Abstract
Aims:
The innervation zone asymmetry of the external anal sphincter (EAS) has been investigated as a risk factor for the development of fecal incontinence (FI). This study aims to utilize an intra-rectal high-density surface electromyogram (HD-sEMG) recording and advanced HD-sEMG decomposition technique to characterize the effects of aging on the asymmetry of EAS functional innervation.
Methods:
HD-sEMG signals were recorded intra-rectally from six healthy young and seven healthy elderly women during voluntary contractions of the EAS. EMG signals were decomposed into constituent motor unit action potentials (MUAPs) and the innervation zone of each decomposed motor unit was identified. Asymmetry index (AI) was defined and calculated for all subjects. The maximum squeezing pressures of the EAS were also measured for all subjects as a comparison.
Results:
The HD-sEMG decomposition and AI calculation were successfully performed from EMG data acquired from all the subjects. The AI values were 28.7 ± 17.0% for the young group and 55.6 ± 18.8% for the elderly group. The AI and EAS contraction strength were found to be negatively correlated (P < 0.05). A two-tailed student’s t-test demonstrated a significant increase in AI with age by comparison between two groups (P < 0.05).
Conclusions:
Our work demonstrates, for the first time, that EAS functional innervation tends to become increasingly asymmetrical with advancing age, and this increase is associated with a compromised anorectal function. Results suggest that the intra-rectal HD-sEMG will serve as an advanced tool for assessing and monitoring the anorectal neuromuscular function minimally invasively under different pathophysiological conditions.
Keywords: aging, asymmetry, external anal sphincter, high-density surface EMG, innervation zone, intra-rectal EMG
1 |. INTRODUCTION
Fecal incontinence (FI) is a common anorectal disorder that affects 7–15% of the population aged 65 years and older.1,2 Accumulated evidence has shown that aging is associated with compromised anorectal strength and innervation. Studies in elderly asymptomatic women indicated that these changes are unrelated to sphincter injury nor other aging-related neurogenic disorders.3 Despite the unclear mechanism and variate etiology of aging-related FI, incontinence can be largely attributed to the neuromuscular alterations that occur with increased age.4 Therefore, the utilization of neurophysiological biomarkers for the diagnosis and prediction of FI in the elderly population holds significant importance.
Functional asymmetry of EAS innervation has long been studied in healthy subjects5 and later for its potential role in the prediction of FI.6 Surgical studies have revealed an asymmetrical distribution of pudendal afferent fibers with respect to side in most reported cases.7 Women with increased functional asymmetry are at an increased risk for FI after sphincter trauma, and the severity of asymmetry has been proposed to predict a patient’s risk for FI.8,9 Reduced EAS innervation symmetry, especially in patients with injuries to the pudendal nerve, contributes to compromised muscle closure strength, and consequently higher risks of exposure to FI.3,10 Early studies on EAS functional asymmetry explored the use of vectormanometry, root mean squared (RMS) amplitude, evoked anorectal EMG, and intramuscular EMG as potential tools to assess EAS innervation symmetry. Vectormanometry has been widely used in research to detect asymmetric pressure zones in the EAS, however, manometry techniques have wide ranges for normal values and are poorly reproducible.11 Furthermore, vectormanometry can only identify the presence of EAS pressure zone asymmetry, and cannot reveal the underlying neuromuscular changes that cause the asymmetry. In addition to manometric techniques, the innervation of the EAS can also be assessed with traditional neurophysiological tools. Evoked muscle action potentials recorded via intramuscular EMG have been used to explore the symmetry of the neuromuscular innervation of the EAS.5 However, pudendal nerve stimulation is a specialized test, and intramuscular EMG suffers from low spatial coverage and is painful to the subject. A correlation between incontinence symptoms and EAS innervation asymmetry using conventional surface EMG has also been reported in post-partum women suffering from FI, suggesting functional EAS asymmetry as a potential risk factor for FI.9 However, the poor spatiotemporal resolution of conventional surface EMG electrodes limits signal analysis to basic global features, such as amplitude and mean frequency, and does not allow for the study of individual motor unit action potentials (MUAPs).
Recent advances in high-density surface electromyography (HD-sEMG) provide a non-invasive method to acquire neuromuscular signals from a target muscle, including broad spatial information related to neuromuscular innervation. These characteristics of HD-sEMG enable the repeated and long-term assessment of neuromuscular function while providing a wealth of information regarding the health of the muscle. However, limited effort has been made to apply HD-sEMG to pelvic muscles, possibly because of the complexity of pelvic anatomy.12–15 A 64-electrode intra-rectal HD-sEMG probe was developed in our lab to provide a direct contact and complete coverage of the EAS, while minimizing physical discomfort. Moreover, the ample circumferential spatial information recorded with our HD-sEMG probe enables an accurate and reliable extraction of MUAPs, by integrating an advanced HD-sEMG decomposition technique.15
A similar HD-sEMG approach has been applied to the EAS to assess changes in the innervation of the EAS after childbirth and/or episiotomy.16 However, no studies have examined the effect of aging on the innervation zone distribution of the EAS. In this study, we employed our non-invasive intra-rectal HD-sEMG probe and advanced EMG decomposition techniques to study the effect of aging on EAS innervation symmetry in healthy women. The clinical significance of innervation asymmetry was also evaluated by correlating it with conventional anorectal manometry pressure recordings. Finally, the innervation asymmetry and manometry results were compared between the young and elderly groups.
2 |. MATERIALS AND METHODS
Continent (for both urine and feces) parous females, with no history of pelvic trauma, pelvic organ prolapse, or neuromuscular disorders, were recruited for this study. Subject fecal continence status was assessed by the Wexner continence grading scale. Subjects that presented with any incontinence symptoms, or reported the loss of liquid or solid stool were excluded. A pelvic floor examination was performed prior to the experiment, and subjects that were discovered to have pelvic organ prolapse and signs of pelvic surgery were further excluded from the study. Subjects between 18 and 40 years were recruited for the young group and subjects over 60 years for the elderly group. Subjects with identifiable perianal scarring or EAS tears presented on transperineal ultrasound were further excluded from participation. All ultrasound, manometry, and HD-sEMG tests were performed by an experienced physician with an approved study protocol reviewed by the Institutional Review Board. All subjects gave informed consent.
The basal and squeezing pressures of the EAS were assessed with a Triton anorectal manometry system (LABORIE Corp., Toronto, Canada) via the rapid-pull through technique. Basal anorectal pressures were subtracted from maximum squeezing pressures to remove the effects of the internal anal sphincter on the analysis. Subjects were then positioned in the lithotomy position and pre-coached to ensure correct contraction of the EAS, while minimizing potential signal interference due to co-contraction of abdominal or leg muscles.17 Surface EMG from the EAS was recorded during voluntary contractions using a 64-channel (8 × 8) intra-rectal probe, as shown in Figures 1A and 1B. The subject’s wrist was cleaned with an alcohol wipe, and a wet ground electrode was affixed to the subject’s wrist with a Velcro strap. A single reference electrode was affixed to the subject’s thigh. Consistent probe insertion was achieved by orienting the company trademark in the anterior direction. Care was taken by the physician to keep the probe in a fixed location, with no longitudinal movement or rotation of the probe during testing to maintain a fixed spatial reference. Three contraction maneuvers were performed under moderate effort for each subject. The subject was instructed to contract for 10 sec. Each contraction was followed by a 5-min rest period to avoid muscle fatigue. Signals were amplified at a sampling rate of 5 kHz through a BrainAmp amplifier (Brain Products GmbH, Germany) and stored for offline processing.
FIGURE 1.
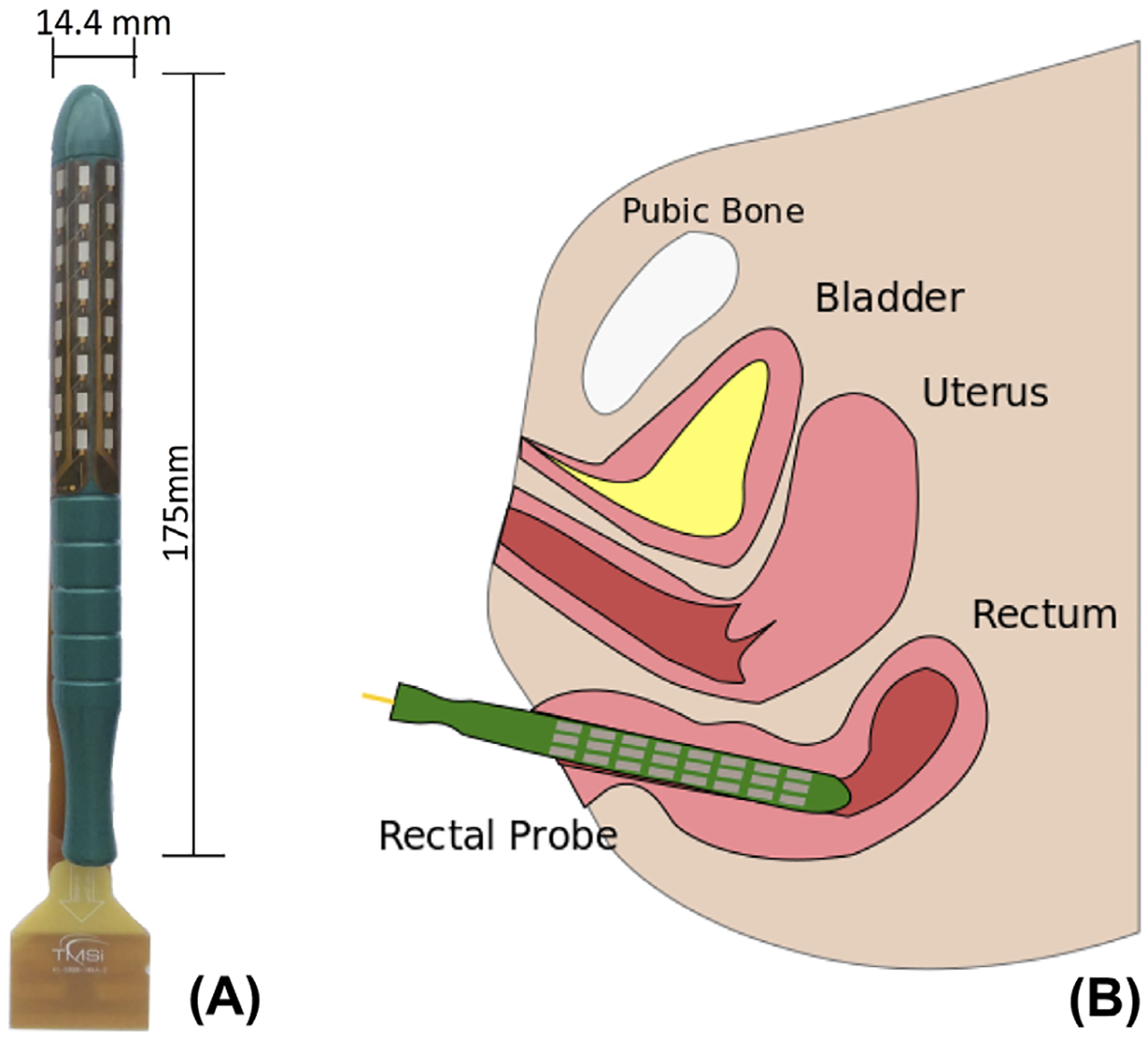
A, The intra-rectal high density surface EMG probe. B, Illustration of the recording protocol
HD-sEMG signals were band-pass filtered with a 2nd-order Butterworth filter at 10–500 Hz and notch filtered at 50 Hz using MATLAB R2016a (Mathworks Inc., Natick, MA). The data was segmented into contraction periods based on the apparent amplitude increase during contraction. EMG RMS was calculated as the square root of the mean of the squares of all samples in a given contraction period. Surface interferential HD-sEMG recordings were then decomposed into constitutive MUAP trains using our recently developed K-means clustering convolution kernel compensation (KmCKC) algorithm.18 The KmCKC algorithm employs k-means clustering to group firing instances to associating motor units (MUs), yielding consecutive firing sequences of MUs. Then, convolution kernel compensation is utilized to improve the accuracy of pulse train clustering and decomposition. Finally, HD-sEMG recordings are temporally averaged with respect to the pulse trains of each MU to produce a 64-channel MU profile.
An innervation zone (IZ), which indicates the location of neuromuscular junctions, was located from the bipolar mapping of the temporally averaged MU profiles. An IZ is readily discernible in bipolar HD-sEMG recordings by recognizing a signal phase inversion in neighboring channels along the muscle fiber direction.15 The location of each IZ was recorded and binned with respect to the electrode nearest to the IZ location to calculate IZhist. The asymmetry index (AI) was further determined from identified IZ locations for each subject. Briefly, the IZ distribution was compiled in a circular manner for each of the eight circumferential channels. The distance of the barycenter from the center of the probe normalized with respect to the radius of the probe was calculated and termed the AI.19 A completely symmetric EAS innervation will have an AI of 0%; conversely, an AI of 100% denotes a completely asymmetric IZ distribution. Equation (1) defines AI,20
| (1) |
3 |. RESULTS
Table 1 summarizes the demographics and calculated AI for both young group (n = 6, 31.5 ± 3.9 years) and elderly group (n = 7, 66.6 ± 4.8 years). The average parity of the elderly group was higher than the average parity of the young group; however, this difference was not significant (p > 0.08). The average RMS value during contraction was 20.7 ± 6.9 μV for the young group, and 21.8 ± 6.6 μV for the elderly group, as shown in Figure 2A, which is consistent with published results.11 Coefficient of variance during three contraction maneuvers was calculated as 18.9%, indicating good signal reproducibility.15 Anorectal manometry showed a higher average change in anorectal squeezing pressure of 48.8 ± 14.7 mmHg for the young group, compared to an average change in squeezing pressure of 33.8 ± 8.9 mmHg in the elderly, illustrated in Figure 2B, yet this difference was not found to be significant by a two-tailed t-test (P > 0.07). To test the applicability of EMG RMS as a potential biomarker for EAS closure function, the relationship between RMS and anorectal pressure was examined with a two-tailed student’s t-test. No significant difference in anorectal pressure and EMG RMS values was observed (P = 0.07).
TABLE 1.
Summary of results
| Age (Years) | Parity | EAS pressure (mmHg) | RMS (uV) | MU decomposed | AI (%) | |
|---|---|---|---|---|---|---|
| Group I | ||||||
| Subject 1 | 37 | 2 | 39.00 | 26.0 (10.7) | 6 | 17.70 |
| Subject 2 | 28 | 2 | 68.00 | 10.9 (2.2) | 7 | 18.00 |
| Subject 3 | 27 | 1 | 64.00 | 25.0 (3.0) | 14 | 38.40 |
| Subject 4 | 36 | 2 | 30.00 | 19.3 (2.0) | 6 | 57.70 |
| Subject 5 | 29 | 1 | 43.00 | 13.0 (3.3) | 4 | 34.60 |
| Subject 6 | 32 | 1 | N/D | 29.9 (6.5) | 9 | 5.90 |
| Mean | 31.5 | 1.5 | 48.80 | 20.68 | 7.67 | 28.71 |
| STD | 3.86 | 0.5 | 14.72 | 6.93 | 3.20 | 17.00 |
| Group II | ||||||
| Subject 1 | 61 | 3 | 47.00 | 11.2 (2.3) | 6 | 23.60 |
| Subject 2 | 76 | 5 | 16.00 | 21.3 (2.4) | 7 | 70.60 |
| Subject 3 | 66 | 2 | 37.00 | 33.4 (9.3) | 9 | 42.90 |
| Subject 4 | 66 | 2 | 38.00 | 23.1 (3.1) | 8 | 67.80 |
| Subject 5 | 67 | 2 | 30.00 | 21.5 (3.1) | 7 | 83.90 |
| Subject 6 | 61 | 1 | 31.00 | 15.9 (4.9) | 9 | 54.80 |
| Subject 7 | 69 | 3 | 38.00 | 26.2 (2.0) | 8 | 45.40 |
| Mean | 66.57 | 2.5 | 33.86 | 21.80 | 7.71 | 55.57 |
| STD | 4.75 | 1.25 | 8.94 | 6.58 | 1.03 | 18.76 |
FIGURE 2.
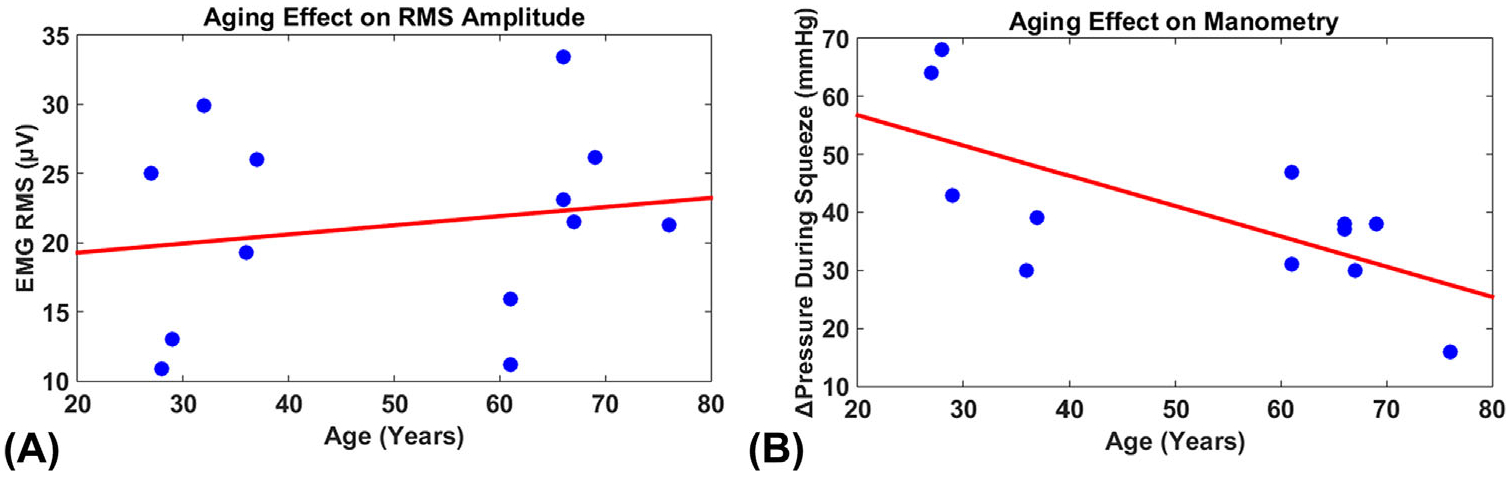
A, The relationship between ages and root mean squared (RMS) amplitudes of electromyogram (EMG) signals. B, The relationship between ages and pressure changes during external anal sphincter (EAS) squeezing
MUAPs were successfully decomposed from HD-sEMG recordings in all thirteen subjects. On average, decomposition yielded spike trains from 7.7 ± 3.2 MUs in the young subjects, and 7.7 ± 1.0 MUs in the elderly. The signals were averaged with respect to spike times determined from KmCKC, to generate a 64-channel MUAP map. Figure 3 shows a representative bipolar MUAP profile, with its propagation patterns detected by one circular row of sEMG sensors. IZs were identified for each detected MUAP in all subjects, and were binned by electrode location onto a cylindrical surface that mimics the axial view of the EAS. The AI values for the young group were 28.7 ± 17.0% and 55.6 ± 18.8% for the elderly group. Figure 4 shows the IZ barycenter locations for the young and aged subjects overlaid on an axial representation of the rectal probe. In young subjects, the EAS IZ distribution did not favor any location, and the resultant barycenter for each subject was distributed near the center of the EAS. Conversely, in aged subjects, the IZ distribution tended to concentrate in specific regions, namely the dorsal left and dorsal right surfaces of the EAS, indicating lessened innervation on the opposite side of the EAS. A significant linear relationship was found between age and AI (P < 0.05), shown in Figure 5A. A two-tailed student’s t-test demonstrated a significant increase in AI with advancing age, with P-value less than 0.05 and power over 0.8. The relationship between AI and anorectal pressure was also tested, and a significant relationship was observed (P < 0.05, CC = 0.65). This relationship is illustrated in Figure 5B.
FIGURE 3.
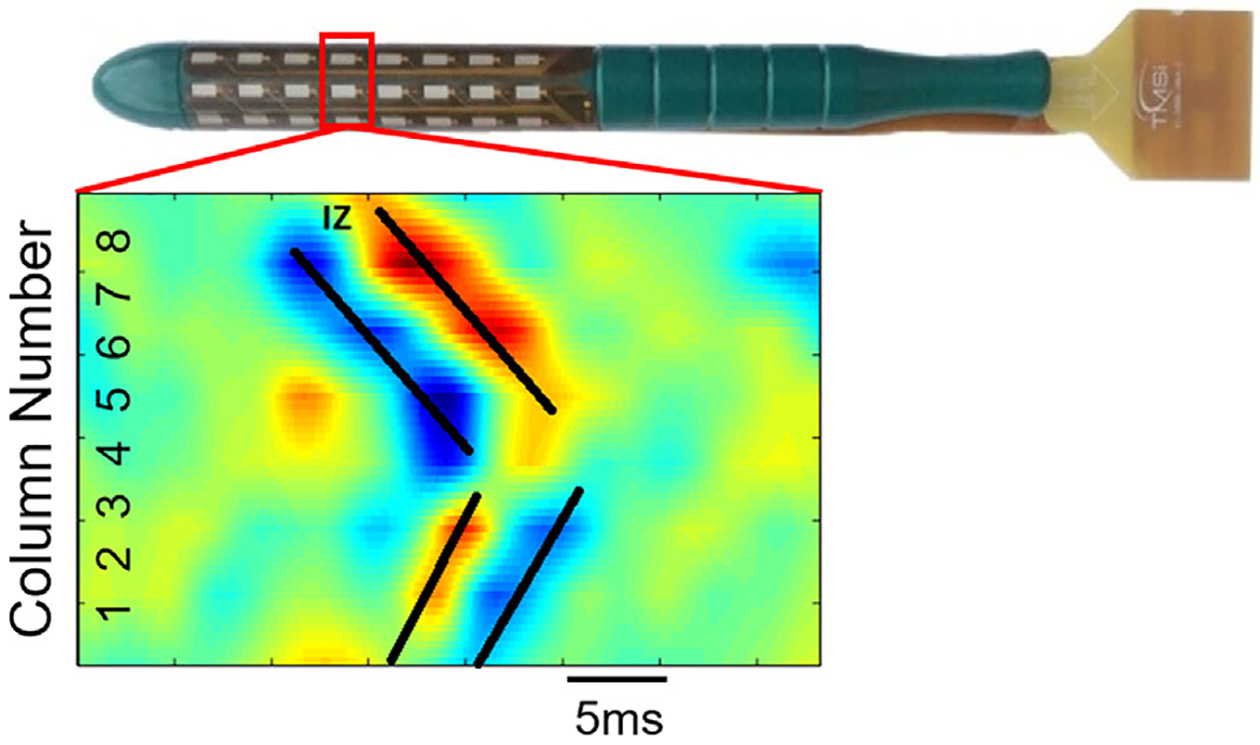
A representative bipolar mapping of the highlighted row of channels (marked by red rectangle). The black traces mark the temporal propagation of motor unit action potential (MUAP) pulses along the circumference of the probe
FIGURE 4.
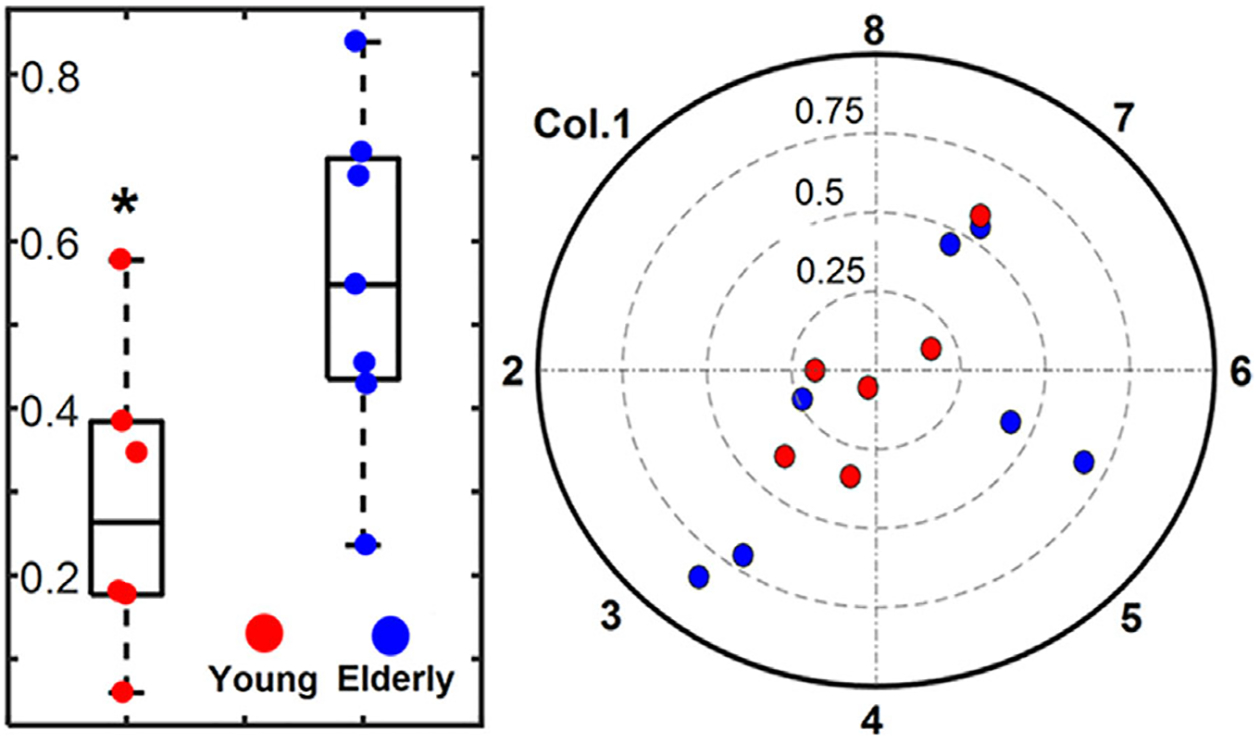
The innervation zone (IZ) distribution barycenters for both young (red) and elderly (blue) group. The distance from the center represents the asymmetry index (AI) value
FIGURE 5.
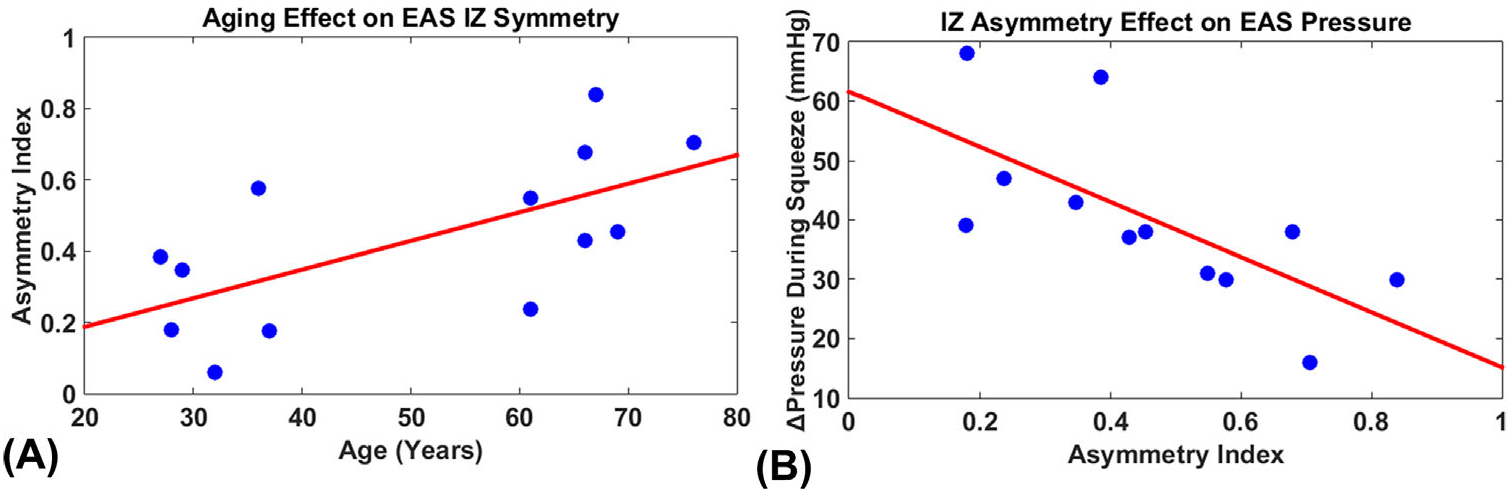
A, The relationship between age and asymmetry index. B, The relationship between asymmetry index and pressure change during external anal sphincter (EAS) squeezing
4 |. DISCUSSION
In this study, we have delivered the first effort to assess the aging-related alterations on EAS innervation symmetry by employing intra-rectal HD-sEMG recording and EMG decomposition techniques. HD-sEMG techniques can capture comparable neuromuscular details compared to intramuscular EMG, while providing greater spatial coverage and minimizing patient discomfort.15,21 To control for the confounding effects of neuromuscular diseases and apparent obstetric sphincter trauma, participants were limited to a healthy population. Subject inclusion was rigorously confirmed by a pelvic health survey including the Wexner Continence Grading Scale, medical history check, and transperineal ultrasound. Anorectal manometry was included as a standard conventional test, to functionally evaluate the consequence of compromised innervating symmetry. AI was determined from electrophysiological measurements and employed as a biomarker to quantify the symmetry of the EAS innervation.
Surface EMG decomposition was successfully performed in all subjects. Slow wave signals from the internal anal sphincter were attenuated by selecting a band-pass filter with a lower cutoff of 10 Hz. MU yield was comparable to similar studies,15,16 and the subsequent IZ identification protocol was successful in identifying an amount of IZs that was similar to past studies.16 The EAS AI values for the young group were calculated as 28.7 ± 17.0% (31.5 ± 3.9 years), which is consistent with published results of 25.03 ± 10.11% (35 ± 11 years).20 Significantly higher AI values in the elderly group suggest that with increasing age, the EAS in healthy women tends to be less symmetrically innervated. These results suggest that aging may lead to a decrease in EAS innervation symmetry, which may further increase one’s risk for FI.
The average RMS values during contraction across all subjects was consistent with published studies.15 However, a comparison of RMS values alone may not be capable of identifying the neuromuscular alterations as the amplitude of the acquired EMG signals was not significantly correlated or sensitive to advanced age (P > 0.05). Anorectal manometry revealed visible yet not significant differences between young and aged groups, which differs from previous studies.22 This potentially controversial result might be caused by the relatively small study size, yet such a result does raise the question regarding the high variability of manometry results in studying the effects of altered neuromuscular innervation on the EAS function. Furthermore, the significant difference observed in AI values suggested a higher sensitivity of the presented approach in detecting neuromuscular alterations of EAS, compared with conventional manometry and simple surface EMG amplitude analysis.
Several EMG and manometry techniques have also been implemented to study anorectal innervation, including EMG decomposition,19 intramuscular motor evoked potential, and vectormanometry.23 These studies employed EMG and manometry approaches to quantify EAS IZ symmetry in healthy and in a variety of patient populations including incontinence, childbirth trauma, and rectal prolapse sufferers.9,24–26 In our study, we have confirmed previous observations that AI values are negatively correlated with anorectal squeezing pressure (Figure 5B), suggesting the contractile capacity of the EAS is impaired in subjects that exhibit increased AI. Asymmetrical innervation may result in unbalanced pressure zones in the rectum, impairing closure efficiency, and patients’ continence.27 Therefore, asymmetrical EAS innervation may help explain the prevalence of FI in elderly than younger populations. An increased AI was found also after unilateral episiotomy with surface EMG recordings,16 indicating a disturbance to the innervation of the EAS during unilateral episiotomy. In addition to women that undergo episiotomy, those who suffer a level three or greater tear during childbirth are significantly more likely to have an elevated AI, and suffer from FI post-delivery.9 Similarly, it has been reported that rectal prolapse patients who suffer from FI also exhibit increased AI.24
Despite such a wealth of research findings in patient populations, little effort has been made to study aging-related changes in the innervation of the EAS. Our findings fill this prior research gap by evidencing increased EAS innervating asymmetry with advancing age. Prior studies employing needle28 and single fiber EMG29 discovered aging-related muscular changes whereby motorneurons are lost and a neuromuscular remodeling process consisting of concurrent denervation and subsequent re-innervation occurs. Our findings suggest that this remodeling may not occur symmetrically in the EAS, resulting in an asymmetrically innervated muscle. Moreover, in subjects over 60 years tested, as shown in Figure 5A, we observed severely asymmetric EAS IZ distributions. These results coincide with previous findings that EAS denervation occurs past sixty years of age.30
It is important to note that the presence of asymmetrical innervation does not necessarily indicate the presence of FI. Correlation between EAS innervation asymmetry, FI, and rectal prolapse is controversial. Multiple pre-clinical research studies report significant correlation between these factors, yet some studies also suggest asymmetry was not relevant to FI severity in nulliparous women.9 Other studies report that the presence of AI in patient groups with sphincter trauma is associated with a more severe symptom phenotype.12 Moreover, when one considers that the cause for FI can be multifactorial, the clinical relevance of AI to the development of FI in the elderly population remains uncovered.
One explanation for this phenomenon is that unilateral trauma to the EAS on the dominant side of innervation may result in anorectal dysfunction at higher rates than trauma to the less innervated side.5 Accordingly, an otherwise healthy person is at a higher risk for FI after trauma if the damage occurs near the location of dominant innervation. This effect increases as the severity of asymmetry increases, since trauma to the innervation zone location affects a higher percentage of muscle fibers at the neuromuscular junction. Taking this theory into consideration, our study may help explain why older women suffer from postpartum FI at higher rates than younger counterparts.31
Our study only included continent women. We chose to only include healthy women to control for the many different etiologies of FI. This may be seen as a limitation of our study; however, we wanted to focus solely on the effect of aging on the innervation symmetry of the EAS. Future efforts can be made to compare AI of FI sufferers and healthy controls. Subject recruitment was only limited to parous women to control for the possible neurogenic damage due to childbirth. The inclusion of multiparous females should not be a concern. Studies have examined the effect of vaginal deliveries on EAS anatomy, and did not find a significant difference between parous and nulliparous women in subjects without childbirth-related EAS trauma.32
5 |. CONCLUSIONS
Our work shows, for the first time, that EAS innervation is significantly associated with age and the EAS innervation tends to become increasingly asymmetrical with advancing age. Results demonstrate a negative correlation between the AI and closure strength of the EAS under voluntary contraction. The non-invasive HD-sEMG probe was successful in detecting high quality signals which provide an advanced phenotyping tool for clinical evaluation and possible prediction of anorectal disorders.
ACKNOWLEDGMENTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases (DK082644, DK113525), National Institute on Aging (AG053778), Society of Urodynamics, Female Pelvic Medicine, and Urogenital Reconstruction Foundation, University of Houston, and Guangdong Provincial Work Injury Rehabilitation Hospital.
Funding information
The University of Houston; National Institute on Aging, Grant number: AG053778; Guangdong Provincial Work Injury Rehabilitation Hospital; Society of Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction Foundation; National Institute of Diabetes and Digestive and Kidney Diseases, Grant numbers: DK082644, DK113525
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Goode PS, Burgio KL, Halli AD, et al. Prevalence and correlates of fecal incontinence in community-dwelling older adults. J Am Geriatrics Soc. 2005;53:629–635. [DOI] [PubMed] [Google Scholar]
- 2.Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox JC, Fletcher JG, Zinsmeister AR, Seide B, Riederer SJ, Bharucha AE. Effect of aging on anorectal and pelvic floor functions in females. Diseases Colon Rectum. 2006;49:1726–1735. [DOI] [PubMed] [Google Scholar]
- 4.Bartolo D, Jarratt J, Read N. The use of conventional electromyography to assess external sphincter neuropathy in man. J Neurol Neurosurg Psychiatr. 1983;46:1115–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamdy S, Enck P, Aziz Q, Uengoergil S, Hobson A, Thompson D. Laterality effects of human pudendal nerve stimulation on corticoanal pathways: evidence for functional asymmetry. Gut. 1999;45:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams N, Barlow J, Hobson A, Scott N, Irving M. Manometric asymmetry in the anal canal in controls and patients with fecal incontinence. Diseases Colon Rectum. 1995;38:1275–1280. [DOI] [PubMed] [Google Scholar]
- 7.Huang JC, Deletis V, Vodusek DB, Abbott R. Preservation of pudendal afferents in sacral rhizotomies. Neurosurgery. 1997;41:411–415. [DOI] [PubMed] [Google Scholar]
- 8.Enck P, Franz H, Azpiroz F, et al. Innervation zones of the external anal sphincter in healthy male and female subjects. Digestion. 2004;69:123–130. [DOI] [PubMed] [Google Scholar]
- 9.Wietek BM, Hinninghofen H, Jehle EC, Enck P, Franz HB. Asymmetric sphincter innervation is associated with fecal incontinence after anal sphincter trauma during childbirth. Neurourol Urodyn. 2007;26:134–139. [DOI] [PubMed] [Google Scholar]
- 10.Clark BC, Manini TM. What is dynapenia? Nutrition. 2012;28: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bansal N, Sachdeva M, Jain P, Ranjan P, Arora A. Anorectal manometry: current techniques and indications. JIMSA. 2013;26: 169–170. [Google Scholar]
- 12.Enck P, Hinninghofen H, Merletti R. Neurophysiology of the pelvic floor: functional asymmetry of pelvic floor innervation. Funct Disorders Gastrointestinal Tract. 2005;3:121. [Google Scholar]
- 13.Merletti R, Bottin A, Cescon C, et al. Multichannel surface EMG for the non-invasive assessment of the anal sphincter muscle. Digestion. 2004;69:112–122. [DOI] [PubMed] [Google Scholar]
- 14.Ullah K, Cescon C, Afsharipour B, Merletti R. Automatic detection of motor unit innervation zones of the external anal sphincter by multichannel surface EMG. J Electromyogr Kinesiol. 2014;24:860–867. [DOI] [PubMed] [Google Scholar]
- 15.Peng Y, He J, Khavari R, Boone TB, Zhang Y. Functional mapping of the pelvic floor and sphincter muscles from high-density surface EMG recordings. Int Urogynecol J. 2016;27:1689–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cescon C, Riva D, Začesta V, et al. Effect of vaginal delivery on the external anal sphincter muscle innervation pattern evaluated by multichannel surface EMG: results of the multicentre study TASI-2. Int Urogynecol J. 2014;25:1491–1499. [DOI] [PubMed] [Google Scholar]
- 17.Flury N, Koenig I, Radlinger L. Crosstalk considerations in studies evaluating pelvic floor muscles using surface electromyography in women: a scoping review. Arch Gynecol Obstet. 2017;1–11. [DOI] [PubMed] [Google Scholar]
- 18.Ning Y, Zhu X, Zhu S, Zhang Y. Surface EMG decomposition based on K-means clustering and convolution kernel compensation. IEEE J Biomed Health Informat. 2015;19:471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mesin L, Gazzoni M, Merletti R. Automatic localisation of innervation zones: a simulation study of the external anal sphincter. J Electromyogr Kinesiol. 2009;19:e413–e421. [DOI] [PubMed] [Google Scholar]
- 20.Enck P, Franz H, Davico E, Mastrangelo F, Mesin L, Merletti R. Repeatability of innervation zone identification in the external anal sphincter muscle. Neurourol Urodyn. 2010;29:449–457. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Munoz A, Lai HH, Boone T, Zhang Y. Non-invasive electromyographic estimation of motor unit number in the external anal sphincter of the rat. Neurourol Urodyn. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bannister J, Abouzekry L, Read N. Effect of aging on anorectal function. Gut. 1987;28:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun JC, Treutner KH, Dreuw B, Klimaszewski M, Schumpelick V. Vectormanometry for differential diagnosis of fecal incontinence. Diseases Colon Rectum. 1994;37:989–996. [DOI] [PubMed] [Google Scholar]
- 24.Damon H, Henry L, Roman S, Barth X, Mion F. Influence of rectal prolapse on the asymmetry of the anal sphincter in patients with anal incontinence. BMC Gastroenterol. 2003;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorge JMN, Habr-Gama A. The value of sphincter asymmetry index in anal incontinence. Int J Colorect Dis. 2000;15:303–310. [DOI] [PubMed] [Google Scholar]
- 26.Willis S, Faridi A, Schelzig S, et al. Childbirth and incontinence: a prospective study on anal sphincter morphology and function before and early after vaginal delivery. Langenbeck’s Arch Surg. 2002;387:101–107. [DOI] [PubMed] [Google Scholar]
- 27.Perry RE, Blatchford GJ, Christensen MA, Thorson AG, Attwood SE. Manometric diagnosis of anal sphincter injuries. Am J Surg. 1990;159:112–117. [DOI] [PubMed] [Google Scholar]
- 28.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve. 2005;31:461–467. [DOI] [PubMed] [Google Scholar]
- 29.Laurberg S, Swash M. Effects of aging on the anorectal sphincters and their innervation. Dis Colon Rectum. 1989;32:737–742. [DOI] [PubMed] [Google Scholar]
- 30.Percy J, Neill M, Kandiah T, Swash M. A neurogenic factor in faecal incontinence in the elderly. Age Ageing. 1982;11: 175–179. [DOI] [PubMed] [Google Scholar]
- 31.Chiarelli P, Murphy B, Cockburn J. Fecal incontinence after high-risk delivery. Obst Gynecol. 2003;102:1299–1305. [DOI] [PubMed] [Google Scholar]
- 32.Murad-Regadas SM, Dealcanfreitas ID, Regadas FSP, Rodrigues LV, Fernandes GO, Pereira Jde J. Do changes in anal sphincter anatomy correlate with anal function in women with a history of vaginal delivery? Arq Gastroenterol. 2014;51:198–204. [DOI] [PubMed] [Google Scholar]


