Abstract
The amount of energy that can be conserved via halorespiration by Desulfitobacterium dehalogenans JW/IU-DC1 was determined by comparison of the growth yields of cells grown with 3-chloro-4-hydroxyphenyl acetate (Cl-OHPA) and different electron donors. Cultures that were grown with lactate, pyruvate, formate, or hydrogen as an electron donor and Cl-OHPA as an electron acceptor yielded 3.1, 6.6, 1.6, and 1.6 g (dry weight) per mol of reduction equivalents, respectively. Fermentative growth on pyruvate yielded 14 g (dry weight) per mol of pyruvate oxidized. Pyruvate was not fermented stoichiometrically to acetate and lactate, but an excess of acetate was produced. Experiments with 13C-labeled bicarbonate showed that during pyruvate fermentation, approximately 9% of the acetate was formed from the reduction of CO2. Comparison of the growth yields suggests that 1 mol of ATP is produced per mol of acetate produced by substrate-level phosphorylation and that there is no contribution of electron transport phosphorylation when D. dehalogenans grows on lactate plus Cl-OHPA or pyruvate plus Cl-OHPA. Furthermore, the growth yields indicate that approximately 1/3 mol of ATP is conserved per mol of Cl-OHPA reduced in cultures grown in formate plus Cl-OHPA and hydrogen plus Cl-OHPA. Because neither formate nor hydrogen nor Cl-OHPA supports substrate-level phosphorylation, energy must be conserved through the establishment of a proton motive force. Pyruvate ferredoxin oxidoreductase, lactate dehydrogenase, formate dehydrogenase, and hydrogenase were localized by in vitro assays with membrane-impermeable electron acceptors and donors. The orientation of chlorophenol-reductive dehalogenase in the cytoplasmic membrane, however, could not be determined. A model is proposed, which may explain the topology analyses as well as the results obtained in the yield study.
Reductive dechlorination of chlorinated compounds can be coupled to growth in a process called halorespiration (6). Desulfomonile tiedjei is the first organism with this ability that has been described. It is also the first organism for which the chemiosmotic coupling of reductive dechlorination and ATP synthesis has been demonstrated (4, 14, 15). Currently, several bacterial strains are known to have the ability to grow on a combination of a chlorinated compound and electron donors like formate and hydrogen (5). Since oxidation of formate and hydrogen cannot result in ATP formation by substrate-level phosphorylation, energy conservation can only occur through formation of an electrochemical gradient across the cytoplasmic membrane. Recently, a model was proposed for 3-chlorobenzoate respiration by D. tiedjei (11). It was suggested that formate oxidation takes place in this organism at the outside of the cytoplasmic membrane, whereas 3-chlorobenzoate is reduced inside the cell. Coupling these two processes would result in charge separation, which can be used for ATP formation. In addition, it has been suggested that protons are actively transported across the membrane during electron transport from formate to the chlorobenzoate-reductive dehalogenase, resulting in extra energy gain by halorespiration (11). However, proton translocation could not be demonstrated for perchloroethylene (PCE) respiration coupled to hydrogen-oxidation in Dehalobacter restrictus (19).
Desulfitobacterium dehalogenans is the first member of the genus Desulfitobacterium to be described (21). Most Desulfitobacterium strains isolated since have the capacity to reductively dechlorinate chloroaromatic compounds, chloroalkenes, or both (5). Furthermore, these organisms are able to use sulfite, fumarate, and nitrate as electron acceptors. Comparison of batch cultures of D. dehalogenans grown with pyruvate and different electron acceptors has indicated that comparable growth yields were obtained when 3-chloro-4-hydroxyphenylacetate (Cl-OHPA), fumarate, sulfite, or nitrate was used as an electron acceptor (12). These authors (12) proposed that chloroaromatic compound respiration yields 1 ATP per reduced chloroaromatic compound by electron transport phosphorylation.
In the present study, we have determined the amount of energy conserved through halorespiration by comparison of the growth yields obtained from batch cultures of D. dehalogenans grown on different electron donors and Cl-OHPA as an electron acceptor. The enzymes involved in electron donation and acceptance were localized, and 13C nuclear magnetic resonance (13C NMR) spectra were used to investigate the contribution of acetate formation by CO2 reduction during pyruvate fermentation and halorespiration. The studies of growth yields and enzyme localization suggest that there is no electron transport phosphorylation when D. dehalogenans grows on lactate and Cl-OHPA or on pyruvate and Cl-OHPA. When formate and hydrogen serve as electron donors for halorespiration, however, a proton motive force is established which can be used for ATP generation.
MATERIALS AND METHODS
Organisms and growth conditions.
D. dehalogenans strain JW/IU-DC1 (DSM 9161) was cultivated at 37°C under anaerobic conditions (100% N2 gas phase) in a medium described previously (22). The medium was buffered with 30 mM bicarbonate and amended with 0.1% peptone. The yield studies were performed with 1l bottles, each containing 500 ml of medium with 10 mM lactate, 20 mM pyruvate, or 20 mM formate as an electron donor and 20 mM Cl-OHPA as an electron acceptor. When hydrogen was used as an electron donor, 10 mmol of hydrogen gas was added to the bottle before autoclaving was done. Pyruvate fermentation was studied with one set of bottles to which 40 mM pyruvate alone was added. Bottles were inoculated with 1% of an actively growing culture. For each growth condition tested, a different inoculum was prepared that was grown for several generations with that specific combination of substrates.
Cells were harvested in late log phase, which was reached after about 48 h for cultures on pyruvate or lactate and after about 72 h for cultures that used formate or hydrogen as an electron donor. These experiments were performed in triplicate. For the NMR experiment, D. dehalogenans was cultivated in 120-ml bottles with 25 ml of medium as described above, with the exception that the bicarbonate was replaced by 13C-labeled bicarbonate. The bacteria were cultivated at 37°C under Cl-OHPA-reducing conditions with 15 mM pyruvate and 16 mM Cl-OHPA or were grown fermentatively on 30 mM pyruvate. The media were inoculated with 1 to 2% of cultures that were adapted to either Cl-OHPA-reducing or fermentative growth conditions.
Dry weight determination.
A total of 400 ml of culture of a 500-ml incubation was concentrated by 10 min of centrifugation at 16,000 × g. The cell pellet was resuspended in 50 ml of 100 mM NaCl in MilliQ water and centrifuged. Thereafter, cells were resuspended in 2 ml of MilliQ water and transferred to a dry aluminum cup of known weight. The cell suspension was dried in a stove at 95°C. The cups were weighed after 24 and 72 h.
NMR experiments.
After 46 h, samples (1 ml each) were taken from the cultures and subsequently centrifuged. Proton-decoupled 13C NMR spectra of a 450-μl sample and 50 μl of D2O in a 5-mm tube (25°C) were recorded at 125.7 MHz on an AMX-500 spectrometer (Bruker GMBH), located at the Wageningen NMR Center. Approximately 50,000 transients were accumulated for one spectrum. Chemical shifts are expressed in parts per million relative to the internal standard of 50 mM succinate at natural abundance. The C-2,3 resonances of succinate were set at 35.1 ppm. 1H NMR spectra were recorded at 500 MHz for the same samples on the same spectrometer at 25°C.
Analytical methods.
Organic acids were analyzed with a SpectraSystem high-performance liquid chromatograph (Thermo Separation Products, Riviera Beach, Fla.) as described previously (20). The samples for Cl-OHPA and OHPA determinations were analyzed with a SpectraSystem high-performance liquid chromatograph with a SpectraSystem P2000 pump, an AS3000 autosampler, and a UV1000 UV detector. A total of 20 μl of sample was injected into a pesticide reversed-phase column (Chrompack, Middelburg, The Netherlands). The mobile phase was acetonitrile–0.01 M H3PO4 with a volume/volume ratio of 10/90. A flow rate of 1 ml·min−1 was applied. Cl-OHPA and OHPA were quantified by their absorption at 206 nm.
Preparation of cell extracts.
All handling was done in an anaerobic glove box with a N2/H2 gas phase at a 95/5 ratio. Cultures (500 ml) of D. dehalogenans were harvested by 10 min of centrifugation at 16,000 × g and resuspended in a total volume of 2 ml of buffer A containing 100 mM potassium phosphate (KPi) (pH 7.5) and 1 mM dithiothreitol. A portion, set aside on ice, was used as a whole-cell suspension. Cells were permealized by incubation of the whole-cell suspension with 0.1% cetyltrimethylammonium bromide (CTAB) for 10 min at 4°C. A few crystals of DNase I were added to a fraction of the whole-cell suspension and the cells were broken in six cycles of 30 s of sonication and 30 s of cooling on ice. Unbroken cells were removed by 5 min of centrifugation at 20,000 × g. A total of 500 μl of this cell extract was stored on ice for enzyme assays. The remaining part was centrifuged for 90 min at 140,000 × g to separate membranes and the cytoplasmic fraction. The supernatant containing the soluble proteins was transferred to a glass vial and stored at 4°C. The pellet containing the membranes was resuspended in buffer A. The fractions were stored at 0°C under a 100% N2 gas phase.
Enzyme assays.
Chlorophenol-reductive dehalogenase activity was determined as described previously (22) by measuring the reduction of methyl viologen (ɛ578= 9.8 mM−1cm−1) in N2-flushed cuvettes at 30°C. The assay mixture was 100 mM Tris-HCl buffer (pH 7.8) and contains 0.3 mM titanium citrate-reduced methyl viologen and extract. The reaction was started by the addition of 10 mM Cl-OHPA. Formate dehydrogenase, hydrogenase, carbon monoxide dehydrogenase, and pyruvate ferredoxin oxidoreductase activities were measured at 30°C in a rubber-stoppered N2-flushed cuvette containing 100 mM Tris-HCl buffer (pH 8.0) and 1 mM methyl viologen. Methyl viologen was slightly reduced with titanium citrate to an optical density at 578 nm of 0.05. A total of 5 to 40 μl of extract was added to the reduced buffer, and the optical density at 578 nm was monitored. Upon detection of a stable signal, 50 μl of substrate was added, and the reduction of methyl viologen was measured. Hydrogenase activity was measured by the addition of 500 μl of oxygen-free hydrogen. Coenzyme A (0.2 mM) was added prior to the addition of pyruvate. Lactate dehydrogenase activity was measured as described by Miller et al. (13). Protein concentrations of the different fractions were determined by the microbiuret method with bovine serum albumin as a standard (8).
Chemicals.
All chemicals were obtained from commercial sources and were of the highest purity available. Yeast extract was obtained from Difco, Detroit, Mich. Peptone, made from trypsin-digested casein, was obtained from Merck, Darmstadt, Germany. 13C-labeled bicarbonate was purchased from Isotec, Inc., Miamisburg, Ohio.
RESULTS
Biomass yield study.
The amount of cell material that is produced during growth of D. dehalogenans on different media has been used in this study to calculate the amount of energy that is conserved through halorespiration. The culture medium contained peptone, instead of yeast extract, to supply certain growth factors, since we had found that approximately 10 mM Cl-OHPA was dechlorinated when medium with only yeast extract (0.1%) and Cl-OHPA was inoculated with 1% of a culture grown with pyruvate plus Cl-OHPA. Replacing yeast extract by peptone did not result in growth and dechlorination. In control experiments where a single electron donor was added to the medium and Cl-OHPA was omitted, growth was observed only in cultures with pyruvate. The biomass yield was determined for cells grown fermentatively with pyruvate (40 mM) or grown with Cl-OHPA (20 mM) as an electron acceptor and lactate (10 mM), pyruvate (20 mM), formate (20 mM), or hydrogen (10 mmol) as an electron donor. We have compared substrate depletion and product formation and calculated the number of electrons that were transferred (Table 1). Determination of substrate and product concentrations showed that all of the Cl-OHPA converted was recovered from the medium as OHPA. The ratio of electron donor oxidized to Cl-OHPA reduced is approximately 1 for the incubations with pyruvate, hydrogen, or formate as an electron donor, indicating that the redox balance was complete. The C balance for the oxidation of pyruvate was complete, but not for lactate where only 80% of the electron donor converted was recovered as acetate. In the latter case, 0.46 mol of acetate was found to be oxidized per mol of Cl-OHPA produced (Table 1). This indicated that there was an extra source of reduction equivalents in these incubations, which has not been taken into account. Growth on lactate and Cl-OHPA reduction yielded 3.1 g of biomass per mol of reduction equivalents, which was approximately half of the biomass yield that was obtained when pyruvate oxidation was coupled to Cl-OHPA reduction (Table 1). The yield per mole of acetate was comparable for both growth substrates, which indicated that oxidation of lactate to acetate and the conversion of pyruvate to acetate yielded a comparable amount of ATP. Oxidation of hydrogen or formate does not support substrate-level phosphorylation, and therefore energy can only be conserved through electron transport phosphorylation. Under these growth conditions the biomass yield was approximately 3.2 g of biomass per mol of Cl-OHPA reduced.
TABLE 1.
Biomass yield and substrate utilization ratio of D. dehalogenans grown under different conditions with Cl-OHPA as an electron acceptora
| Growth substrate | Substrate ratio
|
Yield (g/mol)
|
||||
|---|---|---|---|---|---|---|
| Donor/acetate | Cl-OHPA/OHPA | Acetate/Cl-OHPA | Cl-OHPA | Acetate | [H] | |
| Lactate + Cl-OHPA | 0.82 (±0.09) | 1.02 (±0.05) | 0.46 (±0.05) | 5.54 (±0.53) | 11.85 (±0.36) | 3.06 (±0.05) |
| Pyruvate + Cl-OHPA | 1.00 (±0.21) | 1.02 (±0.03) | 1.02 (±0.06) | 13.11 (±0.64) | 12.75 (±0.60) | 6.59 (±0.28) |
| Formate + Cl-OHPA | 1.08 (±0.01) | 1.08b (±0.01) | 3.20 (±0.49) | 2.97c (±0.49) | 1.60 (±0.23) | |
| Hydrogen + Cl-OHPA | 0.96 (±0.11) | 1.02b (±0.21) | 3.30 (±0.85) | 3.50c (±1.84) | 1.65 (±0.52) | |
The values in parentheses give the deviation from the average. The yield is expressed as grams of biomass per mole of product or of reduction equivalents produced.
The conversion ratio of electron donor/Cl-OHPA is given, since product formation has not been measured.
Yield is given per gram of electron donor used, since the product formation from formate or hydrogen oxidation has not been determined.
The conversion ratio of pyruvate, obtained when D. dehalogenans was grown fermentatively on pyruvate, was different from that reported previously (21). Utkin et al. reported that pyruvate was fermented to equal amounts of lactate and acetate in a HEPES-buffered medium, which had a low bicarbonate concentration (21). However, in our experiments, where D. dehalogenans was cultivated in medium containing 30 mM bicarbonate, 28.1 (±0.7) mM pyruvate was converted to 23.4 (±0.5) mM acetate and 10.9 (±0.6) mM lactate. Growth by pyruvate fermentation yielded 14.2 (±0.1) g of biomass per mol of acetate produced. The concentration of acetate was about twice as high as the lactate concentration. Furthermore, the total concentration of organic acids increased with approximately 5 mM. Fixation of CO2 into acetate is a possible explanation for the 5 mM increase in organic acid concentration during fermentative growth on pyruvate.
CO2-fixation.
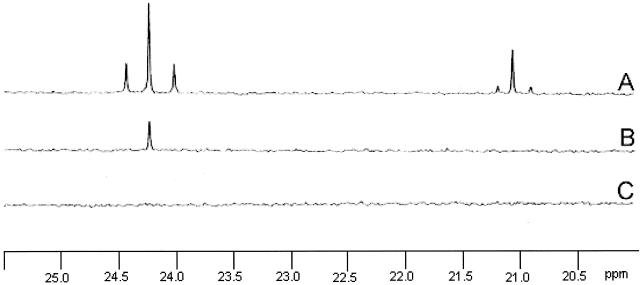
To investigate the possibility of CO2 reduction, we carried out a 13C NMR experiment with 13C-labeled bicarbonate as a source of CO2 and as a buffer. High-performance liquid chromatography analysis of the organic acids in these cultures shows a conversion pattern similar to that obtained in the yield study (Table 2). The 13C NMR spectra of supernatants of cultures of D. dehalogenans grown for 46 h with 13C-labeled bicarbonate and pyruvate as an energy source (Fig. 1A) showed a triplet resonance at the chemical shift position of C2 acetate at 24.2 ppm. The doublet component in this triplet (having a typical coupling constant of 52 Hz) reflects 13C-labeled C2 acetate with a 13C1 carbon as neighbor and thus represents doubly 13C-labeled acetate. The singlet represents singly 13C-labeled C2 acetate next to acetate at natural abundance (1.1% 13C) remaining, for instance, after the decarboxylation of pyruvate. From the 13C NMR spectra, the amount of doubly 13C-labeled C2 acetate was estimated to be about 40% of the total of 13C-labeled acetate and C2 acetate of natural abundance. Also, the C1 resonance of acetate was a triplet (the coupling constant of the doublet being, again, 52 Hz) with a lower intensity due to the lower sensitivity of the C1 carbon (not shown).
TABLE 2.
Substrate conversion and product formation by D. dehalogenansa
| Culture | Substrate conversion and product formation (mmol/liter)
|
||||
|---|---|---|---|---|---|
| Pyruvate degraded | Lactate formed | Acetate formed | Cl-OHPA transformed | OHPA formed | |
| Pyruvate + Cl-OHPAb | 15.3 | 15.0 | 11.5 | 14.5 | |
| Pyruvatec | 29.9 | 8.3 | 23.3 | ||
D. dehalogenans was grown for 46 h at 37°C in medium buffered with 40 mM 13C-labeled bicarbonate.
The initial concentrations were 15 mM pyruvate and 16 mM Cl-OHPA.
The initial pyruvate concentration was 30 mM.
FIG. 1.
13C Nuclear magnetic resonance spectra of culture supernatants of D. dehalogenans incubated for 46 h with 40 mM pyruvate (A) or 20 mM pyruvate and 20 mM Cl-OHPA (B). The spectrum shown in trace C showed the supernatant of the pyruvate incubation just after inoculation (t = 0).
The 1H NMR spectra of the supernatants showed a large singlet at the resonance position of the protons of the methyl group of acetate at 1.905 ppm, reflecting the protons that are bound to 12C2 carbons (not shown). Two triplets surround this singlet with low intensity, the center of each triplet situated at +75 Hz around the large central singlet. These two 1H-13C2 doublet components with a coupling constant of 150 Hz (2 × 75 Hz) typically reflect the protons that are directly bound to 13C-labeled C2 carbons which have no 13C1 as a neighbor. Around the components of this 1H-13C2 doublet, another doublet is present with a coupling constant of 35 Hz (13C2-13C1), representing the 13C2 carbons in acetate that have a 13C1 as neighbor. From the total area of both triplets in this 1H-spectrum, it was estimated that at least 4% of the acetate was 13C labeled at the C2 position, thus exceeding the 1.1% 13C of natural abundance. Both doublets were absent in the 13C NMR spectra when 13CO2 was present during growth on pyruvate and Cl-OHPA (Fig. 1B). From analysis of the 1H NMR spectra, it could be deduced that acetate was not enriched (approximately 1% was found to be labeled) and thus reflects only acetate of natural abundance.
These results demonstrate that CO2 fixation is only important when D. dehalogenans is grown with pyruvate and CO2 alone and not when Cl-OHPA is present as an electron acceptor. The contribution of CO2 fixation to the acetate pool can be calculated from the recovery of organic acids. Approximately 9% of the acetate was produced by CO2 fixation.
In addition to double-labeled acetate, double-labeled lactate was found when D. dehalogenans was grown fermentatively on pyruvate. The C3 of lactate exhibits a resonance at 21.1 ppm (Fig. 1A). Approximately 6% of this signal was split into a doublet because of concomitant labeling at the C2 position. The C1 carboxyl group was found to be labeled to a higher degree. This is due to carboxylation of unlabeled acetate to pyruvate that was reduced to lactate. When these mechanisms are taken into account the mass balance on pyruvate is complete (Table 2).
Enzyme localization.
Lactate dehydrogenase, pyruvate ferredoxin oxidoreductase, formate dehydrogenase, hydrogenase, and chlorophenol-reductive dehalogenase were localized by in vitro enzyme assays with methyl viologen as an artificial electron donor or acceptor (Table 3). The pyruvate ferredoxin oxidoreductase was clearly located in the cytoplasmic fraction. Lactate dehydrogenase, formate dehydrogenase, and hydrogenase activities were recovered for approximately 44% ofthe membrane fraction, which suggests that these activities are membrane associated. Seventy-one percent of the chlorophenol-reductive dehalogenase was located in the membrane fraction, which indicates that chlorophenol-reductive dehalogenase is a membrane-bound enzyme.
TABLE 3.
Localization of enzymes involved in respiration in D. dehalogenansa
| Enzyme | Growth condition | Whole cells
|
Permealized cells
|
Cell extract
|
Membrane fraction
|
Soluble fraction
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sp act (mU/mg) | Total activity (U) | Sp act (mU/mg) | Total activity (U) | Sp act (mU/mg) | Total activity (U) | Sp act (mU/mg) | Total activity (U) | Sp act (mU/mg) | Total activity (U) | ||
| LDH | Lactate + Cl-OHPA | 74 | 6.05 | 69 | 5.57 | 142 | 1.70 | 195 | 1.45 | 356 | 1.87 |
| PFO | Pyruvate + Cl-OHPA | 6 | 0.25 | 270 | 11.11 | 161 | 4.05 | 0 | 0 | 107 | 1.08 |
| FDH | Formate + Cl-OHPA | 14 | 1.25 | 108 | 9.43 | 59 | 5.26 | 190 | 1.57 | 973 | 2.04 |
| HYD | Hydrogen + Cl-OHPA | 81 | 1.52 | 110 | 2.06 | 884 | 3.04 | 206 | 0.54 | 1,726 | 0.69 |
| CPRD | Pyruvate + Cl-OHPA | 55 | 1.14 | ND | ND | 306 | 3.84 | 326 | 2.47 | 194 | 0.98 |
D. dehalogenans was grown with Cl-OHPA as the electron acceptor and different electron donors. Concentrated cells were permealized by the addition of 0.1% (vol/vol) CTAB. one unit is defined as the conversion of 1 μmol of substrate per min. Abbreviations: LDH, lactate dehydrogenase; PFO, pyruvate ferredoxin oxidoreductase; FDH, formate dehydrogenase; HYD, hydrogenase; CPRD, chlorophenol reductive dehalogenase; ND, not detected.
The known inability of methyl viologen to pass through cell membranes was used to localize the active sites of membrane-bound enzymes (10). Enzyme activities in concentrated cell suspensions, CTAB-permealized cells, and cell extracts were measured (Table 3). Pyruvate ferredoxin oxidoreductase could hardly be measured in the whole-cell suspension, but the activity increased 45 times when the cells were permealized. The activity in cell extract was a little lower. This all points to a cytoplasmic location of pyruvate ferredoxin oxidoreductase. The level of formate dehydrogenase activity measured in whole cells was high, but increased over 7.7 times after incubation with CTAB. The total activity of hydrogenase increased little after CTAB treatment, but increased 11 times upon sonication. Lactate dehydrogenase did not react with methyl viologen and had to be localized with phenazine methosulfate. Like methyl viologen, phenazine methosulfate cannot penetrate the membrane (10). A high level of lactate dehydrogenase activity was observed in the whole-cell suspension. No increase was found when cells were incubated with CTAB, but the specific activity did increase twofold upon sonication. Chlorophenol-reductive dehalogenase could be assayed with whole cells but could not be detected in cells that were permealized with CTAB. Furthermore, the level of chlorophenol-reductive dehalogenase activity of cell extract dropped when CTAB was added to the cuvette, indicating that CTAB has an inhibiting effect on chlorophenol-reductive dehalogenase. Chlorophenol-reductive dehalogenase activity increased 5.6 times upon sonication of the whole cells.
DISCUSSION
We have investigated the energy metabolism of D. dehalogenans during halorespiration with different electron donors by analyzing biomass yields and enzyme localization. During growth of D. dehalogenans with pyruvate, formate, or hydrogen as an electron donor and Cl-OHPA as an electron acceptor, the oxidation of 1 mol of pyruvate, formate, or hydrogen was coupled to the reduction of 1 mol of Cl-OHPA. When lactate was used as an electron donor, the conversion of 1 mol of lactate was coupled to the reduction of 2 mol of Cl-OHPA (Table 1). These results confirm the conversion pattern published previously (21). However, the fermentation pattern for growth with pyruvate as sole energy source was different. We did not find conversion of pyruvate into equal amounts of lactate and acetate, but instead a higher amount of acetate. The presence of double-labeled acetate and increased labeling at the C2 position of acetate in the 13C NMR spectrum shows that D. dehalogenans is able to use CO2 as an electron acceptor. Although this is the first report which provides evidence for reduction of CO2 by a Desulfitobacterium sp., other Desulfitobacterium strains are also thought to be able to reduce CO2 to acetate, since acetate was the only product of fermentation of pyruvate (1, 2, 7, 17). Net ATP formation from CO2 to acetate via the CO dehydrogenase-dependent acetyl coenzyme A cleavage pathway (16) can be formed only via a chemiosmotic gradient across the cell membrane. The amount of energy thus conserved is not exactly clear, but ranges between 1/3 to 2/3 mol of ATP per mol of acetate formed (3). This is less than the 1 mol of ATP which would be the result of substrate-level phosphorylation in pyruvate oxidation. As a result of CO2 reduction, less energy is conserved per mole of acetate. When this effect is taken into account, the yield during fermentative growth is 14.6 to 15.0 g of biomass per mol of ATP produced, which is comparable to the biomass yield reported by Mackiewicz and Wiegel for pyruvate fermentation (12).
When D. dehalogenans was cultivated on lactate plus Cl-OHPA or on pyruvate plus Cl-OHPA, we found in both cases a biomass yield of approximately 12 g per mol of acetate produced, which suggests that no electron transport phosphorylation occurred with lactate and pyruvate as electron donors. This yield is close to the amount of biomass that was formed from 1 mol of ATP during fermentative growth but is much lower than the biomass yield that was reported by Mackiewicz and Wiegel for growth on pyruvate plus Cl-OHPA (12). They reported a yield of 24.2 g of biomass per mol of acetate produced, which was found to be comparable to the biomass yield for growth with pyruvate-fumarate, pyruvate-nitrate, or pyruvate-sulfite. The discrepancy with our results can be due to the fact that we used a different growth medium, replaced yeast extract by peptone to supply certain growth factors, and used smaller inocula (1% instead of approximately 10%) to limit the supply of extra growth substrates. For the related bacteria—Desulfitobacterium sp. PCE1 and Desulfitobacterium hafniense, grown in continuous culture with lactate and Cl-OHPA—growth yields of approximately 3.5 g of protein per mol of electrons released have been reported (7). When the protein content is assumed to be 50% (dry weight), the growth yields for D. hafniense and Desulfitobacterium sp. PCE1 are twice those we found for D. dehalogenans.
Formate and hydrogen were found to be poor electron donors for halorespiration, each yielding 1.6 g of biomass per mol of reduction equivalents. Mackiewicz and Wiegel (12) reported the same yield for cultures of D. dehalogenans grown with formate plus Cl-OHPA that were harvested in late exponential growth phase. The growth yields obtained for other halorespiring bacteria grown with formate or hydrogen range between 2.1 and 3.6 g of biomass per mol of chloride released. (7, 9, 14). When comparing these biomass yields, it has to be taken into account that the amount of energy needed for maintenance of cell integrity is only constant when cells grow at the same rate. In our study, the maintenance coefficients for growth with lactate plus Cl-OHPA, pyruvate plus Cl-OHPA, and pyruvate fermentation may be comparable, whereas for growth with hydrogen or formate they may be higher, since D. dehalogenans grows approximately two times more slowly with these two electron donors. A higher-maintenance coefficient results in a lower biomass yield. Continuous culture studies as performed by Gerritse et al. (7) have this advantage: the maintenance coefficient is constant because the growth rate can be controlled. In batch cultures the growth rate is not constant, which results in different yields over time. This was demonstrated by Mackiewicz and Wiegel (12), who reported that the growth yield of D. dehalogenans with formate plus Cl-OHPA varied between 1.6 and 5.7 g of biomass per mol of electrons when cells were harvested in different growth phases. Because of this effect, the growth yields obtained from continuous cultures are likely to be higher than those obtained from cells that are grown in batch culture and harvested in late exponential growth phase.
Pyruvate ferredoxin oxidoreductase was found in the cytoplasm. Lactate dehydrogenase activity did not increase when cells were permealized by CTAB, which indicates that the active site of the enzyme is accessible from the outside. However, lactate dehydrogenase activity increased twofold upon sonication of the cells. Furthermore, lactate dehydrogenase activity in Desulfitobacterium strain PCE-S was localized at the inside of the cytoplasmic membrane (13). Therefore, a clear localization of lactate dehydrogenase was not possible. Hydrogenase activity and formate dehydrogenase activity are localized both inside and outside the cell membrane, suggesting that multiple enzymes are present. For both enzyme activities, it could not be determined at which side of the cytoplasmic membrane the respiratory enzyme is located. Since chlorophenol reductive dehalogenase activity increased 3.4 times after sonication of the whole-cell suspension, it might be argued that chlorophenol-reductive dehalogenase has its active site facing inwards. However, the chlorophenol-reductive dehalogenase activity measured in whole cells is relatively high, indicating that the active site of chlorophenol-reductive dehalogenase must be at least partly accessible for methyl viologen. Contamination of the whole-cell suspension with lysed cells is not likely because pyruvate ferredoxin oxidoreductase activity could not be measured in this cell suspension.
Based on the results presented in this study we propose a model for halorespiration in D. dehalogenans in which the respiratory hydrogenase and formate dehydrogenase activities are facing outwards, analogous to the situation in Escherichia coli (18). With formate or hydrogen, a gradient of two charges (2q+/2e−) can be established when chlorophenol-reductive dehalogenase is located inside the cell without vectorial proton translocation across the cytoplasmic membrane coupled to electron transport. There may be a loss of energy due to unknown processes (e.g., transport of substrates or products or an activation barrier), which then explains the low-growth yield of 3.2 g of biomass per mol of substrate. Similar models have been proposed for 3-chlorobenzoate respiration in D. tiedjei and PCE respiration in Dehalospirillum multivorans and D. restrictus (11, 13, 19). On the other hand, one may also argue that the chlorophenol-reductive dehalogenase is located at the outside of the cytoplasmic membrane, in which case electron transport must be coupled to proton translocation. At present, it is not possible to discriminate between these two possibilities. Both models suggest that no proton motive force is established and no energy is conserved via electron transport phosphorylation during growth on pyruvate plus Cl-OHPA and lactate plus Cl-OHPA, because lactate dehydrogenase and pyruvate ferredoxin oxidoreductase are located at the inside of the cell membrane. This is in accordance with the finding that the yield of ATP per mol of acetate is constant under different growth conditions. Cells that grow on these substrates conserve approximately one mol of ATP per mol of acetate. This amount of energy can be conserved via substrate-level phosphorylation of acetyl-coenzyme A only. When electron transport phosphorylation occurs during halorespiration under the conditions tested, this energy is not used for growth but may be reinvested to drive the process.
Our study indicates that chlorophenol respiration is not an efficient respiration pathway, since only a fraction of the energy can be conserved that is theoretically possible, which is approximately 2 mol of ATP per mol of chloride removed with hydrogen as an electron donor (5). Future study of the composition of the electron transport chain involved in halorespiration may provide better insight in the halorespiration pathway.
REFERENCES
- 1.Bouchard B, Beaudet R, Villemur R, McSween G, Lepine F, Bisaillon J G. Isolation and characterization of Desulfitobacterium frappieri sp. nov., an anaerobic bacterium which reductively dechlorinates pentachlorophenol to 3-chlorophenol. Int J Syst Bacteriol. 1996;46:1010–1015. doi: 10.1099/00207713-46-4-1010. [DOI] [PubMed] [Google Scholar]
- 2.Christiansen N, Ahring B K. Desulfitobacterium hafniense sp.nov., an anaerobic reductively dechlorinating bacterium. Int J Syst Bacteriol. 1996;46:442–448. [Google Scholar]
- 3.Diekert G, Wohlfarth G. Metabolism of homoacetogens. Antonie Leeuwenhoek. 1994;66:209–221. doi: 10.1007/BF00871640. [DOI] [PubMed] [Google Scholar]
- 4.Dolfing J. Reductive dechlorination of 3-chlorobenzoate is coupled to ATP production and growth in an anaerobic bacterium, strain DCB-1. Arch Microbiol. 1990;153:264–266. doi: 10.1007/BF00249079. [DOI] [PubMed] [Google Scholar]
- 5.El Fantroussi S, Naveau H, Agathos S N. Anaerobic dechlorinating bacteria. Biotechnol Prog. 1998;14:167–188. doi: 10.1021/bp980011k. [DOI] [PubMed] [Google Scholar]
- 6.Fetzner S. Bacterial dehalogenases. Appl Microbiol Biotechnol. 1998;50:633–657. doi: 10.1007/s002530051346. [DOI] [PubMed] [Google Scholar]
- 7.Gerritse J, Drzyzga O, Kloetstra G, Keijmel M, Wiersum L P, Hutson R, Collins M D, Gottschal J C. Influence of different electron donors and acceptors on dehalorespiration of tetrachloroethene by Desulfitobacterium frappieri TCE1. Appl Environ Microbiol. 1999;65:5212–5221. doi: 10.1128/aem.65.12.5212-5221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goa J. A micro biuret method for protein determination: determination of total protein of cerebrospinal fluid. Scan J Clin Lab Investig. 1953;5:218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- 9.Holliger C, Schumacher W. Reductive dehalogenation as a respiratory process. Antonie Leeuwenhoek. 1994;66:239–246. doi: 10.1007/BF00871642. [DOI] [PubMed] [Google Scholar]
- 10.Kröger A, Dorrer E, Winkler E. The orientation of the substrate sites of formate dehydrogenase and fumarate reductase in the membrane of Vibrio succinogenes. Biochim Biophys Acta. 1980;589:118–136. doi: 10.1016/0005-2728(80)90136-x. [DOI] [PubMed] [Google Scholar]
- 11.Louie T M, Mohn W W. Evidence for a chemiosmotic model of dehalorespiration in Desulfomonile tiedjei DCB-1. J Bacteriol. 1999;181:40–46. doi: 10.1128/jb.181.1.40-46.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackiewicz N, Wiegel J. Comparison of energy and growth yields for Desulfitobacterium dehalogenans during utilization of chlorophenol and various traditional electron acceptors. Appl Environ Microbiol. 1998;64:352–355. doi: 10.1128/aem.64.1.352-355.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller E, Wohlfarth G, Diekert G. Studies on tetrachloroethene respiration in Dehalospirillum multivorans. Arch Microbiol. 1996;166:379–387. doi: 10.1007/BF01682983. [DOI] [PubMed] [Google Scholar]
- 14.Mohn W W, Tiedje J M. Strain DCB-1 conserves energy for growth from reductive dechlorination coupled to formate oxidation. Arch Microbiol. 1990;153:267–271. doi: 10.1007/BF00249080. [DOI] [PubMed] [Google Scholar]
- 15.Mohn W W, Tiedje J M. Evidence for chemiosmotic coupling of reductive dechlorination and ATP synthesis in Desulfomonile tiedjei. Arch Microbiol. 1991;157:1–6. [Google Scholar]
- 16.Ragsdale S W. CO dehydrogenase and the central role of this enzyme in the fixation of carbon dioxide by anaerobic bacteria. In: Drake H L, editor. Acetogenesis. New York, N.Y: Chapman and Hall; 1994. pp. 88–126. [Google Scholar]
- 17.Sanford R A, Cole J R, Löffler F E, Tiedje J M. Characterization of Desulfitobacterium chlororespirans sp. nov., which grows by coupling the oxidation of lactate to the reductive dechlorination of 3-chloro-4-hydroxybenzoate. Appl Environ Microbiol. 1996;62:3800–3808. doi: 10.1128/aem.62.10.3800-3808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawers G. The hydrogenases and formate dehydrogenases of Escherichia coli. Antonie Leeuwenhoek. 1994;66:57–88. doi: 10.1007/BF00871633. [DOI] [PubMed] [Google Scholar]
- 19.Schumacher W, Holliger C. The proton/electron ratio of the menaquinone-dependent electron transport from dihydrogen to tetrachloroethene in “Dehalobacter restrictus. ” J. Bacteriol. 1996;178:2328–2333. doi: 10.1128/jb.178.8.2328-2333.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stams A J M, Van Dijk J B, Dijkema C, Plugge C M. Growth of syntrophic propionate-oxidizing bacteria with fumarate in the absence of methanogenic bacteria. Appl Environ Microbiol. 1993;59:1114–1119. doi: 10.1128/aem.59.4.1114-1119.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Utkin I, Woese C, Wiegel J. Isolation and characterisation of Desulfitobacterium dehalogenans gen. nov., sp. nov., an anaerobic bacterium which reductively dechlorinates chlorophenolic compounds. Int J Syst Bacteriol. 1994;44:612–619. doi: 10.1099/00207713-44-4-612. [DOI] [PubMed] [Google Scholar]
- 22.van de Pas B A, Smidt H, Hagen W R, van der Oost J, Schraa G, Stams A J M, de Vos W M. Purification and molecular characterization of ortho-chlorophenol reductive dehalogenase, a key enzyme of halorespiration in Desulfitobacterium dehalogenans. J Biol Chem. 1999;274:20287–20292. doi: 10.1074/jbc.274.29.20287. [DOI] [PubMed] [Google Scholar]