Abstract
Micronutrients, namely, vitamins and minerals, are necessary for the proper functioning of the human body, and their deficiencies can have dramatic short‐ and long‐term health consequences. Among the underlying causes, certainly a reduced dietary intake and/or poor absorption in the gastrointestinal tract play a key role in decreasing their bioavailability. Recent evidence from clinical and in vivo studies suggests an increasingly important contribution from the gut microbiome. Commensal microorganisms can in fact regulate the levels of micronutrients, both by intervening in the biosynthetic processes and by modulating their absorption. This short narrative review addresses the pivotal role of the gut microbiome in influencing the bioavailability of vitamins (such as A, B, C, D, E, and K) and minerals (calcium, iron, zinc, magnesium, and phosphorous), as well as the impact of these micronutrients on microbiome composition and functionality. Personalized microbiome‐based intervention strategies could therefore constitute an innovative tool to counteract micronutrient deficiencies by modulating the gut microbiome toward an eubiotic configuration capable of satisfying the needs of our organism, while promoting general health.
Keywords: absorption, biosynthesis, deficiency, gut microbiome, micronutrients, minerals, vitamins
Abbreviations
- DAP
1,3‐diaminopropane
- SCFAs
short‐chain fatty acids
1. INTRODUCTION
Micronutrients include organic and inorganic elements and compounds, such as minerals and vitamins, which are essential for the maintenance of host health and are not used for energy balance. Such micronutrients are commonly found in foods and dietary supplements, and are crucial for the regulation of biosynthetic cellular reactions, for example, those involved in immune and energy functions, 1 as well as in biological processes such as growth, bone health, and fluid balance. 2 Inadequate levels of micronutrients, resulting from reduced intake and/or poor absorption, are known to lead to specific micronutrient deficiency diseases, which represent a major global health concern. 3 , 4 Micronutrient deficiencies can also aggravate infections and noncommunicable chronic diseases, such as osteoporosis, hypothyroidism, cardiovascular disease and cancer, with a potentially dramatic impact on quality of life, morbidity and mortality. 3 , 5 , 6 For example, in children, vitamin D deficiency leads to poor physical and mental development, and contributes to the onset of inflammatory diseases and allergies. 7 , 8 Vitamin D deficiency may contribute to unbalanced immune responses even in adults, resulting in a higher incidence and progression of autoimmune diseases. 7 , 9 Among the most widespread intervention strategies to improve the micronutrient status thus reducing the disease burden, large‐scale fortification and public health programs must certainly be mentioned. Nonetheless, a growing body of evidence has highlighted some concerns about the negative consequences of supplementing certain micronutrients. 4 , 10 Oral iron supplementation has been linked to an increased incidence of constipation, gastric irritation, nausea, and metallic taste. 11 For infants and young children, the consumption of iron‐fortified foods has been associated with decreased growth, 12 impaired cognitive development, 13 , 14 and increased diarrhea probably due to altered to increased levels of intestinal pathogenic bacteria (e.g., Escherichia coli). 15 , 16 In addition, excessive vitamin A intake has been linked to increased bone fracture in both men and women. 17 , 18 Furthermore, despite supplementation, some deficiencies, such as those of iron, vitamin A, and zinc, have been shown to persist in certain individuals, underlining the involvement of other determinants of bioavailability. 4
The gut microbiome, that is, the community of trillions of microorganisms that inhabit our gastrointestinal tract, is known to interact with dietary compounds in a bidirectional way, being impacted in its compositional and functional structure, as well as influencing their metabolism and absorption, therefore their bioavailability. 19 , 20 , 21 As regards specifically micronutrients, these can modulate the diversity and composition of the gut microbiome, leading to beneficial or vice versa detrimental outcomes for the host health. 22 , 23 On the other hand, commensal bacteria in the human gut are able to influence nutrient absorption 24 and to synthesize essential vitamins (e.g., vitamin K and biotin, among others), 25 thus having a potentially considerable impact on the micronutrient status.
In this short narrative review, our aim is to provide up‐to‐date evidence on the two‐way interaction between gut microorganisms and micronutrients. In particular, we summarize the available in vivo and clinical studies dealing with the gut microbiome role in influencing micronutrient bioavailability and the modulation of the microbiome by micronutrients. We will also provide some glimpses on the modulation of the gut microbiome to improve the host micronutrient status. An increased understanding of the underlying mechanisms may indeed pave the way for the development of microbiome‐based intervention strategies to reduce micronutrient imbalances and related diseases, thereby limiting unwanted supplementation and improving overall human health.
2. GUT MICROBIOME–MICRONUTRIENT INTERACTIONS
Microbes utilize micronutrients for their growth and biological functioning. It is therefore not surprising that the intake of micronutrients can influence the compositional and functional structure of the gut microbiome. For example, dietary supplementation with vitamin B, C, D, and E largely contributes to the microbiome composition, by favoring the expansion and colonization of the intestinal mucosa by beneficial genera such as Bifidobacterium, Lactobacillus, and Roseburia. 26 , 27 , 28 , 29 Minerals such as calcium, iron, zinc, magnesium, and phosphorous can also shape the human gut microbiome. 21 , 22 In particular, high calcium intake has been associated with a higher proportion of Clostridium cluster XVIII in men, 30 iron supplementation could induce a depletion of Bifidobacterium and an increase in Lactobacillus levels in children, 31 and phosphorous supplementation leads to an increase in microbial diversity and levels of short‐chain fatty acids (SCFAs) in stool. 30 On the other hand, it has been shown that various members that make up the intestinal microbial ecosystem can influence the bioavailability of micronutrients, by controlling their absorption, particularly phosphorous and calcium, 32 and synthesizing vitamins, such as vitamin K and the water‐soluble fraction of B vitamins. 33
Below we will discuss the most recent in vivo and clinical studies on the bidirectional interaction between gut commensals and micronutrients, that is, minerals (calcium, iron, zinc, magnesium, and phosphorous) and vitamins (A, B, C, D, E, and K). Figure 1 shows the summary of microbiome–micronutrient interactions in the gut.
FIGURE 1.
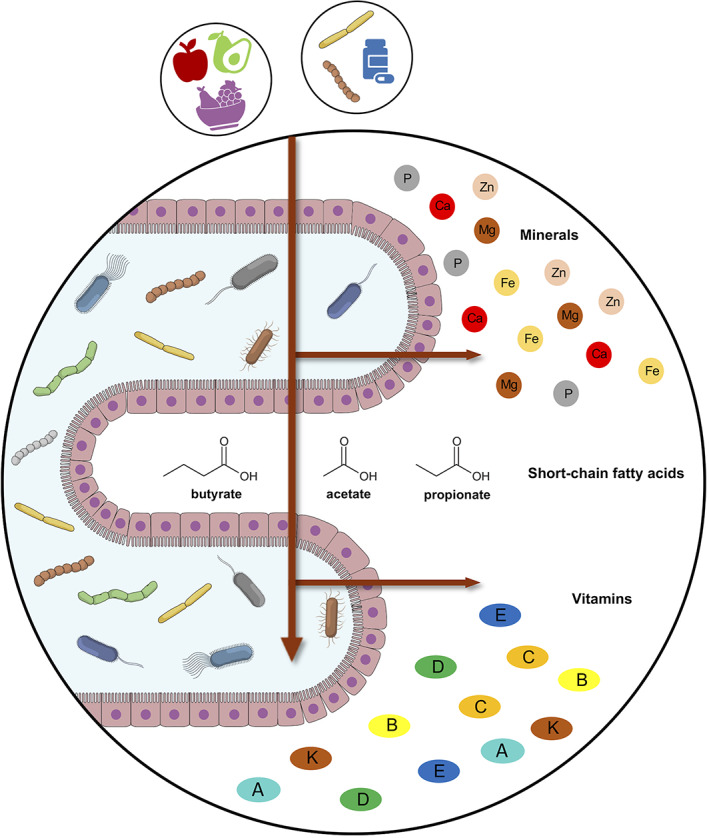
Micronutrient interchange between the gut microbiome and the host. Micronutrients and the gut microbiome interact along a bidirectional axis. Dietary micronutrients can in fact affect the composition and functionality of the microbiome, and the latter can influence the bioavailability of micronutrients, for example, by synthesizing vitamins and controlling the absorption rate. In particular, the absorption seems to be favored by the generation of short‐chain fatty acids, end‐products of the microbial fermentation of polysaccharides. Microbiome‐based intervention strategies, such as prebiotics, probiotics, and postbiotics, could therefore be the key to improving deficiency conditions, thereby limiting potentially harmful supplementation practices
2.1. Minerals
The human gut microbiome comprises several bacteria that possess the ability to affect the host mineral status, both through the synthesis of a wide range of enzymes involved in the release of minerals from dietary sources, and by directly influencing the absorption rate at the gastrointestinal level. 21
Among bacterial enzymes, phytases are capable of releasing bioavailable forms of inorganic minerals, such as calcium, magnesium, iron, and phosphorous, following the hydrolysis of phytic acid present in many plant‐based foods consumed with the diet. 34 Furthermore, a correlation was observed between the relative abundance of SCFA‐producing members of the gut microbiome and the calcium absorption rate in in vivo and clinical studies, as well as with bone mineral density in animal models. 35 , 36 SCFAs (microbial metabolites resulting from polysaccharide fermentation) actually lower the pH of the gastrointestinal tract, facilitating the solubility of calcium and consequently enhancing its transepithelial transport, as demonstrated in vitro with rat cecum and colon preparations. 37 In addition to calcium, Wang et al. recently observed gut microbiome‐dependent amelioration in bone phosphorous content, following oral administration of the probiotic Enterococcus faecium to broilers. 38 Again, the effect was likely attributable to the production of SCFAs, which increase mineral solubilization by lowering the pH and could interact directly with the skeleton (increasing bone mass and preventing bone loss), 39 as well as an increased phytase activity of the microbiome. Calcium and phosphate form amorphous calcium complexes in the small intestine, allowing both bile and fatty acids to bind, 40 , 41 and affecting the composition of the gut microbiome. 41 , 42 Among the major effects, an increase in butyrate and acetate producers such as Clostridium spp. and consequently fecal levels of SCFAs have been observed in a recent clinical trial following oral administration of phosphorous and calcium. 43 However, it is still unclear whether the above associations result from calcium, phosphorous or a combination of the two minerals. 30
Clinical trials focusing on the administration of iron‐containing micronutrient powders to infants at higher risk of diarrhea and respiratory tract infections, have shown that iron supplementation negatively modulates microbiome composition, resulting in decreased relative abundance of health‐associated taxa (i.e., bifidobacteria and lactobacilli), increased proportions of pathogens, and higher levels of plasma intestinal fatty acid‐binding protein, which acts as a biomarker of enterocyte damage. 15 , 16 , 44 , 45 Iron is indeed known to promote the growth and virulence of various enteropathogens, for example, by increasing the production of toxic metabolites or by inducing the biosynthesis of flagella, thus facilitating colonization and invasion of the epithelia, and contributing to an overall proinflammatory state. 46 On the other hand, similarly to what has been discussed above, iron absorption in the intestine is influenced by microbial metabolites, more precisely promoted by SCFAs, 47 , 48 while hindered by 1,3‐diaminopropane and the antimicrobial reuterin (3‐hydroxypropionaldehyde) produced by Lactobacillus reuteri. 49
Low zinc levels represent a significant risk factor for poor health outcomes, with women and children being the most vulnerable targets. 4 , 50 , 51 In vivo studies have shown that zinc deficiency alters the gut microbiome composition, with a decrease in biodiversity, an increase in inflammatory markers and an impairment of the functional potential involved in gut–brain signaling. 52 , 53 As demonstrated in an in vitro digestion model, the gut microbiome could negatively affect zinc bioaccessibility by reducing the dissolution of food‐derived zinc in the colon. 54 It is therefore not surprising that clinical trials on probiotic supplementation for the treatment of zinc deficiency have shown conflicting results. In particular, the first available clinical trial showed no significant changes in mineral bioavailability following probiotic supplementation with Lactobacillus casei and L. reuteri in pediatric cohorts. 55 In contrast, the second clinical trial achieved a significant increase in zinc levels after Lactobacillus plantarum and zinc supplementation, only when the two supplements were administered simultaneously. 56 Despite the small size of the enrolled pediatric cohort, a recent clinical trial by Ballini et al. 57 showed that supplementation with a synbiotic containing the probiotic species L. plantarum, Lactobacillus acidophilus, Bifidobacterium infantis, and Bifidobacterium lactis, in combination with the prebiotic fructooligosaccharide, could be useful for raising blood zinc levels. 57
Finally, several studies in animal models have pointed out associations between magnesium bioavailability and gut microbiome composition. 58 , 59 , 60 The few available studies in humans suggest that gut commensals and probiotic strains belonging to Lactobacillus spp. are effective in increasing the bioavailability of magnesium after cheese and vegetable milk consumption. 61 , 62 , 63 On the other hand, magnesium has been shown to exert a positive impact on the gut microbiome composition, as well as on the metabolism of vitamins B1 and D, in patients with metabolic syndrome, type 2 diabetes and obesity. 64
2.2. Vitamins
Some bacterial genera normally adapted to the human gastrointestinal tract niche, including, Bifidobacterium, Bacteroides, and Enterococcus, are well‐known to biosynthesize vitamin K and those of group B, more precisely the water‐soluble forms of the latter. 23 , 65 , 66 Furthermore, as reported for minerals, the gut microbiome can affect vitamin absorption rates as well as be affected by dietary supplementation, as will be detailed in this section.
With specific regard to B vitamins, in a systematic review, Magnusdottir et al. evaluated in silico the potential of 256 common human gut commensals to produce biotin, cobalamin, folate, niacin, pantothenate, pyridoxine, riboflavin, and thiamine. 67 According to them, human gut microorganisms produce 40 to 65% of these vitamins, supporting host‐microbiome coevolution. Nevertheless, in order to be fully available to the host, the de novo biosynthesis of vitamins must necessarily take place upstream of their absorption site. In this perspective, since vitamin B12 can only be absorbed at the level of the ileum, cobalamin‐producing microorganisms in the large intestine are unlikely to contribute to its actual bioavailability. 68 On the other hand, dietary supplementation of B vitamins has been associated with changes in the diversity and composition of the gut microbiome. For example, vitamin B3 supplementation has been correlated with the expansion of Bacteroidetes, along with improved biomarkers of metabolic inflammation and insulin sensitivity. 69 In addition, clinical trials focusing on the oral administration of vitamin B2 have shown the enrichment of butyrate producers such as Faecalibacterium and Roseburia in healthy individuals, 70 as well as the depletion of Enterobacteriaceae in patients with inflammatory bowel disease. 71 However, in a second clinical trial, Pham et al. did not replicate the increase in Faecalibacterium, but still observed an increase in diversity, 72 reinforcing the hypothesis of a modulatory potential of B vitamins on the human gut microbiome.
Using a model of intestinal enteroids treated with lipopolysaccharide, Subramanian et al. have shown that the gut microbiome may also negatively affect the absorption rate of vitamin C, by downregulating the transcription of sodium‐dependent vitamin C transporters. 73 On the other hand, vitamin C supplementation has been shown to significantly increase the microbial ecosystem biodiversity, along with the relative abundance of Collinsella and fecal levels of SCFAs, particularly butyrate and propionate, when compared to the placebo group. 72 An in vitro association has also been observed with several health‐promoting taxa (i.e., Roseburia, Faecalibacterium, Akkermansia, and Bifidobacterium), but the trend has not yet been confirmed in humans. 72
Although fat‐soluble vitamins are not naturally produced by gut commensals, significant associations have been found between this subgroup of vitamins and the gut microbiome. For instance, in a large‐scale clinical study focusing on the host micronutrient status from the microbiome point of view, serum vitamin D levels were positively correlated with several members of the Firmicutes phylum, such as Ruminococcus, Coprococcus, Mogibacterium, and Blautia. 72 , 74 The modulatory effects of vitamin D are likely due to the control of the expression of antimicrobial peptides and the protective effects on the gut mucosa, that is, the maintenance of barrier integrity and epithelial healing. 75 , 76 , 77 In addition, a previous clinical study by Jones et al. highlighted a relationship between vitamin D and L. reuteri, probably mediated by the metabolism of bile acids, with important therapeutic repercussions given the widespread use of this microorganism as a probiotic. 78 Finally, the dietary intake of vitamin E has been positively associated with SCFA production, as well as the relative abundance of Akkermansia and other health‐promoting taxa such as Lactobacillus, Bifidobacterium, and Faecalibacterium. 79 On the other hand, other in vivo studies have found a negative association between the gut microbiome and the bioavailability of vitamin E. For instance, Ran et al. reported an increase in the absorption rate of vitamin E after antibiotic treatment in a mouse model, suggesting vitamin degradation by intestinal commensals. 80
3. MICROBIOME‐BASED INTERVENTION STRATEGIES TO IMPROVE THE HOST MICRONUTRIENT STATUS
The aforementioned literature on the bidirectional gut microbiome–micronutrient axis strongly suggests that personalized microbiome‐based interventions could be a promising tool for improving host micronutrient status. Tailored interventions aimed at restoring/maintaining a eubiotic microbiome profile, capable of producing vitamins and SCFAs while being low in lipopolysaccharides, could be instrumental to ensure adequate biosynthesis and absorption of micronutrients, for optimal bioavailability for the host. For instance, prebiotic galactooligosaccharides mitigated the adverse effects of iron supplementation on the infant gut, 81 while the administration of L. reuteri in combination with the laxative magnesium oxide ameliorated chronic constipation in children without causing imbalances in the microbiome. 82
Further clinical studies that integrate multiomics approaches are needed to identify the bacterial species involved and assess their actual contribution to the micronutrient balance, hence their role in preventing and/or ameliorating any deficiencies. By elucidating the mechanisms underlying the relationship between our microbial counterpart and micronutrient bioavailability, postbiotic strategies could also be developed to broaden the range of precision personalized interventions aimed at tackling micronutrient deficiencies and related disorders.
4. CONCLUDING REMARKS
The gut microbiome can variously influence the bioavailability of micronutrients, as well as be influenced by micronutrient supplementation, with implications for health even in the long term. Although several mechanisms have been advanced, a thorough characterization of the microbiome–micronutrient bidirectional axis is of utmost importance, as it can guide the design of microbiome‐based precision intervention strategies, aimed at improving micronutrient status and overall health.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Bologna within the CRUI‐CARE Agreement.
Barone M, D'Amico F, Brigidi P, Turroni S. Gut microbiome–micronutrient interaction: The key to controlling the bioavailability of minerals and vitamins? BioFactors. 2022;48:307–314. 10.1002/biof.1835
DATA AVAILABILITY STATEMENT
Data sharing not applicable ‐ no new data generated, or the article describes entirely theoretical research.
REFERENCES
- 1. Yoshii K, Hosomi K, Sawane K, Kunisawa J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front Nutr. 2019;6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huskisson E, Maggini S, Ruf M. The role of vitamins and minerals in energy metabolism and well‐being. J Int Med Res. 2007;35:277–89. [DOI] [PubMed] [Google Scholar]
- 3. Rautiainen S, Manson JE, Lichtenstein AH, Sesso HD. Dietary supplements and disease prevention ‐ a global overview. Nat Rev Endocrinol. 2016;12:407–20. [DOI] [PubMed] [Google Scholar]
- 4. Keats EC, Neufeld LM, Garrett GS, Mbuya MNN, Bhutta ZA. Improved micronutrient status and health outcomes in low‐ and middle‐income countries following large‐scale fortification: evidence from a systematic review and meta‐analysis. Am J Clin Nutr. 2019;109:1696–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson IT. Micronutrients and cancer. Proc Nutr Soc. 2004;63:587–95. [DOI] [PubMed] [Google Scholar]
- 6. Diaz JR, de las Cagigas A, Rodriguez R. Micronutrient deficiencies in developing and affluent countries. Eur J Clin Nutr. 2003;57(suppl 1):S70–2. [DOI] [PubMed] [Google Scholar]
- 7. Clark A, Mach N. Role of vitamin D in the hygiene hypothesis: the interplay between vitamin D, vitamin D receptors, gut microbiota, and immune response. Front Immunol. 2016;7:627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Noriega DB, Savelkoul HFJ. Vitamin D and allergy susceptibility during gestation and early life. Nutrients. 2021;13:1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baye K. Maximising benefits and minimising adverse effects of micronutrient interventions in low‐ and middle‐income countries. Proc Nutr Soc. 2019;78:540–6. [DOI] [PubMed] [Google Scholar]
- 11. Auerbach M, Schrier S. Treatment of iron deficiency is getting trendy. Lancet Haematol. 2017;4:e500–1. [DOI] [PubMed] [Google Scholar]
- 12. Lonnerdal B. Excess iron intake as a factor in growth, infections, and development of infants and young children. Am J Clin Nutr. 2017;106:1681S–7S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wessling‐Resnick M. Excess iron: considerations related to development and early growth. Am J Clin Nutr. 2017;106:1600S–5S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hare DJ, Arora M, Jenkins NL, Finkelstein DI, Doble PA, Bush AI. Is early‐life iron exposure critical in neurodegeneration? Nat Rev Neurol. 2015;11:536–44. [DOI] [PubMed] [Google Scholar]
- 15. Paganini D, Zimmermann MB. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: a review. Am J Clin Nutr. 2017;106:1688S–93S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jaeggi T, Kortman GA, Moretti D, Chassard C, Holding P, Dostal A, et al. Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut. 2015;64:731–42. [DOI] [PubMed] [Google Scholar]
- 17. Michaelsson K, Lithell H, Vessby B, Melhus H. Serum retinol levels and the risk of fracture. N Engl J Med. 2003;348:287–94. [DOI] [PubMed] [Google Scholar]
- 18. Feskanich D, Singh V, Willett WC, Colditz GA. Vitamin A intake and hip fractures among postmenopausal women. JAMA. 2002;287:47–54. [DOI] [PubMed] [Google Scholar]
- 19. Turroni S, Brigidi P, Cavalli A, Candela M. Microbiota‐host transgenomic metabolism, bioactive molecules from the inside. J Med Chem. 2018;61:47–61. [DOI] [PubMed] [Google Scholar]
- 20. Hadadi N, Berweiler V, Wang H, Trajkovski M. Intestinal microbiota as a route for micronutrient bioavailability. Curr Opin Endocr Metab Res. 2021;20:100285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bielik V, Kolisek M. Bioaccessibility and bioavailability of minerals in relation to a healthy gut microbiome. Int J Mol Sci. 2021;22:6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang Q, Liang Q, Balakrishnan B, Belobrajdic DP, Feng QJ, Zhang W. Role of dietary nutrients in the modulation of gut microbiota: a narrative review. Nutrients. 2020;12:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Biesalski HK. Nutrition meets the microbiome: micronutrients and the microbiota. Ann N Y Acad Sci. 2016;1372:53–64. [DOI] [PubMed] [Google Scholar]
- 24. Krajmalnik‐Brown R, Ilhan ZE, Kang DW, DiBaise JK. Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract. 2012;27:201–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24:160–8. [DOI] [PubMed] [Google Scholar]
- 26. Lv Z, Wang Y, Yang T, Zhan X, Li Z, Hu H, et al. Vitamin A deficiency impacts the structural segregation of gut microbiota in children with persistent diarrhea. J Clin Biochem Nutr. 2016;59:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li L, Somerset S. Associations between flavonoid intakes and gut microbiota in a group of adults with cystic fibrosis. Nutrients. 2018;10:1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanhere M, He J, Chassaing B, Ziegler TR, Alvarez JA, Ivie EA, et al. Bolus weekly vitamin D3 supplementation impacts gut and airway microbiota in adults with cystic fibrosis: a double‐blind, randomized, placebo‐controlled clinical trial. J Clin Endocrinol Metab. 2018;103:564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Degnan PH, Barry NA, Mok KC, Taga ME, Goodman AL. Human gut microbes use multiple transporters to distinguish vitamin B12 analogs and compete in the gut. Cell Host Microbe. 2014;15:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trautvetter U, Camarinha‐Silva A, Jahreis G, Lorkowski S, Glei M. High phosphorus intake and gut‐related parameters—results of a randomized placebo‐controlled human intervention study. Nutr J. 2018;17:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sjödin KS, Domellof M, Lagerqvist C, Hernell O, Lonnerdal B, Szymlek‐Gay EA, et al. Administration of ferrous sulfate drops has significant effects on the gut microbiota of iron‐sufficient infants: a randomised controlled study. Gut. 2019;68:2095–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gonzalez A, Galvez N, Martin J, Reyes F, Perez‐Victoria I, Dominguez‐Vera JM. Identification of the key excreted molecule by Lactobacillus fermentum related to host iron absorption. Food Chem. 2017;228:374–80. [DOI] [PubMed] [Google Scholar]
- 33. Engevik MA, Morra CN, Roth D, Engevik K, Spinler JK, Devaraj S, et al. Microbial metabolic capacity for intestinal Folate production and modulation of host folate receptors. Front Microbiol. 2019;10:2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bohn L, Josefsen L, Meyer AS, Rasmussen SK. Quantitative analysis of phytate globoids isolated from wheat bran and characterization of their sequential dephosphorylation by wheat phytase. J Agric Food Chem. 2007;55:7547–52. [DOI] [PubMed] [Google Scholar]
- 35. Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporos Rep. 2015;13:125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Asemi Z, Esmaillzadeh A. Effect of daily consumption of probiotic yoghurt on serum levels of calcium, iron and liver enzymes in pregnant women. Int J Prev Med. 2013;4:949–55. [PMC free article] [PubMed] [Google Scholar]
- 37. Mineo H, Hara H, Tomita F. Short‐chain fatty acids enhance diffusional ca transport in the epithelium of the rat cecum and colon. Life Sci. 2001;69:517–26. [DOI] [PubMed] [Google Scholar]
- 38. Wang W, Cai H, Zhang A, Chen Z, Chang W, Liu G, et al. Enterococcus faecium modulates the gut microbiota of broilers and enhances phosphorus absorption and utilization. Animals. 2020;10:1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lucas S, Omata Y, Hofmann J, Bottcher M, Iljazovic A, Sarter K, et al. Short‐chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat Commun. 2018;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van der Meer R, Lapre JA, Govers MJ, Kleibeuker JH. Mechanisms of the intestinal effects of dietary fats and milk products on colon carcinogenesis. Cancer Lett. 1997;114:75–83. [DOI] [PubMed] [Google Scholar]
- 41. Ditscheid B, Keller S, Jahreis G. Cholesterol metabolism is affected by calcium phosphate supplementation in humans. J Nutr. 2005;135:1678–82. [DOI] [PubMed] [Google Scholar]
- 42. Trautvetter U, Ditscheid B, Kiehntopf M, Jahreis G. A combination of calcium phosphate and probiotics beneficially influences intestinal lactobacilli and cholesterol metabolism in humans. Clin Nutr. 2012;31:230–7. [DOI] [PubMed] [Google Scholar]
- 43. Koh A, De Vadder F, Kovatcheva‐Datchary P, Backhed F. From dietary fiber to host physiology: short‐chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–45. [DOI] [PubMed] [Google Scholar]
- 44. Zimmermann MB, Chassard C, Rohner F, N'Goran EK, Nindjin C, Dostal A, et al. The effects of iron fortification on the gut microbiota in African children: a randomized controlled trial in Cote d'Ivoire. Am J Clin Nutr. 2010;92:1406–15. [DOI] [PubMed] [Google Scholar]
- 45. Tang M, Frank DN, Hendricks AE, Ir D, Esamai F, Liechty E, et al. Iron in micronutrient powder promotes an unfavorable gut microbiota in Kenyan infants. Nutrients. 2017;9:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kortman GA, Raffatellu M, Swinkels DW, Tjalsma H. Nutritional iron turned inside out: intestinal stress from a gut microbial perspective. FEMS Microbiol Rev. 2014;38:1202–34. [DOI] [PubMed] [Google Scholar]
- 47. Yilmaz B, Li H. Gut microbiota and iron: the crucial actors in health and disease. Pharmaceuticals. 2018;11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bougle D, Vaghefi‐Vaezzadeh N, Roland N, Bouvard G, Arhan P, Bureau F, et al. Influence of short‐chain fatty acids on iron absorption by proximal colon. Scand J Gastroenterol. 2002;37:1008–11. [DOI] [PubMed] [Google Scholar]
- 49. Das NK, Schwartz AJ, Barthel G, Inohara N, Liu Q, Sankar A, et al. Microbial metabolite signaling is required for systemic iron homeostasis. Cell Metab. 2020;31:115–30.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wessells KR, Brown KH. Estimating the global prevalence of zinc deficiency: results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One. 2012;7:e50568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Engle‐Stone R, Nankap M, Ndjebayi AO, Allen LH, Shahab‐Ferdows S, Hampel D, et al. Iron, zinc, folate, and vitamin B‐12 status increased among women and children in Yaounde and Douala, Cameroon, 1 year after introducing fortified wheat flour. J Nutr. 2017;147:1426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sauer AK, Grabrucker AM. Zinc deficiency during pregnancy leads to altered microbiome and elevated inflammatory markers in mice. Front Neurosci. 2019;13:1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reed S, Neuman H, Moscovich S, Glahn RP, Koren O, Tako E. Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients. 2015;7:9768–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cai X, Chen X, Yin N, Du H, Sun G, Wang L, et al. Estimation of the bioaccessibility and bioavailability of Fe, Mn, Cu, and Zn in Chinese vegetables using the in vitro digestion/Caco‐2 cell model: the influence of gut microbiota. Food Funct. 2017;8:4592–600. [DOI] [PubMed] [Google Scholar]
- 55. Agustina R, Bovee‐Oudenhoven IM, Lukito W, Fahmida U, van de Rest O, Zimmermann MB, et al. Probiotics Lactobacillus reuteri DSM 17938 and Lactobacillus casei CRL 431 modestly increase growth, but not iron and zinc status, among Indonesian children aged 1‐6 years. J Nutr. 2013;143:1184–93. [DOI] [PubMed] [Google Scholar]
- 56. Surono IS, Martono PD, Kameo S, Suradji EW, Koyama H. Effect of probiotic L. plantarum IS‐10506 and zinc supplementation on humoral immune response and zinc status of Indonesian pre‐school children. J Trace Elem Med Biol. 2014;28:465–9. [DOI] [PubMed] [Google Scholar]
- 57. Ballini A, Gnoni A, De Vito D, Dipalma G, Cantore S, Gargiulo Isacco C, et al. Effect of probiotics on the occurrence of nutrition absorption capacities in healthy children: a randomized double‐blinded placebo‐controlled pilot study. Eur Rev Med Pharmacol Sci. 2019;23:8645–57. [DOI] [PubMed] [Google Scholar]
- 58. Zimowska W, Girardeau JP, Kuryszko J, Bayle D, Rayssiguier Y, Mazur A. Morphological and immune response alterations in the intestinal mucosa of the mouse after short periods on a low‐magnesium diet. Br J Nutr. 2002;88:515–22. [DOI] [PubMed] [Google Scholar]
- 59. Pyndt Jorgensen B, Winther G, Kihl P, Nielsen DS, Wegener G, Hansen AK, et al. Dietary magnesium deficiency affects gut microbiota and anxiety‐like behaviour in C57BL/6N mice. Acta Neuropsychiatr. 2015;27:307–11. [DOI] [PubMed] [Google Scholar]
- 60. Gommers LMM, Ederveen THA, van der Wijst J, Overmars‐Bos C, Kortman GAM, Boekhorst J, et al. Low gut microbiota diversity and dietary magnesium intake are associated with the development of PPI‐induced hypomagnesemia. FASEB J. 2019;33:11235–46. [DOI] [PubMed] [Google Scholar]
- 61. Rekha CR, Vijayalakshmi G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J Appl Microbiol. 2010;109:1198–208. [DOI] [PubMed] [Google Scholar]
- 62. Bergillos‐Meca T, Cabrera‐Vique C, Artacho R, Moreno‐Montoro M, Navarro‐Alarcon M, Olalla M, et al. Does Lactobacillus plantarum or ultrafiltration process improve Ca, Mg, Zn and P bioavailability from fermented goats' milk? Food Chem. 2015;187:314–21. [DOI] [PubMed] [Google Scholar]
- 63. Aljewicz M, Siemianowska E, Cichosz G, Tonska E. The effect of probiotics (Lactobacillus rhamnosus HN001, lactobacillus paracasei LPC‐37, and Lactobacillus acidophilus NCFM) on the availability of minerals from Dutch‐type cheese. J Dairy Sci. 2014;97:4824–31. [DOI] [PubMed] [Google Scholar]
- 64. Piuri G, Zocchi M, Porta MD, Ficara V, Manoni M, Zuccotti GV, et al. Magnesium in obesity, metabolic syndrome, and type 2 diabetes. Nutrients. 2021;13:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Soto‐Martin EC, Warnke I, Farquharson FM, Christodoulou M, Horgan G, Derrien M, et al. Vitamin biosynthesis by human gut butyrate‐producing bacteria and cross‐feeding in synthetic microbial communities. MBio. 2020;11:e00886‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Magnusdottir S, Ravcheev D, de Crecy‐Lagard V, Thiele I. Systematic genome assessment of B‐vitamin biosynthesis suggests co‐operation among gut microbes. Front Genet. 2015;6:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rowley CA, Kendall MM. To B12 or not to B12: five questions on the role of cobalamin in host‐microbial interactions. PLoS Pathog. 2019;15:e1007479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fangmann D, Theismann EM, Turk K, Schulte DM, Relling I, Hartmann K, et al. Targeted microbiome intervention by microencapsulated delayed‐release niacin beneficially affects insulin sensitivity in humans. Diabetes Care. 2018;41:398–405. [DOI] [PubMed] [Google Scholar]
- 70. Steinert RE, Sadaghian Sadabad M, Harmsen HJ, Weber P. The prebiotic concept and human health: a changing landscape with riboflavin as a novel prebiotic candidate? Eur J Clin Nutr. 2016;70:1461. [DOI] [PubMed] [Google Scholar]
- 71. von Martels JZH, Bourgonje AR, Klaassen MAY, Alkhalifah HAA, Sadaghian Sadabad M, Vich Vila A, et al. Riboflavin supplementation in patients with Crohn's disease [the RISE‐UP study]. J Crohns Colitis. 2020;14:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pham VT, Fehlbaum S, Seifert N, Richard N, Bruins MJ, Sybesma W, et al. Effects of colon‐targeted vitamins on the composition and metabolic activity of the human gut microbiome‐ a pilot study. Gut Microbes. 2021;13:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Subramanian VS, Sabui S, Moradi H, Marchant JS, Said HM. Inhibition of intestinal ascorbic acid uptake by lipopolysaccharide is mediated via transcriptional mechanisms. Biochim Biophys Acta Biomembr. 2018;1860:556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Thomas RL, Jiang L, Adams JS, Xu ZZ, Shen J, Janssen S, et al. Vitamin D metabolites and the gut microbiome in older men. Nat Commun. 2020;11:5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vernia F, Valvano M, Longo S, Cesaro N, Viscido A, Latella G. Vitamin D in inflammatory bowel diseases. Mechanisms of action and therapeutic implications. Nutrients. 2022;14:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Qiu F, Zhang Z, Yang L, Li R, Ma Y. Combined effect of vitamin C and vitamin D3 on intestinal epithelial barrier by regulating notch signaling pathway. Nutr Metab. 2021;18:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Akimbekov NS, Digel I, Sherelkhan DK, Lutfor AB, Razzaque MS. Vitamin D and the host‐gut microbiome: a brief overview. Acta Histochem Cytochem. 2020;53:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jones ML, Martoni CJ, Prakash S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25‐hydroxyvitamin D: a post hoc analysis of a randomized controlled trial. J Clin Endocrinol Metab. 2013;98:2944–51. [DOI] [PubMed] [Google Scholar]
- 79. Choi Y, Lee S, Kim S, Lee J, Ha J, Oh H, et al. Vitamin E (alpha‐tocopherol) consumption influences gut microbiota composition. Int J Food Sci Nutr. 2020;71:221–5. [DOI] [PubMed] [Google Scholar]
- 80. Ran L, Liu AB, Lee MJ, Xie P, Lin Y, Yang CS. Effects of antibiotics on degradation and bioavailability of different vitamin E forms in mice. Biofactors. 2019;45:450–62. [DOI] [PubMed] [Google Scholar]
- 81. Paganini D, Uyoga MA, Kortman GAM, Cercamondi CI, Moretti D, Barth‐Jaeggi T, et al. Prebiotic galacto‐oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: a randomised controlled study in Kenyan infants. Gut. 2017;66:1956–67. [DOI] [PubMed] [Google Scholar]
- 82. Kubota M, Ito K, Tomimoto K, Kanazaki M, Tsukiyama K, Kubota A, et al. Lactobacillus reuteri DSM 17938 and magnesium oxide in children with functional chronic constipation: a double‐blind and randomized clinical trial. Nutrients. 2020;12:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable ‐ no new data generated, or the article describes entirely theoretical research.


