Abstract
Non-alcoholic fatty liver disease (NAFLD) is a disorder characterized by the excessive accumulation of lipids in the liver parenchyma. To date, there is no effective pharmacological treatment against NAFLD. Objective: To assess the relationship between the improvement of the intrahepatic fat content (IFC) in patients with NAFLD and metabolic syndrome and biomarkers of oxidative stress and inflammation after 6 months of lifestyle intervention. Patients diagnosed with NAFLD (n = 60 adults; 40–60 years old) residing in the Balearic Islands, Spain, were distributed in tertiles attending the improvement of IFC calculated by magnetic resonance imaging (MRI). Anthropometrics, blood pressure, maximal oxygen uptake, and pro/antioxidant and inflammatory biomarkers were determined in plasma before and after the lifestyle intervention. The improvement in IFC levels was higher in tertile 3 with respect to tertiles 2 and 1. The greatest improvement in IFC is related to cardiorespiratory fitness and adherence to the Mediterranean diet (ADM). Higher reductions in weight, body mass index (BMI), and alanine aminotransferase (ALT) were observed in tertile 3 with respect to tertile 1 after 6 months of intervention. The improvement in catalase, irisin, and cytokeratin 18 plasma levels were higher in tertile 3, whereas no differences were observed in superoxide dismutase activity. Malondialdehyde and protein carbonyl levels, as biomarkers of oxidative damage, remained unchanged in all groups. The present data show that the reduction of IFC is associated with an improvement in pro/antioxidant and pro-inflammatory status and a better cardiorespiratory fitness in NAFLD patients.
Keywords: NAFLD, IFC, aerobic capacity, oxidative stress, inflammation, biomarkers
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is an epidemic liver disorder characterized by excessive accumulation of lipids in the liver parenchyma [1]. This disease is the most common chronic liver disease, and its prevalence has been progressively increased in recent years, like the worldwide increase in diabetes and metabolic syndrome [2]. Nowadays, NAFLD affects about 20–30% of the global population, but it has 90% prevalence among obese individuals [3,4]. If the pathological disorder is not appropriately treated, it can progress from NAFLD to the more advanced stage of non-alcoholic steatohepatitis (NASH), which can, ultimately, lead to cirrhosis and liver cancer [5]. Moreover, NAFLD is related to metabolic disorders with extrahepatic manifestations, such as cardiovascular disease, chronic kidney disease, sleep apnea, obesity, insulin resistance, and diabetes [3,6]. Although the factors responsible for the onset and progression of hepatic steatosis are not well-elucidated, lifestyle, genetics, immunity, and the gut microbiota may be involved [7]. Lifestyle risk factors such as smoking, unhealthy diet, and reduced physical activity significantly increase the risk of hepatic steatosis [8,9].
Numerous evidence suggested that oxidative stress induced by increased production of reactive species (ROS) is involved in the pathogenesis of many diseases, including metabolic disorders such as insulin resistance or diabetes [10]. Oxidative stress (OS) and inflammation induced by the excess of ROS are well-recognized mechanisms that can lead to tissue injury and hepatic cell death [11]. In this sense, NAFLD has been described as a pro-oxidative and pro-inflammatory disease [12]. In NAFLD patients, the accumulation of fatty acids in the liver induces an increase in β-oxidation, which, in turn, causes a ROS overproduction in the respiratory chain [11]. This increase in ROS production can exceed the detoxifying capacity of the antioxidant system, leading to the appearance of lipid peroxidation and oxidative damage [13]. All this leads to impaired mitochondrial and peroxisomal oxidation of fatty acids that can progress and result in reduced hepatic ATP synthesis and caspases-induced apoptosis [14]. ROS also induces an increase of the transforming grow factor β (TGF-β), which activates collagen production by stellate cells, increasing hepatic fibrosis [15]. In addition, previous studies have also demonstrated that NAFLD is related to up-regulation of pro-inflammatory mediators [16,17]. In fact, the excessive accumulation of triglycerides and lipotoxic intermediates causes changes in hepatocyte function and induces the release of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) or interleukin-6 (IL-6), which, together with circulating non-esterified fatty acids, disturb hepatic insulin signaling [18,19].
Consistent evidence support common pathophysiologic mechanisms between MetS and NAFLD, which usually involve visceral obesity and insulin resistance [20]. Currently, there is no drug therapy that can be formulated for treating NAFLD. A combination of a healthy diet, such as Mediterranean diet [21], and increased physical activity [22] remains the mainstay in the management of NAFLD. Thus, lifestyle intervention can be effective for treating NAFLD patients, including a healthy diet and increased physical activity [23]. In this sense, it has been shown that weight reductions could decrease cardiovascular diseases and the risk of diabetes as well as can reverse liver diseases, leading to the improvement of fibrosis [24,25,26,27]. Moreover, it has been shown that the intrahepatic lipid content in people with NAFLD can be reduced by regular exercise training [27]. Regular exercise can improve NAFLD by diverse mechanisms such as decreasing intrahepatic fat content, producing hepato-protective autophagy, rising β-oxidation of fatty acids, overexpressing peroxisome proliferator-activated receptor-γ (PPAR-γ), rising insulin sensitivity, and attenuating hepatocyte apoptosis [28].
Considering the relationship between excessive fat accumulation in tissues and the proinflammatory and pro-oxidative state, the aim of this study was to assess whether a greater improvement in intrahepatic fat contents (IFC) in patients with NAFLD undergoing a lifestyle intervention also leads to an improvement in the proinflammatory and oxidative state.
2. Materials and Methods
2.1. Design and Participants
The current study was included within the frame of the FLIPAN (Prevention and Reversion of NAFLD in Obese Patients with Metabolic Syndrome by Mediterranean Diet and Physical Activity) prospective and randomized control trial (ClinicalTrials.gov Identifier: NCT04442620). It involves 60 participants residing in the Balearic Islands (Spain), aged 40–60 years, with a diagnosis of NAFLD by magnetic resonance imaging (MRI), a BMI of 27–40 kg/m2, and showing at least three of the metabolic syndrome (MetS) criteria as described by the International Diabetes Federation (IDF) consensus [29]. The participants were selected considering the inclusion criteria described elsewhere [12]. Exclusion criteria were previous cardiovascular disease, congestive heart failure, liver diseases (other than NAFLD), cancer or a history of malignancy in the previous 5 years, previous bariatric surgery, acute febrile illnesses, urinary tract infections, post-renal hematuria, hemochromatosis, protein overload, non-medicated depression or anxiety, alcohol and drug abuse, pregnancy, primary endocrinological diseases (other than hypothyroidism and type 2 diabetes mellitus), concomitant therapy with steroids, intense physical exercise, or being unable to provide informed consent.
All procedures and the study protocol were designed according to the ethical standards of the Declaration of Helsinki and were approved by the Ethics Committee of the Balearic Islands (ref. IB 2251/14 PI). The participants were informed of the purposes and the potential risks of the study and signed the informed consent to participate.
After inclusion, participants were randomly allocated to one of the following three groups:
Conventional diet (CD) group: These participants followed the recommendations of American Association for the Study of Liver Disease (AASLD) [30], with energy restrictions to loss of at least 3–5% of the body weight to improve steatosis and 7–10% to improve most of the histopathological features of NASH, following the general guidelines of the U.S. Department of Health and Human Services and U.S. Department of Agriculture (20–35% fat, 10–35% protein, 45–65% carbohydrate) [31].
Mediterranean diet high meal frequency (MD-HMF) group: This group was instructed to follow a Mediterranean diet characterized by a distribution of macronutrients of 40–45% carbohydrates (50–70% of carbohydrates should be low glycemic and rich in fiber), 30–35% fat, and 25% protein. This dietary pattern was previously observed to decrease fat mass and overall weight and improve the oxidative status in subjects with metabolic syndrome [32,33]. Total daily caloric intake was distributed over seven meals, with the highest calorie meals eaten early during the morning.
Mediterranean diet physical activity (MD-PA) group: This group consumed an energy-restricted Mediterranean diet with a meal frequency of four to five meals per day, including snacks. Total calorie intake for this group came from 35–40% from fat (8–10% of saturated fatty acids, >20% of monounsaturated fatty acids, >10% of polyunsaturated fatty acids, and <300 mg/day of cholesterol), about 20% from proteins, and 40–45% or more from carbohydrates (mainly with low glycemic index). Sodium chloride should not reach 6 g/day (2.4 g of sodium), and dietary fiber should be no less than 30–35 g/day [34].
The CD and MD-HMF groups were instructed to perform at least 10,000 steps a day [19], and the MD-PA group was instructed to undergo 35 min interval training session three times a week as previously described [35]. The three nutritional interventions were characterized by an energy reduction of 25–30% of baseline calories intake and increase energy expenditure by 400 kcal/70 kg (5.7 kcal per kg of body weight). The adherence to the Mediterranean diet (ADM) was assessed using a validated 17-item questionnaire at the beginning and after 6 months of intervention [36].
2.2. Anthropometric Characterization
Weight (kg) was determined with a calibrated scale with the patients barefoot and light clothing, therefore subtracting 0.6 kg for their clothing. Height was measured with a mobile anthropometer (Seca 214, SECA Deutschland, Hamburg, Germany) to the nearest millimeter, keeping the patient’s head in the Frankfort horizontal plane position. Body mass index (BMI) was calculated as kg/m2. Intrahepatic fat content (IFC) was determined using a 1.5-T magnetic resonance imaging (MRI) (Signa Explorer 1.5T, General Electric Healthcare, Chicago, IL, USA) equipped with a 12-channel phased-array coil [37]. Blood pressure was determined in triplicate in the sitting position using a validated semi-automatic oscillometer (Omron HEM, 750CP, Hoofddrop, The Netherlands). The maximal oxygen uptake (VO2 max) was measured with Chester step test (CST) [38].
2.3. Blood Collection and Analysis
Venous blood samples were collected from the antecubital vein with vacutainers containing the anticoagulant ethylene diamine tetra acetic acid (EDTA), after 12-hour overnight fasting. Plasma was isolated by centrifuging the fresh blood at 1700× g for15 min at 4 °C. Biochemical parameters were determined using standardized clinical procedures. The hematological parameters and cell counts were analyzed in whole blood (automatic flow cytometer analyzer Technion H2, Bayer, VCS system, Frankfurt, Germany).
2.4. Enzymatic Determinations
Plasma was used to measure the activities of the antioxidant enzymes catalase (CAT) and superoxide dismutase (SOD) using Shimadzu UV-2100 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) at 37 °C. Specifically, the activity of CAT was determined monitoring the decomposition of H2O2 following the method of Aebi [39]. SOD activity was determined by an adapted method of Flohe and Otting based on the inhibition of the reduction of cytochrome C by superoxide anion generated by the xanthine oxidase/hypoxanthine system [40].
2.5. Malondialdehyde Assay
Malondialdehyde (MDA), a marker of lipid peroxidation, was analyzed in plasma using a colorimetric assay kit (Merck Life Science S.L.U., Madrid, Spain). Briefly, samples and MDA standard were positioned in tubes holding n-methyl-2-phenylindole in acetonitrile: methanol (3:1). Then, HCL (12N) was added, and tubes were incubated at 45 °C for 1 h. Finally, the absorbance was determined at 586 nm.
2.6. Protein Carbonyl Determination
Protein carbonyl derivatives were determined by an OxiSelectTM Protein Carbonyl Immunoblot Kit (CELL BIOLABS®, San Jose, CA, USA) following the supplied guidelines for use. The total protein levels in the samples were determined by the Bradford method [41] using a commercial reagent (Merck Life Science S.L.U., Madrid, Spain). Briefly, following the dot blot method (Bio-Rad, Hercules, CA, USA), 10 µg of protein was transferred into a nitrocellulose membrane, which was incubated with 2,4-dinitrophenylhydrazine (DNPH). Then, the membrane was incubated with the primary antibody specific to DNPH (1:1000). This step was followed by incubation with goat antirabbit IgG (1:1000). After that, immunoblot development was carried out using an enhanced chemiluminescence kit (Immun-Star Western C Kit reagent, Bio-Rad Laboratories, Hercules, CA, USA). Finally, the protein carbonyl bands were quantified with the image analysis program, Quantity One (Bio-Rad Laboratories, Hercules, CA, USA).
2.7. Immunoassay Kits
All immunoassay kits were measured in plasma. Myeloperoxidase (MPO) and xanthine oxidase (XOD) (Cusabio Technology LLC®, Houston, TX, USA), irisin (Cell Biolabs®, San Jose, CA, USA), and resolvin D1 (RvD1) (Cayman Chemical®, Ann Arbor, MI, USA) were determined using ELISA kits following the manufacturers’ instructions. Cytokeratin 18 (CK-18) concentration was determined using the M30 Apoptoense® ELISA kit following the guidelines for use (PEVIVA®, in USA, Canada, and Japan). Tumor necrosis factor alpha (TNFα) and Interleukin-6 (IL-6) levels were determined in plasma using Human Custom ProcartaPlexTM (Invitrogen by Thermo Fisher Scientific, Bender MedSystems GmbH, Vienna, Austria) following the manufacturers’ instructions.
2.8. Statistics
Analyses were carried out with the Statistical Package for Social Sciences version 25.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics with mean ± SD (standard deviation) for participants’ baseline characteristics were used. Participants were classified in tertiles according to 6 months changed in IFC (T1: <−0.567, n = 20; T2: −0.567 to −7.13, n = 20; T3: >−7.13, n = 20). Differences among baseline characteristics according to tertiles of IFC were tested with one-way analysis of variance (ANOVA) and Bonferroni’s post hoc analysis when variables followed normal distribution or with Kruskal–Wallis test for non-normally distributed variables. Results were considered statistically significant if p-value < 0.05. Bivariate correlation between the difference of IFC levels and VO2 max were also analyzed with Pearson correlation.
3. Results
3.1. Anthropometric, Biochemical, and Hematological Parameters
The anthropometric and biochemical characteristics of participants with NAFLD categorized by tertiles after 6 months of change in IFC are shown in Table 1. Significant differences were evidenced in weight, BMI, triglycerides, alanine aminotransferase (ALT), and gamma glutamyl transferase (GGT) when comparing the evolution after 6 months of intervention. The patients in tertiles 2 and 3 presented greater decreases with respect to tertile 1 in weight (p = 0.017 and p < 0.001, respectively), BMI (p = 0.025 and p = 0.024, respectively), and triglycerides (p = 0.048 and p = 0.005, respectively), whereas patients in tertile 3 presented better ALT evolution with respect to tertile 1 (p = 0.014). No differences were observed in systolic and diastolic blood pressure or in the rest of the biochemical parameters.
Table 1.
Changes in anthropometric and biochemical characteristics of adults with nonalcoholic fatty liver disease (NAFLD) categorized by tertiles after 6 months of lifestyle intervention in intrahepatic fat content (IFC).
| Tertile 1 (<−0.567) n = 20 |
Tertile 2 (−0.567 to −7.13) n = 20 |
Tertile 3 (>−7.13) n = 20 |
p-Value | ||
|---|---|---|---|---|---|
| Weight (kg) | Baseline | 91.5 ± 15.7 | 97.8 ± 16.2 | 93.9 ± 8.1 | |
| 6 months | 90.4 ± 14.9 | 92.7 ± 13.2 | 87.1 ± 8.53 | ||
| −1.06 ± 3.36 | −5.08 ± 4.83 * | −6.79 ± 5.06 * | <0.001 | ||
| BMI (kg/m2) | Baseline | 32.5 ± 3.30 | 34.4 ± 4.93 | 34.0 ± 2.70 | |
| 6 months | 32.2 ± 2.91 | 32.7 ± 4.48 | 31.5 ± 2.29 | ||
| −0.375 ± 1.17 | −1.71 ± 1.56 * | −2.51 ± 1.87 * | <0.001 | ||
| Systolic blood pressure (mmHg) | Baseline | 137.4 ± 20.5 | 134.0 ± 12.2 | 142.2 ± 16.9 | |
| 6 months | 134.0 ± 12.0 | 135.5 ± 11.1 | 132.7 ± 11.0 | ||
| −3.74 ± 12.6 | 1.56 ± 13.1 | −1.99 ± 30.4 | 0.318 | ||
| Diastolic blood pressure (mmHg) | Baseline | 80.8 ± 9.71 | 81.5 ± 7.03 | 84.3 ± 9.98 | |
| 6 months | 81.4 ± 7.55 | 82.0 ± 7.65 | 78.0 ± 9.05 | ||
| 0.031 ± 9.76 | 1.00 ± 6.83 | −0.353 ± 18.4 | 0.155 | ||
| Glucose (mg/dL) | Baseline | 109.2 ± 23.5 | 116.1 ± 41.9 | 115.1 ± 20.4 | |
| 6 months | 110.1 ± 25.7 | 112.5 ± 43.7 | 111.7 ± 41.0 | ||
| 0.850 ± 17.9 | −2.76 ± 11.0 | −3.40 ± 27.7 | 0.253 | ||
| Hb1Ac (%) | Baseline | 6.09 ± 1.08 | 6.08 ± 1.13 | 6.00 ± 0.557 | |
| 6 months | 6.05 ± 0.886 | 5.85 ± 0.880 | 5.74 ± 0.412 | ||
| −0.026 ± 0.456 | −0.229 ± 0.384 | −0.238 ± 0.447 | 0.235 | ||
| Triglycerides (mg/dL) | Baseline | 178.3 ± 81.8 | 163.0 ± 58.5 | 240.2 ± 140.6 | |
| 6 months | 232.2 ± 160.5 | 128.1 ± 34.2 | 159.4 ± 78.5 | ||
| 53.8 ± 159.7 | −46.8 ± 34.1 * | −80.8 ± 145.5 * | 0.002 | ||
| HDL cholesterol (mg/dL) | Baseline | 44.1 ± 11.9 | 42.2 ± 8.28 | 39.6 ± 6.94 | |
| 6 months | 44.7 ± 14.0 | 45.8 ± 10.9 | 39.9 ± 5.83 | ||
| 0.950 ± 6.59 | 4.24 ± 5.25 | 0.250 ± 4.51 | 0.054 | ||
| LDL cholesterol (mg/dL) | Baseline | 127.3 ± 34.5 | 125.2 ± 27.2 | 129.0 ± 28.8 | |
| 6 months | 128.5 ± 35.1 | 112.4 ± 25.8 | 121.6 ± 33.4 | ||
| 5.88 ± 33.8 | −7.38 ± 25.0 | −7.11 ± 21.3 | 0.266 | ||
| Cholesterol total (mg/dL) | Baseline | 224.0 ± 74.7 | 200.6 ± 32.1 | 214.8 ± 34.1 | |
| 6 months | 212.7 ± 38.5 | 183.6 ± 33.1 | 192.9 ± 39.2 | ||
| −6.05 ± 78.9 | −11.1 ± 26.1 | −21.9 ± 35.5 | 0.616 | ||
| Bilirubin (mg/dL) | Baseline | 0.811 ± 0.527 | 0.650 ± 0.254 | 0.700 ± 0.395 | |
| 6 months | 0.739 ± 0.379 | 0.636 ± 0.267 | 0.820 ± 0.554 | ||
| 0.024 ± 0.371 | 0.003 ± 0.223 | 0.109 ± 0.300 | 0.516 | ||
| AST (U/L) | Baseline | 23.3 ± 8.33 | 22.8 ± 5.71 | 29.4 ± 10.6 | |
| 6 months | 23.9 ± 6.40 | 20.9 ± 5.52 | 23.9 ± 7.68 | ||
| 0.550 ± 6.42 | −1.11 ± 7.83 | −3.28 ± 7.12 | 0.246 | ||
| ALT (U/L) | Baseline | 27.9 ± 10.1 | 32.4 ± 19.1 | 60.8 ± 58.7 | |
| 6 months | 28.7 ± 11.9 | 24.0 ± 9.35 | 29.8 ± 13.0 | ||
| −0.800 ± 8.46 | −8.48 ± 15.4 | −28.0 ± 50.8 * | 0.004 | ||
| GGT (U/L) | Baseline | 44.2 ± 29.2 | 32.5 ± 14.3 | 48.8 ± 25.2 | |
| 6 months | 43.8 ± 23.8 | 27.8 ± 11.3 | 44.7 ± 55.5 | ||
| −0.450 ± 19.5 | −4.68 ± 5.71 | −4.14 ± 40.4 | 0.022 | ||
| CRP (mg/dL) | Baseline | 0.498 ± 0.505 | 0.529 ± 0.509 | 0.529 ± 0.711 | |
| 6 months | 0.356 ± 0.394 | 0.435 ± 0.336 | 0.322 ± 0.272 | ||
| −0.143 ± 0.494 | −0.090 ± 0.318 | −0.220 ± 0.749 | 0.750 |
Values are the mean ± SD. Abbreviations: BMI, body mass index; Hb1Ac, glycated hemoglobin 1A; HDL cholesterol, high-density lipoprotein; LDL cholesterol, low-density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyl transferase; CRP, c-reactive protein. Difference in means of IFC classified in tertiles was tested with one-factor ANOVA and Bonferroni’s post hoc analysis when variables followed a normal distribution or with Kruskal–Wallis test for non-normally distributed variables. * Difference with respect to tertile 1.
Table 2 summarizes information on hematological parameters at baseline and 6 months follow-up according to tertiles of 6 months change in IFC. When changes between tertiles were compared, significant differences were reported in platelets levels, whereas no differences were described in the rest of the hematological variables of the participants.
Table 2.
Changes in hematological parameters of adults with nonalcoholic fatty liver disease (NAFLD) categorized by tertiles of 6 months change in intrahepatic fat content (IFC).
| Tertile 1 (<−0.567) n = 20 |
Tertile 2 (−0.567 to −7.13) n = 20 |
Tertile 3 (>−7.13) n = 20 |
p-Value | ||
|---|---|---|---|---|---|
| Hematocrit (%) | Baseline | 43.7 ± 4.08 | 43.4 ± 4.11 | 44.9 ± 4.15 | |
| 6 months | 43.5 ± 3.45 | 44.3 ± 3.73 | 45.7 ± 2.76 | ||
| −0.250 ± 2.00 | 0.629 ± 1.54 | 0.780 ± 2.58 | 0.244 | ||
| Erythrocytes (106/μL) | Baseline | 4.90 ± 0.349 | 4.84 ± 0.465 | 5.07 ± 0.403 | |
| 6 months | 4.88 ± 0.329 | 4.89 ± 0.407 | 5.12 ± 0.289 | ||
| −0.022 ± 0.216 | 0.040 ± 0.216 | 0.054 ± 0.342 | 0.217 | ||
| Leukocytes (103/μL) | Baseline | 7.18 ± 2.16 | 7.68 ± 1.95 | 7.44 ± 1.50 | |
| 6 months | 6.92 ± 1.85 | 7.77 ± 1.96 | 6.94 ± 1.58 | ||
| −0.253 ± 1.25 | 0.066 ± 1.60 | −0.503 ± 0.691 | 0.533 | ||
| Platelets (103/μL) | Baseline | 228.2 ± 49.4 | 239.1 ± 44.0 | 243.7 ± 53.3 | |
| 6 months | 226.9 ± 55.0 | 242.7 ± 46.7 | 226.2 ± 47.1 | ||
| −1.30 ± 34.8 | 3.62 ± 23.7 | −17.6 ± 27.4 | 0.022 | ||
| Neutrophils (103/μL) | Baseline | 3.85 ± 1.45 | 4.37 ± 1.19 | 3.86 ± 0.953 | |
| 6 months | 3.66 ± 1.48 | 4.44 ± 1.63 | 3.76 ± 1.14 | ||
| −0.189 ± 0.843 | 0.107 ± 1.30 | −0.102 ± 0.616 | 0.914 | ||
| Lymphocytes (103/μL) | Baseline | 2.35 ± 0.672 | 2.62 ± 0.805 | 2.67 ± 0.667 | |
| 6 months | 2.35 ± 0.516 | 2.47 ± 0.695 | 2.36 ± 0.671 | ||
| 0.002 ± 0.497 | −0.151 ± 0.467 | −0.315 ± 0.278 | 0.071 | ||
| Monocytes (103/μL) | Baseline | 0.656 ± 0.266 | 0.599 ± 0.151 | 0.600 ± 0.101 | |
| 6 months | 0.613 ± 0.242 | 0.596 ± 0.159 | 0.566 ± 0.132 | ||
| −0.043 ± 0.112 | −0.003 ± 0.133 | −0.034 ± 0.097 | 0.596 | ||
| Eosinophils (103/μL) | Baseline | 0.266 ± 0.183 | 0.221 ± 0.148 | 0.244 ± 0.111 | |
| 6 months | 0.243 ± 0.139 | 0.207 ± 0.110 | 0.203 ± 0.129 | ||
| −0.023 ± 0.113 | −0.014 ± 0.067 | −0.042 ± 0.094 | 0.310 | ||
| Basophils (103/μL) | Baseline | 0.058 ± 0.026 | 0.058 ± 0.023 | 0.067 ± 0.025 | |
| 6 months | 0.059 ± 0.024 | 0.051 ± 0.024 | 0.054 ± 0.023 | ||
| 0.001 ± 0.026 | −0.006 ± 0.029 | −0.013 ± 0.033 | 0.325 |
Values are the mean ± SD. Difference in means of IFC classified in tertiles was tested with one-factor ANOVA and Bonferroni’s post hoc analysis when variables followed a normal distribution or with Kruskal–Wallis test for non-normally distributed variables.
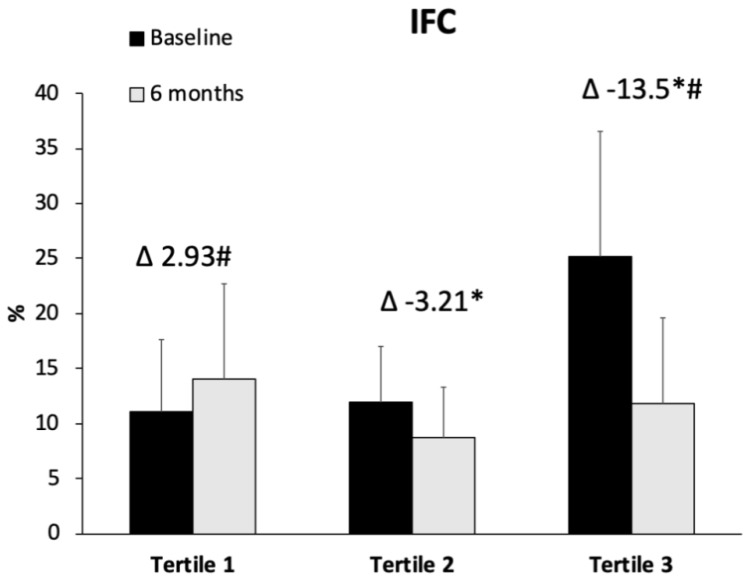
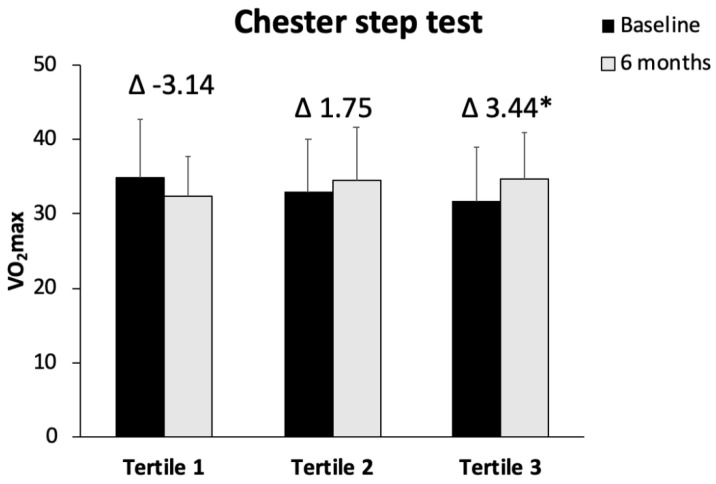
IFC levels at baseline and after 6 months of intervention were shown in Figure 1. The improvement in IFC was significantly higher in tertile 3 with respect to tertiles 2 and 1 (p < 0.001). The ADM was increased in the three group after 6 months when compared with the beginning of the intervention, and this improvement was significantly higher in the tertile 3 with respect to tertile 1 (p = 0.039) (Figure 2). The results of VO2 max in CST are represented in Figure 3. The obtained data reported a significant improvement in tertile 3 with respect to tertile 1 (p = 0.011). A direct correlation between the difference of IFC levels and VO2 max was found (r = 0.386, p < 0.01), whereas an inverse correlation was observed between IFC and ADM (r = −0.332, p < 0.05).
Figure 1.
Values are the mean ± SD. Abbreviations: IFC, intrahepatic fat contents. Differences in means of IFC classified in tertiles was tested with one-factor ANOVA and Bonferroni’s post hoc analysis when variables followed a normal distribution or with Kruskal–Wallis test for non-normally distributed variables. * Differences with respect to tertile 1. # Differences with respect to tertile 2.
Figure 2.
Values are the mean ± SD. Difference in means of ADM classified in tertiles was tested with one-factor ANOVA and Bonferroni’s post hoc analysis when variables followed a normal distribution or with Kruskal–Wallis test for non-normally distributed variables. * Differences with respect to tertile 1.
Figure 3.
Values are the mean ± SD. Difference in means of IFC classified in tertiles was tested with one-factor ANOVA and Bonferroni’s post hoc analysis when variables followed a normal distribution or with Kruskal–Wallis test for non-normally distributed variables. * Differences with respect to tertile 1.
3.2. Oxidative Stress and Inflammatory Biomarkers
Table 3 shows the changes in the enzymatic activities of CAT and SOD and the protein levels of myeloperoxidase (MPO), xanthine oxidase (XOD), resolvin D1, irisin, and cytokeratin 18 (CK-18) and the biomarkers of plasma damage malondialdehyde (MDA) and protein carbonyl derivates. The differences in CAT activity after 6 months of intervention were significantly higher in tertile 3 with respect to tertile 1 (p = 0.002), whereas no significant changes were observed in SOD activity. Irisin and CK-18 levels reported differences in tertile 3 with respect to tertile 1, with higher reductions between baseline and 6 months (p = 0.047 for irisin and p = 0.042 for CK-18). No differences were found in resolvin D1, MPO, XOD, IL-6, TNFα, and MDA and protein carbonyls between groups.
Table 3.
Oxidative stress and inflammatory biomarkers in the plasma of patients with NAFLD categorized by tertiles of 6 months change in IFC.
| Tertile 1 (<−0.567) n = 20 |
Tertile 2 (−0.567 to −7.13) n = 20 |
Tertile 3 (>−7.13) n = 20 |
p-Value | ||
|---|---|---|---|---|---|
| Enzymatic Activities | |||||
| CAT (K/L blood) | Baseline | 41.2 ± 9.41 | 48.0 ± 12.3 | 61.8 ± 16.7 | |
| 6 months | 56.8 ± 29.0 | 37.2 ± 22.6 | 36.7 ± 22.9 | ||
| 13.9 ± 30.2 | −7.84 ± 22.8 | −21.9 ± 26.2 * | 0.003 | ||
| SOD (pkat/L blood) | Baseline | 295.6 ± 60.9 | 283.4 ± 64.3 | 309.9 ± 75.6 | |
| 6 months | 311.9 ± 96.9 | 287.4 ± 65.7 | 287.8 ± 69.8 | ||
| 42.6 ± 156.3 | 9.47 ± 100.9 | −24.9 ± 125.5 | 0.361 | ||
| ELISA assays | |||||
| MPO (ng/mL) | Baseline | 4.07 ± 2.89 | 4.99 ± 2.66 | 4.16 ± 2.22 | |
| 6 months | 3.26 ± 1.06 | 3.67 ± 1.69 | 3.14 ± 1.19 | ||
| −0.874 ± 2.66 | −1.15 ± 3.32 | −1.04 ± 2.00 | 0.680 | ||
| XOD (ng/mL) | Baseline | 0.411 ± 0.122 | 0.370 ± 0.124 | 0.386 ± 0.087 | |
| 6 months | 0.460 ± 0.243 | 0.348 ± 0.161 | 0.350 ± 0.113 | ||
| 0.068 ± 0.261 | −0.017 ± 0.166 | −0.023 ± 0.106 | 0.307 | ||
| Resolvin D1 (pg/mL) | Baseline | 132.9 ± 44.2 | 135.1 ± 43.4 | 140.3 ± 33.8 | |
| 6 months | 147.5 ± 30.7 | 159.5 ± 45.5 | 162.6 ± 32.4 | ||
| 14.4 ± 36.4 | 24.0 ± 62.2 | 23.2 ± 25.8 | 0.706 | ||
| Irisin (ng/mL) | Baseline | 118.6 ± 76.3 | 102.4 ± 63.8 | 132.3 ± 72.7 | |
| 6 months | 124.7 ± 92.9 | 112.2 ± 70.9 | 92.1 ± 59.1 | ||
| 6.3 ± 76.2 | 11.6 ± 65.5 | −39.5 ± 56.4 * | 0.002 | ||
| CK-18 (U/L) | Baseline | 47.1 ± 24.6 | 71.1 ± 44.2 | 96.8 ± 55.4 | |
| 6 months | 42.4 ± 22.3 | 41.0 ± 20.7 | 54.5 ± 41.0 | ||
| −1.04 ± 21.3 | −29.6 ± 50.2 | −44.2 ± 54.0 * | 0.040 | ||
| Multiplex Assay | |||||
| Baseline | 4.25 ± 0.217 | 4.13 ± 0.259 | 4.28 ± 0.520 | ||
| IL-6 (pg/mL) | 6 months | 4.34 ± 0.377 | 4.26 ± 0.424 | 4.34 ± 0.604 | |
| 0.103 ± 0.342 | −0.015 ± 0.117 | 0.035 ± 0.289 | 0.805 | ||
| Baseline | 3.05 ± 0.543 | 3.85 ± 0.466 | 4.25 ± 1.74 | ||
| TNFα (pg/mL) | 6 months | 3.95 ± 0.441 | 3.79 ± 0.381 | 4.19 ± 1.76 | |
| −0.038 ± 0.399 | −0.209 ± 0.284 | −0.120 ± 0.338 | 0.355 | ||
| Oxidative damage | |||||
| MDA (nM) | Baseline | 1.88 ± 0.737 | 1.71 ± 0.640 | 2.01 ± 0.843 | |
| 6 months | 1.69 ± 1.41 | 1.43 ± 0.597 | 1.20 ± 0.483 | ||
| −0.111 ± 1.71 | −0.337 ± 1.01 | −0.874 ± 1.09 | 0.244 | ||
| Protein carbonyl (%) | Baseline | 100.0 ± 67.7 | 123.2 ± 65.0 | 136.8 ± 74.2 | |
| 6 months | 95.9 ± 34.3 | 88.3 ± 31.3 | 84.7 ± 18.6 | ||
| −4.1 ± 88.4 | −35.5 ± 78.4 | −50.9 ± 71.7 | 0.285 |
Values are the mean ± SD. Abbreviations: CAT, catalase; SOD, superoxide dismutase; MPO, myeloperoxidase; CK-18, cytokeratin 18; XOD, xanthine oxidase; MDA, malondialdehyde. Difference in means of IFC classified in tertiles was tested with one-factor ANOVA and Bonferroni’s post hoc analysis when variables followed a normal distribution or with Kruskal–Wallis test for non-normally distributed variables. * Difference with respect to tertile 1.
4. Discussion
The main findings of the current study are that a reduction in IFC regardless of the type of intervention followed is related to better oxidative and inflammatory state and with an improvement in aerobic capacity. The baseline situation of these patients at the beginning of the study in addition to NAFLD diagnosed by MRI showed biochemical parameters out of the described reference values [12]. The reduction of weight and BMI in tertile 3 subjects when compared with tertile 1 is associated with the improvement in IFC. Previous studies suggested that BMI or weight reduction is not essential for decreasing hepatic fat contents or to restore normal liver function [42]; however, current international guidelines stated that the primary goal of nutrition therapy in NAFLD is to reduce energy intake by 500–100 kcal per day to achieve a 7–10% reduction in body weight [30,43,44]. It has been reported that an optimal nutritional therapy for patients with NAFLD could be an initial very-low-calorie diet period of several weeks, characterized by high protein, high-soluble fiber, and low-carbohydrate formula, followed by a structured program of food reintroduction implementing Mediterranean dietary patterns [45].
The intervention groups in the present study were designed to improve the IFC in NAFLD patients. For this reason, three different interventions for the management of NAFLD were selected following international guidelines, which recommend the combination of dietary modifications and physical activity to lose weight [46,47]. At 6 months of intervention, a similar improvement was observed in the three groups, with no statistical differences between them [47]. These results led us to analyze the differences in stress and inflammation markers according to the degree of improvement in the IFC regardless of the intervention followed by the patients. However, when grouping the participants according to the degree of improvement in IFC, it was observed that the groups are different at baseline. In fact, the group that presented the highest IFC at the beginning of the study is the one that improved the most after 6 months of intervention. These data are consistent with studies that report greater weight loss in patients undergoing nutritional intervention as their BMI increases due to the greater ease of losing fat in subjects with a higher initial fat content than in those closer to normal weight [48,49]. In addition, when analyzing the ADM, a greater increase was observed between the start of the intervention and after 6 months in parallel to the greater decrease in IFC. The Mediterranean diet is characterized by its antioxidant and anti-inflammatory properties, so the improvement in diet quality may contribute to the improvement observed in the patients included in tertile 3 [50].
Participants with better improvement in IFC showed fewer levels of triglycerides, ALT, and GGT after 6 months of intervention with respect to basal levels. One of the hallmarks of NAFLD is the appearance of insulin resistance that leads to an increase in plasma glucose concentrations and an excessive production of triglyceride rich VLDL, inducing hypertriglyceridemia [51,52]. ALT is a liver enzyme commonly used as specific marker of liver inflammation and hepatocellular damage and widely used as marker of NAFLD presence in addition to presenting a good correlation with liver fat contents [53,54]. However, it should be considered that up to 25% of patients with NAFLD had normal ALT values; therefore, high ALT serum levels might underestimate the prevalence of NAFLD [55]. The current findings are in accordance with previous results, which showed high levels of triglycerides, ALT, and GGT in NAFLD patients with IFC ≥ 2 in comparison to patients with IFC of 0 and 1 [12]. This improvement was also observed in a dietary intervention study based on the Mediterranean diet, in which the patients reduced their weight by 7% along with a significant improvement in BMI, waist circumference, AST, ALT, GGT, and TG after the intervention period [55].
One of the relevant results of the current study is the direct relationship between the improvement in aerobic capacity (VO2 max) and the improvement in IFC, indicating the importance of physical activity in the reversal of fat accumulation in the liver. It has been evidenced that the CST is a valid, easy, and inexpensive solution for assessing the VO2 max in individuals with hypertension [56]. Increasing physical activity (aerobic physical activity and resistance training) and avoiding a sedentary lifestyle has been shown to exert a beneficial impact on NAFLD by improving liver injury, liver fat, and the histologic features of NAFLD [57]. In this sense, it has been shown that a lifestyle intervention combining diet and physical activity promotion improved functional fitness in patients with NAFLD [58]. However, it has been shown that although patients with NAFLD are aware of the need for physical activity, most described problems for its regular practice, such as lack of resources and education, physical discomfort during exercise, or time constraints [59]. All make it necessary to motivate and work hard with patients with NAFLD so that they perform physical exercise and do not abandon it.
Moreover, current participants also showed a reduction of the activities of enzymatic antioxidants CAT and SOD after 6 months of intervention in tertile 3 subjects. Previous data suggested that the excessive ROS production and inflammation are key actors in the pathogenesis of NALFD and directly related to hepatic cell death and apoptosis [28]. These findings would indicate a reduction in the degree of oxidative stress and points out the importance of the improvement of IFC as a healthy life contributing for a better oxidative stress status. It has been well-established that the activities of enzymatic antioxidants were high in early and advanced NAFLD subjects compared to controls because of a more pro-oxidant state in these patients [12,60]. The alterations in lipid metabolism led to the accumulation of lipids in the liver, which results in an overproduction of ROS within the mitochondria electron transport chain. Moreover, metabolic liver diseases such as NAFLD are associated with increased ROS production during fatty acid β-oxidation, endoplasmic reticulum stress, and NADPH oxidase alterations [61]. The reduction in the activity of antioxidant enzymes is not related to changes in oxidative damage markers, which could indicate that the activation of antioxidant defenses in these patients allows keeping oxidative damage under control.
In addition, the current findings did not show significant differences in the MPO and XOD levels as biomarkers of pro-oxidative states. In the case of MPO, as this enzyme is released mainly by neutrophils, this lack of changes could be related to the fact that the number of these cells did not change after 6 months of intervention [62]. Regarding XOD, there is a trend to decrease, but this change is not significant; therefore, it would be necessary to achieve an additional improvement in hepatic steatosis with a longer intervention time.
Furthermore, irisin levels were significantly reduced in tertile 3 after 6 months with respect to tertile 1. Irisin is a myokine/adipokine induced by exercise in humans, which is proposed to produce “browning” of white adipose tissue, thus increasing thermogenesis and energy expenditure [63]. Irisin is a promising regulator of glucose metabolism, which is involved in glucose homeostasis in muscle, liver, and adipose tissue, contributing to normoglycemia [64]. Previous report showed that the subjects with NAFLD had higher irisin levels than healthy ones [65], and these high levels have been suggested to act as a compensatory mechanism aimed at improving energy metabolism and insulin sensitivity [66]. Thus, the reduction of its levels in the group with more improvement could be indicative of a metabolic normalization of these patients.
The current study revealed that patients with NAFLD, who improved the IFC levels the most, also showed an improvement in CK18 levels. CK18 is a marker for apoptosis and inflammation, which found to be increased in NAFLD patients [67]. Previous studies revealed that CK18 increased with liver steatosis, and it has been described as an adequate and non-invasive marker, which could allow for the identifications of patients with NAFLD [12,68,69]. Moreover, studies have shown a decrease in serum CK18 levels and weight loss in patients with liver fibrosis after 6-month dietary intervention; consequently, this points out the existence of a positive association between changes in CK18 levels and weight loss [70]. However, other more general markers related to inflammation, such as TNFα, IL-6, or resolvin D1, did not show changes in their values. This could be due to the fact that although after 6 months of intervention, there was a reduction in BMI, patients continue to be obese; thus, these more general markers would not be sensitive enough and would require further improvement.
A limitation of the study could derive from the differences in the baseline IFC between the groups, which could make it difficult to interpret the results. However, the objective of the study was to analyze the changes associated with the IFC after a 6-month period to evidence the benefits of liver fat loss rather than comparing the initial values.
5. Conclusions
The present data evidenced that the reduction of IFC is associated with an improvement in oxidative stress and pro-inflammatory status and a better cardiorespiratory fitness in NAFLD patients. Plasma oxidative stress and inflammation biomarkers were mainly reduced in the patients in the tertile that showed a greater reduction in intrahepatic fat levels after 6 months of lifestyle intervention. The relationship between aerobic capacity improvement, the ADM, and IFC reduction highlights the importance of the quality of diet and regular physical activity in the prevention/reversal of NAFLD. In conclusion, the beneficial effects of lifestyle changes on NAFLD development could be applied to prevent incident NAFLD (as cardiovascular diseases) and reduce the future public health burden. However, evidence regarding the effects of this approach on NASH with advanced fibrosis, until now, is insufficient and must be evaluated and verified in future studies.
Acknowledgments
The authors especially thank the participants for their enthusiastic collaboration and the personnel for their outstanding support and exceptional efforts. The authors thank Octavio Barbero from Red Asistencial Juaneda, Palma de Mallorca, Spain, for technical assistance. CIBEROBN is an initiative of Instituto de Salud Carlos III, Spain.
Author Contributions
Conceptualization, A.S., J.A.M. and J.A.T.; methodology, M.M.-M., M.Q.-L., C.B., J.M.G., I.L., A.S., S.M., C.M.M. and S.T.; investigation, M.C., M.M.-M., M.Q.-L., S.M., C.M.M. and S.T.; data curation, M.M.-M., M.Q.-L. and C.B.; writing—original draft preparation, M.M.-M., M.Q.-L., C.B., A.S. and J.A.T. writing—review and editing, M.C., A.S. and J.A.T.; project administration, A.S. and J.A.T.; funding acquisition, J.A.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Balearic Islands (IB 2251/14 PI).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
There are restrictions on the availability of data for this trial, due to the signed consent agreements around data sharing, which only allow access to external researchers for studies following the project purposes. Researchers wishing to access the trial data used in this study can make a request to the corresponding author: pep.tur@uib.es.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Fundació La Marató TV3 (Spain) project ref. 201630.10. Instituto de Salud Carlos III through the Fondo de Investigación para la Salud (CIBEROBN CB12/03/30038 and Proyecto Intramural CIBER OBN18PI03), which are co-funded by the European Regional Development Fund. Other funding received: EU-COST Action CA16112 and IDISBA Grants (FOLIUM, PRIMUS, SYNERGIA, and LIBERI). M.Q.-L. was granted by IDISBA grant. C.M.M. received an FPU Ph.D. Grant from the Spanish Ministry of Education. The funding sponsors had no role in the design of the study, in the collection, analyses, or interpretation of the data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Finck B.N. Targeting metabolism, insulin resistance, and diabetes to treat nonalcoholic steatohepatitis. Diabetes. 2018;67:2485–2493. doi: 10.2337/dbi18-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019;70:531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 4.Ye Q., Zou B., Yeo Y.H., Li J., Huaang D.Q., Wu Y., Yang H., Liu C., Kam L.Y., Tan X.X.E., et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020;5:739–752. doi: 10.1016/S2468-1253(20)30077-7. [DOI] [PubMed] [Google Scholar]
- 5.Ni Than N.A., Newsome P.N. Non-alcoholic fatty liver disease: When to intervene and with what. Clin. Med. 2015;15:186–190. doi: 10.7861/clinmedicine.15-2-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison S.A., Oliver D., Arnold H.L., Gogia S., Neuschwander-Tetri B.A. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 7.Al-Dayyat H.M., Rayyan Y.M., Tayyem R.F. Non-alcoholic fatty liver disease and associated dietary and lifestyle risk factors. Diabetes Metab. Syndr. 2018;12:569–575. doi: 10.1016/j.dsx.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z., Suo C., Zhao R., Yuan H., Jin L., Zhang T., Chen X. Genetic predisposition, lifestyle risk, and obesity associate with the progression of nonalcoholic fatty liver disease. Dig. Liver Dis. 2021;53:1435–1442. doi: 10.1016/j.dld.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Noureddin M., Zelber-Sagi S., Wilkens L.R., Porcel J., Boushey C.J., Marchand L., Le Rosen H.R., Setiawan V.W. Diet Associations With Nonalcoholic Fatty Liver Disease in an Ethnically Diverse Population: The Multiethnic Cohort. Hepatology. 2020;71:1940–1952. doi: 10.1002/hep.30967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rani V., Deep G., Singh R.K., Palle K., Yadav U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016;148:183–193. doi: 10.1016/j.lfs.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Masarone M., Rosato V., Dallio M., Gravina A.G., Aglitti A., Loguercio C., Federico A., Persico M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018;2018:9547613. doi: 10.1155/2018/9547613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monserrat-Mesquida M., Quetglas-Llabrés M., Abbate M., Montemayor S., Mascaró C.M., Casares M., Tejada S., Abete I., Zulet M.A., Tur J.A., et al. Oxidative stress and pro-inflammatory status in patients with non-alcoholic fatty liver disease. Antioxidants. 2020;9:759. doi: 10.3390/antiox9080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alkhouri N., Dixon L.J., Feldstein A.E. Lipotoxicity in nonalcoholic fatty liver disease: Not all lipids are created equal. Expert Rev. Gastroenterol. Hepatol. 2009;3:445–451. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ucar F., Sezer S., Erdogan S., Akyol S., Armutcu F., Akyol O. The relationship between oxidative stress and nonalcoholic fatty liver disease: Its effects on the development of nonalcoholic steatohepatitis. Redox Rep. 2013;18:127–133. doi: 10.1179/1351000213Y.0000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter-Kent C., Zein N.N., Feldstein A.E. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: Implications for treatment. Am. J. Gastroenterol. 2008;103:1036–1042. doi: 10.1111/j.1572-0241.2007.01709.x. [DOI] [PubMed] [Google Scholar]
- 16.El-Din S.H.S., Sabra A.-N.A., Hammam O.A., Ebeid F.A., El-Lakkany N.M. Pharmacological and antioxidant actions of garlic and.or onion in non-alcoholic fatty liver disease (NAFLD) in rats. J. Egypt. Soc. Parasitol. 2014;44:295–308. doi: 10.12816/0006468. [DOI] [PubMed] [Google Scholar]
- 17.Hajighasem A., Farzanegi P., Mazaheri Z. Effects of combined therapy with resveratrol, continuous and interval exercises on apoptosis, oxidative stress, and inflammatory biomarkers in the liver of old rats with non-alcoholic fatty liver disease. Arch. Physiol. Biochem. 2019;125:142–149. doi: 10.1080/13813455.2018.1441872. [DOI] [PubMed] [Google Scholar]
- 18.Patterson R.E., Kalavalapalli S., Williams C.M., Nautiyal M., Mathew J.T., Martinez J., Reinhard M.K., McDougall D.J., Rocca J.R., Yost R.A., et al. Lipotoxicity in steatohepatitis occurs despite an increase in tricarboxylic acid cycle activity. Am. J. Physiol. Endocrinol. Metab. 2016;310:E484–E494. doi: 10.1152/ajpendo.00492.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perdomo C.M., Frühbeck G., Escalada J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients. 2019;11:677. doi: 10.3390/nu11030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dallio M., Romeo M., Gravina A.G., Masarone M., Larussa T., Abenavoli L., Persico M., Loguercio C., Federico A. Nutrigenomics and Nutrigenetics in Metabolic- (Dysfunction) Associated Fatty Liver Disease: Novel Insights and Future Perspectives. Nutrients. 2021;13:1679. doi: 10.3390/nu13051679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abenavoli L., Boccuto L., Federico A., Dallio M., Loguercio C., Di Renzo L., De Lorenzo A. Diet and Non-Alcoholic Fatty Liver Disease: The Mediterranean Way. Int. J. Environ. Res. Public Health. 2019;16:3011. doi: 10.3390/ijerph16173011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cigrovski Berkovic M., Bilic-Curcic I., Mrzljak A., Cigrovski V. NAFLD and Physical Exercise: Ready, Steady, Go! Front. Nutr. 2021;8:734859. doi: 10.3389/fnut.2021.734859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero-Gómez M., Zelber-Sagi S., Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017;67:829–846. doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Wing R.R., Bolin P., Brancati F.L., Bray G.A., Clark J.M., Coday M., Crow R.S., Curtis J.M., Egan C.M., Espeland M.A., et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byers T., Sedjo R.L. Body fatness as a cause of cancer: Epidemiologic clues to biologic mechanisms. Endocr. Relat. Cancer. 2015;22:R125–R134. doi: 10.1530/ERC-14-0580. [DOI] [PubMed] [Google Scholar]
- 26.Lazo M., Solga S.F., Horska A., Bonekamp S., Diehl A.M., Brancati F.L., Wagenknecht L.E., Pi-Sunyer F.X., Kahn S.E., Clark J.M. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156–2163. doi: 10.2337/dc10-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brouwers B., Schrauwen-Hinderling V.B., Jelenik T., Gemmink A., Sparks L.M., Havekes B., Bruls Y., Dahlmans D., Roden M., Hesselink M.K.C., et al. Exercise training reduces intrahepatic lipid content in people with and people without nonalcoholic fatty liver. Am. J. Physiol. Endocrinol. Metab. 2018;314:E165–E173. doi: 10.1152/ajpendo.00266.2017. [DOI] [PubMed] [Google Scholar]
- 28.Farzanegi P., Dana A., Ebrahimpoor Z., Asadi M., Azarbayjani M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019;19:994–1003. doi: 10.1080/17461391.2019.1571114. [DOI] [PubMed] [Google Scholar]
- 29.Alberti K.G.M.M., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P.T., Loria C.M., Smith S.C. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 30.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Department of Health and Human Services. U.S. Department of Agriculture . Dietry Guidlines for Americans 2015–2020. 8th ed. U.S. Department of Agriculture; Washington, DC, USA: 2015. [Google Scholar]
- 32.De La Iglesia R., Lopez-Legarrea P., Abete I., Bondia-Pons I., Navas-Carretero S., Forga L., Martinez J.A., Zulet M.A. A new dietary strategy for long-term treatment of the metabolic syndrome is compared with the American heart association (AHA) guidelines: The MEtabolic Syndrome REduction in NAvarra (RESMENA) project. Br. J. Nutr. 2014;111:643–652. doi: 10.1017/S0007114513002778. [DOI] [PubMed] [Google Scholar]
- 33.Zulet M., Bondia-Pons I., Abete I., de la Iglesia R., López-Legarrera P., Forga L., Navas-Carretero S., Martínez J. The reduction of the metabolyc syndrome in Navarra-Spain (RESMENA-S) study: A multidisciplinary strategy based on chrononutrition and nutritional education, together with dietetic and psychological control. Nutr. Hosp. 2011;26:16–26. [PubMed] [Google Scholar]
- 34.Estruch R., Ros E., Salas-Salvadó J., Covas M.-I., Corella D., Arós F., Gómez-Gracia E., Ruiz-Gutiérrez V., Fiol M., Lapetra J., et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 35.Abbate M., Mascaró C.M., Montemayor S., Barbería-Latasa M., Casares M., Gómez C., Angullo-Martinez E., Tejada S., Abete I., Zulet M.A., et al. Energy Expenditure Improved Risk Factors Associated with Renal Function Loss in NAFLD and MetS Patients. Nutrients. 2021;13:629. doi: 10.3390/nu13020629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Álvarez-Álvarez I., Martinez-Gonzalez M.A., Sánchez-Tainta A., Corella D., Díaz-López A., Fito M., Vioque J., Romaguera D., Martínez J.A., Wärnberg J., et al. Adherence to an energy-restricted Mediterranean diet score and prevalence of cardiovascular risk factors in the PREDIMED-plus: A cross-sectional study. Rev. Española Cardiol. 2019;72:925–934. doi: 10.1016/j.recesp.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Reeder S.B., Sirlin C.B. Quantification of liver fat with magnetic resonance imaging. Magn. Reson. Imaging Clin. N. Am. 2010;18:337–357. doi: 10.1016/j.mric.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buckley J., Sim J., Eston R., Hession R., Fox R. Reliability and validity of measures taken during the Chester step test to predict aerobic power and to prescribe aerobic exercise. Br. J. Sports Med. 2004;38:197–205. doi: 10.1136/bjsm.2003.005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 40.Flohé L., Otting F. Superoxide dismutase assays. Methods Enzymol. 1984;105:93–104. doi: 10.1016/s0076-6879(84)05013-8. [DOI] [PubMed] [Google Scholar]
- 41.Bradford M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 42.Mardinoglu A., Wu H., Bjornson E., Zhang C., Hakkarainen A., Räsänen S.M., Lee S., Mancina R.M., Bergentall M., Pietiläinen K.H., et al. An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell Metab. 2018;27:559–571.e5. doi: 10.1016/j.cmet.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roeb E., Steffen H.M., Bantel H., Baumann U., Canbay A., Demir M., Drebber U., Geier A., Hampe J., Hellerbrand C., et al. [S2k Guideline non-alcoholic fatty liver disease] Z. Gastroenterol. 2015;53:668–723. doi: 10.1055/s-0035-1553193. [DOI] [PubMed] [Google Scholar]
- 44.Marchesini G., Day C.P., Dufour J.F., Canbay A., Nobili V., Ratziu V., Tilg H., Roden M., Gastaldelli A., Yki-Jarvinen H., et al. EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Worm N. Beyond Body Weight-Loss: Dietary Strategies Targeting Intrahepatic Fat in NAFLD. Nutrients. 2020;12:1316. doi: 10.3390/nu12051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Houttu V., Csader S., Nieuwdorp M., Holleboom A.G., Schwab U. Dietary Interventions in Patients With Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front. Nutr. 2021;8:716783. doi: 10.3389/fnut.2021.716783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montemayor S., Bouzas C., Mascaró C.M., Casares M., Llompart I., Abete I., Angullo-Martinez E., Zulet M.Á., Martínez J.A., Tur J.A. Effect of Dietary and Lifestyle Interventions on the Amelioration of NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients. 2022;14:2223. doi: 10.3390/nu14112223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore L.L., Visioni A.J., Qureshi M.M., Bradlee M.L., Ellison R.C., D’Agostino R. Weight loss in overweight adults and the long-term risk of hypertension: The Framingham study. Arch. Intern. Med. 2005;165:1298–1303. doi: 10.1001/archinte.165.11.1298. [DOI] [PubMed] [Google Scholar]
- 49.Greenberg I., Stampfer M.J., Schwarzfuchs D., Shai I., DIRECT group Adherence and success in long-term weight loss diets: The dietary intervention randomized controlled trial (DIRECT) J. Am. Coll. Nutr. 2009;28:159–168. doi: 10.1080/07315724.2009.10719767. [DOI] [PubMed] [Google Scholar]
- 50.Salomone F., Godos J., Zelber-Sagi S. Natural antioxidants for non-alcoholic fatty liver disease: Molecular targets and clinical perspectives. Liver Int. 2016;36:5–20. doi: 10.1111/liv.12975. [DOI] [PubMed] [Google Scholar]
- 51.Fabbrini E., Mohammed B.S., Magkos F., Korenblat K.M., Patterson B.W., Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanni E., Bugianesi E., Kotronen A., Minicis D.S., Yki-Järvinen H., Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa? Dig. Liver Dis. 2010;42:320–330. doi: 10.1016/j.dld.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 53.Williams K., Shackel N., Gorrell M., McLennan S., Twigg S. Diabetes and nonalcoholic Fatty liver disease: A pathogenic duo. Endocr. Rev. 2013;34:84–129. doi: 10.1210/er.2012-1009. [DOI] [PubMed] [Google Scholar]
- 54.Westerbacka J., Cornér A., Tiikkainen M., Tamminen M., Vehkavaara S., Häkkinen A., Fredriksson J., Yki-Järvinen H. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: Implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360–1369. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]
- 55.Xuefeng M., Shousheng L., Zhang J., Mengzhen D., Wang Y., Wang M., Yongning X. Proportion of NAFLD patients with normal ALT value in overall NAFLD patients: A systematic review and meta-analysis. BMC Gastroenterol. 2020;20:10. doi: 10.1186/s12876-020-1165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gelli C., Tarocchi M., Abenavoli L., Di Renzo L., Galli A., De Lorenzo A. Effect of a counseling-supported treatment with the Mediterranean diet and physical activity on the severity of the non-alcoholic fatty liver disease. World J. Gastroenterol. 2017;23:3150–3162. doi: 10.3748/wjg.v23.i17.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Izquierdo M.C., Lopes S., Teixeira M., Polónia J., Alves A.J., Mesquita-Bastos J., Ribeiro F. The Chester step test is a valid tool to assess cardiorespiratory fitness in adults with hypertension: Reducing the gap between clinical practice and fitness assessments. Hypertens. Res. 2019;42:2021–2024. doi: 10.1038/s41440-019-0316-5. [DOI] [PubMed] [Google Scholar]
- 58.Mascaró C.M., Bouzas C., Montemayor S., Casares M., Llompart I., Ugarriza L., Borràs P.A., Martínez J.A., Tur J.A. Effect of a Six-Month Lifestyle Intervention on the Physical Activity and Fitness Status of Adults with NAFLD and Metabolic Syndrome. Nutrients. 2022;14:1813. doi: 10.3390/nu14091813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwak M.-S., Kim D. Non-alcoholic fatty liver disease and lifestyle modifications, focusing on physical activity. Korean J. Intern. Med. 2018;33:64–74. doi: 10.3904/kjim.2017.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stine J.G., Soriano C., Schreibman I., Rivas G., Hummer B., Yoo E., Schmitz K., Sciamanna C. Breaking Down Barriers to Physical Activity in Patients with Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2021;66:3604–3611. doi: 10.1007/s10620-020-06673-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Świderska M., Maciejczyk M., Zalewska A., Pogorzelska J., Flisiak R., Chabowski A. Oxidative stress biomarkers in the serum and plasma of patients with non-alcoholic fatty liver disease (NAFLD). Can plasma AGE be a marker of NAFLD? Oxidative stress biomarkers in NAFLD patients. Free Radic. Res. 2019;53:841–850. doi: 10.1080/10715762.2019.1635691. [DOI] [PubMed] [Google Scholar]
- 62.Chen Z., Tian R., She Z., Cai J., Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020;152:116–141. doi: 10.1016/j.freeradbiomed.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 63.Heinecke J.W. Mechanisms of oxidative damage of low density lipoprotein in human atherosclerosis. Curr. Opin. Lipidol. 1997;8:268–274. doi: 10.1097/00041433-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 64.Polyzos S.A., Anastasilakis A.D., Efstathiadou Z.A., Makras P., Perakakis N., Kountouras J., Mantzoros C.S. Irisin in metabolic diseases. Endocrine. 2018;59:260–274. doi: 10.1007/s12020-017-1476-1. [DOI] [PubMed] [Google Scholar]
- 65.Perakakis N., Triantafyllou G.A., Fernández-Real J.M., Huh J.Y., Park K.H., Seufert J., Mantzoros C.S. Physiology and role of irisin in glucose homeostasis. Nat. Rev. Endocrinol. 2017;13:324–337. doi: 10.1038/nrendo.2016.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu J., Ke Y., Wu F., Liu S., Ji C., Zhu X., Zhang Y. Circulating Irisin Levels in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2020;2020:8818191. doi: 10.1155/2020/8818191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altaf B., Jawed S., Salam R.M.T. Association of apoptotic marker cytokeratin18 with blood pressure in nonalcoholic fatty liver disease patients. J. Pak. Med. Assoc. 2020;70:2128–2131. doi: 10.47391/JPMA.689. [DOI] [PubMed] [Google Scholar]
- 68.Musso G., Gambino R., Cassader M., Pagano G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 69.Kawanaka M., Nishino K., Nakamura J., Urata N., Oka T., Goto D., Suehiro M., Kawamoto H., Yamada G. Correlation between serum cytokeratin-18 and the progression or regression of non-alcoholic fatty liver disease. Ann. Hepatol. 2015;14:837–844. doi: 10.5604/16652681.1171767. [DOI] [PubMed] [Google Scholar]
- 70.Safarian M., Mohammadpour S., Shafiee M., Ganji A., Soleimani A., Nematy M., Bahari A. Effect of diet-induced weight loss on cytokeratin-18 levels in overweight and obese patients with liver fibrosis. Diabetes Metab. Syndr. 2019;13:989–994. doi: 10.1016/j.dsx.2019.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are restrictions on the availability of data for this trial, due to the signed consent agreements around data sharing, which only allow access to external researchers for studies following the project purposes. Researchers wishing to access the trial data used in this study can make a request to the corresponding author: pep.tur@uib.es.