Abstract
Gluconacetobacter xylinus (=Acetobacter xylinum) ATCC 10245 incorporated 2-amino-2-deoxy-d-glucose (glucosamine) and 2-acetamido-2-deoxy-d-glucose (N-acetylglucosamine), but not 3-O-methyl-d-glucose or 2-deoxy-d-glucose into exopolymers. Incorporation was confirmed by gas chromatography with and without mass spectrometry, Fourier transform infrared, and 1H nuclear magnetic resonance. The average molar percentage of glucosamine and N-acetylglucosamine in the exopolymers was about 18%.
Cellulose, (1-4)-linked-β-d-glucan, is a major structural component of the cell walls of higher plants (8) and also generated as an exopolymer by some microorganisms (5, 6, 21). Structurally related polysaccharides such as chitin [(1-4)-linked 2-acetamino-2-deoxy-β-d-glucose] occur as a major cuticular or skeletal component in all arthropods, some invertebrata, and some fungi (11). Chitosan (2-acetamido-2-deoxy-β-d-glucopyranose) is the fully or partially deacetylated form of chitin and is found in the cell walls of some fungi, such as Mucor rouxi (2–4, 13).
The biosynthesis of polysaccharides has traditionally been studied using unmodified simple sugars such as glucose and sucrose or complex carbon sources such as wheat gluten and molasses (14). Alternatively, microbial mutants have been used to manipulate biopolymer molecular weight, yield, and main chain or branch composition (12, 22). Glucose-rich polysaccharides such as cellulose and curdlan have been postbiosynthetically derivatized by nonspecific chemical means to change physical or biological properties (18, 23) and by selective chemical modification under homogeneous conditions (19). Disadvantages to these approaches can include include low yields, side reactions, the use of toxic solvents, and purification requirements. Therefore, it was desirable to explore direct, in vivo incorporation of simple sugar analogs as building blocks for polysaccharides.
Glucose derivatives may be particularly effective as novel carbon sources because glucose is a main component in many polysaccharides and therefore a logical target for direct polymerization by the microorganism. We previously reported the direct incorporation of glucose-related sugars 3-O-methyl-d-glucose (3-O-methylglucose) and 2-acetamido-2-deoxy-d-glucose (N-acetylglucosamine) into curdlan (15), the direct incorporation of specific fatty acid pendant groups on a main chain polysaccharide such as emulsan (9, 10, 24), and the modification of metabolic pathways involved in exopolymer synthesis in the case of pullulan (17).
In the present work, investigations were conducted by selective feeding experiments to determine the flexibility of the polymerization system in Gluconacetobacter xylinum ATCC 10245. Specifically, we report on the ability of this strain to incorporate glucosamine and N-acetylglucosamine into exopolymers.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
G. xylinum (=Acetobacter xylinum) ATCC 10245 was obtained from the American Type Culture Collection. Glucose (>99.5% purity), 3-O-methyl-d-glucose, glucosamine (>99.0% purity), N-acetylglucosamine, 2-deoxy-d-glucose (2-deoxyglucose), and t-butanol (>99.0% purity) were all purchased from Sigma Chemical Co. (St. Louis, Mo.). Methanol (>99.9% purity) was purchased from J. T. Baker Co. (Phillipsburg, N.J.); pyridine (>99.9% purity) was purchased from Aldrich Chemical Co. (Milwaukee, Wis.); acetic anhydride (>97.0% purity) and acetyl chloride (98% purity) were purchased from Fisher Scientific (Fair Lawn, N.J.); and Tri-Sil Z (25% [vol/vol] N-trimethylsilyimidazole in pyridine) for silylation was purchased from Pierce (Rockford, Ill.). The medium (Y3-3) for the production of cellulose was based on that of Johnson and Neogi (13a). Carbon sources included glucose, 3-O-methylglucose, glucosamine, N-acetylglucosamine, and 2-deoxyglucose. Cultures were incubated for 7 to 14 days with either glucose or one of the glucose analogs under the same conditions as for the starter cultures.
Purification and characterization of expolymers.
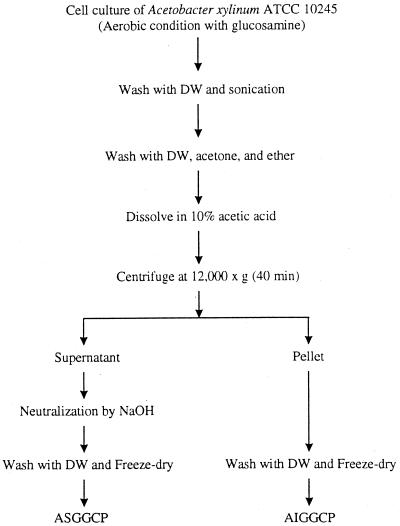
Cell cultures of G. xylinum with the formed exopolymer were centrifuged at 12,000 × g for 40 min at 4°C (Fig. 1). After removing the supernatant, the precipitate was added to an equivalent volume of distilled water, and the mixture was stirred for 10 min at room temperature. The resulting viscous solution was centrifuged at 12,000 × g for 40 min at 4°C. After removing the supernatant, the precipitate was added to an equivalent volume of distilled water, the mixture was stirred for 10 min and sonicated for 5 min at a sonication level of 5 using a Cell Disruptor 350 (Branson Sonic Power Co., Danbury, Conn.) at room temperature. The resulting solution was centrifuged at 12,000 × g for 40 min at 4°C. After removing the supernatant, the precipitate was added to an equivalent volume of distilled water, and the mixture was stirred for 10 min. The resulting viscous solution was centrifuged at 12,000 × g for 40 min at 4°C. After removing the supernatant, the precipitate was repeatedly washed with water, acetone, and ether to remove contaminants. The washed precipitate was freeze-dried to obtain the total exopolymer produced by G. xylinum ATCC 10245.
FIG. 1.
Procedure to purify and fractionate exopolymers synthesized with glucosamine. AIGGCP, acetic acid-insoluble glucose-coglucosamine copolymer; ASGGCP, acetic acid-soluble glucose-coglucosamine copolymer; DW, distilled water.
Further fractionation was accomplished by the addition of 150 ml of aqueous acetic acid (10%, vol/vol) to 300 mg of the exopolymer synthesized with glucosamine. The resulting aqucous acetic acid solutions were centrifuged at 6,000 × g for 20 min. The precipitate was washed with distilled water and freeze-dried. This fraction was termed exopolymer AmG-1 and represented the acetic acid-insoluble glucose-coglucosamine copolymer. The supernatant was neutralized with sodium hydroxide and centrifuged at 15,000 × g for 30 min. The precipitate was also washed with distilled water and freeze-dried. This fraction was termed exopolymer AmG-2 and represented the acetic acid-soluble glucose-coglucosamine copolymer.
Further fractionation was accomplished by addition of 450 ml of distilled water to 300 mg of the exopolymer synthesized with N-acetylglucosamine. The resulting solutions were centrifuged at 6,000 × g for 20 min. The precipitate was washed with distilled water and freeze-dried. This fraction was termed exopolymer AcG-1 and represented the water-insoluble glucose–co-N-acetylglucosamine copolymer. The supernatant was dialyzed against deionized water using dialysis tubing with a molecular weight cutoff of 3,000 and then lyophilized. This fraction was termed exopolymer AcG-2 and represented the water-soluble glucose-coglucosamne copolymer.
The yield of the exopolymer by G. xylinum was determined by direct weighing after purification of exopolymers. Gas chromatographic (GC) analysis after methanolysis of exopolymers and subsequent trimethylsilylation was used to determine the composition of carbohydrates in the exopolymers (7). Samples (approximately 0.5 mg) were dried over phosphorous pentoxide under vacuum in 600-μl Reacti-Vials (Pierce). Methanolic hydrogen chloride (300 μl) prepared by mixing methanol and acetyl chloride in a ratio of 20:1 (vol/vol) was added, and the tubes were sealed with Teflon-lined septum caps. The contents were vortexed and heated at 70°C for 24 h with stirring. t-Butyl alcohol (30 μl) was then added to each tube before evaporating the contents using a stream of dry oxygen-free nitrogen at room temperature.
For incubation on N-acetylglucosamine, complete N-acetylation of amino sugars was ensured by re-N-acetylation by addition of methanol (150 μl), pyridine (15 μl), and acetic anhydride (15 μl) to the above tubes. After standing at room temperature for 30 min, the solutions were evaporated to dryness by the use of a dry oxygen-free nitrogen stream at room temperature, followed by vacuum over phosphorous pentoxide. After this thorough drying, Tri-Sil Z (100 μl) was added, and each mixture was stirred for 2 h at room temperature. GC analyses were performed on a Hewlett Packard (HP) gas chromatograph, model 5890 series II, equipped with a flame ionization detector and HP model 7673 injector. The column was a 30-m by 0.32-mm inner diameter fused silica with cross-linked 0.25-μm 5% phenylmethyl silicon liquid phase (Supelco). Dry oxygen-free nitrogen (2.9 ml/min flow rate) was used as the carrier gas at 10 lb/iu2 head pressure using a temperature program (140°C for 2 min, then increasing at 8°C per min up to 260°C). The injector was purged for 0.8 min after injection.
To quantitate the repeat unit composition of products, response factors were generated from the relative values of GC peak areas using an equimolar mixture of pure sugars and myoinositol as the internal standard. GC/mass spectrometry (GC/MS) analyses were performed on an HP gas chromatograph, model 5890 series II, also equipped with HP model 7673 injector and coupled to a mass selective detector (HP 5971 series). The capillary column was a cross-linked 5% phenylmethyl silicone-fused silica (HP Ultra MS 5; 30 m by 0.25 mm; film thickness, 0.33 μm). Dry oxygen-free helium (0.8 ml/min flow rate) was used as the carrier gas using a temperature program (140°C for 2 min, then increasing at 8°C per min up to 260°C). Sample volumes of 1 μl were injected, and the injector was purged for 0.6 min after injection. Mass spectra were compared based on computer HP computer database, W search version 1/10/99C, and compared with data from methyl esters of known structures. Fourier transform infrared (FTIR) spectra were recorded with a Perkin-Elmer 1720 spectrometer (16 scans; resolution, 2 cm−1) over KBr pellet. The polysaccharide sample (2 mg), which was dried previously at 50°C under reduced pressure, was manually well blended with 100 mg of KBr powder. The powder mixture of the polysaccharide sample and potassium bromide was then desiccated overnight at 50°C under reduced pressure prior to FTIR measurement. Proton nuclear magnetic resonance (1H-NMR) spectra were recorded at 250 MHz using a Bruker model AMX-250. Chemical shifts in parts per million (ppm) were reported with trace amount of acetone (δ = ppm) as an internal reference. The polysaccharide sample (2%, wt/vol) was dissolved in phosphoric acid-d3 (85 wt.% solution in D2O, 99+ atom% D; Aldrich) at room temperature for a minimum of 4 days prior to NMR measurement. The instrumental parameters were as follows: temperature, 300 K; pulse width, 7.8 μs; 32,000 data points, 3.18-s acquisition time; 1-s relaxation delay; and 32 transients.
RESULTS
Incorporation of monosaccharides into exopolymers.
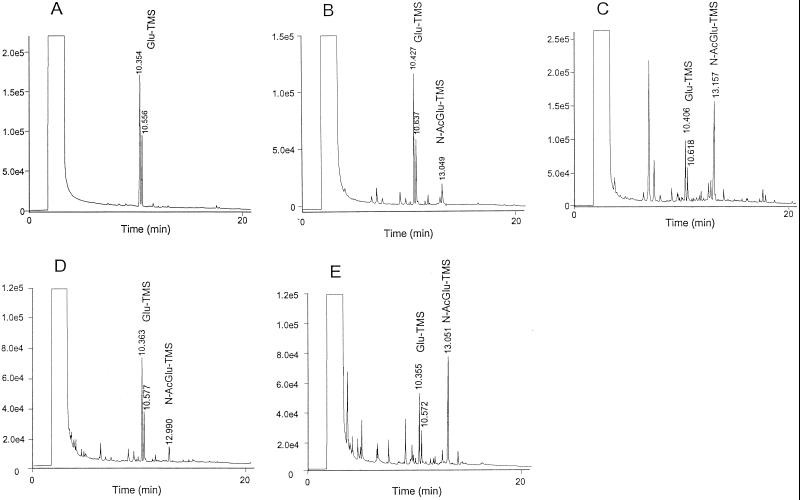
Glucose as the main carbon source resulted in the highest production of exopolymer among the carbon sources used under the conditions of this experiment (Table 1). 3-O-Methylglucose and 2-deoxyglucose as carbon sources were not utilized effectively by G. xylinum ATCC 10245. The yield of exopolymers purified from the culture with glucosamine and N-acetylglucosamine was not as high as with glucose, but higher than that obtained from either 3-O-methylglucose or 2-deoxyglucose. To determine the composition of exopolymers purified from cultures, samples were depolymerized and derivatized as described earlier. The exopolymers fractionated by acetic acid (10%, vol/vol) and distilled water were also analyzed. GC chromatograms corresponding to exopolymer purified from cultures with glucose, glucosamine, and N-acetylglucosamine are shown in Fig. 2. For all the products, the chromatograms show the major component to be glucose, identified by the peak retention times and peak area ratios of the α and β anomers (Fig. 2).
TABLE 1.
Production of exopolymer with glucose and its analogs by G. xylinum ATCC 10245a
| Main carbon | Yield (mg/ml) | Conversionb (%) |
|---|---|---|
| Glucose | 3.83 ± 0.45 | 19.1 |
| 3-O-Methylglucose | 0.21 ± 0.04 | 1.1 |
| Glucosamine | 0.37 ± 0.03 | 1.9 |
| N-Acetylglucosamine | 0.64 ± 0.07 | 3.2 |
| 2-Deoxyglucose | 0.16 ± 0.02 | 0.8 |
Results from 7-day cultures at 30°C. Values are the mean of triplicate experiments. Final pH ranged from 4.2 to 5.3; initial pH was 5.1 to 5.2.
Based on content of carbon in exopolymer versus fed carbon.
FIG. 2.
GC chromatograms of trimethylsilylated (TMS) sugar components of (A) exopolymer made with glucose, (B) 10% acetic acid-insoluble fraction of exopolymer made with glucosamine (the molar ratio of glucose to glucosamine was 6.0 to 1.0), (C) 10% acetic acid-soluble fraction of exopolymer made with glucosamine (the molar ratio of glucose to glucosamine was 0.6 to 1.0), (D) water-insoluble fraction of exopolymer made with N-acetylglucosamine (the molar ratio of glucose to N-acetylglucosamine was 6.2 to 1.0), and (E) water-soluble fraction of exopolymer made with N-acetylglucosamine (the molar ratio of glucose to N-acetylglucosamine was 0.8 to 1.0). Glu, glucose; N-AcGlu, N-acetylglucosamine.
Initial GC identification of glucosamine found in the exopolymers purified from cultures grown on glucosamine and fractionated by acetic acid was based on the elution time of N-acetylglucosamine due to re-N-acetylation of amino sugars during sample preparation for GC (13.05 to 13.16, Fig. 2B and C) (7). The average molar percentage of glucosamine in the exopolymer synthesized with glucosamine was 19%. After fractionation of the glucosamine-incorporated exopolymer, the molar ratios of glucose to glucosamine in the acetic acid-insoluble exopolymer (Fig. 2B) and that in the acetic acid-soluble exopolymer (Fig. 2C) were 6.0:1.0 and 0.6:1.0, respectively (the molar percentages of glucosamine in these exopolymers were 14 and 63%, respectively). GC identification of N-acetylglucosamine found in the exopolymers purified from cultures grown on N-acetylglucosamine and fractionated by distilled water (DW) was also based on the elution time (12.99 to 13.05 min) (Fig. 2D and E). The average molar percentage of N-acetylglucosamine repeat units in the exopolymer synthesized with N-acetylglucosamine was 18%. After fractionation of the N-acetylglucosamine-incorporated exopolymer by DW, the molar ratios of glucose to N-acetylglucosamine in the water-insoluble exopolymer (Fig. 2D) and that in the water-soluble exopolymer (Fig. 2E) were 6.2:1.0 and 0.8:1.0, respectively (the molar percentages of N-acetylglucosamine in these exopolymers were 14 and 56%, respectively).
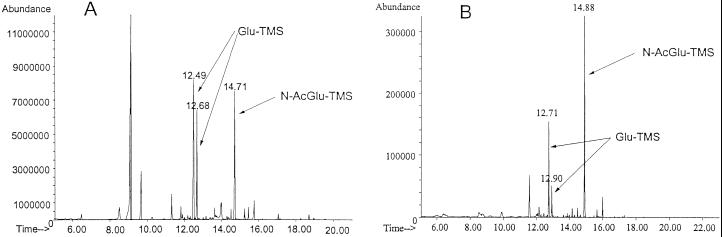
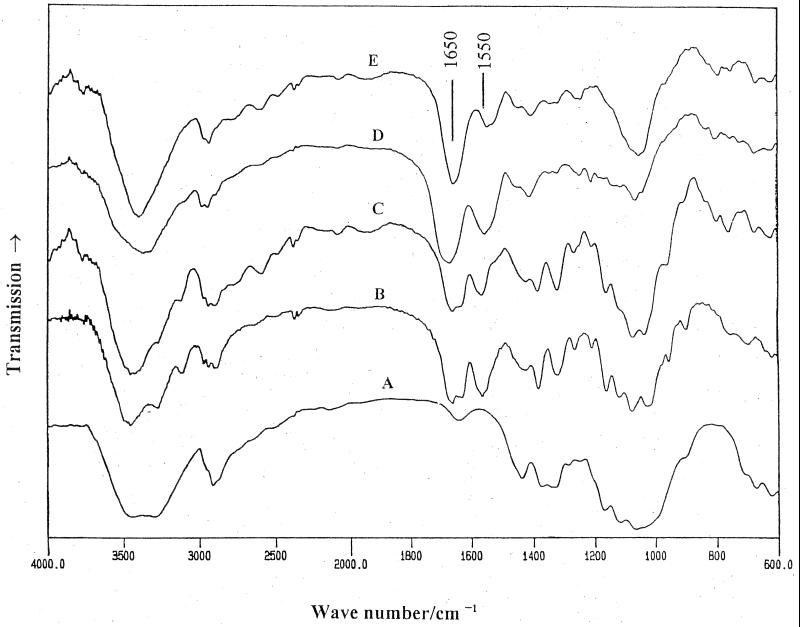
The presence of the trimethylsilyl (TMS) N-acetylglucosamine derivative in the GC/MS chromatogram of exopolymers with glucosamine and N-acetylglucosamine was confirmed by positive identification with reference spectra in the GC/MS data bank (Fig. 3A and B). The FTIR spectra of polysaccharides derived from different carbon sources are shown in Fig. 4. As can be seen, the FTIR spectra of 10% acetic acid-soluble fraction of exopolymer synthesized with glucosamine as the carbon source and water-soluble fraction of exopolymer synthesized with N-acetylglucosamine as the carbon source exhibited similar features as in the chitosan and chitin spectra (17). Specially, the absorbance bands of the amide I (1,650 cm−1) and amide II (1,550 cm−1) signals were clearly observed (Fig. 4D and E). The strong absorption at 1,650 cm−1 in Fig. 4D indicated that the acetic acid-soluble fraction of exopolymer isolated from the culture grown on glucosamine as the main carbon source had some glucosamine repeating units acetylated. Evidence for the production of chitosan- and chitin-like exopolymers from this cellulose-producing system also came from another related experiment in which the water-soluble fraction of exopolymer synthesized with N-acetylglucosamine as the carbon source was treated with 40% (wt/vol) aqueous sodium hydroxide solution at 25°C for 4 days. FTIR spectra of the chitin-like exopolymer before (Fig. 4A) and after alkaline treatment (Fig. 4B) also showed the expected reduction of the acetyl group content after treatment. This result correlated well with the decreased absorption signal intensity of the amide I (1,650 cm−1) and amide II (1,550 cm−1) bands, although quantitative comparisons were difficult.
FIG. 3.
GC chromatograms of trimethylsilylated (TMS) sugar components of (A) 10% acetic acid-soluble fraction of exopolymer made with glucosamine (the molar ratio of glucose to glucosamine was 0.6 to 1.0) and (B) water-soluble fraction of exopolymer made with N-acetylglucosamine (the molar ratio of glucose to N-acetylglucosamine was 0.8 to 1.0). Glu, glucose; N-AcGlu, N-acetylglucosamine.
FIG. 4.
FTIR spectra of (A) cellulose, (B) chitin, (C) chitosan (DS = 0.1, measured by 1H-NMR), (D) 10% acetic acid-soluble fraction of exopolymer made with glucosamine as the carbon source, and (E) water-soluble fraction of exopolymer made with N-acetylglucosamine as the carbon source.
A number of solvents used to dissolve cellulose, chitin, or chitosan were tested for their suitability as an NMR solvent for the samples, including CF3COOD/D2O, DC1/D2O, NaOD/D2O, D2O, dimethyl sulfoxide-d6, DCOOD, 10% (wt/wt) CD3COOD/D2O, 50% (wt/wt) N-methyl morpholine oxide/dimethyl sulfoxide-d6, and 85% (wt/wt) D3PO4/D2O. An 85% (wt/wt) D3PO4/D2O was found to be able to dissolve the chitosan- or chitin-like exopolymers at room temperature. To confirm the incorporation of glucosamine and N-acetylglucosamine into exopolymers, 1H-NMR spectra were recorded for cellulose, chitin, 10% acetic acid-soluble fraction of exopolymer made with glucosamine as the carbon source, and water-soluble fraction of exopolymer made with N-acetylglucosamine as the carbon source. For the spectrum of each sample, the signals between 5.0 and 3.0 ppm were assigned to the sugar hydrogens based on previous work on a related structure and the electron-withdrawing effect of the ring oxygen (19). The signals around 2.0 ppm, which correspond to the acetyl group (21), were found in the spectra of chitin and the water-soluble fraction of exopolymer synthesized with N-acetylglucosamine.
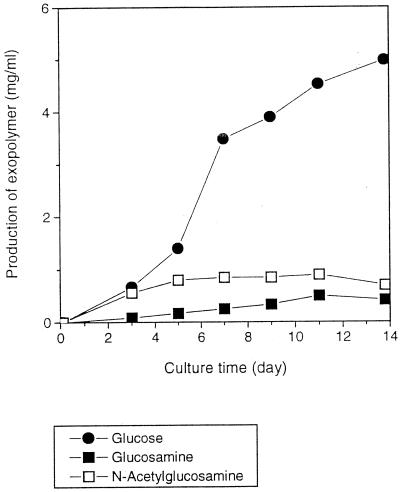
The production of exopolymers with glucose, glucosamine, and N-acetylglucosamine was observed as a function of time up to 14 days (Fig. 5). The production of exopolymer with glucose increased over 14 days and reached 4.98 mg/ml., while with glucosamine and N-acetylglucosime this level was low throughout, likely reflecting the lack of cell growth. The maximum yields of exopolymer found with glucosamine and N-acetylglucosamine were 0.50 and 0.90 mg/ml, respectively. To increase the productivity of glucosamine-incorporated exopolymer, mixtures (2% total) of glucose and glucosamine were used as the carbon source (Table 2). The data illustrate that the higher the relative amount of glucose in the mixture, the higher the production of exopolymer and the lower the mole percent of glucosamine. On the basis of GC analysis, the average mole percent of glucosamine in the exopolymer was 17% and yield of exopolymer was 1.60 mg/ml when the carbon source was the mixture of glucose (0.5%, vol/vol) and glucosamine (1.5%, vol/vol).
FIG. 5.
Production of exopolymers with glucose (●), glucosamine (■), and N-acetylglucosamine (□) as a function of cultivation time of G. xylinum ATCC 10245. Each point represents the average of three samples; standard deviations ranged from 0.002 to 0.005 for the glucosamine and N-acetylglucosamine samples and 0.01 to 0.05 for the glucose samples.
TABLE 2.
Production of exopolymer with mixed carbon of glucose and glucosamine by G. xylinum ATCC 10245a
| Main carbon source (% glucose:% glucosamine) | Yield (mg/ml) | Conversionb (%) | Compositionc (%) |
|---|---|---|---|
| 2.0:0.0 | 3.80 ± 0.55 | 19.0 | 0.1 ± 0.2 |
| 1.5:0.5 | 3.20 ± 0.19 | 16.0 | 4.5 ± 0.2 |
| 1.0:1.0 | 2.47 ± 0.09 | 12.4 | 12.9 ± 0.4 |
| 0.5:1.5 | 1.60 ± 0.21 | 8.0 | 17.4 ± 1.3 |
| 0.0:2.0 | 0.34 ± 0.04 | 1.7 | 19.6 ± 2.2 |
Results from 7-day cultures at 30°C. Values are the mean ± standard deviation of triplicate experiments. Final pH ranged from 4.04 to 4.16; initial pH was 5.1 to 5.2.
Based on content of carbon in exopolymer versus fed carbon.
Molar percentage of glucosamine in the exopolymer.
DISCUSSION
The major components of exopolymers purified from cultures of G. xylinum ATCC 10245 with glucose and its analogs as the main carbon sources were confirmed by GC and GC/MS. In this study, exopolymers generated with glucose, 3-O-methylglucose, and 2-deoxyglucose were confirmed to contain only glucose as the major repeat unit. The exopolymers synthesized with glucosamine or N-acetylglucosamine as the main carbon source were found to contain glucose and glucosamine or Nacetylglucosamine. GC chromatograms of the fractionated exopolymers synthesized with glucosamine or N-acetylglucosamine showed they were a mixture of polymers with different molar ratios of glucose to glucosamine or N-acetylglucosamine. The polymerization of glucosamine and N-acetylglucosamine by G. xylinum ATCC 10245 to form glucose-coglucosamine copolymers and glucose–co-N-acetylglucosamine copolymers, respectively, is one possibility based on these results. This would suggest that the cellulose synthase and other enzymes involved in cellulose synthesis have broader specificities. It is unlikely that the sugars were modified postpolymerization by the synthase, since such an enzyme has not been reported in G. xylinum. Furthermore, no evidence for modified glucose was found in cellulose formed when glucose was the carbon source. The molar ratio of glucose to glucosamine or N-acetylglucosamine in the purified exopolymers after fractionation ranged from 6.0:1.0 to 0.6:1.0 and from 6.2 to 0.8:1.0, respectively. This means that a variety of glucose-coglucosamine copolymers and glucose–co-N-acetylglucosamine copolymers with different contents of glucosamine and N-acetylglucosamine can be generated. Further work is ongoing to investigate the physiological conditions that affect incorporation rates of these sugars as well as optimal production conditions for the modified exopolymers.
The acetic acid-insoluble glucose-coglucosamine copolymer (the molar ratio of glucose to glucosamine was 6.0 to 1.0) was insoluble in distilled water. The acetic acid-soluble glucose-coglucosamine copolymer (the molar ratio of glucose to glucosamine was 0.6 to 1.0) exhibited a solubility similar to that of chitosan, which is insoluble in distilled water but soluble in acetic acid. The water-soluble glucose–co-N-acetylglucosamine copolymer (the molar ratio of glucose to glucosamine was 0.8 to 1.0) seems to be soluble in distilled water under certain conditions, such as a high degree of acetylated exopolymer and low concentration of solute. This observation may be explained by the fact that partially acetylated chitosan can be soluble, depending on the level of acetylation in chitosan and the pH of the chitosan solution (1). The glucose analogs used in this study were highly soluble in water (>20%, wt/vol). Therefore, the procedure used to isolate and purify exopolymers from the cultures also supports the absence of contamination by monosaccharides as well as contaminating lipids and proteins.
Preliminary analysis of the fibers (cellulose and the new copolymers) by environmental scanning electron microscopy suggested similar gross morphology (e.g., diameter and surface smoothness). The formation of chitin-like and chitosan-like polymers by direct bacterial incorporation of glucosamine and N-acetylglucosamine suggests new options in the synthesis and purification of materials that may bridge the properties of the respective homopolymers, cellulose and chitin/chitosan. Control of the level of glucosamine or N-acetylglucosamine incorporated into cellulose would provide new options in tailorability in terms of solubility and reactivity. For example, cellulose with a significant glucosamine content would become soluble in dilute acid, potentially leading to new processing options while maintaining cellulose-like properties, in contrast to cellulose, which has severe processing limitations due to low solubility in most solvents. Cellulose with a significant content of N-acetylglucosamine could be susceptible to lysozyme hydrolysis in the body, leading to new biodegradable biomaterials based on cellulose. This would overcome current limitations with cellulose-based biomaterials that cannot be degraded in the body, thus necessitating the use of lower molecular polymers that can be cleared without degradation. New copolymers of cellulose with glucosamine would generate materials with reactive amine groups on the polymer surface, leading to simple cross-linking schemes and surface modification reactions—e.g., covalent immobilization of dyes, surface treatments to reduce hydrophilicity in pulp and paper, or surface coupling of peptides for biomedical needs to control protein adsorption and cell interactions.
ACKNOWLEDGMENT
Thanks are extended to the NSF (D.K.) for recent support of this work.
REFERENCES
- 1.Aiba S. Studies on chitosan. 2. Solution stability and reactivity of partially N-acetylated chitosan derivatives in aqueous media. Int J Biol Macromol. 1989;11:249–252. doi: 10.1016/0141-8130(89)90077-9. [DOI] [PubMed] [Google Scholar]
- 2.Anthonsen M W, Vårum K M, Smidsrød O. Solution properties of chitosan: conformation and chain stiffness of chitosans with different degrees of N-acetylation. Carbohydr Polym. 1993;22:193–201. [Google Scholar]
- 3.Arcidiacono S, Kaplan D L. Molecular weight distribution of chitosan isolated from Mucor rouxii under different culture and processing conditions. Biotechnol Bioeng. 1992;39:281–286. doi: 10.1002/bit.260390305. [DOI] [PubMed] [Google Scholar]
- 4.Bartnicki-Garcia S, Nickerson W J. Nutrition, growth, and morphogenesis of Mucor rouxii. J Bacteriol. 1962;84:841–858. doi: 10.1128/jb.84.4.841-858.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canale-Parola E, Wolfe R S. Synthesis of cellulose by Sarcina ventriculi. Biochim Biophys Acta. 1964;82:403–405. doi: 10.1016/0304-4165(64)90314-9. [DOI] [PubMed] [Google Scholar]
- 6.Carr J G. A strain of Acetobacter aceti giving a positive cellulose reaction. Nature (London) 1958;182:265–266. doi: 10.1038/182265b0. [DOI] [PubMed] [Google Scholar]
- 7.Chaplin M. A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Anal Biochem. 1982;123:336–341. doi: 10.1016/0003-2697(82)90455-9. [DOI] [PubMed] [Google Scholar]
- 8.Delmer D P, Amor Y. Cellulose biosynthesis. Plant Cell. 1995;7:987–1000. doi: 10.1105/tpc.7.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorkovenko A, Zhang J, Gross R A, Allen A L, Ball D, Kaplan D A. Biosynthesis of emulsan analogs: direct incorporation of exogenous fatty acids. Proc Am Chem Soc Div Poly Sci Eng. 1995;72:92–94. [Google Scholar]
- 10.Gorkovenko A, Zhang J, Gross R A, Allen A L, Ball D, Kaplan D A. Bioengineering of emusifier structure: emulsan analogs. Can J Microbiol. 1997;43:384–390. doi: 10.1139/m97-053. [DOI] [PubMed] [Google Scholar]
- 11.Hackman R H, Goldberg M. Light-scattering and infrared-spectrophotometric studies of chitin and chitin derivatives. Carbohydr Res. 1974;38:35–45. [Google Scholar]
- 12.Hassler R A, Doherty D H. Genetic engineering of polysaccharide structure: production of variants of xanthan gum in Xanthomonas campestris. Biotechnol Prog. 1990;6:182–187. doi: 10.1021/bp00003a003. [DOI] [PubMed] [Google Scholar]
- 13.Hirano S, Ohe Y, Ono H. Selective N-acylation of chitosan. Carbohydr Res. 1976;47:315–320. doi: 10.1016/s0008-6215(00)84198-1. [DOI] [PubMed] [Google Scholar]
- 13a.Johnson, D. C., and A. N. Neogi. 1989. U.S. Patent 4,863,565.
- 14.Kaplan D L, Wiley B J, Mayer J M, Arcidiacono S, Keith J, Lombardi S J, Ball D, Allen A L. Biosynthetic polysaccharides. In: Shalaby S W, editor. Biomedical polymers: designed-to-degrade systems. New York, N.Y: Hanser Publishers; 1994. pp. 189–212. [Google Scholar]
- 15.Lee J W, Yeomans W G, Allen A L, Kaplan D L, Deng F, Gross R A. Exopolymers from curdlan production- incorporation of glucose-related sugars by Agrobacterium sp. ATCC 31749. Can J Microbiol. 1997;43:149–156. [Google Scholar]
- 16.Lee J W, Yeomans W G, Allen A L, Kaplan D L, Deng F, Gross R A. Biosynthesis of novel exopolymers by Aureobasidium pullulans. Appl Environ Microbiol. 1999;65:5265–5271. doi: 10.1128/aem.65.12.5265-5271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishi N, Ebina A, Hishimura S, Tsutsumi A, Hasegawa O, Tokura S. Highly phosphorylated derivatives of chitin, partially deacetylated chitin and chitosan as new functional polymers: preparation and charateization. Int J Biol Macromol. 1986;8:311–317. [Google Scholar]
- 18.Osawa Z, Morota T, Hatanaka K, Akaike T, Matsuzaki K, Nakashima H, Yamamoto N, Suzuki E, Miyano H, Mimura T, Kancko Y. Synthesis of sulfated derivatives of curdlan and their anti-HIV activity. Carbohydr Polym. 1993;21:283–288. [Google Scholar]
- 19.Roesser D S, McCarthy S P, Gross R A, Kaplan D L. Effects of substitution site on acetyl amylose biodegradability by amylase enzymes. Macromolecules. 1996;29:1–9. [Google Scholar]
- 20.Schramm M, Hestrin S. Synthesis of cellulose by Acetobacter xylinum. 1. micromethod for the determination of celluloses. Biochem J. 1954;56:163–166. doi: 10.1042/bj0560163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skjåk-Bræk G, Grasdalen H, Larsen B. Monomer sequence and acetylation pattern in some bacterial alginates. Carbohydr Res. 1986;154:239–250. doi: 10.1016/s0008-6215(00)90036-3. [DOI] [PubMed] [Google Scholar]
- 22.Thorne L, Tansey L, Pollock T J. Clustering of mutations blocking synthesis of xanthan gum by Xanthomonas campestris. J Bacteriol. 1987;169:3593–3600. doi: 10.1128/jb.169.8.3593-3600.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto I, Takayama K, Honma K, Gonda T, Matsuzaki K, Hatanaka K, Uryu T, Yoshida O, Nakashima H, Yamamoto N, Kaneko Y, Mimura T. Synthesis, structure and antiviral activity of sulfates of cellulose and its branched derivatives. Carbohydr Polym. 1991;14:53–63. [Google Scholar]
- 24.Zhang J, Gorkovenko A, Gross R A, Allen A L, Ball D, Kaplan D A. Incorporation of 2-hydroxyl fatty acids by Acinetobacter calcoaceticus RAG-1 to tailor emulsan structure. Int J Biol Macromol. 1997;20:9–21. doi: 10.1016/s0141-8130(97)01147-1. [DOI] [PubMed] [Google Scholar]