Abstract
Attempts were made to engineer the periplasm of Escherichia coli to an expression compartment of heterologous proteins in their native conformation. As a first approach the low-molecular-size additive l-arginine and the redox compound glutathione (GSH) were added to the culture medium. Addition of 0.4 M l-arginine and 5 mM reduced GSH increased the yield of a native tissue-type plasminogen activator variant (rPA), consisting of the kringle-2 and the protease domain, and a single-chain antibody fragment (scFv) up to 10- and 37-fold, respectively. A variety of other medium additives also had positive effects on the yield of rPA. In a second set of experiments, the effects of cosecreted ATP-independent molecular chaperones on the yields of native therapeutic proteins were investigated. At optimized conditions, cosecretion of E. coli DnaJ or murine Hsp25 increased the yield of native rPA by a factor of 170 and 125, respectively. Cosecretion of DnaJ also dramatically increased the amount of a second model protein, native proinsulin, in the periplasm. The results of this study are anticipated to initiate a series of new approaches to increase the yields of native, disulfide-bridged, recombinant proteins in the periplasm of E. coli.
Most therapeutically relevant proteins contain disulfide bridges and cannot be produced in their native conformation in the bacterial cytosol. In vitro refolding of inclusion body material is often laborious and costly. An alternative strategy to obtain these proteins in their native forms is to use their secretion into the periplasmic space. Targeting of proteins to the periplasm has both advantages and disadvantages. A major drawback of the periplasm is that space is limited. Thus, yields of recombinant proteins generally never match those obtained upon cytosolic expression. Also, translocation into the periplasmic space can limit the final yields of recombinant proteins. However, in the case of those proteins that bear multiple disulfide bonds of nonlinear connectivities in their native conformations and that are resilient to renaturation of inclusion body material, expression in the periplasmic space may offer the method of choice. The periplasm is a compartment where oxidation of thiols can occur due to the activity of the disulfide oxidoreductase (Dsb) system (for a review, see reference 28). The overall milieu of the periplasm is strongly oxidizing, with the DsbA protein being the major oxidant. However, Dsb components with disulfide isomerase functions, DsbC and DsbG, have also been described (5, 40). Still, presumably disulfide bond isomerization is insufficient in the periplasm, given that recombinant proteins that carry multiple disulfide bonds in their native conformations have a pronounced tendency to aggregate. Considering this major drawback of the expression of disulfide-containing proteins, the following strategies were devised to optimize folding in the periplasm: (i) modification of the medium composition by the addition of low-molecular-size compounds known to stimulate folding in vitro, (ii) addition of a redox component to allow reshuffling of wrongly formed disulfide bridges, and (iii) cosecretion of ATP-independent chaperones.
As model proteins, a truncated version of tissue-type plasminogen activator, consisting of the kringle-2 and the protease domain (BM 06.022, also known as rPA [23]), proinsulin, and a single-chain antibody fragment were chosen.
Our objective was to improve the yield of native rPA in the periplasm of Escherichia coli. A beneficial effect was observed upon the addition of low-molecular-size folding enhancers and reduced glutathione (GSH) and also upon cosecretion of either DnaJ or Hsp25. The general applicability of an optimized periplasmic expression compartment was confirmed with the two additional model proteins.
MATERIALS AND METHODS
Genetic and protein analytic techniques.
Cloning, transformation of E. coli cells, and DNA preparations were done by standard techniques (1). Oligonucleotides were purchased from Gibco BRL or MWG Biotech AG. Restriction enzymes were obtained from Roche Molecular Biochemicals GmbH, AGS GmbH, or New England Biolabs. Sequences of recloned DNA fragments were routinely confirmed by dideoxy sequencing (LiCor DNA-Sequencer 4000; LiCor, Lincoln, Nebr.). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting were carried out as described in reference 8.
Strains, plasmids, proteins, and chemicals.
E. coli strain BL21(DE3) was obtained from Novagen and used for gene expression; E. coli N4830/pPL-dnaJ-23 as a DnaJ overexpression strain was kindly provided by Thomas Langer (University of Munich, Munich, Germany).
Plasmids of the pIN III ompA (16) series were kindly provided by Masayori Inouye (University of Medicine and Dentistry of New Jersey), pMC111 M1 as a source for hsp25 DNA was provided by Matthias Gaestel (University of Halle-Wittenberg), pA27fd7 (23) as a source for the rPA gene and rPA standard was provided by Ulrich Kohnert (Roche Diagnostics), pUBS520 (6) was provided by Ulrich Brinkmann (Epidauros Biotechnology, Bernried, Germany), and pHEN-scFv-ox (12), containing a pelB signal sequence and a lac promoter as a source for the scFv-ox gene, was provided by Ulrike Fiedler (Scil Proteins). Plasmid pCANTAB5-TSH, a secretion construct for a single-chain Fv fragment against thyroid-stimulating hormone (scFv-TSH) containing a gene 3 signal sequence and the lac promoter, was provided by Alfred Engel (Roche Diagnostics). scFv-TSH is directed against the thyroid stimulating hormone.
Antibody for insulin was a gift of Konrad Kürzinger (Roche Diagnostics), and Hsp25 and DnaJ antibodies were kindly provided by Johannes Buchner (University of Munich, Munich, Germany) and Maciej Zylicz (University of Gdansk, Gdansk, Poland), respectively.
Chemicals were of analytical grade and purchased from Sigma, Roth GmbH, AppliChem GmbH, Biomol GmbH, Fluka, or ICN Pharmaceuticals. Cultivation medium substances were obtained from Becton Dickinson. Other substances and kits were bought from the suppliers as stated below.
Construction of expression plasmids.
For cloning into pET20b(+) (Novagen), the coding sequence of rPA was PCR amplified from pA27fd7 (23) and inserted into pET20b(+). In this construct the second amino acid of rPA (Ser) is replaced by Ala. Proinsulin-encoding DNA was amplified by PCR from plasmid pRK-5-proinsulin (34) and ligated into pET20b(+). This vector mediates secretion via the pelB signal sequence. By QuikChange Mutagenesis (Stratagene), two surplus codons between signal sequence and proinsulin were removed. For coexpression of chaperones and model proteins, a two-plasmid expression system was chosen. After testing secretion of DnaJ and Hsp25, the genes were PCR cloned into pIN III ompA3 (16) and the coding sequences of DnaJ and Hsp25 with the regulatory sequences were recloned into plasmid pUBS520 (6), which bears the p15A replication origin and kanamycin resistance. This vector also carries the dnaY gene encoding the tRNA for the arginine codons AGA and AGG, which are rare in E. coli and thus often limit expression of genes with these codons (the gene for rPA contains seven of these rare arginine codons). The two-plasmid cosecretion system thus includes a vector for the secretion of the disulfide-bridged model protein on the ColE1-based pET vector and the chaperone on a p15A-based plasmid, which also carries the dnaY gene. Both the gene for the model protein and the chaperone were induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG). Via PCR the coding and regulatory regions for scFv-ox were amplified from pHEN-scFv-ox (12) and cloned into pUBS520.
Cultivation of E. coli BL21(DE3) and rPA assay.
In order to test rPA activity, cells were grown in Luria-Bertani medium at 24°C, induced with 1 mM IPTG at mid-log phase, and cultivated for a further 21 h. Medium additives (reduced GSH, 0 to 10 mM, and l-arginine, 0 to 0.4 M; formamide, 0 to 1 M; methylformamide, 0 to 1 M; acetamide, 0 to 1 M; methylurea, 0 to 1 M; or ethylurea, 0 to 1 M) were supplemented at the time of induction. After determination of the optical density at 600 nm (OD600) (Pharmacia Ultrospec 3000; Pharmacia Biotech), 2-ml samples were collected and pelleted. For preparation of periplasmic extracts, the protocol described in reference 18 was downscaled to milliliter volumes. The soluble periplasmic fraction was assayed for rPA activity. For control purposes, cultures of E. coli BL21(DE3), transformed with pET20b(+) and pUBS520, were treated identically. Determination of functional rPA on microplates was performed according to a modified previously described protocol (38) with purified rPA as a standard. The concentration of rPA in the cellular extracts was determined by plotting the extinction against the square of the reaction time. The slope of a linear regression of this plot is directly proportional to the amount of rPA in the assay. The native state of rPA in extracts was tested in parallel assays after addition of 20 μl of 0.6-mg/ml fibrinogen fragments. The slope of the plot after addition of fibrinogen fragments divided by the slope in the absence of fibrinogen fragments defines the stimulation factor (23).
To obtain quantitative values of the influence of cellular components on the activity of rPA, purified rPA was diluted into periplasmic extracts of E. coli BL21(DE3)/pET20b(+)/pUBS520. The measured quenching of rPA activity (1.5-fold) was used as a correction factor for determinations of rPA activities. All determinations of rPA concentrations in the cellular extracts were normalized to 1 ml of cells at an OD600 of 1. Concentrations of l-arginine and glutathione in the cultivation medium were determined with diluted medium sample assays according to the methods described in references 13 and 17, respectively.
Expression studies and determination of scFv-TSH.
E. coli BL21(DE3) transformed with pCANTAB5-TSH and pUBS520 was cultivated as described above in the presence of the indicated concentrations of reduced glutathione and l-arginine. Expression of scFv-TSH was determined via indirect enzyme-linked immunosorbent assay (ELISA) measurements (8) and detected using the ImmunoPure TMB substrate system (Pierce, Rockford, Ill.). The values of cell extracts without scFv-TSH were used for correction of background signal. scFv-TSH purified with the RPAS system (Amersham Pharmacia Biotech) was used as a standard.
Limited proteolysis of periplasmic DnaJ.
E. coli XL1-blue cells, transformed with pIN III ompA3-dnaJ, secreting DnaJ, and N4830/pPL-dnaJ-23 cells, overexpressing DnaJ in the cytosol, were grown to mid-log phase and harvested 3 h after induction by centrifugation. The equivalent of 2 ml of bacteria of an OD600 of 1 were converted to spheroplasts according to the method described in reference 37. The spheroplasts were resuspended in 30 μl of 50 mM Tris-HCl (pH 8.0)–100 mM NaCl with or without 0.1% Triton X-100. For limited proteolysis, aliquots of these fractions were incubated with 25 μg of trypsin per ml. Proteolysis was stopped by the addition of 20 M excess soybean trypsin inhibitor. In a control experiment, 0.1 mg of purified DnaJ per ml was incubated with 6 μg of trypsin per ml and treated in the same way as the spheroplast samples. The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed via Western blotting.
Cosecretion of proinsulin and DnaJ.
E. coli BL21(DE3) cells harboring plasmids for cosecretion of proinsulin and chaperones were grown in Luria-Bertani medium at 25°C. One millimolar IPTG was added at an OD500 of 1, and cells were harvested 6 h after induction. Soluble periplasmic protein was released by osmotic shock according to the method described in reference 22. For analysis and quantification of native proinsulin, an ELISA that specifically detects native (pro)insulin (Enzymun Test Insulin; Roche Diagnostics) was carried out.
RESULTS
Yields of secreted rPA and scFv-TSH in the presence of medium additives.
Tissue-type plasminogen activator (tPA) converts the zymogen plasminogen to plasmin, a serine protease that degrades fibrin networks in thrombi (9). The tPA variant rPA contains nine disulfide bridges and aggregates upon cytosolic synthesis in inclusion bodies. In vitro refolding of rPA from inclusion body material is routinely performed (A. Stern, U. Kohnert, R. Rudolph, S. Fischer, and U. Martin, June 1993, U.S. patent application 5,223,256). As the native state of rPA can easily be assessed, it was chosen as a model protein for expression in the native conformation in the periplasm. For secretion of rPA, plasmid vector pET20b(+) (Novagen), containing the signal sequence of PelB (pectate lyase from Erwinia carotovora), was used. To determine the amount of functional rPA, protease activity was assayed according to the method described in reference 38 with minor modifications (see Materials and Methods).
The characteristic feature of rPA—the stimulation of the protease activity by fibrinogen fragments (23)—was used as an indication of the native state of the two-domain protein. rPA with correctly folded kringle and protease domains possessed proteolytic activity which could be stimulated by a factor of ca. 25 to 35 by fibrinogen fragments (23; Stern et al., October 1992, U.S. patent application 5,223,256). We first verified that stimulation by fibrinogen fragments was not affected when purified rPA was incubated with periplasmic extracts (data not shown), a prerequisite for testing native expression of the protease in the periplasm.
Periplasmic extracts were prepared from cells secreting rPA and control cells. Extracts from the control culture showed only low background protease activity which was not affected by fibrinogen fragments (data not shown). In the strain secreting rPA, 0.023 ng of active rPA per ml was determined in periplasmic extracts. As the activity could be stimulated 35-fold by fibrinogen fragments, rPA was assumed to be responsible for proteolytic activity.
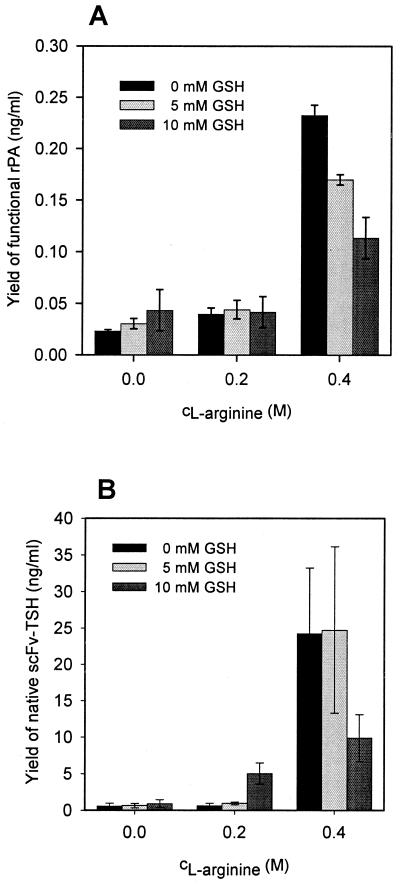
The nine disulfide bridges of rPA are essential for the native conformation and consequently the activity of the protease. To facilitate reshuffling of incorrect disulfide bonds, GSH was added to the culture medium (39; R. Glockshuber, M. Wunderlich, A. Skerra, and R. Rudolph European patent application EPO 510 658) (Fig. 1A). The addition of 5 or 10 mM GSH resulted in a slight increase of protease activity. These results indicate that disulfide shuffling is enhanced when reducing reagents are added to the culture medium.
FIG. 1.
Increases of the yields of secreted rPA and scFv-TSH upon addition of l-arginine to the cultivation medium. (A) Yields of native rPA in the periplasm of E. coli BL21(DE3)/pET20b(+)-rPA after cultivation (24°C) in the presence of the indicated concentrations of reduced GSH and l-arginine. Active rPA was determined as described previously (38). (B) Yields of native single-chain Fv (scFv-TSH) in the periplasm of E. coli BL21(DE3)/pCANTAB5-TSH after cultivation (24°C) in the presence of the indicated concentrations of l-arginine and GSH. Native scFv-TSH was determined using ELISA measurements. Mean values of at least three shake flask cultures and standard deviations are indicated.
l-Arginine is known to effectively improve the yield of native protein during in vitro refolding from inclusion body material (10, 25, 30). Thus, the in vivo effect of l-arginine on the yield of secreted native rPA was investigated. In the absence of GSH and at a concentration of 0.4 M l-arginine, the yield of active plasminogen activator increased about 10-fold (Fig. 1A). Interestingly, in the presence of l-arginine, the addition of GSH had no beneficial effect on the yield of rPA.
The yield of a second secreted model protein, a scFv-TSH (21), was also increased by the presence of l-arginine and reduced GSH. Addition of 0.4 M l-arginine led to the highest yield of native scFv-TSH (Fig. 1B), a 37-fold increase over the control expression. Though absolute yields with 25 ng/ml appear moderate, the results show that l-arginine is a compound that can be used to optimize folding of secreted proteins. A portion of the secreted scFv-TSH was detected in the medium supernatant, and the addition of 0.4 M l-arginine moderately increased the yield of scFv in the supernatant (data not shown). Concentrations of l-arginine higher than 0.4 M inhibited bacterial growth almost completely and led to reduced yields of scFv-TSH and rPA (data not shown). Taken together, these results demonstrate that in vivo structure formation of the two tested model proteins was significantly stimulated by the addition to the growth medium of l-arginine and, to a lesser extent, reduced GSH.
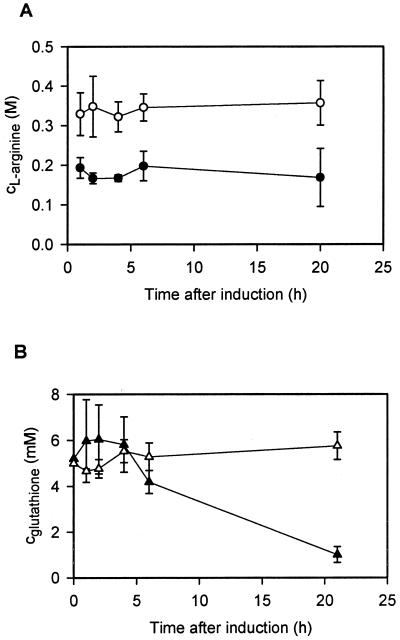
To determine whether GSH or l-arginine would be stably maintained during cell growth, the concentrations of GSH and l-arginine were determined by enzymatic analysis after extended culturing. Concentrations of l-arginine and total GSH in the culture medium remained constant during the entire culture process (20 h; Fig. 2A and B). However, the ratio of reduced GSH to oxidized GSH changed dramatically over 20 h at 24°C. During the first 5 h of cultivation almost all GSH was maintained in the reduced state. This ratio shifted to ca. 20% reduced GSH and 80% oxidized GSH after 20 h of cultivation, due to air oxidation of the thiol groups (Fig. 2B). These data confirm that a disulfide-shuffling system consisting of reduced and oxidized GSH can be maintained for 20 h during fermentation of the E. coli cells under aerobic conditions.
FIG. 2.
Determination of the concentrations of l-arginine and GSH in the medium of E. coli BL21(DE3) after prolonged cultivation according to the methods described in references 13 and 17. (A) Determined l-arginine concentrations in the cultivation medium at the indicated time points after induction. At the time of induction l-arginine was added to the culture medium to final concentrations of either 0.2 M (filled circles) or 0.4 M (open circles). (B) Concentrations of GSH (closed triangles) and total GSH (GSH+GSSG; open triangles) in the cultivation medium after addition of 5 mM GSH.
Construction of a two-plasmid system for cosecretion of DnaJ and Hsp25.
In order to further increase the yield of secreted proteins, cosecretion of ATP-independent chaperones was tested. In a first experiment, the cosecretion of DnaJ was analyzed. This protein belongs to the Hsp70 (DnaK) system of E. coli and is known to suppress aggregation of nonnative proteins also in the absence of Hsp70 (7, 24, 31).
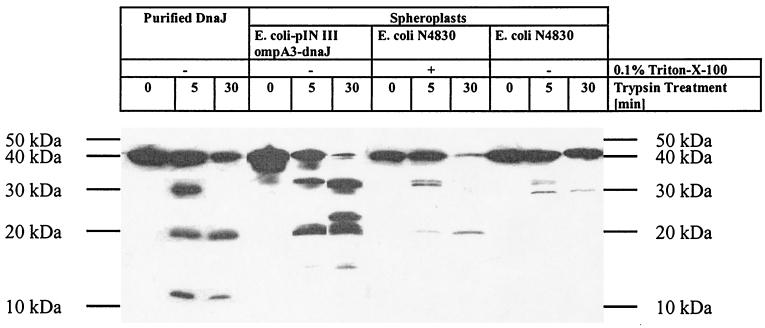
DnaJ, secreted by fusion to the OmpA signal peptide, was detected in the membrane fraction of periplasmic proteins (data not shown). This was expected, as DnaJ is known to associate with membranes (2). To confirm the native conformation of secreted DnaJ, limited proteolysis experiments were performed. Spheroplasts of E. coli XL1-blue/pIN III-dnaJ, secreting the chaperone, and N4830/pPL-dnaJ-23 (41), a control strain which overexpresses DnaJ in the cytoplasm, were prepared (37). Both spheroplast preparations were subjected to limited proteolysis with trypsin (Fig. 3). In intact spheroplasts, intracellular DnaJ of strain N4830 was completely protected from trypsin digestion, whereas secreted DnaJ, expressed in strain BL21(DE3)/pIN III-dnaJ, was susceptible to proteolysis (Fig. 3). The defined products of partial trypsinolysis were similar in size to those obtained by digestion of purified native DnaJ, a fact that suggests the native conformation of secreted DnaJ.
FIG. 3.
Limited proteolysis for determination of native DnaJ. Spheroplasts were incubated with 25 μg of trypsin per ml and purified DnaJ was incubated with 6 μg of trypsin per ml for the indicated times. Proteolysis products of DnaJ and its fragments were detected with a rabbit anti-DnaJ antibody and subsequently with a donkey anti-rabbit immunoglobulin G horseradish peroxidase conjugate (Amersham Pharmacia Biotech).
The effect of a second cosecreted chaperone, murine Hsp25 (14, 19), on the yield of recombinant proteins in the periplasm was investigated. Like DnaJ, Hsp25 has been demonstrated to prevent aggregation of nonnative proteins (11). Translocation of Hsp25 into the periplasm was also mediated by the OmpA signal peptide. Expression and secretion of Hsp25 were confirmed by Western blotting experiments (data not shown).
Yields of native rPA and proinsulin in the periplasm of E. coli upon cosecretion of DnaJ and Hsp25.
Cosecretion of DnaJ yielded a fivefold increase of functional rPA in periplasmic extracts compared to what was observed with the clone without cosecretion. Upon addition of fibrinogen fragments, protease activity was stimulated 35-fold, indicating the native conformation of the secreted rPA. Under optimal expression conditions (0.4 M l-arginine and 5 mM GSH), the yield increased 170-fold (Table 1).
TABLE 1.
Influences of cosecreted proteins and medium additive l-arginine on yield of native rPAa
| Cosecreted protein | Without l-arginine
|
With 0.2 M l-arginine
|
With 0.4 M l-arginine
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| rPAb (ng/ml) | Stimulation factorc | OD600d | rPA (ng/ml) | Stimulation factor | OD600 | rPA (ng/ml) | Stimulation factor | OD600 | |
| None | 0.030 ± 0.001 | 29 | 4.85 | 0.044 ± 0.090 | 20 | 5.05 | 0.170 ± 0.005 | 23 | 3.47 |
| DnaJ | 0.197 ± 0.019 | 29 | 4.31 | 0.730 ± 0.150 | 27 | 5.05 | 3.978 ± 1.000 | 18 | 2.12 |
| Hsp25 | 0.053 ± 0.002 | 27 | 4.81 | 0.140 ± 0.001 | 17 | 4.56 | 2.850 ± 0.214 | 17 | 1.44 |
| scFv-ox | 0.041 ± 0.003 | 13 | 4.23 | 0.144 ± 0.047 | 8 | 3.76 | 0.713 ± 0.113 | 10 | 1.47 |
Cultivations were carried out in the presence of 5 mM GSH; with control cells (no medium additive, no cosecretion), 0.023 ng of active rPA per ml was obtained.
Values for rPA are means ± standard deviations for at least three shake flask culture experiments.
Stimulation factor, stimulation of rPA activity by addition of fibrinogen fragments to the rPA assay (see Materials and Methods); stimulation factors of 25 to 35 are generally considered to indicate native rPA (23; Stern et al., U.S. patent application 5,223,256).
OD600 values were determined at the time of harvest.
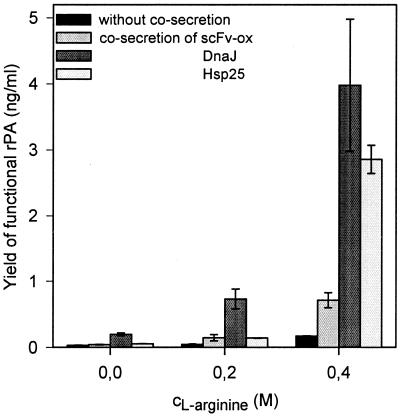
Similarly, cosecretion of Hsp25 increased the yield of native rPA in the periplasm ca. twofold. Under optimal expression conditions, i.e., 5 mM GSH and 0.4 M l-arginine (optimization data not shown), cosecretion of Hsp25 resulted in a 120-fold increase of active plasminogen activator (Table 1 and Fig. 4) compared to what was observed for the strain which did not secrete Hsp25 cultivated in the absence of medium additives.
FIG. 4.
Effects of cosecreted chaperones and scFv-ox (control) on the yields of native rPA at different concentrations of l-arginine. Cells were grown in the presence of 5 mM GSH. Active rPA was determined as described previously (38). Mean values of at least three shake flask culture experiments and standard deviations are indicated.
The fact that both DnaJ and Hsp25 enhanced the yield of native rPA could be due either to the chaperone activities of these proteins or to indirect effects caused by the secretion of a second heterologous protein to the periplasmic space. To test the latter possibility, the effect of cosecretion of scFv-ox (12), a protein which lacks chaperone function, on the yield of native rPA was investigated. Under optimal conditions, cosecretion of scFv-ox yielded a fourfold increase of native rPA compared to the situation when rPA was expressed alone. However, the very low stimulation factor of 10 (Table 1) indicated incomplete folding of rPA. Thus, the huge increase of native rPA upon cosecretion of chaperones is very likely caused by the chaperoning activities of these proteins. Western blot experiments confirmed that the levels of DnaJ and Hsp25 remained constant at the concentrations of l-arginine and GSH tested here. In contrast, increases in scFv-ox levels were observed with increasing concentrations of l-arginine (data not shown).
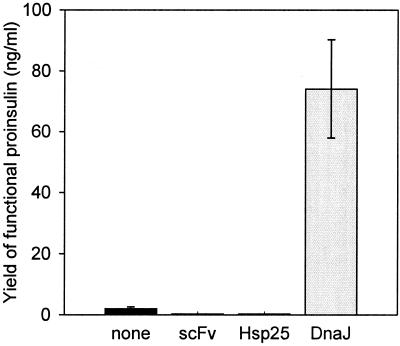
As a second model protein for testing the effects of cosecreted chaperones, proinsulin was secreted to the periplasm. The amounts of native proinsulin in periplasmic fractions were assayed by ELISA using an antibody recognizing selectively native insulin. In the absence of cosecreted chaperones, 2 ng of native proinsulin per ml was detected (Fig. 5). When Hsp25 was cosecreted with proinsulin, no native proinsulin was detectable in the periplasm. In contrast, coexpression of DnaJ resulted in 74 ng of native proinsulin per ml, corresponding to a 37-fold increase of the yield. Upon cosecretion of the negative control, scFv-ox, only 0.3 ng of native proinsulin per ml was detected. Surprisingly, in this case, the presence of 0.4 M l-arginine decreased the amount of native proinsulin to 50% of that of cultivations in the absence of l-arginine (data not shown). With the third model protein, scFv-TSH, cosecretion of DnaJ or Hsp25 did not increase the yield of native scFv-TSH in the periplasm.
FIG. 5.
Yields of proinsulin after cosecretion of DnaJ, Hsp25, and scFv-ox (control). Proinsulin was determined by ELISA (see Materials and Methods). Values represent mean values of at least three shake flask culture experiments. Standard deviations are indicated.
Influence of low-molecular-size additives on the yield of secreted rPA.
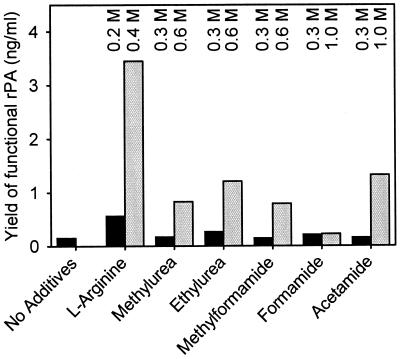
In in vitro refolding experiments, several low-molecular-size additives, especially derivatives of formamide or urea, proved useful for increasing the yield of native rPA (29). We therefore examined the effects of formamide, methylformamide, acetamide, methylurea, and ethylurea on the yield of native rPA. Bacteria were able to grow in media which contained concentrations of up to 1 M formamide or acetamide but only up to 0.6 M methylformamide, methylurea, or ethylurea. The yield of native rPA was tested with the strain E. coli BL21(DE3)/pUBS520-dnaJ/pET20b(+)-rPA cosecreting DnaJ upon cultivation in the presence of these additives and 5 mM GSH. Although l-arginine, which was used for comparison, proved to be the most effective additive, acetamide or ethylurea also had significant beneficial effects on the yield of rPA (Fig. 6).
FIG. 6.
Yields of native rPA in E. coli secreting DnaJ in the presence of 5 mM GSH and low-molecular-size additives at the indicated concentrations. Active rPA was determined as described previously (38).
DISCUSSION
Native expression of disulfide-bridged proteins in prokaryotic host cells remains a scientific challenge (32). Though approaches have been taken to change the cytosolic milieu of E. coli to more oxidizing conditions to allow intracellular formation of disulfide bonds (4, 35), expression of disulfide-bridged proteins in the periplasmic space is an alternative strategy that has not been fully exploited. A major disadvantage of the periplasm as a folding compartment for proteins with multiple disulfide bonds is the strong oxidant DsbA. DsbA has been shown to introduce disulfide bonds into translocating polypeptides as soon as two cysteines have emerged into the periplasm (20, 33). Although disulfide isomerases exist in the periplasm, their function is obviously insufficient to correct wrongly paired cysteines of proteins containing multiple disulfide bonds of nonlinear connectivities (3, 26). The consequence is usually inclusion body formation of these misfolded proteins in the periplasm (15).
Our approach to overcome these problems was to suppress inclusion body formation in the periplasm by adding disulfide-reshuffling reagents and substances known to stabilize folding intermediates to the cultivation medium. Also, the effects of cosecreted ATP-independent molecular chaperones DnaJ and Hsp25, which have been shown to suppress aggregation of nonnative proteins in vitro (11, 31), were analyzed.
We were able to increase the yield of native rPA in the periplasm of E. coli up to 170-fold upon cosecretion of DnaJ and 125-fold upon cosecretion of Hsp25. This huge increase is, to our understanding, mainly due to a synergistic effect of the respective cosecreted chaperone and medium additives on the folding of rPA, as cosecretion of DnaJ or Hsp25 in the absence of medium additives gave rise to ca. fivefold or twofold increases, respectively, of rPA (Table 1). Improvement of the periplasm as an expression compartment for disulfide-bridged proteins has been reported earlier (27). For example, overexpression of DsbC considerably increased the yield of full-length tPA (27). Unfortunately, the yields of functional proteins published in reference 27 and those of our studies cannot be compared, as a variant of tPA has been used in the latter; furthermore, the data of the former study result from high cell density fermentations, whereas here shake flask cultures were used.
The fact that 5 mM GSH was optimal for the folding of rPA under almost all tested conditions confirms previous results that demonstrate that addition of GSH improved folding of an α-amylase-trypsin inhibitor in the periplasm (39).
With proinsulin, a 37-fold increase in the yield of native protein was obtained by cosecretion of DnaJ. Proinsulin secreted to the periplasm has been reported to be degraded by E. coli proteases (36). The presence of DnaJ may prevent the action of proteases and promote native structure formation. Cosecretion of Hsp25 or addition of l-arginine, however, did not improve the yield of native protein.
The increases of native rPA and proinsulin upon cosecretion of DnaJ and Hsp25 are likely to be due to the specific chaperoning activities of these proteins. Our interpretation that we are dealing with specific chaperone functions in cases where we observe increased amounts of folded proteins upon cosecretion of the chaperones is supported by the following observations. (i) If cosecretion of a heterologous protein should unspecifically enhance folding of rPA and proinsulin, cosecreted scFv-ox should have increased the yield. Furthermore, cosecretion of scFv-ox did not result in efficient stimulation of rPA activity by fibrinogen fragments by factors known for the completely folded protease. (ii) The chaperone requirement of a given protein is known to be relatively specific. In accordance with this notion, cosecretion of Hsp25 proved not to be effective in the case of proinsulin and neither DnaJ nor Hsp25 increased the yield of scFv-TSH. Thus, we propose that the beneficial effects of the secreted chaperones reflect the folding activities of DnaJ and Hsp25.
Besides l-arginine, a series of low-molecular-size reagents added to the cultivation medium increased the yield of active rPA. We limited our investigations to additives for which effects on the in vitro refolding of full-length tissue-type plasminogen activator had been demonstrated (29). Although l-arginine was the most effective compound in the case of rPA, other low-molecular-size additives may prove most efficient with different proteins. The effects of the tested additives on the yield of rPA are comparable to those obtained by in vitro refolding experiments (29). Therefore, we consider the effects to be due to the folding-enhancing activities of the compounds and not to their secondary osmolytic effects on cells.
Our results demonstrate that cosecretion of ATP-independent chaperones and the use of low-molecular-size medium additives to the culture medium can dramatically increase the yield of native eukaryotic proteins with complex disulfide patterns in the periplasm of E. coli. The mechanism by which the chaperones act in the periplasm remains unclear and needs further investigation. Still, this study may open new avenues for the production of disulfide-bridged proteins in their native conformation in prokaryotic organisms.
ACKNOWLEDGMENTS
This work was supported by a grant of the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (BMBF) of Germany. J.W. was supported by a grant of the Graduiertenförderung des Landes Sachsen-Anhalt and a project of the European Commission (project no. EU BIO4-CT96-0436). The support of the Fonds der Chemischen Industrie is gratefully acknowledged.
We thank Jason Smith for critically reading the manuscript.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidmann J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Cambridge, United Kingdom: John Wiley & Sons; 1987. –1997. [Google Scholar]
- 2.Bardwell J C A, Tilly K, Craig E, King J, Zylicz M, Georgopoulos C. The nucleotide sequence of the Escherichia coli K12 dnaj+ gene. J Biol Chem. 1986;261:1782–1785. [PubMed] [Google Scholar]
- 3.Bardwell J C A. Building bridges: disulfide bond formation in the cell. Mol Microbiol. 1994;14:199–205. doi: 10.1111/j.1365-2958.1994.tb01281.x. [DOI] [PubMed] [Google Scholar]
- 4.Bessette P H, Aslund F, Beckwith J, Georgiou G. Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc Natl Acad Sci USA. 1999;96:13703–13708. doi: 10.1073/pnas.96.24.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessette P H, Cotto J J, Gilbert H F, Georgiou G. In vivo and in vitro function of the Escherichia coli periplasmic cysteine oxidoreductase DsbG. J Biol Chem. 1999;274:7784–7792. doi: 10.1074/jbc.274.12.7784. [DOI] [PubMed] [Google Scholar]
- 6.Brinkmann U, Mattes R E, Buckel P. High-level expression of recombinant genes in Escherichia coli is dependent on the availability of the dnaY gene product. Gene. 1989;85:109–114. doi: 10.1016/0378-1119(89)90470-8. [DOI] [PubMed] [Google Scholar]
- 7.Bukau B, Horwich A. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 8.Coligan J E, Dunn B M, Ploegh H L, Speicher D W, Wingfield P T, editors. Current protocols in protein science. Washington, D.C.: John Wiley & Sons; 1995. –1997. [Google Scholar]
- 9.Collen D, Lijnen H R. Molecular basis of fibrinolysis, as relevant for thrombolytic therapy. Thromb Haemostasis. 1995;74:167–171. [PubMed] [Google Scholar]
- 10.De Bernandez Clark E, Schwarz E, Rudolph R. Inhibition of aggregation side reactions during in vitro protein folding. Methods Enzymol. 1999;309:217–236. doi: 10.1016/s0076-6879(99)09017-5. [DOI] [PubMed] [Google Scholar]
- 11.Ehrnsperger M, Gräber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiedler U, Conrad U. High-level production and long-term storage of engineered antibodies in transgenic tobacco seeds. Bio/Technology. 1995;13:1090–1093. doi: 10.1038/nbt1095-1090. [DOI] [PubMed] [Google Scholar]
- 13.Gäde G. l-Arginine and l-arginine phosphate. In: Bergmeyer H U, editor. Methods of enzymatic analysis. 3rd ed. VIII. 1989. pp. 425–432. (Metabolites 3). VCH, Weinheim, Germany. [Google Scholar]
- 14.Gaestel M, Gross B, Benndorf R, Strauβ M, Schunk W-H, Kraft R, Otto A, Böhm H, Stahl J, Drabsch H, Bielka H. Molecular cloning, sequencing and expression in Escherichia coli of the 25-kDa growth-related protein of Ehrlich ascites tumor and its homology to mammalian stress proteins. Eur J Biochem. 1989;179:209–213. doi: 10.1111/j.1432-1033.1989.tb14542.x. [DOI] [PubMed] [Google Scholar]
- 15.Georgiou G, Telford J N, Shuler M L, Wilson D B. Localization of inclusion bodies in Escherichia coli overproducing β-lactamase or alkaline phosphatase. Appl Environ Microbiol. 1986;52:1157–1161. doi: 10.1128/aem.52.5.1157-1161.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghayreb J, Kimura H, Takahara M, Hsiung H, Masui Y, Inouye M. Secretion cloning vectors in Escherichia coli. EMBO J. 1984;3:2437–2442. doi: 10.1002/j.1460-2075.1984.tb02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffith O W. Glutathione and glutathione disulphide. In: Bergmeyer H U, editor. Methods of enzymatic analysis. 3rd ed. VIII. 1989. pp. 521–529. (Metabolites 3). VCH, Weinheim, Germany. [Google Scholar]
- 18.Jacobi A, Huber-Wunderlich M, Hennecke J, Glockshuber R. Elimination of all charged residues in the vicinity of the active-site helix of the disulfide oxidoreductase DsbA. J Biol Chem. 1997;272:21692–21699. doi: 10.1074/jbc.272.35.21692. [DOI] [PubMed] [Google Scholar]
- 19.Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- 20.Joly J C, Swartz J R. In vitro and in vivo redox states of the Escherichia coli periplasmic oxidoreductases DsbA and DsbC. Biochemistry. 1997;36:10067–10072. doi: 10.1021/bi9707739. [DOI] [PubMed] [Google Scholar]
- 21.Kaluza, B., and H. Lenz. March 1997. Diagnostic test using chimeric antibodies. U.S. patent 5,614,367.
- 22.Kang Y, Yoon J-W. Effect of modification of connecting peptide of proinsulin on its export. J Biotechnol. 1994;36:45–54. doi: 10.1016/0168-1656(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 23.Kohnert U, Rudolph R, Verheijen J H, Weening-Verhoeff E J D, Stern A, Opitz U, Martin U, Lill H, Prinz H, Lechner M, Kresse G-B, Buckel P, Fischer S. Biochemical properties of the kringle-2 and protease domains are maintained in the refolded t-PA deletion variant BM 06.022. Protein Eng. 1992;5:93–100. doi: 10.1093/protein/5.1.93. [DOI] [PubMed] [Google Scholar]
- 24.Langer T, Lu C, Echols H, Flanagan J, Hayer M K, Hartl F U. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992;356:683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- 25.Lilie H, Schwarz E, Rudolph R. Advances in refolding of proteins produced in E. coli. Curr Opin Biotechnol. 1998;9:497–501. doi: 10.1016/s0958-1669(98)80035-9. [DOI] [PubMed] [Google Scholar]
- 26.Missiakas D, Raina S. Protein folding in the bacterial periplasm. J Bacteriol. 1997;179:2465–2471. doi: 10.1128/jb.179.8.2465-2471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu J, Swartz J R, Georgiou G. Expression of active human tissue-type plasminogen activator in Escherichia coli. Appl Environ Microbiol. 1998;64:4891–4896. doi: 10.1128/aem.64.12.4891-4896.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rietsch A, Beckwith J. The genetics of disulfide bond metabolism. Annu Rev Genet. 1998;32:163–184. doi: 10.1146/annurev.genet.32.1.163. [DOI] [PubMed] [Google Scholar]
- 29.Rudolph, R., S. Fischer, and R. Mattes. January 1997. Process for the activating of gene-technologically produced, heterologous, disulphide bridge-containing eukaryotic proteins after expression in prokaryotes. U.S. patent 5,593,865.
- 30.Rudolph R, Lilie H. In vitro folding of inclusion body proteins. FASEB J. 1996;10:49–56. [PubMed] [Google Scholar]
- 31.Schröder H, Langer T, Hartl F U, Bukau B. DnaK, DnaJ and GrpE form a cellular chaperone machinery capable of repairing heat-induced protein damage. EMBO J. 1993;12:4137–4144. doi: 10.1002/j.1460-2075.1993.tb06097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarz E, Lilie H, Rudolph R. The effect of molecular chaperones on in vivo and in vitro folding processes. Biol Chem. 1996;377:411–416. [PubMed] [Google Scholar]
- 33.Sone M, Akiyama Y, Ito K. Differential in vivo roles played by DsbA and DsbC in the formation of protein disulfide bonds. J Biol Chem. 1997;272:10349–10352. doi: 10.1074/jbc.272.16.10349. [DOI] [PubMed] [Google Scholar]
- 34.Stahl S J, Christiansen L. Selection for signal sequence mutations that enhance production of secreted human proinsulin by Escherichia coli. Gene. 1988;71:147–156. doi: 10.1016/0378-1119(88)90086-8. [DOI] [PubMed] [Google Scholar]
- 35.Stewart E J, Aslund F, Beckwith J. Disulfide bond formation in the Escherichia coli cytoplasm: an in vivo role reversal for the thioredoxins. EMBO J. 1998;17:5543–5550. doi: 10.1093/emboj/17.19.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Talmadge K, Gilbert W. Cellular location affects protein stability in Escherichia coli. Proc Natl Acad Sci USA. 1982;79:1830–1833. doi: 10.1073/pnas.79.6.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorstenson Y, Zhang Y, Olson P S, Mascarenhas D. Leaderless polypeptides efficiently extracted from whole cells by osmotic shock. J Bacteriol. 1997;179:5333–5339. doi: 10.1128/jb.179.17.5333-5339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verheijen J H, Mullaart E, Chang G T G, Kluft C, Wijngaards G A. A simple, sensitive spectrophotometric assay for extrinsic (tissue-type) plasminogen activator applicable to measurements in plasma. Thromb Haemostasis. 1982;48:266–269. [PubMed] [Google Scholar]
- 39.Wunderlich M, Glockshuber R. In vivo control of redox potential during protein folding catalyzed by bacterial protein disulfide-isomerase (DsbA) J Biol Chem. 1993;268:24547–24550. [PubMed] [Google Scholar]
- 40.Zapun A, Missiakas D, Raina S, Creighton T E. Structural and functional characterization of DsbC, a protein involved in disulfide bond formation in Escherichia coli. Biochemistry. 1995;34:5075–5089. doi: 10.1021/bi00015a019. [DOI] [PubMed] [Google Scholar]
- 41.Zylicz M, Yamamoto T, McKittrick N, Sell S, Georgopoulos C. Purification and properties of the dnaJ replication protein of Escherichia coli. J Biol Chem. 1985;260:7591–7598. [PubMed] [Google Scholar]