Abstract
Poultry are considered the major reservoir for Campylobacter jejuni, a leading bacterial cause of human food-borne diarrhea. To understand the ecology of C. jejuni and develop strategies to control C. jejuni infection in the animal reservoir, we initiated studies to examine the potential role of anti-Campylobacter maternal antibodies in protecting young broiler chickens from infection by C. jejuni. Using an enzyme-linked immunosorbent assay (ELISA), the prevalence of anti-C. jejuni antibodies in breeder chickens, egg yolks, and broilers from multiple flocks of different farms were examined. High levels of antibodies to the organism were detected in serum samples of breeder chickens and in egg yolk contents. To determine the dynamics of anti-Campylobacter maternal antibody transferred from yolks to hatchlings, serum samples collected from five broiler flocks at weekly intervals from 1 to 28 or 42 days of age were also examined by ELISA. Sera from the 1-day and 7-day-old chicks showed high titers of antibodies to C. jejuni. Thereafter, antibody titers decreased substantially and were not detected during the third and fourth weeks of age. The disappearance of anti-Campylobacter maternal antibodies during 3 to 4 weeks of age coincides with the appearance of C. jejuni infections observed in many broiler chicken flocks. As shown by immunoblotting, the maternally derived antibodies recognized multiple membrane proteins of C. jejuni ranging from 19 to 107 kDa. Moreover, in vitro serum bactericidal assays showed that anti-Campylobacter maternal antibodies were active in antibody-dependent complement-mediated killing of C. jejuni. Together, these results highlight the widespread presence of functional anti-Campylobacter antibodies in the poultry production system and provide a strong rationale for further investigation of the potential role of anti-C. jejuni maternal antibodies in protecting young chickens from infection by C. jejuni.
Campylobacter jejuni is the most common food-borne bacterial pathogen of humans in the United States and other developed countries, and infection caused by this organism is characterized by self-limiting watery and/or bloody diarrhea (1, 14, 43). Epidemiological studies have also revealed that Campylobacter infection is associated with the development of Guillain-Barré syndrome, an acute neurological disease characterized by ascending paralysis of peripheral nerves, which may lead to respiratory muscle compromise and death (30). The majority of human Campylobacter infections result from consumption of undercooked chicken or food contaminated by raw chicken (1, 14, 45). Although C. jejuni colonizes a variety of wild and domestic animals and birds, commercial poultry is considered the major reservoir of human Campylobacter infections (14). Hence, reduction of the pathogen level in the poultry production system is essential for minimizing the threat of C. jejuni to public health.
In order to reduce or eliminate campylobacters from poultry, it is imperative to understand the ecological aspects of the infection in the reservoir. For the past several decades, a large number of farm-based studies have been performed to determine the epidemiological features of C. jejuni (14, 32, 37, 45). The general consensus is that C. jejuni is highly prevalent in chicken flocks, especially in chickens more than 3 weeks old. The organism is carried in poultry intestinal contents in high numbers, leading to fecal contamination of chicken carcasses in processing plants (32, 37, 45). Despite this high colonization rate, infected chickens show few or no clinical signs of illness (37, 45). Sources of infection and modes of transmission for C. jejuni infection on poultry farms have not been well understood. Many studies suggest that horizontal transmission from environmental sources is the major mode of chicken flock infection by C. jejuni (11, 19, 32, 34, 45). However, several findings suggest that vertical transmission might also play a role in introducing C. jejuni from breeders into broiler flocks (9, 10, 13, 35, 39, 40). The complexity of Campylobacter transmission and the extensive nature of the colonization undermine the effectiveness of management-based intervention measures and highlight the need for alternative strategies, such as vaccination, to control C. jejuni infection in the poultry reservoir and consequently reduce the risk of human campylobacteriosis.
A general observation, and a unique characteristic of C. jejuni colonization in poultry, is that this organism is absent in chicks less than 2 weeks of age (32, 45), suggesting that young chicks may have intrinsic resistance to campylobacter colonization. However, the resistance mechanisms have not been defined. One possible contributing factor for this resistance may be the presence of C. jejuni-specific maternal antibodies in young chicks. It is well known that antibodies can be transferred from hens to their progeny. Maternal antibodies are usually sequestered from the maternal circulation by the developing oocyte and subsequently transported from the egg yolk across the yolk sac membrane into the embryonic circulation (6, 20, 24). Transferred antibodies are predominantly of the immunoglobulin G (IgG) class, while transfer of IgA and IgM usually occurs at substantially lower levels (20, 21, 24, 36). The level of maternal antibodies in young chicks peaks at 3 to 4 days after hatching and thereafter gradually decreases to undetectable levels at 2 to 3 weeks of age (20, 21, 42). In the first week of life, when the level of circulating maternal antibody and intestinal permeability of chicks are high, transport of circulating maternal antibody (mainly IgG) to the intestine occurs, which confers mucosal protection against infectious agents that colonize the intestinal epithelium (16, 17, 28, 42, 44).
Currently, there is a considerable gap in our knowledge concerning poultry immune response to Campylobacter infection under natural conditions. There have been no reported studies examining the level or role of anti-C. jejuni antibodies in chicken populations (including breeders and their progeny) on poultry farms. It is unclear if the anti-Campylobacter maternal antibody is widely present in young broiler chickens on commercial farms and if the maternally derived antibodies would protect young chickens from C. jejuni infection. Elucidation of these aspects of poultry immune response to C. jejuni is critical for understanding the ecology of Campylobacter colonization in the poultry reservoir and may provide new insights into the design of effective intervention measures to control C. jejuni infection in poultry. As a first step to examine the potential role of anti-C. jejuni maternal antibody in protecting young chickens from Campylobacter infection, we examined the prevalence and levels of C. jejuni-specific antibodies in breeder flocks, egg yolks, and young broiler chickens, determined the antigenic specificity of these antibodies by Western blotting, and evaluated the role of maternal antibodies in complement-mediated killing of C. jejuni.
MATERIALS AND METHODS
Bacterial strains.
C. jejuni strain ATCC 33291 and Campylobacter coli ATCC 33559 were obtained from the American Type Culture Collection, Rockville, Md. Other strains, including C1019, S2B, 81-176, 21190 and Turk, have been described previously (2, 50). These strains were chosen for this study because they represented the collection of diverse isolates from human and poultry that are available in our laboratory. Cultures were grown in brucella broth (BB) (Becton Dickinson, Sparks, Md.) in anaerobic jars under microaerophilic conditions produced by CampyPack Plus gas-generating envelopes (BBL Microbiology System, Cockeysville, Md.) at 42°C.
Preparation of Campylobacter outer membrane components.
Outer membrane components of C. jejuni were prepared using the ionic detergent N-lauroyl sarcosine as described previously by Blaser et al. (3). Briefly, cells grown in BB were harvested, washed in phosphate-buffered saline (PBS), and then suspended in 10 mM Tris (pH 7.5) containing phenylmethylsulfonyl fluoride, a protease inhibitor (Pefabloc SC; Boehringer, Mannheim, Germany). The cells were then sonicated on ice using a Vibracell sonicator (Sonics and Materials Inc., Danbury, Conn.). The preparation was then centrifuged two times at 5,000 × g for 20 min to remove nonlysed cells. The supernatant was then centrifuged for 2 h at 100,000 × g at 4°C, followed by suspension of the pellet in 1% (wt/vol) N-lauroyl sarcosine in 10 mM Tris (pH 7.5) and incubation at 25°C for 30 min on a shaker. This suspension was centrifuged again at 100,000 × g for 2 h at 4°C. The pellet was washed and resuspended in 10 mM Tris (pH 7.5) and stored at −80°C in small aliquots.
Preparation of glycine acid extract.
A crude mixture of surface proteins was extracted with 0.2 M glycine-HCl buffer (pH 2.2) as previously described by McCoy et al. (27). Briefly, bacteria were harvested from BB, washed twice in distilled water, and then suspended in 0.2 M glycine-HCl for 15 min at room temperature with stirring. The mixture was centrifuged for 25 min at 7,500 × g, and the supernatant (acid extract) was neutralized, dialyzed at 4°C against distilled water for 2 days, concentrated in a Speed-Vac (Savant Instruments, Holbrook, N.Y.) for 1 h, and kept frozen at −80°C.
Collection of serum samples and egg yolks.
Serum samples were collected from two parent breeder chicken farms, each of which was surveyed with seven different flocks (10 to 20 samples per flock). The age of the sampled birds ranged from 16 weeks to 14 months. Eggs were obtained from three local commercial layer farms for egg production (n = 96) (farms A, B, and C) and from a commercial hatchery for broiler chickens (n = 107). To detect anti-Campylobacter antibodies in eggs, yolk contents were separately collected and diluted 1:5 in PBS for storage at −80°C. To monitor the dynamic change of anti-Campylobacter maternal antibodies, sera were collected from five different broiler chicken flocks on two different farms at weekly intervals starting from day of hatching to 42 days of age.
ELISA.
An enzyme-linked immunosorbent assay (ELISA) was used to determine the level of C. jejuni-specific IgG antibodies in breeder flocks, egg yolks, and commercial broiler chickens. Microtiter plates (Nunc-Immune Plate; Nunc, Roskild, Denmark) were first coated with 100 μl of whole outer membrane components (ca. 60 ng/well) of C. jejuni in coating buffer (sodium carbonate [pH 9.6]) overnight at 25°C. Then, plates were incubated with a blocking buffer (PBS containing 2% milk, 2% bovine serum albumin, and 0.1% Tween 20) at 37°C for 1 h. Serum samples and egg yolks were diluted in the blocking buffer to 1:100, and then 100 μl of each dilution was added to individual wells. Duplicate wells were used for each sample. After incubation at 25°C for 2 h, the plates were washed three times with wash buffer (PBS containing 0.1% Tween 20). Goat anti-chicken IgG conjugated to peroxidase (Kirkegaard & Perry) was diluted to 1:1,000 in blocking buffer and added to the wells (100 μl/well). After 2 h of incubation at 25°C, the plates were washed three times with the wash buffer, and then 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS)-peroxidase substrate (Kirkegaard & Perry) was added. Optical density (OD) values of individual wells were measured using an ELISA reader (Titertek Multiskan MCC∖340) at 405 nm. A cutoff absorbance value for a positive sample was determined by adding 3 standard deviations to the mean absorbance value of negative controls. Sera and yolks from Campylobacter-negative specific-pathogen-free (SPF) chickens and serum samples from Campylobacter-negative broilers (3 to 4 weeks old) were chosen as negative controls. Yolk and serum samples from Campylobacter-colonized breeder chickens which gave an ELISA OD value of 1.5 or higher were used as positive controls.
SDS-PAGE and immunoblotting.
Outer membrane proteins and glycine-HCl extracts of C. jejuni were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by the method of Laemmli (22) with a 4% stacking gel and 10% separating gel. Protein samples were boiled in sample buffer at 100°C for 3 min prior to electrophoresis. The separated proteins were transferred onto nitrocellulose membranes (Bio-Rad). The membrane blots were blocked with the blocking solution used in the ELISA for 2 h at 25°C. Subsequently, the blots were incubated with an appropriate amount (400 μl for each membrane strip or 10 ml for a whole blot) of serum or yolk samples diluted to 1:100 in blocking buffer for 2 h at 25°C. After three washes with PBS containing 0.1% Tween 20, the blots were incubated with peroxidase-conjugated goat anti-chicken-IgG at a dilution of 1:1,000 for 2 h at 25°C. The blots were then washed and developed with the 4-chloro-1-naphthol–peroxidase substrate (Kirkegaard & Perry).
Serum bactericidal assay.
We chose to use C. jejuni strains 33291 (a human isolate) and 21190 (a poultry isolate) to assess the bactericidal activity of poultry maternal antibodies because they were isolated from different host species and were shown to be genetically divergent in a previous study (50). These two strains were grown in BB supplemented with Campylobacter-specific growth supplements (Oxoid, Hampshire, England). Two-day-old cultures were diluted in broth to give approximately 2 × 104 organisms/ml. Sera collected from two Campylobacter-negative SPF chickens were used as the complement source, which were confirmed for the lack of C. jejuni-specific antibodies by immunoblotting, filter sterilized with a 0.45-μm filter, and kept frozen in small aliquots at −80°C until use. To evaluate the role of anti-Campylobacter maternal antibodies in complement-mediated killing of C. jejuni, serum samples from 10 1-day-old chicks which contained high levels of maternal antibodies to C. jejuni, as determined by ELISA and immunoblotting, were pooled and used as the antibody source in the bactericidal assay. Pooled sera derived from 10 21-day-old broilers that were negative for C. jejuni-specific antibody were used as control antibodies. In addition, a commercially available goat anti-Campylobacter spp. antibody (Kirkegaard & Perry) was used as an additional control. These sera were inactivated at 56°C for 30 min prior to use to ablate complement activity. The bactericidal assay was performed in sterile microcentrifuge tubes. Each reaction contained 50 μl of bacterial suspension, 50 μl of 1:5 diluted (in PBS) complement, and 10 μl of undiluted antibodies. Control tubes included (i) bacteria plus complement only; (ii) bacteria plus antibody only; and (iii) bacteria plus PBS only. After the reactions were incubated at 37°C for 1 h, 100 μl of the suspension from each tube was plated onto Mueller-Hinton agar (Becton Dickinson) plates, which were incubated for 2 days at 42°C under microaerophilic conditions. The CFU were counted for each reaction, and percent reduction in the number of live organisms was calculated by the following formula: % reduction = [CFU (bacteria + complement only) − CFU (bacteria + antibody + complement)]/CFU (bacteria + complement only) × 100. For each antibody, the assay was repeated three times, and the mean reduction is presented in Table 1.
TABLE 1.
Bactericidal activity of poultry anti-Campylobacter maternal antibodies
| Strain | Mean % reduction in CFU by serum antibodiesa (SD)
|
||
|---|---|---|---|
| S1 | S21 | SCampy | |
| 33291 | 11 (5.7) | 2.5 (2.3) | 85 (8) |
| 21190 | 61 (10.4) | 7 (3.9) | −3.9 (3.4) |
Values are means for three independent experiments. S1, pooled sera from 1-day-old chickens (with maternal anti-C. jejuni antibody); S21, pooled sera from 21-day-old chickens (no anti-C. jejuni antibody); SCampy, commercial goat anti-Campylobacter spp. antibody (Kirkegaard & Perry).
RESULTS
Prevalence of anti-C. jejuni antibodies in breeder chickens.
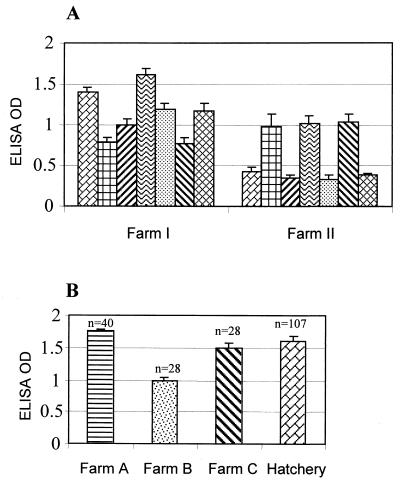
To assess if anti-C. jejuni antibody is present in breeder chickens, serum samples collected from different breeder flocks were examined using an ELISA, in which the microtiter plates were coated with outer membrane proteins extracted from C. jejuni strain 33291. As shown in Fig. 1A, all seven breeder chicken flocks from farm I had very high level of serum IgG antibodies to Campylobacter. In fact, every serum from these flocks (20 samples/flock) was positive for anti-Campylobacter antibodies, as determined by the ELISA. All seven flocks from farm II were also positive for anti-Campylobacter antibodies (Fig. 1A). Four flocks from farm II had relatively lower yet positive antibody levels compared to other flocks in this farm. These results indicated that the levels of anti-Campylobacter antibodies varied among breeder farms as well as among different flocks within a farm. Despite the variation, 100% of the serum samples from farm II were positive, as determined by ELISA. The age of the sampled birds ranged from 16 weeks to 14 months. However, there was no correlation between the antibody level and the age of the birds tested in this study.
FIG. 1.
Prevalence of anti-C. jejuni antibodies in sera of breeder chickens (A) and egg yolks (B) as determined by ELISA. (A) Seven flocks on each of two farms (I and II) were surveyed. Each bar represents the arithmetic mean ± the standard error of the mean for 20 (farm I) or 10 (farm II) serum samples in a single flock. (B) Eggs from three different farms and one hatchery were tested for anti-Campylobacter antibodies. Each bar represents the arithmetic mean ± the standard error of the mean. The number of eggs tested for each farm is shown above each bar. Negative controls (mean OD value = 0.179 ± 0.005) for panel A included five serum samples from 3- to 4-week-old C. jejuni-negative chickens. Five yolk samples (mean OD value = 0.220 ± 0.05) from SPF chickens were used as negative controls for panel B.
Prevalence of anti-C. jejuni IgG antibodies in egg yolks.
To determine if antibodies to C. jejuni are present in eggs, yolk contents of eggs obtained from three layer farms and a hatchery (for broiler chickens) were tested for anti-Campylobacter antibody by ELISA. All yolks from eggs of farms A, B, and C were positive with antibodies to C. jejuni (Fig. 1B). Of 107 eggs from the commercial hatchery for broiler chickens, 105 were also positive with anti-Campylobacter antibodies. These results indicated that anti-Campylobacter antibodies are highly prevalent in egg yolks.
Prevalence of anti-C. jejuni maternal antibodies in young broiler chicks.
To determine the dynamic change of maternal antibodies in broiler chicks, serum samples were collected at weekly intervals from five flocks of broiler chickens and examined by ELISA (Fig. 2). High levels of C. jejuni-specific antibodies were detected in chicks up to 7 days old. The levels of antibodies dropped substantially at 14 days of age and reached background level at the third and fourth week of sampling. The absence of anti-Campylobacter antibodies in the third and fourth weeks coincides with the onset of C. jejuni infection observed in many broiler flocks (32, 45). As observed with flock 1 (Fig. 2), C. jejuni-specific antibodies increased substantially at the fifth week of age and continued to rise at the sixth week of age (Fig. 2). This recurrence of anti-Campylobacter IgG antibodies during the fifth and sixth weeks of age could be due to natural infection by this organism. However, isolation of C. jejuni was not done as part of this study. With flocks 2 to 5 (Fig. 2), no serum samples were collected and analyzed beyond week 4 of age because the focus of this experiment was to monitor the dynamic change of maternal anti-Campylobacter antibodies, which were not detectable after the third week of age.
FIG. 2.
Dynamic change in serum IgG antibody to C. jejuni in broiler chickens. Serum samples collected from five different broiler flocks from days 1 to 28 (flocks 2 to 5) or 42 days of age (flock 1) were analyzed by ELISA. Each bar represents the arithmetic mean ± the standard error of the mean for 23 serum samples.
Antigenic specificity of poultry anti-Campylobacter antibodies.
To examine the antigenic profiles recognized by the poultry anti-Campylobacter IgG antibodies, outer membrane extracts of C. jejuni were separated by SDS-PAGE and immunoblotted with representative egg yolks and serum samples derived from breeder chickens. It was shown that antibody responses in both serum and yolk samples were against multiple membrane components of C. jejuni, ranging from 19 to 107 kDa. There were considerable variations in the banding patterns recognized by antibodies in individual chickens or eggs (data not shown). However, most of the serum and yolk samples showed a strong immune response to lipopolysaccharides (LPS) of C. jejuni, which appeared as a single diffuse band migrating below the 19-kDa marker. The majority of samples also contained antibodies to another unidentified antigen migrating at ca. 37 kDa. ELISA-negative chicken sera and several SPF chicken sera showed no reaction to C. jejuni proteins on immunoblotting. As a positive control, Campylobacter-specific antibody raised in a goat (Kirkegaard & Perry) was included in this experiment. Banding patterns similar to those recognized by the serum and yolk samples were observed with the positive control antibody, indicating that chicken antibody responses to this organism as measured by ELISA were specific to Campylobacter.
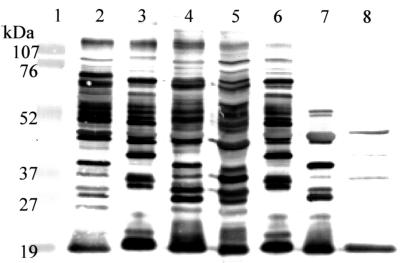
To test if the antibody response is strain or species specific, representative samples (breeder sera and yolks) were tested with antigens prepared from different strains of C. jejuni (ATTC 33291, C1019, S2B, 81-176, 21190, and Turk) and C. coli ATTC 33559. Results showed that individual samples reacted with multiple components in various strains of C. jejuni (Fig. 3). Cross-reactive antigens included the major outer membrane protein, flagellin, LPS, and other unidentified proteins (Fig. 3). Notably, the antibody response to C. coli, which is closely related to C. jejuni, was very limited in the chickens examined in this study, indicating the species specificity of the antibody response. This finding is consistent with previous observations that the majority of Campylobacter infections in poultry are due to C. jejuni instead of C. coli (38).
FIG. 3.
Immunoblot analysis of outer membrane components of thermophilic campylobacters using antibodies from an ELISA-positive egg yolk (Fig. 1B). Lane 1, low-range protein size standards (Bio-Rad). Lanes 2 to 7, C. jejuni isolates Turk, C1019, S2B, 81–176, 21190, and 33291, respectively. Lane 8, C. coli ATTC 33559. Positions of size markers are indicated on the left.
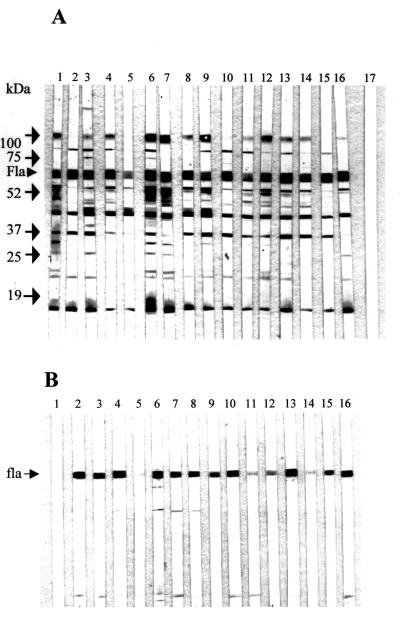
To determine the antigenic specificity of the maternally derived anti-Campylobacter antibodies, outer membrane extracts of C. jejuni were immunoblotted with serum samples from day-old broiler chicks (Fig. 4A). The protein patterns recognized by the tested serum samples were similar to the banding patterns defined by the goal anti-Campylobacter antibody (Fig. 4A, lane 1), indicating the specificity of the poultry maternal antibodies to Campylobacter. As in the case with breeder serum and yolk samples, antibodies in the young broiler chicks were against multiple components of C. jejuni. Although there were variations in the banding pattern among different serum samples tested, most of these sera reacted strongly with several antigens, including the flagellin, LPS, and unidentified antigens migrating between 37 and 52 kDa.
FIG. 4.
Immunoblot analysis of chicken serum maternal IgG antibody response to outer membrane antigens (A) or glycine-HCl extracts (B) of C. jejuni strain 33291 (a human isolate). (A) Lane 1, goat anti-Campylobacter-spp. antibody (Kirkegaard & Perry). Lanes 2 to 16, individual serum samples collected from 1-day-old chickens. Lane 17, serum sample from a 21-day-old chicken. Positions of size markers are indicated on the left. The arrow marked Fla indicates the position of flagellin. (B) Lane 1, serum sample from a 21-day-old chicken. Lanes 2 to 16, Individual serum samples from 1-day-old chickens. The position of flagellin subunits (∼60 kDa) is indicated by an arrow.
To examine the maternal antibody response to surface-associated antigens of C. jejuni, antigens extracted by glycine-HCl were immunoblotted with sera from day-old chicks. Unlike the reactions to the sarcosinate-extracted outer membrane proteins, immunoblotting of the glycine-acid extracts of C. jejuni with the serum samples showed a single immunodominant band of approximately 58 kDa (Fig. 4B), which was previously identified as the flagellin antigen (26).
Maternal antibody-dependent bactericidal activity.
To examine if anti-Campylobacter maternal antibodies were involved in complement-mediated bactericidal activity, C. jejuni organisms were incubated with heat-inactivated sera from 1-day-old (with maternal antibody) or 21-day-old chickens (without maternal antibody) plus an exogenous complement source (Table 1). None of the heat-inactivated sera alone produced killing, indicating the requirement for complement. Two different strains, 33291 (a human strain) and 21190 (a chicken isolate), were evaluated with the bactericidal assay. Strain 33291 was virtually resistant to killing by the chicken antibodies in the presence of complement. However, this strain showed 85% reduction in CFU counts when treated with the commercial goat anti-Campylobacter antibody. On the other hand, a substantial reduction (61%) in CFU counts was observed when strain 21190 was incubated with the sera from 1-day-old chicks (with maternal anti-Campylobacter antibody). However, no significant reduction was observed when sera from 21-day-old chicks (no anti-Campylobacter antibody) were used in the assay (Table 1). Interestingly, the goat anti-Campylobacter antibody did not result in any killing of strain 21190 in the presence of complement. These results indicated that the chicken maternal antibody resulted in complement-mediated killing of C. jejuni in a strain-specific manner.
DISCUSSION
Although several studies have evaluated immune responses to C. jejuni in experimentally infected chickens or in a small number of commercial chickens (8, 29, 48), this work, to our knowledge, is the first extensive study documenting the prevalence of anti-C. jejuni antibodies under natural conditions in the entire poultry production system, including parent breeder chickens, eggs, and young broiler chickens. Results from this study clearly indicated that anti-C. jejuni antibodies were highly prevalent in the poultry production system, possibly due to the extensive nature of C. jejuni colonization in the poultry reservoir. It was also shown in this study that the maternal antibodies in young broiler chickens reacted with multiple outer membrane proteins of C. jejuni and were active in antibody-dependent complement-mediated killing of the organism. These findings underscore the need for further investigation on the potential role of anti-Campylobacter maternal antibodies in protecting young chickens from infection by C. jejuni.
Serum immunoglobulins are readily transferred from hen serum to the egg yolk while the egg is still in the ovary. As the chick embryo develops, it absorbs the yolk immunoglobulins, which then appear in its circulation. The transferred antibodies are predominantly IgG, and transfer of IgA and IgM occurs at substantially lower levels (20, 21). During the first week after hatching, the permeability of the intestine is high, and thus a high level of serum IgG can be transferred from the circulation into the intestinal tract, where it confers mucosal protection against enteric agents in young chickens (16, 17, 28, 42, 44). Unlike mammals in which maternal IgA derived from milk is a key component of maternal immunity against intestinal mucosal infections, young chickens mainly use maternal IgG from the circulation to combat intestinal infections (16, 17, 28, 42, 44). For these reasons, we chose to measure the serum IgG in young broilers to reflect the levels of maternal anti-Campylobacter antibodies.
C. jejuni is generally absent in broiler flocks younger than 2 to 3 weeks of age (32, 45). It seems that there is an intrinsic resistance to natural spread of C. jejuni in young chicks. This speculation is supported by the observation from an experimental study resembling the natural spread of C. jejuni, in which this organism was not detected in 50 birds until 11 days of age, even though these birds were commingled with a C. jejuni-infected seeder bird starting from day 2 of age (18). Although the mechanisms responsible for the intrinsic resistance have not been defined, one possible reason for the lack of C. jejuni infection in young broiler flocks may be related to the presence of anti-Campylobacter maternal antibodies in the chicks. It has been known that maternal antibodies in young chicks confer protection against many enteric agents of poultry during the early stage of their life (16, 17, 28, 42, 44). It was also shown in experimental studies that anti-Campylobacter antibodies in serum or intestinal secretions were associated with resistance to C. jejuni colonization in various species of animals, including chickens (5, 12, 47). Historically, the role of anti-Campylobacter maternal antibodies may have been underestimated because many studies found that young chicks were susceptible to experimental challenge by C. jejuni (8, 41, 46). However, the oral gavage challenge method used in the previous studies did not resemble the natural process of C. jejuni transmission and might overwhelm the protective role of maternal antibodies in the young chickens. There may be a threshold of infection dose required for successful colonization of C. jejuni in chickens (47), which is likely influenced by both the immune status and the genetic background of chickens. It is possible that the high prevalence of anti-Campylobacter maternal antibodies in young chickens results in “flock immunity” against C. jejuni infection. This speculation needs to be examined in future studies.
Immunoblotting revealed that the chicken antibodies reacted with multiple outer membrane components of C. jejuni. The predominant Campylobacter antigens recognized by the chicken antibodies appeared to be flagellin, LPS, and two unidentified proteins of 37 and 40 kDa (Fig. 4). The flagellum of C. jejuni is required for in vivo colonization and induces a strong antibody response in both humans and animals (15). A potential protective role of antiflagellin antibodies against C. jejuni infection has been observed in earlier studies involving humans (15, 31, 33) and chickens (7, 48, 49). Recently, a flagellin-based recombinant vaccine was shown in a mouse model to be protective against challenge by C. jejuni (23). These findings suggest that the flagellin protein of C. jejuni is an important immunogen. Therefore, a prominent antibody response to the flagellin antigen, as maternally derived, in young broilers might contribute to protection against colonization by C. jejuni.
Many C. jejuni strains have been shown to be susceptible to killing by normal or infected human sera, and the killing is mediated by both complement and specific antibody (4). Results from this study (Table 1) indicated that the chicken maternal antibodies significantly reduced the CFU counts when incubated with strain 21190 in the presence of a complement source, but did not have any bactericidal effect on strain 33291. In contrast, the commercial goat anti-Campylobacter antibodies, which were generated by immunization with multiple strains of Campylobacter, had a bactericidal effect on strain 33291 but not on strain 21190. It is unclear what contributed to the difference in killing between the two strains. It is possible that the chickens examined in this study were infected by a Campylobacter strain that was similar to 21190 in antigens inducing complement-fixing antibodies but distinct from strain 33291. Consequently, the chicken maternal antibodies were active in the complement-mediated killing of strain 21190. Likewise, the commercial goat antibodies may contain complement-fixing antibodies that recognize antigens of strain 33291 instead of strain 21190, resulting in the inability to kill strain 21190. Together, these results suggest that the antibody-dependent complement-mediated killing of C. jejuni is strain specific, requiring the presence of specific target antigens on the surface of the organism. It should be pointed out that the in vitro bactericidal activity of anti-Campylobacter maternal antibodies does not necessarily mean that the maternal antibodies confer immune protection against C. jejuni in vivo. To confirm the protective role of anti-Campylobacter antibodies, comprehensive experiments using young chickens with and without maternal anti-Campylobacter antibodies and different challenge strains or doses are required in future studies.
ACKNOWLEDGMENTS
This work is supported in part by the Ohio Poultry Association and USDA NRICGP competitive grant 99-35212-8517. Orhan Sahin is supported by a scholarship from the Ministry of Education of the Turkish Government.
REFERENCES
- 1.Altekruse S F, Stern N J, Fields P I, Swerdlow D L. Campylobacter jejuni—an emerging foodborne pathogen. Emerg Infect Dis. 1999;5:28–35. doi: 10.3201/eid0501.990104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black R E, Levine M M, Clements M L, Hughes T P, Blaser M J. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988;157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 3.Blaser M J, Hopkins J A, Berka R M, Vasil M L, Wang W L. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect Immun. 1983;42:276–284. doi: 10.1128/iai.42.1.276-284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser M J, Smith P F, Kohler P F. Susceptibility of Campylobacter isolates to the bactericidal activity of human serum. J Infect Dis. 1985;151:227–235. doi: 10.1093/infdis/151.2.227. [DOI] [PubMed] [Google Scholar]
- 5.Burr D H, Caldwell M B, Bourgeois A L, Morgan H R, Wistar R, Jr, Walker R I. Mucosal and systemic immunity to Campylobacter jejuni in rabbits after gastric inoculation. Infect Immun. 1988;56:99–105. doi: 10.1128/iai.56.1.99-105.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buxton A. On the transference of bacterial antibodies from hen to the chick. J Gen Microbiol. 1952;7:268–286. doi: 10.1099/00221287-7-3-4-268. [DOI] [PubMed] [Google Scholar]
- 7.Carr M Y, Stern N J, Meinersman R J. Reduction of Campylobacter jejuni colonization in chicks by passive immunisation. Acta GastroenterolBelg. 1993;56(Suppl.):36. [Google Scholar]
- 8.Cawthraw S, Ayling R, Nuijten P, Wassenaar T, Newell D G. Isotype, specificity, and kinetics of systemic and mucosal antibodies to Campylobacter jejuni antigens, including flagellin, during experimental oral infections of chickens. Avian Dis. 1994;38:341–349. [PubMed] [Google Scholar]
- 9.Chuma T, Yamada T, Yano K, Okamoto K, Yugi H. A survey of Campylobacter jejuni in broilers from assignment to slaughter using DNA-DNA hybridization. J Vet Med Sci. 1994;56:697–700. doi: 10.1292/jvms.56.697. [DOI] [PubMed] [Google Scholar]
- 10.Chuma T, Yano K, Omori H, Okamoto K, Yugi H. Direct detection of Campylobacter jejuni in chicken cecal contents by PCR. J Vet Med Sci. 1997;59:85–87. doi: 10.1292/jvms.59.85. [DOI] [PubMed] [Google Scholar]
- 11.Clark A G, Bueschkens D H. Horizantal spread of human and poultry-derived strains of Campylobacter jejuni among chicks held in incubaters and shipping boxes. J Food Prot. 1988;51:438–441. doi: 10.4315/0362-028X-51.6.438. [DOI] [PubMed] [Google Scholar]
- 12.Dolby J M, Newell D G. The protection of infant mice from colonization with Campylobacter jejuni by vaccination of the dams. J Hyg (London) 1986;96:143–151. doi: 10.1017/s0022172400065918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doyle M P. Association of Campylobacter jejuni with laying hens and eggs. Appl Environ Microbiol. 1984;47:533–536. doi: 10.1128/aem.47.3.533-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman C R, Neimann J, Wegener H C, Tauxe R V. Epidemiology of C. jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser M J, editors. Campylobacter. 2nd ed. Washington, D.C.: American Society for Microbiology; 2000. pp. 121–138. [Google Scholar]
- 15.Guerry P. Nonlipopolysaccharide surface antigens of Campylobacter species. J Infect Dis. 1997;176(Suppl. 2):S122–S124. doi: 10.1086/513782. [DOI] [PubMed] [Google Scholar]
- 16.Hassan J O, Curtiss R., III Effect of vaccination of hens with an avirulent strain of Salmonella typhimurium on immunity of progeny challenged with wild-type Salmonella strains. Infect Immun. 1996;64:938–944. doi: 10.1128/iai.64.3.938-944.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornok S, Bitay Z, Szell Z, Varga I. Assessment of maternal immunity to Cryptosporidium baileyi in chickens. Vet Parasitol. 1998;79:203–212. doi: 10.1016/s0304-4017(98)00170-8. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs-Reitsma W. Experimental horizontal spread of Campylobacter amongst one-day-old broilers. In: Lastovica A J, Newell D G, Lastovica E E, editors. Campylobacter, Helicobacter and related organisms. Cape Town, South Africa: University of Cape Town; 1997. pp. 377–378. [Google Scholar]
- 19.Jacobs-Reitsma W F, van de Giessen A W, Bolder N M, Mulder R W A W. Epidemiology of Campylobacter spp. at two Dutch broiler farms. Epidemiol Infect. 1995;114:413–421. doi: 10.1017/s0950268800052122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowalczyk K, Daiss J, Halpern J, Roth T F. Quantitation of maternal-fetal IgG transport in the chicken. Immunology. 1985;54:755–762. [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer T T, Cho H C. Transfer of immunoglobulins and antibodies in the hen's egg. Immunology. 1970;19:157–167. [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Lee L H, Burg E, III, Baqar S, Bourgeois A L, Burr D H, Ewing C P, Trust T J, Guerry P. Evaluation of a truncated recombinant flagellin subunit vaccine against Campylobacter jejuni. Infect Immun. 1999;67:5799–5805. doi: 10.1128/iai.67.11.5799-5805.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linden C D, Roth T F. IgG receptors on feotal chick yolk sac. J Cell Sci. 1978;33:317–328. doi: 10.1242/jcs.33.1.317. [DOI] [PubMed] [Google Scholar]
- 25.Logan S M, Trust T J. Outer membrane characteristics of Campylobacter jejuni. Infect Immun. 1982;38:898–906. doi: 10.1128/iai.38.3.898-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logan S M, Trust T J. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect Immun. 1983;42:675–682. doi: 10.1128/iai.42.2.675-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCoy E C, Doyle D, Burda K, Corbeil L B, Winter A J. Superficial antigens of Campylobacter (Vibrio) fetus: characterization of antiphagocytic component 9. Infect Immun. 1975;11:517–525. doi: 10.1128/iai.11.3.517-525.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Methner U, Steinbach G. Efficacy of maternal Salmonella antibodies and experimental oral infection of chicks with Salmonella enteritidis. Berl Muench Tieraerztl Wochenschr. 1997;110:373–377. [PubMed] [Google Scholar]
- 29.Myszewski M A, Stern N J. Influence of Campylobacter jejuni cecal colonization on immunoglobulin response in chickens. Avian Dis. 1990;34:588–594. [PubMed] [Google Scholar]
- 30.Nachamkin I, Allos B M, Ho T. Campylobacter species and Guillain-Barre syndrome. Clin Microbiol Rev. 1998;11:555–567. doi: 10.1128/cmr.11.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nachamkin I, Hart A M. Western blot analysis of the human antibody response to Campylobacter jejuni cellular antigens during gastrointestinal infection. J Clin Microbiol. 1985;21:33–38. doi: 10.1128/jcm.21.1.33-38.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newell D G, Wagenaar J A. Poultry infections and their control at the farm level. In: Nachamkin I, Blaser M J, editors. Campylobacter. 2nd ed. Washington, D.C.: ASM Press; 2000. pp. 497–509. [Google Scholar]
- 33.Panigrahi P, Losonsky G, DeTolla L J, Morris J G., Jr Human immune response to Campylobacter jejuni proteins expressed in vivo. Infect Immun. 1992;60:4938–4944. doi: 10.1128/iai.60.11.4938-4944.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearson A D, Greenwood M, Healing T D, Rollins D, Shahamat M, Donaldson J, Colwell R R. Colonization of broiler chickens by waterborne Campylobacter jejuni. Appl Environ Microbiol. 1993;59:987–996. doi: 10.1128/aem.59.4.987-996.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson A D, Greenwood M H, Feltham R K, Healing T D, Donaldson J, Jones D M, Colwell R R. Microbial ecology of Campylobacter jejuni in a United Kingdom chicken supply chain: intermittent common source, vertical transmission, and amplification by flock propagation. Appl Environ Microbiol. 1996;62:4614–4620. doi: 10.1128/aem.62.12.4614-4620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rose M E, Orlans E, Buttress N. Immunglobulin classes in the hen's egg: their secretion in yolk and white. Eur J Immunol. 1974;4:521–523. doi: 10.1002/eji.1830040715. [DOI] [PubMed] [Google Scholar]
- 37.Shane S M. The significance of C. jejuni infection in poultry: a review. Avian Pathol. 1992;21:189–213. doi: 10.1080/03079459208418836. [DOI] [PubMed] [Google Scholar]
- 38.Shane S M. Campylobacteriosis. In: Calnek B W, editor. Diseases of poultry. Ames, Iowa: Iowa State University Press; 1997. pp. 235–245. [Google Scholar]
- 39.Shane S M, Gifford D H, Yogasundram K. Campylobacter jejuni contamination of eggs. Vet Res Commun. 1986;10:487–492. doi: 10.1007/BF02214012. [DOI] [PubMed] [Google Scholar]
- 40.Shanker S, Lee A, Sorrell T C. Campylobacter jejuni in broilers: the role of vertical transmission. J Hyg (London) 1986;96:153–159. doi: 10.1017/s002217240006592x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shanker S, Lee A, Sorrell T C. Horizontal transmission of Campylobacter jejuni amongst broiler chicks: experimental studies. Epidemiol Infect. 1990;104:101–110. doi: 10.1017/s0950268800054571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shawky S A, Saif Y M, McCormick J. Transfer of maternal anti-rotavirus IgG to the mucosal surfaces and bile of turkey poults. Avian Dis. 1994;38:409–417. [PubMed] [Google Scholar]
- 43.Skirrow M B, Blaser M J. Clinical aspects of Campylobacter infection. In: Nachamkin I, Blaser M J, editors. Campylobacter. 2nd ed. Washington, D.C.: American Society for Microbiology; 2000. pp. 69–88. [Google Scholar]
- 44.Smith N C, Wallach M, Miller C M, Morgenstern R, Braun R, Eckert J. Maternal transmission of immunity to Eimeria maxima: enzyme-linked immunosorbent assay analysis of protective antibodies induced by infection. Infect Immun. 1994;62:1348–1357. doi: 10.1128/iai.62.4.1348-1357.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stern N J. Reservoirs for C. jejuni and approaches for intervention in poultry. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni: current status and future trends. Washington, D.C.: American Society for Microbiology; 1992. pp. 49–60. [Google Scholar]
- 46.Stern N J, Bailey J S, Blankenship L C, Cox N A, McHan F. Colonization characteristics of Campylobacter jejuni in chick ceca. Avian Dis. 1988;32:330–334. [PubMed] [Google Scholar]
- 47.Stern N J, Meinersmann R J, Dickerson H W. Influence of antibody treatment of Campylobacter jejuni on the dose required to colonize chicks. Avian Dis. 1990;34:595–601. [PubMed] [Google Scholar]
- 48.Widders P R, Perry R, Muir W I, Husband A J, Long K A. Immunisation of chickens to reduce intestinal colonisation with Campylobacter jejuni. Br Poult Sci. 1996;37:765–778. doi: 10.1080/00071669608417906. [DOI] [PubMed] [Google Scholar]
- 49.Widders P R, Thomas L M, Long K A, Tokhi M A, Panaccio M, Apos E. The specificity of antibody in chickens immunised to reduce intestinal colonisation with Campylobacter jejuni. Vet Microbiol. 1998;64:39–50. doi: 10.1016/s0378-1135(98)00251-x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Q, Meitzler J C, Huang S, Morishita T. Sequence polymorphism, predicted secondary structures, and surface-exposed conformational epitopes of Campylobacter major outer membrane protein. Infect Immun. 2000;68:5679–5689. doi: 10.1128/iai.68.10.5679-5689.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]