Abstract
The precursor lesion for the ~30% of colon carcinomas developing along the serrated pathway was first described in detail in 1996, and was named sessile serrated adenoma in 2003. Although the entity itself was controversial initially, over time the concept of a serrated pathway initiated by this lesion has become well accepted in the medical community. The name sessile serrated adenoma, however, has been controversial since the beginning and continues to be controversial. Alternative names, including serrated polyp with abnormal proliferation, sessile serrated polyp and, most recently, sessile serrated lesion, have been proposed. Despite the fact that the term sessile serrated lesion was adopted by the World Health Organization in 2019, none of these terms has received universal acceptance. In this article, arguments for and against adopting the term sessile serrated lesion are discussed in detail.
Keywords: sessile serrated adenoma, sessile serrated lesion, sessile serrated polyp
Abbreviations
- CA
conventional adenoma
- SPAP
serrated polyp with abnormal proliferation
- SSA
sessile serrated adenoma
- SSL
sessile serrated lesion
- SSP
sessile serrated polyp
- TSA
traditional serrated adenoma
1. Introduction
Since the recognition of serrated lesions as precursors of adenocarcinoma in 1996, which was a controversial suggestion at the time, and was not generally accepted until after 2003, the terminology of serrated lesions has been even more controversial. 1 , 2 Although the initial description of this lesion was published in 1996, the first use of the term sessile serrated adenoma (SSA) was published in 2003. 3 In that initial 1996 article, the lesion was misinterpreted as being a flat variant of the serrated adenoma as described by Longacre and Fenoglio‐Preiser, which is usually a very protuberant lesion; hence the term ‘sessile’ serrated adenoma. 4 The term traditional serrated adenoma (TSA) was coined in 2003 to give recognition to the most characteristic of several lesions (including what we now know as TSA as well as what we would now consider to be SSA with cytological dysplasia) described in the initial contribution of Longacre and Fenoglio‐Preiser. 4 Later, it became apparent that SSA and TSA were quite distinct demographically and molecularly. It was recognized that neither SSA or TSA in its earliest form had what is typically recognized as dysplasia in conventional adenomas (CAs) arising secondary to APC mutations (although some authors misinterpret enteric metaplasia in TSA as low‐grade serrated dysplasia); however, both lesions can develop cytological features that are are histologically typical of conventional dysplasia as they progress. Hence, the terms SSA with cytological dysplasia and TSA with conventional dysplasia were coined to represent this more advanced form of these lesions.
From the earliest days, the name SSA was criticized because of the absence of dysplasia as seen in CA, although the term TSA has generally been widely accepted without criticism, despite the fact that it also does not have dysplasia in its early form. 5 , 6 , 7 Alternative terms proposed in the early days included both sessile serrated polyp (SSP) and serrated polyp with abnormal proliferation (SPAP) (a term used by O'Brien et al., recognizing a term coined descriptively in 2003 to describe the main structural difference between SSA and hyperplastic polyps). 8 , 9 The argument proposed in favour of SSP and SPAP was mainly the absence of dysplasia in the lesion, with the dogma at the time being that all adenomas have dysplasia. Although SPAP has some merit as a non‐committal descriptive term, it never received additional support. SSA and SSP, however, both received widespread acceptance by different groups, although the use of two different terms for the same lesion caused considerable confusion in the literature, as it was unclear to some readers whether these were the same or different lesions. Hence, in 2010 the World Health Organization (WHO) agreed to accept either term for routine use and to use the combination SSA/P for publications to prevent confusion. 10
Recently, the term sessile serrated lesion (SSL) has been proposed, mainly in the UK, as an alternative term for the lesion. 11 The rationale for SSL appears to be exactly the same as the rationale for SSP, being based on the absence of dysplasia. In 2019, the WHO chose to adopt the term SSL and recommended that the use of SSA and SSP be discontinued. 12
2. Morphological description
SSAs/SSLs are generally composed of a mixture of goblet cells and microvesicular epithelial cells with bland cytology. Their nuclei are small, round to oval, and basally located. In contrast to that of hyperplastic polyps, the architecture of some of the crypts is distorted, presenting with horizontal growth above the muscularis mucosae, serration and dilatation deep in the basal parts of the crypts, and asymmetrical proliferation. 12 By definition, one unequivocal architecturally distorted crypt is sufficient for the diagnosis of SSA/SSL (Figure 1).
Figure 1.
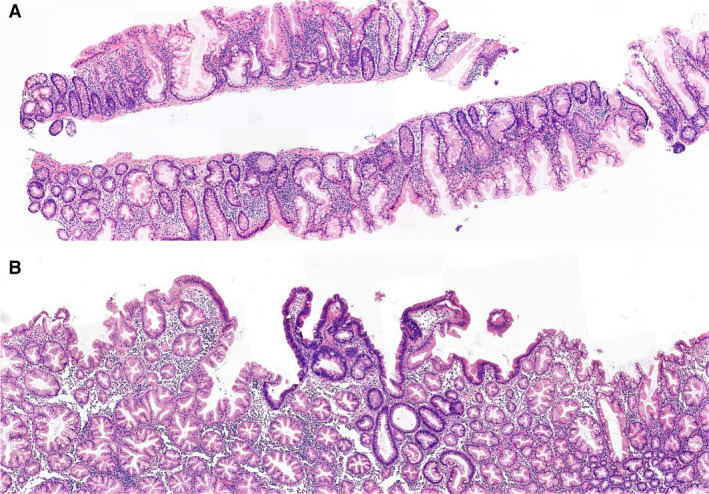
A, Overview of sessile serrated adenoma/sessile serrated lesion with characteristic crypt distortion. B, Focal presence of dysplasia.
SSAs/SSLs are complex lesions with various interactions with the microenvironment. Mucosal herniation can lead to an inverted appearance with additional distortion of the crypt architecture by smooth muscle fibres. 13 A subset of SSLs present with unusual stromal proliferation, either perineurioma‐like 14 or with submucosal or intramucosal accumulation of lipocytes. 15 , 16 The meaning of these phenomena is not clear, but they add to the complexity and heterogeneity of this lesion.
Although, at this point, there is acceptance of the concept of the serrated pathway to colorectal carcinoma (CRC), strong opinions persist regarding the best terminology. Arguments for and against SSL as an alternative term follow.
3. Yes: SSL should be introduced as a unifying term (Iris Nagtegaal)
3.1. What is a Proper Name?
‘The beginning of wisdom is to call things by their proper names’ is a well‐known quotation by Confucius. It can be considered to be the guideline for our work as pathologists, as well as for the classification series of the WHO.
However, in the 1st edition of the WHO series of international classification of tumours (part 15, Histological typing of intestinal tumours) 17 the problem was already clearly stated: ‘Among the prerequisites for comparative studies of cancer are international agreement on histological criteria for the classification of cancer types and a standardized nomenclature. At present, pathologists use different terms for the same pathological entity, and furthermore, the same term is sometimes applied to lesions of different types. An internationally agreed classification of tumours, acceptable alike to physicians, surgeons, radiologists, pathologists and statisticians, would enable cancer workers in all parts of the world to compare their findings and would facilitate collaboration among them’. This points towards the first requirement for a proper name, which is that it should be unifying: one name for one entity.
Although older entities within pathology are known by the names of the persons who first described them, this practice is no longer recommended, 18 , 19 as these terms are less informative than a morphology‐derived or biology‐derived name. In addition, it is helpful to have the opportunity to add information about the biological behaviour of the entity, often by the addition of a description of the grade, e.g. low‐grade dysplasia or borderline malignancy. The latter is extremely helpful in entities that play a role in carcinogenesis.
3.2. All Adenomas in the Gastrointestinal Tract Show Cytological Dysplasia
The definitions of gastrointestinal adenomas, since the first edition of the WHO classification, always implied cytological atypia in the epithelial cells that is sufficient for the diagnosis of dysplasia (Table 1). 10 , 12 , 17 , 20 , 21 This contrasts with the bland cytology of SSL, and is the main argument against the use of the term adenoma for these lesions. For this reason, many pathologists and clinicians were reluctant to use the term SSA. 22 In addition, it was recognized early that SSL would require an additional step of morphological features resembling adenoma before progressing to carcinoma. 2 This intermediate step is now called SSL with dysplasia. The incidence of these overtly dysplastic lesions is estimated at 6% of all SSLs. 23 , 24 These advanced lesions are heterogeneous morphologically, with several patterns of dysplasia having been described, including ‘not otherwise specified’, minimal deviation, conventional and serrated types. 25 , 26 Unlike for CAs, there is no need for grading of dysplasia in SSL. The presence of any histological form of dysplasia in SSL, often associated with loss of MLH1 expression, is considered to be an indicator of a high risk of progression to carcinoma.
Table 1.
Definitions of colorectal adenoma, as derived from the World Health Organisation classification of tumours (various editions)
| WHO classification | Definition |
| 1st edition 17 | A benign pedunculated or sessile neoplasm of glandular epithelium in which there is atypia of varying degrees. |
| 2nd edition 20 | A circumscribed benign neoplasm composed of tubular and/or villous structures lined by dysplastic epithelium. Dysplastic epithelium differs from the normal in including a higher proportion of immature cells containing large, hyperbasophilic and stratified nuclei. |
| 3rd edition 21 | These precursor lesions are defined by the presence of intraepithelial neoplasia, histologically classified by hypercellularity with enlarged hyperchromatic nuclei, varying degrees of nuclear stratification and loss of polarity. |
| 4th edition 10 | Adenomas are defined by the presence of dysplastic epithelium |
| 5th edition 12 | Benign, premalignant neoplasms composed of dysplastic epithelium |
3.3. Biological Behaviour of SSL
The risk of CRC developing in patients with SSL is at least similar to that in patients with CA. Morphological, molecular and epidemiological studies have proven its role as precursor for a significant percentage of CRCs. 27 The serrated pathway, which is a model with stepwise progression towards CRC, has been described in analogy with the adenoma–carcinoma pathway for CA. The sequence and nature of genetic mutations of the serrated pathway are different from those of the adenoma–carcinoma pathway. BRAF mutations are early events, leading to widespread CpG island methylation, resulting in the CpG island methylator phenotype. 28 As a consequence of hypermethylation of the MLH1 promoter, microsatellite instability occurs. This molecular change is present in the majority of SSLs with dysplasia. 25 So, although both CA and SSL are both precursor lesions for CRC, they are not the same and have different developmental pathways.
3.4. Not All Precursor Lesions are Called Adenomas
The fact that SSLs are precursors of CRC does not automatically imply that we should use the term adenoma for SSL. In the tubular gut, several other entities exist that are precursors but are not called adenomas. These include Barrett oesophagus, gastric dysplasia, and inflammatory bowel disease‐associated dysplasia. These lesions are not called adenomas, first because these are not, by definition, polypoid in nature, and second to emphasize their different developmental pathway.
3.5. Why SSL Should Not Be Called SSA, SSP, Or SSA/P
Previous terms for SSL were not ideal. Although SSL is a precursor of CRC, the use of SSA implied the presence of dysplasia, which is absent in the ordinary form of the lesion. There is no obvious need for the use of adenoma to indicate a precursor lesion, because, in gastrointestinal pathology, other terms are in use for different types of precursor lesion. Moreover, the use of the term SSL allows for the addition of ‘with dysplasia’ in order to indicate a high‐risk SSL. The previous term ‘SSA/P with cytological dysplasia’ 10 was confusing when SSA was used.
The main argument against the term SSP is the fact that most SSLs are indeed sessile rather than pedunculated or polypoid. Endoscopic recognition is therefore notoriously difficult. 27 Moreover, the term SSP could potentially be confusing, as both hyperplastic polyps and SSL can described as such. In most cases, this term was used as an alternative for SSA in the absence of dysplasia. 22 However, to emphasize that SSA and SSP were synonyms and to come to a compromise, the hybrid term SSA/P was coined in the 4th edition of the WHO classification. 10 This led to the use of three different terms in clinical practice and the literature, which is far from the ideal unifying nomenclature that is best for patients and research alike.
The term SSL was, before its introduction in the 5th edition of the WHO classification, 12 internationally introduced in Europe in the context of population screening programmes. 29 This term was first used by an international multidisciplinary consortium including pathologists and clinicians in 2008. 30 This unifying term, with possibilities for indicating high‐risk variations, reflects the complexity of the lesion. Clinical acceptance of this term is high 22 and the precursor nature is well understood.
4. No: we should use SSA as the term for this entity (Dale Snover)
4.1. So What Is An Adenoma?
As the argument against the use of the term SSA has been the absence of cytological dysplasia, with the implication that the term adenoma is inappropriate for any lesion which is not dysplastic, we should perhaps explore the meaning of adenoma with particular regard to the need for dysplasia as a defining feature. A typical dictionary definition of an adenoma is a ‘benign tumor of a glandular structure or glandular origin’. 31 Despite the common misconception that adenomas in the gastrointestinal tract by definition have dysplasia, this has not always been true, even in the colon. If one looks at literature in the 1960s, for example, lesions currently considered to be hamartomatous, such as juvenile retention polyps, were considered to be juvenile adenomas of the colon. 32 The concept of dysplasia as a defining feature did not exist, although it became recognized that dysplasia did separate adenomas that were premalignant from those that were not. In essentially all other organs of the body (thyroid, adrenal, liver, upper gastrointestinal tract, or pancreas, for example), the diagnosis of adenoma does not require dysplasia [common examples in the gastrointestinal tract and liver would include hepatocellular adenoma, bile duct adenoma, serous (microglandular) adenoma of the pancreas, and both foveolar and pyloric adenomas of the stomach, as well as TSA in the large intestine]. Perhaps the most ironic point of this is the fact that TSA is also a lesion without typical conventional dysplasia in its non‐advanced form (although, as noted in the Introduction, some authors contend that enteric metaplasia is a form of dysplasia, therefore justifying, in their opinion, the term adenoma), but nobody (including the authors of the 5th edition of the WHO classification) has any difficulty in designating TSA as an adenoma. My point here is not to suggest that any glandular tumour of the colon should now be considered to be an adenoma, as it was in the 1960s, but rather that the definition of adenoma of the colon used through the early 2000s as requiring dysplasia is not an immutable definition, but rather a working definition that can be as easily changed now as it was changed when juvenile retention polyps were no longer considered to be juvenile adenomas. It is important to remember that the concept of dysplasia as part of the definition of adenoma was developed at a time when all carcinomas of the colon were thought to arise from a single precursor lesion, and the alternative serrated pathway was not recognized. Now that we recognize that there is more than one pathway to colon carcinoma, the definition of the precursor adenoma also needs to expand to keep up with our growing knowledge.
So what is the functional meaning of colonic adenoma according to current terminology? The advent of the concept of dysplasia as a defining feature of adenomas was developed to separate those benign glandular lesions of the colon that were prone to progressing to adenocarcinoma from those that were not. Therefore, the functional significance of the term adenoma of the colon is to identify polyps with a propensity towards the development of adenocarcinoma, or ‘premalignant polyps’ if you will, with the attendant management issues related to preventing CRC. The identification of dysplasia as a feature of one type of premalignant polyp was simply a histological marker of this type of polyp, and at the time was considered to be the only marker for identification of premalignant polyps, neglecting the possibility of alternative pathways. This dogma, in retrospect, resulted in incomplete prevention of colon carcinoma, particularly of the right colon, as serrated lesions were treated as non‐worrisome lesions and were ignored. Given this history, it seems more appropriate to consider all polyps with a significant likelihood of becoming malignant as adenomas. If one accepts this concept, it is obvious that the term SSA is a very reasonable name for this lesion, as it is the second most common precursor of adenocarcinoma in the colon after the lesion we now prefer to call CA (tubular, tubulovillous and villous adenomas). Therefore, dysplasia (a term that is actually very difficult to clearly define by any pathologist) should not be a defining feature of adenoma as a category, but just one feature that can be seen as the primary finding in adenomas arising along the APC‐mutated pathway and as a secondary finding (not APC mutation‐related) in advanced serrated lesions. The fact that there seems to be no difficulty in accepting the term TSA without dysplasia should easily apply to SSA, given the absence of dysplasia in both.
As a practical matter, for those of us who have been using the diagnosis of SSA since 2003 or before, the use of this term has not created confusion for our clinical colleagues, who have accepted the concept and term entirely.
4.2. So What Is a ‘Lesion’?
If some authors consider SSL to be a term preferable to SSA, we should explore the meaning of the term lesion. The definition of a lesion is ‘an abnormal change in structure of an organ or part due to injury or disease especially: one that is circumscribed and well defined’. 33 The term lesion has not been used as a specific diagnostic term, but rather as a description of something requiring further evaluation to determine its nature. Lesions can be caused by many things: ischaemia, inflammation, trauma, and neoplasia, among others. A patient presents with a lesion and we biopsy it to determine what it is. It is a term with no connotation of neoplasia or even tumour, let alone any suggestion that it might be a precursor to malignancy. It has approximately the same specificity as calling something a ‘thing’ or a ‘nubbin’ (a small, usually projecting, part or bit 34 ). By the criteria of using sessile serrated ‘lesion’ to replace sessile serrated ‘adenoma’, any discrete neoplastic abnormality in pathology could have its diagnostic term replaced by ‘lesion’. We could have basal cell lesion to replace basal cell carcinoma, or fibroglandular lesion of the breast to replace fibroadenoma. Obviously, these terms would be unacceptable, as apparently would the term traditional serrated lesion for TSA.
It has been suggested that SSL has been an acceptable term since 2008, because it was used in a consensus article at that time. 30 However, prior to the publication of the 5th edition of the WHO classification, 12 there was minimal use of this term in the scientific literature, and the term had not been widely used (if at all) clinically in the USA. Although the term may have been proposed in 2008, in fact SSA continued to be the recommended term in many European countries as well as in North America until 2019. For example, the German Evidence Based Guideline for Colorectal Cancer published in 2014 and updated in 2019 used the term SSA with no mention of SSL. 35 Similarly, a position paper on the management of malignant polyps from the Association of Coloproctology of Great Britain and Ireland published in 2013 used the designation SSA and not SSL. 36 Hence, it does not appear that, until 2019, there was any groundswell of support in favour of SSL as a replacement term for SSA. A PubMed search of the term ‘sessile serrated lesion’ in the titles of papers published from 2008 until 2019 identified only three papers using this term. A search for ‘sessile serrated adenoma’ in the titles during this same time period identified 71 papers. Clearly, SSA has been the preferred term.
4.3. So What is the Best Name for this Lesion?
The term SSA remains the best term for this lesion. Aside from the fact that it was historically the first name, and hence should only be replaced for compelling reasons, it also best reflects the behaviour of the lesion, which is a precursor to a variety of forms of CRC. It therefore directs management decisions. SSP and SSL both suffer from the fact that neither gives the reader any idea of the nature of the lesion, and both of these names could obviously apply to hyperplastic polyps as well as SSA, as both SSA and hyperplastic polyps are sessile, are serrated, and are both polyps and lesions. In addition, to say that the term SSA should be abandoned because the lesion does not have conventional dysplasia implies that the term TSA should be abandoned as well, as the lesion does not necessarily have conventional dysplasia. I have not, however, seen any suggestion that we adopt the term ‘traditional serrated lesion’.
What is the true argument for SSL? As far as I can tell, the only argument proposed is that these lesions cannot be called adenomas because they do not have dysplasia. As pointed out above, in all organ systems, including the gastrointestinal tract, dysplasia in the conventional sense is not a requirement for the diagnosis of adenoma. That distinction seems to be relegated to the colorectum only. One should remember that the definition of adenoma as having dysplasia was something we were all taught in medical school, and arose at a time when there was thought to be only one pathway to carcinoma in the colon. It was a definition created by pathologists to distinguish premalignant polyps from polyps not likely to become malignant. If the serrated pathway to carcinoma had been discovered first, and architectural distortion had been the first histological definition of a premalignant lesion, would the same authors argue that lesions with dysplasia could not be considered to be adenomas because they did not have architectural distortion? Times change, definitions change, and, hopefully, pathologists' minds are plastic enough to change with time as well.
In the end, the term SSL should not be adopted. It does not fulfil the criteria put forward as being required for a good name for a lesion. It is ambiguous regarding the premalignant nature of the lesion and it is unclear that it refers to a specific lesion, as the term itself is more fitting as a description for the family of serrated lesions (hyperplastic polyps, SSAs, and TSAs) than for any one lesion within this family.
5. Discussion
Both authors concur on the morphological description of SSA/SSL and its role in colorectal carcinogenesis. There is no fundamental disagreement regarding the clinical implications of the entity, nor on the current state of knowledge regarding its progression from an early lesion to carcinoma. However, a difference of opinion persists in the terminology for the lesion, with acceptance of the term SSL showing considerable geographical variability. In general, acceptance appears to be greater in Europe and Asia than in the USA. 37 This may well reflect the lack of consideration of the opinions of American pathologists involved in research in this area in the discussion of the advantages and disadvantages of this term prior to the 2019 WHO publication.
Conflicts of interest
The authors have no conflicts of interest to report.
Author contributions
The Introduction and Discussion were written jointly by the authors. The sections arguing for and against adopting SSL were written separately by the authors, as indicated in the article. The final version was approved by both authors. D. C. Snover was senior author of several of the sentinel papers on SSA (Torlakovic and Snover 1 and Torlakovic et al., 3 , 5 ) and senior author of the WHO 4th edition recommendations on serrated lesions of the large intestine (Snover et al. 10 ). I. D. Nagtegaal was senior author of the recent overview article on the serrated pathway (Crockett and Nagtegaal 27 ) and senior editor of the WHO 5th edition chapter on tumours of the colon and rectum.
Acknowledgements
I. D. Nagtegaal would like to thank Christophe Rosty, Envoi Pathology, for useful comments on an early version of her part of the article.
Data availability statement
Data sharing not applicable ‐ no new data generated.
References
- 1. Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996; 110; 748–755. [DOI] [PubMed] [Google Scholar]
- 2. Jass JR. Hyperplastic‐like polyps as precursors of microsatellite‐unstable colorectal cancer. Am. J. Clin. Pathol. 2003; 119; 773–775. [DOI] [PubMed] [Google Scholar]
- 3. Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am. J. Surg. Pathol. 2003; 27; 65–81. [DOI] [PubMed] [Google Scholar]
- 4. Longacre TA, Fenoglio‐Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am. J. Surg. Pathol. 1990; 14; 524–537. [DOI] [PubMed] [Google Scholar]
- 5. Torlakovic EE, Gomez JD, Driman DK et al. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA). Am. J. Surg. Pathol. 2008; 32; 21–29. [DOI] [PubMed] [Google Scholar]
- 6. Bettington ML, Chetty R. Traditional serrated adenoma: an update. Hum. Pathol. 2015; 46; 933–938. [DOI] [PubMed] [Google Scholar]
- 7. Bettington ML, Walker NI, Rosty C et al. A clinicopathological and molecular analysis of 200 traditional serrated adenomas. Mod. Pathol. 2015; 28; 414–427. [DOI] [PubMed] [Google Scholar]
- 8. Vakiani E, Yantiss RK. Pathologic features and biologic importance of colorectal serrated polyps. Adv. Anat. Pathol. 2009; 16; 79–91. [DOI] [PubMed] [Google Scholar]
- 9. O'Brien MJ, Yang S, Clebanoff JL et al. Hyperplastic (serrated) polyps of the colorectum: relationship of CpG Island methylator phenotype and K‐ras mutation to location and histologic subtype. Am. J. Surg. Pathol. 2004; 28; 423–434. [DOI] [PubMed] [Google Scholar]
- 10. Snover DC, Ahnen DJ, Burt RW, Odze RD. Serrated polyps of the colon and rectum and serrated (‘hyperplastic’) polyposis. In Bozman FT, Carneiro F, Hruban RH, Theise N eds World Health Organization classification of tumours. Pathology and genetics. Tumours of the digestive system. 4th ed. Berlin: Springer‐Verlag, 2010. [Google Scholar]
- 11. Bateman AC, Shepherd NA. UK guidance for the pathological reporting of serrated lesions of the colorectum. J. Clin. Pathol. 2015; 68; 585–591. [DOI] [PubMed] [Google Scholar]
- 12. Pai RK, Makinen MJ, Rosty C. Colorectal serrated lesions and polyps. In WHO Classification of Tumours Editorial Board ed. Digestive system tumours, 5th edn. Lyon: IARC Press, 2019. [Google Scholar]
- 13. Huang CC, Frankel WL, Doukides T, Zhou XP, Zhao W, Yearsley MM. Prolapse‐related changes are a confounding factor in misdiagnosis of sessile serrated adenomas in the rectum. Hum. Pathol. 2013; 44; 480–486. [DOI] [PubMed] [Google Scholar]
- 14. Pai RK, Mojtahed A, Rouse RV et al. Histologic and molecular analyses of colonic perineurial‐like proliferations in serrated polyps: perineurial‐like stromal proliferations are seen in sessile serrated adenomas. Am. J. Surg. Pathol. 2011; 35; 1373–1380. [DOI] [PubMed] [Google Scholar]
- 15. Wong N, O'Mahony O. Intramucosal fat is uncommon in large bowel polyps but raises three differential diagnoses. J. Clin. Pathol. 2019; 72; 562–565. [DOI] [PubMed] [Google Scholar]
- 16. Van Weyenberg SJB, Goudkade D. Lipoma of the colon with a sessile serrated adenoma/polyp and adenocarcinoma. Dig. Liver Dis. 2017; 49; 572. [DOI] [PubMed] [Google Scholar]
- 17. Morson BC, Sobin LH. Histological typing of intestinal tumours. International histological classification of tumours, no. 15. Geneva: World Health Organization, 1976. [Google Scholar]
- 18. World Health Organization . WHO issues best practices for naming new human infectious diseases . Available at: https://apps.who.int/iris/bitstream/handle/10665/163636/WHO_HSE_FOS_15.1_eng.pdf2015
- 19. Orphanet . Procedural document: rare disease nomenclature in English . Available at: https://www.orpha.net/orphacom/cahiers/docs/GB/eproc_Disease_naming_rules_in_English_PR_R1_Nom_01.pdf2017
- 20. Jass JR, Sobin LH. Histological typing of intestinal tumours. 2nd ed. Berlin: Springer Verlag, 1989. [Google Scholar]
- 21. Tumours of the digestive system. 3rd ed. Lyon: IARC Press, 2000. [Google Scholar]
- 22. Rex DK, Hassan C, Bourke MJ. The colonoscopist's guide to the vocabulary of colorectal neoplasia: histology, morphology, and management. Gastrointest. Endosc. 2017; 86; 253–263. [DOI] [PubMed] [Google Scholar]
- 23. Yang JF, Tang SJ, Lash RH, Wu R, Yang Q. Anatomic distribution of sessile serrated adenoma/polyp with and without cytologic dysplasia. Arch. Pathol. Lab. Med. 2015; 139; 388–393. [DOI] [PubMed] [Google Scholar]
- 24. Abdeljawad K, Vemulapalli KC, Kahi CJ, Cummings OW, Snover DC, Rex DK. Sessile serrated polyp prevalence determined by a colonoscopist with a high lesion detection rate and an experienced pathologist. Gastrointest. Endosc. 2015; 81; 517–524. [DOI] [PubMed] [Google Scholar]
- 25. Bettington M, Walker N, Rosty C et al. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma. Gut. 2017; 66; 97–106. [DOI] [PubMed] [Google Scholar]
- 26. Liu C, Walker NI, Leggett BA, Whitehall VL, Bettington ML, Rosty C. Sessile serrated adenomas with dysplasia: morphological patterns and correlations with MLH1 immunohistochemistry. Mod. Pathol. 2017; 30; 1728–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crockett SD, Nagtegaal ID. Terminology, molecular features, epidemiology, and management of serrated colorectal neoplasia. Gastroenterology. 2019; 157; 949–966. [DOI] [PubMed] [Google Scholar]
- 28. Weisenberger DJ, Siegmund KD, Campan M et al. CpG Island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat. Genet. 2006; 38; 787–793. [DOI] [PubMed] [Google Scholar]
- 29. Quirke P, Risio M, Lambert R, von Karsa L, Vieth M. International Agency for Research on Cancer. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition—quality assurance in pathology in colorectal cancer screening and diagnosis. Endoscopy. 2012; 44; SE116–SE130. [DOI] [PubMed] [Google Scholar]
- 30. Kudo S, Lambert R, Allen JI et al. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest. Endosc. 2008; 68; S3–S47. [DOI] [PubMed] [Google Scholar]
- 31. Adenoma . Merriam‐Webster.com dictionary . Accessed 6 October 2021. Available at: https://www.merriam‐webster.com/dictionary/adenoma
- 32. Myers TB, Bacon HE. Adenomas of the colon and rectum. Dis. Colon Rectum. 1960; 3; 523–532. [DOI] [PubMed] [Google Scholar]
- 33. Lesion . Merriam‐Webster.com dictionary . Accessed 6 October 2021. Available at: https://www.merriam‐webster.com/dictionary/lesion
- 34. Nubbin . Merriam‐Webster.com dictionary. Accessed 6 October 2021. Available at: https://www.merriam‐webster.com/dictionary/nubbin
- 35. German Guideline Program in Oncology (German Cancer Society GCA, AWMF) . Evidenced‐based guideline for colorectal cancer, long version 2.1 . AWMF—registration number: 021/007OL. Accessed 6 October 2021. Available at: https://www.leitlinienprogramm‐onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Kolorektales_Karzinom/Version_2/GGPO_Guideline_Colorectal_Cancer_2.1.pdf
- 36. Williams JG, Pullan RD, Hill J et al. Management of the malignant colorectal polyp: ACPGBI position statement. Color. Dis. 2013; 15; 1–38. [DOI] [PubMed] [Google Scholar]
- 37. Ono Y, Chiu K, Yantiss RK, Gonzalez RS. Attitudes regarding the World Health Organization‐recommended term sessile serrated lesion: results from an international survey. Arch. Pathol. Lab. Med. 2021; 145; 1189–1190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable ‐ no new data generated.


