Summary
Although the above and belowground sizes and shapes of plants strongly influence plant competition, community structure, and plant–environment interactions, plant sizes and shapes remain poorly characterized across climate regimes. We investigated relationships among shoot and root system size and climate.
We assembled and analyzed, to our knowledge, the largest global database describing the maximum rooting depth, lateral spread, and shoot size of terrestrial plants – more than doubling the Root Systems of Individual Plants database to 5647 observations.
Water availability and growth form greatly influence shoot size, and rooting depth is primarily influenced by temperature seasonality. Shoot size is the strongest predictor of lateral spread, with root system diameter being two times wider than shoot width on average for woody plants.
Shoot size covaries strongly with rooting system size; however, the geometries of plants differ considerably across climates, with woody plants in more arid climates having shorter shoots, but deeper, narrower root systems. Additionally, estimates of the depth and lateral spread of plant root systems are likely underestimated at the global scale.
Keywords: allometry, lateral root spread, plant shape, plant–environment interactions, root systems, rooting depth, shoot height, shoot width
Short abstract
See also the Commentary on this article by Kattge, 235: 821–823.
Introduction
The vertical and horizontal extents of plants partly define plant architecture above and belowground (Lynch, 1995; Schenk & Jackson, 2002a; Hunt, 2016; Pawlik & Kasprzak, 2017). Plant architecture, the three‐dimensional organization of the plant body (Reinhardt & Kuhlemeier, 2002), is plastic; plants compensate for resource limitations by altering allocation among above and belowground organs to optimize growth, survival, and reproduction (Poorter et al., 2012; Díaz et al., 2016). To understand plant responses to changes in resource availability and climate (Dybzinski et al., 2011; Farrior et al., 2015), several global studies have examined plant biomass partitioning across climates (Cheng & Niklas, 2006; Mokany et al., 2006; Reich et al., 2014). However, the vertical and horizontal extents of plants have traditionally been ignored, despite the fact that plants with similar biomass allometries may have different dimensions. In this study we seek to understand how the maxima of plant extents respond to climate through changes among shoot height and width and rooting depth and spread.
Understanding the relationships between the size of plants above and belowground will improve our knowledge of plant form and function. For example, the global spectrum of plant form and function (plant economic spectrum; PES), proposed by Díaz et al. (2016), posits that the size of plants and their organs represents the first major dimension of the PES. Consequently, most PES studies have focused on leaf, seed, and stem traits; however, these studies have typically used only shoot height to represent overall plant size (Verbeeck et al., 2019). Additionally, root traits, such as maximum depth and spread, considered to be an important missing link, have mostly been excluded from such analyses due to a scarcity of data (Joswig et al., 2022). When root traits have been included in studies of the global spectrum of plant form and function, the focus has usually been on fine root traits, not root system size traits, such as maximum depth and spread (Carmona et al., 2021).
Variation in belowground plant traits remains poorly quantified compared with shoot traits (Jackson et al., 1996; Vogt et al., 1996; Norby & Jackson, 2000; Reich, 2014; Iversen & McCormack, 2021). The size and shape of root systems rely, first, on resource demand of the plant (for water and nutrients), depending on overall plant size and growth strategy (Jackson et al., 2000; Enquist & Niklas, 2002; Niklas & Enquist, 2002; Poorter et al., 2012); second, on resource availability belowground (Poorter & Nagel, 2000; Schenk, 2008a); third, on soil constraints, such as horizons, bedrock, hardpans, and groundwater tables (Brantley et al., 2017; Fan et al., 2017; Hasenmueller et al., 2017); and fourth, on the presence, size, and identity of competing root systems (Caldwell et al., 1985; Casper & Jackson, 1997; Schenk et al., 1999; Casper et al., 2003; Dannowski & Block, 2005; Schenk, 2006; van Noordwijk et al., 2015). The complexity of the belowground environment coupled with methodological challenges make quantifying plant–root–environment interactions difficult, especially in the field.
Furthermore, compiled data on root system size are scarce (Guerrero‐Ramírez et al., 2021). Although scarce, estimates of maximum rooting depth remain one of the most sought‐after plant traits, with 10% of the thousands of TRY plant‐trait database inquiries requesting maximum rooting depth data (Kattge et al., 2020). One reason for the demand of rooting depth data is that the depth and lateral placement of roots influences plant–soil interactions, thereby affecting element cycling, plant water uptake, and soil organic matter content (Jobbágy & Jackson, 2000; Poirier et al., 2018; Freschet et al., 2021b). Additionally, rooting depth is a key plant trait used by most terrestrial‐biosphere models to estimate plant water uptake (Warren et al., 2015; Stocker et al., 2021).
Maximum rooting depth has been evaluated through quantitative syntheses such as those of Schenk & Jackson (2002a) and Fan et al. (2017), which acknowledge many important earlier studies (e.g. Weaver, 1919; Phillips, 1963; Canadell et al., 1996). Deeper rooting has been found more often for plants limited by water availability (Freschet et al., 2021a). Relative to plant size, rooting depths increase with aridity and seasonality, and the deepest roots are often found where there is evaporative demand during dry seasons for water available deeper in the soil (Schenk & Jackson, 2005). Additionally, Fan et al. (2017) found that variations in the soil water profile caused by infiltration, drainage, and water table depth helped explain considerable variation in rooting depth. These maximum rooting depth syntheses have led to the following biome‐level characterizations: relatively shallow‐rooted ecosystems tend to be found in boreal and permafrost regions, wetlands, and land covered by annual plants, whereas relatively deeper roots are found in more arid, semi‐arid, and seasonally dry climates (Schenk & Jackson, 2005; Fan et al., 2017). In summary, the distribution of water belowground and the seasonal variation in the amount, location of – and demand for – water strongly affect the depth of plant roots.
Even rarer than rooting depth data are datasets of maximum lateral spread (Klimešová et al., 2018; Guerrero‐Ramírez et al., 2021). Lateral rooting extent is the maximum horizontal distance between roots and the base of the plant. The lateral extent of roots affects nutrient foraging (Cahill & McNickle, 2011; Giehl & von Wirén, 2014), shoot anchorage (Ennos, 1993; Schwarz et al., 2010), and competition (Casper & Jackson, 1997; Schenk et al., 1999; Casper et al., 2003; Schenk, 2006). Lateral rooting extent can also be an extremely plastic trait (Klimešová et al., 2018). Plants have been found to explore large volumes of soil; for example, grasses and trees in the Namib Desert have lateral root extents as great as 12 m and 50 m, respectively (Kutschera, 1997).
To rectify the scarcity of root‐system size data, we assembled, to our knowledge, the largest global database describing the maximum rooting depth, lateral spread, and shoot size of terrestrial plants. The Root Systems of Individual Plants (RSIP) database was developed in 2002 to quantify the maximum depth D R and lateral spread L R of plant root systems (Schenk & Jackson, 2002a; Fig. 1; Supporting Information Fig. S1a). Here, we more than doubled the database to 5647 total observations across a broad range of terrestrial climates and geographies (Figs 2, S1c).
Fig. 1.
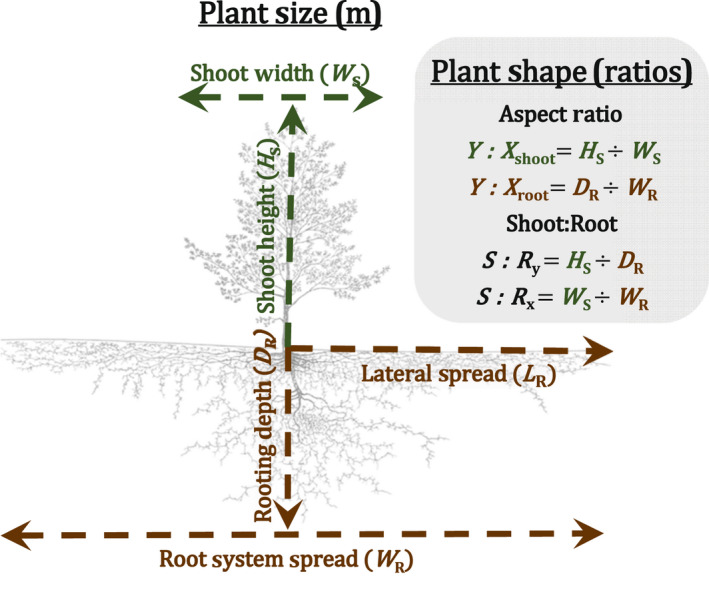
The main plant growth extents as defined in the Root Systems of Individual Plants (RSIP). The plant size measures, or the absolute extents, illustrate the maximum aboveground (in green – shoot width and shoot height) and belowground (in brown – rooting depth, lateral spread, and root system spread) extents in meters. The inset gray box shows the four plant shape ratios used to understand the dimensions or aspect ratio of the shoot (Y : X shoot) and the root system (Y : X root), and the above/belowground vertical (S : Ry ) and horizontal (S : Rx ) allometry. The tree outline was adapted from figure 115 of Wurzelatlas mitteleuropäischer Waldbäume und Sträuche (Kutschera & Lichtenegger, 2002).
Fig. 2.
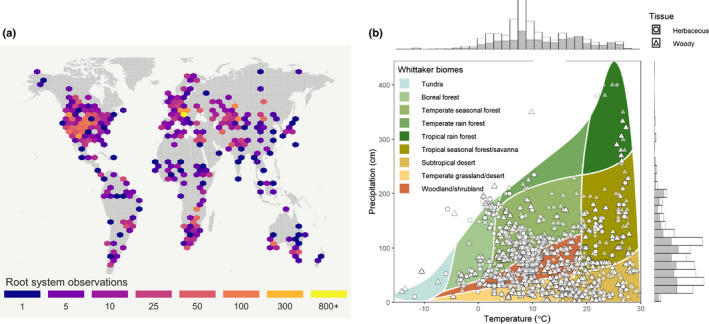
The (a) geographic and (b) climatic distributions of Root Systems of Individual Plants (RSIP) database records. (a) Global hexbin map showing the geographic distribution of RSIP observations, split into 50 hexagonal bins. (b) Whittaker plot of RSIP observations separated into woody (triangles) and herbaceous (circles) plants. The plot shows the distribution of biomes based on mean annual precipitation and temperature (as defined in the figure key), and how the RSIP observations fall within the climate space. The marginal histograms show the relative distribution of woody (white bars) and herbaceous (gray bars) plants across the axes.
We use the expanded RSIP database to examine large‐scale patterns related to plant size and shape both above and belowground. Specifically, we seek to (1) characterize the root and shoot sizes of different plant functional types (PFTs), (2) understand how plant size, climate, and environment influence the vertical and horizontal extents of plants globally, (3) evaluate how plant dimensions shift above and belowground along climatic gradients, and (4) compare individual‐plant‐scale rooting depths to ecosystem‐scale rooting depths across biomes and climates.
Materials and Methods
Dataset
The RSIP dataset integrates observations of the vertical and horizontal extents of individual plants with data for other plant traits. The RSIP data come from published observations of maximum plant root system dimensions, 361 publications (Appendix A1), covering 2989 species from 263 plant families (Fig. 3). The first version of the RSIP (Fig. S1a; Schenk & Jackson, 2002a) included 1305 observations for water‐limited ecosystems, and second version (Fig. S1b; Schenk & Jackson, 2005) included 2449 observations across a broader range of climates. Our expanded RSIP, with 5647 total observations (Fig. S1c), includes a range of root and shoot sizes spanning more than four orders of magnitude (Fig. 4) across most of the Earth’s climates and environments (Fig. 2).
Fig. 3.
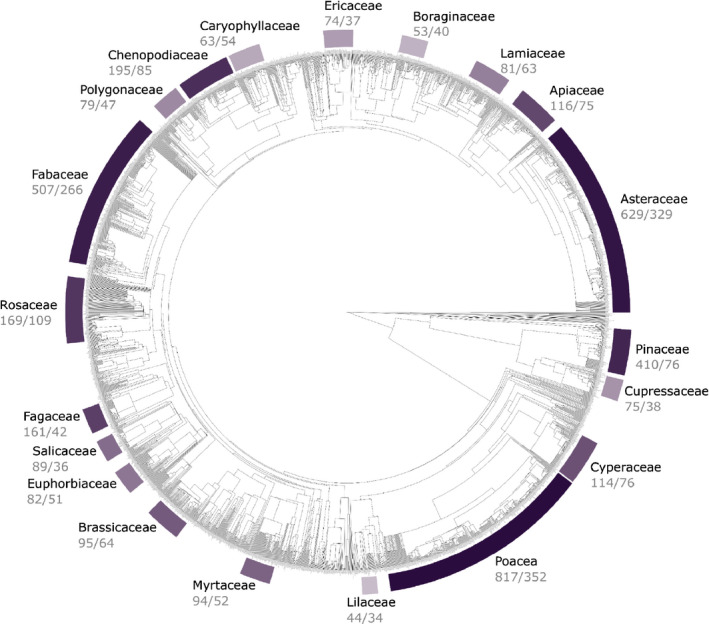
Phylogenetic tree of the 2989 species represented in the Root Systems of Individual Plants (RSIP). The highlighted plant families represent the 20 largest families in the RSIP based on the number of species represented (263 plant families overall). The 20 families represent 71% of all observations in the RSIP. The colors from light purple to dark purple represent the number of observations from each plant family. The labels show the plant family name, followed by the number of observations and the number of species (i.e. family no. of observations/no. of species).
Fig. 4.
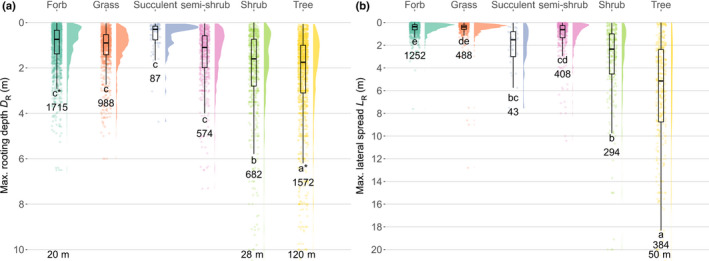
Raincloud plots for (a) maximum rooting depth D R and (b) maximum lateral spread L R across growth forms: forb, grass, succulent, semi‐shrub, shrub, and tree. The lowercase letters represent significantly different treatments for D R and L R across growth forms via Tukey’s honest significance difference tests. The horizontal lines in the boxplots represent the median values. The asterisk indicates the only situation where rooting depth relative to shoot volume differed between growth forms (i.e. the relative depth of forbs was significantly greater than for trees). The number at the end of each whisker indicates the total number of observations for each growth form. The maximum values for growth forms exceeding the plot scales are shown at the bottom.
The RSIP entries are classified by physiology and functional traits (see Tables S1, S2 for a full list of RSIP variables), including six growth forms: forbs (30% of observations), grasses (18%), semi‐shrubs (shrub species and suffrutescent forbs that rarely reach 1 m in height; 10%), shrubs (12%), stem succulents (2%), and trees (28%). We also record coarse‐scale information on the plant’s environment and location, such as biome, elevation, and spatial coordinates (see Tables S1, S2). There are, however, fine‐scale environmental parameters, such as soil traits, that cannot be accurately estimated based on the spatial coordinates for the RSIP entries.
The spatial coordinates allowed us to estimate related climate information, such as mean annual precipitation (MAP), when it was unavailable in the source literature. The estimated climate parameters came from WorldClim2, specifically 1 km spatial resolution climate surfaces for global land areas, providing historical (1970–2000) monthly and annual estimates of temperature and precipitation (Fick & Hijmans, 2017). Estimates for mean annual potential evapotranspiration (MAE) came from the Global Aridity Index and Potential Evapotranspiration Climate Database v.2 (Trabucco & Zomer, 2019). Nineteen additional bioclimatic variables were calculated following Fick & Hijmans (2017), providing long‐term metrics for precipitation and temperature seasonality (Table S1, BIO1‐19).
Bioclimatic variables allowed us to test how seasonality and climate affect the size of plants. Additionally, we calculated the seasonality of precipitation metric described in Schenk & Jackson (2005). To calculate S a, we used long‐term monthly average precipitation (Fick & Hijmans, 2017) and potential evapotranspiration (Trabucco & Zomer, 2019) to calculate the sum of the seasonal surplus P sur or deficit P def of water. See Table S1 for the equations and definitions for S a, P def, and P sur, along with a description of each of the climate metrics, growth extents, plant traits, and environmental metrics.
Describing plant size
The RSIP contains measurements describing the maximum above and belowground dimensions of individual plants at the time of measurement. Maximum rooting depth D R (n = 5633) is defined as the deepest soil depth reached by the roots of an individual plant (Fig. 1; Table 1). Two additional belowground dimensions in the database include lateral spread L R (n = 2874), the maximum one‐sided horizontal distance from the stem of an individual plant reached by its roots (i.e. the radius), and root system width W R (n = 1756), the maximum root system diameter, which is not always the same as 2 × L R because most root systems are asymmetrical (Fig. 1). The main aboveground dimensions in the database are shoot height H S (n = 2373) and shoot width W S (n = 2074; Fig. 1), the maximum shoot diameter. Shoot volume V S was estimated using an ellipsoid shape (). We excluded from the analyses of shoot width W S and lateral spread L R those observations from species known to have clonal, rhizomatous, or stoloniferous growth habits (n = 101), such as Populus tremuloides and Poa pratensis, so as not to give a misleading view of their functional morphology by only measuring the widths of individual ramets. The maximum dimensions of an individual plant at the time of excavation had to be directly measured to be included in the RSIP; observations were excluded from the RSIP if the sampling depth was less than the perceived max rooting depth, if allometric equations or other formulas were used to predict plant dimensions, or if the measurements were an aggregate of multiple observations and were not the dimensions of an individual plant.
Table 1.
A list of commonly used abbreviations (see Supporting Information Table S1 for a list of all RSIP parameters).
| Abbreviation | Explanation |
|---|---|
| Plant size | |
| D R | Maximum rooting depth of plant (m) |
| L R | Maximum lateral root spread, one‐sided (radius) linear distance from stem reached by roots (m) |
| W R | Rooting spread or diameter (m) |
| H S | Height of plant shoot (m) |
| W S | Width of plant shoot (m) |
| V S | Canopy volume, calculated using an ellipsoidal shape: |
|
| |
| DBH | Stem diameter (diameter at breast height) of trees (cm) |
| Plant shape | |
| Y : X shoot | Aboveground dimensional aspect ratio (Y : X shoot = H S/W S) |
| Y : X root | Belowground dimensional aspect ratio (Y : X root = D R/W R) |
| S : Ry | Vertical shoot : root ratio (S : Ry = H S/D R) |
| S : Rx | Horizontal shoot : root ratio (S : Rx = W S/W R) |
| Climate | |
| MAP | Mean annual precipitation (m) |
| MAE | Mean annual potential evapotranspiration (m) |
| A i | Aridity index (A i = MAP/MAE) |
| S a | Seasonality index or annual water storage index: S a = min[P sur, P def] |
| Datasets | |
| RSIP | Root Systems of Individual Plants |
| RPGE | Root Profiles for Global Ecosystems (Schenk & Jackson, 2003) |
Phylogenetic analysis
To understand the importance of phylogeny on the main variables (D R, L R, H S, and W S), we calculated the phylogenetic signal using Pagel’s lambda (Pagel, 1997, 1999) and performed phylogenetically independent contrasts (PICs) between above and belowground plant extents and across the main climate metrics (MAE, MAP, aridity index A i, and S a). The phylogeny of RSIP observations was constructed using the v.phylomaker R package (Jin & Qian, 2019) with the GBOTB.extented mega‐tree (Zanne et al., 2014; Smith & Brown, 2018). The plant names were standardized using the The Plant List (2013; v.1.1; www.theplantlist.org/) to match the nomenclature present in the mega‐tree. Calculating Pagel’s lambda allowed us to estimate the phylogenetic signal of the plant trait in question, by estimating the magnitude by which shared phylogenetic history drives the trait distribution at the tips of the phylogeny (Freckleton et al., 2002). A lambda value of zero indicates no phylogenetic influence on plant traits, whereas a lambda value of one represents high phylogenetic signal. To calculate Pagel’s lambda and the log likelihood statistic we used the phytools::phylosig R function (Revell, 2012) to run 100 simulations for each of the plant extents separated into three groups: (1) all observations, (2) woody plants (trees, shrubs, and semi‐shrubs), and (3) herbaceous plants (forbs and grasses). We performed regressions of phylogenetically independent contrasts (Felsenstein, 1985) for each of the resolved phylogenies using the ape and stats R packages (Paradis & Schliep, 2019; R Core Team, 2020). Phylogenetic relatedness was calculated and used as a predictor variable in the random forest analysis (see next section) via an analysis of the phylogenetic pairwise distance between species using the ape package (Paradis & Schliep, 2019), as suggested in Bergmann et al. (2017).
Evaluating variable importance for shoot and root extents
To determine factors influencing maximum root (D R and L R) and shoot extents (H S and W S), we estimated the importance of covariates using a random forest approach (Breiman, 2001). The list of covariates included aboveground plant traits and climate metrics (see Table S1 for a full list of RSIP parameters). The random forest models for belowground extents were run with (Fig. 6c,d see later) and without (Fig. 6a,b see later) aboveground size (H S, W S, and V S) as predictors; however, belowground extents (D R and L R) were not used as predictors for H S and W S.
Fig. 6.
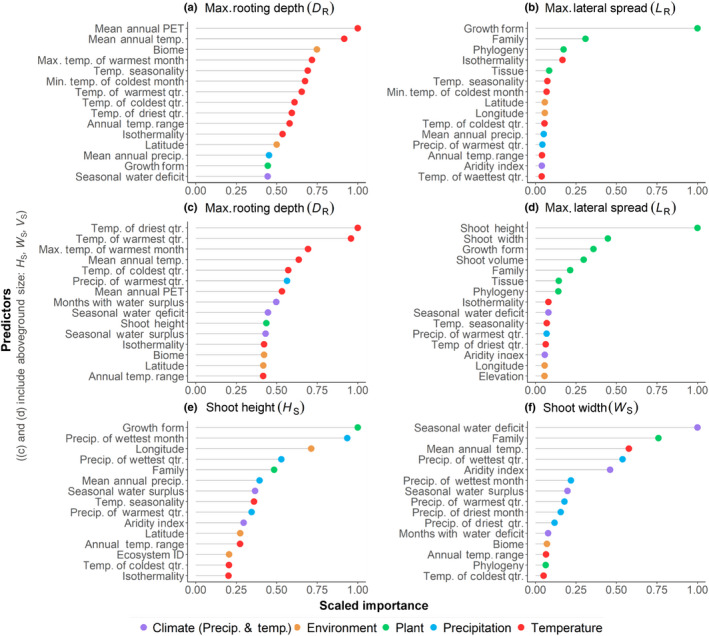
Random forest variable importance for (a) maximum rooting depth D R, (b) maximum lateral spread L R, (c) D R with shoot size included as predictors, (d) L R with shoot size as predictors, (e) shoot height H S, and (f) shoot width W S. The predictors are colored by predictor type, according to the figure key. The y‐axis is the 15 most important predictors in descending order, and the x‐axis is a scaled variable importance. Scaled variable importance = variable importance[i] ÷ max(variable importance). The predictors used for the random forest analysis can be found in the fourth column of Supporting Information Table S1. Measures of aboveground size were not included as predictors in (a) and (b), whereas shoot height, shoot width, and shoot volume V S were included as predictors in (c, d). Belowground extents (D R and L R) were not included as predictors for H S and W S. [Correction added after first publication 8 March 2022: panel (c) in Fig. 6 has been corrected.]
For the random forest approach, we utilized the ranger package (Wright & Ziegler, 2017), which is an implementation of the original random forest (Breiman, 2001) suited for high‐dimensional data (Boehmke & Greenwell, 2020). We split the RSIP dataset using stratified sampling into a model training subset containing 70% of the entries and a model testing subset using the rsample package (Silge et al., 2021). Because random forests cannot handle missing values, we used the missranger package (Mayer, 2019) to impute missing values through a nonparametric approach for mixed‐type data using chains of random forests (Stekhoven & Buhlmann, 2012). The training data were used to adjust the random forest model using a hyperparameter grid to search for the optimal parameter values, resulting in the greatest reduction in root‐mean‐square error (Probst et al., 2019). The hyperparameter tuning resulted in an average 4% improvement compared with the baseline model. The random forest model was then rerun using the selected hyperparameters to calculate the permutation‐based variable importance for each predictor. We chose the permutation‐based method because it is not biased towards variables with high cardinality (Strobl et al., 2008), such as for many climate variables. Although the permutation‐based approach is more computationally intensive (because of the constant shuffling of features across the decision trees), it is generally a more accurate method than the standard mean‐decrease‐in‐impurity importance (Strobl et al., 2007).
Additionally, we sought to determine how plant size differed across categorical variables such as plant characteristics and growth form (Table 2). Significant differences between the plant extents of categorical parameters were tested using ANOVA and post hoc Tukey honest significant difference tests (de Mendiburu, 2021).
Table 2.
Mean belowground (rooting depth (D R), lateral spread (L R), shoot height (H S) and shoot width (W S)) extents across plant traits.
| Belowground extents (m) | Aboveground extents (m) | |||||||
|---|---|---|---|---|---|---|---|---|
| D R | L R | H S | W S | |||||
| Mean | SD and group | Mean | SD and group | Mean | SD and group | Mean | SD and group | |
| Growth form | ||||||||
| Forb | 1.02 | 1.04c | 0.51 | 0.51e | 0.36 | 0.34c | 0.34 | 0.37c |
| Grass | 1.14 | 0.93c | 0.55 | 0.94de | 0.55 | 0.53bc | 0.35 | 0.85c |
| Semi‐shrub | 1.42 | 1.2c | 1.07 | 1.37cd | 0.33 | 0.24c | 0.53 | 0.55c |
| Shrub | 2.36 | 2.85b | 3.33 | 3.46b | 1.47 | 1.5b | 1.48 | 1.68b |
| Succulent | 0.56 | 0.68c | 2.22 | 1.93bc | 0.61 | 0.64bc | 0.78 | 0.55bc |
| Tree | 3.64 | 7.69a | 7.04 | 7a | 8.07 | 9.12a | 3.25 | 4.42a |
| Lifespan | ||||||||
| Annual | 0.76 | 0.6b* | 0.4 | 0.59b | 0.54 | 0.59b | 0.32 | 0.46b |
| Perennial | 2.12 | 4.64a* | 2.05 | 3.93a | 2.06 | 5.14a | 0.92 | 2.07a |
| Tissue | ||||||||
| Herbaceous | 1.06 | 1b* | 0.52 | 0.66b | 0.42 | 0.41b | 0.34 | 0.55b |
| Woody | 2.88 | 6a* | 3.8 | 5.28a | 3.94 | 7.03a | 1.68 | 2.94a |
| Seed category | ||||||||
| Dicot | 2.24 | 5.04a | 1.93 | 3.89b | 1.47 | 4.04b | 0.89 | 1.72b |
| Gymnosperm | 1.92 | 3.74a | 5.57 | 4.76a | 8.63 | 9.49a | 2.56 | 5.35a |
| Monocot | 1.08 | 0.93b | 0.63 | 1.35c | 0.52 | 0.51c | 0.35 | 0.79c |
| Leaf longevity | ||||||||
| Deciduous | 2.96 | 5.5a | 5.67 | 6.27a | 4.48 | 5.71a | 2.6 | 3.06a |
| Evergreen | 3.1 | 6.95a | 3.06 | 4.59b | 4.74 | 8.64a | 1.41 | 3.28b |
| Leaf form | ||||||||
| Broadleaf | 3.59 | 7.43a | 4.71 | 6.01b | 4.12 | 7.12b | 2.08 | 2.86a |
| Needle‐leaf | 1.87 | 3.67b | 5.02 | 5.21b | 7.74 | 9.05a | 2.29 | 4.93a |
| Photosynthetic pathway | ||||||||
| C3 | 2.04 | 4.69a | 1.86 | 3.82a | 2.04 | 5.14a | 0.87 | 2.04a |
| C3–C4 | 1.13 | 0.63ab | 0.85 | 0.69a | 0.36 | 0.2b | 0.37 | 0.24a |
| C4 | 1.75 | 1.85ab | 1.37 | 2.65a | 0.74 | 0.73b | 0.71 | 1.29a |
| CAM | 0.61 | 0.71b | 2.57 | 2.43a | 0.64 | 0.63b | 0.76 | 0.52a |
CAM, Crassulacean acid metabolism.
These are the only two categories where rooting extents relative to aboveground volume showed significant differences between groups, where both annual and herbaceous plants had D R/V S values greater than perennial and woody plants, therefore differing from the pattern shown by D R. D R and L R relative to shoot volume (V S) did not differ across all other classifications. The lowercase letters represent significant differences between groups via Tukey’s honest significance difference tests.
Shifts in plant shape across climate
Whereas our initial analysis focused on factors influencing single measures of plant vertical or horizontal size, we further sought to understand how the shapes or dimensions of plants shift along climatic/resource gradients. To do this, we calculated four new plant shape ratios: two that we call ‘dimensional aspect’ ratios (Y : X shoot and Y : X root) and two ‘shoot : root’ ratios (S : Ry and S : Rx ). We plotted the four indicator ratios (Eqns (Eqn 1), (Eqn 2), (Eqn 3), (Eqn 4)) against a global climate gradient of aridity (Fig. 7, see later). Nonlinear regressions were fit to the mean ratio values for each aridity class.
Fig. 7.
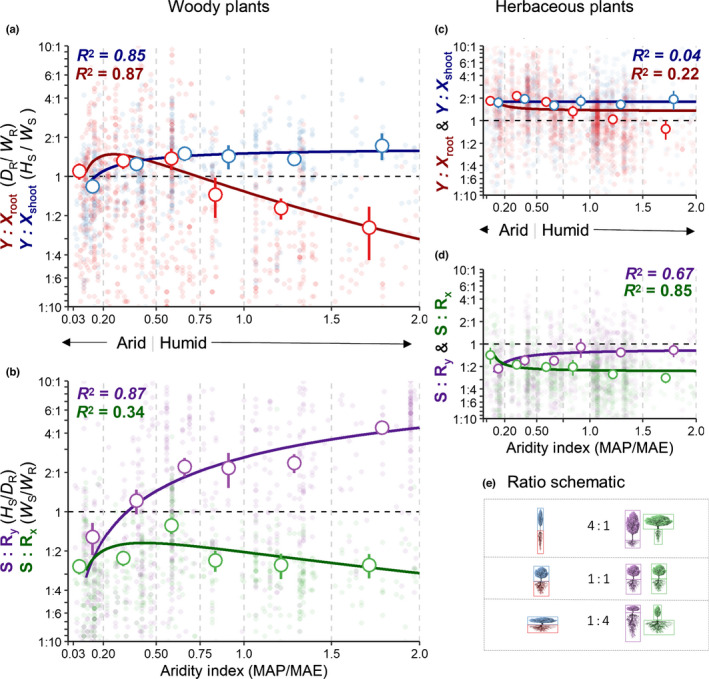
Woody plant shapes diverge across aridity classes. Point range represents the mean and confidence intervals grouped by aridity index categories for the four shape ratios with fitted nonlinear regressions. (a) Dimensional aspect ratios for woody plants, (b) shoot : root ratios for woody plants, (c) dimensional aspect ratios for herbaceous plants, and (d) shoot : root ratios for herbaceous plants. Either log‐normal () or nonlinear saturation curves () were fitted to the means of each shape ratio across aridity classes, and the R 2 values are stated (see Supporting Information Table S5 for full equations). The aridity index categories, delimited by light gray dashed lines, are arid (0–0.2), semi‐arid (0.2–0.5), subhumid (0.5–0.75), humid (0.75–1.0), per‐humid (1.0–1.5), and hyper‐humid (1.5–2.0). The dashed black line represents ratio values equal to one. (e) The color‐coded schematics represent the relative dimensions for each ratio value. MAP, mean annual precipitation; MAE, mean annual potential evapotranspiration; D R, max rooting depth; W R, root system width, H S, shoot height; W S, shoot width.
The two‐dimensional aspect ratios (Y : X shoot and Y : X root) depict a plant’s dimensions shifting towards either lengthening or widening their maximum extents (Eqns 1, 2). A high Y : X ratio represents a relative narrowing of plant morphology, whereas a low ratio represents a widening. As water availability increases, we expect to see relatively shallow plant growth belowground and a narrowing aboveground because plants may not need to root deeply in search of water and shoot heights are less limited by plant water potential. We calculated the dimensional aspect ratios as follows:
| (Eqn 1) |
| (Eqn 2) |
(H S, height of the plant; W S, aboveground width of the plant (shoot diameter); D R, maximum rooting depth; W R: maximum width of the root system). When W R was not reported but L R was, we used 2 × L R in Eqn 2.
The second pair of growth indicator ratios, the shoot : root size ratios (S : Ry and S : Rx ), depict a plant’s vertical and horizontal allometry (Eqns 3, 4). These metrics are similar to traditional shoot‐to‐root biomass ratios, but with biomass replaced by vertical length (S : Ry ) and horizontal width (S : Rx ). A high S : R ratio represents relatively greater aboveground investment, whereas a lower ratio represents relative belowground investment. We calculated the shoot : root size ratios as follows:
| (Eqn 3) |
| (Eqn 4) |
Comparing individual plant rooting depth observations with ecosystem and plant‐functional‐type estimates
Because many terrestrial biosphere models rely on ecosystem‐level estimates of maximum rooting depth (Warren et al., 2015; McCormack et al., 2017; Drewniak, 2019), we compared how our rooting depth estimates for individual plants differ from ecosystem‐level estimates across biomes and climates. For ecosystem‐level data, we used the Root Profiles for Global Ecosystems (RPGE) dataset (Schenk & Jackson, 2002b) available online through the Oak Ridge National Laboratory Distributed Active Archive Center (Schenk & Jackson, 2003). We compared average individual plant rooting depth estimates by biome from the RSIP with (1) the ecosystem rooting depths (D 50 and D 95) by biome from the RPGE, and (2) the PFT rooting depth estimates used by the Energy Exascale Earth System Land Model (ELM; Fig. S2; Drewniak, 2019). ELM uses RPGE data to inform PFT rooting depth estimates (Drewniak, 2019).
To analyze the effect that climate parameters have on individual‐plant (D R) and ecosystem‐level rooting depths (D 50 and D 95), we used linear mixed effect regression models (LMERs) with biome as a random effect, the climate metrics as fixed effects, and rooting depth (D 50, D 95, and D R) as the dependent variable. The LMERs were performed using the lme4 package (Bates et al., 2015). We evaluated the LMERs using likelihood ratio tests, which compare the ANOVA of the full LMER with the fixed effects with the ANOVA of a null LMER with only random effects. Through the likelihood ratio test we computed the corrected Akaike information criterion AICc and P‐values to analyze only significant predictors (Winter, 2013; Hajduk & Bailey, 2017; Mazerolle, 2020). Using the model results for D 50 and D 95, we compared the standardized coefficients with that of individual plant maximum rooting depth D R (Fig. S3).
Results
Rooting extents covary with shoot size
The two main plant rooting extents we examined, D R and L R, differed substantially across growth forms, with woody plants, especially trees (mean D R of 3.64 m), rooting the deepest and the widest (Fig. 4; Table 2). Semi‐shrubs, succulents, forbs, and grasses all had shallower, significantly indistinguishable rooting depths, with D R being only c. 30% as deep as trees on average (Fig. 4a; Table 2; P < 0.001). Trees and shrubs had the widest lateral spreads (average L R of 7.04 m and 3.33 m, respectively), whereas the average L R for succulents was 2.22 m, 4.5 times wider than L R for herbs (forbs and grasses; Fig. 4b; Table 2; P < 0.001). Relative to aboveground volume, L R (L R/V S; Kruskal–Wallis P = 0.173) and D R (D R/V S; Kruskal–Wallis P = 0.053) ratios did not significantly differ across growth forms.
Both rooting depth and spread scaled linearly with shoot size – specifically shoot height and width – across all growth forms (Fig. 5; Table 3). Whereas stem diameter (DBH) had a strong positive linear relationship with both maximum rooting depth and spread for trees (Table 3; P < 0.0001), W S and H S had stronger positive linear relationships with the rooting extents for both woody and herbaceous plants (Table 3).
Fig. 5.
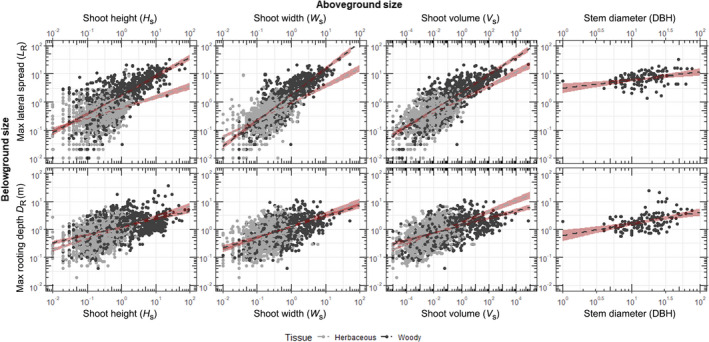
Scatter plots of plant root extents (L R, upper; D R, lower) against aboveground plant extents (H S, shoot height; W S, shoot width; V S, shoot volume; DBH, stem Diameter), with woody plants in dark gray and herbaceous plants in light gray. Shoot volume is calculated using the equation . The dashed lines (woody in dark gray and herbs in light gray) represent a linear regression where P < 0.05 in the form of y = β + α × x, and the red shaded regions are the 95% confidence interval. The statistics and the parameters for the linear regressions are in Table 3. The axes scales are in common log10.
Table 3.
Linear and phylogenetically independent contrast (PIC) regressions of belowground extents (D R and L R) to aboveground extents (H S, W S, V S, and DBH) in the form of y = β 0 + β 1 x, where y is D R or L R, β 0 is the intercept (Int.) and β 1 is the slope.
| Max. rooting depth D R | Max. lateral spread L R | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Linear regression | PIC | Linear regression | PIC | |||||||
| Int. | Slope (SE) | R 2 and P | Slope (SE) | R 2 and P | Int. | Slope (SE) | R 2 and P | Slope (SE) | R 2 and P | |
| Shoot height H S | ||||||||||
| All observations | 0.04 | 0.34 (0.011) | 0.29*** | −0.19 (0.029) | 0.028*** | 0.07 | 0.68 (0.016) | 0.45*** | −0.050 (0.035) | 0.001 |
| Woody | 0.08 | 0.28 (0.016) | 0.24*** | 0.19 (0.024) | 0.118*** | 0.24 | 0.67 (0.024) | 0.5*** | 0.40 (0.020) | 0.504*** |
| Herbaceous | 0.05 | 0.39 (0.021) | 0.2*** | 0.13 (0.045) | 0.008* | −0.2 | 0.39 (0.025) | 0.17*** | 0.51 (0.040) | 0.155*** |
| Shoot width W S | ||||||||||
| All observations | 0.09 | 0.39 (0.014) | 0.28*** | 0.65 (0.020) | 0.454*** | 0.15 | 0.81 (0.015) | 0.6*** | 0.87 (0.019) | 0.627*** |
| Woody | 0.09 | 0.38 (0.023) | 0.27*** | 0.28 (0.038) | 0.127*** | 0.26 | 0.93 (0.024) | 0.68*** | 0.57 (0.031) | 0.449*** |
| Herbaceous | 0.09 | 0.4 (0.022) | 0.2*** | 0.41 (0.030) | 0.173*** | −0.1 | 0.56 (0.022) | 0.34*** | 0.47 (0.027) | 0.251*** |
| Shoot volume V S | ||||||||||
| All observations | 0.15 | 0.16 (0.005) | 0.33*** | 0.23 (0.010) | 0.275*** | 0.27 | 0.3 (0.0053) | 0.63*** | 0.33 (0.010) | 0.459*** |
| Woody | 0.13 | 0.13 (0.0077) | 0.29*** | 0.083 (0.012) | 0.122*** | 0.35 | 0.31 (0.0084) | 0.67*** | 0.18 (0.009) | 0.527*** |
| Herbaceous | 0.23 | 0.19 (0.0084) | 0.29*** | 0.17 (0.013) | 0.161*** | 0.08 | 0.24 (0.0084) | 0.4*** | 0.23 (0.012) | 0.311*** |
| Stem diameter DBH | ||||||||||
| Trees | 1.09 | 0.41 (0.08) | 0.16** | 0.50 (0.10) | 0.221*** | 0.76 | 0.51 (0.11) | 0.16** | 0.31 (0.10) | 0.112* |
***, P < 0.0001; **, P < 0.001; *, P < 0.01. PIC regression intercepts (β 0) set to zero.
High phylogenetic signals for woody plant root system lateral spreads (L R) and aboveground size (H S, W S, V S, and DBH)
Pagel’s lambda values for woody plants showed high phylogenetic signals for aboveground size traits (H S λ = 0.934; W S λ = 0.750; V S λ = 1.0; DBH λ = 0.922) and for root lateral spread (L R λ = 0.865; Table S3). Lambda values for herbaceous plants were much lower than those of woody plants, suggesting a lower phylogenetic signal, except for maximum rooting depth, where the phylogeny of herbs accounted for more of the variation in D R values (herb D R λ = 0.644 and woody D R λ = 0.271; Table S3). For herbaceous L R, W S, and V S the phylogeny accounted for little to no variation in trait values across species (λ < 0.2), whereas phylogeny had a moderate effect on shoot height (H S λ = 0.558).
There remained a positive relationship between shoot size and root system size (Table 3), even when using phylogenetically independent contrasts for woody and herbaceous plants, except for the relationship between H S and belowground extents (D R and L R) when combining woody and herbaceous plants (Figs S4a, S5a). The negative PIC slopes (β 1) for D R (β 1 = −0.19, P < 0.0001) and L R (β 1 = −0.05, P = 0.18) when regressed against H S were due to the strong phylogenetic signal for H S (λ = 0.985), and the large differences between the shoot heights of woody and herbaceous plants (Table 3; Figs S4a, S5a). Overall, the PIC regressions and correlations of above to belowground plant size (Figs S4, S5) tended to be consistent with the linear relationships between plant extents (Fig. 5; Table 3).
Plant size extents across morphological and leaf traits
Plant size extents differed significantly across the leaf and morphological traits we collected (i.e. lifespan, tissue, seed category, leaf longevity, leaf form, and photosynthetic pathway; Table 2). The average absolute extents (D R and L R) of perennials were more than six times greater than for annuals, but their extents relative to shoot volume were four times greater (Table 2). The D R and LR of woody plants were, respectively, six times and 10 times greater than forherbs, but the D R/V S of herbs was 2.3 times greater than for woody plants.
Among woody plants (trees and shrubs), deciduous plants had lateral spreads that were an average of 5.67 m (twice the width of evergreens), and broadleaf plants had an average D R of 3.59 m (two times deeper than needle‐leaf plants) (Table 2). Aboveground, we found similar trends, with perennial and woody plants having greater shoot heights H S and widths W S (Table 2) than annual and herbaceous plants did. Deciduous trees had average shoot widths of 2.6 m, which is twice that of evergreen trees. The H S values of needle‐leaf plants were 7.74 m, also two times the H S of broadleaf plants, whereas D R was two times deeper for broadleaf plants than for needle‐leaf plants.
Deeper roots in drier and more seasonal climates
We found significant linear relationships between the rooting extents and the primary climate metrics (MAE, MAP, A i, and S a; see Table 1 for abbreviation definitions) we analyzed (Fig. S6; Table S4). Rooting depth D R correlated positively with MAE and negatively with A i and MAP (P < 0.0001). Lateral spread L R was positively related to MAP and A i for all plants. L R was negatively related to MAE for herbs, and with S a for woody plants (P < 0.0001; Fig. S6; Table S4).
Though the PIC results tended to agree with the trends shown with the linear regressions (Table S4; Figs S6–S8) there were a few instances where the trends of the PIC results differed from the log‐linear regressions. For example, there was a positive linear relationship between L R and MAP for woody plants (β 1 = 0.21 ± 0.04; P < 0.0001; Table S4), whereas the PIC regression showed a negative relationship (β 1 = −0.45; P < 0.0001; Table S4).
Differences in predictor importance for shoot and root extents
Our random forest approach highlighted the important predictors for each of the plant size extents, with climate and temperature seasonality being important for D R and shoot size and plant characteristics being the most important for L R (Fig. 6a–d). Climate descriptors such as MAE, mean annual temperature, temperature seasonality, and maximum temperature were the most important predictors of D R (Fig. 6a,c), with D R increasing with warmer and more seasonal climates. L R was mostly affected by shoot size (H S and W S, Fig. 6d) and plant descriptors (i.e. growth form and family; Fig. 6b,d). Partial dependencies showed that L R was greatest in woody plants, and in less seasonal climates (i.e. climates where temperature seasonality < 500, annual temperature range < 25°C, and isothermality > 50). When shoot size (W S, H S, and V S) was omitted from the random forest analyses (Fig. 6a,b) it had little effect on the variable importance ranking for D R, but it led to growth form, family, phylogeny, and isothermality becoming the most important variables for predicting L R.
Aboveground, the growth form and family were among the most important predictors of H S and W S, followed by various climate metrics (Fig. 6c,d). The partial dependencies showed that trees, and plant families primarily made up of trees, represented the greatest H S and W S values. Additionally, H S and W S were greatest in less arid climates (A i > 1). H S was greatest in climates with high precipitation and high seasonal water surplus (P sur > 0.3). W S was greatest in climates with low seasonal water deficits (P def < 0.2) and colder climates (mean annual temperature < 10°C).
Divergence in woody plant dimensions across aridity
The dimensions of woody plants shifted towards deeper and narrower root systems in more arid climates and towards taller and narrower shoots in relatively humid climates (Fig. 7a). Significant shifts in Y : X and S : R values with climate were seen only for woody plants (Fig. 7; Table S5). The aspect ratios of shoots and roots (Y : X shoot and Y : X root) for woody plants in arid climates (A i < 0.2) did not differ significantly (P = 0.308) (Fig. 7a). The average Y : X shoot and Y : X root values of woody plants in climates where A i < 0.5 were 1.8 and 1.3, respectively. As A i increased, the aspect ratio curves diverged, crossing at an A i of 0.43, near the arid–humid threshold (A i = 0.5; Fig. 7a). The Y : X shoot curve saturated in humid climates (A i > 0.5). Y : X root decreases as climates become more humid, with root systems being wider relative to their depth (Y : X root < 1) at an A i of 0.73. The average Y : X shoot and Y : X root values of woody plants in humid climates were 1.6 and 0.7, respectively.
Woody plants, on average, had shoots taller relative to rooting depth in humid climates (S : Ry > 1 when A i > 0.34), but in arid climates the rooting depth is generally greater than shoot height (Fig. 7b). In arid climates, woody plants tended to be both wider and deeper belowground than aboveground, with S : R values < 1 (Fig. 7b, purple). Horizontal allometry does not shift much across A i; and the mean S : Rx is 0.44, indicating that woody plants are, on average, more than two times wider belowground than aboveground. Herb S : R and Y : X values do not shift substantially across A i (Fig. 7c,d), with mean S : R values < 1 (S : Ry = 0.654; S : Rx = 0.452), indicating that herbs generally take up more vertical and horizontal space belowground relative to aboveground dimensions.
Comparing RSIP individual plant data to broader scale estimates of maximum rooting depth
We compared the average RSIP maximum rooting depths (D R) across growth forms and biomes with the biome‐based estimates from the RPGE (D 95; Fig. 8). For all biomes with trees, the average tree D R was significantly deeper than D 95 (ecosystem‐scale maximum rooting depth; Table 1), sometimes by several meters, except for boreal forests, where D 95 was deeper. Tropical and seasonally dry climates had the largest disagreement between the RPGE and RSIP values, with D R values for multiple growth forms being significantly deeper than D 95 (Fig. 8). The ELM PFT parameters closely resembled RPGE D 95 estimates, except that ELM assigns tropical forest trees a maximum rooting depth of 3 m (Drewniak, 2019).
Fig. 8.
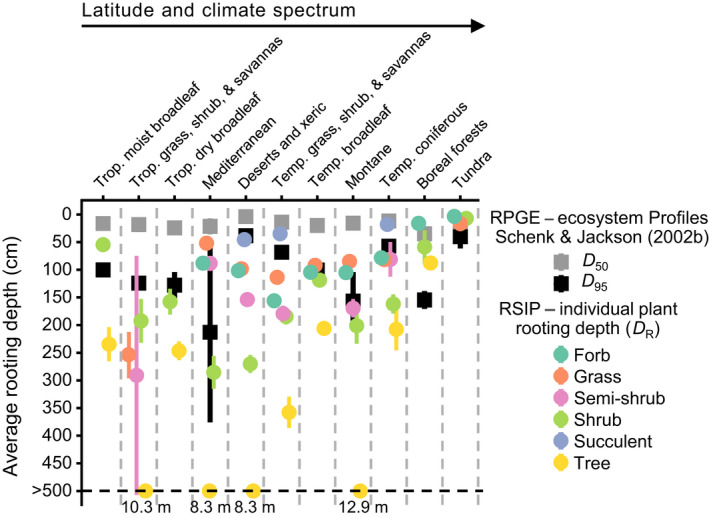
Average maximum rooting depths per biome (±SE) for the Root Profiles for Global Ecosystems (RPGE) in black and gray squares. The Root Systems of Individual Plants (RSIP) maximum rooting depths D R are averaged by biome growth form and biome (colored circles). When the average rooting depth exceeds 5 m, that depth is explicitly stated. D 50 (gray squares) is the 50th percentile rooting depth for ecosystem profiles, and D 95 (black squares) is the effective maximum rooting depth for an ecosystem, the 95th percentile rooting depth (Schenk & Jackson, 2002b).
The ecosystem‐scale D 50 (50th percentile rooting depth) was more sensitive to climate than the individual‐plant (D R) and ecosystem‐scale (D 95) rooting depths were. The D 50 climate coefficients are greater than the D R coefficients, showing that D 50 is more heavily skewed by climate variables than D R is (Fig. S3). This is exemplified by the slope of the linear regression across the coefficients, where a unit slope is a one‐to‐one relationship between D R and D 50 coefficients; however, the slope was 0.52 with an R 2 = 0.81 (y = 0.52x − 0.016; Fig. S3). The opposite was true for D 95 vs D R, where the slope of the regression across the climate coefficients was 2.62 (y = 2.62x − 0.04, R 2 = 0.71; Fig. S3), indicating that individual plant rooting depth D R is more variable across climates than ecosystem‐level rooting depth D 95 is. At both the individual plant and ecosystem scales, roots were deeper in climates with higher mean and maximum temperatures and in climates with greater seasonal deficits of precipitation (Fig. S3). Shallower roots were found in humid climates and in climates with greater surpluses of precipitation (Fig. S3).
Discussion
Using our expanded RSIP database, we found the following patterns in plant size and shape globally: shoot size and root system size strongly covary; water availability and plant characteristics greatly influence shoot size, whereas rooting depth is primarily influenced by temperature seasonality and lateral spread by shoot size; woody plants have deeper, narrower root systems in more arid climates and taller shoots in humid climates; and estimates of the depth and lateral spread of plant root systems are likely underestimated at the global scale.
Shoot size covaries strongly with root system size across plant functional groups
Both rooting depth D R and lateral spread L R scale linearly with aboveground size extents (H S, W S, V S, and DBH; Table 3), as expected with allometric allocation (Niklas & Enquist, 2001; Enquist & Niklas, 2002). Furthermore, multiple tropical forest studies have highlighted the link between shoot size and root system size (Ivanov et al., 2012; Brum et al., 2019; Smith et al., 2019). Of all the aboveground variables, shoot width W S had the strongest positive relationship to rooting depth and spread across all plants (Table 2). Traditionally, stem diameter DBH is the most common metric used for allometric scaling in forestry and plant physiology (Cermák et al., 1998; Ledo et al., 2017), including long‐standing allometric relationships between DBH and crown radius (Dawkins, 1963; O’Brien et al., 1995). DBH has also been used to estimate coarse root biomass (Tobin et al., 2007; Gou et al., 2017) and effective rooting depth (Brum et al., 2019). However, though DBH strongly correlated with shoot height and width, DBH did not correlate with rooting depth in our analysis (Fig. S9). We suggest that shoot width may be better used to estimate the size of root systems, as it correlates positively with both rooting depth and spread (Table 3; Fig. S9).
As expected, woody plants, especially trees, had the largest rooting (D R and L R) and shoot extents (W S, and H S) and herbs had the smallest extents. Succulents have unique root system shapes, having the shallowest root systems yet wider lateral extents than herbs do (Fig. 4). One explanation for the shape of succulent root systems may be that succulents are found in regions with extremely dry soils, where shallow and elongated root systems are adapted to acquiring intermittent rainfall and fog (Jordan & Nobel, 1984; February et al., 2013). Additionally, there can be a large degree of rooting depth plasticity within the same species and environment. For example, a study on the rooting depth of Panicum maximum, a tropical perennial bunchgrass, in the state of São Paulo, Brazil, found rooting depths for mature grasses ranging from 0.85 to 4.85 ms across > 50 observations (Villares et al., 1953).
We found that the rooting depths of annuals and herbs relative to shoot volume (D R/V S) were much greater than that of perennials and woody plants, demonstrating an investment in belowground organs by shorter‐lived plants. John Ernest Weaver (1958) observed the rooting patterns of forbs and found that some forbs can root deeply below the root zone of neighboring plants to avoid competition, quickly occupying depths of greater than 1.2 m in their first growing season and up to 4.6 m at maturity. The deepest‐rooted forb in the RSIP, Alhagi maurorum – commonly known as camelthorn – reached rooting depths of 20 m but rarely exceeded 1.2 m in height (Nechaeva, 1985). The need of some plants to root deeply could also be due to competition in the form of root territoriality or resource depletion (Schenk, 2006, 2008a). The ability of plants such as herbs (which we often think of as being ‘small’ aboveground) to root at times several meters in depth is surprising.
Maximum lateral root spreads were strongly influenced by shoot size, even more than maximum rooting depth (Figs 5, S9; Table 3), further evidenced by the high variable importance of shoot height, width, and volume in the lateral extent random forest model (Fig. 6d). Modeling studies have demonstrated that lateral roots are more efficient at anchoring larger aboveground plants than deep roots are (Ennos, 1993), and a strong linear relationship has been found between lateral spread and stem diameter (Schwarz et al., 2010). The relationship between lateral extent and shoot size highlights the potential importance of lateral root reinforcement for shoot anchorage.
Not surprisingly, there were strong phylogenetic signals for several size and shape traits, especially for aboveground traits, but also for the lateral spread of root systems. Plant species within a genus tend to be similar in growth form, and many plant families consist predominantly of woody plants, herbs, or succulents; and some woody families include mostly trees, whereas others include mostly shrubs and smaller trees. Different environments that favor trees, smaller woody plants, or herbs will therefore cause ecological and evolutionary sorting of genera and families, and historical effects will contribute to this sorting as well (Herrera, 1992), leaving what appears to be a strong phylogenetic signal in plants sizes and shapes (see Table S3).
The effect of temperature and precipitation seasonality on rooting depth
Relationships among above and belowground plant traits that we found are not static across the climate space. Our results agree with Schenk & Jackson (2002a), who found that plants root deeper relative to shoot size in arid climates. A global meta‐analysis of forest biomass allocation found contrasting results, where root biomass decreased with temperature – analogous with MAE – but found no relation with aridity (Reich et al., 2014), potentially highlighting the difference between the space occupied by roots and overall biomass. A decrease in root system size relative to shoot size as climates become less arid would be expected under a plant resource economics framework, where increased water availability would allow plants to invest in aboveground growth when they are no longer limited by water availability belowground (Shipley & Meziane, 2002; Farrior et al., 2015; Anderegg et al., 2016; O'Brien et al., 2017).
Metrics of climate seasonality, specifically temperature seasonality and proxies for water availability, are important for understanding global rooting patterns. The relationship between precipitation seasonality and deep rooting has been well documented in seasonally dry ecosystems (Nepstad et al., 1994; Oliveira et al., 2005; Singh et al., 2020), although predicting deep rooting using global climate metrics is difficult given the complexities of plant–soil–water interactions. However, we provide ample evidence that deeper roots are more likely to occur in arid climates with hotter temperatures and seasonal precipitation (Figs 6a, S3, S6).
The exact relationships between rooting extents and seasonality are still unclear because we need root data at finer scales coupled with measures of seasonality that serve as better proxies for plant‐available water, such as plant‐accessible water storage capacity, dry‐season water drawdown, and climatic water deficit (Fellows & Goulden, 2017; Ledo et al., 2017; Klos et al., 2018). Additionally, as climates change, metrics of interannual seasonality may provide insight on the climates that a plant is adapted to and its rooting response (Fischer et al., 2013; Pratt & Mooney, 2013; Stocker et al., 2013; Knapp et al., 2015). For instance, a study in an arid grassland found that increased interannual variability in precipitation causes a shift in community composition towards deeply rooted shrubs (Weltzin & McPherson, 2000; Gherardi & Sala, 2015). One promising method to understand spatial patterns in maximum rooting depth is to consider the climatology of the cumulative water deficit to estimate the rooting zone water storage capacity to which plants are adapted (Gao et al., 2014; Stocker et al., 2021).
Above and belowground woody plant geometries diverge across climates
Woody plants shift their shapes across climates more than other plant types do (i.e. herbs), with woody root systems being relatively narrower in arid climates and relatively wider in humid climates (Fig. 7a, red). In arid climates, woody plants are short and wide aboveground (Y : X shoot < 1); an important transition occurs at the semi‐arid to subhumid boundary, where plants become taller in relation to their width (Fig. 7a). Shoot height increases as plants compete for light, especially when plants are no longer limited by other resources (Falster & Westoby, 2003; Craine & Dybzinski, 2013). In humid climates, the aspect ratio of shoots (Fig. 7a, blue) does not change much, potentially because of the biological limits to the possible shoot size that plants can support and plants could be limited by other resources (Reich et al., 2003; Koch et al., 2004; Westoby & Wright, 2006; Niklas, 2007; Moles et al., 2009; Krishnamurthy, 2015). Overall, the shapes of woody plants above and belowground diverge across the climate space, where, as aridity decreases, root systems widen and shoots narrow (Fig. 7a). The aspect ratio of root systems decreases with increasing humidity, representing a relative widening of root systems. This could demonstrate a shift in resource priority, where, as plants become less limited by water availability, root systems may prioritize lateral growth to increase nutrient foraging (Lynch, 2005) and to anchor larger aboveground plants (Gilman, 1990; Dupuy et al., 2007).
Woody and herbaceous plants’ root systems exhibit widths that are more than twice their shoot widths on average (S : Rx values of 0.44 and 0.45, respectively). Our results are consistent with the literature review by Schwarz et al. (2010), who found that the lateral radius of tree roots is typically one to three times the shoot radius. The greater widths reached by plants belowground contradicts the common misconception that the width of root systems mirrors the width of shoots (Day et al., 2010; Sinacore et al., 2017). For example, a whole‐tree harvest study found that, unlike the tightly packed crowns of forest trees, roots overlap greatly with their neighbors, resulting in root system radii being twice that of crown radii (Sinacore et al., 2017). We postulate that S : Rx may display plasticity across other resource and competition gradients, such as nutrients belowground (Lynch, 2005), light aboveground (Takenaka, 1994; Cermák et al., 1998; Vieilledent et al., 2010), or increased competition with neighboring plants (Schenk et al., 1999; Schenk, 2006; Cahill et al., 2010; Lepik et al., 2021).
Whereas woody plants growing in more arid climates had deeper, narrower root systems than woody plants in humid climates did, herbs – forbs and grasses – did not show the same trend. Herb root systems may rely on other trait‐based strategies to cope with resource stress (Roumet et al., 2016; Freschet et al., 2018; Wang et al., 2020), such as going dormant or shedding fine roots during the dry season (Eissenstat & Yanai, 1997), increasing root density to avoid dehydration (Norton et al., 2016; Singh et al., 2020), and optimizing for fast resource uptake by having a high specific root length (Roumet et al., 2006). However, we did find that herbs occupy much more space belowground compared with aboveground (Fig. 7), which could be part of a stress or disturbance‐coping strategy (Singh et al., 2020).
Are we underestimating plant rooting depth?
The RPGE dataset has been a primary source of rooting depth data used by many Earth system models, usually incorporated to parameterize biome‐level or PFT rooting depth distributions (Schenk & Jackson, 2002b, 2005; Warren et al., 2015). For example, the US Department of Energy's ELM uses the RPGE to inform its PFT maximum rooting depth, a static parameter, with the exception that tropical tree PFT maximum rooting depths were set to 3 m based on expert opinion (Fig. S2; Drewniak, 2019), considerably deeper than the RPGE estimates. Across several biomes, our analysis found that RSIP rooting depths averaged by growth form were much deeper than the RPGE biome‐level estimates (Fig. 8). This is especially true for biomes with high seasonality and deeply rooted woody plants, such as tropical, Mediterranean, xeric, and forested biomes (Fig. 8).
Comparing RSIP D R values with the RPGE, we found that D 50 was very sensitive to changes in temperature, whereas individual plant maximum rooting depth D R was slightly more sensitive to climate parameters than D 95 was (Fig. S3). The correlations between climate and estimates of rooting depths are important because the estimates are generally used to characterize entire biomes or PFTs without considering environmental changes within biomes. Furthermore, studies have shown that terrestrial‐biosphere models are sensitive to changes in plant rooting depth, leading to significant global variations in gross primary productivity, evapotranspiration, nitrogen uptake, and more – suggesting a more accurate and dynamic approach to modeling the size of plant root systems is needed (Kleidon & Heimann, 1998; Warren et al., 2015; McCormack et al., 2017; Drewniak, 2019). Based on our findings, we suggest the following: rooting depth distribution should be modelled dynamically, by accounting for resource availability and plant optimality, as suggested in previous studies (Schenk, 2008b; Drewniak, 2019), and that the RSIP D R data could be used to parameterize maximum rooting depth across PFTs, whereas the RPGE D 50 could inform the relative distribution of roots within the vertical soil column.
Significance and pitfalls
Our study provides a global synthesis of maximum plant extents and dimensions and shows that the lateral spread of root systems covaries strongly with aboveground plant size, whereas rooting depth is much more influenced by temperature and climate seasonality. As suggested by Tumber‐Dávila & Malhotra (2020), in addition to climate variables, future studies should also focus on root system characteristics across resource gradients. Future studies could also characterize plant volumes above and belowground more explicitly. There are additional environmental constraints on root systems that should be investigated, such as the temporal or vertical availability of plant‐accessible water and plant–soil interactions that we were unable to accurately test at the global scale, leaving a need for additional studies at the ecosystem or individual plant scales (Brantley et al., 2017; Erktan et al., 2018).
We present novel findings on relationships of plant size and shape above and belowground, and across the climate spectrum. Given that aboveground plant size is a major axis of variation in the global spectrum of plant form and function (Díaz et al., 2016; Joswig et al., 2022) and that our results characterized strong links between above‐and belowground plant size, our analysis and the RSIP can contribute to an improved understanding of plant size trade‐offs above and belowground. Better predicting these trade‐offs would have far‐reaching consequences for understanding nutrient, water, and carbon cycling of ecosystems.
Author contributions
SJT‐D wrote the manuscript with critical input and revisions from RBJ, HJS and ED. All authors contributed significantly to the design of the study and the analyses. HJS and RBJ created the original RSIP database that the study builds upon.
Supporting information
Dataset S1 Root systems of individual plants database.
Fig. S1 Map of Root Systems of Individual Plants Database (RSIP) observations by versions.
Fig. S2 Comparison of Root Profiles for Global Ecosystems (RPGE) rooting depth estimates to the plant functional type (PFT) estimates.
Fig. S3 The effect that climate variables have on individual plant rooting depth vs ecosystem‐scale rooting depth.
Fig. S4 PIC of maximum rooting depth (D R) to aboveground plant size (H S, W S, V S, and DBH).
Fig. S5 PIC of maximum lateral spread (L R) to aboveground plant size (H S, W S, V S, and DBH).
Fig. S6 The influence of climate metrics on max rooting depth (D R) and maximum lateral spread (L R).
Fig. S7 PIC of maximum rooting depth (D R) to climate metrics (MAE, MAP, A i and S a).
Fig. S8 PIC of maximum lateral spread (L R) to climate metrics (MAE, MAP, A i and S a).
Fig. S9 Correlation matrix for the above and belowground plant size metrics.
Table S1 Description of RSIP parameters (n is the total number of observations).
Table S2 RSIP categorical groups. The number of total observations n, and unique species, geographic locations, and studies for each class are shown.
Table S3 Pagel’s lambda values for the above and belowground plant measurements.
Table S4 Comparison of absolute extents (D R and L R) with climate metrics.
Table S5 Nonlinear regression curves for the shape ratios plotted in Fig. 7.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Acknowledgements
Thank you to the multiple reviewers and editors at New Phytologist for their valuable insights. We are grateful for the support provided by The Department of Earth System Science at Stanford University. Thank you to the Jackson Lab mates, A Malhotra, and K Harrison, who reviewed the manuscript and provided additional input. Many thanks to the researchers who published the data we used for our analysis; our data come from 361 different publications. We further thank everyone who helped build and expand the database, including R. B. Jackson, H. J. Schenk, and the numerous lab technicians (H. Lu, C. Finnegan, Y. Li, M. Guerrero, L. Kim, V. Rodriguez and L. Villa). SJ Tumber‐Dávila’s research was supported by fellowships from the NASEM Ford Foundation Predoctoral Fellowship and the NSF Graduate Research Fellowship Program. RB Jackson acknowledges support from the US Department of Agriculture/National Institute of Food and Agriculture (USDA/NIFA) grant (2012‐68002‐19795).
Appendix A1.
Citations used in the database
- Abbott ML, Fraley L Jr, Reynolds TD. 1991. Root profiles of selected cold desert shrubs and grasses in disturbed and undisturbed soils. Environmental and Experimental Botany 31: 165–178. [Google Scholar]
- Adams ME. 1967. A study of the ecology of Acacia mellifera, A. seyal and Balanites aegyptiaca in relation to land‐clearing. Journal of Applied Ecology 4: 221–237. [Google Scholar]
- Ahmet Birand H. 1938. Untersuchungen zur Wasserökologie der Steppenpflanzen bei Ankara. Jahrbücher für wissenschaftliche Botanik 87: 93–172. [Google Scholar]
- Albertson FW. 1937. Ecology of mixed prairie in west central Kansas. Ecological Monographs 7: 481–547. [Google Scholar]
- Alexandre DY, Ouédraogo SJ. (1992). Variations in root morphology of Faidherbia albida in relation to soil and agronomic effects. In: Vandenbeldt RJ ed. Faidherbia albida in the West African semi‐arid tropics: proceedings of a workshop, 22–26 April 1991, Niamey, Niger. Patancheru, India: International Crops Research Institute for the Semi‐arid Tropics; Nairobi, Kenya: International Centre for Research in Agroforestry, World Agroforestry Centre, 107–110. [Google Scholar]
- Anderson VL. 1927. Studies of the vegetation of the English Chalk: V. The water economy of the chalk flora. Journal of Ecology 15: 72–129. [Google Scholar]
- Antos JA, Halpern CB. 1997. Root system differences among species: implications for early successional changes in forests of western Orgeon. American Midland Naturalist 138: 97–108. [Google Scholar]
- Ashton DH. 1975. The root and shoot development of Eucalyptus regnans F. Muell. Australian Journal of Botany 23: 867–887. [Google Scholar]
- Baitulin IO. 1979. Kornevaja sistema rastenij aridnoj zony Kazakhstana [Root systems of plants of the arid zone of Kazakhstan]. Alma‐Ata, Russia: Nauka. [Google Scholar]
- Baitulin IO. 1996. Root research in natural plant communities of Kazakhstan. Acta Phytogeographica Suecica 81: 7–10. [Google Scholar]
- Baitulin IO, ed. 1993. Fitoékologicheskie issledovanija v juzhnoj Gobi [Phytoecological investigations in southern Gobi]. Alma‐ata, Russia: Gylim. [In Russian with English summary]. [Google Scholar]
- Bang‐xing W. 1991. Studies on the vertical structure of seasonal rain‐forest in Xishuangbanna of Yunnan. Acta Botanica Sinica 33: 232–239. [Google Scholar]
- Barbour MG, Major J, eds. 1988. Terrestrial vegetation of California. (New expanded edition 1988). Davis, CA, USA: California Native Plant Society. [Google Scholar]
- Batanouny KH, Abdel Wahab AM. 1973. Eco‐physiological studies on desert plants VIII. Root penetration of Leptadenia pyrotechnica (Forsk.) Decne. in relation to its water balance. Oecologia 11: 151–161. [DOI] [PubMed] [Google Scholar]
- Batanouny KH, Batanouny MH. 1969. Formation of phytogenic hillocks. II. Rooting habit of plants forming phytogenic hillocks. Acta Botanica Academiae Scientiarum Hungaricae 15: 1–18. [Google Scholar]
- Becker P, Castillo A. 1990. Root architecture of shrubs and saplings in the understory of a tropical moist forest in lowland Panama. Biotropica 22: 242–249. [Google Scholar]
- Bendali F, Floret C, Le Floc'h E, Ponantier R. 1990. The dynamics of vegetation and sand mobility in arid regions of Tunisia. Journal of Arid Environments 18: 21–32. [Google Scholar]
- Berndt HW, Gibbons RD. (1958) Root distribution of some native trees and understory plants growing on three sites within ponderosa pine watersheds in Colorado. US Dep. Agric. For. Serv. Sta. Pap. RM‐37. [Google Scholar]
- Bertiller MB, Beeskow AM, Coronato F. 1991. Seasonal environmental variation and plant phenology in arid Patagonia (Argentina). Journal of Arid Environments 21: 1–11. [Google Scholar]
- Bhimaya CP, Kaul RN. 1965. Root system of four desert tree species. Annals of Arid Zone 4: 185–194. [Google Scholar]
- Biswell HH. 1935. Effects of environment upon the root habits of certain deciduous forest trees. Botanical Gazette 96: 676–708. [Google Scholar]
- Blagoveshchenskiy EN. 1968. The dry savanna of northwest India. Soviet Geography 9: 519–537. [Google Scholar]
- Bonham CD, Mack SE. 1990. Root distributions of Eurotia lanata in association with two species of Agropyron on disturbed soils. Botanical Gazette 151: 522–527. [Google Scholar]
- Bowns JE Jr, Box TW. 1964. The influence of grazing on the roots and rhizomes of seacoast bluestem. Journal of Range Management 17: 36–39. [Google Scholar]
- Branson FA, Miller RF, McQueen IS. 1976. Moisture relationships in twelve northern desert shrub communities near Grand Junction, Colorado. Ecology 57: 1104–1124. [Google Scholar]
- Breckle SW, Agachanjanz O, Rahmann M. 1994. Spezielle Ökologie der gemäßigten und arktischen Zonen Euro‐Nordasiens, 2 nd edn, vol. Band 3. Stuttgart, Jena, Germany: Gustav‐Fischer Verlag. [Google Scholar]
- Breman H, Kessler JJ. 1995. Woody plants in agro‐ecosystems of semi‐arid regions. Berlin, Germany: Springer‐Verlag. [Google Scholar]
- Briones O, Montaña C, Ezcurra E. 1996. Competition between three Chihuahuan desert species: evidence from plant size–distance relations and root distribution. Journal of Vegetation Science 7: 453–460. [Google Scholar]
- Brisson J, Reynolds JF. 1994. The effect of neighbors on root distribution in a creosotebush (Larrea tridentata) population. Ecology 75: 1693–1702. [Google Scholar]
- Brown JH Jr, Woods FW. 1968. Root extension of trees in surface soils of the North Carolina piedmont. Botanical Gazette 129: 1126–1132. [Google Scholar]
- Brown K. 1992. Prosopis cineraria woodlands in Oman, past, present and future. In: Dutton RW, Powell M, Ridley RJ, eds. Prosopis species, aspects of their value research and development. Rome, Italy: Food and Agriculture Organisation of the United Nations, pp. 131–144. [Google Scholar]
- Büttner V, Leuschner C. 1994. Spatial and temporal patterns of fine root abundance in a mixed oak–beech forest. Forest Ecology and Management 70: 11–21. [Google Scholar]
- Bunger MT, Thomson HJ. 1938. Root development as a factor in the success or failure of windbreak trees in the southern High Plains. Journal of Forestry 36: 790–803. [Google Scholar]
- Burbidge NT. 1945. Morphology and anatomy of the Western Australian species of Triodia R. Br. Transactions of the Royal Society of South Australia 69: 303–308. [Google Scholar]
- Burger JC, Louda SM. 1995. Interaction of diffuse competition and insect herbivory in limiting brittle prickly pear cactus. Opuntia fragilis (Cataceae). American Journal of Botany 82: 1558–1566. [Google Scholar]
- Burgess TL. 1995. Desert grassland. Mixed shrub savanna, shrub steppe or semidesert scrub? The dilemma of coexisting growth forms. In: McClaran MP, Van Devender TR, eds. The desert grassland. Tucson, AZ, USA: University of Arizona Press, 31–67. [Google Scholar]
- Bursova TL. 1983. Ekomorfoz kornevoj sistemy rastenij solonchakovych pochv drevnej Del'ty Cyrdar'i [Ecomorphosis of plant root systems in the solonchak soils of the ancient Syr Darya delta (Kazakh SSR, USSR)]. Izvestiia Akademii Nauk Kazakhskoi SSR. Seriia Biologicheskaia 1983: 6–13. [In Russian]. [Google Scholar]
- Cable DR. 1969. Competition in the semidesert grass‐shrub type as influenced by root systems, growth habits, and soil moisture extraction. Ecology 50: 27–38. [Google Scholar]
- Campion WE. 1926. The depth attained by roots. Australian Forestry Journal 9: 128. [Google Scholar]
- Canadell J, Jackson RB, Ehleringer JR, Mooney HA, Sala OE, Schulze ED. 1996. Maximum rooting depth of vegetation types at the global scale. Oecologia 108: 583–595. [DOI] [PubMed] [Google Scholar]
- Cannon HL. 1960. The development of botanical methods of prospecting for uranium on the Colorado Plateau. US Geological Survey Bulletin 1085‐A: 1–50. [Google Scholar]
- Cannon WA. 1911. The root habits of desert plants, vol. 131. Washington, DC, USA: Carnegie Institution of Washington. [Google Scholar]
- Cannon WA. 1913. Notes on root variation in some desert plants. The Plant World 16: 323–341. [Google Scholar]
- Cannon WA. 1914. Specialization in vegetation and in environment in California. The Plant World 17: 223–243. [Google Scholar]
- Cannon WA. 1921. Plant habits and habitats in the arid portions of South Australia, vol. 308. Washington, DC, USA: Carnegie Institution of Washington. [Google Scholar]
- Cannon WA. 1924. General and physiological features of the vegetation of the more arid portions of southern Africa, with notes on the climatic environment, vol. 354. Washington, DC, USA: Carnegie Institution of Washington. [Google Scholar]
- Carbon BA, Bartle GA, Murray AM, MacPherson DK. 1980. The distribution of root length, and the limits to flow of soil water to roots in a dry sclerophyll forest. Forest Science 26: 656–664. [Google Scholar]
- Carter MR, Gregorich EG. 2010. Carbon and nitrogen storage by deep‐rooted tall fescue (Lolium arundinaceum) in the surface and subsurface soil of a fine sandy loam in eastern Canada. Agriculture, Ecosystems and Environment 136: 125–132. [Google Scholar]
- Clark RB, Alberts EE, Zoel RW, Inclair TR, Miller MS, Kemper WD, Foy CD. 1998. Eastern gamagrass (Tripsacum dactyloides) root penetration into and chemical properties of claypan soils. Plant and Soil 200: 33–45. [Google Scholar]
- Cody ML. 1986. Structural niches in plant communities. In: Diamond J, Case TJ, eds. Community ecology. New York, NY, USA: Harper & Row, 381–405. [Google Scholar]
- Coetzee JA, Page MI, Meredith D. 1946. Root studies in Highveld grassland communities. South African Journal of Science 42: 105–118. [Google Scholar]
- Cole HE, Holch AE. 1941. The root habits of certain weeds of southeastern Nebraska. Ecology 22: 141–147. [Google Scholar]
- Cottle HJ. 1931. Studies in the vegetation of southwestern Texas. Ecology 12: 105–155. [Google Scholar]
- Coughenour MB, Ellis JE, Popp RG. 1990. Morphometric relationships and developmental patterns of Acacia tortilis and Acacia reficiens in southern Turkana, Kenya. Bulletin of the Torrey Botanical Club 117: 8–17. [Google Scholar]
- Coupland RT, Johnson RE. 1965. Rooting characteristics of native grassland species in Saskatchewan. Journal of Ecology 53: 475–507. [Google Scholar]
- Currie PO, Hammer FL. 1979. Detecting depth and lateral spread of roots of native range plants using radioactive phosphorus. Journal of Range Management 32: 101–103. [Google Scholar]
- Dabadghao PM, Marwaha SP, Gupta BS, Das RB, Deb Roy R. 1963. Root ecology of some promising desert grasses of Rajasthan. Annals of Arid Zone 1: 163–173. [Google Scholar]
- Damman AWH. 1971. Effect of vegetation changes on the fertility of a Newfoundland forest site. Ecological Monographs 41: 253–270. [Google Scholar]
- Das DK, Chaturvedi OP. 2008. Root biomass and distribution of five agroforestry tree species. Agroforestry Systems 74: 223–230. [Google Scholar]
- Daubenmire RF. 1941. Some ecologic features of the subterranean organs of alpine plants. Ecology 22: 370–378. [Google Scholar]
- David TS, Pinto CA, Nadezhdina N, Kurz‐Besson C, Henriques MO, Quilhó T, Cermak J, Chaves MM, Pereira JS, David JS 2013. Root functioning, tree water use and hydraulic redistribution in Quercus suber trees: a modeling approach based on root sap flow. Forest Ecology and Management 307: 136–146. [Google Scholar]
- David TS, Ferreira MI, Cohen S, Pereira JS, David JS. 2004. Constraints on transpiration from an evergreen oak tree in southern Portugal. Agricultural and Forest Meteorology 122: 193–205. [Google Scholar]
- Davidson E, Lefebvre PA, Brando PM, Ray DM, Trumbore SE, Solorzano LA, Nepstad DC. 2011. Carbon inputs and water uptake in deep soils of an eastern Amazon forest. Forest Science 57: 51–58. [Google Scholar]
- Davis CB. 1972. Comparative ecology of six members of the Arctostaphylos andersonii complex. PhD dissertation, University of California Davis, Davis, CA, USA. [Google Scholar]
- Davis EA, Pase CP. 1977. Root system of shrub live oak: implications for water yield in Arizona chaparral. Journal of Soil and Water Conservation 32: 174–180. [Google Scholar]
- Davis G, Neilsen W, Mcdavitt J. 1983. Root distribution of Pinus radiata related to soil characteristics in five Tasmanian soils. Australian Journal of Soil Research 21: 165. [Google Scholar]
- Dawson T. 1993. Hydraulic lift and water use by plants: implications for water balance, performance and plant–plant interactions. Oecologia 95: 565–574. [DOI] [PubMed] [Google Scholar]
- Day MW. 1941. The root system of red pine saplings. Journal of Forestry 39: 468–472. [Google Scholar]
- Day MW. 1944. The root system of aspen. American Midland Naturalist 32: 502–509. [Google Scholar]
- Day WR. 1959. Observations on eucalypts in Cyprus. II. Root development in relation to soil conditions. Empire Forest Review 38: 186–197. [Google Scholar]
- Dell B, Bartle JR, Tacey WH. 1983. Root occupation and root channels of jarrah forest subsoils. Australian Journal of Botany 31: 615–627. [Google Scholar]
- Derbel S, Chaieb M. 2012. Growth establishment and phenology of four woody Saharan species. African Journal of Ecology 51: 307–318. [Google Scholar]
- DeSouza J, Silka PA, Davis SD. 1986. Comparative physiology of burned and unburned Rhus laurina after chaparral wildfire. Oecologia 71: 63–68. [DOI] [PubMed] [Google Scholar]
- Dhyani SK, Narain P, Singh RK. 1990. Studies on root distributions of five multipurpose tree species in Doon Valley, India. Agroforestry Systems 12: 149–161. [Google Scholar]
- Dittmer HJ. 1959. A study of the root system of certain sand dune plants in New Mexico. Ecology 40: 265–273. [Google Scholar]
- Do FC, Rocheteau R, Diagne AL, Goudiaby V, Granier A, Lhomme J‐P. 2008. Stable annual pattern of water use by Acacia tortilis in Sahelian Africa. Tree Physiology 28: 95–104. [DOI] [PubMed] [Google Scholar]
- Dobrowolski JP, Caldwell MM, Richards JH. 1990. Basin hydrology and plant root systems. In: Osmond CB, Pitelka LF, Hidy GM, eds. Plant biology of the basin and range, vol. 80. Berlin, Germany: Springer‐Verlag, 243–292. [Google Scholar]
- Dodd J, Heddle EM, Pate JS, Dixon KW. 1984. Rooting patterns of sandplain plants and their functional significance. In: Pate JS, Beard JS, eds. Kwongan: plant life on the sandplain. Nedlands, WA, Australia: University of Western Australia, 146–177. [Google Scholar]
- Donovan LA, Richards JH, Muller MW. 1996. Water relations and leaf chemistry of Chrysothamnus nauseosus ssp. consimilis (Asteraceae) and Sarcobatus vermiculatus (Chenopodiaceae). American Journal of Botany 83: 1637–1646. [Google Scholar]
- Dorji T, Totland Ø, Moe SR, Hopping KA, Pan J, Klein JA 2013. Plant functional traits mediate reproductive phenology and success in response to experimental warming and snow addition in Tibet. Global Change Biology 19: 459–472. [DOI] [PubMed] [Google Scholar]
- Dougherty RL, Lauenroth WK, Singh JS. 1996. Responses of a grassland cactus to frequency and size of rainfall events in a North American shortgrass steppe. Journal of Ecology 84: 177–183. [Google Scholar]
- Douglas DA. 1989. Clonal growth of Salix setchelliana on glacial river gravel bars in Alaska. Journal of Ecology 77: 112–126. [Google Scholar]
- Drexhage M, Gruber F. 1998. Architecture of the skeletal root system of 40‐year‐old Picea abies on strongly acidified soils in the Harz Mountains (Germany). Canadian Journal of Forest Research 28: 13–22. [Google Scholar]
- Du J, Wang N, Alpert P, Yu M‐J, Yu F‐H, Dong M. 2010. Clonal integration increases performance of ramets of the fern Diplopterygium glaucum in an evergreen forest in southeastern China. Flora: Morphology, Distribution, Functional Ecology of Plants 205: 399–403. [Google Scholar]
- Dumortier M. 1991. Below‐ground dynamics in a wet grassland ecosystem. In: Atkinson D, ed. Plant root growth, an ecological perspective. Oxford, UK: Blackwell Scientific Publications. [Google Scholar]
- Duncan WH. 1935. Root systems of woody plants of old fields of Indiana. Ecology 16: 554–567. [Google Scholar]
- Dupuy NC, Dreyfus BL. 1992. Bradyrhizobium populations occur in deep soil under the leguminous tree Acacia albida . Applied and Environmental Microbiology 58: 2415–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye PJ. 1996. Response of Eucalyptus grandis trees to soil water deficits. Tree Physiology 16: 233–238. [DOI] [PubMed] [Google Scholar]
- Eamus D, Chen X, Kelley G, Hutley LB. 2002. Root biomass and root fractal analyses of an open Eucalyptus forest in a savanna of north Australia. Australian Journal of Botany 50: 31–41. [Google Scholar]
- Eis S. 1974. Root system morphology of western hemlock, western red cedar, and Douglas fir. Canadian Journal of Forest Research 4: 28–38. [Google Scholar]
- Eis S. 1987. Root systems of older immature hemlock, cedar, and Douglas‐fir. Canadian Journal of Forest Research 17: 1348–1354. [Google Scholar]
- Elliott GRB. 1924. Relation between the downward penetration of corn roots and water level in peat soil. Ecology 5: 175–178. [Google Scholar]
- Ellsworth PZ, Sternberg LSL. 2015. Seasonal water use by deciduous and evergreen woody species in a scrub community is based on water availability and root distribution. Ecohydrology 8: 538–551. [Google Scholar]
- Esler KJ, Rundel PW. 1999. Comparative patterns of phenology and growth from diversity in two winter rainfall deserts: the Succulent Karoo and Mojave Desert ecosystems. Plant Ecology 142: 97–104. [Google Scholar]
- Estrada‐Medina H, Graham RC, Allen MF, Jimenez‐Osornio JJ, Robles‐Casolco S. 2013. The importance of limestone bedrock and dissolution karst features on tree root distribution in northern Yucatan, Mexico. Plant and Soil 362: 37–50. [Google Scholar]
- Evenari M. 1938. Root conditions of certain plants of the wilderness of Judaea. Journal of the Linnean Society of London. Botany 51: 383–388. [Google Scholar]
- Evenari M, Shanan L, Tadmor N. 1982. The Negev: the challenge of a desert, 2 nd edn. Cambride, MA, USA: Harvard University Press. [Google Scholar]
- Fagg C. 1991. Acacia tortilis: fodder tree for desert sands, vol. 91. Waimanalo, HI, USA: Nitrogen Fixing Tree Association. [Google Scholar]
- Fan Y, Li PF, Hou Z, Ren T. 2012. Water adaptive traits of deep‐rooted C3 halophyte (Karelinia caspica (Pall.) less) and shallow‐rooted C4 halophyte (Atriplex tatarica L.) in an arid region, Northwest China. Journal of Arid Land 4: 469–478. [Google Scholar]
- Fan Y, Miguez‐Macho G, Jobbágy EG, Jackson RB, Otero‐Casal C. 2017. Hydrologic regulation of plant rooting depth. Proceedings of the National Academy of Sciences, USA 114: 10572–10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington P, Greenwood EAN, Bartle GA, Beresford JD, Watson GD. 1989. Evaporation from Banksia woodland on a groundwater mound. Journal of Hydrology 105: 173–186. [Google Scholar]
- Farrish KW. 1991. Spatial and temporal fine‐root distribution in three Louisiana forest soils. Soil Science Society of America Journal 55: 1752–1757. [Google Scholar]
- Fayle DCF. 1965. Rooting habit of sugar maple and yellow birch. Published under the authority of the Minister of Forestry. [Google Scholar]
- February EC, Allsopp N, Shabane T, Hattas D. 2011. Coexistence of a C4 grass and a leaf succulent shrub in an arid ecosystem. The relationship between rooting depth, water and nitrogen. Plant and Soil 349: 253–260. [Google Scholar]
- February EC, Cook GD, Richards AE. 2013. Root dynamics influence tree–grass coexistence in an Australian savanna. Austral Ecology 38: 66–75. [Google Scholar]
- Fenner M. 1980. Some measurements on the water relations of baobab trees. Biotropica 12: 205–209. [Google Scholar]
- Fernández A RJ, Paruelo JM. 1988. Root systems of two Patagonian shrubs: a quantitative description using a geometrical method. Journal of Range Management 41: 220–223. [Google Scholar]
- Fisher RA, Williams M, da Costa AL, Malhi Y, da Costa RF, Almeida S, Meir P 2007. The response of an eastern Amazonian rain forest to drought stress: results and modelling analyses from a throughfall exclusion experiment. Global Change Biology 13: 2361–2378. [Google Scholar]
- Flombaum P, Sala O. 2011. Effects of plant species traits on ecosystem processes. Ecology 93: 227–234. [DOI] [PubMed] [Google Scholar]
- Foldats E, Rutkis E. 1975. Ecological studies of Chaparro (Curatella americana L.) and Manteco (Byrsonima crassifolia H.B.K.) in Venezuela. Ecological Studies 2: 159–178. [Google Scholar]
- Follett R, Allmaras R, Reichman G. 1974. Distribution of corn roots in sandy soil with a declining water table. Agronomy Journal 66: 288–292. [Google Scholar]
- Fonteyn PJ, Mahall BE. 1981. An experimental analysis of structure in a desert plant community. Journal of Ecology 69: 883–896. [Google Scholar]
- Forseth IN, Ehleringer JR, Werk KS, Cook CS. 1984. Field water relations of Sonoran Desert annuals. Ecology 65: 1436–1444. [Google Scholar]
- Frangi JL, Lugo AE. 1985. Ecosystem dynamics of a subtropical floodplain forest. Ecological Monographs 55: 351–369. [Google Scholar]
- Franzluebbers AJ, Stuedemann JA. 2009. Soil‐profile organic carbon and total nitrogen during 12 years of pasture management in the Southern Piedmont USA. Agriculture, Ecosystems and Environment 129: 28–36. [Google Scholar]
- Freckman DW, Virginia RA. 1989. Plant‐feeding nematodes in deep‐rooting desert ecosystems. Ecology 70: 1665–1678. [Google Scholar]
- Freycon V, Wonkam C, Fayolle A, Laclau J‐P, Lucot E, Jourdan C, Cornu G, Gourlet‐Fleury S. 2014. Tree roots can penetrate deeply in African semi‐deciduous rain forests: evidence from two common soil types. Journal of Tropical Ecology 31: 13–23. [Google Scholar]
- Frith AC. 1955. No man's land. Empire Forestry Review 34: 179–187. [Google Scholar]
- Gaines KP, Stanley JW, Meinzer FC, McCulloh KA, Woodruff DR, Chen W, Adams TS, Lin H, Eissenstat DM 2016. Reliance on shallow soil water in a mixed‐hardwood forest in central Pennsylvania. Tree Physiology 36: 444–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary HL. 1963. Root distribution of five‐stamen tamarisk, seepwillow, and arrowweed. Forest Science 9: 311–314. [Google Scholar]
- Gaze SR, Brouwer J, Simmonds LP, Bromley J. 1998. Dry season water use patterns under Guiera senegalensis L. shrubs in a tropical savanna. Journal of Arid Environments 40: 53–67. [Google Scholar]
- Gemmer EW. 1928. The root system of a longleaf pine. The Scientific Monthly 27: 384. [Google Scholar]
- Gentile RM, Martino DL, Entz MH. 2003. Root characterization of three forage species grown in southwestern Uruguay. Canadian Journal of Plant Science 83: 785–788. [Google Scholar]
- George AS, Hopkins AJM, Marchant NG. 1979. The heathlands of western Australia. In: Specht RL, ed. Heathlands and related shrublands, vol. 9A. Amsterdam, the Netherlands: Elsevier, 211–230. [Google Scholar]
- Germon A, Cardinael R, Prieto I, Mao Z, Kim J, Stokes A, Dupraz C, Laclau J‐P, Jourdan C 2016. Unexpected phenology and lifespan of shallow and deep fine roots of walnut trees grown in a silvoarable Mediterranean agroforestry system. Plant and Soil 401: 409–426. [Google Scholar]
- Gibbens RP, Lenz JM. 2001. Root systems of some Chihuahuan Desert plants. Journal of Arid Environments 49: 221–263. [Google Scholar]
- Gile LH, Gibbens RP, Lenz JM. 1995. Soils and sediments associated with remarkable, deeply‐penetrating roots of crucifixion thorn (Koeberlinia spinosa Zucc.). Journal of Arid Environments 31: 137–151. [Google Scholar]
- Gile LH, Gibbens RP, Lenz JM. 1998. Soil‐induced variability in root systems of creosotebush (Larrea tridentata) and tarbush (Flourensia cernua). Journal of Arid Environments 39: 57–78. [Google Scholar]
- Giliberto J, Estay H. 1978. Seasonal water stress in some Chilean matorral shrubs. Botanical Gazette 139: 236–240. [Google Scholar]
- Ginzburg C. 1966. Xerophytic structures in the roots of desert shrubs. Annals of Botany 30: 403–418. [Google Scholar]
- Glover JD, Culman SW, DuPont ST, Broussard W, Young L, Mangan ME, Mai JG, Crews TE, DeHaan LR, Buckley DH 2010. Harvested perennial grasslands provide ecological benchmarks for agricultural sustainability. Agriculture, Ecosystems and Environment 137: 3–12. [Google Scholar]
- Glover PE. 1950. The root systems of some British Somaliland plants – I. East African Agricultural Journal 16: 98–113. [Google Scholar]
- Glover PE. 1951. The root systems of some British Somaliland plants – II. East African Agricultural Journal 16: 154–162. [Google Scholar]
- Glover PE. 1951. The root systems of some British Somaliland plants – III. East African Agricultural Journal 16: 205–217. [Google Scholar]
- Glover PE. 1951. The root systems of some British Somaliland plants – IV. East African Agricultural Journal 17: 38–50. [Google Scholar]
- Glover PE, Trump EC, Waterride LED. 1964. Termitaria and vegetation patterns on the Loita Plains of Kenya. Journal of Ecology 52: 367–377. [Google Scholar]
- Golluscio RA, Sala OE. 1993. Plant functional types and ecological strategies in Patagonian forbs. Journal of Vegetation Science 4: 839–846. [Google Scholar]
- Golovina RD. 1991. Root systems of juniper forest of the Alai Ridge. Soviet Forest Sciences 1991: 64–67. [Google Scholar]
- Goor AY, Barney CW. 1968. Forest tree planting in the arid zones. New York, NY, USA: The Ronald Press Company. [Google Scholar]
- Greenland DJ, Kowal JML. 1960. Nutrient content of the moist tropical forest of Ghana. Plant and Soil 12: 154–174. [Google Scholar]
- Gries D, Zeng F, Foetzki A, Arndt SK, Bruelheide H, Thomas FM, Zhang X, Runge M. 2003. Growth and water relations of Tamarix ramosissima and Populus euphratica on Taklamakan desert dunes in relation to depth to a permanent water table. Plant, Cell & Environment 26: 725–736. [Google Scholar]
- Groeneveld DP. 1989. Shrub rooting and water acquisition on threatened shallow groundwater habitats in the Owens Valley, California. In: Proceedings – Symposium on cheatgrass invasion, shrub die‐off, and other aspects of shrub biology and management, vol. INT‐276. Ogden, UT, USA: U.S.D.A. Forest Service Intermountain Research Station, 221–237. [Google Scholar]
- Groeneveld DP, Crowley DE. 1988. Root system response to flooding in three desert shrub species. Functional Ecology 2: 491–497. [Google Scholar]
- Groot JJR, Koné D, Traoré M, Kamissoko N. 1995. Description du système racinaire de l'Andropogon gayanus, du Vigna unguiculata et du Stylosanthes hamata en zone soudano‐sahélienne, vol. 8. Wageningen, the Netherlands: Production Soudano‐Sahélienne (PSS). [Google Scholar]
- Guinness . 2003. Guinness world records 2004. New York, NY, USA: Guinness World Records Ltd. [Google Scholar]
- Gulmon SL, Rundel PW, Ehleringer JR, Mooney HA. 1979. Spatial relationships and competition in a Chilean desert cactus. Oecologia 44: 40–43. [DOI] [PubMed] [Google Scholar]
- Haag CL, Johnson JE, Erdmann GG. 1989. Rooting depths of red maple (Acer rubrum L.) on various sites in the Lake States. US Dep. Agric. For. Serv. Res. Note NC‐347. [Google Scholar]
- Haase P, Pugnaire FI, Fernández EM, Puigdefábregas J, Clark SC, Incoll LD 1996. An investigation of rooting depth of the semiarid shrub Retama sphaerocarpa (L.) Boiss. by labelling of ground water with a chemical tracer. Journal of Hydrology 177: 23–31. [Google Scholar]
- Haasis FW. 1921. Relations between soil type and root form of western yellow pine seedlings. Ecology 2: 292–303. [Google Scholar]
- Haigh H. 1966. Root development in the sandy soils of Zululand. Forestry in South Africa 7: 31–36. [Google Scholar]
- Hanes TL. 1965. Ecological studies on two closely related chaparral shrubs in southern California. Ecological Monographs 35: 213–235. [Google Scholar]
- Hao Y, Peng S, Mo J, Liu X, Chen Z, Zhou K, Wu J. 2006. Roots of pioneer trees in the lower sub‐tropical area of Dinghushan, Guangdong, China. Journal of Zhejiang University Science B 7: 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GA. 1967. Some competitive relationships between Agropyron spicatum and Bromus tectorum . Ecological Monographs 37: 89–111. [Google Scholar]
- Haworth F. 1953. Observations on the root system of Guatemala grass on an upland tea soil in Ceylon. Tropical Agriculture 30: 116–121. [Google Scholar]
- Hayes FA, Stoeckeler JH. 1935. Soil and forest relationships of the shelter beltzone. In: USFS The Lake States Forest Experiment Station, ed. Possibilities of shelterbelt planting in the Plains Region. Washington, DC, USA: United States Government Printing Office, 111–153. [Google Scholar]
- He WM, Zhang XS. 2003. Responses of an evergreen shrub Sabina vulgaris to soil water and nutrient shortages in the semi‐arid Mu Us Sandland in China. Journal of Arid Environments 53: 307–316. [Google Scholar]
- Hellmers H, Horton JS, Juhren G, O'Keefe J. 1955. Root systems of some chaparral plants in southern California. Ecology 36: 667–678. [Google Scholar]
- Hendrick RL, Pregitzer KS, Hendrick RL, Pregitzer KS. 1996. Temporal and depth‐related patterns of fine root dynamics in northern hardwood forests. Journal of Ecology 84: 167–176. [Google Scholar]
- Heyward F. 1933. The root system of longleaf pine on the deep sands of western Florida. Ecology 14: 136–148. [Google Scholar]
- Higgins KB, Lamb AJ, van Wilgen BW. 1987. Root systems of selected plant species in mesic mountain fynbos in the Jonkershoek Valley, south‐western Cape Province. South African Journal of Botany 53: 249–257. [Google Scholar]
- Hipondoka MHT, Aranibar JN, Chirara C, Lihavha M, Macko SA. 2003. Vertical distribution of grass and tree roots in arid ecosystems of southern Africa: niche differentiation or competition? Journal of Arid Environments 54: 319–325. [Google Scholar]
- Hironaka M. 1961. The relative rate of root development of cheatgrass and medusahead. Journal of Range Management Archives 14: 263–267. [Google Scholar]
- Hnatiuk RJ, Hopkins AJM. 1980. Western Australian species‐rich kwongan (sclerophyllous shrubland) affected by drought. Australian Journal of Botany 28: 573–585. [Google Scholar]
- Hoffmann A. 1978. Root studies in the Chilean matorral. Oecologia 32: 57–69. [DOI] [PubMed] [Google Scholar]
- Holdo RM, Timberlake J. 2008. Rooting depth and above‐ground community composition in Kalahari sand woodlands in western Zimbabwe. Journal of Tropical Ecology 24: 169–176. [Google Scholar]
- Hopkins HH. 1951. Ecology of the native vegetation of the loess hills in central Nebraska. Ecological Monographs 21: 125–147. [Google Scholar]
- Horton KW. 1958. Rooting habits of lodgepole pine. Forest Research Division Technical Note no. 67. Ottawa, ON, Canada: Department of Northern Affairs and National Resources. doi: 10.1038/180270a0. [DOI] [Google Scholar]
- Hosegood PH. 1963. The root distribution of kikuyu grass and wattle trees. East African Agricultural and Forestry Journal 29: 60–61. [Google Scholar]
- Hosegood PH, Howland P. 1966. A preliminary study of the root distribution of some exotic tree crops, evaluated by a rapid sampling method. East African Agricultural and Forestry Journal 32: 16–18. [Google Scholar]
- Howard A. 1925. The effect of grass on trees. Proceedings of the Royal Society of London. Series B: Biological Sciences 97: 284–321. [Google Scholar]
- Hubble TCT, Docker BB, Rutherfurd ID. 2010. The role of riparian trees in maintaining riverbank stability: a review of Australian experience and practice. Ecological Engineering 36: 292–304. [Google Scholar]
- Hulbert LC. 1955. Ecological studies of Bromus tectorum and other annual bromegrasses. Ecological Monographs 25: 181–213. [Google Scholar]
- Hull JC, Muller CH. 1977. The potential for dominance by Stipa pulchra in a California grassland. American Midland Naturalist 97: 147–175. [Google Scholar]
- Humphrey RR. 1937. Ecology of the burroweed. Ecology 18: 1–9. [Google Scholar]
- Ignatenko IV, Knorre AV, Lovelius NV, Norin BN. 1972. Standing crop in plant communities at the station Ary‐Mas. In: Wielgolaski FE, Rosswall T, eds. Tundra biome. Stockholm, Sweden: Tundra Biome Steering Committee, 140–148. [Google Scholar]
- Imai N, Kitayama K, Titin J. 2010. Distribution of phosphorus in an above‐to‐below‐ground profile in a Bornean tropical rain forest. Journal of Tropical Ecology 26: 627–636. [Google Scholar]
- Jackson RB, Banner JL, Jobbágy EG, Pockman WT, Wall DH. 2002. Ecosystem carbon loss with woody plant invasion of grasslands. Nature 277: 623–627. [DOI] [PubMed] [Google Scholar]
- Jackson RB, Moore LA, Hoffmann WA, Pockman WT, Linder CR. 1999. Ecosystem rooting depth determined with caves and DNA. Proceedings of the National Academy of Sciences, USA 96: 11387–11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen DH. 1970. Jaquinia pungens, an heliophile from the understory of tropical deciduous forests. Biotropica 2: 112–119. [Google Scholar]
- Jaramillo VJ, Ahedo‐Hernndez R, Kauffman JB. 2003. Root biomass and carbon in a tropical evergreen forest of Mexico: changes with secondary succession and forest conversion to pasture. Journal of Tropical Ecology 19: 457–464. [Google Scholar]
- Jeffers JNR, Boaler SB. 1966. Ecology of a Miombo site, Lupa North Forest Reserve, Tanzania: I. Weather and plant growth, 1962–64. Journal of Ecology 54: 447–463. [Google Scholar]
- Jeník J. 1971. Root structure and underground biomass in equatorial forests. In: Duvigneaud P, ed. Productivity of forest ecosystems, vol. 4. Paris, France: UNESCO, 323–331. [Google Scholar]
- Jennings CMH. 1974. The hydrology of Botswana. PhD thesis, University of Natal, Pietermaritzburg, South Africa. [Google Scholar]
- Jiménez EM, Moreno FH, Lloyd J, Peñuela MC, Patiño S. 2009. Fine root dynamics forforests on contrasting soils in the Colombian Amazon. Biogeosciences Discussions 6: 3415–3453. [Google Scholar]
- Jipp PH, Nepstad DC, Cassel DK, Reis De Carvalho C. 1998. Deep soil moisture storage and transpiration in forests and pastures of seasonally‐dry Amazonia. Climatic Change 39: 395–412. [Google Scholar]
- Jobbagy EG, Jackson RB. 2004. Groundwater use and salinization with grassland afforestration. Global Change Biology 10: 1299–1312. [Google Scholar]
- Jobbágy EG, Nosetto MD, Villagra PE, Jackson RB. 2011. Water subsidies from mountains to deserts: their role in sustaining groundwater‐fed oases in a sandy landscape. Ecological Applications 21: 678–694. [DOI] [PubMed] [Google Scholar]
- Joffre R, Leiva Morales MJ, Rambal S, Fernández Ales R. 1987. Dynamique racinaire et extraction de l'eau du sol par des graminées pérennes et annuelles méditerranéennes. Acta Oecologica, Oecologia Plantarum 8: 181–194. [Google Scholar]
- Johnson DM, Domec JC, Woodruff DR, McCulloh KA, Meinzer FC. 2013. Contrasting hydraulic strategies in two tropical lianas and their host trees. American Journal of Botany 100: 374–383. [DOI] [PubMed] [Google Scholar]
- Johnson KL, Wright GM, Ashton DH. 1968. Ecological studies of Tunnel Cave, Mt. Eccles. Victoria Naturalist 85: 350–356. [Google Scholar]
- Jones RH, Lockaby BG, Somers GL. 1996. Effects of microtopography and disturbance on fine‐root dynamics in wetland forests of low‐order stream floodplains. American Midland Naturalist 136: 57–71. [Google Scholar]
- Jonsson K, Fidjeland L, Maghembe JA, Högberg P. 1988. The vertical distribution of fine roots of five tree species and maize in Morogoro, Tanzania. Agroforestry Systems 6: 63–69. [Google Scholar]
- Joslin JD, Gaudinski JB, Torn MS, Riley WJ, Hanson PJ. 2006. Fine‐root turnover patterns and their relationship to root diameter and soil depth in a 14C‐labeled hardwood forest. New Phytologist 172: 523–535. [DOI] [PubMed] [Google Scholar]
- Kalisz P, Zimmerman RW, Muller RN. 1987. Root density, abundance, and distribution inthe mixed mesophytic forest of eastern Kentucky. Soil Science Society of America Journal 51: 220–225. [Google Scholar]
- Kalisz PJ, Stringer JW, Volpe JA, Clark DT. 1988. Trees as monitors of tritium in soil water. Journal of Environmental Quality 17: 62–70. [Google Scholar]
- Karasz I. 1996. The root system of Juniperus communis L. in a sandy soil in central Hungary. Acta Phytogeographica Suecica 81: 32–34. [Google Scholar]
- Karizumi N. 1979. Monograph – root system forms and distributions of individual trees in Japan. Tokyo, Japan: Seibundo Shinkosha Publishing. https://www.cabdirect.org/cabdirect/abstract/19870618641 [accessed 20 April 2020]. [In Japanese]. [Google Scholar]
- Karpov VG, ed. 1983. Faktory reguliatsii ekosistem elovykh lesov [Regulation factors of spruce forest ecosystems]. Leningrad, Russia: Nauka. [In Russian]. [Google Scholar]
- Karrfalt EE. 1981. The comparative and developmental morphology of the root system of Selaginella selaginoides (L.) link. American Journal of Botany 68: 244–253. [Google Scholar]
- Kausch W. 1960. Der Einfluß von edaphischen und klimatischen Faktoren auf die Ausbildung des Wurzelwerkes der Pflanzen unter besonderer Berücksichtigung einiger algerischer Wüstenpflanzen. Habilitation. Darmstadt, Germany: Technische Hochschule Darmstadt. [Google Scholar]
- Keller B. 1930. Die Methoden zur Erforschung der Ökologie der Steppen‐ und Wüstenpflanzen. In: Abderhalden E, ed. Handbuch der biologischen Arbeitsmethoden, vol. 11(6). Berlin, Germany: Urban & Schwarzenberg, 1–128. [Google Scholar]
- Kellman M. 1990. Root proliferation in recent and weathered sandy soils from Veracruz, Mexico. Journal of Tropical Ecology 6: 355. [Google Scholar]
- Kenzo T, Ichie T, Hattori D, Itioka T, Handa C, Ohkubo T, Kendawang JJ, Nakamura M, Sakaguchi M, Takahashi N et al. 2009. Development of allometric relationships for accurate estimation of above and below‐ground biomass in tropical secondary forests in Sarawak, Malaysia. Journal of Tropical Ecology 25: 371. [Google Scholar]
- Keresztesi B. 1968. Morphological characteristic of the Robinia root system on different sites of the Great Hungarian Plain. In: Ghilarov MS, Kovda VA, Novichkova‐Ivanova LN, Rodin LE, Sveshnikova VM, eds. Methods of productivity studies in root systems and rhizosphere organisms. International Symposium USSR, August 28–September 12, 1968. Leningrad, Russia: Nauka, 86–96. [Google Scholar]
- Kerfoot O. 1963. The root systems of tropical forest trees. Commonwealth Forestry Review 42: 19–26. [Google Scholar]
- Kimber PC. 1974. The root system of jarrah (Eucalyptus marginata), vol. 10. Perth, WA, Australia: Western Australia Forestry Department. [Google Scholar]
- Kleinhampl FJ, Koteff C. 1960. Botanical Prospecting for U rani urn in the Circle Cliffs Area Garfield County, Utah. USGS Bulletin 1085‐C (USGS). [Google Scholar]
- Klepper EL, Gano KA, Cadwell LL. 1985. Rooting depth and distributions of deep‐rooted plants in the 200 Area Conrol Zone of the Hanford site, vol. PNL‐5247. Richland, WA, USA: Pacific Northwest Laboratory. [Google Scholar]
- Kohzu A, Matsui K, Yamada T, Sugimoto A, Fujita N. 2003. Significance of rooting depth in mire plants: evidence from natural 15N abundance. Ecological Research 18: 257–266. [Google Scholar]
- Kokoreva II. 1996. Root systems of Cratae gus L. in the Trans‐Ili Alatau, Kazakhstan. Acta Phytogeographica Suecica 81: 35–38. [Google Scholar]
- Kong Lingshao MM. 1996. Reserach on bio‐ecological characteristics of Suaeda physophora and its community at the Hutubi cattle farm of Xinjiang, China. Acta Botanica Sinica 38: 475–482. [Google Scholar]
- Köstler JN, Brückner E, Bibelriether H. 1968. Die Wurzeln der Waldbäume: Untersuchungen zur Morphologie der Waldbäume in Mitteleuropa. Hamburg/Berlin, Germany: Paul Parey. [Google Scholar]
- Koteen LE, Baldocchi DD, Harte J. 2011. Invasion of non‐native grasses causes a drop in soil carbon storage in California grasslands. Environmental Research Letters 6: 44001. [Google Scholar]
- Kreutzer K. 1968. The root system of the red alder (Alnus glutinosa Gärtn.). In: Ghilarov MS, Kovda VA, Novichkova‐Ivanova LN, Rodin LE, Sveshnikova VM, eds. Methods of productivity studies in root systems and rhizosphere organisms. International Symposium USSR, August 28–September 12, 1968. Leningrad, Russia: Nauka, 114–119. [Google Scholar]
- Krishnamurthy L, Zaman‐Allah M, Marimuthu S, Wani SP, Kesava Rao AVR. 2012. Root growth in Jatropha and its implications for drought adaptation. Biomass and Bioenergy 39: 247–252. [Google Scholar]
- Kubota M, Tenhunen J, Zimmermann R, Adiku S, Kakubari Y. 2005. Influences of environmental factors on the radial profile of sap flux density in. Tree Physiology 1998: 545–556. [DOI] [PubMed] [Google Scholar]
- Kuiper LC, Coutts MP. 1992. Spatial disposition and extension of the structural root system of Douglas‐fir. Forest Ecology and Management 47: 111–125. [Google Scholar]
- Kummerow J. 1981. Structure of roots and root systems. In: DiCastri F, Goodall DW, Specht RL, eds. Mediterranean‐type shrublands. Amsterdam, the Netherlands: Elsevier, 269–288. [Google Scholar]
- Kutschera L, Lichtenegger E. 1960. Wurzelatlas mitteleuropäischer Ackerunkräuter und Kulturpflanzen. Frankfurt am Main, Germany: DLG‐Verlags GmbH. [Google Scholar]
- Kutschera L, Lichtenegger E, Sobotik M. 1982. Wurzelatlas mitteleuropäischer Grünlandpflanzen. Band 1: Monocotyledonae. Stuttgart, Germany: Gustav Fischer. [Google Scholar]
- Kutschera L, Lichtenegger E, Sobotik M. 1992. Wurzelatlas mitteleuropäischer Grünlandpflanzen. Band 2: Pteridophyta und Dicotyledonae (Magnoliopsida). Teil 1: Morphologie, Anatomie, Ökologie, Verbreitung, Soziologie, Wirtschaft. Stuttgart, Germany: Gustav Fischer. [Google Scholar]
- Kutschera L, Lichtenegger E, Sobotik M. 1997a. Bewurzelung von Pflanzen in verschiedenen Lebensräumen, vol. 49. Land Oberösterreich. Linz, Austria: OÖ Landesmuseum. [Google Scholar]
- Kutschera L, Lichtenegger E, Sobotik M, Haas D. 1997b. Die Wurzel, das neue Organ, ihre Bedeutung für das Leben von Welwitschia mirabilis und anderer Arten der Namib, sowie von Arten angrenzender Gebiete. Klagenfurt, Austria: Pflanzensoziologisches Institut. [Google Scholar]
- Kutschera L, Lichtenegger E, Sobotik M. 2009. Wurzelatlas der Kulturpflanzen gemäßigter Gebiete mit Arten des Feldgemüsebaues. Frankfurt am Main, Germany: DLG‐Verlag. [Google Scholar]
- Kutschera L, Lichtenegger E. 2002. Wurzelatlas mitteleuropäischer Waldbäume und Sträucher. Graz, Austria; Stuttgart, Germany: Leopold Stocker Verlag. [Google Scholar]
- Laclau JP. 2004. The function of the superficial root mat in the biogeochemical cycles of nutrients in Congolese Eucalyptus plantations. Annals of Botany 93: 249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laclau J‐P, da Silva EA, Rodrigues Lambais G, Bernoux M, le Maire G, Stape JL, Bouillet J‐P, Gonçalves JLDM, Jourdan C, Nouvellon Y 2013. Dynamics of soil exploration by fine roots down to a depth of 10 m throughout the entire rotation in Eucalyptus grandis plantations. Frontiers in Plant Science 4: e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laclau J‐P, Arnaud M, Bouillet J‐P, Ranger J. 2001. Spatial distribution of Eucalyptus roots in a deep sandy soil in the Congo: relationships with the ability of the stand to take up water and nutrients. Tree Physiology 21: 129–136. [DOI] [PubMed] [Google Scholar]
- Lamont BB, Lange BJ. 1976. ‘Stalagmiform’ roots in limestone caves. New Phytologist 76: 353–360. [Google Scholar]
- Leduc G, Favreau G, Schroeter P. 2001. Long‐term rise in a Sahelian water‐table: the continental terminal in south‐west Niger. Journal of Hydrology 24: 43–54. [Google Scholar]
- Lee CA, Lauenroth WK. 1994. Spatial distribution of grass and shrub root systems in the shortgrass steppe. American Midland Naturalist 132: 117–123. [Google Scholar]
- Leistner OA. 1967. The plant ecology of the southern Kalahari. Botanical Survey of South Africa Memoirs, vol. 38, Republic of South Africa: Department of Agricultural Technical Services, Government Printers, 172. [Google Scholar]
- Lewis DC, Burgy RH. 1964. The relationship between oak tree roots and groundwater in fractured rock as determined by tritium tracing. Journal of Geophysical Research 69: 2579–2588. [Google Scholar]
- Lichtenegger E. 1996. Root distribution in some alpine plants. Acta Phytogeographica Suecica 81: 76–82. [Google Scholar]
- Link SO, Cadwell LL, Petersen KL, Sackschewsky MR, Landeen DS. 1995. The role of plants and animals in isolation barriers at Hanford, vol. PNL‐10788. Richland, WA, USA: Pacific Northwest Laboratory. [Google Scholar]
- Link SO, Gee GW, Downs JL. 1990. The effect of water stress on phenological and ecophysiological characteristics of cheatgrass and Sandberg's bluegrass. Journal of Range Management 43: 506–513. [Google Scholar]
- Louw GN, Seely MK. 1980. Exploitation of fog water by a perennial Namib dune grass, Stipagrostis sabulicola . South African Journal of Science 76: 38–39. [Google Scholar]
- Ludwig JA. 1977. Distributional adaptations of root systems in desert environments. In: Marshall JK, ed. The belowground ecosystem: a synthesis of plant‐associated processes, vol. 26. Fort Collins, CO, USA: Colorado State University, 85–91. [Google Scholar]
- Lyford WH. 1980. Development of the root system of northern red oak (Quercus rubra L.). Harvard Forest Paper No. 21. Petersham, MA, USA: Harvard Forest, Harvard University. Harvard Forest Bulletin, vol. 21, 30 pp. [Google Scholar]
- Manning SJ, Barbour MG. 1988. Root systems, spatial patterns, and competition for soil moisture between two desert shrubs. American Journal of Botany 75: 885–893. [Google Scholar]
- Manning SJ, Groeneveld DP. 1989. Shrub rooting characteristics and water acquisition on xeric sites in the western Great Basin. In: Proceedings‐–Symposium on cheatgrass invasion. shrub die‐off. and other aspects of shrub biology and management, vol. INT‐276. Ogden, UT, USA: USDA Forest Service Intermountain Research Station, 238–244. [Google Scholar]
- Markle MS. 1917. Root systems of certain desert plants. Botanical Gazette 64: 177–205. [Google Scholar]
- McKell CM, Jones MB, Perrier ER. 1962. Root production and accumulation of root material on fertilized annual range. Agronomy Journal 54: 459–462. [Google Scholar]
- Meinzer OE. 1927. Plants as indicators of groundwater, vol. 577. Washington, DC, USA: Department of the Interior, US Geological Survey. [Google Scholar]
- Mensah KOA, Jenik J. 1968. Root system of tropical tress 2. Features of the root system of iroko (Chlorophora excelsa Benth. et Hook.). Preslia 40: 21–27. [Google Scholar]
- Midwood AJ, Boutton TW, Archer SR, Watts SE. 1998. Water use by woody plants on contrasting soils in a savanna parkland: assessment with δ 2H and δ 18O. Plant and Soil 205: 13–24. [Google Scholar]
- Migahid AM. 1961. The drought resistance of Egyptian desert plants. UNESCO Arid Zone Research 16: 213–233. [Google Scholar]
- Miller PC, Ng E. 1977. Root : shoot biomass ratios in shrubs in southern California and central Chile. Madroño 24: 215–223. [Google Scholar]
- Millikin CS, Bledsoe CS. 1999. Biomass and distribution of fine and coarse roots from blue oak (Quercus douglasii) trees in the northern Sierra Nevada foothills of California. Plant and Soil 214: 27–38. [Google Scholar]
- Miroshnichenko YM. 1975. Kornevye sistemy drevesnych i kustarnikovych rastenij i ich zkologija v vostocnych karakumach [Root systems of trees and bushes and their ecology in eastern Karakum]. Botanicheskii Zhurnal 60: 1776–1795. [In Russian]. [Google Scholar]
- Mooney HA, Gulmon SL, Rundel PW, Ehleringer JR. 1980. Further observations on the water relations of Prosopis tamarugo of the northern Atacama Desert. Oecologia 44: 177–180. [DOI] [PubMed] [Google Scholar]
- Mordelet P, Barot S, Abbadie L. 1996. Root foraging strategies and soil patchiness in a humid savanna. Plant and Soil 182: 171–176. [Google Scholar]
- Moreira MZ, Sternberg LdSL, Nepstad DC. 2000. Vertical patterns of soil water uptake by plants in a primary forest and an abandoned pasture in the eastern Amazon: an isotopic approach. Plant and Soil 222: 95–107. [Google Scholar]
- Morello J. 1955. Estudios botánicos en las regiones áridas de la Argentina. II. Transpiración de los arbustos resinosos de follaje permanente del Monte. Revista Agronomica del Noroeste Argentino 1: 385–524. [Google Scholar]
- Morello J. 1956. Estudios botánicos en las regiones áridas de la Argentina. III. Revista Agronomica del Noroeste Argentino 2: 79–152. [Google Scholar]
- Morello J. 1958. La provincia fitogeografica del Monte. Tucuman, Argentina: University of Tucuman. [Google Scholar]
- Muller CH. 1946. Root development and ecological relations of guayule, vol. 923. Washington, DC, USA: United States Department of Agriculture. [Google Scholar]
- Nakano Y, Brown J. 1972. Mathematical modeling and validation of the thermal regimes in tundra soils, Barrow, Alaska. Arctic and Alpine Research 4: 19–38. [Google Scholar]
- Nechaeva NT. 1985. Description of plants used for vegetative range improvement. In: Nechaeva NT, ed. Improvement of desert ranges in Soviet Central Asia, vol. 4. Chur, Switzerland: Harwood Academic, 55–120. [Google Scholar]
- Nechaeva NT, ed. 1985. Improvement of desert ranges in Soviet Central Asia, vol. 4. Chur, Switzerland: Harwood Academic Publishers. [Google Scholar]
- Nechaeva NT, Prikhod'ko SY, eds. 1968. Sown winter ranges in the foothill deserts of Soviet Central Asia. Jerusalem, Israel: Israel Program for Scientific Translations. [Google Scholar]
- Nesterova SG. 1996. Root structure of plants in the Zailiisky Alatau Range, Kazakhstan. Acta Phytogeographica Suecica 81: 86–87. [Google Scholar]
- Nijland W, van der Meijde M, Addink EA, de Jong SM. 2010. Detection of soil moisture and vegetation water abstraction in a Mediterranean natural area using electrical resistivity tomography. Catena 81: 209–216. [Google Scholar]
- Nobel PS. 1989. Temperature, water availability, and nutrient levels at various soil depths: consequences for shallow‐rooted desert succulents, including nurse plant effects. American Journal of Botany 76: 1486–1492. [Google Scholar]
- Nobel PS, Franco AC. 1986. Annual root‐growth and intraspecific competition for a desert bunchgrass. Journal of Ecology 74: 1119–1126. [Google Scholar]
- Nobel PS, Loik ME, Meyer RW. 1991. Microhabitat and diel tissue acidity changes for two sympatric cactus species differing in growth habit. Journal of Ecology 79: 167–182. [Google Scholar]
- Nobel PS, Zutta BR. 2005. Morphology, ecophysiology, and seedling establishment for Fouquieria splendens in the northwestern Sonoran Desert. Journal of Arid Environments 62: 251–265. [Google Scholar]
- Osman A, Pieper RD. 1988. Growth of Gutierrezia sarothrae seedlings in the field. Journal of Range Management 41: 92–93. [Google Scholar]
- Ourcival JM, Floret C, Le Floc'h E, Ponantier R. 1994. Water relations between two perennial species in the steppes of southern Tunisia. Journal of Arid Environments 28: 333–350. [Google Scholar]
- Pavlychenko TK. 1937. Quantitative study of the entire root systems of weed and crop plants under field conditions. Ecology 18: 62–79. [Google Scholar]
- Peláez DV, Distel RA, Bóo RM, Elia OR, Mayor MD. 1994. Water relations between shrubs and grasses in semi‐arid Argentina. Journal of Arid Environments 27: 71–78. [Google Scholar]
- Phillips WS. 1963. Depth of roots in soil. Ecology 44: 424. [Google Scholar]
- Popov KP. 1979. Fistashka v srednei Azii [The pistachio in central Asia]. Ashkhabad, Turkmenistan: Ylym. [In Russian]. [Google Scholar]
- Preston CE. 1900. Observations on the root system of certain Cactaceae. Botanical Gazette 30: 348–351. [Google Scholar]
- Ramam SS. 1970. Root development in alluvial grasslands of Varanasi. Indian Forester 96: 100–110. [Google Scholar]
- Rawitscher F. 1948. The water economy of the vegetation of the ‘campos cerrados’ in southern Brazil. Journal of Ecology 36: 237–268. [Google Scholar]
- Reynolds TD, Fraley L Jr. 1989. Root profiles of some native and exotic plant species in southeastern Idaho. Environmental and Experimental Botany 29: 241–248. [Google Scholar]
- Roberts EA, Herty SD. 1934. Lycopodium complanatum var. flabelliforme Fernald: its anatomy and a method of vegetative propagation. American Journal of Botany 21: 688–697. [Google Scholar]
- Roupsard O, Ferhi A, Granier A, Pallo F, Depommier D, Mallet B, Joly HI, Dreyer E. 1999. Reverse phenology and dry‐season water uptake by Faidherbia albida (Del.) A. Chev. in an agroforestry parkland of Sudanese west Africa. Functional Ecology 13: 460–472. [Google Scholar]
- Roux J, Hopper S, Smith R. 2009. Isoetes eludens (Isoetaceae), a new endemic species from the Kamiesberg, Northern Cape, South Africa. Kew Bulletin 64: 123–128. [Google Scholar]
- Rundel PW, Ehleringer JR, Mooney HA, Gulmon SL. 1980. Patterns of drought response in leaf‐succulent shrubs of the coastal Atacama Desert in northern Chile. Oecologia 46: 196–200. [DOI] [PubMed] [Google Scholar]
- Rutherford MC. 1983. Growth rates, biomass and distribution of selected woody plant roots in Burkea africana–Ochna pulchra savanna. Vegetatio 52: 45–63. [Google Scholar]
- Saunier RE, Wagle RF. 1967. Factors affecting the distribution of shrub live oak (Quercus turbinella Greene). Ecology 48: 35–41. [Google Scholar]
- Saurina NI, Kamenetskaya IV. 1969. Massa kornej sosny obyknovennoj (Pinus sylvestris L.) v sosnjake mshisto–lishajnikovom juzhnoj tajgi [The root mass of Pinus sylvestris L. in moss–lichen pine forests of the southern taiga]. Biulleten Moskovskogo obshchestva ispytatelei prirody Otdel biologicheskii 74: 96–100. [In Russian with English summary]. [Google Scholar]
- Savory BM. 1963. Site quality and tree root morphology in Northern Rhodesia. Rhodesian Journal of Agricultural Research 1: 55–65. [Google Scholar]
- Schulze E‐D, Mooney HA, Sala OE, Jobbagy E, Buchmann N, Bauer G, Canadell J, Jackson RB, Loreti J, Oesterheld M et al. 1996. Rooting depth. water availability. and vegetation cover along an aridity gradient in Patagonia. Oecologia 108: 503–511. [DOI] [PubMed] [Google Scholar]
- Schuster JL. 1964. Root development of native plants under three grazing intensities. Ecology 45: 63–70. [Google Scholar]
- Scott JD, van Breda NG. 1937a. Preliminary studies on the root system of Galenia africana on the Worcester Veld Reserve. South African Journal of Science 34: 268–274. [Google Scholar]
- Scott JD, van Breda NG. 1937b. Preliminary studies on the root system of the rhenosterbos (Elytropappus rhinocerotis) on the Worcester Veld Reserve. South African Journal of Science 33: 560–569. [Google Scholar]
- Scott JD, van Breda NG. 1939. Preliminary studies on the root system of Euphorbia mauretanica, Euphorbia burmanni and Ruschia multiflora on the Worcester Veld Reserve. South African Journal of Science 36: 227–235. [Google Scholar]
- Sen DN. 1982. Environment and plant life in Indian Desert. Jodhpur, India: Geobios International. [Google Scholar]
- Sen DN, Tanwar GS. 1983. Arid environment and root behaviour. In: Böhm W, Kutschera L, Lichtenegger E, eds. Wurzelökologie und ihre Nutzanwendung [Root ecology and its practical application]. Irdning, Germany: Bundesanstalt für alpenländische Landwirtschaft Grumpenstein, 185–206. [Google Scholar]
- Shachori A, Rosenzweig D, Poljakoff‐Mayber A. 1967. Effect of Mediterranean vegetation on the moisture regime. In: Sopper WE, Lull HW, eds. Forest hydrology. Oxford, UK: Pergamon Press, 291–311. [Google Scholar]
- Shalyt MS. 1950. Podzemnaja cast' nekotorykh lugovykh, stepnykh i pustynnykh rastenyi i fitocenozov. C. I. Travjanistye i polukustarnigkovye rastenija i fitocenozy lesnoj (luga) i stepnoj zon [Belowground parts of some meadow, steppe, and desert plants and plant communities. Part I: herbaceous plants and subshrubs and plant communities of forest and steppe zones]. Trudy Botanicheskogo Instituta im V.L. Komarova. Akademii Nauk SSSR. Seriia III. Geobotanika 6: 205–442. [In Russian]. [Google Scholar]
- Shalyt MS. 1952. Podzemnaja cast' nekotorykh lugovykh, stepnykh i pustynnykh rastenyi i fitocenozov. C. 2. Travjanistye, polukustarnigkovye i kustarnigkovye rastenija i fitocenozy pustynnoj zony [Belowground parts of some meadow, steppe, and desert plants and plant communities. Part 2: Herbaceous plants, subshrubs, and shrubs. and plant communities of the desert zone]. Trudy Botanicheskogo Instituta im. V.L. Komarova. Akademii Nauk SSSR. Seriia III. Geobotanika 8: 71–139. [In Russian]. [Google Scholar]
- Shankarnarayan KA, Dabadghao PM, Pandey PK, Rai P. 1974. Studies on the root system of five principal grasses of Sehima–Dichanthium cover. Annals of Arid Zone 13: 177–186. [Google Scholar]
- Sharifi MR, Nilsen ET, Rundel PW. 1982. Biomass and net primary production of Prosopis glandulosa (Fabaceae) in the Sonoran Desert of California. American Journal of Botany 69: 760–767. [Google Scholar]
- Sharma BM. 1968. Root systems of some desert plants at Churu, Rajasthan. Indian Forester 94: 240–246. [Google Scholar]
- Sharma BM. 1984. Ecophysiological studies of Eleusine indica (L.) Gaertn, Sporobulus pyramidalis P. Beauv. at Ibadan, Nigeria. Journal of Range Management 37: 275–276. [Google Scholar]
- Sharma BM, Chivinge AO. 1982. Contributions to the ecology of Dactyloctenium aegyptiacum (L.) P. Beauv. Journal of Range Management 35: 326–331. [Google Scholar]
- Shaver G, Billings DW. 1975. Root production and root turnover in a wet tundra ecosystem, Barrow, Alaska. Ecology 56: 401. [Google Scholar]
- Sherff E. 1912. The vegetation of Skokie Marsh, with special reference to subterranean organs and their interrelationships. Botanical Gazette 53: 415–435. [Google Scholar]
- Shpak IS. 1971. The effects of forests on the water balance of drainage basins. Jerusalem, Israel: Israel Program for Scientific Translations. [Google Scholar]
- Silva S, Whitford WG, Jarrell WM, Virginia RA. 1989. The microarthropod fauna associated with a deep rooted legume, Prosopis glandulosa in the Chihuahuan Desert. Oecologia 7: 330–335. [Google Scholar]
- Sin'kovsky LP. 1985. Herbs of local flora as used in cultivation. In: Nechaeva NT, ed. Improvement of desert ranges in Soviet Central Asia, vol. 4. Chur, Switzerland: Harwood Academic Publishers, 203–220. [Google Scholar]
- Singh SP. 1964. Cover, biomass, and root‐shoot habit of Larrea divaricata on a selected site in southern New Mexico. MSc Thesis, New Mexico State University, Las Cruces, NM, USA. [Google Scholar]
- Singh V. 1994. Morphology and pattern of root distribution in Prosopis cineraria, Dalbergia sissoo and Albizia lebbek in an arid region of north‐western India. Tropical Ecology 35: 133–146. [Google Scholar]
- Skerman PJ, Riveros F. 1990. Tropical grasses. Rome, Italy: Food and Agriculture Organization of the United Nations. [Google Scholar]
- Soethe N, Lehmann J, Engels C. 2006. Root morphology and anchorage of six native tree species from a tropical montane forest and an elfin forest in Ecuador. Plant and Soil 279: 173–185. [Google Scholar]
- Soriano A. 1983. Deserts and semi‐deserts of Patagonia. In: West NE, ed. Temperate deserts and semi‐deserts, vol. 5. Amsterdam, the Netherlands: Elsevier, 423–460. [Google Scholar]
- Soriano A, Sala OE. 1983. Ecological strategies in a Patagonian arid steppe. Vegetatio 56: 9–15. [Google Scholar]
- Soumaré A, Groot JJR, Koné D, Radersma S. 1994. Structure spatiale du système racinaire de deux arbres du Sahel: Acacia seyal et Sclerocarya birrea, vol. 5. Wageningen, the Netherlands: Production Soudano‐Sahélienne (PSS). [Google Scholar]
- Specht RL. 1957. Dark Island heath (Ninety‐mile Plain, South Australia) III. The root system. Australian Journal of Botany 5: 103–114. [Google Scholar]
- Specht RL. 1957. Dark Island heath (Ninety‐mile Plain, South Australia) IV. Soil moisture patterns produced by rainfall interception and stemflow. Australian Journal of Botany 5: 137–150. [Google Scholar]
- Spence LE. 1937. Root studies of important range plants of the Boise River watershed. Journal of Forestry 35: 747–754. [Google Scholar]
- Sperry TM. 1935. Root systems in Illinois prairie. Ecology 16: 178–202. [Google Scholar]
- Sprackling JA, Read RA. 1979. Tree root systems in eastern Nebraska. Nebraska Conservation Bulletin no. 37. Lincoln, NE, USA: Conservation and Survey Dicvision, Institute of Agriculture and Natural Resources, The University of Nebraska–Lincoln. [Google Scholar]
- Steele SJ, Gower ST, Vogel JG, Norman JM. 1997. Root mass, net primary production and turnover in aspen, jack pine and black spruce forests in Saskatchewan and Manitoba, Canada. Tree Physiology 17: 577–587. [DOI] [PubMed] [Google Scholar]
- Stocker O. 1928. Der Wasserhaushalt ägyptischer Wüsten und Salzpflanzen vom Standpunkt einer experimentellen und vergleichenden Pflanzengeographie aus. Botanische Abhandlungen 13: 200. [Google Scholar]
- Stone EL, Kalisz PJ. 1991. On the maximum extent of tree roots. Forest Ecology and Management 46: 59–102. [Google Scholar]
- Strong WL, La Roi GH. 1983. Rooting depths and successional development of selected boreal forest communities. Canadian Journal of Forest Research 13: 577–588. [Google Scholar]
- Sturges DL. 1977. Soil water withdrawal and root characteristics of big sagebrush. American Midland Naturalist 98: 257–273. [Google Scholar]
- Sveshnikova VM. 1979. Dominanty Kasakhstanskikh stepej [Dominant plants of the Kazakhstan steppe]. Leningrad, Russia: Nauka. [In Russian]. [Google Scholar]
- Tabler RD. 1964. The root system of Artemisia tridentata at 9,500 feet in Wyoming. Ecology 45: 633–636. [Google Scholar]
- Tadmor NH, Orshan G, Rawitz E. 1962. Habitat analysis in the Negev Desert of Israel. Bulletin of the Research Council of Israel 11D: 148–173. [Google Scholar]
- Thomas CM, Davis SD. 1989. Recovery patterns of three chaparral shrub species after wildfire. Oecologia 80: 309–320. [DOI] [PubMed] [Google Scholar]
- Thomas FM. 2000. Vertical rooting patterns of mature Quercus trees growing on different soil types in northern Germany. Plant Ecology 147: 95–103. [Google Scholar]
- Thomas HH. 1921. Some observations on plants in the Libyan desert. Journal of Ecology 9: 75–89. [Google Scholar]
- Thomas WD. 1980. Characteristics of root systems: California oaks. In: Plumb TR, ed. Proceedings of the symposium on the ecology. management. and utilization of California oaks; 1979 June 26‐28; Claremont, CA, vol. PSW‐44. Berkeley, CA, USA: U.S. Department of Agriculture. Forest Service, Pacific Southwest Forest and Range Experiment Station, 178–179. [Google Scholar]
- Tierney GD, Foxx TS. 1982. Floristic composition and plant succession on near‐surface radioactive waste disposal facilities in the Los Alamos National Laboratory, vol. LA‐9212‐MS. Los Alamos, NM, USA: Los Alamos National Laboratory. [Google Scholar]
- Tierney GD, Foxx TS. 1987. Rooting lengths of plants of Los Alamos National Laboratory lands, vol. LA‐10865‐MS. Los Alamos, NM, USA: Los Alamos National Laboratory. [Google Scholar]
- Toky OP, Bisht RP. 1992. Observations on the rooting patterns of some agroforestry trees in an arid region of north‐western India. Agroforestry Systems 18: 245–263. [Google Scholar]
- Tolstead WL. 1942. Vegetation of the northern part of Cherry County, Nebraska. Ecological Monographs 12: 255–292. [Google Scholar]
- Tryon PR, Chapin FS III. 1983. Temperature control over root growth and root biomass in taiga forest trees. Canadian Journal of Forest Research 13: 827–833. [Google Scholar]
- Turner GT, Costello DF. 1942. Ecological aspects of the pricklypear problem in eastern Colorado and Wyoming. Ecology 23: 419–426. [Google Scholar]
- Upendra S, Good ER. 1993. Vertical root distribution in relation to soil properties in New Jersey Pinelands forests. Plant and Soil 150: 87–97. [Google Scholar]
- Van Donselaar‐ten Bokkel Huinink WAE 1966. Structure, root systems and periodicity of savanna plants and vegetations in northern Surinam. Wentia 17: 1–162. [Google Scholar]
- Vandenbeldt RJ. 1991. Rooting systems of western and southern African Faidherbia albida (Del.) A. Chev. (syn. Acacia albida Del.) – a comparative analysis with biogeographic implications. Agroforestry Systems 14: 233–244. [Google Scholar]
- Villares JB, Tundisi A, Becker M. 1953. The subterranean system of colonial grass (Guinea grass) in various soils of the state of São Paulo, Brazil. Journal of Range Management 6: 248–254. [Google Scholar]
- Wallace A, Romney EM. 1972. Radioecology and ecophysiology of desert plants at the Nevada test site, vol. TID‐25954. Washington, DC, USA: US Atomic Energy Commission. [Google Scholar]
- Walter H. 1939. Grasland, Savanne und Busch der arideren Teile Afrikas in ihrer ökologischen Bedingtheit. Jahrbücher für Wissenschaftliche Botanik 87: 750–860. [Google Scholar]
- Walter H. 1963. The water supply of desert plants. In: Rutter AJ, Whitehead FH eds. The water relations of plants, vol. 3. New York, NY, USA: John Wiley & Sons, 199–205. [Google Scholar]
- Walter H, Breckle S‐W. 1984. Spezielle Ökologie der tropischen und subtropischen Zonen, vol. 2. Stuttgart, Jena, Germany: Gustav‐Fischer Verlag. [Google Scholar]
- Walter H, Breckle S‐W. 1986. Ecological systems of the geobiosphere 2: tropical and subtropical zonobiomes. Berlin, Germany: Springer‐Verlag. [Google Scholar]
- Wang P, Mommer L, van Ruijven J, Berendse F, Maximov TC, Heijmans MMPD. 2016. Seasonal changes and vertical distribution of root standing biomass of graminoids and shrubs at a Siberian tundra site. Plant and Soil 407: 55–65. [Google Scholar]
- Weaver JE. 1917. A study of the vegetation of southeastern Washington and adjacent Idaho. University Studies of the University of Nebraska 17: 1–133. [Google Scholar]
- Weaver JE. 1919. The ecological relations of roots, vol. 286. Washington, DC, USA: Carnegie Institution of Washington. [Google Scholar]
- Weaver JE. 1920. Root development in the grassland formation, vol. 292. Washington, DC, USA: Carnegie Institution of Washington. [Google Scholar]
- Weaver JE. 1954. North American prairie. Lincoln, NE, USA: Johnsen. [Google Scholar]
- Weaver JE. 1958. Summary and interpretation of underground development in natural grassland communities. Ecological Monographs 28: 55–78. [Google Scholar]
- Weaver JE, Albertson FW. 1956. Grasslands of the Great Plains. Lincoln, NE, USA: Johnsen Publishing Company. [Google Scholar]
- Weaver JE, Darland RW. 1949. Soil–root relationships of certain native grasses in various soil types. Ecological Monographs 19: 303–338. [Google Scholar]
- Weaver JE, Kramer J. 1932. Root system of Quercus macrocarpa in relation to the invasion of pairie. Botanical Gazette 94: 51–85. [Google Scholar]
- Westman WE, Rogers RW. 1977. Biomass and structure of a subtropical eucalypt forest, North Stradbroke Island. Australian Journal of Botany 25: 171–191. [Google Scholar]
- Williams K, Hobbs RJ. 1989. Control of shrub establishment by springtime soil water availability in an annual grassland. Oecologia 81: 62–66. [DOI] [PubMed] [Google Scholar]
- Wilson RC. 1972. Abronia: I. Distribution, ecology and habit of nine species of Abronia found in California. Aliso 7: 421–437. [Google Scholar]
- Wright CD. 1928. An ecological study of Baccharis pilularis . MS thesis, University of California, Berkeley, CA, USA. [Google Scholar]
- Wright SJ, Machado JL, Mulkey SS, Smith AP. 1992. Drought acclimation among tropical forest shrubs (Psychotria, Rubiaceae). Oecologia 89: 457–463. [DOI] [PubMed] [Google Scholar]
- Wyatt JW, Dollhopf DJ, Schafer WM. 1980. Root distribution in 1 to 48 year‐old strip‐mine spoils in southeastern Montana. Journal of Range Management 33: 101–104. [Google Scholar]
- Yeager AF. 1935. Root systems of certain trees and shrubs grown on prairie soil. Journal of Agricultural Research 51: 1085–1092. [Google Scholar]
- Yeaton RI, Travis J, Gilinsky E. 1977. Competition and spacing in plant communities: the Arizona upland association. Journal of Ecology 65: 587–595. [Google Scholar]
- Zohary M. 1961. On hydro‐ecological relations of the Near East desert vegetation. UNESCO Arid Zone Research 16: 199–212. [Google Scholar]
- Zverev NE, Seiidova RD. 1990. Underground mass of shrub and subshrub plants of the Karakum. Problems of Desert Development 1: 49–54. [Google Scholar]
See also the Commentary on this article by Kattge, 235: 821–823.
Data availability
The RSIP can be found as Dataset S1. The RPGE data (Schenk & Jackson, 2003) are openly available in the ORNL DAAC at https://doi.org/10.3334/ORNLDAAC/660.
References
- Anderegg WR, Klein T, Bartlett M, Sack L, Pellegrini AF, Choat B, Jansen S. 2016. Meta‐analysis reveals that hydraulic traits explain cross‐species patterns of drought‐induced tree mortality across the globe. Proceedings of the National Academy of Sciences, USA 113: 5024–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed‐effects models using lme4. Journal of Statistical Software 67: 1–48. [Google Scholar]
- Bergmann J, Ryo M, Prati D, Hempel S, Rillig MC. 2017. Root traits are more than analogues of leaf traits: the case for diaspore mass. New Phytologist 216: 1130–1139. [DOI] [PubMed] [Google Scholar]
- Boehmke B, Greenwell B. 2020. Hands‐on machine learning with R. Boca Raton, FL, USA: Chapman & Hall/CRC Press. [Google Scholar]
- Brantley SL, Eissenstat DM, Marshall JA, Godsey SE, Balogh‐Brunstad Z, Karwan DL, Papuga SA, Roering J, Dawson TE, Evaristo J. 2017. Reviews and syntheses: on the roles trees play in building and plumbing the critical zone. Biogeosciences 14: 5115–5142. [Google Scholar]
- Breiman L. 2001. Random forests. Machine Learning 45: 5–32. [Google Scholar]
- Brum M, Vadeboncoeur MA, Ivanov V, Asbjornsen H, Saleska S, Alves LF, Penha D, Dias JD, Aragão LEOC, Barros F et al. 2019. Hydrological niche segregation defines forest structure and drought tolerance strategies in a seasonal Amazon forest. Journal of Ecology 107: 318–333. [Google Scholar]
- Cahill JF, McNickle GG, Haag JJ, Lamb EG, Nyanumba SM, St. Clair CC. 2010. Plants integrate information about nutrients and neighbors. Science 328: e1657. [DOI] [PubMed] [Google Scholar]
- Cahill JF, McNickle GG. 2011. The behavioral ecology of nutrient foraging by plants. Annual Review of Ecology, Evolution, and Systematics 42: 289–311. [Google Scholar]
- Caldwell MM, Eissenstat DM, Richards JH, Allen MF. 1985. Competition for phosphorus: differential uptake from dual‐isotope‐labeled soil interspaces between shrub and grass. Science 229: 384–386. [DOI] [PubMed] [Google Scholar]
- Canadell J, Jackson RB, Ehleringer JB, Mooney HA, Sala OE, Schulze E‐D. 1996. Maximum rooting depth of vegetation types at the global scale. Oecologia 108: 583–595. [DOI] [PubMed] [Google Scholar]
- Carmona CP, Bueno CG, Toussaint A, Träger S, Díaz S, Moora M, Munson AD, Pärtel M, Zobel M, Tamme R. 2021. Fine‐root traits in the global spectrum of plant form and function. Nature 597: 683–687. [DOI] [PubMed] [Google Scholar]
- Casper BB, Jackson RB. 1997. Plant competition underground. Annual Review of Ecology and Systematics 28: 545–570. [Google Scholar]
- Casper BB, Schenk HJ, Jackson RB. 2003. Defining a plant’s belowground zone of influence. Ecology 84: 2313–2321. [Google Scholar]
- Cermák J, Riguzzi F, Ceulemans R. 1998. Scaling up from the individual tree to the stand level in Scots pine. I. Needle distribution, overall crown and root geometry. Annales des Sciences Forestières 55: 63–88. [Google Scholar]
- Cheng D‐L, Niklas KJ. 2006. Above and below‐ground biomass relationships across 1534 forested communities. Annals of Botany 99: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craine JM, Dybzinski R. 2013. Mechanisms of plant competition for nutrients, water and light. Functional Ecology 27: 833–840. [Google Scholar]
- Dannowski M, Block A. 2005. Fractal geometry and root system structures of heterogeneous plant communities. Plant and Soil 272: 61–76. [Google Scholar]
- Dawkins HC. 1963. Crown diameters: their relation to bole diameter in tropical forest trees. The Commonwealth Forestry Review 42: 318–333. [Google Scholar]
- Day SD, Wiseman EP, Dickinson SB, Harris RJ. 2010. Contemporary concepts of root system architecture of urban trees. Arboriculture & Urban Forestry 36: 149–159. [Google Scholar]
- Díaz S, Kattge J, Cornelissen JHC, Wright IJ, Lavorel S, Dray S, Reu B, Kleyer M, Wirth C, Prentice IC et al. 2016. The global spectrum of plant form and function. Nature 529: 167–171. [DOI] [PubMed] [Google Scholar]
- Drewniak BA. 2019. Simulating dynamic roots in the energy exascale earth system land model. Journal of Advances in Modeling Earth Systems 11: 338–359. [Google Scholar]
- Dupuy L, Fourcaud T, Stokes A. 2007. A numerical investigation into the influence of soil type and root architecture on tree anchorage. In: Stokes A, Spanos I, Norris JE, Cammeraat E, eds. Developments in plant and soil sciences. Eco‐and ground bio‐engineering: the use of vegetation to improve slope stability. Dordrecht, the Netherlands: Springer, 175–189. [Google Scholar]
- Dybzinski R, Farrior C, Wolf A, Reich PB, Pacala SW. 2011. Evolutionarily stable strategy carbon allocation to foliage, wood, and fine roots in trees competing for light and nitrogen: an analytically tractable, individual‐based model and quantitative comparisons to data. The American Naturalist 177: 153–166. [DOI] [PubMed] [Google Scholar]
- Eissenstat DM, Yanai RD. 1997. The ecology of root lifespan. Advances in Ecological Research 27: 1–60. [Google Scholar]
- Ennos AR. 1993. The scaling of root anchorage. Journal of Theoretical Biology 161: 61–75. [Google Scholar]
- Enquist BJ, Niklas KJ. 2002. Global allocation rules for patterns of biomass partitioning in seed plants. Science 295: 1517–1520. [DOI] [PubMed] [Google Scholar]
- Erktan A, McCormack ML, Roumet C. 2018. Frontiers in root ecology: recent advances and future challenges. Plant and Soil 424: 1–9. [Google Scholar]
- Falster DS, Westoby M. 2003. Plant height and evolutionary games. Trends in Ecology and Evolution 18: 337–343. [Google Scholar]
- Fan Y, Miguez‐Macho G, Jobbágy EG, Jackson RB, Otero‐Casal C. 2017. Hydrologic regulation of plant rooting depth. Proceedings of the National Academy of Sciences, USA 114: 10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrior CE, Rodriguez‐Iturbe I, Dybzinski R, Levin SA, Pacala SW. 2015. Decreased water limitation under elevated CO2 amplifies potential for forest carbon sinks. Proceedings of the National Academy of Sciences, USA 112: 7213–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- February EC, Matimati I, Hedderson TA, Musil CF. 2013. Root niche partitioning between shallow rooted succulents in a South African semi desert: implications for diversity. Plant Ecology 214: 1181–1187. [Google Scholar]
- Fellows AW, Goulden ML. 2017. Mapping and understanding dry season soil water drawdown by California montane vegetation. Ecohydrology 10: e1772. [Google Scholar]
- Felsenstein J. 1985. Phylogenies and the comparative method. The American Naturalist 125: 1–15. [DOI] [PubMed] [Google Scholar]
- Fick SE, Hijmans HJ. 2017. Worldclim 2: new 1‐km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37: 4302–4315. [Google Scholar]
- Fischer EM, Beyerle U, Knutti R. 2013. Robust spatially aggregated projections of climate extremes. Nature Climate Change 3: 1033–1038. [Google Scholar]
- Freckleton RP, Harvey PH, Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. The American Naturalist 160: 712–726. [DOI] [PubMed] [Google Scholar]
- Freschet GT, Pagès L, Iversen CM, Comas LH, Rewald B, Roumet C, Klimešová J, Zadworny M, Poorter H, Postma JA et al. 2021a. A starting guide to root ecology: strengthening ecological concepts and standardising root classification, sampling, processing and trait measurements. New Phytologist 232: 973–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschet GT, Roumet C, Comas LH, Weemstra M, Bengough AG, Rewald B, Bardgett RD, de Deyn GB, Johnson D, Klimešová J et al. 2021b. Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs. New Phytologist 232: 1123–1158. [DOI] [PubMed] [Google Scholar]
- Freschet GT, Violle C, Bourget MY, Scherer‐Lorenzen M, Fort F. 2018. Allocation, morphology, physiology, architecture: the multiple facets of plant above and below‐ground responses to resource stress. New Phytologist 219: 1338–1352. [DOI] [PubMed] [Google Scholar]
- Gao H, Hrachowitz M, Schymanski SJ, Fenicia F, Sriwongsitanon N, Savenije HHG. 2014. Climate controls how ecosystems size the root zone storage capacity at catchment scale. Geophysical Research Letters 41: 7916–7923. [Google Scholar]
- Gherardi LA, Sala OE. 2015. Enhanced precipitation variability decreases grass and increases shrub‐productivity. Proceedings of the National Academy of Sciences, USA 112: 12735–12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giehl RFH, von Wirén N. 2014. Root nutrient foraging. Plant Physiology 166: 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman EF. 1990. Tree root growth and development. I. Form, spread, depth and periodicity. Journal of Environmental Horticulture 8: 215–220. [Google Scholar]
- Gou M, Xiang W, Song T, Lei P, Zhang S, Ouyang S, Zeng Y, Deng X, Fang X, Wang K. 2017. Allometric equations for applying plot inventory and remote sensing data to assess coarse root biomass energy in subtropical forests. Bioenergy Research 10: 536–546. [Google Scholar]
- Guerrero‐Ramírez NR, Mommer L, Freschet GT, Iversen CM, McCormack ML, Kattge J, Poorter H, van der Plas F, Bergmann J, Kuyper TW et al. 2021. Global root traits (GRooT) database. Global Ecology and Biogeography 30: 25–37. [Google Scholar]
- Hajduk GK, Bailey L. 2017. Introduction to linear mixed models. [WWW document] URL https://gkhajduk.github.io/2017‐03‐09‐mixed‐models/ [accessed 23 September 2019]. [Google Scholar]
- Hasenmueller EA, Gu X, Weitzman JN, Adams TS, Stinchcomb GE, Eissenstat DM, Drohan PJ, Brantley SL, Kaye JP. 2017. Weathering of rock to regolith: the activity of deep roots in bedrock fractures. Geoderma 300: 11–31. [Google Scholar]
- Herrera CM. 1992. Historical effects and sorting processes as explanations for contemporary ecological patterns: character syndromes in Mediterranean woody plants. The American Naturalist 140: 421–446. [Google Scholar]
- Hunt AG. 2016. Spatio‐temporal scaling of vegetation growth and soil formation from percolation theory. Vadose Zone Journal 15: vzj2015‐01. [Google Scholar]
- Ivanov VY, Hutyra LR, Wofsy SC, Munger JW, Saleska SR, de Oliveira RC Jr, de Camargo PB. 2012. Root niche separation can explain avoidance of seasonal drought stress and vulnerability of overstory trees to extended drought in a mature Amazonian forest. Water Resources Research 48: W12507. [Google Scholar]
- Iversen CM, McCormack ML. 2021. Filling gaps in our understanding of belowground plant traits across the world: an introduction to a virtual issue. New Phytologist 231: 2097–2103. [DOI] [PubMed] [Google Scholar]
- Jackson RB, Canadell J, Ehleringer JR, Mooney HA, Sala OE, Schulze ED. 1996. A global analysis of root distributions for terrestrial biomes. Oecologia 108: 389–411. [DOI] [PubMed] [Google Scholar]
- Jackson RB, Sperry JS, Dawson TE. 2000. Root water uptake and transport: using physiological processes in global predictions. Trends in Plant Science 5: 482–488. [DOI] [PubMed] [Google Scholar]
- Jin Y, Qian H. 2019. v.phylomaker: an R package that can generate very large phylogenies for vascular plants. Ecography 42: 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobbágy EG, Jackson RB. 2000. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecological Applications 10: 423–436. [Google Scholar]
- Jordan PW, Nobel PS. 1984. Thermal and water relations of roots of desert succulents. Annals of Botany 54: 705–717. [Google Scholar]
- Joswig JS, Wirth C, Schuman MC, Kattge J, Reu B, Wright IJ, Sippel SD, Rüger N, Richter R, Schaepman ME et al. 2022. Climatic and soil factors explain the two‐dimensional spectrum of global plant trait variation. Nature Ecology & Evolution 6: 36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattge J, Bönisch G, Díaz S, Lavorel S, Prentice IC, Leadley P, Tautenhahn S, Werner GDA, Aakala T, Abedi M et al. 2020. TRY plant trait database – enhanced coverage and open access. Global Change Biology 26: 119–188. [DOI] [PubMed] [Google Scholar]
- Kleidon A, Heimann M. 1998. Method of determining rooting depth from a terrestrial biosphere model and its impacts on the global water and carbon cycle. Global Change Biology 4: 275–286. [Google Scholar]
- Klimešová J, Martínková J, Herben T. 2018. Horizontal growth: an overlooked dimension in plant trait space. Perspectives in Plant Ecology, Evolution and Systematics 32: 18–21. [Google Scholar]
- Klos PZ, Goulden ML, Riebe CS, Tague CL, O’Geen AT, Flinchum BA, Safeeq M, Conklin MH, Hart SC, Berhe AA et al. 2018. Subsurface plant‐accessible water in mountain ecosystems with a Mediterranean climate. Wiley Interdisciplinary Reviews: Water 5: e1277. [Google Scholar]
- Knapp AK, Hoover DL, Wilcox KR, Avolio ML, Koerner SE, La Pierre KJ, Loik ME, Luo Y, Sala OE, Smith MD. 2015. Characterizing differences in precipitation regimes of extreme wet and dry years: implications for climate change experiments. Global Change Biology 21: 2624–2633. [DOI] [PubMed] [Google Scholar]
- Koch GW, Sillett SC, Jennings GM, Davis SD. 2004. The limits to tree height. Nature 428: 851–854. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy KV. 2015. Growth and development in plants (textbook series: 21st century biology and agriculture). New Delhi, India: Scientific Publishers. [Google Scholar]
- Kutschera L. 1997. Die Wurzel das neue Organ ihre Bedeutung für das Leben von Welwitschia mirabilis und anderer Arten der Namib sowie von Arten angrenzender Gebiete: mit Erklärung des Geotropen Wachstums der Pflanzen. Bad Goisern, Austria: Eigenverlag, Pflanzensoziologisches Institut. [Google Scholar]
- Kutschera L, Lichtenegger E. 2002. Wurzelatlas_mitteleuropäischer_Waldbäu. Graz, Austria: Leopold Stocker Verlag. [Google Scholar]
- Ledo A, Paul KI, Burslem DFRP, Ewel JJ, Barton C, Battaglia M, Brooksbank K, Carter J, Eid TH, England JR et al. 2017. Tree size and climatic water deficit control root to shoot ratio in individual trees globally. New Phytologist 217: 8–11. [DOI] [PubMed] [Google Scholar]
- Lepik A, Abakumova M, Davison J, Zobel K, Semchenko M. 2021. Spatial mapping of root systems reveals diverse strategies of soil exploration and resource contest in grassland plants. Journal of Ecology 109: 652–663. [Google Scholar]
- Lynch J. 1995. Root architecture and plant productivity. Plant Physiology 109: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2005. Root architecture and nutrient acquisition. In: BassiriRad H, ed. Ecological studies. Nutrient acquisition by plants: an ecological perspective. Berlin, Heidelberg, Germany: Springer, 147–183. [Google Scholar]
- Mayer M. 2019. missranger: fast imputation of missing values. R package v.2.1.3. [WWW document] URL https://CRAN.R‐project.org/package=missRanger [accessed 28 April 2021]. [Google Scholar]
- Mazerolle MJ. 2020. aiccmodavg: model selection and multimodel inference based on (Q)AIC(c). R package v.2.3‐1. [WWW document] URL https://cran.r‐project.org/package=AICcmodavg [accessed 20 September 2020]. [Google Scholar]
- McCormack ML, Guo D, Iversen CM, Chen W, Eissenstat DM, Fernandez CW, Li LE, Ma C, Ma Z, Poorter H et al. 2017. Building a better foundation: improving root‐trait measurements to understand and model plant and ecosystem processes. New Phytologist 215: 27–37. [DOI] [PubMed] [Google Scholar]
- de Mendiburu F. 2021. , agricolae: statistical procedures for agricultural research. R package v.1.3‐5. [WWW document] URL https://CRAN.R‐project.org/package=agricolae [accessed 3 July 2021]. [Google Scholar]
- Mokany K, Raison RJ, Prokushkin AS. 2006. Critical analysis of root : shoot ratios in terrestrial biomes. Global Change Biology 12: 84–96. [Google Scholar]
- Moles AT, Warton DI, Warman L, Swenson NG, Laffan SW, Zanne AE, Pitman A, Hemmings FA, Leishman MR. 2009. Global patterns in plant height. Journal of Ecology 97: 923–932. [Google Scholar]
- Nechaeva N. 1985. Description of plants used for vegetative range improvement. Advances in Desert and Arid Land Technology and Development 4: 55–120. [Google Scholar]
- Nepstad DC, de Carvalho CR, Davidson EA, Jipp PH, Lefebvre PA, Negreiros GH, da Silva ED, Stone TA, Trumbore SE, Vieira S. 1994. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 372: 666–669. [Google Scholar]
- Niklas KJ. 2007. Maximum plant height and the biophysical factors that limit it. Tree Physiology 27: 433–440. [DOI] [PubMed] [Google Scholar]
- Niklas KJ, Enquist BJ. 2001. Invariant scaling relationships for interspecific plant biomass production rates and body size. Proceedings of the National Academy of Sciences, USA 98: 2922–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ, Enquist BJ. 2002. On the vegetative biomass partitioning of seed plant leaves, stems, and roots. The American Naturalist 159: 482–497. [DOI] [PubMed] [Google Scholar]
- van Noordwijk M, Lawson G, Hairiah K, Wilson J. 2015. Root distribution of trees and crops: competition and/or complementarity. In: Ong KC, Black C, Wilson J, eds. Tree‐crop interactions: agroforestry in a changing climate. Wallingford, UK: CABI, 221–257. [Google Scholar]
- Norby RJ, Jackson RB. 2000. Root dynamics and global change: seeking an ecosystem perspective. New Phytologist 147: 3–12. [Google Scholar]
- Norton MR, Malinowski DP, Volaire F. 2016. Plant drought survival under climate change and strategies to improve perennial grasses. A review. Agronomy for Sustainable Development 36: e29. [Google Scholar]
- O’Brien ST, Hubbell SP, Spiro P, Condit R, Foster RB. 1995. Diameter, height, crown, and age relationships in eight neotropical tree species. Ecology 76: 1926–1939. [Google Scholar]
- O'Brien MJ, Engelbrecht BMJ, Joswig J, Pereyra G, Schuldt B, Jansen S, Kattge J, Landhäusser SM, Levick SR, Preisler Y et al. 2017. A synthesis of tree functional traits related to drought‐induced mortality in forests across climatic‐zones. Journal of Applied Ecology 54: 1669–1686. [Google Scholar]
- Oliveira RS, Bezerra L, Davidson EA, Pinto F, Klink CA, Nepstad DC, Moreira A. 2005. Deep root function in soil water dynamics in cerrado savannas of central Brazil. Functional Ecology 19: 574–581. [Google Scholar]
- Pagel M. 1997. Inferring evolutionary processes from phylogenies. Zoologica Scripta 26: 331–348. [Google Scholar]
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401: 877–884. [DOI] [PubMed] [Google Scholar]
- Paradis E, Schliep K. 2019. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35: 526–528. [DOI] [PubMed] [Google Scholar]
- Pawlik Ł, Kasprzak M. 2017. Regolith properties under trees and the biomechanical effects caused by tree root systems as recognized by electrical resistivity tomography (ERT). Geomorphology 300: 1–12. [Google Scholar]
- Phillips WS. 1963. Depth of roots in soil. Ecology 44: 424. [Google Scholar]
- Poirier V, Roumet C, Munson AD. 2018. The root of the matter: linking root traits and soil organic matter stabilization processes. Soil Biology and Biochemistry 120: 246–259. [Google Scholar]
- Poorter H, Nagel O. 2000. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Functional Plant Biology 27: 1191. [Google Scholar]
- Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. 2012. Biomass allocation to leaves, stems and roots: meta‐analyses of interspecific variation and environmental control. New Phytologist 193: 30–50. [DOI] [PubMed] [Google Scholar]
- Pratt JD, Mooney KA. 2013. Variation in Artemisia californica functional traits and plasticity along a steep environmental cline: implications for plant response to predicted climate change. Global Change Biology 19: 2454–2466. [DOI] [PubMed] [Google Scholar]
- Probst P, Wright MN, Boulesteix A. 2019. Hyperparameters and tuning strategies for random forest. Wiley Interdisciplinary Reviews: Data Mining and Knowledge Discovery 9: e1301. [Google Scholar]
- R Core Team . 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [WWW document] URL https://www.R‐project.org/ [accessed 27 July 2020]. [Google Scholar]
- Reich PB. 2014. The world‐wide ‘fast–slow’ plant economics spectrum: a traits manifesto. Journal of Ecology 102: 275–301. [Google Scholar]
- Reich PB, Luo Y, Bradford JB, Poorter H, Perry CH, Oleksyn J. 2014. Temperature drives global patterns in forest biomass distribution in leaves, stems, and roots. Proceedings of the National Academy of Sciences, USA 111: 13721–13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Wright IJ, Cavender‐Bares J, Craine JM, Oleksyn J, Westoby M, Walters MB. 2003. The evolution of plant functional variation: traits, spectra, and strategies. International Journal of Plant Sciences 164: S143–S164. [Google Scholar]
- Reinhardt D, Kuhlemeier C. 2002. Plant architecture. EMBO Reports 3: 846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3: 217–223. [Google Scholar]
- Roumet C, Birouste M, Picon‐Cochard C, Ghestem M, Osman N, Vrignon‐Brenas S, Cao KF, Stokes A. 2016. Root structure–function relationships in 74 species: evidence of a root economics spectrum related to carbon economy. New Phytologist 210: 815–826. [DOI] [PubMed] [Google Scholar]
- Roumet C, Urcelay C, Díaz S. 2006. Suites of root traits differ between annual and perennial species growing in the field. New Phytologist 170: 357–368. [DOI] [PubMed] [Google Scholar]
- Schenk HJ. 2006. Root competition: beyond resource depletion. Journal of Ecology 94: 725–739. [Google Scholar]
- Schenk HJ. 2008a. Soil depth, plant rooting strategies and species' niches. New Phytologist 178: 223–225. [DOI] [PubMed] [Google Scholar]
- Schenk HJ. 2008b. The shallowest possible water extraction profile: a null model for global root distributions. Vadose Zone Journal 7: 1119–1124. [Google Scholar]
- Schenk HJ, Callaway RM, Mahall BE. 1999. Spatial root segregation: are plants territorial? Advances in Ecological Research 28: 145–180. [Google Scholar]
- Schenk HJ, Jackson RB. 2002a. Rooting depths, lateral root spreads and below‐ground/above‐ground allometries of plants in water‐limited ecosystems. Journal of Ecology 90: 480–494. [Google Scholar]
- Schenk HJ, Jackson RB. 2002b. The global biogeography of roots. Ecological Monographs 72: 311–328. [Google Scholar]
- Schenk HJ, Jackson RB. 2003. Global distribution of root profiles in terrestrial ecosystems. Data set. Oak Ridge, TN, USA: Oak Ridge National Laboratory Distributed Active Archive Center. [WWW document] URL http://www.daac.ornl.gov. doi: 10.3334/ORNLDAAC/660. [DOI] [Google Scholar]
- Schenk HJ, Jackson RB. 2005. Mapping the global distribution of deep roots in relation to climate and soil characteristics. Geoderma 126: 129–140. [Google Scholar]
- Schwarz M, Lehmann P, Or D. 2010. Quantifying lateral root reinforcement in steep slopes – from a bundle of roots to tree stands. Earth Surface Processes and Landforms 35: 354–367. [Google Scholar]
- Shipley B, Meziane D. 2002. The balanced‐growth hypothesis and the allometry of leaf and root biomass allocation. Functional Ecology 16: 326–331. [Google Scholar]
- Silge J, Chow F, Kuhn M, Wickham H. 2021. rsample: general resampling infrastructure. R package v.0.1.1. [WWW document] URL https://CRAN.R‐project.org/package=rsample [accessed 20 November 2021]. [Google Scholar]
- Sinacore K, Hall JS, Potvin C, Royo AA, Ducey MJ, Ashton MS. 2017. Unearthing the hidden world of roots: root biomass and architecture differ among species within the same guild. PLoS ONE 12: e0185934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh C, Wang‐Erlandsson L, Fetzer I, Rockström J, van der Ent R. 2020. Rootzone storage capacity reveals drought coping strategies along rainforest‐savanna transitions. Environmental Research Letters 15: e124021. [Google Scholar]
- Smith MN, Stark SC, Taylor TC, Ferreira ML, Oliveira E, Restrepo‐Coupe N, Chen S, Woodcock T, Santos DB, Alves LF et al. 2019. Seasonal and drought‐related changes in leaf area profiles depend on height and light environment in an Amazon forest. New Phytologist 222: 1284–1297. [DOI] [PubMed] [Google Scholar]
- Smith SA, Brown JW. 2018. Constructing a broadly inclusive seed plant phylogeny. American Journal of Botany 105: 302–314. [DOI] [PubMed] [Google Scholar]
- Stekhoven DJ, Buhlmann P. 2012. MissForest – non‐parametric missing value imputation for mixed‐type data. Bioinformatics 28: 112–118. [DOI] [PubMed] [Google Scholar]
- Stocker BD, Tumber‐Dávila SJ, Konings AG, Anderson MB, Hain C, Jackson RB. 2021. Global distribution of the rooting zone water storage capacity reflects plant adaptation to the environment. bioRxiv preprint. doi: 10.1101/2021.09.17.460332. [DOI] [Google Scholar]
- Stocker TF, Qin D, Plattner G‐K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex B, Midgley BM. 2013. IPCC, 2013: climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Strobl C, Boulesteix AL, Zeileis A, Hothorn T. 2007. Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinformatics 8: e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl C, Boulesteix A‐L, Kneib T, Augustin T, Zeileis A. 2008. Conditional variable importance for random forests. BMC Bioinformatics 9: e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka A. 1994. A simulation model of tree architecture development based on growth response to local light environment. Journal of Plant Research 107: 321–330. [Google Scholar]
- The Plant List . 2013. The Plant List: a working list of all plant species. v.1.1. Published on the Internet. [WWW document] URL http://www.theplantlist.org/ [accessed 1 September 2020]. [Google Scholar]
- Tobin B, Čermák J, Chiatante D, Danjon F, Di Iorio A, Dupuy L, Eshel A, Jourdan C, Kalliokoski T, Laiho R et al. 2007. Towards developmental modelling of tree root systems. Plant Biosystems 141: 481–501. [Google Scholar]
- Trabucco A, Zomer RJ. 2019. Global aridity index and potential evapo‐transpiration (ET0) climate database v.2. figshare. Fileset. doi: 10.6084/m9.figshare.7504448.v3 [accessed 10 September, 2020]. [DOI] [Google Scholar]
- Tumber‐Dávila SJ, Malhotra A. 2020. Fast plants in deep water: introducing the whole‐soil column perspective. New Phytologist 225: 7–9. [DOI] [PubMed] [Google Scholar]
- Verbeeck H, Bauters M, Jackson T, Shenkin A, Disney M, Calders K. 2019. Time for a plant structural economics spectrum. Frontiers in Forests and Global Change 2: e43. [Google Scholar]
- Vieilledent G, Courbaud B, Kunstler G, Dhôte JF, Clark JS. 2010. Individual variability in tree allometry determines light resource allocation in forest ecosystems: a hierarchical Bayesian approach. Oecologia 163: 759–773. [DOI] [PubMed] [Google Scholar]
- Villares J, Tundisi M, Becker M. 1953. The subterranean system of colonial grass (Guinea grass) in various soils of the state of Sao Paulo, Brazil. Rangeland Ecology & Management/Journal of Range Management Archives 6: 248–254. [Google Scholar]
- Vogt KA, Vogt DJ, Palmiotto PA, Boon P, O’Hara J, Asbjornsen H. 1996. Review of root dynamics in forest ecosystems grouped by climate, climatic forest type and species. Plant and Soil 187: 159–219. [Google Scholar]
- Wang P, Huang K, Hu S. 2020. Distinct fine‐root responses to precipitation changes in herbaceous and woody plants: a meta‐analysis. New Phytologist 225: 1491–1499. [DOI] [PubMed] [Google Scholar]
- Warren JM, Hanson PJ, Iversen CM, Kumar J, Walker AP, Wullschleger SD. 2015. Root structural and functional dynamics in terrestrial biosphere models – evaluation and recommendations. New Phytologist 205: 59–78. [DOI] [PubMed] [Google Scholar]
- Weaver J. 1919. The ecological relations of roots. Publication no. 286. Washington, DC, USA: Carnegie Institution of Washington. [Google Scholar]
- Weaver JE. 1958. Classification of root systems of forbs of grassland and a consideration of their significance. Ecology 39: 394–401. [Google Scholar]
- Weltzin JF, McPherson GR. 2000. Implications of precipitation redistribution for shifts in temperate savanna ecotones. Ecology 81: 1902–1913. [Google Scholar]
- Westoby M, Wright IJ. 2006. Land‐plant ecology on the basis of functional traits. Trends in Ecology & Evolution 21: 261–268. [DOI] [PubMed] [Google Scholar]
- Winter B. 2013. Linear models and linear mixed effects models in R with linguistic applications. arXiv preprint. arXiv: 1308.5499. [WWW document] URL http://arxiv.org/pdf/1308.5499.pdf [accessed 27 July 2019]. [Google Scholar]
- Wright MN, Ziegler A. 2017. Ranger: a fast implementation of random forests for high dimensional data in C++ and R. Journal of Statistical Software 77: 1–17. [Google Scholar]
- Zanne AE, Tank DC, Cornwell WK, Eastman JM, Smith SA, FitzJohn RG, McGlinn DJ, O’Meara BC, Moles AT, Reich PB et al. 2014. Three keys to the radiation of angiosperms into freezing environments. Nature 506: 89–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dataset S1 Root systems of individual plants database.
Fig. S1 Map of Root Systems of Individual Plants Database (RSIP) observations by versions.
Fig. S2 Comparison of Root Profiles for Global Ecosystems (RPGE) rooting depth estimates to the plant functional type (PFT) estimates.
Fig. S3 The effect that climate variables have on individual plant rooting depth vs ecosystem‐scale rooting depth.
Fig. S4 PIC of maximum rooting depth (D R) to aboveground plant size (H S, W S, V S, and DBH).
Fig. S5 PIC of maximum lateral spread (L R) to aboveground plant size (H S, W S, V S, and DBH).
Fig. S6 The influence of climate metrics on max rooting depth (D R) and maximum lateral spread (L R).
Fig. S7 PIC of maximum rooting depth (D R) to climate metrics (MAE, MAP, A i and S a).
Fig. S8 PIC of maximum lateral spread (L R) to climate metrics (MAE, MAP, A i and S a).
Fig. S9 Correlation matrix for the above and belowground plant size metrics.
Table S1 Description of RSIP parameters (n is the total number of observations).
Table S2 RSIP categorical groups. The number of total observations n, and unique species, geographic locations, and studies for each class are shown.
Table S3 Pagel’s lambda values for the above and belowground plant measurements.
Table S4 Comparison of absolute extents (D R and L R) with climate metrics.
Table S5 Nonlinear regression curves for the shape ratios plotted in Fig. 7.
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Data Availability Statement
The RSIP can be found as Dataset S1. The RPGE data (Schenk & Jackson, 2003) are openly available in the ORNL DAAC at https://doi.org/10.3334/ORNLDAAC/660.


