Abstract
Background:
Although tortuosity of the internal carotid artery (ICA) can pose a significant challenge when performing mechanical thrombectomy, few studies have examined the impact of ICA tortuosity on mechanical thrombectomy outcomes.
Methods:
In a registry-based hospital cohort, consecutive patients with anterior circulation stroke in whom mechanical thrombectomy was attempted were divided into 2 groups: those with tortuosity in the extracranial or cavernous ICA (tortuous group) and those without (nontortuous group). The extracranial ICA tortuosity was defined as the presence of coiling or kinking. The cavernous ICA tortuosity was defined by the posterior deflection of the posterior genu or the shape resembling Simmons-type catheter. Outcomes included first pass effect (FPE; extended Thrombolysis in Cerebral Infarction score 2c/3 after first pass), favorable outcome (3-month modified Rankin Scale score of 0–2), and intracranial hemorrhage.
Results:
Of 370 patients, 124 were in the tortuous group (extracranial ICA tortuosity, 35; cavernous ICA tortuosity, 70; tortuosity at both sites, 19). The tortuous group showed a higher proportion of women and atrial fibrillation than the nontortuous group. FPE was less frequently achieved in the tortuous group than the nontortuous group (21% versus 39%; adjusted odds ratio, 0.45 [95% CI, 0.26–0.77]). ICA tortuosity was independently associated with the longer time from puncture to extended Thrombolysis in Cerebral Infarction ≥2b reperfusion (β=23.19 [95% CI, 13.44–32.94]). Favorable outcome was similar between groups (46% versus 48%; P=0.87). Frequencies of any intracranial hemorrhage (54% versus 42%; adjusted odds ratio, 1.61 [95% CI, 1.02–2.53]) and parenchymal hematoma (11% versus 6%; adjusted odds ratio, 2.41 [95% CI, 1.04–5.58]) were higher in the tortuous group. In the tortuous group, the FPE rate was similar in patients who underwent combined stent retriever and contact aspiration thrombectomy and in those who underwent either procedure alone (22% versus 19%; P=0.80). However, in the nontortuous group, the FPE rate was significantly higher in patients who underwent combined stent retriever and contact aspiration (52% versus 35%; P=0.02).
Conclusions:
ICA tortuosity was independently associated with reduced likelihood of FPE and increased risk of postmechanical thrombectomy intracranial hemorrhage.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02251665.
Keywords: catheters, intracranial hemorrhages, punctures, reperfusion, thrombectomy
Mechanical thrombectomy (MT) is the standard treatment for acute ischemic stroke resulting from anterior circulation large vessel occlusion.1 Achieving an extended Thrombolysis in Cerebral Infarction (eTICI) score of ≥2c with few device passes as possible has been considered crucial to maximize the effectiveness of MT.2–5 Therefore, achieving an eTICI 2c/3 reperfusion with first pass, that is, first pass effect (FPE), is the most important target that MT should achieve.6
Tortuous vascular anatomy is a common cause of the failed thrombectomy.7 In curved vessels, stent retrievers (SRs) are stretched and may collapse during retrieval, which lead to loss of interaction with the clot.8,9 Vessel tortuosity also reduces contact between the tip of the aspiration catheter and the clot and impairs clot aspiration.10 Tortuosity reduces the performance of both SR thrombectomy and contact aspiration (CA) catheters, which increases the number of device passes needed and decreases the likelihood of achieving complete reperfusion.
Since the internal carotid artery (ICA) provides the only access to the anterior circulation, extra- and intracranial ICA tortuosity can pose a significant challenge when performing MT. Although the anatomic configurations of both the cervical and cavernous segments of the ICA have been classified previously,11,12 there are few data on the impact of ICA tortuosity on MT outcomes.13 We hypothesized that the ICA tortuosity adversely affects the likelihood of FPE because of reduced MT device performance. We aimed to investigate the association between the ICA tortuosity and outcomes of MT.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Subjects
All patients with acute ischemic stroke admitted to our institute within 7 days from last known well were prospectively registered in the National Cerebral and Cardiovascular Center Stroke Registry.14–17 For the present study, we retrospectively reviewed consecutive patients enrolled in this registry from January 2014 to June 2021, who met the following criteria: (1) occlusion of the ICA or M1 or M2 segment of the middle cerebral artery and (2) those in whom MT was attempted for the occlusion of the ICA or M1 or proximal M2 segment of the middle cerebral artery. Patients with tandem occlusion (concomitant extracranial and distal intracranial artery occlusion) were also eligible. Patients who underwent intracranial angioplasty or stenting were excluded. We also excluded patients in whom ICA tortuosity could not be angiographically evaluated during the MT procedure. Written informed consent for study registration was waived because the study was retrospective in nature and utilized anonymized data. Ethics approval was obtained from the local institutional review board (M23-073-9). The National Cerebral and Cardiovascular Center Stroke Registry is registered with https://www.clinicaltrials.gov (NCT02251665). The present study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort studies.18 A completed STROBE Statement is included in the Supplemental Material.
Mechanical Thrombectomy
All endovascular procedures were performed by neurointerventionalists certified by the Japanese Society for Neuroendovascular Therapy, as recommended by the American Heart Association/American Stroke Association guidelines.1 MT procedures included SR thrombectomy, CA, or combined SR and CA (retrieval of SR and aspiration catheter as a unit).19 Groin puncture or navigation of a microcatheter for MT without use of thrombectomy devices was considered as an MT attempt. Procedural device selection was at the treating physician’s discretion but limited to those available in Japan. The ones used are listed in Table S1. A balloon guide catheter was routinely used and navigated to the extracranial ICA as much as possible. All patients underwent MT under local anesthesia. Conscious sedation was added if necessary. Written informed consent for MT was obtained from each patient or a relative, if the patients had communication difficulties. Reperfusion status after MT was assessed according to the eTICI scale.20 Follow-up brain computed tomography (CT) or magnetic resonance imaging was routinely performed after MT.
Clinical Data Collection
The following clinical data were collected: age, sex, premorbid modified Rankin Scale (mRS) score, baseline National Institutes of Health Stroke Scale (NIHSS) score, atrial fibrillation, cardiovascular risk factors (hypertension, diabetes, current smoking, dyslipidemia), ischemic heart disease (history of myocardial infarction, angina, or coronary revascularization treatment), previous stroke or transient ischemic attack, and systolic blood pressure on admission. The extent of ischemic change in the middle cerebral artery territory was graded using the Alberta Stroke Program Early CT Score on noncontrast CT or diffusion-weighted magnetic resonance imaging. Occlusion sites were determined using digital subtraction angiography on admission. All digital subtraction angiography studies were performed using biplane angiography units: Integris BV 3000 (Philips Medical Systems Nederland B.V., Best, the Netherlands), Allura Clarity FD 20/20 (Philips Medical System Nederland B.V.), and INX-8000 V/HT or V/JE (Canon Medical Systems, Tochigi, Japan). Intravenous thrombolysis was performed with alteplase at 0.6 mg/kg (the dose approved in Japan).21
Extracranial and Cavernous ICA Tortuosity
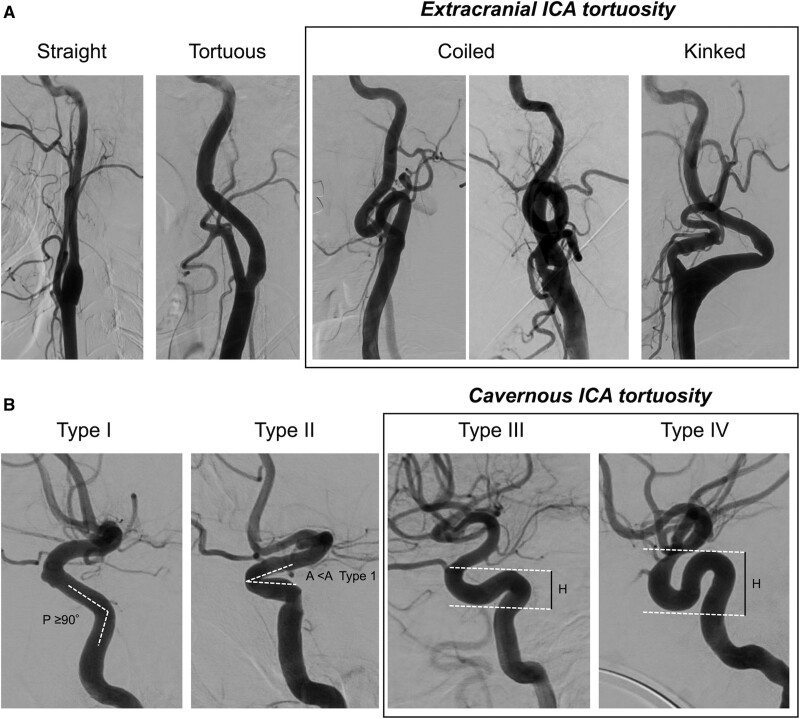
Tortuosity was independently classified by 3 specialists certified by the Japanese Society for Neuroendovascular Therapy (J.K., K.T., and T.Y.) who were blinded to clinical data. Tortuosity of the extracranial ICA was classified based on a previously reported grading system as follows: straight (angle between the centerlines of the common carotid artery and the ICA was <15°), tortuous (angle between the common carotid artery and the ICA centerlines was >15° or S- or C-shaped course of the ICA), coiled (an exaggerated S-shaped curve or circular configuration of the ICA), and kinked (acute [<90°] angulation associated with stenosis).11 The extracranial ICA was considered tortuous if it was coiled or kinked (Figure 1).
Figure 1.
Classification of extracranial and cavernous internal carotid artery (ICA) tortuosity. A, Tortuosity of the extracranial ICA was classified into 4 types as follows: straight (angle between the centerlines of the common carotid artery and the ICA was <15°), tortuous (angle between the common carotid artery and the ICA centerlines was >15; or S- or C-shaped course of the ICA), coiled (an exaggerated S-shaped curve or circular configuration of the ICA), and kinked (acute [<90°] angulation associated with stenosis).11 The extracranial ICA was considered tortuous if it was coiled or kinked. B, Cavernous ICA tortuosity was classified into 4 types based on the geometry of the anterior and posterior genus. Type I has open configurations/angles of anterior and posterior genus (the posterior genu angle [P] ≥90°). Type II is characterized by a closed configuration of the anterior genu (more acute angle of the anterior genu [A] than type I). Type III is defined by posterior deflection of the posterior genu, which gives it a buckled appearance. Type IV is the most tortuous and has a shape characteristic of the Simmons-style angiography catheter where the posterior genu is buckled superiorly compared with the anterior genu.12 H is the height difference of the anterior and posterior genus, measured from the peak of the posterior genu to the trough of the anterior genu. Types III and IV were considered tortuous cavernous ICA.
Cavernous ICA tortuosity was also classified based on a previously reported grading system.12 Briefly, type I has open configurations/angles of the anterior and posterior genus (the posterior genu angle ≥90°). Type II is characterized by a closed configuration of the anterior genu (more acute angle of the anterior genu than type I). Type III is defined by posterior deflection of the posterior genu, which gives it a buckled appearance. Type IV is the most tortuous and has a shape characteristic of the Simmons-style angiography catheter where the posterior genu is buckled superiorly compared with the anterior genu. Types III and IV were considered tortuous cavernous ICA (Figure 1).
Standard anteroposterior and lateral projection angiograms were analyzed for tortuosity classification. Baseline angiograms before MT were used for analysis when possible. For assessment of ICA tortuosity, the data set was divided into 3 subdatasets. In the first session, each of the 3 readers assessed tortuosity in 1 of the 3 subdatasets. In the second, each reader evaluated tortuosity in a sub-dataset different from the first session. The overall interobserver reliability for tortuosity (dichotomized as tortuous or nontortuous) in the extracranial and cavernous ICA was evaluated using the κ-coefficient between the interpretations of the first and second sessions. In cases with a discrepancy between the 2 sessions, disagreements were resolved by discussion among the 3 readers. For analyses, the data on the tortuosity after resolving the disagreements were used.
Patients were divided into 2 groups according to ICA tortuosity (tortuous or nontortuous). The tortuous group included patients with an extracranial or cavernous ICA classified as tortuous. Patients without tortuosity in either the cavernous or extracranial ICA were included in the nontortuous group.
Outcomes
Procedural outcomes were FPE (achievement of eTICI score 2c/3 after first pass), modified FPE (first pass eTICI score ≥2b), final eTICI 2c/3 reperfusion, final eTICI ≥2b reperfusion, time from puncture to eTICI ≥2b reperfusion, procedural time, procedural complications (arterial perforation, arterial dissection, and embolization in a new territory), and failure to reach the target occlusion (failure to navigate MT devices due to difficult access). FPE and modified FPE were assessed only among patients who underwent MT. Clinical outcomes were favorable outcome (mRS score of 0–2 at 3 months), excellent outcome (mRS score of 0–1 at 3 months), poor outcome (mRS score of 5–6 at 3 months), and neurological improvement (a ≥10-point decrease of the NIHSS score from baseline or the score of 0) at 7 days from onset. Safety outcomes were any intracranial hemorrhage (ICH) within 36 hours after the onset, the presence of subarachnoid hemorrhage, parenchymal hematoma (PH), and symptomatic ICH. ICH was assessed using CT or gradient echo magnetic resonance imaging. PH was defined as PH1 and PH2 as described in the hemorrhagic transformation classification system of the European Cooperative Acute Stroke Study.22 Symptomatic ICH was defined as ICH associated with ≥4-point increase in NIHSS score.22
Statistical Analysis
Continuous data are summarized as means with SD or medians with interquartile range. Categorical data are shown as frequencies with percentage. Groups were compared using the Wilcoxon rank-sum test, Kruskal-Wallis test, or Fisher exact test as appropriate. Baseline characteristics and outcomes were compared between groups using univariate analysis. Logistic regression models were then constructed for each binary outcome, and odds ratios with 95% CIs were calculated for the tortuous group using the nontortuous group as reference. The interaction between groups according to ICA tortuosity and the occlusion site regarding FPE was evaluated in the logistic regression model with calculation of P for interaction. Multivariate linear regression models were used to evaluate the association of ICA tortuosity with time from puncture to eTICI ≥2b reperfusion and procedural time. For the procedural and clinical outcomes, the following prespecified variables were included: age, sex, body weight, premorbid mRS score, baseline NIHSS score, occlusion site, first-line MT strategy, and onset-to-puncture time. For the safety outcomes, adjustments for age, sex, body weight, baseline NIHSS score, occlusion site, intravenous thrombolysis, first-line MT strategy, and onset-to-puncture time were performed. Subgroup outcome analyses of patients grouped according to location of ICA tortuosity (extracranial or cavernous) were performed using logistic regression models with the nontortuous group as the reference. Missing data were handled using the pairwise deletion. P<0.05 was considered significant. Statistical analyses were performed using the R Software, version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria), with the following packages: tidyverse, dplyr, sjPlot, magrittr, lmtest, margin, ordinal, and ggplot2.
Results
Patients’ Characteristics
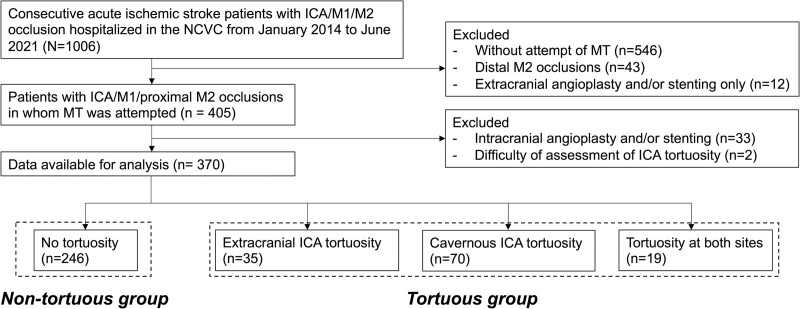
The study flowchart is shown in Figure 2. A total of 370 patients (167 women [45%]; median age, 78 [interquartile range, 71–83] years; median NIHSS score, 19 [interquartile range, 13–24]) were analyzed. Of the 370 patients, 128 patients (35%) had occlusion in the ICA, 157 patients (42%) in the M1 segment, and remaining 85 patients (23%) in the M2 segment. One hundred and twenty patients (32%) were treated by SR thrombectomy, 132 patients (36%) by CA, and 96 patients (26%) by combined SR and CA as the first-line MT strategy. MT was only attempted in 18 patients (5%) due to difficult access in 4 patients, reperfusion before MT in 11 patients, and arterial perforation before MT in 3 patients. Successful reperfusion (final eTICI score ≥2b) was achieved in 315 patients (85%); median time from puncture to reperfusion was 45 minutes (interquartile range, 29–74).
Figure 2.
Study flowchart. ICA indicates internal carotid artery; M1, first segment of the middle cerebral artery; M2, second segment of the middle cerebral artery; MT, mechanical thrombectomy; and NCVC, National Cerebral and Cardiovascular Center.
Tortuosity in the extracranial ICA and cavernous ICA could be assessed by baseline angiography in 269 (73%) and 272 patients (74%), respectively. The overall κ-coefficient for interrater agreement of the dichotomized tortuosity status was 0.70 in the extracranial ICA and 0.79 in the cavernous ICA. For the extracranial ICA, discussion was required to resolve discrepancies in 30 cases; among these, 14 were classified as tortuous and 16 as nontortuous. For the cavernous ICA, discussion was required for 33 cases; 11 were classified as tortuous and 22 as nontortuous.
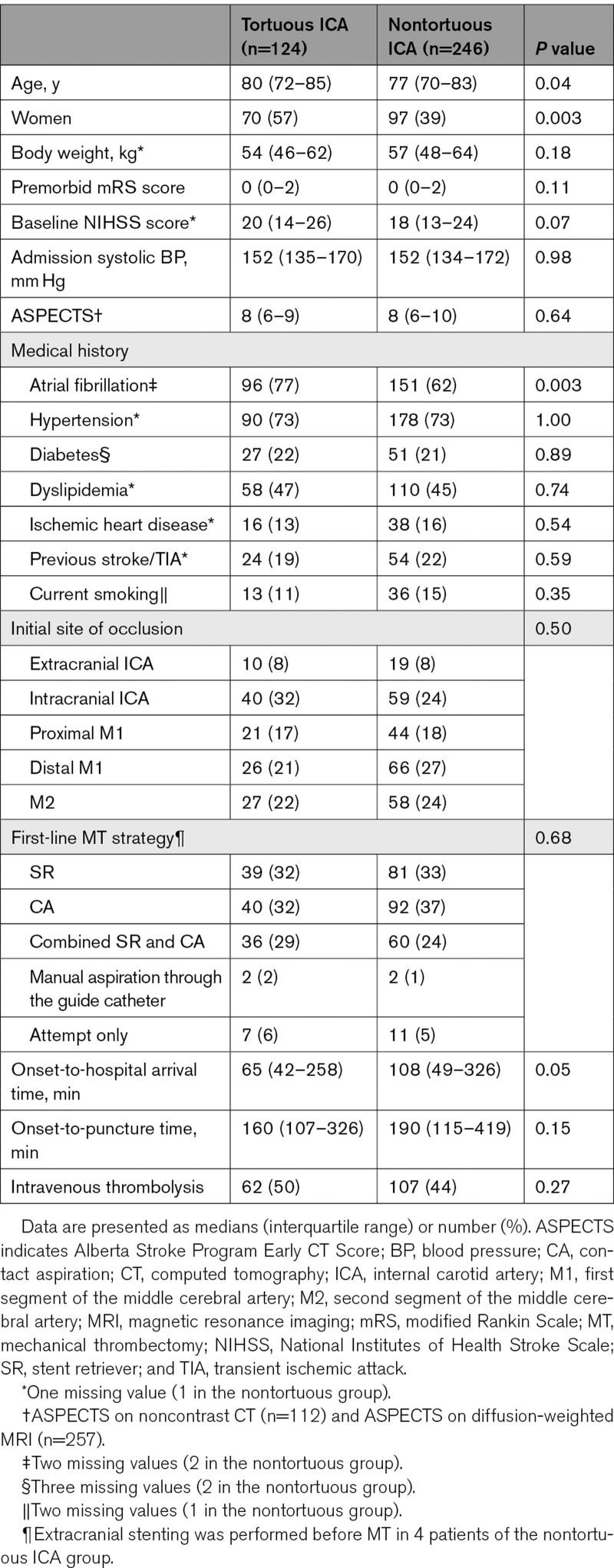
The tortuous group comprised 124 patients (34%). Among these, location of tortuosity was extracranial ICA in 35 patients (9%), cavernous ICA in 70 patients (20%), and both sites in 19 patients (5%). The remaining 246 patients (66%) were assigned to the nontortuous group. Patient characteristics stratified according to the group are shown in Table 1. The tortuous group was older and more female and had shorter time from onset to hospital arrival than the nontortuous group. The tortuous group had the marginally higher baseline NIHSS score. Atrial fibrillation was more frequently observed in the tortuous group than in the nontortuous group. No significant difference was found in the first-line MT strategy (Table 1). Patient characteristics among the 4 subgroups of patients with no tortuosity, with extracranial ICA tortuosity, with cavernous ICA tortuosity, and those with tortuosity at both sites are shown in Table S2.
Table 1.
Patient Characteristics According to the Presence of the ICA Tortuosity
Procedural Outcomes
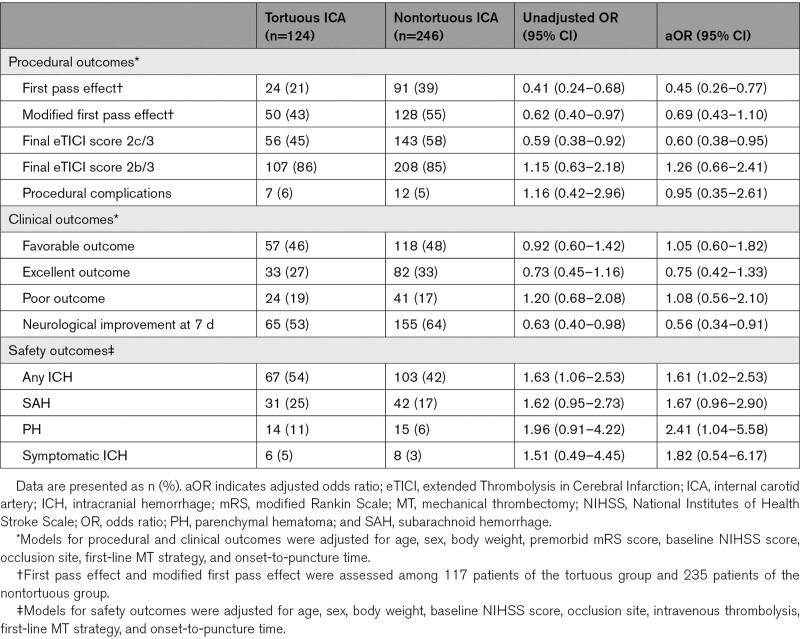
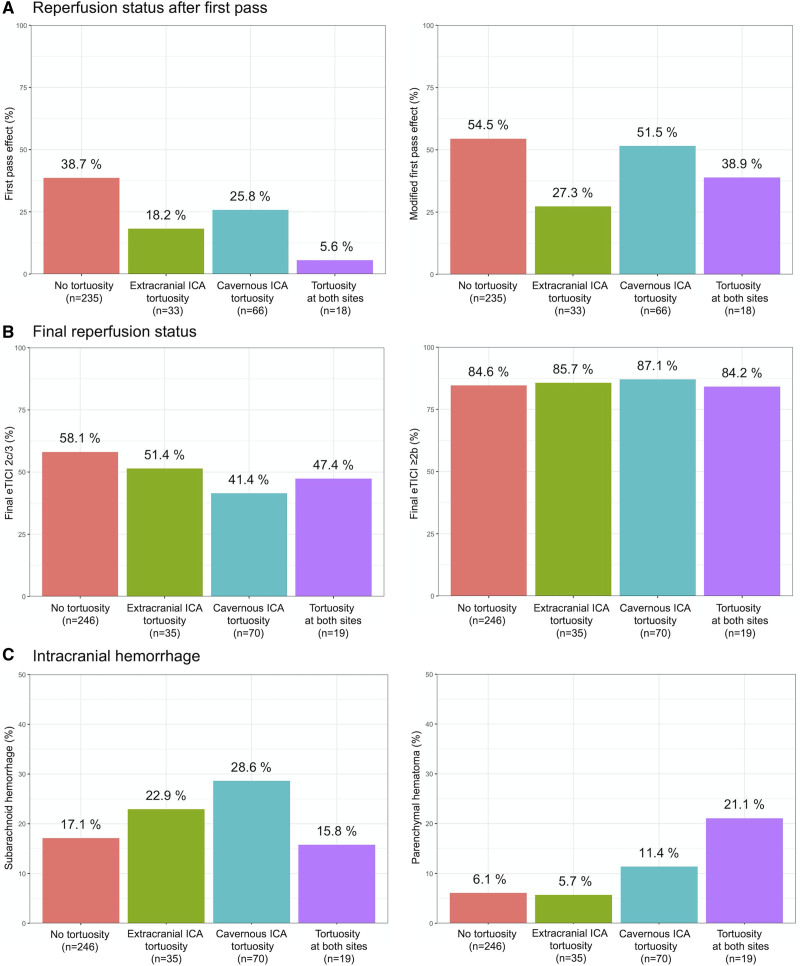
Procedural outcomes according to the group are summarized in Table 2. The rate of FPE was significantly lower in the tortuous group than in the nontortuous group (adjusted odds ratio [aOR], 0.45 [95% CI, 0.26–0.77]). The rate of achieving final eTICI 2c/3 reperfusion was also significantly lower in the tortuous group than in the nontortuous group (aOR, 0.60 [95% CI, 0.38–0.95]). There were no significant differences in the rates of final eTICI ≥2b and procedural complications. Failure to reach the target occlusion was observed in 3 patients (2.5%) of the tortuous group and 1 patient of the nontortuous group (0.4%; P=0.11). The tortuous group had higher number of passes (2 [1–3] versus 1 [1–3]; P=0.04), longer time from puncture to eTICI ≥2b reperfusion (59 [37–89] versus 40 [28–63] minutes; P<0.001), and longer procedural time (80 [56–117] versus 68 [42–112] minutes; P=0.009) than the nontortuous group. On multivariate linear regression analyses, ICA tortuosity was associated with time from puncture to eTICI ≥2b reperfusion (β=23.19 [95% CI, 13.44–32.94]) but not with procedural time (β=9.54 [95% CI, −11.27 to 30.35]). The median time from stroke onset to eTICI ≥2b reperfusion was similar between groups (239 [166–429] versus 232 [171–445] minutes; P=0.90). Procedural outcomes among the 4 subgroups according to location of ICA tortuosity are shown in Figure 3A and 3B.
Table 2.
Outcomes Between Tortuous and Nontortuous Groups
Figure 3.
Procedural outcomes according to the location of internal carotid artery (ICA) tortuosity. Outcomes according to location of ICA tortuosity are shown for (A) reperfusion status after first pass, (B) final reperfusion status, and (C) intracranial hemorrhage. eTICI indicates extended Thrombolysis in Cerebral Infarction.
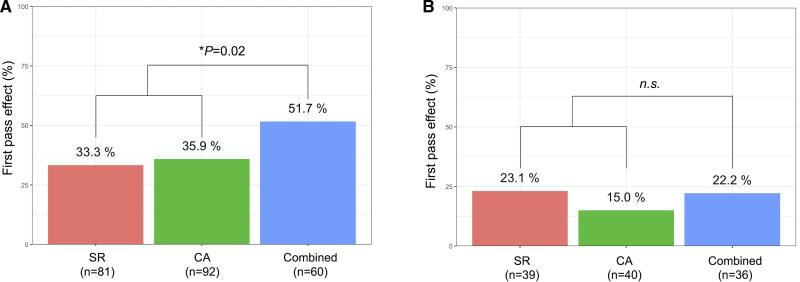
In the tortuous group, the rate of FPE was similar in patients who underwent combined SR and CA and in those who underwent SR thrombectomy or CA alone (22% versus 19%; P=0.80). However, in the nontortuous group, the FPE rate was significantly higher in patients who underwent combined SR and CA (52% versus 35%; P=0.02; Figure 4).
Figure 4.
First pass effect according to the first-line mechanical thrombectomy strategy. The rate of first pass effect according to the first-line mechanical thrombectomy strategy in the (A) nontortuous and (B) tortuous internal carotid artery groups. CA indicates contact aspiration; NS, nonsignificant; and SR, stent retriever. *Fisher exact test.
The rate of FPE was lower in the tortuous group than the nontortuous group in patients with ICA occlusion (aOR, 0.25 [95% CI, 0.10–0.67]) and M1 occlusion (aOR, 0.38 [95% CI, 0.16–0.93]) but not in those with M2 occlusion (aOR, 1.63 [95% CI, 0.49–5.44]; P for interaction, 0.04; Figure S1).
Clinical and Safety Outcomes
The rates of favorable outcome did not differ between groups (Table 2). The excellent outcomes were numerically lower in the tortuous group than in the nontortuous group, though the difference did not reach statistical significance. Neurological improvement at 7 days was less frequent in the tortuous group than in the nontortuous group (aOR, 0.56 [95% CI, 0.34–0.91]). The overall distribution of the mRS scores at 3 months in the tortuous and nontortuous groups is shown in Figure S2.
Regarding safety outcomes, the tortuous group had the higher frequency of any ICH than the nontortuous group (aOR, 1.61 [95% CI, 1.02–2.53]). PH was more frequent in the tortuous group (11%) than in the nontortuous group (6%; aOR, 2.41 [95% CI, 1.04–5.58]). However, frequency of symptomatic ICH did not significantly differ between groups. Frequency of ICH among the 4 subgroups is shown in Figure 3C.
Impact of Tortuosity Location on Outcome
Outcomes according to the location of ICA tortuosity are summarized in Table S3. The FPE rate was lower in patients with extracranial ICA tortuosity alone than in those without tortuosity (aOR, 0.37 [95% CI, 0.14–0.96]). The rate of achieving final eTICI 2c/3 reperfusion was lower in patients with cavernous ICA tortuosity alone than in those without tortuosity (aOR, 0.54 [95% CI, 0.31–0.94]). The frequency of subarachnoid hemorrhage (aOR, 1.96 [95% CI, 1.03–3.71]) was higher in patients with cavernous ICA tortuosity alone than in those without tortuosity. Patients with cavernous ICA tortuosity alone had numerically more PH than those without tortuosity (aOR, 2.65 [95% CI, 0.98–7.21]).
Discussion
The major findings of the present study were that rates of FPE and final eTICI score ≥2c were significantly lower in patients with ICA tortuosity than those without. These patients also had an increased risk of post-MT ICH. The rate of favorable outcome was similar between patients with and without ICA tortuosity. Combined use of SR and CA was associated with a higher FPE rate than SR thrombectomy or CA alone in patients without ICA tortuosity but not in patients with ICA tortuosity.
ICA tortuosity was often present in elderly women with atrial fibrillation. Older age and higher frequency of atrial fibrillation in patients with ICA tortuosity has also been previously reported in other studies.13,23 Moreover, stroke severity tended to be higher in patients with ICA tortuosity than in those without, which may be explained by their older age and multiple comorbidities. Such differences in clinical characteristics between patients with and without ICA tortuosity may influence the relationship between ICA tortuosity and MT outcome.
Prior studies have reported 1.7× higher odds of favorable outcome of FPE compared with non-FPE.6,24 However, the lower FPE rate in patients with ICA tortuosity did not result in a lower favorable outcome rate compared with those without tortuosity in our cohort. Median onset-to-arrival time was 43 minutes shorter in patients with ICA tortuosity, which might compensate the time delay by the failure to achieve FPE; time from stroke onset to eTICI ≥2b reperfusion was similar between groups.
Current devices and techniques would be sufficient to obtain substantial recanalization even for tortuous vessels. A recent study also reported no association between the presence of extracranial ICA tortuosity and TICI ≥2b reperfusion.25 However, the rate of neurological improvement was lower in the tortuous group than in those of the nontortuous group, implicating that there is still room to improve stroke outcomes by improving the FPE rates in patients having tortuous ICA. Achieving the FPE remains challenging in patients with a tortuous ICA.
Our data also demonstrated an association between ICA tortuosity and post-MT ICH. Notably, the odds of a PH developing after MT were twice as high in the tortuous group than the nontortuous group after adjusting for potential confounders. The need to perform more maneuvers during MT because of tortuosity may explain the increased odds of post-MT ICH, as CA and SR both cause endothelial and vessel wall injury.26 Other potential triggers for hemorrhagic transformations could be that the longer procedure time and lower rate of eTICI 2c/3 reperfusion in patients with tortuous ICA.27,28
How to improve procedural outcomes of MT in the tortuous vessel has been poorly documented. In curved vessels, the elongation and flattening of the SR during retrieval maneuvers, described as tapering,9 and the misalignment of the axis between the aspiration catheter and clot can result in poor device-clot interaction.10,29 Consequent fragmentation and distal embolization of the target thrombi in tortuous vessels may lead to the equally lower rate of FPE and final eTICI score 2c/3 in the SR thrombectomy and CA alone. Even use of combined currently available SRs and aspiration catheters could not compensate performance of each device in tortuous vessels. The bendable and segmented design of SR may avoid the tapering phenomenon and improve the rate of FPE in patients with tortuous vessels.8 In addition, the technical tips such as steam shaping of the tip of the aspiration catheter may also be useful to overcome the tortuous vasculature.30 Our study illustrates the unmet need for MT devices that does not lose contact with the clot in the tortuous vasculature.
Angiographic assessment of ICA tortuosity using standard anteroposterior and lateral projections may be useful to predict procedural outcomes in the clinical setting. Prior studies that have investigated the influence of extracranial ICA tortuosity on SR thrombectomy assessed degree of tortuosity using maximal intensity projection images or the tortuosity index in CT angiography.13,23 Although those methods for assessment of the vessel tortuosity are highly accurate, they may be time-consuming for acute clinical setting. The present study also has the strength in the assessment of the cavernous ICA tortuosity and thrombectomy performance in various MT strategies.
The present study has several limitations. First, the inherent bias due to the single-center retrospective nature of the study with relatively small numbers of patients in each group might affect the results. Second, we only assessed curvature in the ICA; curvature in other relevant vessels, including the middle cerebral artery, aorta, and cervical vessels was not assessed. Unmeasured confounding factors with respect to these vessels may have affected our results. Third, we assessed vessel anatomy based on anteroposterior and lateral projections; however, 3-dimensional imaging provides the most precise angle measurements. Fourth, interrater agreement of ICA tortuosity assessed by the present methodology was moderate and may become even lower if assessed in an acute clinical setting, which might limit the clinical applicability of the present findings.
Conclusions
ICA tortuosity was independently associated with lower rates of FPE and higher frequency of post-MT ICH. ICA tortuosity had no significant effect on symptomatic ICH and favorable outcome. For the tortuous ICA, the synergistic effect of combined SR and CA was not observed, and the rate of FPE was equally low among all current MT strategies. To improve outcomes of MT, development of devices and techniques that show promise in the treatment of patients with tortuous vessels will be needed.
Article Information
Acknowledgments
We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this article.
Sources of Funding
This study was supported by an Intramural Research Fund of the National Cerebral and Cardiovascular Center (20-4-3) and Japan Agency for Medical Research and Development (JP21lk0201094, JP21lk0201109).
Disclosures
Dr Koge reports a lecturer’s fee from Medtronic. Dr Tanaka reports lecturer’s fees from Johnson & Johnson and Stryker. Dr Yoshimoto reports lecture’s fees from Takeda Pharmaceutical and Nippon Boehringer Ingelheim, outside the submitted work. Dr Ohta reports receiving honoraria for speaking engagements from Stryker, Medicos Hirata, Kaneka, Terumo, Takeda Pharmaceutical, Tanabe Mitsubishi, Pfizer Japan, Daiichi Sankyo, Otsuka Pharmaceutical, Bayer, MSD Co, Ltd, Kyorin Pharmaceutical, Codman, and Eisai and royalties for publishing from Stryker and Nipro. Dr Satow reports research funding from CANON Medical System and lecture’s fees from Medtronic and Stryker. Dr Kataoka reports research funding from Eizai and lecture’s fee from Daiichi Sankyo, Otsuka Pharmaceutical, and Carl Zeiss. Dr Ihara reports lecturer’s fees from Daiichi Sankyo and Eisai and grant support from Panasonic, GE Precision Healthcare LLC, Bristol Myers Squibb, and Shimadzu Corporation. Dr Koga reports honoraria from Bayer Yakuhin and Daiichi Sankyo; scientific advisory board from Ono; and research supports from Takeda, Daiichi Sankyo, Nippon Boehringer Ingelheim, Astellas, and Shionogi, outside the submitted work. Dr Isobe reports lecturer’s fees from Biogen Japan, Chugai Pharmaceutical, Takeda, Mitsubishi Tanabe Pharma, Novartis, and Alexion Pharma, and research funding from Japan Blood Products Organization. Dr Toyoda reports lecturer’s fees from Daiichi Sankyo, Takeda, Bayer, and Bristol Myers Squibb. The other authors report no conflicts.
Supplemental Material
Tables S1–S3
Figures S1–S2
STROBE Statement
Supplementary Material
Nonstandard Abbreviations and Acronyms
- aOR
- adjusted odds ratio
- CA
- contact aspiration
- CT
- computed tomography
- eTICI
- extended Thrombolysis in Cerebral Infarction
- FPE
- first pass effect
- ICA
- internal carotid artery
- ICH
- intracranial hemorrhage
- mRS
- modified Rankin Scale
- MT
- mechanical thrombectomy
- NIHSS
- National Institutes of Health Stroke Scale
- PH
- parenchymal hematoma
- SR
- stent retriever
- STROBE
- Strengthening the Reporting of Observational Studies in Epidemiology
This manuscript was sent to Harold P. Adams, Jr, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.121.037904.
For Sources of Funding and Disclosures, see page 2466.
Contributor Information
Kanta Tanaka, Email: tanaka19830311kanta@gmail.com.
Takeshi Yoshimoto, Email: yoshimototakeshi1982@ncvc.go.jp.
Masayuki Shiozawa, Email: m.sio@ncvc.go.jp.
Yuji Kushi, Email: kushi.yuji@ncvc.go.jp.
Tsuyoshi Ohta, Email: tsuyoshi@ya2.so-net.ne.jp.
Tetsu Satow, Email: tetsusato213@gmail.com.
Hiroharu Kataoka, Email: hkataoka@ncvc.go.jp.
Masafumi Ihara, Email: ihara@ncvc.go.jp.
Masatoshi Koga, Email: koga@ncvc.go.jp.
Noriko Isobe, Email: isobe.noriko.342@m.kyushu-u.ac.jp.
Kazunori Toyoda, Email: toyoda@ncvc.go.jp.
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. ; American Heart Association Stroke Council. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 2.LeCouffe NE, Kappelhof M, Treurniet KM, Lingsma HF, Zhang G, van den Wijngaard IR, van Es ACGM, Emmer BJ, Majoie CBLM, Roos YBWEM, et al. ; MR CLEAN Registry Investigators. 2B, 2C, or 3: what should be the angiographic target for endovascular treatment in ischemic stroke? Stroke. 2020;51:1790–1796. doi: 10.1161/STROKEAHA.119.028891 [DOI] [PubMed] [Google Scholar]
- 3.Baek JH, Kim BM, Heo JH, Nam HS, Kim YD, Park H, Bang OY, Yoo J, Kim DJ, Jeon P, et al. Number of stent retriever passes associated with futile recanalization in acute stroke. Stroke. 2018;49:2088–2095. doi: 10.1161/STROKEAHA.118.021320 [DOI] [PubMed] [Google Scholar]
- 4.Flottmann F, Brekenfeld C, Broocks G, Leischner H, McDonough R, Faizy TD, Deb-Chatterji M, Alegiani A, Thomalla G, Mpotsaris A, et al. ; GSR Investigators. Good clinical outcome decreases with number of retrieval attempts in stroke thrombectomy: beyond the first-pass effect. Stroke. 2021;52:482–490. doi: 10.1161/STROKEAHA.120.029830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maros ME, Brekenfeld C, Broocks G, Leischner H, McDonough R, Deb-Chatterji M, Alegiani A, Thomalla G, Fiehler J, Flottmann F; GSR Investigators. Number of retrieval attempts rather than procedure time is associated with risk of symptomatic intracranial hemorrhage. Stroke. 2021;52:1580–1588. doi: 10.1161/STROKEAHA.120.031242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaidat OO, Castonguay AC, Linfante I, Gupta R, Martin CO, Holloway WE, Mueller-Kronast N, English JD, Dabus G, Malisch TW, et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke. 2018;49:660–666. doi: 10.1161/STROKEAHA.117.020315 [DOI] [PubMed] [Google Scholar]
- 7.Leischner H, Flottmann F, Hanning U, Broocks G, Faizy TD, Deb-Chatterji M, Bernhardt M, Brekenfeld C, Buhk JH, Gellissen S, et al. Reasons for failed endovascular recanalization attempts in stroke patients. J Neurointerv Surg. 2019;11:439–442. doi: 10.1136/neurintsurg-2018-014060 [DOI] [PubMed] [Google Scholar]
- 8.Kaneko N, Komuro Y, Yokota H, Tateshima S. Stent retrievers with segmented design improve the efficacy of thrombectomy in tortuous vessels. J Neurointerv Surg. 2019;11:119–122. doi: 10.1136/neurintsurg-2018-014061 [DOI] [PubMed] [Google Scholar]
- 9.Schwaiger BJ, Kober F, Gersing AS, Kleine JF, Wunderlich S, Zimmer C, Poppert H, Prothmann S. The pREset stent retriever for endovascular treatment of stroke caused by MCA occlusion: safety and clinical outcome. Clin Neuroradiol. 2016;26:47–55. doi: 10.1007/s00062-014-0329-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyselyova AA, Fiehler J, Leischner H, Flottmann F, Buhk JH, Frölich AM. Vessel diameter and catheter-to-vessel ratio affect the success rate of clot aspiration. J Neurointerv Surg. 2021;13:605–608. doi: 10.1136/neurintsurg-2020-016459 [DOI] [PubMed] [Google Scholar]
- 11.Nagata T, Masumoto K, Hayashi Y, Watanabe Y, Kato Y, Katou F. Three-dimensional computed tomographic analysis of variations of the carotid artery. J Craniomaxillofac Surg. 2016;44:734–742. doi: 10.1016/j.jcms.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 12.Lin LM, Colby GP, Jiang B, Uwandu C, Huang J, Tamargo RJ, Coon AL. Classification of cavernous internal carotid artery tortuosity: a predictor of procedural complexity in Pipeline embolization. J Neurointerv Surg. 2015;7:628–633. doi: 10.1136/neurintsurg-2014-011298 [DOI] [PubMed] [Google Scholar]
- 13.Leker RR, Kasner SE, El Hasan HA, Sacagiu T, Honig A, Gomori JM, Guan S, Choudhry O, Hurst RW, Kung D, et al. Impact of carotid tortuosity on outcome after endovascular thrombectomy. Neurol Sci. 2021;42:2347–2351. doi: 10.1007/s10072-020-04813-8 [DOI] [PubMed] [Google Scholar]
- 14.Sato S, Uehara T, Ohara T, Suzuki R, Toyoda K, Minematsu K; Stroke Unit Multicenter Observational (SUMO) Study Investigators. Factors associated with unfavorable outcome in minor ischemic stroke. Neurology. 2014;83:174–181. doi: 10.1212/WNL.0000000000000572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshimoto T, Inoue M, Tanaka K, Kanemaru K, Koge J, Shiozawa M, Kamogawa N, Kimura S, Chiba T, Satow T, et al. Identifying large ischemic core volume ranges in acute stroke that can benefit from mechanical thrombectomy. J Neurointerv Surg. 2021;13:1081–1087. doi: 10.1136/neurintsurg-2020-016934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koge J, Tanaka K, Yoshimoto T, Shiozawa M, Yamagami H, Satow T, Takahashi JC, Ihara M, Koga M, Kira JI, et al. Early recurrent ischemic events after mechanical thrombectomy: effect of post-treatment intracranial hemorrhage. J Neurol. 2021;268:2810–2820. doi: 10.1007/s00415-021-10449-1 [DOI] [PubMed] [Google Scholar]
- 17.Fujita K, Tanaka K, Yamagami H, Ide T, Ishiyama H, Sonoda K, Satow T, Takahashi JC, Ihara M, Koga M, et al. Detrimental effect of chronic hypertension on leptomeningeal collateral flow in acute ischemic stroke. Stroke. 2019;50:1751–1757. doi: 10.1161/STROKEAHA.119.025142 [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 19.Lapergue B, Labreuche J, Blanc R, Marnat G, Consoli A, Rodesch G, Saleme S, Costalat V, Bracard S, Desal H, et al. Combined use of contact aspiration and the stent retriever technique versus stent retriever alone for recanalization in acute cerebral infarction: the randomized ASTER 2 study protocol. J Neurointerv Surg. 2020;12:471–476. doi: 10.1136/neurintsurg-2019-014735 [DOI] [PubMed] [Google Scholar]
- 20.Liebeskind DS, Bracard S, Guillemin F, Jahan R, Jovin TG, Majoie CB, Mitchell PJ, van der Lugt A, Menon BK, San Román L, et al. ; HERMES Collaborators. eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019;11:433–438. doi: 10.1136/neurintsurg-2018-014127 [DOI] [PubMed] [Google Scholar]
- 21.Toyoda K, Koga M, Iguchi Y, Itabashi R, Inoue M, Okada Y, Ogasawara K, Tsujino A, Hasegawa Y, Hatano T, et al. Guidelines for intravenous thrombolysis (recombinant tissue-type plasminogen activator), the third edition, March 2019: a guideline from the Japan Stroke Society. Neurol Med Chir (Tokyo). 2019;59:449–491. doi: 10.2176/nmc.st.2019-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 23.Mokin M, Waqas M, Chin F, Rai H, Senko J, Sparks A, Ducharme RW, Springer M, Borlongan CV, Levy EI, et al. Semi-automated measurement of vascular tortuosity and its implications for mechanical thrombectomy performance. Neuroradiology. 2021;63:381–389. doi: 10.1007/s00234-020-02525-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Maria F, Kyheng M, Consoli A, Desilles JP, Gory B, Richard S, Rodesch G, Labreuche J, Girot JB, Dargazanli C, et al. ; ETIS Investigators. Identifying the predictors of first-pass effect and its influence on clinical outcome in the setting of endovascular thrombectomy for acute ischemic stroke: results from a multicentric prospective registry. Int J Stroke. 2021;16:20–28. doi: 10.1177/1747493020923051 [DOI] [PubMed] [Google Scholar]
- 25.Benson JC, Brinjikji W, Messina SA, Lanzino G, Kallmes DF. Cervical internal carotid artery tortuosity: a morphologic analysis of patients with acute ischemic stroke. Interv Neuroradiol. 2020;26:216–221. doi: 10.1177/1591019919891295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peschillo S, Diana F, Berge J, Missori P. A comparison of acute vascular damage caused by ADAPT versus a stent retriever device after thrombectomy in acute ischemic stroke: a histological and ultrastructural study in an animal model. J Neurointerv Surg. 2017;9:743–749. doi: 10.1136/neurintsurg-2016-012533 [DOI] [PubMed] [Google Scholar]
- 27.Lee YB, Yoon W, Lee YY, Kim SK, Baek BH, Kim JT, Park MS. Predictors and impact of hemorrhagic transformations after endovascular thrombectomy in patients with acute large vessel occlusions. J Neurointerv Surg. 2019;11:469–473. doi: 10.1136/neurintsurg-2018-014080 [DOI] [PubMed] [Google Scholar]
- 28.Desai SM, Tonetti DA, Morrison AA, Gross BA, Jankowitz BT, Jovin TG, Jadhav AP. Relationship between reperfusion and intracranial hemorrhage after thrombectomy. J Neurointerv Surg. 2020;12:448–453. doi: 10.1136/neurintsurg-2019-015337 [DOI] [PubMed] [Google Scholar]
- 29.Bernava G, Rosi A, Boto J, Brina O, Kulcsar Z, Czarnetzki C, Carrera E, Schaller K, Lovblad KO, Machi P. Direct thromboaspiration efficacy for mechanical thrombectomy is related to the angle of interaction between the aspiration catheter and the clot. J Neurointerv Surg. 2020;12:396–400. doi: 10.1136/neurintsurg-2019-015113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang DH, Park J. Endovascular stroke therapy focused on stent retriever thrombectomy and direct clot aspiration: historical review and modern application. J Korean Neurosurg Soc. 2017;60:335–347. doi: 10.3340/jkns.2016.0809.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.