Abstract
With advances in knowledge and treatment options, pulp regeneration is now a clear objective in clinical dental practice. For this purpose, many methodologies have been developed in attempts to address the putative questions raised both in research and in clinical practice. In the first part of this review, laboratory‐based methods will be presented, analysing the advantages, disadvantages, and benefits of cell culture methodologies and ectopic/semiorthotopic animal studies. This will also demonstrate the need for alignment between two‐dimensional and three‐dimensional laboratory techniques to accomplish the range of objectives in terms of cell responses and tissue differentiation. The second part will cover observations relating to orthotopic animal studies, describing the current models used for this purpose and how they contribute to the translation of regenerative techniques to the clinic.
Keywords: animal models, cell culture, dental pulp, dental pulp regeneration, stem cells
INTRODUCTION
In recent decades, dentistry has witnessed great advances in knowledge and treatment options involving tissue engineering and tissue regeneration strategies. Among the areas that have benefited from these advances, endodontics stands out; it has evolved remarkably, with a broader array of concepts and treatment options becoming routine elements in the endodontist's arsenal.
In this context, much has been discussed about the importance of the reestablishment of the dentine–pulp complex. One of this complex of tissues’ important functions involves the completion of the formation of the root in the case of immature teeth (Schmalz et al., 2020). The continued formation of the root can be encouraged by administering vital pulp therapy; however, the complete development of the apex and root length is not possible in teeth with necrotic pulps, so alternatives to improve the long‐term prognosis must be investigated (Fouad, 2017; Schmalz et al., 2020). The literature provides good examples of current treatment strategies that can be effective, such as the use of mineral trioxide aggregate (MTA) plugs (Torabinejad et al., 2017). On the other hand, the perspective of achieving complete recovery of tooth functions is attractive, as it can benefit patients and promote health by dealing with disease and promoting the regeneration of lost tissues.
The first studies on pulp regeneration focused on the revitalization process, which promotes bleeding into the canal (Banchs & Trope, 2004; Nosrat et al., 2011). Although this procedure has been shown to be effective in promoting an increase in root wall thickness, histological findings demonstrate that, at the moment, the clinician cannot control the type of tissue formed in the root canal; some histological reports show the formation of root cementum and connective tissue resembling those in a periodontal ligament (Lui et al., 2020). On the other hand, studies on cell homing have revealed more complex cellular interactions, with the recruitment of bone marrow stem cells (Widbiller et al., 2018). While the clinical revitalization ultimately results in clinical success, it confirms that regeneration was not achieved. Nonetheless, through advances in tissue engineering in the medical field associated with the discovery of stem cells originating from pulp tissues, new approaches have been developed that lead to an array of viable ways towards regenerating pulp tissue and, consequently, allowing for new dentine formation and pulp protection. This paper will focus on these aspects of the methodologies and strategies, from two‐dimensional (2D) and three‐dimensional (3D) cell culture analyses to studies on human subjects.
TECHNOLOGIES FOR PULP REGENERATION
Two‐dimensional research systems (2DSs) and 3D approaches (3DAs) have resulted in major breakthroughs in the field of dental pulp tissue engineering. Both 2DSs and 3DAs have potential but also limitations. For instance, a 2DS is a reliable and cost‐effective method to set up subculturing procedures that allow for rapid, convenient treatment of cells and high‐yield extraction of genetic and protein material to study differentiation mechanisms (Kapałczyńska et al., 2018). On the other hand, the cells in 2DSs are largely distributed in monolayers, an arrangement that offers good access to culture media ingredients and metabolites that are not equally distributed in living tissues and organs with varying architectures (Gelain et al., 2007; Pampaloni et al., 2007; Rosa et al., 2013). Alternatively, a 3DA aims to mimic the in vivo microarchitectures more closely, allowing for greater cell‐to‐cell contact and signalling networks (Abbott, 2003; Rosa et al., 2013). Nonetheless, the procedures used to produce 3D structures are more labourious and often demand specific skillsets and equipment (Bhargav et al., 2020; Muthusamy et al., 2021; Sriram et al., 2019). The following sections will discuss the potential uses of such technologies for the advancement of dental pulp tissue engineering and regeneration.
TWO‐DIMENSIONAL RESEARCH SYSTEMS
Two‐dimensional systems allow for high testing yields and cost‐effective analyses. The use of 2DSs has been vital to characterizing and unveiling the differentiation potential of dental pulp stem cells (DPSCs) towards odontoblast‐like cells, neurons, and endothelial cells, all of which are essential for the regeneration of functional dental pulp tissues (Gronthos et al., 2000; Madanagopal et al., 2020; Rosa et al., 2013; Sakai et al., 2010).
The establishment of odontoblasts is required for the regeneration of fully functional dental pulps. Nonetheless, maintaining ex vivo odontoblasts in vitro or inducing the promotion of the differentiation of stem cells towards true odontoblasts remain some of the most challenging issues in dental research, due to factors such as the variety of induction protocols with varying compositions and treatment duration used to promote the differentiation of stem cells into odontoblasts in a laboratory setting (Natu et al., 2015; Sabbagh et al., 2020). Notably, the derivation of odontoblast‐like cells able to produce tubular dentine in vivo has been achieved via different strategies (Dissanayaka et al., 2014; Rosa et al., 2013; Sakai et al., 2010; Xie et al., 2018). However, no marker can uniquely identify and distinguish differentiated odontoblasts from other phenotypes. Even dentine matrix acidic phosphoprotein 1 (DMP‐1) and dentine sialophosphoprotein (DSPP), which are commonly used to evaluate odontoblastic differentiation, are also expressed by osteoblasts and osteocytes (Lu et al., 2011; Sabbagh et al., 2020). Hence, many proxy evaluations (i.e., quantification of mineral deposition and expressions of Runt‐related transcription factor 2 (RUNX2), DMP‐1, DSPP, and other genes and proteins are frequently used to characterize the differentiation processes and resultant odontoblastic‐like cells in vitro (Tziafas, 2019).
Two‐dimensional systems have been used to unveil many environmental triggers and processes involved in odontoblastic differentiation. For instance, hydraulic cements can increase environmental alkalinity and promote genetic and protein expression of DMP‐1 and DSPP, and mineralization deposition by stem cells isolated from the dental pulp (Natu et al., 2015; Xie et al., 2018). Alternatively, osteogenic induction media (OM) have commonly been used to convert dental stem cells into odontoblast‐like cells. The OM cocktail can increase the expression of RUNX2 and DSSP by DPSC as early as within three days of treatment (Deng et al., 2021). Many cellular pathways are involved in OM‐induced odontoblastic differentiation, including increased β‐catenin activity and the activation of RUNX2, a transcriptional factor that regulates osteoblastic differentiation (Deng et al., 2021; Han et al., 2014). The RUNX2 is likely to be involved in odontoblastic differentiation during reparative dentine formation through a mechanism that involves β‐catenin activation (Han et al., 2014). Notably, it can also inhibit the terminal differentiation of odontoblasts, promoting the conversion of stem cells into osteoblasts at the late differentiation stage (Li et al., 2011). This transdifferentiation must be controlled when aiming for the regeneration of a dental pulp containing functional odontoblasts able to secrete dentine.
Two‐dimensional systems have also been instrumental in elucidating the role of growth factors, such as bone morphogenetic proteins (BMPs), in odontoblastic differentiation. It is known that BMP‐2 and BMP‐7 control the onset of tooth mineralization and induce reparative dentinogenesis (Malik et al., 2020), and BMP‐2 is required for the differentiation of stem cells from human exfoliated deciduous teeth (SHED) towards odontoblast‐like cells in vitro (Casagrande et al., 2010). Moreover, induced pluripotent stem cells resulted in significant increases in the expression of markers of odontoblastic differentiation such as matrix extracellular phosphoglycoprotein (MEPE), MSX‐1, DMP‐1, and DSPP after being treated for 10 days with OM‐supplemented BMP‐4 (Xie et al., 2018). Likewise, embryonic stem cell‐derived mesenchymal stem cells have acquired an odontoblast‐like phenotype after being stimulated with BMP‐4 and fibroblast growth factor 8 (Kidwai et al., 2014), and mouse embryonic stem cells cultured in a collagen type‐I scaffold combined with BMP‐4 presented high DSPP gene expression and high alkaline phosphatase activity (Kawai et al., 2014). Besides BMP‐4, dentine‐derived BMP‐2 also induces strong expression of MEPE, DMP‐1, and DSPP in SHED (Casagrande et al., 2010).
The regeneration of functional dental pulp requires the reestablishment of vascularized tissue within the root canal (Rosa et al., 2012; Schmalz et al., 2020). It has been shown that stem cells from the dental pulp present positive expression for vascular cell adhesion molecule 1, endostatin, von Willebrand factor domains 1 and 2, and angiotensin‐converting enzyme (d'Aquino et al., 2007; Gronthos et al., 2000; Miura et al., 2003). Indeed, SHED treated with vascular endothelial growth factor (VEGF)‐induced endothelial differentiation through the phosphorylation of extracellular‐signal‐regulated kinase and protein kinases (AKT; Sakai et al., 2010). Likewise, a growth factor milieu obtained from an endothelial conditioned medium increased the expression of Bmi‐1 and induced signal transducer and activator of transcription 3 (STAT3) phosphorylation in both DPSC and SHED (Oh et al., 2020). These results suggest that endothelial factors activate pathways that promote the emergence of functional blood vessels in engineered dental pulps and support differentiation into odontoblasts capable of secreting dentine.
Despite the many achievements outlined in the literature, the lack of unique markers to ascertain odontoblastic differentiation has yet to be resolved. Moreover, the establishment of a standardized odontogenic induction protocol could allow for improved comparisons among the various strategies employed to promote and guide odontoblastic differentiation (Koh et al., 2021). Nonetheless, the protocol diversity in 2DSs also broadens the strategies, promoting the differentiation of stem cells from dental pulp towards phenotypes relevant to the establishment of the functional dental pulp (Casagrande et al., 2010; Natu et al., 2015; Sakai et al., 2010; Xie et al., 2018).
NOVEL 3D APPROACHES FOR TISSUE ENGINEERING APPLICATIONS
Regenerative strategies often employ progenitor cells seeded in 3D scaffolds (supplemented or not with exogenous promoters such as growth factors, bioceramics, and other compounds; Rosa et al., 2012). A valuable 3DA for studying pulp regeneration is the tooth slice/scaffold model in which an extracted tooth crown is sectioned into slices (a few millimetres thick) and a porous biodegradable scaffold is placed in the cavity once occupied by the pulp (Sakai et al., 2011). This 3DA is a cost‐effective model that does not require specialized manpower or advanced technologies for preparation. It has been used to maintain the vitality of human pulp implanted in severe combined immunodeficient mice for up to 7 days (Goncalves et al., 2007) and to demonstrate the feasibility of engineering well‐vascularized pulp‐like tissue using SHED (Cordeiro et al., 2008). Notably, this 3DA enhanced pulp microvessel density when the human pulp was treated with VEGF prior to transplantation (Mullane et al., 2008). A similar angiogenic response was observed in the transplantation of the tooth slice model seeded with DPSC, which enabled the establishment of numerous blood vessels throughout the engineered pulp (Piva et al., 2017). This 3DA revealed the importance of dentine‐derived morphogenic signals (e.g., BMP‐2) in inducing odontoblastic differentiation (Casagrande et al., 2010).
Alternatively, in animal models, cell perfusion chambers may also be used to reconstruct the dentine–pulp interface (pulp analogue) or to test the effects of external stimuli in dental stem cells. In this 3DA, the dentine discs containing scaffolds (nylon, polyamide, and polystyrene) and cells are sandwiched between two chambers, and culture media is perfused on the pulpal side; the dentine side is used for exposure to external stimuli such as dental materials (Galler et al., 2005; Jiang et al., 2017; Schmalz et al., 1999; Sengün et al., 2011). The dynamic perfusion of culture media is intended to mimic pulpal blood and fluid flow. One of the main challenges for this 3DA is the variability in the thickness of dentine discs’ and morphologies, which results in the varied distribution and density of dentinal tubules that potentially affect the permeation of the test substances and experimental outcomes (Galler et al., 2005; Jiang et al., 2017). Similarly, sterilizing the dentine discs with an autoclave prior to testing may cause the denaturation of collagen and other proteins and may impact the diffusion of the test substances (Jiang et al., 2017).
Despite this mimicry of the 3D architecture of the dentine–pulp complex, studies using perfusion chambers have primarily used simple proliferation assays as surrogate tools for the assessment of material cytotoxicity. Future studies should focus on construct miniaturization and high‐end techniques, such as microfluidic technologies, whole‐mount immunostaining, and multiphoton, confocal reflectance, and light‐sheet microscopy, for direct visualization of pulpal responses (Athirasala et al., 2017; Franca et al., 2019; Hartmann et al., 2006; Sriram et al., 2018, 2020).
Organoid‐based reconstruction strategies have emerged as a potential 3DA for reconstructing dental tissues. These strategies involve the replication of the spatial‐temporal cell interactions via co‐culture or the compartmentalization of dental epithelial and mesenchymal cells digested from tooth germs within scaffolds followed by the organoid culture within a bioreactor or in vivo transplantation (Gao et al., 2021). Vascular endothelial cells have been incorporated into the system to develop vascularized tooth germ organoids (Smith et al., 2017). This developmental approach allows tooth germ organoids to develop into natural tooth‐like structures containing dentine and pulp (Cai et al., 2017; Nakao et al., 2007; Smith et al., 2017; Zhang et al., 2017). However, tooth bud organoids, on occasion, fail to produce distinct enamel or dentine (Smith et al., 2017). Nonetheless, the tooth bud model is an innovative approach with the potential to reconstruct both soft and hard tissue, providing many opportunities to study odontogenesis and pulp regeneration. This model demands the development of a certain skillset; specifically, it requires the ability to isolate embryonic tooth tissues and compartmentalize dental epithelial and mesenchymal cells, isolating them from tooth germs. Alternatively, induced pluripotent stem cells can be used to differentiate dental epithelial and mesenchymal cells (Kim et al., 2019; Xie et al., 2018). Another promising 3DA for pulp tissue engineering is the use of DPSC‐derived (with or without endothelial cells) microtissue spheroids, which have been able to produce pulp‐like tissue containing odontoblast‐like cells with positive expression for DSPP, lining the dentine of root canals implanted in animal models (Dissanayaka et al., 2014). Future research on effective induction methods, cell sources, and multifunctional materials that can improve control over the size, shape, and types of tissues formed are essential for the advancement of pulp regeneration reconstruction using this 3DA.
Technological advancements in microfabrication have led to the incorporation of microfluidic (organ‐on‐a‐chip) devices for dental pulp regeneration. These devices are made of microchambers and microchannels linked to pumps. The organ/tooth‐on‐a‐chip devices offer advantages such as:
controlled flow of nutrients, oxygen, and test substances, as well as the drainage of metabolic wastes and the collection of cell‐derived factors for downstream analysis (Alberti et al., 2017; Sriram et al., 2018);
label‐free visualization of structures and cells such as fibroblasts and collagen fibres (Sriram et al., 2018); and
analysis of cell–material interactions and possible cytotoxic responses through live‐cell imaging at a microscopic scale (Franca et al., 2020).
These organ‐on‐a‐chip devices are gaining interest for craniofacial research with the development of tooth‐, oral mucosa‐, skin‐, salivary glands‐, and nasal mucosa‐on‐a‐chip (Alberti et al., 2017; Bal‐Ozturk et al., 2018; Franca et al., 2020; Na et al., 2017; Rahimi et al., 2018; Song et al., 2021; Sriram et al., 2018, 2019; Wang et al., 2014). Hence, the in vivo‐like microenvironment enabled by the organ/tooth‐on‐a‐chip devices can be extended for real‐time monitoring, cellular and tissue responses to biomaterials, and the tracking of differentiation cues and mechanisms through imaging and built‐in biosensors.
The goal of dental pulp tissue engineering strategies is the development of a vascularized and innervated dental pulp that is able to deposit dentine and sustain normal root development. The rigid scaffold cast in the tooth slice model has enabled many advancements but does not take into full account the complex 3D geometry of root canals (Rosa et al., 2013). Hence, 3D hydrogels (e.g., collagen decellularized pulp matrix GelMa, self‐assembling peptides, and others) have been explored and developed for pulp regeneration (Cavalcanti et al., 2013; Chen et al., 2015; Galler et al., 2018; Iohara et al., 2013; Khayat et al., 2017; Rosa et al., 2013). Natural materials such as fibrin, collagen, gelatin, and decellularized matrix offer excellent environments for differentiation and control over biodegradability. Alternatively, hybrid hydrogels containing crosslinking agents provide opportunities to finetune handling characteristics as well as physical and mechanical properties (Hadjichristou et al., 2020; Han et al., 2019; Qu et al., 2015). For instance, the addition of polyethylene glycol (PEG) diacrylate can be used to increase the storage modulus (G′) of PEG/fibrinogen scaffold from 140 to 3601 Pa. This increase in rigidity induces overexpression (>100‐fold) of DSPP and DMP‐1 genes in DPSC (Lu et al., 2015). Likewise, the incorporation of 1 µM simvastatin into a chitosan/calcium hydroxide scaffold, through DPSC using an artificial pulp chamber assay, has been able to increase cell migration and the expression of alkaline phosphatase (ALP), DSPP, and DMP‐1 using an artificial pulp chamber assay (Soares et al., 2021). Scaffolds can be further personalized via additive manufacturing technologies (3D printing). Poly‐caprolactone (PCL) is commonly used to print biocompatible scaffolds, but it is not bioactive, nor does it have natural cell recognition sites to promote cell differentiation (Li et al., 2017). To overcome PCL’s inertness, MTA can be incorporated into the PCL matrix (Lee et al., 2012). Indeed, the addition of 4% MTA to 3D‐printed PCL scaffolds increases their degradation rate, modulus of elasticity cell proliferation rate, and the genetic expression of MSX‐1, ALP, and COL‐I in DPSC after 30 days (Bhargav et al., 2020). Another promising strategy is to bioprint 3D freeform cellular constructs and patient‐specific tooth tissue structures. This can be done via computer‐controlled biofabrication using scaffold materials and bioinks. Recent advances in this 3DA include the odontogenic differentiation of DPSC and the production of dentine–pulp complexes by serial printing PCL (to control shape) and layering two cell‐laden hybrid fibrin‐hyaluronic acid‐based bioinks (Han et al., 2019). However, due to poor cell survival in thick constructs, the reconstruction of tissues through 3D bioprinting remains limited.
Fast‐paced innovations in biotechnology and molecular biology will continue to provide new and exciting routes for the advancement of dental pulp tissue engineering. Combining the benefits of different 2DSs with 3DAs such as microfluidics, perfusion, and 3D bioprinting can allow for fast, cost‐effective, and clinically predictable reconstruction of functional dentine–pulp complexes.
IN VIVO ANIMAL MODELS FOR PULP REGENERATION
As explored above, 2D and 3D methods have come a long way and are capable of simulating more advanced and deeper aspects of cell interactions (Figure 1). The use of animal models in those proof of principle analyses has consolidated the viability of both cell‐dependent (Rosa et al., 2013) and cell homing strategies (Widbiller et al., 2018) and allowed for a better understanding of the effects of different scaffolds and extracellular factors on these ectopic and semiorthotopic approaches. These approaches (Figure 2) refer to the use of other connective tissues (e.g., subcutaneous and renal capsule) to provide vascularization to the pulp constructs, either using a 3D cell culture direct implantation (ectopic) or using a human tooth as a framework for the cell constructs (semiorthotopic). The answers obtained in these types of studies are varied, as explained in the previous section, and they serve as an important background for the application of pulp regeneration strategies in clinical practice, which is the ultimate objective of regeneration research.
FIGURE 1.
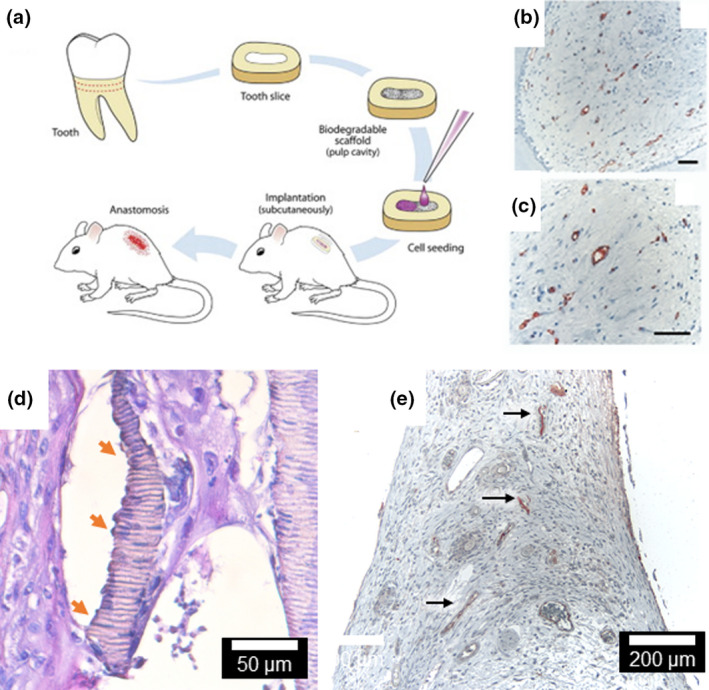
(A) The implantation of tooth slices containing dental pulp stem cells from the dental pulp (exfoliated or permanent teeth) has been instrumental for the development of pulp regeneration strategies (Cordeiro et al. 2008). (B, C)The transplantation of tooth slices containing freshly extracted human pulp pre‐treated with VEGF allowed the development and enhancement of the pulp microvessel density with positive expression of Factor VIII (Gonçalves et al. 2007). (D) The tooth slice model has also been used to produce dentine (orange arrows) containing entrapped odontoblastic‐like projections from induced pluripotent stem cells. (E) Dental pulp tissue engineering with SHED injected into human root canals with microvessel presenting positive expression of Factor VIII (arrows, Rosa et al. 2012, 2013). Figures adapted with permission from Elsevier.
FIGURE 2.
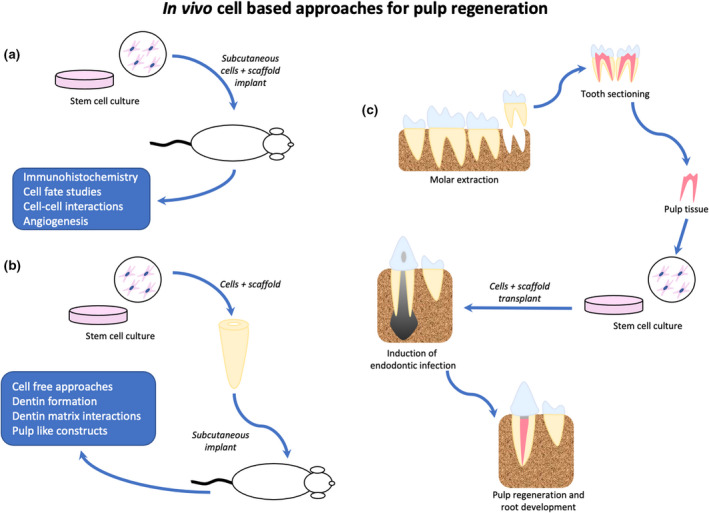
In vivo approaches for cell‐based pulp tissue engineering. (a) Ectopic cell transplantation, based on the use of allogenic stem cells associated with scaffolds, is implanted in different animal tissue. (b) Semi‐orthotopic cell transplantation, based on the same concept as ectopic, but using tooth structures (e.g., tooth slices or roots) to be implanted in different animal tissue. (c) Orthotopic cell transplantation, based on good manufacturing practices to avoid any bacteria or foreign cell contamination, these strategies simulate the clinical practice by obtaining cells from the same species and implanting them in functional teeth
ORTHOTOPIC ANIMAL MODELS
Compared to semiorthotopic animal models, orthotopic (using animal teeth in a technique similar to that involving human teeth) regenerative studies are relevant; they allow for the assessment of the safety and effectiveness of new clinical approaches, and they can provide an assessment of necessary changes or adjustments to clinical protocols (Nakashima et al., 2019). With regard to pulp regeneration, no animal model is completely effective, due to the nature of cell turnover, apical anatomy, or immune system response.
In general, small animal studies are limited; the pulp chambers are small and do not allow for relevant data collection regarding the organization of the newly formed tissue or even for an adequate assessment of clinical protocols (Kim et al., 2015). Moreover, as most small animal models are rodents, there is always the concern about high cell turnover and constant root development, which do not mimic the conditions found in humans. Nonetheless, pulp regeneration studies that specifically address the effects of pulp capping materials and/or pulpotomies are still possible in rodents, as some studies have proven (Minic et al., 2021). However, for full pulp regeneration studies, these species present too many disadvantages to provide meaningful data, leading to the preference for larger animal models (Mangione et al., 2021; Nakashima et al., 2019). Among these larger models, the literature has studies that use various animal groups (e.g., sheep, minipig, ferret, and dog), each with advantages and disadvantages.
STUDIES USING SHEEP AND MINIPIGS
Sheep (Ovis aries) studies are not very common but show good results in pulp regeneration (Altaii et al., 2017) due to the animal size and shape of the teeth. There are disadvantages to these studies, such as the fact that sheep are ruminants, which can affect the oral pH (Mangione et al., 2021), and the fact that not many dental studies used this species. Minipig (Sus scrofa) models are another emerging alternative for the study of pulp regeneration. They have been utilized in dental research for years, mainly due to anatomical similarities between their teeth and human teeth. They also allow for the isolation of dental pulp stem cells (Zhu et al., 2018), and, although the dentine produced by the minipig cells has different characteristics from human dentine (Huang & Garcia‐Godoy, 2014), this model is promising, as their anatomy can simulate both single‐rooted and multi‐rooted teeth (Zhu et al., 2018).
STUDIES USING FERRETS
Among animals with more availability in the literature, ferrets (Mustela poturios furo) are an interesting model, given that they have been used for years in endodontic studies to assess pulp and periapical responses to procedures and materials (Torabinejad et al., 2011). The use of ferrets for pulp regeneration has been limited, and only a few papers have used this model for the assessment of scaffolds or isolation of dental pulp stem cells (Homayounfar et al., 2016). The major advantage of this animal model in orthotopic studies is that its immature canines have very similar features to those observed in immature human teeth (e.g., open apex and narrow dentine walls; Torabinejad et al., 2011). Considering this plus the reproducibility of creating periapical infection (Fouad et al., 1993), ferrets can be considered a step forward in pulp regeneration studies and will allow for better analysis of irrigation and medication protocols as well as comparisons among different types of scaffolds.
STUDIES USING DOGS
The canine model (Canis lupus familiaris) is one of the models of choice for orthotopic pulp regeneration. This model has advantages, such as the wide array of dental pulp analyses that have been made (Nakashima et al., 2019), which brings familiarity and predictability to the responses. In addition, dogs have suitable‐sized teeth, which can facilitate clinical procedures. Their pulps also have dental pulp stem cells, which have proven to be effective in autologous transplantation procedures using good manufacturing practices (Ishizaka et al., 2012), although some of the stemness of canine cells may be lost compared to human cells (Wang et al., 2020). Nonetheless, dog teeth have disadvantages, such as the absence of an apical foramen and the presence of an apical delta, which make the simulation of human anatomy more difficult. Studies have shown that an apical opening needs to be created to mimic the apical opening of human teeth (Ishizaka et al., 2012). In addition, the dog model has proven to be reliable in the induction of periapical lesions (Ferreira et al., 2006), an important factor when studying pulp regeneration in infected root canals.
Clearly, independent of the model, there are difficulties in the translation to human models. There are too many unknowns regarding the correct protocols for instrumentation and disinfection, and the animal models can only go as far as the repair capacity and technical hurdles that these models present. Therefore, studies on humans are still the gold standard, and although such studies are rare and primarily confined to case reports in revitalization, they should be the objective because they allow for adequate translation.
CONCLUSIONS
The increasing number of studies on pulp regeneration brings many answers and an equal number of questions that need to be addressed to allow for the development of this area of interest. Although the ultimate goal is the application of superior techniques that aid human patients, the questions raised cannot be answered in the clinic due to risks and ethical reasons. In this context, in vitro cell culture models and in vivo animal models can assist in addressing these issues and in understanding the biological mechanisms involved in the regeneration process. Consequently, this review has presented many of the methodologies involved, along with the possibilities that each methodology brings. The right answer lies not in the idea that one methodology is better than another but, rather, in the comprehensive application of these different methodologies that can be used in conjunction, offsetting each other's disadvantages, and collectively improving clinical practice.
CONFLICT OF INTEREST
The authors deny any conflicts of interest.
AUTHOR CONTRIBUTION
All authors contributed equally in the literature review, production and revision of this manuscript.
ETHICAL APPROVAL
As a literature review, this manuscript is exempt of ethical approval.
Rosa, V. , Sriram, G. , McDonald, N. & Cavalcanti, B.N. (2022) A critical analysis of research methods and biological experimental models to study pulp regeneration. International Endodontic Journal, 55(Suppl. 2), 446–455. Available from: 10.1111/iej.13712
REFERENCES
- Abbott, A. (2003) Cell culture: biology's new dimension. Nature, 424, 870–872. [DOI] [PubMed] [Google Scholar]
- Alberti, M. , Dancik, Y. , Sriram, G. , Wu, B. , Teo, Y.L. , Feng, Z. et al. (2017) Multi‐chamber microfluidic platform for high‐precision skin permeation testing. Lab on a Chip, 17, 1625–1634. [DOI] [PubMed] [Google Scholar]
- Altaii, M. , Cathro, P. , Broberg, M. & Richards, L. (2017) Endodontic regeneration and tooth revitalization in immature infected sheep teeth. International Endodontic Journal, 50, 480–491. [DOI] [PubMed] [Google Scholar]
- Athirasala, A. , Lins, F. , Tahayeri, A. , Hinds, M. , Smith, A.J. , Sedgley, C. et al. (2017) A novel strategy to engineer pre‐vascularized full‐length dental pulp‐like tissue constructs. Scientific Reports, 7, 3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal‐Öztürk, A. , Miccoli, B. , Avci‐Adali, M. , Mogtader, F. , Sharifi, F. , Çeçen, B. et al. (2018) Current strategies and future perspectives of skin‐on‐a‐chip platforms: innovations, technical challenges and commercial outlook. Current Pharmaceutical Design, 24, 5437–5457. [DOI] [PubMed] [Google Scholar]
- Banchs, F. & Trope, M. (2004) Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? Journal of Endodontics, 30, 196–200. [DOI] [PubMed] [Google Scholar]
- Bhargav, A. , Min, K.S. , Wen Feng, L. , Fuh, J.Y.H. & Rosa, V. (2020) Taguchi's methods to optimize the properties and bioactivity of 3D printed polycaprolactone/mineral trioxide aggregate scaffold: theoretical predictions and experimental validation. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 108, 629–637. [DOI] [PubMed] [Google Scholar]
- Cai, X. , Ten Hoopen, S. , Zhang, W. , Yi, C. , Yang, W. , Yang, F. et al. (2017) Influence of highly porous electrospun PLGA/PCL/nHA fibrous scaffolds on the differentiation of tooth bud cells in vitro. Journal of Biomedical Materials Research Part A, 105, 2597–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande, L. , Demarco, F.F. , Zhang, Z. , Araujo, F.B. , Shi, S. & Nör, J.E. (2010) Dentin‐derived BMP‐2 and odontoblast differentiation. Journal of Dental Research, 89, 603–608. [DOI] [PubMed] [Google Scholar]
- Cavalcanti, B.N. , Zeitlin, B.D. & Nör, J.E. (2013) A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dental Materials, 29, 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G. , Chen, J. , Yang, B.O. , Li, L. , Luo, X. , Zhang, X. et al. (2015) Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration. Biomaterials, 52, 56–70. [DOI] [PubMed] [Google Scholar]
- Cordeiro, M.M. , Dong, Z. , Kaneko, T. , Zhang, Z. , Miyazawa, M. , Shi, S. et al. (2008) Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. Journal of Endodontics, 34, 962–969. [DOI] [PubMed] [Google Scholar]
- d'Aquino, R. , Graziano, A. , Sampaolesi, M. , Laino, G. , Pirozzi, G. , De Rosa, A. et al. (2007) Human postnatal dental pulp cells co‐differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death & Differentiation, 14, 1162–1171. [DOI] [PubMed] [Google Scholar]
- Deng, Z. , Yan, W. , Dai, X. , Chen, M. , Qu, Q. , Wu, B. et al. (2021) N‐Cadherin regulates the odontogenic differentiation of dental pulp stem cells via β‐catenin activity. Frontiers in Cell and Developmental Biology, 9, 661116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayaka, W.L. , Zhu, L. , Hargreaves, K.M. , Jin, L. & Zhang, C. (2014) Scaffold‐free prevascularized microtissue spheroids for pulp regeneration. Journal of Dental Research, 93, 1296–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, F.B. , Campos Rabang, H.R. , Pinheiro, E.T. , Gadê‐Neto, C.R. , Zaia, A.A. , Randi Ferraz, C.C. et al. (2006) Root canal microbiota of dogs’ teeth with periapical lesions induced by two different methods. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics, 102, 564–570. [DOI] [PubMed] [Google Scholar]
- Fouad, A.F. (2017) Microbial factors and antimicrobial strategies in dental pulp regeneration. Jornal of Endodontics, 43, S46–S50. [DOI] [PubMed] [Google Scholar]
- Fouad, A.F. , Walton, R.E. & Rittman, B.R. (1993) Healing of induced periapical lesions in ferret canines. Journal of Endodontics, 19, 123–129. [DOI] [PubMed] [Google Scholar]
- França, C.M. , Riggers, R. , Muschler, J.L. , Widbiller, M. , Lococo, P.M. , Diogenes, A. et al. (2019) 3D‐Imaging of whole neuronal and vascular networks of the human dental pulp via CLARITY and light sheet microscopy. Scientific Reports, 9, 10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França, C.M. , Tahayeri, A. , Rodrigues, N.S. , Ferdosian, S. , Puppin Rontani, R.M. , Sereda, G. et al. (2020) The tooth on‐a‐chip: a microphysiologic model system mimicking the biologic interface of the tooth with biomaterials. Lab on a Chip, 20, 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler, K.M. , Brandl, F.P. , Kirchhof, S. , Widbiller, M. , Eidt, A. , Buchalla, W. et al. (2018) Suitability of different natural and synthetic biomaterials for dental pulp tissue engineering. Tissue Engineering Part A, 24, 234–244. [DOI] [PubMed] [Google Scholar]
- Galler, K. , Hiller, K.A. , Ettl, T. & Schmalz, G. (2005) Selective influence of dentin thickness upon cytotoxicity of dentin contacting materials. Journal of Endodontics, 31, 396–399. [DOI] [PubMed] [Google Scholar]
- Gao, X. , Wu, Y. , Liao, L. & Tian, W. (2021) Oral organoids: progress and challenges. Journal of Dental Research, 100, 454–463. [DOI] [PubMed] [Google Scholar]
- Gelain, F. , Horii, A. & Zhang, S. (2007) Designer self‐assembling peptide scaffolds for 3‐d tissue cell cultures and regenerative medicine. Macromolecular Bioscience, 7, 544–551. [DOI] [PubMed] [Google Scholar]
- Goncalves, S.B. , Dong, Z. , Bramante, C.M. , Holland, G.R. , Smith, A.J. & Nor, J.E. (2007) Tooth slice‐based models for the study of human dental pulp angiogenesis. Journal of Endodontics, 33, 811–814. [DOI] [PubMed] [Google Scholar]
- Gronthos, S. , Mankani, M. , Brahim, J. , Robey, P.G. & Shi, S. (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and invivo. Proceedings of the National Academy of Sciences of the United States of America, 97, 13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjichristou, C. , Papachristou, E. , Bonovolias, I. & Bakopoulou, A. (2020) Three‐dimensional tissue engineering‐based Dentin/Pulp tissue analogue as advanced biocompatibility evaluation tool of dental restorative materials. Dental Materials, 36, 229–248. [DOI] [PubMed] [Google Scholar]
- Han, J. , Kim, D.S. , Jang, H. , Kim, H.R. & Kang, H.W. (2019) Bioprinting of three‐dimensional dentin‐pulp complex with local differentiation of human dental pulp stem cells. Journal of Tissue Engineering, 10, 2041731419845849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, N. , Zheng, Y. , Li, R. , Li, X. , Zhou, M. , Niu, Y. et al. (2014) β‐Catenin enhances odontoblastic differentiation of dental pulp cells through activation of Runx2. PLoS One, 9, e88890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann, A. , Boukamp, P. & Friedl, P. (2006) Confocal reflection imaging of 3D fibrin polymers. Blood Cells, Molecules and Diseases, 36, 191–193. [DOI] [PubMed] [Google Scholar]
- Homayounfar, N. , Verma, P. , Nosrat, A. , El Ayachi, I. , Yu, Z. , Romberg, E. et al. (2016) Isolation, characterization, and differentiation of dental pulp stem cells in ferrets. Journal of Endodontics, 42, 418–424. [DOI] [PubMed] [Google Scholar]
- Huang, G.T. & Garcia‐Godoy, F. (2014) Missing concepts in de novo pulp regeneration. Journal of Dental Research, 93, 717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iohara, K. , Murakami, M. , Takeuchi, N. , Osako, Y. , Ito, M. , Ishizaka, R. et al. (2013) A novel combinatorial therapy with pulp stem cells and granulocyte colony‐stimulating factor for total pulp regeneration. Stem Cells Translational Medicine, 2, 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka, R. , Iohara, K. , Murakami, M. , Fukuta, O. & Nakashima, M. (2012) Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials, 33, 2109–2118. [DOI] [PubMed] [Google Scholar]
- Jiang, R.D. , Lin, H. , Zheng, G. , Zhang, X.M. , Du, Q. & Yang, M. (2017) In vitro dentin barrier cytotoxicity testing of some dental restorative materials. Journal of Dentistry, 58, 28–33. [DOI] [PubMed] [Google Scholar]
- Kapałczyńska, M. , Kolenda, T. , Przybyła, W. , Zajączkowska, M. , Teresiak, A. , Filas, V. et al. (2018) 2D and 3D cell cultures ‐ a comparison of different types of cancer cell cultures. Archives of Medical Science, 14, 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, R. , Ozeki, N. , Yamaguchi, H. , Tanaka, T. , Nakata, K. , Mogi, M. et al. (2014) Mouse ES cells have a potential to differentiate into odontoblast‐like cells using hanging drop method. Oral Diseases, 20, 395–403. [DOI] [PubMed] [Google Scholar]
- Khayat, A. , Monteiro, N. , Smith, E.E. , Pagni, S. , Zhang, W. , Khademhosseini, A. et al. (2017) GelMA‐encapsulated hDPSCs and HUVECs for dental pulp regeneration. Journal of Dental Research, 96, 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwai, F.K. , Movahednia, M.M. , Iqbal, K. , Jokhun, D.S. , Cao, T. & Fawzy, A.S. (2014) Human embryonic stem cell differentiation into odontoblastic lineage: an in vitro study. International Endodontic Journal, 47, 346–355. [DOI] [PubMed] [Google Scholar]
- Kim, E.J. , Yoon, K.S. , Arakaki, M. , Otsu, K. , Fukumoto, S. , Harada, H. et al. (2019) Effective differentiation of induced pluripotent stem cells into dental cells. Developmental Dynamics, 248, 129–139. [DOI] [PubMed] [Google Scholar]
- Kim, S. , Shin, S.J. , Song, Y. & Kim, E. (2015) In vivo experiments with dental pulp stem cells for pulp‐dentin complex Regeneration. Mediators of Inflammation, 2015, 409347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh, B. , Sulaiman, N. , Ismadi, S.N.S.W. , Ramli, R. , Yunus, S.S.M. , Idrus, R.B.H. et al. (2021) Mesenchymal stem cells: a comprehensive methods for odontoblastic induction. Biological Procedures Online, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, W. , Oh, J.H. , Park, J.C. , Shin, H.I. , Baek, J.H. , Ryoo, H.M. et al. (2012) Performance of electrospun poly(ε‐caprolactone) fiber meshes used with mineral trioxide aggregates in a pulp capping procedure. Acta Biomaterialia, 8, 2986–2995. [DOI] [PubMed] [Google Scholar]
- Li, S. , Kong, H. , Yao, N. , Yu, Q. , Wang, P. , Lin, Y. et al. (2011) The role of runt‐related transcription factor 2 (Runx2) in the late stage of odontoblast differentiation and dentin formation. Biochemical and Biophysical Research Communications, 410, 698–704. [DOI] [PubMed] [Google Scholar]
- Li, X. , Zhang, Q. , Ye, D. , Zhang, J. , Guo, Y. , You, R. et al. (2017) Fabrication and characterization of electrospun PCL/Antheraea pernyisilk fibroin nanofibrous scaffolds. Polymer Engineering & Science, 57, 206–213. [Google Scholar]
- Lu, Q. , Pandya, M. , Rufaihah, A.J. , Rosa, V. , Tong, H.J. , Seliktar, D. et al. (2015) Modulation of dental pulp stem cell odontogenesis in a tunable PEG‐fibrinogen hydrogel system. Stem Cells International, 2015, 525367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. , Yuan, B. , Qin, C. , Cao, Z. , Xie, Y. , Dallas, S.L. et al. (2011) The biological function of DMP‐1 in osteocyte maturation is mediated by its 57‐kDa c‐terminal fragment. Journal of Bone and Mineral Research, 26, 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui, J.N. , Lim, W.Y. & Ricucci, D. (2020) An immunofluorescence study to analyze wound healing outcomes of regenerative endodontics in an immature premolar with chronic apical abscess. Journal of Endodontics, 46, 627–640. [DOI] [PubMed] [Google Scholar]
- Madanagopal, T.T. , Franco‐Obregón, A. & Rosa, V. (2020) Comparative study of xeno‐free induction protocols for neural differentiation of human dental pulp stem cells in vitro. Archives of Oral Biology, 109, 104572. [DOI] [PubMed] [Google Scholar]
- Malik, Z. , Roth, D.M. , Eaton, F. , Theodor, J.M. & Graf, D. (2020) Mesenchymal Bmp7 controls onset of tooth mineralization: a novel way to regulate molar cusp shape. Frontiers in Physiology, 11, 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangione, F. , Salmon, B. , EzEldeen, M. , Jacobs, R. , Chaussain, C. & Vital, S. (2021) Characteristics of large animal models for current cell‐based oral tissue regeneration. Tissue Engineering Part B Reviews. [E‐pub ahead of print]. 10.1089/ten.TEB.2020.0384 [DOI] [PubMed] [Google Scholar]
- Minic, S. , Florimond, M. , Sadoine, J. , Valot‐Salengro, A. , Chaussain, C. , Renard, E. et al. (2021) Evaluation of pulp repair after biodentine(TM) full pulpotomy in a rat molar model of pulpitis. Biomedicines, 9, 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura, M. , Gronthos, S. , Zhao, M. , Lu, B. , Fisher, L.W. , Robey, P.G. et al. (2003) SHED: Stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences, 100, 5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane, E.M. , Dong, Z. , Sedgley, C.M. , Hu, J.‐C. , Botero, T.M. , Holland, G.R. et al. (2008) Effects of VEGF and FGF2 on the revascularization of severed human dental pulps. Journal of Dental Research, 87, 1144–1148. [DOI] [PubMed] [Google Scholar]
- Muthusamy, S. , Kannan, S. , Lee, M. , Sanjairaj, V. , Lu, W.F. , Fuh, J.Y.H. et al. (2021) 3D bioprinting and microscale organization of vascularized tissue constructs using collagen‐based bioink. Biotechnology and Bioengineering, 118, 3150–3163. [DOI] [PubMed] [Google Scholar]
- Na, K. , Lee, M. , Shin, H.W. & Chung, S. (2017) In vitro nasal mucosa gland‐like structure formation on a chip. Lab on a Chip, 17, 1578–1584. [DOI] [PubMed] [Google Scholar]
- Nakao, K. , Morita, R. , Saji, Y. , Ishida, K. , Tomita, Y. , Ogawa, M. et al. (2007) The development of a bioengineered organ germ method. Nature Methods, 4, 227–230. [DOI] [PubMed] [Google Scholar]
- Nakashima, M. , Iohara, K. , Bottino, M.C. , Fouad, A.F. , Nor, J.E. & Huang, G.T. (2019) Animal models for stem cell‐based pulp regeneration: foundation for human clinical applications. Tissue Engineering Part B Reviews, 25, 100–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natu, V.P. , Dubey, N. , Loke, G.C.L. , Tan, T.S. , Ng, W.H. , Yong, C.W. et al. (2015) Bioactivity, physical and chemical properties of MTA mixed with propylene glycol. Journal of Applied Oral Science, 23, 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosrat, A. , Seifi, A. & Asgary, S. (2011) Regenerative endodontic treatment (revascularization) for necrotic immature permanent molars: a review and report of two cases with a new biomaterial. Journal of Endodontics, 37, 562–567. [DOI] [PubMed] [Google Scholar]
- Oh, M. , Zhang, Z. , Mantesso, A. , Oklejas, A.E. & Nör, J.E. (2020) Endothelial‐initiated crosstalk regulates dental pulp stem cell self‐renewal. Journal of Dental Research, 99, 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampaloni, F. , Reynaud, E.G. & Stelzer, E.H. (2007) The third dimension bridges the gap between cell culture and live tissue. Nature Reviews Molecular Cell Biology, 8, 839–845. [DOI] [PubMed] [Google Scholar]
- Piva, E. , Tarlé, S.A. , Nör, J.E. , Zou, D. , Hatfield, E. , Guinn, T. et al. (2017) Dental pulp tissue regeneration using dental pulp stem cells isolated and expanded in human serum. Journal of Endodontics, 43, 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, T. , Jing, J. , Ren, Y. , Ma, C. , Feng, J.Q. , Yu, Q. et al. (2015) Complete pulpodentin complex regeneration by modulating the stiffness of biomimetic matrix. Acta Biomaterialia, 16, 60–70. [DOI] [PubMed] [Google Scholar]
- Rahimi, C. , Rahimi, B. , Padova, D. , Rooholghodos, S.A. , Bienek, D.R. , Luo, X. et al. (2018) Oral mucosa‐on‐a‐chip to assess layer‐specific responses to bacteria and dental materials. Biomicrofluidics, 12, 054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa, V. , Della Bona, A. , Cavalcanti, B.N. & Nör, J.E. (2012) Tissue engineering: from research to dental clinics. Dental Materials, 28, 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa, V. , Zhang, Z. , Grande, R.H.M. & Nör, J.E. (2013) Dental pulp tissue engineering in full‐length human root canals. Journal of Dental Research, 92, 970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh, J. , Ghassibe‐Sabbagh, M. , Fayyad‐Kazan, M. , Al‐Nemer, F. , Fahed, J.C. , Berberi, A. et al. (2020) Differences in osteogenic and odontogenic differentiation potential of DPSCs and SHED. Journal of Dentistry, 101, 103413. [DOI] [PubMed] [Google Scholar]
- Sakai, V.T. , Cordeiro, M.M. , Dong, Z. , Zhang, Z. , Zeitlin, B.D. & Nör, J.E. (2011) Tooth slice/scaffold model of dental pulp tissue engineering. Advances in Dental Research, 23, 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai, V.T. , Zhang, Z. , Dong, Z. , Neiva, K.G. , Machado, M. , Shi, S. et al. (2010) SHED differentiate into functional odontoblasts and endothelium. Journal of Dental Research, 89, 791–796. [DOI] [PubMed] [Google Scholar]
- Schmalz, G. , Schuster, U. , Nuetzel, K. & Schweikl, H. (1999) An in vitro pulp chamber with three‐dimensional cell cultures. Journal of Endodontics, 25, 24–29. [DOI] [PubMed] [Google Scholar]
- Schmalz, G. , Widbiller, M. & Galler, K.M. (2020) Clinical perspectives of pulp regeneration. Journal of Endodontics, 46, S161–S174. [DOI] [PubMed] [Google Scholar]
- Sengün, A. , Yalçın, M. , Ülker, H.E. , Öztürk, B. & Hakkı, S.S. (2011) Cytotoxicity evaluation of dentin bonding agents by dentin barrier test on 3‐dimensional pulp cells. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics, 112, e83–e88. [DOI] [PubMed] [Google Scholar]
- Smith, E.E. , Zhang, W. , Schiele, N.R. , Khademhosseini, A. , Kuo, C.K. & Yelick, P.C. (2017) Developing a biomimetic tooth bud model. Journal of Tissue Engineering and Regenerative Medicine, 11, 3326–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares, D.G. , Bordini, E. , Bronze‐Uhle, E.S. , Cassiano, F.B. , Silva, I. , Gallinari, M.O. et al. (2021) Chitosan‐calcium‐simvastatin scaffold as an inductive cell‐free platform. Journal of Dental Research, 100, 1118–1126. [DOI] [PubMed] [Google Scholar]
- Song, Y. , Uchida, H. , Sharipol, A. , Piraino, L. , Mereness, J.A. , Ingalls, M.H. et al. (2021) Development of a functional salivary gland tissue chip with potential for high‐content drug screening. Communications Biology, 4, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram, G. , Alberti, M. , Dancik, Y. , Wu, B. , Wu, R. , Feng, Z. et al. (2018) Full‐thickness human skin‐on‐chip with enhanced epidermal morphogenesis and barrier function. Materials Today, 21, 326–340. [Google Scholar]
- Sriram, G. , Muniraj, G. , Alberti, M. , Cao, T. , Wu, R. & Wang, Z. (2019) Organ‐on‐a‐chip device for culture and testing of skin and oral mucosa equivalents. Journal of Dental Research, 98, 3388. [Google Scholar]
- Sriram, G. , Sudhaharan, T. & Wright, G.D. (2020) Multiphoton microscopy for noninvasive and label‐free imaging of human skin and oral mucosa equivalents. Methods in Molecular Biology, 2150, 195–212. [DOI] [PubMed] [Google Scholar]
- Torabinejad, M. , Corr, R. , Buhrley, M. , Wright, K. & Shabahang, S. (2011) An animal model to study regenerative endodontics. Journal of Endodontics, 37, 197–202. [DOI] [PubMed] [Google Scholar]
- Torabinejad, M. , Nosrat, A. , Verma, P. & Udochukwu, O. (2017) Regenerative endodontic treatment or mineral trioxide aggregate apical plug in teeth with necrotic pulps and open apices: a systematic review and meta‐analysis. Journal of Endodontics, 43, 1806–1820. [DOI] [PubMed] [Google Scholar]
- Tziafas, D. (2019) Characterization of odontoblast‐like cell phenotype and reparative dentin formation in vivo: a comprehensive literature review. Journal of Endodontics, 45, 241–249. [DOI] [PubMed] [Google Scholar]
- Wang, W. , Yan, Y. , Li, C.W. , Xia, H.M. , Chao, S.S. , Wang, D.Y. et al. (2014) Live human nasal epithelial cells (hNECs) on chip for in vitro testing of gaseous formaldehyde toxicity via airway delivery. Lab on a Chip, 14, 677–680. [DOI] [PubMed] [Google Scholar]
- Wang, W. , Yuan, C. , Liu, Z. , Geng, T. , Li, X. , Wei, L. et al. (2020) Characteristic comparison between canine and human dental mesenchymal stem cells for periodontal regeneration research in preclinical animal studies. Tissue and Cell, 67, 101405. [DOI] [PubMed] [Google Scholar]
- Widbiller, M. , Driesen, R.B. , Eidt, A. , Lambrichts, I. , Hiller, K.‐A. , Buchalla, W. et al. (2018) Cell homing for pulp tissue engineering with endogenous dentin matrix proteins. Journal of Endodontics, 44, 956–962. [DOI] [PubMed] [Google Scholar]
- Xie, H. , Dubey, N. , Shim, W. , Ramachandra, C. , Min, K.S. , Cao, T. et al. (2018) Functional odontoblastic‐like cells derived from human iPSCs. Journal of Dental Research, 97, 77–83. [DOI] [PubMed] [Google Scholar]
- Zhang, W. , Vazquez, B. , Oreadi, D. & Yelick, P.C. (2017) Decellularized tooth bud scaffolds for tooth regeneration. Journal of Dental Research, 96, 516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X. , Liu, J. , Yu, Z. , Chen, C.A. , Aksel, H. , Azim, A.A. et al. (2018) A miniature swine model for stem cell‐based de novo regeneration of dental pulp and dentin‐like tissue. Tissue Engineering Part C: Methods, 24, 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]


