Abstract
Background
Salivary duct carcinoma (SDC) is a high‐grade salivary malignancy that frequently occurs as the carcinomatous component of carcinoma ex pleomorphic adenoma. We herein examined the clinical factors affecting outcomes in a large cohort of SDC.
Methods
We selected 304 SDC cases and investigated clinical characteristics and the factors affecting outcomes.
Results
The median age of the cases examined was 68 years, the most common primary site was the parotid gland (238 cases), and there was a male predominance (M/F = 5:1). Outcomes were significantly worse when the primary tumor site was the minor salivary glands (SG) than when it was the major SG. Outcomes were also significantly worse in pN(+) cases (161 cases) than in pN0 cases, particularly those with a metastatic lymph node number ≥11. The cumulative incidence of relapse and distant metastases was significantly higher in stage IV cases than in stage 0–III cases.
Conclusions
The absolute number of lymph node metastases, higher stages, and the minor SG as the primary tumor site were identified as factors affecting the outcome of SDC.
Keywords: competing‐risk model, Japanese patient, outcomes, salivary duct carcinoma
1. INTRODUCTION
Salivary duct carcinoma (SDC) is a high‐grade malignant tumor of the salivary glands (SG). 1 However, it frequently occurs as the carcinomatous component of carcinoma ex pleomorphic adenoma (CXPA). 2 Although SDC shares histological similarities with invasive ductal carcinoma of the breast, it typically shows an apocrine phenotype, which differs from the immunophenotypes (estrogen receptor [ER] + and/or progesterone receptor [PgR]+) of breast cancer; the majority of SDC cases were immunohistochemically negative for ER and/or PgR, but variably positive for the androgen receptor (AR) and gross cystic disease fluid protein‐15. 1 , 3 Boon et al. previously reported that the absolute number of positive lymph nodes (LN) was associated with a poor overall survival (OS) and distant metastasis‐free survival (DMFS) in a multivariable analysis of patients presenting without distant metastases in the Netherlands. 4 In contrast, Otsuka et al. showed that an advanced N stage independently affected both OS and disease‐free survival (DFS). 5 Therefore, the present study investigated the clinical features of SDC and attempted to identify the clinical factors affecting outcomes in the largest cohort of SDC patients in Japan.
2. MATERIALS AND METHODS
2.1. Case selection
We initially collected data on 392 cases of “SDC,” “CXPA,” and “adenocarcinoma” from the pathology files of 18 institutions and a set of consultation files (from K.K.) between 1992 and 2020. Among them, SDC cases, including CXPA cases, were extracted from the central diagnostic system by four expert pathologists (K.K., A.M., K.A., and M.S.; Figure S1, Supporting Information). The following clinical data were collected from the medical records of each institution: age, sex, site, treatments, TNM classification, pathological stage, outcome, and follow‐up data. Tumors were staged according to the eighth edition of the TNM Classification of Malignant Tumors. 6 Hashimoto's classification for T factors and pathological stages was used to stage CXPA 7 : intracapsular (IC), minimally invasive (MinI), and widely invasive (WI), based on the invasive distance from the fibrous capsule, with MinI being ≤2 mm from the fibrous capsule of a co‐existing pleomorphic adenoma (PA) and WI >2 mm from the capsule.
2.2. Statistical analysis
OS was measured from the date of diagnosis until death by any cause. Patients alive at the last known follow‐up date were censored. The cumulative incidence of relapse (CIR) was defined as the number of cases in which local or regional recurrence or distant metastasis occurred after the primary surgery, regardless of which occurred first. Patients that were alive without disease at the last known follow‐up examination were censored for the purposes of the DFS analysis. The cumulative incidence of distant metastasis relapse (CIDMR) was defined as the number of cases in which distant metastasis occurred after the primary surgery. Frequencies and percentages were used for categorical variables. Survival curves were estimated by the Kaplan–Meier method and cumulative incidence curves using a competing‐risk model analysis with Gray's test when the competing‐risk event was death. 8 , 9 A univariate Cox proportional hazards regression model or Fine‐Gray proportional hazard regression model was used for comparisons of patient and tumor characteristics and survival. A multivariate Cox proportional hazards regression model or Fine‐Gray proportion hazard regression model was then performed by adjusting variables with p‐values <0.05 in the univariate analysis. Hazard ratios, 95% confidence intervals (CI), and corresponding p‐values were calculated based on the Wald test. The variables used in regression models for the cumulative accumulation of the overall incidence, relapse incidence, late cervical LN metastasis (CLNM), and distant metastasis incidence included sex, age (categorical), the T‐, N‐, and M‐status, pathological stage, number of positive LN (categorical), CXPA, and the primary tumor site. We also investigated the pattern of treatment failure, including locoregional recurrence and distant metastasis. Patients with metastatic disease at diagnosis and those with missing values for one or more of the variables were excluded from the multivariable analysis. Data were analyzed using R version 3.6.2 software (The R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1. Patient and tumor characteristics
A central pathological review and preserved data led to the inclusion of 304 eligible SDC cases from 392 cases in the initial collection (Figure 1). Patient characteristics are shown in Table 1. Median age was 68 years (range: 27–91) and there was a male predominance (83%). Although the univariate analysis of OS showed poorer outcomes for males than for females, a significant difference was not observed in the multivariate analysis. The most common primary tumor site was the parotid gland in 238 out of 304 cases (78%), followed by the submandibular gland in 55 (18%), and then the sublingual gland (1 case), palate (5 cases), parapharynx (2 cases), buccal gland (1 case), nasal cavity (1 case), and intraoral minor SG (1 case). Sixty‐nine cases (23%) had Tis and T1 as early cancer, whereas 71 (23%), 79 (26%), and 80 (26%) had T2, T3, and T4, respectively, as advanced cancer. CLNM was detected in 161 cases (53%) in the primary surgery. Distant metastases were detected in 19 cases (6.3%). Based on the histological origin, the 304 SDC cases selected for the present study comprised 122 (40%) of de novo SDC and 182 (60%) of SDC arising from PA (CXPA cases), including 47 of the IC subtype, 23 of the MinI subtype, and 112 of the WI subtype. Pathological stages were as follows: stages 0–I in 59 cases (20%), stages II and III in 78 (26%), and stage IV in 156 (51%).
FIGURE 1.
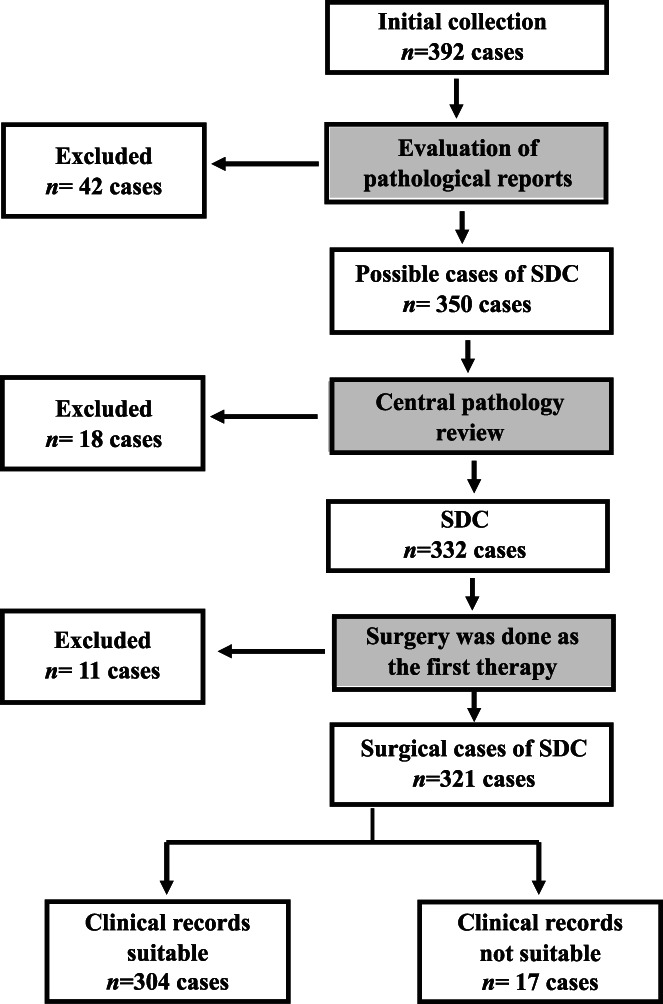
Consort diagram of the inclusion of SDC cases. All data were collected from 18 institutions and consultation cases (KK) and 304 eligible cases of SDC were ultimately selected
TABLE 1.
Characteristics of 304 patients with salivary duct carcinoma
| No. of patients (%) | |
|---|---|
| Age (year); median = 68 (27–91) | |
| ≤49 | 34 (11) |
| 50–59 | 53 (17) |
| 60–69 | 99 (33) |
| 70–79 | 81 (27) |
| ≥80 | 37 (12) |
| Gender | |
| Male | 253 (83) |
| Female | 51 (17) |
| Site | |
| Parotid gland | 238 (78) |
| SMG | 55 (18) |
| Others | 11 (3.6) |
| CXPA | |
| CXPA(−)/de novo cancer | 121 (40) |
| CXPA(+): IC | 47 (15) |
| CXPA(+): MinI | 23 (7.6) |
| CXPA(+): WI | 112 (37) |
| Unknown | 1 (0.3) |
| Stage | |
| Stage 0 | 2 (0.7) |
| Stage I | 58 (19) |
| Stage II | 33 (11) |
| Stage III | 45 (15) |
| Stage IVA | 118 (39) |
| Stage IVB | 19 (6) |
| Stage IVC | 19 (6) |
| Unknown | 10 (3.3) |
| No. of LN metastasis | |
| 0 | 126 (41) |
| 1–10 | 102 (34) |
| ≥11 | 57 (19) |
| Unknown | 19 (6) |
| Therapy | |
| S | 107 (35) |
| S + POT | 197 (65) |
Abbreviations: CXPA, carcinoma ex pleomorphic adenoma; IC, intracapsular; LN, lymph node; MinI, minimally invasive; POT, postoperative therapy; S, surgery; SMG, submandibular gland; WI, widely invasive.
The most frequent target organs for late distant metastases (n = 93) were the lungs (61 cases: 66%), followed by bone (32 cases: 34%), the central nervous system (19 cases: 20%), including the brain, meninges, and spine, distant LN (13 cases: 14%), including the mediastinal, axillary, and/or abdominal LN, the liver (11 cases: 12%), skin (8 cases: 8.6%), and other organs (4 cases), including the thyroid gland, breast, tongue, and kidney.
3.2. Therapy
A total of 107 patients underwent surgery only, while 197 received postoperative radiotherapy (RT; 102 patients: 52%), adjuvant chemotherapy (Ch; 13 patients: 6.6%), adjuvant chemoradiotherapy (CRT; 70 patients: 36%), and additional surgery (5 patients: 2.5%) after the primary surgery (Table S1). After the primary surgery, 25, 30, and 93 patients showed local recurrence, late CLNM (regional relapse), and distant metastasis, respectively. Among 110 patients with recurrence, five underwent additional surgery, while 102, 11, and 70 received additional RT, Ch, and CRT, respectively. Only 3 out of 61 patients with lung metastases recovered from the status of being alive with disease to the status of being alive without disease with additional surgery and RT for metastatic lesion(s).
3.3. Clinical outcomes and survival analysis
The median follow‐up period was 2.93 years (minimum–maximum: 0.01–21.70 years). At the time of the analysis, 149 patients were alive without disease, 66 died of disease, 38 were alive with disease, and 19 died of other causes. Kaplan–Meier curves for OS, DFS, and DMFS are shown in Figure 2. The cumulative incidence rates of 1‐ and 5‐year relapse were 26.2% (95% confidence interval [CI], 20.7–32.1) and 49.0% (95%CI, 41.9–55.7), respectively. The cumulative incidence rates of 1‐ and 5‐year local relapse (CILR), CLNM (CICLNM), and CIDMR were 7.0% (95%CI, 4.2–10.8), 12.0% (95%CI, 8–16.9), and 7.0% (95%CI, 4.2–10.8), and 12.0% (95%CI, 8–16.9), 20.3% (95%CI, 15.4–25.7), and 41.6% (95%CI, 34.7–48.4), respectively (Figures S1 and S2).
FIGURE 2.
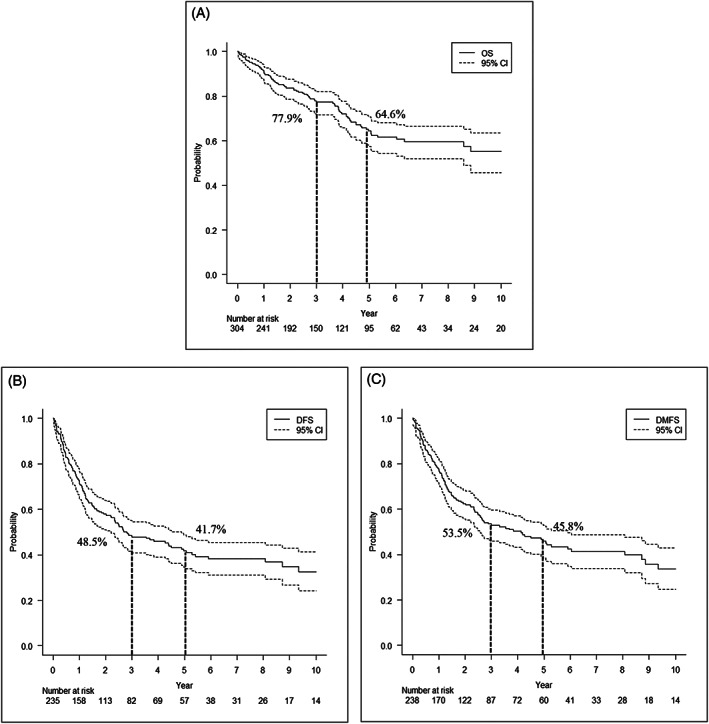
(A) Overall survival (OS), (B) disease‐free survival curve (DFS), and (C) distant metastasis‐free survival (DMFS) in 304 patients with SDC. The non‐dotted line represents survival probability and dotted lines show the 95% confidence interval. Three‐ and five‐year OS, DFS, and DMFS rates were 77.9% and 64.6%, 48.5% and 41.7%, and 53.5% and 45.8%, respectively
Cumulative incidence curves stratifying prognostic factors identified by univariate and multivariate regression models are shown in Figures 3, 4, S2, and S3, whereas those analyzed by the Fine‐Gray proportional hazards model are shown in Tables 2 and 3. OS was significantly worse in patients with a higher pathological stage and larger number of LN metastases (p < 0.001: 0 vs. 1–10 vs. ≥11 cancer‐positive nodes). On the other hand, no significant differences were observed in CIR, CILR, CICLNM, and CIDMR between de novo (CXPA[−]) and CXPA‐WI cases, whereas OS, CIR, CILR, CICLNR, and CIDMR were better in CXPA‐IC/MinI cases than in de novo and CXPA‐WI cases. The multivariate analysis identified stage IV (p < 0.001; vs. stages 0, I, II, and III, respectively) and ≥11 positive LN (p = 0.028; vs. no LN metastasis) as independent prognostic factors for OS. In addition to stage IV, ≥11 positive LN (p < 0.001; vs. no LN metastasis) and minor SG as the primary tumor site (p < 0.001 and p = 0.003; vs. the parotid gland and submandibular gland, respectively) were identified as strongly independent factors for CIR. Similarly, minor SG as the primary tumor site (p < 0.001 and p = 0.012; vs. the parotid gland and submandibular gland, respectively), stage IVA/B (p = 0.005; vs. stages 0, I, II, and III), and ≥11 positive LN (p < 0.001; vs. no LN metastasis) were also independent prognostic factors for CIDMR.
FIGURE 3.
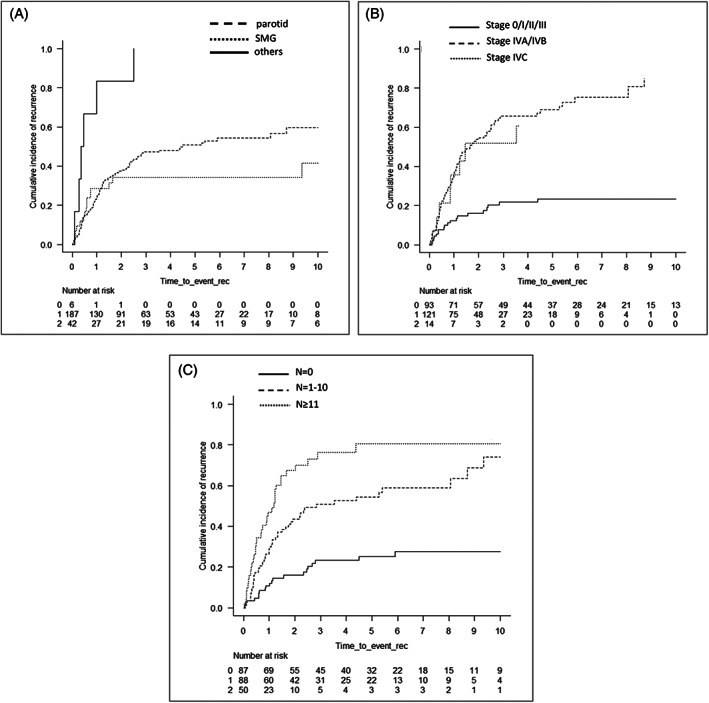
Cumulative incidence of relapse (CIR) curves according to each prognostic factor identified in the univariate analysis and multivariate Fine‐Gray proportional hazard regression model. CIR according to the site (A) (p < 0.001), pStage (B) (p < 0.001) and number of LN metastasis (C) (p < 0.001)
FIGURE 4.
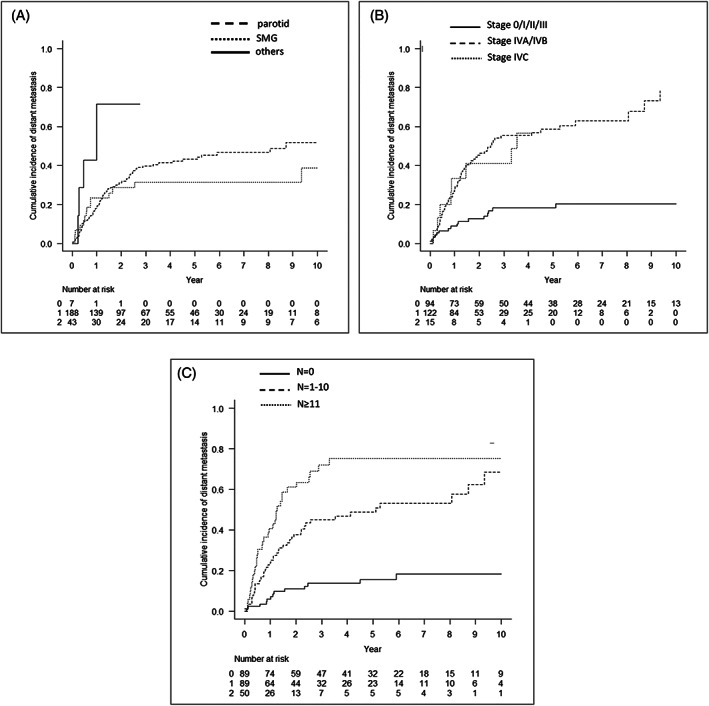
Cumulative incidence of distant metastasis relapse (CIDMR) curves according to each prognostic factor identified in the univariate analysis and multivariate Fine‐Gray proportional hazard regression model. CIDMR according to the site (A) (p = 0.0476), pStage (B) (p < 0.001), and number of LN metastasis (C) (p < 0.001)
TABLE 2.
Univariate analyses for overall survival, cumulative incidence of recurrence, and cumulative incidence of distant metastasis
| N | OS | CIR | CIDMR | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p‐value | HR (95%CI) | p‐value | HR (95%CI) | p‐value | |||
| Age | <65y/o | 131 | 0.83 (0.55–1.26) | 0.380 | 0.79 (0.54–1.14) | 0.200 | 0.83 (0.56–1.25) | 0.27 |
| ≥65y/o | 173 | Ref. | Ref. | Ref. | ||||
| Gender | Female | 51 | Ref. | Ref. | Ref. | |||
| Male | 252 | 2.05 (1.03–4.09) | 0.041 | 1.68 (0.94–3.01) | 0.081 | 1.88 (0.98–3.6) | 0.058 | |
| Site | Parotid | 238 | 2.33 (0.32–16.76) | 0.402 | 0.21 (0.1–0.44) | <0.001 | 0.33 (0.11–1) | 0.050 |
| SMG | 55 | 1.68 (0.22–12.79) | 0.617 | 0.14 (0.05–0.44) | <0.001 | 0.24 (0.07–0.82) | 0.023 | |
| Others | 11 | Ref. | Ref. | Ref. | ||||
| CXPA | (−) de novo | 121 | Ref. | Ref. | Ref. | |||
| (+) IC/MinI | 70 | 0.6 (0.32–1.1) | 0.098 | 0.63 (0.32–1.25) | 0.190 | 0.64 (0.32–1.31) | 0.220 | |
| (+) WI | 112 | 1.12 (0.72–1.75) | 0.615 | 0.91 (0.62–1.34) | 0.630 | 0.77 (0.5–1.17) | 0.220 | |
| T | Tis/pT1 | 69 | Ref. | Ref. | Ref. | |||
| T2/3 | 150 | 2.15 (1.11–4.15) | 0.023 | 2.41 (1.11–5.24) | 0.026 | 3.44 (1.27–9.27) | 0.015 | |
| T4 | 80 | 3.39 (1.69–6.82) | <0.001 | 4.84 (2.24–10.45) | <0.001 | 6.29 (2.23–17.06) | <0.001 | |
| N | N0 | 131 | Ref. | Ref. | Ref. | |||
| N1 | 36 | 0.98 (0.43–2.24) | 0.958 | 2.21 (1.15–4.25) | 0.018 | 3.05 (1.48–6.26) | 0.003 | |
| N2/N3/N(+) | 123 | 2.9 (1.82–4.63) | <0.001 | 4.07 (2.52–6.59) | <0.001 | 5.23 (2.98–9.18) | <0.001 | |
| M | M0 | 281 | Ref. | Ref. | Ref. | |||
| M1 | 19 | 2.578 (1.51–5.12) | <0.001 | 1.32 (0.64–2.74) | 0.460 | 1.43 (0.69–2.95 | 0.330 | |
| Stage | Stage 0/I/II/III | 138 | Ref. | Ref. | Ref. | |||
| Stage IVA/B | 137 | 3.38 (2.05–5.6) | <0.001 | 4.86 (2.9–8.14) | <0.001 | 4.25 (2.47–7.32) | <0.001 | |
| Stage IVC | 19 | 5.61 (2.77–11.35) | <0.001 | 3.56 (1.5–8.14) | 0.004 | 3.6 (1.52–8.53) | 0.004 | |
| No. of LN metastasis | 0 | 126 | Ref. | Ref. | Ref. | 0.001 | ||
| 1–10 | 102 | 1.87 (1.1–3.15) | 0.020 | 2.94 (1.78–4.88) | <0.001 | 4.02 (2.23–7.27) | <0.001 | |
| ≥11 | 57 | 4.14 (2.41–7.11) | <0.001 | 5.39 (3.09–9.39) | <0.001 | 7.32 (3.88–13.81) | <0.001 | |
| Therapy | S | 107 | Ref. | Ref. | Ref. | |||
| S + POT | 197 | 1.1 (0.7–1.74) | 0.669 | 1.63 (0.99–2.69) | 0.055 | 2.27 (1.27–4.05) | 0.006 | |
Note: Bold shows p < 0.05.
Abbreviations: CIDMR, cumulative incidence of distant metastasis relapse; CIR, cumulative incidence of recurrence; CXPA, carcinoma ex pleomorphic adenoma; HR, hazard ratio; 95%CI, 95% confidence interval; IC, intracapsular type; LN, lymph node; MinI, minimally invasive type; POT, postoperative therapy; OS, overall survival; Ref., reference; S, surgery; SMG, submandibular gland; WI, widely invasive type.
TABLE 3.
Multivariate analysis for overall survival, cumulative incidence of recurrence, and cumulative incidence of distant metastasis
| OS HR (95%CI) | p‐value a | CIR HR (95%CI) | p‐value a | CIDMR HR (95%CI) | p‐value a | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 1.54 (0.76–3.09) | 0.230 | ND | ND | ND | ND |
| Female | Ref. | ND | ND | ND | ND | |
| Site | ||||||
| Parotid gland | ND | ND | 0.28 (0.16–0.52) | <0.001 | 0.28 (0.14–0.59) | <0.001 |
| SMG | ND | ND | 0.27 (0.12–0.63) | 0.003 | 0.3 (0.12–0.77) | 0.012 |
| Others | ND | ND | Ref. | Ref. | ||
| Stage | ||||||
| Stage 0/I/II/III | Ref. | Ref. | Ref. | |||
| Stage IVA/B | 2.65 (1.44–4.88) | 0.002 | 3.35 (1.83–6.14) | <0.001 | 2.42 (1.3–4.49) | 0.005 |
| Stage IVC | 3.81 (1.73–8.41) | <0.001 | 2.25 (0.87–5.85) | 0.096 | 1.92 (0.76–4.91) | 0.170 |
| No. of LN metastasis | ||||||
| 0 | Ref. | Ref. | Ref. | |||
| 1–10 | 1.09 (0.59–2.02) | 0.777 | 1.75 (1.03–2.99) | 0.040 | 2.73 (1.45–5.14) | 0.002 |
| ≥11 | 2.07 (1.08–3.94) | 0.028 | 2.86 (1.57–5.2) | <0.001 | 4.63 (2.33–9.22) | <0.001 |
Note: Bold shows p < 0.05.
Abbreviations: CIDMR, cumulative incidence of distant metastasis relapse; CIR, cumulative incidence of recurrence; HR, hazard ratio; 95%CI, 95% confidence interval; LN, lymph node; ND, not done; OS, overall survival; Ref., reference; SMG, submandibular gland.
p‐value of Wald's test relating to recurrence coefficient = 0.
3.4. Patterns of treatment failure
As shown in Figure 5A, treatment failure occurred in 110 cases (36%), including 25 (8.2%) local, 30 (9.9%) regional, and 93 (31%) distant failures, of which 65 (59%) were without locoregional failure. As shown in Figure 5B, the most common sites of distant metastasis were the lungs (n = 61 cases), followed by bone (n = 32 cases), the central nervous system (n = 19 cases), distant LN (n = 13 cases), the liver (n = 11 cases), and skin (n = 8 cases). Preoperative distant metastases were detected in 19 patients (cM1: lungs, 10 cases; liver, 3 cases; bone, 4 cases; axillary LN, 1 case; pleurae 1 case). Among cM1 cases, 11 died of disease and 5 were alive with disease.
FIGURE 5.
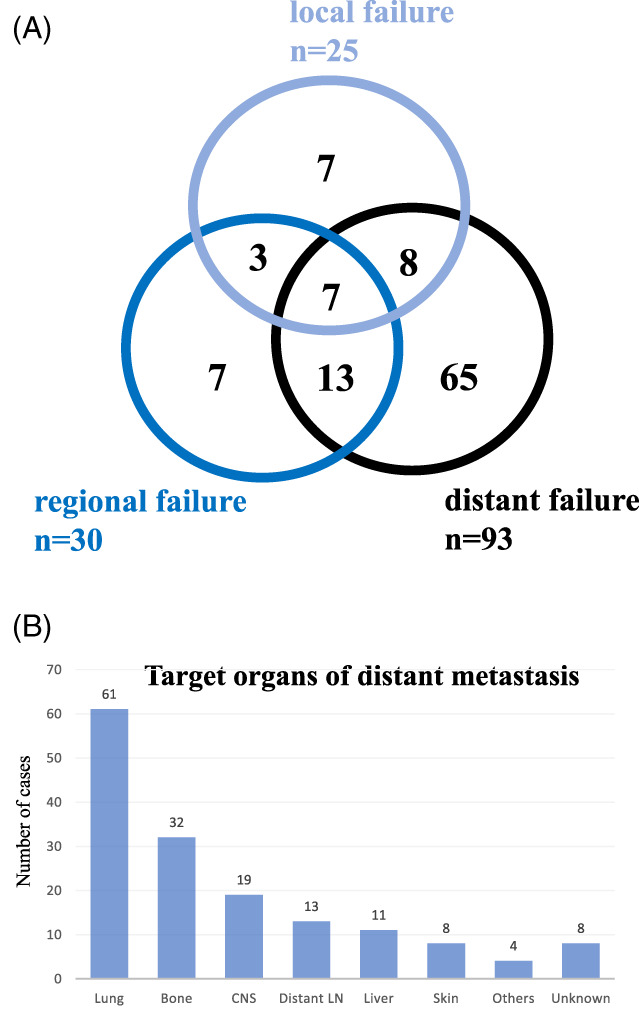
Patterns of disease recurrence. (A) Local and regional recurrence and distant metastases in 110 patients with recurrence. The numbers in the circles represent the absolute number of patients with local and regional recurrence and the presence of distant metastases. Patients with primarily metastatic disease were not included in this figure. (B) Localization of distant metastases sorted by absolute numbers in 93 patients with distant metastases. Patients with primarily metastatic diseases were not included in this figure [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
The present study examined 304 SDC cases, which represents the largest cohort of SDC reported to date, and provides extensive insights into the clinical outcomes, treatment, and prognostic factors of SDC. The results obtained support an aggressive clinical course in spite of the lower rate of distant metastases (31%) than in Boon's retrospective study 4 and a median OS of 11.61 years. In the study by Boon, the number of positive LN was the only factor independently associated with poor OS and DMFS. 4 Previous studies reported that 5‐year OS rates in patients with SDC ranged between 12% and 55%: the weighted average of 5‐year DFS and OS rates were 46% and 35%, respectively. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 The majority of studies on the clinical outcome of SDC presented data from a single institution. However, Jayaprakash et al. 18 conducted an analysis of 228 patients using the Surveillance, Epidemiology, and End Results database. The findings obtained showed that the 10‐year OS rate was 42% and median OS was 79 months, with the majority of deaths occurring within the first 5 years of the diagnosis of SDC. 18 Even in patients with early T stage SDC, the overall prognosis was poor (5‐year DFS and OS rates of 49%). 16 Otsuka et al. 5 reported 3‐year OS and DFS rates of 70.5% and 38.2%, respectively, in 141 SDC cases from multiple institutions, showed that an advanced N stage independently affected both OS and DFS, and identified the most common treatment failure as distant metastasis. In the present study, the most common treatment failure in SDC patients was also distant metastasis. Although another analysis of a larger cohort (n = 56) subsequently showed similar outcomes, with 3‐ and 5‐year OS rates of 42.7% and 26.9%, respectively, recent studies with similar cohort sizes reported a better 5‐year OS rate of 55.1%, suggesting the benefits of the intensification of both surgery and adjuvant RT for treatment outcomes. 12 , 19 , 20 However, marked differences were observed between OS and DFS; the 5‐year DFS was 29% in one study, 19 whereas Otsuka et al. 5 indicated 3‐year OS and DFS rates of 70.5% and 38.2%, respectively. This discrepancy reflects the markedly high ratio of treatment failure for SDC. In the present study, 3‐, 5‐, and 10‐year CIR were 46.3, 49.0, and 57.4%, respectively (3‐, 5‐, and 10‐year DFS rates were 48.5, 41.7, and 32.6%, respectively; data not shown). In our cohort, 3‐year DFS was slightly better in the present study than previously reported, 5 , 12 , 18 , 19 which may be attributed to advances in postoperative therapies.
In the present study, a higher pathological stage, which was associated with advanced T and N factors, and large numbers of cancer‐positive LN were identified as independent prognostic factors. Boon et al. 4 and Otsuka et al. 5 indicated that advanced N factors and/or the number of positive LN correlated with OS and DFS or DMFS. In the present study, an advanced N factor (N0 vs. N2/N3) and ≥11 cancer‐positive LN correlated with poor 5‐year OS, 5‐year CIR, and 5‐year CIDMR. These were consistent with previous studies. 4 , 5 SDC had higher incidences of LN and distant metastases than those reported by Osborn (46.5%) and Jayaprakash et al. (49%), respectively. 18 , 21 In the present study, outcomes were worse in cases with minor SG than in those with the parotid gland and submandibular gland as the primary tumor site. Since standard therapeutic strategies have not yet been established for SDC cases in which minor SG is the primary tumor site, and, thus, adequate therapies were not performed for these cases, their outcomes were worse. Furthermore, a negative surgical margin may not have been achieved in these cases, resulting in incomplete resection. Therefore, clinicians need to consider these factors in cases of SDC arising from minor SG.
In the statistical analyses, we mainly used competing‐risk analysis, in which death was employed as a competing risk, to analyze the cumulative incidence of relapse, local relapse, LN metastasis, and distant metastasis in order to produce more precise statistical results. Kaplan–Meier curve analysis frequently leads to the cumulative risk that patients are exposed to being overestimated, and when a competing risk is present the cumulative risk of patients with certain diseases is not as high as the cumulative risk indicated by the Kaplan–Meier method. 9 , 22 , 23
Otsuka et al. 5 (n = 141) and Jayaprakash et al. 18 (n = 228) identified age and the N factor as independent prognostic factors for OS and DFS/disease‐specific survival, in addition to the tumor size and grade in a multivariate analysis. However, a correlation was not observed between age and outcomes in the 304 SDC cases examined in the present study. However, LN metastasis (N[+]) was associated with worse OS, CIR, CICLNM, and CIDMR than N0 cases, and was one of the independent factors predicting a poor outcome.
In our cohort, the most common form of treatment failure was late distant metastases (n = 93 in our series), which is consistent with the findings from smaller cohorts 11 , 20 , 24 and a larger cohort. 5 Previous studies identified the lungs and bone as the most common sites of distant metastasis in SDC, 5 , 12 , 21 , 25 which is in accordance with the present results. A high ratio of distant metastases is presumed to be the leading cause of high CIR and CIDMR or low DFS and DMFS. Although extended resection with wider margins combined with intensified adjuvant RT appear to have contributed to better treatment outcomes in SDC patients by improving locoregional control, these strategies alone cannot prevent the development of delayed distant metastasis. Therefore, effective systemic therapy after curative surgery is imperative for improving CIR and CIDMR in SDC patients. Immunohistochemical studies revealed the expression of AR in 69%–100% of SDC cases, 3 , 25 , 26 whereas that of HER2 was only observed in 26%–77%, both of which were confirmed in other reports, suggesting a potential role for agents targeting these receptors in molecular‐targeted therapy for SDC. 5 , 27 , 28 Despite the focal or heterogenous expression of AR, androgen deprivation therapy (ADT) was found to be clinically beneficial for patients with AR‐positive SDC, with 18% achieving a partial response and 50% stable disease in addition to longer DSF. 29 , 30 , 31 However, some cases acquire resistance to ADT due to the aberrant expression of SRD5A1 and loss of FOXA1 expression. 32 , 33 The administration of trastuzumab and docetaxel to patients with HER2‐positive SDC achieved a good overall response (70.2%: 95%CI, 56.6–81.6), including partial and complete responses, and was clinically beneficial (84.2%; 95%CI, 72.1–92.5), with increases in OS and progression‐free survival. 34 Since the status of patients with early or late distant metastasis is systemic, novel chemotherapy regimens are needed, such as ADT for AR‐positive SDC and/or trastuzumab therapy for HER2‐positive SDC. 35 , 36 Similar to our cohort, only a few patients have been treated with ADT or trastuzumab and, thus, the therapeutic effects of these agents remain unclear. AR, HER2, and EGFR profiles in SDC patients in our series are currently being investigated.
In the present study, the outcomes of SDC ex‐PA‐WI and de novo SDC were both poor, whereas that of SDC ex‐PA‐IC/MinI was better. Hashimoto's classification was used in the present study to stage CXPA 7 because the TNM classification focused on the extent of invasion of carcinoma and not the tumor size; since CXPA‐IC cases may exhibit large tumors, and CXPA‐WI cases small tumors. Since the extent of invasion of MinI CXPA markedly varies between 1.5 and 8 mm in the 4th WHO classification, we established MinI ≤2 mm from the fibrous capsule of a co‐existing PA for a more practical and easily measurable value. Few studies have investigated differences between CXPA(−) and CXPA(+) cases. 4 , 10 Griffith et al. showed that OS was significantly worse in extracapsular invasive‐type SDC ex‐PA than in IC‐type SDC ex‐PA. 37 IC‐type SDC ex‐PA is an indolent tumor, whereas invasive‐type SDC ex‐PA is an aggressive tumor, similar to de novo SDC; therefore, WI‐type SDC ex‐PA need to be added to the analytical cohort. In our series, 9 out of the 47 cases of IC‐type SDC ex‐PA died mainly due to other diseases except for one case. Therefore, IC‐type SDC ex‐PA has a better outcome than invasive SDC.
In conclusion, SDC frequently occurs in major SG, mostly in the parotid gland; however, outcomes are worse in minor SG cases than in major SG cases. A high N factor, particularly large numbers (≥11) of cancer‐positive LN, or high pathological stage were identified as factors contributing to a worse prognosis, and the main reason for treatment failure was delayed distant metastases.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
AUTHOR CONTRIBUTIONS
Kimihide Kusafuka designed and drafted the manuscript. Kimihide Kusafuka, Aya Muramatsu, Kazumori Arai, and Makoto Suzuki made the histopathological diagnosis for the central pathological review. Kimihide Kusafuka, Satoshi Baba, Matsuyoshi Maeda, Koji Yamanegi, Kaori Ueda, Hiroshi Inagaki, Yoshiro Otsuki, Naoto Kuroda, Kensuke Suzuki, Hiroshi Iwai, Yoshiaki Imamura, Junya Itakura, Shoji Yamanaka, Hideaki Takahashi, Ichiro Ito, Takumi Akashi, Tsutomu Daa, Mei Hamada, Yuri Tachibana, Ryo Kawata, and Hidetaka Yamamoto selected cases and provided samples with pathological and clinical data. Kimihide Kusafuka, Yoko Sato, and Eiji Nakatani performed statistical analyses of all data. Makoto Suzuki supervised this manuscript. All of the authors have read and approved the final manuscript.
ETHICS STATEMENT
The present study was approved by the Institutional Review Board of Shizuoka General Hospital (SGHIRB#2019007). All subjects signed informed consent forms before participating.
Supporting information
Figure S1 (A) Typical histology of a de novo (CXPA[−]) case showing Roman bridge structures of large atypical cells with an eosinophilic cytoplasm, and comedonecrosis (hematoxylin and eosin stain). (B) Typical histology of a CXPA(+) case showing the co‐existence of a pleomorphic adenoma (PA) circumscribed with a fibrous capsule (yellow dotted line). The intracapsular component (IC) showed the growth of atypical glandular cells within the PA component, whereas the invasive component (Inv) showed the extracapsular growth of SDC cells (hematoxylin and eosin stain).
Figure S2 Cumulative incidence of relapse (CIR) (A), cumulative incidence of local relapse (CILR) (B), cumulative incidence of cervical lymph node relapse (CICLNR) (C), and cumulative incidence of distant metastasis relapse (CIDMR) (D). The non‐dotted line represents each incidence and dotted lines show the 95% confidence interval.
Table S1 The summary of postoperative therapy (POT).
Table S2 Univariate and multivariate analyses for cumulative incidence of local relapse (CILR) and cumulative incidence of cervical lymph node metastasis (CICLNM).
ACKNOWLEDGMENTS
The authors thank Mr. Naofumi Ishikawa, Ms. Yoko Yamazaki, Mr. Tomohiro Iwasaki, Ms. Chinatsu Tsuchiya, Ms. Aki Kubota, Mr. Kensuke Shimazaki, Mr. Kazuki Hirata, Mr. Koji Takahashi, Ms. Nobuyo Tsujino, and Mr. Yosuke Saguchi, the co‐medical staff of Department of Pathology, Shizuoka General Hospital, Shizuoka, Japan, for providing technical assistance. We are grateful to Ms. Mamiko Uemura, secretariat, Department of Pathology, Shizuoka General Hospital, Shizuoka, Japan. This work was supported in part by a Grant‐in‐Aid for Science Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (15K08351 to Hiroshi Inagaki) and a Grant‐in‐Aid for Medical Research Support Project of Shizuoka Prefectural Hospital Organization in 2019 of Japan (to Kimihide Kusafuka).
Kusafuka K, Sato Y, Nakatani E, et al. The implicated clinical factors for outcomes in 304 patients with salivary duct carcinoma: Multi‐institutional retrospective analysis in Japan. Head & Neck. 2022;44(6):1430‐1441. doi: 10.1002/hed.27034
[Corrections added after online publication, 06 April 2022: The academic degree of Dr. Hideaki Takahashi and department name in affiliation 9 have been corrected in this version.]
Funding information Shizuoka Prefectural Hospital Organization; Ministry of Education, Culture, Sports, Science and Technology of Japan, Grant/Award Number: 15K08351
DATA AVAILABILITY STATEMENT
The datasets used and analyzed during the present study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Nagao T, Licitra L, Loening T, Vielh P, Williams MD. Salivary duct carcinoma. In: El‐Nagger A, JKC C, Grandis JR, Takata T, Slootweg PJ, eds. WHO Classification of Head and Neck Tumours. IARC Press; 2017:173‐174. [Google Scholar]
- 2. Williams MD, Ihrler S, Seethala R. Carcinoma ex pleomorphic adenoma. In: EL‐Nagger A, Chan JKC, Grandis JR, Takata T, Slootweg PJ, eds. WHO Classification of Head and Neck Tumours. IARC Press; 2017:176‐177. [Google Scholar]
- 3. Kapadia SB, Barns L. Expression of androgen receptor, gross cystic disease fluid protein, and CD44 in salivary duct carcinoma. Mod Pathol. 1998;11:1033‐1038. [PubMed] [Google Scholar]
- 4. Boon E, Bel M, van Boxtel W, et al. A clinicopathological study and prognostic factor analysis of 177 salivary duct carcinoma patients from the The Netherlands. Int J Cancer. 2018;143:758‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Otsuka K, Imanishi Y, Tada Y, et al. Clinical outcomes and prognostic factors for salivary duct carcinoma: a multi‐institutional analysis of 141 patients. Ann Surg Oncol. 2016;23:2038‐2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brierley JD, Gospodarowicz MK, Wittekind C, et al., eds. Union for International Cancer Control: TNM Classification of Malignant Tumours: The 8th ed . John Wiley & Sons; 2017:47‐50. [Google Scholar]
- 7. Hashimoto K, Yamamoto H, Shiratsuchi H, et al. S100P expression in ductal type of carcinoma ex pleomorphic adenoma. Am J Surg Pathol. 2011;35:346‐365. [DOI] [PubMed] [Google Scholar]
- 8. Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13:559‐565. [DOI] [PubMed] [Google Scholar]
- 9. Lacoppidan T, Vogelius IR, Pohl M, Strange M, Persson GF, Nygard L. An investigative expansion of a competing risk model for first failure site in locally advanced non‐small cell lung cancer. Acta Oncol. 2019;58:1386‐1392. [DOI] [PubMed] [Google Scholar]
- 10. Schmitt NC, Kang H, Sharma A. Salivary duct carcinoma: an aggressive salivary gland malignancy with opportunities for targeted therapy. Oral Oncol. 2017;74:40‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breinholt H, Elhakim MT, Godballe C, et al. Salivary duct carcinoma: a Danish national study. J Oral Pathol Med. 2016;45:664‐667. [DOI] [PubMed] [Google Scholar]
- 12. Kim JY, Lee S, Cho KJ, et al. Treatment results of post‐operative radiotherapy in patients with salivary duct carcinoma of the major salivary glands. Br J Radiol. 2012;85:e947‐e952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guzzo M, Di Palma S, Grandi C, Molinari R. Salivary duct carcinoma: clinical characteristics and treatment strategies. Head Neck. 1997;19:126‐133. [DOI] [PubMed] [Google Scholar]
- 14. Shinoto M, Shioyama Y, Nakamura K, et al. Postoperative radiotherapy in patients with salivary duct carcinoma: clinical outcomes and prognostic factors. J Radiat Res. 2013;54:925‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salovaara E, Hakala O, Back L, et al. Management and outcome of salivary duct carcinoma in major salivary glands. Eur Arch Otorhinolaryngol. 2013;270:281‐285. [DOI] [PubMed] [Google Scholar]
- 16. Schimitt NC, Sharma A, Gilbert MR, Kim S. Early T stage salivary duct carcinoma: outcomes and implications for patient counseling. Otolaryngol Head Neck Surg. 2015;153:795‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Villepelt A, Lefevre M, Verillaud B, et al. Salivary duct carcinoma: prospective multicenter study of 61 cases of the Reseau d'Expertise Francais des Cancers ORL Rares. Head Neck. 2018;41:584‐591. [DOI] [PubMed] [Google Scholar]
- 18. Jayaprakash V, Merzianu M, Warren GW, et al. Survival rates and prognostic factors for infiltrating salivary duct carcinoma: analysis of 228 cases from the Surveillance, Epidemiology, and End Results database. Head Neck. 2014;36:694‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roh JL, Lee JI, Choi SH, et al. Prognostic factors and oncologic outcomes of 56 salivary duct carcinoma patients in a single institution: high rate of systemic failure warrants targeted therapy. Oral Oncol. 2014;50:e64‐e66. [DOI] [PubMed] [Google Scholar]
- 20. Johnston ML, Huang SH, Waldron JN, et al. Salivary duct carcinoma: treatment, outcomes, and patterns of failure. Head Neck. 2016;38:E820‐E826. [DOI] [PubMed] [Google Scholar]
- 21. Osborn V, Givi B, Lee A, et al. Characterization, treatment and outcomes of salivary duct carcinoma using the National Cancer Database. Oral Oncol. 2017;7:41‐46. [DOI] [PubMed] [Google Scholar]
- 22. Yang J, Pan Z, He Y, et al. Competing‐risk model for predicting the prognosis of penile cancer based on the SEER database. Cancer Med. 2019;8:7881‐7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu X, Gao S, Xue Q, et al. Development of a predictive nomogram for cause‐specific mortality in surgically resected early‐stage oesophageal cancer: a Surveillance, Epidemiology, and End Results (SEER) analysis. J Thorac Dis. 2020;12:2583‐2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gilbert MR, Sharma A, Schmitt NC, et al. A 20‐year review of 75 cases of salivary duct carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142:489‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jaehne M, Roeser K, Jaeket T, Schepers JD, Albert N, Loning T. Clinical and immunohistochemical typing of salivary duct carcinoma: a report of 50 cases. Cancer. 2005;103:2526‐2533. [DOI] [PubMed] [Google Scholar]
- 26. Locati LD, Perrone F, Losa M, et al. Treatment relevant target immunophenotyping of 139 salivary gland carcinomas (SGCs). Oral Oncol. 2009;45:986‐900. [DOI] [PubMed] [Google Scholar]
- 27. Fan CY, Wang J, Barnes EL. Expression of androgen receptor and prostatic specific markers in salivary duct carcinoma: an immunohistochemical analysis of 13 cases and review of the literature. Am J Surg Pathol. 2000;24:579‐586. [DOI] [PubMed] [Google Scholar]
- 28. Xu B, Dogan S, Haroon Al Rasheed MR, Ghossein R, Katabi N. Androgen receptor immunohistochemistry in salivary duct carcinoma: a retrospective study 188 cases focusing on tumor heterogeneity and temporal concordance. Hum Pathol. 2019;93:30‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Williams MD, Roberts DB, Kies MS, Mao L, Weber RS, El‐Nagger AK. Genetic and expression analysis of HER‐2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res. 2010;16:2266‐2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Boxtel W, Locati LD, van Engen‐van Grunsven ACH, et al. Adjuvant androgen deprivation therapy for poor risk, androgen receptor‐positive salivary duct carcinoma. Eur J Cancer. 2019;110:62‐70. [DOI] [PubMed] [Google Scholar]
- 31. Boon E, van Boxtel W, Buter J, et al. Androgen deprivation therapy for androgen receptor‐positive advanced salivary duct carcinoma: a nationwide case series of 35 patients in The Netherlands. Head Neck. 2018;40:605‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fushime C, Tada Y, Takahashi H, et al. A prospective phase II study of combined androgen blockage in patients with androgen receptor‐positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29:979‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Locati LD, Perrone F, Cartelazz B, et al. Activity of abiraterone in rechallenging two AR‐expressing salivary gland adenocarcinoma, resistant to androgen deprivation therapy. Cancer Biol Ther. 2014;15:678‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wasano K, Sakurai K, Kawasaki T, et al. Acquisition of resistance to androgen deprivation therapy in salivary duct carcinoma: a case report. Rare Tumor. 2018;10:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takahashi H, Tada Y, Saotome T, et al. Phase II trial of Trastuzumab and docetaxel in patients with human epidermal growth factor receptor‐2‐positive salivary duct carcinoma. J Clin Oncol. 2019;37:125‐134. [DOI] [PubMed] [Google Scholar]
- 36. Ujien MJM, Lassche G, van Engen‐van‐Grunsven ACH, et al. Systemic therapy in the management of recurrent or metastatic salivary duct carcinoma: a systemic review. Cancer Treat Rev. 2020;89:102069. doi: 10.1016/j.ctrv2020,102069 [DOI] [PubMed] [Google Scholar]
- 37. Griffith CC, Thompson LD, Assaad A, et al. Salivary duct carcinoma and the concept of early carcinoma ex pleomorphic adenoma. Histopathology. 2014;65:854‐860. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (A) Typical histology of a de novo (CXPA[−]) case showing Roman bridge structures of large atypical cells with an eosinophilic cytoplasm, and comedonecrosis (hematoxylin and eosin stain). (B) Typical histology of a CXPA(+) case showing the co‐existence of a pleomorphic adenoma (PA) circumscribed with a fibrous capsule (yellow dotted line). The intracapsular component (IC) showed the growth of atypical glandular cells within the PA component, whereas the invasive component (Inv) showed the extracapsular growth of SDC cells (hematoxylin and eosin stain).
Figure S2 Cumulative incidence of relapse (CIR) (A), cumulative incidence of local relapse (CILR) (B), cumulative incidence of cervical lymph node relapse (CICLNR) (C), and cumulative incidence of distant metastasis relapse (CIDMR) (D). The non‐dotted line represents each incidence and dotted lines show the 95% confidence interval.
Table S1 The summary of postoperative therapy (POT).
Table S2 Univariate and multivariate analyses for cumulative incidence of local relapse (CILR) and cumulative incidence of cervical lymph node metastasis (CICLNM).
Data Availability Statement
The datasets used and analyzed during the present study are available from the corresponding author upon reasonable request.


