Abstract
Background:
Data from the early pandemic revealed that 0.62% of children hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had an acute arterial ischemic stroke (AIS). In a larger cohort from June 2020 to December 2020, we sought to determine whether our initial point estimate was stable as the pandemic continued and to understand radiographic and laboratory data that may clarify mechanisms of pediatric AIS in the setting of SARS-CoV-2.
Methods:
We surveyed international sites with pediatric stroke expertise to determine numbers of hospitalized SARS-CoV-2 patients <18 years, numbers of incident AIS cases among children (29 days to <18 years), frequency of SARS-CoV-2 testing for children with AIS, and numbers of childhood AIS cases positive for SARS-CoV-2 June 1 to December 31, 2020. Two stroke neurologists with 1 neuroradiologist determined whether SARS-CoV-2 was the main stroke risk factor, contributory, or incidental.
Results:
Sixty-one centers from 21 countries provided AIS data. Forty-eight centers (78.7%) provided SARS-CoV-2 hospitalization data. SARS-CoV-2 testing was performed in 335/373 acute AIS cases (89.8%) compared with 99/166 (59.6%) in March to May 2020, P<0.0001. Twenty-three of 335 AIS cases tested (6.9%) were positive for SARS-CoV-2 compared with 6/99 tested (6.1%) in March to May 2020, P=0.78. Of the 22 of 23 AIS cases with SARS-CoV-2 in whom we could collect additional data, SARS-CoV-2 was the main stroke risk factor in 6 (3 with arteritis/vasculitis, 3 with focal cerebral arteriopathy), a contributory factor in 13, and incidental in 3. Elevated inflammatory markers were common, occurring in 17 (77.3%). From centers with SARS-CoV-2 hospitalization data, of 7231 pediatric patients hospitalized with SARS-CoV-2, 23 had AIS (0.32%) compared with 6/971 (0.62%) from March to May 2020, P=0.14.
Conclusions:
The risk of AIS among children hospitalized with SARS-CoV-2 appeared stable compared with our earlier estimate. Among children in whom SARS-CoV-2 was considered the main stroke risk factor, inflammatory arteriopathies were the stroke mechanism.
Keywords: arteriopathy, child, hospitalization, ischemic stroke, risk factors
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel coronavirus that arose in Wuhan, China in late 2019, is a cause of both arterial and venous thrombotic events in adults and children.1–3 Early reports of young adults presenting with medium and large vessel occlusions leading to arterial ischemic stroke (AIS) raised concern that rates of cerebrovascular events among adults would rise during the pandemic and that there could be a significant increase of pediatric stroke cases as infection rates increased globally.4 However, in a meta-analysis of 145 articles with data from North America, Europe, and Asia that included 108 571 adult patients with COVID-19‚ only 1.4% had acute cerebrovascular diseases including AIS and intracerebral hemorrhage.5 AIS represented 87.4% of all cerebrovascular disease in patients with COVID-19.5
Early in the pandemic‚ an international study identified that ischemic strokes affected 0.82% of pediatric patients hospitalized with evidence of SARS-CoV-2 infection.6 Six of the 8 cases (75%) of ischemic stroke were AIS among children older than 28 days of life. These data were reaffirmed in a large public health database study that included information from 61 sites in the United States.7 Of 1695 patients <21 years with SARS-CoV-2 infection, 12 had stroke, 5 of which were AIS (0.29%).7 Further, case reports of pediatric stroke in the setting of SARS-CoV-2 have emerged due to several stroke mechanisms, including those related to extracorporeal membrane oxygenation, inflammation and thrombosis, arteritis/vasculitis, and inflammatory-type focal cerebral arteriopathy of childhood.7–10 A unique challenge in pediatric stroke is that focal cerebral arteriopathy can take weeks to months after the acute infection to develop,11 raising the question whether the incidence of stroke in children with SARS-CoV-2 may increase during the course of the COVID-19 pandemic.
As the pandemic has continued, we sought to report the frequency of SARS-CoV-2 infection among children with AIS, the frequency of AIS among children hospitalized with SARS-CoV-2, the contribution of the SARS-CoV-2 virus to AIS cases in which the evidence of viral infection was detected, and the associated radiographic and laboratory data that may clarify mechanisms of pediatric AIS in the setting of SARS-CoV-2 infection.
Methods
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design, Participants, and Data Collection
The Publications Committee of the International Pediatric Stroke Study group designed the original data collection tool in April and May 2020.6 The data collection tool was modified in January 2021 (Figure S1). We surveyed International Pediatric Stroke Study and British Paediatric Neurology Association Cerebrovascular Special Interest Group members (52 and 5‚ respectively) as well as 4 non-International Pediatric Stroke Study sites that approached the study group to participate in order to ascertain the following for each month June through December 2020: (1) institutional numbers of pediatric patients <18 years hospitalized with biological evidence of SARS-CoV-2 (polymerase chain reaction, antibody/serology, multisystem inflammatory syndrome in children (MIS-C); symptomatic or asymptomatic from SARS-CoV-2); (2) institutional numbers of incident childhood (age ≥29 days to <18 years) AIS cases; (3) numbers of childhood AIS cases tested for SARS-CoV-2; and (4) institutional numbers of childhood AIS patients positive for SARS-CoV-2. Participating institutions used internal SARS-CoV-2 and stroke tracking systems to obtain AIS and SARS-CoV-2 case numbers to establish this cohort. Sites that reported childhood AIS patients positive for SARS-CoV-2 completed an additional case report form (Figure S2). This form included questions about stroke type and location, presence of established stroke risk factors, severity of COVID-19 illness, and whether the investigator thought SARS-CoV-2 was the main stroke risk factor, a contributory factor, or incidental. The case details were then centrally reviewed by Drs Beslow, Stence, and Dlamini, and a final determination about the virus’s contribution to the stroke was determined by consensus. One survey was completed for each participating site by a site PI with clinical expertise in stroke.
Standard Protocol Approvals, Registrations, and Patient Consents
The survey received an institutional review board waiver from the Hospital for Sick Children, Toronto, Canada. Site institutional review boards approved the case report form for pediatric ischemic stroke patients who tested positive for SARS-CoV-2, and written informed consent was obtained according to institutional guidelines. We adhered to the Strengthening the Reporting of Observational Studies in Epidemiology Guidelines for cohort studies.
Statistical Analysis
Stata 12.0 (StataCorp, College Station, TX) was used for analyses. Counts and frequencies described categorical variables. For the proportion of childhood AISs among pediatric patients hospitalized with SARS-CoV-2, AIS cases positive for SARS-CoV-2 were only included in the numerator if the center provided its number of SARS-CoV-2 hospitalizations. Two sample difference in proportions tests were used to determine whether the proportion of childhood AIS cases tested for SARS-CoV-2, childhood AIS cases positive for SARS-CoV-2, and proportion of SARS-CoV-2 hospitalized pediatric patients with childhood AIS were different in the months June to December 2020 compared with the previously published proportions from March to May 2020. A P of <0.05 was considered statistically significant.
Results
Participating Sites
Sixty-one centers of 74 approached (82.4%) from 21 countries provided AIS data. Participating centers and site investigators are listed in Table S1. Centers are located in Argentina, Australia, Canada (6 sites in 4 provinces), Chile (2 sites), Colombia (4 sites), Egypt, France, Germany, Greece, Hong Kong, India, Israel (2 sites), Italy (2 sites), Philippines, Poland, Serbia, Spain, United Arab Emirates (2 sites), United Kingdom (6 sites), and United States (24 sites in 17 states plus the District of Columbia). Forty-eight centers (78.7%) provided SARS-CoV-2 hospitalization data. Forty-eight sites participated in the first survey, with an additional 13 sites participating in the current survey.
Childhood Arterial Ischemic Stroke and SARS-CoV-2
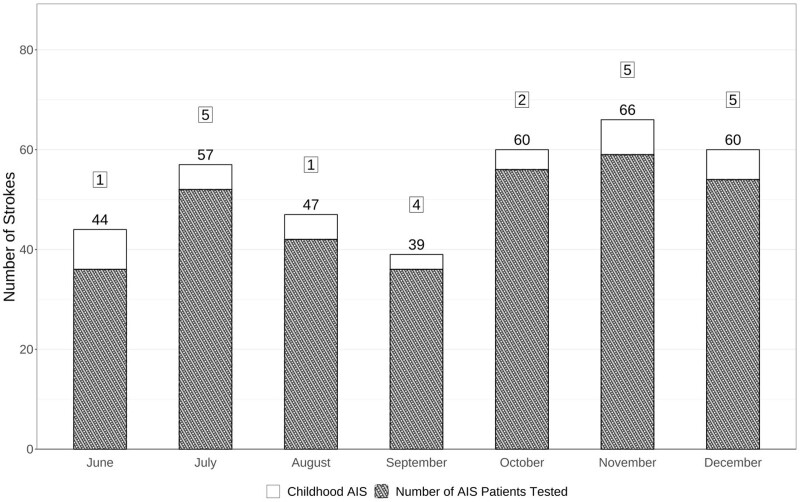
The Figure displays the number of incident acute AIS cases, the number of AIS cases tested for SARS-CoV-2, and the number of AIS cases positive for SARS-CoV-2 each month during the study period. Overall, there were 373 incident acute AIS cases from June to December 2020. SARS-CoV-2 testing was performed in 335/373 (89.8%) compared with 99/166 (59.6%) in March to May, 2020, P<0.0001. Twenty-three of 335 AIS cases tested (6.9%) were positive for SARS-CoV-2 compared with 6/99 tested (6.1%) in March to May 2020, P=0.78.
Figure.
Bar graph with number of children with arterial ischemic strokes (AIS) June 2020 through December 2020. Total number in bar=total number of AIS cases. Hashes=number of AIS cases tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Number in box=number of AIS cases positive for SARS-CoV-2.
Clinical details of 22 of the 23 AIS cases positive for SARS-CoV-2 were available and are presented in Tables S2 and S3. Of 23 AIS cases with SARS-CoV-2 June to December 2020, SARS-CoV-2 was considered the main stroke risk factor in 6 cases (26.1%), a possible contributory factor in 13 (56.5%), incidental in 3 (13.1%), and in 1 (4.3%) clinical details could not be obtained. Ten children (43.5%) had strokes in multiple vascular territories. Five children (21.7%) had large vessel occlusion, 2 in whom SARS-CoV-2 was considered the main stroke risk factor and 3 in whom the virus was contributory to the stroke. Of these 5 children, 3 underwent mechanical thrombectomy. Two children had hemorrhagic transformation of their strokes (Table S2). One of these children had a mechanical thrombectomy, and the other had arteritis/vasculitis.
Thirteen (56.5%) of those with stroke had asymptomatic SARS-CoV-2 infections (Table S2). Of those with symptoms, 7 (30.4%) had COVID-19, and 2 (8.7%) had MIS-C. Three children (13%), including both with MIS-C, were critically ill. Among the 9 with COVID-19 or MIS-C, viral symptoms began a median of 7 days before stroke ictus (range 1–30 days before stroke).
Of those in whom SARS-CoV-2 was considered the main stroke risk factor, 3 had arteritis/vasculitis (1 with MIS-C) and 3 had focal cerebral arteriopathy (Figure S3; one with MIS-C). Of the 16 in whom SARS-CoV-2 was thought to be a possible added risk factor or was not considered likely to have been a major contributor to the stroke, all had at least one other established stroke risk factor. These included cardiac disease, moyamoya arteriopathy, Takayasu arteritis, trauma, critical illness including meningitis, or prothrombotic disorders (Table S2). Inflammatory markers were tested at the discretion of the clinical teams caring for each child. Of the 22 children in whom information on inflammatory markers was available, 17 (77.3%) had elevations in at least 1 value (Table S3). Thirteen of 20 tested (65%) had elevated C-reactive protein, 4 of the 13 tested (30.8%) had elevated ferritin, 3 of the 10 tested (30%) had elevated erythrocyte sedimentation rate, and all 5 (100%) in whom procalcitonin was tested had elevated values. All 13 children in whom anticardiolipin, antiphospholipid, or β2 glycoprotein antibodies were tested were negative, although one was positive for lupus anticoagulant.
Cerebrospinal fluid (CSF) was tested in 8 children (34.8%), 4 of whom had abnormalities. One child with MIS-C and a focal cerebral arteriopathy had normal CSF basic indices but an elevated neopterin of 37 nmol/L (normal <20 nmol/L). Another child with Wiskott Aldrich Syndrome and arteritis had an elevated protein of 232 mg/dL (normal 10–30 mg/dL), 7 white blood cells/mm3, and 7800 red blood cells/mm3. A child with vasospasm versus arteritis and unexplained hydrocephalus had repeated CSF samples from an extraventricular drain with elevated red blood cell counts ranging from 775 to 20 000 red blood cells/mm3. A child who also had cryptococcal meningitis had elevated CSF white blood cell count, red blood cell count, and protein.
Eleven children (47.8%) were tested for varicella infection, including 4 of the 6 with arteriopathic strokes deemed likely to be caused by SARS-CoV-2. All tested for varicella were negative. Testing was by serum polymerase chain reaction in 4 children, CSF by polymerase chain reaction in 4 children (1 also with negative serum serology), and serology in 3 children. Of the remaining 12, 2 were vaccinated against varicella, and 1 was not yet vaccinated due to young age. Two children, both with cardioembolic sources, were unvaccinated. Information about vaccination status against varicella was unavailable in 7.
From 48 centers with SARS-CoV-2 hospitalization data, there were 7231 hospitalized SARS-CoV-2 pediatric patients. Positive tests were by polymerase chain reaction (PCR) in 6385 (88.3%), serology in 378 (5.2%), and were not reported in 468 (6.5%). Twenty-three of 7231 had AIS (0.32% [95% binomial exact CI, 0.20%–0.48%]) compared with 6/971 (0.62% [95% binomial exact CI, 0.23%–1.34%]) from March to May 2020, P=0.14.
Discussion
In this 61-center survey of international pediatric stroke experts, we confirmed that the risk of stroke among over 7000 pediatric patients hospitalized with SARS-CoV-2 was relatively low at 0.32%. This estimate is consistent with results from our first survey study from the early pandemic (0.62%) and with the findings of a study in the United States by LaRovere et al of 5 arterial ischemic strokes among 1695 patients <21 years of age hospitalized with PCR or antibody evidence of SARS-CoV-2 (0.29%).6,7 In the current study‚ the prevalence of SARS-CoV-2 infection among children with AIS tested by PCR or serology was 6.9%. This prevalence is similar to the estimate of 6.1% from March to May 2020 despite the fact that fewer than 60% of childhood AIS patients were tested for SARS-CoV-2 in the earlier study compared with nearly 90% in the current study.6 While the 6.9% SARS-CoV-2 positivity reported in this study could be viewed as low, given the low incidence of pediatric stroke of between 1 and 2 per 100,000 children per year,12 our study findings indicate that SARS-CoV-2 could be an important risk factor for childhood stroke during the pandemic. SARS-CoV-2 infection was considered the likely causative factor in only about 25% of stroke patients positive for SARS-CoV-2, whereas SARS-CoV-2 was considered a possible contributory risk factor in the majority of cases. Notably, many children in this study had established stroke risk factors, making it difficult to assess the contribution of the SARS-CoV-2 infection to stroke in these patients.
After multiple reports of stroke in adults with COVID-19, particularly in young adults,13 numerous studies have sought to understand the mechanisms contributing to this phenomenon. Data comparing patients hospitalized with influenza and stroke compared with patients with SARS-CoV-2 and stroke indicate that there are unique aspects of SARS-CoV-2 infection that contribute to stroke.14 Thus far, clinical and preclinical studies have demonstrated that in the presence of an intact blood-brain barrier, SARS-CoV-2 does not infect the brain.15 However, some individuals with COVID-19 have MRI evidence of cytotoxic lesions of the corpus callosum. These lesions are nonenhancing, diffusion-restricting white matter signal abnormalities isolated to the splenium, which are thought to be caused by a cytokine storm that triggers T cells to breach the blood-brain barrier causing inflammation and intramyelinic edema.16 In the setting of AIS, multiple inflammatory cytokines and other associated markers of inflammation have been identified in both the central nervous system and in the systemic circulation. In a study by McAlpine et al,17 elevated cytokine levels as well as markers of endothelial activation were increased in patients with stroke and COVID-19 compared with patients with stroke without COVID-19. In children, complement activation has also been proposed as a contributing factor to thrombosis and endothelial activation.18 Complement activation in SARS-CoV-2 infection is also under investigation in adults.19 Existing data suggest 2 main consequences of the pro-inflammatory state associated with SARS-CoV-2 infection that may lead to AIS: thrombosis and endothelial dysfunction, both of which are plausibly connected to pediatric AIS as well.20
Inflammatory-mediated thrombosis has been identified as a mechanism for SARS-CoV-2-associated stroke in adults,21 so there is concern that a similar phenomenon may occur in children. In adults, one study identified D-dimer as an independent biomarker for SARS-CoV-2-related AIS, suggesting an association with coagulopathy.22 In a meta-analysis evaluating adult patients, elevated D-dimer and fibrinogen and presence of antiphospholipid antibodies were noted in COVID-19 patients.23 In a pediatric population, Whitworth et al3 found that the risk of thrombotic events in the setting of SARS-CoV-2 infection was the highest among children with MIS-C followed by those with COVID-19, but thrombotic events still occurred among asymptomatic children. All 6 strokes in the current cohort in whom SARS-CoV-2 was considered the likely cause (and in whom we had information) had symptomatic COVID-19 (4) or MIS-C (2) in the month before the stroke. However, of the 13 in whom SARS-CoV-2 was deemed a possible contributory risk factor, ten had asymptomatic infections, suggesting that subclinical inflammation may contribute to strokes among children with SARS-CoV-2. Our data support the findings in another study that 21% of children with SARS-CoV-2 and minimal disease, defined as asymptomatic SARS-CoV-2 infection or those not requiring respiratory support, met criteria for thrombotic microangiopathy.18
Endotheliitis has also been recognized as an important contributor to AIS in SARS-Cov-2 infection, and this was similarly suggested by our data. While 5 of 6 strokes in the current cohort in whom SARS-CoV-2 was the likely cause had evidence of elevated serum inflammatory markers, and one had CSF with elevated neopterin, all 6 had neuroimaging consistent with arteritis (3) or focal cerebral arteriopathy (3). This information indicates that inflammatory arteriopathies may be an important mechanism through which SARS-CoV-2 causes AIS in children. In 2 reported cases of SARS-CoV-2-related stroke in children, 1 with arteritis (also included in this cohort) and 1 with focal cerebral arteriopathy, vessel wall imaging demonstrated enhancement, which further supports an inflammatory mechanism of SARS-CoV-2-related arteriopathies,8,10 and this mirrors findings in adults.24 The hemorrhagic transformation present in 1 child with arteritis may reflect endotheliitis-related vessel fragility. Furthermore, patients with MIS-C present with a different inflammatory milieu than those with COVID-19,25,26 and MIS-C may also contribute to cases of arteriopathy.9 Of note, an older study in Kawasaki disease, a disease that has significant overlap with MIS-C, demonstrated decreased focal cerebral perfusion using single-photon emission computed tomography.27 While focal cerebral arteriopathy is rare in both Kawasaki disease and MIS-C, focal cerebral arteriopathy was an anticipated manifestation of MIS-C based on historical Kawasaki disease literature. These data have provided a basis for consideration of immune-mediated treatments in SARS-CoV-2 arteritis although antithrombotic therapies have remained first line.
Despite the recognition of increased rates of thrombosis-related AIS associated with SARS-CoV-2 infection, the risk of certain treatments in patients with AIS in the setting of COVID-19 may be high. Adult patients with severe COVID-19 and AIS often have concurrent disseminated intravascular coagulation,28 prompting reluctance to administer intravascular thrombolytics. In a small case series of patients who received mechanical thrombectomy, outcomes were overall poor compared with outcomes in patients who underwent mechanical thrombectomy prepandemic.29 Furthermore, in the study by Whitworth et al of pediatric patients, 71% of thromboembolic events not present at hospital admission occurred despite thromboprophylaxis. Future studies are needed both to understand the mechanisms of thrombosis that lead to AIS during SARS-CoV-2 infection better and to improve prevention and treatment of SARS-CoV-2-related-thromboemboli.
Identifying the contribution of SARS-CoV-2 infection to the mechanisms of AIS in children is complex. Definitive determination of stroke etiology can be extremely nuanced in children given the impact of genetic disorders and chronic diseases on the development of cerebrovascular disease. This can make it difficult to understand the contribution of SARS-CoV-2 to a stroke in a child with an underlying disease or condition that is a known predisposition to stroke. Furthermore, not all children were tested for varicella or other viruses that can trigger arteriopathy, so co-infections cannot be ruled out fully. Indeed, the reliance upon clinician expertise to assign SARS-CoV-2 as a risk factor in these patients is a necessary limitation of this study. Given our survey study design with its lack of available data about the non-SARS-CoV-2 stroke patients, the varying timing of the SARS-CoV-2 testing, and the fluctuating and varied prevalence of SARS-CoV-2 infection in the underlying geographic areas, it is not possible to draw firm conclusions about SARS-CoV-2’s contribution to pediatric stroke.
Data from the VIPS I study (Vascular Effects of Infection in Pediatric Stroke) have demonstrated that asymptomatic herpesvirus infections can trigger childhood AIS.30 Similarly, determination of SARS-CoV-2’s role in a stroke is likely to be complex and cannot be based solely on the presence or absence of viral symptoms. In the current study, 13 of 23 cases had asymptomatic SARS-CoV-2 infections, and among those with symptoms, there was a broad range of times between viral symptom onset and stroke. This further complicates the evaluation of mechanisms of pediatric AIS related to SARS-CoV-2 infection, particularly with the knowledge that children are far more likely than adults to have mild or asymptomatic SARS-CoV-2 infections. Case control studies with carefully collected PCR and antibody data along with selective panels of inflammatory markers among both childhood AIS patients and healthy controls are needed to address the virus’s contribution to stroke more completely. The VIPS II study will help to address this important question. Despite the current study’s limitations, the inclusion of children from a large number of international sites provides broadly applicable and generalizable data for the clinician during the diagnostic evaluation of a child presenting with AIS during the ongoing COVID-19 pandemic.
In summary, the risk of AIS among children hospitalized with SARS-CoV-2 appears to be low and similar to the rates reported in previous cohorts. Nearly 7% of incident childhood AIS cases tested were positive for SARS-CoV-2. However, SARS-CoV-2 was the main stroke risk factor in only about a quarter of these, suggesting that SARS-CoV-2 could be incidental in some cases of pediatric stroke or could be an additive factor in children with other known stroke risk factors. When SARS-CoV-2 was considered the primary risk factor for the stroke, inflammatory arteriopathies were the apparent mechanism. Children with elevated inflammatory markers or MIS-C may be at particularly high risk for stroke, and additional studies to determine whether thromboprophylaxis or implementation of other measures to reduce stroke risk are warranted.
Article Information
Acknowledgments
We thank the Auxillium Foundation for their ongoing support of the International Pediatric Stroke Study. We thank the research coordinators at the International Pediatric Stroke Study and The Hospital for Sick Children, Alexandra Linds, Ravianne Tuazon, Daniel Nichol, and Erum Syed, for their project support.
Sources of Funding
None.
Disclosures
Dr Agner reports grant funding from the National Institutes of Health (NIH). Dr Bernard reports grants from the Maternal and Child Health Bureau and from NIH/NINDs.
Supplemental Material
Figures S1–S3
Tables S1–S3
STROBE Checklist
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AIS
- arterial ischemic stroke
- CSF
- cerebrospinal fluid
- MIS-C
- multisystem inflammatory syndrome in children
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- STROBE
- Strengthening the Reporting of Observational Studies in Epidemiology
A list of International Pediatric Stroke Study and British Paediatric Neurology Association Cerebrovascular Special Interest Group participating sites and co-investigators is provided in Table S1.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.038250.
For Sources of Funding and Disclosures, see page 2502.
Contributor Information
Shannon C. Agner, Email: agnersc@wustl.edu.
Jonathan D. Santoro, Email: jdsantoro@chla.usc.edu.
Dipak Ram, Email: dipak.ram@mft.nhs.uk.
Jenny L. Wilson, Email: wilsjen@ohsu.edu.
Dana Harrar, Email: dharrar@childrensnational.org.
Brian Appavu, Email: bappavu@phoenixchildrens.com.
Stuart M. Fraser, Email: stuart.m.fraser@uth.tmc.edu.
Thomas Rossor, Email: trossor@nhs.net.
Marcela D. Torres, Email: marcela.torres@cookchildrens.org.
Manoëlle Kossorotoff, Email: manoelle.kossorotoff@aphp.fr.
Yenny C. Zuñiga Zambrano, Email: yecazuza@gmail.com.
Marta Hernández-Chávez, Email: mhernand@med.puc.cl.
Sahar M.A. Hassanein, Email: saharhassanein@med.asu.edu.eg.
Dimitrios Zafeiriou, Email: Zafeiriou@icloud.com.
Michael M. Dowling, Email: michael.dowling@utsouthwestern.edu.
Ilona Kopyta, Email: ilonakopyta@autograf.pl.
Nicholas V. Stence, Email: nicholas.stence@cuanschutz.edu.
Timothy J. Bernard, Email: Timothy.Bernard@childrenscolorado.org.
Nomazulu Dlamini, Email: nomazulu.dlamini@sickkids.ca.
References
- 1.Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, et al. ; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitworth H, Sartain SE, Kumar R, Armstrong K, Ballester L, Betensky M, Cohen CT, Diaz R, Diorio C, Goldenberg NA, et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood. 2021;138:190–198. doi: 10.1182/blood.2020010218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, et al. Large-Vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2021;16:137–149. doi: 10.1177/1747493020972922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beslow LA, Linds AB, Fox CK, Kossorotoff M, Zuñiga Zambrano YC, Hernández-Chávez M, Hassanein SMA, Byrne S, Lim M, Maduaka N, et al. ; International Pediatric Stroke Study Group. Pediatric ischemic stroke: an infrequent complication of SARS-CoV-2. Ann Neurol. 2021;89:657–665. doi: 10.1002/ana.25991 [DOI] [PubMed] [Google Scholar]
- 7.LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, Walker TC, Singh AR, Dapul H, Hobbs CV, et al. ; Overcoming COVID-19 Investigators. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536–547. doi: 10.1001/jamaneurol.2021.0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulko E, Overby P, Ali S, Mehta H, Al-Mufti F, Gomes W. Vessel wall enhancement and focal cerebral arteriopathy in a pediatric patient with acute infarct and COVID-19 infection. AJNR Am J Neuroradiol. 2020;41:2348–2350. doi: 10.3174/ajnr.A6778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiwari L, Shekhar S, Bansal A, Kumar S. COVID-19 associated arterial ischaemic stroke and multisystem inflammatory syndrome in children: a case report. Lancet Child Adolesc Health. 2021;5:88–90. doi: 10.1016/S2352-4642(20)30314-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appavu B, Deng D, Dowling MM, Garg S, Mangum T, Boerwinkle V, Abruzzo T. Arteritis and large vessel occlusive strokes in children after COVID-19 infection. Pediatrics. 2021;147:e2020023440. doi: 10.1542/peds.2020-023440 [DOI] [PubMed] [Google Scholar]
- 11.Askalan R, Laughlin S, Mayank S, Chan A, MacGregor D, Andrew M, Curtis R, Meaney B, deVeber G. Chickenpox and stroke in childhood: a study of frequency and causation. Stroke. 2001;32:1257–1262. doi: 10.1161/01.str.32.6.1257 [DOI] [PubMed] [Google Scholar]
- 12.Ferriero DM, Fullerton HJ, Bernard TJ, Billinghurst L, Daniels SR, DeBaun MR, deVeber G, Ichord RN, Jordan LC, Massicotte P, et al. ; American Heart Association Stroke Council and Council on Cardiovascular and Stroke Nursing. Management of Stroke in Neonates and Children: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2019;50:e51–e96. doi: 10.1161/STR.0000000000000183 [DOI] [PubMed] [Google Scholar]
- 13.Fifi JT, Mocco J. COVID-19 related stroke in young individuals. Lancet Neurol. 2020;19:713–715. doi: 10.1016/S1474-4422(20)30272-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merkler AE, Parikh NS, Mir S, Gupta A, Kamel H, Lin E, Lantos J, Schenck EJ, Goyal P, Bruce SS, et al. Risk of ischemic stroke in patients with Coronavirus Disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77:1–7. doi: 10.1001/jamaneurol.2020.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farhadian SF, Seilhean D, Spudich S. Neuropathogenesis of acute coronavirus disease 2019. Curr Opin Neurol. 2021;34:417–422. doi: 10.1097/WCO.0000000000000944 [DOI] [PubMed] [Google Scholar]
- 16.Gaur P, Dixon L, Jones B, Lyall H, Jan W. COVID-19-Associated cytotoxic lesions of the corpus callosum. AJNR Am J Neuroradiol. 2020;41:1905–1907. doi: 10.3174/ajnr.A6713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAlpine LS, Zubair AS, Maran I, Chojecka P, Lleva P, Jasne AS, Navaratnam D, Matouk C, Schindler J, Sheth KN, et al. Ischemic stroke, inflammation, and endotheliopathy in COVID-19 patients. Stroke. 2021;52:e233–e238. doi: 10.1161/STROKEAHA.120.031971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diorio C, McNerney KO, Lambert M, Paessler M, Anderson EM, Henrickson SE, Chase J, Liebling EJ, Burudpakdee C, Lee JH, et al. Evidence of thrombotic microangiopathy in children with SARS-CoV-2 across the spectrum of clinical presentations. Blood Adv. 2020;4:6051–6063. doi: 10.1182/bloodadvances.2020003471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma L, Sahu S, Cano M, Kuppuswamy V, Bajwa J, McPhatter J. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. Sci Immunol. 2021;6:eabh2259. doi: 10.1126/sciimmunol.abh2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu SX, Tyagi T, Jain K, Gu VW, Lee SH, Hwa JM, Kwan JM, Krause DS, Lee AI, Halene S, et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2021;18:194–209. doi: 10.1038/s41569-020-00469-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esenwa C, Cheng NT, Luna J, Willey J, Boehme AK, Kirchoff-Torres K, Labovitz D, Liberman AL, Mabie P, Moncrieffe K, et al. Biomarkers of coagulation and inflammation in COVID-19-Associated ischemic stroke. Stroke. 2021;52:e706–e709. doi: 10.1161/STROKEAHA.121.035045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan YK, Goh C, Leow AST, Tambyah PA, Ang A, Yap ES, Tu TM, Sharma VK, Yeo LLL, Chan BPL, et al. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thromb Thrombolysis. 2020;50:587–595. doi: 10.1007/s11239-020-02228-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzacane F, Zito A, Magno S, Persico A, Mazzoleni V, Asteggiano C, Rognone E, Pichiecchio A, Padovani A, Cavallini A, et al. Vessel wall magnetic resonance imaging in COVID-19-associated cryptogenic ischemic stroke. Eur J Neurol. 2022;29:615–619. doi: 10.1111/ene.15128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, Soma VL, Maddux AB, Mourani PM, Bowens C, et al. ; Overcoming COVID-19 Investigators. Characteristics and outcomes of us children and adolescents with Multisystem Inflammatory Syndrome in Children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. doi: 10.1001/jama.2021.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vella LA, Giles JR, Baxter AE, Oldridge DA, Diorio C, Kuri-Cervantes L, Alanio C, Pampena MB, Wu JE, Chen Z, et al. ; UPenn COVID Processing Unit. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci Immunol. 2021;6:eabf7570. doi: 10.1126/sciimmunol.abf7570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ichiyama T, Nishikawa M, Hayashi T, Koga M, Tashiro N, Furukawa S. Cerebral hypoperfusion during acute Kawasaki disease. Stroke. 1998;29:1320–1321. doi: 10.1161/01.str.29.7.1320 [DOI] [PubMed] [Google Scholar]
- 28.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang A, Mandigo GK, Yim PD, Meyers PM, Lavine SD. Stroke and mechanical thrombectomy in patients with COVID-19: technical observations and patient characteristics. J Neurointerv Surg. 2020;12:648–653. doi: 10.1136/neurintsurg-2020-016220 [DOI] [PubMed] [Google Scholar]
- 30.Elkind MS, Hills NK, Glaser CA, Lo WD, Amlie-Lefond C, Dlamini N, Kneen R, Hod EA, Wintermark M, deVeber GA, et al. ; VIPS Investigators*. Herpesvirus infections and childhood arterial ischemic stroke: results of the VIPS study. Circulation. 2016;133:732–741. doi: 10.1161/CIRCULATIONAHA.115.018595 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.