Abstract
Maternal deprivation has been shown to disrupt the development of neonates. Nevertheless, separating the young animals from their dams soon after birth is a common practice in dairy farming. We investigated the effects of maternal deprivation on goat kids’ (Capra hircus) social behavior and social ontogeny before and after weaning. Twenty female kids were raised together with their dams (DR kids) and other lactating goats and kids, whereas 20 female kids were separated from their dams 3 days after birth and artificially reared together (AR kids). At weaning, each treatment group was split in half and moved into two new pens where they were mixed with the other treatment group. Social behaviors were recorded before and after weaning. Before weaning, AR kids were observed performing more play‐fighting, racing, stepping on each other, and standing in contact with each other than DR kids, but AR allogroomed less and spent less time resting alone than DR kids. After weaning and mixing of the treatments, DR kids initiated more and received less agonistic interactions than AR kids, but this difference reduced across the 5 weeks of observations as AR kids appeared to progressively change their social behavior after interacting with DR kids.
Keywords: early experience, infant, mother–infant relations, social, weaning
1. INTRODUCTION
In mammals, maternal care is a prerequisite for the young's survival as the dam is the young's primary source of food, warmth, and security (Newberry & Swanson, 2008; Nowak et al., 2000; Poindron, 2005). Receiving an appropriate level of maternal care is key for the development of the young's social skills, such as the development of proper agonistic interactions in the mice, Mus musculus (Branchi et al., 2013). High levels of licking and allogrooming increased rodent pups’ time spent in contact with an unfamiliar peer (Starr‐Phillips & Beery, 2014), improved its social learning abilities (Lindeyer et al., 2013) and general learning abilities in low‐stress conditions (Champagne et al., 2008), and increased its time spent to evaluate the risks of a social situation (van Hasselt et al., 2012). Receiving high levels of maternal care also reduced a rodent pup's fear response in stressful situations (Beery & Francis, 2011; Menard et al., 2004), its thermal sensitivity (Walker et al., 2008), and slowed down its rate of behavioral changes (Franks et al., 2015).
The complexity of the early social environment has also been shown to alter social ontogeny and to impact neonates’ social behavior in various species. Maternal deprivation increased a goat kid's (Capra hircus) behavioral reactivity to stressful situations and decreased its neophobia toward social and nonsocial stimuli (Lyons et al., 1988; Toinon et al., 2021). The mere absence of the mother and other adults in the social environment heightened the sensitivity to tactile stimulation in squirrel monkeys, Saimiri boliviensis boliviensis, and rhesus monkeys, Macaca mulatta (Mulholland et al., 2020), and increased the amount of self‐directed behavior performed by young rhesus monkeys, interpreted as a substitute for mother‐directed behavior (Champoux et al., 1991). Infant rhesus monkeys raised without their mothers also showed stronger affiliation with other infants, but played less frequently with peers than infants reared with their mothers, and tended to be more often excessively impulsive compared with mother‐reared infants (Suomi, 2009). In chimpanzees, Pan troglodytes, the presence of the mother helped the development of appropriate play behavior (van Leeuwen et al., 2014), reconciliation, and consolation behaviors (Clay & De Waal, 2013). Similarly, calves (Bos taurus) reared with their mother until weaning were more attentive to their social environment and more prone to initiate social play with unknown peers than calves separated from their dams at birth and reared with other calves (Wagner et al., 2013). Calves reared with their mothers for 2 weeks after birth also had a broader social repertoire and interacted more with an unknown peer than calves separated from their mothers at 2 days of age (Flower & Weary, 2001). When reared with their mothers, other ewes, and same‐age peers, lambs (Ovis aries) exchanged less affiliative behavior, such as social play, sniffing, and rubbing each other with peers present in their home‐pen than lambs reared without their mothers, but lambs reared with their mothers showed more appropriate behavior in a social discrimination test than lambs reared without their mothers (Napolitano et al., 2003). Moreover, calves and lambs reared without their mothers often show abnormal oral behavior, such as non‐nutritive sucking activity (Jensen, 2003; Napolitano et al., 2008). Hence, maternal deprivation can impact young's social and nonsocial ontogeny.
The changes in social behavior can also be long lasting. High levels of licking and allogrooming increased rodent pups’ propensity to deliver maternal care to their own pups later in life (Franks et al., 2015). Differences between nursery‐reared rhesus monkeys and monkeys reared with their mothers were still observable when the animals were 18–36 months old (Sackett, 1965; Winslow et al., 2003). Nursery‐reared rhesus monkeys were involved in more agonistic interactions and abnormal behaviors, shorter affiliative behavior, and were less able to benefit from the presence of a cage mate as social support during a stressful situation than monkeys reared with their mothers (Sackett, 1965; Winslow et al., 2003). Maternal deprivation also has long‐term effects on ruminants’ social behavior. Primiparous dairy cows reared with their mothers seemed to have a stronger motivation to reinstate contact with their peers when isolated compared with cows that have been separated at birth (Wagner et al., 2015). Nulliparous dairy cows that had been reared with their mothers for 3 months after birth also showed more lower head postures characteristic of submissive behavior when introduced into the lactating herd than individuals that had been separated from their dam and reared with peers right after birth (Wagner et al., 2012). This suggests that animals reared with their mothers developed higher social skills than cows reared without their mothers (Wagner et al., 2012) supported by similar findings in calves (Buchli et al., 2017). Primiparous goats that had been reared with their dams until weaning also displayed higher social skills by showing greater social cohesion with known peers when integrated into a herd of unknown lactating goats (Szabò et al., 2013). Therefore, the rearing systems of young mammals can alter their social ontogeny, with possible long‐term effects on the social behavior and welfare of these animals. Unfortunately, despite the aforementioned studies, the effects of maternal deprivation on social ontogeny have barely been researched in domestic cattle and goats, for which early separation from the dam a few days after birth is a common farming practice.
In this study, we investigated the effect of the early social environment and maternal deprivation on goat kids’ social behavior. We studied the social behavior of goat kids reared with their mothers in a herd, with adult goats and their kids, compared with goat kids separated from their dams shortly after birth and reared only with peers of a similar age. We hypothesized that before weaning, kids reared with their dams and other individuals would direct most of their affiliative behaviors toward their dams, whereas kids reared without their dams would redirect those social behaviors toward their peers. We also expected that some differences would persist after being weaned and mixed with unknown peers, and kids reared without their dams would interact differently and show lower social skills than kids reared with their dams.
2. ANIMALS, MATERIALS, AND METHODS
All procedures were discussed and approved by the institutional ethics and animal welfare committee of the University of Veterinary Medicine, Vienna, following the guidelines for Good Scientific Practices and the national legislation (project number ETK‐051/03/2019).
2.1. Animals and housing
The study was conducted at a commercial organic dairy goat farm in Austria. The lactating goats were milked twice daily, from 04:30 to 06:00 h and from 16:30 to 18:00 h. Fresh hay was distributed to the animals every 36 h and concentrate three times daily. The animals were housed on deep litter straw in two rectangular pens (36.0 × 4.4 m) facing each other, separated by a raised feeding table. These pens were divided with adjustable 1.1 m high and either 2.75 m or 1.85 m wide metal fences to separate experimental groups (artificially reared [AR], dam reared [DR]—see below), goat kids according to different nutritional stages (preweaning, weaning), or goats in different reproductive stages (preparturient, postnatal, lactating). The adjustable fences allowed visual and physical interactions with other individuals housed in the adjacent pens and were frequently moved to adjust the size of each pen to fit the changing number of individuals in each pen at the beginning of the study. The goats gave birth in the preparturient pens before being brought to the postnatal pen to facilitate the ingestion of colostrum by the kids until allocation to treatment. The number of goats and kids present in the postnatal pen on 1 day depended on the number of goats having given birth within the last 3 days.
The study focused on 40 kids of the Saanen breed born from 25 different mothers. These kids were all healthy females born over 17 days, on the same day as at least another healthy female kid for pair‐matched allocation to the two treatments. At 2 days of age, the kids were weighed and marked using long‐lasting hair‐dye to facilitate individual recognition during behavioral observations. The kids’ ID was alphanumerical, with the letter representing the order of birth within one treatment. The kids excluded from the study because of being male, having health issues, or being the single female born on one day stayed unmarked and were mixed with their dam in the lactating herd at 3 days of age as per usual practice on the farm.
2.2. Treatment allocation
The 40 kids were allocated to one of two treatments 3 days after birth (Figure 1), when they were between 59 and 83 h old, following a randomized matched‐pair design. Treatments were balanced for kids’ age and weight at 2 days of age (average weight ± standard deviation: 4.27 ± .70 kg for the AR kids, 4.30 ± .62 kg for the DR kids). If female twins were born, they were separated and allocated one to each treatment, which occurred for 64.5% of the kids. All the allocation happened in the evening, during evening milking.
FIGURE 1.
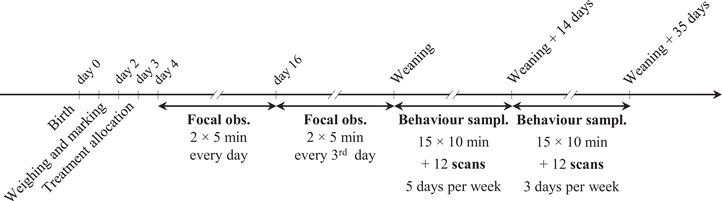
Timeline of the experiment indicating the main procedures and behavioral observations done on the focal kids. obs., observations; sampl., sampling
2.2.1. Artificial rearing
Twenty kids were AR. They were separated from their dams at 3 days of age and reared together in a pen, visually and physically separated from any other individual. They were fed from teat buckets filled with 40°C whole goat milk three times per day, at 06:30, 11:30, and 17:00 h. The amount of milk distributed was calculated for the kids to aim for complete satiation after each meal. Each meal was supervised to ensure that all the kids found a teat to suckle and, after treatment allocation, the kids were helped to feed from the teat buckets until they were able to suckle properly on their own. The group size increased from two to 20 kids over 17 days as the kids were born and allocated to treatment. The adjustable fences were moved to add 0.6 m2 to the original 11.2 m2 for every kid introduced in the group.
2.2.2. Dam rearing
The other 20 kids were DR. They were kept with their dam in the lactating herd along with other adult goats and kids from allocation to treatment until weaning, in a group size fluctuating between five and 56 dams and between 12 and 50 kids. The DR kids fed by suckling their dam ad libitum. When a dam and its kids were introduced in the lactating herd, the adjustable fences were moved to add 1.8 m2 to the lactating pen floor space. The kids reared in the lactating herd also had access to a 10 m2 crèche‐area not accessible to adults. Twice a day, the adults were moved out of the pen for milking and the kids were left only with other DR kids in the pen.
2.3. Weaning
Weaning occurred in two different batches, with half of the kids of each treatment weaned in each batch. To be weaned, the kids had to be at least 6 weeks old and weigh at least 15 kg. Due to losses before weaning, only 35 kids were weaned. Kids of the first batch (N AR = 8, N DR = 8) were weaned at 53 days of age on average (ranging from 43 to 58 days of age), whereas kids of the second batch (N AR = 10, N DR = 9) were weaned at 58 days of age on average (ranging from 55 to 72 days of age). DR kids were weaned at 55 ± 7 days of age and AR kids were weaned at 56 ± 3 days of age. The morning of the weaning day, the kids to be weaned were retrieved from their home pen, brought in a new pen and mixed with kids of the other treatment weaned on the same day. From the weaning day on, the kids remained in their group and pen, and only drank water and fed on the same diet as adult goats.
2.4. Behavioral observations
All behavioral observations were performed by one trained observer using Animal behavior pro 1.4.4 (Newton‐Fisher, 2012). The observer was trained by performing live observation and video coding of alpine goats and kids from another farm until reaching at least 80% intraobserver reliability on each video. During the observation sessions, the observer stood on the feeding table about 1 m outside of the kids’ home‐pen and could move on that line if necessary. The animals were able to see but not interact with the observer. The ethogram (Table 1) was based on preexisting literature (Andersen et al., 2011; Collias, 1956; Miranda‐de la Lama & Mattiello, 2010; Rudge, 1970; Szabò et al., 2013) and preliminary observations on another herd of goats (Toinon et al., 2019), focusing on affiliative behaviors seen between dams and kids or other affiliated goats, such as rubbing, grooming, and staying in physical contact, and social play behavior mainly displayed by kids, such as play‐fighting, mounting‐on, and racing. Agonistic behaviors with and without physical contact were also included in the ethogram as well as the nonsocial and inactive behavior resting alone.
TABLE 1.
Behaviors recorded during focal observation before weaning and behavior sampling after weaning
| Category | Behavior | Definition | Type of observations |
|---|---|---|---|
| Affiliative behaviors | Rubbing a , b | The initiator scrape its head, horns, or neck toward the passive receiver's head, horns, neck, or body, without causing the recipient withdrawal. | Duration before and frequency after weaning |
| Allogrooming a , b | The initiator uses its tongue, lips, or teeth to scrape the head or body, except vulva and anus, of the recipient without causing its withdrawal. | Duration before and frequency after weaning | |
| Standing in contact a | The focal kid is in physical contact with another individual while standing with its four legs in contact with the floor. If one leg is the only part in contact with the other individual, at least the whole half‐leg must be in contact. | Duration before and frequency after weaning | |
| Lying in contact a , b | Two individuals are in physical contact while having their ventral surface at least partially in contact with the floor. | Duration before and number of scans with occurrence after weaning | |
| Sucking a | One kid sucks the udder of an adult individual or groom the inguinal region of another kid. | Duration before weaning | |
| Social play behaviors | Play fighting a , b | Two individuals simulate a fight without causing the withdrawal of one of the individuals, making contact with their foreheads or clashing their foreheads without strength, eventually pushing each other without strength or circling each other, often interspersed with affiliative behavior. | Duration before and frequency after weaning |
| Stepping‐on a , b | The initiator is standing while having one leg or the torso in contact with the back of the receiver's body, without causing its withdrawal. | Duration before and frequency after weaning | |
| Racing | Two kids run side‐by‐side simultaneously. | Duration before and frequency after weaning | |
| Agonistic behaviors | Agonistic without physical contact a , b | Any avoidance behavior (Szabò et al., 2013) where one individual (the receiver) withdraws when another (the initiator) approaches it or threatens it by walking or moving the head quickly toward it, presenting its horns or forehead, or manifesting biting. | Frequency before and after weaning |
| Agonistic with physical contact a , b | Any physical interaction where one individual (the initiator) causes a conspecific (the receiver) to withdraw by biting the receiver, or hitting the receiver's body or clashing the receiver's head with its forehead. | Frequency before and after weaning | |
| Intervention a , b | The intervenor interferes in an agonistic interaction with physical contact between two other individuals by placing itself between the two opponents, without displaying agonistic interaction with physical contact itself. | Frequency before and after weaning | |
| Nonsocial behavior | Resting alone | The focal kid is lying without being in physical contact with any other individuals. | Duration before weaning |
| Other nonsocial | The focal kid is not lying nor interacting with another individual. | Duration before weaning |
Behavior for which the kind of partner involved in the interaction (kid or adult) was recorded before weaning if the focal kid was dam reared.
Behavior for which the identities of the donor and receiver (or partners in case of play‐fight and lying in contact) was recorded after weaning.
2.4.1. Before weaning
Before weaning, social behavior was observed live by continuous focal sampling, where all the behaviors listed in the ethogram (Table 1) and shown by one specific individual during a 5‐min observation session were recorded. Each kid was observed for 5 min twice a day every day for the 12 first days after treatment allocation, and then every third day until weaning. Therefore, the number of observations per day varied throughout the study, but the observation schedule was planned to be able to fit the observation of all 40 kids every day. Observations were split between four periods between 07:00 and 11:10 h, and four periods between 12:10 and 16:30 h (Figure 2). On day 1 of the study, each kid ID was allocated to one period of observation in the morning and one period in the afternoon, with approximately 5 h between observations. Each period included up to five potential sessions of observations of AR kids and five potential sessions of observations of DR kids (Figure 2). Whether AR kids were observed before or after DR kids within one period of observation was randomized as well as the exact time one kid was observed. This allocation alternated in a predetermined order every day so that each kid was observed during all periods over 4 days of observation.
FIGURE 2.
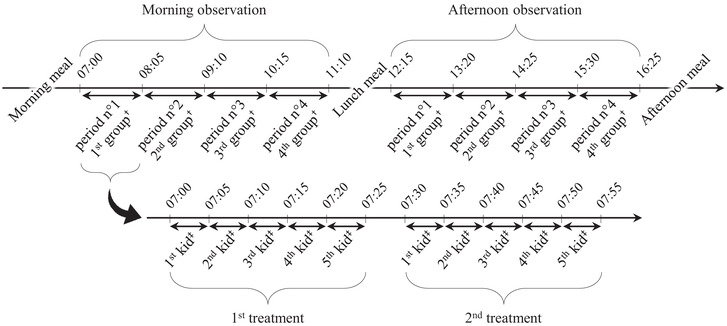
Schedule of one day of observations before weaning. †, Predetermined and alternating every day; ‡, randomized; meal, distribution of milk to the artificially reared kids
For each affiliative, social play and agonistic behavior (Table 1), it was recorded whether the kid's partner was an adult or a kid. For each rubbing, allogrooming, stepping‐on, and agonistic interaction, the role of the focal kid was recorded as initiator or receiver.
2.4.2. After weaning
After weaning, the observations were conducted between 08:00 and 11:10 h in the morning and between 12:10 and 15:20 h in the afternoon. Each batch was observed through 15 sessions of 10 min live continuous behavior sampling and 12 scan sampling sessions per day at 30 min intervals, 5 days per week for the first 2 weeks after weaning, and 3 days per week the following 3 weeks (Figure 1). During the continuous behavioral observations, the whole group of kids was observed at the same time to record all occurrences of rubbing, allogrooming, stepping‐on, social play, and agonistic interactions (Table 1) in one observation session as well as the identities of the donor and receiver for each interaction. During the scan sampling sessions, the identities of the kids lying in physical contact were recorded.
2.5. Statistical analysis
Statistical analyses were performed using RStudio (version 1.2.5033; RStudio Team 2019). Generalized linear models and generalized linear mixed models were used to estimate the effects of treatment on each of the response variables measured (Baayen, 2008). The effects of fixed and random factors detailed below were also estimated but the strength of the effects is not to be discussed in this paper.
2.5.1. Before weaning
Each behavior observed before weaning (Table 1) was considered a distinct response variable. Each response variable was analyzed using a generalized linear mixed‐effects model, with each observation being one data point in each model. The proportions of time lying in contact and resting alone during each observation session were calculated and beta‐transformed to fit in the open interval (0,1) before being analyzed using Beta regression (Cribari‐Neto & Zeileis, 2010). Whether the different behaviors, rubbing, allogrooming, standing in contact, play‐fighting, and stepping‐on, occurred or not in each observation session was analyzed using binomial regressions as these behaviors were not observed frequently enough to be analyzed using a beta‐regression. The frequencies of agonistic interactions with and without physical contact during each observation session were analyzed using a negative‐binomial regression to avoid models’ overdispersion found with Poisson regression.
Each model included treatment (DR; AR), partner (kid; adult), and role (initiator; receiver) as fixed effects as well as age, group size, and time of the day as z‐transformed continuous variables to ease the interpretation of the model (Schielzeth, 2010). Kids and their dams were included as random effects. To avoid the models being overconfident regarding the precision of fixed effects estimates, and to keep type I error rate at the nominal level of 5%, partner (if recorded), role (if recorded), age, group size, and time of the day within kid and dam were included as random slopes (Barr et al., 2013; Schielzeth & Forstmeier, 2009).
For each response variable, the effect of treatment was tested by conducting a full‐null model comparison, with the null model only differing from the full model by lacking the treatment. The full and the null models were compared using a likelihood ratio test (Dobson, 2002). The assumptions of normality and homogeneity of the residuals of each fitted model were checked by visual inspection of a QQ‐plot (Field, 2005) of the residuals and the residuals plotted against the fitted values (Quinn & Keough, 2002). Each model was also checked for overdispersion and collinearity problems between the fixed effects (Quinn & Keough, 2002). The stability and standard deviation of each model on the level of the estimated coefficients were checked by excluding the levels of the random effects one at a time (Nieuwenhuis et al., 2012).
Sucking and suckling were only recorded in one out of the two treatments and racing did not happen often enough to be analyzed using a generalized linear mixed‐effects model and are instead reported descriptively.
2.5.2. After weaning
Whether or not each dyad was observed lying in contact during each scan sampling session was analyzed using a binomial generalized linear mixed‐effects model. The frequency at which every other behavior occurred during each session of behavior sampling was analyzed using negative binomial generalized linear models. The models included treatments of the kids interacting, batch, the z‐transformed variables of day and time, and the interactions between treatments and day as fixed effects. The interaction between treatments and day was included as we expected the difference between treatments to vary across the days after weaning. For all variables, except play‐fight and lying in contact, the interactions were directed, meaning that for each interaction, an initiator and a receiver were identifiable, and the fixed‐effect treatments was a concatenation of the initiators’ and the receivers’ treatments, in that order. For play‐fight and lying in contact, the fixed‐effect treatments was either two AR kids interacting, two DR kids interacting, or one AR kid interacting with a DR kid. For the variable lying in contact, the random effects dyad (goats lying in contact) and dam and the random slopes day and time within kid and dam were also included. After fitting a model, overdispersion and collinearity problems were checked (Quinn & Keough, 2002). The stability and standard deviation of each model were also assessed by excluding the levels of the random effects one at a time (Nieuwenhuis et al., 2012).
3. RESULTS
3.1. Before weaning
Play‐fight occurred more in AR kids than in DR kids (full‐null model comparison: χ 2 = 19.3, df = 1, p < .001; Figure 3(a)), and the occurrence of play‐fight decreased with increasing age and increased with increasing group size (age: χ 2 = 19.9, df = 1, p < .001; group size: χ 2 = 4.0, df = 1, p = .04).
FIGURE 3.
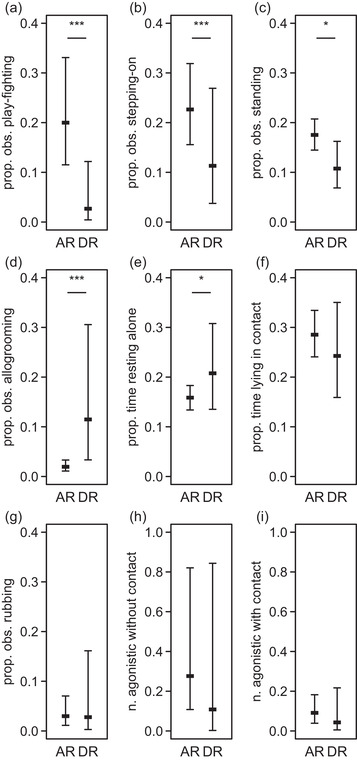
Plot of estimated means and confidence intervals of observed behaviors before weaning. AR, artificially reared kids; DR, dam‐reared kids; n., number; prop., proportion; obs., observation. (a) Proportion of observations with play‐fighting bouts (in total 190 observations with play‐fight bouts, out of 1824 observations on 38 kids). (b) Proportion of observations with stepping‐on bouts (in total 473 observations). (c) Proportion of observations with standing in contact bouts (in total 235 observations). (d) Proportion of observations with allogrooming bouts (in total 422 observations). (e) Proportion of time resting alone (total duration = 860 min). (f) Proportion of time lying in contact with peers (total duration = 3644 min). (g) proportion of observations with rubbing bouts (in total 112 observations). (h) Frequency of agonistic interactions without physical contact per 5 min (in total 166 interactions observed). (h) Frequency of agonistic interactions with physical contact per 5 min (in total 204 interactions observed)
Stepping‐on another individual occurred more in AR kids than in DR kids (full‐null model comparison: χ 2 = 6.4, df = 1, p = .01; Figure 3(b)) occurred less between two DR kids than between one DR kid and one adult (χ 2 = 6.3, df = 1, p = .01), kids were more often stepped‐on than stepping‐on (χ 2 = 21.9, df = 1, p < .001), decreased with increasing age (χ 2 = 8.9, df = 1, p = .003), and tended to increase with group size (χ 2 = 3.3, df = 1, p = .07).
Standing in contact with another individual occurred more in AR kids than in DR kids (full‐null model comparison: χ 2 = 4.2, df = 1, p < .001; Figure 3(c)) happened more between two DR kids than between one DR kid and one adult (χ 2 = 26.2, df = 1, p < .001), and group size and age were not significant.
Allogrooming occurred less in AR kids than in DR kids (full‐null model comparison: χ 2 = 17.5, df = 1, p < .001; Figure 3(d)), kids were receiving these interactions less than they initiating them (χ 2 = 12.5, df = 1, p < .001), the occurrence of allogrooming decreased with increasing age and group size (age: χ 2 = 6.3, df = 1, p < .01; group size: χ 2 = 22.1, df = 1, p < .001), and partner was not significant.
Resting alone was shorter in AR kids than in DR kids (full‐null model comparison: χ 2 = 3.8, df = 1, p = .05; Figure 3(e)), duration of resting alone increased with increased age and decreased with increased group size and (age: χ 2 = 4.3, df = 1, p = .04; group size: χ 2 = 5.4, df = 1, p = .02).
Lying in physical contact with another individual was longer when two DR kids were in contact than when one DR kid was in contact with one adult (χ 2 = 120.9, df = 1, p < .001; Figure 3(f)), lying in contact tended to increase with increased group size (χ 2 = 2.7, df = 1, p = .10), and treatment and age were not significant.
Rubbing another individual was longer when received than when initiated by the focal kid (χ 2 = 10.4, df = 1, p < .001; Figure 3(g)), and treatment, partner, group size, and age were not significant.
Agonistic interactions with and without physical contact happened more frequently between one DR kid and one adult than between two DR kids (with contact: χ 2 = 6.9, df = 1, p = .02; Figure 3(h); without contact: χ 2 = 43.9, df = 1, p < .001; Figure 3(i)), kids were more often receivers than initiator of agonistic interactions with physical contact (χ 2 = 5.8, df = 1, p = .05), and treatment, group size, and age were not significant.
Time of observation was only significant for other nonsocial behaviors (χ 2 = 11.4, df = 1, p = .001) as the kids explored more over the course of the day, and nonsignificant for all other behaviors.
Racing was observed 36 times between AR kids and once between DR kids, sucking was observed eight times in AR kids, and suckling was observed 148 times in DR kids for a total duration of 81 min.
3.2. After weaning
After weaning, treatments were mixed with each other in the groups, and agonistic interactions with physical contact occurred more frequently between a DR kid initiating this interaction and an AR kid receiving it (treatment DR→AR: Z = 5.9, p < .001; Figure 4(a)) and between two DR kids (treatment DR→DR: Z = 5.4, p < .001) than between an AR initiator and a DR receiver or between two AR kids. However, across the weeks, the occurrence of agonistic interactions with physical contact increased between an AR initiator and a DR kid receiver (day × AR→DR: Z = 5.5, p < .001) and, very slightly, between two DR kids (day × DR→DR: Z = 2.7, p = .01), whereas the occurrence of these agonistic interactions decreased between two AR kids and between a DR initiator and an AR receiver (day: Z = 4.8, p < .001; day × DR→AR: Z = 1.7, p = .09; Figure 4(a)). The second batch showed more agonistic interactions with physical contact than the first batch (Z = 4.8, p < .001), and time of the day was not significant.
FIGURE 4.
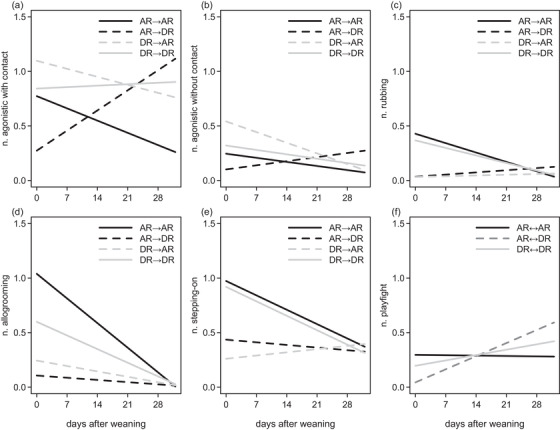
Regression lines of the behaviors observed after weaning. n., number; AR, artificially reared kid; DR, dam‐reared kid; →, orientation of directed interactions between two kids, first treatment is initiator, second is receiver; ↔, undirected interactions between two kids. (a) Frequency of agonistic interactions with physical contact (in total 1475 interactions, out of 570 observations on 35 kids). (b) Frequency of agonistic interactions without physical contact (in total 515 interactions). (c) Frequency of rubbing bouts (in total 207 interactions). (d) Frequency of allogrooming bouts (in total 285 interactions). (e) Frequency of stepping‐on bouts (in total 937 interactions). (f) Frequency of play‐fight bouts (in total 334 interactions)
Agonistic interactions without physical contact occurred more frequently between a DR initiator and an AR receiver (treatment DR→AR: Z = 3.4, p < .001) and between two DR kids (treatment DR→DR: Z = 2.7, p = .01) than between an AR initiator and a DR receiver or between two AR kids. However, the occurrence of agonistic interactions without physical contact increased across the weeks between an AR initiator and a DR kid receiver (day × AR→DR: Z = 3.6, p < .001; Figure 4(b)), whereas it decreased between two AR kids, between a DR initiator and an AR receiver, or between two DR kids (day: Z = −2.5, p < .01). The second batch showed more agonistic interactions without physical contact than the first batch (Z = 6.4, p < .001), and time of the day was not significant.
Rubbing occurred more frequently between two DR kids and between two AR kids than between one AR kid and one DR kid (treatment AR→DR: Z = −3.0, p = .003; treatment DR→AR: Z = −4.0, p < .001), and the frequency of an AR kid stepping on another AR and of a DR kid rubbing another DR kid decreased across the weeks (day: Z = −4.0, p < .001; Figure 4(c)), but the frequency of a DR kid rubbing an AR and of an AR kid rubbing a DR kid, increased across the weeks (day × DR→AR: Z = 3.2, p = .001; day × AR→DR: Z = 4.2, p < .001). Batch and time of the day were not significant.
Allogrooming occurred more frequently between two DR kids, between two AR, and between one DR initiator and one AR receiver than between one AR initiator and one DR receiver (treatment AR→DR: Z = −3.0, p = .002), and the frequency of allogrooming decreased across the weeks (day: Z = −7.5, p < .001; Figure 4(d)), but the frequency of allogrooming including a DR kid decreased less across the weeks than the frequency of allogrooming including two AR kids (day × DR→AR: Z = 2.6, p = .007; day × AR→DR: Z = 3.0, p = .003; day × DR→DR: Z = 2.5, p = .01). The second batch allogroomed more than the first one (Z = 3.1, p = .002) and allogrooming decreased over the course of a day (Z = −3.5, p < .001).
Stepping‐on another kid occurred more frequently between two DR kids and between two AR kids than between one AR kid and one DR kid (treatment AR→DR: Z = −4.1, p < .001; treatment DR→AR: Z = −5.4, p < .001), and the frequency of an AR kid stepping on another AR, of an AR kid stepping on a DR kid, and of DR kid stepping on another DR kid decreased across the weeks (day: Z = −3.1, p = .002; Figure 4(e)), but the frequency of a DR kid stepping on an AR increased across the weeks (day × DR→AR: Z = 3.1, p = .002). The second batch stepped‐on more frequently than the first batch (Z = 3.3, p < .001) and the frequency increased over the course of a day (Z = −2.7, p = .007).
Play‐fighting occurred more frequently between two DR and between two AR kids than between a DR and an AR kid (DR↔AR: Z = −2.9, p = .004), and the frequency of play‐fight bouts between a DR and an AR kid increased across the weeks (day × DR↔AR: Z = 3.1, p < .001; Figure 4(f)), but the frequency of interactions between two DR kids or between two AR kids did not increase across the weeks. Play‐fight frequency decreased over the course of a day (Z = −3.9, p < .001), and batch was not significant.
Lying in contact happened as frequently between two DR kids and two AR kids, but happened less between one DR kid and one AR kid than between two DR kids or two AR kids (full‐null model comparison: χ 2 = 275.9, df = 4, p < .001; Figure 5), and the occurrence of two DR kids and two AR kids lying in contact decreased across the weeks, but the occurrence of one DR kid and one AR kid lying in contact increased (χ 2 = 95.7, df = 2, p < .001). Lying in contact decreased over the course of a day (Z = −4.4, p = .03), and batch was not significant.
FIGURE 5.
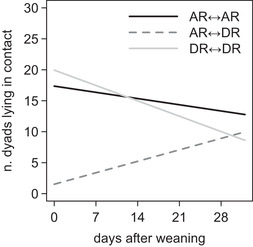
Regression lines of the number of dyads observed lying in contact after weaning on one session of scan sampling. n., number; AR, artificially reared kid; DR, dam‐reared kid; ↔, undirected interactions between two kids
4. DISCUSSION
4.1. Before weaning
Before weaning, AR kids were observed performing more play‐fighting, racing, stepping on each other, and standing in contact with each other than DR kids, but AR kids spent less time resting alone and allogroomed less than DR kids.
After allocation to the treatments at 3 days of age, DR kids experienced maternal care such as allogrooming. AR kids did not experience maternal care, and they did not redirect allogrooming toward their peers either, but play‐fought more than DR kids. Although play behavior is generally considered an indicator of positive welfare in farm animals (Held & Špinka, 2011; Lawrence, 1987), AR kids might have performed play‐fight as a form of coping strategy to deal with the absence of their primary caretakers and the lower level of maternal care received. Indeed, play‐behavior can induce positive affective states (Held & Špinka, 2011; Mason et al., 1963; Pellis & Pellis, 1998, 2013; Trezza et al., 2011; Vanderschuren, 2010; Vanderschuren et al., 2016), and a decrease in maternal care can induce an increase in play behavior in kittens, Felis catus (Bateson et al., 1981; Bateson et al., 1990; Bateson & Young, 1981), rat pups, Rattus norvegicus (Franks et al., 2015; Parent & Meaney, 2008; Smith, 1991; van Hasselt et al., 2012; Veenema & Neumann, 2009), and rhesus monkey yearlings (Devinney et al., 2003). In rhesus monkeys, this increase in play frequency is correlated with a decreased proportion of time in a tense state (Devinney et al., 2003). Receiving high levels of maternal care also reduces playful dominance‐related behavior in rat pups (Parent et al., 2013). It could also be that the presence of adults decreased the propensity of DR kids to play‐fight as play‐fighting could disturb an adult and result in an agonistic interaction, as seen in rhesus monkeys (Harlow, 1969) and mice pups in the presence of their pregnant mothers (Smith, 1991). In contrast, calves reared with access to their dam and other cows showed much more locomotor play compared with calves separated from the dam; possibly due to larger space for play especially while cows were out of the barn for milking (Waiblinger et al., 2020). Unfortunately, we did not observe DR kids’ propensity to play‐fight when the adults were out of the home‐pen during milking. The higher occurrence of play‐fight in AR kids might also be a strategy to compensate for the lack of social enrichment that they experienced compared with DR kids. Indeed, social play facilitates the development of skills necessary for survival in the wild, including social skills (Vanderschuren & Trezza, 2013). In the male rat, experiencing social play is essential to develop appropriate social behavior (Van Den Berg et al., 1999). Rats socially isolated during the period when play behavior normally peaks displayed inappropriate behavior during a social encounter, hence experiencing more aggression (Van Den Berg et al., 1999; Vanderschuren et al., 2016; Von Frijtag et al., 2002). The higher occurrence of play‐fight in AR kids compared with the DR kids could also be the result of the lower amount of time AR kids spent sucking and allogrooming during the observation period. Indeed, DR kids could feed via suckling their dams during the observations, whereas AR could not and had their meals outside of observation time.
The higher amount of time standing in contact with each other and playing with each other in AR kids compared with DR kids may also be a redirection of their social behavior toward peers, as reported in sheep and rhesus monkeys reared in maternal deprivation (Napolitano et al., 2008; Suomi, 2009) and human orphans experiencing low interactions with adults (Kaler & Freeman, 1994). Alternatively, the longer time spent resting alone and the lower occurrence of standing in contact in DR kids compared with AR kids could be the result of the physical and social environment of DR kids. Indeed, DR kids were included in a dominance‐subordinate relationship with all adults of the lactating herd, and kids are usually at the bottom of the hierarchical order (Miranda‐de la Lama & Mattiello, 2010). Therefore, DR kids could potentially experience displacement by any other adult (Miranda‐de la Lama & Mattiello, 2010) and being able to find a location to sleep and stand without being disturbed might have conflicted with the motivation to stay in contact or proximity with the dam or affiliated peers, as the pen size restricted individual space (Bøe et al., 2013). Hence, DR kids could have spent more time resting alone and were less standing in contact with other individuals than AR kids to avoid interacting with the adults present in their home‐pen.
Replicating the study with a larger sample size and with observations of the behavior of DR kids during milking of the adults could help disentangle whether AR kids play‐fought, stood in contact, and lied in contact more than DR kids as a strategy to cope with the absence of dams or whether DR kids reduced their activity to avoid agonistic interactions with adults.
4.2. After weaning
After weaning and mixing with unfamiliar peers, AR kids received more and initiated less agonistic interactions with and without physical contact than DR kids. Receiving more agonistic interactions might have reduced AR kids’ ability to access some resources such as food or preferred lying areas (Correa et al., 2010), thus reducing the welfare of AR kids. The number of agonistic interactions displayed by one DR kid toward one AR kid, or between two AR kids, was highest immediately after weaning and decreased over time. A peak in agonistic interaction between unfamiliar animals after weaning is in agreement with the literature in goats as mixing unfamiliar animals leads to an increased number of agonistic interactions in adults until new relationships are established (Correa et al., 2010; Fernández et al., 2007; Patt et al., 2012, 2013b, 2013a; Waiblinger et al., 2017). The number of agonistic interactions displayed between two DR kids was stable across the weeks, whereas the number of agonistic interactions displayed by an AR kid toward a DR kid increased across weeks. Although the stability of agonistic interactions between DR kids was expected as they already knew each other, it seems that, in contact with DR kids, AR kids developed their agonistic behavioral repertoire and preferentially orientated it toward DR kids, which whom they were less familiar with and thus needed to establish a relationship with (Waiblinger et al., 2017). Such adjustment in social behavior to match mother‐reared peers has been shown in rhesus monkeys reared in social isolation until mixing with mother‐reared individuals (Griffin & Harlow, 1966; Suomi & Harlow, 1972). However, the fact that AR kids had to adjust their agonistic social behavior by increasing it to match DR kids after weaning shows that the higher amount of play‐fight displayed by AR kids compared with DR kids before weaning did not enable AR kids to compensate for the lack of interactions with adults.
Soon after weaning, AR and DR kids displayed a lot of allogrooming, rubbing, and stepping on toward familiar individuals of their own treatment, but the frequency of those behaviors decreased across weeks, and they were rarely observed 4 weeks after weaning. The high frequency of those types of affiliative interactions at weaning might be a coping mechanism to reduce the stress induced by weaning as weaning is a highly stressful period when the kids have to abruptly switch from milk to solid food while being moved in a new environment and mixed with unknown peers (Napolitano et al., 2008). Interacting with peers in an affiliative way may have elicited social support and therefore decreased the level of stress experienced (Rault, 2012). These findings are in accordance with the literature interpreting rubbing and allogrooming as affiliative behaviors (Andersen et al., 2011). Although stepping on peers is not commonly reported as a social behavior in goats, it followed the same pattern of change over time as allogrooming, rubbing, and lying in contact in the present study. We interpret this behavior of stepping on another conspecific as indicative of social tolerance as the kid lying down had at least to tolerate the presence of the mounting kid within its individual distance without moving away or retaliating toward the intruder (Bøe et al., 2013). As it implies for one kid to come in contact with a lying peer, such behavior could be considered as an affiliative contact‐seeking behavior (Andersen et al., 2011; Aschwanden et al., 2008).
Although AR kids play‐fought and stepped on each other more than DR kids before weaning, no difference was found after weaning, suggesting that removing dams from their environment increased DR kids’ propensity to play‐fight. Moreover, play‐fight was the only affiliative and playful behavior that overall increased after weaning. In calves, solitary play peaks between 1 and 6 weeks of age (Duve et al., 2012), but social play peaks around 8 months of age and continues to be performed until 1 year of age (Reinhardt et al., 1978). Although play fighting has been studied in Siberian ibex kids, Capra ibex sibirica, which is classified in the same genus as the domestic goats, the peak period for social play was not studied (Byers, 1977, 1980), and play‐fight in the present study may have peaked after the end of the observations, beyond three months of age.
Affiliative interactions between kids of different treatments were less frequent than affiliative interactions between kids of the same treatments after weaning, but across weeks, kids play‐fought and lay in contact with kids from the other rearing treatment more often, suggesting they had become familiar with each other and affiliative relationships may have developed even between treatments. This is in line with previous studies in cattle, showing that preferential social relationships remain after mixing and take time to form between former unknown peers (Foris & Haas, 2021; Gutmann et al., 2015; Gygax et al., 2010; Nowak & Boivin, 2015; Rocha et al., 2020).
None of the differences between treatments found before weaning was found after weaning, but AR kids received more and initiated less agonistic interaction than DR kids. This difference faded across weeks as AR kids adjusted their agonistic social behavior by increasing it. Further study would be needed to determine if mixing with more aggressive peers such as DR kids is necessary for AR kids to increase their agonistic social behavior or if such an increase would also be shown by AR kids mixed with other AR kids. It would also be interesting to study the social behavior of these individuals in the longer term.
5. CONCLUSION
Goat kids reared without their mothers in groups of same‐age peers expressed different social behaviors compared with kids reared with their dams and other individuals. Before weaning, the kids reared with same‐age peers spent less time resting alone and showed less allogrooming, but play‐fought, raced, stepped‐on each other, and stood in contact with each other more than the kids reared with their dams. These differences were not found after weaning, suggesting that the presence of adults and experiencing maternal care decreases play‐fight and increases allogrooming in kids reared with their dams. However, after weaning and mixing rearing treatments with each other, kids reared with their dams initiated more and received less agonistic interactions than kids reared with same‐age peers, but this difference vanished across weeks, suggesting that kids reared with same‐age peers changed their social behavior after being in contact with kids reared with their dams.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENTS
We thank the farmers for their cooperation and help raising the kids, Mélaine Jannier for her help for the behavioral observations after weaning, Roger Mundry for his statistical advices, and Norbert Sachser for his advices throughout the experiment.
Toinon, C. , Waiblinger, S. , & Rault, J.‐L. (2022). Maternal deprivation affects goat kids’ social behavior before and after weaning. Developmental Psychobiology, 64, e22269. 10.1002/dev.22269
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Phaidra at https://phaidra.vetmeduni.ac.at/o:834, reference number o:834.
REFERENCES
- Andersen, I. L. , Tønnesen, H. , Estevez, I. , Cronin, G. M. , & Bøe, K. E. (2011). The relevance of group size on goats’ social dynamics in a production environment. Applied Animal Behaviour Science, 134(3–4), 136–143. 10.1016/j.applanim.2011.08.003 [DOI] [Google Scholar]
- Aschwanden, J. , Gygax, L. , Wechsler, B. , & Keil, N. M. (2008). Cardiac activity in dairy goats whilst feeding side‐by‐side at two different distances and during social separation. Physiology & Behavior, 95(5), 641–648. 10.1016/j.physbeh.2008.09.016 [DOI] [PubMed] [Google Scholar]
- Baayen, R. H. (2008). Analyzing Linguistic Data: A Practical Introduction to Statistics using R. Cambridge: Cambridge University Press. 10.1017/CBO9780511801686 [DOI] [Google Scholar]
- Barr, D. , Levy, R. , Scheepers, C. , & Tily, H. J. (2013). Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language, 68(3), 1–43. 10.1016/j.jml.2012.11.001.Random [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson, P. , Martin, P. , & Young, M. (1981). Effects of interrupting cat mothers’ lactation with bromocriptine on the subsequent play of their kittens. Physiology & Behavior, 27(5), 841–845. 10.1016/0031-9384(81)90051-2 [DOI] [PubMed] [Google Scholar]
- Bateson, P. , Mendl, M. , & Feaver, J. (1990). Play in the domestic cat is enhanced by rationing of the mother during lactation. Animal Behaviour, 40(3), 514–525. 10.1016/S0003-3472(05)80532-9 [DOI] [Google Scholar]
- Bateson, P. , & Young, M. (1981). Separation from the mother and the development of play in cats. Animal Behaviour, 29(1), 173–180. 10.1016/S0003-3472(81)80163-7 [DOI] [Google Scholar]
- Beery, A. K. , & Francis, D. D. (2011). Adaptive significance of natural variations in maternal care in rats: A translational perspective. Neuroscience and Biobehavioral Reviews, 35(7), 1552–1561. 10.1016/j.neubiorev.2011.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøe, K. E. , Ehrlenbruch, R. , Jørgensen, G. H. M. , & Andersen, I. L. (2013). Individual distance during resting and feeding in age homogeneous vs. age heterogeneous groups of goats. Applied Animal Behaviour Science, 147(1–2), 112–116. 10.1016/j.applanim.2013.04.024 [DOI] [Google Scholar]
- Branchi, I. , Curley, J. P. , D'Andrea, I. , Cirulli, F. , Champagne, F. A. , & Alleva, E. (2013). Early interactions with mother and peers independently build adult social skills and shape BDNF and oxytocin receptor brain levels. Psychoneuroendocrinology, 38(4), 522–532. 10.1016/j.psyneuen.2012.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchli, C. , Raselli, A. , Bruckmaier, R. , & Hillmann, E. (2017). Contact with cows during the young age increases social competence and lowers the cardiac stress reaction in dairy calves. Applied Animal Behaviour Science, 187, 1–7. 10.1016/j.applanim.2016.12.002 [DOI] [Google Scholar]
- Byers, J. A. (1977). Terrain preferences in the play behavior of Siberian Ibex kids (Capra ibex sibirica). Zeitschrift Für Tierpsychologie, 45(2), 199–209. 10.1111/j.1439-0310.1977.tb02117.x [DOI] [PubMed] [Google Scholar]
- Byers, J. A. (1980). Play partner preferences in Siberian Ibex, Capra ibex sibirica. Zeitschrift Für Tierpsychologie, 53(1), 23–40. 10.1111/j.1439-0310.1980.tb00731.x [DOI] [PubMed] [Google Scholar]
- Champagne, D. L. , Bagot, R. C. , van Hasselt, F. , Ramakers, G. , Meaney, M. J. , de Kloet, E. R. , Joëls, M. , & Krugers, H. (2008). Maternal care and hippocampal plasticity: Evidence for experience‐dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. Journal of Neuroscience, 28(23), 6037–6045. 10.1523/JNEUROSCI.0526-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux, M. , Metz, B. , & Suomi, S. J. (1991). Behavior of nursery/peer‐reared and mother‐reared rhesus monkeys from birth through 2 years of age. Primates, 32(4), 509–514. 10.1007/BF02381941 [DOI] [Google Scholar]
- Clay, Z. , & De Waal, F. B. M. (2013). Bonobos respond to distress in others: Consolation across the age spectrum. PLoS ONE, 8(1), e55206. 10.1371/journal.pone.0055206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collias, N. E. (1956). The analysis of socialization in sheep and goats. Ecology, 37(2), 228–239. [Google Scholar]
- Correa, C. M. , Zanela, M. B. , & Schmidt, V. (2010). Comportamento social de cabras em lactação após reagrupamento. Acta Scientiae Veterinariae, 38(4), 425–428. [Google Scholar]
- Cribari‐Neto, F. , & Zeileis, A. (2010). Beta Regression in R. Journal of Statistical Software, 34(2), 1–24. 10.18637/jss.v034.i02 [DOI] [Google Scholar]
- Devinney, B. J. , Berman, C. M. , & Rasmussen, K. L. R. (2003). Individual differences in response to sibling birth among free‐ranging yearling rhesus monkeys (Macaca mulatta) on Cayo Santiago. Behaviour, 140(7), 899–924. 10.1163/156853903770238373 [DOI] [Google Scholar]
- Dobson, A. J. (2002). An introduction to generalised linear models, third edition. Chapman & Hall/CRC. Retrieved from https://www.tandfonline.com/doi/full/10.1080/02664760802695900 [Google Scholar]
- Duve, L. R. , Weary, D. M. , Halekoh, U. , & Jensen, M. B. (2012). The effects of social contact and milk allowance on responses to handling, play, and social behavior in young dairy calves. Journal of Dairy Science, 95(11), 6571–6581. 10.3168/jds.2011-5170 [DOI] [PubMed] [Google Scholar]
- Fernández, M. A. , Alvarez, L. , & Zarco, L. (2007). Regrouping in lactating goats increases aggression and decreases milk production. Small Ruminant Research, 70(2–3), 228–232. 10.1016/j.smallrumres.2006.03.008 [DOI] [Google Scholar]
- Field, A. (2005). Discovering statistics using SPSS (2nd ed.), Sage Publications, Inc. [Google Scholar]
- Flower, F. C. , & Weary, D. M. (2001). Effects of early separation on the dairy cow and calf: 2. Separation at 1 day and 2 weeks after birth. Applied Animal Behaviour Science, 70(4), 275–284. 10.1016/S0168-1591(00)00164-7 [DOI] [PubMed] [Google Scholar]
- Foris, B. , & Haas, H. (2021). Familiarity influences social networks in dairy cows after regrouping. Journal of Dairy Science, 104(3), 3485–3494. 10.3168/jds.2020-18896 [DOI] [PubMed] [Google Scholar]
- Franks, B. , Champagne, F. A. , & Curley, J. P. (2015). Postnatal maternal care predicts divergent weaning strategies and the development of social behavior. Developmental Psychobiology, 57(7), 809–817. 10.1002/dev.21326 [DOI] [PubMed] [Google Scholar]
- Griffin, G. A. , & Harlow, H. F. (1966). Effects of three months of total social deprivation on social adjustment and learning in the rhesus monkey. Child Development, 37(3), 533. 10.2307/1126677 [DOI] [PubMed] [Google Scholar]
- Gutmann, A. K. , Špinka, M. , & Winckler, C. (2015). Long‐term familiarity creates preferred social partners in dairy cows. Applied Animal Behaviour Science, 169, 1–8. 10.1016/j.applanim.2015.05.007 [DOI] [Google Scholar]
- Gygax, L. , Neisen, G. , & Wechsler, B. (2010). Socio‐spatial relationships in dairy cows. Ethology, 116(1), 10–23. 10.1111/j.1439-0310.2009.01708.x [DOI] [Google Scholar]
- Harlow, H. F. (1969). Age‐mate or peer affectional system. Advances in the Study of Behavior, 2, 333–383. 10.1016/S0065-3454(08)60072-8 [DOI] [Google Scholar]
- Held, S. D. E. , & Špinka, M. (2011). Animal play and animal welfare. Animal Behaviour, 81(5), 891–899. 10.1016/j.anbehav.2011.01.007 [DOI] [Google Scholar]
- Jensen, M. B. (2003). The effects of feeding method, milk allowance and social factors on milk feeding behaviour and cross‐sucking in group housed dairy calves. Applied Animal Behaviour Science, 80(3), 191–206. 10.1016/S0168-1591(02)00216-2 [DOI] [Google Scholar]
- Kaler, S. R. , & Freeman, B. J. (1994). Analysis of environmental deprivation: Cognitive and social development in Romanian orphans. Journal of Child Psychology and Psychiatry, 35(4), 769–781. 10.1111/j.1469-7610.1994.tb01220.x [DOI] [PubMed] [Google Scholar]
- Lawrence, A. (1987). Consumer demand theory and the assessment of animal welfare. Animal Behaviour, 35(1), 293–295. 10.1016/S0003-3472(87)80236-1 [DOI] [Google Scholar]
- Lindeyer, C. M. , Meaney, M. J. , & Reader, S. M. (2013). Early maternal care predicts reliance on social learning about food in adult rats. Developmental Psychobiology, 55(2), 168–175. 10.1002/dev.21009 [DOI] [PubMed] [Google Scholar]
- Lyons, D. M. , Price, E. O. , & Moberg, G. P. (1988). Individual differences in temperament of domestic dairy goats: Constancy and change. Animal Behaviour, 36(5), 1323–1333. 10.1016/S0003-3472(88)80201-X [DOI] [Google Scholar]
- Mason, W. A. , Saxon, S. V , & Sharpe, L. G. (1963). Preferential responses of young chimpanzees to food and social rewards. The Psychological Record, 13, 341–345. 10.1007/BF03393535 [DOI] [Google Scholar]
- Menard, J. L. , Champagne, D. L. , & Meaney, M. J. P. (2004). Variations of maternal care differentially influence “fear” reactivity and regional patterns of cFOS immunoreactivity in response to the shock‐probe burying test. Neuroscience, 129(2), 297–308. 10.1016/j.neuroscience.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Miranda‐de la Lama, G. C. , & Mattiello, S. (2010). The importance of social behaviour for goat welfare in livestock farming. Small Ruminant Research, 90(1–3), 1–10. 10.1016/j.smallrumres.2010.01.006 [DOI] [Google Scholar]
- Mulholland, M. M. , Williams, L. E. , & Abee, C. R. (2020). Rearing condition may alter neonatal development of captive Bolivian squirrel monkeys (Saimiri boliviensis boliviensis). Developmental Psychobiology, 62(7), 909–919. 10.1002/dev.21960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napolitano, F. , Annicchiarico, G. , Caroprese, M. , De Rosa, G. , Taibi, L. , & Sevi, A. (2003). Lambs prevented from suckling their mothers display behavioral, immune and endocrine disturbances. Physiology & Behavior, 78(1), 81–89. 10.1016/S0031-9384(02)00892-2 [DOI] [PubMed] [Google Scholar]
- Napolitano, F. , De Rosa, G. , & Sevi, A. (2008). Welfare implications of artificial rearing and early weaning in sheep. Applied Animal Behaviour Science, 110(1–2), 58–72. 10.1016/j.applanim.2007.03.020 [DOI] [Google Scholar]
- Newberry, R. C. , & Swanson, J. C. (2008). Implications of breaking mother‐young social bonds. Applied Animal Behaviour Science, 110(1–2), 3–23. 10.1016/j.applanim.2007.03.021 [DOI] [Google Scholar]
- Newton‐Fisher, N. E. (2012). Animal Behaviour Pro: 1.4.4. [DOI] [PMC free article] [PubMed]
- Nieuwenhuis, R. , te Grotenhuis, M. , & Pelzer, B. (2012). Influence.ME: Tools for detecting influential data in mixed effects models. R Journal, 4(2), 38–47. 10.32614/rj-2012-011 [DOI] [Google Scholar]
- Nowak, R. , & Boivin, X. (2015). Filial attachment in sheep: Similarities and differences between ewe‐lamb and human‐lamb relationships. Applied Animal Behaviour Science, 164, 12–28. 10.1016/j.applanim.2014.09.013 [DOI] [Google Scholar]
- Nowak, R. , Porter, R. H. , Lévy, F. , Orgeur, P. , & Schaal, B. (2000). Role of mother‐young interactions in the survival of offspring in domestic mammals. Reviews of Reproduction, 5(3), 153–163. 10.1530/ror.0.0050153 [DOI] [PubMed] [Google Scholar]
- Parent, C. I. , Del Corpo, A. , Cameron, N. M. , & Meaney, M. J. (2013). Maternal care associates with play dominance rank among adult female rats. Developmental Psychobiology, 55(7), n/a‐n/a. 10.1002/dev.21070 [DOI] [PubMed] [Google Scholar]
- Parent, C. I. , & Meaney, M. J. (2008). The influence of natural variations in maternal care on play fighting in the rat. Developmental Psychobiology, 50(8), 767–776. 10.1002/dev.20342 [DOI] [PubMed] [Google Scholar]
- Patt, A. , Gygax, L. , Wechsler, B. , Hillmann, E. , Palme, R. , & Keil, N. M. (2012). The introduction of individual goats into small established groups has serious negative effects on the introduced goat but not on resident goats. Applied Animal Behaviour Science, 138(1–2), 47–59. 10.1016/j.applanim.2012.02.012 [DOI] [Google Scholar]
- Patt, A. , Gygax, L. , Wechsler, B. , Hillmann, E. , Palme, R. , & Keil, N. M. (2013a). Behavioural and physiological reactions of goats confronted with an unfamiliar group either when alone or with two peers. Applied Animal Behaviour Science, 146(1–4), 56–65. 10.1016/j.applanim.2013.03.009 [DOI] [Google Scholar]
- Patt, A. , Gygax, L. , Wechsler, B. , Hillmann, E. , Palme, R. , & Keil, N. M. (2013b). Factors influencing the welfare of goats in small established groups during the separation and reintegration of individuals. Applied Animal Behaviour Science, 144(1–2), 63–72. 10.1016/j.applanim.2012.11.009 [DOI] [Google Scholar]
- Pellis, S. M. , & Pellis, V. C. (1998). Play fighting of rats in comparative perspective: A schema for neurobehavioral analyses. Neuroscience & Biobehavioral Reviews, 23(1), 87–101. 10.1016/S0149-7634(97)00071-7 [DOI] [PubMed] [Google Scholar]
- Pellis, S. , & Pellis, V. (2013). The Playful Brain: Venturing to the Limits of Neuroscience. Oneworld Publications. [Google Scholar]
- Poindron, P. (2005). Mechanisms of activation of maternal behaviour in mammals. Reproduction Nutrition Development, 45(3), 341–351. 10.1051/rnd:2005025 [DOI] [PubMed] [Google Scholar]
- Quinn, G. P. , & Keough, M. J. (2002). Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge University Press. 10.1017/CBO9780511806384 [DOI] [Google Scholar]
- Rault, J. L. (2012). Friends with benefits: Social support and its relevance for farm animal welfare. Applied Animal Behaviour Science, 136(1), 1–14. 10.1016/j.applanim.2011.10.002 [DOI] [Google Scholar]
- Reinhardt, V. , Mutiso, F. M. , & Reinhardt, A. (1978). Social behaviour and social relationships between female and male prepubertal bovine calves (Bos indicus). Applied Animal Ethology, 4(1), 43–54. 10.1016/0304-3762(78)90092-5 [DOI] [Google Scholar]
- Rocha, L. E. C. , Terenius, O. , Veissier, I. , Meunier, B. , & Nielsen, P. P. (2020). Persistence of sociality in group dynamics of dairy cattle. Applied Animal Behaviour Science, 223(March 2019), 104921. 10.1016/j.applanim.2019.104921 [DOI] [Google Scholar]
- RStudio Team . (2019). RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/
- Rudge, M. R. (1970). Mother and kid behaviour in feral goats (Capra hircus L.). Zeitschrift Für Tierpsychologie, 27(6), 687–692. 10.1111/j.1439-0310.1970.tb01895.x [DOI] [Google Scholar]
- Sackett, G. P. (1965). Effects of rearing conditions upon the behavior of rhesus monkeys (Macaca Mulatta). Child Development, 36(4), 855. 10.2307/1126929 [DOI] [PubMed] [Google Scholar]
- Schielzeth, H. (2010). Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution, 1(2), 103–113. 10.1111/j.2041-210x.2010.00012.x [DOI] [Google Scholar]
- Schielzeth, H. , & Forstmeier, W. (2009). Conclusions beyond support: Overconfident estimates in mixed models. Behavioral Ecology, 20(2), 416–420. 10.1093/beheco/arn145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, E. F. S. (1991). The influence of nutrition and postpartum mating on weaning and subsequent play behaviour of hooded rats. Animal Behaviour, 41(3), 513–524. 10.1016/S0003-3472(05)80854-1 [DOI] [Google Scholar]
- Starr‐Phillips, E. J. , & Beery, A. K. (2014). Natural variation in maternal care shapes adult social behavior in rats. Developmental Psychobiology, 56(5), 1017–1026. 10.1002/dev.21182 [DOI] [PubMed] [Google Scholar]
- Suomi, S. J. (2009). How gene‐environment interactions shape biobehavioural development: Lessons from studies with rhesus monkeys. Development and Prevention of Behaviour Problems: From Genes to Social Policy, London: Psychology Press. pp. 7–23. 10.4324/9780203868706 [DOI] [Google Scholar]
- Suomi, S. J. , & Harlow, H. F. (1972). Social rehabilitation of isolate‐reared monkeys. Developmental Psychology, 6(3), 487–496. 10.1037/h0032545 [DOI] [Google Scholar]
- Szabò, S. , Barth, K. , Graml, C. , Futschik, A. , Palme, R. , & Waiblinger, S. (2013). Introducing young dairy goats into the adult herd after parturition reduces social stress. Journal of Dairy Science, 96(9), 5644–5655. 10.3168/jds.2012-5556 [DOI] [PubMed] [Google Scholar]
- Toinon, C. , Waiblinger, S. & Rault, J.‐L. (2019). Socio‐positive interactions in goats: Prevalence and social network. In N. Ruth, C. , & B. Bjarne, O. (Eds.), ISAE 2019. Proceedings of the 53rd Congress of the ISAE (252).
- Toinon, C. , Waiblinger, S. , & Rault, J.‐L. (2021). Maternal deprivation affects goat kids’ stress coping behaviour. Physiology & Behavior, 239(March), 113494. 10.1016/j.physbeh.2021.113494 [DOI] [PubMed] [Google Scholar]
- Trezza, V. , Campolongo, P. , & Vanderschuren, L. J. M. J. (2011). Evaluating the rewarding nature of social interactions in laboratory animals. Developmental Cognitive Neuroscience, 1(4), 444–458. 10.1016/j.dcn.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Berg, C. L. , Hol, T. , Van Ree, J. M. , Spruijt, B. M. , Everts, H. , & Koolhaas, J. M. (1999). Play is indispensable for an adequate development of coping with social challenges in the rat. Developmental Psychobiology, 34(2), 129–138. 10.1002/(SICI)1098-2302(199903)34:2<129::AID‐DEV6>3.0.CO;2‐L [DOI] [PubMed] [Google Scholar]
- van Hasselt, F. N. , Tieskens, J. M. , Trezza, V. , Krugers, H. J. , Vanderschuren, L. J. M. J. , & Joëls, M. (2012). Within‐litter variation in maternal care received by individual pups correlates with adolescent social play behavior in male rats. Physiology and Behavior, 106(5), 701–706. 10.1016/j.physbeh.2011.12.007 [DOI] [PubMed] [Google Scholar]
- van Leeuwen, E. J. C. , Mulenga, I. C. , & Chidester, D. L. (2014). Early social deprivation negatively affects social skill acquisition in chimpanzees (Pan troglodytes). Animal Cognition, 17(2), 407–414. 10.1007/s10071-013-0672-5 [DOI] [PubMed] [Google Scholar]
- Vanderschuren, L. J. M. J. (2010). How the brain makes play fun. American Journal of Play, 2(3), 315–337. Retrieved from https://eric.ed.gov/?id=EJ1069219 [Google Scholar]
- Vanderschuren, L. J. M. J. , Achterberg, E. J. M. , & Trezza, V. (2016). The neurobiology of social play and its rewarding value in rats. Neuroscience and Biobehavioral Reviews, 70, 86–105. 10.1016/j.neubiorev.2016.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren, L. J. M. J. , & Trezza, V. (2013). What the laboratory rat has taught us about social play behavior: Role in behavioral development and neural mechanisms. In Brain Imaging in Behavioral Neuroscience (pp. 189–212). 10.1007/7854_2013_268 [DOI] [PubMed] [Google Scholar]
- Veenema, A. H. , & Neumann, I. D. (2009). Maternal separation enhances offensive play‐fighting, basal corticosterone and hypothalamic vasopressin mRNA expression in juvenile male rats. Psychoneuroendocrinology, 34(3), 463–467. 10.1016/J.PSYNEUEN.2008.10.017 [DOI] [PubMed] [Google Scholar]
- Von Frijtag, J. C. , Schot, M. , Van Den Bos, R. , & Spruijt, B. M. (2002). Individual housing during the play period results in changed responses to and consequences of a psychosocial stress situation in rats. Developmental Psychobiology, 41(1), 58–69. 10.1002/dev.10057 [DOI] [PubMed] [Google Scholar]
- Wagner, K. , Barth, K. , Hillmann, E. , Palme, R. , Futschik, A. , & Waiblinger, S. (2013). Mother rearing of dairy calves: Reactions to isolation and to confrontation with an unfamiliar conspecific in a new environment. Applied Animal Behaviour Science, 147(1–2), 43–54. 10.1016/j.applanim.2013.04.010 [DOI] [Google Scholar]
- Wagner, K. , Barth, K. , Palme, R. , Futschik, A. , & Waiblinger, S. (2012). Integration into the dairy cow herd: Long‐term effects of mother contact during the first twelve weeks of life. Applied Animal Behaviour Science, 141(3–4), 117–129. 10.1016/j.applanim.2012.08.011 [DOI] [Google Scholar]
- Wagner, K. , Seitner, D. , Barth, K. , Palme, R. , Futschik, A. , & Waiblinger, S. (2015). Effects of mother versus artificial rearing during the first 12 weeks of life on challenge responses of dairy cows. Applied Animal Behaviour Science, 164, 1–11. 10.1016/j.applanim.2014.12.010 [DOI] [Google Scholar]
- Waiblinger, S. , Nordmann, E. M. & Keil, N. M. (2017). Social behaviour and adrenocortical activity in goats up to four weeks after grouping. In Jensen, M. B. , Herskin, M. S. , & Malmkvist, J. (Eds.), ISAE 2019. Proceedings of the 51st Congress of the ISAE (184)
- Waiblinger, S. , Wagner, K. , Hillmann, E. , & Barth, K. (2020). Play and social behaviour of calves with or without access to their dam and other cows. Journal of Dairy Research, 87(S1), 144–147. 10.1017/S0022029920000540 [DOI] [PubMed] [Google Scholar]
- Walker, C. D. , Xu, Z. , Rochford, J. , & Celeste Johnston, C. (2008). Naturally occurring variations in maternal care modulate the effects of repeated neonatal pain on behavioral sensitivity to thermal pain in the adult offspring. Pain, 140(1), 167–176. 10.1016/j.pain.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Winslow, J. T. , Noble, P. L. , Lyons, C. K. , Sterk, S. M. , & Insel, T. R. (2003). Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology, 28(5), 910–918. 10.1038/sj.npp.1300128 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in Phaidra at https://phaidra.vetmeduni.ac.at/o:834, reference number o:834.


