Abstract
The spread of microorganisms causing health-care associated infection (HAI) is contributed to by their intrinsic tolerance to a variety of biocides, used as antiseptics or disinfectants. The natural monomeric stilbenoid resveratrol (RV) is able to modulate the susceptibility to the chlorhexidine digluconate (CHX) biocide in Acinetobacter baumannii. In this study, a panel of reference strains and clinical isolates of Gram-negative bacteria, Gram-positive bacteria and yeasts were analyzed for susceptibility to CHX and benzalkonium chloride (BZK) and found to be tolerant to one or both biocides. The carbonyl cyanide m-chlorophenylhydrazine protonophore (CCCP) efflux pump inhibitor reduced the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of CHX and BZK in the majority of strains. RV reduced dose-dependently MIC and MBC of CHX and BZK biocides when used as single agents or in combination in all analyzed strains, but not CHX MIC and MBC in Pseudomonas aeruginosa, Candida albicans, Klebsiella pneumoniae, Stenotrophomonas maltophilia and Burkholderia spp. strains. In conclusion, RV reverts tolerance and restores susceptibility to CHX and BZK in the majority of microorganisms responsible for HAI. These results indicates that the combination of RV, CHX and BZK may represent a useful strategy to maintain susceptibility to biocides in several nosocomial pathogens.
Keywords: resveratrol, chlorhexidine, benzalkonium, tolerance, Gram-negative bacteria, Gram-positive bacteria, yeasts
1. Introduction
Multi-drug resistant (MDR) bacterial and yeast pathogens have been recognized as a common cause of health care-associated infections. Among the most frightening of the emerging pathogens is a group of six nosocomial pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.) named with the acronym ‘ESKAPE’, because they are capable of ‘escaping’ the biocidal action of antibiotics classified as highly important for human medicine [1,2,3,4,5]. The ESKAPE bacteria are a serious health concern, as they increase the frequency of treatment failures and severity of human infections by adapting to altered environmental conditions and by acquiring resistance determinants [1,2,3,4,5]. Moreover, Stenotrophomonas maltophilia and Burkholderia spp. are emerging pathogens in cystic fibrosis patients [6]. In addition, severe invasive infections caused by Candida spp. that are resistant to antifungal drugs have been increasingly described [7].
The persistence of antimicrobial resistance in MDR pathogens is promoted by a co-selection of antimicrobial resistance with a tolerance to several of the biocides used as antiseptics and disinfectants, such as the bisphenol triclosan (TRI), the quaternary ammonium compounds, benzalkonium chloride (BZK), dequalinium chloride (DQ), cetrimide (CT) and the biguanide chlorhexidine (CHX) [8,9,10]. CHX is a microbicidal agent, which is currently used for hand hygiene, skin antisepsis, oral care and patient washing [9]. BZK has been widely used as a disinfectant in hospitals, or as an antiseptic in antimicrobial soaps [10]. Tolerance to CHX and BZK is emerging in major nosocomial pathogens [9,10,11,12,13,14,15,16].
A reduced susceptibility to biocides in A. baumannii, K. pneumoniae and other pathogens has been correlated with the activation of different efflux pump (EP) systems [13,16,17,18,19,20]. In particular, the inhibition of the AdeB RND superfamily and AceI PACE superfamily EP systems has been demonstrated to restore susceptibility to CHX and BZK in A. baumannii [19,20]. In addition, it has been demonstrated that the natural monomeric stilbenoid resveratrol (RV) [21], which has been demonstrated to possess antimicrobial activity against a wide range of bacterial and fungal species [22,23], is able to inhibit EP expression and restore susceptibility to CHX and BZK biocides in A. baumannii [19,24].
The objectives of the present study were to: (i) analyze the susceptibility to BZK and CHX biocides in a panel of reference strains and clinical isolates of Gram-negative bacteria, Gram-positive bacteria and yeasts; (ii) analyze whether the natural compound RV at non-toxic concentrations may modulate and restore susceptibility to CHX and BZK in the above pathogens.
2. Results and Discussion
2.1. Antimicrobial Activity of CHX and BZK against a Panel of Reference Strains and Clinical Isolates of Gram-Negative Bacteria, Gram-Positive Bacteria and Yeasts
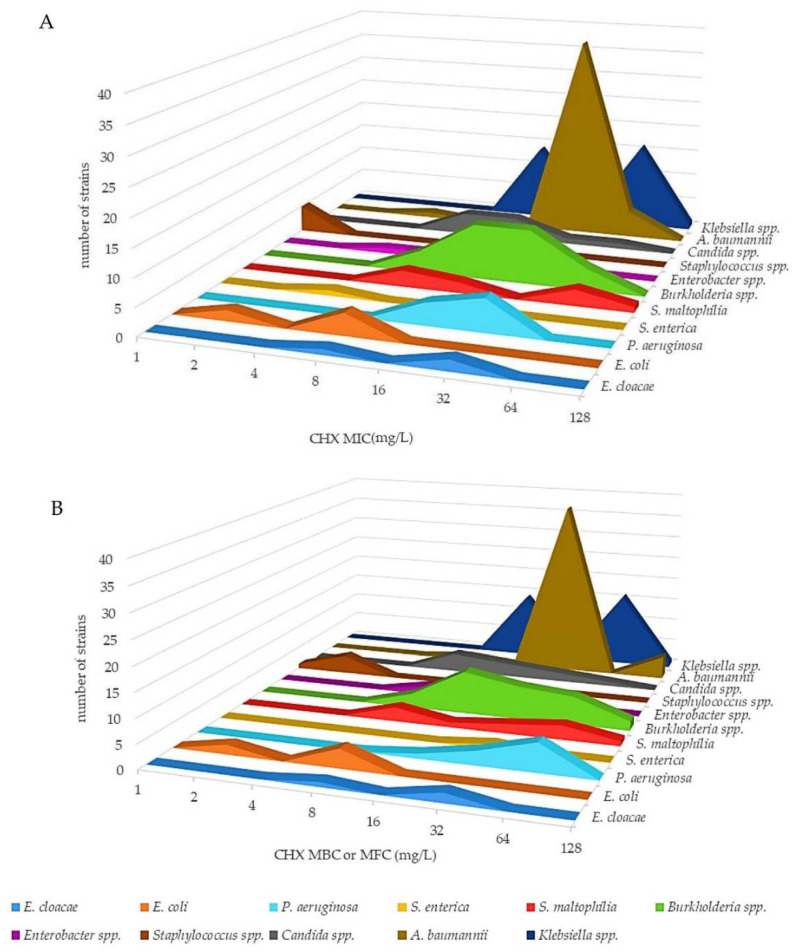
The antimicrobial activity of CHX and BZK was assessed by broth microdilution assay against 151 reference strains and clinical isolates of Gram-negative bacteria, Gram-positive bacteria and yeasts, which included the ESKAPE bacteria, S. maltophilia, Burkholderia spp. and Candida spp. (Figure 1 and Figure 2; Table S1, Supplementary Materials). A. baumannii, K. pneumoniae, K. aerogenes, P. aeruginosa, E. coli EC-Na1-Na4, S. maltophilia, Burkholderia spp., Enterococcus spp. and Candida spp. strains showed CHX minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) or minimum fungicidal concentration (MFC), in the case of Candida spp., values ranging from 4–64 mg/L and 4–128 mg/L, respectively, and were considered tolerant to CHX (Figure 1; Table S1, Supplementary Materials). Instead, E. coli ATCC 25922, E. coli ATCC 35218, S. aureus and S. epidermidis strains showed CHX MIC and MBC values of 1–2 mg/L and were considered susceptible (Figure 1; Table S1, Supplementary Materials). The median MIC and MBC values of CHX were significantly higher in A. baumannii, E. cloacae, Klebsiella spp., S. maltophilia and P. aeruginosa strains compared with those of the susceptible strains (p < 0.05, p < 0.01 and p < 0.001, respectively).
Figure 1.
Three-dimensional aerogram of CHX MICs (mg/L) (A) and MBCs (MFCs in the case of Candida) (mg/L) (B) of reference strains and clinical isolates of Gram-negative bacteria, Gram-positive bacteria and yeasts.
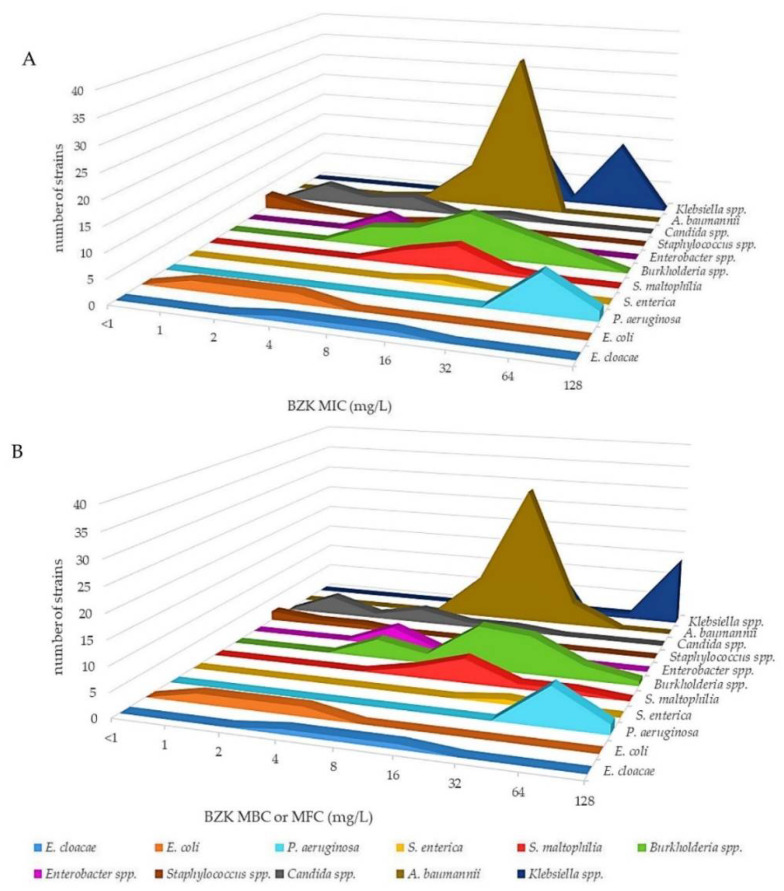
Figure 2.
Three-dimensional aerogram of BZK MICs (mg/L) (A) and MBCs (MFCs in the case of Candida) (mg/L) (B) of reference strains and clinical isolates of Gram-negative bacteria, Gram-positive bacteria and yeasts.
In addition, the A. baumannii, K. pneumoniae, K. aerogenes, P. aeruginosa, E. coli ATCC 25922, E. coli ATCC 35218, S. maltophilia, Burkholderia spp., Enterococcus spp., S. aureus ATCC 43300 and Candida spp. strains showed both MIC and MBC (MFC in the case of Candida spp.) values for BZK within the range of 4–64 mg/L and were considered tolerant (Figure 2; Table S1, Supplementary Materials). Instead, E. coli EC-Na1-Na4, S. aureus ATCC 25923, S. aureus ATCC 29213, S. epidermidis ATCC 12282, C. krusei 81667 and C. tropicalis 61220 showed BZK MIC and MBC (MFC) values of 1–2 mg/L and were considered susceptible (Figure 2; Table S1, Supplementary Materials). The median MIC and MBC values of BZK were significantly higher in the P. aeruginosa strains compared with those of susceptible strains (p < 0.05 and p < 0.001, respectively). The above overall data are in agreement with previous studies showing that microbial pathogens responsible for health care-associated infection, in particular Gram-negative bacteria such as K. pneumoniae and P. aeruginosa, are highly tolerant to CHX and BZK biocides [11,12,13,14,15].
2.2. Effect of Carbonyl Cyanide M-Chlorophenylhydrazine Protonophore (CCCP) EP Inhibitor on CHX and BZK MIC and MBC against Gram-Negative Bacteria, Gram-Positive Bacteria and Yeasts
To evaluate whether the tolerance to CHX and BZK was mediated by the activation of EP, as demonstrated in the Gram-negative and Gram-positive bacteria [13,14,15,16,17,18,19], we analyzed the effect of the EP inhibitor CCCP on CHX and BZK susceptibility. As shown in Table 1, CCCP reduced dose-dependently the CHX MIC and MBC or MFC in A. baumannii, Burkholderia spp., K. pneumoniae, K. aerogenes, Enterobacter spp., Enterococcus spp., S. maltophilia, S. enterica and C. parapsilosis with a decrease between 4- and 64-fold. The inhibitory effect of CCCP was less evident in the P. aeruginosa and Candida spp. strains, in which the MIC and MBC (MCF) of CHX were reduced only by one-fold or not affected (Table 1). In addition, CCCP reduced the MIC and MBC (MFC in the case of Candida spp.) of BZK by one- to four-fold in A. baumannii, Candida spp., B. gladioli, B. dolosa, S. enterica and S. maltophilia strains, while it had no effect on the BZK MIC and MBC in other Burkholderia species, K. pneumoniae and P. aeruginosa strains (Table 1). In accordance with the previous data [19], the reduction in MIC and MBC of CHX and BZK due to CCCP was only four- and two-fold, respectively, in A. baumannii ATCC 19606 carrying the deletion of the adeB EP gene, consistent with the role of AdeB in CHX and BZK extrusion (Table 1). The data shown herein indicate that tolerance to CHX and BZK is mediated by activation of the EPs and are in agreement with previous publications showing that CHX and BZK tolerance in K. pneumoniae and A. baumannii clinical isolates is mediated by RND superfamily EP activation [13,16], and that CHX tolerance in P. aeruginosa clinical isolates is mediated by an increased expression of the mexA, mexC, mexE and mexX EP genes, and a decreased expression of the oprD gene [15].
Table 1.
Effect of CCCP (mg/L) efflux pump inhibitor on CHX and BZK MIC (mg/L) and MBC (MFC in the case of Candida spp.) (mg/L) against Gram-negative bacteria, Gram-positive bacteria and yeasts.
| Strain | CHX MIC (MBC or MFC) | BZK MIC (MBC or MFC) | ||||||
|---|---|---|---|---|---|---|---|---|
| CCCP | CCCP | |||||||
| 0 | 1 | 2 | 4 | 0 | 1 | 2 | 4 | |
| A. baumannii ATCC 19606 | 32 (32) | 8 (8) | 4 (4) | 1 (1) | 16 (32) | 8 (16) | 8 (16) | 8 (16) |
| A. baumannii ∆adeB ATCC 19606 | 4 (4) | 2 (2) | 2 (2) | 2 (2) | 8 (8) | 4 (4) | 4 (4) | 2 (2) |
| A. baumannii ACICU | 64 (128) | 32 (64) | 8 (16) | 8 (16) | 16 (16) | 16 (16) | 16 (16) | 16 (16) |
| A. baumannii AYE | 32 (32) | 8 (8) | 4 (4) | 1 (1) | 16 (32) | 8 (16) | 8 (16) | 8 (16) |
| B. cenocepacia LMG 16654 | 64 (64) | 64 (64) | 32 (32) | 16 (16) | 64 (128) | 64 (128) | 64 (128) | 64 (128) |
| B.cepacia LMG 2161 | 32 (64) | 32 (32) | 8 (8) | 4 (4) | 32 (32) | 32 (32) | 16 (32) | 16 (32) |
| B. vietnamiensis LMG 22486 | 32 (32) | 32 (32) | 8 (8) | 4 (4) | 32 (32) | 32 (32) | 16 (32) | 16 (32) |
| B. gladioli LMG 2121 | 16 (16) | 16 (32) | 8 (16) | 4 (4) | 4 (4) | 4 (4) | 1 (1) | 1 (1) |
| B. dolosa LMG 21443 | 16 (32) | 16 (32) | 8 (16) | 4 (4) | 8 (16) | 8 (16) | 4 (8) | 4 (8) |
| B. multivorans LMG 16665 | 64 (64) | 32 (32) | 16 (16) | 8 (8) | 32 (64) | 32 (32) | 16 (16) | 8 (8) |
| E. cloacae ATCC13047 | 8 (8) | 4 (8) | 2 (4) | 1 (2) | 32 (64) | 32 (64) | 32 (64) | 32 (64) |
| E. cloacae EnC-Na-1 | 32 (32) | 16 (16) | 4 (8) | 2 (4) | 4 (4) | 4 (8) | 4 (8) | 4 (8) |
| K. aerogenes ATCC 13048 | 32 (32) | 8 (16) | 4 (4) | 2 (2) | 32 (32) | 16 (16) | 16 (16) | 16 (16) |
| K. pneumoniae ATCC 700603 | 128 (128) | 32 (32) | 8 (8) | 4 (4) | 32 (64) | 32 (64) | 32 (64) | 32 (64) |
| K. pneumoniae KP-Mo-7 | 64 (64) | 16 (16) | 4 (4) | 1 (1) | 32 (32) | 32 (32) | 32 (32) | 32 (32) |
| K. pneumoniae KP-Mo-6 | 64 (64) | 16 (16) | 4 (4) | 1 (1) | 32 (32) | 32 (32) | 32 (32) | 32 (32) |
| P. aeruginosa RP73 | 32 (32) | 32 (32) | 16 (16) | 16 (16) | 64 (128) | 64 (64) | 64 (64) | 64 (64) |
| P. aeruginosa PAO1 | 16 (16) | 16 (16) | 8 (8) | 8 (8) | 64 (128) | 64 (128) | 64 (128) | 64 (64) |
| P. aeruginosa PA14 | 16 (32) | 16 (16) | 8 (8) | 8 (8) | 64 (64) | 64 (64) | 64 (64) | 64 (64) |
| P. aeruginosa PA-Na-1 | 32 (64) | 32 (32) | 16 (16) | 16 (16) | 64 (128) | 64 (64) | 64 (64) | 64 (64) |
| S. enterica ATCC 13076 | 4 (4) | 1 (1) | 0.5 (1) | 0.5 (1) | 16 (16) | 16 (16) | 16 (16) | 4 (4) |
| S. maltophilia K279 | 32 (32) | 8 (8) | 2 (2) | 2 (2) | 16 (16) | 16 (16) | 16 (16) | 16 (16) |
| S. maltophilia LMG 10853 | 8 (8) | 4 (4) | 2 (2) | 0.5 (1) | 8 (8) | 4 (8) | 4 (8) | 4 (8) |
| S. maltophilia OBGTC20 | 64 (64) | 32 (32) | 8 (8) | 2 (2) | 32 (32) | 16 (16) | 16 (16) | 16 (16) |
| E. faecalis ATCC 29212 | 64 (64) | 16 (16) | 16 (16) | 8 (8) | 4 (8) | 4 (8) | 4 (8) | 4 (8) |
| E. faecium ATCC 6057 | 8 (8) | 4 (4) | 2 (2) | 0.5 (1) | 4 (8) | 4 (8) | 4 (8) | 2 (4) |
| S. aureus ATCC 43300 | 1 (2) | <1 (<1) | <1 (<1) | <1 (<1) | 8 (8) | <1 (<1) | <1 (<1) | <1 (<1) |
| C. albicans ATCC 10231 | 16 (16) | 16 (16) | 16 (16) | 8 (8) | 2 (2) | <1 (<1) | <1 (<1) | <1 (<1) |
| C. albicans 62033 | 16 (16) | 16 (16) | 16 (16) | 8 (8) | 4 (4) | 4 (4) | 1 (1) | 1 (1) |
| C. parapsilosis 4609 | 32 (32) | 16 (16) | 16 (16) | 4 (4) | 2 (2) | <1 (<1) | <1 (<1) | <1 (<1) |
| C. krusei 81667 | 8 (8) | 8 (8) | 8 (8) | 8 (8) | 1 (1) | <1 (<1) | <1 (<1) | <1 (<1) |
| C. glabrata 61112 | 16 (16) | 16 (16) | 16 (16) | 8 (8) | 2 (2) | <1 (<1) | <1 (<1) | <1 (<1) |
| C. tropicalis 61220 | 8 (16) | 8 (8) | 8 (8) | 8 (8) | 1 (1) | <1 (<1) | <1 (<1) | <1 (<1) |
The numbers outside and within the brackets indicate MIC and MBC or MCF values, respectively, and are expressed in mg/L. The numbers 0, 1, 2, and 4 indicate CCCP concentrations and are expressed in mg/L.
2.3. Effect of RV on CHX and BZK MIC and MBC (or MFC) against Gram-Negative Bacteria, Gram-Positive Bacteria and Yeasts
We next analyzed if the natural monomeric stilbenoid RV [21], which was demonstrated to regulate EPs expression and counteract the tolerance to CHX and BZK in A. baumannii [19,24], may restore susceptibility in the Gram-negative bacteria, Gram-positive bacteria and yeasts included in the study. Our previous data demonstrated that RV at >1024 mg/L has no antimicrobial activity against A. baumannii ATCC19606 [19]. In agreement with this, the RV showed no antimicrobial activity against all of the Gram-negative bacteria, Gram-positive bacteria and yeasts included in the study with MIC values > 1024 mg/L (Table S1, Supplementary Materials). On the other hand, the data shown herein are partly in agreement with previous studies showing that RV at high concentrations has antimicrobial activity against S. aureus, E. faecalis, E. faecium, E. coli and Candida spp. strains [22,23]. The discrepancies between our data and previous studies [22,23] may depend on different strains and/or different experimental conditions.
We then analyze the effect of RV in combination with CHX or BZK. The objectives of our experiments were to identify which RV concentrations were able to revert tolerance and restore susceptibility to the CHX and BZK biocides.
As shown in Table 2, RV from 32 to 256 mg/L decreased dose-dependently the MIC and MBC (MCF for Candida spp.) of CHX in 33 selected strains among the Gram-negative bacteria, Gram-positive bacteria and yeasts and restored CHX susceptibility in most of the strains, but not in K. pneumoniae ATCC 700603, all of the P. aeruginosa or Candida spp. strains. Interestingly, a positive correlation was found between the RV effect on CHX MIC and the MBC and CCCP effect on CHX MIC and MBC, P. aeruginosa and Candida spp. strains showing high CHX MIC and MBC values after treatment with RV or CCCP (Table 1 and Table 2; Figure S1, Supplementary Materials) (r = 0.893, p < 0.001). The above data indicate that the RV effect on the inhibition of CHX tolerance in the Gram negative-bacteria, Gram-positive bacteria and yeasts is mediated by the inhibition of EP activity.
Table 2.
Effect of RV (mg/L) on CHX MIC (mg/L) and MBC (MFC in the case of Candida spp.) (mg/L) against Gram-negative bacteria, Gram-positive bacteria and yeasts.
| Strain | CHX MIC (MBC or MFC) | ||||
|---|---|---|---|---|---|
| RV | |||||
| 0 | 32 | 64 | 128 | 256 | |
| A. baumannii ATCC 19606 | 32 (32) | 8 (16) | 4 (8) | 0.5 (2) | 0.125 (0.125) |
| A. baumannii ∆adeB ATCC 19606 | 4 (4) | 4 (4) | 4 (4) | 0.5 (0.5) | 0.125 (0.125) |
| A. baumannii ACICU | 64 (128) | 16 (32) | 4 (8) | 0.5 (0.5) | 0.5 (0.5) |
| A. baumannii AYE | 32 (32) | 4 (8) | 2 (4) | 0.5 (2) | 0.125 (0.125) |
| B. cenocepacia LMG 16654 | 64 (64) | 32 (32) | 32 (32) | 4 (4) | 2 (2) |
| B. cepacia LMG 2161 | 32 (32) | 16 (16) | 16 (16) | 2 (4) | 2 (2) |
| B. vietnamiensis LMG 22486 | 32 (32) | 32 (32) | 16 (16) | 2 (2) | 0.5 (0.5) |
| B. gladioli LMG 2121 | 16 (16) | 4 (4) | 4 (4) | 4 (4) | 1 (1) |
| B. dolosa LMG 21443 | 16 (16) | 8 (8) | 8 (4) | 4 (4) | 2 (2) |
| B. multivorans LMG 16665 | 64 (64) | 64 (64) | 32 (32) | 4 (8) | 2 (2) |
| E. cloacae ATCC 13047 | 8 (8) | 4 (8) | 2 (2) | 0.5 (1) | 0.5 (0.5) |
| E. cloacae EnC-Na-1 | 32 (32) | 8 (8) | 4 (8) | 4 (4) | 2 (2) |
| K. aerogenes ATCC 13048 | 32 (32) | 8 (16) | 8 (16) | 2 (2) | 1 (1) |
| K. pneumoniae ATCC 700603 | 128 (128) | 64 (64) | 32 (32) | 8 (8) | 4 (4) |
| K. pneumoniae KP-Mo-7 | 64 (64) | 32 (32) | 32 (32) | 4 (8) | 2 (4) |
| K. pneumoniae KP-Mo-6 | 64 (64) | 16 (32) | 16 (16) | 8 (16) | 2 (2) |
| P. aeruginosa RP73 | 32 (32) | 8 (16) | 8 (16) | 8 (16) | 4 (8) |
| P. aeruginosa PAO1 | 16 (16) | 4 (8) | 4 (8) | 4 (4) | 4 (4) |
| P. aeruginosa PA14 | 16 (32) | 16 (32) | 8 (32) | 4 (8) | 4 (8) |
| P. aeruginosa PA-Na-1 | 32 (64) | 8 (16) | 4 (16) | 4 (8) | 4 (8) |
| S. enterica ATCC 13076 | 4 (4) | 1 (1) | 0.5 (1) | 0.5 (1) | 0.5 (1) |
| S. maltophilia K279 | 64 (128) | 32 (32) | 16 (16) | 4 (8) | 0.5 (1) |
| S. maltophilia LMG 10853 | 8 (8) | 2 (2) | 0.5 (1) | 0.5 (1) | 0.5 (0.5) |
| S. maltophilia OBGTC20 | 64 (64) | 16 (16) | 16 (16) | 4 (4) | 1 (1) |
| E. faecalis ATCC 29212 | 32 (32) | 16 (16) | 4 (8) | 2 (2) | 0.5 (0.5) |
| E. faecium ATCC 6057 | 8 (8) | 8 (8) | 4 (4) | 2 (2) | 0.5 (0.5) |
| S. aureus ATCC 43300 | 1 (2) | 0.25 (0.25) | 0.25 (0.25) | 0.125 (0.25) | 0.125 (0.25) |
| C. albicans ATCC 10231 | 16 (32) | 16 (32) | 16 (16) | 8 (8) | 8 (8) |
| C. albicans 62033 | 16 (16) | 16 (16) | 16 (16) | 16 (16) | 8 (8) |
| C. parapsilosis 4609 | 64 (64) | 32 (64) | 16 (32) | 16 (32) | 8 (8) |
| C. krusei 81667 | 8 (8) | 4 (8) | 4 (4) | 2 (4) | 2 (4) |
| C. glabrata 61112 | 16 (16) | 8 (16) | 8 (16) | 8 (8) | 8 (8) |
| C. tropicalis 61220 | 8 (16) | 8 (8) | 8 (8) | 4 (4) | 4 (4) |
The numbers outside and within the brackets indicate MIC and MBC or MCF values, respectively, and are expressed in mg/L. The numbers 0, 32, 64, 128 and 256 indicate RV concentrations and are expressed in mg/L.
Furthermore, increasing the doses of RV up to 128 mg/L decreased dose-dependently the BZK MIC and MBC (MCF for Candida spp.) and restored the BZK susceptibility in most of the strains, but not B. cenocepacia LMG16654, B. dolosa LMG21443, B. multivorans LMG16654, E. cloacae ATCC 13047, K. pneumoniae ATCC 700603, S. maltophilia K279 or all of the P. aeruginosa strains (Table 3). A positive correlation was also found between the RV effect on BZK MIC and the MBC and CCCP effect on BZK MIC and MBC (Table 1 and Table 3; Figure S2, Supplementary Materials) (r = 0.775, p < 0.01), thus suggesting that the inhibition of the EPs activation might be involved in the inhibitory effect of RV on tolerance to BZK. This is in agreement with a previous publication that showed that RV inhibited basal and CHX-induced expression of the AdeB RND superfamily and the AceI PACE superfamily EP systems in A. baumannii [19].
Table 3.
Effect of RV (mg/L) on BZK MIC (mg/L) and MBC (MFC in the case of Candida spp.) (mg/L) against Gram-negative bacteria, Gram-positive bacteria and yeasts.
| Strain | BZK MIC (MBC or MFC) | |||
|---|---|---|---|---|
| RV | ||||
| 0 | 32 | 64 | 128 | |
| A. baumannii ATCC 19606 | 16 (32) | 8 (16) | 4 (16) | 0.5 (1) |
| A. baumannii ∆adeB ATCC 19606 | 8 (8) | 2 (4) | 1 (1) | 0.25 (0.5) |
| A. baumannii ACICU | 16 (32) | 2 (4) | 1 (2) | 0.5 (1) |
| A. baumannii AYE | 8 (8) | 1 (2) | 1 (1) | 0.25 (1) |
| B. cenocepacia LMG 16654 | 64 (64) | 32 (32) | 32 (32) | 4 (4) |
| B.cepacia LMG 2161 | 32 (32) | 16 (16) | 16 (16) | 2 (4) |
| B. vietnamiensis LMG 22486 | 32 (32) | 32 (32) | 16 (16) | 2 (2) |
| B. gladioli LMG2121 | 4 (4) | 2 (2) | 0.5 (1) | 0.5 (1) |
| B. dolosa LMG21443 | 32 (32) | 16 (16) | 16 (16) | 8 (8) |
| B. multivorans LMG 16665 | 64 (64) | 64 (64) | 32 (32) | 4 (4) |
| E. cloacae ATCC 13047 | 32 (32) | 16 (32) | 16 (16) | 16 (16) |
| E. cloacae EnC-Na-1 | 4 (4) | 4 (4) | 2 (2) | 2 (2) |
| K. aerogenes ATCC 13048 | 8 (8) | 8 (8) | 4 (4) | 2 (2) |
| K. pneumoniae ATCC 700603 | 32 (32) | 32 (32) | 32 (32) | 16 (16) |
| K. pneumoniae KP-Mo-7 | 16 (16) | 8 (16) | 4 (8) | 2 (2) |
| K. pneumoniae KP-Mo-6 | 16 (16) | 16 (16) | 4 (8) | 2 (2) |
| P. aeruginosa RP73 | 32 (32) | 32 (32) | 32 (32) | 16 (16) |
| P. aeruginosa PAO1 | 64 (64) | 64 (64) | 64 (64) | 32 (32) |
| P. aeruginosa PA14 | 64 (64) | 32 (64) | 32 (64) | 32 (64) |
| P. aeruginosa PA-Na-1 | 64 (128) | 64 (128) | 64 (64) | 64 (64) |
| S. enterica ATCC 13076 | 32 (32) | 32 (32) | 16 (16) | 8 (8) |
| S. maltophilia K279 | 16 (16) | 16 (16) | 8 (16) | 4 (8) |
| S. maltophilia LMG 10853 | 8 (8) | 1 (1) | 0.5 (1) | 0.5 (1) |
| S. maltophilia OBGTC20 | 32 (32) | 8 (16) | 4 (8) | 2 (2) |
| E. faecalis ATCC 29212 | 4 (8) | 4 (8) | 4 (8) | 2 (2) |
| E. faecium ATCC 6057 | 4 (4) | 4 (4) | 1 (1) | 0.5 (1) |
| S. aureus ATCC 43300 | 8 (16) | 0.5 (0.5) | 0.25 (0.25) | 0.125 (0.25) |
| C. albicans ATCC 10231 | 4 (4) | 4 (4) | 2 (2) | 2 (2) |
| C. albicans 62033 | 4 (4) | 4 (4) | 2 (2) | 2 (2) |
| C. parapsilosis 4609 | 4 (8) | 4 (4) | 2 (2) | 2 (2) |
| C. krusei 81667 | 1 (1) | 0.5 (1) | 0.5 (1) | 0.5 (1) |
| C. glabrata 61112 | 2 (2) | 0.5 (1) | 0.5 (1) | 0.5 (1) |
| C. tropicalis 61220 | 1 (1) | 0.5 (1) | 0.125 (0.125) | 0.125 (0.125) |
The numbers outside and within the brackets indicate MIC and MBC or MCF values, respectively, and are expressed in mg/L. The numbers 0, 32, 64 and 128 indicate RV concentrations and are expressed in mg/L.
2.4. Effect of RV on CHX and BZK Combination against Gram-Negative Bacteria, Gram-Positive Bacteria and Yeasts
Because the CHX and BZK biocides/antiseptics are currently used in combination [25,26,27], we analyzed the effect of RV on the susceptibility of Gram-negative bacteria, Gram-positive bacteria and yeasts to the CHX and BZK combined treatment. As shown in Table 4, the CHX and BZK combination inhibited the CHX or BZK MIC in 14 out 21 strains, showing either a synergistic or additive effect in 14 and 5 strains, respectively, but an indifferent effect was observed in the C. albicans 62033 and P. aeruginosa PAO1. The CHX and BZK combination in the presence of 32 mg/L RV inhibited the CHX and BZK MIC and MBC in all of the strains, and restored the CHX or BZK susceptibility in 16 out of 21 strains, resulting in a synergistic or additive effect in 18 and 3 strains, respectively (Table 4). Moreover, the CHX and BZK combination in the presence of RV at 64 mg/L restored the CHX or BZK susceptibility in all of the strains, and showed a synergistic or additive effect in 21 strains (Table 4). In particular, the CHX and BZK combination in the presence of 64 mg/L RV restored the BZK susceptibility in all of the strains, while the combination did not affect the CHX tolerance in three Burkholderia spp. strains, C. albicans 62033, S. maltophilia k279, K. pneumoniae ATCC 700603 and all four of the P. aeruginosa strains were still tolerant to CHX (Table 4). The reduced ability of RV to restore CHX susceptibility compared to BZK susceptibility may be dependent on the elevated EP activation, which was demonstrated to regulate tolerance to CHX in the K. pneumoniae [13] and P. aeruginosa [15] strains. Future experiments are necessary to validate this hypothesis.
Table 4.
Effect of RV (mg/L) on CHX and BZK MIC (mg/L) and MBC (MFC in the case of Candida spp.) (mg/L) in combination against Gram-negative bacteria, Gram-positive bacteria and yeasts.
| Strain | CHX MIC (MBC or MFC) |
BZK MIC (MBC or MFC) |
0 RV | FIC * Index (a) |
32 RV | FIC * Index (b) | 64 RV | FIC * Index (c) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CHX + BZK MIC (MBC or MFC) |
CHX + BZK MIC (MBC or MFC) |
CHX + BZK MIC (MBC or MFC) |
|||||||||
|
A. baumannii ATCC 19606 |
32 (32) | 16 (16) | 8 (8) | 4 (4) | 0.5 | 2 (2) | 2 (2) | 0.187 | 2 (2) | 0.5 (0.5) | 0.093 |
|
A. baumannii ∆adeB ATCC 19606 |
4 (4) | 8 (8) | 1 (1) | 2 (2) | 0.5 | 2 (2) | 0.06 (0.06) | 0.50 | 0.5 (0.5) | 0.5 (0.5) | 0.18 |
|
A. baumannii ACICU |
64 (128) | 16 (32) | 8 (8) | 2 (2) | 0.25 | 2 (2) | 0.5 (0.5) | 0.062 | 2 (2) | 0.5 (0.5) | 0.062 |
|
B. cenocepacia LMG 16654 |
64 (64) | 64 (64) | 8 (8) | 2 (2) | 0.15 | 8 (8) | 2 (2) | 0.15 | 8 (8) | 0.5 (0.5) | 0.13 |
|
B. dolosa LMG 21443 |
16 (16) | 8 (8) | 4 (8) | 2 (2) | 0.31 | 4 (4) | 1 (1) | 0.28 | 4 (4) | 0.5 (0.5) | 0.26 |
|
B. multivorans LMG16665 |
64 (64) | 32 (64) | 8 (8) | 8 (8) | 0.25 | 8 (8) | 8 (8) | 0.25 | 8 (8) | 0.5 (0.5) | 0.13 |
|
E. cloacae ATCC 13047 |
8 (8) | 8 (8) | 2 (2) | 4 (4) | 0.5 | 2 (2) | 4 (4) | 0.5 | 2 (2) | 2 (2) | 0.375 |
|
K. pneumoniae ATCC 700603 |
128 (128) | 16 (16) | 16 (32) | 4 (4) | 0.25 | 4 (4) | 2 (2) | 0.093 | 4 (4) | 2 (2) | 0.093 |
|
K. pneumoniae kp-Mo-7 |
64 (64) | 16 (16) | 2 (2) | 2 (2) | 0.156 | 2 (2) | 1 (1) | 0.092 | 1 (1) | 0.5 (0.5) | 0.062 |
|
P. aeruginosa RP73 |
32 (32) | 64 (64) | 8 (16) | 4 (4) | 0.31 | 4 (8) | 8 (8) | 0.25 | 4 (4) | 1 (1) | 0.14 |
|
P. aeruginosa PAO1 |
16 (16) | 64 (128) | 16 (32) | 2 (2) | 1.03 | 4 (4) | 2 (2) | 0.28 | 4 (4) | 0.5 (0.5) | 0.25 |
|
P. aeruginosa PA14 |
16 (32) | 64 (64) | 4 (8) | 4 (4) | 0.31 | 4 (4) | 4 (4) | 0.31 | 4 (4) | 1 (1) | 0.26 |
|
P. aeruginosa PA-Na-1 |
32 (64) | 64 (128) | 8 (8) | 2 (2) | 0.28 | 4 (4) | 2 (2) | 0.156 | 4 (4) | 0.5 (0.5) | 0.132 |
|
S. enterica ATCC 13076 |
4 (4) | 16 (32) | 1 (1) | 1 (1) | 0.312 | 1 (1) | 1 (1) | 0.312 | 0.5 (0.5) | 0.5 (0.5) | 0.15 |
|
S. maltophilia K279 |
128 (128) | 16 (16) | 16 (32) | 8 (8) | 0.62 | 4 (4) | 4 (8) | 0.281 | 4 (4) | 2 (2) | 0.156 |
|
E. faecalis ATCC 29212 |
32 (32) | 4 (8) | 4 (4) | 2 (2) | 0.625 | 0.5 (0.5) | 0.5 (0.5) | 0.14 | 0.5 (0.5) | 0.5 (0.5) | 0.14 |
|
E. faecium ATCC 6057 |
8 (8) | 4 (4) | 0.125 (0.125) | 0.5 (0.5) | 0.14 | 0.5 (0.5) | 0.125 (0.125) | 0.093 | 0.25 (0.25) | 0.06 (0.06) | 0.046 |
|
S. aureus ATCC43300 |
1 (2) | 8 (16) | 0.125 (0.125) | 0.5 (0.5) | 0.187 | 0.25 (0.25) | 0.06 (0.06) | 0.257 | 0.125 (0.125) | 0.25 (0.25) | 0.156 |
|
C. albicans ATCC 10231 |
16 (32) | 4 (4) | 8 (8) | 1 (1) | 0.75 | 2 (4) | 0.125 (0.125) | 0.156 | 2 (2) | 0.125 (0.0125) | 0.156 |
|
C. albicans 62033 |
16 (16) | 2 (2) | 8 (16) | 2 (2) | 1 | 4 (4) | 2 (2) | 0.75 | 4 (4) | 2 (2) | 0.75 |
|
C. parapsilosis 4609 |
64 (64) | 2 (2) | 8 (16) | 2 (2) | 0.51 | 1 (1) | 2 (2) | 0.26 | 1 (1) | 2 (2) | 0.26 |
* FIC index, Fractional Inhibitory Concentration index; (a) Σ FIC = [(MIC CHX + BZK) + 0 RV/MIC CHX] + [(MIC BZK + CHX) + 0 RV/MIC BZK]; (b) Σ FIC = [(MIC CHX + BZK) + 32 RV/MIC CHX] + [(MIC BZK + CHX) + 32 RV/MIC BZK]; (c) Σ FIC = [(MIC CHX + BZK) + 64 RV/MIC CHX] + [(MIC BZK + CHX) + 64 RV/MIC BZK]. The numbers outside and within the brackets indicate MIC and MBC or MCF values, respectively, and are expressed in mg/L. The numbers 0, 32 and 64 indicate RV concentrations and are expressed in mg/L.
3. Materials and Methods
3.1. Bacterial Strain, Growth Condition and Reagents
A collection of 132 Gram-negative bacteria, 9 Gram-positive bacteria and 10 Candida spp. strains was analyzed in the study (Table S1, Supplementary Materials). The collection included either the reference strains, which were identified with their ATCC number, or clinical isolates, which were identified with their original number (Table S1, Supplementary Materials). The origin and characteristics of all of the strains were described in the references listed in Table S1 (Supplementary Materials). No ethical approval was required for the study because there was no access to patients’ data. The reference and clinical strains were cultured under aerobic conditions in standard selective media at 37 °C, but the S. maltophilia LMG 10991, LMG 10853 and LMG 10871 strains were grown at 30 °C. The chemical reagents, chlorhexidine digluconate (CHX), benzalkonium chloride (alkylbenzyldimethylammonium chloride (BZK), carbonyl cyanide m-chlorophenylhydrazine (CCCP) and resveratrol (3,5,4′-trihydroxy-trans-stilbene, RV), were purchased from Sigma-Aldrich (Sigma, Milan, Italy).
3.2. Determination of Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
The CCCP and RV were dissolved in dimethyl sulfoxide (DMSO), while the CHX and BZK were dissolved in H2O. Two-fold serial dilutions of CHX and BZK, (0.06–1024 mg/L), RV (32–256 mg/L) or CCCP (1–2 and 4 mg/L), were prepared in triplicate and placed into a polystyrene 96-well plate. The bacterial suspensions were prepared by growing overnight in nutrient media with agar and adjusting the turbidity to 0.5 McFarland using a BD PhoenixSpec™ nephelometer. Subsequently, the bacterial cells were diluted in cation-adjusted Mueller–Hinton broth (CAMHB) to a final culture density of approximately 1 × 106 CFU/mL. Only the CAMHB was added into the negative control wells, and wells with no compounds were used on each plate as the positive growth control. The plates were incubated at 30 °C or 37 °C for 18–24 h. The MIC and MBC of CHX, BZK, CCCP or RV were determined by a manual microdilution method, according to the recommended procedures by the European Committee for Antimicrobial Susceptibility Testing (Eucast) of the European Society of Clinical Microbiology and Infectious Diseases (Escmid) [28], and the Clinical and Laboratory Standards (CLSI) [29]. On the other hand, the yeast suspensions were prepared by growing overnight in Sabouraud dextrose agar plates, and adjusting the turbidity to 0.5 McFarland, using a BD PhoenixSpec™ nephelometer. Then, the 5 × 105 CFU/mL yeasts were inoculated in RPMI buffered with morpholinepropane sulfonic acid (MOPS) (pH. 7) containing glucose 2%. The non-treated yeasts were used as the positive controls. The MIC of CHX, BZK, CCCP or RV were determined by a manual microdilution method, according to the recommended procedures by the Subcommittee on Antifungal Susceptibility Testing (AFST) European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the ESCMID [30]. Finally, the plates were incubated at 37 °C for 18–24 h. The susceptibility was assessed to the MIC value < 4 mg/L, as described for A. baumannii [16]. The strains showing MIC values < 4 mg/L were considered susceptible, while the strains having MIC values ≥ 4 mg/L were considered tolerant. In order to evaluate the minimum bactericidal or fungicide concentration, 20 µL of bacteria or yeast suspensions from wells without visible growth were transferred to the respective plates. These plates were incubated at 30 °C or 37 °C and checked for growth after 24 h. All of the tests were performed in triplicate and repeated three times.
3.3. In Vitro Combination Studies
The tests were carried out using the checkerboard method, according to the previously reported method [31]. The serial dilutions of CHX (0.06–164 mg/L) or BZK (0.06–164 mg/L) were prepared and combined with fixed concentrations of resveratrol (32–256 mg/L) or CCCP (1, 2 and 4 mg/L). Subsequently, 1 × 106 CFU/mL bacterial cells in CAMHB and 5 × 105 CFU/mL yeasts in RPMI-MOPS were added to each well of the microtiter plate. Then, the plates were incubated at 30 °C or 37 °C for 18–24 h. Furthermore, the checkerboard method was used to evaluate the MICs for the combination of CHX and BZK from 0.06 to 64 mg/L with RV at fixed concentrations of 32 or 64 mg/L. Afterward, the microtiter was incubated with 1 × 106 CFU/mL bacterial cells in CAMHB or 5 × 105 CFU/mL yeasts in RPMI-MOPS. The plates were then incubated at 37 °C for 18–24 h. The combined effects were then determined by calculating the fractional inhibitory concentration (FIC) index as follows: FICI = FICA + FICB, where FICA is the ratio of the MIC of CHX and BZK with RV (32 or 64 mg/L) combination and the MIC of CHX with RV (32 or 64 mg/L) alone, and FICB is the ratio of the MIC CHX and BZK with RV (32 or 64 mg/L) combination and the MIC of BZK with RV (32 or 64 mg/L) alone. The FIC index was interpreted as synergy (FICI ≤ 0.5), additive (FICI > 0.5 to ≤1.0), indifference (FICI > 1.0 to ≤2.0) and antagonism (FICI > 2.0). All of the experiments were repeated three times [32].
3.4. Statistical Analysis
All of the statistical analyses were performed with GraphPad Prism 8 software (GraphPad, San Diego, CA, USA). The correlations were evaluated by regression analysis, using the Pearson’s correlation coefficient (r). All of the results are presented as arithmetic means ± standard deviations. The significance of the differences was evaluated using one-way ANOVA, followed by Bonferroni’s comparison post-hoc tests. The differences were considered statistically significant if p < 0.05.
4. Conclusions
The tolerance of dangerous Gram-negative bacteria, Gram-positive bacteria and yeasts to commonly used biocides, such as CHX and BZK, is becoming a serious nosocomial problem [6,7,8,11,12,16,17].
Although chemically different, CHX (a cationic poly-biguanide) and BZK (a quaternary ammonium compound) share a cationic nature, that makes them able to bind to the negatively-charged sites on the cell wall; thus, destabilizing it and interfering with osmosis [4,5,6]. However, the bacteria have developed mechanisms to resist the attack of biocides, e.g., extruding them through EPs, resulting in the clinically observed biocide tolerance [9,10,11,12,13,16,17]. This phenomenon has prompted us to search for effective formulations, able to exert their antimicrobial action on the resistant bacterial strains. We have demonstrated synergy in the bactericidal effect of CHX and BZK in a large panel of Gram-negative and Gram-positive bacteria, the highest effects being observed for A. baumannii ACICU, B. dolosa LMG 21443, B. multivorans LMG 16665, B. cenocepacia LMG 16654, P. aeruginosa RP73, P. aeruginosa PA14, K. pneumoniae ATCC 700603, K. pneumoniae kp-Mo-7, S. enterica ATCC 13076 and S. aureus ATCC 43300.
Importantly, a synergistic microbicidal effect was observed when the two biocides were combined with resveratrol, which we previously proved affected the expression level of the EPs [11]. This finding has a strong applicative potential for the preparation of disinfectant/antiseptic formulations containing the three components, to be used against strongly tolerant Gram-negative bacteria, Gram-positive bacteria and yeasts.
Acknowledgments
We thank all colleagues who generously provided strains included in the study: Alessandra Carattoli, Patrice Nordmann and Paolo Visca.
Supplementary Materials
The following are available at https://www.mdpi.com/article/10.3390/antibiotics11070961/s1, Table S1: CHX and BZK MICs (mg/L) and MBCs (MFCs in the case of Candida) (mg/L) in a panel of 151 reference strains and clinical isolates of Gram-negative bacteria, Gram-positive bacteria and yeasts; Figure S1: Effect of RV or CCCP on CHX MICs, Figure S2: Effect of RV or CCCP on BZK MICs.
Author Contributions
Conceptualization, E.D.G. and R.Z.; methodology, A.M., M.S., M.B. and M.T.; formal analysis, A.M., R.B., M.T. and E.D.G.; investigation, A.M., M.S., M.B.; data curation, A.M., E.D.G. and R.Z.; writing—original draft preparation, A.M., E.D.G. and R.Z.; supervision, E.D.G. and R.Z.; funding acquisition, R.B. and R.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by grant from the Italian Ministry of Education, University and Research (MIUR): PRIN2017 (Grant No. 2017SFBFER to RZ and RB).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Durante-Mangoni E., Utili R., Zarrilli R. Combination therapy in severe Acinetobacter baumannii infections: An update on the evidence to date. Future Microbiol. 2014;9:773–789. doi: 10.2217/fmb.14.34. [DOI] [PubMed] [Google Scholar]
- 2.Holt K.E., Wertheim H., Zadoks R.N., Baker S., Whitehouse C.A., Dance D., Jenney A., Connor T.R., Hsu L.Y., Severin J., et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc. Natl. Acad. Sci. USA. 2015;112:E3574–E3581. doi: 10.1073/pnas.1501049112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horcajada J.P., Montero M., Oliver A., Sorlí L., Luque S., Gómez-Zorrilla S., Benito N., Grau S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin. Microbiol. Rev. 2019;32:00031-19. doi: 10.1128/CMR.00031-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David M.Z., Daum R.S. Treatment of Staphylococcus aureus infections. Rev. Microbiol. Immunol. 2017;409:325–383. doi: 10.1007/82_2017_42. [DOI] [PubMed] [Google Scholar]
- 5.De Oliveira D.M.P., Forde B.M., Kidd T.J., Harris P.N.A., Schembri M.A., Beatson S.A., Paterson D.L., Walker M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020;33:00181-19. doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zemanick E.T., Wagner B.D., Robertson C.E., Ahrens R.C., Chmiel J.F., Clancy J.P., Gibson R.L., Harris W.T., Kurland G., Laguna T.A., et al. Airway microbiota across age and disease spectrum in cystic fibrosis. Eur. Respir. J. 2017;50:1700832. doi: 10.1183/13993003.00832-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin-Loeches I., Antonelli M., Cuenca-Estrella M., Dimopoulos G., Einav S., De Waele J.J., Garnacho-Montero J., Kanj S.S., Machado F.R., Montravers P., et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019;45:789–805. doi: 10.1007/s00134-019-05599-w. [DOI] [PubMed] [Google Scholar]
- 8.McDonnell G., Russell A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999;12:147–179. doi: 10.1128/CMR.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milstone A.M., Passaretti C.L., Perl T.M. Chlorhexidine: Expanding the armamentarium for infection control and prevention. Clin. Infect. Dis. 2008;46:274–281. doi: 10.1086/524736. [DOI] [PubMed] [Google Scholar]
- 10.Merchel P.P.B., Tagkopoulos I. Benzalkonium chlorides: Uses, regulatory status, and microbial resistance. Appl. Environ. Microbiol. 2019;85:00377-19. doi: 10.1128/AEM.00377-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kampf G. Acquired resistance to chlorhexidine—Is it time to establish an ‘antiseptic stewardship’ initiative? J. Hosp. Infect. 2016;94:213–227. doi: 10.1016/j.jhin.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Weber D.J., Rutala W.A., Sickbert-Bennett E.E. Use of germicides in health care settings-is there a relationship between germicide use and antimicrobial resistance: A concise review. Am. J. Infect. Control. 2019;47:A106–A109. doi: 10.1016/j.ajic.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Ni L., Zhang Z., Shen R., Liu X., Li X., Chen B., Wu X., Li H., Xie X., Huang S. Disinfection Strategies for Carbapenem-Resistant Klebsiella pneumoniae in a Healthcare Facility. Antibiotics. 2022;11:736. doi: 10.3390/antibiotics11060736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abuzaid A., Hamouda A., Amyes S.G. Klebsiella pneumoniae susceptibility to biocides and its association with cepA, qacDE and qacE efflux pump genes and antibiotic resistance. J. Hosp. Infect. 2012;81:87–91. doi: 10.1016/j.jhin.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Zheng X., Zhang X., Zhou B., Liu S., Chen W., Chen L., Zhang Y., Liao W., Zeng W., Wu Q., et al. Clinical characteristics, tolerance mechanisms, and molecular epidemiology of reduced susceptibility to chlorhexidine among Pseudomonas aeruginosa isolated from a teaching hospital in China. Int. J. Antimicrob. Agents. 2022;60:106605. doi: 10.1016/j.ijantimicag.2022.106605. [DOI] [PubMed] [Google Scholar]
- 16.Rajamohan G., Srinivasan V.B., Gebreyes W.A. Novel role of Acinetobacter baumannii RND efflux transporters in mediating decreased susceptibility to biocides. J. Antimicrob. Chemother. 2010;65:228–232. doi: 10.1093/jac/dkp427. [DOI] [PubMed] [Google Scholar]
- 17.Du D., Wang-Kan X., Neuberger A., Van Veen H.W., Pos K.M., Piddock L.J.V., Luisi B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018;16:523–539. doi: 10.1038/s41579-018-0048-6. [DOI] [PubMed] [Google Scholar]
- 18.Tucker A.T., Nowicki E.M., Boll J.M., Knauf G.A., Burdis N.C., Trent M.S., Davies B.W. Defining gene-phenotype relationships in Acinetobacter baumannii through one-step chromosomal gene inactivation. mBio. 2014;5:01313–01314. doi: 10.1128/mBio.01313-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Migliaccio A., Esposito E.P., Bagattini M., Berisio R., Triassi M., De Gregorio E., Zarrilli R. Inhibition of AdeB, AceI, and AmvA Efflux Pumps Restores Chlorhexidine and Benzalkonium Susceptibility in Acinetobacter baumannii ATCC 19606. Front. Microbiol. 2022;12:790263. doi: 10.3389/fmicb.2021.790263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassan K.A., Jackson S.M., Penesyan A., Patching S.G., Tetu S.G., Eijkelkamp B.A., Brown M.H., Henderson P.J.F., Paulsen I.T. Transcriptomic and biochemical analyses identify a family of chlorhexidine efflux proteins. Proc. Natl. Acad. Sci. USA. 2013;110:20254–20259. doi: 10.1073/pnas.1317052110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattio L.M., Catinella G., Dallavalle S., Pinto A. Stilbenoids: A natural arsenal against bacterial pathogens. Antibiotics. 2020;9:336. doi: 10.3390/antibiotics9060336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma D.S.L., Tan L.T., Chan K.G., Yap W.H., Pusparajah P., Chuah L.H., Chuah L.H., Ming L.C., Khan T.M., Lee L.H., et al. Resveratrol—Potential Antibacterial Agent against Foodborne Pathogens. Front. Pharmacol. 2018;9:102. doi: 10.3389/fphar.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vestergaard M., Ingmer H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents. 2019;53:716–723. doi: 10.1016/j.ijantimicag.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Singkham-In U., Higgins P.G., Wannigama D.L., Hongsing P., Chatsuwan T. Rescued chlorhexidine activity by resveratrol against carbapenem-resistant Acinetobacter baumannii via down-regulation of AdeB efflux pump. PLoS ONE. 2020;15:e0243082. doi: 10.1371/journal.pone.0243082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacour J.P., Castanet J., Boutté P., Ortonne J.P. Antiseptic treatment of the umbilical cord in newborns: Survey and recommendations. Arch. Pediatr. 1999;6:631–634. doi: 10.1016/S0929-693X(99)80293-0. [DOI] [PubMed] [Google Scholar]
- 26.Mimoz O., Villeminey S., Ragot S., Dahyot-Fizelier C., Laksiri L., Petitpas F., Debaene B. Chlorhexidine-based antiseptic solution vs alcohol-based povidone-iodine for central venous catheter care. Arch. Intern. Med. 2007;167:2066–2072. doi: 10.1001/archinte.167.19.2066. [DOI] [PubMed] [Google Scholar]
- 27.Hornschuh M., Zwicker P., Kramer A., Schaufler K., Heiden S., Bohnert J., Becker K., Hübner N.-O. Extensively-drug-resistant Klebsiella pneumoniae ST307 outbreak strain from north-eastern Germany does not show increased tolerance to quaternary ammonium compounds and chlorhexidine. J. Hosp. Infect. 2021;113:52–58. doi: 10.1016/j.jhin.2021.01.032. [DOI] [PubMed] [Google Scholar]
- 28.European Committee for Antimicrobial Susceptibility Testing (Eucast) of the European Society of Clinical Microbiology and Infectious Diseases (Escmid) Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2020;6:503–508. doi: 10.1046/j.1469-0691.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. 29th ed. Clin Lab Stand Institute; Wayne, PA, USA: 2019. CLSI Supplement M100. [Google Scholar]
- 30.EUCAST Definitive Document EDef 7.1. Method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 2008;14:398–405. doi: 10.1111/j.1469-0691.2007.01935.x. [DOI] [PubMed] [Google Scholar]
- 31.Hall M., Middleton R., Westmacott D. The fractional inhibitory concentration (FIC) index as a measure of synergy. J. Antimicrob. Chemother. 1983;11:427–433. doi: 10.1093/jac/11.5.427. [DOI] [PubMed] [Google Scholar]
- 32.Odds F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.