Abstract
Neonatal surgery and concomitant anesthesia coincide with a timeframe of rapid brain development. The speed and complexity of early brain development superimposed on immature regulatory mechanisms that include incomplete cerebral autoregulation, insufficient free radical scavenging and an immature immune response puts the brain at risk. Brain injury may have long‐term consequences for multiple functional domains including cognition, learning skills, and behavior. Neurodevelopmental follow‐up studies have noted mild‐to‐moderate deficits in children who underwent major neonatal surgery and related anesthesia. The present review evaluates neonatal surgery against the background of neurobiological processes that unfold at a pace unparalleled by any other period of human brain development. First, a structured summary of early brain development is provided in order to establish theoretical groundwork. Next, literature on brain injury and neurodevelopmental outcome after neonatal surgery is discussed. Special attention is given to recent findings of structural brain damage reported after neonatal surgery. Notably, high‐quality imaging data acquired before surgery are currently lacking. Third, mechanisms of injury are interrogated taking the perspective of early brain development into account. We propose a novel disease model that constitutes a triad of inflammation, vascular immaturity, and neurotoxicity of prolonged exposure to anesthetic drugs. With each of these components exacerbating the other, this amalgam incites the perfect storm, resulting in brain injury. When examining the brain, it seems intuitive to distinguish between neonates (i.e., <60 postconceptional weeks) and more mature infants, multiple and/or prolonged anesthesia exposure and single, short surgery. This review culminates in an outline of anesthetic considerations and future directions that we believe will help move the field forward.
Keywords: anesthesia, brain development, brain injury, cerebral autoregulation, neonatal surgery, neurotoxicity
1. INTRODUCTION
The human brain is a vastly complex structure, which emerges during embryonic development and verges on its adult form by the time of full‐term birth: a developmental timeframe that typically spans only 260 days. Although short, gestation hosts an elaborate sequence of neurodevelopmental events that is unparalleled by any other period in life. At birth, most structural elements are in place. Postnatally, connectivity between these attributes continues to develop and mature, shaping the brain's connectivity framework over the entire course of development, which extends into adulthood. The speed and complexity of the neurobiological processes governing early brain development render them at risk of developmental adversity. One of such risks is neonatal surgery and related factors such as anesthesia, critical illness, and/or congenital anomalies.*
The past two decades have witnessed a heated debate on postulated neurotoxicity of anesthetic exposure in the neonatal period. Data from nonhuman primate studies have revealed widespread apoptosis of neurons and oligodendrocytes as well as cognitive, motor, and behavioral problems. To date, clinical evidence has pointed to a bilateral paradigm of safety of a single and short surgical procedure and detrimental impact of prolonged and/or repeated surgeries on the developing neonatal brain. The GAS trial meticulously studied the impact of a single short exposure to general anesthesia with sevoflurane vs. regional anesthesia for inguinal hernia repair in the first year after birth in 719 children, demonstrating no difference in intelligence quotient (IQ) at age five. 1 However rigorous its methodology, the GAS study focused on cognition and was not powered to evaluate behavioral problems, nor did it report follow‐up data beyond early school‐going age. Preterm children have been known to grow into their deficits, which may also apply to infants exposed to early‐life surgery given that such exposure would likely inflict subtle brain alterations that would affect higher cognitive functions and socialization skills.
Studies on neurodevelopmental outcome following major noncardiac surgery in the neonatal period have concurrently revealed mild–moderate deficits on various domains including cognitive functioning, motor performance, and behavioral traits. 2 Recent prospective studies noted mild‐to‐moderate neuroimaging abnormalities in up to 75% of babies following surgery for noncardiac congenital anomalies. 3 , 4 Such structural brain abnormalities have not been described in animal studies and are therefore unlikely related to primary neuronal and oligodendrocyte injury associated with general anesthetics. Alternatively, these lesions are more likely related to hemodynamic, respiratory, and metabolic changes associated with general anesthesia and could also be attributable to surgical stress and the primary disease process. Hence, translation of animal data to the clinical setting is difficult to the very least. Collectively, origin and timing of neonatal brain injury in the setting of noncardiac surgery remain unknown. Nevertheless, we can learn from the lesions uncovered in the neonatal brain after surgery as these may provide valuable cues about their potential pathophysiology.
This review aims to describe key principles of early brain development and patterns of brain injury that have been associated with noncardiac neonatal surgery. We discuss clinical evidence and aim to shift attention from neurotoxicity alone to a triad of inflammation, immature vascularization, and anesthetic neurotoxicity.
2. PRENATAL BRAIN DEVELOPMENT
Early brain development constitutes a remarkable complexity of meticulously timed events that are largely orchestrated by genetic programming and governed by signaling factors, extracellular matrix proteins, and early environmental influences. The ontogeny of the human brain originates in neural tube formation between postconceptional days 20–28. Once neurulation is completed, neuroepithelial cells (neural stem cells) start to proliferate rapidly, deriving precursor neurons and supporting glia cells.
In the early fetal period, commencing at 8 postconceptional weeks (PCW), postmigratory neurons start sprouting axons and dendrites, thereby setting the stage for neural circuitry formation. Synaptogenesis emerges in the outer layer of the cerebral wall and presubplate; the latter will soon transform into the subplate (at 13–15 weeks of gestation). The subplate represents the fetal brain's relay station that is of cardinal importance for healthy brain development. The subplate is a transient developmental layer positioned under the premature cerebral cortex (i.e., cerebral wall) and is responsible for early connectivity between brain regions. It is composed of migratory and postmigratory neurons, abundant axons and glia cells and is extensively larger in primates than in other mammals. Afferent axons from the thalamus and basal forebrain have been detected from as early as 8 PCW and form temporary connections in the presubplate and subsequent subplate before targeting their final destinations in the developing cortex. Postmortem studies have identified a distinct primary‐to‐complex sequence of axonal development. Limbic and thalamocortical fibers originate first, followed by commissural fibers of the corpus callosum at around 11 PCW. Intrahemispheric association fibers derive last by 18 PCW and continue to grow well into the early postnatal period (Figure 1).
FIGURE 1.
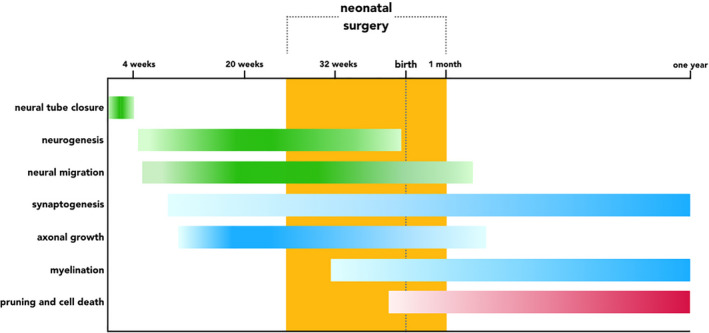
Timeline of early brain development. Schematic diagram of cellular processes governing early brain development from neural tube formation until 1 year after birth. Processes involving neurogenesis are depicted in green, developmental events that result in connection formation are illustrated in blue, and removal of connections is highlighted in red. Intensity of the color bars corresponds with the speed and magnitude of each developmental process. The period during which infants may undergo neonatal surgery is indicated in orange
The mid‐fetal period portrays peak neuronal migration. Although migration culminates between 3 and 5 months of gestation, this critical process does not subside until the third trimester of pregnancy. Extensive migration of neurons to the frontal lobe has recently even been described in the neonatal period.
The early preterm period, that is, 24–32 weeks postmenstrual age (PMA), features a number of events that are vitally important for subsequent neurodevelopment and may be disrupted by exposure to noxious stimuli. First, permanent thalamocortical circuitry is established by the arrival of afferent thalamic axons in the cortical plate. By this time, sensory input including painful stimuli has been noted to elicit a response in the somatosensory cortex. Second, the subplate expands and reaches its peak size and developmental significance. 5 The subplate operates as a waiting compartment for both axonal afferents traveling to the cortical plate and axonal efferents that descend from the immature cortex into deeper gray matter structures. Further, subplate neurons provide axonal guidance for bidirectional pathfinding. Moreover, the subplate is home to transient thalamocortical‐subplate circuits that govern endogenous spontaneous brain activity crucial for brain network formation (i.e., connectivity). As the fetal brain matures, these spontaneous activity transients are gradually replaced by input‐dependent ongoing cortical activity originating from the developing cortex. It is therefore unsurprising that level of brain activity as monitored by early‐life electroencephalography (EEG) recordings has been linked to brain growth in preterm infants. 6 In addition, the subplate was recently demonstrated to be the first to respond to auditory stimuli, well before thalamocortical circuitry comes online. In recent years, the subplate has received increasing attention because of its relevance for typical brain development and its postulated role in neurodevelopmental disorders, including autism, schizophrenia, and hypoxic–ischemic encephalopathy in preterm infants. 5
The third process in the early preterm period that is paramount for healthy brain development involves the oligodendrocyte lineage. Oligodendrocyte precursors (preOLs) are derived from premature glia cells. Following a delicate sequence of maturational events, preOLs advance to mature oligodendrocytes that will subsequently ensheath axons and produce myelin, thereby considerably increasing action potential velocity. Myelination burgeons from around 32 weeks PMA and continues until early adulthood (Figure 1). While these cellular processes derive at a microscopic level, at a macroscopic scale, the brain transforms its layout dramatically, remodeling its smooth surface to a highly convoluted structure that resembles the adult brain by the end of gestation, having all secondary and most tertiary folds in place.
The third trimester of pregnancy is devoted to increasing connectivity in terms of exuberant synaptogenesis, dendritic differentiation, and axonal growth. During this period, long‐range association fibers thrive, and oligodendrocytes develop rapidly. Postnatal brain development is predominantly characterized by ongoing synaptogenesis and elimination of exuberant connections, myelination, and overall brain growth. In the neonatal brain, neuronal architecture matures, glia cells including astrocytes and oligodendrocytes proliferate and short‐range cortico‐cortical association fibers continue to grow into their target sites. The subplate diminishes and transposes into a thin layer at the white and gray matter interface.
2.1. Cerebellar development
Early anatomists described the cerebellum as the arbor vitae, or tree of life, because of its refined anatomical appearance. Traditionally, the cerebellum is considered responsible for motor function and coordination. In the past 15 years, researchers have shifted attention to its pivotal role in cognition and behavior. The cerebellum serves as a control center, modulating and fine‐tuning input and output to and from the cortex. Over the course of development, the cerebellum forms elaborate circuits with various cortical regions, including sensorimotor, limbic, and association cortices. Disruptions to these distinct connectivity frameworks, for example, from early postnatal cerebellar injury have been associated with cognitive impairment, language delay, and behavioral problems. These deficits show similarities with those that have been linked to early‐life surgery and anesthesia. 2 The potential impact of neonatal surgery and related factors is even more worrisome in light of the agility of cerebellar development. While the neocortex develops rapidly, the cerebellum expands at an even faster rate, outpacing all other developmental processes in the brain. Between 24 and 38 weeks PMA, the cerebellum quadruples in volume and multiplies its cortical surface an astonishing 30‐fold.
Granular precursor cells in the cerebellum predominantly determine its expansion rate and harbor its peak growth during the third trimester of pregnancy. Both the intricacy and location of granular cell proliferation jeopardize its developmental trajectory. Being on the outside of the cerebellum and therefore in close proximity to the cerebrospinal fluid (CSF), vulnerable granular precursor neurons may be exposed to potentially toxic CSF compounds including blood (e.g., in case of intraventricular hemorrhage [IVH] and possibly subdural hemorrhage), free radicals, and inflammatory cells. Granular cell damage may impair their proliferation and dendritic arborization, ultimately affecting neural circuit formation and the brain network altogether. Cerebellar injury at birth has been associated with a 36‐fold increased risk of autism, suggesting a brain‐wide imprint of cerebellar damage. 7 Further supporting this postulate, adolescence‐onset schizophrenia has been associated with impaired functional connectivity between the cerebellum and widespread cortical regions. Hence, we have only so much as begun scrutinizing the intricate link between cerebellar development, dysconnectivity and childhood and adolescent disorders of neurodevelopment. In this context, it seems intuitive for preclinical research into anesthetic drug toxicity to attend to the impact on the cerebellum as well. The pace and complexity of cerebellar development likely render it a susceptible target for insult, including (anesthetic) drug toxicity.
3. BRAIN INJURY IN RELATION TO NEONATAL SURGERY
The velocity and complexity of the processes burgeoning in the developing brain around birth render neonatal brain development at risk of injurious events, including early postnatal disease, surgery and related anesthesia and pain. Here, we will describe patterns of brain injury that clinical studies have identified in relation to neonatal surgery mainly focusing on noncardiac surgery that does not involve extracorporeal membrane oxygenation (ECMO) in an effort to avoid indication bias and shed light on the impact of surgery itself. Besides, knowledge gaps predominantly remain in the noncardiac surgery, non‐ECMO population.
In 2014, McCann et al. described a series of six infants who developed serious postoperative encephalopathy after prolonged surgery (>120 min) in the neonatal period. Encephalopathy manifested by seizures within 25 h and a watershed pattern of hypoxic–ischemic brain injury on magnetic resonance imaging (MRI). 8 Watershed injury is typically observed in states of prolonged cerebral hypoperfusion, such as chronic asphyxia, and has also been described in neonatal hypoglycemia. The authors concluded that hypotension was the precedent in all cases and may have been the key player in the pathophysiology of brain injury.
In the following years, the risk of perioperative brain injury was stressed further by three prospective studies. 3 , 4 , 9 , 10 , 11 The first studied 101 newborns who had surgery for noncardiac congenital anomalies and reported mild–moderate brain lesions on postoperative MRI in 72% of preterm infants and 42% of full‐term newborns. Lesions included punctate WMI, stroke, cerebellar hemorrhage, and different stages of IVH including periventricular hemorrhagic infarction (PVHI). WMI was suggested to be ischemic and recent in 69% of preterm infants and 55% of full‐term newborns that were scanned within the time window to detect sub‐acute ischemic injury (>80% of the cohort). No preoperative MRI was performed, and therefore, one cannot rule out that further brain injury was already present prior to surgery.
Remarkably, IVH and related PVHI were found in 9% of full‐term neonates while this pattern of injury is typically observed in extremely preterm infants in the first few days after birth. Similarly, the incidence of cerebellar hemorrhage compared to the numbers reported in the extremely preterm population, suggestive of a mechanism involving vascular immaturity. The notion that subdural hemorrhage was observed in 30% of infants supports this postulate. The authors could not identify risk factors for brain injury other than preterm birth and type of surgery; infants with major gastrointestinal surgery (i.e., gastroschisis, esophageal atresia, and intestinal atresia) were significantly more at risk than those with a minor anomaly (i.e., anorectal malformation). In a separate report, the authors described peri‐ and postoperative seizures in 11 cases. Except for one, all seizures were subclinical, only apparent on amplitude‐integrated EEG recordings and no relationship was found between seizures and brain injury. 12 Infants with genetic diseases and syndromes were included in the study. One may hypothesize that the congenital anomalies requiring neonatal surgery and concomitant brain injury have a common denominator in the child's genetic make‐up. As such, the underpinning genetic abnormality may both be involved in the birth defect and the pathway leading up to postoperatively diagnosed brain injury. Furthermore, the genetic anomaly may harbor comorbidities that add to the chain of events resulting in brain injury.
The second report is a case–control study investigating brain injury, size, and maturation in 39 newborn infants after major surgery that took place within 2 weeks after birth. Cases were matched to 39 controls by gender, gestational age, and PMA at time of MRI. 4 Additionally, the authors evaluated neurodevelopmental outcome at 2 years of age using the Bayley Scales of Infant and Toddler Development (BSITD‐III). Congenital anomalies included esophageal atresia, abdominal wall defects, and congenital diaphragmatic hernia (not requiring ECMO). Infants exposed to neonatal surgery displayed significantly more white matter and cortical gray matter abnormalities assessed using previously reported scoring systems. They also showed smaller brain size and larger ventricles as measured by two‐dimensional brain metrics. Surgical infants were 5.9 times more likely to exhibit delayed cortical maturation and demonstrated significantly poorer language skills and motor performance at age two. The mean difference in language and motor performance was nearly 1 SD, which is generally considered the threshold for neurodevelopmental delay. Infants were scanned 4 weeks after birth or at discharge, whichever came first, and therefore, any inference about timing of brain injury or growth deficits could not be made. The third study described brain volumes in 26 infants with long‐gap esophageal atresia and compared them to healthy control infants in the first year after birth. Overall brain volume and corpus callosum volume were significantly smaller in both preterm (n = 13) and full‐term (n = 13) infants who had undergone esophageal repair using the Foker process (i.e., thoracotomy for staged external traction) compared to healthy control subjects. 10 , 11 Cerebrospinal fluid volumes were reciprocally larger as a result of smaller brain tissue volumes. 9
While studies investigating early brain injury after neonatal surgery are limited to a handful of publications, ample research efforts have been undertaken to assess neurodevelopmental outcome in late infancy and early childhood. Results of such studies were evaluated in a systematic review and meta‐analysis published in 2016. 2 Collating findings from 13 papers reporting on 511 children, the authors found 0.5 SD lower cognitive composite scores and 0.6 SD lower motor scores on the BSITD at age 1 and 2 years. Rates of neurodevelopmental delay defined as BSITD scores <1 SD varied widely, from 3 to 56% regarding cognitive impairment and 0%–77% with respect to motor deficit. Notably, a large proportion of included studies focused on congenital diaphragmatic hernia children with and without ECMO support. The authors identified low birthweight, younger gestational age, multiple congenital anomalies, multiple surgeries, longer hospital stay, and prolonged respiratory support as potential risk factors for neurodevelopmental impairment. Yet, only limited studies described risk factors. The systematic review was further hindered by considerable heterogeneity of studies.
In recent years, efforts to overcome the issue of heterogeneity have surfaced. A number of studies focused on outcomes in cohorts of specific birth defects including gastroschisis 13 and esophageal atresia. 14 School‐aged children with neonatal gastroschisis repair exhibited poorer neurodevelopmental outcome across multiple domains including academic attainment, attention, behavior, and motor skills. Similarly, children with corrected esophageal atresia were noted to have language delay, attention deficit, and motor impairment in early childhood (i.e., 2 and 5 years). However, results have to be interpreted with caution owing to small sample size, loss to follow‐up, a large battery of neurodevelopmental tests, and a wide age range at assessment.
In summary, there is mounting evidence that major surgery for noncardiac congenital anomalies in the neonatal period is associated with mild–moderate brain injury, impaired brain growth, and myriad mild–moderate neurodevelopmental deficits. These findings are offset against results of recent large prospective studies including the hallmark GAS trial. 1 , 15 , 16 Data from these studies provide evidence that a single short exposure to anesthesia before age 3 years in otherwise healthy infants does not lead to cognitive impairment in childhood. However, neonatal surgery frequently involves major surgery of prolonged duration 3 , 4 during a developmental time window that incorporates significant structural changes including widespread synaptogenesis, cortical pathfinding, and proliferation of oligodendrocytes. The neonatal brain is therefore likely to be substantially more susceptible to protracted effects of insult than the more mature brain of older infants whose structural brain network has largely been laid out. 17 , 18 , 19 The arbitrary cutoff of age 3–4 years seems counterintuitive from a brain development point of view. From this perspective, focusing on the neonatal surgery population (i.e., <60 PCW) appears reasonable. We believe that clinical research should aim at unraveling timing of neonatal surgery‐related brain injury, which is best facilitated by pre‐ and postoperative neuroimaging or even prenatal scanning if feasible. The community should be spurred into action to identify risk factors, perform rigorous long‐term follow‐up focusing on (subtle) cognitive and behavioral deficits, improve perioperative monitoring, and ultimately advance perioperative management for our most vulnerable patients.
4. POTENTIAL MECHANISMS OF INJURY AND FUTURE DIRECTIONS
Despite important advances in mapping brain injury following neonatal surgery and defining neurodevelopmental outcome, important questions prevail. As mentioned, origin and potentially preoperative timing of injury remain largely unknown. Most attention has been directed at potential neurotoxicity of anesthetic drugs. Given the observed patterns of WMI, stroke, and IVH/PVHI, inflammation and disrupted cerebral perfusion are likely to be of key relevance (Figure 2). In this final section, we will interrogate potential mechanisms of surgery‐related neonatal brain injury and highlight remaining caveats that we believe are relevant in this context.
FIGURE 2.
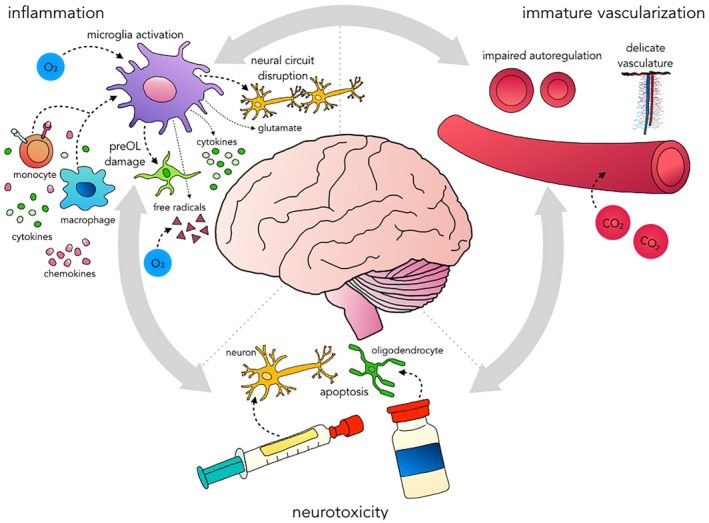
Proposed disease model of brain injury related to noncardiac neonatal surgery. Outline of the triad of inflammation, immature vascularization, and neurotoxicity of prolonged exposure to anesthetic drugs that may ultimately result in brain injury. These attributes are interrelated and may exacerbate each other. Systemic inflammation may be prompted by surgical stress and may have already been set in motion prenatally (e.g., in case of abdominal wall defects). Inflammation and hypoxia–ischemia (e.g., due to hypotension and/or pressure passive perfusion, and desaturation) induce microglia activation, which prompts the release of cytokines, free radicals, and glutamate. The latter instigates excitotoxicity. This cascade as well as microglia activation itself results in damage to preOLs that are abundant in the developing white matter and will ultimately lead to hypomyelination. Microglia orchestrate synaptogenesis, axon formation, and neurogenesis. Their exuberant activation may disrupt neural circuit formation altogether. Finally, (volatile) anesthetics may prompt mitochondrial injury and hyperoxia may enhance injury. Immature vascularization involves underdeveloped autoregulation, incomplete growth of cerebral vessels at the microscopic level and an immature vascular wall. CO2 levels outside their normal range may further compromise cerebral blood flow. Prolonged exposure to various anesthetic agents may induce apoptosis of neurons and oligodendrocytes in the cerebrum and cerebellum
Studies in nonhuman primates have repeatedly shown that prolonged exposure (≥5 h) to various anesthetic drugs including isoflurane, ketamine, and propofol is associated with widespread neuronal apoptosis and oligodendrocyte loss. Recently, 3 h of isoflurane anesthesia was also related to neuronal and oligodendrocyte apoptosis in 6‐day‐old macaques (6–8 months human equivalence). 20 Conjointly, neurotoxicity of anesthesia in the young primate brain has been established but has not been indisputably translated to the human infant, likely underscoring the complexity of such clinical research and the intricacy of human cognitive and behavioral development. Most clinical studies have focused on short and single exposure to general anesthesia. Given the inextricable link between surgery, the underlying condition and prolonged anesthesia exposure, well‐designed prospective studies on the putative impact of lengthy exposure to anesthetic agents are challenging. Despite these challenges, two such efforts are currently underway. A randomized controlled trial (RCT) aims to compare isoflurane against dexmedetomidine and fentanyl anesthesia in neonatal cardiopulmonary bypass surgery (NCT03882788). Another RCT comparing sevoflurane‐based anesthesia to a combination of dexmedetomidine, remifentanil, and a neuraxial agent via caudal block is currently recruiting (NCT03089905), and feasibility results have been published. 21 Dexmedetomidine has been suggested to be less neurotoxic or even neuroprotective and may therefore serve as a valuable comparison. Notably, studies describing brain injury following neonatal surgery reported a wide range of anesthetic regimens, but also of hemodynamic and respiratory management. 3 , 4 , 8 Future research is needed to more extensively investigate the role of anesthetic management as well as respiratory and hemodynamic targets. The putative impact of immature hemodynamic regulation will be discussed in more detail in the last paragraph.
Inflammation is another component that has been suggested in the pathophysiology of perioperative brain injury (Figure 2). Inflammation in addition to hypoxia–ischemia has long been established in the disease model of preterm WMI. 22 These two pillars invoke a cascade of downstream events culminating into an array of WMI. Its most severe form comprises focal necrosis, which is readily seen on modern neuroimaging techniques. Focal necrosis involves destruction of all cellular elements, including glia cells, late migrating neurons, blood vessels, and developing axons as a result of severe energy failure. Small areas of focal necrosis would appear as punctate WMI on MRI. At the other end of the spectrum lies diffuse WMI, which is considered the predominant pattern of WMI in preterm infants. Fundamentally, the downstream mechanisms of inflammation and hypoxia–ischemia lead to oxidative stress, which affects the preOL lineage while leaving axons and other developing cell types intact. Loss of preOLs instigates a wave of preOL renewal but this secondary pool of oligodendrocyte progenitors fails to mature to myelin‐producing oligodendrocytes. Subsequent myelination failure has far‐reaching implications. Lacking trophic support, axons degenerate and growth of their associated gray matter structures in both the cerebrum and cerebellum become impaired. These pathogenic processes translate into functional sequelae across various domains, including attention deficits, motor impairment, and learning disabilities. 22 As a key player in the inflammatory response, microglia dictate preOL demise. Given that microglia have a pivotal role in healthy brain development and are geared to modulate neurogenesis, synaptogenesis, and axonal tract formation, their aberrant activation may fuel disruptive neural circuit formation with potentially long‐term functional consequences. Surgical stress especially in the setting of prolonged procedures and the underlying condition (e.g., abdominal wall defect) may give rise to an inflammatory response that could extend to the brain. Given that the oligodendrocyte lineage is not yet fully mature in the neonatal brain, inflammation may permanently disrupt myelination and related brain development.
The third attribute of the proposed amalgam of surgery‐related neonatal brain injury encompasses cerebral perfusion abnormalities (Figure 2). As mentioned, cerebral hypoxia–ischemia is the mainstay in the pathogenesis of WMI. Cerebral perfusion is mainly regulated by three mechanisms: (i) The arterial baroreflex adjusts heart rate and systemic vascular resistance to systolic arterial blood pressure (ABP) in order to maintain short‐term blood pressure stability; (ii) cerebral blood flow (CBF) autoregulation regulates cerebrovascular tone to compensate for changes in perfusion pressure and maintain CBF more or less constant across a range of perfusion pressures; and (iii) regional CBF is tightly coupled to metabolism (flow‐metabolism coupling) which is mainly reflected in cerebrovascular CO2 reactivity. In preterm neonates, baroreflex sensitivity (BRS) appears to double between 31–32 weeks and 37–39 weeks PMA. 23 Lower birthweight has been associated with diminished BRS. There are no data on BRS during anesthesia in neonatal surgery. However, if analogous to the adult population, common anesthetic drugs including propofol and sevoflurane would depress sympathetic outflow and nearly obliterate BRS.
Diminished autoregulatory capacity reflected by pressure passive perfusion and cerebral hyperperfusion have been established in the etiology of IVH/PVHI in preterm infants. Both patterns of injury have also been linked to neonatal surgery. Indeed, the relative sparsity of vascularization in the developing white matter in addition to immaturity and delicacy of the cerebral vessel walls may give way to both ischemic and hemorrhagic brain injury under fluctuating conditions, especially in preterm infants operated on before full‐term age. CBF autoregulation to systolic and mean ABP develops between 23 and 33 weeks of gestation. 24 However, CBF during diastole is largely pressure passive until 33 weeks PMA. 24 Furthermore, diastole is typically low in newborn infants, (yet also difficult to accurately measure and frequently underestimated). At lower ends of the autoregulatory curve, where diastolic ABP may fall below intracranial pressure, cerebral perfusion may subside, increasing the risk of hypoxic–ischemic brain injury. Such brief episodes of ischemia may also induce inflammation and prime the vasculature to rupture and subsequent hemorrhagic injury. Little is known about CBF autoregulation in neonates during anesthesia. One study measured CBF velocity using transcranial Doppler sonography in 113 infants <2 years during sevoflurane anesthesia and found a significant decrease in CBF velocity when mean NIBP dropped ≥20% from baseline in the subgroup of infants under 6 months of age. Infants older than 6 months did not show such effect, until mean NIBP fell ≥40% from baseline. An obvious major limitation to this study is that blood pressure was not measured by means of indwelling arterial catheters. 25 Another report evaluated cerebral autoregulation in 19 preterm infants undergoing laparotomy for necrotizing enterocolitis and spontaneous intestinal perforation by comparing near infrared spectroscopy (NIRS) metrics extracted every 30 s to mean ABP values measured every 5 s to 5 min by means of indwelling catheters or NIBP. Data were divided into three epochs: the preoperative phase (30 min to 8 h), the surgical timeframe, and the postoperative period (max 8 h). CBF autoregulation was considered absent if the inverse correlation between the NIRS metric of cerebral fractional tissue oxygen extraction and mean ABP was >0.3 across each of these epochs. In this way, 12 (63%) preterm infants demonstrated impaired autoregulation during surgery. Similar to adults, increasing end‐tidal sevoflurane concentration and PCO2 were associated with an increased risk of diminished autoregulatory capacity. 26 To our knowledge, well‐designed studies using high‐frequency sampling to investigate autoregulatory physiology in newborn infants during anesthesia are lacking. Interestingly, lower BRS is associated with greater blood pressure variability and larger swings in cerebral perfusion pressure that may challenge an underdeveloped neonatal CBF autoregulation and put cerebral perfusion at risk of ischemia and hemorrhage.
Cerebral vascular resistance and related CBF are not only modulated by ABP but also under tight control of neurogenic factors, metabolic demand, and chemical compounds including pH, PaCO2, and PaO2. The latter are particularly relevant in the context of neonatal surgery as they may exacerbate injury, both at high and low ends. Comparable risk applies to glucose because hypoglycemia is intrinsically related to neonatal brain injury and both hypo‐ and hyperglycemia may enhance emerging hypoxic–ischemic lesions. Together, these findings suggest a rationale for rigorously stable hemodynamics and respiratory parameters during neonatal surgery. Elaborate monitoring using transcutaneous CO2 sensors, NIRS, and EEG may be relevant in achieving such stability, as well as frequent sampling and administering vasopressors to counteract depressant circulatory effects of common anesthetic agents. Such perioperative regimen seems utmost relevant in newborn infants with pre‐existent brain injury, in preterm infants, if the procedure is estimated to last >1 h and in settings of repeated surgery given that brain lesions are most frequently reported under these conditions. 2 , 3 , 4
5. CONCLUSION
Human brain development is incredibly complex and far from being fully elucidated. The undisputed roles of the fetal subplate and cerebellum in the development of cerebral connectivity have only recently been described. A large body of literature from experimental studies has established the neurotoxic effects of prolonged anesthetic exposure on the young primate brain. Clinical counterparts of these preclinical findings are not as straightforward, underscoring the complexity of such clinical research and the intricacy of human cognitive and behavioral development. Large prospective studies and a landmark RCT revealed the safety of short, single anesthesia exposure in otherwise healthy infants for childhood neurodevelopment. Nonetheless, high‐risk neonatal surgery takes place during a critical period of brain development, against a background of immature regulatory mechanisms including cerebral vasculature, the body's immune response, and free radical scavenging. The neonatal brain is therefore unmistakably at risk. In concert with the abundance of translational evidence for anesthesia‐inflicted neurotoxicity, a small number of recent research efforts have stressed the vulnerability of the neonatal brain and revealed patterns of ischemic and hemorrhagic brain injury in the aftermath of neonatal surgery. To what extent brain injury may already be present before surgery has remained elusive. Neurodevelopmental follow‐up studies in these children have demonstrated mild–moderate deficits across multiple domains. Together, divergent literature findings underscore that much is yet to be deciphered. First, the impact and potential neurotoxicity of prolonged (>1 h) anesthesia remains to be determined. Second, etiology and timing of neonatal brain injury warrant further study. Preterm birth, multiple surgery, congenital anomalies, and low birthweight appear to be associated with surgery/anesthesia‐related brain injury, yet risk factors are to be established. Long‐term follow‐up studies—preferably into adolescence—are key to evaluate the full impact of neonatal surgery on the early developing brain. Given the processes of neural circuit formation and the observed patterns of mild–moderate brain injury in infants undergoing major surgery, one would expect functional sequelae to comprise deficits in higher cognitive functions. These do not manifest until well into childhood and adolescence. We have proposed a disease model that integrates vascular immaturity, inflammation, and putative neurotoxicity of lengthy exposure to anesthetics. Research initiatives are awaited to provide insight into the complex interplay of immature regulatory mechanisms, potentially predisposing factors of aberrant development and perioperative brain injury. Such initiatives should be undertaken in preclinical models as well as in the clinical setting and warrants collaboration within and beyond the pediatric anesthesia community. Until much‐needed answers are available, reasonable anesthetic management seems to encompass maintaining physiologic conditions in terms of cardiac output, heart rate, blood pressure, and metabolism (e.g., glucose, pH, PaO2, and PaCO2) that can be achieved by close monitoring and frequent sampling.
6. REFLECTIVE QUESTIONS
What are the roles of the subplate and cerebellum during fetal and neonatal brain development?
What brain structures are most vulnerable during neonatal surgery and why?
What patterns of brain injury have been associated with neonatal surgery?
What are likely mechanisms of injury and how should we address them?
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors thank Floris Groenendaal and Rogier Immink for fruitful discussions on the topic of neonatal brain injury and cerebral autoregulation.
Keunen KP, Sperna Weiland NH, de Bakker BS, de Vries LS, Stevens MF. Impact of surgery and anesthesia during early brain development: A perfect storm. Pediatr Anesth. 2022;32:697–705. doi: 10.1111/pan.14433
Section Editor: Laszlo Vutskits
Footnotes
From here onwards when neonatal surgery is written, we refer to neonatal surgery and related anesthesia.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this narrative review.
REFERENCES
- 1. McCann ME, De GJC, Dorris L, et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake‐regional anaesthesia in infancy (GAS): an international multicentre randomised controlled equivalence trial. Lancet. 2019;393(10172):664‐677. doi: 10.1016/S0140-6736(18)32485-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stolwijk LJ, Lemmers MA, Harmsen M. Neurodevelopmental outcomes after neonatal surgery for major noncardiac anomalies. Pediatrics. 2016;137(2):e20151728. [DOI] [PubMed] [Google Scholar]
- 3. Stolwijk LJ, Keunen K, De VLS, et al. Neonatal surgery for noncardiac congenital anomalies: neonates at risk of brain injury. J Pediatr. 2016;182:335‐341.e1. doi: 10.1016/j.jpeds.2016.11.080 [DOI] [PubMed] [Google Scholar]
- 4. Moran MM, Gunn‐charlton JK, Walsh JM, et al. Associations of neonatal noncardiac surgery with brain structure. J Pediatr. 2019;212:93‐101.e2. doi: 10.1016/j.jpeds.2019.05.050 [DOI] [PubMed] [Google Scholar]
- 5. Kostović I, Išasegi IŽ, Krsnik Ž. Sublaminar organization of the human subplate: developmental changes in the distribution of neurons, glia, growing axons and extracellular matrix. J Anat. 2019;235(3):481‐506. doi: 10.1111/joa.12920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benders MJ, Palmu K, Menache C, et al. Early brain activity relates to subsequent brain growth in premature infants. Cereb Cortex. 2015;25(9):3014‐3024. doi: 10.1093/cercor/bhu097 [DOI] [PubMed] [Google Scholar]
- 7. Wang SS, Kloth AD, Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83(3):518‐532. doi: 10.1016/j.neuron.2014.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCann ME, Schouten ANJ. Infantile postoperative encephalopathy: perioperative factors as a cause for concern. Pediatrics. 2014;133(3):e751‐e757. doi: 10.1542/peds.2012-0973 [DOI] [PubMed] [Google Scholar]
- 9. Mongerson CRL, Wilcox SL, Goins SM, et al. Infant brain structural MRI analysis in the context of thoracic non‐cardiac surgery and critical care. Front Pediatr. 2019;7:1‐13. doi: 10.3389/fped.2019.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mongerson CRL, Jennings RW, Zurakowski D, Bajic D. Quantitative MRI study of infant regional brain size following surgery for long‐gap esophageal atresia requiring prolonged critical care. Int J Dev Neurosci. 2019;79:11‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mongerson CRL, Jaimes C, Zurakowski D, Jennings RW, Bajic D. Infant corpus callosum size after surgery and critical Care for Long‐gap Esophageal Atresia: qualitative and quantitative MRI. Sci Rep. 2020;10(1):6408. doi: 10.1038/s41598-020-63212-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stolwijk LJ, Weeke LC, de Vries LS, et al. Effect of general anesthesia on neonatal aEEG – a cohort study of patients with non–cardiac congenital anomalies. PLoS One. 2017;12(4):1‐14. doi: 10.1371/journal.pone.0183581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris EL, Hart SJ, Minutillo C, et al. The long‐term neurodevelopmental and psychological outcomes of gastroschisis: a cohort study. J Pediatr Surg. 2016;51(4):549‐553. doi: 10.1016/j.jpedsurg.2015.08.062 [DOI] [PubMed] [Google Scholar]
- 14. Harmsen WJ, Aarsen FJ, Van Der Cammen‐Van Zijp MHM, et al. Developmental problems in patients with oesophageal atresia: a longitudinal follow‐up study. Arch Dis Child Fetal Neonatal ed. 2017;102(3):F214‐F219. doi: 10.1136/archdischild-2015-309976 [DOI] [PubMed] [Google Scholar]
- 15. Warner DO, Zaccariello MJ, Katusic SK, et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the Mayo anesthesia safety in kids (MASK) study. Anesthesiology. 2018;129(1):89‐105. doi: 10.1097/ALN.0000000000002232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun LS, Li G, Miller TLK, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315(21):2312‐2320. doi: 10.1001/jama.2016.6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao W, Alcauter S, Elton A, et al. Functional network development during the first year: relative sequence and socioeconomic correlations. Cereb Cortex. 2014;25(9):1‐10. doi: 10.1093/cercor/bhu088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27(1–2):3‐18. doi: 10.1016/s0149-7634(03)00005-8 [DOI] [PubMed] [Google Scholar]
- 19. Knickmeyer RC, Gouttard S, Kang C, et al. A structural MRI study of human brain development from birth to 2 years. J Neurosci. 2008;28(47):12176‐12182. doi: 10.1523/JNEUROSCI.3479-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noguchi KK, Johnson SA, Dissen GA, et al. Isoflurane exposure for three hours triggers apoptotic cell death in neonatal macaque brain. Br J Anaesth. 2017;119(3):524‐531. doi: 10.1093/bja/aex123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Szmuk P, Andropoulos D, McGowan F, et al. An open label pilot study of a dexmedetomidine‐remifentanil‐caudal anesthetic for infant lower abdominal/lower extremity surgery: the T REX pilot study. Pediatr Anesth. 2019;29(1):59‐67. doi: 10.1111/pan.13544 [DOI] [PubMed] [Google Scholar]
- 22. Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110‐124. doi: 10.1016/S1474-4422(08)70294-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haskova K, Javorka M, Czippelova B, Zibolen M, Javorka K. Baroreflex sensitivity in premature infants ‐ relation to the parameters characterizing intrauterine and postnatal condition. Physiol Res. 2017;66(Supplementum 2):S257‐S264. [DOI] [PubMed] [Google Scholar]
- 24. Rhee CJ, da Costa CS, Austin T, Brady KM, Czosnyka M, Lee JK. Neonatal cerebrovascular autoregulation. Pediatr Res. 2018;84(5):602‐610. doi: 10.1038/s41390-018-0141-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rhondali O, Mahr A, Simonin‐Lansiaux S, et al. Impact of sevoflurane anesthesia on cerebral blood flow in children younger than 2 years. Paediatr Anaesth. 2013;23(10):946‐951. doi: 10.1111/pan.12166 [DOI] [PubMed] [Google Scholar]
- 26. Kuik SJ, van der Laan ME, Brouwer‐Bergsma MT, et al. Preterm infants undergoing laparotomy for necrotizing enterocolitis or spontaneous intestinal perforation display evidence of impaired cerebrovascular autoregulation. Early Hum Dev. 2018;118:25‐31. doi: 10.1016/j.earlhumdev.2018.01.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this narrative review.


