Abstract
Objective
The aim of this study was to determine the diagnostic utility of an MMP‐8 biosensor assay in differentiating periodontal health from gingivitis and periodontitis and compare it with an established time‐resolved immunofluorescence assay (IFMA) and enzyme‐linked immunosorbent assay (ELISA).
Background
Currently available antibody‐based assays display a wide variability in their ability to accurately measure matrix metalloproteinase‐8 (MMP‐8) levels in saliva.
Methods
Salivary MMP‐8 levels were analyzed in 189 systemically healthy participants using an antibody‐based biosensor prototype that operates using a surface acoustic wave technology and compared with IFMA and ELISA antibody assays. Participants were categorized into 3 groups: periodontal health (59), gingivitis (63), and periodontitis (67). A sub‐population of participants (n = 20) with periodontitis received periodontal treatment and were monitored for 6 months.
Results
All the assays demonstrated significantly higher salivary MMP‐8 concentrations in participants with periodontitis versus gingivitis, periodontitis versus health, and gingivitis versus health (all p < .05). The biosensor data demonstrated significant correlations with IFMA (r = .354, p < .001) and ELISA (r = .681, p < .001). Significant reductions in salivary MMP‐8 concentrations were detected by the biosensor (p = .030) and IFMA (p = .002) in participants with periodontitis 6 months after non‐surgical periodontal treatment. IFMA had the best sensitivity (89.2%) for detecting periodontitis and gingivitis versus health and 96.6% for detecting periodontitis versus health and gingivitis. The biosensor had an AUC value of 0.81 and diagnostic accuracy of 74.2% for differentiating periodontitis and gingivitis from health; an AUC value of 0.86 and diagnostic accuracy of 82.8% for periodontitis versus health and gingivitis.
Conclusions
The biosensor, IFMA, and ELISA assays differentiated between periodontal health, gingivitis, and periodontitis based on salivary MMP‐8 levels. Only the biosensor and, particularly, IFMA identified an effect of periodontal treatment in the participants with periodontitis. Our findings support the potential utility of salivary oral fluid aMMP‐8‐based point‐of‐care technology in the future of periodontal diagnostics.
Keywords: active/total matrix metalloproteinase‐8; saliva, biosensor, periodontal disease
1. INTRODUCTION
Periodontitis is a chronic inflammatory disease that results in progressive destruction of the periodontal tissues and remains a significant cause of tooth loss in adults, with concomitant negative impacts on oral health‐related quality of life. 1 Severe periodontitis is the sixth‐most prevalent disease globally, with consequent adverse effects on oral health as well as contributing to systemic inflammation. 2 , 3 , 4 Periodontitis poses a huge health and economic burden globally. 2 , 4 Early diagnosis of periodontitis is therefore a key strategy to facilitate timely and more effective interventions and to achieve a better long‐term prognosis. 5
Periodontitis is diagnosed through clinical and radiographic examination. 6 , 7 However, it is recognized that these traditional diagnostic methods have some shortcomings: often they reflect past disease activity and can be time‐consuming and technically challenging to undertake, as well as being somewhat subjective, being dependent on the expertise and proficiency of the clinician. There is, therefore, potential benefit in developing additional diagnostic methods that can objectively assess current and future periodontal disease activity.
The potential usefulness of disease‐specific inflammatory biomarkers such as matrix metalloproteinase (MMP)‐8 in oral fluids (saliva, gingival crevicular fluid (GCF), peri‐implant sulcular fluid (PISF), and mouth rinses) has been demonstrated in several studies that have correlated MMP‐8 with periodontal disease course and severity. 8 , 9 , 10 , 11 , 12 MMP‐8, otherwise known as neutrophil collagenase or collagenase‐2, is the major collagenolytic enzyme released by neutrophils and is a key mediator in most of the connective tissue destruction in inflammatory periodontal disease and peri‐implantitis. 11 , 13 , 14 Recently, the potential utility of the active form of MMP‐8 (aMMP‐8), as a biomarker in the oral‐systemic link was highlighted, due to the contribution of periodontitis to the inflammatory burden in various systemic diseases and conditions. 15 Active MMP‐8, but not the total or latent form, is related to and predicts the progression of periodontitis due to its catalytic activity in oral fluids. 10 , 16 , 17 , 18 , 19 , 20 , 21
Antibody‐based immunoassays utilizing monoclonal antibodies such as the standard laboratory time‐resolved immunofluorometric assay (IFMA) and enzyme‐linked immunosorbent assay (ELISA) can detect MMP‐8 in oral fluids. 22 , 23 IFMA correlates more strongly with periodontal and peri‐implant tissue destruction than commercially available ELISA kits, which frequently detect total MMP‐8 and cannot readily distinguish between different MMP‐8 forms in periodontal health and disease. 10 , 13 , 19 , 22 , 24 , 25 , 26 , 27 Assays that measure biomarkers could be useful in monitoring the progression of periodontal disease and the response to treatment. 11 , 28 , 29 , 30 Some of these assays can facilitate the rapid detection of aMMP‐8 enzymatic levels in 5–7 minutes, thus offering potential for early diagnosis of periodontal disease (PerioSafe®, ORALyzer®). 31 , 32 The relevance of biomarkers has been highlighted in the staging and grading system for the classification of periodontitis, as potentially improving diagnostic accuracy. 1 In line with this, it was proposed that aMMP‐8 could be the oral biomarker of choice to be used in the staging and grading of periodontitis. 31 , 33 , 34
Laboratory‐based IFMA, ELISA, and chairside lateral flow immunoassays are currently the most widely available methods of quantifying MMP‐8 in oral fluids. 34 , 35 , 36 , 37 Recently, MMP‐8 enzymatic activity was detected and quantified in the GCF of periodontally diseased sites and found to be significantly higher than healthy sites. 20 Also, molecular forms of neutrophilic and mesenchymal‐type MMP‐8 such as 20–27 kDa fragments were shown to be elevated in periodontitis, suggesting a potential role as early diagnostic markers of active periodontal disease. 38 However, these are still at early experimental stages, and the variability in the specificity and sensitivity of the available assays has stimulated the search for other oral fluid point‐of‐care diagnostic methodologies that have greater precision. In this regard, recently, a novel prototype biosensor was developed and utilized to quantify salivary MMP‐8 using specific antibodies and surface acoustic wave (SAW) technology in patients with periodontal disease. 5 Accordingly, in the present study, we aimed to compare the diagnostic utility of the SAW biosensor with other antibody‐based assays (IFMA to measure aMMP‐8 and ELISA to measure total MMP‐8 [tMMP‐8]) in subjects with periodontal health, gingivitis, and periodontitis before and after treatment.
2. MATERIAL AND METHODS
2.1. Study design and setting
The clinical phase of this cohort study was conducted at the Dental Clinical Research Facility of Newcastle Dental Hospital, Newcastle upon Tyne NHS Foundation Trust, UK, from 2012 to 2016. All subjects provided written informed consent, and the ethical approval was received from the National Research Ethics Service North East Newcastle and North Tyneside 1 committee (Ref: 12/NE/0396). SAW analyses were undertaken at Newcastle University, whereas IFMA and ELISA analyses were undertaken at the University of Helsinki.
2.2. Subjects/patients and clinical assessments
Details of the clinical study have been previously published. 5 Briefly, samples from 189 participants were assessed in this study. The inclusion criteria were adults ≥18 years of age, systemically healthy, non‐smokers, with a minimum of 20 natural teeth excluding third molars. Exclusion criteria included periodontal treatment within 6 months prior to the study, removable partial dentures or orthodontic appliances, xerostomia, the use of medications that could affect the periodontal tissues and current use of antibiotics, immunosuppressants, or non‐steroidal anti‐inflammatory drugs. The periodontal parameters assessed included clinical attachment loss (CAL), periodontal probing depth (PPD), modified gingival index (mGI), 39 and percentage bleeding on probing (%BOP), recorded using a manual periodontal probe (UNC‐15, Hu‐Friedy) at six sites per tooth. The patients were allocated into three groups: healthy participants had PPD of ≤3 mm at all sites, no sites with interproximal attachment loss, mGI ≥2.0 in ≤10% of sites and BOP ≤10%; gingivitis patients had mGI of ≥3.0 in ≥30% of sites, no sites with interproximal attachment loss or PPD ≥4 mm, and BOP ≥10%; periodontitis patients had interproximal PPD ≥5 mm at ≥8 teeth and BOP ≥30%.
Participants with periodontitis received non‐surgical periodontal treatment (oral hygiene instruction, root surface debridement using a combination of manual and ultrasonic instruments under local anesthesia) and were re‐assessed after 6 months.
2.3. Saliva sample collection
Unstimulated saliva samples (3–5 ml) were collected by expectoration into sterile plastic centrifuge tubes. The samples were immediately placed on ice after collection and centrifuged for 15 minutes at 1500 g and 4°C. 500 µl aliquots were taken, then frozen in liquid nitrogen, and stored at −80°C until analysis. The saliva samples were collected at baseline from all participants and from twenty (n = 20) periodontitis patients at 6 months following non‐surgical periodontal therapy.
2.4. Salivary MMP‐8 biosensor
The biosensor technology had been described in previous studies. 5 , 40 , 41 In summary, it comprises a disposable Surface Acoustic Wave (SAW) biochip coated with specific antibodies that deliver a signal to a control box upon detection of analyte (MMP‐8). Subsequently, this signal is converted into a digital representation, which is processed by a designated software received by a laptop PC. The biochip has interdigitating input and output gold electrodes connected by a gold‐film‐coated sensing area built on a piezoelectric quartz crystal. This formation enables the excitation of a shear horizontal SAW of specific wavelength and frequency. Capture antibodies on the gold‐film surface thus becomes sensitive to antigen binding by means of amplitude and velocity changes in the SAW signal. Thus, MMP‐8 antigen in the sample binds to the antibodies and the perturbation caused by the antigen/antibody binding is detected by the difference in wave phases between the input and output electrodes (i.e., phase change, Δϕ). The novel prototype device has an assay time of 20 minutes and detection limit of 62.5 ng/ml. 5
2.5. Salivary aMMP‐8 Immunofluorometric assay
A time‐resolved immunofluorometric assay (IFMA) was used to assess aMMP‐8 concentrations in saliva based on the original description. 42 Summarily, the monoclonal MMP‐8‐specific antibodies 8708 and 8706 (Medix Biochemica) were used as catching and tracer antibodies, respectively. The tracer antibody was then labeled using europium‐chelate. The assay buffer contained 20 mM Tris‐HCl (pH 7.5), 0.5 M NaCl, 5 mM CaCl2, 50 mM ZnCl2, 0.5% bovine serum albumin, 0.05% sodium azide, and 20 mg/L diethylenetriaminepentaacetic acid (DTPA). The saliva samples were diluted in assay buffer and incubated with the capture antibody for 1 hour, followed by incubation with the tracer antibody for 1 hour. Enhancement solution was added, and after 5 minutes, fluorescence was measured using EnVision 2105 Multimode Plate Reader (PerkinElmer Finland). The specificity of the monoclonal antibodies against aMMP‐8 corresponded to that of the polyclonal MMP‐8 and the detection limit for the assay is 0.08 ng/ml.
2.6. Salivary total/latent MMP‐8 ELISA
The MMP‐8 concentration in the saliva samples was detected using a commercially available ELISA (Quantikine Human Total MMP‐8 Immunoassay R&D Systems™) according to the manufacturer's instructions and established protocols. 35 , 43 , 44 Duplicate salivary samples were assessed for the enzyme levels in each participant. ELISA has a detection limit of 0.06 ng/ml, and all data points were within the linear range of the assay.
2.7. Statistical analysis
The statistical analyses were carried out with SPSS version 25.0. Differences in continuous variables such as aMMP‐8/total MMP‐8 levels between the three groups were assessed using Kruskal–Wallis tests. In addition, post hoc tests were performed with Dunn–Bonferroni post hoc method while the Spearman's rank correlation was used to determine the correlations between Biosensor, ELISA (total MMP‐8), aMMP‐8 IFMA, and the clinical indices. Wilcoxon signed‐rank test was used to compare differences in salivary MMP‐8 concentrations pre‐ and post‐non‐surgical periodontal treatment. Diagnostic accuracy of MMP‐8 analysis methods for the biosensor, IFMA and ELISA were determined using receiver operating characteristic (ROC) curve analysis. The level of statistical significance was set at p < .05.
3. RESULTS
3.1. Demographic characteristics
Following clinical examination, 59 participants were included in the healthy group, 63 participants were identified as having gingivitis, and 67 participants had periodontitis. Females (n = 118) represented 62.4% of the total study population (n = 189), while the mean age of all participants was 40.4 ± 11.7 years (range 18–62 years).
3.2. Correlation of MMP‐8 of biosensor (phase change, Δφ) with aMMP‐8 IFMA and ELISA
Data related to the salivary measurement of MMP‐8 using the SAW biosensor have been previously published. 5 In the present study, salivary MMP‐8 levels determined by the SAW biosensor had a significant correlation with aMMP‐8 IFMA (ng/ml; r = .354, p < .001) and total MMP‐8 ELISA (ng/ml; r = .681, p = .001) among all the participants (Table 1). With respect to the healthy volunteers, salivary MMP‐8 as measured by the biosensor showed a significant correlation with total MMP‐8 as measured by ELISA (ng/ml; r = .436, p = .002) but not with the measurements from the aMMP‐8 IFMA (Table 1). Among the gingivitis patients, MMP‐8 levels measured using the SAW biosensor had no significant correlation with either the aMMP‐8 IFMA or total MMP‐8 ELISA (Table 1). However, in the periodontitis group, MMP‐8 assayed using the SAW biosensor significantly correlated with total MMP‐8 measured by ELISA (ng/ml; r = .450, p < .001) but not with aMMP‐8 measured by IFMA (ng/ml). Table 2 shows the correlations between aMMP‐8 IFMA and MMP‐8 ELISA. Overall, this correlation was significant and strong (r = .608, p < .001). In periodontal health, the correlation was also significant and strong (r = .700, p < .001) and for gingivitis (r = .482, p < .001) but was not significant for periodontitis.
TABLE 1.
Correlation of salivary MMP‐8 biosensor (Δφ) with IFMA and ELISA assays in study population
| MMP‐8 biosensor (Δφ) vs. |
aMMP‐8 IFMA (ng/ml) |
MMP‐8 ELISA (ng/ml) |
||
|---|---|---|---|---|
| Periodontal status | Spearman's rho | p‐value | Spearman's rho | p‐value |
| Healthy (n = 59) | 0.252 | .081 | 0.436 | .002* |
| Gingivitis (n = 63) | 0.138 | .370 | 0.247 | .106 |
| Periodontitis (n = 67) | −0.068 | .612 | 0.450 | <.001* |
| Total (n = 189) | 0.354 | <.001* | 0.681 | <.001* |
Significant; Spearman's rho (rank correlation test)
TABLE 2.
Correlation of salivary aMMP‐8 IFMA (ng/ml) with MMP‐8 ELISA
| aMMP‐8 IFMA vs. | MMP‐8 ELISA (ng/ml) | |
|---|---|---|
| Periodontal status | Spearman's rho | p‐value |
| Healthy (n = 59) | 0.700 | <.001* |
| Gingivitis (n = 63) | 0.482 | <.001* |
| Periodontitis (n = 67) | 0.177 | .158 |
| Total (n = 189) | 0.608 | <.001* |
Significant; Spearman's rho (rank correlation test).
3.3. Comparative analysis of the performance of salivary MMP‐8 assay methods
The comparative performance of the biosensor and the other assay methods for salivary MMP‐8 in terms of distinguishing periodontal health, gingivitis, and periodontitis was assessed. (Table 3) There were significant differences in the salivary MMP‐8 levels between healthy and gingivitis participants as measured by the biosensor (p < .05, Figure 1A), aMMP‐8 IFMA (p < .001, Figure 1B), and total MMP‐8 ELISA (p < .001, Figure 1C). Salivary MMP‐8 levels were also significantly different between healthy and periodontitis subjects as assayed by the biosensor (p < .001, Figure 1A), aMMP‐8 IFMA (p < .001, Figure 1B), and total MMP‐8 ELISA (p < .001, Figure 1C) and significantly different between gingivitis and periodontitis participants as measured by the biosensor (p < .001, Figure 1A), aMMP‐8 IFMA (p < .01, Figure 1B) and total MMP‐8 ELISA (p < .001, Figure 1C).
TABLE 3.
Comparison of changes in mean rank of salivary MMP‐8 levels in study population
| Change in mean rank Biosensor (Δφ) | p‐value |
Change in mean rank aMMP‐8 IFMA (ng/ml) |
p‐value |
Change in mean rank MMP‐8 ELISA (ng/ml) |
p‐value | |
|---|---|---|---|---|---|---|
| Healthy vs Gingivitis | 22.77 | .037* | 34.99 | .001* | 40.01 | <.001* |
| Gingivitis vs Periodontitis | 41.84 | <.001* | 29.56 | .006* | 45.78 | <.001* |
| Healthy vs Periodontitis vs | 64.60 | <.001* | 64.54 | <.001* | 85.78 | <.001* |
Significant for pairwise post hoc analysis for mean rank of periodontal status.
FIGURE 1.
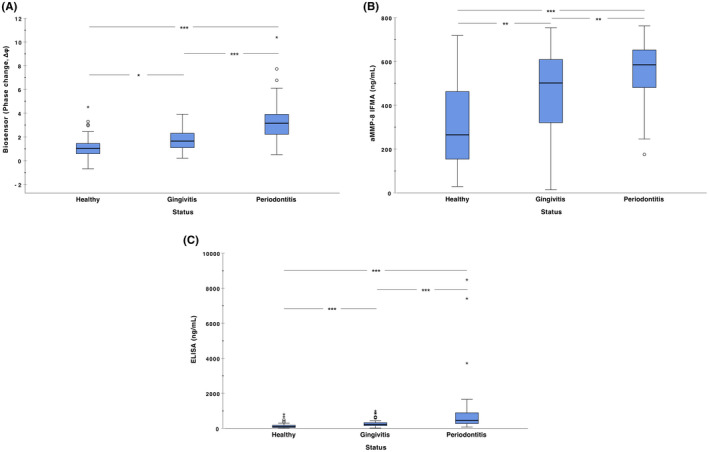
Differences in salivary MMP‐8 levels between the healthy (n = 59), gingivitis (n = 63), and periodontitis (n = 67) participants based on (A) biosensor (B) aMMP‐8 IFMA and (C) ELISA. The data are shown as box and whisker plots and analyzed using Kruskal–Wallis tests, while the post hoc tests were performed with Dunn–Bonferroni post hoc method. *p < .05, **p < .01, ***p < .001
3.4. ROC‐curve analysis
The receiver operating characteristic (ROC) analysis and diagnostic performance of the salivary MMP‐8 assay methods in differentiating between periodontitis, gingivitis, and health, depicted by the area under the curve (AUC) values are shown on Table 4, Figure 2A,B. The biosensor, IFMA, and ELISA assays had high AUC values of 0.808, 0.782, and 0.857, respectively, for differentiating periodontitis and gingivitis versus health. In discriminating periodontitis versus health and gingivitis, the AUC values for biosensor, IFMA and ELISA were 0.857, 0.720, and 0.832, respectively (Table 4). The sensitivities of the biosensor, IFMA, and ELISA assays were 71.6%, 89.2%, and 83.3%, respectively, for periodontitis and gingivitis versus health, while for periodontitis versus health and gingivitis, they were 74.1%, 96.6%, and 56.9%, respectively.
TABLE 4.
ROC analysis and diagnostic performance of MMP‐8 analysis methods Biosensor, IFMA and ELISA in classifying between (A) periodontitis and gingivitis versus health; and (B) periodontitis versus health and gingivitis
| Periodontitis + Gingivitis vs Health | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC | Cutoff | OR | Se(%) | Sp(%) | FN(%) | FP(%) | Acc(%) | MCC | |
| Biosensor | 0.808 (0.736–0.881) | 1.6 | 9.8 | 71.6 | 79.6 | 42.6 | 12.0 | 74.2 | 0.48 |
| IFMA | 0.782 (0.696–0.867) | 347.7 | 14.2 | 89.2 | 63.3 | 26.2 | 16.5 | 80.8 | 0.55 |
| ELISA | 0.857 (0.792–0.922) | 187.6 | 19.5 | 83.3 | 79.6 | 30.4 | 10.5 | 82.1 | 0.61 |
| Periodontitis vs Health + Gingivitis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC | Cutoff | OR | Se(%) | Sp(%) | FN(%) | FP(%) | Acc(%) | MCC | |
| Biosensor | 0.857 (0.793–0.920) | 2.5 | 21.4 | 74.1 | 88.2 | 15.5 | 20.4 | 82.8 | 0.63 |
| IFMA | 0.720 (0.641–0.800) | 378.3 | 27.4 | 96.6 | 49.5 | 4.2 | 45.6 | 67.5 | 0.48 |
| ELISA | 0.832 (0.768–0.896) | 386.5 | 16.2 | 56.9 | 92.5 | 22.5 | 17.5 | 78.8 | 0.54 |
Cutoff calculated by Youden's index.
Abbreviations: Acc, accuracy; FN, false negatives; FP, false positives; MCC, Matthews correlation coefficient; OR, odds ratio; Se, sensitivity; Sp, specificity.
FIGURE 2.
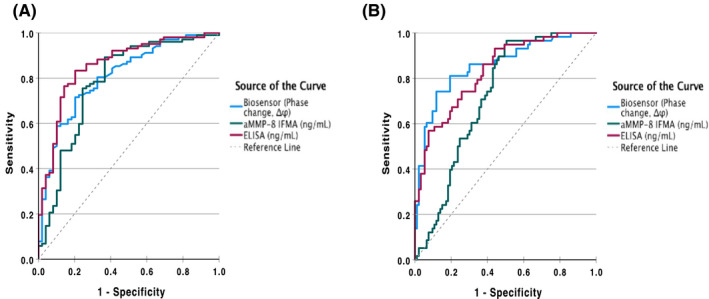
ROC curves of MMP‐8 analysis methods Biosensor, IFMA, and ELISA in classifying between (A) periodontitis and gingivitis versus health; and (B) periodontitis versus health and gingivitis
3.5. Analysis of salivary MMP‐8 before and 6 months after non‐surgical periodontal therapy
The ability of the assays to detect changes in salivary MMP‐8 levels before and 6 months after non‐surgical periodontal treatment was also evaluated in a sub‐set of twenty periodontitis patients (Table 5). The biosensor assay showed a significant reduction in salivary MMP‐8 levels (p = .016; Figure 3A) 6 months after periodontal treatment. This reduction was more significant in aMMP‐8 IFMA after 6 months (p = .002; Figure 3B) but the pre‐ and post‐treatment levels of MMP‐8 as measured by the total MMP‐8 ELISA were not significantly different (p = .221; Figure 3C). It is also important to note that only six and four subjects had persistently elevated MMP‐8 levels as measured by the biosensor and the IFMA assay, respectively.
TABLE 5.
Comparative analysis of mean salivary MMP‐8 levels in periodontitis patients (n = 20) at baseline and 6 months after periodontal treatment (n = 20)
| Assay method | Mean MMP‐8 levels | Mean difference | p‐value | |
|---|---|---|---|---|
| Baseline (t0) | 6 months (t6) | |||
| Biosensor (Δφ) | 2.80 ± 0.99 | 2.29 ± 0.92 | 0.52 ± 0.87 | .030* |
| aMMP‐8 IFMA (ng/ml) | 569.54 ± 95.41 | 445.92 ± 165.07 | 123.62 ± 151.94 | .002* |
| ELISA (ng/ml) | 496.91 ± 347.78 | 418.45 ± 422.63 | 78.46 ± 277.52 | .232 |
Significant by Wilcoxon signed‐rank test.
FIGURE 3.
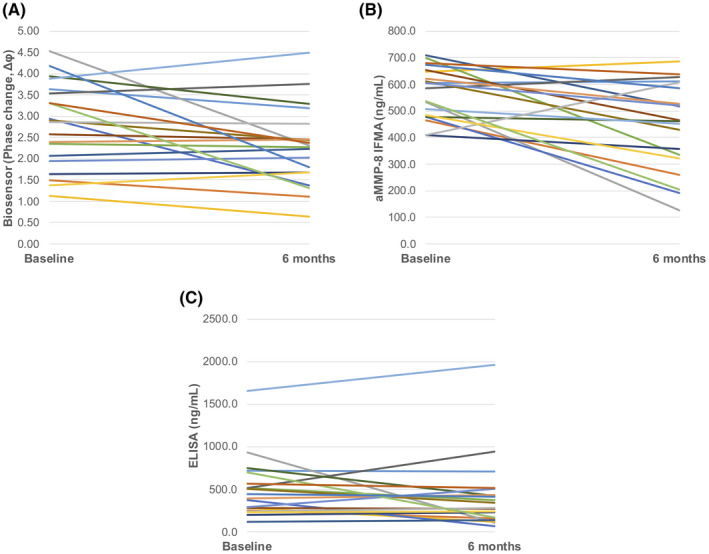
Differences in salivary MMP‐8 levels in periodontitis patients (n = 20) at baseline and 6 months after non‐surgical periodontal treatment. (A) Biosensor (p < .05; B) aMMP‐8 IFMA (p < .01) and (C) ELISA (p > .05)
4. DISCUSSION
In this study, we compared the potential utility of a novel biosensor for detecting MMP‐8 in saliva samples with an established laboratory IFMA which is selective for aMMP‐8, and a commercial ELISA immunoassay which is selective for total/latent MMP‐8. The biosensor utilizes specific antibodies coated on a mini biochip to quantify total MMP‐8 (inactive total/latent pro‐MMP‐8) through a microelectromechanical piezoelectric SAW technology which generates a digital readout to reflect the salivary MMP‐8 levels. 5
The significantly higher salivary MMP‐8 levels detected in gingivitis and periodontitis participants compared with healthy participants by all the three assays, aligns with previous studies that have utilized IFMA, ELISA and quite recently the biosensor. 5 , 11 , 19 , 24 , 33 , 35 , 36 , 45 , 46 Recently, the ability of aMMP‐8 IFMA and PerioSafe® point‐of‐care/chairside assay to distinguish different stages of periodontal disease (gingivitis, periodontitis stages III and IV) from periodontal health was demonstrated using both GCF and saliva. 19 In another study, the aMMP‐8 IFMA assay also differentiated between patients with periodontal health, gingivitis, and periodontitis stage III, grade C based on higher salivary aMMP‐8 concentrations. 33 Higher aMMP‐8 catalytic activity has also been demonstrated in the GCF and saliva of periodontally diseased sites and patients compared with healthy sites and patients. 20 , 47 , 48
The elevated aMMP‐8 levels observed in periodontitis result in active enzymatic degradation of interstitial type I collagen fibers of the periodontal tissues. 24 , 48 The majority (90%–95%) of the collagenolytic activity in GCF originates from aMMP‐8, and its elevation associates closely with disease severity. 11 , 13 , 24 , 32 , 33 , 37 , 48 , 49 In addition, it has been highlighted that the activated form of MMP‐8 released by the polymorphonuclear neutrophils (PMNs), and not the total or latent form of MMP‐8, accurately detects and predicts periodontal tissue destruction. 21 , 24 , 28 , 32 , 50 , 51 , 52 The fact that in the post hoc analyses, the assays clearly demarcated MMP‐8 levels between health and gingivitis, health and periodontitis, and gingivitis and periodontitis is relevant. Potentially, they could be used to facilitate the timing of targeted and personalized treatment.
In this study, we have confirmed the correlation of the salivary MMP‐8 biosensor with MMP‐8 ELISA data. 5 We also report for the first time a correlation between the levels of salivary tMMP‐8 as measured by the biosensor and aMMP‐8 as measured by IFMA, suggesting the SAW biosensor system is capturing quantitatively the active MMP‐8 fraction of the total MMP‐8 in saliva.
The differences in correlations between the biosensor and the other salivary MMP‐8 assays in comparison between healthy, gingivitis, and periodontitis groups in the current study could be partly attributed to different specificities and sensitivities between the antibodies used in the three assays. The affinity of the specific antibodies to detect the active and latent forms of MMP‐8 differs. The biosensor had a stronger correlation for ELISA in the current study, as both assay methods detect total MMP‐8 (active + latent), as ELISA cannot differentiate the active from latent forms of MMP‐8. 16 , 21 , 53 (Gul et al., 2020) In contrast, there was a weak correlation between the biosensor and IFMA in healthy and periodontitis groups. IFMA has a high affinity for the active MMP‐8 which is the molecular form associated with the onset and progression of periodontitis. 10 , 11 , 22
The difference in results from the three MMP‐8 assays following periodontal treatment has been demonstrated previously. 32 , 54 In the present study, measurements using both the IFMA and biosensor assays demonstrated significant differences in salivary MMP‐8 levels in periodontitis patients before, and 6 months after, treatment, although the statistical significance was greater with the IFMA assay. The ability of IFMA to detect a treatment effect in aMMP‐8 levels following periodontal therapy confirms previous findings. 10 IFMA detects mainly aMMP‐8 which is predominant in oral fluids in periodontitis. 10 , 11 , 16 The SAW biosensor, however, detects total MMP‐8 which can also be assessed by the ELISA. 5 The significant reduction in aMMP‐8 post‐treatment detected by the IFMA further corroborates the key role of MMP‐8 in the pathogenesis of periodontitis. 11 , 24 , 32 , 37
The SAW biosensor data correlated more strongly with MMP‐8 ELISA data but less so with aMMP‐8 IFMA possibly because most of the MMP‐8 in saliva is in the total and latent form. 20 , 28 , 48 , 51 , 55 , 56 The significant correlation between salivary MMP‐8 levels measured by the biosensor and ELISA has also been reported. 5 This can also be explained, at least in part, by the fact that the biosensor utilized MMP‐8 antibody that is selective for total MMP‐8 (similar to the ELISA) while IFMA utilized the aMMP‐8 selective antibody. 57
As previously documented, aMMP‐8 measured by IFMA correlates more strongly with periodontitis sites and has a higher diagnostic accuracy than ELISA. 10 , 11 , 22 , 57 , 58 The diagnostic performance of the assay methods to discriminate periodontitis, gingivitis, and health was further determined by a ROC‐curve analysis. IFMA had the best sensitivity (89.2%) for detecting periodontitis and gingivitis versus health, and 96.6% for detecting periodontitis versus health and gingivitis. The AUC value of IFMA in discriminating periodontitis and gingivitis from health was 0.78 and less than those of the biosensor (0.81) and ELISA assays (0.86). In addition, the biosensor and ELISA assays had very good diagnostic AUC values of 0.86 and 0.83, respectively, for periodontitis versus health and gingivitis, which were higher than the IFMA assay (0.72).
The diagnostic performance of the SAW biosensor has been reported previously, 5 and the present study further supports its potential utility in future periodontal diagnostics. We recognize that other oral diseases and conditions, such as caries activity and reduced salivary flow rate, could affect MMP‐8 assays, potentially through the activation of pro‐MMP‐8 by acids produced by cariogenic bacteria. 59 These factors will need to be considered when future studies on the utility of salivary MMP‐8 assays are conducted.
5. CONCLUSION
In summary, this study has reaffirmed the ability of the SAW biosensor, IFMA, and ELISA assays to detect MMP‐8 levels in saliva to distinguish participants with periodontal health, gingivitis, and periodontitis. Both the biosensor and the IFMA (aMMP‐8) detected a periodontal treatment effect among the periodontitis participant, as indicated by the reduced salivary MMP‐8 levels after 6 months. The diagnostic utility of the biosensor and ELISA assays was demonstrated in differentiating between periodontal health, gingivitis, and periodontitis. Overall, the study findings strongly indicate the potential usefulness of, in particular, the oral fluid aMMP‐8‐based technologies for point‐of‐care periodontal assessment in the future.
CONFLICT OF INTEREST
Prof Timo Sorsa is the inventor of U.S. patents 5652223, 5736341, 5864632, 6143476 and US 2017/0023571A1 (issued June 6, 2019), WO 2018/060553A1 (issued May 31, 2018), and 10488415B2, Japanese Patent 2016‐554676 and South Korean patent 10‐2016‐70225378. Other authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
AUTHOR CONTRIBUTIONS
TS, JJT, PP, PMP, ITR, SMB, KAU and SON contributed to the study conception and research design; PP, TT, HL and SMB performed the data collection for the laboratory and clinical aspects; KAU, TT, PMP, JJT, ITR and TS analyzed the data; TS, PP, JJT, SMB, KAU, SON and HL interpreted the data; KAU, PP, PMP, ITR, HL, SMB, SON and TS drafted the manuscript; KAU, PP, PMP, JJT, ITR, SMB, SON and TS revised the manuscript for initial intellectual content; and all the authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
This research was funded by the Helsinki and Uusimaa Hospital District (HUS) (TYH2016251, TYH2017251, TYH2018229, TYH2019319, Y1014SL017, Y1014SL018 and Y1014SULE1), Finland; Finnish Dental Association Apollonia, Finland; Karolinska Institutet, Swedsen. The authors thank Mrs. Maija Kristo for her secretarial assistance.
Umeizudike KA, Lähteenmäki H, Räisänen IT, et al. Ability of matrix metalloproteinase‐8 biosensor, IFMA, and ELISA immunoassays to differentiate between periodontal health, gingivitis, and periodontitis. J Periodont Res. 2022;57:558–567. doi: 10.1111/jre.12985
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 2018;89(suppl 1):159‐172. [DOI] [PubMed] [Google Scholar]
- 2. Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta‐regression. J Dent Res. 2014;93(11):1045‐1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Preshaw PM, Bissett SM. Periodontitis and diabetes. Br Dent J. 2019;227(7):577‐584. [DOI] [PubMed] [Google Scholar]
- 4. Tonetti MS, Jepsen S, Jin L, Otomo‐Corgel J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol. 2017;44(5):456‐462. [DOI] [PubMed] [Google Scholar]
- 5. Taylor JJ, Jaedicke KM, van de Merwe RC, et al. A prototype antibody‐based biosensor for measurement of salivary MMP‐8 in periodontitis using surface acoustic wave technology. Sci Rep. 2019;9(1):11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chapple ILC, Mealey BL, Van Dyke TE, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: consensus report of workgroup 1 of the 2017 world workshop on the classification of periodontal and peri‐implant diseases and conditions. J Clin Periodontol. 2018;45(suppl 20):68‐77. [DOI] [PubMed] [Google Scholar]
- 7. Preshaw PM. Detection and diagnosis of periodontal conditions amenable to prevention. BMC Oral Health. 2015;15(suppl 1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rathnayake N, Gieselmann D‐R, Heikkinen A, Tervahartiala T, Sorsa T. Salivary diagnostics—point‐of‐care diagnostics of MMP‐8 in dentistry and medicine. Diagnostics. 2017;7(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rathnayake N, Akerman S, Klinge B, et al. Salivary biomarkers of oral health: a cross‐sectional study. J Clin Periodontol. 2013;40(2):140‐147. [DOI] [PubMed] [Google Scholar]
- 10. Sorsa T, Hernández M, Leppilahti J, Munjal S, Netuschil L, Mäntylä P. Detection of gingival crevicular fluid MMP‐8 levels with different laboratory and chair‐side methods. Oral Dis. 2010;16(1):39‐45. [DOI] [PubMed] [Google Scholar]
- 11. Sorsa T, Gursoy UK, Nwhator S, et al. Analysis of matrix metalloproteinases, especially MMP‐8, in gingival creviclular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol 2000. 2016;70(1):142‐163. [DOI] [PubMed] [Google Scholar]
- 12. Zhang L, Li X, Yan H, Huang L. Salivary matrix metalloproteinase (MMP)‐8 as a biomarker for periodontitis: a PRISMA‐compliant systematic review and meta‐analysis. Medicine. 2018;97(3):e9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hernández M, Baeza M, Contreras J, et al. MMP‐8, TRAP‐5, and OPG levels in GCF diagnostic potential to discriminate between healthy patients’, mild and severe periodontitis sites. Biomolecules. 2020;10(11):1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Morais EF, Pinheiro JC, Leite RB, Santos PPA, Barboza CAG, Freitas RA. Matrix metalloproteinase‐8 levels in periodontal disease patients: a systematic review. J Periodontal Res. 2018;53(2):156‐163. [DOI] [PubMed] [Google Scholar]
- 15. Umeizudike K, Räisänen I, Gupta S, et al. Active matrix metalloproteinase‐8: a potential biomarker of oral systemic link. Clin Exp Dent Res. 2022;8(1):359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sorsa T, Tjäderhane L, Konttinen YT, et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006;38(5):306‐321. [DOI] [PubMed] [Google Scholar]
- 17. Hernandez‐Rios P, Hernández M, Garrido M, et al. Oral fluid matrix metalloproteinase (MMP)‐8 as a diagnostic tool in chronic periodontitis. Met Med. 2016;3:11–18. [Google Scholar]
- 18. Silbereisen A, Alassiri S, Bao K, et al. Label‐free quantitative proteomics versus antibody‐based assays to measure neutrophil‐derived enzymes in saliva. Proteomics Clin Appl. 2020;14(3):e1900050. [DOI] [PubMed] [Google Scholar]
- 19. Öztürk VÖ, Emingil G, Umeizudike K, et al. Evaluation of active matrix metalloproteinase‐8 (aMMP‐8) chair‐side test as a diagnostic biomarker in the staging of periodontal diseases. Arch Oral Biol. 2021;124: 104955. [DOI] [PubMed] [Google Scholar]
- 20. Mc Crudden MTC, Irwin CR, El Karim I, Linden GJ, Lundy FT. Matrix metalloproteinase‐8 activity in gingival crevicular fluid: development of a novel assay. J Periodontal Res. 2017;52(3):556‐561. [DOI] [PubMed] [Google Scholar]
- 21. Lee W, Aitken S, Sodek J, McCulloch CA. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: role of active enzyme in human periodontitis. J Periodontal Res. 1995;30(1):23‐33. [DOI] [PubMed] [Google Scholar]
- 22. Leppilahti JM, Hernández‐Ríos PA, Gamonal JA, et al. Matrix metalloproteinases and myeloperoxidase in gingival crevicular fluid provide site‐specific diagnostic value for chronic periodontitis. J Clin Periodontol. 2014;41(4):348‐356. [DOI] [PubMed] [Google Scholar]
- 23. Leppilahti JM, Ahonen MM, Hernández M, et al. Oral rinse MMP‐8 point‐of‐care immuno test identifies patients with strong periodontal inflammatory burden. Oral Dis. 2011;17(1):115‐122. [DOI] [PubMed] [Google Scholar]
- 24. Al‐Majid A, Alassiri S, Rathnayake N, Tervahartiala T, Gieselmann DR, Sorsa T. Matrix metalloproteinase‐8 as an inflammatory and prevention biomarker in periodontal and peri‐implant diseases. Int J Dent. 2018;2018:7891323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verhulst MJL, Teeuw WJ, Bizzarro S, et al. A rapid, non‐invasive tool for periodontitis screening in a medical care setting. BMC Oral Health. 2019;19(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Räisänen IT, Lähteenmäki H, Gupta S, et al. An aMMP‐8 point‐of‐care and questionnaire based real‐time diagnostic toolkit for medical practitioners. Diagnostics (Basel). 2021;11(4):711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Romero‐Castro NS, Vázquez‐Villamar M, Muñoz‐Valle JF, et al. Relationship between TNF‐α, MMP‐8, and MMP‐9 levels in gingival crevicular fluid and the subgingival microbiota in periodontal disease. Odontology. 2020;108(1):25‐33. [DOI] [PubMed] [Google Scholar]
- 28. Romanelli R, Mancini S, Laschinger C, Overall CM, Sodek J, McCulloch CA. Activation of neutrophil collagenase in periodontitis. Infect Immun. 1999;67(5):2319‐2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alassiri S, Parnanen P, Rathnayake N, et al. The ability of quantitative, specific, and sensitive point‐of‐care/chair‐side oral fluid immunotests for aMMP‐8 to detect periodontal and peri‐implant diseases. Dis Markers. 2018;2018:1306396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lähteenmäki H, Umeizudike KA, Heikkinen AM, et al. aMMP‐8 point‐of‐care/chairside oral fluid technology as a rapid, non‐invasive tool for periodontitis and peri‐implantitis screening in a medical care setting. Diagnostics (Basel). 2020;10(8):562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sorsa T, Gieselmann D, Arweiler NB, Hernández M. A quantitative point‐of‐care test for periodontal and dental peri‐implant diseases. Nat Rev Dis Primers. 2017;3:17069. [DOI] [PubMed] [Google Scholar]
- 32. Sorsa T, Mäntylä P, Rönkä H, et al. Scientific basis of a matrix metalloproteinase‐8 specific chair‐side test for monitoring periodontal and peri‐implant health and disease. Ann N Y Acad Sci. 1999;878:130‐140. [DOI] [PubMed] [Google Scholar]
- 33. Keles Yucel ZP, Afacan B, Emingil G, Tervahartiala T, Kose T, Sorsa T. Local and systemic levels of aMMP‐8 in gingivitis and stage 3 grade C periodontitis. J Periodontal Res. 2020;55(6):887‐894. [DOI] [PubMed] [Google Scholar]
- 34. Deng K, Pelekos G, Jin L, Tonetti MS. Diagnostic accuracy of a point‐of‐care aMMP‐8 test in the discrimination of periodontal health and disease. J Clin Periodontol. 2021;48(8):1051–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ebersole JL, Nagarajan R, Akers D, Miller CS. Targeted salivary biomarkers for discrimination of periodontal health and disease(s). Front Cell Infect Microbiol. 2015;5:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson N, Ebersole JL, Kryscio RJ, et al. Rapid assessment of salivary MMP‐8 and periodontal disease using lateral flow immunoassay. Oral Dis. 2016;22(7):681‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sorsa T, Alassiri S, Grigoriadis A, et al. Active MMP‐8 (aMMP‐8) as a grading and staging biomarker in the periodontitis classification. Diagnostics (Basel). 2020;10(2):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gürsoy UK, Könönen E, Tervahartiala T, et al. Molecular forms and fragments of salivary MMP‐8 in relation to periodontitis. J Clin Periodontol. 2018;45(12):1421‐1428. [DOI] [PubMed] [Google Scholar]
- 39. Lobene RR, Weatherford T, Ross NM, Lamm RA, Menaker L. A modified gingival index for use in clinical trials. Clin Prev Dent. 1986;8(1):3‐6. [PubMed] [Google Scholar]
- 40. Kogai T, Yoshimura N, Mori T, Yatsuda H. Liquid‐phase shear horizontal surface acoustic wave immunosensor. Jpn J Appl Phys. 2010;49(suppl 7):07HD15. [Google Scholar]
- 41. Turbé V, Gray ER, Lawson VE, et al. Towards an ultra‐rapid smartphone‐ connected test for infectious diseases. Sci Rep. 2017;7(1):11971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hanemaaijer R, Sorsa T, Konttinen YT, et al. Matrix metalloproteinase‐8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor‐alpha and doxycycline. J Biol Chem. 1997;272(50):31504‐31509. [DOI] [PubMed] [Google Scholar]
- 43. Llambés F, Arias‐Herrera S, Caffesse R. Relationship between diabetes and periodontal infection. World J Diabetes. 2015;6(7):927‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ebersole JL, Schuster JL, Stevens J, et al. Patterns of salivary analytes provide diagnostic capacity for distinguishing chronic adult periodontitis from health. J Clin Immunol. 2013;33(1):271‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Räisänen IT, Heikkinen AM, Nwhator SO, Umeizudike KA, Tervahartiala T, Sorsa T. On the diagnostic discrimination ability of mouthrinse and salivary aMMP‐8 point‐of‐care testing regarding periodontal health and disease. Diagn Microbiol Infect Dis. 2019;95(4):114871. [DOI] [PubMed] [Google Scholar]
- 46. Leppilahti JM, Sorsa T, Kallio MA, et al. The utility of gingival crevicular fluid matrix metalloproteinase‐8 response patterns in prediction of site‐level clinical treatment outcome. J Periodontol. 2015;86(6):777‐787. [DOI] [PubMed] [Google Scholar]
- 47. Tatakis DN, Kumar PS. Etiology and pathogenesis of periodontal diseases. Dent Clin North Am. 2005;49(3):491‐516. [DOI] [PubMed] [Google Scholar]
- 48. Uitto VJ, Suomalainen K, Sorsa T. Salivary collagenase. Origin, characteristics and relationship to periodontal health. J Periodontal Res. 1990;25(3):135‐142. [DOI] [PubMed] [Google Scholar]
- 49. Sorsa T, Uitto VJ, Suomalainen K, Vauhkonen M, Lindy S. Comparison of interstitial collagenases from human gingiva, sulcular fluid and polymorphonuclear leukocytes. J Periodontal Res. 1988;23(6):386‐393. [DOI] [PubMed] [Google Scholar]
- 50. Sorsa T, Heikkinen AM, Leppilahti J, et al. Active matrix metalloproteinase‐8: Contributor to periodontitis and a missing link between genetics, dentistry, and medicine. In:In Pathogenesis of Periodontal Diseases. 2018:51‐57. Springer, Cham. [Google Scholar]
- 51. Mancini S, Romanelli R, Laschinger CA, Overall CM, Sodek J, McCulloch CA. Assessment of a novel screening test for neutrophil collagenase activity in the diagnosis of periodontal diseases. J Periodontol. 1999;70(11):1292‐1302. [DOI] [PubMed] [Google Scholar]
- 52. Kiili M, Cox SW, Chen HY, et al. Collagenase‐2 (MMP‐8) and collagenase‐3 (MMP‐13) in adult periodontitis: molecular forms and levels in gingival crevicular fluid and immunolocalisation in gingival tissue. J Clin Periodontol. 2002;29(3):224‐232. [DOI] [PubMed] [Google Scholar]
- 53. Gul SS, Abdulkareem AA, Sha AM, Rawlinson A. Diagnostic accuracy of oral fluids biomarker profile to determine the current and future status of periodontal and peri‐implant diseases. Diagnostics (Basel). 2020;10(10):838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sioustis IA, Martu MA, Aminov L, et al. Salivary metalloproteinase‐8 and metalloproteinase‐9 evaluation in patients undergoing fixed orthodontic treatment before and after periodontal therapy. Int J Environ Res Public Health. 2021;18(4):1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gangbar S, Overall CM, McCulloch CA, Sodek J. Identification of polymorphonuclear leukocyte collagenase and gelatinase activities in mouthrinse samples: correlation with periodontal disease activity in adult and juvenile periodontitis. J Periodontal Res. 1990;25(5):257‐267. [DOI] [PubMed] [Google Scholar]
- 56. Sorsa T, Suomalainen K, Uitto VJ. The role of gingival crevicular fluid and salivary interstitial collagenases in human periodontal diseases. Arch Oral Biol. 1990;(suppl 35):193‐196. [DOI] [PubMed] [Google Scholar]
- 57. Sorsa T, Gieselmann D, Korvuo A, et al. MMP‐8 Activation Product, Its Determination and Use. 2019. U.S. Patent 10,488,415. [Google Scholar]
- 58. Buduneli N, Kinane DF. Host‐derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. J Clin Periodontol. 2011;35(suppl 1):85‐105. [DOI] [PubMed] [Google Scholar]
- 59. Hedenbjörk‐Lager A, Bjørndal L, Gustafsson A, et al. Caries correlates strongly to salivary levels of matrix metalloproteinase‐8. Caries Res. 2015;49(1):1‐8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


