Abstract
Background: Since 2012, few reports on the molecular epidemiology of Pseudomonas aeruginosa were reported in Tunisia. Objectives: This study aimed to evaluate carbapenem-resistance determinants and molecular epidemiology and to compare the carbapenemase-phenotypic detection methods of multidrug-resistant P. aeruginosa isolates. Methods: During a period of four years (2014 to 2017), all imipenem-ceftazidime-resistant P. aeruginosa isolates were retrospectively selected at the microbial laboratory of Charles Nicolle hospital of Tunis. These isolates were examined by the modified Hodge test, modified carbapenem inactivation method (mCIM), and another mCIM, called CIMTris, and their performance was evaluated using PCR analysis as the gold standard. Results: A total of 35 isolates were recovered among patients hospitalized in different units. All strains were colistin-susceptible.All carbapenem-resistant isolates showed a high-level resistance to carbapenems. CIMTris and mCIM showed 96.15% and 46.15% sensitivity and 44.44% and 100% specificity, respectively, for detecting carbapenemase production.Conclusions: CIMTris is a promising approach for detecting carbapenemase activity in P. aeruginosa and merits further testing. Moreover, this study described the first detection of GES-5- and GES-9-producing P. aeruginosa in Tunisia as well as the co-occurrence of the blaGES-5 and blaVIM-11 carbapenemase genes in one isolate. These findings are of great concern because the rapid dissemination of MDR strains represents a major therapeutic and epidemiological threat.
Keywords: Pseudomonas, GES, VIM, ICU
1. Introduction
Pseudomonas aeruginosa is an important pathogen that causes various opportunistic and acute nosocomial-acquired infections, especially in immunocompromised patients. Its high intrinsic resistance and ability to develop multidrug resistance produce serious therapeutic problems. Carbapenems have been considered first-line agents to treat severe cases of P. aeruginosa infections [1]. Resistance to carbapenems can stem from the production of carbapenemases or other mechanisms such as mutation in the oprD gene, the overproduction of cephalosporinases, the over-expression of effluxpumps, or combinations of these mechanisms [2].
The most commonly reported carbapenemases among P. aeruginosa are metallo-β-lactamases (MBLs) (e.g., the Verona Imipenemase (VIM), Imipenemase (IMP), Sao Paulo metallo enzyme (SPM), German imipenemase (GIM), and New Dehli metallo β-lactamase (NDM) types) and, to a lesser extent, Ambler class A carbapenemases (e.g., Klebsiella pneumoniae carbapenemase (KPC) and some Guyana extended-spectrum (GES)-type enzymes [3]. MBL enzymes are also able to hydrolyze penicillin and cephalosporins [4,5]. Moreover, the MBL VIM-2, initially reported in France [4], has emerged and has been reported to be the main MBL determinant in P. aeruginosa isolates in Tunisia and worldwide during the past two decades [6,7,8,9]. Their increase in recent years, which is associated with high mortality, morbidity, long hospital stays, and increased costs, emphasizes the need for the detection of these isolates to avoid therapeutic failures and nosocomial outbreaks [8,9].
It should be noted that detecting carbapenemases is more difficult in P. aeruginosa compared to Enterobacteriaceae [10]. Currently, several phenotypic methods are available, but most of them are unsuitable for clinical laboratories to perform on a routine basis [10,11]. Thus, standardized carbapenemase detection methods using routine phenotypic screening tests are still controversial.
Since 2012, few reports on the molecular epidemiology of P. aeruginosa were reported in Tunisia [6]. The aim of our study was, therefore, to evaluate changes in carbapenem-resistance determinants and molecular epidemiology and to compare carbapenemase-phenotypic detection methods of multidrug-resistant P. aeruginosa isolates recovered at Charles Nicolle Hospital of Tunis, Tunisia, during a 4-year period.
2. Materials and Methods
2.1. Bacterial Isolates
A total of 80 imipenem–ceftazidime-resistant P. aeruginosa (ICRPA) isolates were retrospectively selected from the frozen stocks kept in brain heart infusion with 20% glycerol at −80 °C at the microbial laboratory of Charles Nicolle hospital of Tunis during a period of four years (2014 to 2017).
From frozen stock, each isolate was subcultured twice on tryptic soy agar (Biorad, Marnesta la Coquette, France), incubating each subculture in ambient air at 35 °C ± 2 °C for 18 to 24 h to ensure purity and viability. Thus, only 35 non-duplicated clinical ICRPA isolates were successfully subcultured and included in this study.
Bacterial identification was performed using the API 20NE system (bioMérieux, Marcy l’Etoile, France).
2.2. Antimicrobial Susceptibility Testing
Antibiotic susceptibility testing to 16 antibiotics (ticarcillin, ticarcillin-clavulanic acid, piperacillin, piperacillin–tazobactam, aztreonam, ceftazidime, cefepime, imipenem, meropenem, gentamicin, tobramycin, amikacin, netilmicin, ciprofloxacin, levofloxacin, and fosfomicin) was performed by the agar disk diffusion method on Mueller–Hinton (MH) agar plates (Bio-Rad, Marnesta la Coquette, France), according to the CA-SFM guidelines (http://www.sfm-microbiologie.org/, accessed on 1 January 2017).
The minimum inhibitory concentrations (MICs) of imipenem and meropenem were determined by the agar dilution method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [M100-S25]. Colistin MICs were determined by the broth microdilution method using a commercialized kit (UMIC, Biocentric, Bandol-France).
Escherichia coli ATCC 25922 and P. aeruginosa ATCC 27853 were used as quality control strains in antimicrobial susceptibility testing and MICs.
2.3. Phenotypic Detection of Carbapenemase Production
The phenotypic detection of carbapenemases was performed by a modified Hodge test, modified carbapenem inactivation method (mCIM), and another mCIM, called CIMTris, which uses 0.5 M Tris-HCl buffer rather than water for extraction, as previously reported [10]. All carbapenemase phenotypic methods were assessed twice by different raters.
The performance of the carbapenemase phenotypic tests was evaluated using PCR analysis for blaVIM and blaGES as the gold standard (Table 1) [12]. Phenotypic method sensitivities and specificities were calculated according to Ilstrup [12,13].
Table 1.
Oligonucleotides used in this study.
| Gene | Primer a | Sequence (5′–3′) c | Product Size (bp) b | Reference |
|---|---|---|---|---|
| bla GES | MultiGES-F | AGTCGGCTAGACCGGAAG | 399 | [14] |
| MultiGES-R | TTTGTCCGTGCTCAGGAT | |||
| bla OXA-48 | MultiOXA-48 F | GCTTGATCGCCCTCGATT | 281 | [14] |
| MultiOXA-48 R | GATTTGCTCCGTGGCCGAAA | |||
| bla IMP | MltiIMP-F | TTGACACTCCATTTACDG | 232 | [15] |
| MultiIMP-R | GATYGAGAATTAAGCCACYCT | |||
| bla VIM | MultiVIM-F | GATGGTGTTTGGTCGCATA | 390 | [15] |
| MultiVIM-R | CGAATGCGCAGCACCAG | |||
| bla KPC | MultiKPC-F | CATTCAAGGGCTTTCTTGCT C | 798 | [15] |
| MultiKPC-R | ACGACGGCATAGTCATTTGC | |||
| bla BIC | MultiBIC-F | TATGCAGCTCCTTTAAAGGGC | 537 | [15] |
| MultiBIC-R | TCATTGGCGGTGCCGTACAC | |||
| bla NDM | MultiNDM-F | GGTTTGGCGATCTGGTTTTC | 621 | [15] |
| MultiNDM-R | CGGAATGGCTCATCACGATC | |||
| bla AIM | MultiAIM-F | CTGAAGGTGTACGGAAACAC | 322 | [15] |
| MultiAIM-R | GTTCGGCCACCTCGAATTG | |||
| bla GIM | MultiGIM-F | TCGACACACCTTGGTCTGAA | 477 | [15] |
| MultiGIM-R | AACTTCCAACTTTGCCATGC | |||
| bla SIM | MultiSIM-F | TACAAGGGATTCGGCATCG | 570 | [15] |
| MultiSIM-R | TAATGGCCTGTTCCCATGTG | |||
| bla DIM | MultiDIM-F | GCTTGTCTTCGCTTGCTAACG | 699 | [15] |
| MultiDIM-R | CGTTCGGCTGGATTGATTTG | |||
| bla SPM | SPM-F | AAAATCTGGGTACGCAAACG | 271 | [15] |
| SPM-R | ACATTATCCGCTGGAACAGG |
a F, sense primer; R, antisense primer. b Nucleotide numbering begins at the initiation codons of genes. c D = A or G or T; Y = C or T.
2.4. Detection and Characterization of Beta-Lactamase Genes
The molecular detection of carbapenemase-encoding genes (blaGES, blaKPC, blaOXA-48, blaIMP, blaVIM, blaNDM, blaSPM, blaBIC, blaAIM, blaGIM, blaSIM, and blaDIM) was performedby PCR with previously reported conditions (Table 1) [14,15]. All PCR products were sequenced using a DNAsequencer (ABI PRISM 3130; Applied Biosystems, Foster City, CA, USA) [16].
3. Results
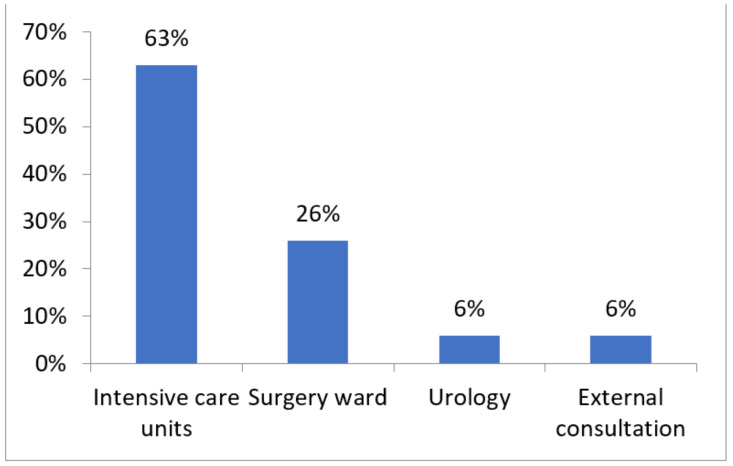
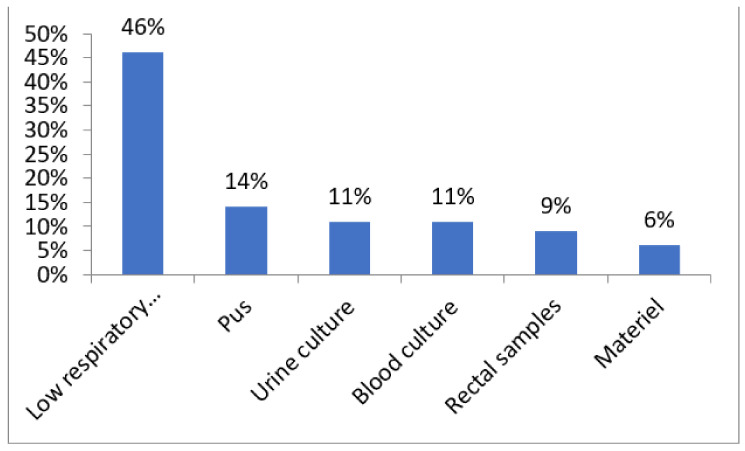
The 35 isolates had been obtained among patients hospitalized in different units (intensive care units, 63%; surgery ward, 26%; urology, 6%; and external consultation, 6%) (Figure 1) and from different types of samples (Figure 2) (low respiratory samples, 46%; pus, 14%; urine culture, 11%; blood culture, 11%; rectal samples, 9%; and material, 6%).
Figure 1.
Distribution of P. aeruginosa isolates according to wards.
Figure 2.
Distribution of P.aeruginosa isolates according to sample types.
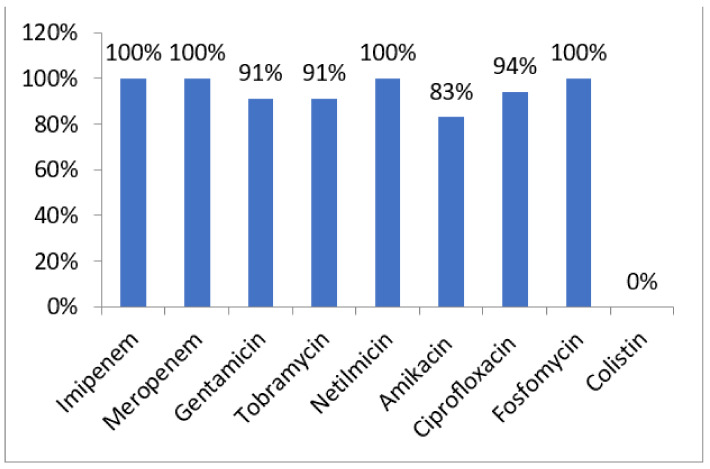
All strains were colistin-susceptible (MIC range 1–4 µg/mL) and were resistant to gentamicin (91%), tobramycin (91%), netilmicin (100%), amikacin (83%), ciprofloxacin (94%), and fosfomycin (100%) (Figure 3). All carbapenem-resistant isolates showed a high-level resistance to carbapenems. The MIC ranges of imipenem and meropenem were 4–512 μg/mL and 4–256 μg/mL, respectively.
Figure 3.
Antibiotic resistance rates among P.aeruginosa isolates.
Carbapenemase-encoding genes were detected in 26 strains (74%) and were identified as: blaGES-5 (n = 14), blaGES-9 (n = 2), blaVIM-1 (n = 1), blaVIM-2 (n = 9), and blaVIM-11 (n = 1). The association between blaGES and blaVIM was found in two strains (S15) (Table 2). None of the strains harbored the genes blaKPC, blaIMP, blaOXA-48, blaSPM, blaNDM, blaBIC, blaAIM, blaGIM, blaSIM, and blaDIM.
Table 2.
Characteristics of imipenem- and ceftazidime-resistant P. aeruginosa isolates (n = 35).
| Strains | Specimen | Ward | Date of Isolation (Day/Month/Year) |
Minimal Inhibitory Concentration (µg/mL) | Resistance to Non β-lactams | Phenotypic Detection of Carbapenemases | bla Genes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Imipenem (4–8) * |
Meropenem (2–8) * |
Colistin (4) * |
mCIM | CIMTris | mHodge | ||||||
| S1 | Pus | Urology | 21 August 2014 | 512 | 128 | 4 | GEN, AMN, NET, TOB, CIP, FOS | - | - | - | - |
| S2 | Urine | ICU | 21 August 2014 | 16 | 4 | 4 | GEN, AMN, NET, TOB, CIP, FOS | - | + | - | bla VIM-2 |
| S3 | Pulmonary | ICU | 21 August 2014 | 32 | 16 | 4 | GEN, AMN, NET, TOB, CIP, FOS | - | + | - | bla VIM-2 |
| S4 | Pulmonary | ICU | 30 August 2014 | 512 | 128 | 4 | GEN, AMN, NET, TOB, CIP, FOS | + | + | + | bla VIM-1 |
| S5 | Pulmonary | ICU | 8 September 2014 | 32 | 16 | 1 | GEN, AMN, NET, TOB, CIP, FOS | - | + | - | bla GES-9 |
| S6 | Material | Surgery | 10 September 2014 | 16 | 8 | 2 | GEN, AMN, NET, TOB, CIP, FOS | - | + | - | bla GES-9 |
| S7 | Pulmonary | ICU | 23 September 2014 | 8 | 4 | 4 | GEN, AMN, NET, TOB, CIP, FOS | - | + | - | bla GES-5 |
| S8 | Pulmonary | Surgery | 30 October 2014 | 16 | 8 | 2 | GEN, AMN, NET, TOB, CIP, FOS | - | + | - | bla GES-5 |
| S9 | Pulmonary | ICU | 24 December 2014 | 512 | 256 | 1 | GEN, AMN, NET, TOB, CIP, FOS | + | + | - | bla VIM-2 |
| S10 | Pulmonary | ICU | 20 December 2014 | 8 | 4 | 1 | GEN, NET, TOB, CIP, FOS | - | + | - | bla GES-5 |
| S11 | Urine | EC | 06 February 2015 | 8 | 8 | 4 | GEN, AMN, NET, TOB, CIP, FOS | - | - | - | - |
| S12 | Pulmonary | Surgery | 27 June 2015 | 16 | 16 | 4 | GEN, AMN, NET, TOB, CIP, FOS | - | + | - | - |
| S13 | Urine | EC | 30 June 2015 | 16 | 8 | 4 | GEN, NET, TOB, CIP, FOS | - | + | - | - |
| S14 | Pulmonary | Surgery | 19 September 2015 | 8 | 8 | 2 | GEN, AMN, NET, TOB, CIP, FOS | - | + | - | bla GES-5 |
| S15 | Pus | Surgery | 30 September 2015 | 128 | 128 | 4 | GEN, AMN, NET, TOB, CIP, FOS | - | + | - | bla GES-5 , bla VIM-11 |
| S16 | Pus | ICU | 18 March 2016 | 16 | 8 | 2 | GEN, NET, TOB, CIP, FOS | + | + | + | bla VIM-2 |
| S17 | Pulmonary | ICU | 06 April 2016 | 16 | 8 | 2 | AMN, NET, TOB, CIP, FOS | - | + | - | bla GES-5 |
| S18 | Pus | ICU | 26 May 2016 | 32 | 32 | 16 | GEN, AMN, NET, TOB, CIP, FOS | - | + | - | bla GES-5 |
| S19 | Blood | ICU | 13 June 2016 | 4 | 4 | 2 | GEN, AMN, NET, TOB, FOS | + | + | - | bla VIM-2 |
| S20 | Blood | ICU | 12 July 2016 | 32 | 8 | 2 | GEN, AMN, NET, TOB, CIP, FOS | - | + | - | blaGES-5, blaVIM-2 |
| S21 | Blood | Surgery | 18 August 2016 | 32 | 16 | 2 | AMN, NET, TOB, CIP, FOS | - | + | - | bla GES-5 |
| S22 | Blood | ICU | 16 August 2016 | 32 | 8 | 4 | GEN, AMN, NET, TOB, CIP, FOS | - | + | - | bla GES-5 |
| S23 | Pulmonary | Surgery | 21 August 2016 | 16 | 8 | 4 | AMN, NET, TOB, CIP, FOS | + | + | - | bla GES-5 |
| S24 | Puncture | Surgery | 13 October 2016 | 16 | 8 | 1 | GEN, AMN, NET, TOB, CIP, FOS | - | + | - | bla GES-5 |
| S25 | Pulmonary | Surgery | 31 October 2016 | 16 | 8 | 2 | GEN, AMN, NET, TOB, CIP, FOS | + | + | - | bla GES-5 |
| S26 | Material | ICU | 25 October 2016 | 16 | 8 | 2 | GEN, AMN, NET, TOB, CIP, FOS | - | - | - | - |
| S27 | Pulmonary | ICU | 15 February 2017 | 128 | 64 | 1 | AMN, NET, TOB, CIP, FOS | + | + | - | bla VIM-2 |
| S28 | Pus | ICU | 17 February 2017 | 16 | 8 | 1 | GEN, AMN, NET, TOB, CIP, FOS | - | + | - | - |
| S29 | Pulmonary | ICU | 24 June 2017 | 16 | 8 | 1 | GEN, AMN, NET, TOB, CIP, FOS | - | - | - | - |
| S30 | Urine | Urology | 28 August 2017 | 16 | 8 | 2 | GEN, AMN, NET, TOB, CIP, FOS | - | + | - | - |
| S31 | Rectal | ICU | 16 September 2017 | 16 | 8 | 1 | GEN, AMN, NET, TOB, CIP, FOS | + | + | - | bla GES-5 |
| S32 | Pulmonary | ICU | 09 October 2017 | 8 | 4 | 1 | GEN, AMN, NET, TOB, CIP, FOS | + | + | - | bla VIM-2 |
| S33 | Rectal | ICU | 09 October 2017 | 32 | 32 | 4 | GEN, AMN, NET, TOB, CIP, FOS | + | + | + | bla VIM-2 |
| S34 | Rectal | ICU | 22 November 2017 | 64 | 32 | 2 | GEN, AMN, NET, TOB, CIP, FOS | + | + | - | bla GES-5 |
| S35 | Pulmonary | ICU | 30 November 2017 | 32 | 16 | 2 | GEN, AMN, NET, TOB, CIP, FOS | + | + | - | bla GES-5 |
EC: External consultation; ICU: Intensive care unit; *: MICs interpretive standard; mCIM: modified carbapenem inactivation method; CIMTris: carbapenem inactivation method Tris; mHodge test: modified Hodge test; +: Positive test; -: Negative; GEN: gentamicin; TOB: tobramycin; AMN: amikacin; NET: netilmicin; CIP: ciprofloxacin; FOS: Fosfomycin.
Of the 35 ICRPA strains tested, 26 (74.2%) harbored acquired carbapenemase-encoding genes, and 24 of these were positive on CIMTris. Four of the nineisolates not harboring acquired carbapenemase genes were negative on CIMTris, whereas for the remaining fivewere positive. Thus, CIMTris showed 96.15% sensitivity and 44.44% specificity for detecting carbapenemase production.
The testing of mCIM in ICRPA isolates showed that 12 of 26 (46.15%) isolates harboring carbapenemase genes were positive on mCIM. All the 14 mCIM-negative isolates harbored acquired carbapenemase genes. Nine of the nine(100%) isolates not harboring acquired carbapenemase genes were negative on mCIM. Thus, mCIM showed a sensitivity of 46.15% and a specificity of 100% for detecting carbapenemase activities (Table 3 and Table 4).
Table 3.
Comparison of three phenotypic methods for carbapenemase detection in Pseudomonas aeruginosa strains.
| mCIM | CIMTris | mHodge Test | |
|---|---|---|---|
| True positive | 12 | 28 | 3 |
| True negative | 8 | 4 | 8 |
| False positive | 0 | 3 | 0 |
| False negative | 15 | 0 | 24 |
| Specificity (%) | 100 | 90,4 | 100 |
| Sensitivity (%) | 34.8 | 100 | 25 |
mCIM: modified carbapenem inactivation method; CIMTris: carbapenem inactivation method Tris; mHodge test: modified Hodge test; %: percentage.
Table 4.
Comparison of three phenotypic methods for carbapenemase detection in Pseudomonas aeruginosa strains according to carbapenemase encoding genes.
| Phenotypic Tests | PCR Results | |||
|---|---|---|---|---|
| Carbapenemase Coding Genes | bla VIM | bla GES | ||
| mHodge test | Positive (n) | 3 | 1 | |
| Negative (n) | 24 | 15 | ||
| Sensitivity (%) | 12.5 | 30 | 5 | |
| Specificity (%) | 100 | 100 | 86.66 | |
| mCIM | Positive (n) | 7 | 5 | |
| Negative (n) | 20 | 10 | ||
| Sensitivity (%) | 46.15 | 63.7 | 26.31 | |
| Specificity (%) | 100 | 83.33 | 62.5 | |
| CIMTris | Positive (n) | 9 | 15 | |
| Negative (n) | 7 | 5 | ||
| Sensitivity (%) | 96.15 | 81.81 | 74 | |
| Specificity (%) | 44.44 | 29 | 21.42 | |
mCIM: modified carbapenem inactivation method; mHodge test: modified Hodge test; CIMTris: carbapenem inactivation method Tris.
4. Discussion
In our study, most of the collected ICRPA strains were isolated from low respiratory samples in ICU patients, confirming the data of a previous study [17]. It has been reported that most of the nosocomial infections caused by carbapenemase-producing P. aeruginosa (CPPA)most frequently affect patients with pneumonia associated with mechanical ventilation, and this is the main cause of chronic respiratory infection in immunocompromised patients.
Our study revealed that all isolated ICRPA remained susceptible only to colistin, indicating the dissemination of multidrug-resistant (MDR) strains and an emerging problem in our hospital. The problem of bacterial resistance to commonly used antibiotics is worldwide [2,5,8,17]. The management of ICRPA infections represents a difficult therapeutic challenge due to the increasing resistance levels of these organisms to most classes of antimicrobial agents.
The acquisition of carbapenemase genes by P. aeruginosa is an important cause of MDR, and therefore, the rapid and correct detection of carbapenemases is crucial [2]. Molecular methods are the gold standard in the identification of carbapenemase-producing strains, but phenotypic methods have been developed due to the high cost of molecular methods and their inability to detect new carbapenemase genes [2]. However, some phenotypic assays are still not accepted for non-fermentative Gram-negative bacilli [10]. In this study, we evaluate the performance of three phenotypic methods in the detection of carbapenemase-producing P.aeruginosa, including the modified Hodge test, mCIM, and CIMTris. The CIMTris differed from the mCIM by the Tris-HCl buffer that was used instead of water during the MEM inactivation step. The CIMTris showed markedly higher sensitivity than the mCIM and modified Hodge test (96.1% vs. 46.1 and 12.5%). The Tris-HCl buffer used in the CIMTris seemed to effectively extract carbapenemases of class A and B. However, the specificity of the CIMTris was lower than the other two methods.This could probably be explained by the degradation of meropenem by other carbapenemases that werenot detected in this study.Our results show that CIMTris is useful, simple, and accessible to clinical laboratories for detecting carbapenemase production in ICRPA, but PCR is needed to confirm the presence of carbapenemase-encoding genes, as previously reported [10].
Of the 35 ICRPA strains, 26 were harboring carbapenemase-encoding genes, and 16 strains were carrying class A beta-lactamases. The coexistence of blaGES-5 and blaVIM-11 was found in one strain. Thus, our results show a predominance of blaGES-5, which is not in agreement with earlier studies carried out in Tunisia [6,8] and worldwide [2,3,4] that showeda dissemination of blaVIM-2. Interestingly, we report here for the first time in North Africa the emergence of blaGES-5 and blaGES-9 harboring CPPA isolates thathad been reported in European [18,19], Asian [20], South African [21], and South American [22] studies.
The high levels of resistance among isolated P. aeruginosa, especially in ICUs where there are critically ill patients who underwent invasive procedures using multiple devices and broader spectrum antibiotics, emphasizes the need for measures to prevent the clinical dissemination of these isolates. A similar scenario was described by Koutsogiannou et al., who also reported the clonal dissemination of MDR P. aeruginosa in a university hospital [23].
5. Conclusions
Carbapenemase enzymes among P. aeruginosa isolates in Tunisia remain poorly investigated. This study reveals new information about carbapenemase enzymes among P. aeruginosa isolates in Tunisia by demonstrating the first detection of of GES-5 and GES-9 carbapenemases as well as the co-occurrence of blaGES-5 and blaVIM-11 in one isolate. Moreover, the CIMTris is a promising approach for detecting carbapenemase activity in P. aeruginosa and merits further testing. These findings are of great concern because the rapid dissemination of MDR strains represents a major therapeutic and epidemiological threat and requires the implementation of strict hygiene procedures and regular surveillance studies.
Author Contributions
Conceptualization, S.F. and I.B.B.B.; methodology, S.F and E.M.; software, S.F. and E.M.; validation, S.F., E.M., A.F., L.K. and I.B.B.B.; formal analysis, S.F. and E.M.; investigation, S.F. and E.M.; resources, A.F and L.K.; data curation, S.F and E.M.; writing—original draft, S.F., E.M. and I.B.B.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Patient consent was waived, only specimen from routine diagnostic were used.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Ministry of Higher Education and Scientific Research of Tunisia.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dantas R.C., Ferreira M.L., Gontijo-Filho P.P., Ribas R.M. Pseudomonas aeruginosa bacteraemia: Independent risk factors for mortality and impact of resistance on outcome. J. Med. Microbiol. 2014;63:1679–1687. doi: 10.1099/jmm.0.073262-0. [DOI] [PubMed] [Google Scholar]
- 2.Gniadek T.J., Carroll K.C., Simner P.J. Carbapenem-resistant non-glucose-fermenting gram-negative bacilli: The missing piece to the puzzle. J. Clin. Microbiol. 2016;54:1700–1710. doi: 10.1128/JCM.03264-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kateete D.P., Nakanjako R., Namugenyi J., Erume J., Joloba M.L., Najjuka C. Carbapenem resistant Pseudomonas aeruginosa and Acinetobacter baumannii at Mulago Hospital in Kampala, Uganda (2007–2009) SpringerPlus. 2016;5:1308. doi: 10.1186/s40064-016-2986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livermore D.M., Woodford N. Carbapenemases: A problem in waiting? Curr. Opin. Microbiol. 2000;3:489–495. doi: 10.1016/S1369-5274(00)00128-4. [DOI] [PubMed] [Google Scholar]
- 5.Poirel L., Naas T., Nicolas D., Collet L., Bellais S., Cavallo J.-D., Nordmann P. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-beta -lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 2000;44:891–897. doi: 10.1128/AAC.44.4.891-897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chairat S., Ben Yahia H., Rojo-Bezares B., Sáenz Y., Torres C., Ben Slama K. High prevalence of imipenem-resistant and metallo-beta-lactamase-producing Pseudomonas aeruginosa in the burns hospital in Tunisia: Detection of a novel class 1 integron. J. Chemother. 2019;31:120–126. doi: 10.1080/1120009X.2019.1582168. [DOI] [PubMed] [Google Scholar]
- 7.Ktari S., Mnif B., Znazen A., Rekik M., Mezghani S., Mahjoubi-Rhimi F., Hammami A. Diversity of β-lactamases in Pseudomonas aeruginosa isolates producing metallo-β-lactamase in two Tunisian hospitals. Microb. Drug Resist. 2011;17:25–30. doi: 10.1089/mdr.2010.0104. [DOI] [PubMed] [Google Scholar]
- 8.Hammami S., Boutiba-Ben Boubaker I., Ghozzi R., Saidani M., Amine S., Ben Redjeb S. Nosocomial outbreak of imipenem-resistant Pseudomonas aeruginosa producing VIM-2 Metallo-β-lactamase in a kidney transplantation unit. Diagn. Pathol. 2011;6:106. doi: 10.1186/1746-1596-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diene S.M., Rolain J.-M. Carbapenemase genes and genetic platforms in gram-negative bacilli: Enterobacteriaceae, Pseudomonas and Acinetobacter species. Clin. Microbiol. Infect. 2014;20:831–838. doi: 10.1111/1469-0691.12655. [DOI] [PubMed] [Google Scholar]
- 10.Uechi K., Tada T., Shimada K., Kuwahara-Arai K., Arakaki M., Tome T., Nakasone I., Maeda S., Kirikae T., Fujita J. A modified carbapenem inactivation method, CIMTris, for carbapenemase production in Acinetobacter and Pseudomonas species. J. Clin. Microbiol. 2017;55:3405–3410. doi: 10.1128/JCM.00893-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucena A., Costa L.M.D., Da Nogueira K.S., Matos A.P., Gales A.C., Raboni S.M. Comparison of phenotypic tests for the detection of metallo-beta-lactamases in clinical isolates of Pseudomonasaeruginosa. Enferm. Infecc. Microbiol. Clínica. 2014;32:625–630. doi: 10.1016/j.eimc.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Leeflang M.M.G., Allerberger F. How to: Evaluate a diagnostic test. Clin. Microbiol. Infect. 2019;25:54–59. doi: 10.1016/j.cmi.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Ilstrup D.M. Statistical methods in microbiology. Clin. Microbiol. Rev. 1990;3:219–226. doi: 10.1128/CMR.3.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dallenne C., Da Costa A., Decré D., Favier C., Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010;65:490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 15.Poirel L., Walsh T.R., Cuvillier V., Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011;70:119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Sanger F., Nicklen S., Coulson A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta R., Malik A., Rizvi M., Ahmed S.M. Incidence of multidrug-resistant Pseudomonas spp. in ICU patients with special reference to ESBL, AMPC, MBL and biofilm production. J. Glob. Infect. Dis. 2016;8:25. doi: 10.4103/0974-777X.176142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malkoçoğlu G., Aktaş E., Bayraktar B., Otlu B., Bulut M.E. VIM-1, VIM-2, and GES-5 carbapenemases among Pseudomonas aeruginosa isolates at a tertiary hospital in Istanbul, Turkey. Microb. Drug Resist. 2017;23:328–334. doi: 10.1089/mdr.2016.0012. [DOI] [PubMed] [Google Scholar]
- 19.Viedma E., Juan C., Acosta J., Zamorano L., Otero J.R., Sanz F., Chaves F., Oliver A. Nosocomial spread of colistin-only-sensitive sequence type 235 Pseudomonas aeruginosa isolates producing the extended-spectrum-lactamases GES-1 and GES-5 in Spain. Antimicrob. Agents Chemother. 2009;53:4930–4933. doi: 10.1128/AAC.00900-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hishinuma T., Tada T., Kuwahara-Arai K., Yamamoto N., Shimojima M., Kirikae T. Spread of GES-5 carbapenemase-producing Pseudomonasaeruginosa clinical isolates in Japan due to clonal expansion of ST235. PLoS ONE. 2018;13:e0207134. doi: 10.1371/journal.pone.0207134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labuschagne C.D.J., Weldhagen G.F., Ehlers M.M., Dove M.G. Emergence of class 1 integron-associated GES-5 and GES-5-like extended-spectrum β-lactamases in clinical isolates of Pseudomonas aeruginosa in South Africa. Int. J. Antimicrob. Agents. 2008;31:527–530. doi: 10.1016/j.ijantimicag.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 22.Picao R.C., Poirel L., Gales A.C., Nordmann P. Diversity of -lactamases produced by ceftazidime-resistant Pseudomonasaeruginosa isolates causing bloodstream infections in Brazil. Antimicrob. Agents Chemother. 2009;53:3908–3913. doi: 10.1128/AAC.00453-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koutsogiannou M., Drougka E., Liakopoulos A., Jelastopulu E., Petinaki E., Anastassiou E.D., Spiliopoulou I., Christofidou M. Spread of multidrug-resistant Pseudomonas aeruginosa clones in a university hospital. J. Clin. Microbiol. 2013;51:665–668. doi: 10.1128/JCM.03071-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.