatelet agents for the treatment of DVT (Stevens 2021), there are widely‐used guidelines regarding antithrombotic therapy for VTE disease that do not indicate any recommendations for antiplatelet agents in people with DVT (Kakkos 2021; NICE 2020). Stevens 2021 suggest that aspirin can be considered for patients who stop anticoagulation treatment, but there is lack of evidence in this setting. In this review, we investigate the evidence for the use of antiplatelet agents in addition to current BMP compared to BMP (e.g. anticoagulation, compression stockings and clinical care, such as physical exercises, skin hydration, etc.) to improve the treatment of DVT concerning the presence of recurrent VTE and PTS, and if there are harms such as bleeding and mortality. We aimed to identify the best available evidence to answer these questions.
Objectives
To assess the effects of antiplatelet agents in addition to current BMP compared to current BMP (with or without placebo) for the treatment of DVT.
Methods
Criteria for considering studies for this review
Types of studies
We considered RCTs examining antiplatelet agents compared to BMP following initial standard anticoagulation treatment for DVT. We considered all RCTs of antiplatelet therapy plus current BMP (including anticoagulants, compression stockings and clinical care such as physical exercises, skin hydration etc.) versus BMP with or without placebo for the treatment of DVT. We considered any antiplatelet agent by any route of administration. As this review focused on proximal DVT, we excluded trials of DVT in other regions (upper limbs, cerebral, etc.).
Types of participants
We included participants of both sexes and of any age as long as they were diagnosed with DVT (acute or chronic) by a medical specialist on clinical assessment and further investigation (duplex ultrasound, multislice computed tomography or angiography). We included both symptomatic or asymptomatic DVT. We considered the DVT as acute if it was diagnosed and treatment started within 21 days of onset of signs/symptoms and chronic if diagnosed and treatment started after 21 days (Flumignan 2015). We considered all participants that received any form of expected intervention or BMP (control group). For chronic DVT, we did not consider studies with anticoagulant treatment of less than 3 months because there is evidence of an increased rate of recurrence after less than 3 months of anticoagulation, but no significant difference with various longer periods of treatment (Boutitie 2011; Robertson 2015).
Types of interventions
We considered the following comparisons.
Acute DVT
antiplatelet agents plus BMP versus BMP alone
antiplatelet agents plus BMP versus BMP plus placebo
Chronic DVT
antiplatelet agents plus BMP versus BMP alone
antiplatelet agents plus BMP versus BMP plus placebo
We considered BMP as anticoagulation, compression stockings and clinical care, as previously defined (Broderick 2021; Flumignan 2015; Kakkos 2021; Robertson 2015; Stevens 2021). It is important to clarify that anticoagulation means any anticoagulation therapy (heparin or oral anticoagulation or both) for more than 2 days. Since the actual role of compression stockings for DVT is still under discussion (Appelen 2017; Stevens 2021), we did not exclude RCTs because they only used compression stockings in their interventions. In this review, we performed a subgroup analysis for the use of compression stockings (with and without compression stockings). In addition, we have to mention that when anticoagulation stops (e.g. 3, 6 or 12 months after the DVT diagnosis), BMP does not necessarily stop, as some BMP components may still continue to be used.
We considered all RCTs utilising antiplatelet therapy either at the same time or after anticoagulation, and we analysed these two distinct groups separately. We did not evaluate comparisons in which antiplatelet agent use is not the only difference.
We intended to include the utilisation of an IVC filter, but in this review we did not find such separate data. In future versions, if we find any data utilising the IVC filter, we will consider this in a subgroup analysis.
Types of outcome measures
We presented the outcomes at three different time points following the start of the intervention.
Early outcomes (≤ 1 year after intervention)
Intermediate outcomes (> 1 year to ≤ 3 years after intervention)
Long‐term outcomes (> 3 years after intervention)
Our time point of primary interest is early; we, therefore, performed primary analysis for this time point, and also planned to report the long‐term outcomes at the longest possible time of follow‐up.
Primary outcomes
Recurrent VTE: including recurrent DVT and PE, first episode or recurrent, fatal or nonfatal. Both outcomes were diagnosed by clinical examination and a diagnostic assessment including ultrasonography, computed tomography or angiography.
Major bleeding: defined by a decreased haemoglobin concentration of ≥ 2 g/dL, a retroperitoneal or intracranial bleed, a transfusion of ≥ 2 units of blood, or fatal haemorrhagic events, as defined by the International Society on Thrombosis and Haemostasis (Schulman 2005; Schulman 2010).
PE (fatal/nonfatal): confirmed by computed tomography pulmonary angiography or ventilation/perfusion (V/Q) scan, or both.
Secondary outcomes
Mortality: all‐cause and VTE‐related.
PTS: diagnosed by objective clinical examination (signs and symptoms), with or without the support of severity ratings such as CEAP (clinical findings, aetiological, anatomical and pathological elements), VCSS (Venous Clinical Severity Score) or Villalta scores (de Wolf 2012).
Adverse events, such as gastrointestinal adverse effects, allergic reaction, renal failure and minor bleeding.
Quality of life (QoL) or participant's subjective perception of improvement (yes or no) as reported by the study authors. If we are unable to pool data on QoL due to the use of different measurements, in future versions of this review, we will attempt to extract data on improvement (Soosainathan 2013).
Duration of hospitalisation (days).
There was no economic information in the included studies, but in future versions of this review we will report on costs or financial impacts if this is reported by study authors.
Search methods for identification of studies
We did not apply any language, publication year or publication status restrictions.
Electronic searches
The Cochrane Vascular Information Specialist conducted systematic searches of the following databases for RCTs and controlled clinical trials without language, publication year or publication status restrictions.
Cochrane Vascular Specialised Register via the Cochrane Register of Studies (CRS‐Web)
Cochrane Central Register of Controlled Trials (CENTRAL; 2021 Issue 11) via the Cochrane Register of Studies Online (CRSO)
MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE
Embase Ovid
CINAHL Ebsco
AMED Ovid
We developed search strategies for other databases from the search strategy designed for MEDLINE. Where appropriate, they were combined with adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2022). Search strategies for major databases and trials registers are provided in Appendix 2 and Appendix 3.
We searched the following trials registries (Appendix 4).
World Health Organization International Clinical Trials Registry Platform (who.int/trialsearch)
ClinicalTrials.gov (clinicaltrials.gov)
We carried out the most recent searches on 7 December 2021.
Authors' searches
We searched Latin American and Caribbean Health Science Information database (LILACS) and the Indice Bibliográfico Español de Ciencias de la Salud (IBECS) (both in lilacs.bvsalud.org/) on 15 December 2021. See Appendix 5 for details of the search strategy we used. We did not use a filter but selected the RCTs manually in the LILACS and IBECS databases. Two review authors (CDQF and RLGF) configured this strategy, which was revised by the Cochrane Brazil Information Specialist and third review author (JCCBS). We searched these databases in collaboration with the Cochrane Brazil Information Specialist.
Searching other resources
We checked the bibliographies of included trials for further references regarding relevant trials. We contacted specialists in the field, manufacturers and authors of the included trials for any possible unpublished data.
Data collection and analysis
Selection of studies
We examined titles and abstracts to select the relevant reports after merging the search results and removing duplicate records. Two review authors (CDQF and RLGF) independently screened the trials identified by the literature search. We retrieved and examined the full text of the selected trials for compliance with the eligibility criteria. We documented the reason for exclusion of individual trials in the Characteristics of excluded studies table. We consulted a third review author (JCCBS) in the case of any disagreement. If trials included more than one type of treatment beyond those specified in the Types of interventions, we used only the specific data that fit our criteria. Any appropriate method of randomisation was eligible, and we took into account any differences in methodological quality in the analyses.
Data extraction and management
Two review authors (CDQF and RLGF) extracted data independently and collected data on a paper data extraction form. We resolved discrepancies by discussion. We consulted a third review author (JCCBS) in the case of any disagreement. We collected the following information.
Study features: publication details (e.g. year, country, authors), study design, population data (e.g. age, comorbidities, severity, duration, history concerning treatments and responses), number of participants randomised into each treatment group, number of participants in each group who failed treatment, number of participants lost to follow‐up, duration of follow‐up period
Outcomes: types of outcomes measured, timing of outcomes, adverse events
Assessment of risk of bias in included studies
Two review authors (CDQF and RLGF) independently evaluated the methodological quality of studies included in the analysis in order to assess the risk of bias according to the domains and criteria of the RoB 1 tool (Higgins 2017). We evaluated the bias of the following domains and characterised them as low risk, high risk or unclear risk.
random sequence generation (randomisation)
allocation ‐ secrecy appropriate
blinding/masking of participants, assessors and results
data on incomplete results
selective publication of results
any other potential threat to validity
We reported these assessments for each study individually in the risk of bias table of the Characteristics of included studies. We contacted author(s) of included studies to seek clarification if in doubt about the data.
Measures of treatment effect
We calculated the risk ratios (RRs) and 95% confidence intervals (CIs) for dichotomous variables such as recurrent VTE and major bleeding, using Review Manager 5 software (Review Manager 2020). We calculated the mean difference (MD) and 95% CI for continuous variables that used similar scales such as duration of hospitalisation. We planned to calculate the standardised mean difference (MD) and 95% CI for continuous variables that used different scales such as QoL. We calculated the number needed to treat (NNT) for the outcomes with direct implication for practice (see Implications for practice), using NNT = 1/(risk difference). The risk difference was obtained with Review Manager 5 software (Review Manager 2020). As recommended by the Cochrane Handbook for Systematic Reviews of Interventions, we expressed the NNT as 'number needed to treat for an additional beneficial outcome' (NNTB) and 'number needed to treat for an additional harmful outcome' (NNTH) to indicate direction of effect (Schünemann 2021). In future versions, if the study authors have not reported all the necessary information for this detail, we will insert the data from these non‐parametric primary studies (e.g. the effects reported as medians, quartiles, etc.) or without sufficient statistical information (e.g. deviations, number of participants, etc.) in an additional table.
Unit of analysis issues
We considered the individual participant as unit of analysis (unit to be distributed randomly for interventions to be compared); that is, the number of observations in the analysis should match the number of randomised participants. We grouped studies evaluating various treatment groups relevant to the review to create a single‐pair comparison. In studies that considered multiple interventions, we analysed only the data of interest. In future versions, if we find cross‐over studies and consider them appropriate for inclusion in the meta‐analysis, we will include data using the results of the paired analysis (Elbourne 2002).
Dealing with missing data
We contacted the study authors for additional information where there were missing or unavailable data. In the case of no response, we reported dropout rates in the Characteristics of included studies tables of the review irrespective of the type of data. We used intention‐to‐treat analysis for assessment of the treatment effects.
Assessment of heterogeneity
We quantified inconsistencies among the pooled estimates using the I2 statistic (where I2 = ((Q ‐ df)/Q) × 100% where Q is the χ2 statistic, and df represents the degrees of freedom). This illustrates the percentage of variability in effect estimates resulting from heterogeneity rather than sampling error (Higgins 2003; Higgins 2021).
As strict thresholds for interpretation of I2 are not recommended, we used the guide to interpretation in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021).
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
When the I² statistic lies in an area of overlap between two categories (e.g. between 50% and 60%), we planned to consider differences in participants and interventions among the studies contributing data to the analysis (Deeks 2021).
Assessment of reporting biases
In future versions, we will assess reporting biases or small‐study effects by drawing a funnel plot (trial effect versus trial size) if we include a sufficient number of studies (more than 10) in the review (Higgins 2021).
Data synthesis
We used Review Manager 2020 software for data analysis for statistical evaluation using an intention‐to‐treat approach.
In accordance with section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions, "The choice between a fixed‐effect and random‐effects meta‐analysis should never be made on the basis of a statistical test for heterogeneity" (Deeks 2021), we used a fixed‐effect model for analyses with very homogenous included studies considering population, intervention, comparator and outcome characteristics, or for analysis when only one study was included. If heterogeneity was detected we used a random‐effects model.
If, in future versions, we are unable to pool data on QoL due to the use of different measurements, we will attempt to extract data on improvement (Soosainathan 2013).
Subgroup analysis and investigation of heterogeneity
In this review version, the subgroup analysis was possible only for types of antiplatelets agents (Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6 and Analysis 3.1), and for compression stockings (Analysis 2.1). However, in future versions, if possible, we will perform subgroup analyses to consider all of the following.
1.3. Analysis.

Comparison 1: Acute DVT ‐ antiplatelet agents plus BMP versus BMP alone, Outcome 3: Post‐thrombotic syndrome
1.4. Analysis.

Comparison 1: Acute DVT ‐ antiplatelet agents plus BMP versus BMP alone, Outcome 4: Post‐thrombotic syndrome
1.5. Analysis.

Comparison 1: Acute DVT ‐ antiplatelet agents plus BMP versus BMP alone, Outcome 5: Adverse events
1.6. Analysis.

Comparison 1: Acute DVT ‐ antiplatelet agents plus BMP versus BMP alone, Outcome 6: Adverse events
3.1. Analysis.

Comparison 3: Chronic DVT ‐ antiplatelet agents plus BMP versus BMP plus placebo, Outcome 1: Recurrent VTE
2.1. Analysis.

Comparison 2: Chronic DVT ‐ antiplatelet agents plus BMP versus BMP alone, Outcome 1: Recurrent VTE
Age
Gender
Different types of antiplatelet agents used
Forms of administration (oral or intravenous) used for antiplatelet agents or the anticoagulation as part of the BMP
Dosage of antiplatelet agents used
Location of DVT
Inferior vena cava filter
Compression stockings
If we find substantial heterogeneity in future, and there are sufficient data, we will investigate the possible causes and further explore the impact of the condition of participants and interventions (i.e. participant characteristics, adjuvant drugs) using subgroup analyses. We will test for subgroup differences using interaction tests.
Sensitivity analysis
In future versions of this review, if there are sufficient studies, we will perform sensitivity analyses using only low risk of bias studies according to allocation concealment and blinding of outcome assessors. We will present these results and compare them with the overall findings.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to interpret the findings of this review (GRADE Working Group). We developed a summary of findings table for each comparison using GRADEpro GDT 2015 software. We included the following outcomes: recurrent VTE, major bleeding, PE, mortality, PTS and adverse events. We assessed the certainty of the body of evidence by considering the overall risk of bias in the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates and the risk of publication bias (GRADE Working Group; Schünemann 2013). We based the tables on methods described in Chapters 11 and 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021); we justified any departures from the standard methods.
Results
Description of studies
Results of the search
Our searches identified 19,241 records from database and author searches. Out of the 19,241 total initial references, we obtained 11,314 records after duplicates were removed. Of these 11,314 records, we assessed 11,254 as not relevant for this review for different reasons (e.g. participants not humans, different design of study, disease is not assessed in this review, etc). For the remaining 60 records (40 studies), we assessed the full text for eligibility. Out of the 40 studies, we excluded seven (Characteristics of excluded studies), we assessed 27 studies as not relevant for this review and six studies as eligible for inclusion (Characteristics of included studies). See Figure 1.
1.
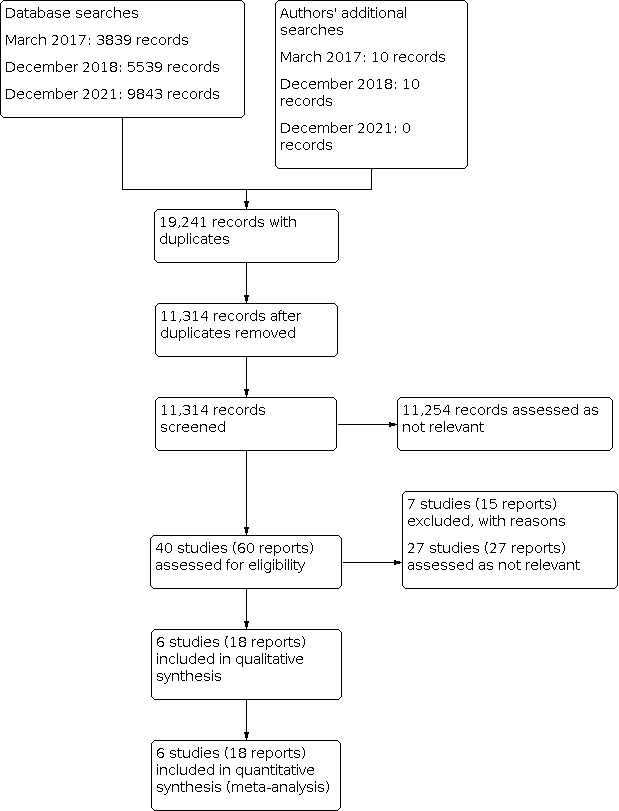
Study flow diagram.
We contacted all authors of the included studies, as well as pharmaceutical companies, in order to find out if there were any ongoing studies. When we received a response, it was negative for new data.
Included studies
See Characteristics of included studies.
Design and setting
We found six studies (18 records) with 1625 eligible participants to include in our analysis (ASPIRE; Indobufen 1993; Moriau 1995; Steele 1980; Sulfinpyrazone 1978; WARFASA). Not all participants in these six studies (2138) were included in this review because only a fraction of them corresponded with our inclusion criteria. In Moriau 1995, the study authors evaluated a group without any treatment (without anticoagulation) in acute DVT (100 participants), which could not be included in our analysis because it is not acceptable BMP. Indobufen 1993 also evaluated a group without treatment, but in the chronic phase of DVT, i.e. the participants had already received an initial treatment of anticoagulants for 6 months before randomisation. WARFASA evaluated participants with DVT, participants with DVT plus PE and participants with PE without diagnosed DVT. The WARFASA participants with PE without diagnosed DVT were not part of this review and therefore not evaluated. Only data for the outcome 'recurrent VTE' were stratified by type of participant and could therefore be included in the analysis in this review. For the ASPIRE trial, the ASPIRE authors provided us with separate data for the group with DVT with or without PE, making it possible to use the study results.
The six included studies provided data for three different comparisons.
Acute DVT ‐ antiplatelet agents plus BMP versus BMP (500 participants; Moriau 1995)
Chronic DVT ‐ antiplatelet agents plus BMP versus BMP (224 participants; Indobufen 1993)
Chronic DVT ‐ antiplatelet agents plus BMP versus BMP plus placebo (901 participants; ASPIRE; Steele 1980; Sulfinpyrazone 1978; WARFASA)
WARFASA provided information about study duration (between May 2004 and August 2010) and setting; the protocol indicated that participants were to be collected from 25 medical centres in Austria, Denmark, France, Great Britain, Italy and the Netherlands. In ASPIRE, study duration was between May 2003 and August 2011, and recruitment was from 56 sites in five countries (Argentina, Australia, India, New Zealand, Canada). All other included studies did not provide details about study period or the collection setting. However, the study reports do mention affiliate countries such as Italy and the UK (Indobufen 1993), Belgium (Moriau 1995), the USA (Steele 1980), and Canada and USA (Sulfinpyrazone 1978).
Participants
All the studies included men and women, with ages ranging from 19 to 77 years, with the majority older than 40. Two studies had a majority of female participants (52% in Indobufen 1993; 65% in Moriau 1995). The four remaining studies had more male participants (54% in ASPIRE, 68% in Steele 1980; 73% in Sulfinpyrazone 1978; and 64% in WARFASA). WARFASA reported that the vein segment with DVT was proximal for all participants, and ASPIRE described enrolment of participants with DVT in the popliteal vein or in proximal leg veins. Moriau 1995 had more than 75% of participants with DVT only in distal settings and less than 20% only in the proximal setting, although the information about DVT setting was not available for each outcome. None of the other included studies mentioned the site of DVT. The participants presented different causes that led to DVT and had variable degrees of signs/symptoms. However, two studies mentioned the proportion of participants without an underlying cause of DVT (45% in Steele 1980; 68% in Sulfinpyrazone 1978).
Indobufen 1993, Steele 1980, Sulfinpyrazone 1978, WARFASA and ASPIRE studied participants with chronic DVT (treatment started after 21 days of symptoms), while Moriau 1995 studied acute DVT (treatment started before 21 days of symptoms) in participants with severe or recurrent DVT.
All studies reported that patients with clinical factors which prohibited antiplatelet agent use or patients who required vitamin K antagonists (VKA) were excluded from participation.
Despite attempts to contact the studies’ authors, not all responded or provided the raw data for analysis. All participants analysed in this review had at least one episode of DVT (with or without PE) confirmed by an objective further investigation, such as Doppler vascular ultrasound, multislice computed tomography or angiography. We did not include in the analysis participants whose confirmation of DVT was not possible. Because of this, we could only analyse part of the data from WARFASA and ASPIRE (the ASPIRE group provided additional data by personal communication).
Interventions and co‐treatments
All included studies evaluated antiplatelet agents in the intervention group with some differences. Moriau 1995 and Indobufen 1993 compared antiplatelet agents plus BMP versus BMP without placebo (Moriau 1995 in the acute phase and Indobufen 1993 in the chronic phase of DVT). Steele 1980, Sulfinpyrazone 1978, WARFASA and ASPIRE compared antiplatelets plus BMP with BMP plus placebo. Moriau 1995 used antiplatelet agents concomitant with anticoagulants (VKA); all other studies used antiplatelet agents only after the initial treatment with anticoagulants was stopped or did not use initial anticoagulation (Sulfinpyrazone 1978).
There are two reports from the same author group that had very similar study aims (Indobufen 1993), specifically the design and number in each group. Since the study authors did not respond to our correspondence, we assumed that both reports show data from the same group of participants and treated both as complementary information to each other. Indobufen 1993 described treatment with antiplatelet agents for the first episode of DVT after 6 months of dicumarol to keep the prothrombin time below 30%. After the discontinuation of VKA and normalisation of coagulation blood rates, the 224 participants were randomised into four groups:
indobufen (200 mg twice/day);
graduated elastic compression stockings;
indobufen (200 mg twice/day) and graduated elastic compression stockings;
no treatment.
In our analyses, we considered the groups separately according to the use of compression stockings (subgroup analysis) and together for meta‐analysis independent of compression stocking use.
Moriau 1995 described six groups in the study without mention of compression stockings. In the acute phase of an episode of DVT classified by the group as severe (described in this study as cases when multiple risk factors were present in healthy participants, and when the three components of Virchow’s triad were present simultaneously, resulting in a formation of mixed thrombi or thrombi richer in platelets than fibrin) or recurrent DVT, 600 participants were randomised as follows.
100 without treatment (excluded from our review because this is not BMP for acute DVT)
100 with acenocoumarol (once/day to maintain prothrombin time of 20% to 30%, international normalised ratio (INR) 2.5 to 3.5, activated recalcification time 1.5 to 2 × normal and activated partial thromboplastin time test 1.5 to 2 × normal)
100 with acenocoumarol (once/day) and piracetam (4 × 2.4 g/day)
100 with acenocoumarol (once/day) and buflomedil (2 × 300 mg/day)
100 with acenocoumarol (once/day) and pentoxifylline (3 × 400 mg/day)
100 with acenocoumarol (once/day) and dipyridamole (2 × 200 mg/day)
Steele 1980 evaluated 38 participants with recurrent DVT (chronic phase with or without PE) during anticoagulation with warfarin, which was withdrawn after recurrence and before randomisation into two groups.
19 with dipyridamole (4 × 25 mg/day) and aspirin (4 × 300 mg/day) by mouth
19 with placebo (4 × a day) by mouth
The treatment was continued for 18 months or until a DVT was confirmed angiographically or an adverse effect was described. Steele 1980 did not mention the utilisation of compression stockings.
Sulfinpyrazone 1978 randomised 41 participants with idiopathic recurrent DVT into three groups:
14 with sulfinpyrazone (1 × 800 mg/day) orally;
ethylestrenol and phenformin (this group was not utilised in this review because they are not antiplatelet agents); and
14 with placebo (no details about dosage).
In the year prior to the study, all participants presented with at least one episode of recurrent DVT with a minimum of one positive venogram. Twenty of these 41 participants were anticoagulated before and during the study. Of these 20, 11 took warfarin and 9 received subcutaneous heparin, but the outcomes were not described separately. After that, 10 additional participants (all men) with recurrent venous thrombosis were studied regarding anticoagulation effects without randomisation and were therefore not included in our review. The study authors did not mention the use of compression stockings.
WARFASA described 403 participants who had their first‐ever unprovoked VTE and completed 6 to 18 months of oral anticoagulant treatment before randomisation into two groups: aspirin 100 mg daily or placebo daily for at least 2 years. The study authors did not mention the use of compression stockings. Since this primary study evaluated an intervention for VTE, they included participants with only DVT, only PE and DVT/PE. Despite the fact that the study authors described the number of participants with these specific characteristics, they did not present all outcomes separately for each index event. We contacted the study authors for clarification but did not receive an answer. Therefore, only the 252 participants for whom the outcomes could be assessed separately in the reports were included in our review.
ASPIRE randomised 822 participants with first‐episode of unprovoked VTE, after the cessation of anticoagulation therapy (duration of anticoagulation from 6 weeks to 24 months). Two groups were randomised: the first received aspirin 100 mg daily, and the second received matching placebo daily, for at least 2 years. The ASPIRE authors kindly provided us with raw data for inclusion in this review. Our group of interest (DVT with or without PE) had 583 participants and was included in this review.
Sample size
Indobufen 1993 and Steele 1980 had all participants included in our review (224 and 38, respectively). From Moriau 1995, Sulfinpyrazone 1978, WARFASA and ASPIRE, we included in our review only a portion of the original participants (500 of 600, 41 of 51, 252 of 403, and 583 of 822, respectively). WARFASA described the power calculation of 80% to detect a relative risk reduction (RRR) of 35% in favour of aspirin compared with placebo in their protocol document, but in their report the RRR was 40% with an alpha error of 5%. ASPIRE reported an 80% power to detect a 32% reduction in major vascular events, their secondary outcome described. All other included studies did not describe a power calculation in the available documents.
Length of follow‐up
Indobufen 1993 randomised the participants 7 months after the first episode of DVT with 6 months of prior anticoagulant treatment. After the initial evaluation, they were studied every 3 months after randomisation and every time they had signs or symptoms (swelling, pain, tenderness, increase in calf/ankle section, or local temperature increase) over a 3‐year period.
Moriau 1995 evaluated participants with severe and/or recurrent DVT in the acute phase, assessed every 8 days for the first month after the randomisation and monthly for the next 3 to 6 months.
Steele 1980 studied subjects with recurrent DVT despite the initial treatment with warfarin for at least 6 months. After 2 to 4 months without warfarin, and after the measurement of baseline platelet survival time normalised, the participants were randomised and followed for up to 18 months or until a venographically‐confirmed DVT or an adverse drug reaction occurred.
Sulfinpyrazone 1978 utilised data of participants with recurrent DVT confirmed by at least one positive venogram. The initial episode of DVT was from 1 year to 6 years before the beginning of the study, and the follow‐up period after randomisation was 3 months.
WARFASA analysed participants with an initial unprovoked DVT after 6 months to 18 months of oral anticoagulant treatment and randomised them in two groups: placebo and aspirin (100 mg once daily). Participants were re‐examined every 3 months during the first year after randomisation and every 6 months thereafter. The median duration of the study treatment was 24 months for the aspirin group and 23.5 months for the placebo group. Exceptional cases were described in the reports.
ASPIRE included participants with their first unprovoked DVT after their anticoagulant treatment finished (from 6 weeks to 24 months). The two groups who were randomised were analysed after 1 month and 6 months until the end of follow‐up. Between visits, they were contacted (email or telephone) quarterly. The duration of treatment had a median of 37.2 months.
Outcomes
The Indobufen 1993 study presented data on recurrent DVT, major bleeding and mortality. Since all subjects finished the study without side effects, we assumed that they did not die or experience any adverse events. There was no explanation as to what was considered major bleeding or if there was any; the trial authors only cited that "no side‐effects were recorded during the three‐year follow‐up period".
Moriau 1995 described some of our primary (major bleeding) and secondary (mortality, PTS at 6 months and adverse events) outcomes of interest. However, there was no explanation about what was considered major bleeding, and the study authors did not utilise a validated method to assess PTS. They used a subjective vision of the participant to assess PTS. The study authors also evaluated other outcomes such as clinical assessments (including embolisation) and ultrasound assessments using a non‐validated 6‐point scale. Individual components of the score were not reported separately. The study authors also studied alterations in laboratory tests, such as platelet count and aggregation tests, ß‐thromboglobulin, platelet factor 4, fibrinogen, factor VIII, fibrinopeptide A, antithrombin III, protein C, protein S, global tests of coagulation and blood and plasma viscosity. In the results, the study authors presented data regarding gastrointestinal symptoms, headache, severe and moderate bleeding that were analysed as adverse events. All participants were cited at the end of the study, so we assumed that all participants completed the follow‐up and no one died.
Steele 1980 described recurrent DVT (objectively confirmed by venography) and PE. The study authors also cited platelet survival time, which is not a relevant outcome for this review. The study authors described adverse side effects, such as peptic ulcers and epigastric pain that were analysed as our adverse event outcome. The study authors did not describe any deaths, and all participants were present in the results. Therefore, we included this study in the assessment of mortality.
Sulfinpyrazone 1978 presented data about recurrent DVT confirmed by venography, adverse events and mortality, which are relevant outcomes for this review. The study authors also reported data on platelet and fibrinogen survival time that are not relevant to our analysis. PE is an important outcome, but was not mentioned by study authors. Sulfinpyrazone 1978 described some adverse side effects, such as gastrointestinal distress (n = 1), emotional difficulties (n = 1) and lethal stroke (n = 1) that were addressed in the adverse events outcome.
WARFASA evaluated several relevant outcomes for this review (recurrence of VTE objectively confirmed, major bleeding, mortality (any cause) and adverse events). However, only data on recurrence of VTE were available in the study reports for the specific population with DVT as an index diagnosis. Despite our attempts to contact the study authors, we did not receive any additional data that could be analysed in our review. We could not analyse results about major bleeding, mortality or adverse events from WARFASA because the available study data were not reported separately for participants with DVT or PE. The study authors also reported the following outcomes: acute ischaemia of the lower limbs, unstable angina, nonfatal myocardial infarction, stroke and transient ischaemic attack, and additional necessity of antiplatelets and anticoagulant therapy that were not relevant for this review.
ASPIRE assessed many outcomes as planned in our protocol, such as recurrent VTE, major bleeding, PE fatal/non‐fatal, mortality separately in all‐cause and VTE‐related, duration of hospitalisation and adverse events (gastrointestinal (n = 26), minor bleeds (n = 6) and respiratory cause (n = 16)). This data were kindly provided by the ASPIRE team through email contact.
None of the included studies presented all outcomes of interest in this review, but all included studies reported at least one primary outcome as planned in our protocol (Flumignan 2016). Costs and financial aspects were not cited in any included study.
Excluded studies
See Characteristics of excluded studies table.
We excluded seven studies (Bick 1981; EINSTEIN CHOICE; Evans 1975; IRCT20200202046344N1; Moriau 1982; Nielsen 1994; Sidhu 2019).
We excluded two studies because the DVT diagnosis was not objectively confirmed, as described in our protocol (Bick 1981; Evans 1975). In Bick 1981, the study authors did not provide an acceptable additional confirmation for DVT diagnosis, and in Evans 1975 the diagnosis of DVT was only by clinical symptoms and signs. Moriau 1982 studied a drug (suloctidil) that was voluntarily withdrawn worldwide in 1985 by the manufacturer due to several cases of hepatitis associated with its use, some of them fatal (UN 2005). In Nielsen 1994 the antiplatelet agent group did not receive BMP with the intervention, as we prespecified in our protocol. Finally, it was not possible to utilise EINSTEIN CHOICE, IRCT20200202046344N1, and Sidhu 2019 data because there is not a comparison of interest for this review; they did not use a control group with BMP or BMP plus placebo.
Risk of bias in included studies
2.

Risk of bias graph: 'Review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: 'Review authors' judgements about each risk of bias item for each included study.
Allocation
WARFASA provided details of randomisation sequence and allocation. The study authors cited a computer‐generated randomisation sequence, with a 1:1 proportion of aspirin/placebo, and they used consecutive box numbers for allocation. Therefore, we classified WARFASA at low risk for selection bias.
We also classified ASPIRE at low risk for selection bias. In the protocol, the study authors described randomisation by a central web‐based system, with stratification by centre and by duration of oral anticoagulation therapy prior to the beginning of study treatment.
Indobufen 1993, Moriau 1995, Steele 1980 and Sulfinpyrazone 1978 did not report details about how randomisation was performed or how allocation was guaranteed. Since they only cited that they were randomised trials, we classified these four studies at ‘unclear risk’ for selection bias.
Blinding
Indobufen 1993 divided the participants into a treatment group (with oral indobufen) and a control group without oral treatment or a placebo. As the participants and personnel were not blinded, we classified it at high risk. The study authors wrote that "all participants were routinely scanned with colour Doppler ultrasound (CDS) every 3 months and every time that they had new signs or symptoms (swelling, pain, tenderness, increase in calf/ankle section, local temperature increase)". Since the participants were not blinded, the outcomes were not blind, and we judged the blinding of outcome assessors to be high risk as well.
Despite the phrase "randomised, prospective blind study", we classified Moriau 1995 at high risk of bias for blinding of participants and personnel because we deemed it almost impossible to maintain blinding of participants in a control group without a placebo, while the intervention groups had several differences in the number of tablets per day (1 to 4 doses per day). We classified detection bias at unclear risk of bias because there were no details provided.
Steele 1980 stated that "patients were randomly assigned (double‐blind)" and all tablets in the two groups were taken four times a day. Therefore, we classified this study at low risk of bias for blinding of participants and personnel. We classified the risk of detection bias of the Steele 1980 study as unclear because this was not mentioned.
We classified Sulfinpyrazone 1978 as unclear for both performance and detection bias because they did not describe any information about blinding of participants and personal or blinding of outcome assessors.
In WARFASA, the treatment regimens (antiplatelets and placebo) were similar, with one tablet once daily. The study authors utilised a central, independent adjudication committee for all suspected study outcome events "whose members were unaware of the group assignments and who reviewed the imaging results". Therefore, we judged this study at low risk for both performance and detection bias.
ASPIRE had similar treatment regimens, with one tablet once daily of aspirin or matching placebo. All suspected outcome events were assessed by an outcome committee who were unaware of the randomisation and treatment. We classified ASPIRE at low risk for performance and detection bias.
Incomplete outcome data
We classified Indobufen 1993 at high risk of attrition bias because the study authors reported that all included participants concluded the analysis, but there were dropouts in all groups with no further explanation.
We classified Moriau 1995 at high risk of attrition bias because losses and exclusions were not detailed. The outcome PE was mentioned as clinically assessed, but it was not presented in the study results.
Although the losses and exclusions were not detailed, we classified both Steele 1980 and Sulfinpyrazone 1978 at low risk of attrition bias because apparently all participants concluded the studies. The same number of participants was observed at the beginning and at the end of the study. Adverse events were not preplanned outcomes but were mentioned in the results in these trials.
We classified WARFASA at low risk of attrition bias, because losses and exclusions were detailed, and the study authors utilised an intention‐to‐treat approach. The adverse events were mentioned and analysed.
We also classified ASPIRE at low risk of attrition bias. ASPIRE described all losses/exclusions, and all participants were analysed by an intention‐to‐treat approach.
Selective reporting
We classified both Indobufen 1993 and Moriau 1995 at high risk of reporting bias because the full protocols were not available (only report information) and Moriau 1995 has not reported prespecified outcomes that are of interest to the review (e.g. PE).
The protocol of the WARFASA study was available and the details were reported. The study authors stated that "the study was performed in accordance with the protocol and with the provisions of the Declaration of Helsinki and local regulations". However, the 'historical versions' in ClinicalTrials.gov record state that the WARFASA protocol was modified for its primary and secondary outcomes prior to the study publication, putting it at high risk of selective reporting.
We classified Sulfinpyrazone 1978 at unclear risk of bias, because the full protocol is not available (only report information), and only one primary outcome that is of interest in the review (recurrent DVT) was prespecified and reported. PE was mentioned as a previous disease, but it was not in the results, possibly due to the lack of events in a short follow‐up (3 months). Bleeding was also not mentioned.
We classified Steele 1980 at low risk of bias because, even though the full protocol is not available (only report information), some primary outcomes that are of interest in the review (PE and recurrent DVT) were preplanned or reported.
ASPIRE's protocol is available in the Australian New Zealand Clinical Trials Registry and, when we assessed the history of modification, there were no great changes in the outcome measures. Because of that, we classified it at low risk of selective reporting bias.
Other potential sources of bias
We judged Indobufen 1993 at high risk of bias because we suspect duplicate publication. Two reports were published in the same year by the same authors' group citing similar data without clear explanation in the text. We contacted the authors, but we have not received additional information.
We suspect Moriau 1995 of pharmaceutical company support (two author affiliations) without clear mention in the report. Because of this, we classified it at high risk of bias.
We classified Sulfinpyrazone 1978 at high risk for several reasons. The study included one group treated with warfarin after randomisation. Moreover, the investigators used different anticoagulation treatments (warfarin or heparin) for some participants during the study, and this was not sufficiently described. There are no details about the numbers of anticoagulated participants in each group.
We classified Steele 1980, WARFASA and ASPIRE at low risk of bias because we did not identify any other reason for bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Acute DVT ‐ antiplatelet agents plus BMP compared to BMP alone for the treatment of DVT.
| Antiplatelet agents plus BMP compared to BMP alone for the treatment of acute DVT | ||||||
| Patient or population: people requiring treatment for acute DVT Setting: outpatient setting Intervention: antiplatelet agents plus BMP Comparison: BMP alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with BMP alone | Risk with antiplatelet agents plus BMP | |||||
| Recurrent VTE follow‐up: range 3 months to 6 months | Study population | Not estimable | 500 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | The study authors reported on a 6‐point scale used for clinical and ultrasonic assessment of efficacy, which included, among other outcomes, embolisation. Components of the score were not reported separately. | |
| ‐ | ||||||
| Major bleeding | Study population | Not estimable | 500 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | The study authors reported no major bleeding. | |
| ‐ | ||||||
| Pulmonary embolism follow‐up: range 3 months to 6 months | Study population | Not estimable | 500 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | The study authors reported on a 6‐point scale used for clinical and ultrasonic assessment of efficacy, which included, among other outcomes, embolisation. Components of the score were not reported separately. | |
| ‐ | ||||||
| Mortality ‐ early and intermediate | Study population | Not estimable | 500 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | The study authors reported no deaths. | |
| ‐ | ||||||
| Post‐thrombotic syndrome | Study population | RR 0.74 (0.61 to 0.91) | 500 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,d | ||
| 580 per 1000 | 151 less per 1000 (226 less to 52 less) | |||||
| Adverse events | Study population | RR 2.88 (1.06 to 7.80) | 500 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 40 per 1000 | 75 more per 1000 (2 more to 272 more) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMP: best medical practice; CI: confidence interval; DVT: deep venous thrombosis; RCT: randomised controlled trial; RR: risk ratio; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded a half level due to imprecision: fewer than 300 events were included in the analysis. bDowngraded two levels due to study limitations. Despite the study described as blinded, the study did not have a control group with placebo, and intervention groups had differences in the number of tablets per day. Losses and exclusion were not mentioned. Some of the study's prespecified outcomes (such as embolisation) that were of interest for this review have not been reported. The full protocol was not available, only information in the study report. There was suspicion of pharmaceutical company support, although not clearly mentioned in the study report. cDowngraded one level due to indirectness: there was no explanation about what was considered major bleeding. dDowngraded a half level due to indirectness: the study authors did not utilise a validated method to assess post‐thrombotic syndrome. Only subjective vision of the participant.
Summary of findings 2. Chronic DVT ‐ antiplatelet agents plus BMP compared to BMP alone for the treatment of DVT.
| Antiplatelet agents plus BMP compared to BMP alone for the treatment of chronic DVT | ||||||
| Patient or population: people requiring treatment for chronic DVT Setting: outpatient setting Intervention: antiplatelet agents plus BMP Comparison: BMP alone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with BMP alone | Risk with antiplatelet agents plus BMP | |||||
| Recurrent VTE ‐ early and intermediate | Study population | RR 0.12 (0.05 to 0.34) | 224 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | Includes recurrent DVT only | |
| 293 per 1000 | 258 less per 1000 (278 less to 193 less) | |||||
| Major bleeding | Study population | Not estimable | 224 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c,d | The study authors reported no major bleeding. | |
| ‐ | ||||||
| Pulmonary embolism follow‐up: range 1 year to 3 years | Study population | Not estimable | 224 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | The study authors reported no pulmonary embolism. | |
| ‐ | ||||||
| Mortality ‐ early and intermediate | Study population | Not estimable | 224 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | The study authors reported no deaths. | |
| ‐ | ||||||
| Post‐thrombotic syndrome | No studies measured this outcome | |||||
| Adverse events | Study population | Not estimable | 224 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | The study authors reported no adverse events. | |
| ‐ | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMP: best medical practice; CI: confidence interval; DVT: deep venous thrombosis; RCT: randomised controlled trial; RR: risk ratio; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded a half level due to imprecision: fewer than 300 events were included in the analysis. bDowngraded two levels due to study limitations: the control group did not have an oral treatment, not even placebo; participants and personnel were not blinded; authors reported that all included participants concluded the analysis but there were dropouts in all groups, without details provided; the full protocol was not available, only report information. cDowngraded one level due to other considerations: there is suspicion of duplicate publication. dDowngraded one level due to indirectness: no explanation as to what was considered major bleeding or if there was any.
Summary of findings 3. Chronic DVT ‐ antiplatelet agents plus BMP compared to BMP plus placebo for the treatment of DVT.
| Antiplatelet agents plus BMP compared to BMP plus placebo for the treatment of chronic DVT | ||||||
| Patient or population: people requiring treatment for chronic DVT Setting: outpatient setting Intervention: antiplatelet agents plus BMP Comparison: BMP plus placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with BMP plus placebo | Risk with antiplatelet agents plus BMP | |||||
| Recurrent VTE ‐ early, intermediate and long term | Study population | RR 0.65 (0.43 to 0.96) | 901 (4 RCTs) |
⊕⊕⊝⊝ Lowa,b,c | ||
| 206 per 1000 | 72 less per 1000 (118 less to 8 less) | |||||
| Major bleeding | Study population | RR 0.98 (0.29 to 3.34) | 583 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Two studies did not assess this outcome (Steele 1980; Sulfinpyrazone 1978) and for one study the data were not available for our population of interest (WARFASA). Only ASPIRE had data for the analysis. | |
| 17 per 1000 | 0 per 1000 (12 less to 41 more) | |||||
| Pulmonary embolism (fatal/non‐fatal) | Study population | RR 0.52 (0.23 to 1.14) | 583 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Two studies did not assess this outcome (Steele 1980; Sulfinpyrazone 1978) and for one study the data were not available for our population of interest (WARFASA). Only ASPIRE had data for the analysis. | |
| 59 per 1000 | 28 less per 1000 (45 less to 8 more) | |||||
| Mortality ‐ intermediate and long term | Study population | RR 0.48 (0.21 to 1.06) | 649 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | ||
| 53 per 1000 | 28 less per 1000 (42 less to 3 more) | |||||
| Post‐thrombotic syndrome | No studies measured this outcome | |||||
| Adverse events | Study population | RR 1.57 (0.34 to 7.19) | 621 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | ||
| 75 per 1000 | 43 more per 1000 (49 less to 464 more) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMP: best medical practice; CI: confidence interval; DVT: deep venous thrombosis; RCT: randomised controlled trial; RR: risk ratio; VTE: venous thromboembolism | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded a half level due to imprecision: fewer than 300 events were included in the analysis. bDowngraded a half level due to study limitations: in the WARFASA protocol, we identified a change in primary and secondary outcomes during the study. cDowngraded a half level due to study limitations: the Sulfinpyrazone 1978 study included one group treated with warfarin, after randomisation. Moreover, the investigators used different anticoagulation treatments (warfarin or heparin) for some participants, and this was not sufficiently described. There are no details of numbers of anticoagulated participants in each group.
None of the studies included in this review contained data for the comparison of ‘acute DVT ‐ antiplatelet agents plus BMP versus BMP plus placebo’.
Acute DVT ‐ antiplatelet agents plus BMP versus BMP alone
For this comparison, we had data from one study with a short follow‐up time (Moriau 1995).
Primary outcomes
Recurrent venous thromboembolism (VTE)
Moriau 1995 did not report data for this outcome. Embolisation was reported as part of a 6‐point scale used for clinical and ultrasonic assessment of efficacy. Components of the score were not reported separately.
Major bleeding
The study authors reported no major bleeding events during the follow‐up period. Therefore, we could not estimate the effect of this outcome with the available data.
Pulmonary embolism (PE) (fatal/nonfatal)
Although the study authors provided information about PE in the baseline characteristics of participants, PE was not an outcome reported in the results. Embolisation was reported as part of a 6‐point scale used for clinical and ultrasonic assessment of efficacy. Components of the score were not reported separately.
Secondary outcomes
Mortality
Since all participants were included in the final results of Moriau 1995, we concluded there were no deaths. We could not estimate the effect of this outcome as follow‐up was limited to 6 months.
Post‐thrombotic syndrome (PTS)
When all types of antiplatelet agents were evaluated together, Moriau 1995 presented data with a lower risk of PTS in the antiplatelet plus BMP group compared with the control group (risk ratio (RR) 0.74, 95% confidence interval (CI) 0.61 to 0.91; 1 study, 500 participants; very low‐certainty evidence). Moderate heterogeneity was observed among the different antiplatelet subgroups (Analysis 1.3).
We performed a subgroup analysis by type of antiplatelet agent, which presented a favourable effect for piracetam and buflomedil but not for pentoxifylline or dipyridamole (Analysis 1.4). In the piracetam (4 x 2.4 g/day) and buflomedil (2 x 300 mg/day) results, there was a difference favouring the antiplatelet plus BMP group without important heterogeneity (RR 0.57, 95% CI 0.42 to 0.79; 1 study, 250 participants; I2 = 0; very low‐certainty evidence; Analysis 1.4). However, the collective results for pentoxifylline (3 x 400 mg/day) and dipyridamole (2 x 200 mg/day), did not indicate a clear difference between the antiplatelet plus BMP and the control groups without important heterogeneity (RR 0.90, 95% CI 0.69 to 1.17; 1 study, 250 participants; I2 = 20%; very low‐certainty evidence; Analysis 1.4). Statistical tests for subgroup differences revealed a difference between the pooled antiplatelet subgroups (P = 0.03).
Adverse events
Moriau 1995 reported adverse events as a percentage interval in each group, e.g. 3% to 4% moderate bleeding (haematoma, haematuria) in each group, 5% to 10% gastrointestinal symptoms in the pentoxifylline group, and 10% to 20% headache in the dipyridamole group. For the estimation of the results in this review, we assumed the biggest value of the reported interval as the number of adverse events in each group. There was a higher risk of adverse events for the antiplatelet plus BMP group than for the control group, without important heterogeneity, when all types of antiplatelet agents were considered together (RR 2.88, 95% CI 1.06 to 7.80; 1 study, 500 participants; I2 = 0%, very low‐certainty evidence; Analysis 1.5). We performed a subgroup analysis by grouping the results by type of antiplatelet agents as we did in the PTS outcome above (Analysis 1.6). There was no evidence of a difference in the risk of adverse events between antiplatelet plus BMP and the control groups when we grouped piracetam and buflomedil together, again without important heterogeneity (RR 1.00, 95% CI 0.22 to 4.56; 1 study, 250 participants; I2 = 0%; very low‐certainty evidence). However, there was a difference in favour of the control group when compared with the combined pentoxifylline and dipyridamole groups, without important heterogeneity (RR 4.75, 95% CI 1.19 to 19.03; 1 study, 250 participants; I2 = 0%; very low‐certainty evidence). Statistical tests for subgroup differences revealed no clear difference between groups (P = 0.14).
Quality of life (QoL)
There were no available data for this outcome.
Duration of hospitalisation
There were no available data for this outcome.
Chronic DVT ‐ antiplatelet agents plus BMP versus BMP alone
One included study evaluated this comparison (Indobufen 1993).
Primary outcomes
Recurrent VTE
Indobufen 1993 did not describe PE or VTE, so data for recurrent VTE only include the reported data of recurrent DVT at 3 years of follow‐up (intermediate time). Indobufen 1993 demonstrated a lower risk of recurrent VTE in the antiplatelet agents plus BMP group (RR 0.12, 95% CI 0.05 to 0.34; 1 study, 224 participants; very low‐certainty evidence; Analysis 2.1). We also evaluated the data by subgroups with and without compression stockings (Analysis 2.1), as planned in the protocol of this review (Flumignan 2016). In the subgroup without compression stockings, the participants receiving antiplatelet agents plus BMP had a lower risk of recurrent VTE than the control group (RR 0.11, 95% CI 0.03 to 0.34; 1 study, 123 participants; very low‐certainty evidence). However, in the subgroup with compression stockings, there was no clear difference between the antiplatelet agents plus BMP and control groups (RR 0.22, 95% CI 0.03 to 1.82; 1 study, 101 participants; very low‐certainty evidence). Statistical tests for subgroup differences did not reveal a clear difference between groups (P = 0.56).
Major bleeding
We could not estimate the effect of antiplatelet agents for major bleeding because the study authors reported no side effects during the 3 years of follow‐up (zero events) (Analysis 2.2).
2.2. Analysis.

Comparison 2: Chronic DVT ‐ antiplatelet agents plus BMP versus BMP alone, Outcome 2: Major bleeding
PE (fatal/nonfatal)
Although the study authors reported no dropout for PE, the outcome was not a preplanned outcome, and it was not reported in the results. Therefore, we could not estimate the effect of the interventions with the available data.
Secondary outcomes
Mortality
We could not estimate this effect with the available data because the study authors reported zero cases of death during 3 years of follow‐up (Analysis 2.3).
2.3. Analysis.

Comparison 2: Chronic DVT ‐ antiplatelet agents plus BMP versus BMP alone, Outcome 3: Mortality
PTS
There were no available data for this outcome.
Adverse events
We could not estimate this effect with the available data (zero events) (Analysis 2.4).
2.4. Analysis.

Comparison 2: Chronic DVT ‐ antiplatelet agents plus BMP versus BMP alone, Outcome 4: Adverse events
QoL
There were no available data for this outcome.
Duration of hospitalisation
There were no available data for this outcome.
Chronic DVT ‐ antiplatelet agents plus BMP versus BMP plus placebo
Four included studies contributes data for this comparison (ASPIRE; Steele 1980; Sulfinpyrazone 1978; WARFASA).
Primary outcomes
Recurrent VTE
All four studies (Steele 1980 at 18 months, Sulfinpyrazone 1978 at 3 months, WARFASA at 24 months, and ASPIRE with a median follow‐up of 37.2 months) had available data for this outcome; meta‐analysis of this data showed a lower risk of recurrent VTE for the antiplatelet agents group compared with the control group (RR 0.65, 95%, CI 0.43 to 0.96; 4 studies, 901 participants; NNTB = 14; low‐certainty evidence; Analysis 3.1). We deemed heterogeneity for the overall effect viewed in Analysis 3.1 as not important (I2 = 19%).
When analysed by antiplatelet subgroups, the antiplatelet arms achieved a lower risk of recurrent VTE for the acetylsalicylic acid (ASA) subgroup (RR 0.71, 95% CI 0.53 to 0.97; 2 studies, 835 participants; NNTB = 18; low‐certainty evidence), but this was not seen in the sulfinpyrazone group (RR 0.14, 95% CI 0.01 to 2.53; 1 study, 28 participants; low‐certainty evidence) nor in the ASA plus dipyridamole group (RR 0.14, 95% CI 0.02 to 1.05; 1 study, 38 participants; low‐certainty evidence) (Analysis 3.1). Statistical tests for subgroup differences did not reveal a clear difference between subgroups (P = 0.17).
Major bleeding
Major bleeding was a prespecified safety outcome in WARFASA, but their data were not available for the participants with confirmed DVT separately from those with only PE. We could therefore not include the WARFASA data in the analysis. Steele 1980 and Sulfinpyrazone 1978 did not specifically report this outcome.
In ASPIRE, this outcome was prespecified and reported. Major bleeding is defined as a minimum of 2 g/dL decreased haemoglobin, intracranial and/or retroperitoneal bleeding, haemorrhagic events with death outcome, transfusion a minimum of two units of blood. These definitions were based on the International Society on Thrombosis and Haemostasis (ISTH) (Schulman 2005). The analysis showed no clear difference between BMP plus placebo and antiplatelet agents plus BMP groups (RR 0.98, 95% CI 0.29 to 3.34; 1 study, 583 participants; moderate‐certainty evidence; Analysis 3.2)
3.2. Analysis.

Comparison 3: Chronic DVT ‐ antiplatelet agents plus BMP versus BMP plus placebo, Outcome 2: Major bleeding
PE (fatal/nonfatal)
WARFASA did not provide isolated information about PE from participants with DVT or with DVT plus PE as the index event. WARFASA reported 3.2% PE events after 24.5 months of follow‐up in the subgroup of DVT as index event, but, in the study report, it is not clear if the group with DVT plus PE is included in this presented data. Because of this, we did not utilise the data in this review's meta‐analysis.
PE was not a preplanned outcome for Steele 1980, but the study authors mentioned one event of pulmonary infarction in the control group. Since the number of events in the intervention group was not mentioned, we could not use the overall data in this review. PE was cited as a previous disease before randomisation in Sulfinpyrazone 1978, but PE was not a preplanned outcome, and it was not mentioned in the results.
ASPIRE provided data about the group of interest for this review, describing the outcome as PE confirmed by computed tomography pulmonary angiography (CTPA) or ventilation/perfusion (V/Q) scan, or both. Results showed no clear difference between the treatment groups (RR 0.52, 95% CI 0.23 to 1.14; 1 study, 583 participants; moderate‐certainty evidence; Analysis 3.3).
3.3. Analysis.

Comparison 3: Chronic DVT ‐ antiplatelet agents plus BMP versus BMP plus placebo, Outcome 3: PE (fatal/nonfatal)
Secondary outcomes
Mortality
Steele 1980 reported no deaths during 18 months of follow‐up. ASPIRE presented separate subgroups (all‐cause and VTE‐related), during a median follow‐up of 37.2 months. In the all‐cause mortality group, it presented a greater number of events in the placebo group, compared with intervention group (16 events of 288 participants in the placebo group, and 8 events of 295 participants in the antiplatelet agent group). In the VTE‐related mortality group, one death occurred in the placebo group. Although WARFASA provided details about mortality in their different groups, we could not extract any relevant data of participants with the index event of DVT. Sulfinpyrazone 1978 had data regarding mortality at 3 months of follow‐up, which could be used in this comparison. The overall effect of antiplatelet agents did not differ from the placebo in the all‐cause mortality subgroup, without important heterogeneity (RR 0.48, 95% CI 0.21 to 1.06; 3 studies, 649 participants; I2 = 0%; moderate‐certainty evidence; Analysis 3.4). In the VTE‐related mortality subgroup, including data from one study (ASPIRE), we found no clear difference between antiplatelet agents plus BMP group and placebo plus BMP group (RR 0.33, 95% CI 0.01 to 7.96; 1 study, 583 participants; moderate‐certainty evidence; Analysis 3.4).
3.4. Analysis.

Comparison 3: Chronic DVT ‐ antiplatelet agents plus BMP versus BMP plus placebo, Outcome 4: Mortality
Post‐thrombotic syndrome
None of the included studies provided data for this outcome.
Adverse events
Steele 1980 reported two participants with peptic ulcers and another participant with epigastric pain in the intervention group during 18 months of follow‐up. They reported no adverse events in the control group. ASPIRE reported six participants with minor bleeds (2 in control group and 4 in the antiplatelet group), 16 participants had respiratory adverse events (8 in the control group and 8 in the antiplatelet group), and 26 participants had adverse events of the gastrointestinal tract (13 in the control group and 13 in the antiplatelet group). Sulfinpyrazone 1978 reported two adverse events in the control group, and no adverse events in the intervention group but did not describe the adverse events. WARFASA did not provide sufficient details about this data because of the limitations already described. The effect estimate of this outcome showed no clear difference between the antiplatelet agents plus BMP and the placebo plus BMP groups when all adverse events were pooled together, with moderate heterogeneity (RR 1.57, 95% CI 0.34 to 7.19; 2 studies, 621 participants; I2 = 38%; moderate‐certainty evidence; Analysis 3.5). Similarly, when each subgroup is analysed, no clear difference was shown: gastrointestinal adverse events (RR 1.58, 95% CI 0.29 to 8.49; 2 studies, 621 participants); respiratory adverse events (RR 0.98, 95% CI 0.37 to 2.57; 1 study, 583 participants); and minor bleeds (RR 1.95, 95% CI 0.36 to 10.58; 1 study, 583 participants; Analysis 3.5).
3.5. Analysis.

Comparison 3: Chronic DVT ‐ antiplatelet agents plus BMP versus BMP plus placebo, Outcome 5: Adverse events
QoL
There were no available data for this outcome.
Duration of hospitalisation
Only ASPIRE provided data about duration of hospitalisation, in days (hospitalised participants: ASA = 69, control = 89). The results showed no clear difference between the antiplatelet agents plus BMP and the placebo plus BMP groups (MD ‐2.10 days, 95% CI ‐9.29 to 5.09; 1 study, 158 participants; moderate‐certainty evidence; Analysis 3.6).
3.6. Analysis.

Comparison 3: Chronic DVT ‐ antiplatelet agents plus BMP versus BMP plus placebo, Outcome 6: Duration of hospitalisation (days)
Discussion
Summary of main results
VTE is the third most common cardiovascular disease in the world and is a growing public health problem, largely due to an ageing population (Goldhaber 2012; Tagalakis 2013). Platelets are the principal effector cells for haemostasis, coagulation and thrombosis that are unique to mammals, and are therefore deeply related to thrombus formation in DVT (Vieira‐de‐Abreu 2012). Because antiplatelet agents act in Virchow’s triad as inhibitors of clot formation, they may be a complement to the current treatment for DVT.
We identified three possible comparisons with the data of the included studies: 1) acute DVT ‐ antiplatelet agents plus BMP versus BMP alone, 2) chronic DVT ‐ antiplatelet agents plus BMP versus BMP alone, and 3) chronic DVT ‐ antiplatelet agents plus BMP versus BMP plus placebo.
Acute DVT
In a setting of acute DVT, antiplatelet agents plus BMP versus BMP alone was compared in one study (Moriau 1995). Moriau 1995 reported four of our relevant outcomes during 6 months of follow‐up (early time point; major bleeding, mortality, post‐thrombotic syndrome (PTS) and adverse events). At the early time point, there were no deaths and no cases of major bleeding reported. The participants that received antiplatelet agents seemed to have a lower risk of PTS (early time point), but the evidence was of very low certainty. The control group had a lower risk of adverse events compared to the intervention group. The results from Moriau 1995 should be viewed with caution, because the study had at least four of the seven risk of bias domains classed as high risk of bias. For instance, there was no blinding of participants and personnel, no details about loss and dropout of participants, no report about PE, and we suspected pharmaceutical company support, although not clearly mentioned in the report.
Chronic DVT
In a setting of chronic DVT where the antiplatelet agents plus BMP were compared with BMP alone, we also identified one study (Indobufen 1993). Despite the study authors reporting four of our relevant outcomes (recurrent VTE, major bleeding, mortality and adverse events), three of them (major bleeding, mortality and adverse events) did not have any events during the 3 years of follow‐up (intermediate time point). Thus, we could only report an effect estimate for recurrent VTE. The effect estimate appeared to be in favour of antiplatelet agents, but the evidence was of very low certainty. The results from Indobufen 1993 should be viewed with caution, because the study had at least four of the seven risk of bias domains classed as high risk of bias. For instance, there was no blinding of participants and personnel, no details about the loss and dropout participants, and suspicions of duplicate publication without clear mention in the report.
In a setting of chronic DVT where the antiplatelet agents plus BMP are compared with BMP plus placebo, we included four RCTs which provided information about six of our relevant outcomes: recurrent VTE, major bleeding, PE fatal/nonfatal, mortality, adverse events and duration of hospitalisation (ASPIRE; Steele 1980; Sulfinpyrazone 1978; WARFASA).
The meta‐analysis showed a lower risk of recurrent VTE for early to long‐term time points, but the evidence was of low certainty. ASPIRE provided data about major bleeding, showing no clear difference between placebo and antiplatelets (moderate‐certainty evidence). The other studies did not provide major bleeding data that could be used in this review. ASPIRE was also the only study with data for the PE fatal/nonfatal outcome showing no clear difference between placebo and antiplatelet groups, also with evidence of moderate certainty.
Two studies provided information about all‐cause mortality up to 37.2 months of follow‐up (ASPIRE; Sulfinpyrazone 1978), showing no clear difference between antiplatelet agents and placebo (moderate‐certainty evidence). VTE‐related mortality, with available data from ASPIRE, showed no clear difference between the antiplatelet agent group and placebo (moderate‐certainty evidence). Two studies described adverse events (ASPIRE; Steele 1980), with no clear difference between antiplatelet agent and placebo groups (moderate‐certainty evidence).
Duration of hospitalisation was reported by ASPIRE, without a clear difference between antiplatelet and placebo groups (moderate‐certainty evidence).
Overall completeness and applicability of evidence
This review assessed whether the addition of antiplatelet agents to BMP for the treatment of DVT could reduce recurrent VTE in a safe manner without major bleeding or PE. It also evaluated other relevant parameters such as mortality, PTS, adverse events, QoL, and time of hospitalisation.
The overall evidence was based on six studies involving 1625 eligible participants in the USA, Canada, many countries in Europe, India, Argentina, Australia and New Zealand.
We found one study for each comparison with BMP as the control group (without placebo). Moriau 1995 (500 participants) evaluated the effects of antiplatelet agents in acute DVT concomitant with the anticoagulant therapy; Indobufen 1993 (224 participants) evaluated the same comparison in a setting of chronic DVT after the interruption of initial anticoagulant therapy. The four other studies, with a total of 901 participants, that could be included in this review (38 in Steele 1980, 28 in Sulfinpyrazone 1978, 252 in WARFASA and 583 in ASPIRE) evaluated the effects of antiplatelet agents as additional intervention compared with BMP plus placebo, in a setting of chronic DVT. All included studies evaluated at least one of our primary outcomes (major bleeding in Moriau 1995; recurrent VTE and major bleeding in Indobufen 1993; recurrent VTE in Steele 1980, Sulfinpyrazone 1978 and WARFASA; and recurrent VTE, major bleeding and PE fatal/nonfatal in ASPIRE). Also, the included studies reported on at least one of our secondary outcomes (mortality, PTS and adverse events in Moriau 1995; mortality and adverse events in Indobufen 1993, Steele 1980, Sulfinpyrazone 1978 and WARFASA; and mortality (divided between VTE‐related and all‐cause), adverse events and duration of hospitalisation in ASPIRE), but there was a lack of mortality events in Moriau 1995 and Indobufen 1993 and a lack of adverse events in Indobufen 1993.
WARFASA had only a portion of data analysed in this review: study data were not stratified by participants with DVT and PE for all outcomes. Our efforts to contact the authors to obtain this data were not successful. We classified only one included study as low risk of bias (ASPIRE), and our efforts to contact the authors to obtain additional data were successful.
We did not identify studies comparing antiplatelet agents plus BMP versus BMP plus placebo for acute DVT.
The key limitations of this review are:
some of the included studies presented a high risk of bias (Indobufen 1993; Moriau 1995; Sulfinpyrazone 1978; WARFASA);
the lack of QoL data;
some data were not available. We did not have access to all of the WARFASA data of interest for this review stratified by the index event (confirmed DVT). Therefore, there were no data regarding major bleeding, PE fatal/non‐fatal, mortality, adverse events and duration of hospitalisation from the WARFASA study included in this review;
short length of follow‐up (from 3 months to 37 months);
age of included studies (from 1978 to 2012).
With the evaluation of all available data, the resulting analysis found low‐certainty evidence to support the use of antiplatelet agents for the treatment of chronic DVT in early, intermediate and long‐term follow‐up, for the reduction of recurrent VTE. However, these results should be viewed with caution, because all other evidence from this review had very low‐ to moderate‐certainty evidence for important outcomes such as major bleeding. Future high‐certainty studies may change the results of all available data.
The short length of follow‐up (from 3 months to 37 months) limited our analysis for the long‐term time point and impacted data for chronic complications of DVT, such as PTS. All included studies were conducted and published more than 10 years ago (from 1978 to 2012) and there have been some changes in what is considered BMP for the treatment of DVT episodes. For instance, recommendations for home treatment and early de‐ambulation for people with acute DVT were not present at the time of those included studies, but are present in the most recent guidelines (Kakkos 2021; Stevens 2021). In the most recent era, another important VTE risk factor, the SARS‐CoV‐2 virus, presents a global issue that was not addressed in the included studies (COVIDSurg 2022; Flumignan 2022; Santos 2022).
We intended to include the utilisation of an IVC filter, but in this review we did not find such separate data. In future versions, if we find any data utilising the IVC filter, we will consider this in a subgroup analysis.
Certainty of the evidence
We created a summary of findings table for each comparison with GRADEpro GDT 2015 software.
For the comparison of antiplatelet agents plus BMP versus BMP for the treatment of acute DVT (Table 1), we found very low‐certainty evidence for the included study (Moriau 1995). We could not assess the effect of the intervention for major bleeding and mortality because there were no events during the 6 months of follow‐up. We downgraded the certainty of the evidence by two levels because of a high risk of bias due to lack of a placebo in the control group and lack of blinding for participants and personnel. The study authors also did not report losses and exclusions and did not mention embolisation, one of the study’s prespecified outcomes that we tried to evaluate in this review. The full protocol was not available, and we suspected pharmaceutical company support, although not clearly mentioned in the report. Following GRADE recommendations we did not downgrade for inconsistency because there was only one study. However, we downgraded a half level for imprecision as there were fewer than 300 events included in the analysis. We downgraded by one level for indirectness because the study authors did not explain the criteria for major bleeding, instead only citing that "severe bleedings were absent, in every group". We downgraded a half level for indirectness because of the assessment of PTS using a non‐validated method.
Table 2 shows the certainty of evidence for the comparison of antiplatelet agents plus BMP versus BMP without placebo for the treatment of chronic DVT. We found very low‐certainty evidence for all outcomes up to 3 years of follow‐up. The single study in this comparison demonstrated a high risk of bias (which was a large contributor to the low certainty of the evidence (Indobufen 1993). We downgraded all reported outcomes by two levels in the risk of bias domain because the control group did not have an oral treatment, there was no blinding of participants and personnel during the study and the dropouts were not cited in detail. In addition, the full protocol of the study was not available. The study authors did not describe PE, which was the study’s prespecified outcome. Following GRADE recommendations we did not downgrade for inconsistency because there was only one study. We downgraded by one level because of indirectness as there was not a clear definition of major bleeding. We downgraded a half level due to imprecision as there were fewer than 300 events included in the analysis. We downgraded further as we suspected duplicate publication.
Table 3 shows the certainty of evidence for the comparison of antiplatelet agents plus BMP versus BMP plus placebo for the treatment of chronic DVT, using the data of four studies (ASPIRE; Steele 1980; Sulfinpyrazone 1978; WARFASA). We found low‐certainty evidence suggesting that the participants that received ASA and BMP as treatment had a lower risk of recurrent VTE in early, intermediate and long‐term follow‐up after randomisation. WARFASA presented, in the 'historical versions' available in ClinicalTrials.gov, changes in its primary and secondary outcomes near publication date of the study, and we classified this as high risk of selective reporting bias. Sulfinpyrazone 1978 included one group treated with warfarin, after randomisation. Moreover, the investigators used different anticoagulation treatments (warfarin or heparin) for some participants, and this was not sufficiently described. There were no details of numbers of anticoagulated participants in each group. Combined, these limitations resulted in us downgrading by one level for risk of bias. We judged PE, major bleeding events, mortality and adverse events as moderate‐certainty evidence. We downgraded the certainty of evidence a half level due to imprecision (fewer than 300 events were included in the analysis) for these outcomes.
The risk of bias differed substantially among the studies (Figure 2 and Figure 3), and it substantially affected the overall certainty of the evidence. The high risk of bias of some of the included studies was deeply correlated to a lack of information in the reporting of the study results. The absence of a prior protocol for the randomised trials and the lack of response from the study authors in order to reduce inconsistencies and queries about methodology are examples of this knowledge gap. It was also reflected by the evidence, because all results from the first two comparisons were deemed to be of very low certainty. All of the results from this very low‐certainty evidence (comparisons without a placebo control (comparisons 1 and 2)) had similar problems of methodology; for example, they did not blind participants or personnel during the studies. This very low‐certainty evidence means that we have very little confidence in the effect estimate, because the true effect is likely to be substantially different from the estimate of effect. We have to note that, it is likely that future versions of this review will not include additional RCTs in comparisons 1 and 2 that did not use a placebo control, as it is expected that new RCTs will have more adequate study designs, with more careful protocols regarding methodology. The use of rigorous methodology and a placebo control is expected in most RCTs, especially in a pharmacological intervention. The use of a placebo medication in a control group with the same features of the intervention drug is important to guarantee blinding of participants and personnel. Since a placebo control is feasible and adds great credence to the results, we hope to find only new RCTs with a placebo or other active drug control.
Potential biases in the review process
We believe that we identified and included all relevant RCTs using a search methodology that included multiple sources, without language restriction, date or status of publication. When duplicate reports of studies were identified during the selection process, we managed to utilise the best information.
We requested relevant data from study authors, but only one responded to our questions. We strictly observed the criteria described in the protocol to include or exclude participants in order to limit any kind of non‐compliance with the protocol (Flumignan 2016).
We performed double‐data extraction with the goal of reducing bias in the review process and ensuring the quality of assessment of included RCTs.
In some of the included studies, we only included a portion of the participants. For instance, we did not include the 'without treatment' group in Moriau 1982 because the lack of anticoagulant treatment is not currently acceptable BMP. For WARFASA, we could only include data for the outcome 'recurrence of VTE' (252 of 403), as this was the only outcome for which we stratified by the study population of interest for this review (participants with DVT with or without PE). The ASPIRE trial team provided us separate data for the group with DVT with or without PE, making it feasible to use their results.
In addition, for Indobufen 1993 we had suspicions of duplicate publication of the same data, although not clearly mentioned in the relevant publications. We tried to contact the study authors without success. Therefore, we analysed both reports as the same study.
We did not perform a funnel plot assessment because of the insufficient number of included studies (less than 10) in each comparison.
Agreements and disagreements with other studies or reviews
We did not find another systematic review of RCTs assessing the effects of antiplatelet agents for the treatment of DVT. There are three systematic reviews that assessed the effects of anticoagulant and antiplatelet interventions for the treatment and for the secondary prevention of VTE (Castellucci 2013; Marik 2015; Sobieraj 2015). These reviews evaluated anticoagulants and antiplatelet agents together and at different time points of the treatment, mainly for the long‐term treatment of DVT. Our review evaluated only the effects of antiplatelet agents for the treatment of DVT. The unique difference among the comparison groups in our review was the presence or absence of antiplatelet agents (Flumignan 2016). There are other essential differences in each identified systematic review that evaluated the treatment of VTE, i.e. they included participants with only PE or with unconfirmed DVT, while in our review we included only participants with confirmed DVT.
Sobieraj 2015 presented a comparison with antiplatelet agents and anticoagulants (VKA and direct oral anticoagulants (DOACs)). Their review also assessed the results from anticoagulants in comparison to antiplatelet agents or a placebo, which is a major difference from our review that did not analyse anticoagulants. Sobieraj 2015 did not include some studies that are in our review (Indobufen 1993; Moriau 1995; Steele 1980; Sulfinpyrazone 1978), and excluded 27 citations without reporting the reasons for exclusion. However, they did include two of our included studies (ASPIRE and WARFASA). Sobieraj 2015 analysed the data from ASPIRE because they evaluated VTE and not just DVT as our review did. Although one of the search strategies was presented in the appendix of the Sobieraj 2015 report, there was no mention of language restrictions in the text, and we did not have access to the protocol of the Sobieraj 2015 review or to the registration number in the PROSPERO database for clarification.
Castellucci 2013 was similar to our review because they provided data of prospective research with a registry number in a database of systematic reviews (PROSPERO), with no limits for language, data or status of publication. However, they evaluated a smaller number of databases (MEDLINE, Embase and CENTRAL) than our review. As well as antiplatelet agents, Castellucci 2013 also evaluated anticoagulants for VTE. They analysed two of our included RCTs (ASPIRE and WARFASA), but they did not evaluate four of our included studies (Indobufen 1993; Moriau 1995; Steele 1980; Sulfinpyrazone 1978).
Marik 2015 also had similarities to our review because they reported no limits for language, data or status of publication and followed a guideline proposed by the PRISMA group (Liberati 2009; Moher 2009), although there is no registration number or an available protocol for the review. Marik 2015 was somewhat more restrictive than the Castellucci 2013 and Sobieraj 2015 reviews, because Marik 2015 studied the use of aspirin and oral anticoagulants (VKA and DOACs) for the secondary prevention of VTE. Marik 2015 deliberately did not seek other types of antiplatelet agents. They were also more restrictive in their database search because they considered only MEDLINE and CENTRAL. This restrictive criteria reflects on the included studies, because they evaluated ASPIRE and WARFASA and did not evaluate four of our included studies (Indobufen 1993; Moriau 1995; Steele 1980; Sulfinpyrazone 1978).
Even though Castellucci 2013, Marik 2015 and Sobieraj 2015 evaluated the same two RCTs with antiplatelet agents in the intervention group (ASPIRE; WARFASA), those reviews presented substantial differences in their effect estimates. Castellucci 2013 and Marik 2015 reported a significant reduction in VTE risk, but Sobieraj 2015 did not report a significant decrease of recurrent VTE risk when aspirin was compared to placebo. The use of indirect statistical analysis (network meta‐analysis) and random‐effect model for all meta‐analyses may have influenced the different effect estimates. Our review, meanwhile, used a direct meta‐analysis with a random or fixed‐effect model for comparisons depending on the studies' heterogeneity (Analysis 3.1; Analysis 3.5).
Simes 2014 made a prospective compilation (INSPIRE) of two primary RCTs (ASPIRE; WARFASA), with a meta‐analysis of data but without a search strategy and without the intention to perform a systematic review. Therefore, we treated the Simes 2014 report as a narrative review with a meta‐analysis of two RCTs. Simes 2014 ascertained that aspirin may reduce the risk of recurrent VTE in participants with VTE after the conclusion of initial treatment with anticoagulants with stronger evidence than the isolated RCTs (ASPIRE; WARFASA). Simes 2014 also concluded that the antiplatelet agent used did not add any risk of bleeding until more than 24 months of follow‐up. Since Simes 2014 evaluated participants with only PE at randomisation time, we cannot directly compare their results with ours.
There are also a number of narrative reviews addressing the use of antiplatelet agents for the treatment and prevention of VTE but not solely DVT (Autar 2006; Boneu 1992; Paikin 2011; Schror 2015; Watson 2008). Autar 2006, Paikin 2011 and Watson 2008 encompassed not only VTE or DVT. For instance, they also evaluated participants with arterial thromboembolism and myeloma. Nevertheless, concerning the VTE treatment and prevention, Paikin 2011 and Watson 2008 conclude that more RCTs were needed for better decision making. We have to note that both Paikin 2011 and Watson 2008 were published in 2011 and 2008, respectively, and two more recent RCTs that we considered had not been published yet (ASPIRE; WARFASA). However, in that time, four of our included RCTs had already been published (Indobufen 1993; Moriau 1995; Steele 1980; Sulfinpyrazone 1978), but those narrative reviews did not mention these studies in their reports.
As another narrative review, Schror 2015 analysed the role of the utilisation of antiplatelet agents in primary and secondary prevention of VTE. They performed a narrative overview of Simes 2014 and, consequently, of two of our included RCTs (ASPIRE; WARFASA). The conclusions did not differ from Simes 2014. Similar to the Paikin 2011 report, we could not directly compare our analysis with Schror 2015 because they evaluated only part of our included RCT and evaluated participants with VTE and not necessarily with confirmed DVT.
Since the 1990s, there has been a hypothesis that antiplatelet agents may be beneficial for people with DVT (Boneu 1992). Our findings are in agreement with this hypothesis. Our certainty of evidence appears to suggest that antiplatelet agents could reduce the risk of recurrent VTE in the chronic phase of DVT with an equipoise between the benefits and harms, such as bleeding.
Authors' conclusions
Implications for practice.
Low‐certainty evidence (4 RCTs, 901 participants) suggests that antiplatelet agents in addition to BMP compared to BMP plus placebo for the treatment of chronic DVT, following the standard initial treatment with anticoagulants, may reduce the risk of recurrent VTE (NNTB = 14) from 3 months to 37.2 months of follow‐up. Moderate‐certainty evidence suggests there is no additional risk of major bleeding, mortality, PE or adverse events in the same setting.
It is uncertain whether antiplatelet agents in addition to BMP in chronic DVT, when compared to BMP without a placebo control, reduce the risk of recurrent VTE or result in additional risks, such as major bleeding, mortality and any adverse events (very low‐certainty evidence).
It is uncertain if antiplatelet agents in addition to BMP, when compared to BMP alone, for the treatment of acute DVT may reduce the risk of PTS but increase the risk of adverse events until 6 months of follow‐up (very low‐certainty evidence). These results should be viewed with caution because none of the primary outcomes (VTE, PE and major bleeding) had estimable data in acute DVT. In addition, PTS was evaluated only at 6 months of follow‐up and data on intermediate, and long‐term outcomes could bring about substantial differences in the results.
Therefore, following initial standard anticoagulation, use of antiplatelet agents in addition to BMP for the treatment of chronic DVT may have some benefits in reducing the risk of recurrent VTE without harm and the risk of mortality, major bleeding and adverse events. However, new high‐certainty evidence may add some important information, especially regarding outcomes without available data (QoL) or estimable effects (major bleeding, mortality, PE and adverse events).
Implications for research.
The majority of included studies in our review are small trials and this type of review has been described as important by others (Handoll 2015). However, our review has limitations such as the possibility of publication bias, the very selective inclusion criteria in the primary studies and a very small number of less frequent events (e.g. PE, mortality and adverse events). Future trials need to be large enough to detect significant clinical outcomes, include all main clinical outcomes (recurrent VTE, PE and major bleeding), report the outcomes separately for different populations (e.g. with confirmed DVT and with only PE) and should ideally last more than 4 years to estimate the long‐term effects of antiplatelet agents. This minimum follow‐up time of 4 years is arbitrary, but it would produce additional data about rare occurrences, such as adverse events following antiplatelet agents, and could highlight the effects on chronic morbidity, such as PTS and QoL. Future trials on VTE should include participants with acute and chronic DVT and PE. Investigators should also provide the outcomes by the index event (DVT and/or PE) and not only for the general population of VTE. Continuous data need to be uniform, using similar scales/scores (especially for PTS and QoL evaluations), or transformed into dichotomous data when at all possible. The control groups should use a placebo in the same posology of the intervention group to avoid the lack of blinding. Since placebo control is feasible and it improves the methodological quality of the study, in the future we hope to find only new RCTs with placebo or other active drug control.
Also, it might be useful to differentiate the effects of antiplatelet agents on younger and older participants, the specific thrombosis level, and whether the DVT was provoked or unprovoked. The use of an IVC filter is also a potential confounder and should be reported. A Cochrane Review assessed DOACs for DVT treatment by demonstrating reduced bleeding when compared to warfarin (Robertson 2015). Therefore, future RCTs should also be subgrouped by anticoagulant type for better interpretation of the results.
History
Protocol first published: Issue 9, 2016
Notes
Parts of the Methods section of this review are based on a standard template established by Cochrane Vascular.
Acknowledgements
We wish to thank Cochrane Vascular, Cochrane Brazil and the Division of Vascular and Endovascular Surgery of Universidade Federal de São Paulo, Brazil for their support.
We wish to thank the ASPIRE group for the raw data provided.
We are grateful to the following peer reviewers for their time and comments: Dr Ehab A Eltahawy (Division of Cardiovascular Medicine, University of Toledo, USA), Awah Cletus Fobuzi, Cathryn Marshal and Peter Cole, and also to the two peer reviewers who wish to remain anonymous.
Appendices
Appendix 1. Glossary of terms
| Term | Definition |
| Anticoagulants | Drugs that suppress, delay or prevent blood from clotting |
| Antiplatelet agents | Drugs that prevent blood clots by inhibiting platelet function |
| Atherosclerosis | A disease characterised by a build up of abnormal fat, cholesterol and platelet deposits on the inner wall of the arteries. This narrows the vessels, reducing blood flow |
| Best medical practice (BMP) | The treatment of a disease with the best available interventions |
| Claudication | A pain or ache in the legs (usually the calves) brought on by walking or exercise, and relieved by rest |
| Compression stockings | Elastic stockings used to compress the limbs and reduce oedema |
| Deep venous thrombosis (DVT) | A blood clot in the veins of the leg |
| Duplex ultrasound | Non‐invasive evaluation of blood flow through the arteries and veins by ultrasound devices |
| Eczema | A general term describing cutaneous inflammation |
| Embolisation | Complete obstruction of a vessel (artery or vein) by material that was not formed at the site of obstruction, but came via the circulatory system |
| Erythaema | When the affected skin becomes redder than expected |
| Heparin | A particular drug used to prevent blood clotting (anticoagulant, blood thinner) |
| Low‐molecular‐weight heparin | A class of drugs used to prevent blood clotting (anticoagulant) |
| Oedema | Excess watery fluid that collects in tissues of the body, causing swelling when fluid leaks out of the body's vessels |
| Placebo | Substance or treatment with no active effect, like a sugar pill |
| Post‐trombotic syndrome | A complication of DVT resulting from damage to the vein caused by a blood clot |
| Pulmonary embolism (PE) | When a blood clot reaches the lungs and blocks the circulation of blood, causing serious breathing difficulties, and in some cases, death. The clot originates in a leg vein (e.g. DVT) and travels to the lung |
| Randomised controlled trial (RCT) | A study in which the participants are divided randomly into separate groups to compare different treatments |
| Thrombosis | Formation of a blood clot within a vessel |
| Ulcer | Open lesion, with loss of substance, in cutaneous or mucosal tissue, causing disintegration and necrosis |
| Unfractioned heparin (UFH) | A mixture of heparins obtained from animals and used to prevent and treat clotting disorders |
| Vascular | Relating to blood vessels (arteries and veins) |
| Vena cava | The largest human vein that returns blood to the heart after it has passed around the body |
| Venous | Relating to a vein |
| Venous thromboembolism (VTE) | A disease involving a blood clot that forms in a vein (DVT) and may migrate to another location (e.g. in the lung it is a PE) |
| Virchow's triad | Three factors that contribute to thrombosis: 1. changes in the vessel wall; 2. changes in the pattern of blood flow; and 3. changes in the blood constituents (hypercoagulability) |
Appendix 2. CENTRAL search strategy March 2017
| Search run on Tue Mar 28 2017 | ||
| #1 | MESH DESCRIPTOR Thrombosis | 1267 |
| #2 | MESH DESCRIPTOR Thromboembolism | 921 |
| #3 | MESH DESCRIPTOR Venous Thromboembolism | 258 |
| #4 | MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES | 2041 |
| #5 | (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*):TI,AB,KY | 18996 |
| #6 | MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES | 748 |
| #7 | (PE or DVT or VTE):TI,AB,KY | 4988 |
| #8 | ((vein* or ven*) near thromb*):TI,AB,KY | 6717 |
| #9 | (blood near3 clot*):TI,AB,KY | 2966 |
| #10 | (pulmonary near3 clot*):TI,AB,KY | 5 |
| #11 | (lung near3 clot*):TI,AB,KY | 4 |
| #12 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 | 24636 |
| #13 | MESH DESCRIPTOR Platelet Aggregation Inhibitors EXPLODE ALL TREES | 8563 |
| #14 | MESH DESCRIPTOR Phosphodiesterase Inhibitors EXPLODE ALL TREES | 5590 |
| #15 | MESH DESCRIPTOR Tetrazoles | 1857 |
| #16 | (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*):TI,AB,KY | 3484 |
| #17 | (((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) near3 (antagonist or inhibitor))):TI,AB,KY | 2436 |
| #18 | ((gp* or glycoprotein* or protease or P2Y12 or TXA2) near3 inhibit*):TI,AB,KY | 3327 |
| #19 | thienopyridine:TI,AB,KY | 284 |
| #20 | (ticlopidine or Ticlid):TI,AB,KY | 1817 |
| #21 | (clopidogrel or Plavix):TI,AB,KY | 3217 |
| #22 | (Prasugrel or Effient or Efient or Prasita):TI,AB,KY | 561 |
| #23 | (ticagrelor or AZD6140 or Brilinta):TI,AB,KY | 521 |
| #24 | (elinogrel or PRT060128 or PRT‐060128):TI,AB,KY | 8 |
| #25 | (cangrelor or AR‐C6993* or ARC6993*):TI,AB,KY | 55 |
| #26 | (SCH530348 or SCH‐530348):TI,AB,KY | 21 |
| #27 | E5555:TI,AB,KY | 6 |
| #28 | (terutroban or Triplion):TI,AB,KY | 14 |
| #29 | (aspirin* or nitroaspirin or ASA):TI,AB,KY | 17514 |
| #30 | (acetylsalicylic acid):TI,AB,KY | 5169 |
| #31 | (acetyl salicylic acid*):TI,AB,KY | 114 |
| #32 | (triflusal or disgren):TI,AB,KY | 99 |
| #33 | (Cilostazol or Pletal or Pletaal):TI,AB,KY | 481 |
| #34 | (dipyridamol* or Persantine):TI,AB,KY | 1136 |
| #35 | (OPC‐13013 or OPC13013):TI,AB,KY | 5 |
| #36 | (picotamide or picotinamide):TI,AB,KY | 41 |
| #37 | satigrel:TI,AB,KY | 3 |
| #38 | vorapaxar:TI,AB,KY | 90 |
| #39 | indobufen:TI,AB,KY | 82 |
| #40 | abciximab.TI,AB,KY | 0 |
| #41 | tirofiban:TI,AB,KY | 406 |
| #42 | #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR#40 OR #41 | 35922 |
| #43 | #12 AND #42 | 3726 |
Appendix 3. Sources searched and search strategies December 2018 onwards
| Source | Search strategy | Hits retrieved |
| VASCULAR REGISTER IN CRSW (Date of most recent search: 7 December 2021) |
#1 DVT AND INREGISTER AND 01/01/2017_TO_19/12/2018:CRSINCENTRAL #2 deep vein thrombosis AND INREGISTER AND 01/01/2017_TO_19/12/2018:CRSINCENTRAL #3 #1 OR #12 #4 antiplatelet OR aspirin AND INREGISTER AND 01/01/2017_TO_19/12/2018:CRSINCENTRAL #5 #13 AND #4 |
Dec 2018: 5 Dec 2021: 21 |
| CENTRAL via CRSO (Date of most recent search: 7 December 2021) |
#1 MESH DESCRIPTOR Thrombosis 1640 #2 MESH DESCRIPTOR Thromboembolism 1139 #3 MESH DESCRIPTOR Venous Thromboembolism 473 #4 MESH DESCRIPTOR Venous Thrombosis EXPLODE ALL TREES 2403 #5 thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* 26461 #6 MESH DESCRIPTOR Pulmonary Embolism EXPLODE ALL TREES 886 #7 (PE or DVT or VTE):TI,AB,KY 6517 #8 ((vein* or ven*) near thromb*):TI,AB,KY 8177 #9 (blood near3 clot*):TI,AB,KY 4047 #10 (lung near3 clot*):TI,AB,KY 7 #11 (pulmonary near3 clot*):TI,AB,KY 9 #12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 33538 #13 MESH DESCRIPTOR Platelet Aggregation Inhibitors EXPLODE ALL TREES 10011 #14 MESH DESCRIPTOR Phosphodiesterase Inhibitors EXPLODE ALL TREES 6569 #15 MESH DESCRIPTOR Tetrazoles 1962 #16 (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*):TI,AB,KY 4672 #17 ((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) near3 (antagonist or inhibitor)):TI,AB,KY 2786 #18 ((gp* or glycoprotein* or protease or P2Y12 or TXA2) near3 inhibit*):TI,AB,KY 3914 #19 thienopyridine:TI,AB,KY 340 #20 (ticlopidine or Ticlid):TI,AB,KY 2256 #21 (clopidogrel or Plavix):TI,AB,KY 4284 #22 (Prasugrel or Effient or Efient or Prasita):TI,AB,KY 836 #23 (ticagrelor or AZD6140 or Brilinta):TI,AB,KY 1061 #24 (elinogrel or PRT060128 or PRT‐060128):TI,AB,KY 8 #25 (cangrelor or AR‐C6993* or ARC6993*):TI,AB,KY 89 #26 (SCH530348 or SCH‐530348):TI,AB,KY 20 #27 E5555:TI,AB,KY 9 #28 (terutroban or Triplion):TI,AB,KY 19 #29 (aspirin* or nitroaspirin or ASA):TI,AB,KY 20443 #30 (acetylsalicylic acid):TI,AB,KY 6269 #31 (acetyl salicylic acid*):TI,AB,KY 126 #32 (triflusal or disgren):TI,AB,KY 107 #33 (Cilostazol or Pletal or Pletaal):TI,AB,KY 626 #34 (dipyridamol* or Persantine):TI,AB,KY 1236 #35 (OPC‐13013 or OPC13013):TI,AB,KY 5 #36 (picotamide or picotinamide):TI,AB,KY 41 #37 satigrel:TI,AB,KY 3 #38 vorapaxar:TI,AB,KY 115 #39 indobufen:TI,AB,KY 82 #40 abciximab:TI,AB,KY 739 #41 tirofiban:TI,AB,KY 454 #42 #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 26746 #43 OR#40 OR #41 454 #44 #12 AND #42 4060 |
Dec 2018: 1024 Dec 2021: 1613 |
| ClinicalTrials.gov (Date of most recent search: 7 December 2021) |
antiplatelet OR aspirin | deep vein thrombosis | Dec 2018: 3 Dec 2021: 2 |
| ICTRP Search Portal (Date of most recent search: 7 December 2021) |
antiplatelet OR aspirin | deep vein thrombosis | Dec 2018: 5 Dec 2021: 9 |
| MEDLINE (Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE) 1946 to present (Date of most recent search: 7 December 2021) |
1 THROMBOSIS/ 2 THROMBOEMBOLISM/ 3 Venous Thromboembolism/ 4 exp Venous Thrombosis/ 5 (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*).ti,ab. 6 exp Pulmonary Embolism/ 7 (PE or DVT or VTE).ti,ab. 8 ((vein* or ven*) adj thromb*).ti,ab. 9 (blood adj3 clot*).ti,ab. 10 (pulmonary adj3 clot*).ti,ab. 11 (lung adj3 clot*).ti,ab. 12 or/1‐11 13 exp Platelet Aggregation Inhibitors/ 14 exp Phosphodiesterase Inhibitors/ 15 exp TETRAZOLES/ 16 (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*).ti,ab. 17 ((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) adj3 (antagonist or inhibitor)).ti,ab. 18 ((gp* or glycoprotein* or protease or P2Y12 or TXA2) adj3 inhibit*).ti,ab. 19 thienopyridine.ti,ab. 20 (ticlopidine or Ticlid).ti,ab. 21 (clopidogrel or Plavix).ti,ab. 22 (Prasugrel or Effient or Efient or Prasita).ti,ab. 23 (ticagrelor or AZD6140 or Brilinta).ti,ab. 24 (elinogrel or PRT060128 or PRT‐060128).ti,ab. 25 (cangrelor or AR‐C6993* or ARC6993*).ti,ab. 26 (SCH530348 or SCH‐530348).ti,ab. 27 E5555.ti,ab. 28 (terutroban or Triplion).ti,ab. 29 (aspirin* or nitroaspirin or ASA).ti,ab. 30 acetylsalicylic acid.ti,ab. 31 acetyl salicylic acid*.ti,ab. 32 (triflusal or disgren).ti,ab. 33 (Cilostazol or Pletal or Pletaal).ti,ab. 34 (dipyridamol* or Persantine).ti,ab. 35 (OPC‐13013 or OPC13013).ti,ab. 36 (picotamide or picotinamide).ti,ab. 37 satigrel.ti,ab. 38 vorapaxar.ti,ab. 39 indobufen.ti,ab. 40 abciximab.ti,ab. 41 tirofiban.ti,ab. 42 or/13‐41 43 12 and 42 44 randomized controlled trial.pt. 45 controlled clinical trial.pt. 46 randomized.ab. 47 placebo.ab. 48 drug therapy.fs. 49 randomly.ab. 50 trial.ab. 51 groups.ab. 52 or/44‐51 53 exp animals/ not humans.sh. 54 52 not 53 55 43 and 54 |
Dec 2018: 1157 Dec 2021: 2549 |
| Embase via Ovid (Date of most recent search: 7 December 2021) |
1 thrombosis/ 2 thromboembolism/ 3 venous thromboembolism/ 4 exp vein thrombosis/ 5 (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*).ti,ab. 6 exp lung embolism/ 7 (PE or DVT or VTE).ti,ab. 8 ((vein* or ven*) adj thromb*).ti,ab. 9 (blood adj3 clot*).ti,ab. 10 (pulmonary adj3 clot*).ti,ab. 11 (lung adj3 clot*).ti,ab. 12 or/1‐11 13 exp antithrombocytic agent/ 14 exp phosphodiesterase inhibitor/ 15 tetrazole derivative/ 16 (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*).ti,ab. 17 ((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) adj3 (antagonist or inhibitor)).ti,ab. 18 ((gp* or glycoprotein* or protease or P2Y12 or TXA2) adj3 inhibit*).ti,ab. 19 thienopyridine.ti,ab. 20 (ticlopidine or Ticlid).ti,ab. 21 (clopidogrel or Plavix).ti,ab. 22 (Prasugrel or Effient or Efient or Prasita).ti,ab. 23 (ticagrelor or AZD6140 or Brilinta).ti,ab. 24 (elinogrel or PRT060128 or PRT‐060128).ti,ab. 25 (cangrelor or AR‐C6993* or ARC6993*).ti,ab. 26 (SCH530348 or SCH‐530348).ti,ab. 27 E5555.ti,ab. 28 (terutroban or Triplion).ti,ab. 29 (aspirin* or nitroaspirin or ASA).ti,ab. 30 acetylsalicylic acid.ti,ab. 31 acetyl salicylic acid*.ti,ab. 32 (triflusal or disgren).ti,ab. 33 (Cilostazol or Pletal or Pletaal).ti,ab. 34 (dipyridamol* or Persantine).ti,ab. 35 (OPC‐13013 or OPC13013).ti,ab. 36 (picotamide or picotinamide).ti,ab. 37 satigrel.ti,ab. 38 vorapaxar.ti,ab. 39 indobufen.ti,ab. 40 abciximab.ti,ab. 41 tirofiban.ti,ab. 42 or/13‐41 43 12 and 42 44 randomized controlled trial/ 45 controlled clinical trial/ 46 random$.ti,ab. 47 randomization/ 48 intermethod comparison/ 49 placebo.ti,ab. 50 (compare or compared or comparison).ti. 51 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 52 (open adj label).ti,ab. 53 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 54 double blind procedure/ 55 parallel group$1.ti,ab. 56 (crossover or cross over).ti,ab. 57 ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 58 (assigned or allocated).ti,ab. 59 (controlled adj7 (study or design or trial)).ti,ab. 60 (volunteer or volunteers).ti,ab. 61 trial.ti. 62 or/44‐61 63 43 and 62 |
Dec 2018: 3148 Dec 2021: 5234 |
| CINAHL via Ebsco (Date of most recent search: 7 December 2021) |
S55 S42 AND S54 S54 S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 S53 MH "Random Assignment" S52 MH "Single‐Blind Studies" or MH "Double‐Blind Studies" or MH "Triple‐Blind Studies" S51 MH "Crossover Design" S50 MH "Factorial Design" S49 MH "Placebos" S48 MH "Clinical Trials" S47 TX "multi‐centre study" OR "multi‐center study" OR "multicentre study" OR "multicenter study" OR "multi‐site study" S46 TX crossover OR "cross‐over" S45 AB placebo* S44 TX random* S43 TX "latin square" S42 S12 AND S41 S41 S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 S40 TX tirofiban S39 TX abciximab S38 TX indobufen S37 TX vorapaxar S36 TX satigrel S35 TX picotamide or picotinamide S34 TX OPC‐13013 or OPC13013 S33 TX dipyridamol* or Persantine S32 TX Cilostazol or Pletal or Pletaal S31 TX triflusal or disgren S30 TX acetyl salicylic acid* S29 TX acetylsalicylic acid S28 TX aspirin* or nitroaspirin or ASA S27 TX terutroban or Triplion S26 TX E5555 S25 TX SCH530348 or SCH‐530348 S24 TX cangrelor or AR‐C6993* or ARC6993* S23 TX elinogrel or PRT060128 or PRT‐060128 S22 TX ticagrelor or AZD6140 or Brilinta S21 TX Prasugrel or Effient or Efient or Prasita S20 TX clopidogrel or Plavix S19 TX ticlopidine or Ticlid S18 TX thienopyridine S17 TX (gp* or glycoprotein* or protease or P2Y12 or TXA2) n3 inhibit*) S16 TX (platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) n3 (antagonist or inhibitor) S15 TX antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg* S14 MH "Phosphodiesterase Inhibitors+" S13 MH "Platelet Aggregation Inhibitors+" S12 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 S11 TX lung n3 clot* S10 TX pulmonary n3 clot* S9 TX blood n3 clot* S8 TX ((vein* or ven*) n thromb*) S7 TX PE or DVT or VTE S6 MH "Pulmonary Embolism" S5 TX thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol* S4 MH "Venous Thrombosis+" S3 MH "Venous Thromboembolism" S2 MH "Thromboembolism" S1 MH "Thrombosis" |
Dec 2018: 197 Dec 2021: 413 |
| AMED via Ovid (Date of most recent search: 7 December 2021) |
1 thrombosis/ 2 thromboembolism/ 3 venous thromboembolism/ 4 exp vein thrombosis/ 5 (thrombus* or thrombopro* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*).ti,ab. 6 exp lung embolism/ 7 (PE or DVT or VTE).ti,ab. 8 ((vein* or ven*) adj thromb*).ti,ab. 9 (blood adj3 clot*).ti,ab. 10 (pulmonary adj3 clot*).ti,ab. 11 (lung adj3 clot*).ti,ab. 12 or/1‐11 13 (antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*).ti,ab. 14 ((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) adj3 (antagonist or inhibitor)).ti,ab. 15 ((gp* or glycoprotein* or protease or P2Y12 or TXA2) adj3 inhibit*).ti,ab. 16 thienopyridine.ti,ab. 17 (ticlopidine or Ticlid).ti,ab. 18 (clopidogrel or Plavix).ti,ab. 19 (Prasugrel or Effient or Efient or Prasita).ti,ab. 20 (ticagrelor or AZD6140 or Brilinta).ti,ab. 21 (elinogrel or PRT060128 or PRT‐060128).ti,ab. 22 (cangrelor or AR‐C6993* or ARC6993*).ti,ab. 23 (SCH530348 or SCH‐530348).ti,ab. 24 E5555.ti,ab. 25 (terutroban or Triplion).ti,ab. 26 (aspirin* or nitroaspirin or ASA).ti,ab. 27 acetylsalicylic acid.ti,ab. 28 acetyl salicylic acid*.ti,ab. 29 (triflusal or disgren).ti,ab. 30 (Cilostazol or Pletal or Pletaal).ti,ab. 31 (dipyridamol* or Persantine).ti,ab. 32 (OPC‐13013 or OPC13013).ti,ab. 33 (picotamide or picotinamide).ti,ab. 34 satigrel.ti,ab. 35 vorapaxar.ti,ab. 36 indobufen.ti,ab. 37 abciximab.ti,ab. 38 tirofiban.ti,ab. 39 or/13‐38 40 12 and 39 41 exp CLINICAL TRIALS/ 42 RANDOM ALLOCATION/ 43 DOUBLE BLIND METHOD/ 44 Clinical trial.pt. 45 (clinic* adj trial*).tw. 46 ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).tw. 47 PLACEBOS/ 48 placebo*.tw. 49 random*.tw. 50 PROSPECTIVE STUDIES/ 51 or/41‐50 52 40 and 51 |
Dec 2018: 0 Dec 2021: 2 |
| TOTAL before deduplication | Dec 2018: 5539 Dec 2021: 9843 |
|
| TOTAL after deduplication | Dec 2018: 4353 Dec 2021: 7864 |
Appendix 4. Trial registries searches March 2017
ClinicalTrials.gov
7 studies found for: antiplatelet AND deep vein thrombosis
20 studies found for: aspirin AND deep vein thrombosis
World Health Organization International Clinical Trials Registry
25 records for 12 trials found for: aspirin AND deep vein thrombosis
1 record for antiplatelet AND deep vein thrombosis
ISRCTN Register
12 results aspirin AND deep vein thrombosis
7 results antiplatelet AND deep vein thrombosis
Appendix 5. LILACS and IBECS search strategy December 2018 onwards
| Source | Search strategy | Hits retrieved |
| LILACS and IBECS via Virtual Health Library | ((MH:(Platelet Aggregation Inhibitors) OR MH:(Inhibidores de Agregación Plaquetaria) OR MH:(Inibidores da Agregação de Plaquetas) OR (Antiagregadores de Plaquetas) OR (Agentes Antiplaquetas) OR (Antagonistas de Plaquetas) OR (Antiagregantes de Plaquetas) OR MH:(Phosphodiesterase Inhibitors) OR MH:(Inhibidores de Fosfodiesterasa) OR MH:(Inibidores de Fosfodiesterase) OR MH:(Antagonistas da Fosfodiesterase) OR MH:(Inibidores de Fosfodiester Hidrolase) OR MH:Tetrazoles OR MH:Tetrazóis OR MH:Thienopyridines OR MH:Tienopiridinas OR MH:D02.886.778.823 OR MH:D03.383.725.849 OR MH:D03.383.903.830 OR MH:D03.438.928 OR MH:Ticlopidine OR MH:Ticlopidina OR MH:D02.886.778.823.500 OR MH:D03.383.725.849.500 OR MH:D03.383.903.830.500 OR MH:D03.438.928.500 OR ticlid OR Plavasc OR Plaketar OR Ticlobal OR Desagreg OR clopidogrel OR Plavix OR Plaq OR Lopigrel OR Iscover OR Prasugrel OR Effient OR Efient OR Prasita OR ticagrelor OR AZD6140 OR Brilinta OR elinogrel OR PRT060128 OR PRT‐060128 OR cangrelor OR AR‐C6993$ OR ARC6993$ OR SCH530348 OR SCH‐530348 OR E5555 OR terutroban OR Triplion OR aspirin$ OR nitroaspirin OR ASA OR AAS OR acetil OR alidor OR aspisin OR buferin OR caas OR ecasil OR ronal OR somalgin OR (acetylsalicylic acid) OR (acetyl salicylic acid$) OR (ácido acetilsalicílico) OR triflusal OR disgren OR Cilostazol OR Pletal OR Pletaal OR vasogard OR vasativ OR elmiron OR claudic OR cebralat OR dipyridamol$ OR Persantine OR dipiridamol OR OPC‐13013 OR OPC13013 OR picotamid$ OR picotinamide OR satigrel OR vorapaxar OR indobufen$ OR antiplatelet$ OR anti‐platelet$ OR antiaggreg$ or anti‐aggreg$ OR ((platelet OR thromboxane OR thrombocyte OR cyclooxygenase OR cyclo‐oxygenase OR phosphodiesterase OR fibrinogen OR PAR‐1) AND (antagonist OR inhibitor)) OR ((gp$ OR glycoprotein$ OR protease OR P2Y12 OR TXA2) AND (inhibit$))) AND (MH:Thrombosis OR MH:Trombosis OR MH:Trombose OR Trombo OR MH:(Embolism and Thrombosis) OR MH:(Embolia y Trombosis) OR MH:(Embolia e Trombose) OR (Trombose e Embolia) OR MH:(Venous Thrombosis) OR MH:(Trombosis de la Vena) OR MH:(Trombose Venosa) OR Flebotrombose OR (Trombose de Veias Profundas) OR (Trombose de Veia Profunda) OR (Trombose Venosa Profunda))) AND (DB:("IBECS" OR "LILACS")) | Dec 2018: 10 Dec 2021: 0 |
Appendix 6. Specialised Register search March 2017
41 records for:
#1 DVT
#2 deep vein thrombosis
#3 #1 OR #2
#4 antiplatelet OR aspirin
#5 #3 AND #4
Data and analyses
Comparison 1. Acute DVT ‐ antiplatelet agents plus BMP versus BMP alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Major bleeding | 1 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.2 Mortality | 1 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.3 Post‐thrombotic syndrome | 1 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.61, 0.91] |
| 1.3.1 piracetam ‐ 4 x 2.4 g/day | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.92] |
| 1.3.2 buflomedil ‐ 2 x 300 mg/day | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.35, 0.87] |
| 1.3.3 pentoxifylline ‐ 3 x 400 mg/day | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.52, 1.13] |
| 1.3.4 dipyridamole ‐ 2 x 200 mg/day | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.72, 1.47] |
| 1.4 Post‐thrombotic syndrome | 1 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.61, 0.91] |
| 1.4.1 piracetam‐buflomedil | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.42, 0.79] |
| 1.4.2 pentoxifylline‐dipyridamole | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.69, 1.17] |
| 1.5 Adverse events | 1 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [1.06, 7.80] |
| 1.5.1 piracetam ‐ 4 x 2.4 g/day | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.12, 8.56] |
| 1.5.2 buflomedil ‐ 2 x 300 mg/day | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.12, 8.56] |
| 1.5.3 pentoxifylline ‐ 3 x 400 mg/day | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.50 [0.48, 25.37] |
| 1.5.4 dipyridamole ‐ 2 x 200 mg/day | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.00 [0.85, 42.25] |
| 1.6 Adverse events | 1 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [1.06, 7.80] |
| 1.6.1 piracetam‐buflomedil | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.22, 4.56] |
| 1.6.2 pentoxifylline‐dipyridamole | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.75 [1.19, 19.03] |
1.1. Analysis.

Comparison 1: Acute DVT ‐ antiplatelet agents plus BMP versus BMP alone, Outcome 1: Major bleeding
1.2. Analysis.

Comparison 1: Acute DVT ‐ antiplatelet agents plus BMP versus BMP alone, Outcome 2: Mortality
Comparison 2. Chronic DVT ‐ antiplatelet agents plus BMP versus BMP alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Recurrent VTE | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.05, 0.34] |
| 2.1.1 without compression stockings | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.34] |
| 2.1.2 with compression stockings | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.03, 1.82] |
| 2.2 Major bleeding | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.3 Mortality | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.4 Adverse events | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 3. Chronic DVT ‐ antiplatelet agents plus BMP versus BMP plus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Recurrent VTE | 4 | 901 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.43, 0.96] |
| 3.1.1 ASA | 2 | 835 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.53, 0.97] |
| 3.1.2 sulfinpyrazone | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.53] |
| 3.1.3 ASA plus dipyridamole | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.05] |
| 3.2 Major bleeding | 1 | 583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.29, 3.34] |
| 3.3 PE (fatal/nonfatal) | 1 | 583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.23, 1.14] |
| 3.4 Mortality | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.4.1 all‐cause | 3 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.21, 1.06] |
| 3.4.2 VTE‐related | 1 | 583 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.96] |
| 3.5 Adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.5.1 gastrointestinal adverse events | 2 | 621 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.29, 8.49] |
| 3.5.2 respiratory adverse events | 1 | 583 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.37, 2.57] |
| 3.5.3 minor bleeds | 1 | 583 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.36, 10.58] |
| 3.5.4 total adverse events | 2 | 621 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.34, 7.19] |
| 3.6 Duration of hospitalisation (days) | 1 | 158 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐9.29, 5.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ASPIRE.
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled trial Exclusions after randomisation: 12 (6 in placebo and 6 in aspirin group) who were enrolled after a diagnosis of the first unprovoked proximal DVT and who were included in the analysis were subsequently found to be ineligible after a review of the records. 12 (10 in placebo and 2 in aspirin) revoked consent Intention‐to‐treat approach mentioned Losses to follow‐up: 6 (4 in placebo and 2 in aspirin group) Study duration: May 2003 to August 2011 Duration of follow‐up: 12 to 48 months |
|
| Participants |
Country: Argentina, Australia, Canada, India and New Zealand Participants: 822 included and analysed (treatment: 411; control: 411) at 56 sites in five countries. Our participant group of interest (DVT with or without PE) had 583 participants. Age: mean 54 ± 15.8 years in the placebo group and 55 ± 16 years in the aspirin group Sex: male 447 (54%); female 375 (46%) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Antiplatelet agents:
Control:
Co‐treatment:
DVT phase:
|
|
| Outcomes |
Primary
Secondary
Safety
Other
|
|
| Notes | Aspirin and placebo were provided by Bayer Healthcare Pharmaceuticals; ‘the company played no other role in the study and was not involved in the collection or analysis of the data or in the preparation of the manuscript’. Other economic information was not available. Additional data provided by trialists (personal communication) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was by a web‐based randomisation system. |
| Allocation concealment (selection bias) | Low risk | Quote: 'After receipt of appropriate baseline data, the patient will be randomised and the completed Randomisation Form will be sent to the central coordinating centre.' Quote: 'Each patient will be provided with a trial card/file containing contact details for their centre/other centres, information to show to their usual doctors and a copy of their baseline scan report.' Comment: information from study protocol document |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Aspirin and placebo were matched, and both were provided by Bayer Healthcare Pharmaceuticals. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: 'All episodes of venous thromboembolism, myocardial infarction, and stroke and the causes of death were adjudicated by an independent outcome assessment committee whose members were unaware of the group assignments'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: 'Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. Aspirin: 6 no qualifying DVT, 2 revoked consent and 2 lost to follow‐up (401/411 = 97% completed); Placebo: 6 no qualifying DVT, 10 revoked consent and 4 lost to follow‐up (391/411 = 95% completed). All 822 participants were analysed by intention‐to‐treat approach'. Comment: data from appendix figure 1 of the study report. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. |
| Other bias | Low risk | Aspirin and placebo were provided by Bayer Healthcare Pharmaceuticals. Quote: 'the company played no other role in the study and was not involved in the collection or analysis of the data or in the preparation of the manuscript' |
Indobufen 1993.
| Study characteristics | ||
| Methods | Randomised control group trial The control group received no treatment or placebo Exclusions after randomisation: study was described as without losses Intention‐to‐treat approach: not mentioned but all participants completed the study; dropout participants were not detailed Losses to follow‐up: none Study duration: not mentioned Duration of follow‐up: 3 years |
|
| Participants |
Country: authors have affiliations from Italy and England. Study authors did not mention where the data were collected. Participants: 224 Participants were randomised into four groups:
For inclusion in this review, we considered all participants, but we divided them into two groups:
Age: mean ± standard deviation was 46 ± 13 years for the control (without antiplatelets) group and 46.7 ± 11 years for the antiplatelet group Sex: male 108 (48%); female 116 (52%) DVT phase: chronic (after 21 days). The study began after 6 months of anticoagulation. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Antiplatelet agents:
Control:
Co‐treatment:
|
|
| Outcomes |
Primary
Safety
Other:
|
|
| Notes | Economic information was not available. There are two different reports apparently about the same study. We tried to contact the authors but no additional information was provided. Additional data not provided by trialists (no response to personal communication) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 'Participants were randomised'. No additional details available |
| Allocation concealment (selection bias) | Unclear risk | There were no detailed data available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The control group did not have an oral treatment or placebo. Therefore, the participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "All patients were routinely scanned with CDS every 3 months and every time that they had new signs or symptoms (swelling, pain, tenderness, increase in calf/ankle section, local temperature increase)". Comment: since the participants were not blinded, the outcomes were not either. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors reported that all participants concluded the analysis, but there were dropouts in all groups, and it was not due to treatment or a PE, but due to causes not related to the thrombosis. There are no details about dropout participants. |
| Selective reporting (reporting bias) | High risk | The full protocol is not available (only the study report information). |
| Other bias | High risk | There is suspicion of duplicate publication. There are two reports in the same year with the same data without clear mention in the texts. We contacted authors for additional information without success (Belcaro 1993). |
Moriau 1995.
| Study characteristics | ||
| Methods | Single centre, randomised, blind, controlled trial. No mention about placebo Exclusions after randomisation: not mentioned Intention‐to‐treat approach: not mentioned Losses to follow‐up: not mentioned but all included participants were presented in results No data regarding study duration Duration of follow‐up: 3 to 6 months |
|
| Participants |
Country: not directly mentioned but all authors are from Belgium Participants: 600 included in the study and 500 analysed as part of this review (reference groups ‐ VKA alone: 100; VKA and piracetam: 100; VKA and buflomedil: 100; VKA and pentoxifylline: 100; VKA and dipyridamole: 100 (group without treatment: 100 participants were not analysed due to lack of treatment, which is not the BMP)) Age: mean 48.9 ± 14.2 years in no‐treatment group, 49.3 ± 13.8 years in VKA alone, 52.0 ± 12.6 years in VKA‐piracetam, 48.9 ± 13.4 years in VKA‐buflomedil, 53.4 ± 10.9 years in VKA‐pentoxifylline and 46.8 ± 10.8 years in VKA‐dipyridamole group Sex: male 210 (35%); female 390 (65%) Inclusion criteria:
Exclusion criteria:
DVT phase:
|
|
| Interventions |
Antiplatelet agents: only in test groups
Participants were asked to take the study drugs for 3 months (proximal DVT) and 6 months (distal DVT). Control:
Co‐treatment:
|
|
| Outcomes | Outcomes were not divided between primary and secondary outcomes.
Safety
Other
|
|
| Notes | There is no mention of financial support or the role of industry, but some authors have pharmaceutical company affiliations. Other economic information was not available. Additional data not provided by trialists (no response to personal communication) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ‘randomised, prospective blind study’ and no more detail |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Despite the sentence ‘randomised, prospective blind study’, we judged it is almost impossible to maintain blinding of participants in a control group without a placebo and intervention groups with several differences in number of tablet per day (1 to 4 doses per day). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: ‘randomised, prospective blind study’ Comment: no more detail provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Losses and exclusions were not mentioned. PE was mentioned as clinically assessed but it was not presented in results |
| Selective reporting (reporting bias) | High risk | The full protocol is not available (only report information) and some of the study’s prespecified outcomes that are of interest in the review have not been reported as planned, e.g. PE. |
| Other bias | High risk | We suspect pharmaceutical company support (two author affiliations), although not clearly mentioned in the report. |
Steele 1980.
| Study characteristics | ||
| Methods | Centre of participants is not clearly indicated but the author has affiliations with two different centres. Randomised, prospective, double‐blind, placebo‐controlled trial Exclusions after randomisation: not mentioned Intention‐to‐treat approach: not mentioned. Losses to follow‐up: not mentioned but all included participants were presented in the results. No data regarding study duration Duration of follow‐up: 18 months (‘Treatment was continued for 18 months or until a venographically confirmed venous thrombosis or an adverse drug reaction occurred’). |
|
| Participants |
Country: not directly mentioned but author affiliations are from USA Participants: 38 included and analysed (ASA + dipyridamole: 19, placebo: 19) Age: ASA + dipyridamole: mean 37 (range from 22 to 47) years, placebo: 34 (range from 21 to 43) years. Sex: male 26 (68%); female 12 (32%). Inclusion criteria:
Exclusion criteria:
Time of DVT:
Participants with clinical conditions which might contribute to DVT:
DVT phase:
|
|
| Interventions |
Antiplatelet agents:
Co‐treatment:
|
|
| Outcomes | Outcomes were not divided into primary and secondary outcomes.
Safety
Other
|
|
| Notes | This work was supported by research funds from the Veteran’s Administration. Other economic information was not available. Additional data not provided by trialists (no response to personal communication) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ‘patients were randomly assigned (double‐blind)’ |
| Allocation concealment (selection bias) | Unclear risk | Quote: ‘patients were randomly assigned (double‐blind)’ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ‘patients were randomly assigned (double‐blind)’ All tablets (intervention and placebo) were taken four times a day. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses and exclusion not detailed but apparently all participants completed the study. Adverse events were foreseen and mentioned in results |
| Selective reporting (reporting bias) | Low risk | The full protocol is not available (only report information) but some primary outcomes that are of interest in the review (PE and recurrent DVT) were preplanned or reported. |
| Other bias | Low risk | Financial support was mentioned and apparently did not interfere with the results. There is no other identified reason for bias. |
Sulfinpyrazone 1978.
| Study characteristics | ||
| Methods | Centre of participants is not clearly indicated but the authors have two different centre affiliations Randomised, prospective, placebo‐controlled trial Exclusions after randomisation: none Intention‐to‐treat approach: not mentioned but all losses were presented and all participants completed the trial Losses to follow‐up: sulfinpyrazone: 0, placebo: 3 (1 due to gastrointestinal disease, 1 due to emotional difficulties and 1 due to lethal stroke) No data about study duration Duration of follow‐up: 3 months |
|
| Participants |
Country: not directly mentioned for all participants but author affiliations are from USA and Canada Participants: 51 in total but only 28 can be included and analysed (sulfinpyrazone: 14, placebo: 14). The other 23 participants could not be included in the review (13 received lytic therapy and 10 were analysed without randomisation) Age: all 51 participants: average age 37 years (range 19 to 56). No details about 28 participants included in the review Sex: male 37 (73%); female 14 (27%). No details about 28 participants included in the review Inclusion criteria:
Exclusion criteria:
Time of DVT: from 1–6 years prior to study Number with precipitating cause for initial DVT:
‘In no patient was there an apparent cause for recurrence of venous thromboembolism after the initial episode.’ No details about 28 participants included in the review DVT phase:
|
|
| Interventions |
Antiplatelet agents:
Control:
Co‐treatment:
|
|
| Outcomes | Outcomes were not divided into primary and secondary outcomes.
Safety
Other
|
|
| Notes | This work was supported by the research funds from the Veteran’s Administration and grants from the Colorado Heart Association, the National Institutes of Health (HL 165X3), CIBAGEIGY Corp, Summit, NJ, and Organon BV, Oss, Netherlands. Other economic information not available. Additional data not provided by trialists (no response to personal communication) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ‘The first 41 patients (27 men, 14 women) were assigned at random to treatment’. No more details |
| Allocation concealment (selection bias) | Unclear risk | No details available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses and exclusion not detailed but apparently all participants concluded the study. Adverse events were not a preplanned outcome but were mentioned in results. |
| Selective reporting (reporting bias) | Unclear risk | The full protocol is not available (only report information). Only one primary outcome that is of interest for this review (recurrent DVT) which was preplanned and reported. PE was mentioned as part of the medical history but not in the results perhaps because of the short follow‐up (3 months). Bleeding is not mentioned |
| Other bias | High risk | The study included one group treated with warfarin after randomisation. Moreover, the investigators used different anticoagulation treatments (warfarin or heparin) for some participants, and this was not sufficiently described. There are no details about numbers of anticoagulated participants in each group. Financial support is mentioned and apparently did not interfere with results. Other economic information not available |
WARFASA.
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled trial Exclusions after randomisation: one participant in the placebo group did not receive the treatment and was excluded after randomisation Intention‐to‐treat approach: "modified intention‐to‐treat principle, with all patients who received at least one dose of the assigned study drug after randomisation included in the analysis." Losses to follow‐up: four participants in the placebo group (2%); three participants in the antiplatelet group (1.4%) were lost to follow‐up. Other treatment dropout: in the control group, 9 withdrew consent, 8 had new indication for aspirin, 2 had new indication other than VTE for VKAs and 6 had adverse events. In the antiplatelet group, 10 withdrew consent, 13 had new indication for aspirin, 3 had new indication other than VTE for VKAs and 7 had adverse events. Study duration: May 2004 to August 2010 Duration of follow‐up: median period of the study duration was 24.8 months for the aspirin group and 24.2 months for the placebo group |
|
| Participants |
Country: authors have affiliations from Italy and Austria. According to protocol information, participants were scheduled to be recruited from 25 European centres in Austria, Denmark, France, Great Britain, Italy, and the Netherlands according protocol information, but nothing was described in the study report. Participants: 403 participants included and randomised but only 402 were analysed in this study. 205 participants received aspirin, 197 received placebo, and one participant, who was assigned to the placebo group, did not receive the study drug (excluded after randomisation). In our review, we could utilise data from 122 participants who received antiplatelet agents and 130 participants who received the placebo because they were described as participants with DVT with or without PE and only for the outcome recurrent VTE ‐ early and intermediate. Data for the other outcomes were not stratified by types of participant and included data from participants with PE only (beyond the scope of this review). Age: mean ± standard deviation was 61.9 ± 15.3 years in the aspirin group and 62.1 ± 15.1 years in the placebo group Sex: male 135/205 (65.8%) in the aspirin group and male 122/197 (61.9%) in the placebo group. Total of 257 (64%) male and 145 (36%) female Inclusion criteria:
Exclusion criteria:
DVT phase:
|
|
| Interventions |
Antiplatelet agent:
Control:
Appendix information:
|
|
| Outcomes |
Primary
Secondary
Safety
|
|
| Notes | Supported by the University of Perugia, a grant‐in‐aid from Bayer Healthcare, and an Aventis Fellowship for Clinical Research from the International Society of Thrombosis and Haemostasis (to Dr Becattini). Dr Agnelli reports receiving consulting fees from Bayer Healthcare, Boehringer Ingelheim, and Daiichi Sankyo and lecture fees from Bayer Healthcare, Bristol‐Myers Squibb, and Sanofi‐Aventis; Dr Eichinger, board memberships from Bayer and Boehringer Ingelheim and lecture fees from Bayer, Boehringer Ingelheim, Pfizer, and Sanofi‐Aventis; and Dr Ageno, board memberships from Bristol‐Myers Squibb, Pfizer, and Bayer Schering Pharma and lecture fees from GlaxoSmithKline, Sanofi‐Aventis, Bayer Schering Pharma, Bristol‐Myers Squibb, Pfizer, and Boehringer Ingelheim. Authors reported that aspirin and placebo tablets were supplied by Bayer Healthcare and Bayer played no role in the design of the study, in data collection or analysis or in manuscript preparation. No other economic information was reported. Although we tried to contact the authors, they did not respond. We were therefore not able to obtain stratified data from the participants with DVT and those with PE alone, preventing the inclusion of the data in this review. Because of this, we only could extract the data for recurrent VTE which was stratified by types of participant from the original paper. Additional data not provided by trialists (no response to personal communication) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: ‘The randomisation sequence will be computer generated. Patients will be randomised according to the consecutive box number assigned to the study centre. Randomisation will be 1/1 (aspirin/placebo)’. Comment: information from protocol document |
| Allocation concealment (selection bias) | Low risk | Quote: ‘Patients will be randomised according to the consecutive box number assigned to the study centre. Randomisation will be 1/1 (aspirin/placebo)’. Comment: information from protocol document Comment: treatment regimens (antiplatelets and placebo) were similar, tablets once daily |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: ‘double‐blind, placebo‐controlled trial’ Comment: information from protocol document Comment: treatment regimens (antiplatelets and placebo) were similar, tablets once daily |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: ‘All suspected study outcome events were assessed by a central, independent adjudication committee whose members were unaware of the group assignments and who reviewed the imaging results.’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses and exclusions were detailed and utilised as intention‐to‐treat. Adverse events were mentioned and analysed. |
| Selective reporting (reporting bias) | High risk | Protocol is available Quote: ‘The study was performed in accordance with the protocol and with the provisions of the Declaration of Helsinki and local regulations’. Comment: in the 'historical versions' in ClinicalTrials.gov, the protocol was modified in its primarily and secondary outcomes near publication of the study, putting it in high risk of selective publication. |
| Other bias | Low risk | Financial support is mentioned and apparently did not interfere with the results. Authors reported that aspirin and placebo tablets were supplied by Bayer Healthcare and Bayer played no role in the design of the study, in data collection or analysis, or in manuscript preparation. There is no other identified reason for bias. |
ASA: acetylsalicylic acid CDS: colour Doppler ultrasound DVT: deep vein thrombosis GECS: graduated elastic compression stockings INR: international normalised ratio PE: pulmonary embolism PTS: post‐thrombotic syndrome tPA: tissue plasminogen activator VKA: vitamin‐K antagonist VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bick 1981 | There was no acceptable additional confirmation for DVT diagnosis as prespecified in our review protocol (e.g. Doppler vascular ultrasound, multi‐slice computed tomography or angiography). |
| EINSTEIN CHOICE | The study used the antiplatelet agent group as the control and rivaroxaban as the intervention group. There is no control group with BMP and/or placebo group. |
| Evans 1975 | Diagnosis of DVT only by clinical aspects. There was no acceptable additional confirmation as prespecified in our review protocol (e.g. Doppler vascular ultrasound, multi‐slice computed tomography or angiography). |
| IRCT20200202046344N1 | The study used the antiplatelet agent group as the control and enoxaparin as the intervention group. There is no control group with BMP and/or placebo group. |
| Moriau 1982 | Suloctidil was voluntarily withdrawn worldwide in 1985 by the manufacturer following several reports of hepatitis associated with its use, some of which were fatal. |
| Nielsen 1994 | The antiplatelet agent group did not receive BMP with the intervention, as we prespecified in our protocol but only the antiplatelet agent and compression stockings in acute DVT. |
| Sidhu 2019 | The study used the antiplatelet agent group as the control and enoxaparin as the intervention group. There is no control group with BMP and/or placebo group. |
BMP: best medical practice DVT: deep vein thrombosis
Differences between protocol and review
We amended the objectives from ‘To assess the effectiveness and safety of’ in the protocol to ‘To assess the effects of’ in the review to be more in accordance with the purpose of our research.
Since the summary of findings tables may incorporate up to seven outcomes, we opted to add the most clinically‐relevant secondary outcomes: mortality, PTS and adverse events.
We amended the method of reporting the ‘summary of findings’ to exclude the necessity of reporting in one time point because reporting all the results (i.e. range of time) better reflected the overall evidence.
We amended the interpretation of I2, following the guide to interpretation in the Cochrane Handbook for Systematic Reviews of Interventions. In accordance with sections 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions, we amended how we used fixed‐effect model and random‐effects model, with the evaluation of the homogeneity of the included studies, considering population, interventions, comparators and outcomes characteristics. This section of the Handbook states 'The choice between a fixed‐effect and random‐effects meta‐analysis should never be made on the basis of a statistical test for heterogeneity.'
We amended the 'Measures of treatment effect' section to detail the NNT calculation, as described in the Cochrane Handbook for Systematic Reviews of Interventions.
Contributions of authors
CDQF is the contact person that co‐ordinated the contributions of the co‐authors and wrote the final version of the review, developed the clinical sections of the Background section, responded to clinical observations of the arbitrators, responded to comments from referees on methodology and statistics, and is responsible for the final review.
LCUN wrote the results section, and contributed to study selection and data extraction.
JCCBS wrote the Methods section and helped write the review.
RLGF wrote the Methods section, responded to comments from referees on methodology and statistics, and helped write the review.
Sources of support
Internal sources
-
Division of Vascular and Endovascular Surgery, Universidade Federal de São Paulo, Brazil
Non‐financial internal sources
-
Cochrane Brazil, Brazil
Non‐financial internal sources
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
CDQF: none known LCUN: none known JCCBS: none known RLGF: none known
New
References
References to studies included in this review
ASPIRE {published and unpublished data}
- ACTRN012605000004662. ASPIRE A multi-centre, randomised, double-blind, placebo-controlled clinical trial examining the efficacy and safety of low-dose aspirin after initial anticoagulation to prevent recurrent venous thromboembolism. ctc.usyd.edu.au/our-work/research-divisions/cardiovascular/closed-trials/aspire/ (first received 1 May 2003).
- ACTRN12605000004662. ASPIRE. A multi-centre, randomised, double-blind, placebo-controlled clinical trial examining the efficacy and safety of low-dose aspirin after initial anticoagulation to prevent recurrent venous thromboembolism. trialsearch.who.int/?trialid=ACTRN12605000004662 (first received 12 July 2005).
- ACTRN12605000004662. ASPIRE A multi-centre, randomised, double-blind, placebo-controlled clinical trial examining the efficacy and safety of low-dose aspirin after initial anticoagulation to prevent recurrent venous thromboembolism. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12605000004662 (first received 12 July 2005).
- Brighton T, Eikelboom J, Mann K, Mister R, Gallus A, Ockelford Petal. Aspirin for the prevention of recurrent venous thromboembolism after a first unprovoked event: results of the ASPIRE randomized controlled trial. In: Late-Breaking Clinical Trial Abstracts. Circulation 2012;126(23):2777. [DOI: 10.1161/CIR.0b013e318278c90d] [DOI] [Google Scholar]
- Brighton TA, Eikelboom JW, Mann K, Mister R, Gallus A, Ockelford P, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. New England Journal of Medicine 2012;367(21):1979-87. [DOI: 10.1056/NEJMoa1210384] [DOI] [PubMed] [Google Scholar]
- Brighton TA, Eikelboom JW, Mann K, Mister R, Gallus A, Ockelford P, et al. Protocol to 'Low-dose aspirin for preventing recurrent venous thromboembolism'. www.nejm.org/doi/suppl/10.1056/NEJMoa1210384/suppl_file/nejmoa1210384_protocol.pdf (accessed 7 December 2016).
- Brighton TA, Eikelboom JW, Mann K, Mister R, Gallus A, Ockelford P, et al. Supplementary Appendix to 'Low-dose aspirin for preventing recurrent venous thromboembolism'. www.nejm.org/doi/suppl/10.1056/NEJMoa1210384/suppl_file/nejmoa1210384_appendix.pdf (accessed 15 August 2018).
- Robledo K. DVT subgroup data used in our trial [personal communication] [ASPIRE results]. Email to: CDQ Flumignan 12 December 2017.
Indobufen 1993 {published data only (unpublished sought but not used)}
- Belcaro G, Errichi BM, De Simone P. Prevention of recurrent deep venous thrombosis with indobufen. A 3-year follow-up study using color duplex scanning. Angiology 1993;44(4):328-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Belcaro G, Laurora G, Cesarone MR, De Sanctis MT. Prophylaxis of recurrent deep venous thrombosis. A randomized, prospective study using indobufen and graduated elastic compression stockings. Angiology 1993;44(9):695-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Moriau 1995 {published data only (unpublished sought but not used)}
- Moriau M, Lavenne-Pardonge E, Crasborn L, Frenckell R, Col-Debeys C. The treatment of severe or recurrent deep venous thrombosis. Beneficial effect of the co-administration of antiplatelet agents with or without rheological effects, and anticoagulants. Thrombosis Research 1995;78(6):469-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Steele 1980 {published data only (unpublished sought but not used)}
- Steele P. Trial of dipyridamole-aspirin in recurring venous thrombosis. Lancet (London, England) 1980;2(8208-8209):1328-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sulfinpyrazone 1978 {published data only (unpublished sought but not used)}
- Steele P, Ellis J Jr, Genton E. Effects of platelet suppressant, anticoagulant and fibrinolytic therapy in patients with recurrent venous thrombosis. American Journal of Medicine 1978;64(3):441-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
WARFASA {published data only (unpublished sought but not used)}
- Becattini C, Agnelli G, Poggio R, Eichinger S, Bucherini E, Silingardi M, et al. Aspirin after oral anticoagulants for prevention of recurrence in patients with unprovoked venous thromboembolism. the WARFASA study. Blood 2011;118(21):543. [online ISSN: 1528-0020] [Google Scholar]
- Becattini C, Agnelli G, Schenone A, Eichinger S, Bucherini E, Silingardi M, et al. Aspirin for preventing the recurrence of venous thromboembolism: correction. New England Journal of Medicine 2012;367(16):1573. [DOI: 10.1056/NEJMx120070] [DOI] [PubMed] [Google Scholar]
- Becattini C, Agnelli G, Schenone A, Eichinger S, Bucherini E, Silingardi M, et al. Aspirin for preventing the recurrence of venous thromboembolism. New England Journal of Medicine 2012;366(21):1959-67. [DOI: 10.1056/NEJMoa1114238] [DOI] [PubMed] [Google Scholar]
- Becattini C, Agnelli G, Schenone A, Eichinger S, Bucherini E, Silingardi M, et al. Supplementary appendix to 'Aspirin for preventing the recurrence of venous thromboembolism'. www.nejm.org/doi/suppl/10.1056/NEJMoa1114238/suppl_file/nejmoa1114238_appendix.pdf 2012;(accessed 7 January 2017).
- NCT00222677. Aspirin after six months or one year of oral anticoagulants for the prevention of recurrent venous thromboembolism in patients with idiopathic venous thromboembolism. The WARFASA Study. clinicaltrials.gov/ct2/show/NCT00222677 (first received 22 September 2005).
References to studies excluded from this review
Bick 1981 {published data only}
- Bick RL, Nix MK, Skondia V. THe effect of piracetam in preventing recurrent deep venous thrombosis. Thrombosis and Haemostasis 1981;46(1):91. [ISSN 0340-6245] [Google Scholar]
EINSTEIN CHOICE {published data only}
- EUCTR2013-000619-26. Reduced-dosed rivaroxaban and standard-dosed rivaroxaban versus ASA in the long-term prevention of recurrent symptomatic venous thromboembolism in patients with symptomatic deep-vein thrombosis and/or pulmonary embolism (The Einstein Choice Study). clinicaltrialsregister.eu/ctr-search/trial/2013-000619-26/AT (first received 12 February 2014).
- EUCTR2013-000619-26-HU. Reduced-dosed rivaroxaban and standard-dosed rivaroxaban versus ASA in the long-term prevention of recurrent symptomatic venous thromboembolism in patients with symptomatic deep-vein thrombosis and/or pulmonary embolism. The Einstein Choice Study - Reduced-dosed rivaroxaban in the long-term prevention of recurrent symptomatic VTE. trialsearch.who.int/?trialid=EUCTR2013-000619-26-HU (first received 11 March 2014).
- NCT02064439. Reduced-dosed rivaroxaban and standard-dosed rivaroxaban versus ASA in the long-term prevention of recurrent symptomatic venous thromboembolism in patients with symptomatic deep-vein thrombosis and/or pulmonary embolism. clinicaltrials.gov/ct2/show/NCT02064439 (first received 17 February 2014).
- Reduced-dosed rivaroxaban and standard-dosed rivaroxaban versus aspirin in the long-term prevention of blood clots from recurring in the leg or lung. explorer.opentrials.net/trials/cc5722b3-d0bc-4a7d-a9d1-35d8391aac9c 2014;(accessed 4 December 2016).
- Weitz JI, Bauersachs R, Beyer-Westendorf J, Bounameaux H, Brighton TA, Cohen AT, et al. Two doses of rivaroxaban versus aspirin for prevention of recurrent venous thromboembolism. Rationale for and design of the EINSTEIN CHOICE study. Thrombosis and Haemostasis 2015;114(3):645-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Weitz JI, Lensing AW, Prins MH, Rupert B, Jan BW, Bounameaux H, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. New England Journal of Medicine 2017;376(13):1211-22. [DOI: 10.1056/NEJMoa1700518] [DOI] [PubMed] [Google Scholar]
Evans 1975 {published data only}
- Evans G, Gent M. Effect of platelet suppressive drugs on arterial and venous thromboembolism. In: Cade JF, Hirsh J, Gallus AS, Schönbaum E, editors(s). Platelets, drugs and thrombosis. Basel: Karger, 1975:258-62. [DOI: 10.1159/000395715] [DOI] [Google Scholar]
IRCT20200202046344N1 {published data only}
- IRCT20200202046344N1. Comparison of the effectiveness of aspirin with enoxaparin sodium for prevention of deep vein thrombosis in tibial fractures. en.irct.ir/trial/49703 (first received 29 September 2020).
Moriau 1982 {published data only}
- Moriau M, Col-De Beys C, Pardonge E, Ferrant A. Clinical and biological activity of the antiplatelet agent suloctidil in treatment of idiopathic recurrent vein thrombosis (I.R.V.T.). Thrombosis and Haemostasis 1982;47(1):27-31. [PMID: ] [PubMed] [Google Scholar]
Nielsen 1994 {published data only}
- Nielsen HK, Husted SE, Krusell L, Fasting H, Charles P, Hansen HH, et al. Anticoagulant therapy in deep venous thrombosis. A randomized controlled study. Thrombosis and Haemostasis 1985;54(1):233. [DOI] [PubMed] [Google Scholar]
- Nielsen HK, Husted SE, Krusell LR, Fasting H, Charles P, Hansen HH, et al. Anticoagulant therapy in deep venous thrombosis. A randomized controlled study. Thrombosis Research 1994;73(3-4):215-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Nielsen HK, Husted SE, Krusell LR, Fasting H, Charles P, Hansen HH. Silent pulmonary embolism in patients with deep venous thrombosis. Incidence and fate in a randomized, controlled trial of anticoagulation versus no anticoagulation. Journal of Internal Medicine 1994;235(5):457-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sidhu 2019 {published data only}
- Sidhu VS, Graves SE, Buchbinder R, Naylor JM, Pratt NL, Steiger RS, et al. CRISTAL: protocol for a cluster randomised, crossover, non-inferiority trial of aspirin compared to low molecular weight heparin for venous thromboembolism prophylaxis in hip or knee arthroplasty, a registry nested study. BMJ Open 2019;9(11):e031657. [DOI: 10.1136/bmjopen-2019-031657] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu VS, Kelly TL, Pratt N, Graves S, Buchbinder R, Naylor J, et al. CRISTAL (a cluster-randomised, crossover, non-inferiority trial of aspirin compared to low molecular weight heparin for venous thromboembolism prophylaxis in hip or knee arthroplasty, a registry nested study): statistical analysis plan. Trials 2021;22(1):564. [DOI: 10.1186/s13063-021-05486-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Anderson 1991
- Anderson FA Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Archives of Internal Medicine 1991;151(5):933-8. [PMID: ] [PubMed] [Google Scholar]
Appelen 2017
- Appelen D, Loo E, Prins MH, Neumann MH, Kolbach DN. Compression therapy for prevention of post‐thrombotic syndrome. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD004174. [DOI: 10.1002/14651858.CD004174.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Autar 2006
- Autar R. Evidence for the prevention of venous thromboembolism. British Journal of Nursing 2006;15(18):980-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Avila 2022
- Ávila RB, Marcondes GB, Dias SV, Silveira BP, Amorim JE, Guedes HJ, et al. External validation of Villalta score in high-middle income country patients with deep vein thrombosis. Medicine 2022;101(24):e29367. [DOI: 10.1097/MD.0000000000029367] [DOI] [PMC free article] [PubMed] [Google Scholar]
Becattini 2012
- Becattini C, Agnelli G, Schenone A, Eichinger S, Bucherini E, Silingardi M, et al. Aspirin for preventing the recurrence of venous thromboembolism. New England Journal of Medicine 2012;366(21):1959-67. [DOI: 10.1056/NEJMoa1114238] [DOI] [PubMed] [Google Scholar]
Belcaro 1993
- Belcaro G, Laurora G, Cesarone MR, De Sanctis MT. Prophylaxis of recurrent deep venous thrombosis. A randomized, prospective study using indobufen and graduated elastic compression stockings. Angiology 1993;44(9):695-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Boneu 1992
- Boneu B. Prevention of venous thrombosis with antiplatelet agents [Prevention des thromboses veineuses par les agents antiplaquettaires]. Annales Francaises d'Anesthesie et de Reanimation 1992;11(3):329. [PMID: ] [DOI] [PubMed] [Google Scholar]
Boutitie 2011
- Boutitie F, Pinede L, Schulman S, Agnelli G, Raskob G, Julian J, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants' data from seven trials. BMJ (Clinical Research ed.) 2011;342:d3036. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brar 2011
- Brar SS, ten Berg J, Marcucci R, Price MJ, Valgimigli M, Kim HS, et al. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data. Journal of the American College of Cardiology 2011;58(19):1945-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Braunwald 2012
- Braunwald E. The rise of cardiovascular medicine. European Heart Journal 2012;33(7):838-45, 845a. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brighton 2012
- Brighton TA, Eikelboom JW, Mann K, Mister R, Gallus A, Ockelford P, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. New England Journal of Medicine 2012;367(21):1979-87. [DOI: 10.1056/NEJMoa1210384] [DOI] [PubMed] [Google Scholar]
Broderick 2021
- Broderick C, Watson L, Armon MP. Thrombolytic strategies versus standard anticoagulation for acute deep vein thrombosis of the lower limb. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD002783. [DOI: 10.1002/14651858.CD002783.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castellucci 2013
- Castellucci LA, Cameron C, Le Gal G, Rodger MA, Coyle D, Wells PS, et al. Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: systematic review and network meta-analysis. BMJ (Clinical research ed.) 2013;347:f5133. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castro 2006
- Castro HC, Ferreira BL, Nagashima T, Schueler A, Rueff C, Camisasca D, et al. Platelets: still a therapeutical target [Plaquetas: ainda um alvo terapêutico]. Jornal Brasileiro de Patologia e Medicina Laboratorial 2006;42(5):321-32. [DOI: 10.1590/S1676-24442006000500004] [DOI] [Google Scholar]
COVIDSurg 2022
- COVIDSurg Collaborative, GlobalSurg Collaborative. SARS-CoV-2 infection and venous thromboembolism after surgery: an international prospective cohort study. Anaesthesia 2022;77(1):28-39. [PMID: 10.1111/anae.15563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
de Wolf 2012
- Wolf MA, Wittens CH, Kahn SR. Incidence and risk factors of the post-thrombotic syndrome. Phlebology/Venous Forum of the Royal Society of Medicine 2012;27 Suppl 1:85-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
El‐Atat 2011
- El-Atat F, Sarkar K, Kodali V, Karajgikar R, Jakkulla M, Mares A, et al. A randomized pilot trial for aggressive therapeutic approaches in aspirin-resistant patients undergoing percutaneous coronary intervention. Journal of Invasive Cardiology 2011;23(1):9-13. [PMID: ] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Falanga 1999
- Falanga A, Rickles FR. Pathophysiology of the thrombophilic state in the cancer patient. Seminars in Thrombosis and Hemostasis 1999;25(2):173-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Flumignan 2015
- Flumignan RL, Flumignan CD, Baptista-Silva JC. Angioplasty for deep venous thrombosis. Cochrane Database of Systematic Reviews 2015, Issue 1. Art. No: CD011468. [DOI: 10.1002/14651858.CD011468] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flumignan 2022
- Flumignan RL, Civile VT, Tinôco JD, Pascoal PI, Areias LL, Matar CF, et al. Anticoagulants for people hospitalised with COVID-19. Cochrane Database of Systematic Reviews 2022, Issue 3. Art. No: CD013739. [DOI: 10.1002/14651858.CD013739.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Galanaud 2012
- Galanaud JP, Kahn SR, Khau Van Kien A, Laroche JP, Quere I. Epidemiology and management of isolated distal deep venous thrombosis [Thromboses veineuses profondes distales isolees des membres inferieurs: epidemiologie et prise en charge]. La Revue de Médecine Interne/fondee par la Societé nationale Française de Médecine Interne 2012;33(12):678-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Goldhaber 2012
- Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Practice and Research. Clinical Haematology 2012;25(3):235-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gomes 2004
- Gomes MP, Deitcher SR. Risk of venous thromboembolic disease associated with hormonal contraceptives and hormone replacement therapy: a clinical review. Archives of Internal Medicine 2004;164(18):1965-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT Guideline Development Tool. Version accessed 6 March 2018. Hamilton (OH): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
GRADE Working Group
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ (Clinical Research ed.) 2004;328(7454):1490-4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guanella 2013
- Guanella R. Post-thrombotic syndrome: the forgotten complication of venous thromboembolism [Syndrome post-thrombotique: la complication negligee de la maladie thromboembolique veineuse]. Revue Médicale Suisse 2013;9(372):321-5. [PMID: ] [PubMed] [Google Scholar]
Handoll 2015
- Handoll HH, Langhorne P. In defence of reviews of small trials: underpinning the generation of evidence to inform practice. Cochrane Database of Systematic Reviews 2015;(11). [DOI: 10.1002/14651858.ED000106] [DOI] [PMC free article] [PubMed]
Heit 2000
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Archives of Internal Medicine 2000;160(6):809-15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Heit 2008
- Heit JA. The epidemiology of venous thromboembolism in the community. Arteriosclerosis, Thrombosis, and Vascular Biology 2008;28(3):370-2. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Research ed.) 2003;327(7414):557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook/archive/v5.2.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
ISTH 2014
- ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Thrombosis and Haemostasis 2014;112(5):843-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kakkos 2021
- Kakkos SK, Gohel M, Baekgaard N, Bauersachs R, Bellmunt-Montoya S, Black SA, et al. Editor's Choice - European Society for Vascular Surgery (ESVS) 2021 Clinical Practice Guidelines on the management of venous thrombosis. European Journal of Vacular and Endovascular Surgery 2021;61(1):9-82. [PMID: 10.1016/j.ejvs.2020.09.023] [DOI] [PubMed] [Google Scholar]
Lefebvre 2022
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI: 10.1136/bmj.b2700] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lugo 2008
- Lugo JJ, Hurtado EF, Calderón LI, Gómez G, Castro P, Estrada G, et al. Resistance to acetylsalicylic acid and to clopidogrel: an emergent clinical entity [Resistencia al ácido acetil salicílico y al clopidogrel: una entidad clínica emergente]. Revista Colombiana de Cardiología 2008;15(4):172-83. [Google Scholar]
Marik 2015
- Marik PE, Cavallazzi R. Extended anticoagulant and aspirin treatment for the secondary prevention of thromboembolic disease: a systematic review and meta-analysis. PloS one 2015;10(11):e0143252. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matielo 2008
- Matielo MF, Presti C, Casella IB, Netto BM, Puech-Leao P. Incidence of ipsilateral postoperative deep venous thrombosis in the amputated lower extremity of patients with peripheral obstructive arterial disease. Journal of Vascular Surgery 2008;48(6):1514-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Matzdorff 2005
- Matzdorff A. Platelet function tests and flow cytometry to monitor antiplatelet therapy. Seminars in Thrombosis and Hemostasis 2005;31(4):393-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
McColl 1997
- McColl MD, Ramsay JE, Tait RC, Walker ID, McCall F, Conkie JA, et al. Risk factors for pregnancy associated venous thromboembolism. Thrombosis and Haemostasis 1997;78(4):1183-8. [PMID: ] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI: 10.1136/bmj.b2535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naess 2007
- Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: a population-based study. Journal of Thrombosis and Haemostasis 2007;5(4):692-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
NICE 2020
- National Institute for Health and Care Excellence. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Available from www.nice.org.uk/guidance/ng158 (accessed 29 March 2022).
Nicolaides 2001
- Nicolaides AN, Breddin HK, Fareed J, Goldhaber S, Haas S, Hull R, et al. Prevention of venous thromboembolism. International Consensus Statement. Guidelines compiled in accordance with the scientific evidence. International Angiology 2001;20(1):1-37. [PMID: ] [PubMed] [Google Scholar]
Paikin 2011
- Paikin JS, Wright DS, Eikelboom JW. Effectiveness and safety of combined antiplatelet and anticoagulant therapy: a critical review of the evidence from randomized controlled trials. Blood Reviews 2011;25(3):123-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Robertson 2015
- Robertson L, Kesteven P, McCaslin JE. Oral direct thrombin inhibitors or oral factor Xa inhibitors for the treatment of deep vein thrombosis. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD010956. [DOI: 10.1002/14651858.CD010956.pub2] [DOI] [PubMed] [Google Scholar]
Robertson 2016
- Robertson L, McBride O, Burdess A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD011536. [DOI: 10.1002/14651858.CD011536.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosendaal 2005
- Rosendaal FR. Venous thrombosis: the role of genes, environment, and behavior. Hematology/the Education Program of the American Society of Hematology. American Society of Hematology. Education Program 2005;2005(1):1-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rossouw 2002
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288(3):321-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Roth 2017
- Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. Journal of the American College of Cardiology 2017;70(1):1-25. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samama 2000
- Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Archives of Internal Medicine 2000;160(22):3415-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Santos 2022
- Santos BC, Flumignan RL, Civile VT, Atallah AN, Nakano LC. Prophylactic anticoagulants for non-hospitalised people with COVID-19. Cochrane Database of Systematic Reviews 2022, Issue 4. Art. No: CD015102. [DOI: 10.1002/14651858.CD015102] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schror 2015
- Schror K. Aspirin and venous thromboses [Acetylsalicylsaure und venose Thrombosen]. Der Internist 2015;56(1):84-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schulman 2005
- Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. Journal of Thrombosis and Haemostasis 2005;3(4):692-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schulman 2010
- Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. Journal of Thrombosis and Haemostasis 2010;8(1):202-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2021
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Sevitt 1961
- Sevitt S, Gallagher N. Venous thrombosis and pulmonary embolism. A clinico-pathological study in injured and burned patients. British Journal of Surgery 1961;48:475-89. [PMID: ] [DOI] [PubMed] [Google Scholar]
Simes 2014
- Simes J, Becattini C, Agnelli G, Eikelboom JW, Kirby AC, Mister R, et al. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation 2014;130(13):1062-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sobieraj 2015
- Sobieraj DM, Coleman CI, Pasupuleti V, Deshpande A, Kaw R, Hernandez AV. Comparative efficacy and safety of anticoagulants and aspirin for extended treatment of venous thromboembolism: a network meta-analysis. Thrombosis Research 2015;135(5):888-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Soosainathan 2013
- Soosainathan A, Moore HM, Gohel MS, Davies AH. Scoring systems for the post-thrombotic syndrome. Journal of Vascular Surgery 2013;57(1):254-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stevens 2021
- Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing GJ, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest 2021;160(6):e545-608. [DOI: 10.1016/j.chest.2021.07.055] [DOI] [PubMed] [Google Scholar]
Tagalakis 2013
- Tagalakis V, Patenaude V, Kahn SR, Suissa S. Incidence of and mortality from venous thromboembolism in a real-world population: the Q-VTE study cohort. American Journal of Medicine 2013;126(9):832.e13-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tsai 2002
- Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Archives of Internal Medicine 2002;162(10):1182-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
UN 2005
- United Nations. Consolidated list of products: whose consumption and/or sale have been banned, withdrawn, severely restricted or not approved by governments. Economic & Social Affairs 2005;(12):251.
Urwin 2000
- Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. British Journal of Anaesthesia 2000;84(4):450-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vedantham 2009
- Vedantham S, Grassi CJ, Ferral H, Patel NH, Thorpe PE, Antonacci VP, et al. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. Journal of Vascular and Interventional Radiology 2009;20(7 Suppl):S391-408. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vieira‐de‐Abreu 2012
- Vieira-de-Abreu A, Campbell RA, Weyrich AS, Zimmerman GA. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Seminars in Immunopathology 2012;34(1):5-30. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 2008
- Watson HG, Chee YL. Aspirin and other antiplatelet drugs in the prevention of venous thromboembolism. Blood Reviews 2008;22(2):107-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Flumignan 2016
- Flumignan CD, Flumignan RL, Baptista-Silva JC. Antiplatelet agents for the treatment of deep venous thrombosis. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD012369. [DOI: 10.1002/14651858.CD012369] [DOI] [PMC free article] [PubMed] [Google Scholar]


