Abstract
Objectives:
We propose a framework of health outcomes modeling with dynamic decision-making and real-world data (RWD) to evaluate the potential utility of novel risk prediction models in clinical practice. Lung transplant (LTx) referral decisions in cystic fibrosis offer a complex case study.
Methods:
We used longitudinal RWD for a cohort of adults (n = 4,247) from the Cystic Fibrosis Foundation patient registry to compare outcomes of an LTx referral policy based on machine learning (ML) mortality risk predictions to referral based on (1) forced expiratory volume in one second (FEV1) alone, and (2) heterogenous usual care (UC). We then developed a patient-level simulation model to project number of patients referred for LTx and 5-year survival, accounting for transplant availability, organ allocation policy, and heterogenous treatment effects.
Results:
Only 12% (95% CI: 11%, 13%) of patients were referred for LTx over 5 years under UC, compared to 19% (18%, 20%) under FEV1 and 20% (19%, 22%) under ML. Of 309 patients who died before LTx referral under UC, 31% (27%, 36%) would have been referred under FEV1 and 40% (35%, 45%) would have been referred under ML. Given a fixed supply of organs, differences in referral time did not lead to significant differences in transplants, pre- or post- transplant deaths, or overall survival in 5 years.
Conclusions:
Health outcomes modelling with RWD may help to identify novel ML risk prediction models with high potential real-world clinical utility, and rule out further investment in models that are unlikely to offer meaningful real-world benefits.
Precis:
Use of a high performing ML-based risk prediction model for clinical decision-making in cystic fibrosis is not expected to improve downstream patient outcomes.
1. Introduction
Despite the rapid development of new risk prediction models (RPMs) using machine learning (ML) methodologies, few RPMs have been implemented for use in clinical practice.1–4 A recent systematic review found only 51 applications of artificial intelligence (AI) in real-world clinical practice in over 15,000 ML or AI publications identified.5 One reason for the gap between development and implementation is a lack of evidence on the real-world clinical utility offered by new RPMs: the expected change in downstream patient outcomes when used for decision-making in clinical practice.6–11 While commonly reported improvements in predictive accuracy are necessary for consideration of novel RPMs, accuracy alone is insufficient for assessing real-world clinical utility because it does not capture the complex clinical context in which the model would be used. Additional consideration is needed for real-world factors that impact RPM utility in clinical practice, including (1) the true, heterogenous current process for making decisions, and (2) the downstream patient outcomes associated with clinical decisions.
Novel RPMs are typically compared to a reference model - an existing risk prediction model, biomarker or clinical guidelines.12,13 However, real-world clinical decision-making is heterogenous and often deviates from the reference model, with different clinicians weighing different factors in decisions, including various pieces of evidence, historical experience, and preferences.4,11,14,15 Clinicians may also have additional pieces of information, such as expensive tests available for a subset of patients and subjective clinical impressions. In such cases, it remains unclear whether an RPM that outperforms a reference model would also outperform usual care (UC).
Furthermore, studies rarely relate changes in the discrimination and calibration properties of an RPM to changes in downstream patient outcomes.16,17 Some approaches have been proposed, such as considering the balance of false positives and false negatives at a given threshold.18,19 However, in many cases, treatment effects are heterogenous, with not all true positives experiencing the same benefits of treatment and not all false negatives and false positives experiencing the same harms of misclassification. In such cases, discrimination will fail to capture the expected patient impacts, for example, that a model which better identifies cases with large treatment benefits offers higher clinical utility than a model that better identifies cases with smaller treatment benefits.
Our objective was to compare the expected real-world patient health outcomes (survival) of using a new RPM for decision-making in clinical practice to those expected under (1) usual care, and (2) a reference model. We propose a health outcomes modeling framework that relies on real-world data (RWD) to estimate changes in real-world clinical decisions and linked downstream outcomes when an RPM is used in clinical practice. We leverage RWD to mimic the clinical context in which a novel RPM model would be used, providing a clearer picture of consequences in clinical practice.
We selected lung transplant (LTx) referral decisions in cystic fibrosis (CF) as a case study for three primary reasons. First, the standard predictor of short-term mortality in CF, forced expiratory volume in one second (FEV1), has low positive predictive value,20–25 and we previously developed an ML-based RPM with better discrimination and calibration (Rodriguez et al, submitted). Second, UC for referral decision-making is heterogenous, so performance improvements relative to FEV1 may not be indicative of performance relative to UC.3,4,26 Third, the relationship between clinical decisions and patient outcomes is complex given limited transplant availability27 and heterogenous benefits,28–30 so additional consideration of downstream outcomes is needed.
2. Methods
Framework
We propose a general framework to evaluate the expected utility of novel RPMs in real-world clinical practice, with respect to patient outcomes. This framework has 3 tenets:
Use of RWD to mimic patterns of real-world clinical practice. Real-world care and decision-making patterns may deviate substantially from expectations (i.e., guidelines), which can impact expected outcomes of RPM model use. Leveraging real-world data allows the RPM evaluation environment to mirror to the real-world context in which the RPM would be used, including patterns of utilization and decision-making practices under UC.
Dynamic decision-making to reflect intended use in clinical practice. Rather than assessing risk model use at a single timepoint, such as baseline, the risk model is applied at each encounter over time, using the most recently collected values.
Health outcomes modeling to evaluate the downstream patient outcomes resulting from a clinical decision. We expand outcomes considered in RPM evaluation to include the clinical decisions resulting from RPM use and subsequent treatment outcomes, accounting for heterogeneity in treatment effects.
While our case study considers an ML-based RPM, the framework is equally applicable to RPMs developed using more traditional methods, such as logistic regression or biomarkers used for decision-making. Similarly, the framework could be used to compare alternative thresholds for decision-making.
Case Study
2.0. Data
We used RWD from the CF Foundation Patient Registry (CFFPR), which collects longitudinal, observational data for all US patients seen at CFF-accredited care centers who consent to participate.31 Data on patient diagnoses, demographics, encounters, care episodes, and annual visits are entered electronically by CF care center staff using information from electronic medical records and patient forms.31 The CFFPR covers approximately 80% the US CF population, and includes 95% of clinic visits and 90% of hospitalizations for participating patients.31 Our cohort included CFFPR adults (≥18 years old) who had not undergone LTx by January 1, 2012, and had at least one encounter in both 2011 and 2012 (n = 10,615). Our cohort was followed until December 31, 2016. We previously split cohort data into training (60%) and validation (40%) sets to develop and evaluate the ML model. The 40% validation set (n = 4,247) was used in this patient-level simulation.
CFFPR data was linked to United Network for Organ Sharing (UNOS) data, which contains additional waitlist, transplant, and post-transplant information for patients listed for LTx. UNOS data also contains information on donated organs. The data linkage was performed at University of Washington in collaboration with University of Toronto.32,33 This study was approved by the University of Washington Institutional Review Board (Study #2270), by St Michael’s Hospital, Toronto, Canada (Research Ethics Board #14–148) and the Seattle Children’s Research Institute (Study #PIROSTUDY15294).
2.1. Patient-level Simulation Model Structure
We developed a patient-level simulation model with 5 mutually exclusive health states: pre-referral, evaluation, waitlist, transplanted, and dead (Figure 1). Patients began in the state corresponding to their status on January 1, 2012: pre-referral, evaluation, or waitlist. Patients transitioned from pre-referral to evaluation at their time referral, which varied between ML, FEV1, and UC policies. Evaluation, modeled as a tunnel state, represents the time between referral and waitlisting when evaluation for LTx occurs. Surviving patients transitioned to the waitlist, where they remained until they were matched with an organ for LTx or died before transplant. Transplanted patients remained in the transplanted state until post-transplant death or model end. Transitions between states were determined by individual-specific transition probabilities, described below, that rely on RWD. We used a cycle time of 1 day and a time horizon of 5 years. Modeling was conducted in R.34
Figure 1: Microsimulation Model.
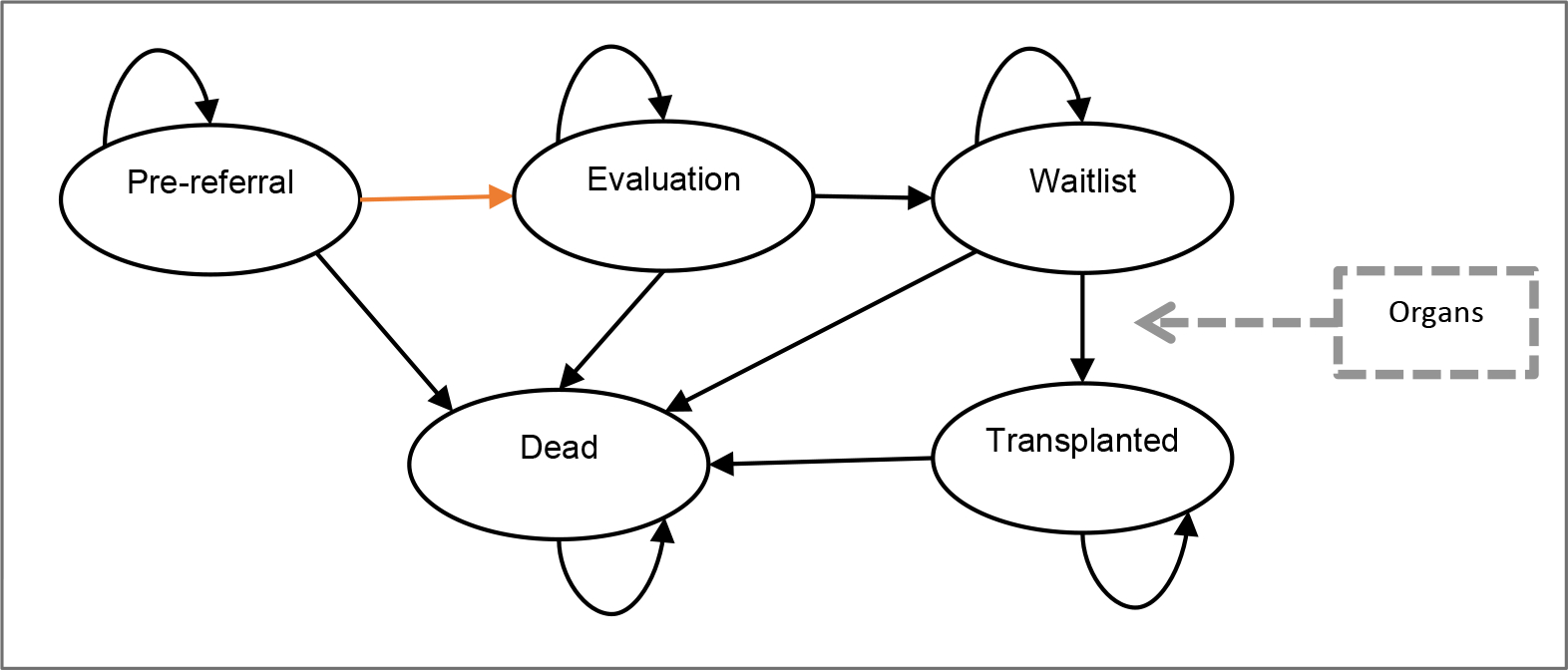
Microsimulation model with 5 mutually exclusive health states: pre-referral, evaluation, waitlist, transplanted, and dead. Patients waitlisted before model start begin in the waitlist state, all other patients begin in pre-referral. A patient moves from pre-referral to evaluation at the time of referral (orange arrow), which varies between policies.
2.2. Interventions: Referral Policies
We considered 3 potential policies for referring patients for LTx: (1) ML-model based, (2) reference model (FEV1) -based, and (3) usual care. The ML policy used individual risk predictions from a previously developed ML model for 2-year mortality. The ML model used Super learner, an ensemble ML approach that optimally combined multiple underlying models (lasso, elastic net, ridge, XGboost, random forest, and support vector machine).35,36 ML had higher discrimination at baseline (AUC: 0.914 (95% CI: 0.898, 0.929)) and over time and better calibration than FEV1 (baseline AUC: 0.876 (0.858, 0.895)). Additional detail is provided in the Appendix.
The ML-based referral policy was intended to reflect the ML model’s likely use in clinical practice. We assumed referral would occur at the first clinic visit where a patient’s predicted ML risk exceeded the threshold corresponding to 95% model specificity at baseline, which matches the specificity of the common FEV1 <30% criteria.21 However, any alternative decision rule could be considered, including different thresholds or more complex rules, such as multiple visits when criteria are met. For the FEV1-based policy, referral occurred at the first clinic visit with a stable FEV1 < 30% predicted based on the Global Lung Initiative equations for % predicted.37 For UC, the referral time was determined using RWD. We describe referral in detail in section 2.5.1 below.
2.3. Simulation Population
Our data contains correlated, longitudinal information on patients’ visit patterns, lung function, other health factors, predicted ML risk, and pre-LTx survival. Simulating a dataset that preserves the complex underlying relationships between these variables would be extremely challenging. Rather than imposing strong and potentially incorrect distributional assumptions to simulate correlated longitudinal data, we use the approach of plasmode simulation, where resampled populations (“plasmodes”) are drawn with replacement from observed data.38,39 In this approach, unmodified RWD for the resampled population are combined with modeling to simulate unknown elements. In our application, we drew 1 resampled population with replacement from observed cohort data on each of 1,000 simulation model runs. Resampled patients retained their true, observed covariate history up to the time of transplant, including visit history, ML risk scores, pulmonary function, and pre-transplant survival. Outcomes of transplant timing and post-transplant survival were then simulated, using models described below. We also used modeling to synthetically extend patients’ pre-transplant covariate history in cases where their actual time of transplant occurred earlier than it would have under an alternative policy (i.e. when pre-transplant covariate history is censored by transplant).40 We summarize resampled versus modeled elements in Appendix table S1.2.
In general, plasmode simulation is a flexible approach that is useful for preserving the underlying relationships between the potentially hundreds of variables in RWD.38 However, it is also more computationally intensive than a completely simulated population.
2.4. Outcomes
We compared referral policies on each of the following outcomes: 5-year overall survival (the sum of time spent in all non-death states), number of 5-year pre-transplant deaths, and number of post-transplant deaths. Overall 5-year survival is intended to capture the population-level impact of using an alternative policy. Because deaths are relatively rare and the impact to overall survival may be small, we separately evaluate 5-year deaths.
2.5. State Transitions
2.5.1. Referral (Pre-referral → evaluation)
Model-based policies
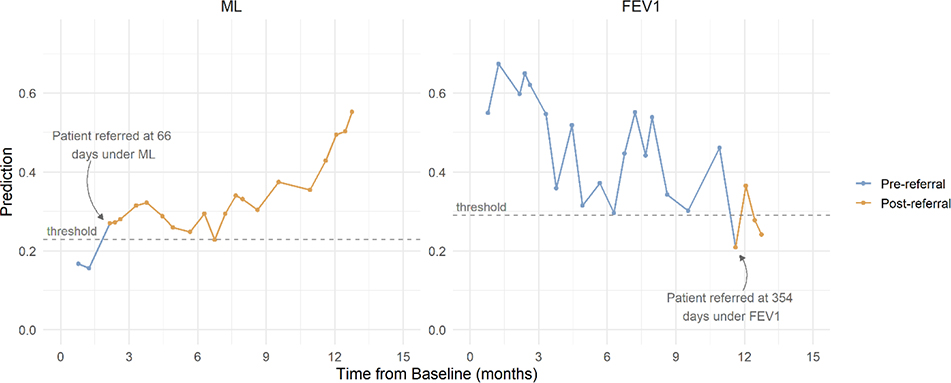
Patients’ dynamically updated ML risk predictions and absolute contraindications to transplant (mycobacterium abscessus and burkholderia cenocepacia) were obtained at each of their pre-transplant clinic visits from 2012 through 2016.41 ML-based referral occurred at the first clinic visit where predicted risk exceeded a fixed threshold corresponding to 95% model specificity and no absolute contraindications to LTx were present. Figure 2 provides an example of ML and FEV1 referral under each model for one patient.
Figure 2: Patient trajectory and referral example.
Risk of 2-year mortality from the ML model and FEV1 % predicted for an example patient at each clinic visit. For the ML model, a patient is referred at the first visit where risk exceeds the threshold, denoted by a change in line color. For FEV1, referral occurs at the first visit where FEV1 is lower than 30%, denoted by a change in line color.
To reflect guidelines, FEV1-based referral occurred at the first clinic visit where stable FEV1 was below 30% and no absolute contraindications were present. FEV1 was considered stable when no pulmonary exacerbation was documented at the same visit.
For patients who actually received LTx, observed pre-transplant visit history, ML risk predictions, and FEV1 were censored at their observed time of transplant, Li. Under an alternative policy, referral may not have occurred by time Li, when pre-transplant history was censored. In such cases, we synthetically extended ML risk and FEV1 trajectories beyond Li.40 We first generated synthetic visit times beyond Li (i.e. referral opportunities), assuming clinic visits would continue at the same frequency observed in the prior 12 months. We then estimated ML risk and FEV1 at each synthetic visit using separate linear mixed effects models. Additional detail is given in the appendix. We separately accounted for pre-transplant deaths (i.e. that an individual may not survive until the synthetic visit) and truncated synthetic visits at the time of pre-transplant death (see section 2.5.4 below).
Usual Care
Exact referral dates observed under UC are not recorded, but categorical transplant status (“not pertinent”, “accepted, on the waitlist”, “evaluated, final decision pending”, “evaluated, rejected”, and “had transplantation”) is recorded annually in the CFFPR. We used the first year with a status other than “not pertinent” as the UC referral year for each patient, then defined a subset of visits where referral could have occurred: clinic visits in the referral year and before the listing date. On each simulation run, we randomly selected one of these visits as the patient’s UC referral date. To test the validity of this assumption, we compared the resulting simulated UC listing time against the observed listing time.
2.5.2. Listing (Evaluation → Waitlist)
After referral for transplant, patients undergo a rigorous evaluation at a lung transplant center to assess whether they are suitable candidates for transplant, including evaluation of their health, medical adherence, emotional well-being, social support, and finances.27 Because evaluation times are not captured in our data, we simulated evaluation times to approximate available estimates26,42 by sampling from a truncated normal distribution (mean 4.5 months, standard deviation 4 months, minimum 3 weeks). Patients’ evaluation time was held constant between policies for each simulation run, but varied between simulation runs.
2.5.3. Organ Allocation (Waitlist → Transplanted)
We simulated population-level organ allocation to reflect current US policy, whereby new organs are allocated to the highest priority, compatible patient on the waitlist. The allocation process is a deterministic function of three components: patients on the waiting list (described above), organs available for transplantation, and the policy for matching organs to patients.40
Organ Flow
We relied on historical organ data from UNOS to define a flow of organs available for transplantation. On each simulation run, we sampled from the average number of organs available annually and their characteristics (ABO, height) using organs matched to patients in our cohort from 2012 – 2016. Organ dates of availability were sampled from a uniform distribution, where all dates were equally likely. We also conducted an expanded organ supply scenario analysis with twice as many organs available annually (Appendix 2).
Organ Matching
Lung allocation policy in the US prioritizes patients based on lung allocation score (LAS), a measure of expected mortality with and without transplant.43 The LAS aims to identify patients with both an urgent need and an expected survival benefit of transplant. LAS is calculated daily to prioritize patients on the waiting list. Observed LAS measures for waitlisted patients were available in UNOS data for each active day on the waiting list. However, alternative policies sometimes resulted in earlier waitlisting and/or the waitlisting of patients not listed under UC, such that LAS values were not available for all patients at all necessary time points. We therefore imputed LAS at all timepoints for all patients listed under any policy using a linear mixed effects model. We relied on LAS components that are measured in the CFFPR, and thus available for all patients regardless of listing status (age, FVC, BMI, diabetes). Large changes in LAS are frequently observed in the days or weeks preceding death or LTx, as patients experience ICU admission or the need for mechanical ventilation. To capture such changes in LAS without access to these variables, we included a fixed and random effect indicator for whether the patient experienced death or transplant in the next 30 days. Additional detail is given in the appendix.
Organ-donor compatibility was determined by blood type (ABO) and body size (height). When unavailable, we imputed patient ABO using the empirical distribution of ABO in each simulation run. While donors and recipients should have similar height (a proxy for lung capacity) no fixed thresholds exist for acceptable donor-recipient height differences.44 We used the 2.5th and 97.5th percentiles of the historically observed distribution of donor-recipient height difference on each simulation run as bounds for height compatibility.
We assumed no organ decline, no re-listing, and all bilateral transplants. We did not account for geographical regions of organ allocation.
2.5.4. Pre-transplant and post-transplant survival
Patients in the pre-referral, evaluation, and waitlist states were at risk of pre-transplant death. For patients who were never transplanted, complete pre-LTx survival was observed. In our simulation, patients observed to die before LTx retained their observed pre-transplant time of death, (Ti | LTx = 0), unless LTx occurred first. Similarly, patients who survived for the full five-year period retained their observed pre-transplant survival, (Ti | LTx = 0) > 5 years, unless LTx occurred first.
Among patients with observed LTx, pre-transplant survival was censored at the observed time of transplant, Li. In such cases, (Ti | LTx = 0) was unknown, but greater than Li. We relied on a potential outcomes model with time-varying transplant exposure to estimate survival in the absence of transplant. Under this model, we assumed that each patient has two potential outcomes at any time: (1) survival without transplant at time t, and (2) survival with transplant at time t. Only one outcome can be realized for each patient, but information from patients with the same likelihood of treatment at time t can inform the counterfactual outcome. Because transplant is allocated using LAS, we assumed transplant assignment was random among waitlist patients with the same LAS.28 That is, we assumed two patients with the same LAS had the same propensity for treatment.
Modeling survival conditional on transplant status using observed data:
We estimated the impact of time-dependent transplant on survival using an exponential survival model, with time-varying covariates for LAS and LTx status. Higher LAS is intended to indicate a greater benefit of treatment. We adjusted for gender, age at waitlisting, BMI at waitlisting. The model was estimated on waitlisted patients in each simulation run, with time measured as time to death since waitlist entry. We provide additional detail in the appendix.
Estimating expected pre- and post-transplant survival for simulation:
For patients with observed transplant whose pre-transplant survival was censored at Li, we obtained expected time of death in the absence of transplant, conditional on survival and history up to Li, (Ti | LTx = 0, Ti > Li, Xt). At t=Li, we use the inverse sampling method to obtain Ti | LTx = 0:
where U∼Uni(0,1), β is a vector of coefficients and Xi (t) is a vector of covariate values for individual i at time t, with the transplant indicator set to 0.
When considering post-transplant survival, a patient’s simulated time of transplant under each policy, Li,p*, may vary across policies and/or simulation runs. For example, a patient could be transplanted at t=100 days under ML and t=150 days under FEV1. If their clinical status declined substantially from 100 to 150 days (e.g. they were admitted to the ICU with respiratory failure requiring mechanical ventilation), their expected post-transplant survival may be lower when transplant occurs at 150 vs. 100 days. To obtain post-transplant survival at each potential transplant time, Li,p*, we again use the inverse sampling method, this time considering transplant at each t=Li,p* and setting the transplant indicator at t to 1.
3. Results
Unless otherwise noted, results are presented as estimate (95% confidence interval).
Validation
Among patients listed for LTx, the simulated UC listing date was a median of 9 days earlier than the observed UC listing date (IQR = 102 days earlier, 157 days later). In observed data, 466 patients died without transplant, compared to 458 (400, 520) in the simulated UC. 309 transplants and 65 post-transplant deaths were observed within the 5-year period, compared to 287 (244, 327) transplants and 41 (24, 59) post-transplant deaths in our simulated UC.
Clinical Decisions
Most patients remained too healthy for referral in the 5-year period, regardless of policy. Only 12.4% (11.4%, 13.4%) of patients were referred for LTx under UC (Table 1). By comparison, a uniform application of FEV1 resulted in significantly more patients referred, 19.2% (18.0%, 20.4%) Referral rates were somewhat higher for ML, 20.4% (19.1%, 21.6%). On average, ML resulted in earlier referral than UC, when patients were relatively healthier. Characteristics were not significantly different (statistically or clinically), including average FEV1 at the time of referral for ML, 31.5% predicted (30.9, 32.2) and UC, 30.9% predicted (29.8, 32) (Table 1). Among patients referred under both ML and UC, ML referral occurred 129 (82, 176) days earlier, on average.
Table 1:
Patient Characteristics at Time of Referral and Transplant, by Policy.
| ML | FEV1 | UC | |
|---|---|---|---|
|
| |||
| Characteristics at Time of Referral | |||
|
| |||
| Patients Referred (n) | 851 (797, 904) | 799 (746, 851) | 518 (477, 560) |
| Age | 32.9 (32.1, 33.6) | 33 (32.3, 33.8) | 33.4 (32.5, 34.4) |
| FEV1 % Predicted | 31.5 (30.9, 32.2) | 26 (25.8, 26.3) | 30.9 (29.8, 32) |
| Risk of 2-year mortality | 34.8% (34.0%, 35.7%) | 27.8% (26.5%, 29.1%) | 30.6% (29.1%, 32.2%) |
|
| |||
| Characteristics at Time of LTx | |||
|
| |||
| Patients Transplanted (n) | 294 (241, 345) | 292 (241, 340) | 287 (241, 325) |
| Age | 35.9 (34.3, 37.5) | 35.6 (33.9, 37.2) | 34.1 (32.8, 35.6) |
| FEV1 % Predicted | 29.2 (26, 32.6) | 28.4 (25.2, 31.7) | 29.6 (27.4, 32.3) |
| LAS | 51.6 (47.3, 57.4) | 50.5 (46.4, 56.3) | 47.9 (44.7, 51.9) |
Mean (95% CI) at the time of referral and transplant by policy. Abbreviations: ML: machine learning; FEV1: forced expiratory volume in 1 second; UC: usual care.
Many patients missed for referral under UC would have been referred by a policy with systematic decision-making using either FEV1 or ML (Figure 3). Of patients who died without being referred for LTx under UC, ML would have referred 40.0% (35.3%, 44.5%) and FEV1 would have referred 31.2% (26.9%, 35.6%).
Figure 3: Patient Referral and Outcome, by UC Referral Status.
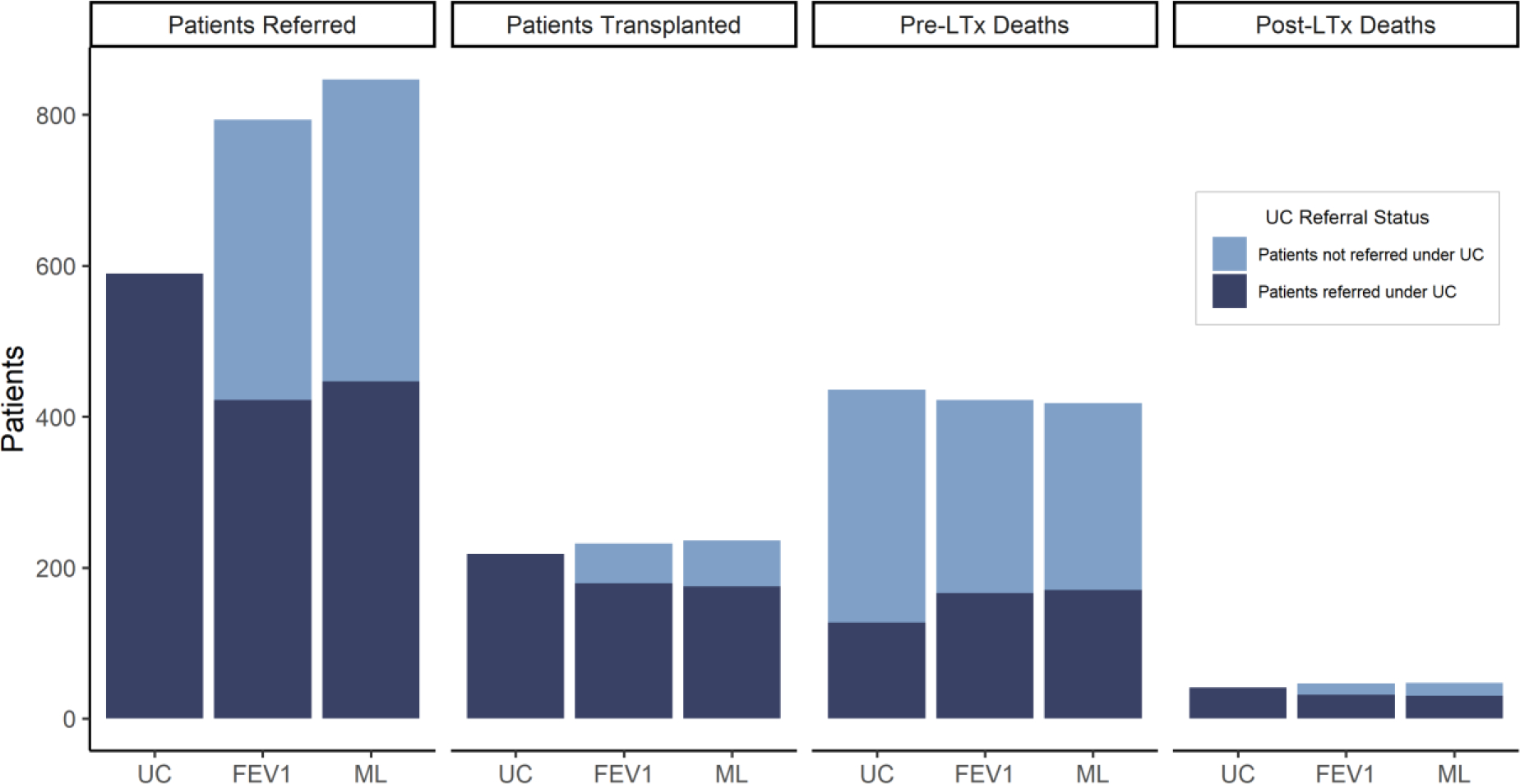
Average number of referrals, transplantations, and pre-LTx deaths, and post-LTx deaths in 5 years, by referral status under UC.
Patient Outcomes
Transplantation
Despite higher referral rates, there was no difference in overall transplantation rates among policies due to real-world constraints in organ supply (Table 1). State membership over time (Figure 4) shows that given a fixed supply of organs available for transplant, relatively higher referral rates under both ML and FEV1 led to increased patients on the waiting list, but no change in patients transplanted. At a population level, 0.39 (0.30, 0.44) years (of 5), on average, were spent on the waiting list under ML, compared to 0.41 (0.36, 0.45) under FEV1 and 0.23 (0.20, 0.27) under UC.
Figure 4: State membership over time, by policy.
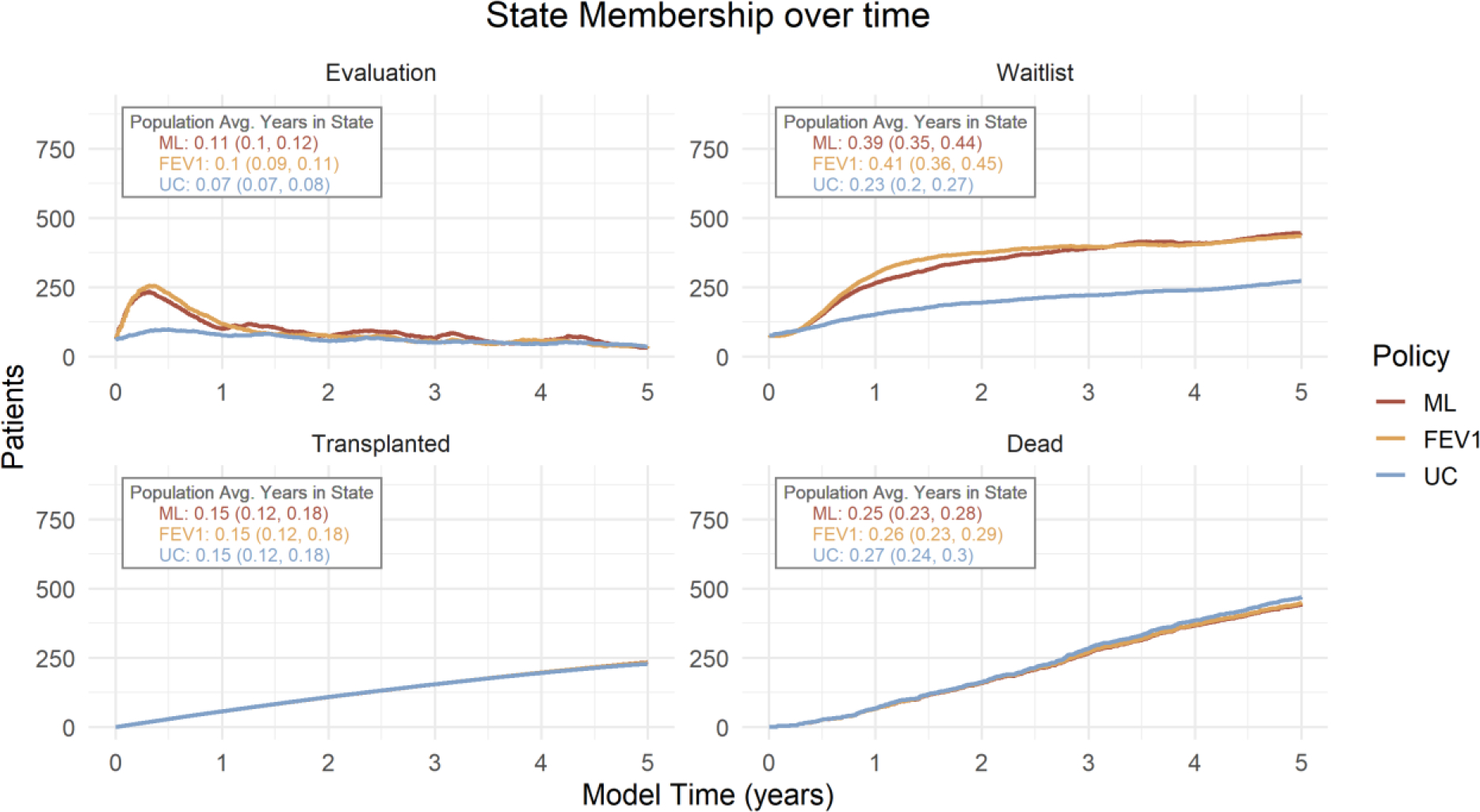
The average number of patients in each state except pre-referral for the 5-year time horizon. Population average years spent in each state (95% CI) is shown.
Patient characteristics at the time of LTx were similar among policies. While confidence intervals overlapped, patients transplanted under ML were slightly older and had slightly higher LAS at the time of transplant, compared to UC. As a measure, higher LAS is intended to indicate a higher expected short-term benefit of LTx.
While characteristics at the time of transplant were similar overall, the specific patients who received transplant and experienced pre-transplant death differed among policies (Figure 3). Under UC, 309 (277, 341) pre-transplant deaths occurred among patients who were never referred for LTx. Approximately 20.1% of these pre-transplant deaths were averted under ML because patients were referred and transplanted. However, this was offset by fewer transplants and more pre-transplant deaths among those who received transplant under UC (Figure 3).
Survival
At a population level, these changes resulted in no significant differences in overall 5-year survival, pre-transplant deaths, or post-transplant deaths (Table 3). Overall, 5-year survival was approximately 4.7 years under all polices.
Table 3:
Expected Outcomes by Policy
| Policy | Model AUC at Baseline* | Pre-transplant Deaths | Post-Transplant Deaths | Overall 5-Year Survival |
|---|---|---|---|---|
| ML | 0.914 (0.898, 0.929) | 383 (332, 436) | 47 (29, 69) | 4.75 (4.72, 4.77) |
| FEV1 | 0.876 (0.858, 0.895) | 389 (339, 442) | 46 (29, 69) | 4.74 (4.71, 4.77) |
| UC | - | 411 (367, 459) | 41 (24, 59) | 4.73 (4.70, 4.76) |
Model area under the receiver operating curve (AUC) at baseline was previously measured in model assessment (Rodriguez et al, submitted). Abbreviations: ML: machine learning; FEV1: forced expiratory volume in 1 second; UC: usual care.
Expanded Organ Availability Scenario
In a scenario with twice as many organs available, 441 (404, 479) transplants occurred under ML, compared to 412 (376, 442) under FEV1 and 367 (338, 394) under UC (Table S2.1). Accordingly, fewer pre-transplant deaths occurred under ML (281 (266, 315)) than UC (359 (345, 381)) (Table S2.2). Overall 5-year survival was slightly higher for ML (4.77 (4.75, 4.79)) than UC (4.74 (4.73, 4.76)), but confidence intervals overlapped.
Discussion
We demonstrated an application of patient-level simulation modeling to estimate the real-world impact of using a novel, ML-based RPM for decision-making in clinical practice. We found that improvements in discrimination and calibration for ML did not yield differences in expected downstream patient outcomes when used for clinical decision-making. While ML did lead to changes in the number of patients referred and referral timing, real-world constraints on organ availability limited the extent to which referral decisions could influence transplant. However, in a scenario of expanded organ availability, higher referral rates under ML led to more transplants and fewer pre-transplant deaths.
We found a significant difference between the clinical decisions expected under FEV1 alone, the reference model, and those observed in clinical practice. While 799 patients (19.2%) would have been referred within the 5-year period under FEV1, only 519 (12.4%) were actually referred in UC. Despite documented differences between clinical decision-making and FEV1,3,4 comparisons to FEV1 are standard for new models in CF.21,24,25
Our work suggests that additional comparisons to UC are needed to assess model performance. While any new RPM must predict better than an existing RPM to add value, improvements relative to a reference model may be a poor proxy for real-world clinical utility when clinical decision-making is heterogenous. RWD can be used to develop a real-world UC comparator.
Currently, the primary approach for assessing a model’s real-world clinical utility is an impact evaluation study - a cluster-randomized trial, where patient outcomes are compared for groups of clinicians with access to a novel model versus those following UC.8,10,45,46 Such studies are typically undertaken as a final step before implementation.14 In contrast, our approach uses RWD to assess the potential clinical impact in the relatively early model evaluation stage. This approach can rule out further investment in models that have limited usefulness in real-world settings. While simulation-based evaluation does not capture the complex ways that clinicians interact with models to make decisions,14,15 it can be used as a first step for demonstrating clinical utility before conducting RCTs. Further, the approach could be extended to include costs and utility measures for cost-effectiveness analysis.
The use of health outcomes modelling to evaluate a new diagnostic test is not new.47 However, health outcomes modelling to evaluate new RPMs specifically remains minimal.16 In contrast, statistical approaches for assessing clinical have gained relatively more popularity,18,19,48 but do not generally capture the clinical context in which models would be used.
Our simulation involves several important assumptions. We considered only absolute contraindications to LTx, which may have resulted in over-referral of patients under FEV1 and ML policies. Many contraindications are relative and vary by center, with larger and more experienced centers willing to accept more complex cases.27,49,50 We assumed a marginal distribution of evaluation time with no rejection for listing, which may not accurately reflect patient-specific factors that influence evaluation times or rejection. However, median simulated listing time was within 10 days of observed times, suggesting this assumption was acceptable on average. Finally, we are unable to distinguish between clinician decision-making and patient preferences using RWD. Lower rates of referral under the UC may represent patient preferences for non-referral, rather than clinician decisions not to refer patients. These complexities can be measured through impact evaluation.
More generally, RWD, including that used in our study, presents issues with missingness and infrequent data collection for some patients. We used imputation approaches to address missingness at multiple levels, including in longitudinal biomarkers and LAS values. However, to the extent individuals with missingness are unlike those with complete data, the results of our analysis may be biased. Imputation strategies for longitudinal measures and time to event outcomes in RWD is an important topic of future research. Additionally, while heterogeneity in our study was established through use of RWD, the impact of heterogeneity on downstream outcomes is an important area for future research.
5. Conclusion
We used a health outcomes modeling framework with RWD to assess the potential real-world clinical utility of a novel, ML-based RPM for LTx referral decisions in CF. We found differences in clinical decisions under the RPM versus UC, but no change in downstream patient outcomes due to constraints in organs available for transplantation. The ML and FEV1 policies effectively increased early referral compared to UC, supporting systematic approaches to referral decisions to increase access to the expertise and treatment available at lung transplant centers. Efforts to expand organ availability may be necessary to reap clinical benefits from earlier referral of CF patients. While constraints in transplant availability are unique to the organ allocation setting, complex real-world factors that impact current clinical decisions and outcomes are common across clinical applications. Health outcomes modeling with RWD can be used to account for these complex real-world factors. When conducted as part of RPM model evaluation, this approach can identify novel, ML-based RPMs that are likely to benefit patients in real-world clinical practice, and rule out further investment in RPMs with limited benefits.
Supplementary Material
Acknowledgements:
We thank the Cystic Fibrosis Foundation for providing data from the Cystic Fibrosis Foundation Patient Registry for this project. Additionally, we would like to thank the patients, care providers, and clinic coordinators at CF Centers throughout the US for their contributions to the CF Foundation Patient Registry.
Funding:
This research was partially supported by the National Cancer Institute of the National Institutes of Health (NIH) (under R37-CA218413). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. CHG was supported by grants from the Cystic Fibrosis Foundation, the NIH (UM1 HL119073, P30 DK089507, U01 HL114589, UL1 TR000423) and the FDA (R01 FD003704, R01 FD006848). KJR was supported by grants from the NIH (K23HL138154) and Cystic Fibrosis Foundation (RAMOS17A0).
Role of Funder:
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr. Veenstra reports grants from NIH during the conduct of the study. Dr. Veenstra is also an editor for Value in Health and had no role in the peer review process of this article. Dr. Heagerty reports grants from NIH during the conduct of the study. Dr. Goss reports grants from Cystic Fibrosis Foundation, European Commission, NIH (NHLBI), and NIH (NIDDK and NCRR) during the conduct of the study, as well as personal fees from Gilead Sciences and Novartis, grants from NIH and FDA, speaking honoraria from Vertex Pharmaceuticals, and is US lead for a clinical trial with Boehringer Ingelheim, outside the submitted work. Dr. Ramos reports grants from National Institutes of Health and Cystic Fibrosis Foundation during the conduct of the study, and grants from CHEST Foundation in partnership with Vertex Pharmaceuticals outside the submitted work. Dr. Bansal reports grants from National Cancer Institute during the conduct of the study. No other disclosures were reported.
References
- 1.Wessler BS, Paulus J, Lundquist CM, et al. Tufts PACE clinical predictive model registry: update 1990 through 2015. Diagnostic and prognostic research. 2017;1(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly CJ, Karthikesalingam A, Suleyman M, Corrado G, King D. Key challenges for delivering clinical impact with artificial intelligence. BMC medicine. 2019;17(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramos KJ, Quon BS, Psoter KJ, et al. Predictors of non-referral of patients with cystic fibrosis for lung transplant evaluation in the United States. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2016;15(2):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramos KJ, Somayaji R, Lease ED, Goss CH, Aitken ML. Cystic fibrosis physicians’ perspectives on the timing of referral for lung transplant evaluation: a survey of physicians in the United States. BMC Pulm Med. 2017;17(1):21–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin J, Ngiam KY, Teo HH. Role of Artificial Intelligence Applications in Real-Life Clinical Practice: Systematic Review. Journal of medical Internet research. 2021;23(4):e25759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dekker FW, Ramspek CL, van Diepen M. Con: Most clinical risk scores are useless. Nephrology Dialysis Transplantation. 2017;32(5):752–755. [DOI] [PubMed] [Google Scholar]
- 7.Vollmer S, Mateen BA, Bohner G, et al. Machine learning and artificial intelligence research for patient benefit: 20 critical questions on transparency, replicability, ethics, and effectiveness. BMJ. 2020;368:l6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moons KG, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. Bmj. 2009;338. [DOI] [PubMed] [Google Scholar]
- 9.Steyerberg EW, Moons KG, van der Windt DA, et al. Prognosis Research Strategy (PROGRESS) 3: prognostic model research. PLoS medicine. 2013;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reilly BM, Evans AT. Translating Clinical Research into Clinical Practice: Impact of Using Prediction Rules To Make Decisions. Annals of Internal Medicine. 2006;144(3):201–209. [DOI] [PubMed] [Google Scholar]
- 11.Khalifa M, Magrabi F, Luxan BG. Evaluating the Impact of the Grading and Assessment of Predictive Tools Framework on Clinicians and Health Care Professionals’ Decisions in Selecting Clinical Predictive Tools: Randomized Controlled Trial. Journal of medical Internet research. 2020;22(7):e15770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto T, Camargo CA, Faridi MK, Freishtat RJ, Hasegawa K. Machine learning–based prediction of clinical outcomes for children during emergency department triage. JAMA network open. 2019;2(1):e186937–e186937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osawa I, Goto T, Yamamoto Y, Tsugawa Y. Machine-learning-based prediction models for high-need high-cost patients using nationwide clinical and claims data. NPJ digital medicine. 2020;3(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kappen TH, Van Loon K, Kappen MA, et al. Barriers and facilitators perceived by physicians when using prediction models in practice. Journal of clinical epidemiology. 2016;70:136–145. [DOI] [PubMed] [Google Scholar]
- 15.Bate L, Hutchinson A, Underhill J, Maskrey N. How clinical decisions are made. Br J Clin Pharmacol. 2012;74(4):614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Giessen A, Peters J, Wilcher B, et al. Systematic review of health economic impact evaluations of risk prediction models: stop developing, start evaluating. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2017;20(4):718–726. [DOI] [PubMed] [Google Scholar]
- 17.Siontis KC, Siontis GC, Contopoulos-Ioannidis DG, Ioannidis JP. Diagnostic tests often fail to lead to changes in patient outcomes. Journal of clinical epidemiology. 2014;67(6):612–621. [DOI] [PubMed] [Google Scholar]
- 18.Vickers AJ, Cronin AM. Traditional statistical methods for evaluating prediction models are uninformative as to clinical value: towards a decision analytic framework. Paper presented at: Seminars in oncology2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Medical Decision Making. 2006;26(6):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. New England Journal of Medicine. 1992;326(18):1187–1191. [DOI] [PubMed] [Google Scholar]
- 21.Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. American journal of respiratory and critical care medicine. 2002;166(12 Pt 1):1550–1555. [DOI] [PubMed] [Google Scholar]
- 22.Aaron SD, Chaparro C. Referral to lung transplantation--too little, too late. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society. 2016;15(2):143–144. [DOI] [PubMed] [Google Scholar]
- 23.Buzzetti R, Alicandro G, Minicucci L, et al. Validation of a predictive survival model in Italian patients with cystic fibrosis. Journal of Cystic Fibrosis. 2012;11(1):24–29. [DOI] [PubMed] [Google Scholar]
- 24.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. American journal of epidemiology. 2001;153(4):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nkam L, Lambert J, Latouche A, Bellis G, Burgel P-R, Hocine M. A 3-year prognostic score for adults with cystic fibrosis. Journal of Cystic Fibrosis. 2017;16(6):702–708. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Vela M, Rudakevych T, Wigfield C, Garrity E, Saunders MR. Patient factors associated with lung transplant referral and waitlist for patients with cystic fibrosis and pulmonary fibrosis. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2017;36(3):264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell AB, Glanville AR. Lung transplantation: a review of the optimal strategies for referral and patient selection. Therapeutic advances in respiratory disease. 2019;13:1753466619880078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thabut G, Christie JD, Mal H, et al. Survival benefit of lung transplant for cystic fibrosis since lung allocation score implementation. American journal of respiratory and critical care medicine. 2013;187(12):1335–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vock DM, Tsiatis AA, Davidian M, et al. Assessing the causal effect of organ transplantation on the distribution of residual lifetime. Biometrics. 2013;69(4):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vock DM, Durheim MT, Tsuang WM, et al. Survival benefit of lung transplantation in the modern era of lung allocation. Annals of the American Thoracic Society. 2017;14(2):172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knapp EA, Fink AK, Goss CH, et al. The Cystic Fibrosis Foundation Patient Registry. Design and Methods of a National Observational Disease Registry. Annals of the American Thoracic Society. 2016;13(7):1173–1179. [DOI] [PubMed] [Google Scholar]
- 32.Ramos KJ, Sykes J, Stanojevic S, et al. Survival and lung transplant outcomes for individuals with advanced cystic fibrosis lung disease in the United States and Canada: an analysis of national registries. Chest. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephenson AL, Ramos KJ, Sykes J, et al. Bridging the survival gap in cystic fibrosis: An investigation of lung transplant outcomes in Canada and the United States. The Journal of Heart and Lung Transplantation. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.R: A language and environment for statistical computing [computer program]. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 35.Polley E, LeDell E, Kennedy C, Laan Mvd. SuperLearner: Super Learner Prediction. In:2019.
- 36.Van der Laan MJ, Polley EC, Hubbard AE. Super learner. Statistical applications in genetics and molecular biology. 2007;6(1). [DOI] [PubMed] [Google Scholar]
- 37.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. In: Eur Respiratory Soc; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franklin JM, Schneeweiss S, Polinski JM, Rassen JA. Plasmode simulation for the evaluation of pharmacoepidemiologic methods in complex healthcare databases. Computational statistics & data analysis. 2014;72:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaughan LK, Divers J, Padilla MA, et al. The use of plasmodes as a supplement to simulations: a simple example evaluating individual admixture estimation methodologies. Computational statistics & data analysis. 2009;53(5):1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson D, Waisanen L, Wolfe R, Merion RM, McCullough K, Rodgers A. Simulating the allocation of organs for transplantation. Health Care Management Science. 2004;7(4):331–338. [DOI] [PubMed] [Google Scholar]
- 41.Bansal A, Heagerty PJ. A Tutorial on Evaluating the Time-Varying Discrimination Accuracy of Survival Models Used in Dynamic Decision Making. Medical Decision Making. 2018;38(8):904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alkhateeb AA, Lease ED, Mancl LA, Chi DL. Untreated dental disease and lung transplant waitlist evaluation time for individuals with cystic fibrosis. Special Care in Dentistry. 2021. [DOI] [PubMed] [Google Scholar]
- 43.Egan TM, Murray S, Bustami R, et al. Development of the new lung allocation system in the United States. American Journal of Transplantation. 2006;6(5p2):1212–1227. [DOI] [PubMed] [Google Scholar]
- 44.Chambers DC, Yusen RD, Cherikh WS, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult lung and heart-lung transplantation report—2017; focus theme: allograft ischemic time. The Journal of Heart and Lung Transplantation. 2017;36(10):1047–1059. [DOI] [PubMed] [Google Scholar]
- 45.Moons KG, Kengne AP, Grobbee DE, et al. Risk prediction models: II. External validation, model updating, and impact assessment. Heart. 2012;98(9):691–698. [DOI] [PubMed] [Google Scholar]
- 46.Wallace E, Smith SM, Perera-Salazar R, et al. Framework for the impact analysis and implementation of Clinical Prediction Rules (CPRs). BMC medical informatics and decision making. 2011;11(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaafsma JD, van der Graaf Y, Rinkel GJ, Buskens E. Decision analysis to complete diagnostic research by closing the gap between test characteristics and cost-effectiveness. Journal of clinical epidemiology. 2009;62(12):1248–1252. [DOI] [PubMed] [Google Scholar]
- 48.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for some traditional and novel measures. Epidemiology (Cambridge, Mass). 2010;21(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weill D Lung transplantation: indications and contraindications. Journal of thoracic disease. 2018;10(7):4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lynch JP III, Sayah DM, Belperio JA, Weigt SS. Lung transplantation for cystic fibrosis: results, indications, complications, and controversies. Paper presented at: Seminars in respiratory and critical care medicine2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.