Abstract
Selenoprotein K (SELENOK) is an endoplasmic reticulum stress (ERS)-regulated protein required for the calcium (Ca2+) flux-mediated migration of T cells and neutrophils, and the migration and phagocytosis of macrophages and microglia. However, the effect of SELENOK on the regulation of the immune function of dendritic cells (DCs), including immature DCs (imDCs) and mature DCs (mDCs), is still unclear. In this study, imDCs prepared from SELENOK knockout mice were used to evaluate the effect of SELENOK on the migration and phagocytosis of imDCs. The results showed that ERS-induced downregulation of imDCs phenotypic markers led to a reduction in Ras homolog gene family member A (RhoA)-dependent migration and enhanced Ca2+/CD205-mediated phagocytosis. SELENOK deficiency-induced upregulation of selenoprotein S (SELENOS) attenuated ERS levels in imDCs. An increase in Ca2+ levels resulted in increased migration and decreased phagocytosis with or without ERS conditions. The migration was RhoA-dependent, and Ca2+ or CD205 was associated with regulating phagocytosis in imDCs. Our study found that SELENOK is required for imDC migration and phagocytosis.
Keywords: selenoprotein K, dendritic cells, endoplasmic reticulum stress
1. Introduction
Dendritic cells (DCs) are proficient antigen-presenting cells that play an essential role in connecting innate and adaptive immune responses. In accordance with the levels of co-stimulatory molecules, such as the cluster of differentiation (CD)40 and CD80, DCs are divided into immature DCs (imDCs) and mature DCs (mDCs), which play an essential role in antigen phagocytosis and presentation, respectively [1]. Several previous studies have demonstrated that the immune function of chicken DCs was regulated by selenium [2,3]. Our recent studies have also illustrated that selenium can regulate the differentiation and maturation of human DCs [4,5], and its deficiency and excess impaired the DCs’ immune function [6] and reduced the number of splenic DCs in mice [7]. It is well known that selenium exerts its biological effect through selenoprotein, and the effect of selenium on the DCs’ immune function in chickens [2,3], humans [5] and mice [6,7] was related to changes in the levels of selenoprotein. It was found that selenium regulated the mRNA levels of selenoprotein K (SELENOK) in human imDCs and mDCs [5], and the protein levels involved in the regulation of DCs in mice [6]. We hypothesise that SELENOK may be involved in regulating the DCs’ immune function.
SELENOK is widely distributed in human and mouse tissues, with high expression levels in the immune system, and it is an endoplasmic reticulum (ER) transmembrane protein that regulates endoplasmic reticulum stress (ERS) and calcium (Ca2+) signalling [8]. Previous studies have shown that SELENOK deficiency reduces the proliferation and migration of T cells and neutrophils [9]. Furthermore, SELENOK is essential for Toll-like receptor-mediated activation [10] and FcγR-mediated phagocytosis [11] in macrophages. Recently, SELENOK was shown to be involved in the apoptosis of mouse neuronal cells [12] and the recovery of synaptic defects in Alzheimer’s disease model mice [13] through ERS or Ca2+ signalling. However, the role of SELENOK in ERS-induced alterations in the DCs’ immune function is still unclear. Numerous studies have shown that ERS can regulate the immune function of DCs, including cytokine production, DC maturation, antigen presentation and tumour immune response, by using an inositol-requiring enzyme 1 α/X-box-binding protein 1 (IRE1α/XBP1) signalling axis and PKR-like ER kinase (PERK) [14,15,16,17]. However, the role of activating transcription factor 4 (ATF4) and ATF6 in DCs is unclear. Therefore, we hypothesised that SELENOK could regulate ERS and, thus, affect the DCs’ immune function.
In this study, we evaluated the effect of SELENOK on the immune function of imDCs prepared from wild-type (WT) and SELENOK knockout (KO) mice. The effect of the ERS inducer, tunicamycin (Tm), on the migration, phagocytosis and phenotypic markers in WT imDCs was examined. Furthermore, ERS-induced migration and the phagocytosis of SELENOK-deficient imDCs were evaluated, and the ERS markers and phenotypic markers were detected.
2. Materials and Methods
2.1. Mice
Male WT mice (C57BL/6J) were purchased from the Guizhou Laboratory Animal Engineering Technology Centre, and the SELENOK knockout (KO) mice were obtained from the Saiye Biotechnology Co., Ltd. (Suzhou, China). The Institutional Animal Care and Use Committee at the Guizhou Medical University approved all the research protocols (No. 1800238).
2.2. Preparation of imDCs and mDCs
Fifty-one WT and twenty-four KO male mice aged eight weeks were used for the preparation of the imDCs and the mDCs, as previously described [6]. Briefly, bone marrow suspensions treated with erythrocyte lysate were cultured in RPMI 1640 medium containing foetal bovine serum (Hyclone, Logan, UT, USA), granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) (PeproTech, Rocky Hill, NJ, USA); the imDCs were obtained after 7 days. Lipopolysaccharides (Sigma–Aldrich, Darmstadt, Germany) were used to induce mDCs for another 2 days.
2.3. Cell Counting Kit-8 (CCK8) Assay
The imDCs and the mDCs prepared from the WT mice were treated with the ERS inducer, Tm (0.1, 0.5, 1, 2, 5, 10, 20 μg/mL) (Sigma–Aldrich, Darmstadt, Germany), for 24 h, and the control group was treated with dimethyl sulfoxide (DMSO) for the same time. Subsequently, a CCK8 assay was used to determine cell viability by using a microplate reader (Cytation5, Biotek, VT, USA) at 450 nm. For the SELENOK KO imDCs, the cells were treated with or without Tm (0.5 μg/mL), followed by a CCK8 assay.
2.4. Migration Capability Detection
The free migration of the imDCs prepared from the WT mice was assayed by Transwell (Millipore, MA, USA) according to the previous description [6] after treatment with Tm (0.1, 0.5, 1 μg/mL) for 24 h. In addition, the imDCs were pre-treated with 30 μM Rhosin (MedChemExpress, Rocky Hill, NJ, USA), a RhoA inhibitor, for 24 h, followed by 1 μg/mL of Tm, and then the migration ability was detected. To evaluate the effect of SELENOK on the imDCs’ migration, the imDCs prepared from the KO mice were treated with or without Tm (0.5 μg/mL). Then, the migration ability was assayed by Transwell.
2.5. Phagocytosis Assay
Phagocytosis was assayed in terms of the cells’ ability to internalise FITC-dextran. The imDCs prepared from the WT mice were treated with Tm (0.1, 0.5, 1 μg/mL) for 24 h and co-cultured with FITC-dextran (1 mg/mL) (40 KDa, Sigma, Darmstadt, Germany) for 1.5 h, as previously described [6]. For the SELENOK KO imDCs, the cells were treated with or without Tm (0.5 μg/mL), followed by FITC-dextran treatment. The phagocytosis ability was then measured by flow cytometry at Ex./Em. = 488 nm/520 nm (NovoCyte, ACEA Biosciences, San Diego, CA, USA).
2.6. Calcium Measurement
Cytosolic Ca2+ levels in the WT and SELENOK-deficient imDCs treated with or without 0.5 μg/mL Tm were measured by flow cytometry [18]. After collecting the imDCs, they were incubated with 1 μM calcium fluorescent dye, Fluo-4 AM (Beyotime, Beijing, China), for 30 min at room temperature and then washed with phosphate buffer solution. Fluorescence intensity was detected by flow cytometry and the Ca2+ levels were monitored.
2.7. Real-Time PCR
The total RNA was isolated from the WT imDCs after treatment with Tm (0.1, 0.5, 1 μg/mL) for 24 h by the TRIzol method (Invitrogen, Carlsbad, CA, USA). The cDNA synthesis and RT-PCR were performed according to our previous description [6]. The primers used in this study are listed in Table 1. The ERS genes included the C/EBP homologous protein (CHOP), the binding immunoglobulin protein (BIP), ATF4 and ATF6. The reference gene was glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the mRNA levels of target genes were calculated using the 2−ΔΔCT method [19].
Table 1.
Real-time PCR primers.
| Symbol | Accession Number | Forward Primer | Reverse Primer | Refs. |
|---|---|---|---|---|
| GAPDH | NM_001289726 | tgacatcaagaaggtggtgaagc | ccctgttgctgtagccgtattc | [20] |
| SELENOK | NM_019979 | tccacgaagaatgggtagga | gcttctcagagcagacatttacct | [20] |
| SELENOS | NM_024439 | cagaagattgaaatgtgggacagc | cctttggggatgacagatgaagtag | [20] |
| CHOP | NM_007837 | ccaacagaggtcacacgcac | tgactggaatctggagagcga | [21] |
| BIP | NM_001163434 | tgtggtacccaccaagaagtc | ttcagctgtcactcggagaat | [21] |
| ATF4 | NM-009716 | cgagatgagcttcctgaacagc | ggaaaaggcatcctccttgc | [21] |
| ATF6 | NM_001081304 | taccacccacaacaagacca | tgatgatcccggagataagg | [21] |
2.8. Western Blot
The RIPA lysates were used to prepare protein extracts of DCs and Western blotting was performed. The antibodies RhoA (2117T), ATF4 (11815S), CHOP (5554T), BIP (3177T) and GAPDH (5174T) were purchased from CST; ATF6 (ab227830) and SELENOK (ab139949) were obtained from Abcam; SELENOS (15591-1-AP) was obtained from Proteintech; CD11c (GB11059) was obtained from Servicebio; CD40 (ER1803-54), CD80 (M1007-10) and CD205 (ET7107-58) were obtained from Huabio.
2.9. Statistical Analysis
The data were analysed by GraphPad Prism 8.0, and an analysis of variance (ANOVA) was applied to determine the statistical difference, followed by Tukey’s test. The data were presented as mean ± SD. The p values of less than 0.05 indicated statistical significance.
3. Results
3.1. ERS Resulted in Decreased DCs Cell Viability
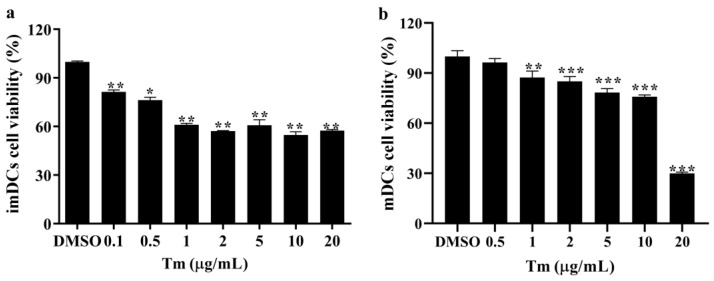
The effect of the ERS inducer, Tm, on the cell viability of imDCs and mDCs was determined by the CCK8 assay. The results in Figure 1a show that the cell viability of imDCs was significantly reduced after Tm (0.1, 0.5, 1, 2, 5, 10, 20 μg/mL) treatment. Moreover, treatment with 0.5 μg/mL Tm did not affect mDCs cell viability, while the cell viability decreased significantly after treatment with 1, 2, 5, 10 or 20 μg/mL of Tm (Figure 1b).
Figure 1.
ERS resulted in decreased DC cell viability. The effect of the ERS inducer, tunicamycin (Tm), on the cell viability of imDCs (a) and mDCs (b) was determined by the CCK8 assay (n = 6). * p < 0.05, ** p < 0.01 and *** p < 0.001 were compared with DMSO-treated imDCs or mDCs. ERS, endoplasmic reticulum stress; DCs, dendritic cells; imDCs, immature dendritic cells; mDCs, mature dendritic cells; CCK8, Cell Counting Kit-8; DMSO, dimethyl sulfoxide.
3.2. ERS Decreased Migration and Increased Phagocytosis in imDCs
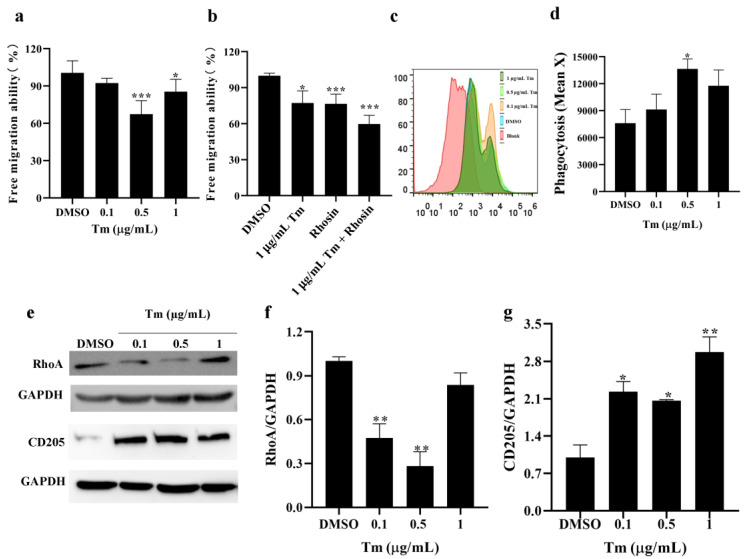
The migration and phagocytosis of imDCs treated with Tm were assayed by Transwell and flow cytometry, respectively. As shown in Figure 2a, the free migration of imDCs was reduced after treatment with 0.5 and 1 μg/mL of Tm, and Rhosin, a RhoA inhibitor, which could also lead to a decrease in the imDCs migration capacity (Figure 2b). In addition, Tm (0.1, 0.5 μg/mL) treatment downregulated the protein levels of RhoA (Figure 2e,f), indicating that a decrease in the RhoA protein level or activity is partly responsible for the decrease in Tm-induced imDC migration. The phagocytosis ability of imDCs was significantly enhanced by 0.5 μg/mL of Tm (Figure 2c,d), and elevated CD205 protein levels, an endocytic receptor of DC, were observed after the Tm (0.1, 0.5, 1 μg/mL) treatment (Figure 2e,g).
Figure 2.
ERS decreased migration and increased phagocytosis in imDCs. (a) The free migration ability of imDCs treated with Tm (0.1, 0.5, 1 μg/mL) was assayed by Transwell (n = 3). (b) The free migration ability of imDCs treated with or without Rhosin, a RhoA inhibitor, under Tm treatment was assayed by Transwell (n = 3). (c,d) The phagocytosis ability in imDCs after treatment with Tm was measured by flow cytometry, and the fluorescence intensity was calculated (n = 3). (e–g) The protein levels of RhoA and CD205 in imDCs after treatment with Tm were assayed by Western blotting, and the density values were calculated (n = 3). * p < 0.05, ** p < 0.01 and *** p < 0.001 were compared with DMSO-treated imDCs. ERS, endoplasmic reticulum stress; imDCs, immature dendritic cells; Tm, tunicamycin; Ras homolog gene family member A, RhoA; CD205, cluster of differentiation 205; DMSO, dimethyl sulfoxide.
3.3. ERS Downregulated Phenotypic Markers in imDCs
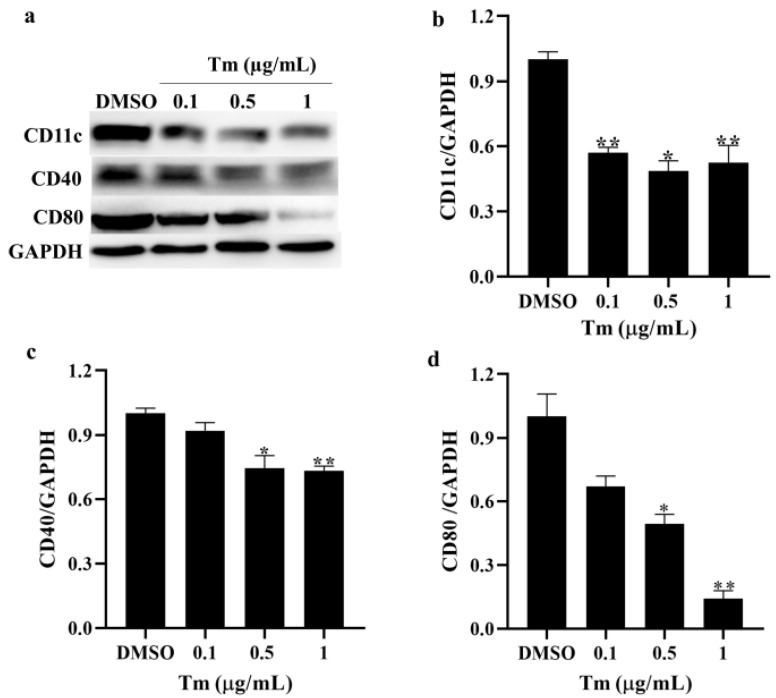
The phenotypic markers of the imDCs treated with Tm were measured by Western blotting. It was shown that the phenotypic markers CD11c, CD40 and CD80 were downregulated (Figure 3), suggesting that Tm induces a decrease in the total protein levels of imDC phenotypic markers, thereby affecting their function.
Figure 3.
ERS downregulated the phenotypic markers in imDCs. (a–d) The protein levels of CD11c, CD40 and CD80 in imDCs after treatment with Tm were assayed by Western blotting, and the density values were calculated (n = 3). * p < 0.05 and ** p < 0.01 were compared with DMSO-treated imDCs. ERS, endoplasmic reticulum stress; imDCs, immature dendritic cells; CD11c, cluster of differentiation 11c; CD40, cluster of differentiation 40; CD80, cluster of differentiation 80; Tm, tunicamycin; DMSO, dimethyl sulfoxide.
3.4. ERS Increased the Levels of ERS Markers in imDCs
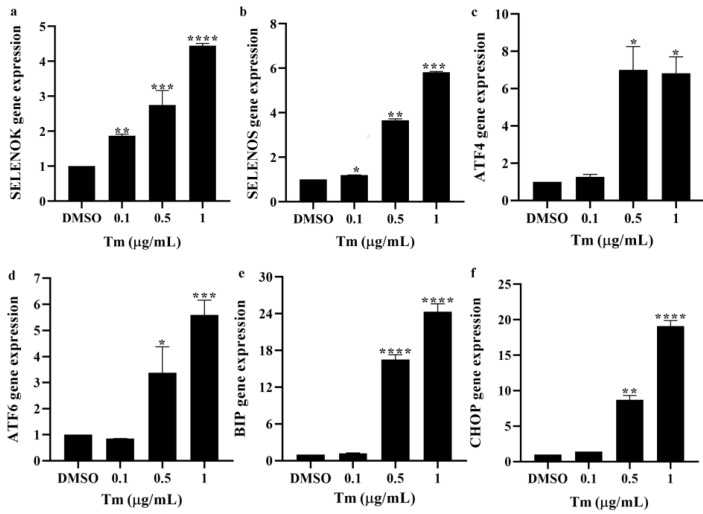
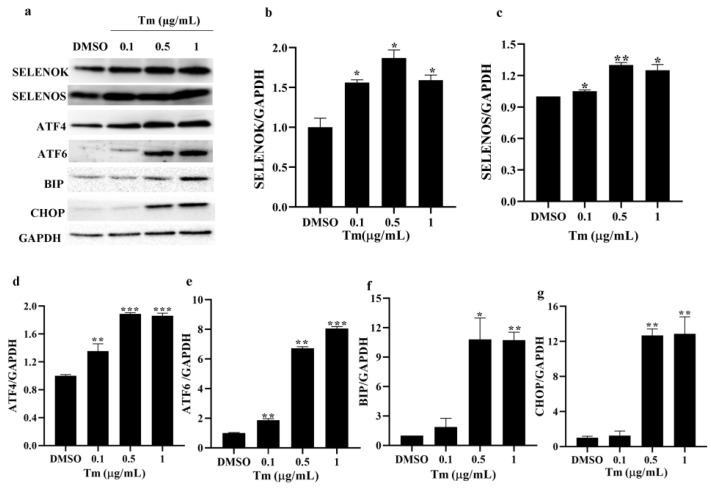
The mRNA and protein levels of the ERS markers SELENOK and SELENOS were assayed by RT-PCR and Western blotting, respectively. The results showed that the gene (Figure 4) and protein (Figure 5) levels of SELENOK, SELENOS, ATF4, ATF6, BIP and CHOP were gradually increased in Tm-treated imDCs, suggesting that altered immune function was due to an alteration in the imDCs markers, which is associated with increased ERS levels, and SELENOK and SELENOS were involved in the regulation of ERS.
Figure 4.
ERS increased the mRNA levels of the ERS markers in imDCs. (a,b) The mRNA levels of SELENOK and SELENOS, as well as (c–f) the ERS markers ATF4, ATF6, BIP and CHOP in imDCs after treatment with Tm, were assayed by RT-PCR (n = 3). * p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001 were compared with DMSO-treated imDCs. ERS, endoplasmic reticulum stress; imDCs, immature dendritic cells; SELENOK, selenoprotein K; SELENOS, selenoprotein S; ATF4, activating transcription factor 4; ATF6, activating transcription factor 6; BIP, binding immunoglobulin protein; CHOP, C/EBP homologous protein; Tm, tunicamycin; DMSO, dimethyl sulfoxide.
Figure 5.
ERS increased the protein levels of the ERS markers in imDCs. (a–c) The protein levels of SELENOK and SELENOS, as well as (a,d–g) the ERS markers ATF4, ATF6, BIP and CHOP in imDCs after treatment with Tm, were assayed by Western blotting, and the density values were calculated (n = 3). * p < 0.05, ** p < 0.01 and *** p < 0.001 were compared with DMSO-treated imDCs. ERS, endoplasmic reticulum stress; imDCs, immature dendritic cells; SELENOK, selenoprotein K; SELENOS, selenoprotein S; ATF4, activating transcription factor 4; ATF6, activating transcription factor 6; BIP, binding immunoglobulin protein; CHOP, C/EBP homologous protein; Tm, tunicamycin; DMSO, dimethyl sulfoxide.
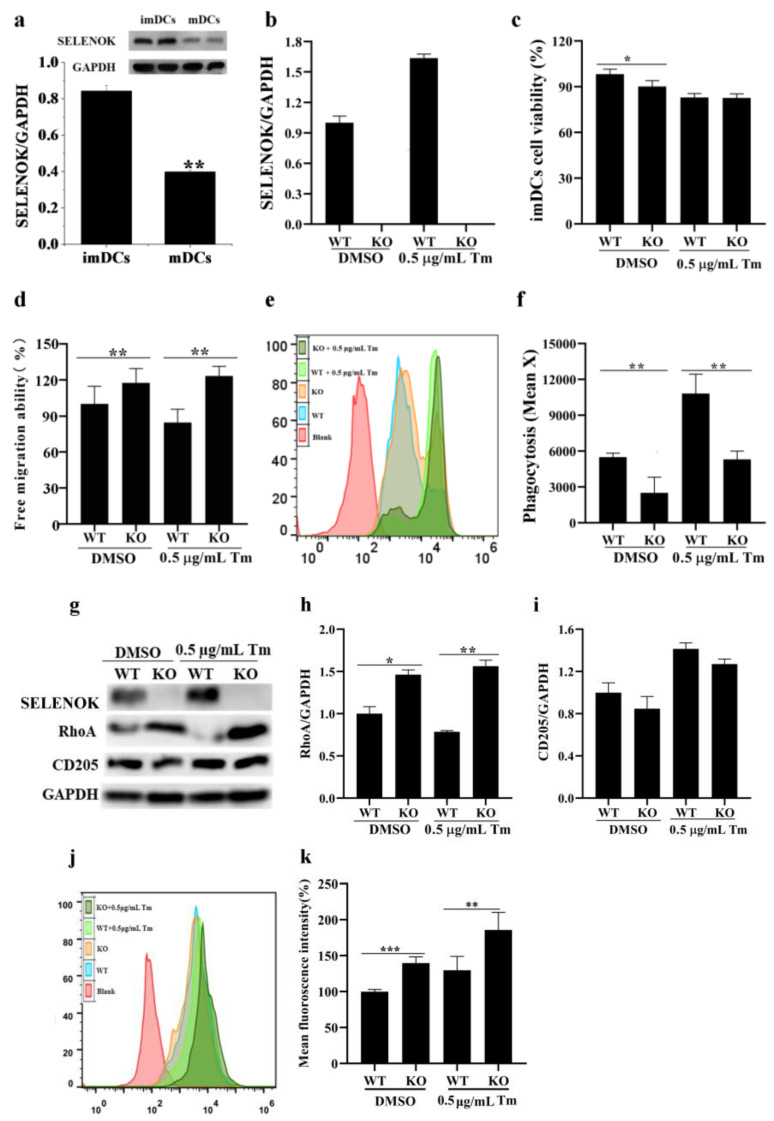
3.5. SELENOK Deficiency Induced Increased Migration and Decreased Phagocytosis in imDCs
The results in Figure 6a indicate that SELENOK protein levels were downregulated in mDCs when compared to imDCs, and SELENOK deficiency in imDCs of SELENOK KO mice was observed (Figure 6b,g). SELENOK-deficient imDCs showed reduced viability (Figure 6c), increased migratory capacity (Figure 6d) and reduced antigen phagocytosis (Figure 6e,f). Compared with Tm-treated WT imDCs, Tm-treated SELENOK-deficient imDCs showed unchanged cell viability (Figure 6c), increased migratory capacity (Figure 6d) and decreased antigen phagocytosis (Figure 6e,f); changes in migration capacity were RhoA-dependent (Figure 6d,g,h). Compared with WT and KO controls, Tm treatment showed increased phagocytosis and CD205. Still, the decrease in the phagocytosis induced by SELENOK deficiency did not lead to significant downregulation of CD205 (Figure 6e–g,i). Furthermore, SELENOK deficiency led to elevated Ca2+ levels with or without Tm treatment (Figure 6j,k).
Figure 6.
SELENOK deficiency induced increased migration and decreased phagocytosis in imDCs. (a) The protein levels of SELENOK in imDCs and mDCs were assayed by Western blotting, and the density values were calculated (n = 3). (b) The density value analysis of SELENOK KO validation in imDCs after treatment with Tm (n = 3). (c) Effect of SELENOK KO on the viability of imDCs after treatment with Tm (n = 3). (d) Effect of SELENOK KO on the migration of imDCs after treatment with Tm (n = 3). (e,f) Effect of SELENOK KO on the phagocytosis ability of imDCs after treatment with Tm (n = 3). (g–i) Effect of SELENOK KO on the protein levels of RhoA and CD205 in imDCs after treatment with Tm (n = 3). (j,k) Effect of SELENOK KO on the levels of Ca2+ in imDCs after treatment with Tm (n = 3). * p < 0.05, ** p < 0.01 and *** p < 0.001 were compared with DMSO- or Tm-treated WT imDCs. SELENOK, selenoprotein K; imDCs, immature dendritic cells; mDCs, mature dendritic cells; Tm, tunicamycin; RhoA, Ras homolog gene family member A; CD205, cluster of differentiation 205; DMSO, dimethyl sulfoxide; WT, wild-type; KO, knockout.
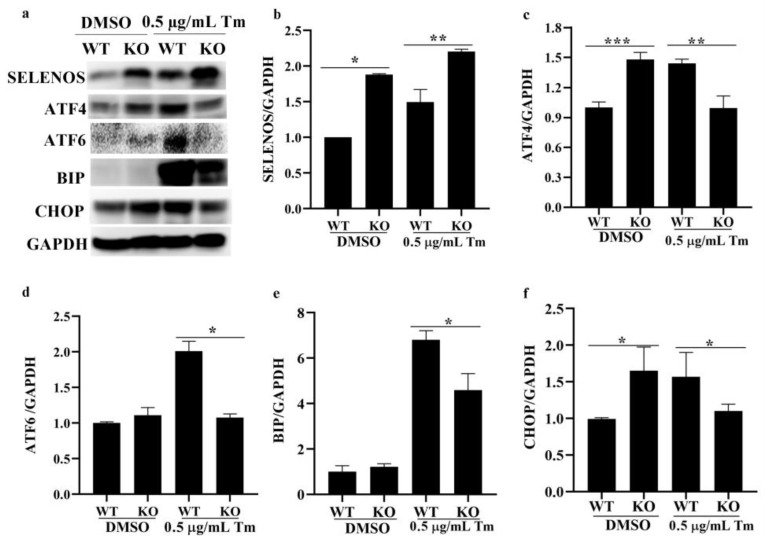
3.6. SELENOK Deficiency-Induced SELENOS Upregulation Was Involved in ERS
As shown in Figure 7, SELENOK deficiency upregulated the expression of SELENOS, ATF4 and CHOP in control imDCs. In Tm-treated imDCs, SELENOK deletion induced significant upregulation of SELENOS and significant downregulation of ATF4, ATF6, BIP and CHOP, indicating that SELENOK deficiency-induced SELENOS upregulation is involved in the regulation of ERS.
Figure 7.
SELENOK deficiency-induced SELENOS upregulation was involved in ERS in imDCs. (a–f) The protein levels of SELENOS and the ERS markers ATF4, ATF6, BIP and CHOP in WT and KO imDCs after treatment with Tm were assayed by Western blotting, and the density values were calculated (n = 3). * p < 0.05, ** p < 0.01 and *** p < 0.001 were compared with DMSO- or Tm-treated WT imDCs. SELENOK, selenoprotein K; SELENOS, selenoprotein S; ERS, endoplasmic reticulum stress; imDCs, immature dendritic cells; ATF4, activating transcription factor 4; ATF6, activating transcription factor 6; BIP, binding immunoglobulin protein; CHOP, C/EBP homologous protein; Tm, tunicamycin; DMSO, dimethyl sulfoxide; WT, wild-type; KO, knockout.
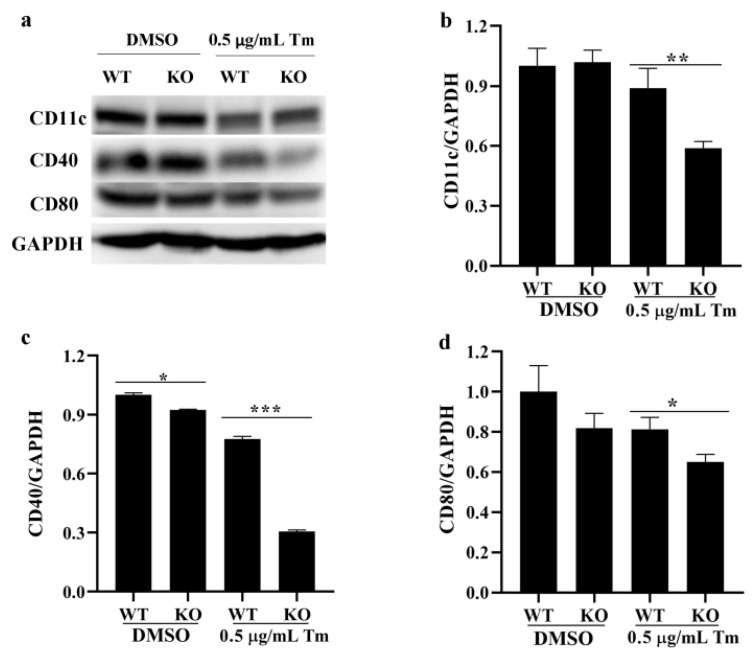
3.7. SELENOK Deficiency Downregulated Phenotypic Markers in imDCs
It was shown that SELENOK deficiency reduced the expression of CD40, and the expression of CD11c, CD40 and CD80 was further reduced in Tm-treated SELENOK-deficient imDCs compared to Tm-treated WT imDCs (Figure 8), suggesting that SELENOK deficiency can downregulate the phenotypic markers of imDCs and affect their immune function.
Figure 8.
SELENOK deficiency downregulated the phenotypic markers in imDCs. (a–d) The protein levels of CD11c, CD40 and CD80 in WT and KO imDCs after treatment with Tm were assayed by Western blotting, and the density values were calculated (n = 3). * p < 0.05, ** p < 0.01 and *** p < 0.001 were compared with DMSO- or Tm-treated WT imDCs. SELENOK, selenoprotein K; imDCs, immature dendritic cells; CD11c, cluster of differentiation 11c; CD40, cluster of differentiation 40; CD80, cluster of differentiation 80; Tm, tunicamycin; DMSO, dimethyl sulfoxide; WT, wild-type; KO, knockout.
4. Discussion
Our study showed that the ERS inducer, Tm, can downregulate the phenotypic markers in WT imDCs through ERS, leading to decreased migration and increased phagocytosis. Furthermore, SELENOK deficiency downregulated the phenotypic markers in imDCs by SELENOS-regulated ERS, which led to increased migration and decreased phagocytosis.
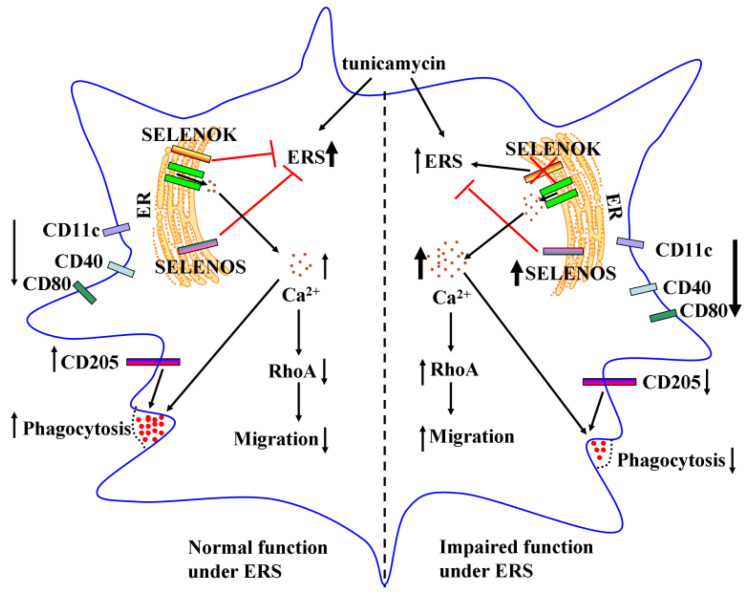
Several previous studies suggested that SELENOK plays an essential role in the immune function of T cells, neutrophils and macrophages [9,10,11]. However, how SELENOK regulates the function of DCs is still unknown. In the present study, SELENOK deficiency downregulated the phenotypic markers of imDCs under ERS conditions. It modulated RhoA-dependent migration and Ca2+/CD205-mediated phagocytosis (Figure 9), indicating that SELENOK is essential for imDCs’ migration and phagocytosis.
Figure 9.
Schematic diagram of the mechanism of imDCs dysfunction mediated by SELENOK. Under ERS conditions, SELENOK, SELENOS and ERS markers were upregulated, and the phenotypic markers were downregulated. The downregulation of RhoA inhibited migration and increased Ca2+ or CD205 enhanced phagocytosis in WT imDCs. In SELENOK KO imDCs, SELENOS upregulation attenuated ERS levels, decreased phenotypic markers, upregulated RhoA due to increased Ca2+, which promoted migration, increased Ca2+ or decreased CD205, which inhibited phagocytosis. imDCs, immature dendritic cells; SELENOK, selenoprotein K; ERS, endoplasmic reticulum stress; SELENOS, selenoprotein S; RhoA, Ras homolog gene family member A; CD205, cluster of differentiation 205; WT, wild-type; KO, knockout.
It was found that reduced SELENOK levels increased ERS-induced apoptosis in the HepG2 cells [22]. The deficiency of SELENOK increased West Nile virus-induced death in mice [9] and induced apoptosis in mice neurons [12]. Overexpression of SELENOK could decrease the viability and increase apoptosis in the BGC-823 cells, but did not affect the HEK-293 cells [23]. In this study, the cell viability of imDCs and mDCs was decreased after the ERS inducer treatment (Figure 1), and SELENOK and SELENOS were upregulated by Tm in imDCs (Figure 4 and Figure 5). In addition, SELENOK deficiency induced a decrease in imDCs cell viability, but had no changes after Tm treatment (Figure 6), which was related to the increase in SELENOS (Figure 7). It was shown that SELENOK [9,12,22] and SELENOS [24,25] have a protective role in ERS-induced cell death or apoptosis. In addition, SELENOK and SELENOS can form a complex involved in regulating ERS [26], which is consistent with the Tm-induced upregulation of SELENOK and SELENOS.
SELENOK deficiency inhibits the migration of human melanoma cells by reducing the Ca2+ flux [27]. Still, overexpression of SELENOK inhibits the migration of human gastric cancer cells by increasing Ca2+ levels [23]. It reduces the migration of choriocarcinoma cells by ERK, p38 MAPK and Akt pathways [28], suggesting that SELENOK has different effects on the migration of different tumours. For immune cells, the migration of T cells and neutrophils and the migration and phagocytosis of macrophages in SELENOK-deficient mice were decreased via deficiency in Ca2+ flux [9,29]. Furthermore, SELENOK overexpression enhances microglia migration and phagocytosis through an inositol triphosphate receptor (IP3R)-induced increase in Ca2+ levels [30]. In this study, we found that SELENOK deficiency induced an increase in cytosolic Ca2+ levels, resulting in increased migration and decreased phagocytosis. In addition, changes in the migration due to Ca2+ levels were RhoA-dependent, and Ca2+ or CD205 were associated with the regulation of phagocytosis in imDCs (Figure 2 and Figure 6). Our previous study also revealed that a decrease in RhoA led to a decrease in imDCs migration [6]. Previous reports have also shown that increased Ca2+-dependent RhoA could lead to increased migration in intestinal epithelial cells [31]. This study consistently showed that increased Ca2+ levels increased RhoA-mediated migration in SELENOK-deficient imDCs. Recently, human CD205 has not been predicted to bind to Ca2+, which contributes to oligosaccharide binding to other endocytic receptors [32,33], suggesting that Ca2+, CD205 or other endocytic receptors are jointly involved in the phagocytosis of imDCs. In addition, mechanical stiffness-induced PIEZO1-mediated increases in Ca2+ levels promoted the antitumor response in DCs [18]. In contrast, the increase in Ca2+ levels inhibited antigen phagocytosis of imDCs in this study, which is probably due to the developmental state of DCs and different treatment conditions.
An increase in misfolded proteins and reactive oxygen species or disruptions in Ca2+ homeostasis induce ERS, leading to unfolded protein reactions that include the IRE1, PERK and ATF6 pathways [12,34]. ERS plays an essential role in the immune response of T cells, B cells, macrophages and DCs [34]. Reports showed that IRE1/XBP1 and PERK/CHOP could affect maturation, antigen presentation, cytokine secretion and antitumor response in DCs [14,15,16,17,34,35,36,37,38,39]. In this study, the ERS markers ATF4, ATF6, BIP and CHOP were increased after treatment with Tm in WT imDCs, leading to decreased migration and enhanced phagocytosis (Figure 2, Figure 3, Figure 4 and Figure 5). In contrast, in SELENOK-deficient imDCs, SELENOS upregulation diminished ERS levels, leading to increased migration and decreased phagocytosis (Figure 6, Figure 7 and Figure 8). However, impaired ER function resulted in a decrease in the phenotypic markers of the WT or KO imDCs. Studies have shown that ERS in the tumour microenvironment impairs the antitumor immune response of DCs [36,37,38]. Combined with the results in this study, we suggest that SELENOK-deficient DCs in tumour tissues would attenuate their antitumor immune response. The human and mouse SELENOK proteins have high sequence homology [8], and we hypothesise that SELENOK performs the same role in human DCs, but further experimental verification is required.
5. Conclusions
In conclusion, this study found that SELENOK is required for imDC migration and phagocytosis. SELENOK has essential functions in tumour cell invasion and migration [23,27,28], and the migration and phagocytosis of immune cells [9,29,30]. SELENOK is associated with the prognosis of lung adenocarcinoma and melanoma [8,27,40] and can be a potential target for tumour immunotherapy. Previous studies have only focused on the effect of SELENOK on tumour cells or immune cell function. We anticipate that it is essential to investigate the effect of SELENOK on immune cell infiltration in the tumour microenvironment and on tumour cell–immune cell interactions. Importantly, SELENOK, in addition to regulating ERS, is a palmitoylation-modifying protease. It can promote DHHC6-catalysed palmitoylation of CD36, which is required for macrophage function [8,29,41,42]. In addition, SELENOK plays an important role in the differentiation of myogenic cells mediated by satellite cells [43], and the degradation of SELENOK is required for adipocyte differentiation [44]. The effects of SELENOK-mediated palmitoylation on DCs’ function and SELENOK deficiency on DCs’ differentiation and maturation are worthy of future investigation.
Acknowledgments
The authors would like to thank the Research Center for Basic Sciences of Medicine of Guizhou Medical University for providing large-scale instruments and equipment.
Author Contributions
Conceptualization, H.X., Y.W. and Y.J.; methodology, H.X., Y.W., J.D., X.Z. and Y.J.; validation, J.D. and Y.J.; data curation, H.X., Y.W., J.D. and X.Z.; writing—original draft preparation, H.X. and Y.W.; writing—review and editing, J.Z., Z.Z. and Y.J.; visualization, H.X. and Y.W.; supervision, J.D. and Y.J.; funding acquisition, J.D., J.Z., Z.Z. and Y.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Guizhou Medical University (protocol code: 1800238, date of approval: 8 March 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China (21867007, 22167008 and 12132006), Shenzhen Fundamental Research Program (JCYJ20200109105836705), Key Laboratory of Infectious Immune and Antibody Engineering of Guizhou Province (KY [2020]012), Guizhou Provincial Natural Science Foundation ([2019]1258), Opening fund of Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica (BCMM202002), Science and Technology Fund of Guizhou Provincial Health Commission (gzwkj2022-514) and the Guizhou Provincial Natural Science Foundation for High-Level Innovative Talents and Teams (2016-5676, 2015-4021).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Balan S., Saxena M., Bhardwaj N. Dendritic cell subsets and locations. Int. Rev. Cell Mol. Biol. 2019;348:1–68. doi: 10.1016/bs.ircmb.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Sun Z., Liu C., Pan T., Yao H., Li S. Selenium accelerates chicken dendritic cells differentiation and affects selenoproteins expression. Dev. Comp. Immunol. 2017;77:30–37. doi: 10.1016/j.dci.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Sun Z., Xu Z., Wang D., Yao H., Li S. Selenium deficiency inhibits differentiation and immune function and imbalances the Th1/Th2 of dendritic cells. Metallomics. 2018;10:759–767. doi: 10.1039/C8MT00039E. [DOI] [PubMed] [Google Scholar]
- 4.Xia H., Zhang L., Dai J., Liu X., Zhang X., Zeng Z., Jia Y. Effect of selenium and peroxynitrite on immune function of immature dendritic cells in humans. Med. Sci. Monit. 2021;27:e929004. doi: 10.12659/MSM.929004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia Y., Zhang L., Liu X., Zhang S., Dai J., Huang J., Chen J., Wang Y., Zhou J., Zeng Z. Selenium can regulate the differentiation and immune function of human dendritic cells. Biometals. 2021;34:1365–1379. doi: 10.1007/s10534-021-00347-4. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L., Xia H., Xia K., Liu X., Zhang X., Dai J., Zeng Z., Jia Y. Selenium regulation of the immune function of dendritic cells in mice through the ERK, Akt and RhoA/ROCK pathways. Biol. Trace Elem. Res. 2021;199:3360–3370. doi: 10.1007/s12011-020-02449-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X., Zhang L., Xia K., Dai J., Huang J., Wang Y., Zhu G., Hu Z., Zeng Z., Jia Y. Effects of dietary selenium on immune function of spleen in mice. J. Funct. Foods. 2022;89:104914. doi: 10.1016/j.jff.2021.104914. [DOI] [Google Scholar]
- 8.Marciel M.P., Hoffmann P.R. Molecular mechanisms by which selenoprotein K regulates immunity and cancer. Biol. Trace Elem. Res. 2019;192:60–68. doi: 10.1007/s12011-019-01774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma S., Hoffmann F.W., Kumar M., Huang Z., Roe K., Nguyen-Wu E., Hashimoto A.S., Hoffmann P.R. Selenoprotein K knockout mice exhibit deficient calcium flux in immune cells and impaired immune responses. J. Immunol. 2011;186:2127–2137. doi: 10.4049/jimmunol.1002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Z., Hoffmann F.W., Norton R.L., Hashimoto A.C., Hoffmann P.R. Selenoprotein K is a novel target of m-calpain, and cleavage is regulated by Toll-like receptor-induced calpastatin in macrophages. J. Biol. Chem. 2011;286:34830–34838. doi: 10.1074/jbc.M111.265520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norton R.L., Fredericks G.J., Huang Z., Fay J.D., Hoffmann F.W., Hoffmann P.R. Selenoprotein K regulation of palmitoylation and calpain cleavage of ASAP2 is required for efficient FcγR-mediated phagocytosis. J. Leukoc. Biol. 2017;101:439–448. doi: 10.1189/jlb.2A0316-156RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia S.Z., Xu X.W., Zhang Z.H., Chen C., Chen Y.B., Huang S.L., Liu Q., Hoffmann P.R., Song G.L. Selenoprotein K deficiency-induced apoptosis: A role for calpain and the ERS pathway. Redox Biol. 2021;47:102154. doi: 10.1016/j.redox.2021.102154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z.H., Chen C., Jia S.Z., Cao X.C., Liu M., Tian J., Hoffmann P.R., Xu H.X., Ni J.Z., Song G.L. Selenium restores synaptic deficits by modulating NMDA receptors and selenoprotein K in an Alzheimer’s disease model. Antioxid. Redox Signal. 2021;35:863–884. doi: 10.1089/ars.2019.7990. [DOI] [PubMed] [Google Scholar]
- 14.Salvagno C., Cubillos-Ruiz J.R. The impact of endoplasmic reticulum stress responses in dendritic cell immunobiology. Int. Rev. Cell Mol. Biol. 2019;349:153–176. doi: 10.1016/bs.ircmb.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Betts B.C., Locke F.L., Sagatys E.M., Pidala J., Walton K., Menges M., Reff J., Saha A., Djeu J.Y., Kiluk J.V., et al. Inhibition of human dendritic cell ER stress response reduces T cell alloreactivity yet spares donor anti-tumor immunity. Front. Immunol. 2018;9:2887. doi: 10.3389/fimmu.2018.02887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medel B., Costoya C., Fernandez D., Pereda C., Lladser A., Sauma D., Pacheco R., Iwawaki T., Salazar-Onfray F., Osorio F. IRE1α activation in bone marrow-derived dendritic cells modulates innate recognition of melanoma cells and favors CD8+T cell priming. Front. Immunol. 2019;9:3050. doi: 10.3389/fimmu.2018.03050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govindarajan S., Verheugen E., Venken K., Gaublomme D., Maelegheer M., Cloots E., Gysens F., De Geest B.G., Cheng T.Y., Moody D.B., et al. ER stress in antigen-presenting cells promotes NKT cell activation through endogenous neutral lipids. EMBO Rep. 2020;21:e48927. doi: 10.15252/embr.201948927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakraborty M., Chu K., Shrestha A., Revelo X.S., Zhang X., Gold M.J., Khan S., Lee M., Huang C., Akbari M., et al. Mechanical stiffness controls dendritic cell metabolism and function. Cell Rep. 2021;34:108609. doi: 10.1016/j.celrep.2020.108609. [DOI] [PubMed] [Google Scholar]
- 19.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann P.R., Höge S.C., Li P.A., Hoffmann F.W., Hashimoto A.C., Berry M.J. The selenoproteome exhibits widely varying, tissue-specific dependence on selenoprotein P for selenium supply. Nucleic Acids Res. 2007;35:3963–3973. doi: 10.1093/nar/gkm355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamieh A., Cartier D., Abid H., Calas A., Burel C., Bucharles C., Jehan C., Grumolato L., Landry M., Lerouge P., et al. Selenoprotein T is a novel OST subunit that regulates UPR signaling and hormone secretion. EMBO Rep. 2017;18:1935–1946. doi: 10.15252/embr.201643504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du S., Zhou J., Jia Y., Huang K. SelK is a novel ER stress-regulated protein and protects HepG2 cells from ER stress agent-induced apoptosis. Arch. Biochem. Biophys. 2010;502:137–143. doi: 10.1016/j.abb.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Ben S.B., Peng B., Wang G.C., Li C., Gu H.F., Jiang H., Meng X.L., Lee B.J., Chen C.L. Overexpression of selenoprotein SelK in BGC-823 cells inhibits cell adhesion and migration. Biochemistry. 2015;80:1344–1353. doi: 10.1134/S0006297915100168. [DOI] [PubMed] [Google Scholar]
- 24.Men L., Yu S., Yao J., Li Y., Ren D., Du J. Selenoprotein S protects against adipocyte death through mediation of the IRE1α-sXBP1 pathway. Biochem. Biophys. Res. Commun. 2018;503:2866–2871. doi: 10.1016/j.bbrc.2018.08.057. [DOI] [PubMed] [Google Scholar]
- 25.Men L., Yao J., Yu S., Li Y., Cui S., Jin S., Zhang G., Ren D., Du J. Selenoprotein S regulates adipogenesis through IRE1α-XBP1 pathway. J. Endocrinol. 2020;244:431–443. doi: 10.1530/JOE-19-0292. [DOI] [PubMed] [Google Scholar]
- 26.Lee J.H., Park K.J., Jang J.K., Jeon Y.H., Ko K.Y., Kwon J.H., Lee S.R., Kim I.Y. Selenoprotein S-dependent selenoprotein K binding to p97(VCP) protein is essential for endoplasmic reticulum-associated degradation. J. Biol. Chem. 2015;290:29941–29952. doi: 10.1074/jbc.M115.680215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marciel M.P., Khadka V.S., Deng Y., Kilicaslan P., Pham A., Bertino P., Lee K., Chen S., Glibetic N., Hoffmann F.W., et al. Selenoprotein K deficiency inhibits melanoma by reducing calcium flux required for tumor growth and metastasis. Oncotarget. 2018;9:13407–13422. doi: 10.18632/oncotarget.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M., Cheng W., Nie T., Lai H., Hu X., Luo J., Li F., Li H. Selenoprotein K mediates the proliferation, migration, and invasion of human choriocarcinoma cells by negatively regulating human chorionic gonadotropin expression via ERK, p38 MAPK, and Akt signaling pathway. Biol. Trace Elem. Res. 2018;184:47–59. doi: 10.1007/s12011-017-1155-3. [DOI] [PubMed] [Google Scholar]
- 29.Meiler S., Baumer Y., Huang Z., Hoffmann F.W., Fredericks G.J., Rose A.H., Norton R.L., Hoffmann P.R., Boisvert W.A. Selenoprotein K is required for palmitoylation of CD36 in macrophages: Implications in foam cell formation and atherogenesis. J. Leukoc. Biol. 2013;93:771–780. doi: 10.1189/jlb.1212647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng X.L., Chen C.L., Liu Y.Y., Su S.J., Gou J.M., Huan F.N., Wang D., Liu H.S., Ben S.B., Lu J. Selenoprotein SELENOK enhances the migration and phagocytosis of microglial cells by increasing the cytosolic free Ca2+ level resulted from the up-regulation of IP3R. Neuroscience. 2019;406:38–49. doi: 10.1016/j.neuroscience.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 31.Rao J.N., Li L., Golovina V.A., Platoshyn O., Strauch E.D., Yuan J.X., Wang J.Y. Ca2+-RhoA signaling pathway required for polyamine-dependent intestinal epithelial cell migration. Am. J. Physiol. Cell Physiol. 2001;280:C993–C1007. doi: 10.1152/ajpcell.2001.280.4.C993. [DOI] [PubMed] [Google Scholar]
- 32.Gully B.S., Venugopal H., Fulcher A.J., Fu Z., Li J., Deuss F.A., Llerena C., Heath W.R., Lahoud M.H., Caminschi I., et al. The cryo-EM structure of the endocytic receptor DEC-205. J. Biol. Chem. 2021;296:100127. doi: 10.1074/jbc.RA120.016451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor M.E. Evolution of a family of receptors containing multiple C-type carbohydrate-recognition domains. Glycobiology. 1997;7:v–viii. doi: 10.1093/glycob/7.3.323. [DOI] [PubMed] [Google Scholar]
- 34.So J.S. Roles of endoplasmic reticulum stress in immune responses. Mol. Cells. 2018;41:705–716. doi: 10.14348/molcells.2019.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodall J.C., Wu C., Zhang Y., McNeill L., Ellis L., Saudek V., Gaston J.S. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc. Natl. Acad. Sci. USA. 2010;107:17698–17703. doi: 10.1073/pnas.1011736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahadevan N.R., Anufreichik V., Rodvold J.J., Chiu K.T., Sepulveda H., Zanetti M. Cell-extrinsic effects of tumor ER stress imprint myeloid dendritic cells and impair CD8+ T cell priming. PLoS ONE. 2012;7:e51845. doi: 10.1371/journal.pone.0051845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cubillos-Ruiz J.R., Silberman P.C., Rutkowski M.R., Chopra S., Perales-Puchalt A., Song M., Zhang S., Bettigole S.E., Gupta D., Holcomb K., et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161:1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garris C.S., Pittet M.J. ER stress in dendritic cells promotes cancer. Cell. 2015;161:1492–1493. doi: 10.1016/j.cell.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Márquez S., Fernández J.J., Terán-Cabanillas E., Herrero C., Alonso S., Azogil A., Montero O., Iwawaki T., Cubillos-Ruiz J.R., Fernández N., et al. Endoplasmic reticulum stress sensor IRE1α enhances IL-23 expression by human dendritic cells. Front. Immunol. 2017;8:639. doi: 10.3389/fimmu.2017.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia Y., Dai J., Zeng Z. Potential relationship between the selenoproteome and cancer. Mol. Clin. Oncol. 2020;13:83. doi: 10.3892/mco.2020.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fredericks G.J., Hoffmann F.W., Hondal R.J., Rozovsky S., Urschitz J., Hoffmann P.R. Selenoprotein K increases efficiency of DHHC6 catalyzed protein palmitoylation by stabilizing the Acyl-DHHC6 intermediate. Antioxidants. 2017;7:4. doi: 10.3390/antiox7010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fredericks G.J., Hoffmann P.R. Selenoprotein K and protein palmitoylation. Antioxid. Redox Signal. 2015;23:854–862. doi: 10.1089/ars.2015.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S., Zhao X., Liu Q., Wang Y., Li S., Xu S. Selenoprotein K protects skeletal muscle from damage and is required for satellite cells-mediated myogenic differentiation. Redox Biol. 2022;50:102255. doi: 10.1016/j.redox.2022.102255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee J.H., Jang J.K., Ko K.Y., Jin Y., Ham M., Kang H., Kim I.Y. Degradation of selenoprotein S and selenoprotein K through PPARγ-mediated ubiquitination is required for adipocyte differentiation. Cell Death. Differ. 2019;26:1007–1023. doi: 10.1038/s41418-018-0180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.