ABSTRACT
Background
Solid organ transplant recipients (SOTR) are at high risk of keratinocyte carcinoma (KC). Long‐term evidence for acitretin as chemoprophylaxis in this population is lacking.
Objective
To determine the benefit of long‐term acitretin for KC chemoprevention in SOTR.
Methods
A retrospective cohort study of SOTR treated with acitretin at an Australian transplant dermatology clinic was performed. General estimating equations were used to evaluate change in rates of histologically confirmed KC in the 6–12 months prior to acitretin and following a minimum 6 months of treatment. A control group of patients within the same service was included, comprising SOTR who were not treated with acitretin.
Results
Twenty‐two patients received acitretin treatment for at least 6 months, eighteen for at least 5 years and four for at least 9 years. The median KC rate pretreatment was 3.31 per year (IQR 1.93, 5.40). There was a significant reduction in the rate of KC in the first year of acitretin treatment (IRR 0.41, 95% CI 0.22, 0.76, P = 0.005), and this effect was observed for 5 years (IRR at 5 years 0.34, 95% CI 0.17, 0.67, P = 0.002). The control group had no statistically significant change in KC rate over time in the study.
Conclusions
Acitretin appears to be well‐tolerated and effective in reducing KC in SOTR for at least 5 years. Study limitations include its retrospective nature, small sample size and lack of blinding.
Keywords: acitretin, chemoprevention, immunosuppression, organ transplants, retinoids, skin neoplasms, squamous cell carcinoma
INTRODUCTION
Solid transplant recipients (SOTR) treated with immunosuppressant medications are at significantly increased risk of keratinocyte carcinoma (KC). 1 Compared to the general population, they are also more likely to have aggressive disease with higher rates of invasion and mortality. 2
Management of skin cancer in SOTR includes close surveillance, surgery, radiotherapy, cryotherapy and topical chemotherapeutic agents. Strategies for prevention include photoprotection, systemic acitretin chemoprophylaxis, reduction of immunosuppression and substitution of immunosuppressive agent(s) with mammalian target of rapamycin (mTOR) inhibitors. 3 A recent Delphi consensus statement recommended acitretin as chemoprophylaxis in SOTR who develop one high‐risk cutaneous squamous cell carcinoma (SCC) after multiple low‐risk SCCs, or more than 10 low‐risk SCCs per year. 4
Acitretin is a synthetic retinoid thought to affect cell‐cycle control, induce apoptosis, promote cellular differentiation and immunomodulation, and inhibit ornithine decarboxylase, cellular proliferation and keratinisation. 5 Retinoids also inhibit growth of human papilloma virus (HPV)‐16‐immortalised keratinocytes compared with noninfected cells, and there is a high prevalence of HPV in KC of SOTR. 6 , 7
A randomised controlled trial of 6‐month duration and a randomised crossover trial of 2‐year duration have shown acitretin reduces KC in SOTR. 8 , 9 A retrospective before‐after study demonstrated benefit with up to 3 years of treatment but was unable to show a significant reduction beyond 3 years as only 11 patients were followed beyond that point. 10 A similar study suggested benefit with up to 4 years of treatment, but only 10 patients were followed up for that duration. 11 A ‘rebound effect’ has been observed with rapid increases in KC following discontinuation of acitretin; however, its long‐term efficacy is unclear. 8 , 10
Common side effects of acitretin include mucocutaneous xerosis, alopecia, headaches and myalgia. 12 Hypercholesterolaemia, liver impairment and bony abnormalities are also of concern, particularly with high‐dose treatment. 13 Considering the adverse side‐effect profile of acitretin, further evidence is needed to establish its long‐term utility.
OBJECTIVE
To assess the short‐ and long‐term efficacy of acitretin in reducing the rate of KC in SOTR.
METHODS
Ethics approval was obtained from the St Vincent's Hospital (Melbourne) Human Research Ethics Committee.
A retrospective cohort study of SOTR at the Skin Health Institute's specialised organ transplant dermatology service in Melbourne was performed. Data including patients' age, sex, Fitzpatrick skin phototype, transplantation history, age at transplantation, immunosuppressive regimen, acitretin treatment (including dose) and histological diagnosis of skin cancers at each visit were prospectively collected in the clinic and recorded in a central database. SOTR with at least one invasive SCC, significant actinic field damage and no contraindications were commenced on acitretin. Transplant physician approval was sought prior to commencement of acitretin. Contraindications to acitretin included hypersensitivity, potential pregnancy, breastfeeding, severely impaired liver or kidney function, uncontrolled hyperlipidaemia or medication interactions. Dosing started at 10 mg daily and was titrated up to a maximum of 35 mg daily according to clinical response and tolerability. Acitretin was discontinued if there were unacceptable side effects or significant derangement in laboratory tests. Patients were assessed by dermatologists for monitoring adverse effects and a full skin examination every 3 to 6 months. Suspicious lesions were biopsied or excised, and premalignant lesions were treated with cryotherapy or topical 5‐fluorouracil. Education regarding photoprotection and self skin surveillance was reinforced. Immunosuppression may have been altered by transplant physicians for various reasons.
Patients who received a minimum of 6 months of acitretin between February 2004 and February 2018 with a history of at least one KC were included. KC was defined as histologically confirmed SCC, squamous cell carcinoma in situ (SCCIS) or basal cell carcinoma (BCC). Each patient served as his/her own control. A period of 6 to 12 months prior to commencement of acitretin was included in the analysis. Data cleaning was performed by verification with medical records. We compared the rate of KC development before and after commencing acitretin. Another control group of patients within the same service was included, comprising SOTR with at least one KC who were observed for a minimum of 2 years and who were not treated with acitretin. Patients in the control group either did not meet prescription criteria for acitretin (including contraindications) or declined acitretin.
Descriptive analyses were presented as median (interquartile range) and frequency (percentage). Characteristics of patients receiving acitretin and the control group were compared using the rank‐sum test (continuous variables) and the Fisher's exact test (categorical variables). To compare the patients' pretreatment KC rate with each year on acitretin, general estimating equations with negative binomial distribution were used, calculating incidence rate ratios (IRR) with 95% confidence intervals. A P‐value of <0.05 was considered statistically significant. Confounding factors including age, gender, time since transplantation and skin phototype were adjusted for in the analyses. All available follow‐up data were used in the analysis. Missing data were accounted for within the model using maximum likelihood. Analyses were conducted using Stata 16.1 (StataCorp 2019, TX:StataCorp LLC).
RESULTS
During the study period, 456 SOTR were seen, of which 54 were commenced on acitretin. Twenty‐two patients met inclusion criteria (Fig. 1). Of the other 32 patients excluded, 21 commenced acitretin without 6 months of pretreatment observation, and 3 did not have at least one KC in the 12 months prior to acitretin. Nine of the 54 patients (16.7%) stopped acitretin due to side effects (6 with mucocutaneous xerosis, 1 with peripheral sensory neuropathy, 1 with visual hallucinations and 1 with diarrhoea). Eight of these patients stopped acitretin within the first 6 months of treatment.
Figure 1.
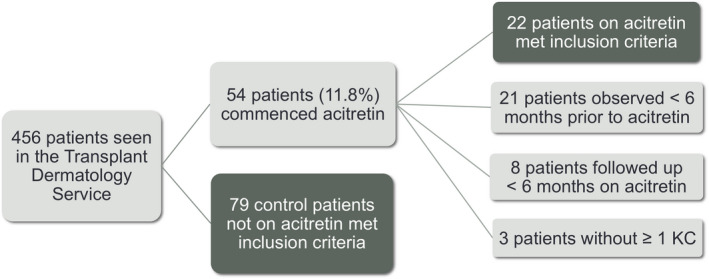
Flowchart of patient inclusion in study. KC, keratinocyte carcinoma. [Colour figure can be viewed at wileyonlinelibrary.com]
All 22 patients included had at least 2 years of acitretin treatment; 18 had at least 5 years and 4 at least 9 years. All 22 patients who completed 6 months on acitretin remained on treatment for their duration of follow‐up.
Nineteen patients (86%) were male, and 3 patients (14%) were female. Patients were commenced on acitretin at an average age of 58 years (IQR 53, 66) and, on average, 12 years post‐transplant (IQR 8, 15). Organs transplanted included 16 (73%) renal, 4 (18%) heart, 1 (5%) liver and 1 (5%) lung. One renal transplant patient had graft failure after 5 years on acitretin with subsequent re‐transplantation and ongoing immunosuppression. The graft failure was not attributed to acitretin, and she remained on acitretin for a further 5 years.
The median acitretin dose was 10 mg daily (IQR 10.00, 10.71). The lowest average patient dose throughout treatment was 8.10 mg daily, and the highest was 22.50 mg daily. The mode was 10 mg daily, but dosing regimens ranged from 10 mg every 4 days to 35 mg daily, determined by clinical benefit and side effects.
Immunosuppressive regimens consisted of 13 different combinations including prednisolone, mycophenolate mofetil, cyclosporin, azathioprine, tacrolimus, everolimus and sirolimus. Nine patients changed regimens between 1 and 3 times during the study period. The commonest regimen was prednisolone, mycophenolate mofetil and cyclosporin (8 patients), followed by prednisolone, mycophenolate mofetil and tacrolimus (6 patients). Four patients were changed to regimens involving mTOR inhibitors during the study.
Seventy nine control patients who were not on acitretin met the inclusion criteria. Table 1 summarises the demographic characteristics of the acitretin and control groups. The control group was significantly younger (median age 54 vs 58.5 years, P = 0.037) and fewer were male (63% vs 86%, P = 0.042). Median time post‐transplantation in the control and acitretin groups was 4 and 12 years respectively (P = 0.001). Otherwise, there were no significant differences, including age at transplant, skin phototype or organ transplanted.
Table 1.
Patient characteristics
| Acitretin | Control | P‐value | ||
|---|---|---|---|---|
| Number of patients | 22 | 79 | ||
| Age at first transplant, median (IQR) | 49.5 (44, 55) | 50 (39, 58) | 0.80 | |
| Age at acitretin commencement/control study commencement, median (IQR) | 58.5 (53, 66) | 54 (47, 62) | 0.037 | |
| Years since first transplant, median (IQR) | 12 (8, 15) | 4 (1, 9) | <0.001 | |
| Years of follow‐up after acitretin commencement/control study commencement, median (IQR) | 5.5 (4.7, 7.7) | 5.9 (3.8, 8.8) | 0.64 | |
| Sex | Male | 19 (86%) | 50 (63%) | 0.042 |
| Female | 3 (14%) | 29 (37%) | ||
| Skin phototype | 1 | 4 (18%) | 11 (14%) | 0.92 |
| 2 | 7 (32%) | 26 (33%) | ||
| 3 | 7 (32%) | 23 (29%) | ||
| 4 | 2 (9%) | 6 (8%) | ||
| Not documented | 2 (9%) | 13 (16%) | ||
| Organ(s) transplanted | Renal | 16 (73%) | 66 (84%) | 0.33 |
| Heart | 4 (18%) | 5 (6%) | ||
| Liver | 1 (5%) | 3 (4%) | ||
| Lung | 1 (5%) | 2 (3%) | ||
| Renal and pancreas | 0 (0%) | 3 (4%) | ||
IQR, interquartile range.
Figure 2 and Table S1 show the annual KC rates for patients on acitretin. Prior to acitretin treatment, the median KC rate was 3.31 per year (IQR 1.93, 5.40). This then decreased between 0 and 1 KC per year for the first 5 years on acitretin. This decrease was also seen for the SCC and SCCIS subgroups. The rates of BCC were low, with a median of 0.44 pretreatment (IQR 0.00, 1.93).
Figure 2.
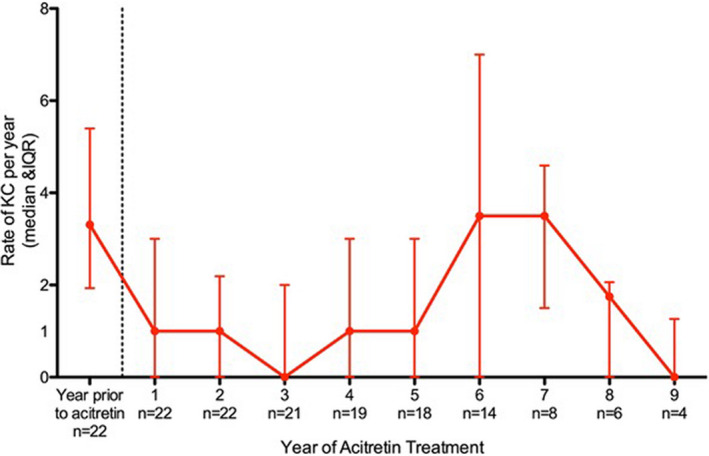
Annual rate of keratinocyte carcinoma on acitretin treatment. Rate of keratinocyte carcinoma (median and interquartile range) for each year of follow‐up before and after commencing acitretin with the corresponding number (n) of patients followed up for that duration. KC, keratinocyte carcinoma; IQR, interquartile range. [Colour figure can be viewed at wileyonlinelibrary.com]
A 50% reduction in KC development was observed for the first 5 years of acitretin treatment (IRR < 0.5, P ≤ 0.023) (Fig. 3 and Table S2). At years 6, 7 and 8, a reduction compared with the pretreatment baseline was maintained, but this was no longer significant. Similar findings were found for the SCC and SCCIS subgroups. IRR in the BCC subgroup was significantly reduced in the first year only (IRR 0.27; 95% CI 0.10, 0.70; P = 0.008).
Figure 3.
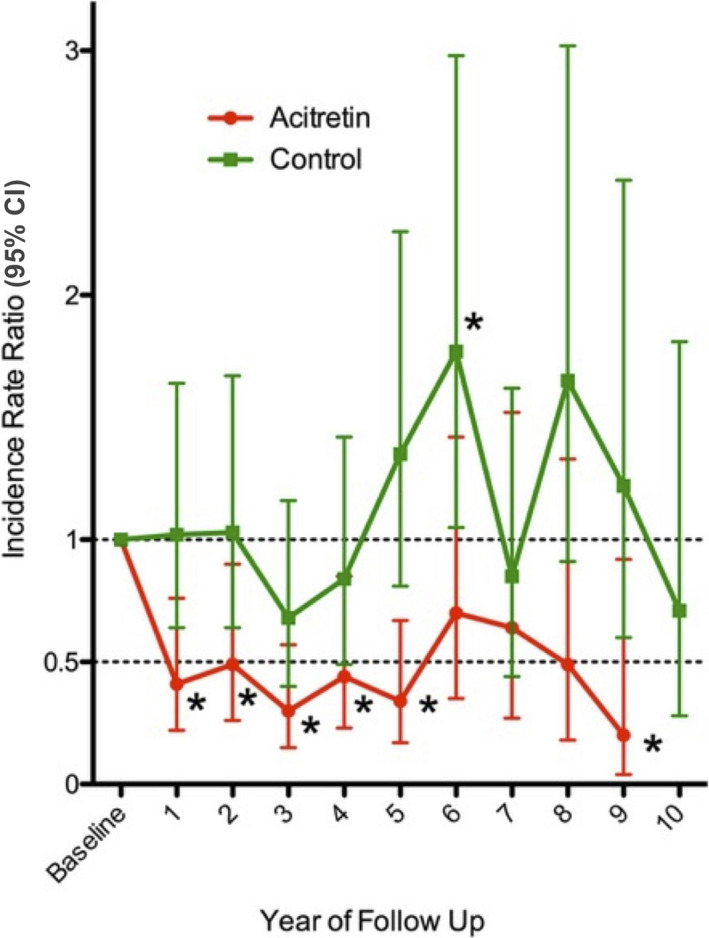
Incidence rate ratio of keratinocyte carcinoma in the acitretin and control groups compared with baseline. General estimating equations were calculated to compare the patients' yearly baseline keratinocyte carcinoma rate with each subsequent year of follow‐up (baseline corresponds to the year prior to acitretin treatment in the acitretin group). Incidence rate ratio = 1 denotes no change in keratinocyte carcoma rate from baseline. CI, confidence interval. *P < 0.05. [Colour figure can be viewed at wileyonlinelibrary.com]
Adjusting for variables including patient sex, age, skin phototype and time since transplantation showed there was a significant effect for skin phototype only. Compared with skin phototype 1, skin phototypes 3 and 4 had 51% (P = 0.048) and 68% (P = 0.031) lower rates of KC respectively. An increase in age of 1 year increased KC rate by 3% (P = 0.08). Adjusting for these variables did not significantly affect the trends in the rate of KC over time. It was not possible to adjust for all variables simultaneously due to the small sample size.
There was no significant change in IRR of KC over time in the control group (Fig. 3 and Table S3). This contrasts with a significant reduction in IRR for the first 5 years for those on acitretin (P ≤ 0.023).
The acitretin group's KC rate was compared with the control group (Fig. 4 and Table S4). These analyses were adjusted for sex, age and time since transplantation, as there were significant differences in these characteristics between groups. Prior to treatment, the acitretin group had a significantly higher rate of KC (IRR 6.31; 95% CI, 1.44–11.8; P < 0.001). The comparison of KC rates showed approximately a halving of the pretreatment IRR for the first 6 years after acitretin commencement (IRR ≤ 3.89). At year 5, the rate of KC in the acitretin group was similar to the control group (IRR 1.73, 95% CI 0.83, 3.63, P = 0.146).
Figure 4.
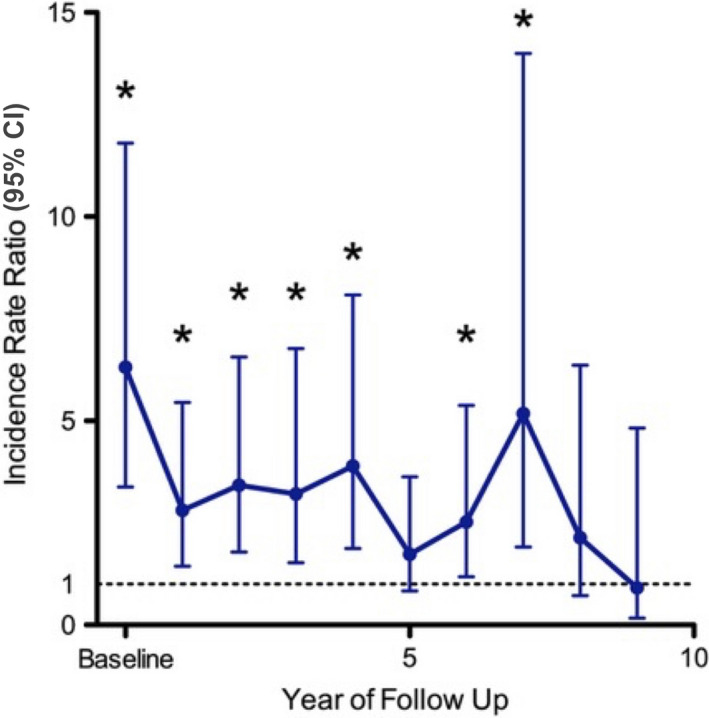
Incidence rate ratio of keratinocyte carcinoma in the acitretin group compared with the control group. General estimating equations were used to compare the acitretin and control groups' keratinocyte carcinoma rates with adjustment for sex, age and time since transplantation. IRR = 1 denotes no change in keratinocyte carcoma rate from baseline. CI, confidence interval. *P < 0.05. [Colour figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
Our study demonstrated a significant treatment effect of acitretin to at least 5 years. This is longer than the previously reported duration of the effect of up to 4 years. 11 While the effect continues to 9 years, the loss of significance may reflect the small sample size of patients followed up beyond 5 years. The IRR in our study was less than 0.50, suggesting a significant 50% reduction in KC for the first 5 years of treatment (P < 0.05). This reflects similar findings in George and colleagues' randomised crossover trial, where the average number of SCC in the acitretin‐free period increased by 42%. 9 In a retrospective before‐after study where the mean SCC number was 2.9 in the 12‐month pretreatment interval, Harwood and colleagues showed a mean difference of SCC of 1.46 in the first year of treatment (P = 0.006), 2.20 in the second (P < 0.001) and 2.14 in the third (P = 0.02). 10
The overall KC rates in our study mirrored closely the SCC and SCCIS subgroup rates. BCCs developed less often, and the IRR was significantly reduced only for the first year on acitretin. This initial effect on BCC rate is unlikely to be of clinical significance given it was not sustained.
In their randomised double‐blinded placebo‐controlled trial, Bavinck and colleagues demonstrated benefit within the first month of commencing acitretin at a dose of 30 mg daily, and our study also showed benefit from the first analysed time point at 1 year. 8 Considerable improvement in widespread epidermal dysplasia was observed for many patients in this trial; however, this was not quantified in our study.
Our results showed skin phototype impacted the IRR of KC with skin phototype 1 having an increased rate of KC. Our analyses also correlated increasing age with higher KC rate, but this was not significant. Other studies have shown that age (≥ 55 years vs < 55 years) had no major effect on retinoid efficacy. 10 We found time since transplantation did not impact acitretin effect, consistent with prior literature. 10
Acitretin dosing in our cohort, titrated according to clinical benefit and side effects, was at a median of 10 mg daily, lower than most studies, which included doses up to 50 mg daily. 8 , 10 , 11 , 14 Nine of 54 (16.7%) patients ceased acitretin in our clinic due to side effects. This compares with reported rates of 39% where dosing was 25–50 mg alternate daily. 9 In another study, only 3 of 14 patients could tolerate a dose of 0.4 mg/kg daily. 14 Although there is theoretical concern for graft rejection associated with the immunopotentiating effects of retinoids, acitretin was not implicated in the one patient who developed graft rejection 5 years into acitretin treatment.
As expected, the control group had characteristics that confer a lower skin cancer risk including younger age and less time post‐transplantation. The control group had no statistically significant change in KC rate over time in the study. Compared with the decreased rate of KC in the acitretin group, this control group finding supports a treatment effect of acitretin beyond that from increased surveillance within our service alone, which included treatment of premalignant lesions and education regarding photoprotection.
All KCs in our study were histologically proven, providing more accurate data than studies based on clinical diagnoses. Interobserver variation was restricted by having a small number of dermatologists assessing patients. Our study was inclusive of SOTR with organs other than renal transplant making these finding generalizable to a wider transplant population; however, subgroup analyses of each organ transplanted was not performed. KC incidence increases with decreasing latitude, with studies showing higher rates in Australian SOTR than those in Europe and the United Kingdom, 15 so these findings may not directly translate to other countries.
Our study was limited by its retrospective nature, small sample size and lack of blinding. The sample size decreased for the later years of our study, which could explain why there was no significant effect observed beyond 5 years. Acitretin dosage and immunosuppressive regimens varied between and within individual patients over time in our study. We were therefore not powered to assess the impact of these in our analysis. Four patients changed immunosuppressive regimens to include an mTOR inhibitor during the study, which may have decreased their rate of KC development independently of acitretin. Patients who discontinued acitretin if their perceived benefit did not outweigh the side effects may have contributed to bias against the therapeutic effect of acitretin. It is possible that our study method underestimated the extent and duration of benefit, as data have shown an increase in KC with advancing age and increased time on immunosuppression following transplant. 16 Bavinck and colleagues demonstrated the effect of acitretin is more pronounced in those with a history of KC. 8 The effect of acitretin may have therefore been greater than observed in our study as 21 high‐risk patients with multiple previous KCs were excluded because they were started promptly on acitretin without 6 months prior observation within our service.
CONCLUSION
Acitretin appears to reduce KC development in SOTR by at least 50% for the first 5 years of treatment. Side effects can be a limiting factor, but lower doses, such as the median dose of 10 mg daily in our study, are relatively well‐tolerated. Clinical response should be assessed and the risk–benefit ratio considered in determining maintenance dosing. Further research with a larger patient population is needed to determine optimal dosing, timing of intervention, and long‐term safety and efficacy of acitretin for chemoprophylaxis in SOTR.
CONFLICTS OF INTEREST
The authors have no conflict of interest to declare.
Funding information
Funding was provided by the Skin Health Institute, Carlton, Victoria, Australia.
PERMISSIONS
All figures and tables are original, and reprint permission is not required.
IRB APPROVAL STATUS
Reviewed and approved by St Vincent's Hospital (Melbourne) Human Research Ethics Committee, reference # LRR 009/18.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the Transplant Dermatology Clinic at the Skin, Health Institute, Melbourne, Australia.
REFERENCES
- 1. Ng JC, Cumming S, Leung V et al. Accrual of non‐melanoma skin cancer in renal‐transplant recipients: Experience of a Victorian tertiary referral institution. Aust. J. Dermatol. 2014; 55: 43–8. [DOI] [PubMed] [Google Scholar]
- 2. Garrett GL, Lowenstein SE, Singer JP et al. Trends of skin cancer mortality after transplantation in the United States: 1987 to 2013. J. Am. Acad. Dermatol. 2016; 75: 106–12. [DOI] [PubMed] [Google Scholar]
- 3. Perez HC, Benavides X, Perez JS et al. Basic aspects of the pathogenesis and prevention of non‐melanoma skin cancer in solid organ transplant recipients: a review. Int. J. Dermatol. 2017; 56: 370–8. [DOI] [PubMed] [Google Scholar]
- 4. Massey PR, Schmults CD, Li SJ et al. Consensus‐based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a delphi consensus statement. JAMA Dermatol. 2021; 157: 1219–26. 10.1001/jamadermatol.2021.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lens M, Medenica L. Systemic retinoids in chemoprevention of non‐melanoma skin cancer. Expert Opin. Pharmacother. 2008; 9: 1363–74. [DOI] [PubMed] [Google Scholar]
- 6. Berkhout RJM, Tieben LM, Smits HL et al. Nested PCR approach for detection and typing of epidermodysplasia verruciformis‐associated human papillomavirus types in cutaneous cancers from renal transplant recipients. J. Clin. Microbiol. 1995; 33: 690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harwood CA, Proby CM. Human papilloma viruses and non‐melanoma skin cancer. Curr. Opin. Infect. Dis. 2002; 15: 101–14. [DOI] [PubMed] [Google Scholar]
- 8. Bavinck JN, Tieben LM, Van der Woude FJ et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double‐blind, placebo‐controlled study. J. Clin. Oncol. 1995; 13: 1933–8. [DOI] [PubMed] [Google Scholar]
- 9. George R, Weightman W, Russ GR et al. Acitretin for chemoprevention of non‐melanoma skin cancers in renal transplant recipients. Aust. J. Dermatol. 2002; 43: 269–73. [DOI] [PubMed] [Google Scholar]
- 10. Harwood CA, Leedham‐Green M, Leigh IM et al. Low‐dose retinoids in the prevention of cutaneous squamous cell carcinomas in organ transplant recipients: a 16‐year retrospective study. Arch. Dermatol. 2005; 141: 456–64. [DOI] [PubMed] [Google Scholar]
- 11. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years experience using low‐dose acitretin. Br. J. Dermatol. 1999; 140: 656–60. [DOI] [PubMed] [Google Scholar]
- 12. Chen K, Craig JC, Shumack S. Oral retinoids for the prevention of skin cancers in solid organ transplant recipients: a systematic review of randomized controlled trials. Br. J. Dermatol. 2005; 152: 518–23. [DOI] [PubMed] [Google Scholar]
- 13. Otley C, Stasko T, Tope W et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol. Surg. 2006; 32: 562–8. [DOI] [PubMed] [Google Scholar]
- 14. De Sévaux R, Smit J, de Jong E et al. Acitretin treatment of premalignant and malignant skin disorders in renal transplant recipients: clinical effects of a randomized trial comparing two doses of acitretin. J. Am. Acad. Dermatol. 2003; 49: 407–12. [DOI] [PubMed] [Google Scholar]
- 15. Berg D, Otley CC. Skin cancer in organ transplant recipients: epidemiology, pathogenesis, and management. J. Am. Acad. Dermatol. 2002; 47: 1–20. [DOI] [PubMed] [Google Scholar]
- 16. Howard MD, Su JC, Chong AH. Skin cancer following solid organ transplantation: a review of risk factors and models of care. Am. J. Clin. Dermatol. 2018; 19: 585–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


