Abstract
The relationship between antibiotic use and Clostridioides difficile (C. difficile) has been well established in adults and older children but remains unclear and is yet to be fully examined in infant populations. This study aimed to determine the separate and cumulative impact from antibiotics and household cleaning products on C. difficile colonization in infants. This study included 1429 infants at 3–4 months of age and 1728 infants at 12 months of age from the Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort. The levels of infant antimicrobial exposure were obtained from hospital birth charts and standardized questionnaires. Infant gut microbiota was characterized by Illumina 16S ribosomal ribonucleic acid (rRNA) gene sequencing. Analysis of C. difficile was performed using a quantitative polymerase chain reaction (qPCR). Overall, C. difficile colonized 31% and 46% of infants at 3–4 months and 12 months, respectively. At 3–4 months, C. difficile colonization was significantly higher in infants exposed to both antibiotics and higher (above average) usage of household cleaning products (adjusted odds ratio (aOR) 1.50, 95% CI 1.03–2.17; p = 0.032) than in infants who had the least antimicrobial exposure. This higher colonization persisted up to 12 months of age. Our study suggests that cumulative exposure to systemic antibiotics and higher usage of household cleaning products facilitates C. difficile colonization in infants. Further research is needed to understand the future health impacts.
Keywords: antibiotics, cleaning products, antimicrobials, Clostridioides difficile, C. difficile, infant, gut microbiota
1. Introduction
Global antibiotic consumption has risen substantially over the past two decades [1]. While the administration of antibiotics directly to infants has reduced by more than 50% in Canada [2], an overlooked source of infant antibiotic exposure is the administration of antibiotics to mothers during childbirth. The guidelines of the Society of Obstetricians and Gynecologists of Canada (SOGC) recommend antibiotic prophylaxis before caesarean section or during labor (intrapartum antibiotic prophylaxis, IAP) for women who are positive for Group B Streptococcus (GBS) or who have other risk factors [3]. In accordance with these recommendations, up to 40% of newborns are exposed before or during delivery to maternal IAP [4]. Furthermore, about 2% to 5% of vaginally delivered newborns receive intravenous (IV) antibiotics after birth for treatment of suspected neonatal sepsis [4,5]. Outside of the hospital setting, household standards of cleaning have also evolved over the years, in response to various socio-cultural factors. The commercialization of the cleaning industry has encouraged the increased use of cleaning products in the home, contributing to antimicrobial exposure [6].
Antimicrobial exposure during infancy is not without consequences. Epidemiological studies have shown that early life antimicrobial exposure is associated with disruption of gut microbiota [7,8,9,10] and influences future asthma and allergic diseases [11,12]. Multiple courses of antibiotics have a great influence on the composition of infant gut microbiota [13]; however, cumulative antimicrobial exposure from additional sources, such as household cleaning products, have not been studied. Clostridiodes (formerly Clostridium) difficile (C. difficile) is a gram-positive spore forming bacteria. It is a major pathogen that is responsible for the clinical manifestations of antibiotic-induced diarrhea in adults and older children [14]. Although the colonization rate is high (over 40%) in infants below the age of 1 year, the biological relevance of C. difficile in this age group remains uncertain, as most colonized infants do not manifest clinical symptoms [15]. However, colonization in infancy may serve as a reservoir for adult C. difficile infections (CDI) or a marker for reduced colonization resistance and delayed gut microbiota maturation [16,17,18]. Disruption of the gut microbiome early in life may be associated with conditions such as allergy and asthma, inflammatory bowel disease (IBD), and obesity later in life [19]. Antimicrobial exposure can destroy the diversity of the gut microbiome, limiting the number of microbes that are in competition for growth, thereby allowing C. difficile colonization and overgrowth. Previous studies proposed clear effects of antimicrobial exposure on the infant gut microbiota, but those studies were limited to small samples or reporting at the genus level [7,8,9,10]. The aim of our study was to determine the separate and cumulative impact of antibiotics and environmental antimicrobials (i.e., household cleaning products) on C. difficile colonization, and to understand how these antimicrobial exposures modify the infant gut microbiota. This area of research is currently understudied, and the issues are not fully understood.
2. Results
2.1. Study Population
In study infants at 3 months of age, 29% of them were exposed to no antibiotics and lower (below average) usage of cleaning products (NALC); 24% were exposed to any antibiotics and lower (below average) usage of cleaning products (AALC); 22% were exposed to no antibiotics and higher (above average) usage of cleaning products (NAHC); and 25% were exposed to any antibiotics and higher (above average) usage of cleaning products (AAHC) (Table 1). All caesarean section (CS) deliveries by participants in the Canadian Healthy Infant Longitudinal Development (CHILD) study received antibiotic prophylaxis, in accordance with Canadian practice guidelines. Fecal samples were collected from 1429 infants at 3–4 months of age (mean age 3.6 ± 1.04 months) and 1728 infants at 12 months of age (mean age 12.2 ± 1.48 months). Of note, the 12-month sample was larger because stool samples from participants were easier to collect for analyses. In general, our sample of infants at both 3–4 months of age and 12 months of age did not differ from the overall CHILD cohort (Supplementary Table S1).
Table 1.
Population characteristics according to antimicrobial exposure at 3 months (3–4 months-old sample; N = 1429).
| Row Percentages | Total a | NALC * n = 406 |
AALC * n = 346 |
NAHC * n = 318 |
AAHC * n = 359 |
p Value |
|---|---|---|---|---|---|---|
| 29% | 24% | 22% | 25% | |||
| Maternal age | ||||||
| 18–29 | 503 | 148 (29%) | 105 (21%) | 137 (27%) | 113 (22%) | <0.001 |
| 30–39 | 874 | 241 (26%) | 220 (25%) | 177 (20%) | 236 (27%) | |
| ≥40 | 52 | 17 (33%) | 21 (40%) | 4 (8%) | 10 (19%) | |
| Maternal race | ||||||
| Caucasian | 1072 | 270 (25%) | 271 (25%) | 246 (23%) | 285 (27%) | <0.001 |
| Asian | 203 | 108 (53%) | 38 (18%) | 33 (16%) | 24 (12%) | |
| Other | 141 | 27 (19%) | 34 (24%) | 35 (25%) | 45 (32%) | |
| Family income | ||||||
| <50,000 | 199 | 34 (17%) | 31 (16%) | 54 (27%) | 80 (40%) | <0.001 |
| 50,000–99,999 | 487 | 134 (27%) | 121 (25%) | 111 (23%) | 121 (25%) | |
| ≥100,000 | 516 | 207 (40%) | 158 (31%) | 105 (20%) | 46 (9%) | |
| Prefer not to answer | 130 | 26 (20%) | 25 (19%) | 33 (26%) | 46 (35%) | |
| Birth method | ||||||
| Vaginal | 1096 | 406 (37%) | 211 (19%) | 317 (29%) | 162 (15%) | <0.001 |
| CS-elective | 134 | 0 | 52 (39%) | 0 | 82 (61%) | |
| CS-emergency | 194 | 0 | 82 (42%) | 0 | 112 (58%) | |
| Gestational age | ||||||
| <39 weeks | 372 | 73 (20%) | 107 (29%) | 73 (20%) | 119 (32%) | <0.001 |
| ≥39 weeks | 1052 | 333 (32%) | 238 (23%) | 245 (23%) | 236 (22%) | |
| Infant Sex | ||||||
| Male | 766 | 206 (27%) | 196 (26%) | 161 (21%) | 203 (27%) | 0.172 |
| Female | 663 | 200 (30%) | 150 (23%) | 157 (24%) | 156 (24%) | |
| Breastfeeding at 3 Months | ||||||
| Exclusive | 791 | 262 (33%) | 209 (26%) | 153 (19%) | 167 (21%) | <0.001 |
| Mixed | 384 | 98 (26%) | 88 (23%) | 85 (22%) | 113 (29%) | |
| Formula | 251 | 45 (18%) | 48 (19%) | 80 (32%) | 78 (31%) | |
| Furry pet | ||||||
| No | 777 | 258 (33%) | 205 (26%) | 258 (20%) | 205 (21%) | <0.001 |
| Yes | 648 | 146 (23%) | 141 (22%) | 166 (26%) | 195 (30%) | |
| Older sibling | ||||||
| No | 712 | 189 (27%) | 194 (27%) | 125 (18%) | 204 (29%) | <0.001 |
| Yes | 712 | 216 (30%) | 150 (21%) | 193 (27%) | 153 (21%) | |
| Smoke exposure | ||||||
| No | 1160 | 352 (30%) | 290 (25%) | 251 (22%) | 267 (23%) | <0.001 |
| Yes | 248 | 51 (21%) | 50 (20%) | 62 (25%) | 85 (34%) |
* Antimicrobial exposure (NALC: no antibiotics and lower usage of cleaning products; AALC: any antibiotics and lower usage of cleaning products; NAHC: no antibiotics and higher usage of cleaning products; AAHC: any antibiotics and higher usage of cleaning products). a Total may not add up due to missing data; IAP: intrapartum antibiotic prophylaxis; p-value calculated using Pearson chi-square test and displayed in bold when statistically significant.
2.2. Study Population and C. difficile Colonization
2.2.1. C. difficile Colonization at 3–4 Months of Age
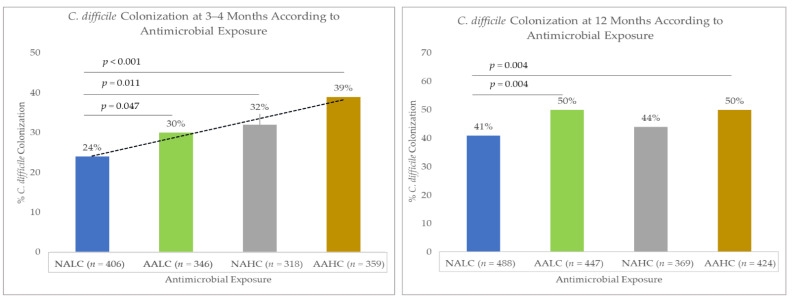
In our study, the prevalence of C. difficile colonization in infants at 3–4 months of age was 31% (445/1429). The C. difficile colonization of infants differed according to antimicrobial exposure: 24% for NALC, 30% for AALC, 32% for NAHC, and 39% for AAHC (p < 0.001; Figure 1). Before adjusting for covariates, the odds of C. difficile colonization were 38% higher (odds ratio (OR): 1.38, 95% confidence interval (CI) 1.00–1.91; p = 0.047) in the AALC infants, 52% higher (OR: 1.52, 95% CI 1.10–2.11; p = 0.011) in the NAHC infants, and 103% higher (OR: 2.03, 95% CI 1.49–2.78; p < 0.001) in the AAHC infants, compared with the NALC infants (Table 2). The initial selection of covariates for model testing is shown in the directed acyclic graph (DAG; Supplementary Figure S1). After adjusting for covariates in the final model (i.e., maternal age, birth method, and breastfeeding), C. difficile colonization remained significantly higher only for infants in the cumulative exposure (AAHC) group (adjusted odds ratio (aOR): 1.50, 95% CI 1.03–2.17; p = 0.032), compared with those infants with no antibiotics and lower usage of cleaning products (NALC). C. difficile colonization was also higher in the AALC and NAHC infants, compared with the NALC infants, but did not attain statistical significance at p < 0.05 (Table 2).
Figure 1.
C. difficile colonization according to antimicrobial exposure (NALC: no antibiotics and lower usage of cleaning products; AALC: any antibiotics and lower usage of cleaning products; NAHC: no antibiotics and higher usage of cleaning products; AAHC: any antibiotics and higher usage of cleaning products).
Table 2.
Univariable and multivariable logistic regression for antimicrobial exposure and C. difficile colonization at 3–4 months of age.
| Crude (Unadjusted) | Model 1 (Adjusted for Maternal and Birth Characteristics) | Model 2 (Adjusted for Postnatal Characteristics) | Model 3 (Adjusted for Maternal Age, Birth Method, Breastfeeding, Furry Pet, Older Siblingship, and Smoke Exposure) | Final Model (Adjusted for Maternal Age, Birth Method, and Breastfeeding) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) |
p Value | aOR (95% CI) |
p Value | aOR (95% CI) |
p Value | aOR (95% CI) |
p value | aOR (95% CI) |
p Value | |
| Antimicrobial Exposure * (ref = NALC) | ||||||||||
| AALC | 1.38 (1.00–1.91) |
0.047 | 1.23 (0.86–1.76) |
0.238 | 1.29 (0.92–1.80) |
0.136 | 1.17 (0.81–1.69) |
0.376 | 1.21 (0.84–1.73) |
0.293 |
| NAHC | 1.52 (1.10–2.11) |
0.011 | 1.48 (1.06–2.06) |
0.020 | 1.22 (0.86–1.72) |
0.252 | 1.19 (0.84–1.69) |
0.304 | 1.27 (0.90–1.78) |
0.166 |
| AAHC | 2.03 (1.49–2.78) |
<0.001 | 1.67 (1.16–2.41) |
0.006 | 1.53 (1.10–2.14) |
0.010 | 1.33 (0.90–1.95) |
0.141 | 1.50 (1.03–2.17) |
0.032 |
| Block 1: Maternal and birth characteristics | ||||||||||
| Maternal age (ref = 30–39) | ||||||||||
| 18–29 | 1.60 (1.34–2.13) |
<0.001 | 1.79 (1.41–2.27) |
<0.001 | 1.66 (1.29–2.14) |
<0.001 | 1.71 (1.34–2.18) |
<0.001 | ||
| ≥40 | 0.80 (0.41–1.55) |
0.513 | 0.79 (0.40–1.55) |
0.505 | 0.90 (0.45–1.79) |
0.768 | 0.86 (0.43–1.71) |
0.688 | ||
| Birth method (ref = Vaginal) | ||||||||||
| CS-elective | 1.42 (1.02–2.15) |
0.039 | 1.44 (0.93–2.22) |
0.095 | 1.29 (0.82–2.03) |
0.260 | 1.28 (0.83–1.99) |
0.251 | ||
| CS-emergency | 1.63 (1.19–2.24) |
0.002 | 1.54 (1.06–2.24) |
0.022 | 1.50 (1.02–2.22) |
0.038 | 1.49 (1.02–2.18) |
0.038 | ||
| Gestational age (ref ≤ 39weeks) | ||||||||||
| ≥39 weeks | 1.00 (0.77–1.29) |
0.979 | 1.08 (0.83–1.41) |
0.550 | ||||||
| Block 2: Postnatal characteristics | ||||||||||
| Breastfeeding a (ref = Exclusive) | ||||||||||
| Mixed | 1.96 (1.50–2.55) |
<0.001 | 1.88 (1.43–2.47) |
<0.001 | 1.89 (1.43–2.49) |
<0.001 | 1.88 (1.43–2.46) |
<0.001 | ||
| Formula | 3.11 (2.31–4.19) |
<0.001 | 2.78 (2.03–3.80) |
<0.001 | 2.65 (1.93–3.63) |
<0.001 | 2.71 (1.99–3.69) |
<0.001 | ||
| Furry pet (ref=No) | ||||||||||
| Yes | 1.38 (1.10–1.73) |
0.005 | 1.17 (0.92–1.50) |
0.180 | 1.19 (0.93–1.52) |
0.148 | ||||
| Older sibling (ref = No) | ||||||||||
| Yes | 0.77 (0.62–0.97) |
0.030 | 0.80 (0.63–1.02) |
0.073 | 0.87 (0.68–1.13) |
0.316 | ||||
| Smoke exposure (ref = No) | ||||||||||
| Yes | 1.77 (1.33–2.35) |
<0.001 | 1.43 (1.06–1.93) |
0.018 | 1.31 (0.97–1.78) |
0.075 | ||||
* Antimicrobial exposure in infants by 3 months of age (NALC: no antibiotics and lower usage of cleaning products; AALC: any antibiotics and lower usage of cleaning products, NAHC: no antibiotics and higher usage of cleaning products; AAHC: any antibiotics and higher usage of cleaning products). a Breastfeeding at 3 months of age; OR: odds ratio, aOR: adjusted odds ratio; CI: confidence interval; statistically significant p-values displayed in bold.
2.2.2. C. difficile Colonization at 12 Months of Age
In our study, the prevalence of C. difficile colonization in infants at 12 months of age was 46% (797/1728). C. difficile colonization rates in infants were different, depending on antimicrobial exposure: 41% for the NALC infants, 50% for the AALC infants, 44% for the NAHC infants, and 50% for the AAHC infants (p = 0.009; Figure 1). Before adjusting for covariates, the odds of colonization with C. difficile were 46% higher for both the AALC infants (OR: 1.46, 95% CI 1.12–1.88; p = 0.004) and the AAHC infants (OR: 1.46, 95% CI 1.12–1.90; p = 0.004), but not different for the NAHC infants (OR: 1.17, 95% CI 0.87–1.54; p = 0.256), compared with the NALC infants (Table 3). The covariates that were initially selected for testing in the models are shown in the directed acyclic graph (DAG; Supplementary Figure S2). After adjusting for covariates in the final model (i.e., birth method, breastfeeding, and older siblingship), C. difficile colonization remained significantly higher in both the AALC infants (aOR: 1.36, 95% CI 1.02–1.83; p = 0.035) and the AAHC infants (aOR: 1.37, 95% CI 1.00–1.86; p = 0.043), compared with the NALC infants (Table 3). To account for antibiotic administration between 3 months and 12 months of age, we performed sensitivity analysis that adjusted models for oral antibiotic treatment during the 3 to 12 month period, as well as excluded infants with this usage. Similar results were obtained (Supplementary Table S2).
Table 3.
Univariable and multivariable logistic regression for antimicrobial exposure and C. difficile colonization at 12 months of age.
| Crude (Unadjusted) | Model 1 (Adjusted for Birth Characteristics) | Model 2 (Adjusted for Postnatal Characteristics) | Final Model (Adjusted for Birth Method, Breastfeeding, and Older Siblingship) | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) |
p Value | aOR (95% CI) |
p Value | aOR (95% CI) |
p Value | aOR (95% CI) |
p Value | |
|
Antimicrobial Exposure *
(ref = NALC) |
||||||||
| AALC | 1.46 (1.12–1.88) |
0.004 | 1.44 (1.08–1.92) |
0.012 | 1.34 (1.02–1.75) |
0.030 | 1.36 (1.02–1.83) |
0.035 |
| NAHC | 1.17 (0.89–1.54) |
0.256 | 1.16 (0.88–1.53) |
0.272 | 1.13 (0.85–1.51) |
0.371 | 1.11 (0.84–1.48) |
0.442 |
| AAHC | 1.46 (1.12–1.90) |
0.004 | 1.47 (1.09–1.99) |
0.012 | 1.37 (1.04–1.80) |
0.023 | 1.37 (1.00–1.86) |
0.043 |
|
Block 1:
Birth characteristics |
||||||||
| Birth method (ref = Vaginal) | ||||||||
| CS-elective | 1.17 (0.86–1.61) |
0.305 | 0.90 (0.63–1.28) |
0.575 | 1.13 (0.78–1.63) |
0.511 | ||
| CS-emergency | 1.22 (0.93–1.59) |
0.148 | 0.97 (1.71–1.33) |
0.892 | 0.86 (0.63–1.18) |
0.374 | ||
| Gestational age (ref ≤ 39weeks) | ||||||||
| ≥39 weeks | 0.81 (0.66–1.01) |
0.067 | 0.85 (0.68–1.06) |
0.170 | ||||
| Block 2: Postnatal characteristics | ||||||||
| Breastfeeding a (ref = Yes) | ||||||||
| No | 1.10 (0.91–1.34) |
0.297 | 1.09 (0.89–1.33) |
0.369 | 1.08 (0.89–1.32) |
0.399 | ||
|
Furry pet
(ref = No) |
||||||||
| Yes | 0.89 (0.73–1.07) |
0.236 | 0.84 (0.68–1.02) |
0.091 | ||||
| Older sibling (ref = No) | ||||||||
| Yes | 0.60 (0.49–0.72) |
<0.001 | 0.60 (0.49–0.73) |
<0.001 | 0.59 (0.48–0.73) |
<0.001 | ||
| Smoke Exposure (ref = No) | ||||||||
| Yes | 0.94 (0.73–1.22) |
0.690 | 0.91 (0.70–1.19) |
0.533 | ||||
Notes: * Antimicrobial exposure in infants by 3 months of age (NALC: no antibiotics and lower usage of cleaning products; AALC: any antibiotics and lower usage of cleaning products; NAHC: no antibiotics and higher usage of cleaning products; AAHC: any antibiotics and higher usage of cleaning products); a Breastfeeding at 12 months; OR: odds ratio, aOR: adjusted odds ratio; CI: confidence interval; statistically significant p-values displayed in bold.
2.2.3. Persistent C. difficile Colonization
In a smaller subset of 653 infants, 28% (184/653) were colonized with C. difficile at both 3–4 of age and 12 months of age. We classified this process as “persistent colonization”. Persistent C. difficile colonization was present in 18% of the NALC infants, 28% of the AALC infants, 26% of the NAHC infants, and 42% of the AAHC infants. Overall, antimicrobial exposure influenced persistent C. difficile colonization (p < 0.001). After adjusting for covariates in the final model (i.e., birth method, breastfeeding, and older siblingship), persistent C. difficile colonization remained significantly higher only in the AAHC infants (aOR: 2.40, 95% CI 1.33–4.35; p = 0.004), compared with the NALC infants (Table 4).
Table 4.
Univariable and multivariable logistic regression for antimicrobial exposure and persistent C. difficile colonization.
| Crude (Unadjusted) | Model 1 (Adjusted for Maternal and Birth Characteristics) | Model 2 (Adjusted for Postnatal Characteristics) | Model 3 (Adjusted for Maternal Age, Birth Method, Breastfeeding, Older Siblingship, and Smoke Exposure) | Final Model (Adjusted for Birth Method, Breastfeeding, and Older Siblingship) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) |
p Value | aOR (95% CI) |
p Value | aOR (95% CI) |
p Value | aOR (95% CI) |
p Value | aOR (95% CI) |
p Value | |
| Antimicrobial Exposure * (ref = NALC) | ||||||||||
| AALC | 1.75 (1.06–2.89) |
0.028 | 1.57 (0.91–2.71) |
0.104 | 1.38 (0.81–2.35) |
0.235 | 1.33 (0.75–2.37) |
0.322 | 1.31 (0.74–2.13) |
0.337 |
| NAHC | 1.59 (0.95–2.67) |
0.075 | 1.61 (0.96–2.71) |
0.071 | 1.36 (0.77–2.39) |
0.481 | 1.39 (0.79–2.44) |
0.241 | 1.50 (0.87–2.57) |
0.142 |
| AAHC | 3.20 (1.96–5.23) |
<0.001 | 2.77 (1.55–4.92) |
0.001 | 2.55 (1.51–4.33) |
<0.001 | 2.42 (1.32–4.44) |
0.004 | 2.40 (1.33–4.35) |
0.004 |
| Block 1: Maternal and birth characteristics | ||||||||||
| Maternal age (ref = 30–39) | ||||||||||
| 18–29 | 1.48 (1.04–2.13) |
<0.030 | 1.61 (1.11–2.34) |
0.011 | 1.24 (0.82–1.85) |
0.294 | ||||
| ≥40 | 0.76 (0.27–2.09) |
0.598 | 0.74 (0.26–2.11) |
0.584 | 0.99 (0.34–2.92) |
0.998 | ||||
| Birth method (ref = Vaginal) | ||||||||||
| CS-elective | 1.66 (0.94–2.94) |
0.079 | 1.03 (0.53–2.01) |
0.921 | 1.21 (0.60–2.45) |
0.579 | 1.19 (0.59–2.39) |
0.609 | ||
| CS-emergency | 2.10 (1.32–3.35) |
0.002 | 1.51 (0.87–2.61) |
0.141 | 1.18 (0.65–2.12) |
0.574 | 1.16 (0.65–2.06) |
0.601 | ||
| Gestational age (ref ≤ 39 weeks) | ||||||||||
| ≥39 weeks | 0.75 (0.51–1.10) |
0.151 | 0.77 (0.52–1.15) |
0.217 | ||||||
| Block 2: Postnatal characteristics | ||||||||||
| Breastfeeding a (ref = Exclusive) | ||||||||||
| Mixed | 2.30 (1.53–3.44) |
<0.001 | 2.42 (1.58–3.72) |
<0.001 | 2.35 (1.53–3.62) |
<0.001 | 2.22 (1.45–3.39) |
<0.001 | ||
| Formula | 3.34 (2.11–5.28) |
<0.001 | 2.55 (1.55–4.21) |
<0.001 | 2.44 (1.48–4.03) |
<0.001 | 2.83 (1.74–4.58) |
<0.001 | ||
| Furry pet (ref = No) | ||||||||||
| Yes | 1.26 (0.89–1.77) |
0.180 | 0.93 (0.63–1.37) |
0.749 | ||||||
| Older sibling (ref = No) | ||||||||||
| Yes | 0.38 (0.26–0.54) |
<0.001 | 0.36 (0.24–0.54) |
<0.001 | 0.38 (0.25–0.58) |
<0.001 | 0.40 (0.27–0.59) |
<0.001 | ||
| Smoke exposure (ref = No) | ||||||||||
| Yes | 1.63 (1.05–2.54) |
0.029 | 1.58 (0.97–2.59) |
0.064 | 1.50 (0.92–2.45) |
0.103 | ||||
* Antimicrobial exposure in infants by 3 months of age. (NALC: no antibiotics and lower usage of cleaning products; AALC: any antibiotics and lower usage of cleaning products; NAHC: no antibiotics and higher usage of cleaning products; AAHC: any antibiotics and higher usage of cleaning products); a Breastfeeding at 3 months of age; OR: odds ratio, aOR: adjusted odds ratio; CI: confidence interval; statistically significant p-values displayed in bold.
2.2.4. Stratified Analysis of Antimicrobial Effects on C. difficile Colonization
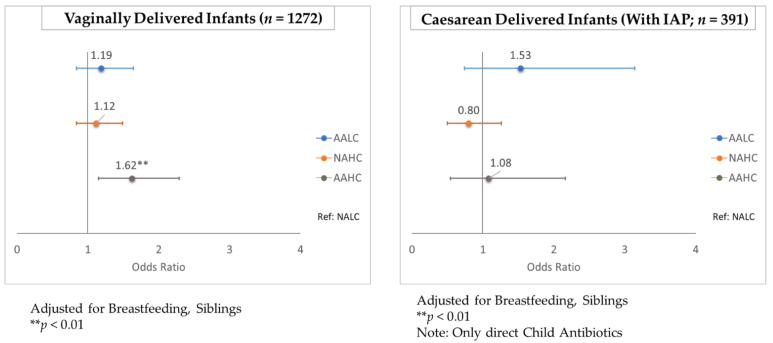
In a subset of vaginally-delivered infants, C. difficile colonization was higher for the AAHC infants compared with the NALC infants at both 3–4 months of age and 12 months of age (aOR: 1.59, 95% CI 1.06–2.38; p = 0.025 and aOR: 1.62, 95% CI 1.15–2.29; p = 0.005, respectively). This association was not observed in caesarean-delivered infants, for whom there was already a greater risk of C. difficile colonization (Figure 2 and Figure 3; Supplementary Table S3). All caesarean-delivered infants were exposed to maternal IAP, so the reference group (NALC) for the vaginal-birth subanalysis consisted of infants who did not receive any direct antibiotics (Figure 2 and Figure 3). In a stratified analysis by sex, no difference was observed between boys and girls with respect to the impact of antimicrobial exposure on C. difficile colonization (Supplementary Figure S3). In infants without an older sibling, C. difficile colonization at 12 months of age was higher for the AALC infants (aOR: 1.58, 95% CI 1.07–2.35; p = 0.021) and the AAHC infants (aOR: 1.77, 95% CI 1.16–2.70; p = 0.008), compared with the NALC infants. This association was not observed in infants who had an older sibling (Supplementary Figure S4). Individual adjustments for each covariate showed that none were strong enough alone to remove the statistical significance from antimicrobial exposure on C. difficile colonization (Supplementary Table S4).
Figure 2.
Stratified analysis by birth method for antimicrobial exposure and C. difficile colonization at 3–4 months of age. (NALC: no antibiotics and lower usage of cleaning products; AALC: any antibiotics and lower usage of cleaning products; NAHC: no antibiotics and higher usage of cleaning products; AAHC: any antibiotics and higher usage of cleaning products).
Figure 3.
Stratified analysis by birth method for antimicrobial exposure and C. difficile colonization at 12 months of age. (NALC: no antibiotics and lower usage of cleaning products; AALC: any antibiotics and lower usage of cleaning products; NAHC: no antibiotics and higher usage of cleaning products; AAHC: any antibiotics and higher usage of cleaning products).
2.2.5. Antimicrobial Exposure, C. difficile Colonization and Other Gut Microbes
Infant gut microbiota composition differed across groups of antimicrobial exposure at 3–4 months of age and to a lesser extent at 12 months of age. Of note, most changes occurred in the cumulative exposure group (AAHC), where the highest C. difficile colonization was observed. At 3–4 months of age, the relative abundance of Bifidobacteriaceae and Bacteroidaceae decreased, while the relative abundance of Clostridaceae, Lachnospiraceae, Veillonellaceae, and Enterobacteriaceae increased in the AALC and AAHC infants, compared with the NALC infants. In the NAHC infants, the relative abundance of Lachnospiraceae and Ruminococcaceae increased and the relative abundance of Bifidobacteriaceae and Clostridaceae decreased, compared with the NALC infants (Figure 4). At 12 months of age, the most obvious changes were a lower abundance of Bacteroidaceae in the AALC and AAHC infants, compared with the NALC infants, although Clostridaceae, Lachnospiraceae, Veillonellaceae, and Enterobacteriaceae were still higher. In the NAHC infants, the relative abundance of Bifidobacteriaceae and Clostridaceae decreased, compared with the NALC infants (Figure 4).
Figure 4.
Stacked bar plots of mean relative abundance of dominant taxa (family level) according to antimicrobial exposure. Comparisons were performed using Kruskal–Wallis test with Dunn’s post hoc test. Positive false discovery rate (FDR) was used to adjust p-values for multiple testing.
3. Discussion
3.1. Main Findings
In a general population of 1429 infants, C. difficile colonization of infant gut microbiota was affected by antimicrobial exposure. At 3–4 months of age, colonization rates were 1.5 times greater (95% CI 1.03–2.17; p = 0.032) following cumulative infant exposure to systemic and household antimicrobials (AAHC) than the colonization rates after minimal antimicrobial exposure (NALC). C. difficile colonization in infants aged 3–4 months persisted until the age of 12 months. Further, stratification by birth mode showed that in vaginally delivered infants, the colonization rates were 1.59 times greater (95% CI 1.06–2.38; p = 0.025) and 1.62 times greater (95% CI 1.15–2.29; p = 0.005) in the cumulative exposure group, compared with the rates in those with minimal antimicrobial exposure at 3–4 months of age and 12 months of age, respectively. This association was not observed in infants born via caesarean section, indicating that post-caesarean antimicrobial exposure did not further increase the risk of C. difficile colonization. At the older infant age, the association with antimicrobials was strongest from perinatal (maternal IAP and newborn IV) antibiotic exposure (odds ratio (OR):1.33, 95% confidence interval (CI) 1.10–1.62; p = 0.003). Although C. difficile colonization of infants is asymptomatic [15], its presence in gut microbiota at this early age is associated with future asthma and allergic diseases [20,21]. Our study is the first to evaluate both the systemic antibiotic exposure and the environmental antimicrobial exposure of infants, with separate consideration of infants delivered vaginally and by caesarean section.
3.2. Interpretation
Importantly, the cumulative effect of antimicrobial exposure on C. difficile colonization remained in vaginally-delivered infants, even after adjusting for covariates, showing that the vulnerability of full-term newborns to antibiotic exposure is not related to caesarean delivery. Consistent with what others have reported [15], we found that up to 31% of infants were colonized with C. difficile at a time when gut microbiota are being established. Antimicrobial exposure significantly increased C. difficile colonization at both 3–4 months of age and 12 months of age. The biological or clinical relevance of C. difficile colonization in infants is yet to be fully understood. In a small group of infants (n = 65), C. difficile was associated with an increased risk of allergic diseases in early childhood [20]. An earlier study of 957 infants linked the presence of C. difficile in infants at 1 month of age with atopic manifestations at 2 years of age [21]. Further, colonization with C. difficile in infancy may promote a dysbiotic gut environment by modifying the composition of the microbial ecosystem [18], or serve as a reservoir for adult C. difficile infection [16,17]. Early microbial dysbiosis may also be associated with inflammatory bowel disease (IBD), allergy and asthma, obesity, and other metabolic disorders [19].
Several studies have evaluated the impact of maternal IAP or postnatal antibiotics on infant gut microbiota [7,8,22]. Similar to our results, Tapiainen et al. [8] reported changes in infant gut microbiota from both IAP exposure and IV antibiotics that were still observed at 6 months of age, including the enrichment of Clostridium and the depletion of Bacteroides species. Consistent with what others have reported [8,23,24,25,26], we found a reduction of Bifidobacteriaceae and Bacteroidaceae in gut microbiota following antibiotic exposure, as well as an increase in Clostridaceae, Lachnospiraceae, Veillonellaceae, and Enterobacteriaceae at both 3–4 months of age and 12 months of age. Colonization of more Proteobacteria (phylum to which Enterobacteriaceae belongs) may be a signal for gut dysbiosis and inflammation [27], while a reduction in important gut microbes may provide room for C. difficile colonization and overgrowth. We observed the most significant changes in infants with the highest antimicrobial exposure (the AAHC group), who were also identified as having the highest C. difficile colonization.
While many household cleaning products contain antimicrobials that can give rise to resistant bacteria [28], the evidence of their impact on infant gut microbiota is limited. Consistent with a previous report on frequent use of household disinfectants in a smaller sample of CHILD study infants [9], this study found a higher abundance of Lachnospiraceae in infants of 3–4 months of age who were exposed to the higher usage of cleaning products. Moreover, our results further demonstrated that the combined effect of systemic antibiotic exposure and additional antimicrobial exposure from household cleaning products increases the likelihood of C. difficile colonization. This cumulative antimicrobial effect on C. difficile colonization at 3–4 months of age persisted until at least 12 months of age, demonstrating that the collateral damage inflicted on the gut microbiota is not rapidly repaired [29]. This effect was even greater in infants who were persistently colonized with C. difficile at both 3–4 months of age and 12 months of age.
We found few other early life variables that influenced C. difficile colonization at 3–4 months of age or 12 months of age. As previously reported by others [30,31], infants born via emergency caesarean section (CS) were more likely to be colonized by C. difficile at 3–4 months of age, compared with vaginally delivered infants. Many women who undergo emergency CS delivery planned to give birth vaginally and are positive for GBS [32], increasing the cumulative antimicrobial exposure of the newborn. Previous epidemiological studies have identified breastfeeding as an important contributor to the infant gut microbiota [22], [31,33]. In our study, exclusively breastfed infants were less likely to be colonized with C. difficile at 3–4 months of age than were mixed-fed infants or exclusively formula-fed infants. We found that having an older sibling seemed to strongly influence C. difficile colonization at 12 months of age. Infants with an older sibling had lower C. difficile colonization. This finding was similar to that of a systematic review comprising six studies that reported a decreased abundance of Clostridium in infants with an older sibling [34]. It is suggested that the influence of other children or siblings on the infant gut microbiota is in line with the “hygiene hypothesis”, in that other children or siblings increase the infant’s exposure to early gut colonizers that prime the infant’s immune system and provide colonization resistance against pathogens and C. difficile [33,35].
3.3. Strengths and Limitations
Our study has several strengths, including the application of high throughput sequencing and qPCR to profile gut microbiota and C. difficile colonization in a prospective birth cohort with representative and large sample size. To the best of our knowledge, this study is the first to examine the influence of household cleaning products and their cumulative effect, together with systemic antibiotics, on C. difficile colonization and the infant gut microbiota. Unique to our study was the capture of both perinatal (maternal IAP and newborn IV) and postnatal antibiotic use. We also performed a sensitivity analysis for C. difficile colonization at age 12 months to account for antibiotics given during the period from 3 months to 12 months of age.
Our findings should be considered with some limitations. Exposure status to cleaning products was dependent on self-reported questionnaires, and we were not able to determine the specifics of cleaning product brands, ingredients, or how much product was used per application. In addition, we were not able to perform analysis based on chemical composition. However, we assigned a score based on the frequency of use of each product and classified all infants as either living in a home with higher (above average) or lower (below average) usage of cleaning products. Moreover, we did not report on the longitudinal colonization of C. difficile, as our sample collection was limited to two time points. However, we performed analysis to examine persistent colonization at both 3–4 months of age and 12 months of age.
4. Materials and Methods
4.1. Study Design
This study included a subsample of 1429 infants at 3–4 months of age and 1728 infants at 12 months of age from families that were enrolled in the Canadian Healthy Infant Longitudinal Development (CHILD) Cohort (www.childstudy.ca; accessed on 10 January 2022). Women were enrolled into CHILD during the second or third trimester of pregnancy between 2009 to 2012, from study sites in Vancouver, Edmonton, and Manitoba. Written informed consent was obtained from the mothers upon enrollment. This study was approved by the Human Research Ethics Boards of the University of Alberta, the University of Manitoba, and the University of British Columbia.
4.2. Exposures
This prospective population-based cohort study examined the effect of antimicrobial exposures, before or at 3 months of age, on C. difficile colonization in the gut of the infants. Data on maternal intrapartum antibiotic prophylaxis (IAP) and newborn antibiotic treatment were obtained from hospital birth charts for each of the participants. At 3 months post-partum, the mothers completed validated questionnaires regarding infant usage of antibiotics and the frequency of use of various household cleaning products. Parents were asked about their usage of household cleaning products from a list of 26 cleaning products (Supplementary Figure S5). The cleaning products questionnaire was validated with visual inspection of the products by research assistants during home visits [9]. The frequency of use for each product was assigned a score: 0 for never (not used), 1 for less than a month usage, 2 for monthly usage, 3 for weekly usage, and 4 for daily usage. The scores for each respondent were added together to obtain a total score. The total scores were split at the median into two groups of higher usage (i.e., living in a home with above average cleaning product use) and lower usage (i.e., living in a home with below average cleaning product use) of household cleaning products to make comparisons of the effect of sizes during the analyses. To determine the impact of the antibiotics and the household cleaning products, infants were assigned to one of four groups: (1) no antibiotic exposure and lower usage of cleaning products (NALC), (2) any antibiotic exposure and lower usage of cleaning products (AALC), (3) no antibiotic exposure and higher usage of cleaning products (NAHC) and (4) any antibiotic exposure and higher usage of cleaning products (AAHC). The exposure to “any” antibiotics indicated exposure to maternal IAP and/or the administration of antibiotics directly to infants from birth to 3 months of age.
4.3. Fecal Microbiota Analysis
Infant stool samples (fresh or frozen) were collected at approximately 3–4 months of age and 12 months of age after a home assessment or in the clinic. Samples were stored at −80 °C prior to analysis. Fecal samples were characterized with Illumina MiSeq using the bacterial 16S ribosomal ribonucleic acid (rRNA) gene hypervariable V4 region, as previously described [36]. Analysis of C. difficile was performed using quantitative polymerase chain reaction (qPCR) with appropriate primers, as previously described [7]. Primers and probe efficiency were determined by a standard curve procedure by establishing five 1:10 serial dilutions of C. difficile American Type Culture Collection (ATCC) 9689D-5 genomic DNA, starting at 1 ng/uL. For each plate, a non-template control was used. An efficiency between 90% and 110%, and an R2 greater than or equal to 0.9 for the primers and probes combination were used as quality control parameters for each run. A Quantitative Insights Into Microbial Ecology (QIIME) pipeline (www.qiime.org, accessed on 15 June 2017) was used to group microbiota into taxonomic order and to summarize Operational Taxonomic Unit (OTU) data within infant fecal samples.
4.4. Statistical Analysis
C. difficile colonization (outcome) at 3–4 months of age and 12 months of age was analyzed as a binary variable (yes or no). Non-parametric tests were carried out as appropriate to compare C. difficile colonization, taxon mean relative abundance, antimicrobial exposure, and demographic variables. Statistical significance was defined as a two-sided p or q-value ≤ 0.05, after a false discovery rate (FDR) correction for multiple comparisons. Potential confounding variables were identified from the literature [31,33,37]. They included maternal age and race, family income, birth method, gestational age, breastfeeding status at 3 months of age, furry pet ownership, having an older sibling(s), and tobacco smoke exposure from birth to 3 months of age. Thereafter, a reduced set of these variables was selected using the directed acyclic graph (DAG) method; these variables were tested in models to prevent over-adjustment [38] (See Supplementary Figures S1 and S2). Logistic regression analysis was used to determine the association between antimicrobial exposure and C. difficile colonization. Model 1 was adjusted for maternal and birth characteristics (maternal age and birth method), model 2 was adjusted for postnatal characteristics (breastfeeding, furry pet, older siblingship, and smoke exposure). Model 3 was adjusted for variables from models 1 or 2 that had a p-value ≤ 0.05 or caused a ≥15% change in the estimate of antimicrobial exposure. Confounding variables from model 3 were retained in the final model if they had a p-value ≤ 0.05 or if they caused a ≥15% change in the estimate of antimicrobial exposure. It is noteworthy that model 3 was not included for the 12-month analysis, because most covariates were not significant at p-value ≤ 0.05 in models 1 or 2; confounding variables retained in the final model were those from models 1 or 2 that had a p-value ≤ 0.05 or caused a ≥15% change in estimate of antimicrobial exposure. Statistical analysis was conducted using STATA 13.0 software (64-bit); Stata Corp 4905 Lakeway Drive College Station, Texas 77845 USA.
5. Conclusions
In Canada, oral antibiotic treatment of young infants is not common; however, indirect neonatal exposure to intrapartum antibiotics administered to the mother is increasing, due to current recommendations for GBS and the growing prevalence of caesarean delivery. Our results support ongoing efforts to reduce antimicrobial exposure during infancy, especially in the neonatal period. These results show that cumulative exposure to antibiotics and household cleaning products (environmental antimicrobials) is not without consequence. Previous epidemiological studies have linked early life antimicrobial exposure to the development of childhood asthma and allergic diseases, but the mechanisms for these associations are unknown. C. difficile colonization and/or gut microbiota composition may or may not be a pathway. Further studies are required to replicate these findings in other populations and to determine the impact on future health outcomes.
Acknowledgments
The authors would like to acknowledge that this work could not have been completed without the cooperation of all members, staff, and participants of the CHILD Cohort Study. They include research staff, administrative staff, study families and participants, volunteers, laboratory technicians, statisticians, and clinical staff.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11070981/s1. Table S1: Distribution (%) of Antibiotic Exposure, Household Cleaning Products Use and Selected Covariates at 3–4 Months, 12 Months and Both Time Points; Compared to the Entire Canadian Healthy Infant Longitudinal Development (CHILD) Cohort at 3 Sites (Edmonton, Winnipeg, Vancouver); Table S2: Sensitivity Analysis for Antimicrobial Exposure and C. difficile Colonization at 12 Months; Table S3: Population Characteristics and C. difficile colonization at 3–4 Months and 12 Months; Table S4: Individual Adjustment for Covariates on Antimicrobial Exposure and C. difficile Colonization; Figure S1: Directed Acyclic Graph for Antimicrobial Exposure and C. difficile Colonization at 3–4 Months (minimal sufficient adjustment sets for estimating the total effect of exposure on outcome: Maternal age, Birth method, Gestational age, Breastfeeding, Older sibling, Furry pet, Smoke exposure); Figure S2: Directed Acyclic Graph for Antimicrobial Exposure and C. difficile Colonization at 12 Months (minimal sufficient adjustment sets for estimating the total effect of exposure on outcome: Birth method, Gestational age, Breastfeeding, Older sibling, Furry pet, Smoke exposure); Figure S3: Stratified Analysis by Infant Sex for Antimicrobial Exposure and C. difficile Colonization at 12 Months; Figure S4: Stratified Analysis by Older Siblingship for Antimicrobial Exposure and C. difficile Colonization at 12 Months; Figure S5: Questionnaire on household cleaning products use (Frequency of use score: 0 for never (not used), 1 for less than a month, 2 for monthly, 3 for weekly and 4 for daily).
Author Contributions
Conceptualization, C.V.O. and A.L.K.; data curation, P.J.M., T.J.M., E.S., S.E.T., P.S., J.A.S. and A.L.K.; formal analysis, C.V.O.; funding acquisition, P.J.M., T.J.M., E.S., S.E.T., P.S., J.A.S. and A.L.K.; investigation, P.J.M., T.J.M., E.S., S.E.T., P.S. and J.A.S.; methodology, C.V.O., J.P., T.K.T., H.M.T., N.M.-L., M.B.A., J.A.S. and A.L.K.; supervision, A.L.K.; writing—original draft, C.V.O.; writing—review & editing, C.V.O., J.P., T.K.T., H.M.T., N.M.-L., M.B.A., P.J.M., T.J.M., E.S., S.E.T., P.S., J.A.S. and A.L.K. All the authors reviewed the manuscript content, provided feedback, and approved the final version. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This microbiome study of the CHILD cohort was approved by the Human Research Ethics Boards of the University of Alberta (Pro00010073).
Informed Consent Statement
Written informed consent was obtained from the mothers upon enrollment into the CHILD cohort study.
Data Availability Statement
The data and the analysis code that support the findings of this study can be made available from the corresponding author and from CHILD Cohort Study coordinators upon reasonable request. These data, including study participant data, are securely stored in the https://childdb.ca database; accessed on 10 January 2022.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by grant number 108028 from the Canadian Institutes of Health Research Canadian Microbiome Initiative (Dr Kozyrskyj). The Canadian Institutes of Health Research and the Allergy, Genes, and Environment (AllerGen) Network of Centres of Excellence provided core support for the Canadian Healthy Infant Longitudinal Development (CHILD) Cohort Study.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van Boeckel T.P., Gandra S., Ashok A., Caudron Q., Grenfell B.T., Levin S., Laxminarayan R. Global antibiotic consumption 2000 to 2010: An analysis of national pharmaceutical sales data. Lancet Infect. Dis. 2014;14:742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 2.Patrick D.M., Sbihi H., Dai D.L., Al Mamun A., Rasali D., Rose C., Marra F., Boutin R.C.T., Pettersen C., Stiemsa L.T., et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: Evidence from population-based and prospective cohort studies. Lancet Respir. Med. 2020;8:1094–1105. doi: 10.1016/S2213-2600(20)30052-7. [DOI] [PubMed] [Google Scholar]
- 3.Money D., Allen V.M., Yudin M.H., Bouchard C., Boucher M., Caddy S., Castillo E., Murphy K.E., Ogilvie G., Paquet C., et al. The Prevention of Early-Onset Neonatal Group B Streptococcal Disease. J. Obstet. Gynaecol. Can. 2013;35:939–948. doi: 10.1016/S1701-2163(15)30818-5. [DOI] [PubMed] [Google Scholar]
- 4.Persaud R.R., Azad M.B., Chari R.S., Sears M.R., Becker A.B., Kozyrskyj A.L. Perinatal antibiotic exposure of neonates in Canada and associated risk factors: A population-based study. J. Matern. Neonatal Med. 2014;28:1190–1195. doi: 10.3109/14767058.2014.947578. [DOI] [PubMed] [Google Scholar]
- 5.Fjalstad J.W., Stensvold H.J., Bergseng H., Simonsen G.S., Salvesen B., Rønnestad A.E., Klingenberg C. Early-onset Sepsis and Antibiotic Exposure in Term Infants: A Nationwide Population-based Study in Norway. Pediatr. Infect. Dis. J. 2016;35:1–6. doi: 10.1097/INF.0000000000000906. [DOI] [PubMed] [Google Scholar]
- 6.Williams H., Moyns E., Bateman D., Thomas S.H., Thompson J.P., Vale J.A. Hazard of household cleaning products: A study undertaken by the UK National Poisons Information Service. Clin. Toxicol. 2012;50:770–775. doi: 10.3109/15563650.2012.709936. [DOI] [PubMed] [Google Scholar]
- 7.Azad M., Konya T., Persaud R., Guttman D., Chari R., Field C., Sears M.R., Mandhane P., Turvey S., Subbarao P., et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: A prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2015;123:983–993. doi: 10.1111/1471-0528.13601. [DOI] [PubMed] [Google Scholar]
- 8.Tapiainen T., Koivusaari P., Brinkac L., Lorenzi H.A., Salo J., Renko M., Pruikkonen H., Pokka T., Li W., Nelson K., et al. Impact of intrapartum and postnatal antibiotics on the gut microbiome and emergence of antimicrobial resistance in infants. Sci. Rep. 2019;9:10635. doi: 10.1038/s41598-019-46964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tun M.H., Tun H., Mahoney J.J., Konya T.B., Guttman D.S., Becker A.B., Mandhane P.J., Turvey S., Subbarao P., Sears M.R., et al. Postnatal exposure to household disinfectants, infant gut microbiota and subsequent risk of overweight in children. Can. Med Assoc. J. 2018;190:E1097–E1107. doi: 10.1503/cmaj.170809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei S., Mortensen M.S., Stokholm J., Brejnrod A.D., Thorsen J., Rasmussen M.A., Trivedi U., Bisgaard H., Sorensen S.J. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: A double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265–272. doi: 10.1016/j.ebiom.2018.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni J., Friedman H., Boyd B.C., McGurn A., Babinski P., Markossian T., Dugas L.R. Early antibiotic exposure and development of asthma and allergic rhinitis in childhood. BMC Pediatr. 2019;19:1–8. doi: 10.1186/s12887-019-1594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obiakor C.V., Tun H.M., Bridgman S.L., Arrieta M.-C., Kozyrskyj A.L. The association between early life antibiotic use and allergic disease in young children: Recent insights and their implications. Expert Rev. Clin. Immunol. 2018;14:841–855. doi: 10.1080/1744666X.2018.1521271. [DOI] [PubMed] [Google Scholar]
- 13.Korpela K., Zijlmans M.A.C., Kuitunen M., Kukkonen K., Savilahti E., Salonen A., De Weerth C., De Vos W.M. Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome. 2017;5:1–9. doi: 10.1186/s40168-017-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crobach M.J.T., Vernon J.J., Loo V.G., Kong L.Y., Péchiné S., Wilcox M.H., Kuijper E.J. Understanding Clostridium difficile Colonization. Clin. Microbiol. Rev. 2018;31:1–29. doi: 10.1128/CMR.00021-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tougas S.R., Lodha N., Vandermeer B., Lorenzetti D.L., Tarr P.I., Tarr G.A., Chui L., Vanderkooi O.G., Freedman S.B. Prevalence of detection of clostridioides difficile among asymptomatic children: A systematic review and meta-analysis. JAMA Pediatr. 2021;175:1–11. doi: 10.1001/jamapediatrics.2021.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoesser N., Eyre D.W., Quan T.P., Godwin H., Pill G., Mbuvi E., Vaughan A., Griffiths D., Martin J., Fawley W., et al. Epidemiology of Clostridium difficile in infants in Oxfordshire, UK: Risk factors for colonization and carriage, and genetic overlap with regional C. difficile infection strains. PLoS ONE. 2017;12:e0182307. doi: 10.1371/journal.pone.0182307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adlerberth I., Huang H., Lindberg E., Åberg N., Hesselmar B., Saalman R., Nord C.E., Wold A.E., Weintraub A. Toxin-Producing Clostridium difficile Strains as Long-Term Gut Colonizers in Healthy Infants. J. Clin. Microbiol. 2014;52:173–179. doi: 10.1128/JCM.01701-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rousseau C., Levenez F., Fouqueray C., Doré J., Collignon A., Lepage P. Clostridium difficile Colonization in Early Infancy Is Accompanied by Changes in Intestinal Microbiota Composition. J. Clin. Microbiol. 2011;49:858–865. doi: 10.1128/JCM.01507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ihekweazu F.D., Versalovic J. Development of the Pediatric Gut Microbiome: Impact on Health and Disease. Am. J. Med Sci. 2018;356:413–423. doi: 10.1016/j.amjms.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S.H., Gong Y.N., Ryoo E. Clostridium difficile colonization and/or infection during infancy and the risk of childhood allergic diseases. Korean J. Pediatrics. 2017;60:145–150. doi: 10.3345/kjp.2017.60.5.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penders J., Thijs C., van den Brandt P.A., Kummeling I., Snijders B., Stelma F., Adams H., van Ree R., Stobberingh E.E. Gut microbiota composition and development of atopic manifestations in infancy: The KOALA Birth Cohort Study. Gut. 2007;56:661–667. doi: 10.1136/gut.2006.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bokulich N.A., Chung J., Battaglia T., Henderson N., Jay M., Li H., Lieber A.D., Wu F., Perez-Perez G.I., Chen Y., et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016;8:343ra82. doi: 10.1126/scitranslmed.aad7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fouhy F., Guinane C.M., Hussey S., Wall R., Ryan C.A., Dempsey E.M., Murphy B., Ross R.P., Fitzgerald G.F., Stanton C., et al. High-Throughput Sequencing Reveals the Incomplete, Short-Term Recovery of Infant Gut Microbiota following Parenteral Antibiotic Treatment with Ampicillin and Gentamicin. Antimicrob. Agents Chemother. 2012;56:5811–5820. doi: 10.1128/AAC.00789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stearns J.C., Simioni J., Gunn E., McDonald H., Holloway A.C., Thabane L., Mousseau A., Schertzer J.D., Ratcliffe E.M., Rossi L., et al. Intrapartum antibiotics for GBS prophylaxis alter colonization patterns in the early infant gut microbiome of low risk infants. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-017-16606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coker M.O., Hoen A.G., Dade E., Lundgren S., Li Z., Wong A.D., Zens M.S., Palys T.J., Morrison H.G., Sogin M.L., et al. Specific class of intrapartum antibiotics relates to maturation of the infant gut microbiota: A prospective cohort study. BJOG: Int. J. Obstet. Gynaecol. 2019;127:217–227. doi: 10.1111/1471-0528.15799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazzola G., Murphy K., Ross R., Di Gioia D., Biavati B., Corvaglia L.T., Faldella G., Stanton C. Early Gut Microbiota Perturbations Following Intrapartum Antibiotic Prophylaxis to Prevent Group B Streptococcal Disease. PLoS ONE. 2016;11:e0157527. doi: 10.1371/journal.pone.0157527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin N.-R., Whon T.W., Bae J.-W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496–503. doi: 10.1016/j.tibtech.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Bondi C.A. Applying the precautionary principle to consumer household cleaning product development. J. Clean. Prod. 2011;19:429–437. doi: 10.1016/j.jclepro.2010.07.008. [DOI] [Google Scholar]
- 29.Yassour M., Vatanen T., Siljander H., Hämäläinen A. Natural history of the infant gut microbiome and impact of antibiotic treatments on strain-level diversity and stability. Sci. Transl. Med. 2016;8:343. doi: 10.1126/scitranslmed.aad0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adlerberth I., Strachan D.P., Matricardi P.M., Ahrné S., Orfei L., Åberg N., Perkin M.R., Tripodi S., Hesselmar B., Saalman R., et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J. Allergy Clin. Immunol. 2007;120:343–350. doi: 10.1016/j.jaci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Penders J., Thijs C., Vink C., Stelma F.F., Snijders B., Kummeling I., Van den Brandt P.A., Stobberingh E.E. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 32.Zalaski L., Chari R.S., Kozyrskyj A.L. Indications for Perinatal Antibiotic Exposure and Atypical Use Patterns in Canada: A Retrospective Analysis (abstract); Proceedings of the WCHRI Research Day; Edmonton, AB, Canada. 25 October 2017. [Google Scholar]
- 33.Martin R., Makino H., Yavuz A.C., Ben-Amor K., Roelofs M., Ishikawa E., Kubota H., Swinkels S., Sakai T., Oishi K., et al. Early-Life Events, Including Mode of Delivery and Type of Feeding, Siblings and Gender, Shape the Developing Gut Microbiota. PLoS ONE. 2016;11:e0158498. doi: 10.1371/journal.pone.0158498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan C.W.H., Wong R.S., Law P.T.W., Wong C.L., Tsui S.K.W., Tang W.P.Y., Sit J.W.H. Environmental Factors Associated with Altered Gut Microbiota in Children with Eczema: A Systematic Review. Int. J. Mol. Sci. 2016;17:1147. doi: 10.3390/ijms17071147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azad M.B., Konya T., Maughan H., Guttman D.S., Field C.J., Sears M.R., Becker A.B., Scott J.A., Kozyrskyj A.L., CHILD Study Investigators Infant gut microbiota and the hygiene hypothesis of allergic disease: Impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin. Immunol. 2013;9:15. doi: 10.1186/1710-1492-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azad M.B., Konya T., Guttman D.S., Field C., Sears M.R., HayGlass K.T., Mandhane P.J., Turvey S., Subbarao P., Becker A.B., et al. Infant gut microbiota and food sensitization: Associations in the first year of life. Clin. Exp. Allergy. 2015;45:632–643. doi: 10.1111/cea.12487. [DOI] [PubMed] [Google Scholar]
- 37.Stewart C.J., Ajami N.J., O’Brien J.L., Hutchinson D.S., Smith D.P., Wong M.C., Ross M.C., Lloyd R.E., Doddapaneni H., Metcalf G.A., et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562:583–588. doi: 10.1038/s41586-018-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Textor J., van der Zander B., Gilthorpe M.S., Liśkiewicz M., Ellison G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty. Int. J. Epidemiol. 2016;5:1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and the analysis code that support the findings of this study can be made available from the corresponding author and from CHILD Cohort Study coordinators upon reasonable request. These data, including study participant data, are securely stored in the https://childdb.ca database; accessed on 10 January 2022.