Abstract
Background
The central molecular mechanisms of nonorganic erectile dysfunction remains unknown.
Objective
This study aimed to investigate the association of dopaminergic neurons projecting to the nucleus accumbens of male rats with nonorganic erectile dysfunction.
Materials/methods
Nonorganic erectile dysfunction was induced by chronic mild stress. The sucrose consumption test, sexual behavior test, and apomorphine test were carried out to select depression‐like rats with erectile dysfunction. These rats were considered as nonorganic erectile dysfunction model rats. Dopamine D1/D2 receptor agonist/antagonist was infused into the nucleus accumbens to observe the effect on sexual behavior. Dopaminergic projections to the nucleus accumbens were labeled with both the retrograde tracer FluoroGold injected into the nucleus accumbens and tyrosine hydroxylase. The expression level of tyrosine hydroxylase in dopaminergic neurons projecting to the nucleus accumbens in the ventral tegmental area was measured. The expression levels of dopamine D1/D2 receptors and tyrosine hydroxylase in the nucleus accumbens were also measured.
Results
Nonorganic erectile dysfunction was proved by the sucrose consumption test, sexual behavior test, and apomorphine test in model rats. After central infusion of the dopamine D2 receptor agonist into the nucleus accumbens, the recovery of erectile function, sexual arousal, and motivation were indicated by increased intromission ratio and decreased mount latency. Decreased expression levels of dopamine D2 receptors and tyrosine hydroxylase in the nucleus accumbens and decreased expression level of tyrosine hydroxylase in the dopaminergic neurons projecting to the nucleus accumbens were observed in model rats.
Discussion
These results suggest the impairment of dopaminergic neurons projecting to the nucleus accumbens and dopamine D2 signaling in the nucleus accumbens, causing the suppression of erectile function, sexual arousal, and motivation.
Conclusion
These results suggest that the impaired dopamine D2 receptor pathway in the nucleus accumbens may be one of the main pathway involved in the development of nonorganic erectile dysfunction in the present model.
Keywords: dopamine D2 receptor, nonorganic erectile dysfunction, nucleus accumbens, rat model, ventral tegmental area
1. INTRODUCTION
Nonorganic erectile dysfunction (nED) (also known as psychogenic erectile dysfunction (ED)) is a medical condition characterized by the inability to achieve and/or maintain a penile erection sufficient for successful vaginal intercourse predominantly or exclusively owing to psychological or interpersonal conditions. 1 , 2 Forty‐five percent of patients with ED are affected by nED. 3 nED is common in young adults. The incidence of nED is 13%–85.2% in adult patients under 40 years with ED, while the incidence in patients older than 40 years with ED is about 40%. 4 Although the prevalence of nED is high, the central mechanism of nED remains to be explored. Recently, neuroimaging studies have revealed both structural and functional alternations in nED patients. 5 , 6 , 7 However, the molecular mechanisms of these alternations are poorly understood because human brain tissue is not easily accessible for research.
Based on animal experiments (mainly rats), central neurotransmitters and neuropeptides (including dopamine, oxytocin, serotonin, excitatory amino acids, nitric oxide, adrenocorticotropin, and opioid peptides) that control penile erection have been discovered. 8 Among these neurotransmitters and neuropeptides, dopamine is one of the most important neurotransmitter system for sexual excitation. 9 Chronic and acute stressors (such as immobilization, electric foot shocks, and immersion in cold water) were used to develop nED animal models. After exposure to these stressors, decreased intromission ratio (IR), increased mount latency (ML), and changes in hormone (such as corticosterone, testosterone, beta‐endorphin, and gonadotropin‐releasing hormone) levels in plasma was observed. 10 , 11 Wang et al. 12 induced nED in rats with prenatal immobilization and observed decreased copulatory activity. During exposure to estrous female rats, no significant changes in dopamine release in the nucleus accumbens (NAcc) were observed in prenatally stressed male rats. However, in nED animal models, research on central nerve system is rather limited.
In our previous study, we developed a nED rat model based on chronic mild stress (CMS). 13 Our rat model exhibited similar symptoms of nED to human nED patients. The changes of brain activity observed by functional magnetic resonance imaging in this rat model was also similar to human patients. Lower expression levels of tyrosine hydroxylase (TH) and the dopamine D2 receptor in the basolateral amygdala were observed. Central infusion of a dopamine D2 receptor agonist into the basolateral amygdala partially restored the impaired erectile function. These results suggested decreased activity of dopaminergic neurons, that originate in the ventral tegmental area (VTA). 14 Partial recovery of erectile function after central infusion also suggested the existence of other central mechanisms of nED. Therefore, we hypothesized that other dopaminergic neurons (such as projections to the NAcc) originating in the VTA was also impaired in this nED rat model. As dopaminergic neurons projecting to the NAcc is involved in sexual motivation. 15 In this study, we aimed to investigate the dopaminergic neurons projecting to the NAcc in a nED rat model. To achieve this goal, we induced nED with CMS in rats as previously reported. 16 Then, the sexual behavior of the nED rats after central infusion of a dopamine D1/D2 receptor agonist/antagonist was observed. The activity of dopaminergic neurons projecting to the NAcc and the expression levels of dopamine D1/D2 receptors in the NAcc were also measured in nED rats.
2. METHODS AND MATERIALS
2.1. Subjects
Male Wistar rats (6–7 weeks, 180–220 g) and female Wistar rats (6–7 weeks, 160–180 g) (Nanjing Medical University, Nanjing, China) were caged at 22°C and humidity 40%–60%, with lights on from 08:00 to 20:00 h. The male rats were housed in groups of five at first. One week later, they were housed as singles until the end of the experiment. The female rats were housed in groups of five. Three weeks later, the female rats were ovariectomized bilaterally under isoflurane (Sigma‐Aldrich, Inc., St. Louis, MO, USA) anesthesia. A subcutaneous injection of 20 μg of estradiol benzoate (Aladdin Co., Shanghai, China, dissolved in 0.2 ml of sesame oil) 52 h before the sexual behavior test and 1 mg of progesterone (Aladdin Co., dissolved in 0.2 ml of sesame oil) 4 h before the sexual behavior test were given to female rats to induce artificial estrous. During the sexual behavior test, the female rats showing no proceptive or receptive behavior were replaced by different female rats. All animal experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Experimental Animals Welfare and Ethics Committee of Nanjing Medical University, Nanjing, China.
2.2. Male rat sexual behavior
The sexual behavior tests adopted from Agmo 17 started 2 h after the onset of darkness in a quiet and dimly lit room. After the habituation of a male rat for 10 min in the testing cage (60 × 40 × 30 cm with sawdust bedding), a receptive female was placed in the testing cage. Then, the rats were allowed to copulate for 30 min. During copulation the following parameters were recorded: ML, the latency to the first mount; intromission (vaginal penetration) latency (IL), the latency to the first intromission; mount frequency (MF), the number of mounts until the first ejaculation; intromission frequency (IF), the number of intromissions until the first ejaculation; and IR (IR = IF/(MF + IF)). The male sexual behavior test began when the male rats reached 10 weeks. Any male showing no mount or intromission throughout the whole experiment was excluded from the experiment.
2.3. nED modeling
The nED modeling procedure described by Chen et al. 13 is shown in Figure 1. CMS adapted from Wiborg 18 (shown in Table 1) was used to induce ED in male rats. The sucrose consumption test 18 was used to assess the hedonic state. After food and water deprivation for 14 h, the rats were exposed to 1.5% sucrose solution (in bottles) for 1 h. The sucrose consumption test value was defined as the weight of the consumed sucrose solution. The sucrose consumption test was conducted twice per week during the first 5 weeks and once per week during CMS exposure. The mean of the last three tests before CMS exposure was considered the baseline value of each rat.
FIGURE 1.
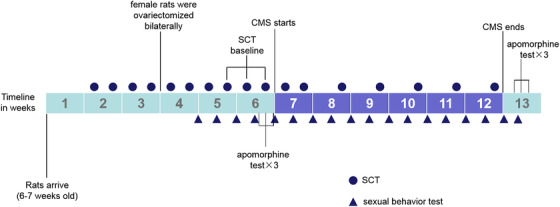
A timeline of nonorganic erectile dysfunction (nED) modeling adopted from Chen et al. 13
TABLE 1.
Stress protocol
| Day | Evening | |
|---|---|---|
| Monday | Intermittent illumination | No stress |
| Tuesday | Water deprivation | Cage tilting |
| Wednesday | Strobe | Wetting |
| Thursday | No stress | Food and water deprivation |
| Friday | No noise test | Grouping |
| Saturday | Food deprivation | Cage tilting |
| Sunday | Cage tilting | Wetting |
Note: Intermittent illumination: light on/off every 2 h; cage tilting: 45°; strobe: stroboscopic lightning (5 Hz); wetting: wetting the bedding by pouring water into the cage; grouping: pairing rats with unfamiliar partners.
The apomorphine (Medchemexpress, Shanghai, China) test was used to assess erectile function. 19 After 10 min of habituation in a test cage (40 × 25 × 20 cm stainless cage) in a quiet observation room, the rat was injected with apomorphine (100 μg/kg) subcutaneously on the back of the neck. A digital camera underneath the test cage recorded erections for 30 min.
Before CMS exposure, the apomorphine test was conducted three times. Any rat showing no erection in any of these apomorphine tests was excluded from the experiment. Then, based on baseline sucrose consumption test values, the rats were divided into two matched groups (control group: n = 20, CMS group: n = 70). The CMS group was exposed to CMS for 6 weeks. After 6 weeks of CMS, the rats with sucrose consumption test value lower than 70% of baseline were regarded as depression‐like rats. Rats with a low IR (<0.5) that showed no erection in any of the three apomorphine tests after 6 weeks of CMS were regarded as rats with ED. Depression‐like rats with ED were considered as nED rats (nED group) and divided into seven matched groups for immunofluorescence analysis (two groups: nED‐IF group, for immunofluorescence analysis; nED‐IFG group, central infused of FluoroGold for immunofluorescence analysis) or central infusion of different drugs (five groups: nED‐vehicle, nED‐D1+, nED‐D1‐, nED‐D2+, and nED‐D2‐ groups) according to the IR. According to the IR, rats in control group were divided into two matched groups: Con‐IF group (for immunofluorescence analysis) and Con‐IFG group (central infused of FluoroGold for immunofluorescence analysis).
2.4. Surgery
Before central drug infusion, nED‐vehicle, nED‐D1+, nED‐D1‐, nED‐D2+, nED‐D2‐, nED‐IFG, and Con‐IFG groups were anesthetized by intraperitoneal injection of ketamine (75 mg/kg, Merck KGaA, Darmstadt, Germany) and xylazine (10 mg/kg, Merck KGaA). The subjects were fixed in a stereotaxic frame (1404, Kopf Instruments, Tujunga, USA). Twenty‐six gauge stainless steel guide cannulas (PlasticsOne, Roanoke, VA, USA) were implanted bilaterally 1 mm above the NAcc (1.6 mm anterior, ±0.7 mm lateral, and 2.0 mm ventral to dura). 20 Acrylic resin and dental cement were used to secure the cannulas to the skull. In order to prevent clogging, each guide cannula was sealed with a stainless steel wire.
2.5. The effect of drugs into the NAcc on sexual behavior
Five days after surgery, sexual behavior before and after central infusion was tested in nED‐vehicle, nED‐D1+, nED‐D1‐, nED‐D2+, nED‐D2‐, nED‐IFG, and Con‐IFG groups. All drugs were dissolved in vehicle (saline), and 0.5 μl was injected in either side. The following drugs were used for the different groups: the dopamine D1‐like receptor agonist D1+ SKF82957 (0.5 μg/0.5 μl/side, Merck KGaA) (nED‐D1+ group, n = 9), the dopamine D1‐like receptor antagonist SCH23390 (1 μg/0.5 μl/side, Merck KGaA) (nED‐D1‐ group, n = 9), the dopamine D2‐like receptor agonist quinpirole (0.5 μg/0.5 μl/side, Merck KGaA) (nED‐D2+ group, n = 9), the dopamine D2‐like receptor antagonist raclopride (2.5 μg/0.5 μl/side, Merck KGaA) (nED‐D2‐ group, n = 8), vehicle (nED‐vehicle group, n = 8), and FluoroGold (10 μg/0.5 μl/side, Abcam, Cambridge, MA, USA) (nED‐IFG group, n = 9 and Con‐IFG group, n = 10). The doses were adopted from previous research on NAcc. 21 , 22 , 23 , 24 , 25 The infusions were carried out 15 min before the sexual behavior test. The stainless steel wire in the guide cannula was removed before infusion. Then the drug or vehicle was infused bilaterally over a period of 2 min with injectors (33 gauge, PlasticsOne) extended 1 mm beyond the tips of the guide cannula. These injector cannulas were connected by polyethylene tubing to 1‐μl Hamilton syringes. The injectors were left in the injection site for 1 min. Then they were replaced by a stainless steel wire after the infusion. Note that 0.5 μl of 2% Evans Blue was infused bilaterally into the NAcc over a period of 30 s in nED‐vehicle, nED‐D1+, nED‐D1‐, nED‐D2+, and nED‐D2‐ groups after the sexual behavior test. Five days after infusion, 0.5 μl of 2% Evans Blue was infused bilaterally into the NAcc over a period of 30 s in nED‐IFG and Con‐IFG groups.
2.6. Histology
Five days after infusion, rats from the Con‐IF, Con‐IFG, nED‐IF, and nED‐IFG groups were selected for immunofluorescence. All rats were deeply anesthetized with isoflurane and perfused intracardially with 200 ml of saline and 500 ml of 4% paraformaldehyde (pH = 7.2–7.4). The brains were removed and stored in 4% paraformaldehyde. The whole NAcc (bregma levels 0.48–2.7 mm, Con‐IF and nED‐IF groups) and VTA (bregma levels ‐6.8 to ‐4.8 mm, Con‐IFG and nED‐IFG groups) were acquired on a vibratome in the coronal plane. 26 Ethanol and xylene were used to dehydrated tissue slices. Then, tissue slices were embedded in paraffin. The tissues were incubated with rabbit anti‐TH (dilution 1:200, Abcam), rabbit anti‐dopamine D1 receptor (D1R, dilution 1:100, Abcam), or rabbit anti‐dopamine D2 receptor (D2R, dilution 1:200, Proteintech, Rosemont, IL, USA) antibodies at 4°C overnight after they were blocked with 5% bovine serum albumin for 30 min. Then, the tissues were incubated with CY3/fluorescein‐conjugated goat anti‐rabbit secondary antibody (dilution 1:300, Jackson, Bar Harbor, ME, USA) for 1 h at room temperature. 4,6‐Diamidino‐2‐phenylindole was used for nucleus staining. Images of the whole brain were acquired by a fluorescence scanner (Pannoramic MIDI, 3D HISTECH, Budapest, Hungary). NAcc and VTA were located according to stereotaxic coordinates. 26 FluoroGold labeling was detected with a Zeiss LSM880 confocal microscope (Zeiss USA, Thornwood, NY, USA). The whole NAcc/VTA in each section (five sections from each subject) was analyzed. The cell bodies of dopaminergic projections to the NAcc were located in the VTA while the axons were located in the NAcc. 9 , 24 Therefore, for statistical analysis of the TH content in Con‐IF and nED‐IF groups, the percentage of TH‐positive area (the sum of five sections for each rat, in pixels) in the whole NAcc (the sum of five sections for each rat, in pixels) was calculated. For statistical analysis of the D1R/D2R content in Con‐IF and nED‐IF groups, the percentage of D1R/D2R‐positive cells (the sum of five sections for each rat)/total cells (the sum of five sections for each rat) in the whole NAcc was calculated. Dopaminergic projections to the NAcc were labeled with both the retrograde tracer FluoroGold injected into the NAcc and TH. For statistical analysis of the TH content in Con‐IFG and nED‐IFG groups, the percentage of cells labeled by both TH and FluoroGold (the sum of five sections for each rat)/total cells (the sum of five sections for each rat) in the whole VTA was calculated. The microinjection sites were located according to stereotaxic coordinates by tissue slices that were embedded in paraffin (shown in Figure 2B).
FIGURE 2.
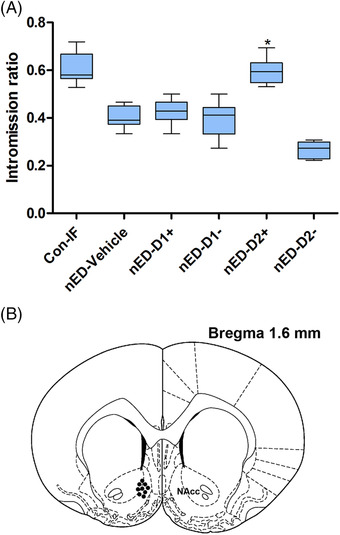
(A) Intromission ratios from the control group for immunofluorescence analysis (Con‐IF), nonorganic erectile dysfunction (nED)‐vehicle, nED‐D1+, nED‐D1‐, nED‐D2+, and nED‐D2‐ groups after central infusion. * p < 0.05; ** p < 0.005 different from the nED‐vehicle group (Con‐IF group was not included in the comparison). Whiskers: maximum and minimum. (B) Schematic representation of the rat brain showing the position of the tip of the cannula (filled circles) for drugs into the nucleus accumbens (NAcc)
2.7. Statistical analysis
Mann–Whitney U‐test was used to analyze sexual behavior data after CMS in control and CMS group to compare differences between the groups. Kruskal–Wallis test was used to analyze sexual behavior data after central infusion in all groups to compare differences among the groups. Student's t‐test was used to analyze the immunofluorescence data to compare differences between the groups. Data were presented as the mean ± standard error of mean (SEM). p < 0.05 was considered to be significant. Data were analyzed by spss 19 (SPSS Inc., Chicago, IL, USA).
3. RESULTS
3.1. nED modeling
Sucrose consumption test values decreased in the CMS group after 6 weeks of exposure to CMS. The rats with sucrose consumption test value lower than 70% of baseline were regarded as depression‐like rats. After 6 weeks of exposure to CMS, no rats exhibited erections during the apomorphine test. Depression‐like rats with a low IR (<0.5) that showed no erection in any of the three apomorphine tests after 6 weeks of CMS were assigned to the nED group (n = 60). ML (p < 0.005) and IL (p < 0.005) were longer in nED group than the control group (shown in Table 2). MF (p < 0.05), IF (p < 0.005), and IR (p < 0.005) were lower in nED group than the control group (shown in Table 2).
TABLE 2.
Sexual behavior after 6 weeks of exposure to chronic mild stress (CMS) (data from control and nonorganic erectile dysfunction (nED) group)
| Control group | nED group | |
|---|---|---|
| ML (s) | 42.0 ± 2.3 | 246.5 ± 29.7** |
| IL (s) | 44.2 ± 2.3 | 316.3 ± 30.3** |
| MF | 16.1 ± 1.7 | 12.4 ± 0.7* |
| IF | 24.9 ± 2.1 | 8.4 ± 0.5** |
| IR | 0.611 ± 0.014 | 0.398 ± 0.006** |
The data are presented as the mean ± standard error of mean (SEM).
Abbreviations: :IF, intromission frequency; IL, intromission latency; IR, intromission ratio; MF, mount frequency; ML, mount latency.
p < 0.05.
p < 0.005 different from the controls.
3.2. The effect of dopamine on sexual behavior
Sexual behavior was tested before and after central drug infusion. Three of the 62 rats were excluded from analysis because of inaccurate cannula placement or equipment failure. Comparing to sexual behavior before surgery, no significant change in any parameters were observed after surgery. Comparing to sexual behavior before central infusion, no significant differences were observed after central infusion of the vehicle (data not shown). These results indicated that surgery or central infusion of the vehicle does not affect sexual behavior. After central infusion of the dopamine D1/D2 receptor agonist/antagonist, a few sexual parameters changed (shown in Table 3 and Figure 2A). After central infusion, ML was shorter (p < 0.05) and IR (p < 0.05) was higher in nED‐D2+ group than nED‐vehicle group. MF (p < 0.005) and IF (p < 0.05) were lower in nED‐D2‐ group than nED‐vehicle group after central infusion.
TABLE 3.
Effects of central drug infusion on sexual behavior (after central drug infusion)
| Con‐IF | nED‐vehicle | nED‐D1+ | nED‐D1‐ | nED‐D2+ | nED‐D2‐ | |
|---|---|---|---|---|---|---|
| ML (s) | 40.3 ± 2.1 | 239.9 ± 68.4 | 226.5 ± 84.3 | 249.3 ± 72.2 | 52.9 ± 8.7* | 378.9 ± 60.8 |
| IL (s) | 42.5 ± 2.0 | 303.4 ± 78.8 | 312.6 ± 86.8 | 319.4 ± 76.3 | 67.8 ± 10.3 | 478.1 ± 56.7 |
| MF | 16.4 ± 1.6 | 13.9 ± 1.3 | 12.1 ± 1.4 | 11.1 ± 0.8 | 14.8 ± 1.6 | 8.1 ± 0.3** |
| IF | 25.4 ± 3.0 | 9.5 ± 1.0 | 9.4 ± 1.6 | 7.7 ± 1.1 | 21.3 ± 1.5 | 3.0 ± 0.3* |
| IR | 0.610 ± 0.014 | 0.403 ± 0.016 | 0.426 ± 0.018 | 0.393 ± 0.024 | 0.595 ± 0.018* | 0.266 ± 0.012 |
Note: The data are presented as the mean ± standard error of mean (SEM).
Abbreviations: Con‐IF, control group for immunofluorescence analysis; IF, intromission frequency; IL, intromission latency; IR, intromission ratio; nED, nonorganic erectile dysfunction; MF, mount frequency; ML, mount latency.
p < 0.05.
p < 0.005 different from nED‐vehicle group (control group was not included in the comparison).
3.3. Dopaminergic neurons and dopamine receptors in the VTA and NAcc
The TH content in the NAcc was lower in the nED rats (nED‐IF) than in the control rats (Con‐IF) (p < 0.005; Figures 3 and 4). No significant difference was observed in the D1R content in the NAcc between the nED‐IF and Con‐IF groups (p > 0.05). The D2R content in the NAcc was lower in the nED‐IF group than in the Con‐IF group (p < 0.005; Figures 3 and 4). The percentage of both TH‐ and FluoroGold‐positive cells in the VTA was lower in the nED rats (nED‐IFG) than in the control rats (Con‐IFG) (p < 0.05; Figures 5 and 6).
FIGURE 3.
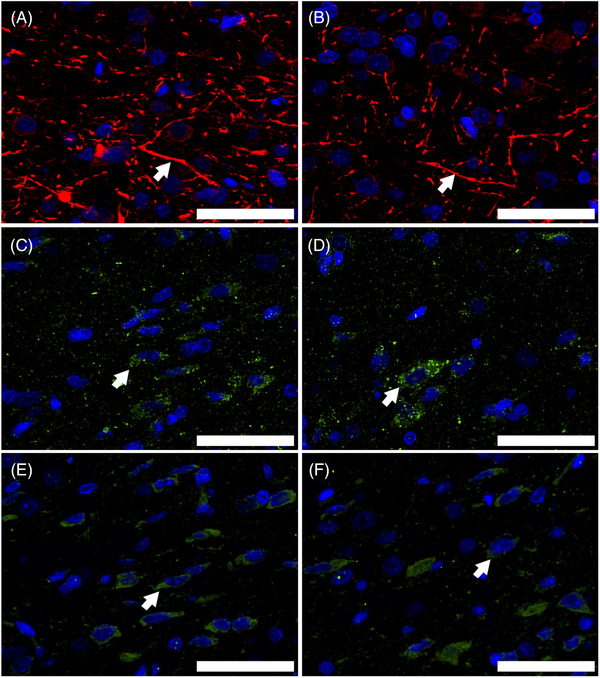
The expression level of tyrosine hydroxylase (TH), dopamine D1 receptor (D1R), and dopamine D2 receptor (D2R) in the nucleus accumbens (NAcc). Representative immunofluorescence staining of TH‐positive axons ((A) control group for immunofluorescence analysis (Con‐IF), (B) nonorganic erectile dysfunction for immunofluorescence analysis (nED‐IF)), D1R‐positive neurons ((C) Con‐IF, (D) nED‐IF), and D2R‐positive neurons ((E) Con‐IF, (F) nED‐IF) (indicated by arrows). Scale bar indicates 50 μm
FIGURE 4.
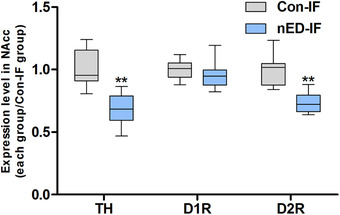
Semi‐quantification results of tyrosine hydroxylase (TH), dopamine D1 receptor (D1R), and dopamine D2 receptor (D2R) expression levels. The percentage of TH‐positive area in the whole nucleus accumbens (NAcc) area (in pixels) was calculated. For the D1R or D2R content analysis, the percentage of positive cells/total cells was calculated. Expression levels are presented as fold‐changes relative to those of the control group for immunofluorescence analysis (Con‐IF). Whiskers: maximum and minimum. * p < 0.05; ** p < 0.005 different from the Con‐IF group
FIGURE 5.
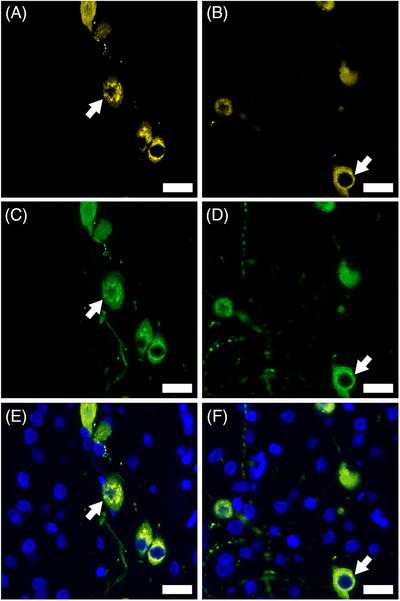
The expression level of tyrosine hydroxylase (TH) in FluoroGold‐labeled cells in the ventral tegmental area (VTA). Dopaminergic projections to the nucleus accumbens (NAcc) were labeled with both the retrograde tracer FluoroGold injected into the NAcc and TH. Representative immunofluorescence staining of FluoroGold‐positive neurons ((A) control group for central infused of FluoroGold for immunofluorescence analysis (Con‐IFG), (B) nonorganic erectile dysfunction for immunofluorescence analysis (nED‐IFG)), TH‐positive neurons ((C) Con‐IFG, (D) nED‐IFG), and FluoroGold + TH‐positive neurons ((E) Con‐IFG, (F) nED‐IFG) (indicated by arrows). Scale bar indicates 20 μm
FIGURE 6.
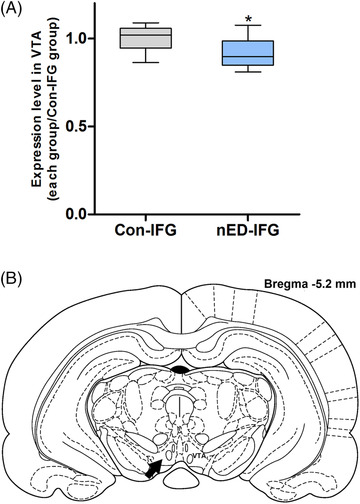
(A) Semi‐quantification results of tyrosine hydroxylase (TH) expression levels in FluoroGold‐labeled cells in the ventral tegmental area (VTA). The percentage of FluoroGold + TH‐positive cells/total cells was calculated. Expression levels are presented as fold‐changes relative to those of the control group for central infused of FluoroGold for immunofluorescence analysis (Con‐IFG). Whiskers: maximum and minimum. * p < 0.05; ** p < 0.005 different from the Con‐IFG group. (B) Schematic representation of the rat brain showing the position of the VTA (indicated by an arrow)
4. DISCUSSION
In this study, CMS was used to induce nED. Depression‐like behavior was suggested by decreased values in the sucrose consumption test. Impaired erectile function was suggested by the decrease in the IR and failure in the apomorphine test. Depression‐like male rats with impaired erectile function were considered as nED rats. The role of dopamine signaling within the NAcc in the development of nED in this model was investigated by central drug infusion and histology.
The nED rats in this study exhibited increased ML and IL, indicating suppression of sexual arousal and motivation. The decrease of IF and IR indicated impaired erectile function, 17 , 27 , 28 which was also proved by apomorphine test. The suppression of erectile function, sexual arousal, and motivation is similar to the symptoms of human nED patients. 9 The impaired erectile function following the induction of depression‐like behavior was similar to the etiology of human nED patients, as depression was one of the major psychiatric factors of nED. 2 , 4 , 29 With similarities in symptoms and etiology, the nED model in our study had good face validity. 30
Dopamine agonists such as apomorphine have been reported to be effective for nED treatment, indicating the impairment of dopamine signaling in nED patients. 31 However, the lack of effect on desire and the side effect such as emesis remains to be problems. 32 , 33 Therefore, the specific site and subtype of dopamine signaling involved in the development of nED can favor the treatment of nED. Neuroanatomy and neurochemical studies have found that, in the paraventricular nucleus of the hypothalamus, oxytocin modulate erectile responses through oxytocinergic neurons projecting to extra‐hypothalamic brain areas (including the VTA) and the spinal cord. 34 , 35 Oxytocin released in the VTA activates dopaminergic projections to the NAcc, leading to an increase of extra‐cellular dopamine concentration in the NAcc. 15 , 25 Dopamine released in the NAcc in turn activates neural pathways that lead to the activation of incerto‐hypothalamic dopaminergic neurons projecting to the paraventricular nucleus. Then, incerto‐hypothalamic dopaminergic neurons stimulate paraventricular oxytocinergic neurons projecting to the spinal cord which mediates penile erection. 8 , 25 In line with this mechanism, oxytocin antagonist injected into the VTA before oxytocin, abolished the increase in extra‐cellular dopamine concentration in the NAcc and penile erection induced by oxytocin. 36 Dopamine D2 receptor antagonist which was injected into the shell of the NAcc reduced penile erection induced by oxytocin injected into the VTA. 25 These findings suggested the important role of dopaminergic projections to the NAcc and dopamine D2 signaling in the NAcc in the process of penile erection. Additionally, mesolimbic dopaminergic neurons (including dopaminergic projections to the NAcc) play a key role in the motivational and rewarding properties of stimuli, such as food, water, and sexual activity. 22 Particularly, dopamine released from these neurons is thought to mediate motivational aspect of sexual activity to reach reward and satisfaction. 37
In the present study, the expression level of TH in dopaminergic projections to the NAcc (labeled with both TH and the retrograde tracer FluoroGold injected into the NAcc) in the VTA was lower in nED rats than control. In the NAcc, the expression levels of TH and D2R was lower in nED rats than control. These results suggest the impairment of dopamine release system and D2R signaling in the NAcc. The suppression of sexual arousal and motivation observed in sexual behavior test in nED rats also suggests the impairment of mesolimbic dopaminergic neurons (including dopaminergic projections to the NAcc). These results are consistent with previous study reporting decreased dopamine release in the NAcc in response to motivational stimuli in rats exposed to CMS than control rats. 38 After central infusion of the D2R agonist, the decrease of ML suggests recovery of sexual arousal and motivation while the increase of IR indicates recovery of erectile function. After central infusion of the D2R antagonist, the decrease of MF and IF indicates further impairment of erectile function. Particularly, IR after central infusion of the D2R agonist was close to health control, indicating a nearly total recovery of erectile function. These results suggest that D2R pathway in the NAcc may be the main pathway involved in the development of nED in the present model. Dopamine release decreased in the NAcc and D2R pathway in the NAcc was inhibited in nED rats, causing the suppression of sexual arousal and motivation. At the same time, neural pathways that lead to the activation of incerto‐hypothalamic dopaminergic neurons projecting to the paraventricular nucleus were also inhibited, reducing dopamine released in the paraventricular nucleus. Eventually, the activity of paraventricular oxytocinergic neurons projecting to the spinal cord was down regulated, which lead to ED.
Previous study on nED rat model also reported lower expression levels of TH and D2R in the basolateral amygdala. Central infusion of a dopamine D2 receptor agonist into the basolateral amygdala partially restored IR in nED model rats. 13 Combining these two studies, our results suggest dopaminergic neurons originating in the VTA and projecting to the NAcc and basolateral amygdala are impaired in nED rats. Additionally, D2R pathways in the NAcc and basolateral amygdala was inhibited in nED rats. The dysfunction of these dopamine pathways contribute to the development of nED from erection, sexual arousal, motivation, and information processing and transmission aspect. 39 , 40
The present study had the following limitations. Before central infusion, nED rats were divided into different matched groups according to IR. However, other sexual behavior parameters such as ML, IL, and IF varied among groups although no significant difference was observed between groups before central infusion. This may lead to the absence of statistical changes in these sexual behavior parameters after central infusion. Further research on groups divided according to other sexual behavior parameters should promote a better understanding of development of nED. In our experiment, there were some rats showed IR > 0.5 despite absence of erection during apomorphine test after 6 weeks of exposure to CMS. Actually, during the CMS exposure, the failure of apomorphine test occurred before the decrease of IR. Erection during apomorphine test require normal function of central dopamine pathway which was impaired in the present nED rat model. 19 We thought normal IR in some rats may be supported by the compensation of other central pathways such as oxytocin and excitatory amino acids. Further studies on other central pathways may provide a better understanding of central mechanisms nED.
5. CONCLUSION
To summarize, we induced nonorganic erectile dysfunction in rats with chronic mild stress and observed decreased expression levels of tyrosine hydroxylase and dopamine D2 receptor in the nucleus accumbens and decreased expression level of tyrosine hydroxylase in the dopaminergic neurons projecting to the nucleus accumbens. After central infusion of the dopamine D2 receptor agonist into the nucleus accumbens, the recovery of erectile function, sexual arousal, and motivation were observed. Our results suggest that the impaired dopamine D2 receptor pathway in the nucleus accumbens may be one of the main pathway involved in the development of nonorganic erectile dysfunction in the present model. Dopamine D2 receptor agonist can recover the impaired erectile function, sexual arousal, and motivation in the present nonorganic erectile dysfunction model. The impaired dopamine D2 receptor pathway in the nucleus accumbens should be helpful for further research on the central mechanism and treatment of nonorganic erectile dysfunction.
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
Conception and design: Guotao Chen, Deshui Yu, Ninghan Feng, and Jian Dong. Acquisition of data: Guotao Chen, Deshui Yu, Yunhong Wu, Lei Hu, and Ninghan Feng. Analysis and interpretation of data: Guotao Chen, Deshui Yu, and Yunhong Wu. Drafting the article: Guotao Chen and Deshui Yu. Revising it for intellectual content: Ninghan Feng and Jian Dong. Final approval of the completed article: Ninghan Feng and Jian Dong.
ACKNOWLEDGMENTS
This work was supported by grants of Special for key research and development plan of Jiangsu Province (social development) (BE2018629), Natural Science Foundation of Jiangsu Province (ZD2021002), “Double innovation project”—double innovation doctor of Jiangsu Province, Top Talent Support Program for young and middle‐aged people of Wuxi Health Committee.
Chen G, Yu D, Wu Y, Dong J, Hu L, Feng N. Dopamine D2 receptors in the nucleus accumbens modulate erectile function in a rat model of nonorganic erectile dysfunction. Andrology. 2022;10:808–817. 10.1111/andr.13171
REFERENCES
- 1. Burnett AL, Nehra A, Breau RH, et al. Erectile dysfunction: AUA guideline. J Urol. 2018;200:633–641. [DOI] [PubMed] [Google Scholar]
- 2. Lizza EF, Rosen RC. Definition and classification of erectile dysfunction: report of the Nomenclature Committee of the International Society of Impotence Research. Int J Impot Res. 1999;11:141–143. [DOI] [PubMed] [Google Scholar]
- 3. Aydin S, Ünal D, Erol H, et al. Multicentral clinical evaluation of the aetiology of erectile dysfunction: a survey report. Int Urol Nephrol. 2001;32:699–703. [DOI] [PubMed] [Google Scholar]
- 4. Zou Z, Lin H, Zhang Y, Wang R. The role of nocturnal penile tumescence and rigidity (NPTR) monitoring in the diagnosis of psychogenic erectile dysfunction: a review. Sex Med Rev. 2019;7:442–454. [DOI] [PubMed] [Google Scholar]
- 5. Chen J, Huang X, Liu S, et al:. Disrupted topological properties of brain networks in erectile dysfunction patients owing predominantly to psychological factors: a structural and functional neuroimaging study. Andrology. 2020;8(2):381–391. [DOI] [PubMed] [Google Scholar]
- 6. Jin C, Guan M, Dong M, et al. Aberrant baseline brain activity in psychogenic erectile dysfunction patients: a resting state fMRI study. Brain Imaging Behav. 2018;12:1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang P, Liu J, Li G, et al. White matter microstructural changes in psychogenic erectile dysfunction patients. Andrology. 2014;2:379–385. [DOI] [PubMed] [Google Scholar]
- 8. Melis MR, Argiolas A. Central control of penile erection: a re‐visitation of the role of oxytocin and its interaction with dopamine and glutamic acid in male rats. Neurosci Biobehav Rev. 2011;35:939–955. [DOI] [PubMed] [Google Scholar]
- 9. Pfaus JG. Pathways of sexual desire. J Sex Med. 2009;6:1506–1533. [DOI] [PubMed] [Google Scholar]
- 10. Bidzinska B, Petraglia F, Angioni S, et al. Effect of different chronic intermittent stressors and acetyl‐l‐carnitine on hypothalamic beta‐endorphin and GnRH and on plasma testosterone levels in male rats. Neuroendocrinology. 1993;57:985–990. [DOI] [PubMed] [Google Scholar]
- 11. Retana‐Márquez S, Bonilla‐Jaime H, Vázquez‐Palacios G, Martínez‐García R, Velázquez‐Moctezuma J. Changes in masculine sexual behavior, corticosterone and testosterone in response to acute and chronic stress in male rats. Horm Behav. 2003;44:327–337. [DOI] [PubMed] [Google Scholar]
- 12. Wang CT, Shui HA, Huang RL, Tai MY, Peng MT, Tsai YF. Sexual motivation is demasculinized, but not feminized, in prenatally stressed male rats. Neuroscience. 2006;138:357–364. [DOI] [PubMed] [Google Scholar]
- 13. Chen G, Chen J, Yang B, Yu W, Chen Y, Dai Y. Dopamine D2 receptors in the basolateral amygdala modulate erectile function in a rat model of nonorganic erectile dysfunction. Andrologia. 2019;51:1–10. [DOI] [PubMed] [Google Scholar]
- 14. Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Succu S, Sanna F, Melis T, Boi A, Argiolas A, Melis MR. Stimulation of dopamine receptors in the paraventricular nucleus of the hypothalamus of male rats induces penile erection and increases extra‐cellular dopamine in the nucleus accumbens: involvement of central oxytocin. Neuropharmacology. 2007;52:1034–1043. [DOI] [PubMed] [Google Scholar]
- 16. Che G, n, Yang B, Chen J, et al. Changes in male rat sexual behavior and brain activity revealed by functional magnetic resonance imaging in response to chronic mild stress. J Sex Med. 2018;15:136–147. [DOI] [PubMed] [Google Scholar]
- 17. Agmo A. Male rat sexual behavior. Brain Res Protoc. 1997;1:203–209. [DOI] [PubMed] [Google Scholar]
- 18. Wiborg O. Chronic mild stress for modeling anhedonia. Cell Tissue Res. 2013;354:155–169. [DOI] [PubMed] [Google Scholar]
- 19. Heaton JP, Varrin SJ, Morales A. The characterization of a bio‐assay of erectile function in a rat model. J Urol. 1991;145:1099–1102. [DOI] [PubMed] [Google Scholar]
- 20. Melis MR, Succu S, Sanna F, Boi A, Argiolas A. Oxytocin injected into the ventral subiculum or the posteromedial cortical nucleus of the amygdala induces penile erection and increases extracellular dopamine levels in the nucleus accumbens of male rats. Eur J Neurosci. 2009;30:1349–1357. [DOI] [PubMed] [Google Scholar]
- 21. Guadarrama‐Bazante IL, Rodríguez‐Manzo G. Nucleus accumbens dopamine increases sexual motivation in sexually satiated male rats. Psychopharmacology. 2019;236:1303–1312. [DOI] [PubMed] [Google Scholar]
- 22. Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–232. [DOI] [PubMed] [Google Scholar]
- 23. Bialy M, Kalata U, Nikolaev‐Diak A, Nikolaev E. D1 receptors involved in the acquisition of sexual experience in male rats. Behav Brain Res. 2010;206:166–176. [DOI] [PubMed] [Google Scholar]
- 24. Farzinpour Z, Mousavi Z, Karimi‐Haghighi S, Haghparast A. Antagonism of the D1‐ and D2‐like dopamine receptors in the nucleus accumbens attenuates forced swim stress‐ and morphine priming‐induced reinstatement of extinguished rats. Behav Brain Res. 2018;341:16–25. [DOI] [PubMed] [Google Scholar]
- 25. Melis MR, Melis T, Cocco C, et al. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur J Neurosci. 2007;26:1026–1035. [DOI] [PubMed] [Google Scholar]
- 26. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. CA, USA: Academic Press; 1997. [Google Scholar]
- 27. Sachs BD, Barfield RJ. Temporal patterning of sexual behavior in the male rat. J Comp Physiol Psychol. 1970;73:359–364. [DOI] [PubMed] [Google Scholar]
- 28. Ferraz MMD, Sab IM, Silva MA, Santos DAS, Ferraz MR. Prenatal hypoxia ischemia increases male rat sexual behavior. J Sex Med. 2015;12:2013–2021. [DOI] [PubMed] [Google Scholar]
- 29. Brotto L, Atallah S, Johnson‐Agbakwu C, et al. Psychological and interpersonal dimensions of sexual function and dysfunction. J Sex Med. 2016;13:538–571. [DOI] [PubMed] [Google Scholar]
- 30. Willner P, Mitchell PJ. The validity of animal models of predisposition to depression. Behav Pharmacol. 2002;13:169. [DOI] [PubMed] [Google Scholar]
- 31. Segraves RT, Bari M, Segraves K, Spirnak P. Effect of apomorphine on penile tumescence in men with psychogenic impotence. J Urol. 1991;145:1174–1175. [DOI] [PubMed] [Google Scholar]
- 32. Proctor JD, Chremos AN, Evans EF, Wasserman AJ. An apomorphine‐induced vomiting model for antiemetic studies in man. J Clin Pharmacol. 2013;18:95–99. [DOI] [PubMed] [Google Scholar]
- 33. Giuliano F, Allard J. Apomorphine SL (Uprima (R)): preclinical and clinical experiences learned from the first central nervous system‐acting ED drug. Int J Impot Res. 2002;14:S53–S56. [DOI] [PubMed] [Google Scholar]
- 34. Argiolas A, Melis MR. The role of oxytocin and the paraventricular nucleus in the sexual behaviour of male mammals. Physiol Behav. 2004;83:309–317. [DOI] [PubMed] [Google Scholar]
- 35. Argiolas A, Melis MR. Central control of penile erection: role of the paraventricular nucleus of the hypothalamus. Prog Neurobiol. 2005;76:1–21. [DOI] [PubMed] [Google Scholar]
- 36. Succu S, Sanna F, Cocco C, Melis T, Melis MR. Oxytocin induces penile erection when injected into the ventral tegmental area of male rats: role of nitric oxide and cyclic GMP. Eur J Neurosci. 2010;28:813–821. [DOI] [PubMed] [Google Scholar]
- 37. Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal‐directed behavior. Nat Neurosci. 2005;8:805–812. [DOI] [PubMed] [Google Scholar]
- 38. Chiara GD, Loddo P, Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatry. 1999;46:1624–1633. [DOI] [PubMed] [Google Scholar]
- 39. Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317. [DOI] [PubMed] [Google Scholar]
- 40. Ferretti A, Caulo M, Del Gratta C, et al. Dynamics of male sexual arousal: distinct components of brain activation revealed by fMRI. Neuroimage. 2005;26:1086–1096. [DOI] [PubMed] [Google Scholar]


