Abstract
Dietary calcium deficiency is considered to be widespread globally, with published estimates suggesting that approximately half of the world's population has inadequate access to dietary calcium. Calcium is essential for bone health, but inadequate intakes have also been linked to other health outcomes, including pregnancy complications, cancers, and cardiovascular disease. Populations in low‐ and middle‐income countries (LMICs) are at greatest risk of low calcium intakes, although many individuals in high‐income countries (HICs) also do not meet recommendations. Paradoxically, many LMICs with lower calcium intakes show lower rates of osteoporotic fracture as compared with HICs, though data are sparse. Calcium intake recommendations vary across agencies and may need to be customized based on other dietary factors, health‐related behaviors, or the risk of calcium‐related health outcomes. The lack of standard methods to assess the calcium status of an individual or population has challenged efforts to estimate the prevalence of calcium deficiency and the global burden of related adverse health consequences. This paper aims to consolidate available evidence related to the global prevalence of inadequate calcium intakes and associated health outcomes, with the goal of providing a foundation for developing policies and population‐level interventions to safely improve calcium intake and status where necessary.
Keywords: calcium, calcium deficiency, osteoporosis, calcium paradox
This paper aims to consolidate available evidence related to the global prevalence of inadequate calcium intakes and associated health outcomes, with the goal of providing a foundation for developing policies and population‐level interventions to safely improve calcium intake and status where necessary.
Purpose
In March and April 2021, the Nutrition Science Program of the New York Academy of Sciences, in partnership with the Children's Investment Fund Foundation, convened a Calcium Task Force and hosted two virtual meetings. This task force is composed of experts in micronutrients, malnutrition, pediatrics, gynecology and obstetrics, biochemistry, public health, and strategies for supplementation and fortification. During these meetings, the task force assessed evidence on global calcium deficiency and its health consequences, calcium status indicators, calcium supplementation for pregnant women to improve pregnancy outcomes, and associated implementation challenges, as well as food‐based interventions to improve the intake of this vital micronutrient, especially in populations with low calcium intake. The group identified research gaps and provided guidance for interventions and policies based on available evidence. This paper describes discussions and conclusions on the global prevalence of inadequate calcium intakes and their related health outcomes.
Dietary calcium requirements
Calcium is an essential mineral with critical functions in the skeletal, cardiovascular, endocrine, and neurological systems. Approximately 99% of total body calcium is in bone, where it provides rigidity and structure to the skeletal system and acts as a calcium reservoir. The remaining fraction participates in metabolic processes, including vascular and muscle contraction, nervous system transmission, transmembrane transport, enzymatic activation, and hormonal function. The majority of studies of long‐term consequences of inadequate calcium intake are related to bone health, especially rickets in children and fractures, osteopenia, and osteoporosis in older adults. 1 , 2
A variety of methods have been used to estimate calcium requirements; most recent references for calcium intake were set for healthy individuals based on evidence for bone health outcomes (Table 1). The Institute of Medicine/National Academy of Medicine (IOM/NAM) dietary reference intake values for calcium in North America (United States and Canada) were published in 2011. Requirements were based on the effect of calcium intake on bone health, as evidence for the effect of calcium intake on cancer, cardiovascular disease, diabetes, and autoimmune disorders was too inconsistent to inform nutritional requirements. 3 , 4 However, future calcium panels may want to consider adding dietary reference intakes (i.e., for chronic disease risk reduction) to include some of the emerging data on calcium and these and other disease outcomes.
Table 1.
Dietary calcium recommended intakes by life stage and region
| Region/organization | Recommendation | Infants | Children | Adolescent males | Adolescent females | Men | Women | Men 50+ | Women 50+ | Pregnancy | Lactation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| United States/Canada (IOM) 5 | EAR (mg/day) | – | 500−800 | 1100 | 1100 | 800 | 800 | 800 | 1000 | No change | No change |
| Europe (EFSA) 6 | AR (mg/day) | – | 390−680 | 960 | 960 | 860−750 | 860−750 | 750 | 750 | No change | No change |
| United Kingdom (SACN) 95 | EAR (mg/day) | 400 | 275−425 | 750 | 625 | 525 | 525 | 525 | 525 | No change | +550 |
| India 7 | EAR (mg/day) | 300 (AI) | 400−650 | 800 | 800 | 800 | 800 | 800 | 1000 | No change | 1000 |
| Southeast Asia 96 | RDA (mg/day) | 300−400 | 500−700 | 1000 | 1000 | 700 | 700 | 1000 | 1000 | 1000 | 1000 |
| Taiwan 97 | DRI (mg/day) | – | – | – | – | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| Mexico 98 | mg/day | – | – | 1200 | 1200 | 800 | 800 | 800 | 800 | 1200 | 1200 |
| South Africa 99 | mg/day | – | – | 1000 | 1000 | 1000 | 1000 | 1000 | – | – | – |
| FAO/WHO 100 | EAR (mg/day) | 240−300 | 440 | 1040 | 1040 | 840 | 840 | 840 | 840 | 940 | 1040 |
AI, adequate intake; AR, average requirement; DRI, dietary reference intake; EAR, estimated average requirement; EFSA, European Food Safety Authority; FAO/WHO, Food and Agriculture Organization/World Health Organization; IOM, Institute of Medicine; RDA, recommended dietary allowance; SACN, Scientific Advisory Committee on Nutrition.
Current requirements are intended to maximize bone accretion during the growth periods of childhood and adolescence, and promote bone retention later in life, particularly among postmenopausal women. 5 Requirements peak in adolescence to support the period of rapid growth and then decrease in adulthood. Guidelines recommend calcium intakes between 700 and 1200 mg/day for individuals >19 years of age, with some authorities recommending an increase during lactation. Recommendations rise again for women following menopause and in populations >70 years of age, as calcium bioavailability decreases with age. 5 Average requirements established by the European Food Safety Authority (EFSA) in 2015 are generally slightly lower than those recommended by the IOM/NAM, and EFSA emphasized the inconsistency of the available evidence to support causal relationships between calcium intake and health outcomes, including bone health. 6 Some low‐ and middle‐income countries (LMICs) have calcium recommendations that are specific to their populations, and several agencies have reference values for calcium that were developed some time ago (Table 1). 7
The IOM/NAM established a tolerable upper intake level for calcium as the highest average daily intake likely to pose no risk of adverse effects for nearly all persons in the general population and it was set at a calcium intake of 2500 mg/day for adults 19–50 years of age and 2000 mg/day for adults >50 years of age. More recently, EFSA set an upper limit of 2500 mg/day for all adults, including during pregnancy and lactation, based on evidence from existing randomized controlled trials (RCTs) that showed an absence of adverse effects at the specified intake levels. IOM/NAM upper intake levels for children and adolescents range from 1000 mg/day (birth to 6 months) to 3000 mg/day (9–18 years). Conversely, EFSA did not establish a calcium upper limit for infants, children, and adolescents due to insufficient information for these age groups. Adverse effects of calcium supplementation have also been systematically investigated and are covered in greater detail elsewhere in this special issue. 8
Factors influencing calcium requirements
Dietary factors
Several nutrients are known to influence calcium requirements. Plant nutrient and antinutrient factor composition and soil properties (pH, water content, and microbial activity) in which foods are grown, vitamin D intake, and the presence of phytic acid and oxalic acid can all influence calcium available through the diet. 9 Phytates and oxalates inhibit calcium absorption as they bind calcium to form unabsorbable salts. 9 Dietary sodium intake has been shown to increase urinary calcium loss, thus resulting in increased calcium requirements among individuals with high sodium intakes. 2 Previously, high protein intakes were thought to negatively affect calcium balance by increasing urinary calcium excretion; 10 however, recent studies have shown that diets rich in protein may increase intestinal calcium absorption leading to calcinuria but without affecting calcium balance. 11 Dietary cofactors that influence calcium bioavailability will be covered in detail elsewhere in a related paper on food‐based interventions in this special issue. 12
Vitamin D is essential for intestinal calcium absorption by the active, transcellular pathway and is involved in maintaining normocalcemia and thus bone mineralization; therefore, dietary calcium requirements depend, in part, on vitamin D status. 2 In most adult populations, vitamin D deficiency is unlikely to be a rate‐limiting constraint on calcium absorption, as only very low concentrations of 25‐hydroxyvitamin D (25(OH)D) are associated with impaired calcium absorption. 13 , 14 The effect of vitamin D on calcium absorption in children is not clearly established. 15 Calcium retention is higher in children than adults, especially during periods of rapid growth when bone mineral accretion is high. Fractional intestinal calcium absorption is higher in children than adults, possibly due their higher 1,25‐dihydroxyvitamin D (1,25(OH)2D) levels. Thus, vitamin D may play a more important role in maintaining calcium homeostasis in children, especially when calcium intakes are low. 16
Vitamin D and calcium interact in the regulation of parathyroid hormone (PTH), such that suppression of PTH to low (normal) concentrations depends on the adequacy of calcium intake and vitamin D status, indicated by the serum 25(OH)D concentration. Vitamin D–deficient populations may have higher PTH and, therefore, higher bone turnover and calcium requirements. In South Korea, where average calcium intakes are low (∼485 mg/day), the 2009–2010 Korea National Health and Nutrition Examination Survey found that PTH levels were inversely associated with calcium intakes at both lower (<50 nmol/L) and higher (>75 nmol/L) 25(OH)D levels. 13 In Iceland, where calcium intakes are higher, a cross‐sectional study of 944 healthy participants with a mean calcium intake of >1000 mg/day found that calcium intakes of <800 mg/day were significantly associated with higher serum PTH compared with calcium intakes of >1200 mg/day in people with low 25(OH)D (<25 nmol/L). However, in people without vitamin D deficiency, calcium intake was not related to PTH. 14 These are representative findings in that observational studies generally show an inverse relationship between calcium intake and PTH when calcium intakes are low, particularly in the context of low vitamin D status, but the relationship is attenuated at higher calcium intake levels and/or when vitamin D status is adequate. 15
Physical activity
Weight‐bearing exercises (e.g., walking and running) and resistance exercise support bone health. As IOM/NAM dietary reference values for calcium intake are based on maximizing calcium retention for bone health independent of exercise, the level and type of physical activity of a population may affect bone health and is, therefore, relevant to recommendations for calcium intake. 17 In children, a synergistic effect of dietary calcium intake and exercise on bone mass has been reported. 17 However, in a study of adolescent gymnasts, calcium supplements had no effect on bone mass, possibly because they were already taking sufficient dietary calcium prior to the supplementation. 18
Population‐level variations in calcium requirements
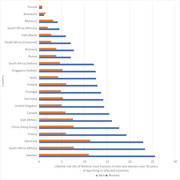
Some studies have shown racial and ethnic differences in calcium retention and bone mineral density (BMD). Among American adolescents, Black female adolescents have higher calcium retention, more efficient absorption, and lower urinary calcium excretion than white male and female adolescents, a trend that persists into adulthood. 19 , 20 Some Black African populations may have lower bone mass compared with Black Americans, perhaps owing to lower calcium intakes. 21 , 22 Although we do not know the distributions of bone mass or fracture incidence rates across Africa, available studies suggest that there is a relatively low incidence of osteoporotic fractures among Black Africans (Fig. 1). 23 , 24 , 25 A lower incidence of fracture may be an adaptative response to low calcium intake, ultraviolet sun exposure, or genetic differences in calcium absorption. 26 , 27
Figure 1.
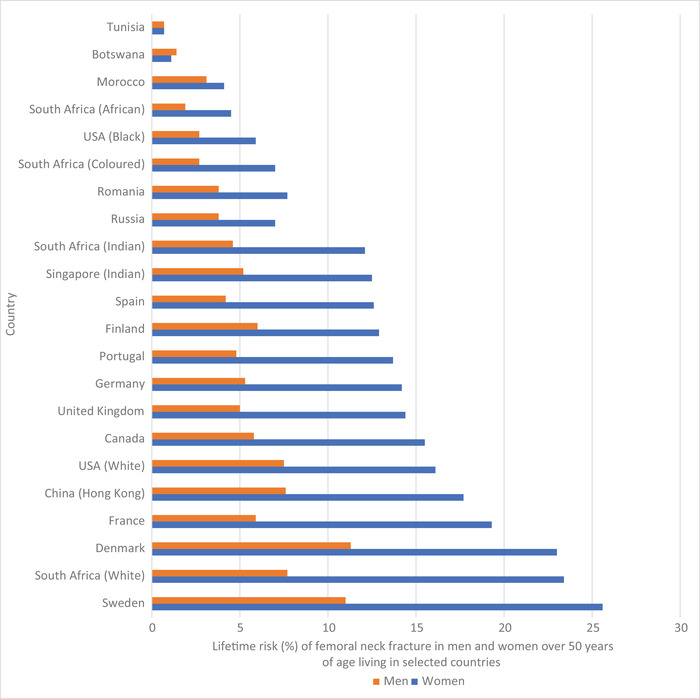
Lifetime risk (%) of femoral neck fracture in men and women over 50 years of age living in selected countries. Based on data compiled by Ref. 25.
Numerous studies have identified single nucleotide polymorphisms associated with calcium homeostasis. Differences in polymorphism expression showed changes in calcium levels or renal excretion of calcium, and, therefore, may be relevant if calcium requirements depend on the genotypes expressed. Understanding the frequency of these different genotypes in various populations and if they can be used to guide dietary calcium recommendations requires further research. 28 Additional cross‐national studies are necessary to provide convincing evidence of racial or geographic differences in bone health, including fracture risks.
Risk factors for inadequate calcium intake
Global distribution of calcium intake and prevalence of inadequate intake
Due to the lack of a reliable biomarker of calcium intake or status in population surveys, calcium intake data are often used to assess calcium adequacy at the population level. Dietary reference guidelines set by several agencies are used to measure the prevalence of inadequate calcium intake in a population comparing the calcium intake with the age‐specific estimated average requirement. 2 , 6 However, it may be problematic to assume inadequacy in one population using dietary reference standards established in another population, where calcium needs may vary due to the aforementioned factors.
Where calcium intake data are unavailable, risk of calcium intake inadequacy may be estimated from national food balance sheets. 29 Food balance sheets provide the available foods for consumption in a population from which the average amount of calcium available for that population can be derived. 30 The risk of inadequate calcium intake is then estimated considering calcium available and age‐specific requirements for that population. This information usually underestimates the risk of inadequate intake as it does not consider household waste or inter and intrahousehold variation in food intake. 29 Moreover, calcium availability is estimated per capita without considering inequities in food access or distribution. Despite these limitations, available data suggest substantial variability in calcium intake worldwide. 29 , 31 A major reason low calcium intakes are widespread globally is the low availability of or preference for dairy products in many regions of the world, particularly in LMICs, as well as the limited number of countries in which commercial food products are calcium fortified. 31 For example, wheat flour, breakfast cereals, and fruit juices are fortified with calcium in some high income countries (HICs), but less frequently in LMICs. 32 More information on calcium‐fortified foods can be found in a related paper on food‐based interventions in this special issue. 12
Low‐ and middle‐income countries
Of the 3.5 billion people at risk of inadequate calcium intake, approximately 90% live in Africa and Asia. 29 Many countries in South and East Asia, including India, have average dietary calcium intakes that are far lower than those in Western nations, and some are <500 mg/day, 31 although recommendations are similar (Table 1). 7 For example, calcium intake in Malaysian adolescents has been reported as low as 377 mg/day, a third of the IOM/NAM recommended daily intake for this age group. 33 Though most countries in Africa do not have calcium intake data available, those with data have average dietary calcium intakes of between 400 and 700 mg/day. 31 A systematic review of global calcium intake during pregnancy showed that calcium intakes <800 mg/day were reported in 5 (29%) HICs and in 14 (82%) LMICs studied. 34 Mean calcium intake was 622 mg/day in Latin America (10 studies), 653 mg/day for Asia Pacific (32 studies), and 566 mg/day in African countries (5 studies). This systematic review highlighted that many LMICs do not have estimates of calcium intake during pregnancy. 34
High‐income countries
Although overall average calcium intake is greater in HICs compared with LMICs, 1 numerous subgroups in HICs are at high risk of insufficient calcium intake, including adolescents, postmenopausal women, women with amenorrhea, women involved in high‐performance athletics, individuals with lactose intolerance or cow's milk allergy, and people who maintain a vegan diet. 2 In the United States, at‐risk age groups include boys and girls 9–13 years of age, girls 14–18 years of age, women 51–70 years of age, and both men and women ≥70 years of age. 2 Still, those living in the United States have mean total calcium intakes from food and supplements ranging from 918 to 1296 mg/day, 2 and Northern European countries have national calcium intakes >1000 mg/day. 31
Outcomes of inadequate calcium intake
Since the most recent systematic review of calcium‐related health outcomes, published in 2009, was based mainly on bone health, 35 new evidence on the effect of adequate calcium intake on other health outcomes has been published. 1 One of the most well‐documented benefits of calcium supplementation beyond bone health is a significant reduction in the risk of preeclampsia and maternal morbidity in pregnant women and preterm birth. This topic is, therefore, discussed in detail elsewhere in this special issue. 8
In nonpregnant adults, calcium supplementation may have a small effect in reducing blood pressure, especially in young adults, 36 , 37 but the broader public health impact on the prevalence of hypertension is unclear. Calcium supplementation has also been associated with favorable changes in cholesterol metabolism, including a reduction in low‐density lipoprotein and increase in high‐density lipoprotein. 38 Although less studied, unabsorbed calcium in the intestinal lumen may bind and impair absorption of oxalates, thereby reducing the risk of renal stones, and bind to triglycerides and bile acids, which may reduce low‐density lipoprotein cholesterol concentrations. 39 The same mechanisms have been postulated to reduce the risk of recurrent colorectal adenomas by reducing bile‐induced mucosal damage. 40 These studies are described in greater detail in Table 2.
Table 2.
Effect of calcium intake on health outcomes
| Health outcomes | Outcome | Population group | Research evidence | Effect size |
|---|---|---|---|---|
| Hypertensive disorders of pregnancy | Preeclampsia | Pregnant women | Meta‐analysis | Calcium supplementation compared to placebo reduced the risk of preeclampsia, RR = 0.45 (95% CI: 0.31–0.65) 101 |
| Pregnant women with low basal calcium intake | Meta‐analysis | Calcium supplementation compared to placebo reduced the risk of preeclampsia, RR = 0.36 (95% CI: 0.20–0.65) 101 | ||
| High blood pressure | Pregnant women | Meta‐analysis | Calcium supplementation compared to placebo reduced the high blood pressure RR to 0.65 (95% CI: 0.530.81) 101 | |
| Blood pressure | Blood pressure | Normotensive adults | Meta‐analysis | Calcium supplementation reduced SBP in adults by 1.14 mmHg (95% CI: −2.01 to −0.27) with doses of calcium 1000–1500 mg/day and by 2.79 mmHg (95% CI: −4.71 to −0.86) with doses of calcium equal to or over 1500 mg/day. Calcium supplementation had the greatest effect in young adults of less than 35 years as their SBP was reduced by 2.11 mmHg (95% CI: −3.58 to −0.64) 102 |
| Blood pressure | Hypertensive adults | Calcium supplementation reduced SBP by −1.86 mmHg (95% CI: −2.91 to −0.81) and DBP by −0.99 mmHg (95% CI: −1.61 to −0.37) 37 | ||
| Blood pressure | Hypertensive adults with low basal calcium intake | In people with relatively low calcium intake (≤800 mg/day) calcium supplementation reduced SBP by −2.63 (95% CI: −4.03 to −1.24) and DBP by −1.30 (95% CI: −2.13 to −0.47) 37 | ||
| Blood pressure | Hypertensive adults | Calcium supplementation as compared to control induced a statistically significant reduction in SBP (mean difference: −2.5 mmHg, 95% CI: −4.5 to −0.6, I(2)= 42%) but not DBP (mean difference: −0.8 mmHg, 95% CI: −2.1 to 0.4, I(2) = 48%) 103 | ||
| Progeny blood pressure | High blood pressure | Pregnant women/children | RCT | Calcium supplementation showed that children whose mothers received calcium supplementation had, at 7 years of age, a reduction in the risk of high blood pressure (above the 90th percentile) in comparison with children whose mothers were in the placebo group (RR = 0.59; 95% CI: 0.39–0.90) 36 |
| Cholesterol | LDL and HDL cholesterol | Adults | Meta‐analysis | Calcium supplementation reduced low‐density lipoprotein (LDL) cholesterol (−0.12 mmol/L (95% CI: −0.22 to −0.02)) and increased high‐density lipoprotein (HDL) cholesterol (0.05 mmol/L (95% CI: 0.00–0.10)) 104 |
| Colorectal adenomas | Recurrent colorectal adenomas | Adults with previous adenomas | Meta‐analysis | Calcium supplementation with doses from 1200 to 2000 mg/day and treatment duration from 36 to 60 months reduced the risk of recurrent colorectal adenomas, RR = 0.89 (95%CI: 0.82−0.96) 105 |
| Bone health | Bone mineral density | Children | Meta‐analysis | Calcium supplementation had a small effect on total body bone mineral content (standardized mean difference = 0.14, 95% CI: 0.01–0.27) and upper limb bone mineral density (0.14, 95% CI: 0.04–0.24), and this effect persisted after the end of supplementation only in the upper limb (0.14, 95% CI: 0.01–0.28) 106 |
| Renal stones | Urolithiasis | Individuals with osteoporosis | Meta‐analysis | Calcium supplementation compared to placebo, RR = 0.66 [95% CI 0.19, 2.34]; five studies in postmenopausal or elderly women, including 2038 subjects 107 |
| Urolithiasis | Pregnant women | Meta‐analysis | Calcium supplementation during pregnancy did not increase the risk of urolithiasis, RR = 1.52 [95% CI: 0.06, 40.67] or renal colic, RR = 1.75 [95% CI; 0.51, 5.99] in two studies with 12,901 women 108 |
Note: Evidence from randomized controlled trials (RCTs) and systematic reviews of RCTs. Table taken from Ref. 1, with permission of the authors.
CI, confidence interval; DBP, diastolic blood pressure; RR, relative risk; SBP, systolic blood pressure.
Bone outcomes
Bone increases in size and mass during periods of growth, with peak bone mass achieved by around 30 years of age. Osteopenia (low bone mass) and osteoporosis characterized by porous, fragile bones is a public health problem in most parts of the world. When calcium intake is low or when calcium is poorly absorbed, bone resorption occurs since the stored calcium in bone is used to maintain normal biological functions. Bone loss is part of the normal aging process, particularly in postmenopausal women with reduced circulating estrogen. Other factors that increase the risk of developing osteoporosis include inactivity, smoking, drinking excessive alcohol, and a family history of osteoporosis. 41
The calcium paradox
Despite lower calcium intakes in most LMICs, osteoporotic fracture rates are also lower in some LMICs, from which data are available for comparison to North America and Europe (Fig. 1). This has been described as the calcium paradox. 42 In the following sections, we review data from three general regions to explore the relationship between calcium intakes and bone outcomes. These data are summarized in Table 3 and at the end of this section.
Table 3.
Data summary of South Asian and African regions exploring the relationship between calcium intakes and bone outcomes
| Bone health data | Calcium intake data | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region and population type | Study year | Number of participants | Age (years) | Sex | Method of bone assessment | Bone health outcome (%) | Ca intake data available, or other source used for Ca intake data. (Ref. #) | Method of calcium intake assessment | Population (#, age, sex, and population type) | Calcium intake (mg/day) |
| India, Mumbai; urban 109 | 2002−2003 | 200 | >40 | Females | DXA, PF, and spine | OP ‐ 0.34 OS ‐ 0.08 | Ref. 31 | 24‐h diet recall | 306,329; ≥18 years; male and female | 429 |
| India, Hyderabad; urban, slum‐dwelling 63 | nd | 289 | 30−60 | Females | Hologic DXA, LS, hip, and total body | OP ‐ 0.52 OS ‐ 0.29 | Available | 24‐h diet recall and FFQ | n/a | 270 ± 54 |
| India, Jammu; urban 110 | 2004−2005 | 158 | 25−65 | Females | Calcaneal QUS | OP ‐ 0.37 OS ‐ 0.20 | ||||
|
India, Delhi, low SES (55), high SES (250); and rural Haryana (125) 111 |
nd | 430 | 60−80 | Females | Hologic DXA, hip, and LS | OP ‐ 0.29 OS ‐ 0.62 | ||||
| India, Vellore; urban 65 | nd | 150 | ≥50 | PostM females | Hologic DXA, LS, and PF | OP: LS ‐ 0.35, PF ‐ 0.57; OS: LS ‐ 0.48, PF ‐ 0.17 | Available | Oral semiquantitative FFQ | n/a | 398.8 ± 190.13 |
| India, Kerala; rural 112 | 2005−2007 | 609 | >18 |
Males (71) Females (538) |
QUS and distal radius | OP: M ‐ 0.37, F ‐ 0.44; OS: M ‐ 0.28, F ‐ 0.44 | ||||
| India, Pune; urban 113 | 2008 | 105 | 40−72 | Females | DXA lunar and LS | OP ‐ 0.31 OS ‐ 0.14 | ||||
| India, Pune; urban 64 | 2008 | 172 | 40−75 | Females PreM: 80 PostM: 92 | Lunar DXA, LS, and dual femurs | OP, PreM: LS‐0.44, PF‐0.46, TH‐0.27 OP, PostM: LS‐0.48, PF‐0.62, TH‐0.45 OS, PreM: LS‐0.08, OS, PostM: LS‐0.26, PF‐0.09, TH‐0.02 | Available | 24‐h diet recall | n/a |
PreM: 416 + 154 PostM: 434 + 160 |
| India, Chandigarh; urban 114 | nd | 200 | >45 | Females | Lunar DXA and LS | OP/OS: 0.53 | Available | 24‐h diet recall | n/a | 516.8 ± 208.9 |
| India, Delhi; urban 115 | nd | 1600 | >50 |
Males (792) Females (808) |
Lunar DXA, LS, femur, and forearm | OP: M‐0.54, F‐0.45 OS: M‐0.25, F‐0.43 | ||||
| India, Pune; urban low SES (54), high SES (58) 116 | 2008 | 112 | 39−70 | Females | Lunar DXA, LS, and total femur |
OP, Low‐SES: LS‐0.33, femur‐0.11 OP, high‐SES: LS‐0.12, femur‐0.0 |
Available | 3‐day diet recall with 2 weekdays and a Sunday | n/a | Low SES: 231.4 ± 120.9 High SES: 342.2 ± 128.3 |
| India; urban 117 | 2010 | 158 | >35 | Females | Calcaneal QUS | OP ‐ 0.48 OS ‐ 0.13 | ||||
| India, Varanasi; urban 118 | 2010−2011 | 200 | 50−84 | Males | Lunar DXA and right PF | OP: FN‐0.42, PF‐0.37, Hip‐0.41 OS: FN‐0.09, PF‐0.08 | ||||
| India, Vellore; urban 119 | nd | 250 | 51−74 | Males | Hologic DXA, LS, and PF | OP ‐ 0.58 OS ‐ 0.20 | ||||
| Delhi; urban 58 | nd | 760 | >50 | Males (345) Females (415) | Lateral X‐rays of the LS and thoracic spine, Genant's semiquantitative method for fracture assessment |
Vertebral fracture: M: 0.19 F: 0.17 |
||||
| Bangladesh, Dhaka; urban 66 | 2010−2011 | 500 | 16−65 | Females | DXA, LS, and PF | OP: LS ‐ 0.41, FN ‐ 0.21; OS: LS ‐ 0.03, FN ‐ 0.04 | Ref. 120 | National household diet survey | 31,066; <5 to >80 years; male and female; urban and rural | 529 |
| Nepal, Kathmandu; urban 67 | 2017−2018 | 169 | >50 |
Males (38) Females (131) |
Lunar Prodigy DXA, LS, and femur | OP: M‐0.45, F‐0.37 OS: M‐0.24, F‐0.41 | Assessed | 24‐h diet recall | n/a | 520.4 ± 297.0 |
| India, Bharatpur, Pokhara, and Bhairahawa; urban 68 | 2018 | 465 | >20 |
Males (201) Females (264) |
Calcaneal QUS |
OP: M‐0.59, F‐0.62 OS: M‐0.24, F‐0.22 |
||||
| Pakistan, Quetta; urban 121 | 2007 | 334 | >20 | Females | Calcaneal QUS | OS: 0.43, OP:0.13 | Ref. 122 | 24‐h diet recall and FFQ | 144; ≥18 years; female; urban | 462 ± 176 |
| Pakistan, Karachi; urban 123 | 2004 | 925 | >35 | Females | Heel ultrasound | OS: 0.32, OP:0.07 | ||||
| Pakistan, Karachi; urban 124 | 2009 | 170 | 18−80 | Females | Calcaneal QUS | OS: 0.52, OP:0.11 | ||||
| Pakistan, Nahaqi; rural 125 | nd | 107 | 40−65 | PostM females | Broadband ultrasound attenuation of the calcaneus | OS: 0.43, OP:0.27 | Available | 24‐h diet recall | n/a | 360.9 ± 74.2 |
| Pakistan, Karachi; urban 126 | 2013 | 203 | 40−60 | PostM Females | Hologic DXA, LS, hip, and femur | OS: 0.49, OP:0.29 | ||||
| Sri Lanka, Gampaha district, near Colombo; urban 72 | 2007 | 700 | 35−64 |
Males (279) Females (421) |
AccuDexa scanner peripheral DXA, middle phalanx of the middle finger of the nondominant hand | OS: M‐0.06, F‐0.20 | ||||
| Sri Lanka, seven different provinces; urban (1150), rural (492) 74 | 2004−2005 | 1642 | 56.5 ± 6.8 | PostM females | AccuDEXA scanner BMD and BMC of the middle phalanx of the middle finger of the nondominant hand | OS: 0.45 | ||||
| Sri Lanka, seven different provinces; urban and rural 73 | 2004−2005 | 1147 | 50−84 | Males | AccuDEXA scanner, middle phalanx of the middle finger of the nondominant hand | OS: 0.06 | ||||
| Sri Lanka, Galle 75 | 2017−2018 | 355 | 20−70 | Females | Hologic DXA, LS, and femur | OS: PostM ‐ 0.37 using manufacturer's Asian reference data; 0.17 using local reference data | ||||
| South Africa, Baragwanath, Hillbrow, and Johannesburg; urban 21 | nd | 367 | 20−64 | Female | Hologic QDR 1000 DXA, spine, and femur. Single‐photon absorptiometry using a Norland densitometer, radial bone at distal third radius on nondominant side | Spinal bone density: Black‐ 0.94, White‐ 0.97; femoral bone density Black‐0.74, White‐0.90 | Ref. 127 | 24‐h diet recall | 3231; ≥15 years; male and female | 479 |
| Botswana, two private hospitals in the capital city; three tertiary‐level public hospitals in southern, central, and northern parts of the country; three private insurance companies 25 | 2009−2011 | 435 | ≥40 |
Males (196) Females (239) |
Used retrospective patient chart, including radiology reports, digital radiology files, surgical ward notes, postoperative theater notes, and discharge summaries. FRAX used to calculate fracture probabilities |
Hip fracture: M‐0.03, F‐0.03 | Ref. 128 | 24‐h diet recall and FFQ | 79; 18−75 years; male and female | 588 |
| Uganda, Kampala and Zimbabwe, Harare, Chitungwiza; urban 76 | 2009−2012 | 518 | 18−45 | PreM women | DXA, TH, and LS | OP: LS‐0.35, TH‐0.10 | Ref. 129 | 24‐h diet recall and FFQ | 173; male and female; local | 238 |
| South Africa, Bantu; urban 81 | 1960 | 117 | ≥30 | Male and female | Radiographic examination of the pelvis with a portable unit | Hip fracture: 0.03; osteoarthrtis of the hip: 0.13 | ||||
| Nigeria, Ibadan and UK, Southampton and Newcastle; urban 82 | 1988−1989 | 746,700 | ≥50 | Male (385,200) Female (361,500) | All fractures were radiologically confirmed | Hip fracture: Nigeria‐0.003, UK‐0.131 | Ref. 130 | FFQ | 13,142; all ages; male and female | 636 |
| South Africa, Durban; urban and rural 83 | 1966−1967 | 300 | 50−90 | Female | Lateral X‐ray films of LS | OS: 0.06 | ||||
| South Africa, Cape Town; urban 84 | nd | 189 | ≥40 | Female | DXA, LS, and PF; postero‐anterior standing radiographs of the thoracic and LS were assessed | Vertebral fracture: White‐0.05, Black‐0.09 hip fracture: White‐0.01 | ||||
| South Africa, Durban; urban 85 | 2010−2013 | 197 | ≥60 | Male and female | DXA, hip, and spine | OP: hip‐0.45, spine‐0.38 OS: hip‐0.16, spine‐0.21 vertebral fracture: M‐0.13, F‐0.24 | Available | Calcium intake diary | n/a | 474.1 (reported) |
| Congo,Kinshasa; urban 86 | 2011−2016 | 430 | 47–87 | PostM black women | Computerized tomography scanners | Vertebral fracture ‐ 0.69 | ||||
| Gambia, Keneba, Kanton Kunda, and Manduar; rural 79 | nd | 195 | >44 | Female | Dual‐photon absorptiometer bone mineral status at LS and PF | LS‐0.29, midshaft of the radius‐0.35, PF‐0.30, distal radius‐0.60 | ||||
BMD, bone mineral density; DXA, dual X‐ray absorptiometry; FFQ, food frequency questionnaire; LS, lumbar spine; OP, osteopenia; OS, osteoporosis; PF, proximal femur; PostM, postmenopausal; PreM, premenopausal; QUS, qualitative ultrasound; SES, socioeconomic status; TH, total hip; UK, United Kingdom.
There are limitations in assuming an ecological association between average calcium intake and prevalence of osteoporosis or fracture. There are limited high‐quality, relevant datasets, including both dietary calcium intake and bone health parameters. Moreover, data related to osteoporosis are more likely to be available in well‐resourced health systems located in HICs, which also typically have higher proportions of older adults. Regional sources of calcium may differ, and dietary calcium estimates do not take bioavailability and other dietary factors into account, which may influence calcium bioavailability or metabolism. Similarly, other factors, such as physical activity and genetics, may modify relationships between calcium intake and osteoporosis. Lastly, it is important to consider associations that may be real at the individual level but might not be apparent—or even be reversed—at the group level.
Regional considerations
High‐income countries
Osteoporosis is a public health issue for greater than 10 million U.S. adults, of whom 80% are women. An estimated 1.5 million fractures occur yearly due to osteoporosis, 43 with most occurring in the hip, vertebrae, wrist, pelvis, and ribs. 44 The Centers for Disease Control and Prevention reports that 4.2% of men and 18.8% of women ≥50 years of age have osteoporosis of the femoral neck or lumbar spine, as defined by BMD measurements. 45
Supplementation with calcium and vitamin D has been effective in reducing fractures and falls in institutionalized women >50 years of age. 46 However, among community‐dwelling older adults >50 years of age, the benefits of calcium supplementation for reducing fracture rates are less clear. A systematic review of 26 RCTs published in 2015 found that calcium supplements (most studies used a dose of ≥1000 mg/day) modestly, though significantly, reduced the risk of total and vertebral fractures, but not hip or forearm fractures. 47 When the analysis was limited to the four trials with the lowest risk of bias, involving 44,505 individuals, it showed no effect of calcium supplementation on risk of fracture at any site. Another meta‐analysis assessing the effect of calcium intake on BMD (with 51 studies and 12,257 individuals) found supplementation produced only a small increase (0.7–1.8%) in BMD, with little additional effect after a year, which is unlikely to produce a clinically significant reduction in fracture risk. According to the authors of this systematic review, increases in BMD by 1–2% due to increased calcium intake would be predicted to produce a relatively small reduction of 5–10% in fracture risk. 48 In 2013, the U.S. Preventive Services Task Force concluded that there was insufficient evidence to assess the balance of benefits and harms of combined vitamin D and calcium supplementation to prevent bone fractures in premenopausal women and men. 49
South Asia
Among the important factors contributing to low calcium intakes are a lack in availability of calcium‐rich foods, traditional dietary habits, food insecurity, and gender discrimination. 50 , 51 There is considerable disparity among Southeast Asians with regard to dairy consumption, but principally dairy consumption is low due to traditional dietary practices. While East Asians do not consume much dairy due to traditional dietary customs, 51 South Asians consume some dairy products like curd, buttermilk, and clarified butter, but the practice of milk drinking is uncommon among South Asians, with milk consumption limited to that added to tea or coffee. 52 , 53 Many Indians consume very little dairy and follow a vegetarian diet, which often includes cereals and vegetables with significant phytate and oxalate levels, known to inhibit calcium absorption. Food insecurity and poor purchasing power also limit milk and milk product consumption by most of the population in LMICs. 52 This is compounded by gender discrimination in many communities, resulting in girls and women being at a disadvantage for being allocated milk and milk products for consumption in their households. 50 , 54
While osteoporosis is an important public health concern globally, the overwhelming burden of malnutrition and infectious diseases overshadows its importance in South Asian countries. Data on osteoporosis prevalence come from studies conducted in small groups across the region as there are no national data from any South Asian country (Table 3).
In 2013, approximately 50 million Indians were estimated to have osteopenia or osteoporosis. 55 Prevalence of osteoporosis among Indian women >25 years of age was reported as 8–62%. 56 Studies among Indian men ≥50 years of age report osteoporosis prevalence rates of 8.5–25%. 57 Vertebral fractures are commonly reported among Indians, with a prevalence of at least one reported among 15–20% of urban adults ≥50 years of age. 55 For example, the Delhi Vertebral Osteoporosis Study reported a prevalence of 17.9% of vertebral fractures in older adults. 58 These rates are similar to those reported among whites worldwide, though osteoporotic fracture rates among Indian men were highest. 58 Crude incidence of hip fractures among Indians from Rohtak, North India was reported as 105 and 159/100,000 in men and women, respectively, >50 years of age, with an increasing trend in rates noted with increasing age in both sexes. 59 Population prevalence of low trauma hip, spine, and wrist fractures in a questionnaire‐based study was reported as 34.3/100,000. 55
Multiple reports show that while hip fracture rates have stabilized or decreased in HICs, they have been steadily increasing in LMICs. 60 Projections on incidence of hip fractures in India indicated that by 2050, there would be a 2.39‐fold increase in the total number of hip fractures compared with 2018. 60 However, increased rates may be attributed to increased life expectancy among Asians. Compared with other Asian populations, Indian women (≥45 years) residing in Singapore have higher hip BMD than Chinese women. 61 Zengin et al. reported that South Asian and white men residing in the UK have lower areal BMD compared with Black men. While there are no differences in areal BMD between white and South Asian men, South Asian men had thinner radial and tibial cortices than white men; despite this, no differences were noted in bone strength between these two groups. 62
Few studies have reported determinants of low BMD or osteoporosis among Indians. Of slum‐dwelling women 30–60 years of age from Hyderabad, India, 29% had osteoporosis and calcium intake was significantly and positively associated with hip BMD. 63 A positive correlation of calcium intake with BMD at lumbar spine, femoral neck, and total hip, and a positive, yet weak, association of serum vitamin D with BMD at all three sites was reported in urban Indian women >40 years of age living in Pune, India. 64 Paul et al. reported a positive correlation between vitamin D and femoral neck BMD, but failed to find a correlation between dietary calcium intake and BMD, which they attributed to overall inadequate calcium consumption among their sample of 150 ambulatory postmenopausal South Indian women ≥50 years of age. 65
Begum et al. reported among urban and suburban Bangladeshi women 16–45 years of age attending a tertiary care public hospital that prevalence of osteopenia was 43.6% and osteoporosis was 5.5%. Among women 46–65 years of age attending the same hospital, 40.7% had osteopenia and 41.8% had osteoporosis. 66 Limited reports from Nepal indicate considerable prevalence of osteopenia and osteoporosis among the population. In a hospital‐based study in Kathmandu, 23.7% of men and 41.2% of women had osteoporosis as assessed by dual‐X‐ray absorptiometry (DXA) scans; daily calcium intake was inversely associated with osteoporosis 67 In a cross‐sectional study among residents of three major metropolitan cities of Nepal, the prevalence of osteopenia and osteoporosis, assessed by calcaneal ultrasound in 465 participants ≥20 years of age, was reported to be 60.6% and 22.4%, respectively. 68
In Pakistan, data on the prevalence of osteoporosis are available from hospital‐based studies using heel ultrasound; DXA data are scarce. Limited data suggest a prevalence of osteoporosis ranging from 5.6% to 17.8% in women who are premenopausal and 20–49.3% in those who are postmenopausal, mostly from urban areas. 69 Furthermore, in a retrospective study on patients with low impact hip fractures admitted to a tertiary care center in Pakistan, health providers rarely evaluated patients for osteoporosis despite typical presentations. 70 Data are also lacking on osteoporosis prevalence in Sri Lanka. While the Galle Prospective Osteoporosis survey reported that 61.5% of women >50 years of age were found to be osteoporotic based on DXA assessments, 71 a study using a peripheral DXA found that 27% of women and 7% of men >50 years of age had osteoporosis. 72 Another study in men ≥50 years of age living in Sri Lanka reported that 5.8% had osteoporosis based on phalangeal BMD. 73 In postmenopausal Sri Lankan women, one report indicates that osteoporosis is likely to be prevalent among 45% of the participants via phalangeal BMD, 74 while more recently, a study of trabecular bone score of the lumbar spine via DXA found osteoporosis in approximately 33% using the Asian reference data provided by the manufacturer, and approximately 20% using local reference data. 75
Africa
Few studies have addressed the prevalence of osteoporosis in Africa, particularly among populations in the sub‐Saharan region (Table 3). Though osteoporosis and fragility fractures are widely considered to be uncommon among Black African communities despite low calcium intakes, extensive data to support this hypothesis are lacking. In South Africa, BMD at the lumbar spine in pre and postmenopausal Black women was similar or lower to that in South African whites after adjusting for differences in anthropometry, while femoral neck BMD was greater in Black women than in their white counterparts. 21 Similar findings were reported from Zimbabwe, where Black women have lower lumbar spine BMD than U.S. Blacks and whites, but similar or higher femoral neck BMD to U.S. whites. 76 The latter study suggests that much of the difference could be explained by differences in weight and body mass index. 77
A recent study from Kampala, Uganda, in East Africa, comparing the BMD of premenopausal Black women with the NHANES reference database, found that mean lumbar spine BMD was 1.2 SD below the mean for U.S. Black women and 0.8 SD below that of U.S. white women, while at the femoral neck, the BMD was 0.4 SD below that of U.S. Black women but 0.1 SD above that of U.S. white women. 78 In West Africa, a study of pre and postmenopausal Gambian women revealed that after correcting for age, weight, and height, bone mineral content at the lumbar spine was on average 24% lower than that of white women in the UK. The reduction in bone mineral content increased to 42% in those >64 years of age. 79
Somali women who had immigrated to Sweden from Africa were found to have lumbar spine BMD almost 1 SD below that of U.S. white women, although the hip BMD was similar. Compared with U.S. Black women, both lumbar spine and hip BMDs were between 0.9 and 1.6 SD below Black reference means, respectively. A small study in the United States found that immigrant Somali women had lower lumbar spine BMD (4%) than white women, but higher femoral neck BMD (11%). 80
The incidence of femoral neck and vertebral fractures in Africa has also been infrequently studied. In 1968, Solomon calculated the femoral neck fracture rates for African Blacks in Johannesburg at 4.5 and 4.2/100,000 for men and women, respectively. 81 The rate for women in Malmö, Sweden at that time was more than 10‐fold higher at 46.9/100,000. A more recent study confirmed lower incidence of hip fractures in South African Black men and women compared with their white, Indian, and mixed‐race compatriots (Fig. 2). 24 In Nigeria, hip and forearm fracture incidences in adults >50 years of age were studied in Ibadan. As in other sub‐Saharan African countries, calculated incidence varied between 1.5 and 8/100,000, substantially lower than rates found in Southampton and Newcastle in the UK. 82 A study from Botswana confirmed the low risk of hip fracture in Black Africans living there (Fig. 1). 25 Countries with the lowest risk of hip fracture for local adults include Tunisia, Botswana, Morocco, South Africa (Black Africans), Ukraine, and the United States (African Americans).
Figure 2.
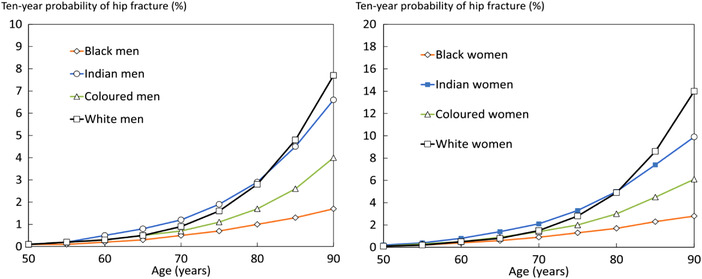
Ten‐year probability of hip fracture in South African men and women by ethnic group. Reproduced from Ref. 24.
Less information is available about the incidence of osteoporotic vertebral fractures in African countries, but like hip fractures, they are thought to be uncommon. In 1968, Dent reported the low prevalence of lumbar vertebral fractures in radiographs of African patients >50 years of age compared with similarly aged white patients from the same Durban, South Africa, hospital. 83 More recently, however, a small study of thoraco‐lumbar radiographs in a convenience sample of 189 women (47% Black) >40 years of age found similar prevalence of radiographic vertebral fractures in Black and white women living in Western Cape, South Africa. 84 Both studies suffer from small numbers and lack of random sampling. In a study of 197 study volunteers >60 years of age living in Durban, South Africa, 20.8% had morphometric vertebral fractures on thoraco‐lumbar radiographs. There was no statistical difference in fracture prevalence between Black and Indian subjects in that study. 85 In a cohort of 430 postmenopausal women living in Kinshasa, Democratic Republic of Congo, who complained of back pain, vertebral fractures were diagnosed in 11% of the thoraco‐lumbar vertebrae; the prevalence of fractures rose with age. 86 In the Gambia, a cross‐sectional study of 488 healthy men and women >40 years of age from Kiang West were found to have a prevalence of 9% moderate or severe vertebral fractures on DXA. 62
Regional considerations summary
Evidence is mixed in North America and other HICs that calcium supplementation is beneficial for the prevention of bone fractures in premenopausal women and men. In postmenopausal women, the benefits of supplementation on fracture rate are also still unclear.
Reports from South Asian countries suggest a significant prevalence of osteopenia and osteoporosis, with women being investigated more commonly than men. However, data are primarily from hospital‐based studies or small community studies and are unlikely to be representative of the broad population. Moreover, assessment methods for BMD differ, with few reports using DXA; as fracture data are inadequately captured, current incidence rates may be an under‐representation of the prevailing incidence. Still, reports from South Asian countries indicate poor calcium intake, with low consumption of dairy. Together, these data suggest that the prevalence of osteoporosis among South Asians is similar to those of North Americans and Europeans despite lower calcium intakes. Further studies investigating community prevalence of low BMD or osteoporosis, using standard assessment methods, and fractures among South Asian populations are required.
In African populations, there is increasing evidence that BMD in Black women living in sub‐Saharan Africa is lower than that of Black American women, but hip BMD Z‐scores are consistently higher than lumbar spine Z‐scores. Lumbar spine BMD in African Black women is well below that of U.S. Blacks and more approximate to that of U.S. or UK whites (except for Gambian women who had lower bone mineral content than UK white women), while femoral neck BMD is above that of U.S. whites but below that of U.S. Black women. 22 Notably, hip fracture rates among Black Africans are among the lowest in the world, but information on vertebral fractures, although limited, suggests that the difference in rates between Black Africans and whites is less marked. Further studies are needed from more African countries to confirm these findings and more information is needed on men in these populations. Consistently lower dietary calcium intakes in Black Africans than the recommended allowances in the face of low femoral neck fracture rates suggest that other factors, such as physical activity and genotype, may play important roles in maintaining bone health.
Rickets
Nutritional rickets is the most frequent cause of pediatric bone disease in the world and is most common in LMICs, especially in India, Africa, and the Middle East. 87 Calcium intake along with vitamin D status influence the risk of developing nutritional rickets (Fig. 3). Risk of rickets appears to be highest for children with both low vitamin D levels and calcium intake, 15 but a severe deficiency of either nutrient can also lead to rickets. Evidence suggests that as calcium intakes decrease, there is a greater necessity for a child to meet vitamin D requirements to maintain normal calcium homeostasis. This interaction between calcium and vitamin D is central to both vitamin D–deficiency rickets and calcium–deficiency rickets. 88 Young children with vitamin D–deficiency rickets are most likely to be those born prematurely or with very low birth weight and those who were exclusively breastfed infants or toddlers with low endogenous vitamin D production due to dark skin pigmentation or insufficient sunlight exposure. As infants are generally weaned onto the same foods consumed by the whole family, dietary calcium deficiency becomes a greater contributor to the pathogenesis of nutritional rickets. 87 Beyond early infancy, calcium‐deficiency rickets mainly afflicts children and teenagers with normal or slightly low vitamin D levels and extremely low calcium intakes (<300 mg/day). Extended breastfeeding without sufficient vitamin D and complementary food sources of calcium, as well as extremely restrictive diets, including vegan diets, also increase the risk of nutritional rickets. 87
Figure 3.
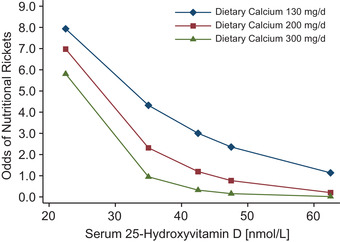
Synergistic effects of dietary calcium intake and serum 25‐hydroxyvitamin D concentrations on the odds of having rickets in young Nigerian children. Reproduced from Ref. 15.
Research efforts are ongoing to elucidate potential links between dietary factors for rickets and other risk factors, including genetic and environmental influences. 89 A study of Nigerian children revealed a nearly 15% incidence of family history of rickets among a cohort of rachitic children, compared with 3% in the control group. 90 Similarly, mothers who had previously had a child with rickets had lower calcium concentration in breastmilk than control mothers. 91 Whether these findings are the result of a genetic difference in calcium homeostasis in mothers of rachitic children or some other predisposing factor remains to be seen.
Additionally, preterm birth prevents optimal newborn calcium accrual in utero because calcium and phosphorus accrual peak during the third trimester. As a result, preterm babies, especially those born at <28 weeks gestation and weighing <1500 g, have the highest incidence of rickets worldwide. Rickets in this population is distinct etiologically from the disease in toddlers and young children, the former being the result of both dietary phosphorus and calcium deficiency needed for rapid bone mineralization rather than isolated calcium and/or vitamin D deficiency. Babies who are small for gestational age, approximately a third of babies born in LMICs, are also at elevated risk for rickets, as in utero growth failure is associated with a decreased transfer of bone minerals to the fetus. 92 , 93
Challenges and limitations
There is no global consensus on the definitions of low calcium intake or calcium deficiency. At a population level, the prevalence of low calcium intake can be estimated by comparing calcium intake or calcium availability in a population with age‐specific dietary requirements provided by different agencies. Information is needed on calcium intake from dairy and nondairy sources, namely, in LMICs where data are limited or of poor quality.
Evidence from HICs suggests that intakes below 800 mg/day in adults are suboptimal, although most populations in LMICs have intakes closer to 400–500 mg/day without strong evidence that these levels cause adverse bone outcomes.
Since much of the information on the effects of calcium on bone health comes from observational studies, more evidence from RCTs is necessary. As calcium effects on bone health are more likely to be evident in the long term, it would be desirable to start these trials in a variety of age groups. Proxies of bone health, such as BMD and bone status biomarkers, could be measured. Likewise, it would be desirable that existing RCTs of calcium intake follow up the randomized individuals for evaluation of long‐term outcomes, such as fracture rates.
Options for assessing calcium status are limited by the absence of a well‐validated, specific biomarker of calcium status that is field‐friendly, and therefore, feasible at population level and priced for use in LMICs. Several emerging methodologies may hold promise in this area, but more research is needed.
Research gaps and priorities
Questions remain not just regarding the true global prevalence of inadequate calcium intakes, but also on what should be considered related health outcomes when making dietary goals and recommendations. More complete and consistent data for global calcium intake or status, specifically in LMICs where these data are especially lacking, are necessary to review a benefit to these populations.
Evidence on the effects of calcium on bone health in understudied regions thought to be inadequate in calcium intake must be obtained from RCTs. This could begin within the populations outlined above when describing the calcium paradox. Research to determine a potential mechanism of low fracture rates and adequate BMD in the face of low calcium intakes within these populations is also needed. Ultimately, better clarity on these questions would inform and support development of policies and population‐level interventions to safely improve calcium intake and status where necessary.
Finally, the absence of a standard calcium status indicator has long hampered efforts to define the population burden of inadequate calcium intake and its associated health effects. Concentrations of total and ionized calcium in circulation are tightly regulated and do not accurately reflect calcium intake or physiological status. 26 , 94 Meanwhile, as calcium is just one determinant of bone health and is associated with many other aspects of health beyond bone, no single biomarker or health outcome offers a perfect indicator of calcium status. The quest for both sensitive and specific markers of calcium status, which are also field‐friendly, remains.
Competing interests
The authors declare no competing interests.
Acknowledgments
Development of this paper, its open access, and the assembly of and meetings of the Calcium Task Force were supported by funding from The Children's Investment Fund Foundation to the Nutrition Science Program of the New York Academy of Sciences.
References
- 1. Cormick, G. & Belizán J.M.. 2019. Calcium intake and health. Nutrients 11: E1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Office of Dietary Supplements ‐ Calcium. Accessed July 7, 2021. https://ods.od.nih.gov/factsheets/Calcium‐HealthProfessional/.
- 3. Anderson, J.J.B. , Kruszka B., Delaney J.A.C., et al. 2016. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10‐year follow‐up of the multi‐ethnic study of atherosclerosis (MESA). J. Am. Heart Assoc. 5: e003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prentice, R.L. , Pettinger M.B., Jackson R.D., et al. 2013. Health risks and benefits from calcium and vitamin D supplementation: Women's Health Initiative clinical trial and cohort study. Osteoporos. Int. 24: 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ross, A.C. , Manson J.E., Abrams S.A., et al. 2011. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J. Clin. Endocrinol. Metab. 96: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Food Safety Authority . Scientific opinion on dietary reference values for calcium. Accessed July 10, 2021. https://www.efsa.europa.eu/en/efsajournal/pub/4101.
- 7.ICMR‐National Institute of Nutrition. Recommended Dietary Allowances and Estimated Average Requirements Nutrient Requirements for Indians‐2020: A Report of the Expert Group Indian Council of Medical Research National Institute of Nutrition. Accessed August 4, 2021. https://www.nin.res.in/RDA_Full_Report_2020.html.
- 8. Gomes, F. & Ashorn P.. 2022. Calcium supplementation for the prevention of hypertensive disorders of pregnancy: current evidence and programmatic considerations. Ann. N.Y. Acad. Sci. 10.1111/nyas.14733. [DOI] [PMC free article] [PubMed]
- 9. Weaver, C.M. 2020. Chapter 19 ‐ Calcium. In Present Knowledge in Nutrition (11th ed.) Marriott B.P., Birt D.F., Stallings, V.A. et al., Eds.: 321–334. Academic Press. [Google Scholar]
- 10. Calvez, J. , Poupin N., Chesneau C., et al. 2012. Protein intake, calcium balance and health consequences. Eur. J. Clin. Nutr. 66: 281–295. [DOI] [PubMed] [Google Scholar]
- 11. Wallace, T.C. 2019. Optimizing dietary protein for lifelong bone health: a paradox unraveled. Nutr. Today 54: 107–115. [Google Scholar]
- 12. Bourassa, M.W. & Abrams S.A.. 2021. Interventions to improve calcium intake through foods in populations with low intake. Ann. N.Y. Acad. Sci. 10.1111/nyas.14743. [DOI] [PMC free article] [PubMed]
- 13. Joo, N.‐S. , Dawson‐Hughes B., Kim Y.‐S., et al. 2013. Impact of calcium and vitamin D insufficiencies on serum parathyroid hormone and bone mineral density: analysis of the fourth and fifth Korea National Health and Nutrition Examination Survey (KNHANES IV‐3, 2009 and KNHANES V‐1, 2010). J. Bone Miner. Res. 28: 764–770. [DOI] [PubMed] [Google Scholar]
- 14. Steingrimsdottir, L. , Gunnarsson O., Indridason O.S., et al. 2005. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 294: 2336–2341. [DOI] [PubMed] [Google Scholar]
- 15. Sempos, C.T. , Durazo‐Arvizu R.A., Fischer P.R., et al. 2021. Serum 25‐hydroxyvitamin D requirements to prevent nutritional rickets in Nigerian children on a low‐calcium diet—a multivariable reanalysis. Am. J. Clin. Nutr. 114: 231–237. [DOI] [PubMed] [Google Scholar]
- 16. Pettifor, J.M. 2014. Calcium and vitamin D metabolism in children in developing countries. Ann. Nutr. Metab. 64(Suppl. 2): 15–22. [DOI] [PubMed] [Google Scholar]
- 17. Specker, B. & Binkley T.. 2003. Randomized trial of physical activity and calcium supplementation on bone mineral content in 3‐ to 5‐year‐old children. J. Bone Miner. Res. 18: 885–892. [DOI] [PubMed] [Google Scholar]
- 18. Ward, K.A. , Roberts S.A., Adams J.E., et al. 2007. Calcium supplementation and weight bearing physical activity–do they have a combined effect on the bone density of pre‐pubertal children? Bone 41: 496–504. [DOI] [PubMed] [Google Scholar]
- 19. Braun, M. , Palacios C., Wigertz K., et al. 2007. Racial differences in skeletal calcium retention in adolescent girls with varied controlled calcium intakes. Am. J. Clin. Nutr. 85: 1657–1663. [DOI] [PubMed] [Google Scholar]
- 20. Ettinger, B. , Sidney S., Cummings S.R., et al. 1997. Racial differences in bone density between young adult black and white subjects persist after adjustment for anthropometric, lifestyle, and biochemical differences. J. Clin. Endocrinol. Metab. 82: 429–434. [DOI] [PubMed] [Google Scholar]
- 21. Daniels, E.D. , Pettifor J.M., Schnitzler C.M., et al. 1995. Ethnic differences in bone density in female South African nurses. J. Bone Miner. Res. 10: 359–367. [DOI] [PubMed] [Google Scholar]
- 22. Nelson, D.A. , Pettifor J.M., Barondess D.A., et al. 2004. Comparison of cross‐sectional geometry of the proximal femur in white and black women from Detroit and Johannesburg. J. Bone Miner. Res. 19: 560–565. [DOI] [PubMed] [Google Scholar]
- 23. Paruk, F. , Matthews G. & Cassim B.. 2017. Osteoporotic hip fractures in Black South Africans: a regional study. Arch. Osteoporos. 12: 107. [DOI] [PubMed] [Google Scholar]
- 24. Johansson, H. , Dela S.S., Cassim B., et al. 2021. FRAX‐based fracture probabilities in South Africa. Arch. Osteoporos. 16: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kebaetse, M. , Nkhwa S., Mogodi M., et al. 2021. Epidemiology of hip fracture in Botswana. Arch. Osteoporos. 16: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aloia, J.F. 2008. African Americans, 25‐hydroxyvitamin D, and osteoporosis: a paradox. Am. J. Clin. Nutr. 88: 545S–550S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ilich, J.Z. & Kerstetter J.E.. 2000. Nutrition in bone health revisited: a story beyond calcium. J. Am. Coll. Nutr. 19: 715–737. [DOI] [PubMed] [Google Scholar]
- 28. Da Silva Lopes, K. & Abe S.K.. 2021. Polymorphisms contributing to calcium status: a systematic review. Nutrients 13: 2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumssa, D.B. , Joy E.J.M., Ander E.L., et al. 2015. Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Sci. Rep. 5: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization & Food and Agriculture Organization of the United Nations . 1962. Calcium requirements: report of an FAO/WHO Expert Group, Rome, Italy, 23 to 30 May 1961 . World Health Organization.
- 31. Balk, E.M. , Adam G.P., Langberg V.N., et al. 2017. Global dietary calcium intake among adults: a systematic review. Osteoporos. Int. 28: 3315–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palacios, C. , Hofmeyr G.J., Cormick G., et al. 2021. Current calcium fortification experiences: a review. Ann. N.Y. Acad. Sci. 1484: 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suriawati, A.A. , Majid H.A., Al‐Sadat N., et al. 2016. Vitamin D and calcium intakes, physical activity, and calcaneus BMC among school‐going 13‐year old Malaysian adolescents. Nutrients 8: E666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cormick, G. , Betrán A.P., Romero I.B., et al. 2019. Global inequities in dietary calcium intake during pregnancy: a systematic review and meta‐analysis. BJOG 126: 444–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chung, M. , Balk E.M., Brendel M., et al. 2009. Vitamin D and calcium: a systematic review of health outcomes. Evid. Rep. Technol. Assess. (Full Rep.) 2009 Aug (183): 1–420. [PMC free article] [PubMed] [Google Scholar]
- 36. Villa‐Etchegoyen, C. , Lombarte M., Matamoros N., et al. 2019. Mechanisms involved in the relationship between low calcium intake and high blood pressure. Nutrients 11: E1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cormick, G. , Ciapponi A., Cafferata M.L., et al. 2015. Calcium supplementation for prevention of primary hypertension. Cochrane Database Syst. Rev. CD010037. [DOI] [PMC free article] [PubMed]
- 38. Chen, C. , Ge S., Li S., et al. 2017. The effects of dietary calcium supplements alone or with vitamin D on cholesterol metabolism: a meta‐analysis of randomized controlled trials. J. Cardiovasc. Nurs. 32: 496–506. [DOI] [PubMed] [Google Scholar]
- 39. Taylor, E.N. & Curhan G.C.. 2008. Determinants of 24‐hour urinary oxalate excretion. Clin. J. Am. Soc. Nephrol. 3: 1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bonovas, S. , Fiorino G., Lytras T., et al. 2016. Calcium supplementation for the prevention of colorectal adenomas: a systematic review and meta‐analysis of randomized controlled trials. World J. Gastroenterol. 22: 4594–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. General Facts . National Osteoporosis Foundation. Accessed July 10, 2021. https://www.nof.org/preventing‐fractures/general‐facts/.
- 42. Polzonetti, V. , Pucciarelli S., Vincenzetti S., et al. 2020. Dietary intake of vitamin D from dairy products reduces the risk of osteoporosis. Nutrients 12: 1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Riggs, B.L. & Melton L.J.. 1995. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 17: 505S–511S. [DOI] [PubMed] [Google Scholar]
- 44. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy . 2001. Osteoporosis prevention, diagnosis, and therapy. JAMA 285: 785–795.11176917 [Google Scholar]
- 45. FastStats. 2021. April 14, 2021 Accessed July 11, 2021. https://www.cdc.gov/nchs/fastats/osteoporosis.htm.
- 46. Cranney, A. , Horsley T., O'Donnell S., et al. 2007. Effectiveness and safety of vitamin D in relation to bone health. Evid. Rep. Technol. Assess. (Full Rep.) 2007 Aug (158): 1–235. [PMC free article] [PubMed] [Google Scholar]
- 47. Bolland, M.J. , Leung W., Tai V., et al. 2015. Calcium intake and risk of fracture: systematic review. BMJ 351: h4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tai, V. , Leung W., Grey A., et al. 2015. Calcium intake and bone mineral density: systematic review and meta‐analysis. BMJ 351: h4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moyer, V.A. & U.S. Preventive Services Task Force* . 2013. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 158: 691–696. [DOI] [PubMed] [Google Scholar]
- 50. Aurino, E. 2017. Do boys eat better than girls in India? Longitudinal evidence on dietary diversity and food consumption disparities among children and adolescents. Econ. Hum. Biol. 25: 99–111. [DOI] [PubMed] [Google Scholar]
- 51. Ohta, H. , Uenishi K. & Shiraki M.. 2016. Recent nutritional trends of calcium and vitamin D in East Asia. Osteoporos. Sarcopenia 2: 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Harinarayan, C.V. , Akhila H. & Shanthisree E.. 2021. Modern India and dietary calcium deficiency—half a century nutrition data‐retrospect‐introspect and the road ahead. Front. Endocrinol. (Lausanne) 12: 583654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sharma, M. , Kishore A., Roy D., et al. 2020. A comparison of the Indian diet with the EAT‐Lancet reference diet. BMC Public Health 20: 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Blum, L.S. , Khan R., Sultana M., et al. 2019. Using a gender lens to understand eating behaviours of adolescent females living in low‐income households in Bangladesh. Matern. Child Nutr. 15: e12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mithal, A. , Bansal B., Kyer C.S., et al. 2014. The Asia‐Pacific Regional Audit‐Epidemiology, Costs, and Burden of Osteoporosis in India 2013: a report of International Osteoporosis Foundation. Indian J. Endocrinol. Metab. 18: 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Khadilkar, A.V. & Mandlik R.M.. 2015. Epidemiology and treatment of osteoporosis in women: an Indian perspective. Int. J. Womens Health 7: 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bhadada, S.K. , Chadha M., Sriram U., et al. 2021. The Indian Society for Bone and Mineral Research (ISBMR) position statement for the diagnosis and treatment of osteoporosis in adults. Arch. Osteoporos. 16: 102. [DOI] [PubMed] [Google Scholar]
- 58. Marwaha, R.K. , Tandon N., Gupta Y., et al. 2012. The prevalence of and risk factors for radiographic vertebral fractures in older Indian women and men: Delhi Vertebral Osteoporosis Study (DeVOS). Arch. Osteoporos. 7: 201–207. [DOI] [PubMed] [Google Scholar]
- 59. Dhanwal, D.K. , Siwach R., Dixit V., et al. 2013. Incidence of hip fracture in Rohtak district, North India. Arch. Osteoporos. 8: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cheung, C.‐L. , Ang S.B., Chadha M., et al. 2018. An updated hip fracture projection in Asia: the Asian Federation of Osteoporosis Societies study. Osteoporos. Sarcopenia 4: 16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thambiah, S.C. & Yeap S.S.. 2020. Osteoporosis in South‐East Asian countries. Clin. Biochem. Rev. 41: 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zengin, A. , Pye S.R., Cook M.J., et al. 2016. Ethnic differences in bone geometry between White, Black and South Asian men in the UK. Bone 91: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shatrugna, V. , Kulkarni B., Kumar P.A., et al. 2005. Bone status of Indian women from a low‐income group and its relationship to the nutritional status. Osteoporos. Int. 16: 1827–1835. [DOI] [PubMed] [Google Scholar]
- 64. Kadam, N. , Chiplonkar S., Khadilkar A., et al. 2010. Low bone mass in urban Indian women above 40 years of age: prevalence and risk factors. Gynecol. Endocrinol. 26: 909–917. [DOI] [PubMed] [Google Scholar]
- 65. Paul, T.V. , Thomas N., Seshadri M.S., et al. 2008. Prevalence of osteoporosis in ambulatory postmenopausal women from a semiurban region in Southern India: relationship to calcium nutrition and vitamin D status. Endocr. Pract. 14: 665–671. [DOI] [PubMed] [Google Scholar]
- 66. Begum, R.A. , Ali L., Akter J., et al. 2014. Osteopenia and osteoporosis among 16–65 year old women attending outpatient clinics. J. Community Health 39: 1071–1076. [DOI] [PubMed] [Google Scholar]
- 67. Chaudhary, N.K. , Timilsena M.N., Sunuwar D.R., et al. 2019. Association of lifestyle and food consumption with bone mineral density among people aged 50 years and above attending the hospitals of Kathmandu, Nepal. J. Osteoporos. Article ID 1536394. [DOI] [PMC free article] [PubMed]
- 68. Bagudai, S. & Upadhayay H.. 2019. Prevalence of osteoporosis and osteopenia status among Nepalese population using calcaneal ultrasonography method. J. Coll. Med. Sci. Nepal 15: 249–255. [Google Scholar]
- 69. Khan, A.H. , Jafri L., Ahmed S., et al. 2018. Osteoporosis and its perspective in Pakistan: a review of evidence and issues for addressing fragility fractures. Ann. Med. Surg. 29: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Arshad, A. , Ibrahim M.T., Arshad H., et al. 2021. Clinical characteristics and outcomes of patients presenting with hip fractures at a tertiary care hospital in Pakistan. Arch. Osteoporos. 16: 25. [DOI] [PubMed] [Google Scholar]
- 71. Siribaddana, S. & Lekamwasam S.. 2004. Osteoporosis in Sri Lanka. Clin Calcium 14: 128–133. [PubMed] [Google Scholar]
- 72. Karunanayake, A.L. , Pinidiyapathirage M.J. & Wickremasinghe A.R.. 2010. Prevalence and predictors of osteoporosis in an urban Sri Lankan population. Int. J. Rheum. Dis. 13: 385–390. [DOI] [PubMed] [Google Scholar]
- 73. Lekamwasam, S. , Wijayaratne L., Rodrigo M., et al. 2009. Prevalence and determinants of osteoporosis among men aged 50 years or more in Sri Lanka: a community‐based cross‐sectional study. Arch. Osteoporos. 4: 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lekamwasam, S. , Wijayaratne L., Rodrigo M., et al. 2007. Prevalence of osteoporosis among postmenopausal women in Sri Lanka: a cross‐sectional community study. APLAR J. Rheumatol. 10: 234–238. [Google Scholar]
- 75. Rathnayake, H. , Lekamwasam S., Wickramatilake C., et al. 2019. Trabecular bone score and bone mineral density reference data for women aged 20–70 years and the effect of local reference data on the prevalence of postmenopausal osteoporosis: a cross‐sectional study from Sri Lanka. Arch. Osteoporos. 14: 1–7. [DOI] [PubMed] [Google Scholar]
- 76. Mgodi, N.M. , Kelly C., Gati B., et al. 2015. Factors associated with bone mineral density in healthy African women. Arch. Osteoporos. 10: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mukwasi, C. , Chibanda L.S., Banhwa J., et al. 2015. US white and black women do not represent the bone mineral density of sub‐Saharan black women. J. Clin. Densitom. 18: 525–532. [DOI] [PubMed] [Google Scholar]
- 78. Matovu, F.K. , Nabwana M., Kiwanuka N., et al. 2021. Bone mineral density in antiretroviral therapy‐naive HIV‐1–infected young adult‐women using depot medroxyprogesterone acetate or nonhormonal contraceptives in Uganda. JBMR Plus 5: e10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Aspray, T.J. , Prentice A., Cole T.J., et al. 1996. Low bone mineral content is common but osteoporotic fractures are rare in elderly rural Gambian women. J. Bone Miner. Res. 11: 1019–1025. [DOI] [PubMed] [Google Scholar]
- 80. Melton III, L.J. , Marquez M.A., Achenbach S.J., et al. 2002. Variations in bone density among persons of African heritage. Osteoporos. Int. 13: 551–559. [DOI] [PubMed] [Google Scholar]
- 81. Solomon, L. 1968. Osteoporosis and fracture of the femoral neck in the South African Bantu. J. Bone Joint Surg. Br. 50: 2–13. [PubMed] [Google Scholar]
- 82. Adebajo, A.O. , Cooper C. & Evans J.G.. 1991. Fractures of the hip and distal forearm in West Africa and the United Kingdom. Age Ageing 20: 435–438. [DOI] [PubMed] [Google Scholar]
- 83. Dent, C.E. , Engelbrecht H.E. & Godfrey R.C.. 1968. Osteoporosis of lumbar vertebrae and calcification of abdominal aorta in women living in Durban. Br. Med. J. 4: 76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Conradie, M. , Conradie M.M., Scher A.T., et al. 2015. Vertebral fracture prevalence in black and white South African women. Arch. Osteoporos. 10: 203. [DOI] [PubMed] [Google Scholar]
- 85. Esaadi, M. , Paruk F. & Cassim B.. 2021. Prevalence and clinical risk factors for morphometric vertebral fractures in older subjects in KwaZulu‐Natal. J. Endocrinol. Metab. Diabetes South Afr. 26: 29–33. [Google Scholar]
- 86. Kabenkama, J.M.K. , Banza L., Tshibola J.M., et al. 2018. Morphometric semi‐quantitative assessment of vertebral fractures in postmenopausal black women in Central Africa. Arch. Osteoporos. 13: 13. [DOI] [PubMed] [Google Scholar]
- 87. Creo, A.L. , Thacher T.D., Pettifor J.M., et al. 2017. Nutritional rickets around the world: an update. Paediatr. Int. Child Health 37: 84–98. [DOI] [PubMed] [Google Scholar]
- 88. Carpenter, T.O. , Shaw N.J., Portale A.A., et al. 2017. Rickets. Nat. Rev. Dis. Primers 3: 17101. [DOI] [PubMed] [Google Scholar]
- 89. Thacher, T.D. , Pettifor J.M., Tebben P.J., et al. 2019. Rickets severity predicts clinical outcomes in children with X‐linked hypophosphatemia: utility of the Radiographic Rickets Severity Score. Bone 122: 76–81. [DOI] [PubMed] [Google Scholar]
- 90. Thacher, T.D. , Fischer P.R., Pettifor J.M., et al. 2000. Case–control study of factors associated with nutritional rickets in Nigerian children. J. Pediatr. 137: 367–373. [DOI] [PubMed] [Google Scholar]
- 91. Thacher, T.D. , Pettifor J.M., Fischer P.R., et al. 2006. Case–control study of breast milk calcium in mothers of children with and without nutritional rickets. Acta Paediatr. 95: 826–832. [DOI] [PubMed] [Google Scholar]
- 92. Nutritional Care of Preterm Infants. Karger Book. [Google Scholar]
- 93. Tihtonen, K. , Korhonen P., Isojärvi J., et al. 2021. Calcium supplementation during pregnancy and maternal and offspring bone health: a systematic review and meta‐analysis. Ann. N.Y. Acad. Sci. 10.1111/nyas.14705. [DOI] [PMC free article] [PubMed]
- 94. Sadideen, H. & Swaminathan R.. 2004. Effect of acute oral calcium load on serum PTH and bone resorption in young healthy subjects: an overnight study. Eur. J. Clin. Nutr. 58: 1661–1665. [DOI] [PubMed] [Google Scholar]
- 95. Wiseman, M. 1992. The COMA Report: dietary reference values for food energy and nutrients for the United Kingdom. Br. Food J. 94: 7–9. [Google Scholar]
- 96. ILSI . Recommended Dietary Allowances (RDA) – Harmonization in Southeast Asia. Accessed September 12, 2021. https://ilsisea‐region.org/publication/recommended‐dietary‐allowances‐rda‐harmonization‐in‐southeast‐asia/.
- 97. Wai, J. 2012. Taiwan Osteoporosis Practice Guidelines.
- 98. Chávez, A. 2017. Comer bien para vivir mejor . Primera edición. Ciudad de México: Instituto Nacional de Ciencias Médicas y Nutrición “Salvador Zubirán.”
- 99. Vorster, H.H. , Badham J.B. & Venter C.S.. 2013. 1. An introduction to the revised food‐based dietary guidelines for South Africa. South Afr. J. Clin. Nutr. 26: S5–S12. [Google Scholar]
- 100.Chapter 11. Calcium. Accessed October 3, 2021. http://www.fao.org/3/y2809e/y2809e0h.htm.
- 101. Dickinson, H.O. , Nicolson D.J., Cook J.V., et al. 2006. Calcium supplementation for the management of primary hypertension in adults. Cochrane Database Syst. Rev. CD004639. [DOI] [PMC free article] [PubMed]
- 102. Furspan, P.B. , Rinaldi G.J., Hoffman K., et al. 1989. Dietary calcium and cell membrane abnormality in genetic hypertension. Hypertension 13: 727–730. [DOI] [PubMed] [Google Scholar]
- 103. van Mierlo, LaJ. , Arends L.R., Streppel M.T., et al. 2006. Blood pressure response to calcium supplementation: a meta‐analysis of randomized controlled trials. J. Hum. Hypertens. 20: 571–580. [DOI] [PubMed] [Google Scholar]
- 104. Bergel, E. & Belizán J.M.. 2002. A deficient maternal calcium intake during pregnancy increases blood pressure of the offspring in adult rats. BJOG 109: 540–545. [PubMed] [Google Scholar]
- 105. Winzenberg, T. , Shaw K., Fryer J., et al. 2006. Effects of calcium supplementation on bone density in healthy children: meta‐analysis of randomised controlled trials. BMJ 333: 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Omotayo, M.O. , Martin S.L., Stoltzfus R.J., et al. 2018. With adaptation, the WHO guidelines on calcium supplementation for prevention of pre‐eclampsia are adopted by pregnant women. Matern. Child Nutr. 14: e12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Newmark, H.L. , Wargovich M.J. & Bruce W.R.. 1984. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. J. Natl. Cancer Inst. 72: 1323–1325. [PubMed] [Google Scholar]
- 108. Pence, B.C. & Buddingh F.. 1988. Inhibition of dietary fat‐promoted colon carcinogenesis in rats by supplemental calcium or vitamin D3. Carcinogenesis 9: 187–190. [DOI] [PubMed] [Google Scholar]
- 109. Gandhi, A.B. & Shukla A.K.R.. 2005. Evaluation of BMD of women above 40 years of age. J. Obstet. Gynecol. India 55: 265–267. [Google Scholar]
- 110. Sharma, S. , Tandon V.R., Mahajan A., et al. 2006. Preliminary screening of osteoporosis and osteopenia in urban women from Jammu using calcaneal QUS. Indian J. Med. Sci. 60: 183–189. [PubMed] [Google Scholar]
- 111. Ammini, A.C. 2013. Endocrine Society of India (2006–2007).
- 112. Babu, A.S. , Ikbal F.M., Noone M.S., et al. 2009. Osteoporosis and osteopenia in India: a few more observations. Indian J. Med. Sci. 63: 76–77. [PubMed] [Google Scholar]
- 113. Unni, J. , Garg R. & Pawar R.. 2010. Bone mineral density in women above 40 years. J. Midlife Health 1: 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Aggarwal, N. , Raveendran A., Khandelwal N., et al. 2011. Prevalence and related risk factors of osteoporosis in peri‐ and postmenopausal Indian women. J. Midlife Health 2: 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Marwaha, R.K. , Tandon N., Garg M.K., et al. 2011. Bone health in healthy Indian population aged 50 years and above. Osteoporos. Int. 22: 2829–2836. [DOI] [PubMed] [Google Scholar]
- 116. Vaidya, S.V. , Ekbote V.H., Khadilkar A.V., et al. 2012. Bone status of women over 40 years of age from two socioeconomic strata. Endocr. Res. 37: 25–34. [DOI] [PubMed] [Google Scholar]
- 117. Agrawal, T. & Verma A.K.. 2013. Cross sectional study of osteoporosis among women. Med. J. Armed Forces India 69: 168–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Agrawal, N.K. & Sharma B.. 2013. Prevalence of osteoporosis in otherwise healthy Indian males aged 50 years and above. Arch. Osteoporos. 8: 116 [DOI] [PubMed] [Google Scholar]
- 119. Shetty, S. , Kapoor N., Naik D., et al. 2014. Osteoporosis in healthy South Indian males and the influence of life style factors and vitamin D status on bone mineral density. J. Osteoporos. 2014: e723238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Akhtaruzzaman, M. , Nazrul Islam Khan M., Aktar F. & Islam S.N.. 2013. Nutrition, health and demographic survey of Bangladesh‐2011.
- 121. Fatima, M. , Nawaz H., Kassi M., et al. 2009. Determining the risk factors and prevalence of osteoporosis using quantitative ultrasonography in Pakistani adult women. Singapore Med. J. 50: 20–28. [PubMed] [Google Scholar]
- 122. Iqbal, R. , Haroon M.A., Dar F.J., et al. 2014. Validation of a food frequency questionnaire for assessing macronutrient and calcium intake in adult Pakistani population. J. Coll. Physicians Surg. Pak. 24: 224–227. [PubMed] [Google Scholar]
- 123. Baig, L. , Mansuri F.A. & Karim S.A.. 2009. Association of menopause with osteopenia and osteoporosis: results from population based study done in Karachi. J. Coll. Physicians Surg. Pak. 19: 240–244. [PubMed] [Google Scholar]
- 124. Keramat, A. , Patwardhan B., Larijani B., et al. 2008. The assessment of osteoporosis risk factors in Iranian women compared with Indian women. BMC Musculoskelet. Disord. 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lowe, N.M. , Ellahi B., Bano Q., et al. 2011. Dietary calcium intake, vitamin D status, and bone health in postmenopausal women in rural Pakistan. J. Health Popul. Nutr. 29: 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Naeem, S.T. , Hussain R., Raheem A., et al. 2016. Bone turnover markers for osteoporosis status assessment at baseline in postmenopausal Pakistani females. J. Coll. Physicians Surg. Pak. 26: 408–412. [PubMed] [Google Scholar]
- 127. Steyn, N.P. , Wolmarans P., Nel J.H., et al. 2008. National fortification of staple foods can make a significant contribution to micronutrient intake of South African adults. Public Health Nutr. 11: 307–313. [DOI] [PubMed] [Google Scholar]
- 128. Jackson, M.D. , Motswagole B.S., Kwape L.D., et al. 2013. Validation and reproducibility of an FFQ for use among adults in Botswana. Public Health Nutr. 16: 1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wamono, E.B.K. , Kaaya A.N., Ng'ang'a Z., et al. 2011. Nutrient‐enhancement of Matooke banana for improved nutrient intake of people living with HIV/AIDS in Rakai District, Uganda. African J. Food Agric. Nutr. Dev. 11. [Google Scholar]
- 130. Akerele, D. 2015. Household food expenditure patterns, food nutrient consumption and nutritional vulnerability in Nigeria: implications for policy. Ecol. Food Nutr. 54: 546–571. [DOI] [PubMed] [Google Scholar]


