Abstract
Objectives
Effective antiretroviral therapy (ART) has improved the life expectancy of women living with HIV (WLWH). This population is now experiencing age‐related comorbidities. This systematic review presents the current understanding of the prevalence and impact of comorbidities in WLWH in the modern ART era.
Methods
MEDLINE and Embase were searched for studies (1 January 2010 to 1 September 2020) reporting the prevalence of cardiovascular, bone, renal and neurocognitive disease in WLWH aged > 18 years. Studies were included if at least 100 participants (or > 50%) were female and data analysis included prevalence by sex.
Results
In all, 3050 articles were identified and screened; 153 full‐text articles were assessed for eligibility and 38 were included in the final review. Significant gaps in the literature were identified, notably a lack of data on WLWH aged > 50 years. The data suggest a high burden of cardiovascular, bone, renal and neurocognitive disease in WLWH compared with HIV negative women. Traditional risk factors, such as hypertension, diabetes and dyslipidaemia, were common and often poorly managed. Generalizability of the results was limited, as many studies were conducted in the USA. Comparisons between WLWH and men with HIV were limited by marked differences in demographic and socioeconomic factors.
Conclusions
Women living with HIV experience a high burden of comorbid disease. Traditional risk factors are common and often poorly managed. This review also highlights the magnitude of differences between women and men living with HIV beyond the pathophysiological. Future research must unpick the complex drivers of morbidity in WLWH, to improve the holistic management of this population.
Keywords: ageing, comorbidities, HIV, review, women
INTRODUCTION
Over half of all people living with HIV (PLWH) globally are female [1]. While this proportion is lower in many resource‐rich settings, 35.3% of new HIV diagnoses in Europe in 2018 were in women [2]. Despite this, our understanding of HIV in women is limited, with both publicly funded and industry‐sponsored HIV trials often failing to demonstrate adequate female representation [3].
Effective combination antiretroviral therapy (ART) has markedly improved the life expectancy of PLWH [4]. Consequently, PLWH are experiencing an increasing burden of age‐related comorbid disease [5] with likely contributions from immune activation, exhaustion, senescence and chronic inflammation [6]. The ageing process is complex, and numerous clinical and lifestyle factors add to the challenge of apportioning causation.
Ageing and multimorbidity in PLWH create a unique set of challenges for those providing HIV‐related care. Additional expertise in the screening and management of a greater number of conditions is required. Careful consideration of ART selection is needed to avoid compounding comorbidity risk and drug–drug interactions that could jeopardize the successful management of both HIV and the comorbid condition. It is essential that care providers understand, and are prepared for, the changing needs of PLWH.
Evidence suggests that the experience of women ageing with HIV differs from that of men. An analysis of the AIDS Therapy Evaluation in the Netherlands (ATHENA) cohort found that women living with HIV (WLWH) experienced greater multimorbidity at a younger age than their male counterparts and that every individual comorbidity, except for non‐AIDS malignancies, carried an excess mortality risk [7]. Marked disparities are projected among key populations of PLWH in the US, with the highest burden of multimorbidity predicted among black women who inject drugs and the greatest increase in multimorbidity among Hispanic heterosexual women.
A notable physiological difference between men and women which may contribute to differing health outcomes, is the difference between sex hormones, reproductive capability and transition through the menopause. The oestrogen‐deficient postmenopausal state is a well‐recognized driver of cardiovascular disease (CVD), metabolic syndrome, bone density loss and decline in cognitive function in the general population [8, 9, 10, 11], although little is known about the impact of reproductive ageing on the development of age‐related comorbidities in WLWH [12]. In addition, greater systemic immune activation [13], sex‐specific differences in immune response to HIV infection [14] and differing pharmacokinetic profiles may further explain the different outcomes between women and men with HIV [15]. There are also marked demographic and socioeconomic differences between women and men with HIV which may impact on the success of health promotion and disease prevention activities, and on the ability to access and engage with health services.
To address this evidence gap, this systematic review presents a summary of our current understanding of the prevalence and impact of cardiovascular, bone, renal and neurocognitive disease in WLWH, in resource‐rich settings, in the modern ART era.
METHODS
Search strategy and study selection
We conducted a systematic literature search of MEDLINE and Embase for cohort, case–control and cross‐sectional studies published from 1 January 2010 to 1 September 2020 (Appendix 1 for data sources and search terms). We also screened reference lists from relevant articles. One reviewer (SR) screened all identified titles and abstracts for studies presenting data on the prevalence of cardiovascular, bone, renal and neurocognitive disease. The remaining studies were reviewed for eligibility, with all authors agreeing on the final studies for inclusion.
Only original articles of human studies published in the English language were included. In acknowledgment of global discrepancies in access to HIV and wider medical care, only studies based in resource‐rich settings were included. Studies of women under the age of 18 were excluded, as were studies primarily reporting on the outcomes of pregnancy in WLWH.
A minimum of 100 female participants were required for a study to be included or, in the case of smaller studies, at least 50% of the participants needed to be women. Studies containing both men and women were only included if data disaggregated by sex were presented. Studies were evaluated to avoid duplication of data from the same participants in multiple studies, with the most recent or comprehensive study selected for inclusion for each outcome. We excluded reviews, comments and letters. Our systematic review follows the recommendations from the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) statement (Appendix 2 for PRISMA checklist).
Quality assessment and data extraction
Extracted data included author, year of publication, geographical location, number of study participants, number/proportion of female participants, mean age, study design, measures/definitions used, relevant risk association examined for, variables adjusted for and major findings (predominately prevalence of a comorbidity, incidence of new diagnosis of comorbidity ± hazard or risk ratio or relative risk). Quality and risk of bias were assessed using the Newcastle‐Ottawa scale.
RESULTS
Study selection
Our initial database search identified 3050 documents. An additional 35 articles were identified through other sources. After removal of duplicates, 2075 articles remained for abstract review, with 153 full‐text articles assessed for eligibility and 38 included in the final review (Figure 1). Article exclusion was predominately due to inadequate women in the cohort studied or lack of sex‐based analysis.
FIGURE 1.
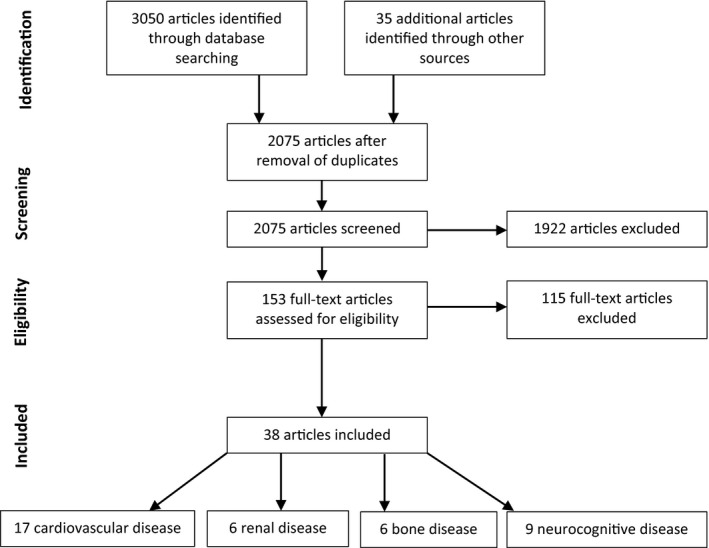
Flow chart of study selection process
Cardiovascular disease
Seventeen articles relating to CVD met eligibility criteria (Table 1).
TABLE 1.
Cardiovascular disease: characteristics and outcomes of eligible studies
| Author (year) | Study design | Location | Aim/Outcome | Population | Sample size (n/% female) | Mean age of women (years) | Measures used | Relevant risk association examined for | Major findings |
|---|---|---|---|---|---|---|---|---|---|
| Aboud et al. (2010)a | Cross‐sectional analysis of prospective cohort study (CREATE 1) | UK | To describe the CVD risk factor burden in a large HIV cohort and to compare with two other cohorts: (1) an HIV‐negative control population from the HEART‐UK study; and (2) a cohort of PLWH (DAD) |
PLWH HEART‐UK (HIV‐negative cohort) DAD (cohort of PLWH) |
990 (253/26%) 71 037 (43 261/61%) 23 468 (5867/25%) |
38.8 51 39 |
FRS Clinical assessment |
HIV status | WLWH were more likely to smoke (16% vs. 12.7%) and have a waist circumference > 88 cm (64% vs. 40.1%) than HIV‐negative women. The prevalence of hypertension, high total cholesterol and diabetes was similar between women living with and without HIV |
| Womack (2014)b | Prospective, longitudinal, observational cohort (VACS‐VC) | USA | To determine if HIV and ART are associated with CVD events (acute MI, unstable angina, ischaemic stroke or congestive heart failure) |
WLWH HIV‐negative women |
710 (710/100%) 1477 (1477/100%) |
43.2 44 |
Risk factors as defined by the ICD‐9‐CM Clinical assessment |
HIV status |
Prevalence of risk factors differed by HIV status. WLWH were more likely to have raised triglycerides (33.6% vs. 23.4%; p < 0.001), low HDL cholesterol (53.8% vs. 41.1%; p < 0.001), be HCV‐coinfected (24.4% vs. 5.7%; p < 0.001), to be current smokers (59.2% vs. 40.4%; p < 0.001) or to have a history of alcohol (13.8% vs. 5%; p < 0.001) or cocaine (13.5% vs. 3.6%; p < 0.001) abuse/dependence HIV‐negative women were significantly more likely to have a BMI > 30 kg/m2 (44.5% vs. 25.3%; p < 0.001) and to be hypertensive (28% vs. 22.9%; p < 0.02) Incident CVD per 1000 person‐years was significantly higher among WLWH (13.5; 95% CI: 10.1–18.1) compared withHIV‐negative women (5.3%; 95% CI: 3.9–7.3) After adjusting for Framingham risk factors, comorbidities, substance use and demographics, WLWH had a significantly increased risk of total CVD compared with HIV‐negative women (HR = 2.8; 95% CI: 1.7–4.6; p < 0.001). WLWH with HIV RNA > 500 copies/mL were at greatest risk (HR = 4.4; 95% CI: 2–9.9) WLWH had an increased risk of death compared with HIV‐negative women (HR = 2.6; 95% CI: 1.7–3.9; p < 0.001) |
| Tariq et al. (2007)c | Prospective cohort study (POPPY) | UK | To describe the prevalence of CVD risk factors in women aged > 50 years and explore the effects of HIV and menopausal status |
WLWH > 50 HIV‐negative women > 50 |
86 (86/100%) 109 (109/100%) |
54 57 |
FRS Clinical assessment |
HIV status |
No significant difference between the prevalence of CVD risk factors in WLWH vs. HIV‐negative women; hypertension (30.2% vs. 26.6%; p0.69), BMI > 30 kg/m2 (37.2 vs. 25.7%; p = 0.12), total cholesterol: HDL ratio > 5 (7 vs. 8.3%; p = 0.95) or glucose > 5.5mmol/L (12.8 vs. 16.5%; p = 60) Many WLWH and HIV‐negative women who met eligibility criteria of lipid‐lowering drugs (79% and 89%) or antihypertensives (56% and 71%) were not on them |
| Zachary (2012)d | Cross‐sectional study | USA | To determine cardiovascular health among African‐American women, and examine how well HIV care providers screen and manage CVD risk factors | African‐American WLWH >20 | 161 (161/100%) | 42 |
Framingham Risk Score Risk factors as defined by the American Diabetes Association, American Heart Association and National Cholesterol Education Program Clinical assessment (history, examination and laboratory results) |
N/A |
The mean CD4 count in the cohort was 483 cells/µL, 78% were on ART and 75% had an HIV RNA load < 75 copies/mL Mean Framingham risk scores were 7.6 for CVD, 5.5 for CHD, 2.9 for MI and 1 for stroke There was a high prevalence of modifiable risk factors among the WLWH; 33% had a diagnosis of diabetes, 40% dyslipidaemia, 42% hypertension, 42% were current smokers and 63% overweight or obese |
| Cortés et al. (2017)e | Cross‐sectional analysis of two longitudinal cohort studies | USA | To characterize and compare CVD risk in HIV‐infected and uninfected postmenopausal minority women using the Framingham Risk Score (FRS) |
Postmenopausal, Hispanic or African‐American WLWH Postmenopausal, Hispanic or African American HIV‐negative women |
109 (109/100%) 43 (43/100%) |
56.2 60 |
Framingham Risk Score Clinical assessment (case note review and laboratory results) |
HIV status |
Both groups of women were predominantly Hispanic and overweight‐obese. Age at menopause was similar in both groups (mean age 46.2 years in both groups) WLWH were younger, more likely to be African‐American (39.4% vs. 20.9%) and had a lower BMI (median 27.8 vs. 30.2) Median FRS did not differ between groups [14.6 (IQR: 9.1–21.6] vs. 15.5 (IQR: 12.3–22.1); p = 0.73]. In a subgroup of age‐matched controls, more WLWH with a history of CVD met FRS criteria of low risk compared with HIV‐negative women although this was not statistically significant (13% vs. 8%; p = 0.72) In WLWH, older age at HIV diagnosis was associated with worse FRS In both WLWH and HIV‐negative women, many women meeting criteria for statin therapy were not receiving this treatment (52% vs. 67%; p = 0.26) |
| Frazier et al. (2019)f | Retrospective complex sample, cross‐sectional study (MMP) | USA | To understand differences in cardiovascular comorbid conditions in PLWH aged > 50 and to understand differences between WLWH and MLWH |
WLWH 50‐64 MLWH 50‐64 WLWH > 65 MLWH > 65 |
1681 (1681/100%) 4987 (0/0%) 166 (166/100%) 602 (0/0%) |
Clinical assessment (case note review and laboratory results) | Sex |
After adjustment for socioeconomic and behavioural factors, WLWH aged 50–64 years were more likely to be obese (adjusted prevalence difference 8.4%, 95% CI: 4.4 – 12.3%), have hypertension (3.9%, 95% CI: 1–7.6%) or have high total cholesterol (9.9%, 95% CI: 6.2–13.6%) than MLWH WLWH > 65 years were more likely to be diabetic (13.1%, 95% CI: 3.4–22.8%) and have a high total cholesterol (18.8, 95% CI: 6.1–31.5%) than MLWH |
|
| Hatleberg et al. (2018)g | Prospective cohort study (DAD) | Europe, USA, Australia | To investigate potential gender differences in the use of CVD‐related interventions | PLWH | 49 049 (12 955/26%) | 34 |
MIs classified with a Dundee score using criteria from the WHO MONICA study Clinical assessment (case note review and laboratory results) |
Sex |
Of the participants, WLWH were younger (34 vs. 39 years; p < 0.0001) and more likely to be black African (20.2% vs. 6.2%; p < 0.0001) and less likely to smoke (29.4% vs. 37.6%; p < 0.0001) than MLWH. Both groups had a similar median CD4 count (405 vs. 400 cells/µL) but fewer WLWH were virologically suppressed (27.5% vs. 29.1%; p = 0.0007) Many CVD risk factors were more common in MLWH, including hypertension (7% vs. 10.8%; p = 0.0001) and diabetes (1.7% vs. 2.7%; p = 0.0001). MLWH were also more likely to have undergone previous invasive cardiac interventions (0.0% vs. 0.3%; p = 0.001) |
| Reinsch et al. (2011)h | Cross‐sectional analysis of a longitudinal cohort study | Germany | To calculate the prevalence of an estimated 10‐year CHD risk in PLWH and to assess management of risk factors |
PLWH > 18 |
761 (128/16.8%) | 40.4 |
Framingham Risk Score Risk factors as defined by the National Committee on Prevention, Detection, Evaluation and Treatment of High BP, National Cholesterol Program and the German Society of Cardiology Clinical assessment (history, examination and laboratory results) |
Sex |
The prevalence rates of low, moderate and high 10‐year CHD risk in WLWH were 106 (82.8%), 5 (3.9%) and 17 (13.3%), respectively, compared with MLWH where prevalence rates of low, medium and high 10‐year CHD risk were 353 (55.8%), 159 (25.1%) and 121 (19.1%), respectively The WLWH in the cohort were younger than the MLWH (40.4 vs. 45 years; p <0.001) Overall, sufficient treatment of risk factors was poor but no sex‐specific data were provided |
| Thompson‐Paul et al. (2019)i | Cross‐sectional analysis of a prospective cohort study (HOPS) | USA | To investigate the heart age (an estimate of the physiological age of a person’s vascular system) of PLWH | PLWH | 3086 (619/20%) | 49.1 |
Heart age was calculated as the age of a person with the same predicted risk but with all other risk factors in the normal range. Clinical assessment (case note review and laboratory results) |
HIV status Sex |
The MLWH and WLWH were of a similar age but the women were more likely to be non‐Hispanic/Latino black (54.6% vs. 23.6%), be coinfected with HCV (25.7% vs. 14.9%) and have a BMI > 30 kg/m2 (41.8% vs. 20%) than the men. The WLWH were less likely than the MLWH to be educated beyond high school (27.9% vs. 54.4%) The median CD4 count was lower in WLWH and MLWH (500 vs. 527 cells/µL) and more MLWH were virally suppressed than WLWH (50.4% vs. 65.7%). Use of NRTIs (89.9% vs. 89.7%) and boosted protease inhibitors (42.1% vs. 42.7%) was similar in WLWH and MLWH but fewer WLWH were prescribed NNRTIs (33.1% vs. 37.9%) In WLWH mean chronological age was 49.1, mean heart age 62.2 and excess heart age 13.1 years. This was greater than in MLWH (chronological age 49.3, heart age 60.8, excess heart age 11.5 years). The excess heart age for the general US population is 5.4 years in women and 7.8 years in men Excess heart age was greatest in PLWH aged 50–59 years (16.4; 95% CI: 14.8–18 in WLWH, 13.7; 95% CI: 13–14.4 in MLWH) |
| Shahmanesh et al. (2016)j | Prospective, observational cohort (EuroSIDA) | Europe, Israel, Argentina | To describe the patterns of modifiable cardiovascular risk and explore predictors of successful medical management of modifiable risk in PLWH | PLWH | 8762 (2078/23.7%) | 42 |
DAD risk equation Framingham Risk Score Modifiable risk factors as defined by the European AIDS Clinical Society (EACS) guidelines Clinical assessment (history and laboratory results) |
Sex |
Overall prevalence of traditional risk factors was high, and at baseline 17.2% had a moderate‐to‐high CV risk according to DAD risk assessment and 19.7% according to Framingham’s. Having a high cardiovascular risk at baseline was associated with male sex and older age In this cohort, WLWH were more likely to successfully modify their blood pressure than MLWH (RR = 0.68, 95% CI: 0.57–0.96; p < 0.001). There was no difference in smoking cessation (RR = 0.93; 95% CI: 0.8–1.1; p = 0.407) or in reducing cholesterol (RR = 1.33; 95% CI: 0.84–2.09) between WLWH and MLWH |
| Quiros‐Roldan et al. (2016)k | Retrospective cohort study | Italy | To investigate the incidence of CV events in PLWH and factors associated with CV events |
PLWH HIV‐negative controls |
3766 (1081/28.7%) | 38.1 |
CVD end‐points as defined by the ICD‐9‐CM Clinical assessment (case note review and laboratory results) |
HIV status | The risk of CV event was double [standard incidence ratio (SIR) = 2.02] in PLWH compared wi the general population. WLWH were at particular risk of MI (SIR = 2.91) when compared with stroke (SIR = 2.07) which was the opposite to MLWH (MI: SIR = 1.89, stroke SIR = 2.25) |
| Triant et al. (2014)l | Observational cohort | USA | To identify incidence of major adverse cardiac events in PLWH compared with matched controls |
PLWH HIV‐negative controls |
3109 (1467/33%) 23327 (12 782/34%) |
42 42 |
CVD end‐points as defined by the ICD‐9‐CM Clinical assessment (case note review and laboratory results) |
HIV status Sex |
While the HIV‐negative women were well matched as a control group for WLWH in terms of age and race, the women were more likely to be black than the men (31% vs. 17%) Major adverse cardiac events were more common in PLWH than in the control group. Incidence rate ratio for a composite CVD end‐point (MI, stroke, angina or coronary revascularization) was 1.56 (95% CI: 1.4–1.75) for all PLWH, 2.19 (95% CI: 1.76–2.7) for WLWH and 1.35 (95% CI: 1.17–1.54) for MLWH IRR values for acute MI were 1.58 (95% CI: 1.35–1.85) for all PLWH, 2.35 (95% CI: 1.74–3.12) for WLWH and 1.33 (95% CI: 1.1–1.6) for MLWH For stroke the overall IRR values were 1.53 (95% CI: 1.28–1.83) for all PLWH, 2.1 (95% CI: 1.49–2.9) for WLWH and 1.32 (95% CI: 1.06–1.64) for MLWH |
| Chow et al. (2012)m | Cohort study | USA | To determine the incidence of ischaemic stroke in PLWH, compare this with the general population and to investigate whether HIV is independently associated with stroke |
PLWH HIV‐negative controls |
4308 (1350/31%) 32 423(11 204/35%) |
41.6 40.8 |
Diagnosis of stroke as defined by the ICD‐9‐CM Clinical assessment (case note review and laboratory results) |
HIV status Sex |
HR for HIV as a risk factor for stroke was significant for women (HR = 1.21, 95% CI: 1.53 – 3.04, p < 0.001) even after adjusting for demographics and traditional risk facto |
| Lang et al. (2010)n | Nested case–control study ‐ Cohort (FHDH‐ANRS CO4) | France | To estimate the incidence of MI in PLWH compared with the general population |
WLWH MLWH |
90 856 person‐years (100%) 207 300 person‐years (0%) |
Myocardial infarction defined by American College of Cardiology and European Society of Cardiology Clinical assessment (case note review and laboratory results) |
HIV status Sex |
There were 360 cases of MI in PLWH (325 in men, 35 in women) corresponding to an incidence rate of 1.24/1000 person‐years When compared with the general population, risk of MI was higher overall (SMR = 1.5, 95% CI: 1.3–1.7) and in both MLWH (SMR = 1.4, 95% CI 1.3–1.6) and WLWH (SMR = 2.6, 95% CI: 1.8–3.9) |
|
| Knudsen et al. (2018)o | Longitudinal cohort study (COCOMO) | Denmark | To determine the prevalence and risk factors for peripheral arterial disease (PAD) in PLWH compared with uninfected controls |
PLWH > 40 HIV‐negative controls |
908 (135/15%) 11 106 (1932/17%) |
Ankle–brachial pressure index Clinical assessment (history, examination and laboratory results) |
HIV status |
PLWH were less likely to be of Scandinavian ancestry (76% vs. 89%; p < 0.0001), more likely to smoke (28% vs. 13%; p < 0.0001), to have a lower mean BMI (25 vs. 27; p < 0.0001) and less likely to be hypertensive (48% vs. 61%; p < 0.0001) than the HIV‐negative controls PAD was more common in PLWH (12% vs. 6%; p < 0.001) even after adjusting for risk factors Female sex was associated with PAD (OR = 1.49; 95% CI: 1.19–1.87) particularly WLWH (OR = 2.24; 95% CI: 1.06–4.73) |
|
| Ogunbayo et al. (2018)p | Observational cohort, retrospective (National Inpatient Survey) | USA | To evaluate if differences exist in the management of acute MI between WLWH and MLWH | PLWH | 10810 (2043/18.9%) | 54.1 |
MI as defined by the ICD‐9‐CM Clinical assessment (case note review and laboratory results) |
Sex |
Of those with a diagnosis of acute MI, WLWH were younger (53.1 vs. 54.3), more likely to be black (57.2 vs. 36%; p < 0.01), more likely to have diabetes (38.5% vs. 24.1%; p < 0.001), be obese (14.1% vs. 5.2%; p < 0.001), and be anaemic (26.7% vs. 16.9%; p < 0.001) than MLWH. They were less likely to have had a previous PCI (10.7% vs. 16.9%; p < 0.001) or a known history of CAD (72.8% vs. 83%; p < 0.001) WLWH were less likely to have an ST‐segment elevation MI than MLWH (23.4% vs. 34.6%; p < 0.001). WLWH were less likely to undergo coronary angiography than MLWH (73% vs. 80%) but this was not statistically significant WLWH were significantly less likely to have PCI than MLWH (54.2% vs. 69.7%; p < 0.01) and more likely to have no revascularization intervention (37.8% vs. 21.8%; p <0.1). The rate of CABG was similar in both women and MLWH |
| Fitch et al. (2013)q | Cross‐sectional, case–control study | USA | To examine atherosclerotic plaque features and detailed indices of immune activation among WLWH and investigate the relationship of age, sex and HIV infection |
WLWH HIV‐negative women |
60 (60/100%) 30 (30/100%) |
47 47 |
Cardiac computed tomography (CT) angiography Framingham Risk Score Clinical assessment (history, examination and laboratory results) Markers of immune activation (sCD163, MCP‐1, CXCL10, sCD14, hsIL‐6, hsCRP) |
HIV status |
Age, race, BMI and traditional risk factors did not differ between WLWH and HIV‐negative women. The number of postmenopausal women was similar (47% WLWH vs. 43% HIV‐negative women; p = 0.76) The prevalence of coronary plaque was similar between WLWH and controls (37% vs. 38%; p = 0.88), WLWH demonstrated a significantly higher prevalence of non‐calcified coronary plaque (35% vs. 12%; p = 0.04). This remained after controlling for CVS risk factors (p = 0.004) WLWH demonstrated a lower prevalence of calcified plaque (6% vs. 26%; p = 0.01) and a higher percentage of HIV‐negative women had a calcium score > 100 (15% vs. 2%; p = 0.02) Markers of immune activation (sCD163 (p = 0.006), sCD14 (p = 0.5), CXCL10 (p =0.002) and percentage CD8 (< 0.0001), CD8 HLA‐DR+ (p = 0.0004), HLA‐DR+ CD38 CD4 (p < 0.0001) and CD14+CD16+ (p = 0.008) were all significantly higher in WLWH, while markers of inflammation [hsCRP (p = 0.005) and hsIL‐6 (p = 0.92)] did not differ by HIV status |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CABG, coronary arterial bypass graft; CHD, coronary heart disease; CVD, cardiovascular disease; CVS, cardiovascular system; DAD, Data Collection on Adverse events of Anti‐HIV Drugs; FRS, Framingham Risk Score; HCV, hepatitis C virus; HDL, high‐density lipoprotein; HOPS, HIV Outpatient Study; hsCRP, high‐sensitivity C‐reactive protein; hsIL, high sensitivity interleukin‐6; ICD‐9‐CM, International Classification of Diseases, Ninth Revision, Clinical Modification; IRR, incidence rate ratio; MI, myocardial infarction; MLWH, men living with HIV; NNRTI, nonnucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; PI, protease inhibitors; PLWH, people living with HIV; RR, risk ratio; SMR, standardized morbidity ratio; VACS‐VC, Veterans Aging Cohort Study‐Virtual Cohort; WIHS, Women’s Interagency HIV Study; WLWH, women living with HIV.
aAboud M, Elgalib A, Pomeroy L, Panayiotakopoulos G, Skopelitis E, Kulasegaram R, et al. Cardiovascular risk evaluation and antiretroviral therapy effects in an HIV cohort: implications for clinical management: the CREATE 1 study. Int J Clin Pract. 2010;64(9):1252–9.
bWomack JA, Chang CH, So‐Armah KA, Alcorn C, Baker JV, Brown ST, et al. HIV Infection and Cardiovascular Disease in Women. J Am Heart Assoc. 2014;3(5):e001035.
cTariq S, Winston A, Bagkeris E, Asboe D, Johnson M, Anderson J, et al. Prevalence of cardiovascular risk factors in women ageing with HIV: an analysis of data from the POPPY Study (Pharmacokinetic and Clinical Observations in People Over Fifty). In: 22nd Annual Conference of the British HIV Association. 2016.
dZachary D, Gillani FS, Najfi N, Casarella R, Tashima K. Cardiovascular Health of HIV‐infected AfricanAmerican Women at the Miriam Hospital Immunology Center in Providence, RI. Medicine and health, Rhode Island. 2012;96.
eCortés YI, Reame N, Zeana C, Jia H, Ferris DC, Shane E, et al. Cardiovascular Risk in HIV‐Infected and Uninfected Postmenopausal Minority Women: Use of the Framingham Risk Score. J Women’s Heal. 2017;26(3):241–8.
fFrazier EL, Sutton MY, Tie Y, Fagan J, Fanfair RN. Differences by Sex in Cardiovascular Comorbid Conditions Among Older Adults (Aged 50–64 or ≥65 Years) Receiving Care for Human Immunodeficiency Virus. Clin Infect Dis. 2019;69(12):2091–100.
gHatleberg CI, Ryom L, El‐Sadr W, Mocroft A, Reiss P, Wit SD, et al. Gender differences in the use of cardiovascular interventions in HIV‐positive persons; the D:A:D Study. J Int Aids Soc. 2018;21(3):e25083.
hReinsch N, Neuhaus K, Esser S, Potthoff A, Hower M, Mostardt S, et al. Are HIV patients undertreated? Cardiovascular risk factors in HIV: results of the HIV‐HEART study. Eur J Prev Cardiol. 2011;19(2):267–74.
iThompson‐Paul AM, Palella FJ, Rayeed N, Ritchey MD, Lichtenstein KA, Patel D, et al. Excess heart age in adult outpatients in routine HIV care. Aids. 2019;33(12):1935–42.
jShahmanesh M, Schultze A, Burns F, Kirk O, Lundgren J, Mussini C, et al. The cardiovascular risk management for people living with HIV in Europe. Aids. 2016;30(16):2505–18.
kQuiros‐Roldan E, Raffetti E, Focà E, Brianese N, Ferraresi A, Paraninfo G, et al. Incidence of cardiovascular events in HIV‐positive patients compared to general population over the last decade: a population‐based study from 2000 to 2012. Aids Care. 2016;28(12):1–8.
lTriant VA, Regan S, Grinspoon SK. MACE Incidence Among HIV and Non‐HIV‐Infected Patients in a Clinical Care Cohort. In: Conference on Retroviruses and Opportunistic Infections. 2014.
mChow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of Ischemic Stroke Incidence in HIV‐Infected and Non–HIV‐Infected Patients in a US Health Care System. Jaids J Acquir Immune Defic Syndromes. 2012;60(4):351–8.
nLang S, Mary‐Krause M, Cotte L, Gilquin J, Partisani M, Simon A, et al. Increased risk of muocardial infarction in HIV‐infected patients in France, relative to the general population. AIDS. 2010;24(8):1228–30.
oKnudsen AD, Gelpi marco, Afzal S, Ronit A, Roen A. Prevalence of Peripheral Artery Disease is Higher in Persons Living with HIV Compared to Uninfected Controls. JAIDS. 2018;79(3):381–5.
pOgunbayo GO, Bidwell K, Misumida N, Ha LD, Abdel‐Latif A, Elayi CS, et al. Sex differences in the contemporary management of HIV patients admitted for acute myocardial infarction. Clin Cardiol. 2018;41(4):488–93.
qFitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, et al. Noncalcified Coronary Atherosclerotic Plaque and Immune Activation in HIV‐Infected Women. J Infect Dis. 2013;208(11):1737–46.
Comparison of CVD risk factors between WLWH and HIV‐negative women
Three studies compared prevalence of traditional risk factors between WLWH and HIV‐negative women. The first, a cross‐sectional analysis of the Cardiovascular Risk Evaluation and Antiretroviral Therapy Effects (CREATE) cohort, compared the burden of traditional CVD risk factors in a cohort of ethnically and socioeconomically diverse PLWH in London, with a control population from UK general practice. The 253 WLWH in the cohort were more likely to smoke (16% vs. 12.7%) and have a waist circumference > 88 cm (64% vs. 40.1%) than women in the general population. Prevalence rates of hypertension (9% vs. 13%) and diabetes (2% vs. 3%) as well as mean total cholesterol (TC; 4.74 vs. 5.2mmol/L) and high‐density lipoprotein cholesterol (HDL‐C) (1.54 vs. 1.5 mmol/L) were similar regardless of HIV status. However, WLWH were younger than the women in the general population (mean age 38.8 vs. 52.1 years) and, while 9% of the WLWH were white, ethnicity data were unavailable for the general population [16].
In a US study, the 710 WLWH in the cohort were more likely to have elevated triglycerides (> 150 mg/dL, 33.6% vs. 23.4%), reduced HDL‐C (< 50 mg/dL, 53.8% vs. 41.1%) and to be smokers (59.2% vs. 40.4%) compared with HIV‐negative women, but were less likely to have a body mass index (BMI) > 30 kg/m2 (25.3% vs. 44.5%) or to be hypertensive (22.9% vs. 28%) [17]. By contrast, Tariq et al. [18] found no difference in prevalence of hypertension (30.2% vs. 26.6%; p = 0.69), BMI > 30 kg/m2 (37.2% vs. 25.7%; p = 0.12), TC:HDL‐C ratio > 5 mg/dL (7% vs. 8.3%; p = 0.95) or glucose > 5.5 mmol/L (12.8% vs. 16.5%; p = 60) between WLWH and HIV‐negative women aged > 50 years. Notably, this UK‐based cohort was smaller (86 WLWH and 109 HIV‐negative women), the WLWH were younger (median age 54 vs. 57 years), more likely to be black African (67.4% vs. 20.2%; p = 0.0001), single (79.1% vs. 48.6%; p = 0.0001) and unemployed (62.8 vs. 41.3%; p = 0.005). A fourth study, without an HIV‐negative control group, reported a high prevalence of modifiable CVD risk factors in 161 African‐American WLWH in the USA (diabetes, 33%; dyslipidaemia, 40%; hypertension, 42%; current smokers, 42%; overweight/obese, 63%) [19]
Cortés et al. reported that among postmenopausal Hispanic and African‐American women, the median Framingham Risk Score (FRS) did not differ between the 109 women living with, and 43 women without, HIV [20] (14.6 vs. 15.5; p = 0.73) although the WLWH were younger and more likely to be African‐American (39.4% vs. 20.9%). In WLWH, older age at HIV diagnosis was associated with higher FRS.
Comparison of CVD risk factors between WLWH and men living with HIV (MLWH)
Four studies compared the prevalence of CVD risk factors between WLWH and MLWH. In a US cohort of 7436 (25% female) PLWH aged > 50 years [21], WLWH were significantly more likely to be black (age 50–64 years: 62% vs. 33%; age ≥ 65 years: 58.6% vs. 29.7%) and live‐in poverty (50–64 years: 59.9% vs. 35%; ≥ 65 years: 47.3% vs. 27.1%) than MLWH. WLWH were less likely to have private health insurance (50–64 years: 22% vs. 36.1%; ≥ 65 years: 19.5% vs. 41.1%) or to have educational attainment beyond high school (50–64 years: 37.7% vs. 58.3%; ≥ 65 years: 28.8% vs. 53.3%). Following adjustment for these factors, WLWH aged 50–64 years were more likely to have a BMI > 24 kg/m2 (adjusted prevalence difference = 8.4%), hypertension (3.9%) or TC > 200 mg/dL (9.9%); WLWH aged ≥ 65 years were more likely to be diabetic (13.1%) and to have a high TC (18.8) than were MLWH.
By contrast, the international D:A:D cohort of > 49 000 PLWH found many CVD risk factors to be more common in MLWH, including hypertension (7% vs. 10.8%) and diabetes (1.7% vs. 2.7%) [22].
When comparing overall CVD risk between 128 WLWH and 633 MLWH in Germany, WLWH had lower rates of moderate and high 10‐year cardiovascular risk, although the women in this cohort were significantly younger (mean age 40.4 vs. 45 years) [23].
A cross‐sectional analysis of the HIV Outpatient Study (HOPS) cohort used excess heart age [chronological age – heart age (age of a person with the same predicted risk but with all other risk factors in the normal range)] to estimate the physiological age of the vascular system of 3086 (20% female) PLWH [24]. The excess heart age of the WLWH in the cohort of 13.1 years was greater than that of both the MLWH (11.5 years) and women in the general US population (5.4 years). Although the MLWH and WLWH had a similar age, the WLWH were more likely to be non‐Hispanic/Latino black (54.6% vs. 23.6%), coinfected with HCV (25.7% vs. 14.9%) and have a BMI > 30 kg/m2 (41.8% vs. 20%) but were less likely to be educated beyond high school (27.9% vs. 54.4%).
Incidence of CVD events
Six studies considered the incidence of CVD events. Womack et al. found a higher incidence of CVD [acute myocardial infarction (MI), unstable angina, ischaemic stroke or congestive heart failure] among 710 WLWH (13.5/1000 person‐years) compared with 1477 HIV‐negative women (5.3/1000 person‐years) [17]. This risk remained after adjusting for potential confounders [hazard ratio (HR) = 2.8, 95% confidence interval (CI): 1.7–4.6]. WLWH with HIV RNA > 500 copies/mL were at greatest risk (HR = 4.4, 95% CI: 2–9.9). The mortality risk was also higher in WLWH than in HIV‐negative women (HR = 2.6, 95% CI: 1.7–3.9).
A 12‐year Italian cohort study of 3766 PLWH found a two‐fold increased risk of CVD events overall [standardized incidence ratio (SIR) = 2.02] in PLWH as compared with the general population [25], although the pattern of events appeared to differ by sex, with the relative risk of coronary artery disease being higher in WLWH than in MLWH (WLWH: SIR = 2.91; MLWH: SIR = 1.82) but that of stroke being lower in WLWH (WLWH, 2.07; MLWH, 2.3). Triant et al. also found an increased risk of major adverse cardiac events (acute MI, stroke, angina or coronary revascularization) in PLWH in a cohort of a similar size in the USA compared with HIV‐negative controls [incidence rate ratio (IRR) = 1.56], with a greater risk in WLWH (IRR = 2.19) than in MLWH (IRR = 1.35) [26]. By contrast with the Italian cohort, WLWH were at greater risk of both MI (IRR = 2.35 vs. 1.33) and stroke (IRR = 2.1 vs. 1.32) than were MLWH. A further US‐based cohort study including 1350 WLWH reported that HIV was a risk factor for stroke in women, even after adjusting for established risk factors (HR = 1.21; p < 0.001) [27] and a French cohort also found an increased risk of MI in PLWH compared with the general population [standardized morbidity ratio (SMR) = 1.5, 95% CI: 1.3–1.7], particularly in WLWH (SMR = 2.6, 95% CI: 1.8–3.9) [28].
Peripheral artery disease (PAD) was seen more frequently in Danish women (OR = 1.49, 95% CI: 1.19–1.87) and particularly WLWH (OR = 2.24, 95% CI: 1.06–4.73), compared with the Danish general population. The PLWH were less likely to be of Scandinavian ancestry (76% vs. 89%), more likely to smoke (28% vs. 13%) and have a lower mean BMI (25 vs. 27 kg/m2) and less likely to be hypertensive (48% vs. 61%) than the general population although risk of PAD remained after adjustment [29].
Management of CVD risk factors and acute events
Little evidence was available regarding how successfully traditional cardiovascular risk factors are managed in WLWH. Tariq et al. found that regardless of HIV status, few women eligible for lipid‐lowering or antihypertensives were receiving treatment, although the numbers in this study were small [18]. One study found that female sex was associated with successful modification of blood pressure among PLWH but with no difference between women and men in smoking cessation or cholesterol reduction [30].
Ogunbayo et al. evaluated sex differences in management of MI in PLWH in a large US‐based cohort [31]. Among those with an acute MI, WLWH were younger (53.1 vs. 54.3), more likely than MLWH to be black (57.2 vs. 36%), to have diabetes (38.5% vs. 24.1%), to be obese (14.1% vs. 5.2%) and to be anaemic (26.7% vs. 16.9%). The WLWH were less likely to have an ST‐segment elevation MI (23.4% vs. 34.6%; p < 0.001) and were significantly less likely to have received a percutaneous coronary intervention than were MLWH (54.2% vs. 69.7%; p < 0.01). They were more likely to have no revascularization intervention (37.8% vs. 21.8%; p < 0.01) although the rate of coronary artery bypass grafting was similar.
Pathophysiology of CVD in WLWH
Fitch et al. used cardiac computed tomography (CT) angiography and markers of immune activation to examine the atherosclerotic plaque features and detailed indices of immune activation among 60 WLWH compared with 30 well‐matched female HIV‐negative controls [13]. The prevalence of coronary plaque was similar between WLWH and controls (37% vs. 38%; p = 0.88) but WLWH demonstrated a significantly higher prevalence of noncalcified coronary plaque (35% vs. 12%; p = 0.04) and a lower prevalence of calcified plaque (6% vs. 26%; p = 0.01) than did the controls. A calcium risk score > 100 was also less frequent in WLWH (15% vs. 2%; p = 0.02). Markers of inflammation (hsCRP and hsIL‐6) did not differ by HIV status but markers of immune activation [sCD163 (p = 0.006), sCD14 (p = 0.5), CXCL10 (p = 0.002), percentage CD8+ ( < 0.0001), CD8+ HLA‐DR+ (p = 0.0004), HLA‐DR+CD38+CD4+ (p < 0.0001) and CD14+CD16+ (p = 0.008)] were all significantly higher in WLWH.
Renal disease
No studies looking specifically at renal disease in WLWH, and only six studies that presented data disaggregated by sex were identified (Table 2).
TABLE 2.
Renal disease: characteristics and outcomes of eligible studies
| Author (year) | Study design | Location | Aim/outcome | Population | Sample size (n/% female) | Mean age (years) | Measures used | Relevant risk association examined for | Major findings |
|---|---|---|---|---|---|---|---|---|---|
| Abraham et al. (2015)a | Observational cohort study (NA‐ACCORD) | USA and Canada | To assess the relative contributions of clinical and demographic factors to ESRD incidence and to describe recent trends in ESRD in PLWH |
PLWH General population |
38 354(7703/20%) N/A |
41 63 |
Clinical assessment (history, examination and laboratory results). eGFR calculated using the CKD‐EPI equation. The Kidney Disease: Improving Global Outcomes (KDIGO) thresholds were used to categorize severity of CKD |
HIV status |
The incidence rate of ESRD in PLWH was 179 (95% CI: 160–201)/100 000 person‐years compared with 35/100 000 in the general population. Incidence of ESRD in PLWH declined between 2001 and 2008 from 317 to 119/100 000 person‐years. Risk factors for ESRD in PLWH also changed over time with those diagnosed after 2005 more likely to have hypertension than those diagnosed prior to 2005 (84 vs. 68%) ESRD was seen more frequently in WLWH than MLWH both overall (259 vs. 157) and in those with a well‐suppressed HIV RNA (127 vs. 56/100,000 person‐years) black race was a significant risk factor for ESRD in PLWH with an overall incidence of 437 (95% CI: 384–498)/100 000 person‐years compared with 45 (95% CI: 32–64) in non‐black participants. The rate of viral suppression in PLWH with ESRD was markedly different in black and non‐black patients. Prior to 2005, 26% of black patients were suppressed compared with 48% of non‐black patients. After 2005, 48% of black patients were suppressed compared with 73% of non‐black patients |
| Schoffelen et al. (2015)b | Observational cohort study (ATHENA) | The Netherlands | To assess the impact of ethnicity on the development of CKD in PLWH | PLWH | 16 836 (2823/16.8%) | 42.2 |
Clinical assessment (history, examination and laboratory results). eGFR calculated using the Cockcroft–Gault equation. The Kidney Disease: Improving Global Outcomes (KDIGO) thresholds were used to categorize severity of CKD |
Ethnicity |
At baseline, prevalence of CKD was 2.7%. The prevalence among those from SSA was similar to that among patients of western European origin (2.8% vs. 2.6%, OR = 1.10, 95% CI: 0.85–1.45; p = 0.5). However, on multivariate analysis SSA origin was associated with CKD (aOR = 1.49, 95% CI: 1.04–2.13) as was originating from Asia or the Pacific (aOR = 2.27, 95% CI: 1.46–3.55) when compared with those originating from western Europe Interestingly, on subgroup analysis of patients previously in care and new to care, there was no significant association between SSA origin and CKD (aOR = 1.25, 95% CI: 0.8−1.93; p = 0.33). There was also no association between SSA origin and development of CKD over time (aHR = 1.00, 95% CI: 0.63−1.59) Patients of SSA origin were more likely to be female (50.1% vs. 8.9%; p < 0.05), younger (36.9 vs. 44 years; p < 0.05), have lower CD4 counts (380 cells/µL vs. 460 cells/ µL; p < 0.05) |
| Cristelli et al. (2018)c | Observational, cross‐sectional study | Spain | To assess the influence of sex, ART and classical risk factors on the occurrence of mild decreased renal function in PLWH |
WLWH MLWH |
819 (100%) 3518 (0%) |
47 44 |
Clinical assessment (history, examination and laboratory results). eGFR calculated using the CKD‐EPI equation |
Sex |
The WLWH were older (47 vs. 44 years; p < 0.001), more likely to be black (5.3% vs. 1.4%; p < 0.001), have a higher prevalence of HCV coinfection (32.8% vs. 19.4%; p < 0.001), longer duration of HIV (17.5 vs. 9.2 years; p < 0.001), have a prior AIDS diagnosis (23.4% vs. 18.3%; p = 0.001), more frequent exposure to PI (60.6% vs. 47.2%; p < 0.001) or boosted PI with tenofovir (52.5% vs. 40.8%; p < 0.001) but less likely to be currently on tenofovir (58.1% vs. 66.8%; p < 0.001) than the MLWH The overall prevalence rate of mildly reduced renal function (eGFR 60–89 mL/min) was 20%. WLWH were significantly more likely to have mild renal impairment than MLWH (29.2% vs. 23.9%; p = 0.002). Following multivariate analysis, female sex remained a risk factor for mildly decreased renal function (OR 1.02−1.48; p = 0.031) |
| Ibrahim et al. (2012)d | Prospective, observational cohort study (UK CHIC) | UK | To examine the effect of baseline eGFR on all‐cause mortality and to assess the risk of progression to stages 4−5 CKD in a cohort of PLWH | PLWH | 20 132 (4317/21.4%) | 34 |
Clinical assessment (history, examination and laboratory results). eGFR calculated using the CKD‐EPI equation |
At baseline, WLWH were over‐represented among PLWH with an eGFR between 44 and 30 mL/min (29.2% vs. 70.8%) and an eGFR < 30 mL/min (42.5% vs. 57.5%) By the end of the study, 118 (0.6%) had stages 4−5 CKD. In 62 (53%) of these, CKD stages 4−5 was already established at baseline |
|
| Mocroft et al. (2014)e | Prospective, observational, longitudinal cohort study (EuroSIDA) | International | To determine the relationship between measures of renal function and proportion of follow‐up with a low eGFR and fatal/non‐fatal AIDS, non‐AIDs events and all‐cause mortality in PLWH | PLWH | 12 155 (3128/25.7%) | 42 |
Clinical assessment (history, examination and laboratory results). eGFR calculated using the CKD‐EPI equation |
Baseline prevalence eGFR < 60 mL/min in WLWH (109/3128). WLWH were over‐represented in those with a baseline eGFR < 60 mL/min (28.2%; p = 0.0004) Both current eGFR and proportion of follow‐up with a low eGFR (< 60 mL/min) were associated with death and non‐AIDS events |
|
| Mocroft et al. (2015)f | Multicentre, prospective cohort collaboration (D:A:D) | International | To develop a simple, externally validated long‐term risk score model for CKD in PLWH with a baseline eGFR > 60 mL/min/1.73 m2 | PLWH | 17 954 (4824/26.9%) | 40 |
Clinical assessment (history, examination and laboratory results). eGFR calculated using the Cockcroft–Gault formula |
Overall, at 2, 5 and 8 years after baseline, 1.1% (95% CI: 0.9−1.2), 2.7% (95% CI: 2.4−2.9) and 5.3% (95% CI: 4.9−5.8) were estimated to have developed CKD. The incidence of CKD was 6.2/1000 person‐years of follow‐up (95% CI: 5.7−6.7) Female sex was a significant predictor of CKD and was included in the risk score model |
Abbreviations: aHR, adjusted hazard ratio; aOR, adjusted odds ratio; ART, antiretroviral therapy; ATHENA, AIDS Therapy Evaluation in the Netherlands; CKD, chronic kidney disease; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; D:A:D, Data Collection on Adverse events of Anti‐HIV Drugs; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease; EuroSIDA, Clinical and Virological Outcome of European Patients Infected with HIV; MLWH, men living with HIV; NA‐ACCORD, North American Cohort Collaboration on Research and design; PI, protease inhibitor; PLWH, people living with HIV; SSA, sub‐Saharan Africa; WLWH, women living with HIV.
aAbraham AG, Althoff KN, Jing Y, Estrella MM, Kitahata MM, Wester CW, et al. End‐Stage Renal Disease Among HIV‐Infected Adults in North America. Clin Infect Dis. 2015;60(6):941–9.
bSchoffelen AF, Smit C, Lelyveld SFL van, Vogt L, Bauer MP, Reiss P, et al. Diminished Impact of Ethnicity as a Risk Factor for Chronic Kidney Disease in the Current HIV Treatment Era. J Infect Dis. 2015;212(2):264–74.
cCristelli MP, Trullàs JC, Cofán F, Rico N, Manzardo C, Ambrosioni J, et al. Prevalence and risk factors of mild chronic renal failure in HIV‐infected patients: influence of female gender and antiretroviral therapy. Braz J Infect Dis. 2018;22(3):193–201.
dIbrahim F, Hamzah L, Jones R, Nitsch D, Sabin C, Post FA, et al. Baseline Kidney Function as Predictor of Mortality and Kidney Disease Progression in HIV‐Positive Patients. Am J Kidney Dis. 2012;60(4):539–47.
eMocroft A, Ryom L, Begovac J, Monforte AD, Vassilenko A, Gatell J, et al. Deteriorating renal function and clinical outcomes in HIV‐positive persons. Aids. 2014;28(5):727–37.
fMocroft A, Lundgren JD, Ross M, Law M, Reiss P, Kirk O, et al. Development and Validation of a Risk Score for Chronic Kidney Disease in HIV Infection Using Prospective Cohort Data from the D:A:D Study. Plos Med. 2015;12(3):e1001809.
Incidence of renal disease
Only one eligible study compared the incidence of kidney disease in PLWH with data from an HIV‐negative population. The North American AIDS Cohort Collaboration on Research and Design (NA‐ACCORD) group reported that the incidence of end‐stage renal disease (ESRD) [32] was higher in all PLWH (179/100 000 person‐years) but particularly in WLWH (259/100 000 person‐years) than in the general population (35/100 000).
An analysis of the ATHENA cohort assessed the impact of ethnicity on the development of chronic kidney disease (CKD) in PLWH [33], finding a significantly higher prevalence of CKD in those born in sub‐Saharan Africa (adjusted OR = 1.49) or Asia and the Pacific region (adjusted OR = 2.27) than in those born in Western Europe. However, the risk of developing CKD over time was similar regardless of country of birth (adjusted HR =1.00, 95% CI: 0.63–1.59). Although no sex‐specific data were presented, those born in sub‐Saharan Africa were more likely to be female (50.1% vs. 8.9%), younger (36.9 vs. 44 years) and to have lower CD4 count (380 vs. 460 cells/µL).
A 2018 study of 4337 (19% female) PLWH in Spain found that WLWH were significantly more likely to have mild renal impairment than MLWH (29.2% vs. 23.9%; p = 0.002) [34]. WLWH were older (47 vs. 44 years), more likely to be black (5.3% vs. 1.4%), had a higher prevalence of HCV coinfection (32.8% vs. 19.4%), had lived with HIV for longer (17.5 vs. 9.2 years) and had a prior AIDS diagnosis. However, female sex remained a risk factor for mildly decreased renal function on multivariate analysis (OR = 1.02–1.48; p = 0.031).
Several large cohort studies of PLWH, while primarily looking at longer‐term outcomes in PLWH with impaired renal function also found an association between female sex and renal impairment. A baseline analysis of the UK Collaborative HIV Cohort (UK CHIC) Study found that WLWH were over‐represented among PLWH with an estimated glomerular filtration rate (eGFR) between 44 and 30 mL/min (29.2% vs. 70.8%) and an eGFR < 30 mL/min (42.5% vs. 57.5%) [35]. The EuroSIDA study group also found a disproportionate number of women with a baseline eGFR < 60 mL/min (28.2%; p = 0.0004) [36] and, in a sub‐study, female sex was associated with an increased incidence of developing CKD [relative hazard (RH) = 1.68; p = 0.0013] [37]. In the development of a validated long‐term risk score model for CKD in PLWH, the D:A:D Study also identified female sex as a significant predictor of CKD [38].
Bone disease
Six studies looking at bone health in WLWH were included (Table 3).
TABLE 3.
Bone disease: characteristics and outcomes of eligible studies
| Author (year) | Study design | Location | Aim/Outcome | Population | Sample size (n/% female) | Mean age (years) | Measures used | Relevant risk association examined for | Major findings |
|---|---|---|---|---|---|---|---|---|---|
| Yin et al. (2012)a | Longitudinal analysis of prospective cohort study | USA | To assess the effects of HIV infection and ART on change in BMD in postmenopausal Hispanic and African‐American women |
WLWH HIV‐negative women |
73 (100%) 55 (100%) |
56 59 |
DEXA Clinical assessment |
HIV status |
WLWH had significantly lower BMD at the lumbar spine (p < 0.01), total hip (p < 0.01), distal radius (p = 0.02) and ultra‐distal radius (p = 0.02), with a trend towards a lower adjusted z‐score at the femoral neck (p = 0.008) than HIV‐negative women at index visit The annualized percentage decrease in BMD adjusted for baseline BMD was greater in WLWH than in HIV‐negative women by 2.4‐fold at the lumbar spine (p = 0.0009), 3.7‐fold at the distal radius (p = 0.006) and 1.7‐fold at the ultra‐distal radius (p = 0.02) After adjustment for traditional risk factors, HIV status remained a significant risk factor for declining BMD at the lumbar spine, total hip and ultra‐distal radius but not at the femoral neck When those not on ART were excluded from the analysis, the annualized rate of bone loss was still greater in WLWH than HIV‐negative women. Among those on ART, the annualized rates of bone loss did not differ between those on PI‐based vs. NNRTI‐based ART at any site (p = 0.1–0.93). The rate of bone loss was higher in those on tenofovir than those on non‐tenofovir containing ART at the lumbar spine (−2.8 vs. 0.7; p = 0.001), the distal radius (−2.3 vs. −0.8; p = 0.02) and the ultra‐distal radius (−2.2 vs. −1; p = 0.001). This relationship remained following adjustment for age, ethnicity, BMI, index CD4 and duration of ART exposure. Bone loss did not differ by tenofovir exposure at the total hip or femoral neck Incidence of fractures did not differ between WLWH and HIV‐negative women (10% vs. 8%) |
| Sharma et al. 2012b | Prospective, multicentre observational study (WIHS) | USA | To understand how regional body composition changes, including lean mass and regional body fat, affect bone mineral density in WLWH vs. HIV‐negative women |
WLWH HIV‐negative women |
318 (100%) 122 (100%) |
44 37 |
DEXA BIA Clinical assessment |
HIV status |
The WLWH had a lower BMI (27 vs. 30.2) and lower trunk (12.7 vs. 15.6 kg), leg (8.7 vs. 11.9 kg) and total body fat (26.4 vs. 32.1 kg) than the HIV‐negative women. There was little difference in absolute changes over time for trunk fat, leg fat, fat‐free mass, total body fat and percentage body fat between WLWH and HIV‐negative women HIV infection was associated with decreased BMD at all three sites as was being postmenopausal and HCV coinfection. Increased total lean mass was associated with increased BMD, regardless of HIV status In the WLWH, greater log HIV RNA was associated with greater BMD at all three sites and a significant association between cumulative NNRTI use and increased BMD at the hip and femoral neck. No association between recent or nadir CD4 count, cumulative use of tenofovir, PIs or other ART with BMD at any site |
| Young et al. 2011c | Prospective cohort study (HOPS) and population data (NHAMCS‐OPD) | USA | To compare rates of bone fracture in PLWH vs. the general population and to explore risk factors |
PLWH HIV‐negative controls |
5826 (1223/21%) | 40 | Clinical assessment | HIV status |
There was no statistically significant difference in crude fracture rate per 10 000 population between WLWH and HIV‐negative women at any time point WLWH had a higher incidence of vertebral (18% vs. 4%; p < 0.01) and femoral neck (5% vs. 1%; p = 0.004) fractures than HIV‐negative women and significantly fewer fractures at a non‐fragility site (69% vs. 86%; p < 0.01) Among all PLWH, CD4 cell count nadir < 200 cells/µL, AIDS diagnosis, current smoking, HCV infection, diabetes, substance misuse and peripheral neuropathy were associated with increased fracture risk |
| Sharma et al. 2015d | Prospective, multicentre observational study (WIHS) | USA | To compare incidence rates and determinants of fracture among WLWH and HIV‐negative women |
WLWH HIV‐negative women |
1713 (100%) 662 (100%) |
40 35 |
Clinical assessment | HIV status |
Unadjusted incidence rates of fracture at any site were higher in WLWH than in HIV‐negative women (2.19 vs. 1.54/100 person‐years; p = 0.002) although rates of fragility fracture were similar (0.56 vs. 0.39/100 person‐years; p = 0.13) In bivariate analysis of the WLWH, ART use at index or cumulative use, including index (1.24, 95% CI: 0.98–1.56; p = 0.08) or cumulative (1, 95% CI: 0.95–1.05; p = 0.98) tenofovir use, were not associated with incident fracture In multivariate analysis, age per 10‐year increase (1.25, 95% CI: 1.1–1.43; p = 0.0007), white race (1.37, 95% CI: 1.06–1.78; p = 0.06), smoking (1.44, 95% CI: 1.14–1.81; p = 0.002) and an AIDS‐defining diagnosis (1.57, 95% CI: 1.24–1.99; p = 0.0002) were associated with incident fracture |
| Gedmintas et al. 2014e | Retrospective cohort study | USA | To compare the incidence ratios of fracture between WLWH and MLWH |
WLWH > 18 MLWH > 18 |
869 (100%) 2292 (0%) |
40.9 44.3 |
Clinical assessment | Sex |
Incidence rates of fracture at any site were similar between WLWH (43.6/100 person‐years, 95% CI: 36.9–50.2) and MLWH (43.4/100 person‐years, 95% CI: 39.3–47.6). The IRR of all fractures between WLWH and MLWH was 1.00 (95% CI: 0.83–1.19) Incidence rates of fracture at any osteoporotic site were 12.1/100 person‐years (95% CI: 8.6–15.1) in WLWH and 15.2/100 person‐years (95% CI: 12.7–17.6) in MLWH, resulting in an IRR for fragility fracture of MLWH compared with WLWH of 1.26 (95% CI: 0.9–1.75) |
| Libois et al. 2010f | Cross‐sectional study | Belgium | To evaluate the relationship between BMD and ART in pre‐menopausal WLWH |
ART‐naïve NRTI/NNRTI regimen PI‐containing regimen |
37 (100%) 25 (100%) 27 (100%) |
36.5 37 37 |
DEXA Clinical assessment |
ART regimen |
Overall prevalence of osteopenia or osteoporosis was high at 31.5% with no difference between the three groups. This was consistent at both the lumbar spine (13.5% vs. 16% vs. 25.9%; p = NS) and femoral neck (21.6% vs. 32% vs. 33.3%) In univariate analysis, osteopenia/porosis was significantly associated with Caucasian race (p = 0.001), low BMI (p = 0.0065) and a high prolactin (p = 0.03). There was no association between osteopenia/porosis and duration of ART In multivariate analysis, only low BMI was a risk factor for osteopenia/porosis (p = 0.004) |
Abbreviations: ART, antiretroviral therapy; BIA, bioimpedance analysis; BMD, bone mineral density; BMI, body mass index; DEXA, dual‐energy x‐ray absorptiometry; HOPS, HIV Outpatient Study; IRR, incidence rate ratio; MLWH, men living with HIV; NHAMCS‐OPD, National Hospital Ambulatory Medical care Survey – Outpatient department; NNRTI, nonnucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; PI, protease inhibitors; PLWH, people living with HIV; WIHS, Women’s Interagency HIV Study; WLWH, women living with HIV.
aYin MT, Zhang CA, McMahon DJ, Ferris DC, Irani D, Colon I, et al. Higher Rates of Bone Loss in Postmenopausal HIV‐Infected Women: A Longitudinal Study. J Clin Endocrinol Metabolism. 2012;97(2):554–62.
bSharma A, Tian F, Yin MT, Keller MJ, Cohen M, Tien PC. Association of Regional Body Composition With Bone Mineral Density in HIV‐Infected and HIV‐Uninfected Women. Jaids J Acquir Immune Defic Syndromes. 2012;61(4):469–76.
cYoung B, Dao CN, Buchacz K, Baker R, Brooks JT, Investigators HOS (HOPS). Increased Rates of Bone Fracture Among HIV‐Infected Persons in the HIV Outpatient Study (HOPS) Compared With the US General Population, 2000–2006. Clin Infect Dis. 2011;52(8):1061–8.
dSharma A, Shi Q, Hoover DR, Anastos K, Tien PC, Young MA, et al. Increased Fracture Incidence in Middle‐Aged HIV‐Infected and HIV‐Uninfected Women. Jaids J Acquir Immune Defic Syndromes. 2015;70(1):54–61.
eGedmintas L, Wright EA, Losina E, Katz JN, Solomon DH. Comparative Risk of Fracture in Men and Women with HIV. J Clin Endocrinol Metabolism. 2014;99(2):486–90.
fLibois A, Clumeck N, Kabeya K, Gerard M, Wit SD, Poll B, et al. Risk factors of osteopenia in HIV‐infected women: No role of antiretroviral therapy. Maturitas. 2010;65(1):51–4.
Bone mineral density in WLWH compared with HIV‐negative women
Dual‐energy X‐ray absorptiometry (DXA) was used in two studies to compare bone mineral density (BMD) between women with and without HIV. Among 128 postmenopausal Hispanic and African‐American women [39], WLWH had a lower baseline BMD at many sites (lumbar spine, total hip and distal radius) than HIV‐negative women. The study also reported a 2.4‐fold greater annualized percentage decrease in BMD in WLWH at the lumbar spine (p = 0.0009), 3.7‐fold at the distal radius (p = 0.006) and 1.7‐fold at the ultra‐distal radius (p = 0.02), even after adjustment for traditional risk factors. HIV status was associated with reduced BMD in an analysis of 440 women from the Women’s Interagency HIV Study (WIHS) cohort, although the WLWH in this cohort were older (44 vs. 37 years), more likely to be HCV‐coinfected (32% vs. 14%) and to be postmenopausal (26% vs. 3%) than the HIV‐negative comparison group [40].
Fracture incidence
Yin et al. found no significant difference in fracture incidence between postmenopausal African‐American women living with or without HIV (10% vs. 8%) [39]. Similarly, an analysis of 5826 (21% female) PLWH from the HOPS cohort found no significant difference in crude fracture rate by HIV status [41], although authors reported a higher incidence of vertebral (18% vs. 4%; p < 0.01) and femoral neck (5% vs. 1%; p = 0.004) fractures in WLWH but significantly fewer fractures at non‐fragility sites (69% vs. 86%; p < 0.01) than among HIV‐negative women. By contrast, among 2375 women in the WIHS cohort, although the overall incidence of fracture was higher (2.19 vs. 1.54/100 person‐years; p = 0.002) in WLWH than in HIV‐negative women, the incidence of fragility fractures was similar (0.56 vs. 0.39/100 person‐years; p = 0.13) despite the WLWH being older (40 vs. 35 years), of lower weight (74.5 vs. 79.7kg), more likely to be HCV‐coinfected (24% vs. 15%) and to be postmenopausal (19% vs. 11%) than the HIV‐negative women [42]. Gedmintas et al. found no significant difference in incidence rate of either any (IRR = 1.00) or fragility (IRR = 1.26) fractures between 869 WLWH and 2292 MLWH [43], although no adjustment was made for potential confounders.
HIV‐related factors and bone disease
HIV‐related risk factors associated with increased fracture incidence included CD4 T‐cell count nadir < 200 cells/µL and an AIDS‐defining diagnosis [41, 42]. Traditional risk factors such as being postmenopausal, HCV coinfection, current smoking, diabetes, substance misuse and decreased total lean mass were also associated with decreased BMD and fracture incidence regardless of HIV status [40, 41].
Libois et al. reported a high prevalence of osteopenia/osteoporosis (31.5%) among 89 premenopausal WLWH [44], with no difference between ART‐naïve women and women on ART, regardless of drug class received. Findings were consistent at both the lumbar spine (13.5% vs. 16% vs. 25.9%) and femoral neck (21.6% vs. 32% vs. 33.3%). After adjustment, only low BMI was a risk factor for osteopenia/osteoporosis (p = 0.004), although notably only two women in the cohort had received ART containing tenofovir disoproxil fumarate (TDF). Sharma et al. reported a significant association between use of nonnucleoside reverse transcriptase inhibitor (NNRTI) and increased BMD at the hip and femoral neck but no association between cumulative use of TDF, protease inhibitors (PIs) or other ART with BMD loss at any site [40]. Among postmenopausal Hispanic and African‐American women, 78% of the WLWH were on ART with a mean duration of exposure of 4.5 years [39], of whom 21% were on a TDF‐containing regimen. After exclusion of those not on ART, the annualized rate of bone loss remained greater in WLWH than in HIV‐negative women, although this did not differ between those on PI‐based vs. NNRTI‐based ART at any site. The rate of bone loss was, however, higher in those on TDF than those on non‐TDF‐containing ART at the lumbar spine (–2.8 vs. 0.7; p = 0.001), the distal radius (–2.3 vs. –0.8; p = 0.02) and the ultra‐distal radius (–2.2 vs. –1; p = 0.001), with these differences remaining after confounder adjustment. Bone loss did not differ by TDF exposure at the total hip or femoral neck.
Neurocognitive disease
Nine studies reporting aspects of neurocognitive disease in WLWH were identified (Table 4).
TABLE 4.
Neurocognitive disease: characteristics and outcomes of eligible studies
| Author (year) | Study design | Location | Aim/outcome | Population | Sample size (n/% female) | Mean age (years) | Measures used | Relevant risk association examined for | Major findings |
|---|---|---|---|---|---|---|---|---|---|
| Chow, F et al. (2019)a | Cross‐sectional analysis of a prospective, multicentre observational study (HAILO) | USA | To evaluate the association between cardiometabolic risk factors and prevalent cognitive impairment in PLWH | PLWH > 40 | 795 (195/20%) | 52 |
Brief neurocognitive screen International Physical Activity Questionnaire Clinical assessment |
Sex |
A greater proportion of WLWH were cognitively impaired compared with MLWH (36% vs. 26%; p = 0.003). Increased physical activity (OR = 0.33 for ≥ 3 days/week; p = 0.003) was protective against cognitive impairment in WLWH but not MLWH. Higher HDL cholesterol was also associated with a lower risk of cognitive impairment in WLWH but not MLWH (OR = 0.78 for every 10 mg/dL higher HDL; p = 0.028). |
| Gustafson et al. (2013)b | Cross‐sectional analysis of a prospective, multicentre observational study (WIHS) | USA | To explore the relationship between BMI, waist circumference and waist‐to‐hip ratio with cognition in WLWH and HIV‐negative women |
WLWH HIV‐negative women |
1196 (1196/100%) 494 (494/100%) |
42.5 38.4 |
Standardized neurocognitive battery Anthropomorphic measurements |
HIV status | In WLWH, but not HIV‐negative women, a BMI < 18.5 kg/m2 was associated with worse performance in some domains of cognitive testing compared with WLWH with a BMI in the healthy range (BMI 18.5–25 kg/m2). |
| Maki et al. (2015)c | Cross‐sectional analysis of a prospective, multicentre observational study (WIHS) | USA |
1. To examine the association between HIV status and cognition in relation to other determinants of cognitive function 2. To examine the pattern and magnitude of impairment across cognitive outcomes |
WLWH HIV‐negative women |
1019 (1019/100%) 502 (502/100%) |
47.48 43.48 |
Standardized neurocognitive battery Clinical assessment |
HIV status |
WLWH performed worse on measures of verbal learning and memory, speed of information processing and attention (0.05–0.09 SD units). The effect of HIV on cognitive function was less than the effect of years of education, age, race/ethnicity, household income and reading level. WLWH with a low CD4 count, high VL, low education or an AIDS‐defining illness were more vulnerable to cognitive deficit. |
| Rubin (2018)d | Cross‐sectional analysis of a prospective, multicentre observational study (WIHS) | USA | To assess the cognitive effects of medications with known NC‐AE on WLWH and HIV negative women |
WLWH HIV‐negative women |
1037 (1037/100%) 521 (521/100%) |
47 43 |
Standardized neurocognitive battery Self‐reported NC‐AE medication history Clinical assessment |
HIV status |
WLWH performed worse than HIV‐negative women on global function (p = 0.01), memory (p = 0.04), attention/working memory (p = 0.02) and executive function (p = 0.02). WLWH reported using more NC‐AE medications than HIV‐negative women (p < 0.05). Opioid and anticonvulsant use was similar in both groups. Anticholinergic burden was negatively associated with learning and executive function. This association was greater in WLWH than in HIV‐negative women. |
| Meyer et al. (2013)e | Cross‐sectional analysis of a prospective, multicentre observational study (WIHS) | USA | To investigate the impact of HIV infection and illicit drug use on cognition in WLWH and HIV‐negative women |
WLWH HIV‐negative women |
952 (952/100%) 443 (443/100%) |
42.8 |
Neuropsychological testing, HVLT and Comalli Stroop test Self‐reported history of recreational drug use |
HIV status Recreational drug use |
Regardless of reported drug use, WLWH performed worse on total learning, learning slope, delayed recall and recognition (p‐values < 0.05). In WLWH, recent drug use was associated with a worse performance on learning slope (p = 0.04), delayed recall (p = 0.007) and recognition (p = 0.002) than non‐users. This was not seen in HIV‐negative women. |
| Rubin et al. (2015)f | Cross‐sectional analysis of a prospective, multicentre observational study (WIHS) | USA | To investigate the association between perceived stress and cognitive performance in WLWH and HIV‐negative women |
WLWH HIV‐negative women |
1003 (1003/100%) 496 (496/100%) |
46.2 |
Neuropsychological testing including HVLT PSS |
HIV status Higher vs. lower PSS |
Overall WLWH performed worse on verbal learning (p = 0.003), memory (p < 0.001), attention and concentration (p = 0.004), executive functioning (p = 0.005) and psychomotor speed (p = 0.01) than HIV‐negative women. A similar proportion of WLWH and HIV‐negative women reported higher perceived stress (38% vs. 36%; p = 0.41). Among WLWH, those with higher PSS had lower CD4 counts and higher plasma HIV RNA levels. Regardless of HIV status, PSS was inversely associated with cognitive performance, with those with higher perceived stress performing worse on all HVLT indices. In the WLWH, those with higher PSS performed worse on the verbal memory domain (B = −2.24, SE = 0.62; p < 0.001), specifically driven by delayed recall (B = −2.24, SE = 0.65; p < 0.001). This persisted after controlling for CD4 count, HIV VL, ART use and ART duration. This was not seen in HIV‐negative women. |
| Rubin et al. (2016)g | Cross‐sectional analysis of a prospective, multicentre observational study (WIHS) | USA | To investigate the association between PTSD and verbal learning and memory in WLWH and HIV‐negative women |
WLWH HIV‐negative women |
1004 (1004/100%) 496 (496/100%) |
47 43 |
PTSD Checklist‐Civilian version Neuropsychological testing including HVLT |
HIV status |
The proportion of women with PTSD was similar in WLWH and HIV‐negative women (18% vs. 16%; p = 0.49). Regardless of HIV status, PTSD was significantly associated with poor cognitive performance; with women performing worse on verbal learning (p < 0.001), memory (p < 0.001) and psychomotor speed (p < 0.001). Women |
| Rubin et al. (2016)h | Cross‐sectional analysis of a prospective, multicentre observational study (WIHS) | USA | To examine the association between stress, verbal memory and brain volumes in WLWH |
WLWH |
38 (38/100%) |
43.9 |
PSS PTSD Checklist‐Civilian version CES‐D scale Substance use history HVTL Structural MRI |
Higher vs. lower PSS |
Depressive symptoms were reported by 26% and an elevated PTSD symptom burden in 26%. 84% reported ever having experience abuse. Women with higher PSS performed worse than those with lower PSS on the verbal memory domain (p < 0.005). They had higher CD4 counts than those with lower PSS (p = 0.002). Higher PSS was associated with smaller volumes bilaterally in the medial temporal region (parahippocampal gyri) and prefrontal cortex regions, regions involved in verbal memory performance. |
| Rubin et al. (2016)i | Cross‐sectional analysis of a prospective, multicentre observational study (WIHS) | USA | To examine the association between stress and prefrontal cortical activation during verbal memory tasks | WLWH | 36 (36/100%) | 43.7 |
PSS PTSD Checklist‐Civilian version CES‐D scale Functional MRI In‐scanner verbal memory task similar to the HVLT |
Higher vs. lower PSS |
Women with higher PSS performed worse than those with lower PSS on the verbal memory domain (p < 0.005). They had higher CD4 counts than those with lower PSS (p = 0.002). Patterns of brain activation during recognition, but not encoding, differed between women with higher PSS than lower PSS. Women with higher PSS demonstrated greater deactivation in medial prefrontal cortex. |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CES‐D, Center for Epidemiological Studies‐Depression; HAILO, Long‐term follow‐up of older HIV‐infected adults; HVLT, Hopkins Verbal Learning Test; MLWH, men living with HIV; MRI, magnetic resonance imaging; NC‐AE, neurocognitive‐adverse effects; OR, odds ratio; PLWH, people living with HIV; PSS, Perceived Stress Scale; PTSD, post‐traumatic stress disorder; VL, viral load; WIHS, Women’s Interagency HIV Study; WLWH, women living with HIV.
aChow FC, Makanjuola A, Wu K, Berzins B, Kim K‐YA, Ogunniyi A, et al. Physical Activity Is Associated with Lower Odds of Cognitive Impairment in Women but Not Men Living With Human Immunodeficiency Virus Infection. J Infect Dis. 2018;219(2):264–74.
bGustafson DR, Mielke MM, Tien PC, Valcour V, Cohen M, Anastos K, et al. Anthropometric measures and cognition in middle‐aged HIV‐infected and uninfected women. The Women’s Interagency HIV Study. J Neurovirol. 2013;19(6):574–85.
cMaki PM, Rubin LH, Valcour V, Martin E, Crystal H, Young M, et al. Cognitive function in women with HIV. Neurology. 2015;84(3):231–40.
dRubin LH, Radtke KK, Eum S, Tamraz B, Kumanan KN, Springer G, et al. Cognitive Burden of Common Non‐antiretroviral Medications in HIV‐Infected Women. Jaids J Acquir Immune Defic Syndromes. 2018;79(1):83–91.
eMeyer VJ, Rubin LH, Martin E, Weber KM, Cohen MH, Golub ET, et al. HIV and Recent Illicit Drug Use Interact to Affect Verbal Memory in Women. Jaids J Acquir Immune Defic Syndromes. 2013;63(1):67–76.
fRubin LH, Cook JA, Weber KM, Cohen MH, Martin E, Valcour V, et al. The association of perceived stress and verbal memory is greater in HIV‐infected versus HIV‐uninfected women. J Neurovirol. 2015;21(4):422–32.
gRubin LH, Pyra M, Cook JA, Weber KM, Cohen MH, Martin E, et al. Post‐traumatic stress is associated with verbal learning, memory, and psychomotor speed in HIV‐infected and HIV‐uninfected women. J Neurovirol. 2016;22(2):159–69.
hRubin LH, Meyer VJ, Conant RJ, Sundermann EE, Wu M, Weber KM, et al. Prefrontal cortical volume loss is associated with stress‐related deficits in verbal learning and memory in HIV‐infected women. Neurobiol Dis. 2016;92(Pt B):166–74.
iRubin LH, Wu M, Sundermann EE, Meyer VJ, Smith R, Weber KM, et al. Elevated stress is associated with prefrontal cortex dysfunction during a verbal memory task in women with HIV. J Neurovirol. 2016;22(6):840–51.
Risk factors for neurocognitive disease
Only one study, a cross‐sectional analysis of cardiometabolic risk factors and cognitive impairment in a cohort of 795 (20% female) older PLWH (> 40 years), included a male comparison group [45]. Although a greater proportion of WLWH than MLWH met the criteria for cognitive impairment (36% vs. 26%; p = 0.003) there were significant differences between the women and men in terms of ethnicity (52% women vs. 25% men were black; p < 0.001) and educational attainment (median 12 vs. 14 years of education; p < 0.001). Increased physical activity (OR = 0.33 for > 3 days/week of physical activity; p = 0.003) and higher HDL‐C (OR = 0.78 for every 10 mg/dL higher; p = 0.28) were protective against cognitive impairment in the female participants but not among men. No significant associations between other traditional cardiovascular risk factors or socioeconomic factors were reported in this cohort.
By contrast, Gustafson et al. found that a BMI < 18.5 kg/m2 was associated with poorer performance across a limited battery of cognitive tests in WLWH compared with those with a BMI in the healthy range (18.5–25 kg/m2) [46]. This relationship was not seen in HIV‐negative women. Obesity (BMI > 30 kg/m2) was related to better performance on some aspects of neurocognitive testing but a worse performance on others in both WLWH and HIV‐negative women. No consistent relationships between waist circumference or waist‐to‐hip ratio and cognitive performance were seen. Of note, most women (69%) in the study had BMI > 25 kg/m2 with only 3.1% of WLWH and 1% of HIV‐negative women having a BMI < 18.5 kg/m2.
Pattern of cognitive impairment in WLWH
In 2015, Maki et al. [47] examined the association between HIV status and cognition in the WIHS cohort and explored the impact of HIV on the pattern and magnitude of cognitive impairment. In this cohort of 1521 women (13% of the 1019 WLWH had CD4 T‐cell count < 200 cells/µL and 53% had an undetectable plasma HIV RNA), HIV status had a small but significant (0.05–0.09 SD units) negative impact on verbal learning and memory, speed of information processing and attention. Of note, the impact of HIV on cognitive function was less than the effect of years of education, age, race/ethnicity, household income and reading level. WLWH with a low CD4 count, high viral load, low education or an AIDS‐defining illness were more vulnerable to cognitive deficit.
Drugs and neurocognitive disease in WLWH
Two studies examined the impact of drugs on cognitive performance. The first considered medications with known neurocognitive‐adverse effects (NC‐AE) on 1037 WLWH and 521 HIV‐negative women [48]. The WLWH were older (47 vs. 43 years), more likely to have positive HCV antibody (Ab) serology (23% vs. 13%) but less likely to report recent heavy alcohol (14% vs. 22%), marijuana (15% vs. 22%) or recreational drug (6% vs. 8%) use. The WLWH reported using more NC‐AE medications than HIV‐negative women (p < 0.05), although opioid and anticonvulsant use was similar in both groups.
The WLWH performed worse than HIV‐negative women on global function (p = 0.01), memory (p = 0.04), attention/working memory (p = 0.02) and executive function (p = 0.02), but measures of fluency and motor skills were similar. NC‐AE medication use was not associated with worse cognitive performance nor did it moderate the association between HIV and performance. However, anticholinergic burden was negatively associated with learning and executive function; a greater association in WLWH than in HIV‐negative women suggested an increased cognitive vulnerability.
Meyer et al. investigated the impact of illicit drug use on 1003 women with and 496 women without HIV [49]. Reported drug use was high regardless of HIV status, with recent recreational drug use reported by 9.6% of WLWH and 11% HIV‐negative women, and former use by 48.6% and 42.4%, respectively. Regardless of reported drug use, WLWH performed worse than HIV‐negative women on total learning, learning slope, delayed recall and recognition (p‐values < 0.05). While recent drug users performed worse on learning slope (p = 0.04), delayed recall (p = 0.007) and recognition (p = 0.002), former users did not perform differently to never‐users on any measure. Recent drug use (compared with never‐use) had a negative impact on verbal learning and memory in WLWH but not in HIV‐negative women, suggesting a potential synergistic neurotoxicity between HIV serostatus and recent drug use which remained following adjustment for HIV‐specific characteristics.
Stress and neurocognitive disease in WLWH
Four studies from the WIHS cohort examined the impact of stress on cognitive impairment. The first of these by Rubin et al. considered the relationship between perceived stress and cognitive performance [50], demonstrating similar proportions of WLWH and HIV‐negative women reporting high perceived stress (38% vs. 36%; p = 0.41). Regardless of HIV status, those with higher perceived stress performed worse on all indices of the Hopkins Verbal Learning test. In WLWH, those with higher levels of perceived stress performed worse on the verbal memory domain (p < 0.001), specifically driven by delayed recall (p < 0.001). This was not seen among the HIV‐negative women, where there were no significant differences in either the verbal domain (p = 0.99) or delayed recall (p = 0.76) between those with higher and lower perceived stress. Among the WLWH, those with higher perceived stress had lower CD4 counts and higher plasma HIV RNA; however, the relationship with poor verbal memory and delayed recall persisted after controlling for these factors.
A further analysis of the WIHS cohort examined the relationship between post‐traumatic stress disorder (PTSD) and verbal learning and memory in women with and without HIV [51]. Again, the proportions of women with PTSD (defined by the PTSD Checklist‐Civilian version) were similar in WLWH and HIV‐negative women (18% vs. 16%; p = 0.49) and, regardless of HIV status, PTSD was significantly and inversely associated with cognitive performance, particularly verbal learning (p < 0.001), memory (p < 0.001) and psychomotor speed (p < 0.001). There was no relationship between HIV status and the impact of PTSD on verbal learning or memory, although WLWH meeting criteria for PTSD performed worse on assessment of fine motor skills (p = 0.003). Of note, in this cohort the WLWH were significantly older (p < 0.001), had a higher minority representation, and were more likely to be HCV‐Ab‐positive (31% vs. 19%; p < 0.001) and to use antidepressants (17% vs. 10%; p < 0.001) and less likely to report excessive alcohol (15% vs. 25%; p < 0.001) or marijuana use (15% vs. 22%; p < 0.001) compared with HIV‐negative women.
A small sub‐study of 38 predominately African‐American WLWH used structural magnetic resonance imaging (MRI) to examine the association between stress, verbal memory and brain volume [52]. In this cohort, 26% reported depressive symptoms, 26% had an elevated PTSD burden and 84% reported having experienced abuse (sexual, physical or domestic). Women with a higher perceived stress score performed worse on testing of verbal memory (p < 0.005). As established in the HIV‐negative population, high perceived stress score was associated with smaller volumes bilaterally in the medial temporal region (parahippocampal gyri) and prefrontal cortex regions, both involved in verbal memory. A further study, utilizing functional MRI to explore cortical activation during verbal memory tasks in 36 WLWH, found that patterns of brain activation during recognition, but not encoding, were associated with perceived stress score [53]. Women with higher perceived stress score demonstrated greater deactivation in medial prefrontal cortex.
DISCUSSION
This review confirms the high burden of age‐related morbidity experienced by WLWH. As compared with HIV‐negative women, WLWH are at an increased risk of an acute cardiovascular event, reduced BMD and impaired cognitive performance. Female sex was also consistently identified as a risk factor for renal disease in PLWH.
However, this review also highlights the inadequacy of our understanding of the drivers behind this increased disease burden. During screening and assessment of eligibility, multiple large studies were excluded due to poor female representation among participants or a failure to present any sex‐based analysis. Only 21 studies focused specifically on comorbidities in women (five cardiovascular, four bone and eight neurocognitive disease); notably no studies looked specifically at renal disease in WLWH.
There may be underlying, HIV‐driven biological mechanisms contributing to this increased burden of comorbid disease. Fitch et al. [13] identified differing patterns of coronary calcification between women living with and without HIV, which raised questions as to the efficacy of our current tools for screening for ischaemic heart disease in WLWH. Similarly, to ensure the safe use of medication that acts on the central nervous system in WLWH, we need a better understanding of the increased cognitive vulnerability highlighted by Rubin et al. [48]. To ensure we are able to screen and manage comorbidities effectively in WLWH, it is essential that future research explores these mechanisms by focusing specifically on women.
A number of other limitations impair our ability to draw conclusions from this review. The studies lack geographical diversity, restricting our ability to generalize the results. The marked differences in population demographics, behaviours, healthcare systems and approach to HIV treatment between settings also impair our ability to draw any meaningful comparisons between different studies. Few studies focused on postmenopausal women which may not capture the true impact of ageing and comorbid disease in WLWH.
However, by far the greatest factor limiting our ability to draw conclusions from this review is the number of both acknowledged and unacknowledged confounding factors. Control groups were often poorly matched, particularly in terms of ethnicity. This is profoundly important when we consider how readily different populations are able to access healthcare. Abraham et al. [32] noted that the rate of HIV viral suppression was markedly lower in black than in non‐black participants (47% vs. 73%). Ogunbayo et al. [31] also found that WLWH, of whom a higher proportion were black compared with the MLWH, were significantly less likely to undergo coronary revascularization following MI. These two studies, both from North America, highlight the need to explore sex and race‐related inequality in the healthcare setting as a barrier to WLWH accessing care.
Very few studies attempted to report on wider socioeconomic factors that influence health and access to healthcare. Those that did found striking differences in measures such as educational attainment [21, 45], household income [21] and unemployment [18] between WLWH and either MLWH or HIV‐negative controls.
In order to provide the best possible care and support for women as they age with HIV, it is essential that more research, focusing specifically on women, explores the complex and multiple drivers behind the increased burden of age‐related comorbidities in this population. Potential biological mechanisms must be explored, but equally it is essential that we gain a greater understanding of the multiple factors that drive disparities in healthcare to improve the holistic management of WLWH.
CONFLICT OF INTEREST
CS has received funding for membership of Data Safety and Monitoring Boards and Advisory Boards, and for preparation of educational materials from Gilead Sciences, ViiV Healthcare and Janssen‐Cilag. YG has received funding for membership of Advisory Boards and for preparation of educational materials from Gilead Sciences, ViiV Healthcare and Janssen‐Cilag.
AUTHOR CONTRIBUTIONS
SR, CS and YG were involved in systematic review topic design, and SR led on review of articles with contributions from CS and YG. All authors contributed to drafting of the manuscript.
ACKNOWLEDGEMENTS
We thank Tom Roper and his team at Brighton and Sussex NHS Library and Knowledge Service for their support with the literature search. CS was supported by the National Institute for Health Research (NIHR) Health Protection Research Unit (HPRU) in Blood Borne and Sexually Transmitted Infections at University College London in partnership with the UK Health Security Agency (UKHSA). The views expressed are those of the authors and not necessarily those of the NIHR, the Department of Health and Social Care or UKHSA. We acknowledge the support of the HPRU Steering Committee: Caroline Sabin (HPRU Director), John Saunders (PHE Lead), Catherine Mercer, Hamish Mohammed, Greta Rait, Ruth Simmons, William Rosenberg, Tamyo Mbisa, Rosalind Raine, Sema Mandal, Rosamund Yu, Samreen Ijaz, Fabiana Lorencatto, Rachel Hunter, Kirsty Foster and Mamooma Tahir.
APPENDIX 1.
MEDLINE search strategy
"HIV Infections"/ep, mo (42749)
(hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or "hiv infect*").ti,ab. (304957)
("human immunodeficiency virus" or "human immunedeficiency virus" or "human immuno‐deficiency virus" or "human immune‐deficiency virus").ti,ab. (85384)
("human immun*" and "deficiency virus").ti,ab. (627)
("AIDS virus" or "HIV/AIDS").ti,ab. (29158)
1 or 2 or 3 or 4 or 5 (327266)
exp Women/ (35746)
Female/ (8535316)
(wom?n* or female* or girl* or mother* or widow*).ti,ab. (2192690)
7 or 8 or 9 (8929644)
exp *Pregnancy/ (148784)
10 not 11 (8782685)
6 and 12 (135636)
exp Comorbidity/ (104912)
exp Aging/ (241043)
exp Chronic Disease/ (260908)
(comorbidit* or non‐AIDS‐related).ti,ab. (115382)
(aging or ageing).ti,ab. (210046)
(chronic disease* or chronicity).ti,ab. (68863)
14 or 15 or 16 or 17 or 18 or 19 (844741)
13 and 20 (6600)
Morbidity/ or Incidence/ or Prevalence/ (539398)
exp Mortality/ (372714)
(morbidity or incidence or prevalence or mortality or cause of death).ti,ab. (2010947)
exp Epidemiologic Studies/ (2432397)
ep.fs. (1623791)
22 or 23 or 24 or 25 or 26 (4680495)
21 and 27 (5314)
28 (5314)
limit 29 to yr="2000 ‐Current" (4815)
exp *neoplasms/ (2860074)
(cancer* or sarcoma* or neoplasm* or oncolog* or malignan* or carcinoma* or lymphoma* or melanoma* or tumor* or tumour* or leukemia* or leukaemia*).ti,ab. (3458503)
*Developing Countries/ (27369)
(Africa or Asia or Caribbean or West Indies or South America or Latin America or Central America).ti,ab. (186319)
(Afghanistan or Albania or Algeria or Angola or Antigua or Barbuda or Argentina or Armenia or Armenian or Aruba or Azerbaijan or Bahrain or Bangladesh or Barbados or Benin or Byelarus or Byelorussian or Belarus or Belorussian or Belorussia or Belize or Bhutan or Bolivia or Bosnia or Herzegovina or Hercegovina or Botswana or Brasil or Brazil or Bulgaria or Burkina Faso or Burkina Fasso or Upper Volta or Burundi or Urundi or Cambodia or Khmer Republic or Kampuchea or Cameroon or Cameroons or Cameron or Camerons or Cape Verde or Central African Republic or Chad or Chile or China or Colombia or Comoros or Comoro Islands or Comores or Mayotte or Congo or Zaire or Costa Rica or Cote d'Ivoire or Ivory Coast or Croatia or Cuba or Cyprus or Czechoslovakia or Czech Republic or Slovakia or Slovak Republic or Djibouti or French Somaliland or Dominica or Dominican Republic or East Timor or East Timur or Timor Leste or Ecuador or Egypt or United Arab Republic or El Salvador or Eritrea or Estonia or Ethiopia or Fiji or Gabon or Gabonese Republic or Gambia or Gaza or Georgia Republic or Georgian Republic or Ghana or Gold Coast or Greece or Grenada or Guatemala or Guinea or Guam or Guiana or Guyana or Haiti or Honduras or Hungary or India or Maldives or Indonesia or Iran or Iraq or Isle of Man or Jamaica or Jordan or Kazakhstan or Kazakh or Kenya or Kiribati or Korea or Kosovo or Kyrgyzstan or Kirghizia or Kyrgyz Republic or Kirghiz or Kirgizstan or Lao PDR or Laos or Latvia or Lebanon or Lesotho or Basutoland or Liberia or Libya or Lithuania or Macedonia or Madagascar or Malagasy Republic or Malaysia or Malaya or Malay or Sabah or Sarawak or Malawi or Nyasaland or Mali or Malta or Marshall Islands or Mauritania or Mauritius or Agalega Islands or Mexico or Micronesia or Middle East or Moldova or Moldovia or Moldovian or Mongolia or Montenegro or Morocco or Ifni or Mozambique or Myanmar or Myanma or Burma or Namibia or Nepal or Netherlands Antilles or New Caledonia or Nicaragua or Niger or Nigeria or Northern Mariana Islands or Oman or Muscat or Pakistan or Palau or Palestine or Panama or Paraguay or Peru or Philippines or Philipines or Phillipines or Phillippines or Poland or Portugal or Puerto Rico or Romania or Rumania or Roumania or Russia or Russian or Rwanda or Ruanda or Saint Kitts or St Kitts or Nevis or Saint Lucia or St Lucia or Saint Vincent or St Vincent or Grenadines or Samoa or Samoan Islands or Navigator Island or Navigator Islands or Sao Tome or Saudi Arabia or Senegal or Serbia or Montenegro or Seychelles or Sierra Leone or Slovenia or Sri Lanka or Ceylon or Solomon Islands or Somalia or South Africa or Sudan or Suriname or Surinam or Swaziland or Syria or Tajikistan or Tadzhikistan or Tadjikistan or Tadzhik or Tanzania or Thailand or Togo or Togolese Republic or Tonga or Trinidad or Tobago or Tunisia or Turkey or Turkmenistan or Turkmen or Uganda or Ukraine or Uruguay or USSR or Soviet Union or Union of Soviet Socialist Republics or Uzbekistan or Uzbek or Vanuatu or New Hebrides or Venezuela or Vietnam or Viet Nam or West Bank or Yemen or Yugoslavia or Zambia or Zimbabwe or Rhodesia).ti,ab. (1188643)
((developing or less* developed or under developed or underdeveloped or middle income or low* income or underserved or under served or deprived or poor*) adj (countr* or nation? or population? or world)).ti,ab. (96280)
((developing or less* developed or under developed or underdeveloped or middle income or low* income) adj (economy or economies)).ti,ab. (516)
(low* adj (gdp or gnp or gross domestic or gross national)).ti,ab. (238)
(low adj3 middle adj3 countr*).ti,ab. (15068)
(lmic or lmics or third world or lami countr*).ti,ab. (7110)
transitional countr*.ti,ab. (157)
31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 (5284091)
30 not 42 (2884)
exp *Bacterial Infections/ (736756)
(multiple or recurrent).ti,ab. (1465160)
44 and 45 (32017)
((mutilple or recurrent) adj1 bacterial infection*).ti,ab. (585)
exp *Candidiasis/ (22433)
(bronchus or bronchi or trachea* or lung* or pulmonary or espohag* or oesophag*).ti,ab. (1072622)
48 and 49 (1027)
((bronchus or bronchi or trachea* or lung* or pulmonary or espohag* or oesophag*) and candidiasis).ti,ab. (1120)
*Coccidioidomycosis/ (2505)
(disseminated or extrapulmonary).ti,ab. (66572)
52 and 53 (510)
((disseminated or extrapulmonary) adj1 coccidioidomycosis).ti,ab. (345)
*Cryptococcosis/ (5788)
extrapulmonary.ti,ab. (8232)
56 and 57 (41)
extrapulmonary cryptococcosis.ti,ab. (17)
*Cryptosporidiosis/ (4443)
chronic intestinal.ti,ab. (2497)
60 and 61 (3)
chronic intestinal cryptosporidiosis.ti,ab. (2)
exp *Cytomegalovirus Infections/ (19394)
(cytomegalovirus Infections or cytomegalovirus disease*).ti,ab. (2280)
*AIDS Dementia Complex/ (2835)
(AIDS Dementia Complex or "HIV encephalopath*").ti,ab. (910)
exp *Herpes Simplex/ (18427)
"herpes simplex".ti,ab. (39802)
*histoplasmosis/ (4997)
(disseminated or extrapulmonary).ti,ab. (66572)
70 and 71 (1201)
((disseminated or extrapulmonary) adj1 histoplasmosis).ti,ab. (1174)
*Isosporiasis/ (196)
chronic intestinal.ti,ab. (2497)
74 and 75 (1)
chronic intestinal isosporiasis.ti,ab. (2)
lymphoid interstitial pneumonia.ti,ab. (217)
pulmonary lymphoid hyperplasia complex.ti,ab. (1)
*Mycobacterium avium Complex/ (1701)
*Mycobacterium kansasii/ (329)
(disseminated or extrapulmonary).ti,ab. (66572)
81 and 82 (45)
mycobacterium avium complex.ti,ab. (2878)
((pulmonary or disseminated) adj1 mycobacterium kansasii).ti,ab. (84)
*Mycobacterium tuberculosis/ (36014)
(pulmonary or disseminated or extrapulmonary).ti,ab. (566676)
86 and 87 (4402)
((pulmonary or disseminated or extrapulmonary) adj1 mycobacterium tuberculosis).ti,ab. (138)
*Mycobacterium/ (12240)
(disseminated or extrapulmonary).ti,ab. (66572)
90 and 91 (198)
((disseminated or extrapulmonary) adj1 mycobacterium).ti,ab. (774)
*Pneumocystis carinii/ (1207)
pneumonia.ti,ab. (110638)
94 and 95 (856)
((Pneumocystis carinii or Pneumocystis jirovecii) and pneumonia).ti,ab. (6000)
recurrent pneumonia.ti,ab. (971)
*Leukoencephalopathy, Progressive Multifocal/ (2354)
progressive multifocal leukoencephalopathy.ti,ab. (3013)
salmonella septic?emia.ti,ab. (98)
*Toxoplasmosis, Cerebral/ (936)
cerebral toxoplasmosis.ti,ab. (834)
(brain adj1 toxoplasmosis).ti,ab. (40)
*HIV Wasting Syndrome/ (368)
HIV wasting syndrome.ti,ab. (74)
46 or 47 or 50 or 51 or 54 or 55 or 58 or 59 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 72 or 73 or 76 or 77 or 78 or 79 or 80 or 83 or 84 or 85 or 88 or 89 or 92 or 93 or 96 or 97 or 98 or 99 or 100 or 101 or 102 or 103 or 104 or 105 or 106 (124759)
(case report or case series).ti. (248880)
Case Reports.pt. (2073732)
108 or 109 (2128812)
107 or 110 (2228141)
43 not 111 (2683)
APPENDIX 2.
PRISMA 2020 checklist
| Section and Topic | Item no. | Checklist item | Location where item is reported |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review. | P1 |
| Abstract | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | P1 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | P3 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | P4 |
| Methods | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | P5 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | P5 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | Appendix 1 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | P5 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | P5 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g. for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | P5 |
| 10b | List and define all other variables for which data were sought (e.g. participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | Tables 1, 2, 3, 4 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | P6 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g. risk ratio, mean difference) used in the synthesis or presentation of results. | Tables 1, 2, 3, 4 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis [e.g. tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item no. 5)]. | N/A |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | N/A | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | Tables 1, 2, 3, 4 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta‐analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | N/A | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g. subgroup analysis, meta‐regression). | N/A | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | N/A | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | N/A |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | N/A |
| Results | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | P7 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | P6 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Tables 1, 2, 3, 4 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Appendix 3 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate); and (b) an effect estimate and its precision (e.g. confidence/credible interval), ideally using structured tables or plots. | Tables 1, 2, 3, 4 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | N/A |
| 20b | Present results of all statistical syntheses conducted. If meta‐analysis was done, present for each the summary estimate and its precision (e.g. confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | N/A | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | N/A | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | N/A | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | N/A |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | N/A |
| Discussion | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | P21 |
| 23b | Discuss any limitations of the evidence included in the review. | P21 | |
| 23c | Discuss any limitations of the review processes used. | P21 | |
| 23d | Discuss implications of the results for practice, policy and future research. | P22 | |
| Other information | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | Not registered |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | Not prepared | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | N/A | |
| Support | 25 | Describe sources of financial or non‐financial support for the review, and the role of the funders or sponsors in the review. | WAVE. No input |
| Competing interests | 26 | Declare any competing interests of review authors. | None |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | Supplementary information |
Raffe S, Sabin C, Gilleece Y; Women Against Viruses in Europe (WAVE), European AIDS Clinical Society . Comorbidities in women living with HIV: A systematic review. HIV Med. 2022;23:331–361. doi: 10.1111/hiv.13240
Funding information
This work was supported by European AIDS Clinical Society, Women Against Viruses in Europe. The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication. EACS WAVE acknowledges the following industry sponsors for their financial contributions in the form of unrestricted educational grants in 2021 from Gilead Sciences Europe, MSD and ViiV Healthcare.
References
- 1. UNAIDS . Fact sheet 2021 [Internet]. 2021. Available from: https://www.unaids.org/en/resources/fact‐sheet.
- 2. Mårdh O, Quinten C, Kuchukhidze G, et al. HIV among women in the WHO European Region – epidemiological trends and predictors of late diagnosis, 2009‐2018. Eurosurveillance. 2019; 24(48):24–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Curno MJ, Rossi S, Hodges‐Mameletzis I, Johnston R, Price MA, Heidari S. A systematic review of the inclusion (or Exclusion) of women in HIV research. Jaids J Acquir Immune Defic Syndromes. 2016; 71(2):181–188. [DOI] [PubMed] [Google Scholar]
- 4. May MT, Gompels M, Delpech V, et al. Impact on life expectancy of HIV‐1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28(8):1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pelchen‐Matthews A, Ryom L, Borges ÁH, et al. Aging and the evolution of comorbidities among HIV‐positive individuals in a European cohort. AIDS. 2018;32(16):2405–2416. [DOI] [PubMed] [Google Scholar]
- 6. Appay V, Almeida JR, Sauce D, Autran B, Papagno L. Accelerated immune senescence and HIV‐1 infection. Exp Gerontol. 2007;42(5):432–437. [DOI] [PubMed] [Google Scholar]
- 7. Wit F, van der Valk M, Gisolf J, Bierman W, Reiss P. Multimorbiditiy and risk of death differs by gender in people living with HIV in the Netherlands: the ATHENA cohort study. J Int AIDS Soc. 2018;21:25187. [Google Scholar]
- 8. Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metabolism. 2008;93(3):861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Henderson VW. Cognitive changes after menopause: influence of estrogen. Clin Obstet Gynecol. 2008;51(3):618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khoudary SRE, Aggarwal B, Beckie TM, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the american heart association. Circulation. 2020;142(25):e506–e532. [DOI] [PubMed] [Google Scholar]
- 11. Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metabolism. 2003;88(6):2404–2411. [DOI] [PubMed] [Google Scholar]
- 12. High KP, Brennan‐Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. Jaids J Acquir Immune Defic Syndromes. 2012;60(Supplement 1):S1–S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fitch KV, Srinivasa S, Abbara S, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV‐infected women. J Infect Dis. 2013;208(11):1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Addo MM, Altfeld M. Sex‐Based Differences in HIV Type 1 Pathogenesis. J Infect Dis. 2014;209(suppl 3):S86–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mathad JS, Gupte N, Balagopal A, et al. Sex‐related differences in inflammatory and immune activation markers before and after combined antiretroviral therapy initiation. Jaids J Acquir Immune Defic Syndromes. 2016;73(2):123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aboud M, Elgalib A, Pomeroy L, et al. Cardiovascular risk evaluation and antiretroviral therapy effects in an HIV cohort: implications for clinical management: the CREATE 1 study. Int J Clin Pract. 2010;64(9):1252–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Womack JA, Chang CH, So‐Armah KA, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc. 2014;3(5):e001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tariq S, Winston A, Bagkeris E, Asboe D, Johnson M, Anderson J, et al. Prevalence of cardiovascular risk factors in women ageing with HIV: an analysis of data from the POPPY Study (Pharmacokinetic and Clinical Observations in People Over Fifty). In: 22nd Annual Conference of the British HIV Association. 2016
- 19. Zachary D, Gillani FS, Najfi N, Casarella R, Tashima K. Cardiovascular Health of HIV‐infected AfricanAmerican Women at the Miriam Hospital Immunology Center in Providence. RI Medicine and health, 2012: 96. [PMC free article] [PubMed] [Google Scholar]
- 20. Cortés YI, Reame N, Zeana C, et al. Cardiovascular Risk in HIV‐infected and uninfected postmenopausal minority women: use of the framingham risk score. J Women’s Heal. 2017;26(3):241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frazier EL, Sutton MY, Tie Y, Fagan J, Fanfair RN. Differences by sex in cardiovascular comorbid conditions among older adults (Aged 50–64 or ≥65 Years) receiving care for human immunodeficiency virus. Clin Infect Dis. 2019;69(12):2091–2100. [DOI] [PubMed] [Google Scholar]
- 22. Hatleberg CI, Ryom L, El‐Sadr W, et al. Gender differences in the use of cardiovascular interventions in HIV‐positive persons; the D:A: D Study. J Int Aids Soc. 2018;21(3):e25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reinsch N, Neuhaus K, Esser S, et al. Are HIV patients undertreated? Cardiovascular risk factors in HIV: results of the HIV‐HEART study. Eur J Prev Cardiol. 2011;19(2):267–274. [DOI] [PubMed] [Google Scholar]
- 24. Thompson‐Paul AM, Palella FJ, Rayeed N, et al. Excess heart age in adult outpatients in routine HIV care. Aids. 2019;33(12):1935–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quiros‐Roldan E, Raffetti E, Focà E, et al. Incidence of cardiovascular events in HIV‐positive patients compared to general population over the last decade: a population‐based study from 2000 to 2012. Aids Care. 2016;28(12):1–8. [DOI] [PubMed] [Google Scholar]
- 26. Triant VA, Regan S, Grinspoon SK. MACE Incidence Among HIV and Non‐HIV‐Infected Patients in a Clinical Care Cohort. In: Conference on Retroviruses and Opportunistic Infections. 2014.
- 27. Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV‐infected and non–HIV‐infected patients in a US Health Care System. Jaids J Acquir Immune Defic Syndromes. 2012;60(4):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lang S, Mary‐Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV‐infected patients in France, relative to the general population. AIDS. 2010;24(8):1228–1230. [DOI] [PubMed] [Google Scholar]
- 29. Knudsen AD, Gelpi M, Afzal S, Ronit A, Roen A. Prevalence of peripheral artery disease is higher in persons living with HIV compared to uninfected controls. JAIDS. 2018;79(3):381–385. [DOI] [PubMed] [Google Scholar]
- 30. Shahmanesh M, Schultze A, Burns F, et al. The cardiovascular risk management for people living with HIV in Europe. Aids. 2016;30(16):2505–2518. [DOI] [PubMed] [Google Scholar]
- 31. Ogunbayo GO, Bidwell K, Misumida N, et al. Sex differences in the contemporary management of HIV patients admitted for acute myocardial infarction. Clin Cardiol. 2018;41(4):488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abraham AG, Althoff KN, Jing Y, et al. End‐stage renal disease among HIV‐Infected adults in North America. Clin Infect Dis. 2015;60(6):941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schoffelen AF, Smit C, van Lelyveld SFL, et al. Diminished impact of ethnicity as a risk factor for chronic kidney disease in the current HIV treatment era. J Infect Dis. 2015;212(2):264–274. [DOI] [PubMed] [Google Scholar]
- 34. Cristelli MP, Trullàs JC, Cofán F, et al. Prevalence and risk factors of mild chronic renal failure in HIV‐infected patients: influence of female gender and antiretroviral therapy. Braz J Infect Dis. 2018;22(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ibrahim F, Hamzah L, Jones R, et al. Baseline kidney function as predictor of mortality and kidney disease progression in HIV‐positive patients. Am J Kidney Dis. 2012;60(4):539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mocroft A, Ryom L, Begovac J, et al. Deteriorating renal function and clinical outcomes in HIV‐positive persons. Aids. 2014;28(5):727–737. [DOI] [PubMed] [Google Scholar]
- 37. Mocroft A, Kirk O, Reiss P, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV‐positive patients. Aids. 2010;24(11):1667–1678. [DOI] [PubMed] [Google Scholar]
- 38. Mocroft A, Lundgren JD, Ross M, et al. Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A: D study. PLoS Medicine. 2015;12(3):e1001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yin MT, Zhang CA, McMahon DJ, et al. Higher rates of bone loss in postmenopausal HIV‐infected women: a longitudinal study. J Clin Endocrinol Metabolism. 2012;97(2):554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sharma A, Tian F, Yin MT, Keller MJ, Cohen M, Tien PC. Association of regional body composition with bone mineral density in HIV‐infected and HIV‐uninfected women. Jaids J Acquir Immune Defic Syndromes. 2012;61(4):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Young B, Dao CN, Buchacz K, Baker R, Brooks JT. Increased Rates of Bone Fracture Among HIV‐Infected Persons in the HIV Outpatient Study (HOPS) Compared With the US General Population, 2000‐2006. Clin Infect Dis. 2011;52(8):1061–1068. [DOI] [PubMed] [Google Scholar]
- 42. Sharma A, Shi Q, Hoover DR, et al. Increased fracture incidence in middle‐aged hiv‐infected and HIV‐uninfected women. Jaids J Acquir Immune Defic Syndromes. 2015;70(1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gedmintas L, Wright EA, Losina E, Katz JN, Solomon DH. Comparative risk of fracture in men and women with HIV. J Clin Endocrinol Metabolism. 2014;99(2):486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Libois A, Clumeck N, Kabeya K, et al. Risk factors of osteopenia in HIV‐infected women: No role of antiretroviral therapy. Maturitas. 2010;65(1):51–54. [DOI] [PubMed] [Google Scholar]
- 45. Chow FC, Makanjuola A, Wu K, et al. Physical activity is associated with lower odds of cognitive impairment in women but not men living with human immunodeficiency virus infection. J Infect Dis. 2018;219(2):264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gustafson DR, Mielke MM, Tien PC, et al. Anthropometric measures and cognition in middle‐aged HIV‐infected and uninfected women. The Women’s Interagency HIV Study. J Neurovirol. 2013;19(6):574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maki PM, Rubin LH, Valcour V, et al. Cognitive function in women with HIV. Neurology. 2015;84(3):231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rubin LH, Radtke KK, Eum S, et al. Cognitive burden of common non‐antiretroviral medications in HIV‐infected women. Jaids J Acquir Immune Defic Syndromes. 2018;79(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meyer VJ, Rubin LH, Martin E, et al. HIV and recent illicit drug use interact to affect verbal memory in women. Jaids J Acquir Immune Defic Syndromes. 2013;63(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rubin LH, Cook JA, Weber KM, et al. The association of perceived stress and verbal memory is greater in HIV‐infected versus HIV‐uninfected women. J Neurovirol. 2015;21(4):422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rubin LH, Pyra M, Cook JA, et al. Post‐traumatic stress is associated with verbal learning, memory, and psychomotor speed in HIV‐infected and HIV‐uninfected women. J Neurovirol. 2016;22(2):159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rubin LH, Meyer VJ, Conant RJ, et al. Prefrontal cortical volume loss is associated with stress‐related deficits in verbal learning and memory in HIV‐infected women. Neurobiol Dis. 2016;92(Pt B):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rubin LH, Wu M, Sundermann EE, et al. Elevated stress is associated with prefrontal cortex dysfunction during a verbal memory task in women with HIV. J Neurovirol. 2016;22(6):840–851. [DOI] [PMC free article] [PubMed] [Google Scholar]


