Abstract
Bacterial ghosts (BGs) are nonliving empty bacterial shells without cytoplasm retaining original morphology and identical antigenicity of natural bacteria, making them high potential and promising vaccine candidates and delivery vehicles. However, the low yield of commonly used BGs preparation methods limits its mass production and widely application. In order to improve BGs production, E. coli phage ID52 lysis protein E was introduced to generating BGs for the first time. Above all, we compared the lysis activity of lysis protein of E. coli phage φX174 and E. coli phage ID52 as well as the effects of promoters on the lysis activity of ID52-E, which shown that the lysis activity and BGs formation rate of protein ID52-E was significantly higher than protein φX174-E. Further, the lysis activity of ID52-E was significantly improved under the control of L-arabinose inducible promoter which initial induction OD600 reached as high as 2.0. The applicability of lysis protein ID52-E induced by L-arabinose was proved by preparing probiotic E. coli Nissle 1917 BGs and pathogenic Salmonella typhimurium BGs in mass production. This paper introduced a novel and highly efficient method for BGs preparation depending on recombinant expression of E. coli phage ID52-E under eco-friendly and reasonable price inducer L-arabinose.
Keywords: bacterial ghosts, E. coli phage ID52, lysis protein E, lysis activity, L-arabinose
1. Introduction
Bacterial ghosts (BGs) mainly refer to the inactive hollow cell envelopes derived from Gram-negative bacteria without cytoplasmic contents, which retain the intact cellular morphology and natural surface antigenic structures such as pathogen-associated molecular patterns (PAMPs), which include lipopolysaccharides (LPS), peptidoglycan, monophosphoryl lipid A (MPL), flagella, adhesins and other immune-related stimulating elements of natural bacteria [1]. Therefore, BGs inherit the characteristics of bacterial surface antigenicity and adhesivity [2], making them easy to be recognized and captured by immune cells, and effectively stimulating strong mucosal immunity [3,4], specific humoral and cellular immunity [1,5,6], indicating that BGs can be good candidates for vaccine. And compared with other vaccines that require additional adjuvants [7], BGs have inherent adjuvant components such as LPS and MPL, so BGs don’t need to add additional adjuvants. Since the internal genetic materials were discharged out of the bacteria, BGs don’t have the pathogenicity of natural bacteria, avoiding the problem of virulence reversion, and reducing the risk of virulence gene transfer [8,9]. Furthermore, the special empty cavity structure of BGs has a wide loading capacity, which can load with proteins [10,11], drugs [12,13] and foreign DNA [14]. In addition, antigens can be expressed to the inner membrane, outer membrane and periplasmic space of BGs [15], giving an opportunity to design novel types of polyvalent vaccines and to be an advanced drug delivery system [16]. The simplicity of preparation process and the long shelf life without the need of cold-chain storage on account of the freeze-dry status, make the cost of BGs as vaccines or delivery systems greatly reduced [17].
BGs have so many advantages that they have broad application prospect and great research value, while the low yield of BGs limits the large-scale production and application of BGs. In the short term, BGs were mainly prepared by inducing the expression of E. coli phage phiX174 lysis protein gene E (φX174-E), which under the control of the temperature-inducible expression plasmid pBV220 without adding other chemicals. Contrary to lysis proteins derived from other phages, gene φX174-E has no inherent enzymatic function, which encodes a 91-amino acid membrane protein with the competence to fuse the inner and outer membranes of Gram-negative bacteria, giving rise to forming an E-specific transmembrane tunnel structure with a size in diameter between 40~400 nm [18] by oligomerizing and co-translationally integrating into the cell membranes [1]. Owing to the difference of osmotic pressure between cytoplasm and surrounding medium, the cytoplasmic contents were expelled to external through transmembrane pores [19]. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) revealed that the specific transmembrane channels structure of protein E is not randomly distributed on the envelope, but is limited to the potential division sites, mainly in the center or poles of the bacteria [19,20].
However, lysis activity of protein φX174-E depended on the growth phase of bacteria [21]. In a general way, when the optical density value at 600 nm (OD600) approximated 0.2~0.6, host bacteria can be lysed effectively by a temperature elevation to 42 °C [22], resulting in a very low yield of BGs, incomplete utilization of medium, and high cost of production. Therefore, if initial induction OD600 can be improved during the production of BGs, not only can medium components be fully utilized, but also can the yield of BGs be significantly increased. In order to improve the yield of BGs, Yu et al. [23] obtained a mutant mE of lysis gene φX174-E using gene recombination technology, whose initial induced lysis OD600 was up to 1.1. Further, Zhu et al. [22] constructed a fusion expression vector containing the fusion gene of mutant gene mE and Staphylococcus aureus nuclease A gene, which could effectively induce host bacteria to lysis with the intracellular degradation of genetic material of host cells, and initial induced lysis OD600 could reach as high as 1.2.
However, the BGs yield of above preparation methods was still far from large scale production. Based on this, Ma et al. [24] screened a mutant protein EM by site-directed mutation of φX174-E, and the initial induced OD600 could reach 2.0 with the joint control of T7 promoter and pLysS plasmid, achieving efficient preparation of E. coli and Salmonella enteritidis. However, the inducer IPTG had a certain toxic effect, and the high price and cost of IPTG were not conducive to the large-scale production and application of BGs. Furthermore, Ma et al. [24] continued to mutate on the basis of the mutant protein EM without screening the mutant protein with high lysis activity. Therefore, it can be seen that the possibility of improving the yield of BGs by mutating lysis gene φX174-E was low, and this method has been in a bottleneck period. Some chemical agents such as NaOH, H2O2 and SDS have been proved to be an alternative method to commonly method involving protein E for producing BGs [25]. But the antigenic components LPS was destroyed after treated with NaOH, which may reduce the immunogenicity [26]. Therefore, there is still a long way to go to find a safer, effective and low-cost method for the preparation of BGs.
The E. coli Nissle 1917 (EcN), a widely used probiotic, not only possesses the special lipopolysaccharide (LPS) in outer membrane, which exhibits a good immunomodulating property, but also can colonize and replicate in tumors efficiently [27]. Therefore, EcN ghosts can be excellent drug delivery vehicles in targeting therapy of tumor. Salmonella typhimurium (ST), an important food-borne non-host-adapted Gram-negative intracellular pathogen, has competence to infect a variety of animal hosts and humans around the world. Vaccination is one of the effective methods to reduce the infection of ST in animals [28], and it also reduces the risk of human infection from food infected with ST. Studies have shown that ST ghosts can effectively protect animals from infection by ST [29]. However, there is no effective method to produce EcN ghosts and ST ghosts with high yield. Therefore, it is of great significance to improve the yield of EcN ghosts and ST ghosts to facilitate the large-scale application of BGs.
Like E. coli phage phiX174, E. coli phage ID52 also belongs to the Microvirus family, and it also contains lysis protein gene gpE (ID52-E), which encodes a 103-amino acid [30]. According to the protein homology comparison, the homology of lysis protein ID52-E and φX174-E was 55.34%, so protein ID52-E also has the potential to forming BGs. However, there are few reports on the lysis activity of protein ID52-E. Thus, this paper focuses on the lysis effect of E. coli phage ID52 lysis protein ID52-E to detect whether it can be applied to produce BGs and improve the yield of BGs. First, the lysis gene φX174-E and lysis gene ID52-E were cloned into pBV220-Chl plasmid to compare the lysis effects of two lysis proteins, whose results showed that the lysis effect of lysis protein ID52-E was significantly higher than that of φX174-E. In order to test whether the yield of BGs could be further improved, the temperature inducible promoter of pBV220 was replaced with L-arabinose inducible promoter and the results manifested that the OD600 initiation can reach as high as 2.0, while the minimum OD600 can be reduced to 0.3~0.4 after lysis. Finally, in order to test the feasibility of the efficient BGs preparation system by protein ID52-E under control of L-arabinose inducible promoter, the system was applied to prepare EcN ghosts and ST ghosts, respectively. And the results manifested that protein ID52-E could be applied to the high-efficiency and high-yield production of EcN ghosts And ST ghosts, whose initial induced OD600 could be as high as 2.5.
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Culture Conditions
The Bacterial strains, plasmids, and primers used in this research were listed in Table 1. LB (Luria-Bertani) medium used in this research was purchased from Beijing Land Bridge (Beijing, China) for bacterial growth. Bacteria containing pBV220 plasmids were cultured at 30 °C and 220 rpm, and protein expression was induced at 42 °C and 220 rpm. All other bacteria were cultured at 37 °C and 220 rpm unless otherwise indicated. Furthermore, L-arabinose, chloramphenicol (Chl+), and other reagents used in this study were purchased from Sangon Biotech (Shanghai, China) unless otherwise specified. The final concentrations of chloramphenicol, kanamycin and ampicillin in LB were 25 μg/mL (Chl+), 50 μg/mL (Kan+) and 100 μg/mL (Amp+), respectively.
Table 1.
Bacterial strains, plasmids and primers used in this research.
| Strains Plasmids Primers | Description | Source |
|---|---|---|
| Strains | ||
| DH5α | Host cells for plasmid amplification | TIANGEN BIOTECH, Beijing, China |
| BL21(DE3) | Host cells for protein expression | TIANGEN BIOTECH, Beijing, China |
| E. coli Nissle 1917 | Wild type | Mutaflor, Germany |
| E. coli Nissle 1917 ΔaraBAD::FRT | Deletion of araBAD gene and insertion of FRT locusin E. coli Nissle 1917 | This study |
| ST | Wild type of Salmonella enterica subsp. enterica serovarTyphimurium str. ATCC 14028 | Institute of Microbiology, Guangdong Academy of Science, China |
| ST ΔaraBAD::FRT | Deletion of araBAD gene and insertion of FRT locus in ST | This study |
| Plasmids | ||
| pBV220-sGFP-Chl | The template of constructing expression vector of lysis gene E | Our lab |
| pET29a-φX174-E | The template of constructing expression vector ofgene φX174-E | Our lab |
| pET29a-ID52-E | The template of constructing expression vector of gene ID52-E | Our lab |
| pBV220-φX174-E | Lysis plasmid used in this study | This study |
| pBV220-ID52-E | Lysis plasmid used in this study | This study |
| araC-ParaBAD-ID52-E | Lysis plasmid used in this study | This study |
| pKD4 | Plasmid for λ Red homologous recombination | Our lab |
| pKD46 | Plasmid for λ Red homologous recombination | Our lab |
| pCP20 | Plasmid for λ Red homologous recombination | Our lab |
| Primers | ||
| φX174-E-F | TTGGTTAAAAATTAAGGAGGAATTCATGGTACGCTGGACTTTGTG | |
| φX174-E-R | ACAGCCAAGCTTGGCTGCAGTTATTTTTCAAACTGCGGATG | |
| ID52-E-F | TAAAAATTAAGGAGGAATTCATGGAACGCTGGACCTTAAG | |
| ID52-E-R | ACAGCCAAGCTTGGCTGCAGTTATTTTTCAAACTGCGGATG | |
| araC-ParaBAD-F | TGCGCCGACCAGAACACCTTGCCGATTATGACAACTTGACGGCTACA | |
| araC-ParaBAD-R | TGCCGCTTAAGGTCCAGCGTTCCATTTTTTATAACCTCCTTAGAGCTCG | |
| linearized pBV220-ID52-E-F | ATGGAACGCTGGACCTTAAG | |
| linearized pBV220-ID52-E-R | TCGGCAAGGTGTTCTGGT | |
| P1 | ATGACACCGGACATTATCCTG | |
| P2 | GTGCTTTCAGTGGATTTCGG | |
| P3 | TTTTTCGCAACTCTCTACTGTTTCTCCATACCCGTTTTTTTGGATGGAGTGAAACGGTGTAGGCTGGAGCTGCTTC | |
| P4 | CTGGTTTCGTTCCAAAACCAAAATTTATTTTGATTGGCTGTGGTTTTATACAGTCACATATGAATATCCTCCTTAG | |
| P5 | TTTGCCGCGACTCTCTACTGTTTCTCCATACCTGTTTTTCTGGATGGAGTAAGACGGTGTAGGCTGGAGCTGCTTC | |
| P6 | TATATCACCGACCAGATTCATCAACGCGCCCCCCATGGGAGCGTTTTTAGAGGCACATATGAATATCCTCCTTAG | |
| P7 | TTAGCGGATCCAGCCTGA | |
| P8 | TGCAGCATTCGCAGATCG | |
| P9 | GATTAGCGGATCCTGCCTGA | |
| P10 | TATCAAAGCGCATTTGCTGAA | |
| P11 | AAGGGATAAATATCTAACACCGTGC | |
| P12 | ACGGCATAGTGCGTGTTTATG | |
Red letters indicate the 50-nt homology arms targeting the araBAD gene.
2.2. Lysis Protein E Expressing Plasmid Construction
Plasmid pBV220-φX174-E and pBV220-ID52-E were constructed by restriction-free cloning technology (RF cloning) [31,32] (Figure S1A,B in Supplementary Materials). First, using plasmid pET29a-φX174-E and pET29a-ID52-E as template, lysis gene φX174-E and lysis gene ID52-E were amplified by PCR with primer pairs of φX174-E-F/φ174-E-R and ID52-E-F/ID52-E-R, respectively. Then PCR products were detected by 1% agarose gel and purified and recovered by DNA Gel Extraction Kit (TIANGEN BIOTECH, Beijing, China). Next, using the purified lysis gene φX174-E and the purified lysis gene ID52-E fragment as a pair of primers in a linear amplification reaction around a circular plasmid pBV220-sGFP-Chl, respectively. Later, the methylated parental plasmid was digested by treating the linear amplification reaction products with Dpn I enzyme. Then the reaction mixture was transformed into E. coli DH5α competent cells. Positive clones were confirmed by DNA sequencing (TIANYI HUIYUAN, Guangzhou, China). The resulting plasmids were designated as pBV220-φX174-E and pBV220-ID52-E.
Plasmid araC-ParaBAD-ID52-E was constructed by seamless assembly cloning with Seamless Assembly Cloning kit (Taihe Biotechnology, Beijing, China) (Figure S1C in Supplementary Materials). Firstly, using the plasmid pBV220-ID52-E as template, a pair of primer linearized pBV220-ID52-E-F/linearized pBV220-ID52-E-R was used for amplifying linearized fragment of pBV220-ID52-E, whose temperature-sensitive lambda-repressor CI857 gene was deleted. Next, the L-arabinose inducible promoter araC-ParaBAD was amplified from the plasmid pKD46 using primers “araC-ParaBAD-F/araC-ParaBAD-R”. Finally, seamless Assembly Cloning kit was used for seamless assembly cloning of linearized fragment pBV220-ID52-E and L-arabinose inducible promoter araC-ParaBAD. Then the reaction mixture was transformed into E. coli DH5α competent cells. Positive clones were confirmed by DNA sequencing (TIANYI HUIYUAN, Guangzhou, China). The resulting plasmids were designated as araC-ParaBAD-ID52-E. Primers required to the above vectors were listed in Table 1.
2.3. Induction Expression of Lysis Protein E
The three aforementioned plasmids were transformed into E. coli BL21(DE3) competent cells, respectively. Next, the recombinant strains containing different plasmid were cultured to different OD600 values, and the expression of lysis proteins in bacteria containing plasmid pBV220-φX174-E or pBV220-ID52-E was activated by shift the temperature to 42 °C, while L-arabinose with final concentration of 0.5 mg/mL was added to activate the expression of lysis protein ID52-E in bacteria containing plasmid araC-ParaBAD-ID52-E.
On the one hand, the decrease degree of OD600 value is one of the indicators to evaluate the lysis activity of different lysis proteins E in E. coli BL21(DE3). The values of OD600 were detected at initial induction OD600 approximately 0.8, 1.2, 1.6, 2.0 and detected every 30 min after inducing the expression of lysis protein E. And the OD600 decrease degree was calculated by the following formula: OD600 decrease degree % = (1 − post-lysis OD600/pre-lysis OD600) × 100%. On the other hand, the lysis efficiency of different lysis proteins E was evaluated by detecting viable cell counts before and after lysis, which was conducted by serial dilution and plate count methods. And the lysis efficiency was calculated by the following formula: lysis efficiency % = (1 − post-lysis CFU/pre-lysis CFU) × 100%.
Furthermore, the outflow of genome and intracellular protein after lysis were another indicator to evaluate the lysis activity of lysis protein. In order to detect the outflow of the genome after lysis, E. coli BL21(DE3) harboring pBV220-φX174-E, pBV220-ID52-E and araC-ParaBAD-ID52-E plasmid respectively was cultured to OD600 approximately 1.6 to induce the expression of lysis protein. Then 2 mL bacterial liquid before and after lysis were collected respectively and centrifuged at 5000 rpm for 20 min to collect the bacterial deposits whose genome were extracted by Rapid Bacterial Genomic DNA Isolation Kit (Sangon Biotech, Guangzhou, China) and the genome concentration was measured. The degree of genome leakage was calculated by the following formula: genome leakage degree % = (1 − post-lysis genome concentration/pre-lysis genome concentration) × 100%. All experiments were conducted with three biological replicates.
At the same time, the protein leakage after lysis was detected by SDS-PAGE. E. coli BL21(DE3) harboring pBV220-φX174-E, pBV220-ID52-E and araC-ParaBAD-ID52-E plasmid respectively was cultured to OD600 approximately 1.6 to induce the expression of lysis protein. Then 5 mL bacterial liquid before and after lysis were collected respectively and centrifuged at 5000 rpm for 20 min to collect the bacterial deposits and culture supernatant. The bacterial deposits were re-suspended with 500 μL PBS and ultrasonic crushing was performed. Then the bacterial deposits sample after crushing and the culture supernatant sample were detected by SDS-PAGE.
2.4. Scanning Electron Microscopy (SEM)
E. coli BL21(DE3) harboring different plasmids respectively was cultured to OD600 approximately 1.6 to induce the expression of lysis protein Then 10 mL bacterial liquid was collected before lysis and after lysis and the bacteria cells were pelleted by centrifuging at 5000 rpm for 20 min at 4 °C, respectively. Then the precipitate was washed three times with sterile phosphate-buffered saline (PBS) to fully remove the medium. Next, the pellets were resuspended in 5 mL 2.5% glutaraldehyde special electron microscopic fixative and fixed at 4 °C for 8 h. After fixation, cells were washed 3 times with deionized water and eventually resuspended in 1 mL deionized water. Drop samples to the dried cell strip and embed them in filter paper. Subsequently, samples were dehydrated with 70%, 85% and 95% ethanol step by step for 15 min each time, and soaked with 100% ethanol for 15 min (repeat this step 3 times). Next, samples were dried with critical point dryer named Autosamdri-815 and following were coated by a gold sputter coater. Finally, the SEM images were acquired using Merlin SEM (Zeiss, Oberkochen, Germany).
2.5. Transmission Electron Microscopy (TEM)
E. coli BL21(DE3) harboring different plasmids respectively was cultured to OD600 approximately 1.6 to induce the expression of lysis protein Then 10 mL bacterial liquid was collected before lysis and after lysis and the bacteria cells were pelleted by centrifuging at 5000 rpm for 20 min at 4 °C, respectively. Same as SEM sample pretreatment, the pellets were washed three times with sterile PBS and fixed with 5 mL 2.5% glutaraldehyde special electron microscopic fixative at 4 °C for 8 h. Then samples were washed three times with deionized water to get rid of fixative. Eventually, 1 mL sterile ddH2O was used to resuspend the sample. Placed a clean piece of filter paper in the culture dish and placed the carbon coated copper mesh (face up) on top of the filter paper. Added 20 µL bacterial droplets to the carbon film copper mesh for 5~10 min at room temperature. Gently moved the copper mesh with tweezers so that the excess bacteria droplets on the copper net were absorbed by the filter paper. Then dropped 20 µL of 3% phosphotungstic acid staining solution to the carbon coated copper mesh with bacteria. Finally, the samples were visualized under a transmission electron microscope (ThermoFisher scientific, Talos L120C, Waltham, MA, USA).
2.6. Western Blot Analysis
Western blot analysis in this research was performed according to an established procedure with slight modifications [24]. In brief, the samples at different induction time points was harvested by centrifuging at 5000 rpm for 15 min. Then the pellets were resuspended in PBS and the precipitates were ultrasonic broken for 100 W, 5 min. The crushed bacterial liquid was centrifuged at 17,000× g for 90 min at 4 °C to obtain supernatant and precipitation. Added 5× loading buffer to the whole liquid, supernatant and precipitation in proportion, and boiled for 10 min at 100 °C, respectively. 10 µL protein samples of E. coli BL21(DE3) was separated by 16% tricine-SDS-PAGE. After electrophoresis, proteins bands on the gel were blotted onto a nitrocellulose (NC) membrane (0.2 µm, GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) using the Bio-Rad Transblot Turbo system at 80 V for 1.5 h. Following washed with TBST buffer (Sangon Biotech, Shanghai, China) for 10 min, the NC membrane was incubated with blocking buffer (5% (w/v) skimmed milk powder in TBST) for 2 h to block the reactive sites of the NC membrane. Then the NC membrane was washed with TBST for three times to get rid of superfluous milk, 10 min each time. Later, the NC membrane was incubated with Anti-Strep-Tag II Monoclonal Antibody 8C12 produced in mouse (AmyJet Scientific, Wuhan, China) at 1:5000 in TBST at 4 °C overnight. The following day, the NC membrane was washed with TBST for three times to remove surplus primary antibody, 10 min each time. The NC membrane was incubated subsequently with horseradish peroxidase (HRP)-conjugated Goat Anti-Mouse IgG H&L (Abcam, Cambridge, MA, USA) at 1:3000 in TBST at room temperature for 2 h. Following repeated washing with TBST, the Super Signal West Pico Chemiluminescent Substrate (ThermoFisher scientific, Waltham, MA, USA) was used to visualize the labeled bands on the NC membrane.
2.7. Construction of EcN with araBAD Deletion Mutation and ST with araBAD Deletion Mutation
E. coli Nissle 1917 and Salmonella Typhimurium engineering strain with araBAD gene deletion were constructed by using λ Red homologous recombination system. Firstly, the pKD46 plasmid was transformed into EcN or ST competent cells by electroporation with 2.0 kV and the recombinant positive clones were screened by ampicillin and colony PCR by P1/P2. Then the FRT-kanamycin-FRT linear fragment was amplified by using P3/P4 (EcN) and P5/P6 (ST) containing 56-nt homology arms targeting the araBAD gene as primers and pKD4 plasmid as PCR template following purified by DNA Gel Extraction Kit (Sangon Biotech, Guangzhou, China). The EcN or ST recombination clones containing pKD46 plasmid was inoculated into LB liquid medium with 100 µg/mL Amp+ at 30 °C. The next day, the cells were transferred to fresh LB broth and cultured at 30 °C and 220 rpm until OD reached 0.3~0.4, then L-arabinose with final concentration of 0.5 mg/mL was added to continue culturing until OD reached 0.5~0.6. Subsequently, the cells were made into electrocompetent cells and were transformed with 500 ng purified FRT-kanamycin-FRT linear fragment. Colony PCR was performed to verify whether araBAD gene was successfully replaced by kanamycin fragment by primers P7/P8 (EcN) and P9/P10 (ST) on the colonies grown on ampicillin and kanamycin double resistant plates, respectively. Nextly, the strain EcNΔaraBAD::kanamycin and the strain STΔaraBAD::kanamycin were obtained by culturing at LB broth at 42 °C for 12 h to remove the pKD46 plasmid. Then the plasmid pCP20 was transformed into the strain EcNΔaraBAD::kanamycin and STΔaraBAD::kanamycin competent cells by electroporation with 2.0 kV, respectively, which could express FLP recombinase at 42 °C to remove kanamycin resistance genes inserted into the genome and disappeared with the cell division and proliferation. The successful transfer of pCP20 plasmid was achieved by colony PCR using P11/P12 as primers. Finally, the EcN engineering strain and ST engineering strain with araBAD gene deletion named EcNΔaraBAD::FRT and STΔaraBAD::FRT were identified by PCR using primers P7/P8 and P9/P10 according to the nucleotide sequence of EcN and ST available in NCBI (EcN: NZ_CP007799.1; ST: NZ_CP034230.1). The lysis curves of EcNΔaraBAD:FRT engineering bacteria and STΔaraBAD::FRT engineering bacteria were plotted by measuring OD600. The morphological observation and identification of EcNΔaraBAD:FRT BGs and STΔaraBAD::FRT BGs were performed by SEM and TEM.
2.8. Statistical Analysis
All statistical analyses were performed with GraphPad Prism 8.0.2 software and OriginPro 2021. The Data were expressed in the format of mean ± S.D, and the significance level was analyzed by ordinary one-way ANOVA with Tukey multiple comparison.
3. Results
3.1. Lysis Protein E Expressing Plasmid Construction
The specific primers were used to conduct colony PCR on the positive clones obtained from the transformation products to verify whether the construction of lysis protein E expressing plasmid was successful. Bands of approximately 343 bp and bands of approximately 376 bp were visualized in positive clones harboring pBV220-φX174-E and pBV220-ID52-E by PCR using φX174-E-F/φX174-E-F and ID52-E-F/ID52-E-R primers, respectively (Figure S2A in Supplementary Materials). Bands of approximately 1631 bp were visualized in positive clones containing araC-ParaBAD-ID52-E by PCR using araC-ParaBAD-F and ID52-E-R primers (Figure S2B in Supplementary Materials).
3.2. Lysis Activity of Protein φ174-E and ID52-E in E. coli BL21(DE3)
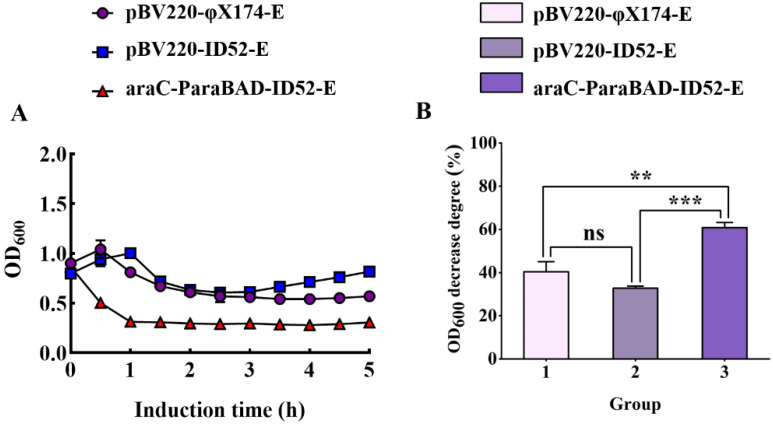
The most visual way to assess the difference in lysis activity between φX174-E and ID52-E was OD600 value decrease degree. Therefore, different initial induction OD600 values (low OD and high OD) were controlled to be the same before inducing the expression of lysis proteins E. When the initial induction OD600 value was low, there was no significant difference between lysis protein φX174-E and lysis protein ID52-E under the control of thermo-inducible lambda pL/pR-cI857 promoter, while there were significant differences between lysis protein ID52-E under the control of L-arabinose inducible promoter and the other two (Figure 1A,B). Furthermore, with the increase of initial induction OD600 value, the OD600 value of lysis protein ID52-E under the control of L-arabinose inducible promoter decreased the most, indicating that its lysis activity was the most optimal (Figure 1). As was shown in Table 2, contrary to the OD600 decrease degree, the lysis efficiency of lysis protein ID52-E under the control of L-arabinose inducible promoter was slightly lower than the other two, but there was no significant difference in the lysis efficiency among the three. Because the expression of lysis protein in bacteria harboring pBV220 plasmid was activated at 42 °C, while the high temperature affected bacterial growth, cell membrane composition and fluidity [33]. Moreover, as a membrane protein, the insertion of lysis protein into the cell membrane could reduce the cell growth rate and had a toxic effect on cell growth [34]. Therefore, it was speculated that the reason for this phenomenon was that the high temperature and the insertion of lysis protein on the cell membrane may cause a double burden on the bacteria, leading to a significant decrease in viable bacteria after induction.
Figure 1.
The lysis curves of E. coli BL21(DE3) harboring different lysis plasmids. The OD600 value of bacteria cells was measured to assess the lysis activity of bacteria (A,C,E,G). At the same time, the significance analysis of OD600 decrease degree of different lysis plasmids was conducted (B,D,F,H). The OD600 values at different times were expressed as mean ± standard deviation (S.D). The initial induction OD600 values were 0.8 (A,B), 1.2 (C,D), 1.6 (E,F), and 2.0 (G,H), respectively. Note: "ns" meant there was no significant difference. And “*”, “**”, “***” and “****” represented “p < 0.05”, “p < 0.01”, “p < 0.001”, and “p < 0.0001”, respectively, which had significant differences.
Table 2.
The lysis efficiency of E. coli BL21(DE3) harboring different lysis plasmids.
| Initial Induction OD600 Values | Lysis Efficiency (%) | ||
|---|---|---|---|
| pBV220-φX174-E | pBV220-ID52-E | araC-ParaBAD- ID52-E |
|
| 0.8 | 100 | 99.984 | 99.755 |
| 1.2 | 100 | 99.994 | 99.994 |
| 1.6 | 100 | 99.998 | 99.997 |
| 2.0 | 100 | 100 | 99.998 |
Note: the data were presented in the format of mean values.
And since the death of bacteria did’t mean the formation of BGs, the lysis efficiency can’t be used as the absolute standard of the lysis effect of lysis protein. As was shown in Table 2, the lysis efficiency of lysis protein ID52-E increased gradually with the increase of initial induction OD600 value. Although the initial induction OD600 value differed, the ultimate OD600 value after the lysis protein ID52-E expression dropped to similar levels. Therefore, the yield of BG was significantly increased.
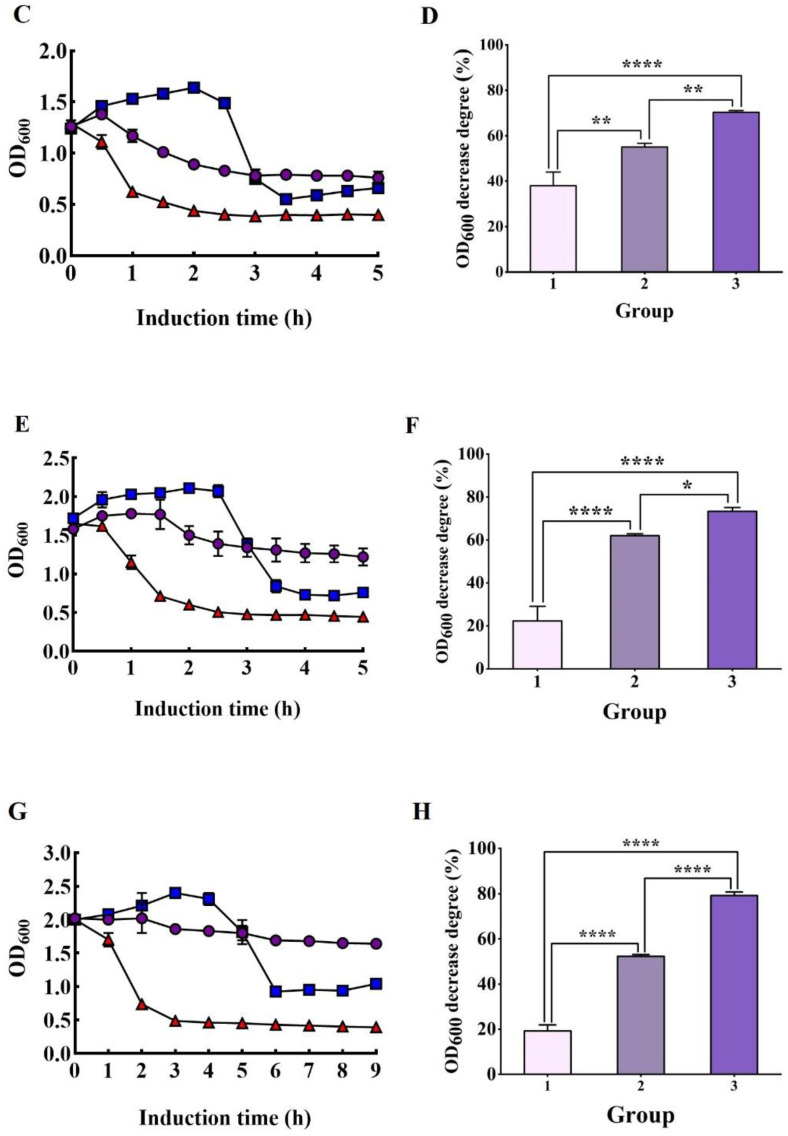
Consistent with the trend of lysis curve, the characterization results of bacterial genome leakage showed that E. coli BL21(DE3) containing araC-ParaBAD-ID52-E plasmid had the least residual amount of genome and the highest degree of genome leakage, while E. coli containing pBV220-φX174-E plasmid had the most residual amount of genome and the lowest degree of genome leakage. These results indicated that lysis protein ID52-E under the control of L-arabinose inducible promoter had the best lysis effect, which resulted in more genome flow to external media (Figure 2A,B). The results of SDS-PAGE also showed that E. coli BL21(DE3) containing araC-ParaBAD-ID52-E plasmid had the most intracellular protein leakage (Figure 2C).
Figure 2.
Characterization of bacterial genome and intracellular protein leakage. (A) Genomic concentrations of bacterial pellets before and after lysis, bacterial genome leakage (B) and intracellular protein leakage (C) in E. coli BL21(DE3) containing different plasmids. Note: “ns” represented "ns" meant there was no significant difference. And “***” represented “p < 0.001”, which had significant differences.
3.3. Morphological Observation of E. coli BL21(DE3) BGs by SEM and TEM
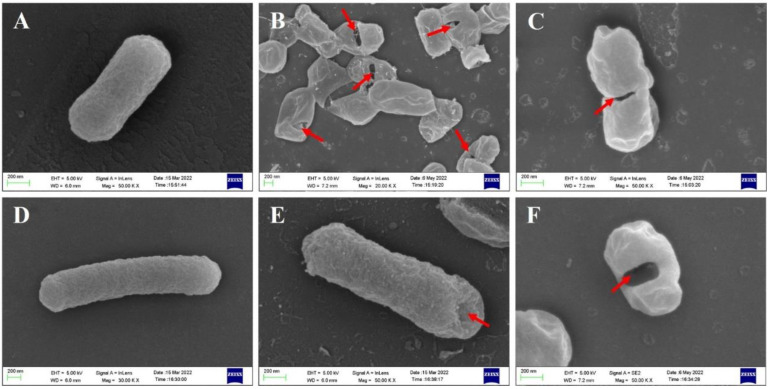
BGs retained the natural surface morphological and structural characteristics of bacteria and could be observed by SEM, while effluent of bacterial contents could be identified by TEM. SEM images revealed that the overall morphology of BGs formed by lysis protein φX174-E and lysis protein ID52-E was not much different from that of intact bacterial cells, and both retained the same membrane structure as that of natural living bacteria (Figure 3). At the beginning of lysis, the cell membrane at the lysis pore shrinked into the lumen of the cell, but the cell membrane may collapse inward when the cell contents were all expelled. Similar to the reported lysis protein φX174-E [19,20], transmembrane channels of ID52-E were not randomly distributed on the envelope, but mainly formed at the equatorial center, poles or position near the poles of the bacteria. Moreover, except for a few BGs that formed two transmembrane channels, most of BGs formed only one pore. From SEM images, it was observed that the transmembrane lysis channels formed by ID52-E was larger than those formed by φX174-E, which might be the reason why the lysis activity of ID52-E was stronger than φX174-E.
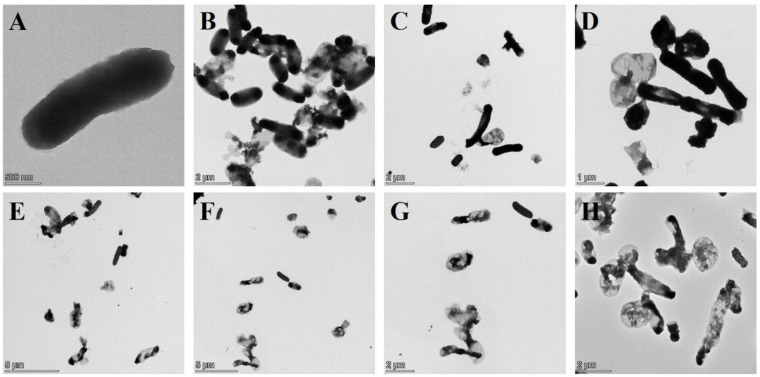
Figure 3.
Morphological Observation of E. coli BL21(DE3) BGs by SEM. (A): Intact morphology of wild-type E. coli BL21(DE3); (B,C): E. coli BL21(DE3) ghost formed after lysis induced by plasmid pBV220-φX174-E; (D–F): E. coli BL21(DE3) ghost formed after lysis induced by plasmid pBV220-ID52-E; (G–L): E. coli BL21(DE3) ghost formed after lysis induced by plasmid araC-ParaBAD-ID52-E. The red arrow indicates the transmembrane tunnels.
TEM images manifested that the untreated wild-type E. coli BL21(DE3) showed uniform dark color due to containing DNA, protein and other cell contents (Figure 4A), while the color of the lumen of E. coli BL21 ghosts were relatively light due to the devoid of intracellular cytoplasm (Figure 4B–P). As shown in Figure 4B–D, BGs formation rate was the lowest after the lysis induced by plasmid pBV220-φX174-E where most of the bacteria had not excreted cell cytoplasm. However, BGs formation rate was the highest after lysis induced by plasmid araC-ParaBAD-ID52-E where bacteria basically expelled cytoplasm to form BGs, except for a few bacteria (Figure 4I–P). TEM results were consistent with the characterization results of DNA leakage and protein leakage in Figure 2.
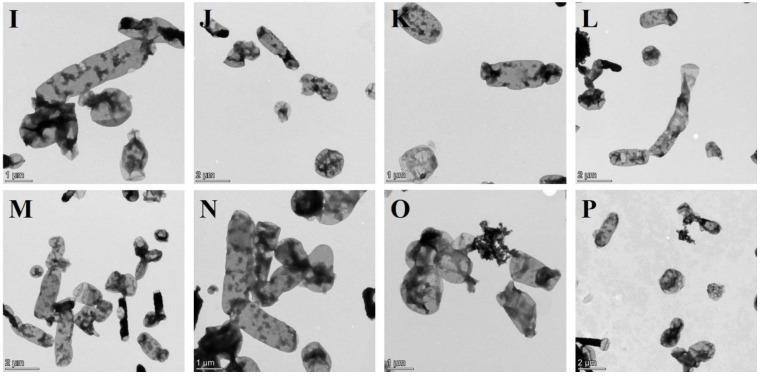
Figure 4.
Morphological Observation of E. coli BL21(DE3) BGs by TEM. (A): Intact morphology of wild-type E. coli BL21(DE3); (B–D): E. coli BL21(DE3) ghost formed after lysis induced by plasmid pBV220-φX174-E; (E–H): E. coli BL21(DE3) ghost formed after lysis induced by plasmid pBV220-ID52-E; (I–P): E. coli BL21(DE3) ghost formed after lysis induced by plasmid araC-ParaBAD-ID52-E.
3.4. Detecting the Expression Level and Expression Location of Lysis Protein E by Western Blot
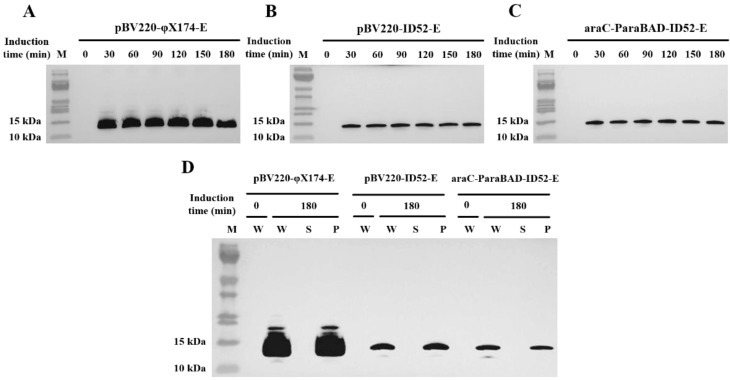
Western blot of the samples manifested that the expression levels of different lysis protein E were significantly different in E. coli BL21(DE3) containing different plasmid (Figure 5A–C). The expression levels of lysis protein in E. coli BL21(DE3) containing pBV220-φX174-E was significantly higher than E. coli BL21(DE3) containing pBV220-ID52-E or araC-ParaBAD-ID52-E plasmid, while the expression levels of different plasmids containing lysis protein ID52-E were almost the same. It was speculated that the molecular weight of the lysis protein φX174-E (11.64 kDa) was lower than that of ID52-E (12.77 kDa), so the expression quantity of lysis protein φX174-E was higher in the same induction time. Further, there is possible that the amount of protein required by lysis protein ID52-E to lyse host bacteria was far less than that required by lysis protein φX174-E to lyse host bacteria. When the number of lysis protein ID52-E synthesis reached the threshold of lysis host bacteria, lysis pores formed on the cell membrane, and the host bacteria began to lyse and die, and the lysis protein ID52-E synthesis was terminated, resulting that the expression of ID52-E was lower than φX174-E. And contrary to the expression levels of the two lysis proteins E, the lysis activity of protein ID52-E with lower expression level was significantly higher than those of φX174-E with higher expression level, indicating that the lysis activity of protein ID52-E was so stronger that lysis bacteria required less protein ID52-E.
Figure 5.
The expression levels and expression location of lysis protein E at different induction times were detected by Western blot. (A–C): The whole lysis solution of E. coli BL21(DE3) harboring pBV220-φX174-E, pBV220-ID52-E and araC-ParaBAD-ID52-E plasmid, respectively; (D) The expression location of lysis protein E. M: 26616 prestained protein marker, W: whole lysis solution; S: supernatant; P: precipitate.
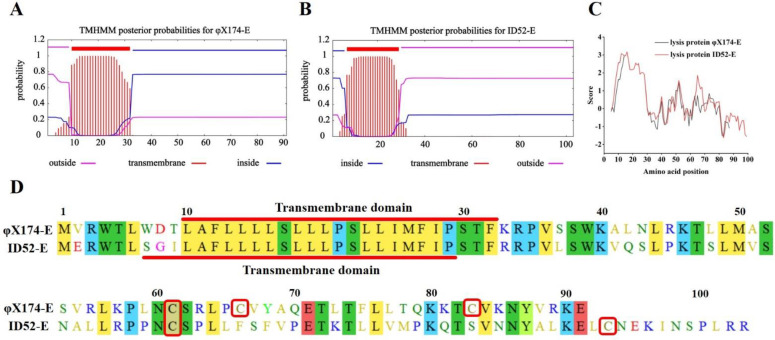
In order to understand the possible causes of the difference in the lysis activity of the two lysis proteins, sequence alignment of amino acids of lysis protein was performed by Molecular Evolutionary Genetics. At the same time, TMHMM Server 2.0 was used to predict and analyze the transmembrane region of lysis protein, and ExPASy online tool Protscale was used to further analyze the hydrophilicity and hydrophobicity of lysis protein. It was found that the transmembrane domain of the lysis protein φX174-E was the 10~32 amino acids, and that of the lysis protein ID52-E was the 7~29 amino acids (Figure 6A–C). The length and location of the transmembrane domain of the two proteins were similar, and the 21th amino acid was proline, indicating that the transmembrane domain was very conserved in lysis protein of Microviridae (Figure 6A,B,D), while the hydrophilicity of the C-terminal of the two lysis proteins was significantly different (Figure 6C). Previous experiments [35,36,37] showed that transmembrane domain was a necessary condition for the lysis activity of the protein φX174-E, and the C-terminal affected the lysis effect of the protein, significantly. In addition, the C-terminal of φX174-E has three cysteines, while the C-terminal of ID52-E has only two cysteines. As is known to all, cysteine is the prerequisite for the formation of disulfide bonds, and previous studies have shown that the lysis protein φ174-E played a role as a polymer [38]. Because more cysteine may mean that the lysis protein may have a more complex structure. So it was reasonable to speculate that this may be one of the reasons for the significant difference in the lysis activity of the two proteins. In general, C-terminal may play an important role in the lysis activity of lysis proteins, in which the hydrophilicity and hydrophobicity of C-terminal and cysteine content may play a major role.
Figure 6.
Transmembrane domain prediction, hydrophilicity analysis and amino acids sequence alignment of lysis proteins E. (A,B): Transmembrane domain prediction of proteins φX174−E and ID52−E performed by TMHMM Server 2.0, respectively; (C): The hydrophilicity analysis of proteins φX174−E and ID52−E performed by ExPASy online tool Protscale; (D): The amino acids sequence alignment of lysis protein performed by Molecular Evolutionary Genetics. The NCBI Reference Sequence of lysis protein φX174−E: NP_040709.1; The NCBI Reference Sequence of lysis protein ID52−E: YP_512455.1. The color represented the similarity of amino acids, and the same color meant that the amino acid residues of the two lysis proteins were the same or the characteristics were very similar. The red box represented the cysteine contained in the lysis proteins.
According to the report, the lysis protein φX174-E is a membrane protein and should be detected in the precipitate after centrifugation [39]. In accordance with the report, Western blot results revealed that both the protein φX174-E and protein ID52-E could be detected in the whole solution and precipitation, but not in the supernatant, suggesting that both lysis protein φX174-E and lysis protein ID52-E were expressed on the membranes (Figure 5D).
3.5. Construction of Engineered Strain EcN with araBAD Deletion Mutation and ST with araBAD Deletion Mutation
In order to test the application scope of the recombinant expression of protein ID52-E under the control of L-arabinose inducible promoter to prepare BGs, the system was applied to EcN and ST. However, different from E. coli BL21(DE3), EcN and ST have a faster consumption rate of inducer L-arabinose, so it was easy to cause insufficient supply of inducer and thus reduced the lysis rate. At the same time, a certain amount of L-arabinose can promote the growth of EcN and ST, and finally leaded to the phenomenon that the growth rate was faster than the lysis rate and the lysis curve OD600 value rised, resulting in a diminished output of BGs. Therefore, in order to reduce the use of inducers and increase the yield of BG, it was necessary to knock out araBAD gene related to L-arabinose consumption. And the λ Red homologous recombination system was used for gene knockout.
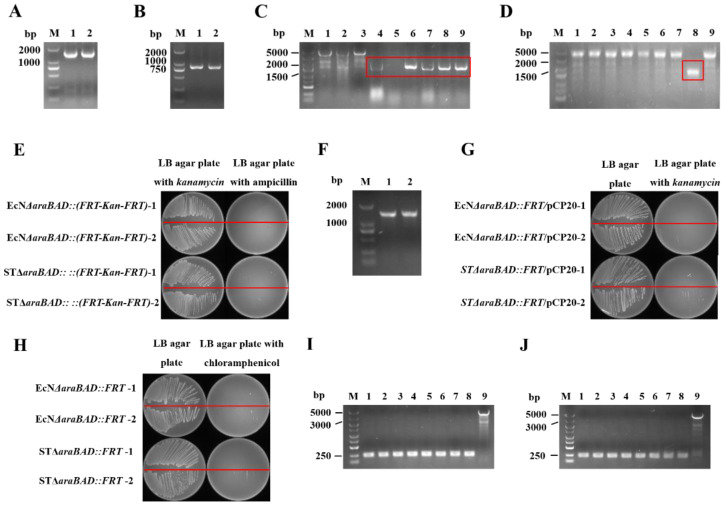
The linear FRT-kanamycin-FRT fragment of 1477 bp containing 56-nt homology arms targeting the araBAD gene was amplified and then was identified by 1% agar electrophoresis analysis (Figure 7A). The detection of 792 bp bands showed that pKD46 plasmids were successfully transited into EcN and ST (Figure 7B). And a band of approximately 1600 bp were visualized in the strain with the deletion of araBAD gene and the insertion of kanamycin, while bands of approximately 4200 bp were detected in the wild-type (Figure 7C,D). Clones that were able to grow on LB agar plates containing kanamycin but not LB agar plates containing ampicillin proved successful in losing the pKD6 plasmid (Figure 7E). Next, the pCP20 plasmid was successfully transferred into the ECNΔaraBAD::kanamycin and STΔaraBAD::kanamycin, which were verified by the detection of a 1387 bp band (Figure 7F). After induction at 42 °C, clones sensitive to kanamycin were screened, which proved that kanamycin fragment was successfully excised (Figure 7G). As with the loss of pKD46 plasmid, the pCP20 plasmid was lost by culturing kanamycin-sensitive clones at 42 °C, which could be verified by measuring the sensitivity to chloramphenicol (Figure 7H). Eventually, bands of approximately 250 bp were visualized in the engineered strains EcNΔaraBAD::FRT and STΔaraBAD::FRT, while bands of approximately 4200 bp were detected in wild-type, indicating that the araBAD gene was deleted from wild-type strains (Figure 7I,J). For further verification, PCR products were sent for sequencing (data not shown).
Figure 7.
Construction of engineering strain EcN with araBAD deletion mutation and ST with araBAD deletion mutation. (A) The amplification of linear FRT-kanamycin-FRT fragment, band 1: linear FRT-kanamycin-FRT fragment containing 56-nt homology arms targeting the araBAD gene of EcN, band 2: linear FRT-kanamycin-FRT fragment containing 56-nt homology arms targeting the araBAD gene of ST; (B) The electrotransformation of pKD46, band 1: EcN clone, band 2: ST clone; (C,D) Colony PCR to identify the deletion of araBAD gene and the insertion of kanamycin gene of EcN or ST, and positive bands are marked in a red box; (E): The loss of pKD46 plasmid; (F): The electrotransformation of pCP20, band 1: EcNΔaraBAD::kanamycin strain, band 2: ST ΔaraBAD::kanamycin strain; (G): Excision of kanamycin gene; (H): The loss of pCP20 plasmid; (I,J): Colony PCR to identify the deletion of araBAD gene engineered strain of EcN or ST, bands 1-8: engineered strain of EcN or ST, band 9: EcN wild type or ST wild type.
3.6. Growth, Lysis, and Characterization of EcN BGs and ST BGs
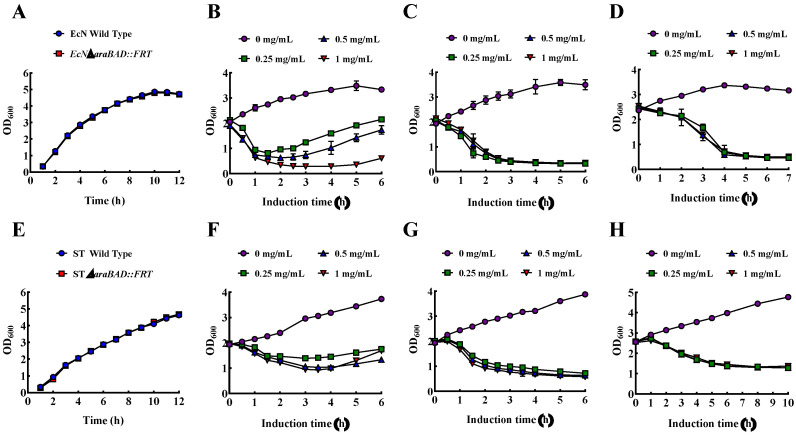
There was no significant difference between the growth rate of EcN wild-type and EcN engineering strain, and the growth rate of ST wild-type and ST engineering strain, indicating that araBAD gene knockout had no significant effect on the growth of EcN and ST strain in LB broth (Figure 8A,E). When the initial OD600 was about 2.0, under the induction of different L-arabinose concentration, the wild type E. coli Nissle 1917 decreased to a certain OD600 and began to rise (Figure 8B). However, the concentration of L-arabinose had no effect on the lysis of E. coli Nissle 1917ΔaraBAD::FRT, and the lowest OD600 value was about 0.3 (Figure 8C), indicating that the knockout of L-arabinose consumption related genes araBAD effectively reduced the consumption of inducer L-arabinose in E.coli Nissle 1917. What’s more, effective lysis can still occur when the initial induction was up to 2.5 (Figure 8D). The lysis of STΔaraBAD::FRT engineering strain was similar to that of E.coli Nissle 1917ΔaraBAD::FRT (Figure 8F–H). The efficient and high yield preparation of EcN BGs and ST BGs demonstrated the feasibility of recombinant expression of E. coli phage ID52-E under the control of L-arabinose inducible promoter, significantly improving the yield of BGs.
Figure 8.
The growth curves and lysis curves of E. coli Nissle 1917ΔaraBAD::FRT containing plasmid araC-ParaBAD-ID52-E and STΔaraBAD::FRT containing plasmid araC-ParaBAD-ID52-E engineering strain. (A): The growth curves of EcN WT and EcNΔaraBAD::FRT; (B) The lysis curves of EcN WT when the initial OD600 reached 2.0; (C) The lysis curves of EcNΔaraBAD::FRT when the initial OD600 reach 2.0; (D) The lysis curves of EcNΔaraBAD::FRT when the initial OD600 reached 2.5; (E): The growth curves of ST WT and STΔaraBAD::FRT; (F) The lysis curves of ST WT when the initial OD600 reached 2.0; (G) The lysis curves of STΔaraBAD::FRT when the initial OD600 reached 2.0; (H) The lysis curves of STΔaraBAD::FRT when the initial OD600 reached 2.5.
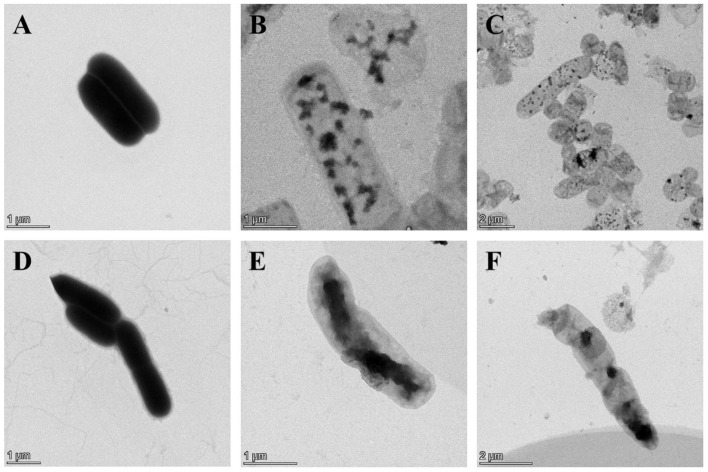
As were shown in Figure 9, the EcN BGs and ST BGs maintained their intact cell morphology and membrane structures whose transmembrane tunnels were located at the equator or poles of bacteria cells (Figure 9B,C,E,F). In comparison with the untreated EcN and ST, the discharge of cellular contents of the EcN BGs and ST BGs were visualized by TEM (Figure 10B,C,E,F).
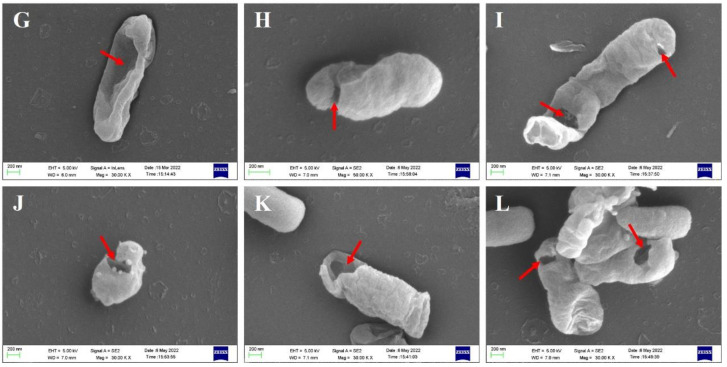
Figure 9.
Morphological observation and cellular contents observation of EcN BGs and ST BGs by SEM. (A): Untreated control EcN cells; (B,C): EcN ghosts; (D) untreated control ST cells; (E,F): ST ghosts. The red arrows indicated the lysis transmembrane tunnels.
Figure 10.
Morphological observation and cellular contents observation of EcN BGs and ST BGs by TEM. (A): untreated control EcN cells; (B,C): EcN ghosts; (D) untreated control ST cells; (E,F): ST ghosts.
4. Discussion
BGs referred to the nonliving empty bacterial shell without cytoplasmic contents, but retained original cell morphology and the same antigenicity of living counterparts, which could evoke potent humoral immunity response, cellular immunity response and mucosal immunity response [1,2]. As potential vaccine candidates, BGs had inherent adjuvant properties without adding extra adjuvant, while commercial inactivated vaccines were weak in immunogenicity and required additional adjuvants to enhance the immune response [40]. Compared with live attenuated vaccines, due to the lack of cytoplasm, BGs did’t have the possibility of reverting to strong virulence and horizontal genetic recombination [40]. Further, BGs conferred an efficient delivery vehicle that enhance immunogenicity of protein subunits vaccines and DNA-encoded antigens [18]. Moreover, not only can the empty cavity of BGs be loaded with drug [41], DNA [42] and pesticide [43], but also can the protein antigens be expressed at the inner membrane, outer membrane [10] and periplasmic space [44] of BGs before lysis. The good qualities of BGs made them have a broad and good application prospect in immunology and drug delivery.
The vaccine control effect and drug delivery performance of BGs have been studied, which showed that BGs were excellent vaccine candidates and delivery systems. However, no BGs has been officially approved and commercialized. One of the reasons was that the production of BGs was very low. In recent years, how to effectively improve the production of BGs has been perplexing many scholars, and some scholars have conducted researches on this [22,23,24], but all of them focused on the lysis protein E of E. coli phage φX174. However, whether other phage lysis proteins can be applied to the preparation of BGs has not been reported.
Like E. coli phage φX174, E. coli phage ID52 belongs to the Microviridae family with a ubiquitous small isometric ssDNA genome, which can infect numerous bacterial species in different bacterial phyla [30,45]. Contrary to the multi-gene lysis system of double-strand nucleic acid phages, which need at least one muralytic enzyme, the endolysin, and one cytoplasmic membrane protein, the holin, that actively attack the integrality of the membrane at a genetically determined time, lysis systems of phages in the Microviridae family only require encode a single lysis protein, which does not encode degradation activity but induces host cells to undergo autolysis [46,47]. Thus, the lysis of Microviridae family is not precisely scheduled, but occurs at a time dependent on the cell cycle and/or the phase of host growth [46].
Previous studies have shown that the lysis activity of protein φX174-E was affected by the growth stage of host bacteria. Only when the host bacteria were in the logarithmic phase, can protein φX174-E effectively lyse host bacteria [24], resulting the low yield of BGs. In order to improve the yield of BGs, the lysis protein of E. coli phage ID52 were introduced into the production of BGs to investigate whether the lysis activity of protein ID52-E was also affected by the growth phase of host bacteria. The results showed that the lysis effect of protein φX174-E was gradually decreased with the increase of initial induction OD600, while the lysis effect of protein ID52-E was not significantly affected, indicating that the growth stage of host bacteria had no significant effect on the lysis of protein ID52-E. In contrast to the lysis activity of the protein φX174-E and ID52-E, Western blot analysis revealed that the expression level of protein φX174-E was significantly higher than ID52-E, indicating that the protein φX174-E required for the lysis of same number bacteria was significantly higher than that required by ID52-E. The results of amino acid sequence alignment, transmembrane domain prediction and hydrophilicity prediction showed that the N-terminal and transmembrane domain sequences of protein φX174-E and ID52-E were similar, but the length, hydrophilicity and cysteine content of C-terminal were different, suggesting that the difference in lysis activity of the two proteins was mainly attributed to the C-terminal which required more experimental verifications to confirm in spite of that it has been confirmed that the C-terminal has a great influence on the lysis of protein φX174-E [35,36,37]. As it was reported, protein φX174-E formed transmembrane channel in the form of polymer [38], so it was speculated that the number of cysteines at C-terminal played an important role by affecting the conformation of the polymer.
Studies have shown that the lysis activity of the protein φX174-E depends on the lipid composition and fluidity of the cell membranes [48]. The results showed that the lysis activity of protein ID52-E under the control of L-arabinose-inducible promoter were significantly higher than that of ID52-E under the control of temperature-inducible promoter despite almost the same amount of protein being expressed, which was speculated that high temperature affected the cell growth, cell membrane composition and fluidity [33], thus affecting the membrane insertion of lysis protein ID52-E. The successful preparation of EcN BGs and ST BGs confirmed the feasibility of L-arabinose-inducible ID52-E lysis system, and it was speculated that the lysis system can also be applied to the preparation of other Enterobacteriaceae BGs. Recently, a novel and efficient high-yield method for preparing BGs by the mutant of lysis protein φX174-E under the control of T7 promoter and plasmid pLysS were constructed, whose initial induction OD600 also can reached 2.5 [24]. However, the T7 promoter needed to be induced by IPTG, which was not only costly, but also had a certain toxic effect [49]. On the contrary, the inducer L-arabinose had the advantages of low cost and edible, so the preparation method of BGs in this paper is safer and cheaper.
However, although some achievements have been obtained in this study, there were still some shortcomings to be improved. Whether the lysis system of lysis protein ID52-E under the control of L-arabinose inducible promoter could be applied to the efficient preparation of other host bacterial ghosts remains to be further studied. On the one hand, it is necessary to consider whether the protein ID52-E can lyse host bacteria, and on the other hand, it is necessary to consider whether the host bacteria can initiate the L-arabinose inducible promoter, although the lysis plasmid araC-ParaBAD-ID52-E contains regulatory elements regulating the L-arabinose inducible promoter. In addition, whether the L-arabinose consumption related gene araBAD needs to be knocked out can be determined according to the rate of L-arabinose consumption by host bacteria.
And it has been reported that protein φX174-E mediated cell lysis mechanism was related to cell division, and its kinetics was species-specific, which was speculated to be the same for protein ID52-E [21,24]. Therefore, the maximum initial lysing OD600 and minimum lysing OD600 of different host bacteria were also different, which needed to be optimized according to different host bacteria. Further, the recombinant expression of lysis protein ID52-E was used to successfully prepare E. coli BL21(DE3) ghosts, EcN ghosts and ST ghosts with high efficiency and high yield, but the immune effect was not performed, which still needs to be further expanded. And the lysis efficiency of protein ID52-E under control of L-arabinose inducible promoter was lower than that of protein φX174-E under control of temperature-inducible promoter, but the death of host bacteria didn’t represent the formation of BGs. As observed by TEM (Figure 4), protein φX174-E under control of temperature-inducible promoter has a high lysis efficiency (Table 2), but most of the cell inclusions of host bacteria have not been discharged, manifesting the BGs formation rate was low, while protein ID52-E under control of L-arabinose inducible promoter, whose BGs formation rate was high because the inclusions of host bacteria in the majority have been discharged, has a slightly lower lysis efficiency (Table 2). Therefore, it was necessary to find other methods for quantitative detection of BGs formation rate.
Finally, inspired by other scholars’ research on targeted tumor therapy with EcN BGs [50], because EcN live bacteria can colonize and replicate in tumors, it was speculated that it was possible that some anticancer factors were expressed in EcN, which colonize directed to the tumors, and then L-arabinose, a five carbons sugar [51], was administered to induce EcN lysis to release drugs, so as to achieve the purpose of targeted tumor therapy.
5. Conclusions
In summary, this study not only described a novel and excellent lysis protein ID52-E for BGs preparation for the first time, but established an efficient high-yield BGs preparation system with the recombinant expression of E. coli phage ID52-E under the control of L-arabinose inducible promoter, which provided the possibility for the efficient preparation of Enterobacteriaceae BGs and significantly improved the yield of BGs, laying a technical foundation for the large-scale production and application of BGs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bioengineering9070300/s1, Figure S1: The construction of lysis protein E expressing Plasmid pBV220-φX174-E (A), pBV220-ID52-E (B) and araC-ParaBAD-ID52-E (C), Figure S2: Agarose gel electrophoresis analysis of colony PCR of E. coli DH5α colonies containing plasmids pBV220-φX174-E (A, lane 1-2), pBV220-ID52-E (A, lane 3-4) and araC-ParaBAD-ID52-E (B), respectively.
Author Contributions
Y.M. conceived and designed the experiments. Y.M., W.Z., G.Z., Y.X. and S.L. performed the experiments, analyzed the data. Y.M. and W.Z. wrote the manuscript. Y.M., R.C. and L.C. helped to analyze the data and revised the manuscript. Y.M. and J.W. supervised the experiments. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was financially supported by the Natural Science Foundation of Guangdong Province (2022A1515010716, 2019A1515011251), Guangdong Key Areas R&D Program (2022B1111080007) and the Fundamental Research Funds for the Central Universities (2018MS54).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Langemann T., Koller V.J., Muhammad A., Kudela P., Mayr U.B., Lubitz W. The Bacterial Ghost platform system: Production and applications. Bioeng. Bugs. 2010;1:326–336. doi: 10.4161/bbug.1.5.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganeshpurkar A., Ganeshpurkar A., Pandey V., Agnihotri A., Bansal D., Dubey N. Harnessing the potential of bacterial ghost for the effective delivery of drugs and biotherapeutics. Int. J. Pharm. Investig. 2014;4:1–4. doi: 10.4103/2230-973X.127733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abtin A., Kudela P., Mayr U.B., Koller V.J., Mildner M., Tschachler E., Lubitz W. Escherichia coli ghosts promote innate immune responses in human keratinocytes. Biochem. Biophys. Res. Commun. 2010;400:78–82. doi: 10.1016/j.bbrc.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 4.Tu F.P., Chu W.H., Zhuang X.Y., Lu C.P. Effect of oral immunization with Aeromonas hydrophila ghosts on protection against experimental fish infection. Lett. Appl. Microbiol. 2010;50:13–17. doi: 10.1111/j.1472-765X.2009.02746.x. [DOI] [PubMed] [Google Scholar]
- 5.Riedmann E.M., Kyd J.M., Cripps A.W., Lubitz W. Bacterial ghosts as adjuvant particles. Expert Rev. Vaccines. 2007;6:241–253. doi: 10.1586/14760584.6.2.241. [DOI] [PubMed] [Google Scholar]
- 6.Peng W., Si W., Yin L., Liu H.F., Yu S.Y., Liu S.G., Wang C.L., Chang Y.H., Zhang Z., Hu S.P., et al. Salmonella enteritidis ghost vaccine induces effective protection against lethal challenge in specific-pathogen-free chicks. Immunobiology. 2011;216:558–565. doi: 10.1016/j.imbio.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Di Pasquale A., Preiss S., Tavares Da Silva F., Garcon N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines. 2015;3:320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kudela P., Koller V.J., Lubitz W. Bacterial ghosts (BGs)—Advanced antigen and drug delivery system. Vaccine. 2010;28:5760–5767. doi: 10.1016/j.vaccine.2010.06.087. [DOI] [PubMed] [Google Scholar]
- 9.Hu J., Zuo J., Chen Z., Fu L., Lv X., Hu S., Shi X., Jing Y., Wang Y., Wang Z., et al. Use of a modified bacterial ghost lysis system for the construction of an inactivated avian pathogenic Escherichia coli vaccine candidate. Vet. Microbiol. 2019;229:48–58. doi: 10.1016/j.vetmic.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Jechlinger W., Haller C., Resch S., Hofmann A., Szostak M.P., Lubitz W. Comparative immunogenicity of the hepatitis B virus core 149 antigen displayed on the inner and outer membrane of bacterial ghosts. Vaccine. 2005;23:3609–3617. doi: 10.1016/j.vaccine.2004.11.078. [DOI] [PubMed] [Google Scholar]
- 11.Gong S.S., Nan N., Sun Y.K., He Z.L., Li J.J., Chen F.H., Li T., Ning N.Z., Wang J.X., Li Z., et al. Protective Immunity Elicited by VP1 Chimeric Antigens of Bacterial Ghosts against Hand-Foot-and-Mouth Disease Virus. Vaccines. 2020;8:61. doi: 10.3390/vaccines8010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paukner S., Kohl G., Jalava K., Lubitz W. Sealed bacterial ghosts--novel targeting vehicles for advanced drug delivery of water-soluble substances. J. Drug Target. 2003;11:151–161. doi: 10.1080/10611860310001593366. [DOI] [PubMed] [Google Scholar]
- 13.Paukner S., Kohl G., Lubitz W. Bacterial ghosts as novel advanced drug delivery systems: Antiproliferative activity of loaded doxorubicin in human Caco-2 cells. J. Control. Release. 2004;94:63–74. doi: 10.1016/j.jconrel.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Ebensen T., Paukner S., Link C., Kudela P., de Domenico C., Lubitz W., Guzman C.A. Bacterial ghosts are an efficient delivery system for DNA vaccines. J. Immunol. 2004;172:6858–6865. doi: 10.4049/jimmunol.172.11.6858. [DOI] [PubMed] [Google Scholar]
- 15.Lubitz W., Witte A., Eko F.O., Kamal M., Jechlinger W., Brand E., Marchart J., Haidinger W., Huter V., Felnerova D., et al. Extended recombinant bacterial ghost system. J. Biotechnol. 1999;73:261–273. doi: 10.1016/S0168-1656(99)00144-3. [DOI] [PubMed] [Google Scholar]
- 16.Paukner S., Stiedl T., Kudela P., Bizik J., Al Laham F., Lubitz W. Bacterial ghosts as a novel advanced targeting system for drug and DNA delivery. Expert Opin. 2006;3:11–22. doi: 10.1517/17425247.3.1.11. [DOI] [PubMed] [Google Scholar]
- 17.Mayr U.B., Walcher P., Azimpour C., Riedmann E., Haller C., Lubitz W. Bacterial ghosts as antigen delivery vehicles. Adv. Drug Deliv. Rev. 2005;57:1381–1391. doi: 10.1016/j.addr.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 18.Batah A.M., Ahmad T.A. The development of ghost vaccines trials. Expert Rev. Vaccines. 2020;19:549–562. doi: 10.1080/14760584.2020.1777862. [DOI] [PubMed] [Google Scholar]
- 19.Witte A., Wanner G., Blasi U., Halfmann G., Szostak M., Lubitz W. Endogenous Transmembrane Tunnel Formation Mediated by pX174 Lysis Protein E. J. Bacteriol. 1990;172:4109–4114. doi: 10.1128/jb.172.7.4109-4114.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witte A., Wanner G., Sulzner M., Lubitz W. Dynamics of PhiX174 protein E-mediated lysis of Escherichia coli. Arch. Microbiol. 1992;157:381–388. doi: 10.1007/BF00248685. [DOI] [PubMed] [Google Scholar]
- 21.Witte A., Brand E., Mayrhofer P., Narendja F., Lubitz W. Mutations in cell division proteins FtsZ and FtsA inhibit φX174 protein-E-mediated lysis of Escherichia coli. Arch. Microbiol. 1998;170:259–268. doi: 10.1007/s002030050641. [DOI] [PubMed] [Google Scholar]
- 22.Zhu W.X., Zhang Y.Y., Liu X.L. Efficient production of safety-enhanced Escherichia coli ghosts by tandem expression of PhiX 174 mutant gene E and staphylococcal nuclease A gene. Microbiol. Res. 2015;176:7–13. doi: 10.1016/j.micres.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Yu S.Y., Peng W., Si W., Yin L., Liu S.G., Liu H.F., Zhao H.L., Wang C.L., Chang Y.H., Lin Y.Z. Enhancement of bacteriolysis of Shuffled phage PhiX174 gene E. Virol. J. 2011;8:206. doi: 10.1186/1743-422X-8-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Y., Cui L., Wang M., Sun Q., Liu K., Wang J. A Novel and Efficient High-Yield Method for Preparing Bacterial Ghosts. Toxins. 2021;13:420–431. doi: 10.3390/toxins13060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amara A.A., Salem-Bekhit M.M., Alanazi F.K. Sponge-like: A new protocol for preparing bacterial ghosts. Sci. World J. 2013;2013:545741–545747. doi: 10.1155/2013/545741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park H.J., Oh S., Vinod N., Ji S.M., Noh H.B., Koo J.M., Lee S.H., Kim S.C., Lee K.S., Choi C.W. Characterization of Chemically-Induced Bacterial Ghosts (BGs) Using Sodium Hydroxide-Induced Vibrio parahaemolyticus Ghosts (VPGs) Int. J. Mol. Sci. 2016;17:1904–1918. doi: 10.3390/ijms17111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu W., Hao L., Liu X., Borras-Hidalgo O., Zhang Y. Enhanced anti-proliferative efficacy of epothilone B loaded with Escherichia coli Nissle 1917 bacterial ghosts on the HeLa cells by mitochondrial pathway of apoptosis. Drug Dev. Ind. Pharm. 2018;44:1328–1335. doi: 10.1080/03639045.2018.1449855. [DOI] [PubMed] [Google Scholar]
- 28.Herrero-Fresno A., Olsen J.E. Salmonella Typhimurium metabolism affects virulence in the host—A mini-review. Food Microbiol. 2018;71:98–110. doi: 10.1016/j.fm.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Jawale C.V., Lee J.H. Evaluation of immunogenicity and protective efficacy of adjuvanted Salmonella Typhimurium ghost vaccine against salmonellosis in chickens. Vet. Q. 2016;36:130–136. doi: 10.1080/01652176.2016.1138248. [DOI] [PubMed] [Google Scholar]
- 30.Rokyta D.R., Burch C.L., Caudle S.B., Wichman H.A. Horizontal gene transfer and the evolution of microvirid coliphage genomes. J. Bacteriol. 2006;188:1134–1142. doi: 10.1128/JB.188.3.1134-1142.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Ent F., Lowe J. RF cloning: A restriction-free method for inserting target genes into plasmids. J. Biochem. Biophys. Methods. 2006;67:67–74. doi: 10.1016/j.jbbm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Bond S.R., Naus C.C. RF-Cloning.org: An online tool for the design of restriction-free cloning projects. Nucleic Acids Res. 2012;40:W209–W213. doi: 10.1093/nar/gks396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mykytczuk N.C., Trevors J.T., Leduc L.G., Ferroni G.D. Fluorescence polarization in studies of bacterial cytoplasmic membrane fluidity under environmental stress. Prog. Biophys. Mol. Biol. 2007;95:60–82. doi: 10.1016/j.pbiomolbio.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Gubellini F., Verdon G., Karpowich N.K., Luff J.D., Boel G., Gauthier N., Handelman S.K., Ades S.E., Hunt J.F. Physiological Response to Membrane Protein Overexpression in E. coli. Mol. Cell. Proteom. 2011;10:M111.007930. doi: 10.1074/mcp.M111.007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuller A., Harkness R.E., Ruther U., Lubitzl W. Deletion of C-terminal amino acid codons of PhiX174 gene E: Effect on its lysis inducing properties. Nucleic Acids Res. 1985;13:4143–4153. doi: 10.1093/nar/13.11.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maratea D., Young K., Young R. Deletion and fusion analysis of the phage φX174 lysis gene E. Gene. 1985;40:39–46. doi: 10.1016/0378-1119(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 37.Bläsi U., Lubitz W. Influence of C-terminal Modifications of φX174 Lysis Gene E on its Lysis inducing Properties. J. Gen. Virol. 1985;66:1209–1213. doi: 10.1099/0022-1317-66-6-1209. [DOI] [PubMed] [Google Scholar]
- 38.Bläsi U., Linke R.P., Lubitz W. Evidence for membrane-bound oligomerization of bacteriophage ϕ X174 lysis protein-E. J. Biol. Chem. 1989;264:4552–4558. doi: 10.1016/S0021-9258(18)83778-4. [DOI] [PubMed] [Google Scholar]
- 39.Bläsi U., Linke R.P., Lubitz W. Proline 21, a residue within the alpha-helical domain of phiX174 lysis protein E, is required for its function in Escherichia coli. Biol. Chem. 1989;264:2552–2558. doi: 10.1046/j.1365-2958.1997.5781941.x. [DOI] [PubMed] [Google Scholar]
- 40.Ma J., Bruce T.J., Jones E.M., Cain K.D. A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches. Microorganisms. 2019;7:569. doi: 10.3390/microorganisms7110569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koller V.J., Dirsch V.M., Beres H., Donath O., Reznicek G., Lubitz W., Kudela P. Modulation of bacterial ghosts—induced nitric oxide production in macrophages by bacterial ghost-delivered resveratrol. FEBS J. 2013;280:1214–1225. doi: 10.1111/febs.12112. [DOI] [PubMed] [Google Scholar]
- 42.Paukner S., Kudela P., Kohl G., Schlapp T., Friedrichs S., Lubitz W. DNA-loaded bacterial ghosts efficiently mediate reporter gene transfer and expression in macrophages. Mol. Ther. 2005;11:215–223. doi: 10.1016/j.ymthe.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 43.Hatfaludi T., Liska M., Zellinger D., Ousman J.P., Szostak M., Ambrus A., Jalava K., Lubitz W. Bacterial ghost technology for pesticide delivery. J. Agric. Food Chem. 2004;52:5627–5634. doi: 10.1021/jf049489w. [DOI] [PubMed] [Google Scholar]
- 44.Walcher P., Cui X.L., Arrow J.A., Scobie S., Molinia F.C., Cowan P.E., Lubitz W., Duckworth J.A. Bacterial ghosts as a delivery system for zona pellucida-2 fertility control vaccines for brushtail possums (Trichosurus vulpecula) Vaccine. 2008;26:6832–6838. doi: 10.1016/j.vaccine.2008.09.088. [DOI] [PubMed] [Google Scholar]
- 45.Doore S.M., Fane B.A. The microviridae: Diversity, assembly, and experimental evolution. Virology. 2016;491:45–55. doi: 10.1016/j.virol.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 46.Chamakura K.R., Young R. Single-gene lysis in the metagenomic era. Curr. Opin. Microbiol. 2020;56:109–117. doi: 10.1016/j.mib.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cahill J., Young R. Phage Lysis: Multiple Genes for Multiple Barriers. Adv. Virus Res. 2019;103:33–70. doi: 10.1016/bs.aivir.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wadle D., Henrich B., Plapp R. Effects of mutations in genes fadR, fabB, fadE, and envC of Escherichia coli on the action of the lysis gene of bacteriophage φ X174. Curr. Microbiol. 1986;14:65–69. doi: 10.1007/BF01568405. [DOI] [Google Scholar]
- 49.Dvorak P., Chrast L., Nikel P.I., Fedr R., Soucek K., Sedlackova M., Chaloupkova R., de Lorenzo V., Prokop Z., Damborsky J. Exacerbation of substrate toxicity by IPTG in Escherichia coli BL21(DE3) carrying a synthetic metabolic pathway. Microb. Cell Fact. 2015;14:201–215. doi: 10.1186/s12934-015-0393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie S., Zhang P., Zhang Z., Liu Y., Chen M., Li S., Li X. Bacterial navigation for tumor targeting and photothermally-triggered bacterial ghost transformation for spatiotemporal drug release. Acta Biomater. 2021;131:172–184. doi: 10.1016/j.actbio.2021.06.030. [DOI] [PubMed] [Google Scholar]
- 51.Ye S., Kim J.W., Kim S.R. Metabolic Engineering for Improved Fermentation of L-Arabinose. J. Microbiol. Biotechnol. 2019;29:339–346. doi: 10.4014/jmb.1812.12015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not Applicable.