Abstract
Purpose of Review:
Dipeptidyl peptidase 4 (DPP4) is a regulatory serine protease that has many diverse functions in a variety of different cell types, both healthy and diseased. However, there is still much unknown about the mechanisms and targets of DPP4. Our purpose is to discuss new studies exploring DPP4-mediated cellular regulation, provide an updated list of potential peptide targets of DPP4, and to discuss possible clinical implications of each.
Recent Findings:
Recent studies have built on the known role of DPP4 in regulating hematopoietic stem and progenitor cell function to improve the potential to modulate these functions in a clinical application. Further studies have identified and explored new roles of DPP4 in different cellular compartments and have demonstrated possible ways to target this protein in malignancy. These findings, together with an expanded list of putative extracellular, cell surface, and intracellular DPP4 targets, provide insight into a variety of new ways that DPP4 may regulate cell functions.
Summary:
DPP4 post translationally modifies proteins and peptides that have essential roles in hematopoietic cell regulation, stem cell transplantation, and malignancy. Targets include secreted factors that drive signaling and may include membrane proteins and transcription factors that are critical for different hematopoietic functions. Knowing these targets and functions can provide insight into possibly new regulatory roles for DPP4 that can then be targeted to enhance transplantation, to treat disease, and to better understand different regulatory pathways of hematopoiesis.
Keywords: Dipeptidyl peptidase 4, hematopoietic stem and progenitor cell regulation, intracellular signaling regulation, transcription regulation, hematopoietic cell transplantation
Introduction
Hematopoiesis is dynamic process regulated by a variety of different mechanisms [1]. Under homeostatic conditions, hematopoietic stem cells (HSCs) undergo self-renewal and differentiation to continuously replenish the mature blood cells in the body. Under stress conditions, such as pathogenic insult, the hematopoietic compartment must be rapidly activated to proliferate and produce increased amounts of myeloid cells to fight infection [2]. Maintenance of the hematopoietic system as well as induction of changes to hematopoiesis, as in the context of emergency myelopoiesis, are tightly regulated processes influenced by a variety of factors. These factors include responses to external stimuli such as cytokines, chemokines, interferons, and other regulatory proteins and signals [3–5]. Additionally, hematopoiesis is regulated by dynamic changes in transcription, which in turn is affected by the expression and post-translational modifications of nuclear proteins such as transcription factors [6–8]. Changes in these regulatory processes also play important roles in increasing the expansion of functional HSCs and hematopoietic progenitor cells (HPCs) for the purposes of HSC/HPC cell transplant to treat malignant and non-malignant disorders. This is particularly so in the context of cord blood transplant where numbers of engraftable cells are often a limiting factor [9–12]. To fully understand the manner in which hematopoiesis is regulated as well as the ways it can become deregulated in context of disease or to be manipulated to enhance transplants as a mode of treatment, we need to fully explore how these regulatory factors can be modulated by other regulatory proteins.
One manner in which proteins involved in hematopoietic processes are regulated is through post translational modifications. A specific post translational cleavage of N-terminal dipeptides from several of these modulatory factors has been identified as an important avenue of regulating HSC/HPC function [13–18]. Dipeptidyl peptidase 4 (DPP4) is a serine protease that that cuts 2 amino acids from the N-termini of proteins, which can have profound effects on the function of at least some of these targets [19–21]. This peptidase specifically targets proteins and peptides that have proline or alanine in the penultimate amine position, though DPP4 has also been reported to cleave peptides with a penultimate serine, glycine, or valine residue, but with a much lower affinity for these substrates [19,22]. DPP4 exists as both a Type II membrane protein and as a soluble protein [*23]. As a membrane anchored protein, its catalytic C-terminus extends into the extracellular space, allowing for it to target extracellular peptides. When its transmembrane domain is proteolytically cleaved, the soluble form of the protein is shed into the extracellular space, allowing for it to exert its enzymatic activity non-proximally to its cell of origin.
This review provides an update on the role DPP4 plays in the regulation of HSC/HPC function, as well as novel regulatory functions that have been revealed in other cell types that may be relevant to the study of DPP4 in HSCs/HPCs. We also provide an updated list of peptides with putative DPP4 sites and new roles that these potential DPP4 targets might reveal
DPP4 in hematopoietic cell regulation and HSC transplant
A series of papers published over the last ~20 years, including many from this laboratory, have explored the role of DPP4 in regulating HSC/HPC growth, self-renewal, homing, and engraftment [13–18]. DPP4 has been shown to truncate the hematopoietic chemotactic agent CXCL12 (Stromal cell factor-1; SDF-1), and preventing this truncation by DPP4 depletion or inhibition enhanced the chemotactic function of SDF-1 [13,16,18,24]. Recently, a new study has built on this and shown that lymphocytes are a primary source for soluble DPP4, suggesting that activating T-cells may lead to DPP4 modulated changes in the chemotactic ability of specific cells, including the same T-cell compartment that shed the DPP4 [*23]. Further, growth factors such as IL-3, G-CSF, and GM-CSF are truncated by DPP4, which leads to a dramatic decrease in the colony forming potential of cells stimulated with them compared to the full-length proteins [16,24]. Blocking DPP4 activity also leads to higher engraftment capacity for HSCs [16,18,25]. From a clinical perspective, chemical inhibition of DPP4 has been shown to enhance both hematopoietic cell expansion as well as the engraftment capacity of HSCs during transplantation. Sitagliptin, an FDA approved inhibitor of DPP4 used to treat type 2 diabetes, has been used at high doses to increase the efficiency of cord blood transplantation in patients with leukemia or lymphoma [26–28]. Currently, additional progress is being made on increasing the efficacy of DPP4 inhibition with respect to enhancing transplantation efficiency. In vivo treatment with the orally available DPP4 inhibitor Vildagliptin at doses that are typically used to treat diabetic patients has recently been shown to enhance the engraftment capacity of stem cells expanded ex vivo in the presence trepostinil, a prostaglandin agonist [*29]. This is consistent with previous reports that prostaglandin agonists and DPP4 inhibition used in a stepwise regimen can synergize to increase engraftment of HSCs. This potentially opens the door for a more targeted treatment window to avoid off-target effects that may occur with higher doses of DPP4 inhibition [28–30].
Proteins with putative DPP4 cleavage sites
Previous work has identified mature proteins containing putative DPP4 truncations sites, with a particular focus on chemokines, cytokines, and other secreted factors [17]. To obtain a more comprehensive look at additional factors that may be modified by DPP4, we performed a search to identify all mature proteins and small active peptides that contain putative DPP4 truncation sites. This search was run on reviewed Uniprot entries and included both mature protein chains and active peptides, focusing on the human proteome. Mature protein sequences exclude any regions that are removed during protein processing, as in signal peptide cleavages, initiator methionine removals, or other known annotated processing, in order for the protein to become the functional peptide chain as defined by Uniprot. Active peptides are small polypeptides that have 40–50 amino acids or less and have known biological functions [31]. Out of a background list of 21,175 mature proteins and active peptides, 1425 molecules had a penultimate proline residue and 3531 had a penultimate alanine residue for a total of 4956 candidates. An additional 2384 molecules contained a penultimate serine residue, which has also been shown to be a substrate of DPP4, though one that the active site of DPP4 has a much lower affinity for. To focus this search, we subsetted out three different groups of proteins based on their annotated cellular compartment: secreted, membrane bound, and nuclear proteins. Potential implications of truncation of the different types of factors are discussed (See Figure 1 for selected examples).
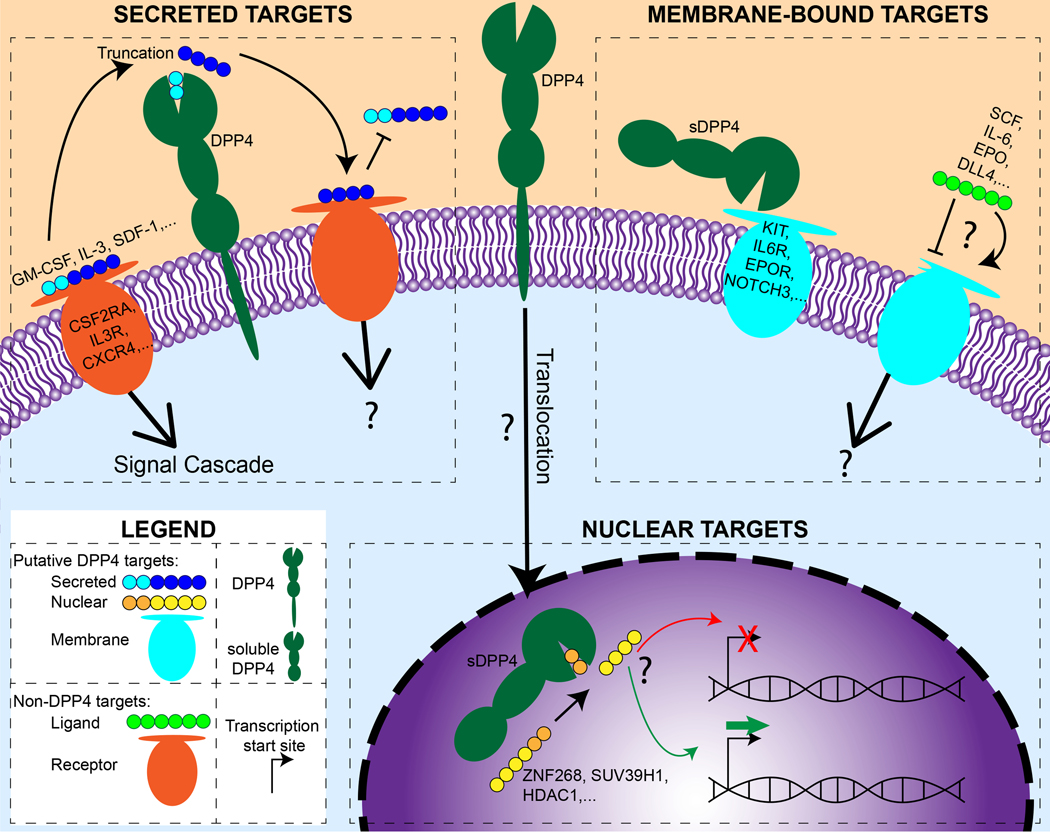
Figure 1:
A graphical representation of the known roles of DPP4 and our proposed new roles. Both membrane bound and soluble DPP4 (dark green protein) has been shown to act on extracellular proteins (polypeptide chain indicated by dark blue and teal circles; the teal circles represent the 2 N-terminal amino acids that are cleaved by DPP4), affecting the binding of these proteins to their receptors (orange membrane protein) and impacting the signals induced by the ligand-receptor interaction. We propose that DPP4 might also cleave membrane bound proteins (teal membrane bound protein) to have similar or novel effects on the signaling pathways. Further, we propose that DPP4 may act on a variety of nuclear factors, including transcription factors (polypeptide chain indicated by yellow and orange circles; the two yellow circles represent the 2 N-terminal amino acids that would be cleaved by DPP4), that may either repress or promote transcription driven by the putative DPP4 targets. Question marks in the diagram denote mechanisms of action that are unknown or have not yet had their mechanism of action elucidated.
Secreted protein and peptide targets
The first subgroup consists of secreted proteins and peptides and consists of 518 mature human proteins and small active peptides with unique molecular functions (see selected targets in Table 1; new putative targets not identified previously [17] are highlighted in red text). Acting on these secreted peptides is the most well-known role of DPP4, and as expected, we identified many of the previously reported experimentally proven DPP4 substrates, including chemokines and cytokines such as GM-CSF, IL-3, SDF-1, EPO, several members of the CCL family of proteins and several members of the CXCL family of proteins. It has been shown that truncation of these factors can affect cell growth, colony formation capacity, and engraftment in mouse transplant models as well as human clinical studies [10,16,18,24,27]. Importantly, at least some of these truncated proteins can also bind to their respective receptors with higher affinity than the full-length proteins [14,16,24]. Therefore, we must discern whether these truncated protein-receptor interactions simply block signaling or induce a different signaling cascade that affects cellular function. We also identified molecules known to be important in other cellular contexts, such as Glucagon like peptide 1 (GLP-1) and Neuropeptide Y (NPY), two well-known substrates that are important in the contexts of diabetes and depression, respectively [32–34]; interestingly the truncation of these factors also have reported roles in regulating HSCs/HSPs [35,36]. A more comprehensive list of hematopoiesis associated regulatory cytokines and chemokines with putative DPP4 truncation sites can be found [17].
Table 1:
Shown are the protein names, N-terminal sequence of the mature protein, and the penultimate residue that would be recognized and cleaved by DPP4, as annotated by Uniprot. The final column indicates whether there are multiple truncation sites available to DPP4. What YES indicates is that after the first truncation, there is another putative DPP4 site remaining on the truncated protein that may be cleaved again. Additional cleavages of the protein may further alter the functions of these molecules, and there may be differences in the function of a singly truncated protein versus a doubly (triply, etc.) truncated peptide. YES *(Serine) indicates that the protein has a second putative DPP4 truncation site, but that this second site is a Serine residue, for which DPP4 has a lower affinity. Proteins highlighted in red are newly identified putative targets of DPP4 that were not listed in a previous review that gave a more comprehensive description specifically of secreted DPP4 targets [17].
| Protein | N-term sequence | Penultimate AA (substrate) | Multiple truncation sites? | Protein | N-term sequence | Penultimate AA (substrate) | Multiple truncation sites? |
|---|---|---|---|---|---|---|---|
|
| |||||||
| CCL11 | GPASVP | P | YES (*Serine) | CXCL8/IL8 | SAKELR | A | NO |
| CCL13 | LAQPDA | A | YES | CXCL9 | TPVVRK | P | NO |
| CCL13 | QPDALN | P | YES | GCG/GLP-1 | HADGSF | A | NO |
| CCL14 | GPYHPS | P | NO | IFNL1/IL29 | GPVPTS | P | YES |
| CCL15 | AADCCT | A | NO | IFNL2/IL28A | VPVARL | P | YES |
| CCL16 | QPKVPE | P | NO | IFNL3/IL28B | VPVARL | P | YES |
| CCL2 | QPDAIN | P | YES | IFNL4 | AAPRRC | A | NO |
| CCL22 | GPYGAN | P | NO | IL10 | SPGQGT | P | NO |
| CCL23 | NPVLLD | P | NO | IL16 | SAASAS | A | YES (*Serine) |
| CCL23 | HATSAD | A | YES (*Serine) | IL17B | QPRSPK | P | YES (*Serine) |
| CCL23 | SADCCI | A | NO | IL18BP | TPVSQT | P | YES (*Serine) |
| CCL3L1 | APLAAD | P | YES | IL1A | SAPFSF | A | NO |
| CCL3L1 | LAADTP | A | NO | IL1B | APVRSL | P | NO |
| CCL4 | APMGSD | P | NO | IL1RA | RPSGRK | P | NO |
| CCL4L1 | APMGSD | P | NO | IL2 | APTSSS | P | YES (*Serine) |
| CCL5 | SPYSSD | P | YES (*Serine) | IL22 | APISSH | P | YES (*Serine) |
| CCL7 | QPVGIN | P | NO | IL23A | RAVPGG | A | YES |
| CCL8 | QPDSVS | P | YES (*Serine) | IL27 | FPRPPG | P | YES |
| CXCL10 | VPLSRT | P | YES (*Serine) | IL3 | APMTQT | P | NO |
| CXCL11 | FPMFKR | P | NO | IL5 | IPTEIP | P | NO |
| CXCL12/ SDF-1 |
KPVSLS | P | YES (*Serine) | IL6 | VPPGED | P | NO |
| CXCL2 | APLATE | P | YES | LIF | SPLPIT | P | YES |
| CXCL6 | GPVSAV | P | YES (*Serine) | NPY | YPSKPD | P | NO |
Membrane protein targets
The next subgroup we looked at consisted of cell membrane localized proteins (see selected targets in Table 2). In order to focus on targets that are most likely to be physiological targets of DPP4, we narrowed this subset to Type I membrane proteins (160 candidates). Because Type I cell membrane proteins have their amine terminus targeted to the extracellular space, the amine terminal dipeptides that serve as putative substrates of DPP4 are available to both membrane-anchored (if the proteins are proximal to each other) and to soluble DPP4 that exists in the extracellular space. Of particular interest, we found that many of these putative DPP4 substrates are receptors involved in development, differentiation, and proliferation, including some well-known hematopoietic regulatory receptors like KIT, EPOR and NOTCH3. Interestingly, the ligand for EPOR (Erythropoietin; EPO) has a putative DPP4 truncation site, but the ligand for KIT (Stem cell factor; SCF) does not [16], which suggests that these signaling pathways may be affected not just by cleavage of the secreted regulatory factors, but potentially by cleavage of the receptors as well.
Table 2:
Shown are the protein names, N-terminal sequence of the mature protein, the penultimate residue that would be recognized and cleaved by DPP4, and the basic molecular function of the protein, as annotated by Uniprot. The final column indicates whether there are multiple truncation sites available to DPP4. What YES indicates is that after the first truncation, there is another putative DPP4 site remaining on the truncated protein that may be cleaved again. Additional cleavages of the protein may further alter the functions of these molecules, and there may be differences in the function of a singly truncated protein versus a doubly (triply, etc.) truncated peptide.
| Protein | Description | N-term sequence | Penultimate AA (substrate) | Multiple truncation sites? |
|---|---|---|---|---|
|
| ||||
| CSF1R/M-CSF-R | M-CSF Receptor | IPVIEP | P | NO |
| DOCK5 | Guanine Nucleotide Exchange Factor | MARWIP | A | NO |
| EPOR | EPO Receptor | APPPNL | P | YES |
| FGFR1 | Fibroblast Growth Receptor | RPSPTL | P | YES |
| FGFR2 | Fibroblast Growth Receptor | RPSFSL | P | NO |
| IL6RA | IL-6 Receptor subunitalpha | LAPRRC | A | NO |
| KIT | Stern Cell Factor (SCF) Receptor | QPSVSP | P | NO |
| MMP16 | Matrix Metalloproetinase | YALTGQ | A | NO |
| MMP24 | Matrix Metalloproetinase | YALTGQ | A | NO |
| NOTCH3 | Notch Signalling Receptor | APPCLD | P | NO |
| TLR5 | Toll-Like Receptor | IPSCSF | P | NO |
| TNFRSF11A | Tumor Necrosis Factor Ligand | IAPPCT | A | YES |
| TNFRSF14 | Tumor Necrosis Factor Ligand | LPSCKE | P | NO |
| TNFRSF1B | Tumor Necrosis Factor Ligand | LPAQVA | P | NO |
| TNFRSF1B | Tumor Necrosis Factor Ligand | VAFTPY | A | NO |
| TNFRSF21 | Tumor Necrosis Factor Ligand | QPEQKA | P | NO |
| TNFRSF8 | Tumor Necrosis Factor Ligand | FPQDRP | P | NO |
| TNFSF10 | Tumor Necrosis Factor Ligand | MAMMEV | A | NO |
| TNFSF12 | Tumor Necrosis Factor Ligand | MAARRS | A | NO |
| TNFSF12 | Tumor Necrosis Factor Ligand | SAPKGR | A | NO |
Nuclear protein targets:
Finally, we also examined potential substrates of DPP4 that are annotated as nuclear proteins (see selected targets in Table 3). Though DPP4 is classically thought of as being either a Type I membrane protein or a soluble protein that resides in the extracellular space after being shed from the membrane, it has recently been demonstrated that DPP4 can also be translocated into the nucleus to bind transcription factors and influence the transcriptional activity of RNA Polymerase II (RNAPII) [37,38,**39]. Further, DPP4 enzymatic activity in the nucleus is reduced but detectable in at least one diseased cellular context [40]. While this nuclear function has as of yet only been shown in certain malignant cells, it will be important to see if there are other physiological contexts in which DPP4 is found to play a role in nuclear function. In our search, we identified 1451 mature nuclear proteins and 1 nuclear active peptide that contains putative DPP4 sites. The mature proteins are highly enriched for Zinc finger (ZNF) domain proteins, such as Znf268, which helps drive erythropoietic differentiation [41]. There is also a high enrichment of proteins associated with epigenetic modifications, such as EHMT1/2 and SUV39H1/2, which are histone methyltransferases with known roles in driving chromatin conformation changes during the process of differentiation [42]. The potential for DPP4 to cleave nuclear factors, though yet unverified, could largely expand the role of DPP4 in regulating hematopoiesis and other cell systems.
Table 3:
Shown are the protein names, N-terminal sequence of the mature protein, the penultimate residue that would be recognized and cleaved by DPP4, and the basic molecular function of the protein, as annotated by Uniprot. The final column indicates whether there are multiple truncation sites available to DPP4. What YES indicates is that after the first truncation, there is another putative DPP4 site remaining on the truncated protein that may be cleaved again. Additional cleavages of the protein may further alter the functions of these molecules, and there may be differences in the function of a singly truncated protein versus a doubly (triply, etc.) truncated peptide. YES *(Serine) indicates that the protein has a second putative DPP4 truncation site, but that this second site is a Serine residue, for which DPP4 has a lower affinity.
| Protein | Description | N-term sequence | Penultimate AA (substrate) | Multiple truncation sites? |
|---|---|---|---|---|
|
| ||||
| ATF7IP2 | Histone methyltransferase cofactor | MASPDR | A | YES |
| BCL2/BAD | Pro-apoptotic factor | MAHAGR | A | YES |
| BCL2L13 | Pro-apoptotic factor | MASSST | A | YES(*Serine) |
| BCL3 | Anti-apoptotic factor | M PRC PA | P | NO |
| BCL6 | Anti-apoptotic factor | MASPAD | A | YES |
| BEX3 | Pro-apoptotic factor | MANIHQ | A | NO |
| EHMT1 | Histone methyltransferase | AAADAE | A | NO |
| EHMT2 | Histone methyltransferase | AAAAGA | A | YES |
| HDAC1 | Histone deacetylase | MAQTQG | A | NO |
| HDAC2 | Histone deacetylase | MAYSQG | A | YES(*Serine) |
| HDAC3 | Histone deacetylase | MAKTVA | A | NO |
| KDM1B | Histone demethylase | MATPRG | A | YES |
| KDM2B | Histone demethylase | MAGPQM | A | YES |
| KDM5A | Histone demethylase | MAGVGP | A | NO |
| KDM7A | Histone demethylase | MAGAAA | A | YES |
| KDM8 | Histone demethylase | MAGDTH | A | NO |
| P0LA1 | DNA polymerase component | MAPVHG | A | NO |
| P0LE2 | DNA polymerase component | MAPERL | A | NO |
| P0LE4 | DNA polymerase component | AAAAAA | A | YES |
| POLH | DNA polymerase component | MATGQD | A | NO |
| P0LR1C | RNA polymerase I component | AASQAV | A | NO |
| P0LR1E | RNA polymerase I component | MAAEVL | A | NO |
| POLR2D | RNA polymerase II component | MAAGGS | A | NO |
| POLR3E | RNA polymerase III component | MANEED | A | NO |
| POLR3G | RNA polymerase III component | MAGNKG | A | NO |
| POLR3GL | RNA polymerase III component | MASRGG | A | NO |
| SET | Transcription factor | APKRQS | P | NO |
| SETD6 | Histone methyltransferase | MATQAK | A | NO |
| SUV39H1 | Histone methyltransferase | MAENLK | A | NO |
| SUV39H2 | Histone methyltransferase | MAAVGA | A | NO |
| TMPO | Thymopoeitin | MPEFLE | P | NO |
| ZNF16 | Zinc fingertranscriptional regulator | MPSLRT | P | NO |
| ZNF268 | Zinc fingertranscriptional regulator | MATRVR | A | NO |
| ZNF91 | Zinc fingertranscriptional regulator | MPGTPG | P | NO |
Overlapping and unique substrates of the DPP4 family
It is highly likely that many of the proteins that we have identified here are not bona fide targets of DPP4. However, it is important to note that other members of the DPP4 family share certain substrate preferences with DPP4, namely the existence of a penultimate proline or alanine. DPP4 is a member of the DPP4 protein family which includes three other exopeptidases that also cleave amine terminal dipeptides, DPP8, DPP9, and Fibroblast activation protein alpha (FAP) [43]. While the shared preferences for a penultimate proline or alanine in the N-terminus of DPP4 family protein substrates is known, whether there is a strong overlap in bona fide substrates or whether there are further considerations that separate substrates of one protein in this family from another have not been clearly elucidated. Additionally, while the prevailing thought has been that the different subunits exist in different cellular compartments, the studies previously mentioned in this review have made apparent that the different DPP4 family proteins cannot always be subdivided by localization. A full understanding of the substrate specificity of the different members of the DPP4 family, as well as a thorough study of the localization of the different members of this family in different cell types and in different physiological contexts, will help us to more fully realize the impact that N-terminal dipeptidase activity has on various cellular functions.
DPP4 in malignancies
DPP4 is known to play a role in a variety of diseases, including diabetes, cardiovascular disease, and Alzheimer’s disease, among others [44,45,*46,47]. DPP4 also plays a major role in several different solid tissue malignancies, including melanoma, lung cancer, and thyroid cancer. There has also been an emerging recognition of DPP4 as an important player in a variety of hematology malignancies, including CML, AML, and ALL [24,48,49]. In fact, a novel drug targeting DPP4 nuclear functions was shown to induce toxicity in leukemic cell lines [**39]. Recently, DPP4 was also revealed as a potential therapeutic target in multiple myeloma (MM) [*50]. Interestingly, a parolog of DPP4, DPP8, was shown to be a potential therapeutic target in MM [*51]. This suggests that the exopeptidase function of both proteins may play a crucial part of the development or progression of this disease. Furthermore, a new study has demonstrated that eosinophil migration in response to tumor growth is regulated in a DPP4 dependent manner [**52]. This study showed that DPP4 inhibition leads to reduced tumor growth by enhancing CCL11-mediated migration of eosinophils. This suggests that the impact of DPP4 driven regulation of hematopoietic cells is wide ranging and may affect many tissues or many different subtypes of disease.
An emerging role for DPP4 in viral infection
The recent outbreak of the novel coronavirus (known as COVID-19 or SARS-CoV-2) has underscored the importance of understanding the host cell proteins involved in viral infection and replication and the implications of targeting these proteins therapeutically. DPP4 is known to act as a functional receptor to which the spike proteins found on MERS-CoV can bind, facilitating host cell invasion and activation of viral replication [53]. Recent work has demonstrated that polymorphisms in DPP4 may affect who can be infected and by what degree by MERS-CoV [54], however the role of DPP4 appears to be largely a protein-protein interaction role, as its catalytic activity does not seem to be required for viral entry to host cells [53]. Currently, there are conflicting reports as to the role of DPP4 in the emerging novel coronavirus outbreak. A new predictive modelling study was able to resolve a high-resolution model for DPP4 bound to the COVID-19 spike protein, suggesting that DPP4 may also play a role in facilitating host cell infection by the novel coronavirus [**55]. However, a separate study showed that only expression of ACE2, a separate cell surface protein, and not DPP4, was able to facilitate host cell infection by COVID-19 in an ex-vivo setting [**56]. These studies highlight the critical need to fully understand the role of DPP4 and other cell surface proteins in facilitating viral entry to host cells. Further, it is tempting to suggest that DPP4 may be a good therapeutic target for the treatment of these viral infections. However, we believe that it is critical to fully elucidate 1) the role of DPP4 catalytic activity in facilitating or preventing viral entry to host cells; 2) the role of DPP4 catalytic activity in host response to viral insult; 3) how therapeutically targeting DPP4 may disrupt these functions; and 4) whether this presents a therapeutic option for treatment or not.
Important considerations when dealing with confirmed or potential DPP4 targets:
It is becoming clearer as we learn more about DPP4 and the DPP4 family of proteins that this group of peptidases can play a critical role in the regulation of a variety of normal cellular processes and during disease, and these proteins are important players in a variety of different tissue contexts. We should therefore be conscious of the fact that many proteins may be targets of DPP4/8/9 activity and that because it is so difficult to resolve the truncated protein from the full length protein due to a difference of only 2 amino acids, observed phenomena involving these proteins may be attributed to the full length protein, the truncated protein, or some combination thereof. To that end, it will be beneficial to continually make physiologically relevant identification of targets of DPP4’s exopeptidase activity more sensitive and accurate. Strong progress been made on this front using mass spectrometry based approaches [57,58], but it would be beneficial to develop new strategies to confirm regulatory roles of the full-length proteins versus the truncated targets of DPP4, such as by developing antibodies that can distinguish between the two. We should also be cautious about the factors that we introduce into our experimental systems that may be normally affected by DPP4, particularly when they are exogenous in nature. For instance, many investigators in the field of hematology rely on recombinant cytokines such as G-CSF or IL-3 for a variety of assays. These cytokines both contain putative DPP4 truncation sites, have been verified to be truncated by DPP4 in vitro, and have significant effects on hematopoietic cell function in vitro and in vivo when they are cleaved. However, some productions of these cytokines that are commercially available are not cleavable by DPP4, and they are not always annotated as such. This is usually the result of an initiator methionine or a tag that is not removed which results in a shift of the penultimate proline or away from the amine terminus, or the deposition of a protein purification associated N-terminal post translational modification which does not exist in nature that protects from DPP4 truncation. These molecules may have completely different effects when they are incapable of being truncated than they normally would have, and this confounding variable should be taken into account during experimental design when cells that express DPP4 are involved. From our experience, the identity of proteins must be confirmed using Mass spectrometry before being used in DPP4-centric assays. Furthermore, there is even some evidence that proteins that contain a proline in the third position from the N-terminus can actually inhibit DPP4 activity [59], suggesting that shifting the proline from the penultimate position to this position could have dramatic effects on DPP4 activity. For this reason, when at all possible, the natural mature protein sequence of these potential and confirmed DPP4 targets should be used and steps should be taken to confirm an assumed truncation event in all studies involving DPP4 with exogenous proteins.
Conclusions
Much of the work involving DPP4 has focused on its role in regulating secreted factors that play regulatory roles in cell proliferation, self-renewal, proliferation, differentiation, and metabolism, to name a few. However, there are emerging new roles for DPP4 in different cellular contexts that have not been fully elucidated. By better understanding the many roles of DPP4 and the DPP4 family of proteins, we may better be able to target them in a therapeutic manner.
Key points.
DPP4 is a protease that truncates many regulatory molecules to dramatically affect cellular functions.
DPP4 is an important therapeutic target in hematologic disorders, hematopoietic stem cell transplant, and other clinical contexts.
Newly discovered functions of DPP4 may highlight novel targets of its peptidase activity, expanding its role in cell regulation.
DPP4 may be an important player in the newly emerging COVID-19 pandemic.
Acknowledgements
We thank Scott Cooper for his helpful discussions regarding the use of exogenous protein constructs in studies involving DPP4 targets and Dr. Pratibha Singh for her helpful discussion regarding DPP4 and viral replication.
Financial support and sponsorship
This work was supported by US Public Health Service Grants R35HL139599 and U54DK106846 to H.E.B and T32DK007519 to J.R. (PI H.E.B).
Footnotes
Conflicts of interest
The authors have no conflicts to declare
Bibliography
- 1.Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: a human perspective. Cell Stem Cell 2012; 10:120–136. [DOI] [PubMed] [Google Scholar]
- 2.Mitroulis I, Kalafati L, Hajishengallis G, Chavakis T. Myelopoiesis in the context of innate immunity. J Innate Immun 2018; 10:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broxmeyer HE. Chemokines in hematopoiesis. Curr Opin Hematol 2008; 15:49–58. [DOI] [PubMed] [Google Scholar]
- 4.Robb L Cytokine receptors and hematopoietic differentiation. Oncogene 2007; 26:6715–6723. [DOI] [PubMed] [Google Scholar]
- 5.Metcalf D Hematopoietic cytokines. Blood 2008; 111:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson NK, Calero-Nieto FJ, Ferreira R, Göttgens B. Transcriptional regulation of haematopoietic transcription factors. Stem Cell Res Ther 2011; 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwasaki H, Mizuno S, Arinobu Y et al. The order of expression of transcription factors directs hierarchical specification of hematopoietic lineages. Genes Dev 2006; 20:3010–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosan C, Godmann M. Genetic and epigenetic mechanisms that maintain hematopoietic stem cell function. Stem Cells Int 2016; 2016:5178965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broxmeyer HE. Cord blood hematopoietic stem cell transplantation. (May 26, 2010), StemBook, ed. The Stem Cell Research Community, StemBook. [Google Scholar]
- 10.Broxmeyer HE. Enhancing the efficacy of engraftment of cord blood for hematopoietic cell transplantation. Transfus Apher Sci 2016; 54:364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang X, Guo B, Capitano M, Broxmeyer HE. Past, present, and future efforts to enhance the efficacy of cord blood hematopoietic cell transplantation. [version 1; peer review: 3 approved]. F1000Res 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, Broxmeyer HE. Progress towards improving homing and engraftment of hematopoietic stem cells for clinical transplantation. Curr Opin Hematol 2019; 26:266–272 [DOI] [PubMed] [Google Scholar]
- 13.Christopherson KW, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol 2002; 169:7000–7008. [DOI] [PubMed] [Google Scholar]
- 14.Broxmeyer HE, Capitano M, Campbell TB, Hangoc G, Cooper S. Modulation of Hematopoietic Chemokine Effects In Vitro and In Vivo by DPP-4/CD26. Stem Cells Dev 2016; 25:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Leary H, Ou X, Broxmeyer HE. The role of dipeptidyl peptidase 4 in hematopoiesis and transplantation. Curr Opin Hematol 2013; 20:314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broxmeyer HE, Hoggatt J, O’Leary HA et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med 2012; 18:1786–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ou X, O’Leary HA, Broxmeyer HE. Implications of DPP4 modification of proteins that regulate stem/progenitor and more mature cell types. Blood 2013; 122:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christopherson KW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science 2004; 305:1000–1003. [DOI] [PubMed] [Google Scholar]
- 19.Martin RA, Cleary DL, Guido DM, Zurcher-Neely HA, Kubiak TM. Dipeptidyl peptidase IV (DPP-IV) from pig kidney cleaves analogs of bovine growth hormone-releasing factor (bGRF) modified at position 2 with Ser, Thr or Val. Extended DPP-IV substrate specificity? Biochim Biophys Acta 1993; 1164:252–260. [DOI] [PubMed] [Google Scholar]
- 20.Mentlein R Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides. Regul Pept 1999; 85:9–24. [DOI] [PubMed] [Google Scholar]
- 21.Metzemaekers M, Van Damme J, Mortier A, Proost P. Regulation of Chemokine Activity - A Focus on the Role of Dipeptidyl Peptidase IV/CD26. Front Immunol 2016; 7:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen HB, Branner S, Wiberg FC, Wagtmann N. Crystal structure of human dipeptidyl peptidase IV/CD26 in complex with a substrate analog. Nat Struct Biol 2003; 10:19–25. [DOI] [PubMed] [Google Scholar]
- 23. Casrouge A, Sauer AV, Barreira da Silva R et al. Lymphocytes are a major source of circulating soluble dipeptidyl peptidase 4. Clin Exp Immunol 2018; 194:166–179. * This study demonstrates the importance of hematopoeitic cell produced DPP4 as a major source of soluble DPP4 for other tissue types. This gives the study of DPP4 in hematopoeitic cells, as well as the new putative targets of DPP4, expanded importance.
- 24.O’Leary HA, Capitano M, Cooper S et al. DPP4 truncated GM-CSF and IL-3 manifest distinct receptor-binding and regulatory functions compared with their full-length forms. Leukemia 2017; 31:2468–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell TB, Hangoc G, Liu Y, Pollok K, Broxmeyer HE. Inhibition of CD26 in human cord blood CD34+ cells enhances their engraftment of nonobese diabetic/severe combined immunodeficiency mice. Stem Cells Dev 2007; 16:347–354. [DOI] [PubMed] [Google Scholar]
- 26.Vélez de Mendizábal N, Strother RM, Farag SS et al. Modelling the sitagliptin effect on dipeptidyl peptidase-4 activity in adults with haematological malignancies after umbilical cord blood haematopoietic cell transplantation. Clin Pharmacokinet 2014; 53:247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farag SS, Srivastava S, Messina-Graham S et al. In vivo DPP-4 inhibition to enhance engraftment of single-unit cord blood transplants in adults with hematological malignancies. Stem Cells Dev 2013; 22:1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farag SS, Nelson R, Cairo MS et al. High-dose sitagliptin for systemic inhibition of dipeptidylpeptidase-4 to enhance engraftment of single cord umbilical cord blood transplantation. Oncotarget 2017; 8:110350–110357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zebedin-Brandl E, Themanns M, Kazemi Z et al. Regimen-dependent synergism and antagonism of treprostinil and vildagliptin in hematopoietic cell transplantation. J Mol Med 2020; 98:233–243. * This study used mouse modelling to enhance the efficacy of low-dose DPP4 inhibition using combinatorial treatment with a prostaglandin antagonist. Enhancing the efficacy of low-dose DPP4 inhibition may make clinical application of DPP4 inhibtion in transplantation more successful.
- 30.Broxmeyer HE, Pelus LM. Inhibition of DPP4/CD26 and dmPGE₂ treatment enhances engraftment of mouse bone marrow hematopoietic stem cells. Blood Cells Mol Dis 2014; 53:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Consortium UniProt. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 2019; 47:D506–D515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner L, Kaestner F, Wolf R et al. Identifying neuropeptide Y (NPY) as the main stress-related substrate of dipeptidyl peptidase 4 (DPP4) in blood circulation. Neuropeptides 2016; 57:21–34. [DOI] [PubMed] [Google Scholar]
- 33.Mentlein R I Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides. Regul Pept 1999; 85: 9–24. [DOI] [PubMed] [Google Scholar]
- 34.Dipeptidyl Peptidase– IV: A Brief Review. Research & Reviews: Journal of Chemistry 2014; 2. [Google Scholar]
- 35.Singh P, Hoggatt J, Kamocka MM et al. Neuropeptide Y regulates a vascular gateway for hematopoietic stem and progenitor cells. J Clin Invest 2017; 127:4527–4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu E, Hu L, Wu H et al. Dipeptidyl Peptidase-4 Regulates Hematopoietic Stem Cell Activation in Response to Chronic Stress. J Am Heart Assoc 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Angevin E, Isambert N, Trillet-Lenoir V et al. First-in-human phase 1 of YS110, a monoclonal antibody directed against CD26 in advanced CD26-expressing cancers. Br J Cancer 2017; 116:1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada K, Hayashi M, Madokoro H et al. Nuclear localization of CD26 induced by a humanized monoclonal antibody inhibits tumor cell growth by modulating of POLR2A transcription. PLoS One 2013; 8:e62304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hayashi M, Madokoro H, Yamada K et al. Novel Antibody-Drug Conjugate with Anti-CD26 Humanized Monoclonal Antibody and Transcription Factor IIH (TFIIH) Inhibitor, Triptolide, Inhibits Tumor Growth via Impairing mRNA Synthesis. Cancers (Basel) 2019; 11. ** This study describes a novel drug that specifically targets the nuclear function of DPP4 in leukemia and other cancers. This expansion of the role of DPP4 and the fact that it may be therapeutically relevant opens the door to many new and potentially clinically important roles for DPP4 in cell regulation.
- 40.Xie Y, Zhu S, Song X et al. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep 2017; 20:1692–1704. [DOI] [PubMed] [Google Scholar]
- 41.Zeng Y, Wang W, Ma J et al. Knockdown of ZNF268, which is transcriptionally downregulated by GATA-1, promotes proliferation of K562 cells. PLoS One 2012; 7:e29518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ugarte F, Sousae R, Cinquin B et al. Progressive chromatin condensation and H3K9 methylation regulate the differentiation of embryonic and hematopoietic stem cells. Stem Cell Rep 2015; 5:728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamson EJ, Keane FM, Tholen S, Schilling O, Gorrell MD. Understanding fibroblast activation protein (FAP): substrates, activities, expression and targeting for cancer therapy. Proteomics Clin Appl 2014; 8:454–463. [DOI] [PubMed] [Google Scholar]
- 44.Röhrborn D, Wronkowitz N, Eckel J. DPP4 in Diabetes. Front Immunol 2015; 6: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angelopoulou E, Piperi C. DPP-4 inhibitors: a promising therapeutic approach against Alzheimer’s disease. Ann Transl Med 2018; 6:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deacon CF. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front Endocrinol (Lausanne) 2019; 10:80. * A very thorough review on the role of DPP4 in modulating small peptide functions in the context of Type 2 Diabetes.
- 47.Xia C, Goud A, D’Souza J et al. DPP4 inhibitors and cardiovascular outcomes: safety on heart failure. Heart Fail Rev 2017; 22:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Andrade CFCG, Bigni R, Pombo-de-Oliveira MS, Alves G, Pereira DA. CD26/DPPIV cell membrane expression and DPPIV activity in plasma of patients with acute leukemia. J Enzyme Inhib Med Chem 2009; 24:708–714. [DOI] [PubMed] [Google Scholar]
- 49.Valent P, Sadovnik I, Ráčil Z et al. DPPIV (CD26) as a novel stem cell marker in Ph+ chronic myeloid leukaemia. Eur J Clin Invest 2014; 44:1239–1245. [DOI] [PubMed] [Google Scholar]
- 50. Nishida H, Hayashi M, Morimoto C, Sakamoto M, Yamada T. CD26 is a potential therapeutic target by humanized monoclonal antibody for the treatment of multiple myeloma. Blood Cancer J 2018; 8:99. * This work discusses a new role for DPP4 in driving multiple myeloma and underscores the importance of studying DPP4 in the context of hematologic malignancies.
- 51. Sato T, Tatekoshi A, Takada K et al. DPP8 is a novel therapeutic target for multiple myeloma. Sci Rep 2019; 9:18094. * This work presents DPP8 as a potential target for treating multiple myeloma, and highlights the need to understand the overlapping roles of the DPP4 family of proteins both in homeostasis and in the context of disease.
- 52. Hollande C, Boussier J, Ziai J et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat Immunol 2019; 20:257–264. ** This study explored the effects of inhibiting DPP4 to block truncation of cytokines that are critical for inducing immune cell chemotaxis to the site of insult. This work demonstrated that DPP4’s function in modulating secreted cytokines has implications not only for hematopoeisis but also for patient response to distant tumors.
- 53.Raj VS, Mou H, Smits SL et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013; 495:251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kleine-Weber H, Schroeder S, Krüger N et al. Polymorphisms in dipeptidyl peptidase 4 reduce host cell entry of Middle East respiratory syndrome coronavirus. Emerg Microbes Infect 2020; 9:155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vankadari N, Wilce JA. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect 2020; 9:601–604. ** This group generated a high resolution model of DPP4 docked to the spike proteins of the novel coronavirus. This demonstrates the critical need to understand the role of DPP4 in the context of this infection to better understand potential mechanisms that can be thereapeutically exploited.
- 56. Hoffmann M, Kleine-Weber H, Schroeder S et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor [published online ahead of print 5 Mar 2020]. Cell 2020. ** This group showed that DPP4 is not a critial protein involved in host cell uptake of the novel coronavirus. This stands in contrast to Raj, et al. and again undescores the need to elucidate the true role of DPP4 in viral infection.
- 57.Tinoco AD, Kim Y-G, Tagore DM et al. A peptidomics strategy to elucidate the proteolytic pathways that inactivate peptide hormones. Biochemistry 2011; 50:2213–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang H, Maqsudi S, Rainczuk A et al. Identification of novel dipeptidyl peptidase 9 substrates by two-dimensional differential in-gel electrophoresis. FEBS J 2015; 282:3737–3757. [DOI] [PubMed] [Google Scholar]
- 59.Wrenger S, Reinhold D, Hoffmann T et al. The N-terminal X-X-Pro sequence of the HIV-1 Tat protein is important for the inhibition of dipeptidyl peptidase IV (DP IV/CD26) and the suppression of mitogen-induced proliferation of human T cells. FEBS Lett 1996; 383:145–149. [DOI] [PubMed] [Google Scholar]