Abstract
New Findings
-
What is the topic of this review?
This review focuses on the physiological impact of abdominal aortic aneurysm (AAA) on cardiorespiratory fitness and the negative consequences of low fitness on clinical outcomes in AAA. We also discuss the efficacy of exercise training for improving cardiorespiratory fitness in AAA.
-
What advances does it highlight?
We demonstrate the negative impact of low fitness on disease progression and clinical outcomes in AAA. We highlight potential mechanistic determinants of low fitness in AAA and present evidence that exercise training can be an effective treatment strategy for improving cardiorespiratory fitness, postoperative mortality and disease progression.
Abstract
An abdominal aortic aneurysm (AAA) is an abnormal enlargement of the aorta, below the level of the renal arteries, where the aorta diameter increases by >50%. As an aneurysm increases in size, there is a progressive increase in the risk of rupture, which ranges from 25 to 40% for aneurysms >5.5 cm in diameter. People with AAA are also at a heightened risk of cardiovascular events and associated mortality. Cardiorespiratory fitness is impaired in people with AAA and is associated with poor (postoperative) clinical outcomes, including increased length of hospital stay and postoperative mortality after open surgical or endovascular AAA repair. Although cardiorespiratory fitness is a well‐recognized prognostic marker of cardiovascular health and mortality, it is not assessed routinely, nor is it included in current clinical practice guidelines for the management of people with AAA. In this review, we discuss the physiological impact of AAA on cardiorespiratory fitness, in addition to the consequences of low cardiorespiratory fitness on clinical outcomes in people with AAA. Finally, we summarize current evidence for the effect of exercise training interventions on cardiorespiratory fitness in people with AAA, including the associated improvements in postoperative mortality, AAA growth and cardiovascular risk. Based on this review, we propose that cardiorespiratory fitness should be considered as part of the routine risk assessment and monitoring of people with AAA and that targeting improvements in cardiorespiratory fitness with exercise training might represent a viable adjunct treatment strategy for reducing postoperative mortality and disease progression.
Keywords: abdominal aortic aneurysm, aneurysm progression, cardiorespiratory fitness, oxygen delivery, oxygen utilisation
1. INTRODUCTION
Abdominal aortic aneurysm (AAA) is characterized by an abnormal progressive dilatation of the aorta, below the level of the renal arteries, surpassing the normal diameter of the aorta by >50% (Upchurch & Schaub, 2006). The burden of AAA is significant, with a reported global prevalence of ∼6% and a mortality rate that accounts for ∼2% of all annual deaths in males aged >60 years (Ashton et al., 2002). Development of AAA is regarded as a local manifestation of a systemic inflammatory disease, whereby gradual degeneration of the aortic wall leads to weakening, enlargement and, ultimately, a high risk of rupture (Brady et al., 2004). Aneurysm rupture is often life threatening, and the associated mortality rate surpasses 90% (Van ’t Veer et al., 2008). To date, treatment options for AAA consist of open surgical or endovascular aneurysm repair, which are generally available only for patients with a large AAA (>5.5 cm diameter). People with a small AAA (<5.5 cm) typically undergo regular imaging surveillance, and there are no viable treatment options (Wanhainen et al., 2018). Beyond the risk of AAA rupture, common causes of mortality among people with AAA are the postoperative mortality associated with AAA repair (Eslami et al., 2017) and cardiovascular disease‐related mortality (Bath et al., 2017). Of particular note, the prevalence of cardiovascular disease and associated events (e.g., ischaemic heart disease ∼45%, myocardial infarction ∼27% and stroke ∼14%) is very high in people with AAA and has been reported to increase by ∼3% year‐on‐year after AAA diagnosis (Bath et al., 2017).
Cardiorespiratory fitness, measured as the maximal capacity to take up and utilize oxygen, relies on the health and coordinated responses of various physiological systems and organs (Arena et al., 2007). Among the general population, there is a strong association between cardiorespiratory fitness and the risk of morbidity and mortality, particularly that associated with cardiovascular disease (Lee et al., 2010). Current evidence demonstrates that cardiorespiratory fitness is impaired in people with AAA, relative to those without AAA (Rose et al., 2018) and age‐related normative reference values (Ferguson, 2014). Moreover, the association between cardiorespiratory fitness and cardiovascular‐related risk (Kodama et al., 2009) and factors related to AAA growth and rupture, such as increased arterial stiffness (Arena et al., 2007) and endothelial dysfunction (Montero, 2015), raises the possibility that cardiorespiratory fitness might be a viable marker or determinant of clinical outcomes in people with AAA.
In this review, we explore the premise that the inclusion of cardiorespiratory fitness as a treatment target has the potential to mitigate cardiovascular risk, aneurysm progression and the morbidity and mortality associated with AAA. The aim of the review is to provide a comprehensive overview of the importance of cardiorespiratory fitness in people with AAA, and it includes three related sections: (1) the physiological impact of AAA on cardiorespiratory fitness; (2) the association of low cardiorespiratory fitness with clinical outcomes in AAA; and (3) the effect of exercise training interventions on cardiorespiratory fitness and cardiovascular risk in people with AAA.
2. THE IMPACT OF ABDOMINAL AORTIC ANEURYSM ON CARDIORESPIRATORY FITNESS
2.1. The assessment of cardiorespiratory fitness
Cardiorespiratory fitness reflects the capacity of the body to take up and utilize oxygen. It is dependent on the synergistic function of key organ systems, particularly the respiratory, cardiovascular and muscle‐metabolic systems, to deliver oxygen from the ambient air to the mitochondria in the working skeletal muscles (Lee et al., 2010). Oxygen consumption is described by the Fick equation, where oxygen utilization () = cardiac output × arteriovenous oxygen difference (Levine, 2008). These parameters provide an insight into the physiological determinants of oxygen consumption, whereby cardiac output is primarily dependent on central factors, including heart rate, stroke volume and aortic function, and arteriovenous oxygen difference depends largely on peripheral factors, such as peripheral blood flow, blood oxygen‐carrying capacity, capillary supply and mitochondrial volume and density, and the matching of oxygen perfusion and diffusion between the capillaries and the mitochondria (Del Torto et al., 2017).
Cardiopulmonary exercise testing (CPET) is used to assess functional capacity and cardiorespiratory fitness (Albouaini et al., 2007). During CPET, expired ventilatory gasses are collected and analysed while the test participant undertakes incremental exercise to their maximal effort (i.e., the point at which they are not volitionally able to sustain the exercise load and continue). The maximal rate of oxygen uptake during exercise () is considered to be the gold‐standard measure of cardiorespiratory fitness. The is commonly defined as a plateau in oxygen consumption for a sustained period (e.g., 30–60 s) during maximal incremental exercise. However, given that this plateau in oxygen consumption is often not observed, (i.e., the highest rate of oxygen consumption during a test) is commonly used as a measure of cardiorespiratory fitness (Arena et al., 2007; Green & Askew, 2018). Cardiopulmonary exercise testing with expired gas analysis also enables an assessment of gas exchange thresholds (GETs), including the ventilatory threshold (VT), where expired ventilation increases disproportionately to the increase in . The VT provides a submaximal measure of functional capacity, occurring at ∼45–65% of the (Sato et al., 1989). Although its estimation can be subjective (e.g., using the V‐slope, ventilatory equivalent or excess carbon dioxide methods), it has been shown to be interpreted reliably between clinicians (Vainshelboim et al., 2017). Exercise beyond VT is associated with metabolic acidosis, hyperventilation and reduced capacity to perform work; therefore, its assessment is useful in clinical populations when a maximal CPET might be contraindicated (Ferguson, 2014).
2.2. Cardiorespiratory fitness in people with AAA
Cardiorespiratory fitness has been demonstrated to be impaired in people with AAA. A recent large retrospective study reported a mean reduction of 13.6 ml kg−1 min−1 [95% confidence interval (CI) 12.0−15.2, P < 0.001] in in people with AAA (n = 124) compared with apparently healthy age‐matched individuals (n = 108) (Rose et al., 2018). In support of this finding, a recent comparative study reported that people with a small AAA (<5.5 cm) demonstrate significantly lower cardiorespiratory fitness (n = 22, 19.0 ± 3.5 ml kg−1 min−1) when compared with those without an AAA (n = 22, 24.5 ± 2.8 ml kg−1 min−1, P ≤ 0.001) (Perissiou et al., 2019). These findings from comparative studies suggest there is at least a ∼25% deficit in cardiorespiratory fitness in people with AAA. Although to date these are the only two studies to compare cardiorespiratory fitness directly between people with and without AAA, there have been 17 studies that have reported estimates of cardiorespiratory fitness in patients with AAA. These studies are summarized in Table 1 and include cross‐sectional and exercise training investigations in people with a small or large AAA. Across the 2,259 study participants with small or large AAA (aged 69–76 years), these studies report means ranging between 13.3 and20.0 ml kg−1 min−1 and a VT range of 9.4–12.5 ml kg−1 min−1. According to age‐related normative data, the and VT of people with AAA are categorized as ‘very poor’ and within the lowest (25th) percentile of the general population (Ferguson, 2014; Vainshelboim et al., 2020). Importantly, current evidence demonstrates that a < 15 ml kg−1 min−1 and a VT < 10 ml kg−1 min−1 are associated with reduced functional capacity and severe cardiovascular risk (Kodama et al., 2009). The reported mean in the eight studies that included people with a large AAA (n = 1,859; 13.3–17.5 ml kg−1 min−1; Table 1B) was generally lower when compared with the nine studies that included people with a small AAA (n = 700; 18.0–20.0 ml kg−1 min−1; Table 1A). To date, there have been no studies directly comparing cardiorespiratory fitness levels between those with small and large AAA.
TABLE 1.
Cardiorespiratory fitness in people with a small (A) or large (B) abdominal aortic aneurysm
| Authors | Group/site | n | Age (years) | AAA size (cm) | Body mass index (kg m−2) | Type of exercise |
|
VT (ml kg−1 min−1) | |
|---|---|---|---|---|---|---|---|---|---|
| A. Reported cardiorespiratory fitness in people with a small AAA (<5.5 cm) | |||||||||
| Kothmann et al. (2009) | Exercise | 17 | 70 | <5.5 | – | Cycling | – | 10.5 ± 2 | |
| Usual care | 8 | 70 | – | – | 10.4 ± 2 | ||||
| Myers et al. (2011) | – | 306 | 72 ± 7.5 | <5.5 | 28.2 ± 4 | Treadmill | 20.0 ± 6.3 | – | |
| Tew et al. (2012) | Exercise | 11 | 71 ± 8 | <5.5 | 27.9 ± 3 | Cycling | 19.3 ± 4.5 | – | |
| Usual care | 14 | 74 ± 6 | 28.3 ± 3 | 18.0 ± 5.7 | 12.4 ± 3.1 | ||||
| Barakat et al. (2014) | – | 20 | 75 ± 6 | – | – | Treadmill | 18.2 ± 2.8 | 12.2 ± 2.1 | |
| Myers et al. (2014) |
Exercise |
72 |
72 ± 7 |
<5.5 |
29.1 ± 4 |
Treadmill |
19.5 ± 5.8 |
– |
|
| Usual care | 68 | 71 ± 8 | 27.0 ± 3 | 20.0 ± 6.4 | – | ||||
| West et al. (2015) | – | 48 | 70 ± 6 | <5.5 | 28.7 ± 4 | Cycling | 18.2 ± 5.3 | 11.4 ± 2.7 | |
| Lima et al. (2018) |
Exercise |
33 |
73 ± 6 |
<5.5 |
28.8 ± 3 |
Cycling |
18.8 ± 4.8 |
13.3 ± 3.3 |
|
| Usual care | 32 | 73 ± 6 | 27.6 ± 3 | 19.7 ± 5.5 | 15.6 ± 4.7 | ||||
| Nakayama et al. (2018) | – | 49 | 72 ± 8 | <5.5 | 24.1 ± 3 | Cycling | 18.0 ± 6.0 | 12.0 ± 3.0 | |
| Perissiou et al. (2019) | – | 22 | 74 ± 6 | <5.5 | 28.0 ± 9 | Cycling | 19.0 ± 3.5 | – | |
| B. Reported cardiorespiratory fitness in people with a large AAA (>5.5 cm) | |||||||||
| Prentis et al. (2012) | – | 185 | 74 ± 8 | >5.5 | 27.7 ± 4 | Cycling | 14.0 ± 3.5 | 11.3 ± 2.7 | |
| Barakat et al. (2015) | – | 130 | 75 ± 7 | >5.5 | 27.8 ± 4 | Treadmill | 16.6 ± 2.2 | 11.8 ± 1.5 | |
| Carlisle et al. (2015) | Newcastle | 283 | 74 ± 8 | >5.5 | 27.8 ± 5 | Cycling | 14.7 ± 3.6 | 11.6 ± 2.6 | |
| Sheffield | 358 | 74 ± 7 | >5.5 | 27.5 ± 5 | Cycling | 17.8 ± 3.8 | 11.5 ± 2.5 | ||
| South Tees | 153 | 74 ± 7 | >5.5 | – | Cycling | 13.3 ± 3.6 | 9.4 ± 2.3 | ||
| Torbay | 302 | 73 ± 7 | >5.5 | 27.2 ± 4 | Cycling | 15.7 ± 3.7 | 11.0 ± 2.3 | ||
| Barakat et al. (2017) | – | 124 | 73 ± 7 | >5.5 | – | Treadmill | 17.5 ± 4.5 | 12.5 ± 3.9 | |
| Tew et al. (2017) | Exercise | 27 | 75 ± 6 | >5.5 | 26.5 ± 4 | Cycling | 16.5 ± 3.7 | 11.0 ± 2.1 | |
| Usual care | 26 | 75 ± 6 | 26.8 ± 3 | 15.7 ± 3.1 | 10.9 ± 2.7 | ||||
| Weston et al. (2017) | – | 27 | 74 ± 6 | >5.5 | – | Cycling | 16.5 ± 3.7 | 11.0 ± 2.1 | |
| Rose et al. (2018) | – | 124 | 70 ± 7 | >5.5 | 27.1 ± 3 | Cycling | 14.4 ± 3.2 | – | |
| Straw et al. (2020) | – | 120 | 76 ± 8 | >5.5 | – | Cycling | 14.7 ± 3.6 | 10.9 ± 2.8 | |
Note. Data were derived from studies that assessed cardiorespiratory fitness in people with an abdominal aortic aneurysm (AAA) using the measures peak oxygen consumption () and/or ventilatory threshold (VT). Data that are extracted from exercise training studies include separate sets of data for the exercise training group and the comparator group (usual care, control). The large study by Carlisle et al. (2015) reported separate sets of means for each of the four hospital sites.
2.3. The potential impact of AAA on oxygen delivery and utilization
Although there have been no direct investigations to understand the impact of AAA on the physiological determinants of cardiorespiratory fitness, impairments in cardiorespiratory fitness can broadly be explained by limitations in factors associated with oxygen delivery and/or oxygen utilization (Burtscher, 2013). A primary physiological determinant of cardiorespiratory fitness is the ability of the blood and the vasculature to carry oxygen efficiently from the heart to the periphery, in order to meet the oxygen requirements of working muscles (Levine, 2008). Chronic systemic inflammation, a primary determinant of AAA (Dale et al., 2015), plays a key role in the formation of vascular lesions and remodelling, which consequently leads to endothelial dysfunction and increased arterial stiffness, both markers of arterial wall damage (Castellon & Bogdanova, 2016) and main characteristics of AAA (Kadoglou et al., 2012; Siasos et al., 2015). Importantly, vascular endothelial dysfunction and elevated arterial stiffness are factors known to impact blood flow and oxygen delivery directly (Kadoglou et al., 2012; Siasos et al., 2015). We recently demonstrated that aortic stiffness and endothelial dysfunction are associated with lower cardiorespiratory fitness () in people with a small AAA (Bailey et al., 2017; Perissiou et al., 2019). There is evidence that increased arterial stiffness is directly associated with impaired muscle oxygenation during exercise in hypertensive patients (Dipla et al., 2017). In addition, endothelial dysfunction is widely associated with hypoperfusion of regional vasculature, including limb and muscle blood flow during exercise (Vallet, 2002). Likewise, at the microvasculature, endothelial dysfunction and a disturbed production of nitric oxide derivatives is associated with impaired capillary blood flow and altered oxyhaemoglobin binding (Iankovskaia & Zinchuk, 2007), which potentially limit oxygen delivery to working muscles.
Impaired function and structure of the aorta are also associated with deterioration in aortic Windkessel function (Belz, 1995). The Windkessel effect dampens the phasic systolic surges in blood flow produced by ventricular ejection into a smoother, more continuous outflow to the peripheral vessels. Interestingly, Swillens et al. (2008) demonstrated in computer‐constructed models of AAA that the aneurysm itself is responsible for a deterioration in Windkessel wave reflection, leading to an impairment in cardiac output and reduced blood flow to the periphery (Swillens et al., 2008). Reduced blood flow is commonly reported at the site of aortic aneurysms (White & Dalman, 2008), and Suh et al. (2011) demonstrated a reduction in aneurysmal blood flow during cycling exercise (Suh et al., 2011). This has been interrogated further with three‐dimesional computer models of large AAA, in which during rest and exercise conditions there is recirculation of blood within the aneurysm, which contributes to reduce blood distribution to the periphery throughout the cardiac cycle (Varshney et al., 2020). These impairments in vascular function and haemodynamics are potentially compounded by an altered blood oxygen‐carrying capacity in people with AAA. Specifically, Zhang et al. (2012) retrospectively reviewed haemoglobin levels in 255 people with AAA and reported a high prevalence of anaemia (34.5%) and that the haemoglobin concentration was independently and inversely associated with aneurysm diameter (Zhang et al., 2012).
Oxygen extraction and the efficient utilization of oxygen by the mitochondria are fundamental determinants of cardiorespiratory fitness (Jacobs & Lundby, 2013). To date, there have been no direct investigations of muscle oxygen utilization in people with AAA. However, several studies have established that mitochondrial dysfunction is evident in the smooth muscle of the aneurysm wall. It has also been reported that there is differential expression of a number of genes associated with mitochondrial function and oxidative phosphorylation within the aneurysm wall (Yuan et al., 2015). The reduced mitochondrial function might also be accompanied by increased glycolysis and increased lactate production (Prado‐Garcia et al., 2020). Indeed, Tsuruda et al. (2012) reported an increased glycolytic activity in aneurysmal mouse models (Tsuruda et al., 2012), and Modrego et al. (2012) showed in vitro that lactate content is elevated in AAA compared with control participants.
Chronic inflammation, which characterizes AAA (Dale et al., 2015), is known to contribute further to a hypoxic microenvironment within tissues, a phenomenon known as inflammatory hypoxia (Biddlestone et al., 2015). Indeed, studies have reported that AAAs demonstrate inflammation‐induced tissue hypoxia and attenuated oxygen diffusion (Blassova et al., 2019). In addition, systemic chronic inflammation in AAA has been shown to favour a pro‐oxidant microenvironment in people with AAA (Meital et al., 2020), a state that is associated with impairments in muscle oxygen utilization and exercise capacity (König et al., 2001). Indeed, Menteşe et al. (2016) reported that compared with control subjects, individuals with AAA demonstrate elevated oxidative stress levels with no change in antioxidant capacity (the ability of inhibiting molecules with high redox potential).
Morphometric analyses of muscle biopsy samples from the anterior tibialis muscle show a predominance of atrophic type I muscle fibres in people with AAA (Albani et al., 2000). Interestingly, current evidence suggests that type I muscle fibre atrophy is a consequence of injury induced by reactive oxygen species and is associated with impaired oxygen utilization (Bonaldo & Sandri, 2013). Hence, we could speculate that muscle atrophy contributes indirectly to impaired muscle oxygen utilization in people with AAA. The studies presented here provide only indirect evidence of the potential impact of AAA on muscle oxygen utilization. There is a need for future studies directly to assess the determinants of muscle oxygen utilization (e.g., mitochondrial volume and function, capillary supply, aerobic enzyme activities and muscle oxygen extraction) at rest and during exercise in people with AAA.
2.4. The potential impact of AAA co‐morbidities on the cardiorespiratory fitness of people with AAA
There are several co‐morbidities commonly observed in people with AAA that might contribute to their impairment in cardiorespiratory fitness. Coronary artery disease (CAD) is one of the most prevalent co‐morbidities, with 25–37% of people with AAA reported also to have a diagnosis of CAD (Van Kuijk et al., 2009). There is a well‐established impairment in cardiorespiratory fitness associated with CAD (Gander et al., 2015), because myocardial ischaemia associated with the stenosis of coronary arteries leads to a reduction in cardiac output and therefore limits oxygen delivery to the working skeletal muscles. Likewise, peripheral arterial disease, which is present in ∼20% of people with AAA (Kent et al., 2010), is associated with low levels of cardiorespiratory fitness (Hou et al., 2002). Peripheral arterial disease is characterized by blood flow impairment to the muscles of the lower limbs. There is also evidence of skeletal muscle changes, including alterations in capillary supply, mitochondrial density and function, and muscle fibre morphology and metabolism, all of which potentially contribute to impaired oxygen extraction and utilization (Baum et al., 2016; Hamburg & Creager, 2017). These skeletal muscle changes can also be exacerbated by the presence of type 2 diabetes, which is diagnosed in ∼15% of people with AAA (De Rango et al., 2014; Green et al., 2007). Furthermore, diabetes potentially limits the efficient use of glucose as a substrate during exercise, which is associated with impaired oxygen economy during exercise (Bauer et al., 2007) and a reduction in cardiorespiratory fitness (Nesti et al., 2020). Finally, people with AAA commonly present with impaired pulmonary function, and chronic obstructive pulmonary disease is reported in up to ∼28% of AAA patients (Lederle et al., 2015). Chronic obstructive pulmonary disease primarily causes a diffusion limitation at the lungs and has been shown to limit the capacity for oxygen delivery to peripheral tissues and working muscles (Broxterman et al., 2020; Nakamura et al., 2004).
Sections 2.3 and 2.4 outline the systemic and local cardiovascular alterations that occur with AAA, in addition to several common co‐morbidities, that are likely to contribute to the impaired cardiorespiratory fitness in these individuals. A theoretical overview of the association between these mechanisms is depicted in Figure 1. Although this provides a plausible basis for understanding the limits associated with AAA, studies that interrogate the physiological mechanisms of cardiorespiratory fitness in people with AAA are lacking, and there is a need for further research in this area.
FIGURE 1.
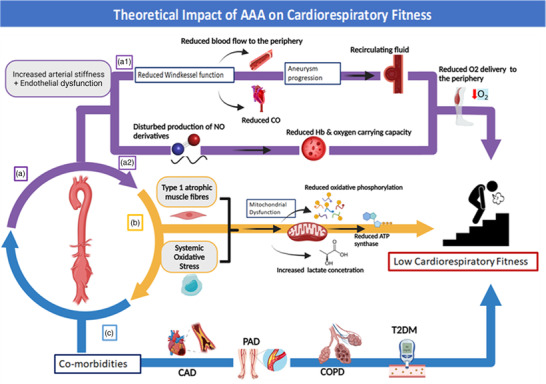
Theoretical impact of AAA on cardiorespiratory fitness. (a) Abdominal aortic aneurysm affects oxygen delivery throughout the body. (a1) Increased arterial stiffness contributes to the reduced Windkessel function observed in AAA, leading to reduced cardiac output and blood flow to the periphery (Kadoglou et al., 2012; Swillens et al., 2008). Furthermore, as AAA diameter increases, the recirculating fluid in the aneurysmal site leads to further disruption of blood distribution to the periphery (Suh et al., 2011; Varshney et al., 2020). (a2) Endothelial dysfunction and disturbed production of NO derivatives contribute to a reduced oxygen‐carrying capacity by the blood, leading to reduced blood flow to the periphery (Iankovskaia & Zinchuk, 2007). (b) People with AAA demonstrate increased systemic oxidative stress (Menteşe et al., 2016) and predominance of atrophic type I muscle fibres (Albani et al., 2000), factors associated with oxygen utilization determinants, such as mitochondrial dysfunction (Handy & Loscalzo, 2012), reduced oxidative phosphorylation and ATP synthase and increased lactate production (Bonaldo & Sandri, 2013). (c) Abdominal aortic aneurysms are characterized by co‐morbidities that create an ischaemic environment in the central (CAD) (Kent et al., 2010) and peripheral (PAD) circulatory system (Kent et al., 2010) and affect oxygen distribution by the lungs (COPD) (Lederle et al., 2015) and oxygen utilization by the muscles (T2DM) (De Rango et al., 2014). Abbreviations: AAA, abdominal aortic aneurysm; BF, blood flow; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; Hb, haemoglobin; NO, nitric oxide; PAD, peripheral arterial disease; T2DM, type 2 diabetes mellitus. The figure was created with BioRender.com
3. CARDIORESPIRATORY FITNESS AS A PREDICTOR OF CLINICAL OUTCOMES IN PEOPLE WITH ABDOMINAL AORTIC ANEURISM UNDERGOING OPEN SURGICAL AND ENDOVASCULAR REPAIR
Open surgical or endovascular repair are currently the only recognized effective treatments for AAA to prevent rupture and aneurysm‐related death, according to the National Institute for Health & Care Excellence (NICE) AAA guidelines [NG156] (National Institute for Health & Care Excellence, 2020). Open repair is considered a major surgical procedure that requires an abdominal incision to be made in order to repair the aneurysm with a graft. Endovascular aneurysm repair (EVAR) is considered less invasive. It involves a small incision in the groin and femoral artery, through which a stent graft is deployed intra‐arterially in order to exclude circulation from the aneurysm sac effectively. Regardless of the method used, aneurysm repair is associated with a significant risk of mortality (open ∼3%; EVAR ∼1%), and for this reason, it is generally reserved for those with a large AAA (>5.5 cm in diameter) and for those where the risk of rupture is greatest (Locham et al., 2017). Abdominal aortic aneurysm repair places considerable metabolic demands on patients during the repair procedure and the short‐term (i.e., 3 months) postoperative period (Salartash et al., 2001). This is thought to be attributable to a strong inflammatory response that leads to an increase in basal oxygen demand of ∼110–170 ml min−1 during the postoperative period (Older et al., 1999). The increased energy requirements are reported to be necessary for wound healing and the resolution of inflammation and are associated with significant elevations in ventilation and cardiac activity (Davies & Wilson, 2004). Failure of the cardiorespiratory system to meet these increased metabolic requirements is suggested to contribute to intra‐ and postoperative complications and mortality in AAA (Struthers et al., 2008).
Several studies have established that an impairment in preoperative cardiorespiratory fitness is closely associated with the risk of death in the short‐term (≤3 months) period after open AAA repair (Table 2A). Specifically, Hartley et al. (2012) demonstrated that people with a ≤ 15 ml kg−1 min−1 and a VT ≤ 10.2 ml kg−1 min−1 were at increased risk of both 30‐ and 90‐day mortality after open repair. Likewise, Goodyear et al. (2013) found that people with a VT ≤ 11 ml kg−1 min−1 demonstrated a 9.9% higher 30‐day mortality rate after open repair compared with people who achieved a VT ≥ 11 ml kg−1 min−1. Finally, Barakat et al. (2015) reported that different measures of fitness are associated with specific perioperative complications. A significant relationship was demonstrated between a VT ≤ 10.2 ml kg−1 min−1 and cardiac complications and between a ventilatory equivalent for carbon dioxide ≥ 42 ml kg−1 min−1 (, a GET variable associated with elevated pulmonary pressures) and respiratory complications (Barakat et al., 2015). These results highlight the clinical importance and impact of cardiorespiratory fitness on the short‐term postoperative mortality and morbidity of people with AAA after open surgical repair.
TABLE 2.
Cardiorespiratory fitness as a predictor of short‐term (≤3 months; A) and long‐term (>3 months; B) postoperative clinical outcomes after abdominal aortic aneurysm repair
| Survival analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors | n | Male (%) | AAA repair method assessed | Mean follow‐up (months) | Primary outcome | Fitness threshold | Effect of fitness on postoperative outcome | Odds ratio | Hazard ratio | P‐value |
| A. Cardiorespiratory fitness relationship with short‐term (≤3 months) postoperative clinical outcomes | ||||||||||
| Hartley et al. (2012) a,b | 415 | 84 | OPEN+EVAR | 1 | Postoperative mortality | ≤ 15.0 | Both subthreshold CPET values identified patients at increased risk of early death after AAA repair | 5.41 | – | 0.013 |
| VT ≤ 10.2 | 4.50 | – | 0.013 | |||||||
| Hartley et al. (2012) a,b | 415 | 84 | 3 | Postoperative mortality | ≤ 15.0 | 8.00 | – | 0.001 | ||
| Prentis et al. (2012) | 185 | 87 | OPEN+EVAR | 2 | Postoperative mortality | VT ≤ 10.2 | A VT ≤ 10.2 was associated with postoperative complications and mortality | 3.46 | – | 0.013 |
| Goodyear et al. (2013) | 230 | – | OPEN+EVAR | 1 | Postoperative mortality | VT ≤ 11.0 | A preoperative VT ≤ 11.0 was associated with increased postoperative mortality and length of hospital stay | 3.06 | – | 0.015 |
| Barakat et al. (2015) | 130 | 89 | OPEN+EVAR | 1 | Postoperative cardiac complications | ≥ 42 |
A VT ≤ 10.2 was associated only with postoperative mortality and cardiac complications after AAA repair. |
1.02 | – | 0.054 |
| VT ≤ 10.2 | 0.55 | – | 0.005 | |||||||
| ≥ 42 | 0.96 | – | 0.540 | |||||||
| Postoperative pulmonary complications | ≤ 15.0 |
A ≥ 42 was associated with pulmonary complications after AAA repair |
0.89 | – | 0.363 | |||||
| VT ≤ 10.2 | 0.85 | – | 0.317 | |||||||
| ≥ 42 | 1.18 | – | 0.005 | |||||||
| B. Cardiorespiratory fitness relationship with long‐term postoperative clinical outcomes | ||||||||||
| Nugent et al. (1998) | 30 | 75 | OPEN | 12 | Postoperative complications | ≤ 20.0 | A ≤ 20.0 identified patients at increased risk of complications after AAA repair | 2.33 | – | 0.001 |
| Carlisle et al. (2007) | 130 | – | OPEN | 24 | Postoperative mortality | ≤ 15.0 |
Subthreshold CPET variables identified patients unlikely to survive in the 24‐month period after AAA repair |
– | 0.83 | 0.002 |
| VT ≤ 10.0 | 0.74 | 0.001 | ||||||||
| > 42 | 1.12 | 0.001 | ||||||||
| Thompson et al. (2011) | 102 | 93 | OPEN | 30 | Postoperative mortality | VT≤ 11.0 | A VT ≤ 11.0 was successful in predicting 30‐month mortality after AAA repair | 3.20 | – | 0.001 |
| Grant et al. (2015) | 506 | 83 | OPEN+EVAR | 36 | Postoperative mortality | ≤ 15.0 |
Subthreshold CPET variables were independent predictors of reduced survival after AAA repair |
– | 1.63 | 0.046 |
| ≥ 42 | – | 1.68 | 0.049 | |||||||
| Carlisle et al. (2015) | 1,096 | 90 | OPEN+EVAR | 60 | Postoperative mortality | Age‐expected | Subthreshold CPET values identified patients who were at long‐term (1–5 years) increased risk of death after AAA repair | – | 0.88 | ≥0.001 |
| Age‐expected VT | – | 0.88 | ≥0.001 | |||||||
| Age‐expected | – | 1.05 | ≥0.001 | |||||||
| Rose et al. (2018) | 124 | 83 | OPEN+EVAR | 24 | Postoperative mortality | ≤ 13.1 | A ≤ 13.1 and a ≥ 34 were associated with increased risk of postoperative mortality | 0.81 | 0.010 | |
| VT ≤ 10.2 | 0.74 | 0.030 | ||||||||
| ≥ 34 | 1.11 | 0.010 | ||||||||
| Straw et al. (2020) | 120 | 86 | EVAR | 36 | Postoperative mortality |
≤ 15.0 ≥ 42 |
A ≤ 15.0 and a ≥ 42 were associated with reduced survivorship after AAA repair | – | 1.34 | 0.283 |
| – | 1.88 | 0.016 | ||||||||
Abbreviations: AAA, abdominal aortic aneurism; CPET, cardiopulmonary exercise testing; , ventilatory equivalent for carbon dioxide; , peak oxygen consumption; VT, ventilatory threshold.
Note. Data are from published studies that assessed the risk using the hazard ratio (the relative risk of an event happening at a specific time) or odds ratio (which quantifies the strength of the association between two events) of mortality or adverse outcomes after open surgical (OPEN) or endovascular (EVAR) AAA repair based on preoperative cardiorespiratory fitness. The VT and are presented in relative units (millilitres per kilogram per minute).
Hartley et al. (2012) reported mortality at a1 month and b3 months after repair.
Endovascular AAA repair was originally developed as a lower‐risk non‐invasive procedure that would also accommodate people who were considered physically ineligible (unfit) for open surgical repair (Parodi et al., 1991). It is associated with a significantly lower rate of aneurysm‐related mortality than no repair (Greenhalgh, 2005). Indeed, studies to date have also reported that EVAR demonstrates significantly reduced short‐term postoperative mortality (Goodyear et al., 2013; Hartley et al., 2012) and morbidity (Prentis et al., 2012) in unfit (VT ≤ 11 ml kg−1 min−1) people with AAA compared with open repair. However, it seems likely that the early benefit of EVAR with respect to short‐term postoperative mortality is abolished in the long term, owing largely to fatal endograft leaks and ruptures (Patel et al., 2016). Indeed, a committee of clinicians appointed by the UK National Institute for Health and Care Excellence recently published a scientific report recommending the use of open repair over EVAR repair (Bradbury et al., 2021). Interestingly, evidence demonstrates that besides endograft ruptures, cardiorespiratory fitness is one of the main determinants of the increased long‐term postoperative mortality observed after EVAR. Specifically, Straw et al. (2020) assessed the 3‐year postoperative mortality in people undergoing EVAR and reported that patients with a ≤ 15 ml kg−1 min−1 and a ≥ 42 ml kg−1 min−1 have an increased risk of long‐term postoperative mortality compared with patients who have higher measures of fitness. These results reinforce the impact of cardiorespiratory fitness as a significant determinant of long‐term survival in people with AAA undergoing EVAR.
To date, several studies have demonstrated that cardiorespiratory fitness is associated with long‐term postoperative mortality regardless of the AAA repair modality used (Table 2B). Specifically, a VT ≤ 10.2 ml kg−1 min−1 and a ≤ 15 ml kg−1 min−1 were found successfully to predict 3‐year postoperative survival (Grant et al., 2015) and length of hospital stay (Prentis et al., 2012) regardless of whether EVAR or open repair was used. Importantly, the use of a combination of cardiorespiratory fitness and GET variables, including , VT and , might strengthen the prediction of mortality following AAA repair and help to assess the risk versus benefit before AAA repair (Grant et al., 2015). Finally, in a large multicentre study (n = 1,096), Carlisle et al. (2015) used presurgical values of , VT and in a survival calculator that strongly predicted 5‐year postoperative mortality in patients that underwent either EVAR or open repair.
In summary, there is a plethora of evidence demonstrating that cardiorespiratory fitness is a significant predictor of short‐ and long‐term postoperative mortality and morbidity, regardless of the repair modality, in people with AAA. However, heterogeneity among available studies and the use of retrospective study designs does not allow for a suitable comparison, because the reported CPET variables and cut‐off values are not reported universally between studies. This is also noted in the latest guidelines for people with AAA from the National Institute of Health and Care Excellence (NICE guideline 156), published in 2020, in which it is highlighted that current evidence is not sufficiently robust and homogeneous to form official guidelines for using cardiorespiratory fitness in order to identify patients at risk for postoperative mortality (National Institute for Health & Care Excellence, 2020). Future studies should address the need for more homogeneous fitness data that will aid in the development of universal clinical thresholds to aid in the management of people with AAA.
4. THE BENEFICIAL EFFECTS OF EXERCISE TRAINING IN PEOPLE WITH ABDOMINAL AORTIC ANEURISM
Over the last decade, there has been growing interest in the use of exercise training (therapy) as an adjunct treatment for both surgical (large AAA) and non‐surgical (small AAA) management of people with AAA. This is based on the many benefits that improved cardiorespiratory fitness seems to have on postoperative outcomes (as highlighted in Section 3) and in reducing cardiovascular‐related mortality (Kodama et al., 2009). This section provides a detailed review of evidence of the effect of exercise training on cardiorespiratory fitness, postoperative outcomes, cardiovascular health parameters and disease progression in people with AAA.
4.1. The effect of exercise training on cardiorespiratory fitness
Several studies have reported improvements in cardiorespiratory fitness after short‐term (6–12 weeks) exercise training in patients with a small AAA (Table 3). All studies have used aerobic or combined exercise (aerobic plus resistance exercise) at a moderate intensity, with a frequency of two to three in‐hospital exercise sessions per week. No exercise‐induced adverse events have been reported in any of the studies to date. Overall, short‐term exercise interventions were able to evoke significant increases in VT [change (Δ) ranging from 1.1 to 3.0 ml kg−1 min−1] (Kothmann et al., 2009; Tew et al., 2012) and (Δ1.2–1.7 ml kg−1 min−1) (Lima et al., 2018; Tew et al., 2012) in patients with small AAA (< 5.5 cm) when compared with a usual care group. Importantly, most of the studies reported that the improvements in cardiorespiratory fitness met the criteria for a minimum clinically important difference (i.e., 0.5 × SD of the reported change in or VT) (Lima et al., 2018; Tew et al., 2012).
TABLE 3.
Summary of studies investigating the effect of exercise training in patients with a small or large abdominal aortic aneurysm
| Author(s) | Group | n | Age (years) | AAA size (cm) | Exercise training | Exercise intensity | Duration and frequency | Primary outcomes (reported change in the exercise group compared with usual care group a or baseline b ) |
|---|---|---|---|---|---|---|---|---|
| Kothmann et al. (2009) |
Exercise Usual care |
17 8 |
70 70 |
<5.0 | 30 min of static bicycling | Moderate | 6 weeks; three sessions per week | VT ↑ 1.1 ml kg−1 min−1a |
| Tew et al. (2012) |
Exercise Usual care |
11 14 |
71 ± 8 74 ± 6 |
<5.0 | 35–45 min of treadmill walking and cycle ergometry | Moderate | 12 weeks; three sessions per week |
VT ↑ 2.5 ml kg−1 min−1 a ↑ 1.7 ml kg−1 min−1 SBP ↓ 9.0 mmHg hs‐CRP ↓ 0.8 mg L−11 a |
|
Myers et al. (2014) 3 months |
Exercise Usual care |
60 61 |
72 ± 7 71 ± 8 |
<5.0 | 60 min of treadmill, cycle ergometry, stair climbing, elliptical training | Moderate | 3 months; three sessions per week |
↑ 1.1 ml kg−1 min−1 b AAA growth Non‐applicable |
| 12 months |
Exercise Usual care |
53 58 |
12 months; three sessions per week |
↑ 1.7 ml kg−1 min−1 b AAA growth ↓ 0.03 cm |
||||
| 24 months |
Exercise Usual care |
36 46 |
24 months; three sessions per week |
↑ 1.7 ml kg−1 min−1 b AAA growth −0.06 cm b |
||||
| 36 months |
Exercise Usual care |
21 24 |
36 months; three sessions per week |
↑ 2.5 ml kg−1 min−1 b AAA growth ↑ 0.07 cm b |
||||
| Barakat et al. (2016) |
Exercise Usual care |
33 15 |
74 ± 7 73 ± 8 |
>5.0 | 60 min of cycle ergometer, knee extensions, biceps/arm curls, knee bends | Moderate | 6 weeks; three sessions per week |
↑ 2.8 ml kg−1 min−1 b VT ↑ 2.1 ml kg−1 min−1 b |
| Tew et al. (2017) |
Exercise Usual care |
27 26 |
75 ± 6 75 ± 6 |
>5.0 | 8 × 2 min cycling intervals with 2 min active recovery | High | 4 weeks; three sessions per week |
↑ 0.5 ml kg−1 min−1 VT ↑ 0.3 ml kg−1 min−1 |
| Lima et al. (2018) |
Exercise Usual care |
33 32 |
73 ± 6 72 ± 8 |
<5.0 | 45 min treadmill, cycle ergometry, stair climbing, elliptical rowing and resistance exercises | Moderate | 12 weeks; three sessions per week |
↑ 1.2 ml kg−1 min−1 b VT ↑ 3.0 ml kg−1 min−1 b |
| Nakayama et al. (2018) |
CR Non‐CR |
44 44 |
72 ± 8 72 ± 8 |
<5.0 | 30–40 min bicycle ergometer and limb resistance training | Low | 150 days; one to three sessions per week |
AAA growth rate ↓ 0.24 cm per year a hs‐CRP ↓ 1.6 mg L−1 a |
| Nakayama et al. (2019) |
CR Non‐CR |
15 25 |
77 ± 4 74 ± 6 |
<5.0 |
30–40 min bicycle ergometer and limb resistance training |
Low | 150 days; one to three sessions per week | AAA growth rate ↓ 0.13 cm per year a |
| Niebauer et al. (2021) |
Exercise Usual care |
42 54 |
73 ±8 74 ± 8 |
<5.0 | 60 min of treadmill, cycle ergometry, stair climbing, elliptical training | Moderate | 12 months; three sessions per week |
SBP ↓ 9.0 mm Hg−1 LAP ↓ 9.61 cm mmol L−1 a |
Abbreviations: AAA, abdominal aortic aneurism; CR, cardiac rehabilitation; hs‐CRP, high sensitivity C‐reactive protein; LAP, lipid accumulation product; SBP, systolic blood pressure; VT, ventilatory threshold; , peak oxygen consumption.
Change in the exercise group is reported compared with the usual care group (P < 0.05).
Change in the exercise group is reported compared with baseline.
Conversely, in studies of people with a large AAA, findings from studies of short‐term exercise are more variable. Tew et al. (2017) reported no significant increase in cardiorespiratory fitness after 4 weeks of high‐intensity interval aerobic exercise in people with a large AAA (>5.5 cm). The authors reported that only 63% of the study cohort was considered adherent to the exercise intervention and that during the intervention period the exercise intensity occasionally had to be reduced for the majority of the cohort (∼74%) owing to triggered exercise safety criteria, which might have resulted in limited exercise progression. In contrast, Barakat et al. (2016) reported significant increments in (Δ1.6 ml kg−1 min−1) and VT (Δ1.9 ml kg−1 min−1) after 6 weeks of combined moderate‐intensity exercise training in people with a large AAA (>5.5 cm) compared with the usual care group. Importantly, it was also reported that people with a large AAA randomized to the control group demonstrated a decrease of 1.2 ml kg−1 min−1 in their during the 6‐week period, indicating that exercise training potentially mitigates a deterioration in cardiorespiratory fitness over time. Overall, studies to date indicate that exercise training might induce significant improvements in cardiorespiratory fitness in people with a large AAA, but it might be that longer‐duration moderate‐intensity training is preferential and more feasible in this population.
The effect of long‐term exercise training (>12 weeks) on cardiorespiratory fitness in people with AAA has been assessed in only one study to date. Myers et al. (2014) assessed the effect of a long‐term combined training programme (≤3 years follow‐up) on cardiorespiratory fitness in people with a small AAA (<5.5 cm). The results demonstrated significant increases in in the exercise group at the 3‐month (Δ 0.9 ml kg−1 min−1) and 1‐year (Δ 1.3 ml kg−1 min−1) evaluations. Although at the 2‐ and 3‐year evaluations the remained stable in the exercise group, the authors reported a significant decrease for the usual care group (second year, Δ −1.6 ml kg−1 min−1; third year, Δ −2.3 ml kg−1 min−1). A potential limitation of their study was the use of a home‐based exercise intervention; however, current evidence in general clinical populations supports the use of home‐based programmes compared with supervised in‐centre programmes (Anderson et al., 2017). Importantly, these results demonstrate that despite advanced age (72 ± 7 years) and multiple co‐morbidities (CAD, peripheral arterial disease and type 2 diabetes), training ≤3 years was well tolerated and feasible in patients with a small AAA.
4.2. The effect of exercise training on postoperative outcomes
Exercise training‐induced improvements in cardiorespiratory fitness have been associated with favourable postoperative outcomes in people undergoing AAA repair (Barakat et al., 2016). The study by Barakat et al. (2016) was the first to demonstrate that an increase of 1.6 ml kg−1 min−1 in and 1.9 ml kg−1 min−1 in VT in the exercise group was associated with a lower rate of postoperative complications (cardiac 8.1%, pulmonary 11.3% and renal 6.5%) when compared with the usual care group who underwent open surgery alone. Likewise, Hayashi et al. (2016) reported that increased levels of preoperative self‐reported physical activity were associated with early ambulation and reduced length of hospital stay after AAA repair. Importantly, the authors also reported that individuals who engaged in exercise at the earlier stages of the disease had superior postoperative outcomes (reduced mortality and length of hospital stay) compared with those who became physically active at a later stage. Conversely, Tew et al. (2017) reported no impact on postoperative mortality after 4 weeks of high‐intensity interval training in people with a large AAA. It is important to note that this was not a full‐scale trial (the authors characterized it as an external pilot trial) and that no significant increases in cardiorespiratory fitness were reported after the exercise intervention. To date, these are the only studies that have assessed the effect of an exercise intervention on postoperative clinical outcomes in people with AAA. A recent meta‐analysis (Wee & Choong, 2020) and a Cochrane review (Fenton et al., 2021) assessed the impact of preoperative exercise training for people with AAA. Both those studies reported that preoperative exercise training appears to be beneficial for people with AAA; however, owing to methodological heterogeneity among studies, it remains premature to conclude that exercise training as a preoperative intervention improves postoperative outcomes.
4.3. The effects of exercise training on cardiovascular parameters and aneurysm progression
Exercise training‐induced increases in cardiorespiratory fitness are accompanied by a cardiovascular health benefit in people with AAA. Tew et al. (2012) reported a decrease of 10 mmHg in systolic blood pressure in the exercise group after the completion of a short‐term (12 weeks) exercise intervention in people with a small AAA. In addition, the authors reported a corresponding decrease in high‐sensitivity C‐reactive protein in the exercise group that was deemed clinically important. With these changes, the risk stratification of the exercise group changed from ‘moderate’ to ‘low’. Likewsie, Nakayama et al. (2018) reported that a reduction in high‐sensitivity C‐reactive protein, observed in people with a small AAA who underwent cardiac rehabilitation, was associated with slower aneurysm growth. Recently, Niebauer et al. (2021) reported sub‐analysis of data stemming from the AAA Stop Trial (Myers et al., 2014). The authors reported a significant reduction in systolic blood pressure and in lipid accumulation product (a biomarker of atherosclerosis) in people with AAA after a year of exercise training compared with a usual care group. These results are promising, given that exercise‐induced reductions in chronic inflammation are associated with corresponding improvements in endothelial function, blood flow and cardiorespiratory fitness in other chronic diseases, such as type 2 diabetes mellitus (Okada et al., 2010) and CAD (Cwikiel et al., 2018). Interestingly, it was recently demonstrated that even a single bout of exercise is able transiently to improve the cardiovascular profile of people with a small AAA by reducing aortic stiffness (Perissiou et al., 2019) and inflammation (Windsor et al., 2018) and improving endothelial function (Bailey et al., 2017). These parameters have all been associated with cardiovascular risk and aneurysm progression. It is apparent from present evidence that exercise can favourably influence markers of cardiovascular risk and aneurysm progression in patients with AAA; however, larger‐scale clinical trials are needed in order to establish exercise as adjunct treatment modality for addressing cardiovascular risk in this population.
4.4. Risks and concerns of exercise in people with AAA
To date, the available studies report that only a low percentage of the AAA population is engaged in regular physical activity (Hayashi et al., 2016), with a recent meta‐analysis associating physical inactivity with the risk of AAA development (Aune et al., 2020). Concerns regarding the risks of exercise in people with AAA have been expressed in the past, although this is mostly based on opinion or medical concern rather than empirical evidence. Available data indicate that exercise is safe in people with AAA. In a total of 294 patients who underwent exercise training in the available nine studies that were reviewed, only one adverse event (cardiac arrest) was reported in the exercise group, which was not aneurysm or exercise related (Kothmann et al., 2009). A recent meta‐analysis that assessed the safety of exercise training in patients with AAA reported that the cardiovascular event rate was 0.8% (Kato et al., 2019), which is remarkably less than that reported (1.5%) for healthy older individuals without AAA (Goodrich et al., 2007). Further to this, it was reported that exercise training did not increase aneurysm diameter in patients with a small AAA. Importantly, our recent study reported a similar acute haemodynamic response to moderate‐ and higher‐intensity exercise between people with a small AAA and older individuals without an AAA (Perissiou et al., 2019), suggesting that exercise can be undertaken safely to improve cardiorespiratory fitness and cardiovascular health in this population.
5. SUMMARY AND FUTURE DIRECTIONS
This review highlights the clinical and physiological importance of cardiorespiratory fitness in people with AAA. Although we have identified several potential mechanisms and factors that are likely to contribute to the low levels of fitness observed in people with AAA, studies directly exploring the determinants of cardiorespiratory fitness in this population are lacking. Further studies are needed, in order to understand the impact of AAA on oxygen delivery and utilization in the working muscles. Current evidence demonstrates that cardiorespiratory fitness is a significant predictor of short‐ and long‐term postoperative mortality and morbidity in people undergoing AAA repair. Studies to date have used a wide range of GET methods for assessing and reporting cardiorespiratory fitness, and there is a need for more robust and homogeneous reporting of data that will aid in the development of preoperative fitness thresholds and inform clinical guidelines. Finally, this review synthesizes the current available evidence of the benefits of exercise training on cardiorespiratory fitness for people with AAA. Exercise‐induced improvements in fitness are associated with markers of cardiovascular health and a reduction in postoperative mortality. However, current evidence regarding the safety and clinical effectiveness of exercise in people with AAA is still considered limited, and there is a need for larger‐scale clinical trials to establish clear exercise training guidelines for patients with AAA that take into consideration disease severity, high‐risk patients and the presence of co‐morbidities.
COMPETING INTERESTS
None declared.
AUTHOR CONTRIBUTIONS
All authors contributed to the intellectual content of the manuscript; M.P., C.D.A. and T.G.B. conceived and planned the work; M.P. drafted the manuscript; all authors revised the manuscript and provided critical input to specific sections. All authors read and approved the final manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Biography
Dr Maria Perissiou is a Senior Lecturer in Clinical Exercise Physiology in the School of Sports, Exercise and Health Science at the University of Portsmouth. Her PhD work was focused on the effect of exercise in vascular health of people with abdominal aortic aneurysm. Her research interests explore the effect of different stimuli (exercise, physical inactivity, posture, nutrition) on cardiovascular health in an effort to help improve and inform clinical treatment.
Perissiou, M. , Bailey, T. G. , Saynor, Z. L. , Shepherd, A. I. , Harwood, A. E. , & Askew, C. D. (2022). The physiological and clinical importance of cardiorespiratory fitness in people with abdominal aortic aneurysm. Experimental Physiology, 107, 283–298. 10.1113/EP089710
Edited by: Jeremy Ward
REFERENCES
- Albani, M. , Kiskinis, D. , Natsis, K. , Megalopoulos, A. , Gigis, P. , & Guiba‐Tziampiri, O. (2000). Histochemical and ultrastructural characteristics of leg muscle fibres in patients with repairative abdominal aortic aneurysm (AAA). The Anatomical Record, 260(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Albouaini K., Egred M., Alahmar A., Wright D. J. (2007). Cardiopulmonary exercise testing and its application. Postgraduate Medical Journal, 83(985), 675–682. 10.1136/hrt.2007.121558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, L. , Sharp, G. A. , Norton, R. J. , Dalal, H. , Dean, S. G. , Jolly, K. , Cowie, A. , Zawada, A. , & Taylor, R. S. (2017). Home‐based versus centre‐based cardiac rehabilitation. Cochrane Database of Systematic Reviews (Online), 6(6), CD007130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arena, R. , Fei, D. Y. , Arrowood, J. A. , & Kraft, K. A. (2007). Influence on aerobic fitness on aortic stiffness in apparently healthy Caucasian and African‐American subjects. International Journal of Cardiology, 122(3), 202–206. 10.1016/j.ijcard.2006.11.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arena, R. , Myers, J. , Williams, M. A. , Gulati, M. , Kligfield, P. , Balady, G. J. , Collins, E. , & Fletcher, G. (2007). Assessment of functional capacity in clinical and research settings. Circulation, 116(3), 329–343. 10.1161/CIRCULATIONAHA.106.184461 [DOI] [PubMed] [Google Scholar]
- Ashton, H. A. , Buxton, M. J. , Day, N. E. , Kim, L. G. , Marteau, T. M. , Scott, R. A. , Thompson, S. G. , & Walker, N. M. (2002). The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: A randomised controlled trial. Lancet, 360(9345), 1531–1539. [DOI] [PubMed] [Google Scholar]
- Aune, D. , Sen, A. , Kobeissi, E. , Hamer, M. , Norat, T. , & Riboli, E. (2020). Physical activity and the risk of abdominal aortic aneurysm: A systematic review and meta‐analysis of prospective studies. Scientific Reports, 10(1), 22287. 10.1038/s41598-020-76306-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, T. G. , Perissiou, M. , Windsor, M. T. , Schulze, K. , Nam, M. , Magee, R. , Leicht, A. S. , Green, D. J. , Greaves, K. , Golledge, J. , & Askew, C. D. (2018). Effects of acute exercise on endothelial function in patients with abdominal aortic aneurysm. American Journal of Physiology‐Heart and Circulatory Physiology, 314(1), H19–H30. 10.1152/ajpheart.00344.2017 [DOI] [PubMed] [Google Scholar]
- Barakat, H. M. , Shahin, Y. , Khan, J. A. , McCollum, P. T. , & Chetter, I. C. (2016). Preoperative supervised exercise improves outcomes after elective abdominal aortic aneurysm repair: A randomized controlled trial. Annals of Surgery, 264(1), 47–53. 10.1097/SLA.0000000000001609 [DOI] [PubMed] [Google Scholar]
- Barakat, H. M. , Shahin, Y. , McCollum, P. T. , & Chetter, I. (2014). Hospital based exercise programme improves preoperative aerobic capacity in abdominal aortic aneurysm patients. British Journal of Surgery, 101, 7–8. [Google Scholar]
- Barakat, H. M. , Shahin, Y. , McCollum, P. T. , & Chetter, I. C. (2015). Prediction of organ‐specific complications following abdominal aortic aneurysm repair using cardiopulmonary exercise testing. Anaesthesia, 70(6), 679–685. 10.1111/anae.12986 [DOI] [PubMed] [Google Scholar]
- Bath, M. F. , Saratzis, A. , Saedon, M. , Sidloff, D. , Sayers, R. , & Bown, M. J. (2017). Patients with Small Abdominal Aortic Aneurysm are at Significant Risk of Cardiovascular Events and this Risk is not Addressed Sufficiently. Journal of Vascular Surgery, 65(3), 928. 10.1016/j.jvs.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Bauer, T. A. , Reusch, J. E. B. , Levi, M. , & Regensteiner, J. G. (2007). Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care, 30(11), 2880–2885. 10.2337/dc07-0843 [DOI] [PubMed] [Google Scholar]
- Baum, O. , Torchetti, E. , Malik, C. , Hoier, B. , Walker, M. , Walker, P. J. , Odriozola, A. , Graber, F. , Tschanz, S. A. , Bangsbo, J. , Hoppeler, H. , Askew, C. D. , & Hellsten, Y. (2016). Capillary ultrastructure and mitochondrial volume density in skeletal muscle in relation to reduced exercise capacity of patients with intermittent claudication. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 310(10), R943–R951. 10.1152/ajpregu.00480.2015 [DOI] [PubMed] [Google Scholar]
- Belz, G. G. (1995). Elastic properties and Windkessel function of the human aorta. Cardiovascular Drugs and Therapy, 9(1), 73–83. 10.1007/BF00877747 [DOI] [PubMed] [Google Scholar]
- Biddlestone, J. , Bandarra, D. , & Rocha, S. (2015). The role of hypoxia in inflammatory disease (Review). International Journal of Molecular Medicine, 35(4), 859–869. 10.3892/ijmm.2015.2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blassova, T. , Tonar, Z. , Tomasek, P. , Hosek, P. , Hollan, I. , Treska, V. , & Molacek, J. (2019). Inflammatory cell infiltrates, hypoxia, vascularization, pentraxin 3 and osteoprotegerin in abdominal aortic aneurysms – A quantitative histological study. PLoS One, 14(11), e0224818. 10.1371/journal.pone.0224818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaldo, P. , & Sandri, M. (2013). Cellular and molecular mechanisms of muscle atrophy. Disease Models & Mechanisms, 6(1), 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury, A. W. , Davies, A. H. , Dhesi, J. K. , Hammond, C. J. , Hampshire, M. , Jellett, K. , Lindridge, J. , Pichel, A. C. , Ribbons, T. , Ruffell, L. , Slater, M. , Smith, A. H. , Trender, H. , Tang, S. , & Wilson, N. V. (2021). Recommendations on the use of open surgical and endovascular aneurysm repair for the management of unruptured abdominal aortic aneurysm from the guideline development committee appointed by the UK National Institute for Health and Care Excellence. European Journal of Vascular and Endovascular Surgery, 61(6), 877–880. 10.1016/j.ejvs.2021.01.047 [DOI] [PubMed] [Google Scholar]
- Brady, A. R. , Thompson, S. G. , Fowkes, F. G. , Greenhalgh, R. M. , & Powell, J. T. (2004). Abdominal aortic aneurysm expansion: Risk factors and time intervals for surveillance. Circulation, 110(1), 16–21. 10.1161/01.CIR.0000133279.07468.9F [DOI] [PubMed] [Google Scholar]
- Broxterman, R. M. , Hoff, J. , Wagner, P. D. , & Richardson, R. S. (2020). Determinants of the diminished exercise capacity in patients with chronic obstructive pulmonary disease: Looking beyond the lungs. The Journal of Physiology, 598(3), 599–610. 10.1113/JP279135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtscher, M. (2013). Exercise limitations by the oxygen delivery and utilization systems in aging and disease: Coordinated adaptation and deadaptation of the lung‐heart muscle axis – a mini‐review. Gerontology, 59(4), 289–296. 10.1159/000343990 [DOI] [PubMed] [Google Scholar]
- Carlisle, J. B. , Danjoux, G. , Kerr, K. , Snowden, C. , & Swart, M. (2015). Validation of long‐term survival prediction for scheduled abdominal aortic aneurysm repair with an independent calculator using only pre‐operative variables. Anaesthesia, 70(6), 654–665. 10.1111/anae.13061 [DOI] [PubMed] [Google Scholar]
- Carlisle, J. , & Swart, M. (2007). Mid‐term survival after abdominal aortic aneurysm surgery predicted by cardiopulmonary exercise testing. British Journal of Surgery, 94(8), 966‐969. 10.1002/bjs.5734 [DOI] [PubMed] [Google Scholar]
- Castellon, X. , & Bogdanova, V. (2016). Chronic inflammatory diseases and endothelial dysfunction. Aging and Disease, 7(1), 81–89. 10.14336/AD.2015.0803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cwikiel, J. , Seljeflot, I. , Berge, E. , Njerve, I. U. , Ulsaker, H. , Arnesen, H. , & Flaa, A. (2018). Effect of strenuous exercise on mediators of inflammation in patients with coronary artery disease. Cytokine, 105, 17–22. 10.1016/j.cyto.2018.02.006 [DOI] [PubMed] [Google Scholar]
- Dale, M. A. , Ruhlman, M. K. , & Baxter, B. T. (2015). Inflammatory cell phenotypes in AAAs: Their role and potential as targets for therapy. Arteriosclerosis, Thrombosis, and Vascular Biology, 35(8), 1746–1755. 10.1161/ATVBAHA.115.305269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, S. J. , & Wilson, R. J. T. (2004). Preoperative optimization of the high‐risk surgical patient. British Journal of Anaesthesia, 93(1), 121–128. 10.1093/bja/aeh164 [DOI] [PubMed] [Google Scholar]
- De Rango, P. , Farchioni, L. , Fiorucci, B. , & Lenti, M. (2014). Diabetes and abdominal aortic aneurysms. European Journal of Vascular and Endovascular Surgery, 47(3), 243–261. 10.1016/j.ejvs.2013.12.007 [DOI] [PubMed] [Google Scholar]
- Del Torto, A. , Corrieri, N. , Vignati, C. , Gentile, P. , Cattadori, G. , Paolillo, S. , & Agostoni, P. (2017). Contribution of central and peripheral factors at peak exercise in heart failure patients with progressive severity of exercise limitation. International Journal of Cardiology, 248, 252–256. 10.1016/j.ijcard.2017.07.071 [DOI] [PubMed] [Google Scholar]
- Dipla, K. , Triantafyllou, A. , Koletsos, N. , Papadopoulos, S. , Sachpekidis, V. , Vrabas, I. S. , Gkaliagkousi, E. , Zafeiridis, A. , & Douma, S. (2017). Impaired muscle oxygenation and elevated exercise blood pressure in hypertensive patients. Hypertension, 70(2), 444–451. 10.1161/HYPERTENSIONAHA.117.09558 [DOI] [PubMed] [Google Scholar]
- Eslami, M. H. , Rybin, D. V. , Doros, G. , & Farber, A. (2017). Description of a risk predictive model of 30‐day postoperative mortality after elective abdominal aortic aneurysm repair. Journal of Vascular Surgery, 65(1), 65–74.e2. 10.1016/j.jvs.2016.07.103 [DOI] [PubMed] [Google Scholar]
- Fenton, C. , Tan, A. R. , Abaraogu, U. O. , & McCaslin, J. E. (2021). Prehabilitation exercise therapy before elective abdominal aortic aneurysm repair. Cochrane Database of Systematic Reviews (Online), 7, CD013662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, B. (2014). ACSM's guidelines for exercise testing and prescription 9th Ed. 2014. The Journal of the Canadian Chiropractic Association, 58(3), 328. [Google Scholar]
- Gander, J. C. , Sui, X. , Hébert, J. R. , Hazlett, L. J. , Cai, B. , Lavie, C. J. , & Blair, S. N. (2015). Association of cardiorespiratory fitness with coronary heart disease in asymptomatic men. Mayo Clinic proceedings, 90(10), 1372–1379. 10.1016/j.mayocp.2015.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich, D. E. , Larkin, A. R. , Lowery, J. C. , Holleman, R. G. , & Richardson, C. R. (2007). Adverse events among high‐risk participants in a home‐based walking study: A descriptive study. The International Journal of Behavioral Nutrition and Physical Activity, 4, 20. 10.1186/1479-5868-4-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear, S. J. , Yow, H. , Saedon, M. , Shakespeare, J. , Hill, C. E. , Watson, D. , Marshall, C. , Mahmood, A. , Higman, D. , & Imray, C. H. E. (2013). Risk stratification by pre‐operative cardiopulmonary exercise testing improves outcomes following elective abdominal aortic aneurysm surgery: A cohort study. Perioperative Medicine, 2(1). 10.1186/2047-0525-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, S. W. , Hickey, G. L. , Wisely, N. A. , Carlson, E. D. , Hartley, R. A. , Pichel, A. C. , Atkinson, D. , & McCollum, C. N. (2015). Cardiopulmonary exercise testing and survival after elective abdominal aortic aneurysm repair†. British Journal of Anaesthesia, 114(3), 430–436. 10.1093/bja/aeu383 [DOI] [PubMed] [Google Scholar]
- Green, S. , & Askew, C. (2018). V̇o2peak is an acceptable estimate of cardiorespiratory fitness but not V̇o2max . Journal of Applied Physiology, 125(1), 229–232. 10.1152/japplphysiol.00850.2017 [DOI] [PubMed] [Google Scholar]
- Green, S. , Askew, C. D. , & Walker, P. J. (2007). Effect of type 2 diabetes mellitus on exercise intolerance and the physiological responses to exercise in peripheral arterial disease. Diabetologia, 50(4), 859–866. 10.1007/s00125-006-0587-7 [DOI] [PubMed] [Google Scholar]
- Greenhalgh, R. M. (2005). Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. The Lancet, 365(9478), 2187–2192. 10.1016/s0140-6736(05)66628-7 [DOI] [PubMed] [Google Scholar]
- Hamburg, N. M. , & Creager, M. A. (2017). Pathophysiology of intermittent claudication in peripheral artery disease. Circulation Journal, 81(3), 281–289. 10.1253/circj.CJ-16-1286 [DOI] [PubMed] [Google Scholar]
- Handy, D. E. , & Loscalzo, J. (2012). Redox regulation of mitochondrial function. Antioxidants & Redox Signaling, 16(11), 1323–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley, R. A. , Pichel, A. C. , Grant, S. W. , Hickey, G. L. , Lancaster, P. S. , Wisely, N. A. , McCollum, C. N. , & Atkinson, D. (2012). Preoperative cardiopulmonary exercise testing and risk of early mortality following abdominal aortic aneurysm repair. British Journal of Surgery, 99(11), 1539–1546. 10.1002/bjs.8896 [DOI] [PubMed] [Google Scholar]
- Hayashi, K. , Hirashiki, A. , Kodama, A. , Kobayashi, K. , Yasukawa, Y. , Shimizu, M. , Kondo, T. , Komori, K. , & Murohara, T. (2016). Impact of preoperative regular physical activity on postoperative course after open abdominal aortic aneurysm surgery. Heart and Vessels, 31(4), 578–583. 10.1007/s00380-015-0644-6 [DOI] [PubMed] [Google Scholar]
- Hou, X. Y. , Green, S. , Askew, C. D. , Barker, G. , Green, A. , & Walker, P. J. (2002). Skeletal muscle mitochondrial ATP production rate and walking performance in peripheral arterial disease. Clinical Physiology and Functional Imaging, 22(3), 226–232. 10.1046/j.1475-097X.2002.00423.x [DOI] [PubMed] [Google Scholar]
- Iankovskaia, A. V. , & Zinchuk, M. A. (2007). Oxygen‐transport function of the blood and endothelial dysfunction in patients with angina pectoris and arterial hypertension. Kardiologiia, 47(4), 22–27. [PubMed] [Google Scholar]
- Jacobs, R. A. , & Lundby, C. (2013). Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. Journal of Applied Physiology, 114(3), 344–350. 10.1152/japplphysiol.01081.2012 [DOI] [PubMed] [Google Scholar]
- Kadoglou, N. P. E. , Papadakis, I. , Moulakakis, K. G. , Ikonomidis, I. , Alepaki, M. , Moustardas, P. , Lampropoulos, S. , Karakitsos, P. , Lekakis, J. , & Liapis, C. D. (2012). Arterial stiffness and novel biomarkers in patients with abdominal aortic aneurysms. Regulatory Peptides, 179(1–3), 50–54. 10.1016/j.regpep.2012.08.014 [DOI] [PubMed] [Google Scholar]
- Kato, M. , Kubo, A. , Green, F. N. , & Takagi, H. (2019). Safety and efficacy of exercise training in patients with abdominal aortic aneurysm: A meta‐analysis of randomized controlled trials. Journal of Vascular Surgery, 69(3), 933–943. [DOI] [PubMed] [Google Scholar]
- Kent, K. C. , Zwolak, R. M. , Egorova, N. N. , Riles, T. S. , Manganaro, A. , Moskowitz, A. J. , Gelijns, A. C. , & Greco, G. (2010). Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. Journal of Vascular Surgery, 52(3), 539–548. 10.1016/j.jvs.2010.05.090 [DOI] [PubMed] [Google Scholar]
- Kodama, S. , Saito, K. , Tanaka, S. , Maki, M. , Yachi, Y. , Asumi, M. , Sugawara, A. , Totsuka, K. , Shimano, H. , Ohashi, Y. , Yamada, N. , & Sone, H. (2009). Cardiorespiratory fitness as a quantitative predictor of all‐cause mortality and cardiovascular events in healthy men and women: A meta‐analysis. JAMA, 301(19), 2024–2035. 10.1001/jama.2009.681 [DOI] [PubMed] [Google Scholar]
- König, D. , Wagner, K. H. , Elmadfa, I. , & Berg, A. (2001). Exercise and oxidative stress: Significance of antioxidants with reference to inflammatory, muscular, and systemic stress. Exercise Immunology Review, 7, 108–133. [PubMed] [Google Scholar]
- Kothmann, E. , Batterham, A. M. , Owen, S. J. , Turley, A. J. , Cheesman, M. , Parry, A. , & Danjoux, G. (2009). Effect of short‐term exercise training on aerobic fitness in patients with abdominal aortic aneurysms: A pilot study. British Journal of Anaesthesia, 103(4), 505–510. 10.1093/bja/aep205 [DOI] [PubMed] [Google Scholar]
- Lederle, F. A. , Noorbaloochi, S. , Nugent, S. , Taylor, B. C. , Grill, J. P. , Kohler, T. R. , & Cole, L. (2015). Multicentre study of abdominal aortic aneurysm measurement and enlargement. British Journal of Surgery, 102(12), 1480–1487. 10.1002/bjs.9895 [DOI] [PubMed] [Google Scholar]
- Lee, D.‐C. , Artero, E. G. , Sui, X. , & Blair, S. N. (2010). Review: Mortality trends in the general population: The importance of cardiorespiratory fitness. Journal of Psychopharmacology, 24(4 suppl), 27–35. 10.1177/1359786810382057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, B. D. (2008). : What do we know, and what do we still need to know? The Journal of Physiology, 586(1), 25–34. 10.1113/jphysiol.2007.147629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima, R. M. , Vainshelboim, B. , Ganatra, R. , Dalman, R. , Chan, K. , & Myers, J. (2018). Exercise training improves ventilatory efficiency in patients with a small abdominal aortic aneurysm: A randomized controlled study. Journal of Cardiopulmonary Rehabilitation and Prevention, 38(4), 239–245. 10.1097/HCR.0000000000000270 [DOI] [PubMed] [Google Scholar]
- Locham, S. , Lee, R. , Nejim, B. , Dakour Aridi, H. , & Malas, M. (2017). Mortality after endovascular versus open repair of abdominal aortic aneurysm in the elderly. Journal of Surgical Research, 215, 153–159. 10.1016/j.jss.2017.03.061 [DOI] [PubMed] [Google Scholar]
- Meital, L. T. , Windsor, M. T. , Maynard, A. E. , Schulze, K. , Magee, R. , O'Donnell, J. , Jha, P. , Meital, C. Y. , Perissiou, M. , Coverdale, S. , Golledge, J. , Kuballa, A. V. , Bailey, T. G. , Askew, C. D. , & Russell, F. D. (2020). Endotoxin tolerance in abdominal aortic aneurysm macrophages, in vitro: A case–control study. Antioxidants, 9(9), 896. 10.3390/antiox9090896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menteşe, U. , Turan, I. , Usta, S. , Demir, S. , Koral, Ö. , Öztaş Menteşe, S. , Çavuşoğlu, I. G. , Karahan, S. C. , Alver, A. , Doğan, O. V. , & Aykan, A. Ç. (2016). Systemic oxidant/antioxidant balance in human abdominal aortic aneurysm. Perfusion, 31(4), 288–294. 10.1177/0267659115598856 [DOI] [PubMed] [Google Scholar]
- Modrego, J. , López‐Farré, A. J. , Martínez‐López, I. , Muela, M. , Macaya, C. , Serrano, J. , & Moñux, G. (2012). Expression of cytoskeleton and energetic metabolism‐related proteins at human abdominal aortic aneurysm sites. Journal of Vascular Surgery, 55(4), 1124–1133. 10.1016/j.jvs.2011.10.033 [DOI] [PubMed] [Google Scholar]
- Montero, D. (2015). The association of cardiorespiratory fitness with endothelial or smooth muscle vasodilator function. European Journal of Preventive Cardiology, 22(9), 1200–1211. 10.1177/2047487314553780 [DOI] [PubMed] [Google Scholar]
- Myers, J. , McElrath, M. , Jaffe, A. , Smith, K. , Fonda, H. , Vu, A. , Hill, B. , & Dalman, R. (2014). A randomized trial of exercise training in abdominal aortic aneurysm disease. Medicine and Science in Sports and Exercise, 46(1), 2–9. 10.1249/MSS.0b013e3182a088b8 [DOI] [PubMed] [Google Scholar]
- Myers, J. , Powell, A. , Smith, K. , Fonda, Holly , Dalman, R. L. , & Stanford AAA SCCOR Investigators . (2011). Cardiopulmonary exercise testing in small abdominal aortic aneurysm: Profile, safety, and mortality estimates. European Journal of Cardiovascular Prevention and Rehabilitation, 18(3), 459–466. [DOI] [PubMed] [Google Scholar]
- Nakamura, Y. , Tanaka, K. , Shigematsu, R. , Homma, T. , & Sekizawa, K. (2004). Determinants of cardiorespiratory fitness in patients with chronic obstructive pulmonary disease, focusing on activities parallel to daily living. Respirology (Carlton, Vic.), 9(3), 326–330. 10.1111/j.1440-1843.2004.00605.x [DOI] [PubMed] [Google Scholar]
- Nakayama, A. , Morita, H. , Nagayama, M. , Hoshina, K. , Uemura, Y. , Tomoike, H. , & Komuro, I. (2018). Cardiac rehabilitation protects against the expansion of abdominal aortic aneurysm. Journal of the American Heart Association, 7(5), e007959. 10.1161/JAHA.117.007959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence . (2020). Abdominal aortic aneurysm: Diagnosis and management Evidence review G: Tests for predicting outcomes after repair of unruptured abdominal aortic aneurysms. NICE guideline; Evidence reviews. https://www.nice.org.uk/guidance/ng156/documents/evidence‐review‐7 [PubMed]
- Nesti, L. , Pugliese, N. R. , Sciuto, P. , & Natali, A. (2020). Type 2 diabetes and reduced exercise tolerance: A review of the literature through an integrated physiology approach. Cardiovascular Diabetology, 19(1), 134. 10.1186/s12933-020-01109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebauer, S. , Niebauer, J. , Dalman, R. , & Myers, J. (2021). Effects of exercise training on vascular markers of disease progression in patients with small abdominal aortic aneurysms. American Journal of Medicine, 134(4), 535–541. 10.1016/j.amjmed.2020.07.029 32835687 [DOI] [PubMed] [Google Scholar]
- Nugent, A. M. , Riley, M. , Megarry, J. , O'Reilly, M. J. , MacMahon, J. , & Lowry, R. (1998). Cardiopulmonary exercise testing in the pre‐operative assessment. Irish Journal of Medical Science, 167(4), 238–241. [DOI] [PubMed] [Google Scholar]
- Okada, S. , Hiuge, A. , Makino, H. , Nagumo, A. , Takaki, H. , Konishi, H. , Goto, Y. , Yoshimasa, Y. , & Miyamoto, Y. (2010). Effect of exercise intervention on endothelial function and incidence of cardiovascular disease in patients with type 2 diabetes. Journal of Atherosclerosis and Thrombosis, 17(8), 828–833. 10.5551/jat.3798 [DOI] [PubMed] [Google Scholar]
- Older, P. , Hall, A. , & Hader, R. (1999). Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest, 116(2), 355–362. 10.1378/chest.116.2.355 [DOI] [PubMed] [Google Scholar]
- Parodi, J. C. , Palmaz, J. C. , & Barone, H. D. (1991). Transfemoral Intraluminal Graft Implantation for Abdominal Aortic Aneurysms. Annals of Vascular Surgery, 5(6), 491–499. 10.1007/bf02015271 [DOI] [PubMed] [Google Scholar]
- Patel, R. , Sweeting, M. J. , Powell, J. T. , & Greenhalgh, R. M. (2016). Endovascular versus open repair of abdominal aortic aneurysm in 15‐years' follow‐up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): A randomised controlled trial. The Lancet, 388(10058), 2366–2374. 10.1016/s0140-6736(16)31135-7 [DOI] [PubMed] [Google Scholar]
- Perissiou, M. , Bailey, T. G. , Windsor, M. , Greaves, K. , Nam, M. C. Y. , Russell, F. D. , O'Donnell, J. , Magee, R. , Jha, P. , Schulze, K. , Leicht, A. S. , Golledge, J. , & Askew, C. D. (2019). Aortic and systemic arterial stiffness responses to acute exercise in patients with small abdominal aortic aneurysms. European Journal of Vascular and Endovascular Surgery, 58, 708–718. 10.1016/j.ejvs.2019.02.021 [DOI] [PubMed] [Google Scholar]
- Prado‐Garcia, H. , Campa‐Higareda, A. , & Romero‐Garcia, S. (2020). Lactic acidosis in the presence of glucose diminishes Warburg effect in lung adenocarcinoma cells. Frontiers in Oncology, 10, 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentis, J. M. , Trenell, M. I. , Jones, D. J. , Lees, T. , Clarke, M. , & Snowden, C. P. (2012). Submaximal exercise testing predicts perioperative hospitalization after aortic aneurysm repair. Journal of Vascular Surgery, 56(6), 1564–1570. 10.1016/j.jvs.2012.05.097 [DOI] [PubMed] [Google Scholar]
- Rose, G. A. , Davies, R. G. , Appadurai, I. R. , Lewis, W. G. , Cho, J. S. , Lewis, M. H. , Williams, I. M. , & Bailey, D. M. (2018). Cardiorespiratory fitness is impaired and predicts mid‐term postoperative survival in patients with abdominal aortic aneurysm disease. Experimental Physiology, 103(11), 1505–1512. 10.1113/EP087092 [DOI] [PubMed] [Google Scholar]
- Salartash, K. , Sternbergh, W. C. 3rd , York, J. W. , & Money, S. R. (2001). Comparison of open transabdominal AAA repair with endovascular AAA repair in reduction of postoperative stress response. Annals of Vascular Surgery, 15(1), 53–59. 10.1007/BF02693801 [DOI] [PubMed] [Google Scholar]
- Sato, I. , Matsumura, N. , Nishijima, H. , & Yasuda, H. (1989). Relation between anaerobic threshold and maximal oxygen consumption during graded treadmill exercise. Journal of Cardiology, 19(1), 257–262. [PubMed] [Google Scholar]
- Siasos, G. , Mourouzis, K. , Oikonomou, E. , Tsalamandris, S. , Tsigkou, V. , Vlasis, K. , Vavuranakis, M. , Zografos, T. , Dimitropoulos, S. , Papaioannou, T. G. , Kalampogias, A. , Stefanadis, C. , Papavassiliou, A. G. , & Tousoulis, D. (2015). The role of endothelial dysfunction in aortic aneurysms. Current Pharmaceutical Design, 21(28), 4016–4034. 10.2174/1381612821666150826094156 [DOI] [PubMed] [Google Scholar]
- Straw, S. , Waduud, M. A. , Drozd, M. , Warman, P. , Bailey, M. A. , Hammond, C. J. , Abdel‐Rahman, S. E. D. , Witte, K. K. , & Scott, D. J. A. (2020). The role of cardiopulmonary exercise testing and echocardiography prior to elective endovascular aneurysm repair. The Annals of The Royal College of Surgeons of England, 102(5), 383–390. 10.1308/rcsann.2020.0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struthers, R. , Erasmus, P. , Holmes, K. , Warman, P. , Collingwood, A. , & Sneyd, J. R. (2008). Assessing fitness for surgery: A comparison of questionnaire, incremental shuttle walk, and cardiopulmonary exercise testing in general surgical patients. British Journal of Anaesthesia, 101(6), 774–780. 10.1093/bja/aen310 [DOI] [PubMed] [Google Scholar]
- Suh, G.‐Y. , Les, A. S. , Tenforde, A. S. , Shadden, S. C. , Spilker, R. L. , Yeung, J. J. , Cheng, C. P. , Herfkens, R. J. , Dalman, R. L. , & Taylor, C. A. (2011). Hemodynamic Changes Quantified in Abdominal Aortic Aneurysms with Increasing Exercise Intensity Using MR Exercise Imaging and Image‐Based Computational Fluid Dynamics. Annals of Biomedical Engineering, 39(8), 2186–2202. 10.1007/s10439-011-0313-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillens, A. , Lanoye, L. , De Backer, J. , Stergiopulos, N. , Verdonck, P.R. , Vermassen, F. , & Segers, P. (2008). Effect of an Abdominal Aortic Aneurysm on Wave Reflection in the Aorta. IEEE Transactions on Biomedical Engineering, 55(5), 1602–1611. 10.1109/tbme.2007.913994 [DOI] [PubMed] [Google Scholar]
- Tew, G. A. , Batterham, A. M. , Colling, K. , Gray, J. , Kerr, K. , Kothmann, E. , Nawaz, S. , Weston, M. , Yates, D. , & Danjoux, G. (2017). Randomized feasibility trial of high‐intensity interval training before elective abdominal aortic aneurysm repair. British Journal of Surgery, 104(13), 1791–1801. 10.1002/bjs.10669 [DOI] [PubMed] [Google Scholar]
- Tew, G. A. , Moss, J. , Crank, H. , Mitchell, P. A. , & Nawaz, S. (2012). Endurance exercise training in patients with small abdominal aortic aneurysm: A randomized controlled pilot study. Archives of Physical Medicine and Rehabilitation, 93(12), 2148–2153. 10.1016/j.apmr.2012.07.012 [DOI] [PubMed] [Google Scholar]
- Thompson, A. R. , Peters, N. , Lovegrove, R. E. , Ledwidge, S. , Kitching, A. , Magee, T. R. , & Galland, R. B. (2011). Cardiopulmonary exercise testing provides a predictive tool for early and late outcomes in abdominal aortic aneurysm patients. Annals of the Royal College of Surgeons of England, 93(6), 474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruda, T. , Hatakeyama, K. , Nagamachi, S. , Sekita, Y. , Sakamoto, S. , Endo, G. J. , Nishimura, M. , Matsuyama, M. , Yoshimura, K. , Sato, Y. , Onitsuka, T. , Imamura, T. , Asada, Y. , & Kitamura, K. (2012). Inhibition of development of abdominal aortic aneurysm by glycolysis restriction. Arteriosclerosis, Thrombosis, and Vascular Biology, 32(6), 1410–1417. 10.1161/ATVBAHA.111.237065 [DOI] [PubMed] [Google Scholar]
- Upchurch, G. R. Jr. , & Schaub, T. A. (2006). Abdominal aortic aneurysm. American Family Physician, 73(7), 1198–1204. [PubMed] [Google Scholar]
- Vainshelboim, B. , Arena, R. , Kaminsky, L. A. , & Myers, J. (2020). Reference standards for ventilatory threshold measured with cardiopulmonary exercise testing: The fitness registry and the importance of exercise: A national database. Chest, 157(6), 1531–1537. 10.1016/j.chest.2019.11.022 [DOI] [PubMed] [Google Scholar]
- Vainshelboim, B. , Rao, S. , Chan, K. , Lima, R. M. , Ashley, E. A. , & Myers, J. (2017). A comparison of methods for determining the ventilatory threshold: Implications for surgical risk stratification. Canadian Journal of Anesthesia/Journal canadien d'anesthésie, 64(6), 634–642. 10.1007/s12630-017-0862-8 [DOI] [PubMed] [Google Scholar]
- Vallet, B. (2002). Endothelial cell dysfunction and abnormal tissue perfusion. Critical Care Medicine, 30(5 Suppl), S229–S234. 10.1097/00003246-200205001-00010 [DOI] [PubMed] [Google Scholar]
- Van Kuijk, J. P. , Flu, W. J. , Dunckelgrun, M. , Bax, J. J. , & Poldermans, D. (2009). Coronary artery disease in patients with abdominal aortic aneurysm: A review article. Journal of Cardiovascular Surgery, 50(1), 93–107. [PubMed] [Google Scholar]
- van ’t Veer, M. , Buth, J. , Merkx, M. , Tonino, P. , van den Bosch, H. , Pijls, N. , & van de Vosse, F. (2008). Biomechanical properties of abdominal aortic aneurysms assessed by simultaneously measured pressure and volume changes in humans. Journal of Vascular Surgery, 48(6), 1401–1407. 10.1016/j.jvs.2008.06.060 [DOI] [PubMed] [Google Scholar]
- Varshney, M. , Haani Farooqi, M. , & Usmani, A. Y. (2020). Quantifying hemodynamics within an aneurysm exposed to prolonged exercise levels. Computer Methods and Programs in Biomedicine, 184, 105124. 10.1016/j.cmpb.2019.105124 [DOI] [PubMed] [Google Scholar]
- Wanhainen, A. , Verzini, F. , Van Herzeele, I. , Allaire, E. , Bown, M. , Cohnert, T. , Dick, F. , van Herwaarden, J. , Karkos, C. , Koelemay, M. , Kölbel, T. , Loftus, I. , Mani, K. , Melissano, G. , Powell, J. , Szeberin, Z. , ESVS Guidelines Committee, de Borst, G. J. , Chakfe, N. , … Verhagen, Hence (2019). Editor's Choice – European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto‐iliac Artery Aneurysms. European Journal of Vascular and Endovascular Surgery, 57(1), 8–93. 10.1016/j.ejvs.2018.09.020 [DOI] [PubMed] [Google Scholar]
- Wee, I. J. Y. , & Choong, A. M. T. L. (2020). A systematic review of the impact of preoperative exercise for patients with abdominal aortic aneurysm. Journal of Vascular Surgery, 71(6), 2123–2131.e1. 10.1016/j.jvs.2018.09.039 [DOI] [PubMed] [Google Scholar]
- West, M. A. , Parry, M. , Asher, R. , Key, A. , Walker, P. , Loughney, L. , Pintus, S. , Duffy, N. , Jack, S. , & Torella, F. (2015). The Effect of beta‐blockade on objectively measured physical fitness in patients with abdominal aortic aneurysms–A blinded interventional study. British Journal of Anaesthesia, 114(6): 878–885. [DOI] [PubMed] [Google Scholar]
- Weston, M. , Batterham, A. M. , Tew, G. A. , Kothmann, E. , Kerr, K. , Nawaz, S. , Yates, D. , & Danjoux, G. (2017). Patients Awaiting Surgical Repair for Large Abdominal Aortic Aneurysms Can Exercise at Moderate to Hard Intensities with a Low Risk of Adverse Events. Frontier Physiology, 7, 684. 10.3389/fphys.2016.00684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J. J. , & Dalman, R. L. (2008). Gaining new insights into early abdominal aortic aneurysm disease. The Permanente Journal, 12(2), 10–14. 10.7812/TPP/07-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor, M. T. , Bailey, T. G. , Perissiou, M. , Greaves, K. , Jha, P. , Leicht, A. S. , Russell, F. D. , Golledge, J. , & Askew, C. D. (2018). Acute inflammatory responses to exercise in patients with abdominal aortic aneurysm. Medicine and Science in Sports and Exercise, 50(4), 649–658. 10.1249/MSS.0000000000001501 [DOI] [PubMed] [Google Scholar]
- Yuan, K. , Liang, W. , & Zhang, J. (2015). A comprehensive analysis of differentially expressed genes and pathways in abdominal aortic aneurysm. Molecular Medicine Reports, 12(2), 2707–2714. 10.3892/mmr.2015.3709 [DOI] [PubMed] [Google Scholar]
- Zhang, X.‐yY. , Zhang, J. , Xia, Q. , Han, Y.‐s. , Liu, Z.‐m. , Wang, F.‐y. , Xin, S.‐j. , & Duan, Z.‐q. (2012). Correlation of hemoglobin concentration with the diameter of abdominal aortic aneurysm and long‐term patient survival. Zhonghua Yi Xue Za Zhi, 92(43), 3050–3053. [PubMed] [Google Scholar]


