Abstract
Primary prostate cancer (PCa) can show marked molecular heterogeneity. However, systematic analyses comparing primary PCa and matched metastases in individual patients are lacking. We aimed to address the molecular aspects of metastatic progression while accounting for the heterogeneity of primary PCa. In this pilot study, we collected 12 radical prostatectomy (RP) specimens from men who subsequently developed metastatic castration‐resistant prostate cancer (mCRPC). We used histomorphology (Gleason grade, focus size, stage) and immunohistochemistry (IHC) (ERG and p53) to identify independent tumors and/or distinct subclones of primary PCa. We then compared molecular profiles of these primary PCa areas to matched metastatic samples using whole‐exome sequencing (WES) and amplicon‐based DNA and RNA sequencing. Based on combined pathology and molecular analysis, seven (58%) RP specimens harbored monoclonal and topographically continuous disease, albeit with some degree of intratumor heterogeneity; four (33%) specimens showed true multifocal disease; and one displayed monoclonal disease with discontinuous topography. Early (truncal) events in primary PCa included SPOP p.F133V (one patient), BRAF p.K601E (one patient), and TMPRSS2:ETS rearrangements (eight patients). Activating AR alterations were seen in nine (75%) mCRPC patients, but not in matched primary PCa. Hotspot TP53 mutations, found in metastases from three patients, were readily present in matched primary disease. Alterations in genes encoding epigenetic modifiers were observed in several patients (either shared between primary foci and metastases or in metastatic samples only). WES‐based phylogenetic reconstruction and/or clonality scores were consistent with the index focus designated by pathology review in six out of nine (67%) cases. The three instances of discordance pertained to monoclonal, topographically continuous tumors, which would have been considered as unique disease in routine practice. Overall, our results emphasize pathologic and molecular heterogeneity of primary PCa, and suggest that comprehensive IHC‐assisted pathology review and genomic analysis are highly concordant in nominating the ‘index’ primary PCa area. © 2022 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Keywords: castration‐resistant, CRPC, genomics, heterogeneity, metastasis, multifocal, NEPC, pathology, prostate cancer, transcriptomics
Introduction
Primary prostate cancer (PCa) is a multifocal disease in up to 80% of PCa patients [1, 2]. In addition, it has been shown to display marked inter‐ and intratumor heterogeneity at the genomic, transcriptomic, and DNA methylation levels [3, 4, 5, 6, 7, 8]. Conversely, metastatic castration‐resistant prostate cancer (mCRPC) appears to be of clonal origin, even though it can acquire molecular heterogeneity through subclonal evolution [4, 7, 9]. There is a critical knowledge gap regarding the molecular mediators of PCa progression to mCRPC in individual patients. While pathology criteria associated with aggressive disease, e.g. Gleason grade or focus size, are typically used to nominate the dominant (index) focus in radical prostatectomy (RP) specimens, the validity of this approach has not been addressed by systematic studies, and has even been challenged by occasional case reports [10].
Due to the long time between primary therapy and development of metastatic disease, only a few studies have analyzed matched primary and metastatic samples. As part of two precision oncology trials, the CRPC500 [11, 12] and the Weill Cornell Medicine (WCM) Precision Oncology cohort [13], we present a comprehensive pathology and genomic analysis of RP specimens from 12 men who subsequently developed mCRPC. We compare the genomics of multiple areas of primary PCa with matched metastatic disease, with the aim of nominating the index focus and identifying the histopathological and molecular criteria that could be associated with metastatic outcome.
Materials and methods
Ethics approval statement
The study included patients enrolled in the Weill Cornell Medicine precision cancer care program and/or in the Stand‐Up‐To‐Cancer (SU2C) CRPC500 study with appropriate Institutional Review Board (IRB) approval and written informed consent.
Patient consent statement
Written informed consent for molecular studies was previously obtained from all patients as part of the Weill Cornell Medicine precision cancer care program and/or in the Stand‐Up‐To‐Cancer (SU2C) CRPC500 study.
Sample collection and pathology review
Medical records of patients with mCRPC enrolled in the WCM precision cancer care program and/or in the Stand‐Up‐To‐Cancer (SU2C) CRPC500 study [12] were interrogated to identify patients who had previously undergone radical prostatectomy (RP). Only patients for whom complete RP pathology material (all hematoxylin and eosin [H&E] slides and blocks) could be retrieved were included. Three pathologists (JMM, BR, and JC) jointly reviewed all H&E slides for each RP specimen. Immunohistochemistry (IHC) for ETS‐related gene (ERG) and p53 was performed on all tumor areas. Potentially distinct areas (i.e. either subclones of the same primary disease or truly independent tumors) were selected for sequencing based on topography (i.e. discontinuous nature of tumor areas), individual Gleason score, histomorphology, and/or IHC. The putative index focus was nominated based on the highest individual Gleason score (primary criterion) and focus size (secondary criterion). Seminal vesicle invasion and metastatic regional lymph nodes removed at the time of RP were also included in this evaluation and, by principle, considered as putative index disease. Pathology review of metastatic samples (frozen sections) was previously performed as part of the WCM precision cancer care and/or the SU2C CRPC500 study [12]. Both studies were performed with appropriate written patient consent and IRB approval.
Immunohistochemistry
IHC was performed on sections of formalin‐fixed paraffin‐embedded (FFPE) tissue using a Bond III automated immunostainer and the Bond Polymer Refine detection system (Leica Microsystems, Buffalo Grove, IL, USA), with the following antibodies and conditions (dilution, heat‐mediated antigen retrieval solution, retrieval time): anti‐ERG (clone EPR386, 1/100, H1, 30 min; Abcam, Cambridge, MA, USA); anti‐p53 (clone DO‐7, 1/100, H1, 20 min; Cell Signaling Technology, Danvers MA, USA).
Whole‐exome sequencing
WES was performed using previously validated WCM protocols [14]. In brief, DNA was extracted from macrodissected unstained slides of FFPE tissue cut at 10 μm (for each primary tumor focus); from cored frozen, Optimal Cutting Temperature (OCT) compound‐embedded tissue (for metastases); and from peripheral blood lymphocytes (for germline control). DNA extraction was accomplished using Promega Maxwell 16 MDx (Promega, Madison, WI, USA). DNA quality was confirmed using a Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Libraries were prepared using exome capture of 21,522 genes with the HaloPlex System (Agilent). Sequencing was performed on Illumina HiSeq 2500 (Illumina, San Diego, CA, USA) in 100 bp paired‐end mode [14]. Reads were aligned to GRC37/hg19 reference using BWA [15] and processed accordingly to the Whole Exome Sequencing Test for Cancer – ExaCT‐1 – pipeline v0.9 [14].
To identify single‐nucleotide variants (SNVs), we applied both MuTect [16] and SNVseeqer [17] to nominate putative aberrant genomic positions. Identified genomic positions were filtered, requiring coverage of at least 10 reads, a read count of the alternative base of at least 3, and a minimum variant allelic fraction (VAF) of 5%. Filtered positions were inspected with ASEQ [18], which provides a read count for each of the four bases in tumor and matched normal samples. Genomic positions where the read count of the alternative base in a normal matched sample was greater than 0 were considered not aberrant. The list of aberrant genes was divided into three tiers: tier1 containing genes in the EXaCT‐2 WCM test, tier2 containing genes in the COSMIC Cancer Gene Census, tier3 containing all the remaining genes.
For metastatic samples for Patients 11 and 12, WES data from fresh‐frozen tissue were obtained through the CRPC500 study and generated as described previously [11].
Targeted DNA and RNA sequencing
DNA and RNA extraction, amplicon‐based next generation sequencing, and bioinformatics analysis were performed as previously described [5].
Phylogenetic reconstruction and clonality analysis
Based on SNV calls, primary and metastatic samples were analyzed for clonal relatedness through phylogenetic tree reconstruction and clonality score. The final list of aberrant genomic positions was composed of: (1) mutations in genes in tier1 or tier2 called by either Mutect or SNVseeqer, and (2) mutations in genes in tier3 called by both Mutect and SNVseeqer. Maximum‐parsimony trees were inferred using the phangorn package [19]. The germline sample was designated as the root. Primary and metastatic tumor samples were plotted as descendent nodes, with edge lengths indicating the number of alterations newly accumulated in descendant nodes. Samples sharing common mutations formed a node. Clonality scores were determined as previously described [20] by performing the conditional test on sites with a mutation in one or both tumors. Marginal probabilities at each locus were estimated via empirical relative frequencies from the somatic mutations dataset from the publicly available TCGA PRAD study [21].
Results
Pathology and clinical findings
Twelve patients were included in this pilot cohort (nine enrolled in the WCM precision cancer care and three in the SU2C CRPC500 study [12]). The number and type of samples per patient, pathology results, and IHC are summarized in Figure 1 and in supplementary material, Table S1. We assigned individual Gleason scores to each discrete sample. Samples showed a range from Gleason score 6 (3 + 3) to 9 (5 + 4). Disease stage per patient ranged from pT2N0 to pT3bN1. Partial neuroendocrine features (positive synaptophysin expression with maintained AR expression and an adenocarcinoma morphology) were noted in primary PCa from one patient (Patient 7) and in the metastasis of another patient (Patient 6). Neuroendocrine prostatic carcinoma (NEPC) with adequate morphology [22] and loss of AR expression was seen in the RP specimen from one patient (Patient 1) and in metastases from two patients (Patients 1 and 7). ERG expression was seen in all or a subset of samples in seven (58%) patients.
Figure 1.
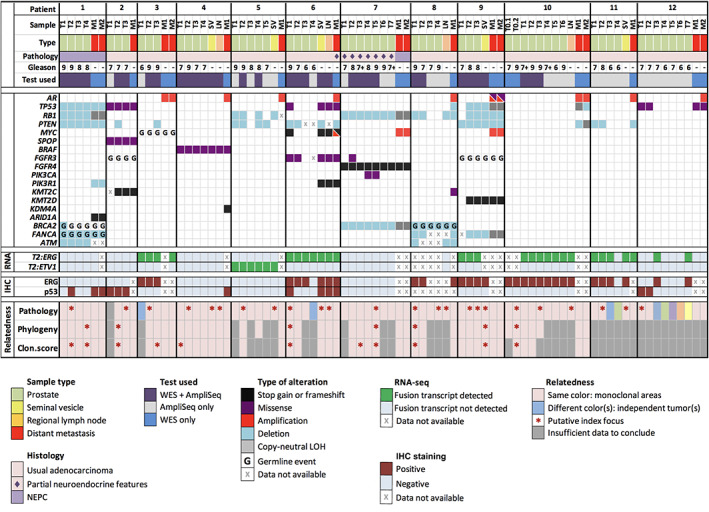
A summary of all analyzed samples, including their histopathology characteristics, a schematic representation of selected genomic findings, immunohistochemistry (IHC) results, and clonal relatedness as interpreted by pathology and by genomics, i.e. phylogenetic reconstruction (‘Phylogeny’) and clonality score (‘Clon.score’); 7+ indicates Gleason score 7 with tertiary 5 pattern; T2:ERG stands for TMPRSS2:ERG, T2:ETV1 for TMPRSS2:ETV1. Selected genomic alterations are shown. To account for tumor content variability, the genomic annotation of figure panel genes was manually curated.
The time from prostatectomy to metastatic sample collection (per sample) ranged from 16 to 230 months (mean, 77.8 months); the shortest time interval was measured for a patient with NEPC primary tumor. Time intervals for patients whose primary disease harbored at least one focus with an individual Gleason score of 9 were significantly shorter compared to other patients (mean, 46 months versus 115.5 months, p = 0.046, unpaired two‐tailed t‐test). Metastatic sites included bone, lymph node, liver, and brain. The available information about patient treatments is summarized in supplementary material, Table S2.
Initial pathology review of RP specimens, including IHC results for ERG and p53, but blinded to the genomic data, interpreted eight (67%) RP specimens as monoclonal disease with intratumor heterogeneity and four (33%) RP specimens as harboring true multiclonal disease (Patients 3, 6, 11, and 12). In three of the multiclonal cases, the additional tumors were only small‐volume Gleason grade 6 foci (Patients 3, 6, and 11). In one case (Patient 12), as many as six potentially independent tumors were identified, including three Gleason grade 7 and three Gleason grade 6 lesions (Figure 2). Using Gleason grade, tumor size, and stage, one or multiple index tumor areas were nominated by pathology for all cases.
Figure 2.
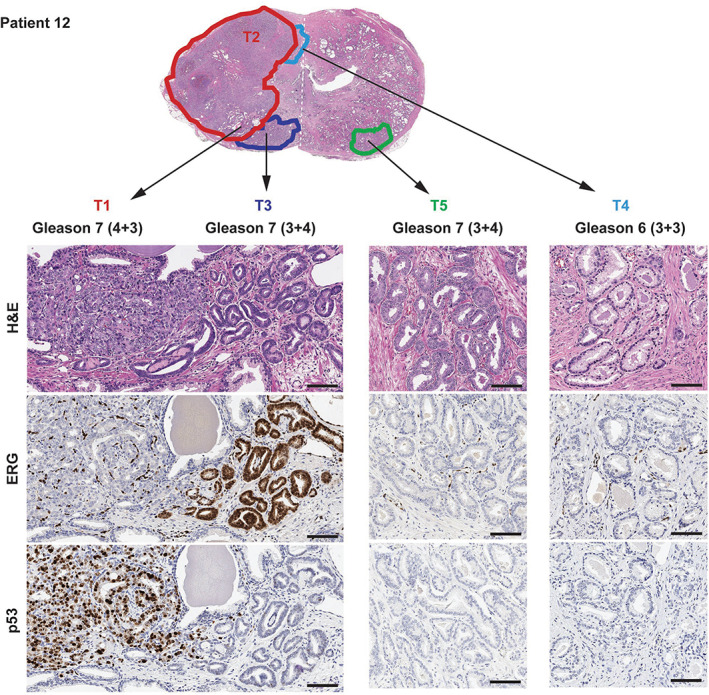
An example of pathology annotation in a case of multiclonal prostate cancer (Patient 12), including collision tumors. Pathology review and immunohistochemistry (IHC) identified six potentially independent tumors: a large, Gleason grade 7 focus which was ERG−/p53+ by IHC (samples T1 and T2); a Gleason grade 7 focus, which was ERG+/p53− by IHC (sample T3) and in collision with T1; a topographically distinct Gleason grade 7 focus which was ERG−/p53− by IHC (sample T5); and 3 additional low‐volume distinct Gleason grade 6 foci (sample T4, pictured, and samples T6 and T7, not shown). Topographical confluence (tumor collision) was noted between areas T1 and T3, and between areas T2 and T4. The low‐power view at the top is a montage of low‐power views from two distinct pathology slides, taken at the same level and thus representative of one complete section of the specimen. The white dashed line indicates where the two images have been assembled.
Genomic landscape of primary PCa and paired metastatic samples
WES was performed on 36 primary tumor areas from 10 patients (2–6 samples per patient) (supplementary material, Tables S3–S5, Figures 3 and 4, supplementary material, Figures S1–S8). Targeted DNA sequencing was performed on 63 primary samples from 12 patients (3–7 per patient) (supplementary material, Tables S6 and S7). The results were compared to metastatic tumor samples (18 samples from 12 patients, 1–2 samples per patient), which previously underwent WES [12, 13].
Figure 3.
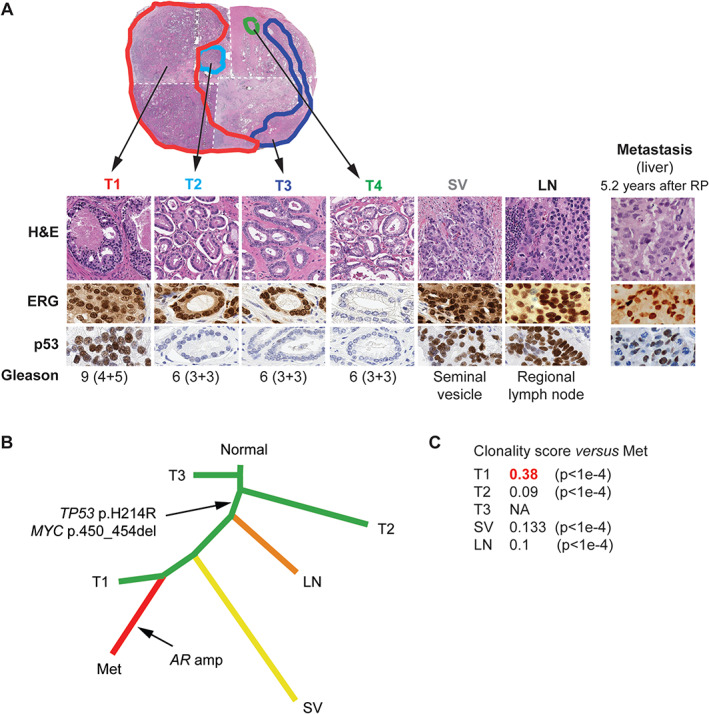
A summary of pathology features, phylogeny and clonality analyses for Patient 6. (A) Pathology features of each sample, including histomorphology, ERG, and p53 immunohistochemistry (IHC). Tumor focus 1 (T1) is the dominant (index) focus with highest Gleason grade and size. The T4 focus has a different ERG IHC status from other foci, and was thus regarded as an independent tumor on pathology evaluation. Aberrant p53 immunostaining is shared by T1, seminal vesicle (SV), lymph node (LN), and metastasis (Met). H&E: hematoxylin–eosin. This low‐power view is a montage of low‐power views from four distinct pathology slides, taken at the same level and thus representative of one complete section of the specimen. The white dashed line indicates where the images have been assembled. (B) Phylogenetic reconstruction using 79 genes and 110 events confirms that samples T1, SV, and LN are more closely related to the metastasis than samples T2 or T3, as evidenced by a common ‘trunk,’ which includes the TP53 mutation. (C) A summary of clonality score results. Sample T1 shows the highest clonality score (highlighted in red) with respect to the metastatic sample.
Figure 4.
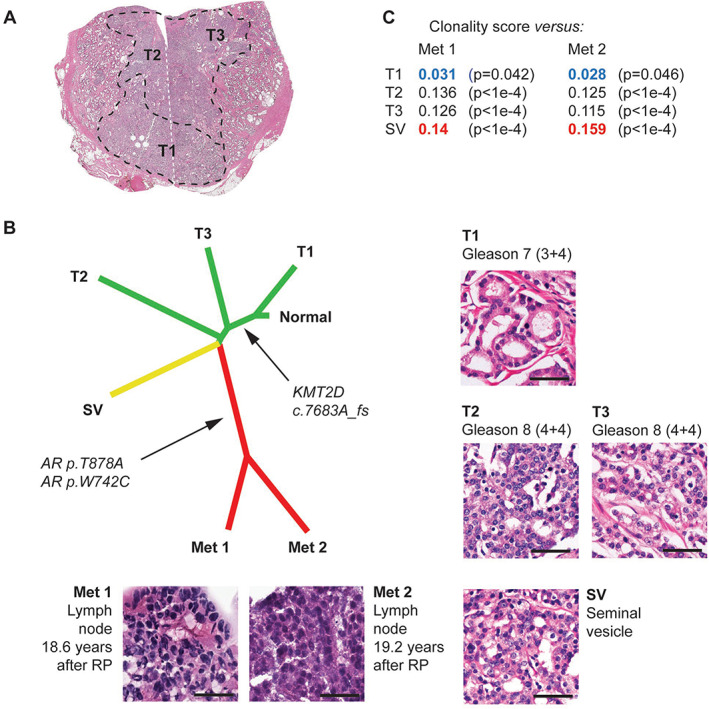
A summary of pathology features, phylogeny, and clonality analyses for Patient 9. (A) Low‐power pathology view. This case was interpreted as a unifocal, monoclonal tumor, albeit with the T1 tumor area showing a lower Gleason grade than areas T2 and T3. This low‐power view is a montage of low‐power views from two distinct pathology slides, taken at the same level and thus representing one complete section of the specimen. The white dashed line indicates where the two images have been assembled. (B) Phylogenetic reconstruction using 71 genes and 103 events, confirming that samples T2, T3, and seminal vesicle (SV) are more closely related to the metastasis than T1. Detailed histomorphology for all foci is also shown. (C) A summary of clonality score results. Sample SV shows the highest clonality score (highlighted in red) with respect to both metastatic samples. Note that sample T1 (with the lowest individual Gleason score) also shows the lowest clonality score (highlighted in blue).
The most frequent genomic event found in the metastases, but not in the matching primary samples, were AR activating alterations, seen in nine (75%) patients (amplification in eight cases, and activating point mutations with amplification in one case). The second most frequent alteration type found in metastases were TP53 missense hotspot mutations and MYC amplifications, each identified in three (25%) patients. Importantly, in contrast to AR activating alterations, matched analysis confirmed that these TP53 alterations were present in some (two cases) or all (one case) areas of primary PCa in each patient (Figures 1, 2, 3).
Comparative analysis of paired samples from individual patients identified some early (‘truncal’) events, present at high allelic frequencies in all primary samples and in the metastases. These included: a hotspot p.F133V SPOP mutation in Patient 2, and a BRAF p.K601E mutation in Patient 4. In addition, RNA sequencing identified TMPRSS2:ERG and TMPRSS2:ETV1 fusion transcripts in six patients and in one patient, respectively. These fusion transcripts were detected across multiple samples and, when data were available, in the matched metastasis (except for Patient 12). The TMPRSS2:ERG status was consistent with ERG IHC in all but one sample. This one sample (Patient 6, T4) was ERG‐negative on IHC and displayed a relatively low TMPRSS2:ERG signal on RNA‐seq, likely due to contamination with cells from the nearby ERG‐positive tumor focus T3 (supplementary material, Figure S9). The time from RP to metastatic biopsy was not significantly different between patients with ERG‐positive and ERG‐negative primary disease (unpaired two‐tailed t‐test).
Several cases harbored alterations in genes encoding epigenetic modifiers. KMT2D (MLL2/4) c.7683A_fs in Patient 9 was shared by all primary samples, except for one area with a lower Gleason score, and by the two metastases (Figure 4). Other alterations included: KMT2C (MLL3) p.C1013* in Patient 2, shared by all primary samples and the metastasis (supplementary material, Figure S2); KDM4A (JMJD2) p.L696_fs in Patient 4, detected in the metastasis only (supplementary material, Figure S4); and p.1479_fs in ARID1A, encoding a member of the SWI/SNF complex, detected in both metastatic samples in Patient 1, but not in the primary samples (supplementary material, Figure S1).
BRCA2 germline mutations were found in two (16.7%) cases (Patients 1 and 8), one of which showed neuroendocrine features in the primary and metastatic tumors.
Assessing clonal relatedness between primary and metastatic samples
Phylogenetic reconstruction using WES SNV calls allowed nominating an index focus/foci in seven (70%) of the 10 patients for whom WES on the primary samples was available, and clonality scores could be obtained for nine patients (90%) (Figure 1, supplementary material, Table S1). Both approaches showed highly concordant results.
In six of the nine cases (67%), the index focus nominated by phylogenetic reconstruction and/or by the highest clonality score was consistent with the one proposed by the pathology review. Discordance was seen in three cases (Patients 2, 3, and 4), all of which were monoclonal tumors with continuous spread.
In addition, although phylogeny and clonality analyses were not available for Patient 12, concordance between pathology and genomics could be inferred owing to the presence of a shared TP53 mutation (p.R248W) and the absence of a TMPRSS2:ERG fusion in the index focus and the metastases (Figure 1).
Gene expression analysis
Targeted RNA sequencing was performed on 58 primary and 5 metastatic samples (Figure 5). Unsupervised hierarchical clustering using gene expression levels from RNA‐seq further supported molecular similarity between genomically similar PCa areas, especially in monoclonal primary tumors. Conversely, sample clustering results for Patients 3, 6, 11, and 12 were potentially consistent with multiclonality. Of note, mCRPC samples from four patients (two bone and two liver metastases) formed a distinct transcriptomic cluster, characterized by high expression of proliferation genes (Figure 5, box), consistent with results using this approach on a separate compendium of localized treatment‐naive PCa and CRPC metastases [5].
Figure 5.
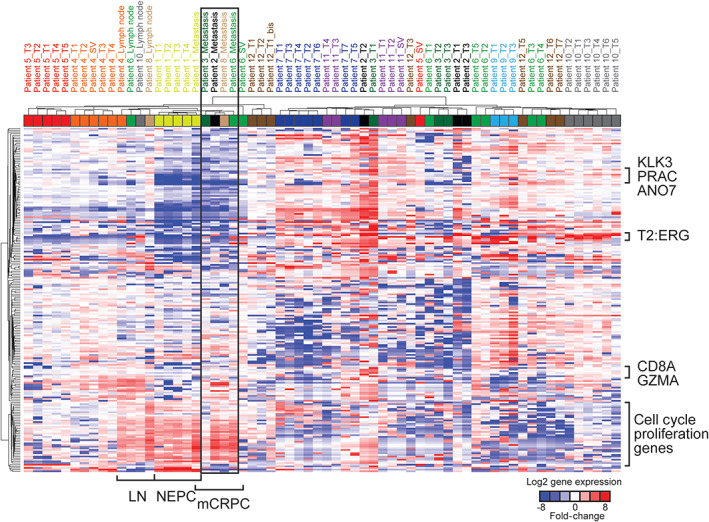
Unsupervised sample clustering and heatmap representation of RNA‐seq gene expression levels using 204 target expression amplicons. Only samples that have passed quality control as detailed in Salami et al [5] are shown. LN, regional lymph node; mCRPC, metastatic castration‐resistant prostatic carcinoma; NEPC, neuroendocrine prostatic carcinoma; T2:ERG, TMPRSS2:ERG fusion transcripts. The black box indicates mCRPC samples from four patients.
Discussion
Genomic heterogeneity of PCa and different scenarios for PCa clonality
Primary PCa has previously been shown to represent a molecularly heterogeneous disease [3, 4, 5, 6]. When considering the clonality of primary PCa, four scenarios can be proposed, all of which were encountered in our pilot cohort (Figure 6, supplementary material, Table S1).
Figure 6.
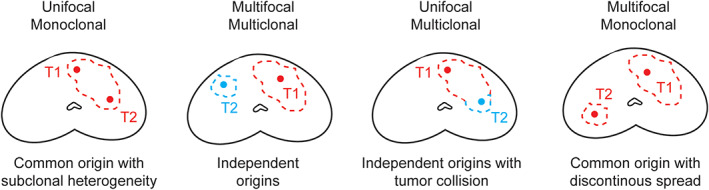
Possible scenarios for clonality interpretation of primary prostate cancer.
In one scenario, tumors are monoclonal (all areas have a common origin) and show topographically continuous spread, but still demonstrate intratumor heterogeneity due to the emergence of distinct subclones [3, 4]. This was the most common scenario in our study, seen in seven (58%) cases. It has previously been shown that evolutionary branching usually precedes metastatic seeding, and genomic heterogeneity of localized PCa has even been suggested as a potential marker of aggressive disease [3, 7].
Another possibility is the presence of true multiclonal tumors with independent origins in the RP specimen [23, 24, 25, 26]. Only four (33.3%) cases from our cohort showed true multiclonal disease. The rate of true multiclonal disease in our study was lower than the 50–80% previously reported in a large RP series [1, 2]. This could be due to selection bias, as we only included patients who subsequently developed metastases, thus selecting for aggressive primary disease, which may potentially override synchronous low‐volume lesions or not leave the time for such lesions to develop. This is in keeping with a study by Wise et al, who found that a greater number of ‘lesser cancers’ correlates with a smaller volume of the index cancer [2]. In most instances, and in three (25%) of our patients, such additional foci are low‐grade and low‐volume (<0.5 cm3), and represent an incidental finding rather than clinically relevant disease [1, 2, 27].
Critically, multiclonal tumors may either be topographically distinct (multifocal), which represents the second scenario, or confluent (collision tumors), which represents the third scenario (Figures 2 and 6). We observed collision of clonally independent tumors, confirmed by discordant ERG IHC status, in three RP specimens (Patients 6, 11, and 12). This phenomenon of collision tumors suggests that topography alone is not reliable in determining the relatedness of PCa areas.
Lastly, the disease may have a common clonal origin, but discontinuous topography (monoclonal–multifocal), potentially due to a particular form of tumor dissemination or to RP specimen processing errors; however, contrary to what has been proposed by some authors [28], this appears to be a rare scenario (one patient in our study).
Overall, some degree of molecular heterogeneity of PCa (either in a ‘subclonal’ or ‘multiclonal’ form) was observed in all cases in our cohort. This suggests that random sampling of a single primary tumor area may not be sufficient to capture the entire genomic landscape of a patient's primary disease [5].
In our study, the index tumor focus nominated by pathology in RP specimens was concordant with the one nominated by genomics in most cases where a conclusion could be reached. The three instances of discordance (Patients 2, 3, and 4) pertained to monoclonal and topographically continuous tumor areas (supplementary material, Figures S2–S4), which would have been considered a unique tumor in routine pathology practice.
In contrast to our findings, Haffner et al reported a case in which an organ‐confined tumor area with Gleason pattern 3, and not the areas with predominant pattern 4, was found to be most closely related to the metastasis [10]. However, this was not an independent low‐volume Gleason grade 6 tumor, but an area within a larger lesion showing various proportions of patterns 3 and 4. In addition, this specific area also harbored a TP53 mutation.
Comparative analysis of primary PCa and matched metastases
Paired analysis of multiple primary samples and matched metastases from individual patients is particularly helpful for determining at which point of disease progression genomic alterations of interest appear. Activating AR alterations were found only in metastatic samples, in agreement with the previously reported major enrichment of such alterations in metastatic cohorts [9, 11, 29, 30, 31, 32], although exceptional instances of AR alterations in primary disease have been reported [9]. This is likely due to the fact that metastatic samples from our study represented castration‐resistant disease with AR alterations driving treatment resistance [11, 31]. The metastatic NEPC in Patients 1 and 7 did not show AR amplification, consistent with ‘indifference’ of the NEPC to AR signaling [30].
In addition, we were able to demonstrate in individual patients that some alterations reported to be enriched in metastatic cohorts readily arise in the primary disease. In particular, TP53 alterations, which are more frequent in metastatic PCa cohorts compared to primary PCa cohorts [11, 31, 32, 33], were found in metastatic samples from three patients in our study. In all three patients, these alterations were also detected in the primary cancer, and were subclonal in two cases. This could warrant further assessment of TP53 alterations as a predictive factor for metastatic relapse in patients with localized PCa, and appears consistent with the case reported by Haffner et al [10]. In addition, for Patient 1, one primary tumor area (T2) and the brain metastases showed abnormal staining for P53, which usually indicates an underlying mutation (supplementary material, Figure S1). However, although all samples from this patient displayed a hemizygous TP53 loss, no point mutations in TP53 were detected by targeted DNA sequencing or by WES, despite adequate coverage. Nevertheless, we cannot exclude that accumulation of the P53 protein could be a result of alterations in other pathways that interact with P53, or of a cryptic deleterious variant in TP53.
Conversely to subclonal TP53 mutations, some alterations seem to represent early (‘truncal’) oncogenic driver events, as they were clonally present throughout all samples from the primary disease and also shared by the metastases: SPOP p.F133V (a well‐described hotspot mutation in PCa [34, 35]), BRAF p.K601E (a potentially activating variant, which has previously been reported in PCa [21]), and TMPRSS2:ERG or ETV1 rearrangements, in keeping with the early and clonal nature of this alteration [36]. Although to date, there are no therapies targeting these specific alterations, these examples suggest that in cases where the genomic assessment of the metastatic disease is not possible, sequencing of the primary tumor may still provide valuable information on driver mutations.
Among other recurrent alterations in PCa, FOXA1 mutations have been reported at a frequency of 8–9% in primary disease and 12–13% in metastatic disease [37]. In our cohort, only one sample‐ one of the two metastases in Patient 3‐carried a FOXA1 heterozygous p.P297 frameshift. This alteration could have an activating effect on FOXA1, as it truncates the C‐terminal regulatory domain [37, 38]; interestingly, this specific type of FOXA1 alteration has been reported as significantly enriched in mCRPC compared to primary PCa [37].
Our results also highlight the potential role of epigenetic processes in metastatic PCa progression. Multiple studies have found alterations in KMT2C and KMT2D, which encode histone methyltransferases, to be enriched in metastatic PCa cohorts as compared to primary PCa [9, 11, 31, 32, 39]. Similar to TP53, through paired analysis, we showed that truncating KMT2C and KMT2D alterations detected in the metastases (Patients 2 and 9, respectively) were already present in the matched primaries. It could be important to investigate in larger cohorts whether the presence of such alterations in primary PCa is a risk factor for metastatic recurrence. Alterations in KDM4A and ARID1A could also warrant further investigation [3, 21, 39].
The frequency of pathogenic BRCA2 germline alterations in mCRPC patients has previously been reported at 5.3% [40]. In our cohort, germline BRCA2 alterations were found in two patients (16.7%); in one patient (Patient 1), the primary tumor and the metastases were NEPC.
This pilot study has several limitations. Because of the limited size of this pilot cohort, findings from these ‘n‐of‐1’ cases need to be confirmed in larger studies. Nevertheless, collecting archival RP specimens from patients with mCRPC is exceedingly difficult, as the period of time between primary therapy and metastatic disease can be very long (up to 18 years in our cohort, Patient 9). In addition, single‐cell approaches are currently the gold standard for exploring intratumor heterogeneity and clonal evolution, especially since some mutations may be present in the primary tumor at low allele frequencies [41]. While this cohort of archival FFPE specimens was not suitable for single‐cell approaches, they should be considered in future prospective studies. Such sensitive methods would also allow detecting rare clones that were readily present in the primary tumor and selected under treatment pressure [42]. Another important hurdle in analyzing genomic relatedness between multiple samples are variations in tumor DNA content. Because of this caveat, copy number alterations could not be included in the phylogeny analyses, and SNV‐based analyses may have been negatively impacted for some samples (e.g. regional lymph node samples) in which tumor purity was typically low. Further, although a recent study on multiple FFPE tissue biopsies from active surveillance PCa patients had shown consistent results between matched WES and IHC [43], the ability to compare genomic aberrations from frozen and from FFPE tissue‐extracted DNA could be impaired by FFPE‐related artifacts [44, 45]; therefore, introducing an additional source of uncertainty. Lastly, other IHC biomarkers previously used in assessing PCa heterogeneity, such as PTEN and SPINK1 [46], could potentially be employed in future studies to even better discriminate tumor populations on pathology review. In particular, multiple studies have shown PTEN loss in PCa to be associated with recurrence after prostatectomy, metastatic progression, and/or death [47]. In addition, the Gleason pattern 3 tumor area, which proved to be the ‘index’ focus in the case report by Haffner et al, displayed PTEN loss [10]. Thus, PTEN IHC should be employed in future study protocols of PCa heterogeneity.
Conclusion
Our study confirms that primary PCa is characterized by marked pathologic and genomic heterogeneity, which must be considered when selecting tumor areas for precision medicine studies. This is one of the first studies to formally address the still controversial concept of the ‘index lesion’ in primary PCa. Our findings in this pilot cohort show high concordance between a comprehensive, IHC‐assisted pathology review and genomic analyses for nominating the ‘index focus,’ although the specificity of these approaches should be validated in larger cohorts.
Supporting information
Figure S1. A summary of pathology features, phylogeny and clonality analyses for Patient 1
Figure S2. A summary of pathology features, phylogeny and clonality analyses for Patient 2
Figure S3. A summary of pathology features, phylogeny and clonality analyses for Patient 3
Figure S4. A summary of pathology features, phylogeny and clonality analyses for Patient 4
Figure S5. A summary of pathology features, phylogeny and clonality analyses for Patient 5
Figure S6. A summary of pathology features, phylogeny and clonality analyses for Patient 7
Figure S7. A summary of pathology features, phylogeny and clonality analyses for Patient 8
Figure S8. A summary of pathology features, phylogeny and clonality analyses for Patient 10
Figure S9. Patient 6, primary tumor focus T4
Table S1. Sample summary: A summary of samples for each patient, together with pathology findings and interpretation of clonality based on pathology and on genomics
Table S2. Treatments: A summary of the therapies received by each patient before metastatic biopsy, when available
Table S3. WES SNV‐clonality: A list of WES‐based SNV calls per sample, as used for phylogeny and clonality analyses (after applying additional filters described in Materials and methods)
Table S4. WES indels: A list of WES‐based short insertions and deletions per sample
Table S5. WES CNA: A list of WES‐based copy number alterations (CNA) by CLONET
Table S6. AmpliSeq SNV: A list of targeted sequencing‐based SNV and indel calls per sample
Table S7. AmpliSeq CNA: A list of targeted sequencing‐based CNA calls per sample
Acknowledgements
We thank the patients and their families for participating in genomics, transcriptomics, and precision cancer care studies. We are thankful for expert assistance from the Translational Research Program at WCM Pathology and Laboratory Medicine (Bing He, Leticia Dizon, Yifang Liu, Mai Ho) and the WCM CLC Genomics Core Facility (Jenny Xiang). We thank Drs. Samaneh Motanagh (Weill Cornell Medicine) and Wassim Abida (Memorial Sloan Kettering Cancer Center) for their help with retrieving clinical information for some patients. We are grateful to Makayla DeJong (Seattle Cancer Care Alliance) for her help with retrieving archival pathology material. We thank Dr. Christopher Barbieri (Weill Cornell Medicine) for his valuable suggestions regarding this study. We acknowledge expert assistance from Mariana Ricca (University of Bern) in preparing the article for submission. This study was supported by the 2015–2017 Movember Foundation‐PCF Challenge Award. It has also received funding from the NIH/NCI WCM SPORE in Prostate Cancer P50‐CA211024 (H.B., F.D., M.A.R., J.M.M., B.D.R.), the Nuovo‐Soldati Foundation (J.C.), the National Science Foundation Project grants number R37 CA241486 (H.B.), and R01 CA125612 (F.D.), the Pacific Northwest Cancer Spore Award P50CA097186 and the CDMRP Award W81XWH‐18‐1‐0406 (P.N.), and the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No 648670) (F.D.). Open Access Funding provided by Universitat Bern. [Correction added on May 19, 2022, after first online publication: CSAL funding statement has been added.]
Conflict of interest statement: The University of Michigan and Harvard have a patent in the field of diagnostics and therapeutics for ETS gene fusion prostate cancer; ST, FD, and MAR are named as co‐inventors. The diagnostic field of use was licensed to Hologic/Gen‐Probe (who sublicensed some rights to Ventana/Roche) and is licensed to LynxDx. Cornell University has a patent on SPOP prostate cancer mutations in the field of diagnostics and therapeutics; MAR is a co‐inventor. MAR serves on the Scientific Advisory Board of Neogenomics. ST is a co‐inventor on a patent issued to Strata Oncology related to MSI determination and checkpoint inhibitor benefit. ST has the following other conflicts of interest: Employment (Strata Oncology); Leadership (Strata Oncology), Stock and Other Ownership Interests (Strata Oncology, Javelin Oncology); Consulting or Advisory Roles (Janssen, Astellas Medivation, Strata Oncology); Research Funding (Astellas Medivation); Travel, accommodation, and expenses (Strata Oncology, Genzyme).
Data availability statement
RNA‐sequencing data were generated using a custom targeted amplicon‐based panel and, as such, do not represent transcriptome‐wide gene expression profiles and were not deposited in a public repository. However, they can be made available to researchers upon request.
WES data are being deposited to the European Genome‐Phenome Archive (EGA). In the meantime, processed data are available in Supplementary tables, and raw data can be shared upon request by contacting A.S. (ans2077@med.cornell.edu) or F.D. (f.demichelis@unitn.it).
References
- 1. Villers A, McNeal JE, Freiha FS, et al. Multiple cancers in the prostate. Morphologic features of clinically recognized versus incidental tumors. Cancer 1992; 70: 2313–2318. [DOI] [PubMed] [Google Scholar]
- 2. Wise AM, Stamey TA, McNeal JE, et al. Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology 2002; 60: 264–269. [DOI] [PubMed] [Google Scholar]
- 3. Espiritu SMG, Liu LY, Rubanova Y, et al. The evolutionary landscape of localized prostate cancers drives clinical aggression. Cell 2018; 173: 1003, .e15–1013. [DOI] [PubMed] [Google Scholar]
- 4. Rubin MA, Demichelis F. The Genomics of Prostate Cancer: emerging understanding with technologic advances. Mod Pathol 2018; 31: S1–S11. [DOI] [PubMed] [Google Scholar]
- 5. Salami SS, Hovelson DH, Kaplan JB, et al. Transcriptomic heterogeneity in multifocal prostate cancer. JCI Insight 2018; 3: e123468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brocks D, Assenov Y, Minner S, et al. Intratumor DNA methylation heterogeneity reflects clonal evolution in aggressive prostate cancer. Cell Rep 2014; 8: 798–806. [DOI] [PubMed] [Google Scholar]
- 7. Woodcock DJ, Riabchenko E, Taavitsainen S, et al. Prostate cancer evolution from multilineage primary to single lineage metastases with implications for liquid biopsy. Nat Commun 2020; 11: 5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmidt L, Møller M, Haldrup C, et al. Exploring the transcriptome of hormone‐naive multifocal prostate cancer and matched lymph node metastases. Br J Cancer 2018; 119: 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gundem G, Van Loo P, Kremeyer B, et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015; 520: 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haffner MC, Mosbruger T, Esopi DM, et al. Tracking the clonal origin of lethal prostate cancer. J Clin Invest 2013; 123: 4918–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015; 162: 454. [DOI] [PubMed] [Google Scholar]
- 12. Abida W, Cyrta J, Heller G, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A 2019; 116: 11428–11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beltran H, Eng K, Mosquera JM, et al. Whole‐exome sequencing of metastatic cancer and biomarkers of treatment response. JAMA Oncol 2015; 1: 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rennert H, Eng K, Zhang T, et al. Development and validation of a whole‐exome sequencing test for simultaneous detection of point mutations, indels and copy‐number alterations for precision cancer care. NPJ Genom Med 2016; 1: 16019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li H, Durbin R. Fast and accurate short read alignment with Burrows‐Wheeler transform. Bioinformatics 2009; 25: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cibulskis K, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 2013; 31: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang Y, Soong TD, Wang L, et al. Genome‐wide detection of genes targeted by non‐Ig somatic hypermutation in lymphoma. PLoS One 2012; 7: e40332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Romanel A, Lago S, Prandi D, et al. ASEQ: fast allele‐specific studies from next‐generation sequencing data. BMC Med Genomics 2015; 8: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schliep KP. phangorn: phylogenetic analysis in R. Bioinformatics 2011; 27: 592–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ostrovnaya I, Seshan VE, Begg CB. Using somatic mutation data to test tumors for clonal relatedness. Ann Appl Stat 2015; 9: 1533–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cancer Genome Atlas Research Network . The molecular taxonomy of primary prostate cancer. Cell 2015; 163: 1011–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Epstein JI, Amin MB, Beltran H, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol 2014; 38: 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cooper CS, Eeles R, Wedge DC, et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nat Genet 2015; 47: 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lindberg J, Klevebring D, Liu W, et al. Exome sequencing of prostate cancer supports the hypothesis of independent tumour origins. Eur Urol 2013; 63: 347–353. [DOI] [PubMed] [Google Scholar]
- 25. Cheng L, Song SY, Pretlow TG, et al. Evidence of independent origin of multiple tumors from patients with prostate cancer. J Natl Cancer Inst 1998; 90: 233–237. [DOI] [PubMed] [Google Scholar]
- 26. Qian J, Bostwick DG, Takahashi S, et al. Chromosomal anomalies in prostatic intraepithelial neoplasia and carcinoma detected by fluorescence in situ hybridization. Cancer Res 1995; 55: 5408–5414. [PubMed] [Google Scholar]
- 27. Karavitakis M, Ahmed HU, Abel PD, et al. Anatomically versus biologically unifocal prostate cancer: a pathological evaluation in the context of focal therapy. Ther Adv Urol 2012; 4: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boyd LK, Mao X, Xue L, et al. High‐resolution genome‐wide copy‐number analysis suggests a monoclonal origin of multifocal prostate cancer. Genes Chromosomes Cancer 2012; 51: 579–589. [DOI] [PubMed] [Google Scholar]
- 29. Liu W, Laitinen S, Khan S, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med 2009; 15: 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beltran H, Prandi D, Mosquera JM, et al. Divergent clonal evolution of castration‐resistant neuroendocrine prostate cancer. Nat Med 2016; 22: 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abida W, Armenia J, Gopalan A, et al. Prospective genomic profiling of prostate cancer across disease states reveals germline and somatic alterations that may affect clinical decision making. JCO Precis Oncol 2017; 2017: PO.17.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wedge DC, Gundem G, Mitchell T, et al. Sequencing of prostate cancers identifies new cancer genes, routes of progression and drug targets. Nat Genet 2018; 50: 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hong MK, Macintyre G, Wedge DC, et al. Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer. Nat Commun 2015; 6: 6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barbieri CE, Baca SC, Lawrence MS, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet 2012; 44: 685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baca SC, Prandi D, Lawrence MS, et al. Punctuated evolution of prostate cancer genomes. Cell 2013; 153: 666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perner S, Mosquera JM, Demichelis F, et al. TMPRSS2‐ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol 2007; 31: 882–888. [DOI] [PubMed] [Google Scholar]
- 37. Parolia A, Cieslik M, Chu SC, et al. Distinct structural classes of activating FOXA1 alterations in advanced prostate cancer. Nature 2019; 571: 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Annala M, Taavitsainen S, Vandekerkhove G, et al. Frequent mutation of the FOXA1 untranslated region in prostate cancer. Commun Biol 2018; 1: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Armenia J, Wankowicz SAM, Liu D, et al. The long tail of oncogenic drivers in prostate cancer. Nat Genet 2018; 50: 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA‐repair gene mutations in men with metastatic prostate cancer. N Engl J Med 2016; 375: 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y, Waters J, Leung ML, et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature 2014; 512: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim C, Gao R, Sei E, et al. Chemoresistance evolution in triple‐negative breast cancer delineated by single‐cell sequencing. Cell 2018; 173: 879, .e13–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gandellini P, Casiraghi N, Rancati T, et al. Core biopsies from prostate cancer patients in active surveillance protocols harbor PTEN and MYC alterations. Eur Urol Oncol 2019; 2: 277–285. [DOI] [PubMed] [Google Scholar]
- 44. Oh E, Choi YL, Kwon MJ, et al. Comparison of accuracy of whole‐exome sequencing with formalin‐fixed paraffin‐embedded and fresh frozen tissue samples. PLoS One 2015; 10: e0144162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haile S, Corbett RD, Bilobram S, et al. Sources of erroneous sequences and artifact chimeric reads in next generation sequencing of genomic DNA from formalin‐fixed paraffin‐embedded samples. Nucleic Acids Res 2019; 47: e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fontugne J, Davis K, Palanisamy N, et al. Clonal evaluation of prostate cancer foci in biopsies with discontinuous tumor involvement by dual ERG/SPINK1 immunohistochemistry. Mod Pathol 2016; 29: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jamaspishvili T, Berman DM, Ross AE, et al. Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol 2018; 15: 222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A summary of pathology features, phylogeny and clonality analyses for Patient 1
Figure S2. A summary of pathology features, phylogeny and clonality analyses for Patient 2
Figure S3. A summary of pathology features, phylogeny and clonality analyses for Patient 3
Figure S4. A summary of pathology features, phylogeny and clonality analyses for Patient 4
Figure S5. A summary of pathology features, phylogeny and clonality analyses for Patient 5
Figure S6. A summary of pathology features, phylogeny and clonality analyses for Patient 7
Figure S7. A summary of pathology features, phylogeny and clonality analyses for Patient 8
Figure S8. A summary of pathology features, phylogeny and clonality analyses for Patient 10
Figure S9. Patient 6, primary tumor focus T4
Table S1. Sample summary: A summary of samples for each patient, together with pathology findings and interpretation of clonality based on pathology and on genomics
Table S2. Treatments: A summary of the therapies received by each patient before metastatic biopsy, when available
Table S3. WES SNV‐clonality: A list of WES‐based SNV calls per sample, as used for phylogeny and clonality analyses (after applying additional filters described in Materials and methods)
Table S4. WES indels: A list of WES‐based short insertions and deletions per sample
Table S5. WES CNA: A list of WES‐based copy number alterations (CNA) by CLONET
Table S6. AmpliSeq SNV: A list of targeted sequencing‐based SNV and indel calls per sample
Table S7. AmpliSeq CNA: A list of targeted sequencing‐based CNA calls per sample
Data Availability Statement
RNA‐sequencing data were generated using a custom targeted amplicon‐based panel and, as such, do not represent transcriptome‐wide gene expression profiles and were not deposited in a public repository. However, they can be made available to researchers upon request.
WES data are being deposited to the European Genome‐Phenome Archive (EGA). In the meantime, processed data are available in Supplementary tables, and raw data can be shared upon request by contacting A.S. (ans2077@med.cornell.edu) or F.D. (f.demichelis@unitn.it).


