Background:
More than one-fifth of the world’s population consumes Chinese cuisines regularly, but no evidence-based healthy diets fitting the Chinese food culture are available for implementation.
Methods:
A multicenter, patient- and outcome assessor–blind, randomized feeding trial was conducted among 265 participants with 130 to 159 mm Hg baseline systolic blood pressure (SBP) for 4 major Chinese cuisines (Shangdong, Huaiyang, Cantonese, Szechuan). After a 7-day run-in period on a control diet matching the usual local diets, participants were randomized to continue with the control diet or the cuisine-based Chinese heart-healthy diet for another 28 days. The primary outcome was SBP, and secondary outcomes included diastolic blood pressure and food preference score. Linear regression models were used to estimate the intervention effects and adjustments for the center. The incremental cost per 1 mm Hg reduction in SBP was also calculated.
Results:
A total of 265 participants were randomized (135 on the Chinese heart-healthy diet and 130 on the control diet), with 52% women, mean age of 56.5±9.8 years, and mean SBP and diastolic blood pressure of 139.4±8.3 and 88.1±8.0 mm Hg, respectively, at baseline. The change in SBP and diastolic blood pressure from baseline to the end of the study in the control group was –5.0 (95% CI, –6.5 to –3.5) mm Hg and –2.8 (95% CI, –3.7 to –1.9) mm Hg, respectively. The net difference of change between the 2 groups in SBP and diastolic blood pressure were –10.0 (95% CI, –12.1 to –7.9) mm Hg and –3.8 (95% CI, –5.0 to –2.5) mm Hg, respectively. The effect size did not differ among cuisines (P for interaction=0.173). The mean food preference score was 9.5 (with 10 the best preferred) at baseline, and the net change during intervention was 0.1 (95% CI, –0.1 to 0.2; P=0.558). The incremental cost-effectiveness ratio per 1 mm Hg SBP reduction was CNY 0.4 (USD 0.06) per day. No difference in the number of adverse events was found between the 2 groups (P=0.259), and none of the adverse events was associated with the intervention.
Conclusions:
The Chinese heart-healthy diet is effective, palatable, and cost-effective in reducing blood pressure in Chinese adults with high blood pressure, with a clinically significant effect applicable across major Chinese cuisine cultures.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03882645.
Keywords: adult, blood pressure, China, healthy diet, hypertension
Clinical Perspective.
What Is New?
This study developed the first heart-healthy diet that fits Chinese food culture and emphasizes its palatability by involving master chefs in developing recipes.
With a patient- and assessor-blinded randomized feeding trial, the study demonstrated that the blood pressure–lowering effect of the Chinese heart-healthy diet could be substantial and compatible with medications while palatable and affordable in Chinese adults with high blood pressure.
What Are the Clinical Implications?
Our results support the idea that “food is medicine” and will give many patients with high blood pressure the confidence to adopt a healthy diet as their lifestyle treatment.
According to the study, clinicians should recommend that their Chinese patients with high blood pressure adopt a healthy diet with low sodium and high potassium, fiber, vegetables, and fruits as the first-line treatment.
Restaurants and cafeterias should consider adopting the Chinese heart-healthy diet menu, particularly those providing services to institutional living populations.
Cardiovascular disease (CVD) remains the leading cause of global deaths and a major contributor to disability.1,2 High systolic blood pressure (SBP) ranked as the top modifiable risk factor for CVD burden.3 Enormous data have shown the causal relationship between dietary factors and blood pressure or hypertension.4 In 2017, 11 million deaths and 255 million disability-adjusted life years were attributable to dietary risk factors globally.5
In China, the CVD burden has increased rapidly in the past decades.6 Among the major factors driving the rise, the changes in diet toward an unhealthy pattern are undoubtedly among the most important ones.6 The 2012 China National Nutrition Survey showed that grain foods, tubers and legumes, and vegetables and fruits decreased by 34%, 80%, and 15%, respectively, compared with 1982 data; in contrast, meat, eggs, and edible oil increased by 162%, 233%, and 132%, respectively (Report on China National Nutrition Survey Data Collection, unpublished data, 1985). The CVD burden, in conjunction with the departure from the once healthier traditional Chinese diet toward an unhealthy pattern, is increasing and has become a growing concern.7 It is clear that China, as the most populous country in the world, needs effective dietary strategies to improve the health status of its population.
Strong evidence supporting the effective blood pressure– and CVD risk–lowering properties of healthy diets such as the Dietary Approach to Stop Hypertension (DASH) diet, Mediterranean diet, etc, have been obtained collectively during the past decades.8–12 In particular, the DASH study established the blood pressure–lowering effect of healthy diets by providing the most substantial evidence with a rigorously controlled randomized feeding trial, which successfully reduced SBP by 5.5 mm Hg in 8 weeks.8 Since the creation of the DASH diet, numerous trials have demonstrated that it consistently lowers blood pressure across a diverse range of patients with hypertension and prehypertension, globally.13 However, implementing these healthy diets is challenging and unsatisfactory, and they are often criticized as unpalatable.14
Because of the complexity of Chinese cuisines and the obvious differences between Chinese and Western diets,15 the current evidence-based diets proven mostly in Western populations are hard to implement in China. Even within the same Chinese food culture, people living in different regions consume diets differently with different cuisines that bear strong local characters. “One size fits all” may not work in this situation, and thus a Chinese heart-healthy (CHH) diet incorporating characters of major Chinese cuisines has to be developed. In the DECIDE-Diet trial (Diet, Exercise and Cardiovascular Health), we aimed to develop and rigorously evaluate the CHH diet’s palatability, affordability, and health efficacy across major Chinese cuisines.16
Methods
The data that support the findings of this study are available from the corresponding author (Yangfeng Wu) on reasonable request.
Study Design
The DECIDE-Diet study design and protocol have been published previously.16 Briefly, it was a multicenter, randomized, patient- and outcome assessors–blind, parallel controlled feeding trial to evaluate the CHH diet’s efficacy, preference, and cost-effectiveness in lowering blood pressure and other cardiovascular risks among community residents with mild hypertension. The trial was conducted at 4 study centers across China (Beijing, Shanghai, Guangzhou, and Chengdu), representing 4 major Chinese cuisines: Shandong, Huaiyang, Cantonese, and Szechuan. These cuisines represent the dietary styles of >80% of the total population in China.17 Each center chose a collaborative local catering organization that was able to provide a kitchen with enough space, equipment, and dining rooms to run the study. The chefs were selected from the catering organizations, trained, and overseen by the research staff. The Management Committee designed and oversaw the trial in consultation with the Advisory Committee. The trial was supported by the National Key Research and Development Program, Ministry of Science and Technology of China. The Peking University Institutional Review Board approved the trial protocol. The trial was performed in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent. The trial was registered (URL: https://www.clincialtrials.gov; Unique identifier: NCT03882645) for the first time on March 18, 2019; the first patient was enrolled on March 22, 2019. The inclusion criteria were updated to ensure the evaluation of the intervention effect on blood pressure by requiring all participants to have SBP ≥130 mm Hg and extending the upper limit of the age range to 75 years because of difficulties in completing the first batch of 8 patients. We also replaced 24-hour urine collection with spot urine collection to increase the feasibility. The registry information on these key modifications was first updated on July 5, 2019, but no significant changes were made afterward. The final follow-up of the last patient was completed on January 31, 2021.
Study Population and Screening
Men and women aged 25 to 75 years old, living in the target communities for at least 6 months before the study, with SBP between 130 to 159 mm Hg, were eligible for this trial. More detailed inclusion and exclusion criteria were presented in the design article. Meetings were held, and advertisement posters were placed in the relevant communities to recruit potential participants. The responders were first screened physically by a trained recruiter using a simple questionnaire and underwent blood pressure measurements. The potential participants were then invited to the study sites for the second screening by trained research staff to confirm their eligibility.
Run-In (7 Days), Baseline Assessment, and Recruitment
The eligible participants were invited to a 7-day run-in phase during which the usual local diets (control diets) were provided and further adjusted to fit best the participants’ usual diet taste and food preference. The baseline data were collected at the end of the run-in phase, including measurement of blood pressure, pulse rate, height, and weight; a questionnaire interview was conducted on demography, lifestyle and health behaviors, medical history, current medication use, and the food preference of the run-in diet. In addition, the spot urine was collected to test for urinary sodium and potassium excretion.
Specifically, blood pressure was measured 3 times within 24 hours: 8:00 to 10:00 am, 2:00 to 4:00 pm, and 6:00 to 8:00 pm. All blood pressure measurements were taken at least half an hour after each meal to ensure quality. Three readings were taken at each time point on the upper right arm with at least a 1-minute interval using an Omron HEM-7136 blood pressure monitor (Omron Health Care Co, Ltd; Matsusaka, Japan). The mean of 9 readings was used in the primary analysis. The pulse rate was measured with the same device for blood pressure measurement. Data on demography, lifestyle and health behaviors, medical history, and current medication use were collected with interviews by trained staff using a questionnaire.18 Body weight was measured using a standard protocol, and the food preference was measured by the participants using a visual assessment scale with a score of 10 indicating most preferred.
The participants who consumed more than 18 meals in the 7 days and whose baseline mean SBP was still within 130 to 159 mm Hg would be formally enrolled in the trial.
Randomization
All enrolled participants were randomly assigned in a 1:1 ratio to the CHH intervention and control groups using a central randomization procedure stratified by study center and enrollment batch. A statistician based at the study coordinating center in Peking University Clinical Research Institute was responsible for generating the random allocation sequence using SAS V9.4 (SAS Institute, Cary, NC). The allocation sequence was concealed from researchers and participants.
Interventions (28 Days)
Immediately after completing the 7-day run-in phase, participants in the CHH intervention group started to consume the CHH diet, whereas those in the control group continued with the same usual diet as provided during the run-in phase. All participants were asked to consume the study meals at 3 meal times (breakfast, lunch, and dinner) for the next 28 days. No other intervention was provided to the participants in both groups.
CHH Diet
As previously reported,16 the CHH diet encompasses 4 versions of Chinese cuisines, and each version contains a set of menus for breakfast, lunch, and dinner, with nonrepetitive dishes within a cycle of at least 2 weeks. The recipe of each dish was developed jointly by the study nutritionists, dietitians, and chefs in the study team in advance, according to the CHH diet daily nutrients and energy composition targets,16 and the availability and interchangeability of foods during the season. The daily nutrients and energy composition targets of the CHH diet were based on the Chinese Dietary Guidelines,19 knowledge obtained from previous successful healthy diets, particularly the DASH diet,8 Chinese food and nutrient intake levels from the recent national nutrition survey,20 and the most common local foods and dishes from our surveys. Compared with the nutrient composition of a usual Chinese diet in urban China, the CHH diet had reduced energy from fat by 5 to 8%, increased energy intake from protein by 3.5 to 5.5%, and increased energy from carbohydrates by 0 to 5%, respectively. Further, dietary fiber intake increased by 2-fold (from 11 to 30 g/d); potassium intake increased from <1700 mg/d to 3700 mg/d; and it is important that sodium intake reduced from nearly 6000 mg/d to 3000 mg/d. More details of the CHH diet composition can be found in the design article.16
Control Diet
The menu of the usual local diet was developed according to our survey of common local foods. It was adjusted to the participants’ usual taste and flavor during the run-in period and was used during the 28 days of the randomized period.
Blinding
Participants in both groups were unaware of the intervention allocation and arranged to take their meals in separate dining rooms. Although the nutrients targets differed between the control and CHH diets, each meal’s menu on the same day was kept as similar as possible between intervention groups, with the same dish name (eg, Kongbao chicken) and interchangeable raw food ingredients. In addition, research staff blinded to the intervention allocation conducted outcome measurements. Blood pressure was measured by the trained research staff blinded to the interventions, and laboratory measurements were performed by central laboratory staff without awareness of the randomization codes. Our assessment at the end of the study showed that the blinding was successful with a James’s blinding index of 0.68 (95% CI, 0.59 to 0.77).21
Outcomes and Follow-Ups
All the data measurements conducted at baseline were repeated at the end of the trial with the same methods. In addition, morning blood pressure, body weight, and food preference were measured weekly at the end of the intervention’s first, second, and third weeks. Morning blood pressure was measured using the same device and method used at the baseline and the end of the intervention.
The primary outcome was the change in SBP from baseline to the end of the study. The secondary outcomes included the change in diastolic blood pressure (DBP) and food preference. The mean costs of the CHH and control diets per participant during the intervention period (28 days) were calculated, and the incremental cost of the CHH diet was estimated per mm Hg SBP reduction.
The participants were reminded daily to keep their medications unchanged unless their physicians instructed it.
Quality Assurance and Quality Control
During the run-in and randomized intervention periods, the leftover foods of each participant for every meal, as well as the food not provided by the study, were recorded to estimate their exact daily intakes of food and nutrients.
All participants were required to consume all of their meals on-site during the entire study period, with <20% of the study meals allowed to be taken home to eat with the investigators’ permission and to accommodate the participants when they were unable to consume the meal on-site. The amounts of leftovers were assessed by trained study staff after each meal by weighing the leftovers or visual estimation of either real leftovers or photos of leftovers submitted by the participants. The visual estimation method was validated by 19 staff before the study began. For 5 samples of study foods leftover, each had <20%, 20% to 39%, 40% to 59%, 60% to 79% and >80% of the original foods. Mean differences (SD) between estimated and weighted amount were +1.4 (4.2) g, +2.6 (21.0) g, +1.7 (27.5) g, –1.3 (34.3) g, and –9.7 (29.1) g, respectively, for visual estimation of real foods; and were +2.5 (6.4) g, –2.5 (17.8) g, –1.4 (26.0) g, –0.6 (32.1) g, and –16.4 (34.3) g, respectively, for visual estimation of photos. The interobserver correlation coefficient ranged from 0.91 to 0.99 among regions for the real food estimation and 0.98 to 0.99 for the photo estimation.
If a participant’s body weight changed by >2 kg from baseline, their energy intake was adjusted immediately to stabilize weight.
In addition, information on foods outside of the study was collected before every meal. Overall, the participants who consumed <80% of the study meals had their medications (medications to lower blood pressure, glucose, or serum lipids) adjusted by either themselves or their physicians, and body weight changes >2 kg were considered a deviation from the study protocol and hence were excluded from the per-protocol analysis.
We established an electronic data capture system that was centrally managed, allowed prompt data collection as the study moved forward, and enabled remote monitoring of the study implementation quality.
We determined 24-hour urinary sodium and potassium excretion by the measured concentration of the electrolytes in spot urine samples using the method developed in the China Salt Substitute and Stroke Study,22 in which individual spot urine sodium and potassium concentrations were multiplied by a single estimated 24-hour urine volume that represents the average 24-hour urine volume of the study population. Our study obtained the mean volume (2.5 L) of 24-hour urine samples in the first batch of 8 participants in the Beijing study center.
Statistical Analysis
The study was designed to recruit 180 participants in each arm that would allow us to detect an intervention effect size of 3.0 mm Hg in SBP in 4 weeks, assuming a SD change in SBP of 8.0 mm Hg, with 90% power and a type I error rate of 5%.16 The effect size was conservatively estimated on the basis of data from the DASH trial.8
The primary analyses followed the intention-to-treat principle and were conducted among randomized participants with blood pressure taken at baseline and final follow-up (or at withdrawal). The linear regression model was used to estimate the net differences between the 2 groups for primary and secondary outcomes, reported as least square means after adjusting for the center as a fixed effect. We did not impute primary or secondary outcomes for the minimal dropout rate (3.7% in CHH and 1.7% in control).
The baseline characteristics were compared between the 2 groups to examine the comparability. Sensitivity analyses were performed to repeat the analyses in the per-protocol set and adjust for the change in body weight from baseline to end of the intervention, as prespecified in the statistical analysis plan. More sensitivity analyses were conducted to explore further the possible effect of other factors on the intervention effects, including a linear mixed model to adjust for the center’s clustering effect and the baseline blood pressure values, another linear mixed model to adjust further for all possible confounders measured in our study (ie, age, sex, antihypertension medication use, alcohol drinking, and weight change from baseline to the end of study), and pooling the center-specific estimations by the random-effect meta-approach to account for different centers. The per-protocol set excluded participants who consumed <80% of the study meals, had their medications (medications to lower blood pressure, glucose, or serum lipids) changed, or had body weight change >2 kg during the intervention period.
The mean of the 3 morning blood pressure readings at all visits (baseline and end of first, second, third, and last week) was used as the outcome variable to test the temporal trend of effect. A linear mixed model was used to adjust for the center as a random effect, time of visit, and the interaction term of dietary intervention with the time of visit. Prespecified subgroup analyses were performed to identify potential modifiers of the intervention effect, including the type of Chinese cuisine, sex, age, hypertension status, baseline SBP, antihypertension medication use, and estimated 10-year risk of ischemic cardiovascular disease.
Each participant’s everyday nutrient and food intakes were estimated according to the standard daily menus, meal recipes, recorded amount of leftovers, and foods consumed outside of the study, using the China Food Composition Table.23 The baseline intakes of nutrients for each participant were defined as the means during the last 3 days of run-in, and the intervention intakes were defined as the means during the 28 days of intervention. The same model as for primary analyses was used to compare the differences in change in nutrients and foods between the intervention groups.
Results
Implementation of the Study
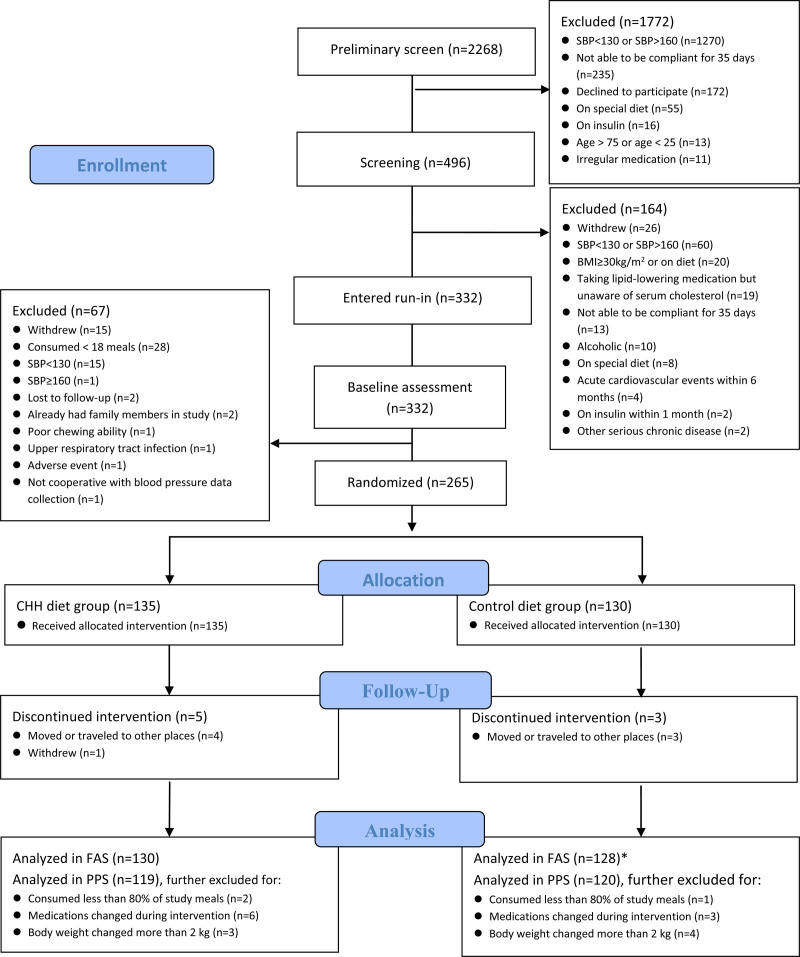
Among the 2268 individuals screened, the eligible 332 started the run-in phase. Of 332 initial participants, 265 passed the run-in and were randomized (Figure 1). Because of the COVID-19 epidemic and the quarantine policy, the study had to be terminated early, achieving 74% of the planned sample size (360). However, a high percentage (97%) of the participants completed the study, and on average, they consumed 97% of the meals provided by the study. The study recruited at least 20% of participants from each cuisine area.
Figure 1.
Flow diagram. BMI indicates body mass index; CHH, Chinese heart-healthy diet; FAS, full analysis set; PPS, per-protocol set; and SBP, systolic blood pressure. *One participant discontinued the intervention but had blood pressure measured at the withdrawal and was included in FAS.
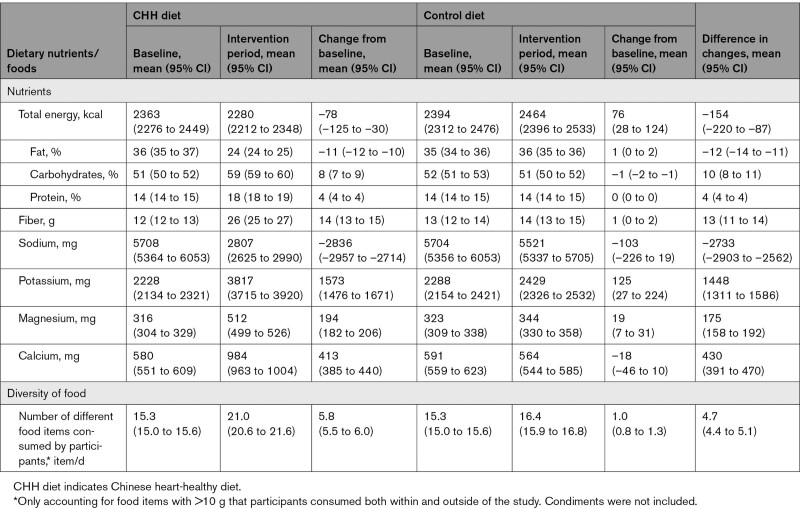
The actual mean nutrients intake and energy composition of participants in the CHH intervention group were similar to the targets of the CHH diet (Table S1). Compared with the baseline intakes, the percentage of energy intake from carbohydrates and protein increased, whereas it decreased for fat in the CHH diet group. There were also increased fiber, potassium, magnesium, and calcium and decreased sodium intakes (all P values <0.05, Table 1). Meanwhile, the control group’s major nutrient intakes were almost unchanged compared with the baseline.
Table 1.
Baseline, Intervention Period, Change From the Baseline, and Difference in Changes in Daily Average Intake of Nutrients in Participants, by CHH Diet and Control Diet
The net change in estimated 24-hour urinary sodium and potassium excretion was –2194 mg (95% CI, –2870 to –1517) and 941 mg (95% CI, 363 to 1519), respectively, in line with the changes in dietary sodium and potassium intake.
The mean change in weight from the baseline to the end of the intervention was –0.6 (±1.0) kg and 0.1 (±1.0) kg in the CHH diet and control diet groups, respectively (P<0.001).
Baseline Characteristics of the Participants
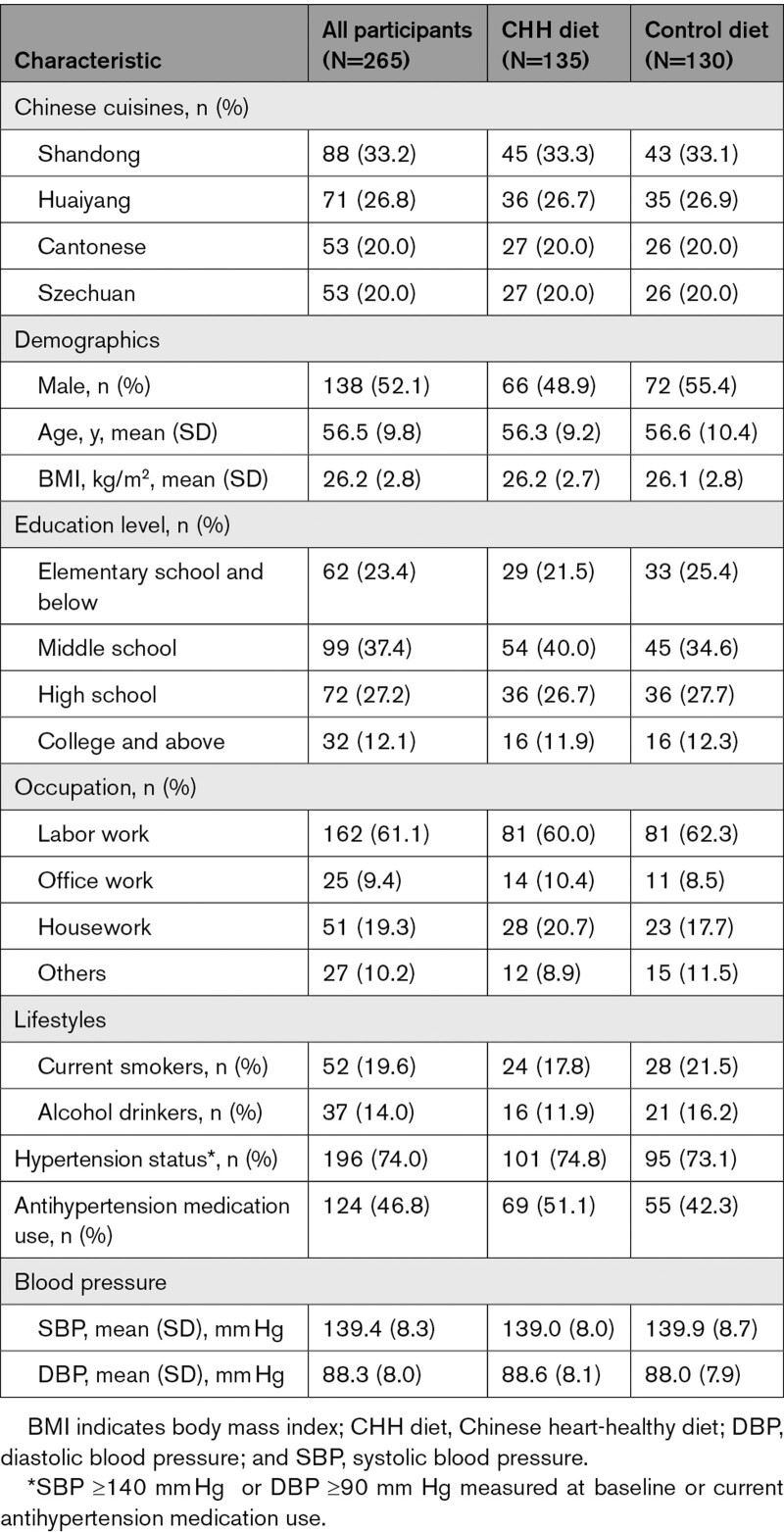
The baseline characteristics, including the type of cuisine, demography, lifestyle, blood pressure, history of hypertension, antihypertension medication use, nutrients intake, and food pattern, were compatible (all P values >0.05) between the 2 study groups (Tables 1 and 2). The baseline characteristics of participants in the primary analysis were almost the same as those randomized.
Table 2.
Baseline Characteristics of Participants Randomized
Effect of CHH Diet on Blood Pressure
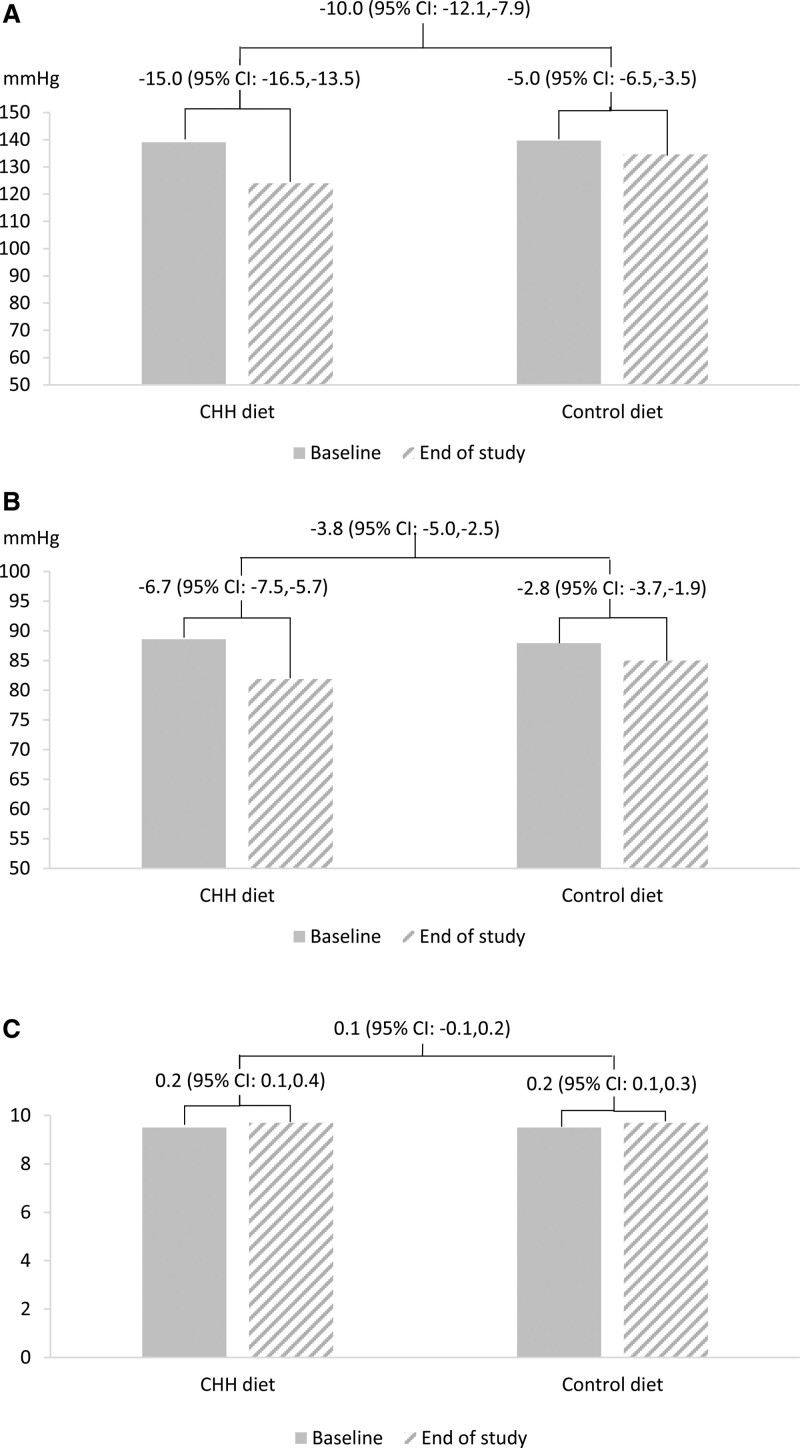
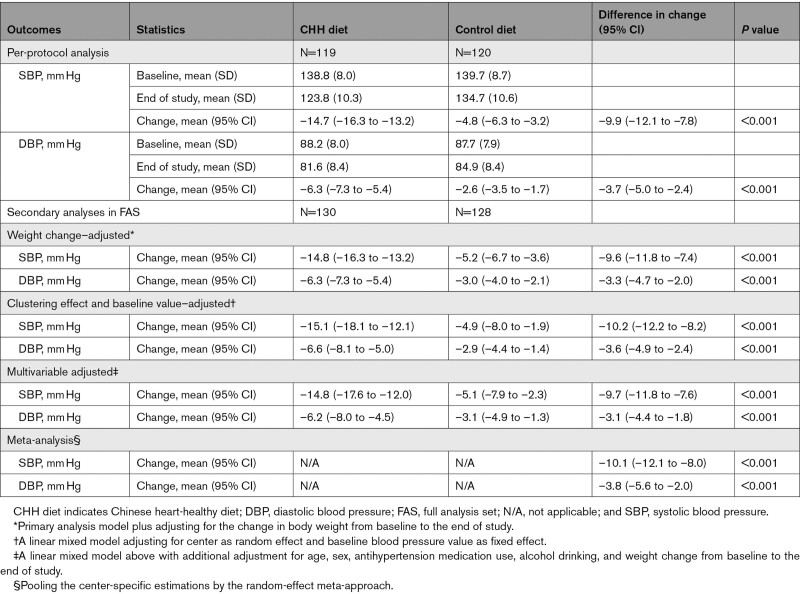
The primary analysis showed that the changes in SBP from baseline to the end of the intervention in the CHH diet and control diet groups were –15.0 (95% CI, –16.5 to –13.5) mm Hg and –5.0 (95% CI, –6.5 to –3.5) mm Hg, respectively. The net difference in the change of SBP in the CHH diet group from baseline to the end of the intervention was –10.0 mm Hg (95% CI, –12.1 to –7.9) compared with the control diet group (P<0.001). For DBP, the corresponding changes in the CHH diet and control diet groups were –6.7 (95% CI, –7.5 to –5.7) mm Hg and –2.8 (95% CI, –3.7 to –1.9) mm Hg, respectively. The net difference in the CHH diet group was –3.8 mm Hg (95% CI, –5.0 to –2.5) compared with the control diet group (Figure 2). The sensitivity analyses in the per-protocol set, with weight change adjusted, adjustment for clustering and baseline values, adjustment for all possible confounders, and meta-analysis, showed similar results (Table 3).
Figure 2.
The effects of Chinese heart-healthy (CHH) diet on blood pressure and food preference score. Data shown are center-adjusted least squares mean differences of change between the 2 groups from linear regression model. A, Systolic blood pressure; B, diastolic blood pressure; C, food preference score.
Table 3.
Sensitivity Analyses of the Effects of CHH Diet on Systolic and Diastolic Blood Pressure
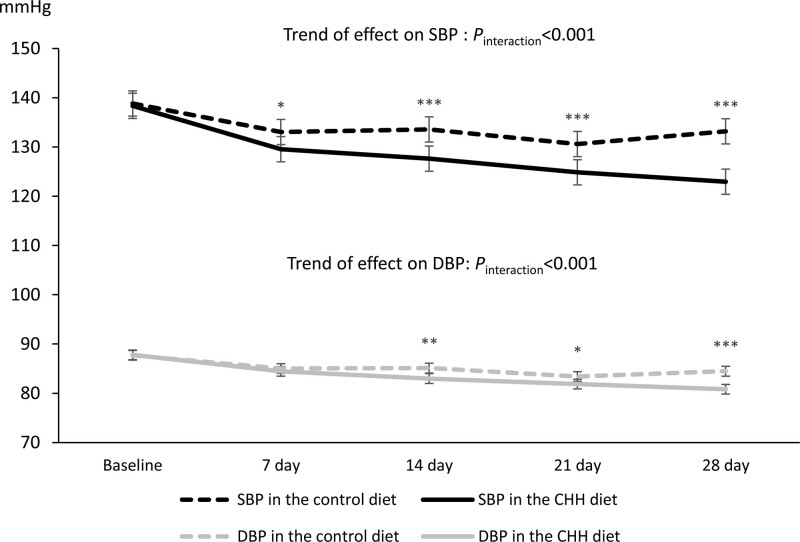
Figure 3 shows the change in morning blood pressure measured weekly from baseline to the end of the intervention. Morning SBP started to differ between the 2 groups from the end of the first week, and the difference became wider until the end of the intervention. The mean DBP showed a similar trend and a significant difference at the end of the study.
Figure 3.
Trends in systolic and diastolic blood pressure measured in the morning during whole study period by intervention group. The error bars represent 95% CI. CHH diet indicates Chinese heart-healthy diet; DBP, diastolic blood pressure; and SBP, systolic blood pressure. Comparison between groups: *P<0.05, **P<0.01, ***P<0.001.
Subgroup Analysis
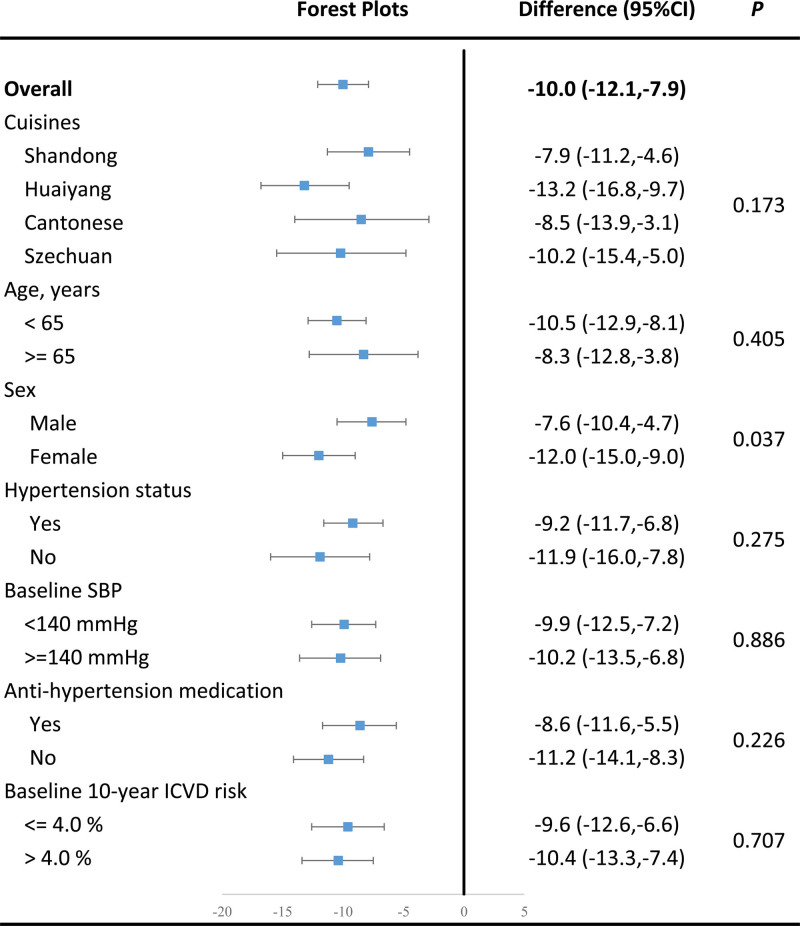
The prespecified subgroup analyses showed that female participants experienced a greater reduction in SBP (–12.0 [95% CI, –15.0 to –9.0] mm Hg) than the men (–7.6 [95% CI, –10.4 to –4.7] mm Hg), with a P for interaction of 0.037. All other prespecified factors did not significantly influence the effects of the CHH diet on SBP (Figure 4).
Figure 4.
Subgroup analysis on prespecified factors that may affect the intervention effect on primary outcome. In subgroups defined by cuisines, the difference data showed are the mean difference of change between the 2 groups and P value for Cochran’s Q test. In other subgroups, the difference data shown are center-adjusted least squares mean differences of change between the 2 groups from linear regression model and P values for interactions. ICVD indicates ischemic cardiovascular disease; and SBP, systolic blood pressure.
Effects on Food Preference and Food Variety
The food preference visual analytic score was 9.5 in both groups at baseline. The change from baseline to the end of the trial was 0.2 in both groups, and the net difference in the change between the 2 groups was 0.1 (95% CI, –0.1 to 0.2; P=0.558).
The food variety defined by the number of food items consumed by study participants was, on average, 4.7 (95% CI, 4.4 to 5.1) more in the CHH diet group than in the control diet group.
Cost of the CHH Diet in Comparison With the Control Diet
The average cost for food materials was CNY 24 and CNY 21 per day for the CHH and control diets, respectively. Other costs, such as the expenses of chefs and assistants who worked for the study, were about the same between the 2 groups and were cancelled out between the CHH and local diets. The incremental cost-effectiveness ratio for 1 mm Hg SBP reduction was CNY 0.4 (USD 0.06) per day.
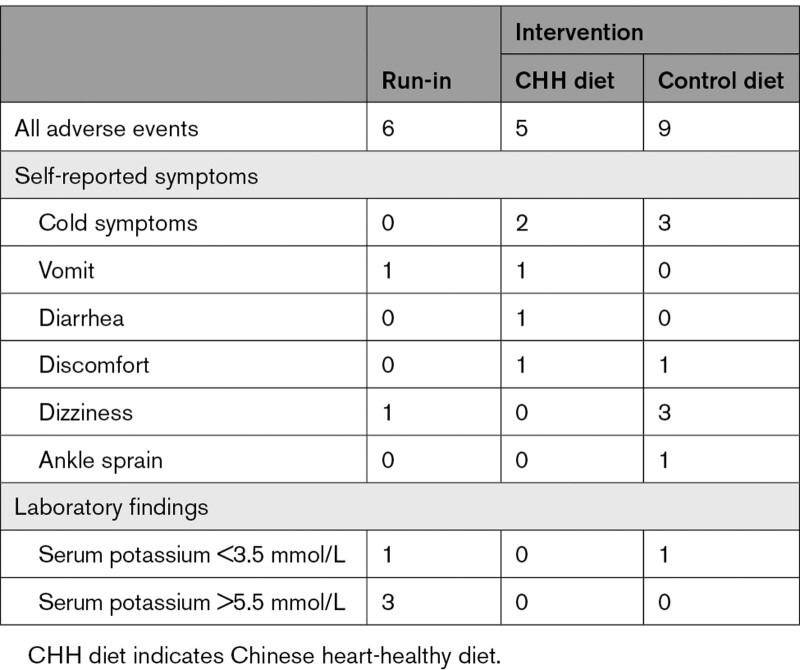
Safety Outcomes
Six adverse events were reported during the run-in phase and 14 during the intervention phase. None of them was a serious adverse event, and none was related to the intervention. No difference in the number of events was found between the 2 groups (P=0.259; Table 4). No participant withdrew because of adverse events.
Table 4.
Adverse Events Reported in the CHH Diet and Control Diet Groups
Discussion
The DECIDE-Diet trial showed that the cuisine-based CHH diet effectively reduced SBP and DBP by a mean of 10 mm Hg and 3.8 mm Hg, respectively, among Chinese adults with mild hypertension. The participants’ flavor preference for the CHH diet was as good as the usual local diet. The incremental cost of the CHH diet per person per day (USD 0.6 for 10 mm Hg) was low and affordable for common people. Our study also found that the intervention effect applied to all 4 major cuisines of Chinese culture. According to the systematic review and meta-analysis,24 the effect achieved by the CHH diet, if sustainable, would lead to a 20% reduction in major cardiovascular disease, 28% reduction in heart failure, and 13% reduction in all-cause death. Considering the already high and still increasing prevalence of hypertension in China25 and the size of the population affected, scaling the CHH diet up countrywide bears great significance in preventing and controlling hypertension and cardiovascular disease, which currently accounts for >40% of total deaths.25 Our study provides a strong scientific base for implementing the “healthy eating” campaign in China, which is the second priority among the Healthy China Initiatives.26
To the best of our knowledge, the CHH diet is the first healthy diet developed according to the Chinese food culture. The DECIDE-Diet study, with a rigorously randomized controlled feeding trial design, demonstrated that the CHH diet could produce a considerable blood pressure–lowering effect comparable with antihypertensives among patients with hypertension. Our findings supported the concept that “food is medicine” and highlighted the importance and practical value of healthy diets in controlling hypertension and related cardiovascular diseases. The study menus were carefully planned while considering the needs for practical implementation and achievement of the nutrient targets, including choosing the right food materials, adjusting the energy composition, using a potassium-enriched salt substitute, and replacing regular milk with low-fat milk, etc. The CHH diet was designed with 2 weeks of nonrepeating essential menu dishes to ensure palatability. In addition, the involvement of master chefs during its development guaranteed the flavor of dishes through professional cooking to meet the CHH diet targets without sacrificing food flavor. In addition, seasonal vegetables were used to increase the feasibility and generalizability.
The observed blood pressure effect of the CHH diet may be attributed to nutrients that have been proven effective in reducing blood pressure in previous studies. Specifically, mean dietary sodium intake per estimated 24-hour urinary excretion decreased by >2000 mg/d, and mean dietary potassium intake increased by nearly 1000 mg/d, corresponding to a 38% reduction from the baseline sodium intake level and a 42% increase from the baseline potassium intake level. In addition to the differences in sodium and potassium intake, the CHH diet had other vital differences from the control diet, which might also contribute to the blood pressure effect. The CHH diet increased energy from protein by 4%, more than doubled soluble fiber, and largely increased fruit intake. Previous meta-analyses of randomized trials have shown a significant association between reducing SBP and DBP and increased intake of soluble fiber,27 protein,28 and fruit and vegetables.29 In addition, the variety of foods increased significantly in the CHH diet compared with the control diet. It has been reported previously that food variety was associated with blood pressure level.30
The sex differences in response to the CHH diet observed in our study might be explained by women’s better adherence to the intervention. In our study, 52% and 42% of female and male participants consumed 100% of the study meals.
Our study has several strengths. First, like the DASH study, we used a rigorously controlled feeding protocol in which all raw food materials and condiments were weighted. The meals were also measured before and after consumption, thus ensuring high precision in estimating nutrient intakes. Second, adherence was high for both the dietary interventions and attendance at the follow-up visits. More than 98% of the participants consumed >80% of all study meals in both the CHH diet and control diet groups. Only 3% of the participants were lost at the final study visit. Third, to ensure high quality of the blood pressure outcome and high adherence of the participants to the measurement schedule, we measured blood pressure 3 times within 24 hours, with 3 readings each time.31–33 To further reduce the burden on the study participants, we measured blood pressure only before and after the 4 weeks of intervention using this protocol. We measured the blood pressure only in the morning for the rest of the study visits.
Our study has some limitations. First, our study did not reach the planned recruitment target because of the COVID-19 pandemic and the strict quarantine policy. Although the sample size was adequate statistically in detecting the main intervention effect, the study may not have sufficient power to detect the effect of cuisine type on the effect. Second, we collected spot urine only in the morning for feasibility consideration. With the method proposed by Huang et al,22 our estimated 24-hour urinary sodium and potassium excretion confirmed the corresponding changes in the dietary intakes of these 2 minerals in the CHH and control diets. Third, the intervention period lasted only 4 weeks because of budget constraints. Because our weekly monitoring of morning blood pressure showed the intervention effect increasing with the study duration, we cannot conclude if the effect size would be further increased if the intervention is prolonged. Fourth, we did not use whole food analysis to measure nutrients contents in the actual meals. This limited our ability to measure the actual intake of nutrients such as vitamins significantly affected by heating during food preparation. Fifth, unlike the DASH trial, our study could not separate the effect of reducing sodium intake from those caused by changes in diet by design. We believe that this is an important question that should be addressed in future studies. Sixth, we did not achieve the targets of calcium in the CHH diet during our intervention. Typically, Chinese diet culture includes no or little dairy products, and thus to include dairy intake similar to that of Western populations would have been hard for the participants to comply with.34 In addition to increasing moderately the intake of dairy products in the CHH diet, we used other strategies to increase calcium intake, including increasing other calcium-rich foods such as legumes and tofu, etc. Our study showed that participants in the CHH diet group reached a mean calcium intake of 983 mg/d, which is about 80% of our target and more than the 800 mg/d recommended in the Chinese Dietary Guidelines issued by the Chinese Nutrition Society. Further, data from the China National Nutrition Surveys showed that the average dietary intake of calcium in China remained <500 mg/d.20 Last, because this is a controlled feeding trial, its generalizability to the real world requires further implementation trials to confirm. However, its excellent preference assessed by the participants, low incremental cost, and implementation in community kitchens all suggest high scalability.
To conclude, the DECIDE-Diet trial demonstrated that the CHH diet is effective, palatable, and affordable in reducing blood pressure in Chinese adults with high blood pressure. The effect size was more than expected and applicable across major Chinese cuisines. Scaling up with the CHH diet in the enormous population predominantly on Chinese cuisines throughout the world bears tremendous significance in preventing and controlling hypertension and its related diseases in China26 and globally.35
Article Information
Acknowledgments
Contributor and guarantor information: Y. Wu, Y. Wang, and J.C. developed the research idea and designed the study; Y. Wang, G.Z., H.Z., J.S., X. Li, and Z.Y. developed the CCH diet with advices from J.C. and Y. Wu; Y. Wang, L.F., J.Y., X. Lan, S.L., Y.Z., H.D., S.C., and Z.L. collected data; L.F. and Y.Z. analyzed data with advice from P.G.; X.C., L.F., and G.X. were responsible for the field and data quality control; Y. Wang, L.F., and Y. Wu wrote the first draft of the article, which was reviewed by all authors. Y. Wu and Y. Wang are the guarantors. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Study Management Committee: Yangfeng Wu (Chair), Yanfang Wang (Cochair), Jianqin Sun, Huilian Zhu, Guo Zeng, Zhenquan Yang.
Study Advisory Committee: Junshi Chen (Chair), Pao-Hwa Lin, Wenyi Niu, Pei Gao, Hai Fang, Guansheng Ma, Ming Li.
Participating centers and staff information:
Peking University Clinical Research Institute (study coordinating center): Yangfeng Wu (Project Principal Investigator), Yanfang Wang (Study Principal Investigator), Huijuan Li (Project Manager), Lin Feng (Study Coordinator), Gaoqiang Xie (Data Management Supervisor), Wenyao Ma (Data Manager), Jiarong Li (Assistant Data Manager), Xiayan Chen (Clinical Research Associate), Yidan Zhu, Wuxiang Xie, Shulan Zhu, Xue Bai, Yuqing Gao, Chenglong Li, Yanjun Ma, Yiyu Hu, Ke Miao, Shujing Zhu, Caiyun Zhao, Yunqing Zhu.
School of Public Health, Sichuan University: Guo Zeng (Key Investigator), Wenya Yin, Ming Li, Yiqi Zhang, Congjie Cai, Xinxin Pang, Hong Sun, Haiying Zhang, Dan Bai.
School of Public Health, Sun Yat-sen University: Huilian Zhu (Key Investigator), Aiping Fang, Rongzhu Huang, Yun Luo, Zhaoyan Liu, Xinlei Lin.
Huadong Hospital Affiliated to Fudan University: Jianqin Sun (Key Investigator), Yanfang Zhao, Huijing Bai, Mengyao Ye, Zhen Li, Qing Fan, Jun Tang, Fei Xiao, Jianming Wang, Yanguo Zhang, Guixiang Zhang, Weiping Chen, Weigang Zhao.
Sichuan Tourism University Culinary College: Xiang Li (Key Investigator), Guangsen Tong, Kun Zhang.
The Center for Disease Control and Prevention of Yuexiu District, Guangzhou: Bin Xu, Ting Zhang.
Yangzhou University College of Tourism and Cuisine: Zhenquan Yang (Key Investigator), Xinchi Wang, Yunlong Zhu, Jing Peng, Haifeng Zhang, Lu Gao, Shengqi Rao.
Chinese Center for Disease Control and Prevention: Jianguo Xu, Jing Yang, Dong Jin, Ji Pu, Juan Zhou, Yuanmeihui Tao, Yifan Jiao.
Qingdao Yilianhua Catering Co, Ltd: Zhiheng Wang, Yiying Xu.
Health Hope (Beijing) Technology Co, Ltd: Yuxin Li, Shi Qiu.
Sources of Funding
The study is funded by the National Key Research and Development Program, Ministry of Science and Technology of China (2016YFC1300201) and the Chinese Nutrition Society Nutrition Science Foundation-Yum China Dietary Health Foundation (CNS-YUM2019B16). The funders have no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The researchers are independent from the funders. Y. Wu and Y. Wang had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
None.
Supplemental Material
Table S1 (Targets and means of nutrients intakes consumed by participants in the CHH diet group during the 28-day intervention phase)
DECIDE-Diet Study Group
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CHH
- Chinese heart-healthy diet
- CVD
- cardiovascular disease
- DASH
- Dietary Approach to Stop Hypertension
- DBP
- diastolic blood pressure
- DECIDE-Diet
- Exercise and Cardiovascular Health-Diet
- SBP
- systolic blood pressure
Circulation is available at www.ahajournals.org/journal/circ
Y. Wang and L. Feng contributed equally.
A complete list of the investigators in the DECIDE-Diet Study Group is provided in the Appendix.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.122.059045.
For Sources of Funding and Disclosures, see page 314.
Contributor Information
Yanfang Wang, Email: zyf17317821245@163.com.
Lin Feng, Email: april1022@163.com.
Guo Zeng, Email: zg_huaxi2016@126.com.
Huilian Zhu, Email: yidanzhu@bjmu.edu.cn.
Jianqin Sun, Email: jianqins@163.com.
Pei Gao, Email: peigao@bjmu.edu.cn.
Jihong Yuan, Email: 1393624655@qq.com.
Xi Lan, Email: 1016729148@qq.com.
Shuyi Li, Email: pucri_lihj@bjmu.edu.cn.
Yanfang Zhao, Email: zyf17317821245@163.com.
Xiayan Chen, Email: jshchen@ilsichina.org.
Hongli Dong, Email: 2949971729@qq.com.
Si Chen, Email: jshchen@ilsichina.org.
Zhen Li, Email: pucri_lihj@bjmu.edu.cn.
Yidan Zhu, Email: yidanzhu@bjmu.edu.cn.
Ming Li, Email: pucri_lihj@bjmu.edu.cn.
Xiang Li, Email: pucri_lihj@bjmu.edu.cn.
Zhenquan Yang, Email: yangzq@yzu.edu.cn.
Huijuan Li, Email: pucri_lihj@bjmu.edu.cn.
Hai Fang, Email: hfangjournal@163.com.
Gaoqiang Xie, Email: gxie@bjmu.edu.cn.
Pao-Hwa Lin, Email: pao.hwa.lin@dm.duke.edu.
Junshi Chen, Email: jshchen@ilsichina.org.
References
- 1.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang L, Trieu K, Yoshimura S, Neal B, Woodward M, Campbell NRC, Li Q, Lackland DT, Leung AA, Anderson CAM, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020;368:m315. doi: 10.1136/bmj.m315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393:1958–1972. doi: 10.1016/S0140-6736(19)30041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y, Benjamin EJ, MacMahon S. Prevention and control of cardiovascular disease in the rapidly changing economy of China. Circulation. 2016;133:2545–2560. doi: 10.1161/CIRCULATIONAHA.115.008728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao SC, Huang ZD, Wu XG, Zhou BF, Xiao ZK, Hao JS, Li YH, Cen RC, Rao XX. CHD and its risk factors in the People’s Republic of China. Int J Epidemiol. 1989;18(3 suppl 1):S159–S163. doi: 10.1093/ije/18.3_Supplement_1.S159 [PubMed] [Google Scholar]
- 8.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601 [DOI] [PubMed] [Google Scholar]
- 9.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779 [DOI] [PubMed] [Google Scholar]
- 10.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, et al.; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389 [DOI] [PubMed] [Google Scholar]
- 11.Obarzanek E, Sacks FM, Vollmer WM, Bray GA, Miller ER, 3rd, Lin PH, Karanja NM, Most-Windhauser MM, Moore TJ, Swain JF, et al.; DASH Research Group. Effects on blood lipids of a blood pressure-lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Clin Nutr. 2001;74:80–89. doi: 10.1093/ajcn/74.1.80 [DOI] [PubMed] [Google Scholar]
- 12.Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr. 2015;113:1–15. doi: 10.1017/S0007114514003341 [DOI] [PubMed] [Google Scholar]
- 13.Chiavaroli L, Viguiliouk E, Nishi SK, Blanco Mejia S, Rahelić D, Kahleová H, Salas-Salvadó J, Kendall CW, Sievenpiper JL. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11:E338. doi: 10.3390/nu11020338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macdiarmid JI, Loe J, Kyle J, McNeill G. “It was an education in portion size”. Experience of eating a healthy diet and barriers to long term dietary change. Appetite. 2013;71:411–419. doi: 10.1016/j.appet.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 15.Yang S, Zhang Y. The research of the differences between Chinese and Western diet cultures. Cross Cult Commun. 2010;6:75–83. doi: 10.3968/j.ccc.1923670020100602.009 [Google Scholar]
- 16.Xie W, Wang Y, Sun J, Zeng G, Zhu H, Yang Z, Gao P, Yang J, Feng L, Lin PH, et al. Protocol of a multicenter, single-blind, randomised, parallel controlled feeding trial evaluating the effect of a Chinese Healthy Heart (CHH) diet in lowering blood pressure and other cardiovascular risk factors. BMJ Open. 2020;10:e036394. doi: 10.1136/bmjopen-2019-036394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan Y. Discussion on Chinese cuisines. Sci Tech Vis. 2016;4:202–206. doi: 10.3969/j.issn.2095-2457.2016.04.150 [Google Scholar]
- 18.Li N, Yan LL, Niu W, Labarthe D, Feng X, Shi J, Zhang J, Zhang R, Zhang Y, Chu H, et al. A large-scale cluster randomized trial to determine the effects of community-based dietary sodium reduction–the China Rural Health Initiative Sodium Reduction Study. Am Heart J. 2013;166:815–822. doi: 10.1016/j.ahj.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinese Nutrition Society. The Chinese Dietary Guidelines. People’s Medical Publishing House Press; 2016. [Google Scholar]
- 20.National Health Family Planning Commission Disease Prevention Control Bureau. Report on the Nutrition and Chronic Diseases Status of Chinese Residents. People’s Medical Publishing House Press; 2015. [Google Scholar]
- 21.Chen X, Wang Y, Wu Y, Li S, Zhao Y, Miao K, Feng L, Li H. The Diet, ExerCIse and CarDiovascular hEalth (DECIDE)-Diet study was taken as a case to discuss the methods of blinding and blinding assessment for feeding trials. Chin J Clin Nutr. 2022;30:49–52. doi: 10.3760/cma.j.cn115822-20211130-00230 [Google Scholar]
- 22.Huang L, Woodward M, Stepien S, Tian M, Yin X, Hao Z, Li Z, Sun J, Yu Y, Zhou B, et al. Spot urine samples compared with 24-h urine samples for estimating changes in urinary sodium and potassium excretion in the China Salt Substitute and Stroke Study. Int J Epidemiol. 2018;47:1811–1820. doi: 10.1093/ije/dyy206 [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Wang G, Pan X. China Food Composition Tables. Standard ed. Book 1. 2nd ed. Beijing, China: Peking University Medical Press; 2009. [Google Scholar]
- 24.Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–967. doi: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 25.National Center for Cardiovascular Disease Control. Annual Report on Cardiovascular Health and Diseases in China (2020). Science Press; 2021. [Google Scholar]
- 26.The Promotional Committee of The Healthy China Initiative. The Healthy China Initiative (2019-2030). July 9, 2019. http://www.gov.cn/xinwen/2019-07/15/content_5409694.htm. Accessed July 15, 2019. [Google Scholar]
- 27.Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, He J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J Hypertens. 2005;23:475–481. doi: 10.1097/01.hjh.0000160199.51158.cf [DOI] [PubMed] [Google Scholar]
- 28.Rebholz CM, Friedman EE, Powers LJ, Arroyave WD, He J, Kelly TN. Dietary protein intake and blood pressure: a meta-analysis of randomized controlled trials. Am J Epidemiol. 2012;176(suppl 7):S27–S43. doi: 10.1093/aje/kws245 [DOI] [PubMed] [Google Scholar]
- 29.Hartley L, Igbinedion E, Holmes J, Flowers N, Thorogood M, Clarke A, Stranges S, Hooper L, Rees K. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev. 2013;2013:Cd009874. doi: 10.1002/14651858.CD009874.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira EP, Camargo KF, Castanho GK, Nicola M, Portero-McLellan KC, Burini RC. Dietary variety is a protective factor for elevated systolic blood pressure. Arq Bras Cardiol. 2012;98:338–343. doi: 10.1590/s0066-782x2012005000024 [DOI] [PubMed] [Google Scholar]
- 31.Ringrose JS, Bapuji R, Coutinho W, Mouhammed O, Bridgland L, Carpenter T, Padwal R. Patient perceptions of ambulatory blood pressure monitoring testing, tolerability, accessibility, and expense. J Clin Hypertens (Greenwich). 2020;22:16–20. doi: 10.1111/jch.13760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vischer AS, Burkard T. Principles of blood pressure measurement - current techniques, office vs ambulatory blood pressure measurement. Adv Exp Med Biol. 2017;956:85–96. doi: 10.1007/5584_2016_49 [DOI] [PubMed] [Google Scholar]
- 33.Hu J, Zhao L, Thompson B, Zhang Y, Wu Y. Effects of salt substitute on home blood pressure differs according to age and degree of blood pressure in hypertensive patients and their families. Clin Exp Hypertens. 2018;40:664–672. doi: 10.1080/10641963.2018.1425415 [DOI] [PubMed] [Google Scholar]
- 34.He Y, Yang X, Xia J, Zhao L, Yang Y. Consumption of meat and dairy products in China: a review. Proc Nutr Soc. 2016;75:385–391. doi: 10.1017/S0029665116000641 [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Ensure Healthy Lives and Promote Well-Being for All at All Ages. September 25, 2015. https://www.who.int/news/item/25-09-2015-ensure-healthy-lives-and-promote-well-being-for-all-at-all-ages. Accessed July 15, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.