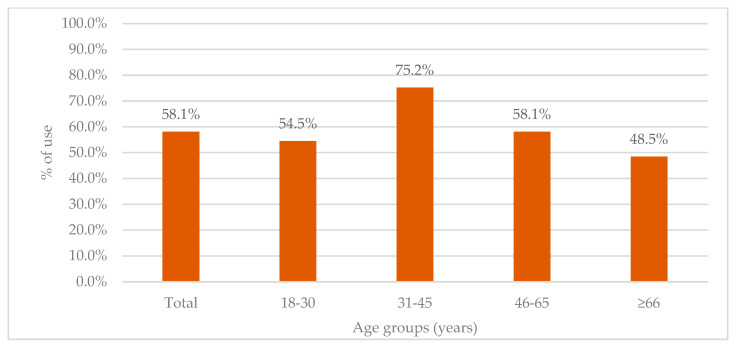Abstract
Antimicrobial resistance (AMR) is a global concern, and antibiotic use has risen throughout the COVID-19 pandemic. Up to 75% of COVID-19 patients are treated with antibiotics despite little evidence for their use. A retrospective study from 6 March 2020 (the start of the pandemic in Serbia) to 31 December 2021 was conducted at the Clinic for Infectious and Tropical Diseases, University Clinical Centre of Serbia. In total, 523 patients with a microbiological diagnosis of COVID-19 were included. Patient data were analysed, including antibiotic use before and after admission. Pre-admission use of antibiotics for COVID-19 treatment was documented in more than half of patients (58.1%), of which a third (34.1%) used more than one antibiotic. Macrolides, cephalosporins, and fluoroquinolones were mainly used, most frequently among patients aged between 31–45 years (75.2%). Prior antibiotic use was associated with a longer duration of illness at admission (8.8 vs. 5.7, p < 0.001), oxygen therapy upon admission (27.6% vs. 16.0%, p = 0.002), and a lower vaccination rate (60.7% vs. 50.7%, p = 0.04). When hospitalised, 72.1% of patients received antibiotics, primarily cephalosporins (71.9%). Significant efforts are needed to reduce antibiotic use in the community and improve prescribing rates by healthcare professionals.
Keywords: COVID-19, Serbia, antibiotic use, cephalosporins, adults
1. Introduction
Antimicrobial resistance (AMR) is responsible for five million deaths every year and its contribution to global mortality will only continue to grow in the coming decades [1]. The COVID-19 pandemic has facilitated the problem of AMR through significant increases in antibiotic prescribing. Up to 75% of patients with COVID-19 are treated with antibiotics, even though the rate of coinfections is low and no clinical benefit has been confirmed [2,3,4]. Most countries in the European Union (EU) have managed to reduce antibiotic prescribing in the years prior to the pandemic [5], but the opposite is true for Serbia [6]. Higher AMR rates are seen in Serbia compared to almost all EU countries in the latest European Centre for Disease Prevention and Control (ECDC) and the World Health Organisation (WHO) reports [7]. For example, Serbia is in the group of several countries with the highest prevalence rate (25–50%) of Staphylococcus aureus isolates resistant to methicillin (MRSA) and is one of a very few countries where carbapenem resistance in Pseudomonas aeruginosa and vancomycin resistance in Enterococcus faecium isolates are detected in ≥50% strains, while the majority of European countries report rates of <25% [7]. In Serbia, it is illegal to buy antibiotics without a prescription, but the sale of antibiotics without a prescription in private pharmacy chains, as well as self-medication, represent significant gaps in better antimicrobial stewardship [8], which is required to reduce the burden of AMR, both in the community and in the hospital setting.
Through our retrospective study covering over 500 patients, we sought to investigate antibiotic use among patients hospitalised with mild-to-moderate COVID-19, before and after admission. We further investigated antibiotic use among major age groups and analysed various clinical features with respect to prior antibiotic use.
2. Results
The clinical characteristics of 523 patients included in the study are shown in Table 1. The mean age was 56.7 years and 214 (40.9%) patients were female. A large proportion of patients (325, 62.1%) had at least one comorbidity, most frequent being hypertension (227, 43.4%), obesity (138, 26.4%), and diabetes (70, 13.4%). Coinfections were present in 22 (4.0%) patients, half of which were urinary tract infections (UTIs; 10, 45.4%). 132 patients (25.2%) were vaccinated. The majority of patients recovered (476, 91.0%).
Table 1.
Clinical characteristics of study patients with COVID-19.
| Patient Characteristics (n = 523) | N (%) | Patient Characteristics (n = 523) | N (%) |
|---|---|---|---|
| Age (mean ± SD) | 56.7 ± 16.0 | Coinfections | 22 (4.0) |
| Gender (female) | 214 (40.9) | Bacterial | 16 (72.7) |
| Vaccinated | 132 (25.2) | UTI | 10 (45.4) |
| LOS (median, IQR) | 9 (5) | SSI | 3 (13.6) |
| Clostridium difficile | 2 (9.0) | ||
| Comorbidities | 325 (62.1) | Syphilis | 1 (4.5) |
| Hypertension | 227 (43.4) | Viral/Fungal | 6 (27.2) |
| Obesity | 138 (26.4) | Primary EBV infection | 1 (4.5) |
| Diabetes | 70 (13.4) | Hepatitis B | 1(4.5) |
| Atrial fibrillation | 40 (7.6) | CMV | 1 (4.5) |
| Coronary heart disease | 39 (7.5) | Herpes zoster | 1(4.5) |
| Solid Tumour | 42 (8.0) | Candidiasis | 1 (4.5) |
| COPD | 11 (26.2) | Pulmonary aspergillosis | 1(4.5) |
| Connective Tissue Disease | 31 (5.9) | ||
| Neurological disease | 30 (5.7) | Outcome | |
| Leukaemia/Lymphoma | 23 (4.4) | Recovered | 476 (91.0) |
| Cardiomyopathy | 20 (3.8) | Transferred to ICU | 20 (3.8) |
| Paralysis | 19 (3.6) | Transferred to another hospital | 19 (3.6) |
| Hashimoto thyroiditis | 15 (2.9) | Died | 3 (0.6) |
| Liver disease | 10 (1.9) | Discharged at personal request | 5 (1.0) |
| Chronic kidney disease | 10 (1.9) | ||
| Congestive heart failure | 9 (1.7) | ||
| Dementia | 8 (1.5) | ||
| HIV | 6 (1.1) | ||
| Peptic ulcer disease | 4 (0.8) |
SD: standard deviation. LOS: length of stay; IQR: interquartile range; COPD: Chronic obstructive pulmonary disease; HIV: human immunodeficiency virus; UTI: urinary tract infection; SSI: skin and soft tissue infection; EBV: Epstein-Barr virus; CMV: cytomegalovirus; ICU: intensive care unit.
More than half of admitted patients (304, 58.1%) used antibiotics prior to admission, with differences in use across major age groups (Figure 1). The lowest rates were seen in patients ≥66 years (48.5%), whereas the highest rate of prior antibiotic use was observed for the 31–45-year group (75.2%).
Figure 1.
Pre-admission antibiotic use stratified across age groups.
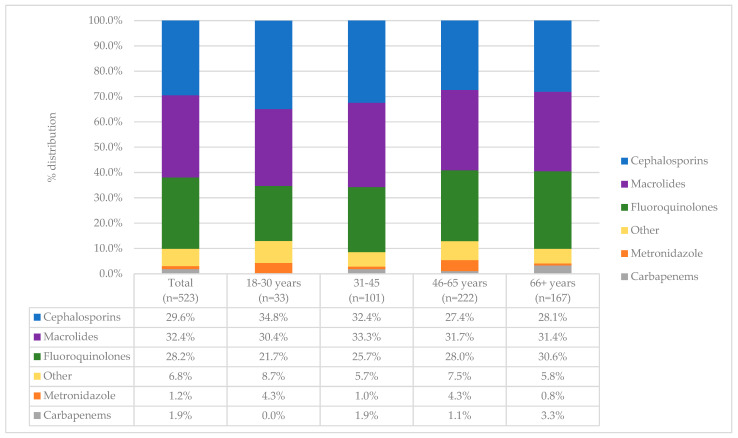
The most frequently used antibiotics used for COVID-19 treatment were macrolides (32.4%) cephalosporins (29.6%) and fluoroquinolones (28.2%). A third of patients (105, 34.5%) reported using more than one antibiotic. When analysing prior antimicrobial use across major age groups, three antibiotic groups emerged with a similar percentage of use (Figure 2). Cephalosporin use ranged from 27.4% in 46–65 years to 34.8% in 18–30 years, macrolide use ranged from 30.4% in 18–30 years to 33.3% in 31–45 years, whereas fluoroquinolones use ranged from 21.7% in 18–30 years to 30.6% in 66+ years.
Figure 2.
Pre-admission profile of antibiotics used across major age groups.
Various patient characteristics at admission were analysed in relation to prior antibiotic use. Associations with a longer duration of illness at admission (8.8 ± 5.3 vs. 5.7 ± 3.7, p < 0.001), a more frequent use for oxygen therapy upon admission (27.6% vs. 16.0%, p = 0.002) and a lower vaccination rate (60.7% vs. 50.7%, p = 0.04) were found (Table 2). Additionally, male gender (62.7% vs. 51.4%, p = 0.009) and younger age (55.1 ± 15.8 vs. 59.0 ± 16.2, p = 0.006) were also associated with prior antibiotic use. Several symptoms on admission, including fever (95.7% vs. 79.0%, p < 0.001), cough (78.0% vs. 58.0%, p < 0.001), malaise/fatigue (72.0% vs. 62.6%, p = 0.019), diarrhoea (22.0% vs. 15.1%, p = 0.044) and nausea (21.0% vs. 12.3%, p = 0.009) were more frequently encountered in patients who used antibiotics before admission.
Table 2.
Patient characteristics in relation to antibiotic use prior to admission.
| Variables | Pre-Admission Antibiotic Use | ||
|---|---|---|---|
| Yes (n = 304) |
No (n = 219) |
p | |
| Gender (male) | 194 (62.7) | 110 (51.4) | 0.009 |
| Comorbidities | 180 (59.2) | 145 (66.2) | 0.103 |
| Vaccinated | 68 (50.7) | 153 (60.7) | 0.045 |
| Age | 55.1 ± 15.8 | 59.0 ± 16.2 | 0.006 |
| Day of illness at admission (median, IQR) | 8.0 (4) | 5.0 (4) | <0.001 |
| O2 therapy upon admission | 84 (27.6) | 35 (16.0) | 0.002 |
| Symptoms | |||
| Fever | 291 (95.7) | 173 (79.0) | <0.001 |
| Cough | 237 (78.0) | 127 (58.0) | <0.001 |
| Malaise/fatigue | 219 (72.0) | 137 (62.6) | 0.019 |
| Dyspnoea | 79 (26.0) | 43 (19.6) | 0.088 |
| Myalgia | 73 (24.0) | 41 (18.7) | 0.145 |
| Diarrhoea | 67 (22.0) | 33 (15.1) | 0.044 |
| Nausea | 64 (21.0) | 27 (12.3) | 0.009 |
| Vomiting | 22 (7.2) | 12 (5.5) | 0.418 |
IQR—interquartile range.
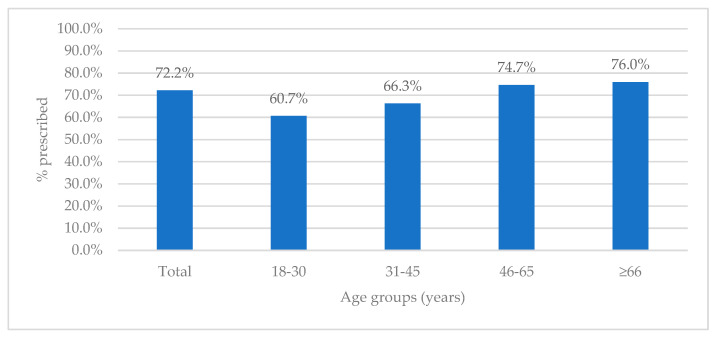
The number of patients who received antibiotics after admission was even higher (377, 72.2%), with slightly lower differences in prescribing across age groups (Figure 3). The lowest rate of prescribing was seen in the 18–30-year group (60.7%), with the highest rates among patients ≥ 66 years (76.0%) and for patients 46–65 years old (74.7%).
Figure 3.
Antibiotic prescribing upon admission across age groups.
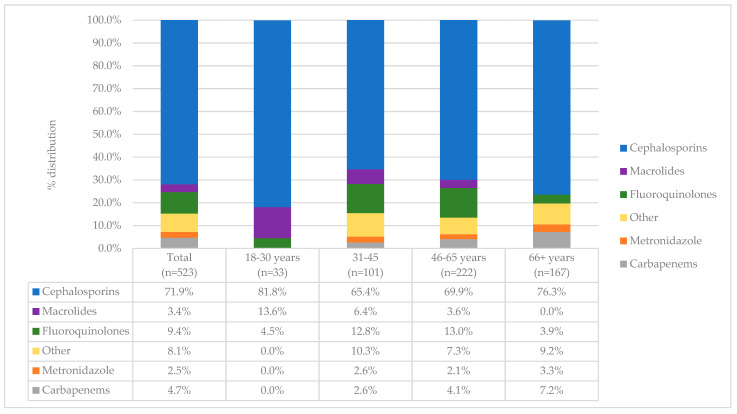
More than one antibiotic was used in 89 (23.6%) patients after hospitalisation, and the vast majority of patients received cephalosporins (276, 71.9%), of which ceftriaxone (231, 83.7%) was by far the most frequently prescribed (Figure 4). Cefuroxime and cefepime were other notable cephalosporins, though used in much lower rates (in 23.1% and 15.5% of patients, respectively). Fluoroquinolone (49, 9.4%) and carbapenem (24, 4.7%) prescribing was significantly lower compared to cephalosporins. When observing post-admission prescribing across age groups, cephalosporin use was highest in the 18–30 year group (428, 81.8%).
Figure 4.
Post-admission profile of antibiotics used across major age groups.
3. Discussion
This is the first study from Serbia looking at antibiotic use in COVID-19 patients, revealing a very high prescribing rate. Despite the fact that only a small number of patients have coinfections (4.0% in our case), our results support numerous studies indicating disproportionately high rates of antibiotic prescribing without a clear indication [9,10,11,12]. Antibiotic use in Serbia is plagued by the poor health education of the general public which leads to frequent self-medication and overall misuse [8,13,14,15]. It is not surprising that overall antibiotic use has increased in the past decade in Serbia compared to virtually all countries in the European Union (EU) [6], with significant efforts needed on a national level to curb the development of AMR.
In our study, pre-admission antibiotic use was associated with a longer duration of illness before admission, a lower vaccination rate, and a more frequent need for oxygen therapy. Such findings hint at either self-treatment or a lack of awareness of the reasons for antibiotic usage, reinforcing the case for health education and more rigorous antibiotic access in the outpatient context. In the outpatient context, we discovered that the age group 31–45 received more antibiotics than the age group > 66. This might be explained by a lack of medical education, the minimization of subjective symptoms, the failure to report to the doctor, and, as a result, the freedom to treat oneself. A different symptom profile, including more frequent nausea and diarrhoea in patients who used antibiotics prior to admission, requires further analysis. This might be explained by the fact that persons with more severe symptoms, such as nausea and diarrhoea, sought out medications, including antibiotics, in the hope that they would alleviate their symptoms.
Regardless of the fact that antibiotics have no clinical benefit for COVID-19 patients and their use should be avoided unless indicated [3,16,17,18], in-patient prescribing rates reach as high as 75% in numerous countries [2], as was the case in our study. Serbian guidelines for COVID-19, from its initial versions, have clearly advised not to use antibiotic treatment unless there is a clear indication (Supplementary Table S1), but we still found very high rates of prescribing, particularly of cephalosporins. Although inappropriate overprescribing could be the primary reason for such high rates, it is still the primary drug of choice for empirical treatment of community acquired pneumonia, predominantly caused by Streptococcus pneumoniae, while atypical pneumonia is quite rarely encountered in our practice. Furthermore, the absence of an accompanying drug such as a macrolide can be explained by its overall avoidance in clinical practice due to high resistance rates, seen both in Serbia and in neighbouring countries, [6,7,19,20]. De-escalation of therapy after excluding pneumococcal pneumonia through microbiological testing, however, is a significant challenge at our hospital, as gold-standard bronchoalveolar lavage (BAL) is not available. Thus, improving microbiological capabilities would certainly optimise antibiotic prescribing, particularly early de-escalation. Nevertheless, further efforts are needed to prevent antibiotic misuse in the hospital setting and studies in Canada and the US have shown successful reductions in antibiotic prescribing [21,22]. In our country, it seems evident that recommendations for antibiotic prescribing and use are not being followed. More rigorous mechanisms of control and surveillance are needed, coupled with education of the general public, but also healthcare professionals.
Our study had a few important limitations. First, the single-centre study prevents the ability to generalize results on a population level. Second, the retrospective design of the study limited us in analysing pre-admission use of antibiotics, including duration of use, the mode of acquiring antibiotics and the indications (if present) for use. The same limitation applies for post-admission use, where a better understanding of in-hospital prescribing was prevented by the study design.
4. Materials and Methods
This retrospective study was conducted at the Department of Clinical pharmacotherapy of the Clinic for Infectious and Tropical Diseases, University Clinical Centre of Serbia. Throughout the COVID-19 pandemic, the Department of Clinical pharmacotherapy was responsible for treating mild-to-moderate patients suffering from COVID-19. Doctors in Serbia are obliged to follow the National Guidelines for Antimicrobial therapy [23], which was used as the main guideline in this study, in addition to the Serbian National COVID-19 Treatment Guidelines that were introduced since the beginning of the pandemic (Supplementary Table S1). From 6 March 2020 (the beginning of the COVID-19 pandemic in Serbia) to 31 December 2021, a total of 523 patients with a microbiological diagnosis of COVID-19 who were hospitalised and treated at our department were included in the study. Patients with incomplete medical records and those in whom a microbiological diagnosis was not made were excluded from the study.
Patient data were collected from the hospital’s electronic medical records system and the following variables were extracted: age, gender, vaccination status, presence of comorbidities and coinfections, length of stay (LOS), and outcome. Since the start of widespread vaccination in Serbia on 19 January 2021, three types of vaccines have become available, with the possibility of individual choice: messenger RNA (mRNA), viral vector vaccines, and inactivated vaccines. Information about antibiotic use, prior to and after hospitalisation, was collected as well, including the number of antibiotics used upon admission.
Mean and standard deviation was used to describe data with normal distribution, whereas median with minimum and maximum values was used for data that were not normally distributed. For categorical variables, numbers and percentages were used. The Chi-square test and the independent t-test were used to compare variables among patients who used antibiotics prior to admission. The level of significance p was established at <0.05.
5. Conclusions
More than half of patients used antibiotics prior to admission, while 72.2% of patients were prescribed antibiotics upon admission despite a very low number of coinfections and no evidence for clinical benefit. As the problem of AMR continues to grow, significant efforts are needed to limit antibiotic use in the community and educate the general public about the proper use of antibiotics. The same applies to the healthcare setting, where better strategies are needed to ensure adherence to guidelines for rational antibiotic prescribing.
Acknowledgments
We would like to thank the entire staff of the Clinic for Infectious and Tropical Diseases, University Clinical Centre of Serbia, for their dedication and selfless commitment throughout the COVID-19 pandemic.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11070847/s1, Table S1. Serbian National COVID-19 Treatment Guidelines Version 11.
Author Contributions
Conceptualization, A.D. and A.B.; methodology, G.S.; formal analysis, A.D., K.C. and T.C.; data curation K.C., T.C.; writing—original draft preparation, A.D.; writing—review and editing, A.D., A.B. and G.S.; visualization, A.D. and A.B.; supervision, G.S.; project administration, A.D.; funding acquisition A.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University Clinical Centre of Serbia (decision no. 847/I, titled “Antibiotic use in COVID-19 patients before and after hospitalisation in a tertiary care hospital”).
Data Availability Statement
Data supporting the results of this study are not publicly available but can be made available on request of the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langford B.J., So M., Raybardhan S., Leung V., Soucy J.-P.R., Westwood D., Daneman N., MacFadden D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021;27:520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chedid M., Waked R., Haddad E., Chetata N., Saliba G., Choucair J. Antibiotics in treatment of COVID-19 complications: A review of frequency, indications, and efficacy. J. Infect. Public Health. 2021;14:570–576. doi: 10.1016/j.jiph.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng T.M., Ong S.W.X., Loo A.Y.X., Tan S.H., Tay H.L., Yap M.Y., Lye D.C., Lee T.H., Young B.E. Antibiotic Therapy in the Treatment of COVID-19 Pneumonia: Who and When? Antibiotics. 2022;11:184. doi: 10.3390/antibiotics11020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Centre for Disease Prevention and Control . Antimicrobial Consumption in the EU/EEA (ESAC-Net)–Annual Epidemiological Report 2020. ECDC; Stockholm, Sweden: 2021. [Google Scholar]
- 6.Tomas A., Pavlović N., Stilinović N., Horvat O., Paut-Kusturica M., Dugandžija T., Tomić Z., Sabo A. Increase and Change in the Pattern of Antibiotic Use in Serbia (2010–2019) Antibiotics. 2021;10:397. doi: 10.3390/antibiotics10040397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Centre for Disease Prevention and Control & World Health Organization . Regional Office for Europe. (Antimicrobial Resistance Surveillance in Europe 2022–2020 Data. World Health Organization. Regional Office for Europe; Geneva, Switzerland: 2022. [Google Scholar]
- 8.Tomas A., Paut Kusturica M., Tomić Z., Horvat O., Djurović Koprivica D., Bukumirić D., Sabo A. Self-medication with antibiotics in Serbian households: A case for action? Int. J. Clin. Pharm. 2017;39:507–513. doi: 10.1007/s11096-017-0461-3. [DOI] [PubMed] [Google Scholar]
- 9.Al-Hadidi S.H., Alhussain H., Abdel Hadi H., Johar A., Yassine H.M., Al Thani A.A., Eltai N.O. The Spectrum of Antibiotic Prescribing During COVID-19 Pandemic: A Systematic Literature Review. Microb. Drug Resist. 2021;27:1705–1725. doi: 10.1089/mdr.2020.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdela S.G., Liesenborghs L., Tadese F., Abegaz S.H., Bayuh F.B., Asmamaw E.A., Mebrate T.A., Mamo A.E., Embiale W., Hunegnaw S., et al. Antibiotic Overuse for COVID-19: Are We Adding Insult to Injury? Am. J. Trop. Med. Hyg. 2021;105:1519–1520. doi: 10.4269/ajtmh.21-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llor C., Ouchi D., Giner-Soriano M., García-Sangenís A., Bjerrum L., Morros R. Correlation between Previous Antibiotic Exposure and COVID-19 Severity. A Population-Based Cohort Study. Antibiotics. 2021;10:1364. doi: 10.3390/antibiotics10111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elsayed A.A., Darwish S.F., Zewail M.B., Mohammed M., Saeed H., Rabea H. Antibiotic misuse and compliance with infection control measures during COVID-19 pandemic in community pharmacies in Egypt. Int. J. Clin. Pract. 2021;75:e14081. doi: 10.1111/ijcp.14081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvat O., Tomas A., Paut Kusturica M., Bukumiric D., Blagojevic B., Kovacevic Z. Serbian students’ knowledge, attitudes and behaviour towards antibiotic use: Is there room for improvement? Int. J. Public Health. 2020;65:1257–1267. doi: 10.1007/s00038-020-01448-6. [DOI] [PubMed] [Google Scholar]
- 14.Kusturica M.P., Tomić Z., Bukumirić Z., Horvat O., Pavlović N., Mikov M., Sabo A. Antibiotics in Serbian Households: A Source of Potential Health and Environmental Threats? Cent. Eur. J. Public Health. 2015;23:114–118. doi: 10.21101/cejph.a4093. [DOI] [PubMed] [Google Scholar]
- 15.Horvat O., Mijatović V., Milijasević B., Tomas A., Kusturica M.P., Tomić Z., Sabo A. Are There Striking Differences in Outpatient Use of Antibiotics Between South Backa District, Serbia, and Some Scandinavian Countries? Front. Public Health. 2018;6:91. doi: 10.3389/fpubh.2018.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin X., Xu X., Li H., Jiang N., Wang J., Lu Z., Xiong N., Gong Y. Evaluation of early antibiotic use in patients with non-severe COVID-19 without bacterial infection. Int. J. Antimicrob. Agents. 2022;59:106462. doi: 10.1016/j.ijantimicag.2021.106462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popp M., Stegemann M., Riemer M., Metzendorf M.-I., Romero C.S., Mikolajewska A., Kranke P., Meybohm P., Skoetz N., Weibel S. Antibiotics for the treatment of COVID-19. Cochrane Database Syst. Rev. 2021;10:CD015025. doi: 10.1002/14651858.CD015025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atić A., Sorić M., Stojić J., Andrilović A., Grabovac V. The effect of outpatient antibiotic treatment of coronavirus disease 2019 on the outcomes in the emergency department: A propensity score matching study. Croat. Med. J. 2022;63:53–61. doi: 10.3325/cmj.2022.63.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mijac V., Opavski N., Markovic M., Gajic I., Vasiljevic Z., Sipetic T., Bajcetic M. Trends in macrolide resistance of respiratory tract pathogens in the paediatric population in Serbia from 2004 to 2009. Epidemiol. Infect. 2015;143:648–652. doi: 10.1017/S0950268814001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kastrin T., Paragi M., Erčulj V., Čretnik T.Ž., Slovenian Meningitidis Study Group. Bajec T., Čižman M. Lack of correlation between reduced outpatient consumption of macrolides and macrolide resistance of invasive Streptococcus pneumoniae isolates in Slovenia during 1997–2017. J. Glob. Antimicrob. Resist. 2019;16:242–248. doi: 10.1016/j.jgar.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Knight B.D., Shurgold J., Smith G., MacFadden D.R., Schwartz K.L., Daneman N., Gravel Tropper D., Brooks J. The impact of COVID-19 on community antibiotic use in Canada: An ecological study. Clin. Microbiol. Infect. 2022;28:426–432. doi: 10.1016/j.cmi.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staub M.B., Beaulieu R.M., Graves J., Nelson G.E. Changes in antimicrobial utilization during the coronavirus disease 2019 (COVID-19) pandemic after implementation of a multispecialty clinical guidance team. Infect. Control Hosp. Epidemiol. 2021;42:810–816. doi: 10.1017/ice.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radna Grupa za Izradu Nacionalnog Vodica Dobre Klinicke Prakse Ministarstva Zdravlja Republike Srbije . Nacionalni Vodic Dobre Klinicke Prakse. Racionalna Upotreba Antibiotika. Ministarstvo Zdravlja Republike Srbije; Belgrade, Serbia: 2018. [(accessed on 30 April 2022)]. Available online: https://www.zdravlje.gov.rs/tekst/335899/novi-nacionalni-vodic-za-racionalnu-upotrebu-antibiotika-.php. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the results of this study are not publicly available but can be made available on request of the corresponding author.